- 1Office of Medical Student Research, Oklahoma State University Center for Health Sciences, Tulsa, OK, United States
- 2Department of Surgery, University of Kansas Medical Center, Kansas City, KS, United States
- 3Department of Obstetrics, Gynecology and Reproductive Sciences, UTHealth Houston, Houston, TX, United States
- 4Department of Obstetrics and Gynecology, Baylor Scott and White Medical Center, Temple, TX, United States
- 5Department of Internal Medicine, Oklahoma State University Medical Center, Tulsa, OK, United States
- 6Department of Anesthesiology, University of Oklahoma College of Medicine, Oklahoma City, OK, United States
- 7Department of Psychiatry and Behavioral Sciences, Oklahoma State University Center for Health Sciences, Tulsa, OK, United States
Background: Naltrexone is a pharmacological intervention widely used for alcohol use disorder (AUD), opioid use disorder (OUD), and several off-label conditions. Systematic reviews (SRs) play a critical role in synthesizing data on the efficacy and safety of such interventions to inform clinical guidelines and decision-making. However, adequate reporting of harms in SRs remains inconsistent, limiting the ability to fully assess the safety profile of naltrexone. This study evaluates completeness of harms reporting and methodological quality in SRs focusing on naltrexone.
Methods: A comprehensive search of MEDLINE, EMBASE, Epistemonikos, and the Cochrane Database of Systematic Reviews was conducted. The study employed masked, duplicate screening and data extraction. Included SRs were evaluated for completeness of harms reporting using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) harms checklist and other established frameworks. Methodological quality was appraised using the A MeaSurement Tool to Assess Systematic Reviews-2 (AMSTAR-2) tool, and primary study overlap among SRs was assessed through corrected covered area (CCA) analysis.
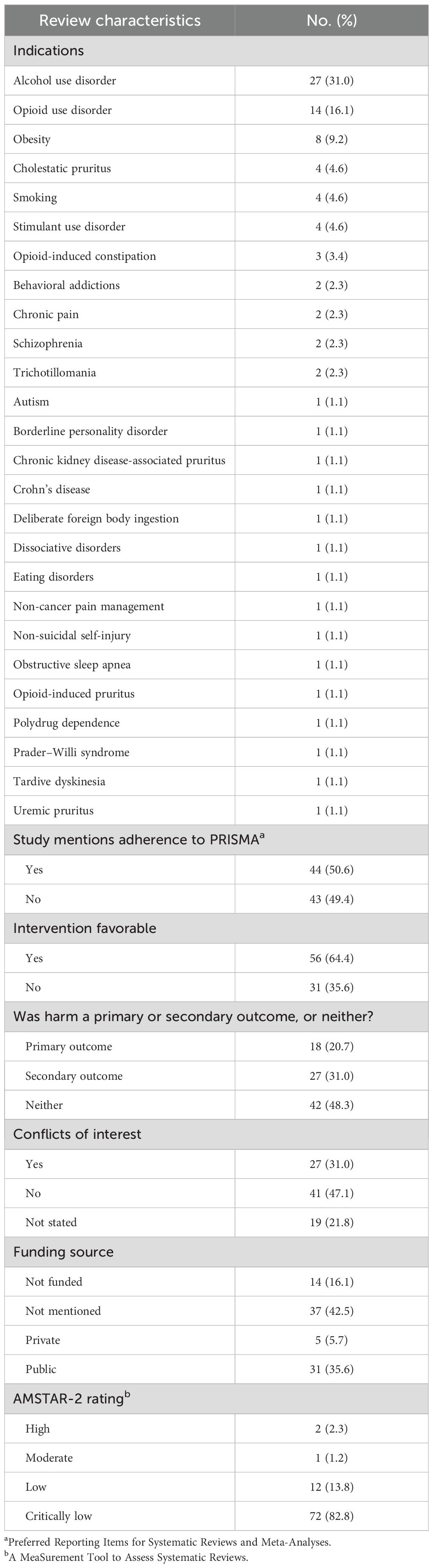
Results: A total of 87 SRs were included in the analysis. Only 1.1% (1/87) utilized severity scales to classify harms, and 4.6% (4/87) defined harms in their methods. Nearly half (48.3%) of SRs failed to address harms as either a primary or secondary outcome. A total of 82.8% (72/87) of SRs were rated as “critically low” quality by AMSTAR-2. Statistical analysis revealed a significant relationship between “critically low” AMSTAR-2 ratings and incomplete harms reporting (p = 0.0486). Additionally, four SR pairs demonstrated “high” overlap (>50%) of primary studies, accompanied by inconsistencies in harms reporting.
Conclusion: Our findings underscore the critical need for improved and standardized harms reporting in SRs on naltrexone. Inconsistent and incomplete reporting limits the ability of clinicians to fully assess the safety profile of naltrexone within systematic reviews. Adopting established frameworks such as PRISMA harms extensions and severity scales is imperative to enhance transparency and reliability in SRs. This study advocates for methodological improvements in SRs to support comprehensive safety evaluations and evidence-based prescribing of naltrexone.
1 Introduction
Harms reporting is crucial for interventions with rapidly expanding indications and recently updated literature. For example, naltrexone has been approved by the Food and Drug Administration (FDA) as an oral formulation for the treatment of alcohol use disorder (AUD) since 1984 and as an extended-release intramuscular injectable to treat both AUD and opioid use disorder (OUD) since 2006 (1, 2). Importantly, newer indications such as obesity and dermatologic conditions have been documented (3, 4). Given the growing list of possible indications for naltrexone therapy, medical literature, specifically systematic reviews (SRs), must provide a balanced reporting of benefits and harms, as SRs commonly underpin clinical practice guidelines, which guide clinical decision-making. Reporting complications of naltrexone is important for clinicians to adequately interpret the drug’s full safety profile. Furthermore, it has been documented that patients with higher levels of the urinary metabolite of naltrexone, 6-beta-naltrexol, experienced several side effects (including nausea, headache, anxiety, and erection), necessary information for physicians to consider when prescribing naltrexone (5).
SRs are the highest form of evidence offered within medical literature. However, SRs have demonstrated several inconsistencies, especially with regard to reporting outcomes data (6–8). Qureshi et al. also reported on such inconsistencies, finding that SRs often fail to capture the entirety of adverse events, such as rate, severity, and timing (9). Omitting results or failing to completely report information is critical, as harms data may allow readers to reach inaccurate conclusions that have downstream effects on clinical decision-making and, ultimately, patient care.
