- 1Department of Psychiatry, Wonkwang University Hospital, Iksan, Republic of Korea
- 2Department of Psychiatry, Soonchunhyang University Cheonan Hospital, Cheonan, Republic of Korea
- 3Department of Applied Artificial Intelligence, Hanyang University, Ansan, Republic of Korea
Introduction: Non-suicidal self-injury (NSSI) is a serious concern in adolescents and is associated with impairments in impulsivity and social functioning. However, the underlying neural mechanisms remain unclear. This study aimed to examine inhibitory control and its association with depressive symptoms and interpersonal distress in adolescents with NSSI using event-related potentials (ERPs) and source-level analysis.
Methods: A total of 51 adolescents with NSSI and 50 HC were recruited. Psychological characteristics were assessed using standardized scales including the Interpersonal Needs Questionnaire (INQ) and Short UPPS-P Impulsivity Scale (SUPPS-P). EEG were recorded during a go/no-go task to measure P3 amplitudes. Source analysis was performed to localize the neural activity. Group differences were analyzed using RMANOVA, followed by Pearson correlation and mediation analyses to evaluate the relationships among the variables.
Results: The NSSI group showed significantly lower accuracy than HCs. The interaction between group and electrode site was significant (p = .032, ηp² = .010), indicating spatially specific reductions in no-go P3 amplitude in the NSSI group. No-go P3 at Fz was negatively correlated with depressive symptoms and interpersonal distress. Source-level analysis revealed marginally reduced activation in the right superior frontal gyrus in the NSSI group but diminished after adjusting for depressive symptoms. Mediation analysis indicated that depressive symptoms significantly mediated the relationship between both neural indices and interpersonal distress.
Conclusions: Adolescents with NSSI exhibit impaired inhibitory control that is associated with depressive symptoms and social distress. These findings highlight the role of affective dysregulation in linking cognitive control deficits to interpersonal functioning in NSSI.
1 Introduction
Non-suicidal self-injury (NSSI), defined as the direct and deliberate destruction of one’s own bodily tissue in the absence of suicidal intent, has recently increased among young people, raising significant alarm among psychiatric researchers (1, 2). This behavior encompasses a range of self-inflicted actions such as cutting, burning, or hitting oneself, and is primarily used as a maladaptive coping mechanism to relieve emotional distress or regulate emotional dysregulation (3).
Although NSSI is defined by the absence of suicidal intent, its clinical significance should not be underestimated, as it is strongly associated with elevated risks of suicidal ideation and attempts (4, 5). These findings highlight that NSSI is not a benign behavior, but rather a marker of severe emotional distress, interpersonal dysfunction, and frequent psychiatric comorbidities such as depression and anxiety (6). These findings emphasize the need for early identification and a deeper understanding of underlying mechanisms to inform effective prevention strategies.
Theoretical models suggest that NSSI is driven by multiple, interacting psychological processes. One prominent framework is the Interpersonal-Psychological Theory of Suicide (IPTS), which proposes that perceived burdensomeness (PB) and thwarted belongingness (TB) increase the risk of self-injurious thoughts and behaviors (7). PB, defined as the perception of being a burden to others and being worth more dead than alive, and TB, defined as a feeling of disconnection from others (8), are key components of the IPTS. Recent studies have suggested a relationship between interpersonal distress and NSSI among adolescents (9–11). Adolescents who report greater interpersonal distress are more likely to engage in self-injury as a maladaptive coping mechanism to alleviate emotional pain or seek social connection (11). Therefore, interventions based on the IPTS are suggested to improve the symptoms of NSSI in adolescence.
In addition to interpersonal difficulties, deficits in inhibitory control have been widely implicated in the emergence and maintenance of NSSI behaviors. Adolescents engaging in NSSI often exhibit heightened impulsivity and difficulty regulating their actions in response to emotionally charged situations (12). Neurocognitive studies using event-related potentials (ERPs) point to the no-go P3 as a fronto-cingulate marker of conflict monitoring and response suppression. This component—and thus the capacity to allocate attentional and control resources under changing demands—has been shown to be attenuated in both impulsive and affective disorder (13, 14). Studies have shown that individuals with NSSI exhibit altered brain activity patterns, such as reduced no-go P3 amplitudes, which are associated with impaired cognitive control and increased impulsivity (15).
Furthermore, depressive symptoms are not only prevalent among adolescents with NSSI, but are also significantly associated with impairments in inhibitory control (16, 17). Individuals with depressive mood demonstrate attenuated no-go P3 amplitudes and longer no-go reaction times in the go/no-go task, suggesting deficits in cognitive control processes (18–20). In addition, depressive symptomatology has been shown to correlate with increased interpersonal distress (21). These findings align with theoretical perspectives suggesting that depressed mood may bridge the gap between impaired inhibitory control and social-affective impairments. However, empirical research directly testing such mediation models in NSSI populations remains scarce.
Although several studies have documented altered ERP responses in populations with NSSI, the direct relationship between neural markers of inhibitory control and interpersonal distress which are measured by psychological scales has rarely been examined. Understanding how these psychological and neurophysiological factors interact may provide critical insights into the mechanisms underlying NSSI and inform targeted interventions. Therefore, the present study aimed to investigate the cognitive and neural correlates of NSSI among adolescents, focusing on both psychological and electrophysiological indices. Addressing this gap, the present study examined both psychological characteristics, including depression and interpersonal distress, and electrophysiological features represented inhibitory control in adolescents with NSSI.
We hypothesized that no-go P3 amplitude at the midline would be negatively associated with interpersonal distress. Furthermore, we proposed that depressive symptoms would mediate this association, such that reduced P3 amplitude would be linked to heightened interpersonal distress indirectly through elevated depressive symptoms.
To strengthen anatomical inferences, we additionally performed source-level analyses to identify cortical generators supporting inhibitory control during the no-go task.
2 Materials and methods
2.1 Participants
Altogether, 50 adolescents with NSSI and 51 HC were enrolled between January 2020 and May 2023. All patients were between the ages of 12 and 19 years and right-handed, with normal hearing ability. All participants were recruited from outpatient psychiatric clinics at Soonchunhyang University Hospital via clinician referral. Participants were interviewed using the Korean version of the MINI International Neuropsychiatric Interview. All adolescents in the NSSI group met diagnostic criteria for Major Depressive Disorder (MDD) as their primary diagnosis, based on the MINI International Neuropsychiatric Interview. Participants with bipolar disorders, psychotic disorders, neurodevelopmental disorders such as intellectual disabilities and autism spectrum disorder, neurological or severe medical diseases, a history of alcohol or substance abuse/dependence, head trauma, or who were currently pregnant were excluded from the study through screening interviews. In the case of NSSI, EEG was performed when the patients were drug naïve. Only participants who did not take psychiatric drugs were recruited, and EEG was performed during their first visit. This study was approved by the Institutional Review Board and Ethics Committee of Soonchunhyang University Cheonan Hospital, and all experimental protocols were approved by the committee (2020-07-042). Participants were informed that they could end the study at any time if they wanted to, and the study was performed in accordance with approved guidelines. Informed consent was obtained from all study participants, and all consent forms were completed by the participants and their parents.
2.2 Clinical measures
All participants were assessed for psychiatric symptoms such as depressive mood, anxiety, impulsivity, and emotional dysregulation. To assess the clinical characteristics of the abovementioned psychiatric symptoms, the Center for Epidemiologic Studies Depression scale (CES-D), State-Trait Anxiety Inventory (STAI), Short version of UPPS-P impulsive behavior scale (SUPPS-P), Acquired Capability for Suicide Scale (ACSS), Childhood Trauma Questionnaire (CTQ), Interpersonal Needs Questionnaire (INQ), Difficulties in Emotion Regulating Scale-16 (DERS-16) and Pain Catastrophizing Scale (PCS) were administered. The CES-D is a 20-item self-report scale designed to measure depressive symptoms over the past week, with each question scored on a scale of 0 to 3 points. Higher scores are positively correlated with severe depression. We used the Korean version of the CES-D, which has been validated for Korean adolescents (22). The STAI distinguishes between state anxiety, which is a temporary emotional state, and trait anxiety, which is defined as the general tendency to experience anxiety. Each item is scored on a 4-point Likert scale, with higher scores indicating greater levels of anxiety. The SUPPS-P measures impulsivity across five dimensions: negative urgency, lack of perseverance, lack of premeditation, sensation seeking, and positive urgency (23). All items are scored on a Likert scale from 1 to 4. The ACSS is a 20-item self-reported measure of the extent to which individuals perceive themselves as capable of performing or being exposed to potentially dangerous or fatal situations, including suicide, with scores ranging from 0 to 4 (24). We also used the CTQ to measure the patients’ traumatic experiences during childhood. The CTQ is a self-reported scale that defined as 5 different types of childhood abuse and neglect, which are rated on a scale from 1 to 5 (25). The INQ is a self-reported tool designed to assess two constructs central to the IPTS: TB and PB (26). It consists of 15 items rated on a 7-point Likert scale ranging from 1 to 7. Higher total scores on each subscale indicate greater levels of PB or TB. The DERS-16 is a self-report tool that consists of 16 items, with each item’s score ranging from 1 to 5, higher score means participants experience more emotional dysregulation (27). Finally, we used the PCS, a 13-item self-report scale ranged from 0 to 5, to measure the tendency to magnify and ruminate about pain experiences, often linked to emotional dysregulation and self-injurious behaviors (28). And also, to assess the characteristics and motivations of NSSI, we used the Korean version of the Inventory of Statements About Self-Injury (K-ISAS). The K-ISAS consists of two sections: Section 1 assesses the lifetime frequency and types of NSSI behaviors, and Section 2 evaluates 13 functional motivations for NSSI, including affect regulation, self-punishment, anti-dissociation, anti-suicide, interpersonal influence, interpersonal boundaries, sensation seeking, peer bonding, marking distress, toughness, autonomy, revenge, and self-care, through 39 items rated on a 3-point Likert scale (29). In this study, we examined both the behavioral patterns and functional subscales of NSSI.
