Abstract
Objective:
To understand the current status of research and development (R&D) of psychotropic drugs.
Methods:
Retrieved psychotropic drugs clinical trials (PDCTs) registered in China from 2019 to 2024 using the platform of chinadrugtrials.org.cn, and systematically analyzed the data.
Results:
Included four perspectives: 1) for general information, we screened 1377 PDCTs, with phase bioequivalency (BE) accounting for the majority (78.5%), covering 411 pharmaceutical companies and 212 leading institutions, and the start-up time in 2024 was significantly shortened (P < 0.05); 2) for indications, 11 indications were involved, with the highest number of PDCTs for depression (30.9%); 3) for drugs, 222 drugs were involved, of which 52 were innovative drugs (33 with disclosed targets), and 13 were improved new drugs with six administration routes; 4) for trial design, four exploratory designs were retrieved, including population pharmacokinetics (9), pharmacogenomics (12), biomarker detection (3), and drug combination (3).
Conclusions:
In recent years, clinical trials of psychotropic drugs in China have been developing. Innovative targets discovery, dosage forms/drug delivery systems optimization, and exploratory designs have the potential to break the current treatment dilemma. This study introduced the hotspots and potential development directions of psychotropic drugs R&D in China from the above aspects, providing new ideas for psychiatric treatment, drug development, and clinical trial methods.
1 Introduction
Psychiatric disorders have become one of the leading causes of disability in the world due to the high prevalence (1, 2). According to the World Health Organization (WHO), approximately 1 billion people worldwide suffer from psychiatric disorders, accounting for about 13.0% of the total population, with anxiety (ANX, 3.8%) and depression (DEP, 3.4%) being the most common (3). As the world’s second most population country, the lifetime prevalence of psychiatric disorders in China has risen from 1.3% in 1982 and 1.4% in 1993 to 16.6% in 2019, of which ANX (7.6%) and DEP (6.8%) being the main types (4). Worryingly, with the acceleration of urbanization process and the change of social structure, the global prevalence of psychiatric disorders still has the risk of continued rapid growth (5). Psychiatric disorders pose an increasing disease, societal, and economic burden, and have become a major health issue of common concern throughout the world.
However, in the face of such enormous and urgent demands for psychiatric disorders treatment, the clinical medication strategy update remain slowly, which is intrinsically linked to the complex nature of psychiatric disorders: (1) the etiology of psychiatric disorders is the results of the combined effects of biological, social, psychological, and environmental factors, making the mechanism complex and not fully revealed; (2) the therapeutic targets for psychiatric disorders are mainly distributed in the central nervous system (CNS), which means that drugs need to cross the blood-brain barrier (BBB) to exert their effects, and conventional administration routes may reduce efficacy while increasing side effects (6–8). Given these inherent biological challenges, despite investing huge funds and time, research and development (R&D) of drugs for psychiatric disorders has lagged far behind other disease areas over the past decades (9). Most of the psychotropic drugs currently in clinical practice still act through the mechanisms of the first- or second-generation drugs, targeting dopamine receptor (DR) or 5-hydroxytryptamine (5-HT) receptor, but due to significant individual differences and frequent side effects, patients have limited benefits (10–12). The rapid growth of prevalence and the slow progress of drug development mean that the existing psychotropic drugs have been difficult to meet the clinical needs, but also mean that there is still a lot of room for exploration in the pathological mechanism and treatment methods of psychiatric disorders. Therefore, for both the healthcare industry and pharmaceutical companies, developing psychotropic drugs with new mechanisms or optimization measures is of great significance.
Good Clinical Practice (GCP) provide evidence-based evaluation of drug efficacy and safety, and have been incorporated into many countries’ laws as the gold standard for new drugs development (13, 14). The changing trends and implementation status of clinical trials can provide significant information for the direction of new drug development, research resource allocation, and trial design. With the globalization of the pharmaceutical industries, the clinical trials field in Asia is flourishing (15). China has a large market for psychotropic drugs and promising prospects for R&D of new drugs, due to the increasing prevalence of psychiatric disorders (4, 16). Based on the above, it is an interesting topic to use clinical trials information to explore the current development status and research hot spots of drugs for psychiatric disorders in China. However, there are few related reports.
In this study, we systematically analyzed the psychiatric drug clinical trials (PDCTs) registered in China from 2019 to 2024, and evaluated research progress and development prospects through innovative targets, optimization approaches, and exploratory trial designs. By integrating these perspectives, we elucidated the latest R&D trends in China’s psychiatric drugs field, and provided the reference and decision-making basis for mechanism exploration, drug development, and trial planning.
2 Methods
2.1 Information sources and search strategy
Logged in to the http://www.chinadrugtrials.org.cn (http://www.chinadrugtrials.org.cn), and as of December 31, 2024, the platform has registered a total of 27902 drug clinical trials. The inclusion criteria for the trials were as follows: (1) the time range for the first announcement date is from January 1, 2019 to December 31, 2024; (2) the target indications for clinical trial drugs should include the psychiatric term(s) in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V). The exclusion criteria were as follows: (1) physiological disorders related to psychological factors (such as feeding and eating disorders, sleep-wake disorders, and sexual dysfunctions); (2) partial neurodevelopmental disease (such as intellectual disabilities, communication disorder, and specific learning disorder).
There were 1481 trials that met the inclusion criteria. After excluding 91 physiological disorders related to psychological factors and 13 neurodevelopmental disease, 1377 trials were ultimately selected for further analysis.
Logged in to the Center for Drug Evaluation (CDE) of China National Medical Products Administration (NMPA) (https://www.cde.org.cn/) to confirm the registration classification of the experimental drugs.
The http://www.chinadrugtrials.org.cn is a platform for the registration and public disclosure of drug clinical trials in China, which is supervised by the CDE. According to the Good Clinical Practice (GCP)-2020, a Chinese law jointly issued by the NMPA and the National Health Commission (NHC), which is used to regulate the entire process of drug clinical trials applied for registration in China (https://www.gov.cn/gongbao/content/2020/content_5525106.htm), all the trials disclosed on this platform have been approved by the Ethics Committee of the leading institutions. All raw data of this study were obtained from this platform.
2.2 Information extraction
Information extracted covered drug name, indication, drug category, phase, trial status, scope, sponsor, leading institution, registration classification, start-up time and so on. Among them, drug categories include chemical drug, biological agent, or traditional Chinese medicine (TCM)/natural medicine; phases include I, II, III, IV, bioequivalence (BE), or others (I/II or II/III); trial status includes in-progress (not recruited, recruiting, or recruitment completed), completed, or suspended/terminated; the scope includes domestic or international multi-center clinical trials; registration classification includes innovative drugs, improved new drugs, or generic drugs;start-up time = completion date of the first informed consent - approval date by the ethics committee of the leading institution.