Systematic reviews have the unique ability to synthesize relevant studies on a particular topic and can draw timely and informative summary effects (10). They are often a reference source for physicians to ensure that their clinical decisions are high-quality and evidence-based (11–13). Several established reporting guidelines specifically address adverse effect reporting—Consolidated Standards of Reporting Trials (CONSORT) harms for randomized trials, Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) harms for systematic reviews, and the Cochrane Handbook chapter on adverse effects—which specify key items such as prespecifying adverse events, ascertainment methods, appropriate denominators, severity grading, and balanced presentation. However, adherence remains inconsistent (14, 36–39). To our knowledge, no studies thus far have analyzed the extent to which SRs on naltrexone address harms. Thus, we aim to 1) evaluate harms reporting in SRs on naltrexone, 2) determine if any relationships exist between completeness of harms reporting and study characteristics, and 3) evaluate the reporting of harms between SRs with common primary studies.
2 Methods
2.1 Study design
This cross-sectional analysis followed the PRISMA guidelines (15, 16). Our study was not subject to Institutional Review Board (IRB) approval, as it did not involve human subjects.
2.2 Harms terminology
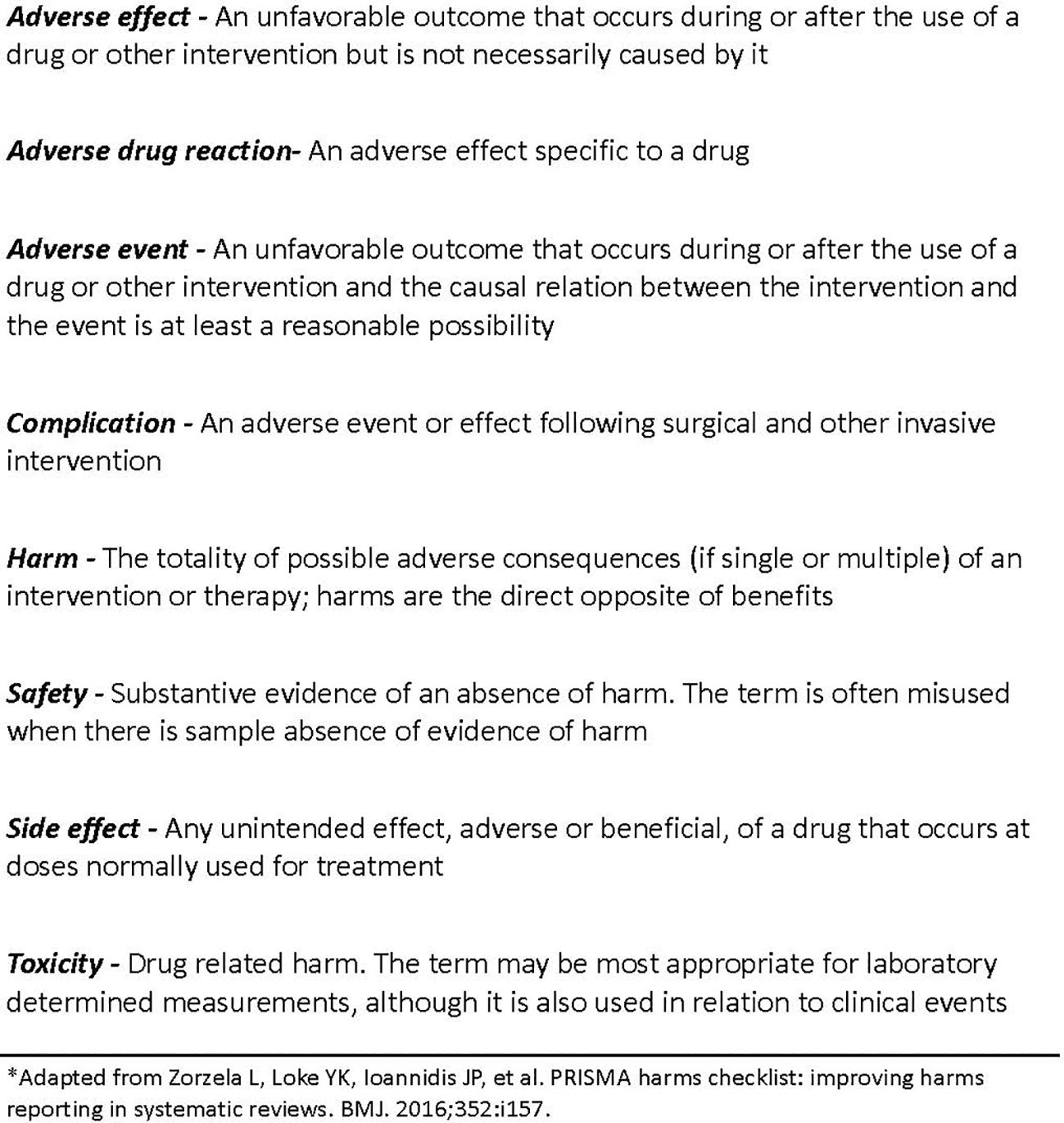
In accordance with the PRISMA harms group, we used terms and definitions for harms displayed in Figure 1 (17).
2.3 Search strategy
An SR librarian developed a search string to search the databases MEDLINE (PubMed and Ovid), EMBASE, Epistemonikos, and Cochrane Database of Systematic Reviews. The strategies combined controlled vocabulary (e.g., MeSH Naltrexone in MEDLINE; Emtree naltrexone in EMBASE) with text words for the generic name, chemical synonyms (e.g., “naltrexone hydrochloride” and “N-cyclopropylmethylnoroxymorphone”), and brand names (e.g., ReVia, Vivitrol, Depade, Nodict, Trexan, and Vivitrex). Where available, we applied systematic review limits/filters (e.g., PubMed “Systematic Review” filter and database-specific SR limits). Afterward, we uploaded the records obtained to Rayyan (https://rayyan.qcri.org/), an SR screening platform. Two investigators (JS and LP) independently screened records in a masked, duplicate fashion for inclusion and removed all duplicates. Following title and abstract screening, investigators were unmasked, and any disagreements were resolved by a third-party investigator (MG).
2.4 Search string
The search string was uploaded to the Open Science Framework (OSF) (18).
2.5 Eligibility criteria
To be included in our sample, we required the following criteria: 1) the publication must be an SR regardless of having a meta-analysis or not, and 2) the SR must be designated to evaluate naltrexone for both FDA-approved uses (AUD and OUD) and off-label uses. Studies had to be in English and only include human subjects. Studies were excluded if they were not related to naltrexone or were not SRs.
2.6 Training
Two investigators (JS and LP) were trained on SRs via the Johns Hopkins Systematic Review course (19). Investigators were instructed on how to extract harms items from SRs in other fields of medicine using a pilot-tested Google Form. Training on A MeaSurement Tool to Assess Systematic Reviews-2 (AMSTAR-2) in video and lecture format was also provided. Data from the AMSTAR-2 tool were compiled and interpreted using a pilot-tested Google Form. Senior author MV—who has published a multitude of studies evaluating the methodology of SRs—led all training (20–23).