2.3 EEG data acquisition and analysis
EEG data were collected while the participants were seated approximately 60 cm away from a computer monitor in a soundproof EEG room. Signals were recorded using a NeuroScan SynAmps amplifier (Compumedics USA, El Paso, TX, USA) with 64 Ag/AgCl electrodes positioned on a QuickCap adhering to the extended 10–20 electrode placement system. Key electrodes were positioned at the frontal (Fz), central (Cz), and parietal (Pz) sites, with the Earth electrode at FPz. Electrodes for monitoring eye movements were placed infraorbitally and mastoid electrodes were used as references. The impedance was maintained below 10 kΩ throughout the sessions. EEG signals were band-pass filtered (0.1–100 Hz) and sampled at 1000 Hz. The CURRY 8 software (Compumedics USA, Charlotte, NC, USA) was used for preprocessing. Large artifacts, such as those caused by muscle movements or gross body shifts, were visually inspected and manually excluded by a licensed clinical neurophysiology technologist who was blinded to participants’ group assignments Eye movement-related artifacts were corrected using automated preprocessing methods. Independent component analysis (ICA) was subsequently performed using CURRY 8 to identify and remove artifacts. Following artifact correction, the EEG data were re-referenced to a reference electrode standardization technique (REST) to achieve a more neutral reference (30). The data were further band-pass filtered (1.0–30 Hz) and segmented into epochs from 500 ms pre-stimulus to 900 ms post-stimulus. Baseline corrections were performed at pre-stimulus intervals. Epochs with residual artifacts exceeding ±75 µV at any electrode site were excluded from further analysis. Only clean epochs were averaged across trials and participants for event-related potential (ERP) analysis. Based on prior research identifying no-go ERPs as indicators of behavioral inhibition (15), this study focused on no-go trials for ERP analyses.
2.4 Behavioral task paradigm
An auditory go/no-go task using an oddball paradigm was employed to elicit ERPs. Participants wore headphones and were instructed to press the spacebar as quickly and accurately as possible in response to the target tone (go condition) and to withhold responses to the non-target tone (no-go condition). A total of 400 trials were presented, comprising 85% go trials and 15% no-go trials. The target tone (no-go) was 1,500 Hz, while the non-target tone (go) was 1,000 Hz, with a 1,500 ms inter-trial interval. Stimuli were generated using E-Prime software (Psychology Software Tools; Pittsburgh, PA, USA). This study analyzed the P300 ERP components (the most positive peak between 250 and 500 ms post-stimulus, P3) at the frontal (Fz), fronto-central (FCz), central (Cz), and parietal (Pz) electrodes. The time windows for the analysis were based on previous studies (20). Behavioral data, including go accuracy, no-go accuracy, and reaction times were collected using E-Prime software.
2.5 Statistical analysis
Group differences in demographic characteristics, clinical measures, and behavioral task performance were analyzed using the chi-square test for categorical variables and independent t-tests for continuous variables, following the verification of normality assumptions. For behavioral task, go accuracy, no-go accuracy, and go reaction time were compared between groups using independent t-tests. To account for three parallel comparisons, Benjamini-Hochberge false discovery rate (FDR) was applied. For ERP data, repeated-measures analysis of variance (RMANOVA) was performed on no-go P3 amplitudes across midline electrode sites (Fz, FCz, Cz, and Pz), with electrode as a within-subject factor and group (NSSI vs. HC) as a between-subject factor, CES-D entered as a covariate. After conducting RMANOVA, we performed Pearson correlation analyses to examine the associations between no-go P3 amplitude at frontal electrode sites (Fz and FCz) and psychological variables, including depressive symptom severity (CES-D scores) and interpersonal distress (INQ scores). Based on extensive prior literature implicating frontal midline sites in inhibitory control measured by no-go p3, a priori follow-up correlation analysis was restricted to Fz and FCz (31, 32) and FDR was used for adjust p-value. Statistical significance was defined as p ≤ 0.05. All analyses were performed using SPSS version 26.0 (SPSS Inc., Chicago, IL, USA) and R statistical software.
2.6 Source analysis
Source analysis was conducted to identify the neural generators of the no-go P3 component using the Brainstorm toolbox (33). A depth-weighted L2 norm solution was applied to estimate cortical current density time series. The forward model was computed using a three-layer boundary element model (BEM) based on the MNI/Colin27 anatomy template. Source activity was estimated over 15,002 cortical vertices, and cortical regions were defined using the Desikan–Killiany atlas, which partitions the cortex into 34 anatomical regions per hemisphere. For each region of interest (ROI), representative values were obtained by applying principal component analysis (PCA) to source signals within a 5 mm radius of each ROI centroid (34). Based on prior literature linking fronto-cingulate and parietal regions to inhibitory control and affective processing, a subset of midline and adjacent ROIs was selected for group comparisons. These included the bilateral rostral and caudal anterior cingulate cortices, posterior cingulate cortices, medial orbitofrontal cortices, superior frontal gyri, and paracentral lobules (35, 36). To complement the frequentist approach and provide a graded measure of evidence for or against group differences, Bayesian t-tests were also performed using the BayesFactor package in R (37). Because we tested a large number of a priori ROIs, conventional family-wise error corrections risk masking genuine effect trends. Instead, we used Bayesian independent-samples t-tests to quantify evidence directly via Bayes factors, which obviate the need for ad-hoc α-level adjustments while preserving sensitivity to meaningful patterns (38). BF10 values greater than 3 were interpreted as moderate evidence for the alternative hypothesis, while values below 1/3 were taken as moderate evidence for the null.
2.7 Mediation analysis
To examine potential mediation effects, a causal mediation analysis was conducted using the R package mediation (39). The model tested whether depressive symptoms mediated the relationship between no=go p3 activity and Interpersonal needs (INQ). Two linear models were specified: one predicting CES-D from Fz amplitude, and the other predicting INQ from both CES-D and Fz amplitude. The indirect (mediation) and direct effects were estimated using nonparametric bootstrapping with 1,000 simulations to generate percentile-based confidence intervals.