Some clinical trials involved more than one indication, sponsor, or leading institution, whose information was independently collected.
2.3 Statistical analyses
Statistical analyses and graphs were performed by SPSS 22 software and GraphPad Prism 10 software. Normality test is performed using the Shapiro-Wilk (n ≤ 50) or Kolmogorov-Smirnov (n > 50) methods. Normal distribution data is represented by mean ± SD, while non-normal distribution data is represented by median (25th - 75th), and the t-test and the Mann-Whitney U test are used to calculate P values, respectively. P < 0.05 means statistically significance (two-tailed).
3 Results
3.1 General information on clinical trials
The flowchart diagram of this study was shown (Figure 1). A total of 1377 PDCTs were included in the study. Except for a slightly decrease in 2020, the number of registered PDCTs and their proportion in the total number of the CDE registered trials the same year showed an increasing trend (Figure 2A). The trials phases were mainly BE (78.5%, Figure 2B). There were 1184 trials registered the first informed consent time, with a start-up time ranging from 1–693 days and an average start-up time of 80 days. Compared with 2019-2023, the start-up time for phase III, IV, BE, and other trials in 2024 was significantly shortened (Table 1, P < 0.05).
Figure 1

Flowchart for screening and analysis of clinical trials for psychotropic drugs.
Figure 2
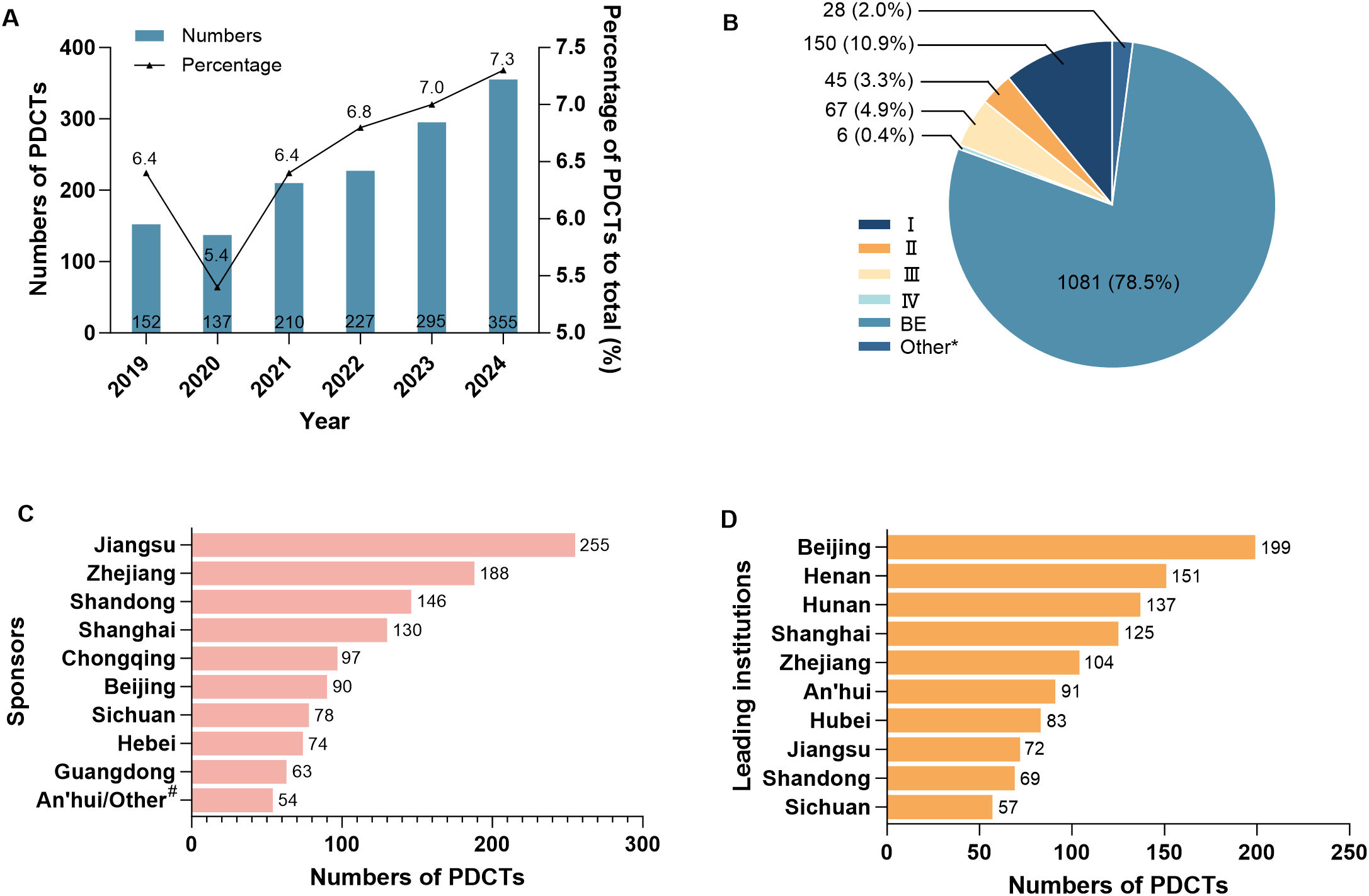
The general information on psychotropic drug clinical trials in China in 2019-2024. The registration trends (A) and phases (B) of PDCTs, as well as the geographical distribution of the top 10 sponsors (C) and leading institutions (D) in terms of registration numbers of PDCTs were displayed, respectively. A total of 1377 PDCTs were included, and some of which involved more than one sponsor or leading institution. *: phase I/II or II/III; #: global sponsors; PDCTs, psychiatric drug clinical trials; BE, bioequivalency.
Table 1
| Phase | Year (days) | P value | |
|---|---|---|---|
| 2019-2023 | 2024 | ||
| I, median (25th-75th) | 73 (43-120) | 59 (27-89) | 0.157 |
| II, median (25th-75th) | 95 (62-133) | 76 (21-147) | 0.447 |
| III, median (25th-75th) | 156 (97-217) | 55 (16-90) | 0.001 |
| IV, mean ± SD | 153 ± 54 | 58* | 0.040 |
| BE, median (25th-75th) | 57 (38-86) | 49 (36-64) | 2.40×10-5 |
| Other, median (25th-75th) | 118 (98-208) | 34 (1-75) | 0.002 |
The start-up time of psychiatric drug clinical trials in China in 2019-2024.