2.7 Data extraction
Two investigators (JS and LP) extracted study characteristics using a pilot-tested Google Form. The characteristics included title, journal, Rayyan ID, and nine variables to evaluate studies (e.g., whether harms were evaluated as an outcome and whether the SR mentioned adherence to PRISMA guidelines) (16). Using methods similar to those of Mahady and colleagues, the same investigators extracted the data items listed in Table 1 from included SRs, coding each item as “yes” or “no” (24). Using methods similar to those of Qureshi and colleagues, they also extracted the items listed in Table 2, again coding “yes” or “no” unless free response or multiple choice was required (9, 25, 26). All extraction was performed independently in masked duplicates; disagreements were resolved by discussion, with MG adjudicating as needed.
To quantify how much the included SRs relied on the same primary studies, we calculated the corrected covered area (CCA), which standardizes overlap by accounting for both the number of SRs and the number of unique studies (27). We first constructed a citation matrix listing all included SRs (columns) against all primary studies (rows), marking presence/absence. We then computed CCA = (C − U)/[(U × R) − U], where C is the total number of primary study citations across all SRs (sum of matrix entries), U is the number of unique primary studies, and R is the number of SRs. Higher CCA indicates greater redundancy of evidence across reviews. Following published guidance, we interpreted overlap as minimal (<20%), moderate (20%–50%), or high (>50%). For pairs of SRs with ≥50% overlap (high), we performed targeted, side-by-side comparisons (“dyads”) of harms reporting to evaluate consistency (e.g., whether similar adverse events, definitions, and severities were presented despite drawing on largely the same primary evidence).
The authors performed a quality appraisal of each SR using the AMSTAR-2 instrument (28). Each of the 16 items was scored as “yes”, “partial yes”, or “no” depending on whether all criteria were met, some criteria were met, or the criteria were insufficiently met to warrant “yes” or “partial yes”. Items 11, 12, and 15 pertain to SRs with a meta-analysis; reviews without a meta-analysis were therefore scored out of 13 rather than 16. AMSTAR-2 assigns overall confidence ratings based on the presence of critical and non-critical flaws: reviews with no or only one non-critical weakness were rated high, those with more than one non-critical weakness but no critical flaws were rated moderate, those with one critical flaw (with or without non-critical weaknesses) were rated low, and those with more than one critical flaw (with or without non-critical weaknesses) were rated critically low. Using these criteria, each SR in our sample was classified into a quality category using the AMSTAR-2 quality assessment generator.
2.8 Data analysis
The characteristics of included studies, harms data, and AMSTAR-2 data for all included SRs were reported in frequency and percentage. A bivariate analysis was performed to determine if any associations existed between quality rating, general characteristics, and harms reporting. The nature of the data (i.e., statistical assumptions and distributional qualities) influenced the choice of statistical test. A p-value less than or equal to 0.05 was considered significant. For the CCA, the following were reported: the number of primary studies across all SRs, the range of primary studies used by an included SR, and the number of primary studies reported in one, two or more, and five or more included SRs (26). Overall, CCA was calculated across all SRs. Lastly, in all pairs of SRs with a high overlap of primary studies, individual harms and reporting items were compared (27). Stata 16.1 (StataCorp, LLC, College Station, TX) was used for data analysis. Data scrubbing was conducted using Microsoft Excel.
2.9 Reproducibility
To maximize transparency and reproducibility, all study materials were publicly archived on the OSF (https://osf.io/zae45/) (18). The repository includes the full protocol with prespecified objectives, eligibility criteria, outcomes, and analysis plans; complete database search strategies; the deidentified, raw screening and extraction datasets; the pilot-tested extraction forms used by investigators; and the statistical code used for the corrected covered area analysis. Screening and data extraction were conducted independently in masked duplicates, with disagreements resolved by consensus or third-party adjudication; AMSTAR-2 assessments followed the same process. Version history is preserved in the OSF to document any updates to methods or data, and all materials are available to enable verification, replication, and extension of our analyses.
3 Results
3.1 Screening process
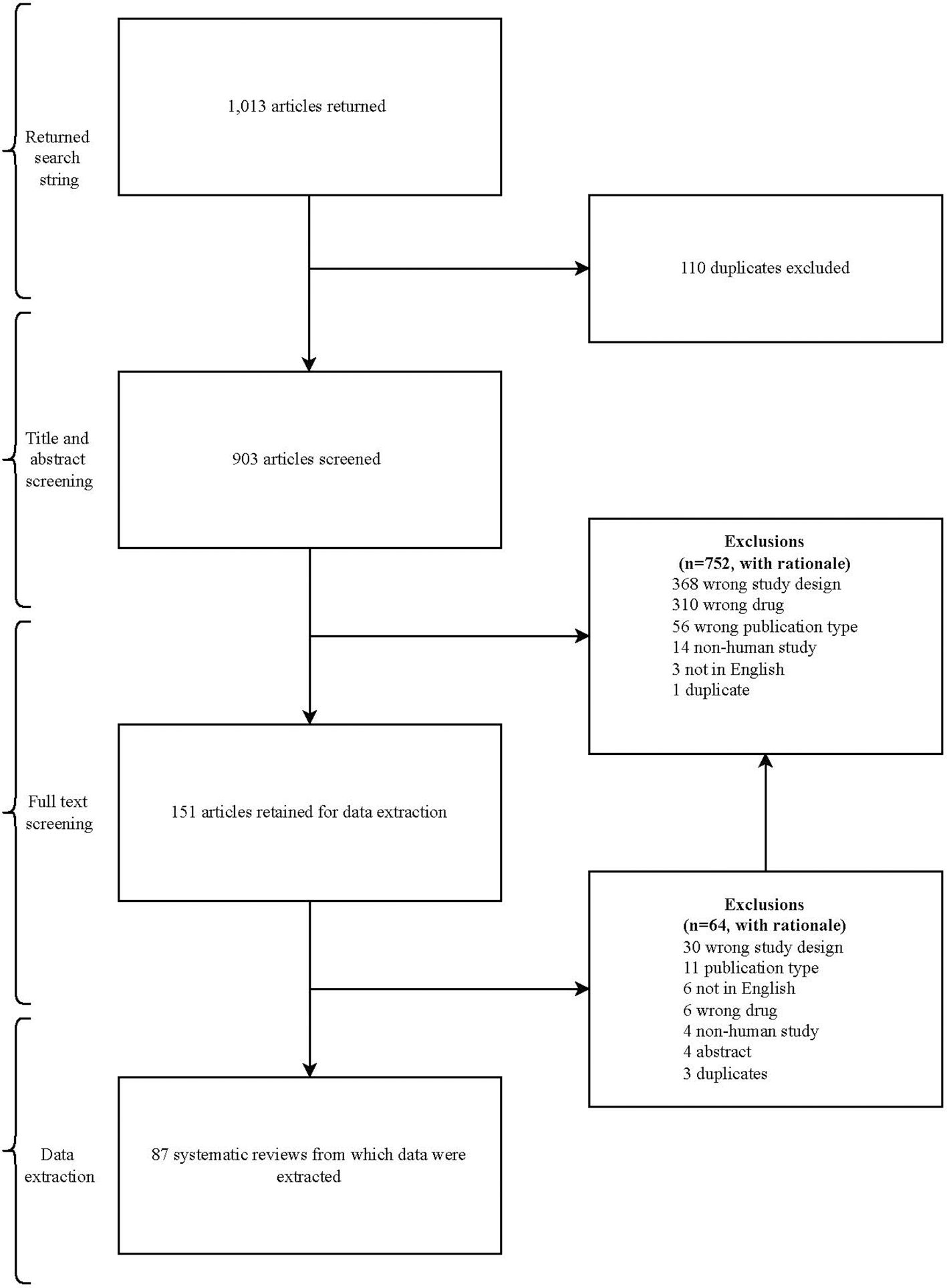
Our search returned 1,013 articles. After duplicates were removed, 903 articles were eligible for title and abstract screening. An additional 752 articles were excluded, leaving 151 articles eligible for full-text review. The reasons for exclusion in each phase of the screening process are presented in Figure 2.