3 Results
3.1 Participants
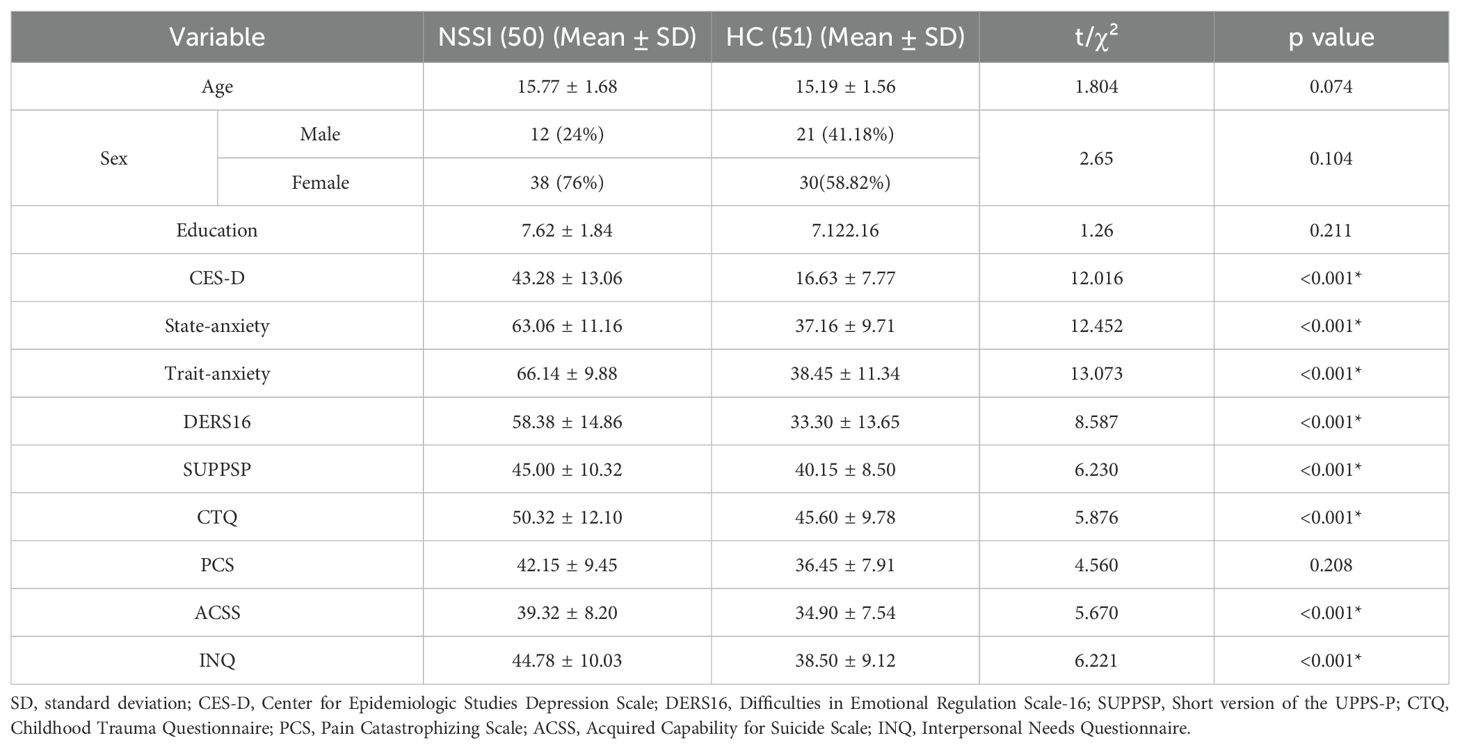
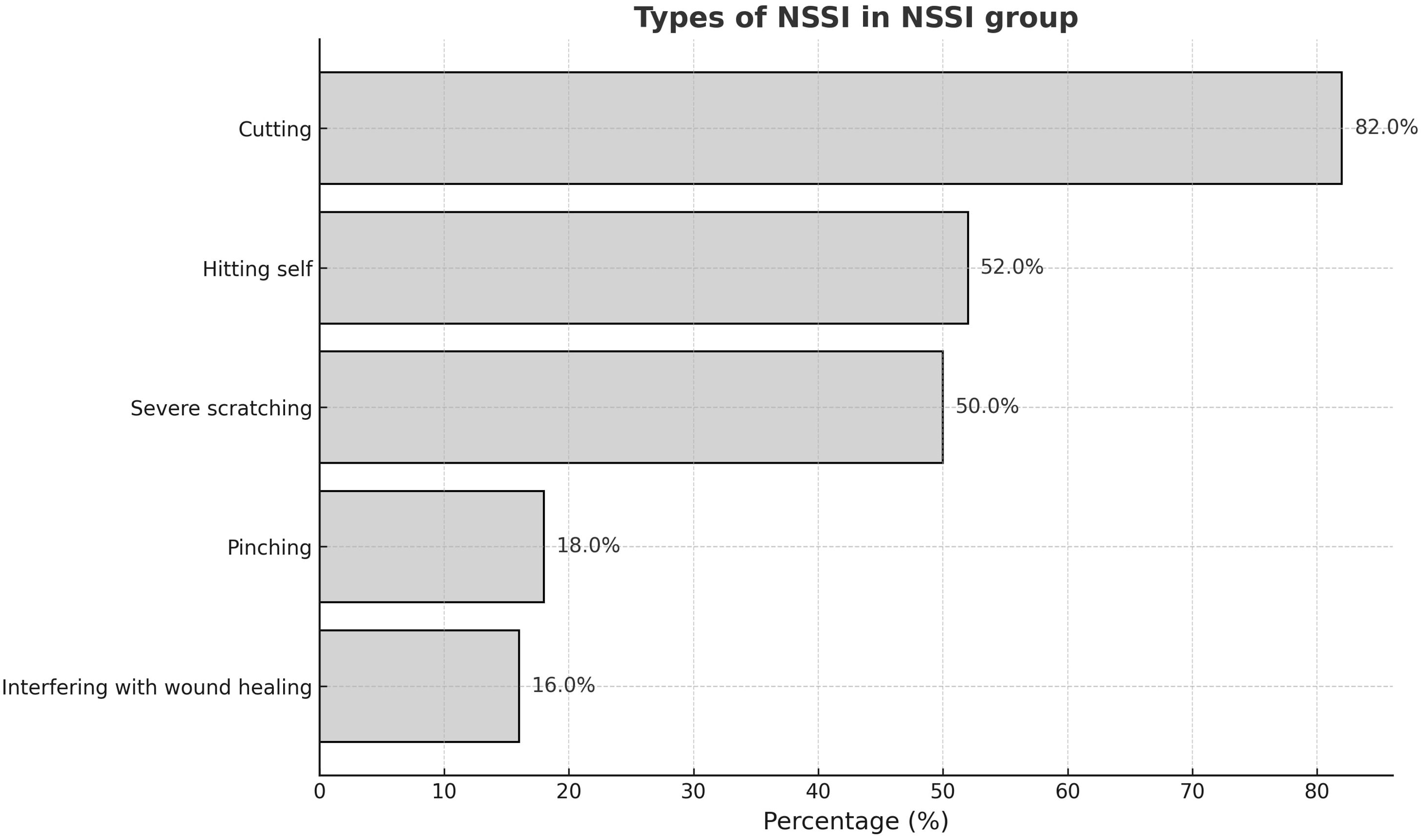
All adolescents in the NSSI group met diagnostic criteria for Major Depressive Disorder (MDD) according to the MINI. Table 1 presents the demographic data and clinical measurements of all participant groups. No significant differences in age (p = 0.074), sex (p =0.104), or educational level (p = 0.211) were observed between the two groups. The NSSI group had significantly higher scores on all psychological scales than the HC group, except for the PCS (p = 0.208). Table 2 shows that after adjusting for CES-D and STAI scores, only ACSS and INQ scores were significantly different between the NSSI and HC groups (p < 0.001). In the NSSI group, cutting was the most reported self-injurious behavior, endorsed by 82.0% of participants (Figure 1). This was followed by hitting oneself (52.0%) and severe scratching (50.0%). Less frequently endorsed behaviors included pinching (18.0%) and interfering with wound healing (16.0%). From the perspective of motivation of NSSI, affect regulation, self-punishment, and anti-dissociation were the most highly endorsed NSSI functions based on responses to the K-ISAS Section 2.
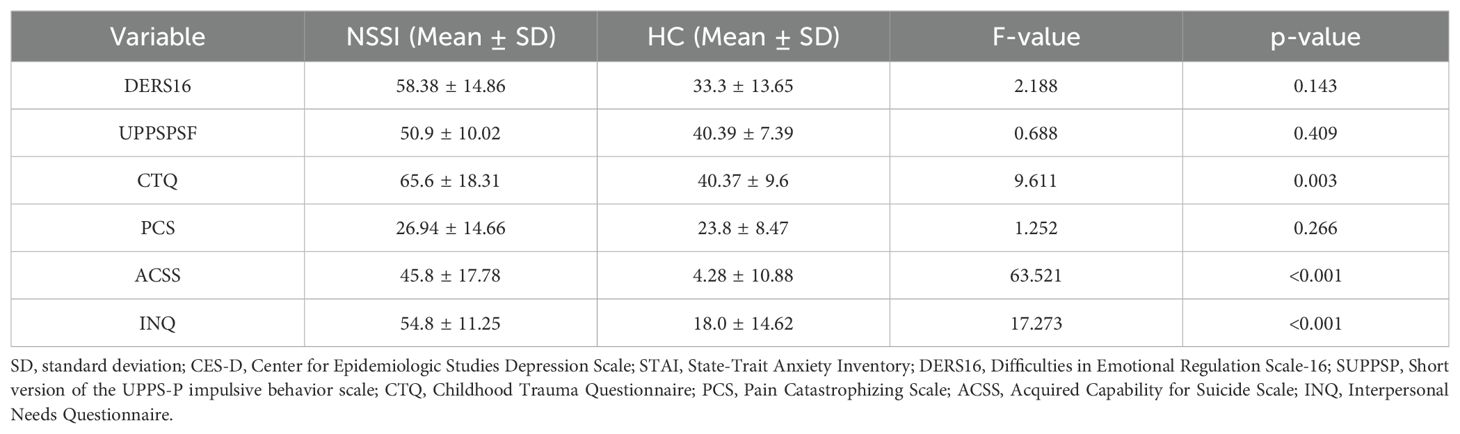
Table 2. Comparison of psychological test results between NSSI and HC groups after adjusting CES-D and STAI score.
3.2 ERP
3.2.1 Behavioral outcomes
Table 3 presents the behavioral outcomes of the no-go paradigm. Compared to the HC, NSSI group showed significantly lower accuracy on both go trials (t (96) = 4.26, p <.001) and no-go trials (t (96) = 5.24, p < .001). Reaction times were longer in the NSSI group than in the HC group, though this difference did not reach statistical significance (t (96) = 1.85, 0.68).