There were 1184 trials registered the first informed consent time. *: n = 1; BE, bioequivalency; Other: phase I/II or II/III. The mean ± SD and median (25th-75th) were used to present normal and non-normal distribution data, respectively. The One-sample t-test and the Mann-Whitney U test were used to calculate P values, respectively. Bold value indicates the statistical significance.
1346 of the 1377 clinical trials were initiated by domestic sponsors (alone or in collaboration), involving 370 domestic pharmaceutical companies distributed in 28 provinces and regions, with the highest number of the pharmaceutical companies located in Jiangsu (68). 53 trials were initiated by global sponsors (alone or in collaboration), involving 41 global pharmaceutical companies distributed in 13 countries and regions, with the highest number of pharmaceutical companies coming from the U.S. (17). There were 26 international multi-center trials, of which four were initiated by domestic sponsors alone, involving three companies located in Shanghai (2) and An’hui (1), respectively.
There were 212 leading institutions distributed in 27 provinces and regions, with the highest number of the leading institutions located in Beijing (199). Eight leading institutions undertook international multi-center trials, located in three provinces which were Beijing (5), Shanghai (2), and Shandong (1), respectively.
The geographical distribution of the top 10 sponsors (Figure 2C) and leading institutions (Figure 2D) in terms of the number of PDCTs was shown, respectively.
3.2 Indications
The 1377 clinical trials involved 11 indications, including 426 for DEP (30.9%), 306 for schizophrenia (SC, 22.2%), 195 for parkinsonism (PD, 14.2%), 155 for ANX(11.3%), 117 for Alzheimer’s disease (AD, 8.5%), 91 for bipolar disorder (BD, 6.6%), 51 for obsessive-compulsive disorder (OCD, 3.7%), 11 for withdrawal symptoms (WS, 0.8%), 11 for tourette syndrome (TS, 0.8%), 10 for delirium (0.7%), and four for autism (0.3%). The PDCTs information for those indications was presented (Table 2).
Table 2
| Indication | n * | Classification | Phase | Status | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemical drug | Biological agent | TCM/Natural medicine | I | II | III | IV | BE | Other | In Progress | Completed | Terminated/ Suspended | ||
| DEP | 426 | 418 | 0 | 8 | 43 | 19 | 12 | 1 | 346 | 5 | 122 | 291 | 13 |
| SC | 306 | 306 | 0 | 0 | 36 | 5 | 14 | 1 | 241 | 9 | 94 | 199 | 13 |
| PD | 195 | 193 | 2 | 0 | 30 | 8 | 10 | 0 | 144 | 3 | 68 | 127 | 0 |
| ANX | 155 | 151 | 0 | 4 | 3 | 2 | 6 | 1 | 141 | 2 | 35 | 117 | 3 |
| AD | 117 | 97 | 14 | 6 | 35 | 10 | 17 | 2 | 46 | 7 | 52 | 57 | 8 |
| BD | 91 | 91 | 0 | 0 | 1 | 0 | 5 | 0 | 84 | 1 | 22 | 63 | 6 |
| OCD | 51 | 51 | 0 | 0 | 0 | 0 | 2 | 0 | 49 | 0 | 7 | 44 | 0 |
| WS | 11 | 10 | 0 | 1 | 1 | 0 | 0 | 0 | 10 | 0 | 4 | 7 | 0 |
| TS | 11 | 9 | 0 | 2 | 0 | 0 | 1 | 1 | 9 | 0 | 3 | 7 | 1 |
| Delirium | 10 | 10 | 0 | 0 | 1 | 0 | 0 | 0 | 8 | 1 | 2 | 8 | 0 |
| Autism | 4 | 4 | 0 | 0 | 0 | 1 | 0 | 0 | 3 | 0 | 1 | 3 | 0 |
Drug clinical trial information on psychiatric disorders in China in 2019-2024.
DEP, depression; SC, schizophrenia; PD, parkinsonism; ANX, anxiety; AD, Alzheimer’s disease; BD, bipolar disorder; OCD, obsessive-compulsive disorder; WS, withdrawal symptoms; TS, tourette syndrome; TCM, traditional Chinese medicine; BE, bioequivalency; Other, phase I/II or II/III.
There were a total of 11 indications. Some clinical trials involved more than one indication. *: number of clinical trial registrations.
The categories of drugs for AD clinical trials were chemical drug (82.9%), biological agent (12.0%), and TCM/natural medicine (5.1%); the categories of drugs for DEP, ANX, WS, and TS clinical trials were chemical drug (98.1%, 97.4%, 90.9%, and 81.8%, respectively) and TCM/natural medicine (1.9%, 2.6%, 9.1%, and 18.2%, respectively); the categories of drugs for PD clinical trials were chemical drug (99.0%) and biologics agent (1.0%). The category of drugs for the remaining indications was chemical drug.
Clinical trials for AD had a high proportion of phase BE (39.3%) and I (30.0%), while other indications were dominated by phase BE. The completion rate of clinical trials for OCD and delirium was high, at 86.3% and 80.0%, respectively. The terminated/suspended rate of clinical trials for TS was the highest, at 9.1%.
3.3 Drugs
The 1377 clinical trials involved 222 drugs, and the drug categories was shown in Figure 3A. The indication distribution of the top 10 drugs with the highest number of PDCTs was shown, all of which were generic drugs (Figure 3B).
Figure 3

Information of drugs involved in psychiatric drug clinical trials in China in 2019-2024. The distribution of categories for all psychiatric drugs (A), indication distribution for the top 10 drugs with the highest number of PDCTs (B), and indication distribution for innovative psychiatric drugs (C) were displayed, respectively. The total number of psychiatric drugs was 222, of which 52 were innovative drugs. Some drugs participated in two or more indications. TCM, traditional Chinese medicine; DEP, depression; SC, schizophrenia; PD, parkinsonism; ANX, anxiety; AD, Alzheimer’s disease; BD, bipolar disorder; OCD, obsessive-compulsive disorder; PDCTs, psychiatric drug clinical trials.
Selected innovative drugs from the 222 drugs based on the screening criteria as follows: (1) being approved for investigational new drug application in China for the first time between 2019 and 2024; and (2) being registered as Class 1 (innovative drugs) in the CDE. The 52 (23.4%) innovative drugs were screened, participated in 97 PDCTs for six indications (some drugs participated in two or more indications). The indication with the highest number of innovative drugs was DEP (15), followed by SC (14) and AD (14) (Figure 3C).