3.2 Characteristics of included studies
A total of 87 SRs were included. Of the 87 SRs, 44 (44/87, 50.6%) reported adherence to PRISMA, 56 (56/87, 64.4%) found naltrexone as a favorable intervention, and 37 (37/87, 42.5%) did not report a funding source. Additionally, 18 SRs (18/87, 20.7%) reported harms as a primary outcome, 27 (27/87, 31.0%) reported harms as a secondary outcome, and 42 (42/87, 48.3%) did not report harms as a primary or secondary outcome. The general characteristics of included SRs can be found in Table 3.
3.3 Harms extraction
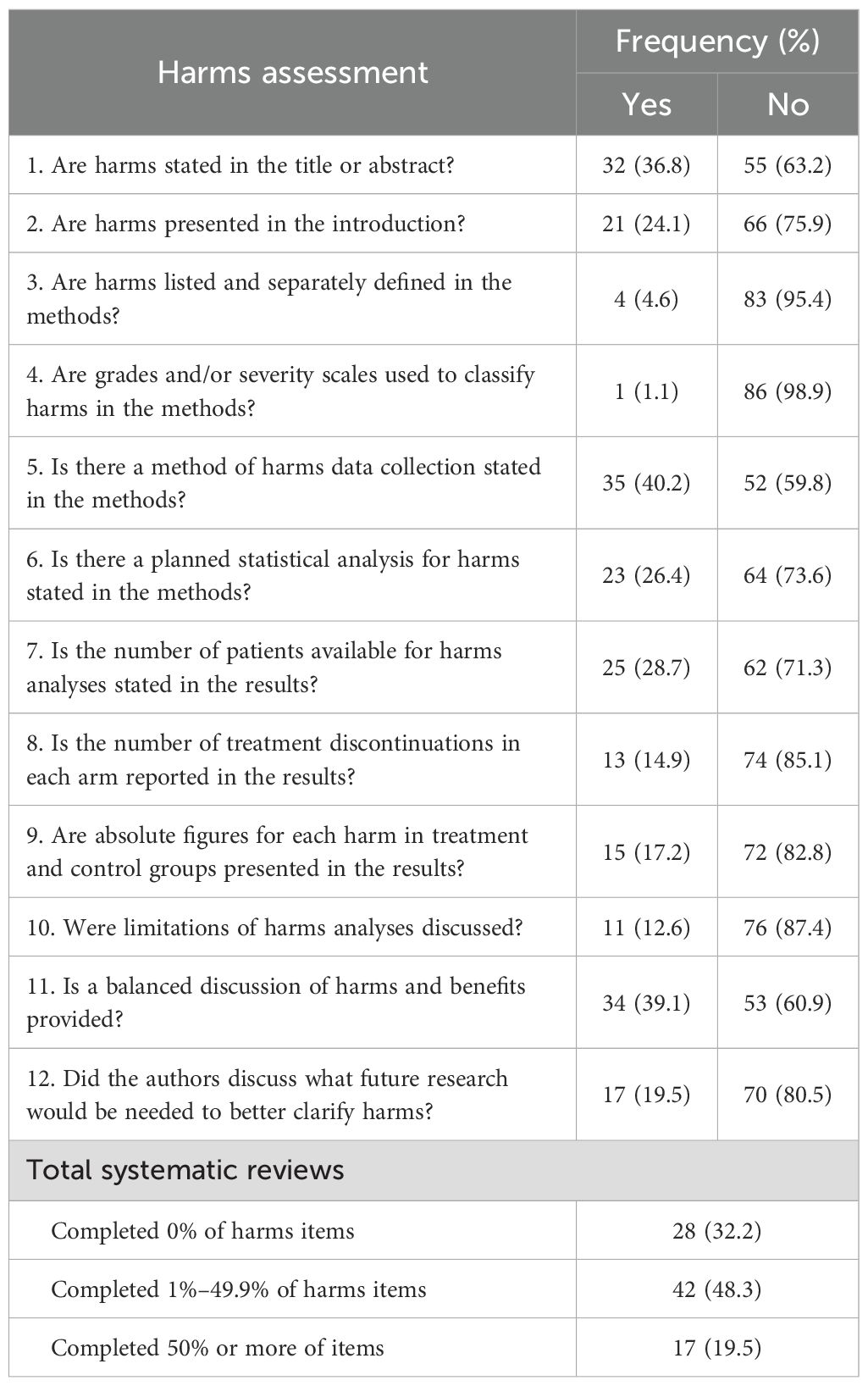
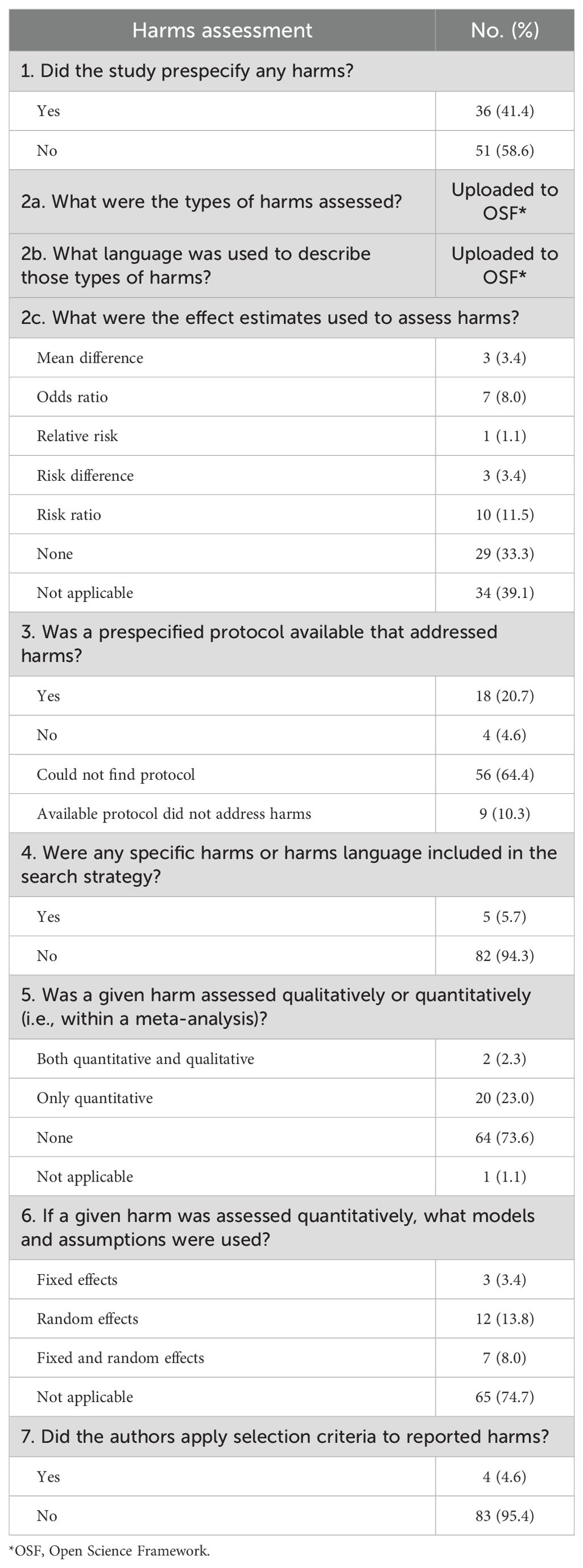
Of the 87 SRs in our analysis, one SR (1/87, 1.1%) classified grades/severity scales for harms in the methods, and four SRs (4/87, 4.6%) listed and separately defined harms in the methods. We found that 11 SRs (11/87, 12.6%) of the included studies discussed limitations to assessing harms. Five SRs (5/87, 5.7%) included harms language in their search strategies, 18 SRs (18/87, 20.7%) followed a protocol that addressed harms, and 36 (36/87, 41.4%) prespecified harms. A total of 17 SRs completed 50% or more of harms items (17/87, 19.5%). A comprehensive list of evaluated harms items can be found in Tables 1 and 2.
3.4 Corrected covered area