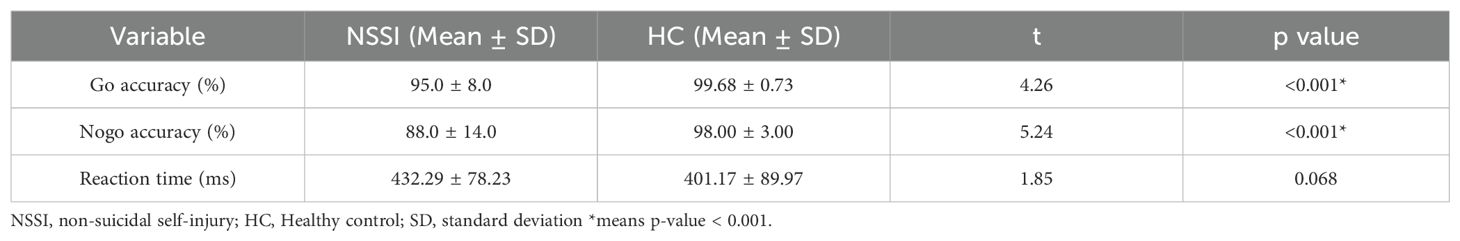
Table 3. Behavioral performance in the go/no-go task for adolescents with non-suicidal self-injury (NSSI) and healthy controls (HC).
3.2.2 Amplitude and latency
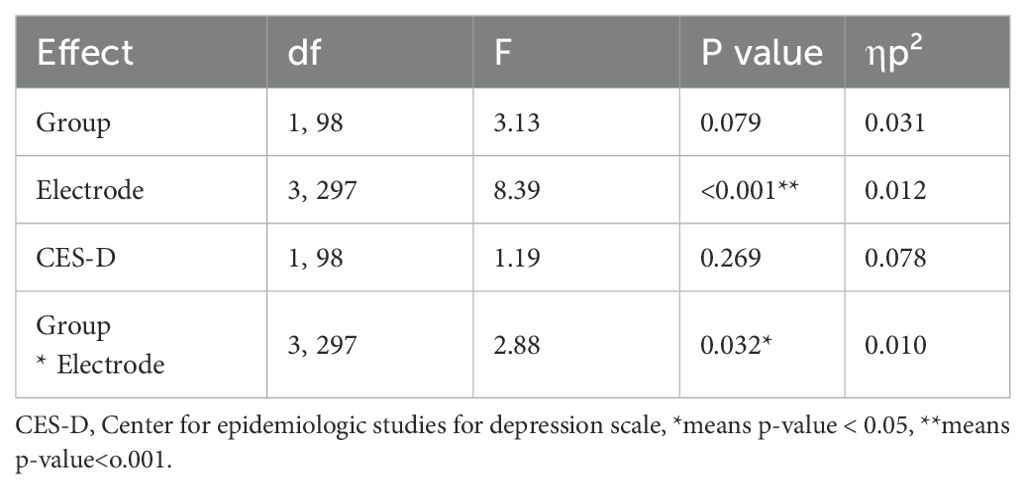
A repeated-measures ANCOVA was conducted on no-go P3 amplitudes with Group (NSSI vs. HC) as a between-subjects factor, Electrode (Fz, FCz, Cz, Pz) as a within-subjects factor, and CES-D score as a covariate. There was a significant main effect of Electrode emerged (F (3, 297) = 8.39, p <.001, ηp² = .078). Crucially, the Group × Electrode interaction was also significant (F (3, 297) = 2.88, p = .0361, ηp² = .010) indicating that the pattern of group differences varied across electrode sites (Table 4). Although there was no overall main effect of Group on no-go P3 amplitude, the significant Group × Electrode interaction indicates that group differences depend on electrode site. RMANOVA showed no significant main effect of group on no-go P3 latency (F (1, 98) = 0.02, p = .89).
3.2.3 Source analysis
Bayesian model comparison indicated that most frontal regions favored the null model (BF10 < 1), only the Rt. superior frontal gyrus showed a BF10 > 1 (BF10 ≈ 1.38), indicating weak evidence favoring group differences in that ROI. NSSI group showed decreased brain activity in Rt. superior frontal gyrus. However, after adjusting for depressive symptoms, the evidence for group differences substantially decreased across all ROIs. In particular, the BF10 for the right superior frontal gyrus dropped to 0.33 (Table 5).
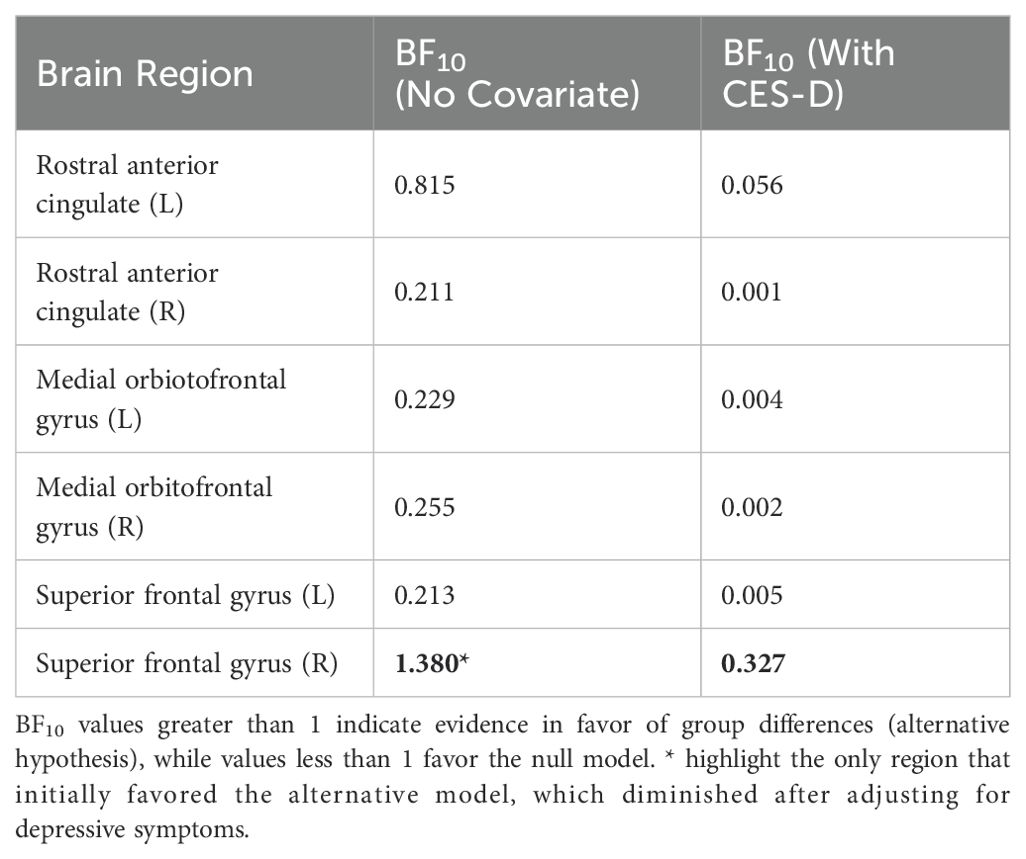
Table 5. Bayesian model comparison (BF10) for group differences with and without CES-D as covariate.
3.3 Correlations
In the full sample, the total score of CES-D was positively correlated with those of INQ, indicating higher depressive symptoms are closely linked to greater interpersonal distress. Within the NSSI group, affective regulation was positively correlated with CES-D (r = 0.27, p < 0.001, q < 0.001) and with INQ (r = 0.26, p <0.001, q < 0.001). Self-Punishment showed weaker but still significant correlations with CES-D (r = 0.15, p = 0.031, q = 0.031) and INQ (r = 0.18, p = 0.009, q = 0.011).
3.3.1 Scalp-level ERP with psychological scales
No-go P3 at Fz showed moderate negative correlations with both depressive symptoms (CES-D: r = –0.23, p = 0.019, q = 0.048) and interpersonal distress (INQ: r = –0.22, p = 0.024, q = 0.048) after FDR correction (Figure 2). Correlation between No-go P3 at FCz and psychological scales did not reach significance after FDR correction (CES-D: r = –0.21, p = 0.040, q = 0.080; INQ: r = –0.18, p = 0.072, q = 0.144).