There were 33 innovative drugs that publicized the targets, as basic characteristics in Table 3. A total of 28 targets were disclosed, which were classified into five categories based on molecular types: G protein-coupled receptors (GPCR, 53.6%), enzymes (14.3%), ion channel receptors (ICR, 7.1%), transporters (7.1%), and others (17.9%). There were 6 (18.2%) drugs targeting traditional DR and/or 5-HT receptors only, while other drugs involved novel targets. The innovative targets were mainly focused on amyloid β-protein (Aβ, 5), γ-aminobutyric acid A receptor subunit (GABAAR, 3), muscarinic M1/M4 receptors (mAChRM1/M4, 2), and brain derived neurotrophic factor (BDNF, 2). The proportion of drugs acting on these targets was 12/33 (36.4%).
Table 3
| Drug | Target | Classification | Indication (n*) | Phase | Drug | Target | Classification | Indication (n) | Phase |
|---|---|---|---|---|---|---|---|---|---|
| SIPI6398 | 5-HT1A;5-HT2A; DRD2; DRD3 | GPCR | SC (4) | II | NORA520 | GABAAR | ICR | DEP (1) | I |
| JX11502MA | 5-HT1A;5-HT2A; DRD2; DRD3 | GPCR | SC (2) | II | KH607 | GABAAR | ICR | DEP (1) | I |
| HS-10380 | 5-HT2A; DRD2; DRD3 | GPCR | SC (4) | II | GW201 | NMDAR | ICR | DEP (1) | I |
| GW117 | 5-HT2C; MT1; MT2 | GPCR | DEP (3) | II | PQ912 | QPCT | Enzymes | AD (1) | II |
| LPM787000048 | 5-HT2C; TAAR1 | GPCR | SC (2); AD (2) | I | WXWH0226 | LRRK2 | Enzymes | PD (1) | I |
| AM006 | DR; DRIP | GPCR | PD (2) | II | HEC12 2505MsOH |
MAO-B | Enzymes | PD (1) | I |
| NH300231 | 5-HT2A; DR | GPCR | SC (1) | I | MK-8189 | PDE 10 | Enzymes | SC (1) | I |
| Aticaprant | KOR | GPCR | DEP (3) | III | BI 425809 | GlyT1 | Transporter | SC (3) | III |
| KarXT | mAChRM1/M4 | GPCR | SC (1); AD (1) | III | SHR-1707 | Aβ | Others (amyloid protein) | AD (3) | II |
| VG081821AC | A2aR | GPCR | PD (2) | II | Remternetug | Aβ | Others (amyloid protein) | AD (2) | III |
| NH130 | 5-HT2A | GPCR | PD (2) | I | RP902 | Aβ | Others (amyloid protein) | AD (2) | I |
| HS-10506 | OX2R | GPCR | DEP (2) | I | BAN2401 | Aβ | Others (amyloid protein) | AD (1) | III |
| NS-136 | mAChRM4 | GPCR | SC (1) | I | OAB-14 | Aβ | Others (amyloid protein) | AD (1) | I |
| NH102 | 5-HT2A; SLC6A3 |
GPCR Transporter |
DEP (2) | I | JS1-1-01 | BDNF | Others (neurotrophic factors) | DEP (3) | II |
| Liafensine | 5-HT; Dopamine; NE |
GPCR Others (neurotransmitter) |
DEP (1) | II | BrAD-R13 | BDNF | Others (neurotrophic factors) | AD (1) | I |
| HS-10353 | GABAAR | ICR | DEP (8) | II | QD202 | LYZ; SYP | Others (synaptic function regulation) | AD (1) | I |
| MN-08 | NMDAR | ICR | AD (1) | II |
Basic characteristics of innovative drugs in psychiatric drug clinical trials in China in 2019-2024.
There were 33 innovative drugs for psychiatric disorders that have disclosed a total of 28 targets. The targets were classified into five categories based on molecular types. *: number of clinical trial registrations; 5-HT, 5-hydroxytryptamine receptor; DR, dopamine receptor; MT1, melatonin MT1; MT2, melatonin MT2; TAAR1, trace amine-associated receptor 1; DRIP, dopamine receptor interacting protein; KOR, kappa-type opioid receptor; mAChRM1/M4, muscarinic M1/M4 receptors; A2aR, adenosine A2a receptor; OX2R, orexin type 2 receptor; SLC6A3, solute carrier family 6 member 3; NE, norepinephrine; GABAAR, γ-aminobutyric acid A receptor subunit; NMDAR, N-methyl-D-aspartate receptor; QPCT, glutaminyl-peptide cyclotransferase; LRRK2, leucine rich repeat kinase 2; MAO-B, monoamine oxidase B; PDE 10, phosphodiesterase 10; GlyT1, glycine transporter 1; Aβ, amyloid β-protein; BDNF, brain derived neurotrophic factor; LYZ, lysozyme; SYP, synaptophysin; GPCR, G protein-coupled receptors; ICR, ion channel receptors; DEP, depression; SC, schizophrenia; AD, Alzheimer’s disease; PD, parkinsonism.
Selected improved new drugs from the 222 drugs based on the screening criteria as follows: (1) same as the criterion (1) for innovative drugs; and (2) being registered as Class 2 (improved new drugs) in the CDE. There were 13 drugs were screened out involved in 12 dosage forms and six administration routes (Table 4). Traditional oral and injection administration routes account for a large proportion (75%). Additionally, there are four other routes of administration, namely: intranasal, transmucosal, rectal, and transdermal.
Table 4
| Drug | Indication | Phase | Dosage form | Administration Route |
|---|---|---|---|---|
| Olanzapine | SC/BD | BE/I | Oral dissolving film | Oral |
| Brexpiprazole | SC/DEP | BE/I | Oral dissolving film | Oral |
| BCM857 | SC | I | Oral dissolving film | Oral |
| N2106 | SC | I | Oral dissolving film | Oral |
| Donepezil | AD | I | Oral dissolving film | Oral |
| Huperzine A | AD | I | Controlled-release tablet | Oral |
| HRG2010 | PD | III | Sustained-release capsule | Oral |
| TV-44749 (Olanzapine) | SC | I | Sustained-release suspension | Injection |
| Aripiprazole | SC | I | Sustained-release microspheres | Injection |
| Rotigotine | PD | I/III | Sustained-release microspheres | Injection |
| Brexpiprazole | SC | I | Long-acting injection | Injection |
| Donepezil | AD | I | Injection | Injection |
| (R)-Ketamine | DEP | I | Nasal spray | Intranasal |
| Rasagiline | PD | I | Sublingual film | Transmucosal |
| Midazolam | ANX | I | Gel | Rectal |
| Asenapine | SC | Other (pharmacokinetics) | Patch | Transdermal |
Basic characteristics of improved new drugs in psychiatric drug clinical trials in China in 2019-2024.