Of our 87 included SRs, our CCA analysis included primary studies from 85 SRs. In total, 2,475 primary studies were cited. The total number of unique primary studies included across all SRs was 1,791. The fewest number of primary studies cited by an SR was 2, and the most was 151. Of our 85 included SRs for CCA analysis, there were 1,463 primary studies cited once. There were 284 primary studies cited in two to four SRs and 44 primary studies cited in five or more SRs. For the eligible 85 SRs, the overall CCA was 0.45%. Four dyads were considered “high” overlap, 35 dyads were considered “moderate” overlap, and the remaining dyads were considered “minimal” overlap. The results of CCA are found in Table 4.
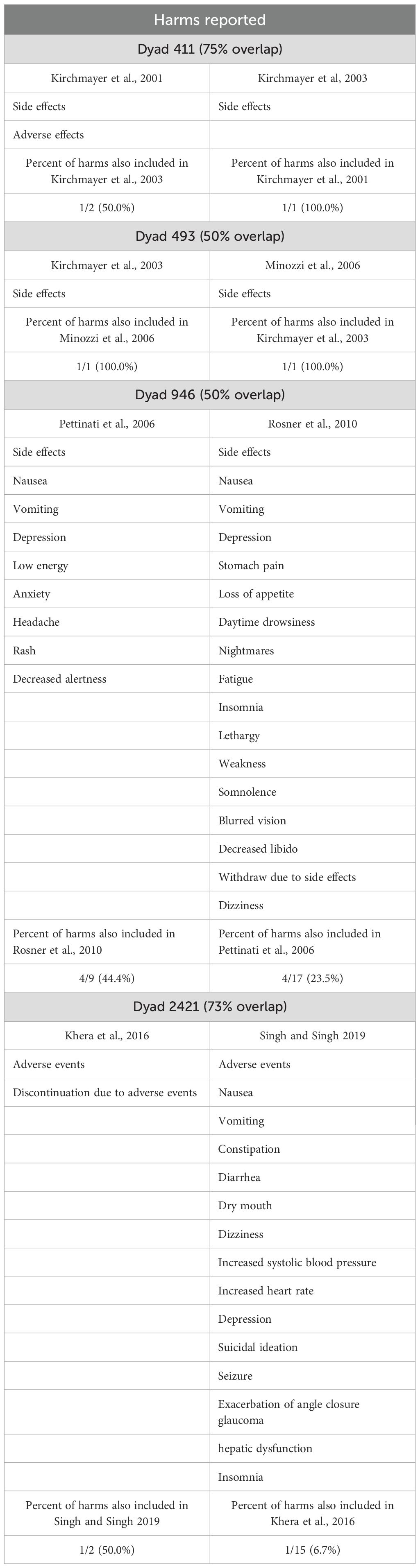
Table 4. Naltrexone harms reported by the paired reviews with a corrected covered area (CCA) >50% (n = 4 pairs of reviews).
3.5 AMSTAR-2 assessment
Of the 87 included SRs, two SRs (2/87, 2.3%) were graded as “high” quality, one SR (1/87, 1.1%) was graded as “moderate” quality, 12 SRs (12/87, 13.8%) were graded as “low” quality, and 72 (72/87, 82.8%) were graded as “critically low” quality (Table 3).
3.6 Associations
A Kruskal–Wallis test showed a significant relationship between studies graded “critically low” via AMSTAR-2 and completeness of harms reporting (p = 0.0486). Also, a significant relationship was found between studies that specified harms as an outcome and completeness of harms reporting (p = 0.0001). No significant association was determined between completeness of harms reporting and whether the SR reported adherence to PRISMA.