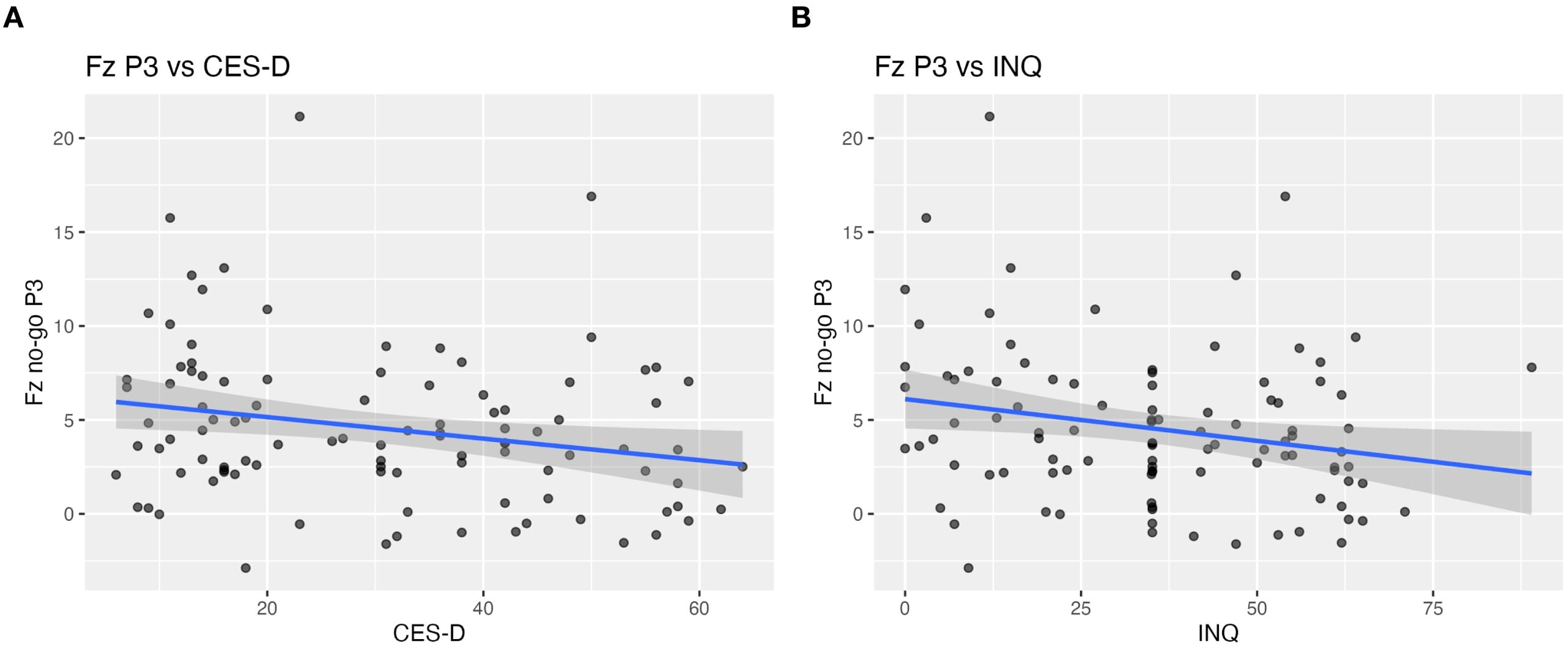
Figure 2. Scalp-level correlations between Fz no-go P3 amplitude and clinical measures. Scatter plots illustrating the relationship between frontal no-go P3 amplitude at Fz and (A) depressive symptoms (CES-D) and (B) interpersonal distress (INQ) across all participants. Reduced P3 amplitude at Fz was moderately associated with higher CES-D scores (r = –0.23, p = 0.019, q = 0.048) and higher INQ scores (r = –0.22, p = 0.024, q = 0.048).
3.3.2 Source-level ROI with psychological scales and ISAS
Right superior frontal gyrus (SFG) source activity showed negative correlations with depressive symptoms (CES-D: r = –0.21, p = 0.036, q = 0.036) and with interpersonal distress (INQ: r = –0.23, p = 0.022, q = 0.036) after FDR correction (Figure 3). Within the NSSI group, SFG activity was marginally associated with affective regulation (r = –0.25, p = 0.081, q = 0.162) but not with self-punishment (r = –0.04, p = 0.66, q = 0.66).
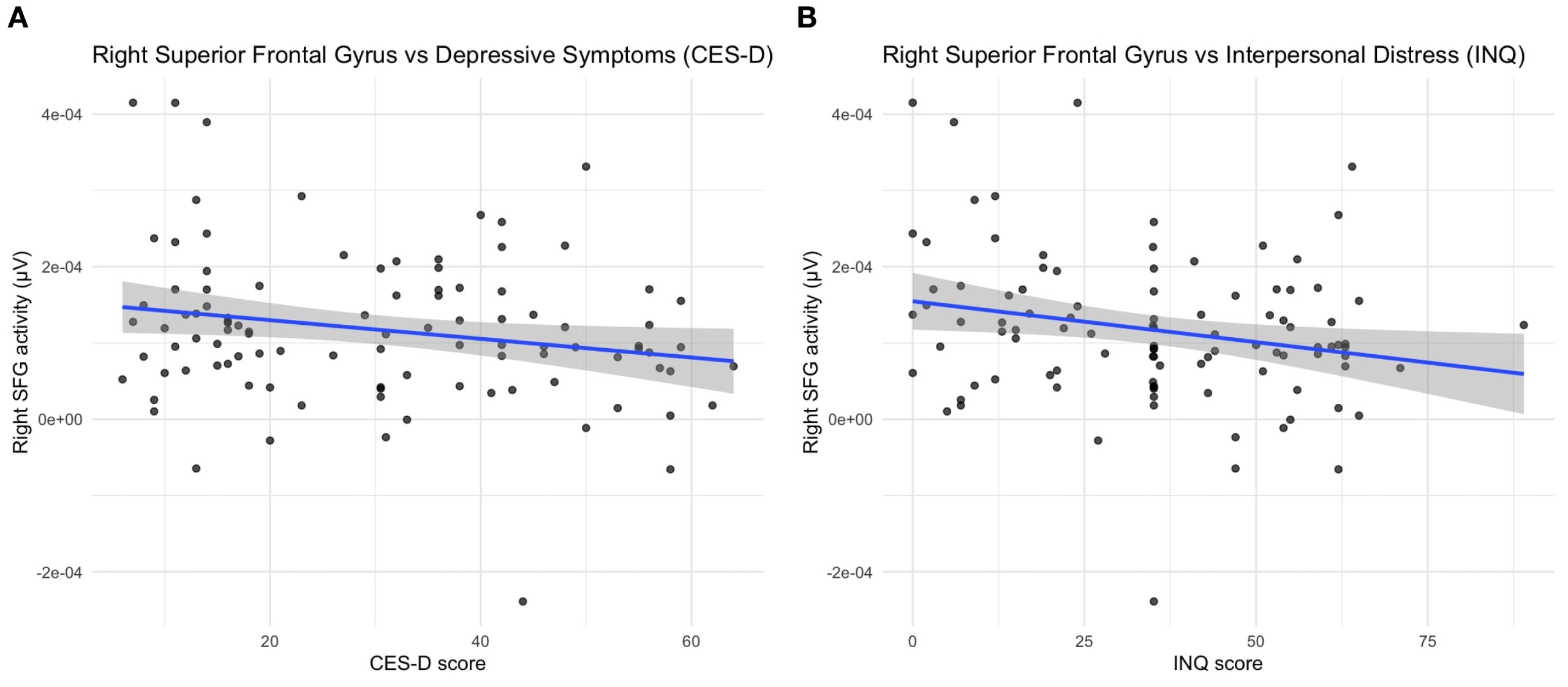
Figure 3. Source-level correlations between right superior frontal gyrus activity and clinical measures. Scatter plots illustrating the relationship between right superior frontal gyrus (SFG) source activity and (A) depressive symptoms (CES-D) and (B) interpersonal distress (INQ) across all participants. Reduced SFG activity was moderately associated with higher CES-D scores (r = –0.21, p = 0.036, q = 0.036) and higher INQ scores (r = –0.23, p = 0.022, q = 0.036).
3.4 Mediation analysis
To examine whether depressive symptoms mediated the relationship between inhibitory control and interpersonal distress, a mediation analysis was conducted with Fz no-go P3 amplitude as the predictor, CES-D as the mediator, and INQ as the outcome.
The indirect effect was statistically significant (ACME = -0.17, 95% CI [-0.32, -0.03], p = .018), whereas the direct effect was non-significant (ADE = -0.05, p = .474). The total effect remained significant (p = .040), with approximately 76% of the effect of Fz no-go P3 on INQ being mediated through depressive symptoms (proportion mediated = 0.76, p = .042). Similarly, a mediation analysis was performed using right superior frontal gyrus (SFG) source activity as the predictor, CES-D as the mediator, and INQ as the outcome. The indirect effect was also significant (ACME = -0.15, 95% CI [-0.28, -0.01], p = .030), while the direct effect was not significant (ADE = -0.08, p = .216). The total effect remained significant (p = .018), and approximately 67% of the effect of right SFG activity on INQ was mediated through depressive symptoms (proportion mediated = 0.67, p = .024) (Figure 4).