There were 13 improved new drugs involved in 12 dosage forms and six administration routes. Brexpiprazole and donepezil had two dosage forms, respectively. SC, schizophrenia; BD, bipolar disorder; DEP, depression; AD, Alzheimer’s disease; PD, parkinsonism; ANX, anxiety.
3.4 Trial design
The 1377 clinical trials were screened for trial objective. Some of the trials had further exploratory design, including population pharmacokinetics (PopPK), pharmacogenomics (PGx), biomarker detection, and drug combination (Table 5).
Table 5
| Objective | Indication | Registration ID | Drugs | Objective | Indication | Registration ID | Drugs |
|---|---|---|---|---|---|---|---|
| PopPK | AD | CTR20210187 | GV-971 | PGx | DEP | CTR20220758; CTR20222884; CTR20240090 |
SAL0114 |
| CTR20243157 | 50561 | ||||||
| CTR20243461 | HRG2010 | ||||||
| ANX | CTR20231392; CTR20231405 |
Buagafuran | CTR20221896 | Liafensine | |||
| CTR20222150 | JS1-1-01 | ||||||
| CTR20231959 | Toludesvenlafaxine | CTR20233032 | LV232 | ||||
| DEP | CTR20211677; CTR20230246 |
Ammoxetine | SC | CTR20213168; CTR20213353 | SEP-363856 | ||
| SC | CTR20213353 | SEP-363856 | CTR20192086 | Pomaglumetad methionil | |||
| Biomarker | AD | CTR20210187 | GV-971 | CTR20201189 | WenDanPian | ||
| PD | CTR20243324 | TJ0113 | CTR20201480 | Aripiprazole | |||
| DEP | CTR20211677 | Ammoxetine | CTR20231088 | KarXT | |||
| Drug combination | DEP | CTR20233237; CTR20233277 | Aticaprant | ||||
| CTR20222884 | SAL0114 |
Exploratory design of psychiatric drug clinical trials in China in 2019-2024.
PopPK, population pharmacokinetics; PGx, pharmacogenetic; AD, Alzheimer’s disease; ANX, anxiety; DEP, depression; SC, schizophrenia; PD, parkinsonism.
Nine trials were designed with PopPK involved four indications which were DEP, SC, ANX, and AD.
12 trials were designed with PGx involved two indications which were DEP and SC. Among them, six trials explored the relationship between CYP2D6 gene polymorphisms and pharmacokinetics, with registration ID was CTR20220758, CTR20222884, CTR20240090, CTR20201480, CTR20213168, and CTR20213353, respectively; one trial explored the norepinephrine transporter protein (net) T182C locus gene polymorphisms, with registration ID was CTR20201189.
Three trials proposed biomarker detection involved three indications which were DEP, PD, and AD. Among them, one trial explored the changes in inflammatory factors, with registration ID was CTR20243324, and the other one trial detected biomarkers of intestinal metabolites, with registration ID was CTR20210187.
Three trials were designed with drug combination, and all for DEP. Among them, two trials explored the efficacy and safety of combining Aticaprant with SSRI or SNRI therapy, with registration ID was CTR20233277 and CTR20233237, respectively; the other one trial explored the efficacy and safety of combining SAL0114 with bupropion, with registration ID was CTR20222884.
4 Discussion
Since the 1990s, psychiatric disorders have been recognized as one of the top 10 global burdens, with a consistently high prevalence rate that is thought to be closely related to disability and increased economic burdens (17). However, because of the complex etiology, although great progress has been made in the research on the pathogenesis of psychiatric disorders, strategies that have a significant impact on disease diagnosis and treatment have not been developed. Therefore, designing effective drug therapies for psychiatric disorders is a common challenge and urgent need worldwide, which can be reflected in our research findings that the number of PDCTs in China has shown an increasing trend over the past few years. At the same time, with the WHO announcing the end of the COVID-19 global emergency in 2023, the trial team cooperation as well as the subject recruitment has become more convenient, so we showed a significant shorten in start-up time in our study, which provides favorable conditions for the efficient implementation of PDCTs and the acceleration of the new drugs R&D progress. It is worth noting that whether in terms of the number of PDCTs or the number of innovative drugs, the vast majority of involved indications were DEP, SC, and PD from our results. It suggested that the current research still focus on common psychiatric disorders, and the development of uncommon psychiatric diagnosis and treatment is slow and needs more attention.
There are several aspects that can contribute to the success of therapeutic drugs: (1) discovery and rational design of new compounds with better efficacy and safety; (2) development of improved dosage forms and alternative drug delivery systems to increase compliance; and (3) adoption of exploratory strategies such as PopPK, genomics, combination therapy, and biomarker detection to achieve precision pharmacotherapy (18–22). This study will also discuss the current status of R&D of psychotropic drugs in China from the above aspects.
4.1 Innovative targets
The first- and second-generation psychotropic drugs are still the mainstream in clinical practice currently for limited R&D progress. Due to the complexity and heterogeneity of psychiatric disorders, developing effective and fewer side effects drugs has become a challenge. It is encouraging that approximately 80% of the innovative drugs with disclosed targets in our study were novel mechanisms (Table 3). Among them, Aβ, GABAAR, mAChRM1/M4, and BDNF were designed as targets by two or more drugs.
AD is a progressive neurodegenerative disorder with characteristics are memory loss, cognitive impairment, and behavioral disturbances (23). One of the mainstream views regarding the pathogenesis of AD currently is amyloid cascade hypothesis, which posits that the deposition of Aβ is a key driving factor for early disease progression in AD, and leads to subsequent pathological changes (24). Recent studies on genetics, pathology, and biomarkers have also provided evidence for this hypothesis (25–27). Therefore, targeting the reduction of Aβ levels in the brain of patients has been a foundational strategy to improve AD symptoms in the last few decades (28). In the past four years, the U.S. Food and Drug Administration (FDA) has approved three anti-Aβ monoclonal antibodies (aducanumab, lecanemab, and donanemab) consecutively, ushering in a new era of targeted therapy for AD (29–31). Among them, lecanemab (BAN2401) was approved by the FDA in 2023, becoming the first fully approved Aβ-antibody drug for AD treatment. This drug is conducting a phase III trial (CTR20200005) in China as shown in our study. From the results, 10 of 33 innovative drugs were targeted for AD, with half acting on Aβ, which suggests that the current focus of R&D for AD treatment in China is also centered on Aβ.