4 Discussion
We observed a lack of harms reporting in SRs concerning naltrexone—19.5% of our included SRs reported on half or more of the assessed harms items, and 28 SRs made no mention of harms (24). Most SRs in our sample failed to address harms within the methodology, specifically in regard to classifying and listing harms. Of concern, only one SR used a grade or severity scale for classifying harms. Our findings suggest that harms reporting is scarce, and improvements are needed to provide clinicians with accurate and complete safety profiles regarding naltrexone. Here, we discuss our findings along with relevant studies, give examples of underreported harms items as well as their implications, and provide recommendations to improve reporting.
In accordance with our findings, studies have previously shown that harms reporting is deficient in SRs. For example, Papanikolaou and Ioannidis conducted a study examining SRs published in the Cochrane Database and found that of the 138 SRs with at least 4,000 subjects, 77 SRs reported no harms data (29). Furthermore, the authors found that when harms reporting was deficient in a given SR, specific harms were presented adequately in 29% of the primary studies, suggesting that failure to report harms took place not only at the SR level (29). Additionally, Mahady and their colleagues looked at 78 gastroenterology SRs and found that one-third of the included SRs did not address harms at all and that the number of figures on harms was lacking, especially compared to the number of figures on efficacy (24). The results of these studies, along with ours, suggest that underreporting of harms is prevalent.
In our CCA analysis, we found that many of our included SRs cited the same primary studies. For example, Dyad 2421 shared 73% of the cited primary studies. However, harms reporting was very different. This dyad discussed adverse events and discontinuations due to adverse events in one SR, while the other SR discussed adverse events, such as nausea, vomiting, constipation, diarrhea, dry mouth, dizziness, increased blood pressure and heart rate, depression, suicidal ideation, seizure, exacerbation of angle closure glaucoma, hepatic dysfunction, and insomnia. This suggests the possibility of reporting bias of harms among SRs concerning naltrexone and that improvements in harms reporting in SRs are needed to reduce such inconsistency.
In our study, almost all SRs failed to use grades or severity scales to classify harms. This finding is not benign and may have multiple downstream effects. The Substance Abuse and Mental Health Services Administration (SAMHSA) reports “common” side effects (nausea, headache, etc.) and “serious” side effects (pain, tissue death requiring surgery, etc.) of naltrexone (30). Interestingly, “serious” side effects are not defined. Thus, clinicians and researchers are left to speculate on the true severity of a “serious” side effect. To mitigate this uncertainty, other studies have applied severity scales to classify and define harms. For example, a study evaluating brodalumab for the treatment of psoriasis used the Columbia-Suicide Severity Rating Scale (C-SSRS) to determine if suicidal ideations and behaviors were related to initiating pharmacotherapy (31). By reviewing results provided by this scale, the authors were able to conclude that suicidal ideations and behaviors were likely unrelated to brodalumab. We argue that the implementation of standardized scales is crucial to SRs owing to the ease of data synthesis when combining similar harms from primary studies. Moreover, the use of severity scales allows SR authors to provide a meaningful discussion on harms along with the translation of harms into clinical decision-making.
Furthermore, the classification of harms provides clinicians with additional information when determining the best plan of care for a patient. For example, one side effect of naltrexone classified as “serious” is a depressed mood. This particular harm poses unique challenges to clinicians, as depressed mood may be a side effect of treatment or related to a given diagnosis. Linden expanded on these challenges by stating that within the field of psychiatry, there is inherent difficulty in differentiating side effects, as they may be attributable to patient behavior. Linden also described the use of a checklist Unwanted Event–Adverse Treatment Reaction checklist (UE-ATR) to record, monitor, and classify adverse events related to psychotherapy (32). Applying a similar checklist to pharmacological therapy may encourage clinicians to account for harms with great accuracy and allow for a standard comparison of harms. Use of a standardized checklist would likely reduce the burden of characterizing ambiguous harms, especially in higher-complexity cases that require multiple therapies.
4.1 Recommendations
Because our study found deficiencies in harms reporting on naltrexone, we first recommend an overall improvement in harms reporting. This could be attained by adherence to standardized methods of harms reporting, such as PRISMA and CONSORT harms extensions (17, 33). Second, we suggest improvements be made to SRs using grades or severity scales when reporting harms to reduce ambiguity. Petrova et al. and Koh et al. discussed potential methods and tools for reporting the severity of harms of medical interventions (34, 35).
Furthermore, to reduce ambiguity and improve comparability, systematic reviews could prespecify and apply standardized grading frameworks for adverse events [e.g., map events to Common Terminology Criteria for Adverse Events (CTCAE) (5-point grades) and use the Naranjo Algorithm for causality when attribution is unclear], classify suicidal ideation/behavior using C-SSRS, and use systems such as ABACUS for general drug reaction classification (40–42). Practically, protocols could name target scales a priori; define how non-standard labels (e.g., “serious”, “severe”, and “clinically significant”) will be mapped to scale grades, extract, and report both counts and grade distributions (e.g., Grade ≥3); and use consistent denominators and exposure windows for grade-stratified summaries. Applying these frameworks standardizes terminology, clarifies thresholds for seriousness, and supports meta-analysis where appropriate, thereby improving clarity, reproducibility, and clinical interpretability of harms reporting. Notably, 82.7% of SRs in our sample were rated “critically low” using AMSTAR-2, underscoring the need for better methods; until harms reporting improves, clinicians should exercise caution with naltrexone and monitor patients closely.
To operationalize these recommendations, future SRs could register a protocol that prespecifies adverse-event definitions/lists, ascertainment windows, severity grading (with protocol-listed scales and explicit mapping rules for non-standard terms), denominators/time-at-risk, and rules for zero-event data to reduce selective reporting and clarify rate calculations; expand information sources beyond trials to include long-term extensions, observational cohorts/registries, and post-marketing surveillance to better capture long-latency or infrequently collected harms; use dual, standardized extraction that records adverse event (AE) definition, assessment method, grade, timing window, denominator, exposure duration, and whether the AE was prespecified to increase accuracy and comparability; synthesize using both counts and rates and, for rare events, prespecify effect measures and sensitivity analyses or provide a structured narrative when meta-analysis is inappropriate to yield stable, transparent estimates; and report according to PRISMA harms with balanced presentation and public sharing of extraction sheets/code to strengthen transparency and reproducibility. Collectively, these steps improve capture of long-latency or infrequently collected harms, increase completeness and comparability, and enhance interpretability and reproducibility for clinicians.
4.2 Strengths and limitations
Addressing study strengths, we executed a study design created specifically for transparency and reproducibility. Documenting our strategies prior to starting the project, we uploaded a detailed protocol to the OSF for reference (18). We routinely uploaded any changes, updates, or modifications. Additionally, we worked with an SR librarian to develop a search strategy including numerous bibliographic databases responsible for routinely cataloging reviews. Screening for harms and AMSTAR-2 in a masked, duplicate fashion allowed the authors to extract accurate data. While there were many strengths within our study, some limitations are noted.