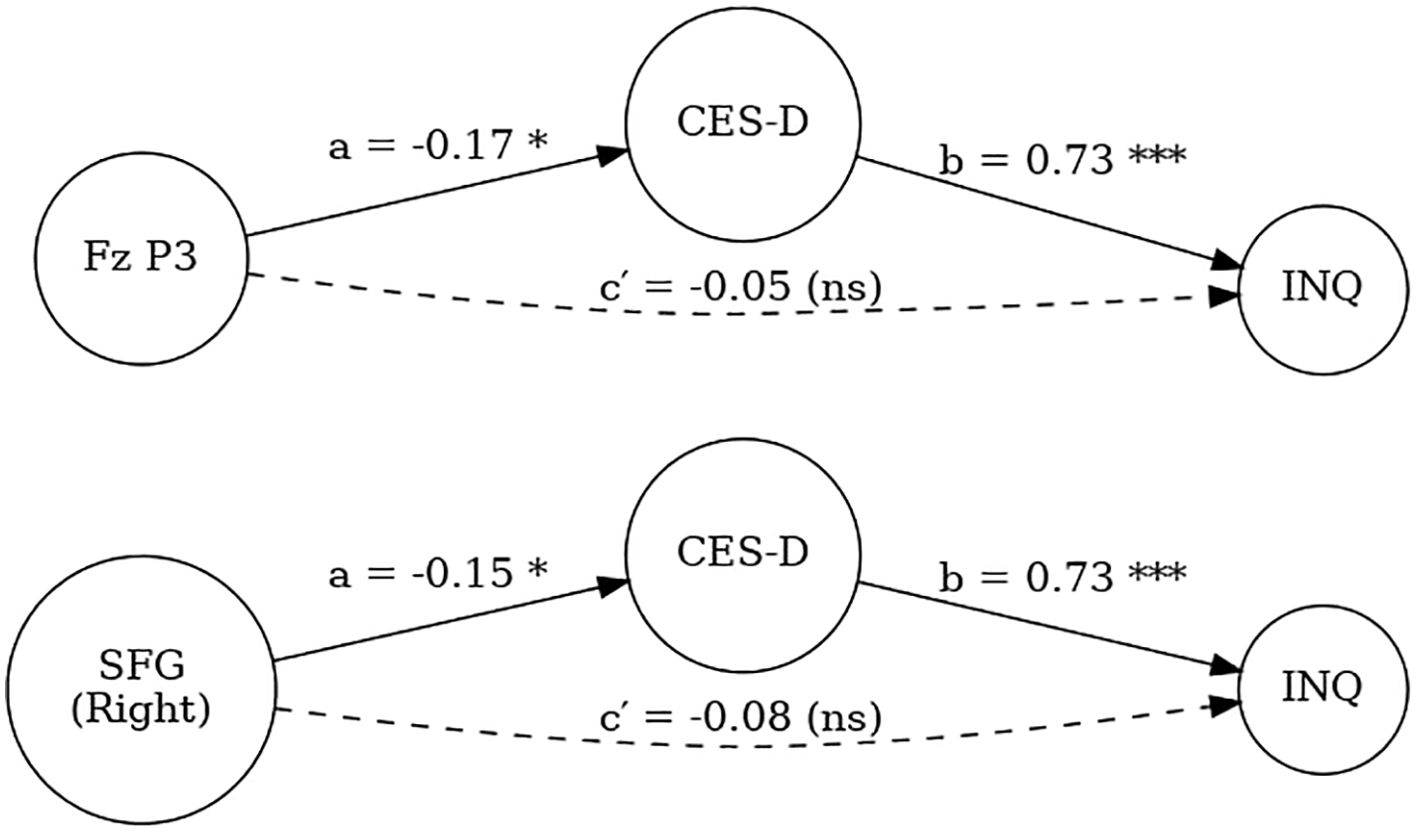
Figure 4. Mediation models testing the indirect effects of Fz no-go P3 amplitude (top) and right superior frontal gyrus (SFG) activity (bottom) on interpersonal distress (INQ) through depressive symptoms (CES-D). Path a represents the effect of neural indices on CES-D, path b represents the effect of CES-D on INQ, and path ca represents the direct effect of neural indices on INQ after accounting for CES-D. Indirect effects were statistically significant, while direct effects were non-significant. (p <.05*, **p <.001, ns = not significant).
4 Discussion
This study investigated the electrophysiological and psychological characteristics of adolescents engaging in non-suicidal self-injury (NSSI), focusing on no-go P3 amplitude and its relationship with impulsivity and interpersonal distress. Our key findings were: (1) the NSSI group showed significantly lower accuracy in the go/nogo task, (2) the NSSI group showed reduced no-go P3 amplitude at Fz compared to those of the HC, (3) this reduction was associated with increased depressed symptoms and interpersonal distress, (4) source-level activity in the right superior frontal gyrus (SFG) was marginally lower in the NSSI group but diminished after adjusting for depressive symptoms, (5) this reduction was also associated with increased depression and interpersonal distress, and (6) mediation analysis revealed that depressive symptoms significantly mediated the relationship between inhibitory neural markers and interpersonal distress.
4.1 Behavioral inhibition deficits in NSSI
The NSSI group showed poorer behavioral performance in both go and no-go trials, reflecting diminished recruitment of cognitive resources (40). In previous studies, no-go P3 has been shown to be related to impulsivity (15, 20, 41). Consistent with previous studies, the NSSI group exhibited reduced no-go P3 amplitudes at Fz electrodes compared to HC, suggesting impaired inhibitory control and reduced allocation of attentional resources in this population. This result also aligns with previous findings in which diminished P3 amplitudes during no-go tasks were associated with impaired impulse control (20, 41). However, it is noteworthy that after controlling for depressive symptoms, no significant group differences were found in self-reported measures of impulsivity (UPPS-P) or emotion regulation difficulties (DERS). These results suggest that the observed differences in neural markers may not fully correspond to self-perceived deficits in these domains, underscoring the need for cautious interpretation and further investigation into potential dissociations between subjective and neurophysiological indices. Notably, the reduction in no-go P3 amplitude was confined to the Fz site, with no significant group differences observed at other midline electrodes. This spatial specificity is consistent with prior electrophysiological studies indicating that response inhibition related P3 components are most robustly expressed over frontal midline regions, particularly Fz and FCz, which are closely associated with prefrontal executive functioning (42, 43).
4.2 Neural-affective interaction: P3 and interpersonal distress
In our study, no-go P3 amplitude at Fz was negatively correlated with both depressive symptoms and interpersonal distress, as measured by the INQ. These findings suggest that impaired inhibitory control and depressed mood frequently co-occur with interpersonal difficulties in adolescents engaging in NSSI. These findings are consistent with the IPTS, which posits that TB and PB, core elements captured by the INQ, are critical contributors not only to suicidal ideation, but also to self-injurious behavior (44, 45). Within this framework, reduced inhibitory capacity may impair the regulation of socially aversive impulses, thereby fostering feelings of interpersonal alienation (13). Together, our findings underscore a constellation of overlapping vulnerabilities—neurophysiological, affective, and social—that characterize NSSI, and point to the importance of integrated interventions targeting both inhibitory control and mood to alleviate interpersonal pain.
4.3 Source analysis and social-cognitive control
Although Bayes factors provided only weak evidence for group differences in right SFG activity (BF10 ≈ 1.38), source‐level findings enriched our ERP results by implicating higher‐order social–cognitive control mechanisms. The dorsal SFG, often grouped with dorsomedial prefrontal cortex, is a core node of the mentalizing network, has been implicated in top-down regulation of emotional interference and conflict, particularly in tasks requiring suppression of affective distractors and the resolution of socially salient cues (46–48). Recent neuroimaging studies in individuals with MDD have reported SFG dysfunction associated with emotion dysregulation and self-injurious behavior, raising the possibility that similar mechanisms may be present in adolescents with NSSI (48). In the present study, decreased right SFG activation was significantly associated with both depressive symptoms and interpersonal distress, and mediation analysis indicated that depressive symptoms accounted for the relationship between frontal hypoactivation and INQ scores. While the right SFG has not been consistently identified as a direct neural substrate of interpersonal distress, the reduced right SFG activation observed in adolescents with NSSI may reflect an upstream deficit in inhibitory control and depressive symptoms. Prior research has implicated the SFG in the modulation of emotional responses during interpersonal conflict and social competition (47, 49), suggesting that diminished engagement in this region may compromise the capacity to regulate affectively charged interpersonal interactions.
4.4 Depressive symptoms as a mediator of neural-social links
When we included depressive symptoms (CES-D) as a covariate in our scalp‐level RMANOVA, the Group × Electrode interaction for no-go P3 remained significant, indicating frontal‐midline inhibition deficits in NSSI beyond mood effects. In contrast, applying the same covariate adjustment to our source‐level analyses abolished all group differences in right SFG activity. This pattern suggests that source-localized activity largely reflects depressive severity.