SC is considered as one of the most serious psychiatric disorders (32). Although drug development for SC has been ongoing for 70 years, all antipsychotics currently approved are antagonists or partial agonists at the dopamine D2 receptor (DRD2) (33). So, limitations of therapeutic efficacy and frequent side effects are pervasive shortcoming. In the 1990s, the mAChRsM1/M4 receptor agonist xanomeline demonstrated its potential for the first time in treating AD and SC (34, 35). The mechanism of this drug may be to activate acetylcholine signaling by stimulating mAChRs, rather than blocking DRD2 (36). However, due to the similarity in structure among the five subtypes, mAChR-targeted therapy can affect receptor subtypes distributed in different tissues, causing serious side effects and hindering further drug development (37–39). The successful development of the drug named KarXT (Cobenfy) solved this problem. It can not only selectively excite mAChRsM1/M4 receptor to exert pharmacological effects, but also inhibit muscarine receptors to suppress peripheral side effects (40, 41). KarXT was full approved by the FDA in 2024, becoming the first mAChR-targeted drug for adult SC (42). It is conducting a phase III trial (CTR20231088) in China now. In addition, a small molecule M4 selective agonist named NS-136, independently developed by China, is currently undergoing Phase 1 trials (CTR20244269).
GABAAR is an ICR that inhibits neural excitability by promoting chloride ion influx (43). Its signal dysregulation is related to various neuropsychiatric disorders, such as ANX, DEP, and AD (44). Brexanolone is a positive allosteric modulator of GABAA, which can enhance the inhibitory effects of GABAA. It was approved by the FDA in 2019 as the first drug for postpartum depression (45, 46). BDNF promotes neuroprotection and neuroregeneration (47). Neurodegenerative diseases and neuropsychiatric disorders may be related to insufficient neuronal supply of BDNF, and over the past 20 years, BDNF has been regarded as a key factor in the treatment of neuropsychiatric disorders (48, 49). The evidence indicating the relationship between decrease of BDNF level and the progression of PD is increasing (50, 51). Due to the lack of a cure for PD, BDNF has a promising prospect in this area. Besides, the targets mentioned in this study, such as kappa-type opioid receptor (KOR), orexin type 2 receptor (OX2R), and N-methyl-D-aspartate receptor (NMDAR) and so on, may also become new mechanisms for treating psychiatric disorders (52–54). The launch of clinical trials for these potential drugs is expected to break the decades-long treatment dilemma, heralding the arrival of a new era of non-traditional targeted therapy for psychiatric disorders.
4.2 Drug optimization
Many psychotropic drugs are administered via traditional oral routes, which face challenges such as first-pass effect, limited BBB penetration, low bioavailability, and systemic side effects, making it difficult to achieve optimal drug level (55). To solves these issues, several optimization strategies for existing drugs are proposed. In our research, there were some improved new drugs that adopted methods such as changing drug delivery system (DDS) and administration route to avoid BBB and eliminate first-pass effect, in order to improve the pharmacokinetic process and enhance drug efficacy.
Due to BBB, most substances in the blood are difficult to enter the brain, making it very challenging for drugs to cross the BBB distribution, especially in neurological diseases that may cause BBB dysfunction and make this process even more complex (56). So how to avoid the BBB and directly deliver drugs to the brain is an important idea to improve the distribution of psychotropic drugs in the CNS. As an alternative DDS, the mechanism of nose-to-brain delivery is that drugs are first absorbed through the nasal mucosa, then transported retrogradely along the axons of the trigeminal nerve, and ultimately direct reach the brainstem and spinal cord (57). This approach provides a non-invasive method to avoid BBB while also avoiding first-pass effect. It has advantages including rapid onset, high bioavailability, and reduced systemic exposure, making it highly valuable for clinical applications (58, 59). Ketamine is an anesthetic and analgesic drug that is commonly administered by injection (60). The S(+)-isomer named esketamine was approved for market in 1997, and received initial approval by the FDA for treatment-resistant depression in 2019 (61). The bioavailability of its nasal spray reached about 50%, which can rapidly improve DEP symptoms and provide golden-hour window intervention for patients at risk of acute suicide (62). (R)-ketamine hydrochloride is the levorotatory form of ketamine, and the phase I clinical trials (CTR20212471 and CTR20191785) of its nasal spray for treatment-resistant depression has been completed in China. However, nose-to-brain also has certain shortcomings. On the one hand, this method has limited drug loading capacity, which restricts the amount of drug that can be delivered at once; On the other hand, the nasal cavity has a self-defense function that limits the efficiency of drug delivery (63).
The traditional oral route has problems such as first-pass effect, enzyme digestion attack, and gastrointestinal irritation, etc. In addition, some patients with psychiatric disorders are accompanied by poor compliance and swallowing difficulties. Altering drug dosage form/administration route provides a conventional solution, such as injectables, transdermal delivery (gel, ointment, patch, etc.), and mucosal administration (oral, nasal, rectal, etc.), as shown in Table 4 (64–66). Among them, transdermal delivery, as a non-invasive and self-administration method, allows drugs to be absorbed through the skin and minimizes the liver first-pass effect (67). Compared with traditional semisolid dosage forms (such as ointments and gels), transdermal patches overcome the limitations in dose accuracy and application consistency, while enabling reduced the dosing frequency through sustained drug release (68). Combining the above, patches are well suited as alternative formulations to traditional oral or injectable psychotropic drugs (69). Asenapine transdermal patch (Secuado®) was approved by the FDA in 2019, and it is the only FDA-approved patch for SC in adults (70). An asenapine transdermal patch clinical trial (CTR20240253) conducted in China employs sustained-release technology to extends the dosing interval, which is expected to significantly enhance patient compliance compared with Secuado®. However, patches also exhibit certain limitations, such as constrained drug loading capacity, requirements for drug solubility and molecular weight, and skin barrier, which restrict the improvement of some psychotropic drug formulations (71).
At present, multiple systematic technological paths are expected to break through the bottleneck of dosage forms in drug optimization schemes. With the supports of emerging technologies such as molecular structure modification, nanoformulation technologies, solvent casting systems, 3D printing, and artificial intelligence, traditional delivery routes of psychotropic drugs are expected to undergo revolutionary changes (72–74).