Our analyses are limited to harms collected and reported in the included trials and SRs; because randomized trials often have restricted eligibility and short follow-up, long-term or infrequently captured adverse events may be underrepresented, and complementary sources (e.g., long-term extensions, observational cohorts, registries, and post-marketing surveillance) may be required to detect them. Unclear or unreported items were coded as not reported per prespecified rules (no imputation), which likely biases completeness estimates downward and may amplify between-review differences. Although extraction and AMSTAR-2 ratings were performed in masked duplicates with adjudication, some judgments remain partially subjective. Two SRs lacked full primary study lists and were excluded from the CCA, which could modestly affect overlap estimates and dyad composition. Generalizability is limited due to the cross-sectional nature of our study. Additionally, the quality assessment used AMSTAR-2, a checklist developed in 2012; therefore, studies published prior to this could not follow this set of guidelines.
5 Conclusion
Our analysis found the harms of naltrexone to be underreported in SRs. Considering the important role of SRs in medicine, harms should be well-reported. Standardized reporting methods currently exist that could improve harms reporting, but adherence to them is lacking. The benefits and harms of naltrexone should influence clinical decision-making when using the medication. However, until harms reporting is more complete, including defined grades/severity scales, properly informed decisions on the use of naltrexone are deficient.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
JS: Writing – original draft, Writing – review & editing. LP: Writing – original draft, Writing – review & editing. MG: Writing – original draft, Writing – review & editing. HF: Writing – original draft, Writing – review & editing. AW: Writing – original draft, Writing – review & editing. MK: Writing – original draft, Writing – review & editing. BR: Writing – original draft, Writing – review & editing. AK: Writing – original draft, Writing – review & editing. JB: Project administration, Supervision, Writing – original draft, Writing – review & editing. MV: Conceptualization, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We are grateful to Dr. Courtney Kennedy for assisting who assisted in the development of our search strategy and to the OSU Medical Library for their procurement of relevant literature. We are grateful for the CCA guidance and code provided by Dr. Riaz Qureshi.
Conflict of interest
MV reports receipt of funding from the National Institute on Drug Abuse, the National Institute on Alcohol Abuse and Alcoholism, the US Office of Research Integrity, the Oklahoma Center for Advancement of Science and Technology, and internal grants from the Oklahoma State University Center for Health Sciences—all outside of the present work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2025.1597019/full#supplementary-material
Abbreviations
AUD, alcohol use disorder; OUD, opioid use disorder; SR, systematic review; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; AMSTAR-2, A MeaSurement Tool to Assess Systematic Reviews-2; CCA, corrected covered area.
References
1. Substance Abuse and Mental Health Services Administration (US). Chapter 3C: Naltrexone. Rockville, MD: Substance Abuse and Mental Health Services Administration (US) (2018).
2. Stewart J. Vivitrol (naltrexone) FDA Approval History. Auckland, New Zealand: Drugs.com (2021). Available online at: https://www.drugs.com/history/vivitrol.html.
3. Coulter AA, Rebello CJ, and Greenway FL. Centrally acting agents for obesity: Past, present, and future. Drugs. (2018) 78:1113–32. doi: 10.1007/s40265-018-0946-y
4. Lee B and Elston DM. The uses of naltrexone in dermatologic conditions. J Am Acad Dermatol. (2019) 80:1746–52. doi: 10.1016/j.jaad.2018.12.031
5. King AC, Volpicelli JR, Gunduz M, O’Brien CP, and Kreek MJ. Naltrexone biotransformation and incidence of subjective side effects: a preliminary study. Alcohol Clin Exp Res. (1997) 21:906–9. doi: 10.1097/00000374-199708000-00020
6. Ernst E and Pittler MH. Assessment of therapeutic safety in systematic reviews: literature review. BMJ. (2001) 323:546. doi: 10.1136/bmj.323.7312.546
7. Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomized controlled trials on a cohort of systematic reviews. BMJ. (2010) 340:c365. doi: 10.1136/bmj.c365
8. McIntosh HM, Woolacott NF, and Bagnall A-M. Assessing harmful effects in systematic reviews. BMC Med Res Methodol. (2004) 4:19. doi: 10.1186/1471-2288-4-19
9. Qureshi R, Mayo-Wilson E, and Li T. Harms in systematic reviews paper 1: An introduction to research on harms. J Clin Epidemiol. (2022) 143:186–96. doi: 10.1016/j.jclinepi.2021.10.023
10. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. (2015) 4:1. doi: 10.1186/2046-4053-4-1
11. Barry HC, Ebell MH, Shaughnessy AF, Slawson DC, and Nietzke F. Family physicians’ use of medical abstracts to guide decision making: style or substance? J Am Board Fam Pract. (2001) 14:437–42.
12. Marcelo A, Gavino A, Isip-Tan IT, Apostol-Nicodemus L, Mesa-Gaerlan FJ, Firaza PN, et al. A comparison of the accuracy of clinical decisions based on full-text articles and journal abstracts alone: a study among residents in a tertiary care hospital. Evid Based Med. (2013) 18:48–53. doi: 10.1136/eb-2012-100537
13. Morrow AS, Whiteside SP, Sim LA, Brito JP, Wang Z, and Murad MH. Developing tools to enhance the use of systematic reviews for clinical care in health systems. BMJ Evid Based Med. (2018) 23:206–9. doi: 10.1136/bmjebm-2018-110995
14. Cornelius VR, Perrio MJ, Shakir SAW, and Smith LA. Systematic reviews of adverse effects of drug interventions: a survey of their conduct and reporting quality. Pharmacoepidemiol Drug Saf. (2009) 18:1223–31. doi: 10.1002/pds.1844
15. Moher D, Liberati A, Tetzlaff J, Altman DG, and PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. (2009) 339:b2535. doi: 10.1136/bmj.b2535
16. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
17. Zorzela L, Loke YK, Ioannidis JP, Golder S, Santaguida P, Altman DG, et al. PRISMA harms checklist: improving harms reporting in systematic reviews. BMJ. (2016) 352:i157. doi: 10.1136/bmj.i157
18. Wise A, Kee MD, Rucker B, Flores H, Garrett M, Purcell L, et al. Naltrexone. Open Science Framework. Charlottesville, VA (USA): Open Science Framework (Center for Open Science) (2022). doi: 10.17605/OSF.IO/ZAE45.
19. Coursera. Introduction to Systematic Review and Meta-Analysis . Available online at: https://www.coursera.org/learn/systematic-review (Accessed February 22, 2022).