Crucially, because no-go P3 amplitude at Fz correlated with both depressive symptoms and interpersonal distress, we pursued a mediation model in which depressive symptoms bridge the neural inhibition (no-go P3 amplitude and right SFG activation) and interpersonal distress. Mediation analysis revealed that depressive symptoms significantly mediated the association between diminished no-go P3 amplitude, Rt. SFG activation and interpersonal distress. This result supports the interpretation that inhibitory deficits may contribute to social distress primarily through their impact on affective dysregulation (50).
4.5 Limitations
Our study had some limitations. First, due to the cross-sectional design, we could not assess causal relationships between neural responses and clinical features. Longitudinal studies are needed to clarify the temporal dynamics of these associations. Second, various scales for measuring clinical characteristics were evaluated using self-report measures. Despite the self-reported scales used in this study having good stability and validity, they could not reflect the neural or cognitive basis of clinical characteristics such as impulsivity and emotional regulation. Third, our results are limited to patients with MDD, requiring further studies on accompanying self-harm in other psychiatric diseases. Fourth, because our participants were adolescents, systematic assessments of stable personality traits (e.g., borderline or avoidant features) were not conducted. This may limit interpretation of trait-level contributors to interpersonal distress and inhibitory control.
4.6 Conclusions
In summary, adolescents engaging in NSSI exhibit co-occurring deficits in fronto-midline inhibitory control, elevated depressive symptoms, and heightened interpersonal distress, with depressive mood mediating the link between neural inhibition markers and social pain. These findings underscore the value of integrated interventions that simultaneously target cognitive control and mood regulation to alleviate interpersonal suffering and reduce self-injurious behaviors. Future longitudinal and experimental studies should test whether strengthening inhibitory capacity or alleviating depressive symptoms can disrupt this vulnerability cluster and inform tailored prevention and treatment strategies.
Data availability statement
The datasets presented in this article are not readily available because of ethical and privacy concerns. Requests to access the datasets should be directed to JK, aWRlYWw5MUBoYW5tYWlsLm5ldA==.
Ethics statement
The studies involving humans were approved by the Institutional Review Board and Ethics Committee of Soonchunhyang University Cheonan Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
SY: Visualization, Writing – original draft, Methodology, Writing – review & editing. JK: Writing – review & editing, Conceptualization, Supervision, Methodology, Validation. HL: Supervision, Writing – review & editing, Validation, Data curation, Formal analysis. WL: Writing – review & editing, Conceptualization. YS: Writing – review & editing, Data curation, Formal analysis, Methodology. SS: Data curation, Conceptualization, Supervision, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (2020R1I1A3A04036435). This research was also supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant number: RS-2022-KH125605).
Acknowledgments
This study was supported by Soonchunhyang University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ACSS, Acquired Capability for Suicide Scale; ANOVA, analysis of variance; ANCOVA, analysis of covariance; CES-D: center of epidemiology studies for depressions scale; CTQ, childhood trauma questionnaire; Cz, central; DERS, Difficulties in Emotion Regulation Scale; EEG, electroencephalography; ERP, event-related potential; Fz, frontal; FCz, fronto-central; HC, healthy control; INQ, interpersonal need questionnaire; IPTS, interpersonal theory of suicide; MDD, major depressive disorder; MNE, minimum norm estimation; NSSI, non-suicidal self-injury; PB, perceived burdensomeness; PCS, pain catastrophizing scale; Pz, parietal; RMANOVA, repeated measures ANOVA; STAI, state-trait anxiety inventory; TB, thwarted belongingness.
References
1. Swannell SV, Martin GE, Page A, Hasking P, and St John NJ. Prevalence of nonsuicidal self-injury in nonclinical samples: Systematic review, meta-analysis and meta-regression. Suicide Life Threatening Behav. (2014) 44:273–303. doi: 10.1111/sltb.12070
2. Xiao Q, Song X, Huang L, Hou D, and Huang X. Global prevalence and characteristics of non-suicidal self-injury between 2010 and 2021 among a non-clinical sample of adolescents: a meta-analysis. Front Psychiatry. (2022) 13:912441. doi: 10.3389/fpsyt.2022.912441
3. Cipriano A, Cella S, and Cotrufo P. Nonsuicidal self-injury: A systematic review. Front Psychol. (2017) 8:1946. doi: 10.3389/fpsyg.2017.01946
4. Plener PL, Schumacher TS, Munz LM, and Groschwitz RC. The longitudinal course of non-suicidal self-injury and deliberate self-harm: a systematic review of the literature. Borderline Pers Disord Emotion Dysregulat. (2015) 2:1–11. doi: 10.1186/s40479-014-0024-3
5. Hawton K, Bergen H, Cooper J, Turnbull P, Waters K, Ness J, et al. Suicide following self-harm: findings from the multicentre study of self-harm in England, 2000–2012. J Affect Disord. (2015) 175:147–51. doi: 10.1016/j.jad.2014.12.062
6. Serra M, Presicci A, Quaranta L, Caputo E, Achille M, Margari F, et al. Assessing clinical features of adolescents suffering from depression who engage in non-suicidal self-injury. Children. (2022) 9:201. doi: 10.3390/children9020201
7. Assavedo BL and Anestis MD. The relationship between non-suicidal self-injury and both perceived burdensomeness and thwarted belongingness. J Psychopathol Behav Assess. (2016) 38:251–7. doi: 10.1007/s10862-015-9508-8
8. Marco JH, Pérez S, and García-Alandete J. Meaning in life buffers the association between risk factors for suicide and hopelessness in participants with mental disorders. J Clin Psychol. (2016) 72:689–700. doi: 10.1002/jclp.22285
9. Marco JH, Gallego-Hernández de Tejada B, Guillén V, Baños RM, and Pérez S. Meaning in life buffers the association between perceived burdensomeness, thwarted belongingness, and frequency of non-suicidal self-injuries in Spanish adolescents. J Clin Med. (2021) 10:4867. doi: 10.3390/jcm10214867
10. Chu C, Rogers ML, and Joiner TE. Cross-sectional and temporal association between non-suicidal self-injury and suicidal ideation in young adults: The explanatory roles of thwarted belongingness and perceived burdensomeness. Psychiatry Res. (2016) 246:573–80. doi: 10.1016/j.psychres.2016.07.061
11. Wang D, Zhao J, and Wang Y. Cumulative interpersonal risk, non-suicidal self-injury, and suicide attempts in early adolescence: between-person differences and within-person effects. J Youth Adolesc. (2024) 54:1–14. doi: 10.1007/s10964-024-02101-8
12. Lockwood J, Daley D, Townsend E, and Sayal K. Impulsivity and self-harm in adolescence: a systematic review. Eur Child Adolesc Psychiatry. (2017) 26:387–402. doi: 10.1007/s00787-016-0915-5
13. Kim JS, Kim S, Jung W, Im C-H, and Lee S-H. Auditory evoked potential could reflect emotional sensitivity and impulsivity. Sci Rep. (2016) 6:37683. doi: 10.1038/srep37683
14. Congdon E, Mumford JA, Cohen JR, Galvan A, Canli T, and Poldrack RA. Measurement and reliability of response inhibition. Front Psychol. (2012) 3:37. doi: 10.3389/fpsyg.2012.00037
15. Zhou D-D, Zhao L, Ma L-L, Hu J-H, Chen R, Jiang Z-H, et al. Altered neural reactivity in adolescents with nonsuicidal self-injury during exposure to self-injury related cues: electrophysiological evidence from a two-choice oddball paradigm. Front Psychiatry. (2022) 13:827480. doi: 10.3389/fpsyt.2022.827480
16. Hu R, Peng L-L, Du Y, Feng Y-W, Xie L-S, Shi W, et al. Reciprocal effect between non-suicidal self-injury and depressive symptoms in adolescence. Front Public Health. (2024) 11:1243885. doi: 10.3389/fpubh.2023.1243885
17. Dhami P, Quilty LC, Schwartzmann B, Uher R, Allen TA, Kloiber S, et al. Alterations in the neural correlates of affective inhibitory control following cognitive behavioral therapy for depression: A Canadian biomarker integration network for depression (CAN-BIND) study. J Affect Disord Rep. (2022) 10:100413. doi: 10.1016/j.jadr.2022.100413
18. Ruchsow M, Groen G, Kiefer M, Beschoner P, Hermle L, Ebert D, et al. Electrophysiological evidence for reduced inhibitory control in depressed patients in partial remission: a Go/Nogo study. Int J Psychophysiol. (2008) 68:209–18. doi: 10.1016/j.ijpsycho.2008.01.010
19. Kim M, Lee YJ, Hwang J, S-i W, and Hahn S-W. Impulsivity in major depressive disorder patients with suicidal ideation: event-related potentials in a GoNogo task. Clin Psychopharmacol Neurosci. (2023) 21:787. doi: 10.9758/cpn.23.1064
20. Yoon SH, Shim S-H, and Kim JS. Electrophysiological changes between patients with suicidal ideation and suicide attempts: an event-related potential study. Front Psychiatry. (2022) 13:900724. doi: 10.3389/fpsyt.2022.900724
21. Triscoli C, Croy I, and Sailer U. Depression predicts interpersonal problems partially through the attitude towards social touch. J Affect Disord. (2019) 246:234–40. doi: 10.1016/j.jad.2018.12.054
22. Heo E-H, Choi K-S, Yu J-C, and Nam J-A. Validation of the center for epidemiological studies depression scale among Korean adolescents. Psychiatry Invest. (2017) 15:124. doi: 10.30773/pi.2017.07.19
23. Lim SY and Kim SJ. Validation of a short Korean version of the UPPS-P Impulsive Behavior Scale. Asia Pacif Psychiatry. (2018) 10:e12318. doi: 10.1111/appy.12318
24. Seo JW and Kwon SM. Preliminary validation of a Korean version of the acquired capability for suicide scale-fearlessness about death. Suicide Life Threatening Behav. (2018) 48:305–14. doi: 10.1111/sltb.12360
25. Bernstein DP, Fink L, Handelsman L, and Foote J. Childhood trauma questionnaire. In: Assessment of family violence: A handbook for researchers and practitioners (1998) (Washington, DC: American Psychological Association (APA)).