4.3 Exploratory design
There are still significant gaps in the diagnosis and treatment of psychiatric disorders that needs to be filled. Drug therapy is an important way of psychiatric disorders treatment. The efficacy and tolerability of drugs are key factors in determining whether patients can benefit from their treatment. However, due to individual differences, it usually takes several months to years to adjust the dosing regimen in clinical practice in order to find suitable therapeutic drugs (75). This has led to a series of problems such as poor disease prognosis and reduced compliance. These issues are driving the pharmaceutical industry to shift from traditional trial design to patient-centered exploratory design. The four exploratory designs in this study also suggest the occurrence of this transformation.
Distinct from traditional pharmacokinetics, PopPK utilizes a large number of clinical samples to quantify the factors contributing to drug concentration differences in the target population, in order to determine and optimize drug dosage (76, 77). Through Bayesian forecasting, PopPK analysis can be used to estimate individual pharmacokinetic parameters and customize personalized dosing regimens (78). The approved dosage strategies for many drugs used in different therapeutic fields are based on PopPK analysis. A study on PopPK of BAN2401 for AD used data from three clinical trials, including two phase I trials and one phase II trial, to identified individual differences in BAN2401 pharmacokinetic parameters, and the synergy of factors such as height and gender (79). In our study, nine trials were described as conducting PopPK. Among them, Toludesvenlafaxine, an innovative anti-anxiety drug with independent intellectual property rights in China, has been approved for market by the CDE in 2022 (80). The drug is currently undergoing a phase III trial in China to evaluate PopPK in patients with generalized anxiety disorder (CTR20231959), in order to develop a more scientific dosing regimen.
Cytochrome P450 enzymes (CYPs) play key roles in the metabolism of psychotropic drugs, especially CYP2C19, CYP2D6, and CYP3A4 (81). However, because of the high polymorphisms of CYPs, the therapeutic effects on patients are different, and approximately 30% of patients showing no response to SC (82). Thus, it is necessary to design PGx strategies in new drugs development to achieve personalized treatment. The current drug development and design strategies mainly focus on the population level, and personalized genomics research is relatively lacking and lagging behind (83). The FDA has added genomic labels for some psychotropic drugs and approved in 2020 that patients should test CYP2C19 genotype when applying citalopram (75). It’s the first FDA-approved PGx-test for psychotropic drugs, indicating a breakthrough in PGx research on psychotropic drugs. Six clinical trials in our study were targeted to detect CYP2D6 genetic polymorphisms, and the related drugs including SAL0114, SEP-363856, and aripiprazole. Besides, a phase II trial of TCM WenDanPian was aimed at net T182C locus polymorphisms. Those suggest that PGx is becoming a trend in drug development across various drug categories to ensure precision medication.
In addition to the above, some studies we displayed also employed biomarker detection and/or drug combination strategies. The determination of plasma biomarkers, especially inflammatory factors, provides a non-invasive way to explore the possible pathological changes and diagnostic indicators of psychiatric disorders (84–86). Currently, drug combination strategy is quite common in the treatment of cancer and chronic diseases. Considering the polygenic and complex etiology of psychiatric disorders, using drugs with multiple pharmacological effects or drug combinations with different molecular mechanisms may be a new approach to improve the therapeutic effect of psychotropic drugs (87).
4.4 Future directions
Psychiatric disorders have become a huge challenge threatening human health. The monoamine hypothesis, as the mainstream theory, is the basis for the development of a large number of drugs, especially antidepressants (88). However, the mere 30% effectiveness of antidepressants indicates that the monoamine hypothesis is not sufficient to explain the entire pathogenesis of psychiatric disorders (89). The increasing prevalence and limited progress have prompted a transformation in the direction of R&D for psychiatric disorders, namely the development of treatment strategies with novel mechanisms driven by new technologies. Our study has demonstrated a part of innovative targets, delivery systems, and design ideas involved in clinical trials conducted in China. Here we briefly overview the possible future R&D directions in this field worldwide, including emerging mechanism theories and upgraded delivery systems.
In addition to the traditional monoamine hypothesis, new theories proposed in recent decades mainly include the gene-environment interaction and the neuroplasticity. The increasing evidence suggests that the pathogenesis of psychiatric disorders is not only caused by individual gene mutations, but also the result of the interaction between genes and environmental risk factors, with clear biological characteristics (90). Thus, a new concept of gene-environment interaction was proposed, which may be mediated by epigenetics (91, 92). Epigenetic modifications play a crucial role in the human CNS physiology by regulating tissue-specific gene expression through DNA methylation, histone modification, and RNA interference (93, 94). Histone deacetylase (HDAC) is a key enzyme that maintains neuronal morphology and brain stability partly by catalyzing the deacetylation of histones (95). A series of inhibitor compounds such as RGFP-966 (HDAC3 inhibitor), T-518 (HDAC6 inhibitor), etc. have been proven to improve brain cognitive ability in AD mouse models (96–98). MeCP2 and ALKBH5 may affect the disease processes of AD and depression respectively by regulating methylation, which have been validated in animal models (99, 100).
Neuroplasticity refers to the ability of the brain to respond to internal and external stimuli through neurobiological changes (101). This theory overturns the traditional view of a “static brain” and believes that the nervous system undergoes lifelong dynamic changes. These changes can be adaptive and support the recovery of high-risk populations; otherwise, they may lead to neuropathology and psychiatric disorders (102). The AMPA receptor diffusional trapping machinery improves brain learning and memory function by regulating synaptic plasticity, making it a potential target for AD (103). Berberine reduces neuroinflammation by inhibiting the activation of NLRP3 inflammasome, thereby reversing neuroplastic damage and improved symptoms in depression model mice (104).
One of the reasons hindering the progress of psychiatric disorders treatment is related to delivery, and DDS technology innovation may significantly improve drug efficacy. The direction of future drug delivery mainly focuses on intelligent systems, including stimulus-responsive DDS and pulsatile DDS. Traditional delivery techniques suffer from non-specific biological distribution and uncontrollable release, leading to systemic side effects. Stimulus-responsive DDS can overcome the aforementioned issues. The design principle is to load drugs onto specific materials, and provide endogenous and exogenous stimuli (105). By changing or disrupting the carrier, drugs can be released at specific times and locations. Endogenous stimuli are triggered by the disease microenvironment, including pH, enzymes overexpressed at the lesion site, etc (106, 107); exogenous stimuli are external physical stimuli applied, such as light, temperature, ultrasound, etc (108, 109). By combining multiple stimuli, drug delivery efficiency and accuracy can be further improved (110). The hydrogel of Paliperidone (an antipsychotic drug) first enters the nasal cavity through intranasal route in the form of spray, and changes into a mucosal gel after exposure to physiological PH, thus playing a long-term controlled release role (94). Pulsatile DDS can release drugs based on preset time intervals or external signals (such as electricity, magnetism, ultrasound, etc.), making them suitable for drugs with intermittent dosing needs. Psychotropic drugs can use this system to enhance compliance (111).