20. Aran G, Hicks C, Demand A, Johnson AL, Beaman J, Bailey Y, et al. Treating schizophrenia: the quality of evidence behind treatment recommendations and how it can improve. BMJ Evid Based Med. (2020) 25:138–42. doi: 10.1136/bmjebm-2019-111233
21. Detweiler BN, Kollmorgen LE, Umberham BA, Hedin RJ, and Vassar BM. Risk of bias and methodological appraisal practices in systematic reviews published in anaesthetic journals: a meta-epidemiological study. Anaesthesia. (2016) 71:955–68. doi: 10.1111/anae.13520
22. Jacobsen SM, Douglas A, Smith CA, Roberts W, Ottwell R, Oglesby B, et al. Methodological quality of systematic reviews comprising clinical practice guidelines for cardiovascular risk assessment and management for noncardiac surgery. Br J Anaesth. (2021) 127:905–16. doi: 10.1016/j.bja.2021.08.016
23. Scott J, Howard B, Sinnett P, Schiesel M, Baker J, Henderson P, et al. Variable methodological quality and use found in systematic reviews referenced in STEMI clinical practice guidelines. Am J Emerg Med. (2017) 35:1828–35. doi: 10.1016/j.ajem.2017.06.010
24. Mahady SE, Schlub T, Bero L, Moher D, Tovey D, George J, et al. Side effects are incompletely reported among systematic reviews in gastroenterology. J Clin Epidemiol. (2015) 68:144–53. doi: 10.1016/j.jclinepi.2014.06.016
25. Qureshi R, Mayo-Wilson E, Rittiphairoj T, McAdams-DeMarco M, Guallar E, and Li T. Summaries of harms in systematic reviews are unreliable (Part 1 of 2): Methods used to assess harms are neglected in systematic reviews of gabapentin. New York, NY (USA): Elsevier Inc. (2021). doi: 10.31219/osf.io/7g4ez.
26. Qureshi R, Mayo-Wilson E, Rittiphairoj T, McAdams-DeMarco M, Guallar E, and Li T. Summaries of harms in systematic reviews are unreliable Paper 3: Given the same data sources, systematic reviews of gabapentin have different results for harms. J Clin Epidemiol. (2021) 143:224–41. doi: 10.1016/j.jclinepi.2021.10.025
27. Hennessy EA and Johnson BT. Examining overlap of included studies in meta-reviews: Guidance for using the corrected covered area index. Res Synth Methods. (2020) 11:134–45. doi: 10.1002/jrsm.1390
28. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomized or non-randomized studies of healthcare interventions, or both. BMJ. (2017) 358:j4008. doi: 10.1136/bmj.j4008
29. Papanikolaou PN and Ioannidis JP. Availability of large-scale evidence on specific harms from systematic reviews of randomized trials. Am J Med. (2004) 117:582–9. doi: 10.1016/j.amjmed.2004.04.026
30. Naltrexone. Available online at: https://www.samhsa.gov/medication-assisted-treatment/medications-counseling-related-conditions/naltrexone (Accessed June 22, 2022).
31. Lebwohl MG, Papp KA, Marangell LB, Koo J, Blauvelt A, Gooderham M, et al. Psychiatric adverse events during treatment with brodalumab: Analysis of psoriasis clinical trials. J Am Acad Dermatol. (2018) 78:81–9.e5. doi: 10.1016/j.jaad.2017.08.024
32. Linden M. How to define, find and classify side effects in psychotherapy: from unwanted events to adverse treatment reactions. Clin Psychol Psychother. (2013) 20:286–96. doi: 10.1002/cpp.1765
33. Ioannidis JP, Evans SJ, Gøtzsche PC, O’Neill RT, Altman DG, Schulz K, et al. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Ann Intern Med. (2004) 141:781–8. doi: 10.7326/0003-4819-141-10-200411160-00009
34. Koh Y, Yap CW, and Li SC. Development of a combined system for identification and classification of adverse drug reactions: Alerts Based on ADR Causality and Severity (ABACUS). J Am Med Inform Assoc. (2010) 17:720–2. doi: 10.1136/jamia.2010.006882
35. Petrova G, Stoimenova A, Dimitrova M, Kamusheva M, Petrova D, and Georgiev O. Assessment of the expectancy, seriousness and severity of adverse drug reactions reported for chronic obstructive pulmonary disease therapy. SAGE Open Med. (2017) 5:2050312117690404. doi: 10.1177/2050312117690404
36. Ioannidis JPA, Evans SJW, Gøtzsche PC, O’Neill RT, Altman DG, Schulz K, et al. CONSORT Group. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Ann Intern Med. (2004) 141:781–8. doi: 10.7326/0003-4819-141-10-200411160-00009
37. Junqueira DR, Zorzela L, Golder S, Loke Y, Gagnier JJ, Julious SA, et al. CONSORT Harms Group. CONSORT Harms 2022 statement, explanation, and elaboration: updated guideline for reporting harms in randomized trials. J Clin Epidemiol. (2023) 158:149–65. doi: 10.1016/j.jclinepi.2023.04.005
38. Zorzela L, Loke YK, Ioannidis JPA, Golder S, Santaguida P, Altman DG, et al. PRISMA Harms Group. PRISMA harms checklist: improving harms reporting in systematic reviews. BMJ. (2016) 352:i157. doi: 10.1136/bmj.i157
39. Peryer G, Golder S, Junqueira D, Vohra S, Loke YK, and on behalf of the Cochrane Adverse Effects Methods Group. Chapter 19: adverse effects. In: Higgins JPT, Thomas J, Chandler J, et al, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.5. London, United Kingdom: Cochrane (2024).
40. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). Version 5.0. Bethesda, MD, USA: National Cancer Institute (2017). Available online at: https://dctd.cancer.gov/research/ctep-trials/trial-development.
41. Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. (2011) 168:1266–77. doi: 10.1176/appi.ajp.2011.10111704
Keywords: naltrexone, systematic reviews, harms reporting, AMSTAR-2, PRISMA harms, adverse effects, cross-sectional analysis
Citation: Schnitker J, Purcell L, Garrett M, Flores H, Wise A, Kee M, Rucker B, Khan A, Beaman J and Vassar M (2025) Reporting of harms in systematic reviews focused on naltrexone: a cross-sectional study. Front. Psychiatry 16:1597019. doi: 10.3389/fpsyt.2025.1597019
Received: 20 March 2025; Accepted: 01 September 2025;
Published: 22 September 2025.
Edited by:
Stephen Lewis, Case Western Reserve University, United StatesReviewed by:
Siddharth Sarkar, All India Institute of Medical Sciences, IndiaTakuma Inagawa, National Center of Neurology and Psychiatry, Japan
Copyright © 2025 Schnitker, Purcell, Garrett, Flores, Wise, Kee, Rucker, Khan, Beaman and Vassar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adam Khan, QWRhbS5raGFuQG9rc3RhdGUuZWR1
 Joseph Schnitker1
Joseph Schnitker1 Adam Khan
Adam Khan