26. Park Y and Kim HS. Validation of the Korean version interpersonal needs questionnaire. Suicide Life Threatening Behav. (2019) 49:739–58. doi: 10.1111/sltb.12473
27. Kim G, Yim M, Bae H, and Hur J-W. Factor structures and psychometric properties of three brief versions of the difficulties in emotion regulation scale in the Korean population. BMC Psychol. (2024) 12:1–13. doi: 10.1186/s40359-024-02261-z
28. Cho S, Kim H-Y, and Lee J-H. Validation of the Korean version of the Pain Catastrophizing Scale in patients with chronic non-cancer pain. Qual Life Res. (2013) 22:1767–72. doi: 10.1007/s11136-012-0308-2
29. Kim S, Kim Y, and Hur J-W. Nonsuicidal self-injury among Korean young adults: a validation of the Korean version of the inventory of statements about self-injury. Psychiatry Invest. (2019) 16:270. doi: 10.30773/pi.2019.01.23
30. Dong L, Li F, Liu Q, Wen X, Lai Y, Xu P, et al. MATLAB toolboxes for reference electrode standardization technique (REST) of scalp EEG. Front Neurosci. (2017) 11:601. doi: 10.3389/fnins.2017.00601
31. Albert J, López-Martín S, Hinojosa JA, and Carretié L. Spatiotemporal characterization of response inhibition. Neuroimage. (2013) 76:272–81. doi: 10.1016/j.neuroimage.2013.03.011
32. Hong X, Sun J, Bengson JJ, and Tong S. Age-related spatiotemporal reorganization during response inhibition. Int J Psychophysiol. (2014) 93:371–80. doi: 10.1016/j.ijpsycho.2014.05.013
33. Baillet S, Mosher JC, Pantazis D, and Leahy RM. Brainstorm: a user-friendly application for MEG/EEG analysis. Comput Intell Neurosci. (2011) 2011. doi: 0.1155/2011/879716
34. Choi K-M, Kim J-Y, Kim Y-W, Han J-W, Im C-H, and Lee S-H. Comparative analysis of default mode networks in major psychiatric disorders using resting-state EEG. Sci Rep. (2021) 11:22007. doi: 10.1038/s41598-021-00975-3
35. Schmaal L, van Harmelen A-L, Chatzi V, Lippard ET, Toenders YJ, Averill LA, et al. Imaging suicidal thoughts and behaviors: a comprehensive review of 2 decades of neuroimaging studies. Mol Psychiatry. (2020) 25:408–27. doi: 10.1038/s41380-019-0587-x
36. Auerbach RP, Pagliaccio D, Allison GO, Alqueza KL, and Alonso MF. Neural correlates associated with suicide and nonsuicidal self-injury in youth. Biol Psychiatry. (2021) 89:119–33. doi: 10.1016/j.biopsych.2020.06.002
37. Rouder JN, Speckman PL, Sun D, Morey RD, and Iverson G. Bayesian t tests for accepting and rejecting the null hypothesis. Psychonom Bull Review. (2009) 16:225–37. doi: 10.3758/PBR.16.2.225
38. Gelman A, Hill J, and Yajima M. Why we (usually) don’t have to worry about multiple comparisons. J Res Educ Effectiveness. (2012) 5:189–211. doi: 10.1080/19345747.2011.618213
39. Tingley D, Yamamoto T, Hirose K, Keele L, and Imai K. Mediation: R package for causal mediation analysis. J Stat Software. (2014) 59:1–38. doi: 10.18637/jss.v059.i05
40. Clark CA, Cook K, Wang R, Rueschman M, Radcliffe J, Redline S, et al. Psychometric properties of a combined go/no-go and continuous performance task across childhood. psychol Assess. (2023) 35:353. doi: 10.1037/pas0001202
41. Liu H, Wen Y, Liang X, Xu Y, Qiao D, Yang C, et al. Prefrontal cortex neural activity predicts reduction of non-suicidal self-injury in adolescents with major depressive disorder: An event related potential study. Front Neurosci. (2022) 16:972870. doi: 10.3389/fnins.2022.972870
42. Anokhin AP, Golosheykin S, Grant JD, and Heath AC. Heritability of brain activity related to response inhibition: A longitudinal genetic study in adolescent twins. Int J Psychophysiol. (2017) 115:112–24. doi: 10.1016/j.ijpsycho.2017.03.002
43. Smith JL, Johnstone SJ, and Barry RJ. Movement-related potentials in the Go/NoGo task: the P3 reflects both cognitive and motor inhibition. Clin Neurophysiol. (2008) 119:704–14. doi: 10.1016/j.clinph.2007.11.042
44. Mbroh H, Zullo L, Westers N, Stone L, King J, Kennard B, et al. Double trouble: Nonsuicidal self-injury and its relationship to suicidal ideation and number of past suicide attempts in clinical adolescents. J Affect Disord. (2018) 238:579–85. doi: 10.1016/j.jad.2018.05.056
45. Joiner TE Jr., Van Orden KA, Witte TK, Selby EA, Ribeiro JD, Lewis R, et al. Main predictions of the interpersonal–psychological theory of suicidal behavior: Empirical tests in two samples of young adults. J Abnormal Psychol. (2009) 118:634. doi: 10.1037/a0016500
46. Egner T, Etkin A, Gale S, and Hirsch J. Dissociable neural systems resolve conflict from emotional versus nonemotional distracters. Cereb Cortex. (2008) 18:1475–84. doi: 10.1093/cercor/bhm179
47. Etkin A, Egner T, and Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. (2011) 15:85–93. doi: 10.1016/j.tics.2010.11.004
48. Huang Y, Yan R, Zhang Y, Wang X, Sun H, Zhou H, et al. Abnormal fractional amplitude of low-frequency fluctuations and regional homogeneity in major depressive disorder with non-suicidal self-injury. Clin Neurophysiol. (2024) 157:120–9. doi: 10.1016/j.clinph.2023.11.016
49. Koban L, Pichon S, and Vuilleumier P. Responses of medial and ventrolateral prefrontal cortex to interpersonal conflict for resources. Soc Cogn Affect Neurosci. (2014) 9:561–9. doi: 10.1093/scan/nst020
Keywords: interpersonal relations, non-suicidal self-injury, event related potentials, electroencephalography, depression
Citation: Yoon S-H, Kim JS, Lee H-A, Lee W-S, Song YW and Shim S-H (2025) Electrophysiological characteristics in adolescents with non-suicidal self-injury: an event-related potential study and source analysis. Front. Psychiatry 16:1596035. doi: 10.3389/fpsyt.2025.1596035
Received: 19 March 2025; Accepted: 16 July 2025;
Published: 07 August 2025.
Edited by:
Matt Dobbertin, Boys Town National Research Hospital, United StatesReviewed by:
Kristina Meyer, Charité University Medicine Berlin, GermanyDezhong Yao, University of Electronic Science and Technology of China, China
Copyright © 2025 Yoon, Kim, Lee, Lee, Song and Shim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Se-Hoon Shim, c2hzaGltMmtAZGF1bS5uZXQ=
†These authors have contributed equally to this work and share first authorship
‡ORCID: Sung-Hoon Yoon, orcid.org/0009-0000-1375-7414
Ji Sun Kim, orcid.org/0000-0003-2472-4591
Hyeon-Ah Lee, orcid.org/0000-0002-7178-9200
Woo-Seung Lee, orcid.org/0009-0001-5919-7632
Young Wook Song, orcid.org/0009-0008-7289-3262
Se-Hoon Shim, orcid.org/0000-0002-3137-6591
 Sung-Hoon Yoon
Sung-Hoon Yoon Ji Sun Kim
Ji Sun Kim Hyeon-Ah Lee
Hyeon-Ah Lee Woo-Seung Lee
Woo-Seung Lee Young Wook Song
Young Wook Song Se-Hoon Shim
Se-Hoon Shim