4.5 Limitations
This study has certain limitations. First, according to the NMPA, clinical trials that meet one of the following criteria must be registered on the http://www.chinadrugtrials.org.cn platform prior to the start of the trial, and continuously updated on the progress: (1) approved by the NMPA and conducted in China; (2) approved for filing for bioequivalence testing of chemical drugs; and (3) phase IV clinical trials and post-marketing studies as required by Chinese regulations. However, there are still some trials that have not been included in this study due to not being required to register on this platform, such as trials in the early stages of development or investigator-initiated trials (IIT). Second, the platform did not provide detailed research stages and trial subjects information, so we were unable to conduct in-depth analysis and evaluation of treatment efficacy and adverse events. Third, due to the complex mechanisms of psychiatric disorders and the lack of detailed mechanism description, we did not further classify and analyze these drugs.
In conclusion, this study intuitively demonstrates the latest R&D progress of psychotropic drugs in China by analyzing the information of projects, indications, drugs, and trial design of PDCTs. It also introduced the research hotspots of psychotropic drugs from the perspectives of innovative drug target, drug optimization approach, and trial design strategies. Besides, we also introduced emerging mechanisms and delivery systems with promising applications. We are committed to providing insights into the latest stages of psychotropic drugs, delivering valuable information and references for the direction of psychotropic drugs development, the conduct of clinical trials, and individualized clinical dosing regimens.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
CX: Conceptualization, Writing – review & editing, Writing – original draft. YZ: Data curation, Writing – review & editing. XY: Writing – review & editing. JZ: Writing – review & editing. QT: Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
- R&D
research and development
- PDCT
psychotropic drugs clinical trial
- BE
bioequivalency
- WHO
World Health Organization
- ANX
anxiety
- DEP
depression
- CNS
central nervous system
- BBB
blood-brain barrier
- DR
dopamine receptor
- 5-HT
5-hydroxytryptamine
- GCP
Good Clinical Practice
- DSM-V
Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition
- CDE
Center for Drug Evaluation
- NMPA
National Medical Products Administration
- NHC
National Health Commission
- TCM
traditional Chinese medicine
- SC
schizophrenia
- PD
parkinsonism
- AD
Alzheimer’s disease
- BD
bipolar disorder
- OCD
obsessive-compulsive disorder
- WS
withdrawal symptoms
- TS
tourette syndrome
- MT1
melatonin MT1
- MT2
melatonin MT2
- TAAR1
trace amine-associated receptor 1
- DRIP
dopamine receptor interacting protein
- KOR
kappa-type opioid receptor
- mAChR
muscarinic receptor
- A2aR
adenosine A2a receptor
- OX2R
orexin type 2 receptor
- SLC6A3
solute carrier family 6 member 3
- NE
norepinephrine
- GABAAR
γ-aminobutyric acid A receptor subunit
- NMDAR
N-methyl-D-aspartate receptor
- QPCT
glutaminyl-peptide cyclotransferase
- LRRK2
leucine rich repeat kinase 2
- MAO-B
monoamine oxidase B
- PDE 10
phosphodiesterase 10
- GlyT1
glycine transporter 1
- Aβ
amyloid β-protein
- BDNF
brain derived neurotrophic factor
- LYZ
lysozyme
- SYP
synaptophysin
- GPCR
G protein-coupled receptors
- ICR
ion channel receptors
- PopPK
population pharmacokinetics
- PGx
pharmacogenomics
- FDA
Food and Drug Administration
- DDS
drug delivery system
- HDAC
Histone deacetylase
References
1
Plana-Ripoll O Pedersen CB Agerbo E Holtz Y Erlangsen A Canudas-Romo V et al . A comprehensive analysis of mortality-related health metrics associated with mental disorders: a nationwide, register-based cohort study. Lancet. (2019) 394:1827–35. doi: 10.1016/S0140-6736(19)32316-5
2
McGrath JJ Al-Hamzawi A Alonso J Altwaijri Y Andrade LH Bromet EJ et al . Age of onset and cumulative risk of mental disorders: a cross-national analysis of population surveys from 29 countries. Lancet Psychiatry. (2023) 10:668–81. doi: 10.1016/S2215-0366(23)00193-1
3
World Health Organization . World mental health report: Transforming mental health for all. (2022). Available online at: https://www.who.int/publications/i/item/9789240049338 (Accessed June 16, 2022).
4
Huang Y Wang Y Wang H Liu Z Yu X Yan J et al . Prevalence of mental disorders in China: a cross-sectional epidemiological study. Lancet Psychiatry. (2019) 6:211–24. doi: 10.1016/S2215-0366(18)30511-X
5
van der Wal JM van Borkulo CD Deserno MK Breedvelt JJF Lees M Lokman JC et al . Advancing urban mental health research: from complexity science to actionable targets for intervention. Lancet Psychiatry. (2021) 8:991–1000. doi: 10.1016/S2215-0366(21)00047-X
6
Liston C Roberts A Dzirasa K Geschwind D Ahmari SE Luscher C . Understanding the biological basis of psychiatric disease: What's next? Cell. (2022) 185:1–3. doi: 10.1016/j.cell.2021.12.010
7
Wu D Chen Q Chen X Han F Chen Z Wang Y . The blood-brain barrier: structure, regulation, and drug delivery. Signal Transduct Target Ther. (2023) 8:217. doi: 10.1038/s41392-023-01481-w
8
Mehrdadi S . Drug delivery of solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) to target brain tumors. Adv Pharm Bull. (2023) 13:512–20. doi: 10.34172/apb.2023.062
9
Nutt DJ . Drug development in psychiatry: 50 years of failure and how to resuscitate it. Lancet Psychiatry. (2025) 12:228–38. doi: 10.1016/S2215-0366(24)00370-5
10
Paul SM Potter WZ . Finding new and better treatments for psychiatric disorders. Neuropsychopharmacology. (2024) 49:3–9. doi: 10.1038/s41386-023-01690-5
11
Siafis S Wu H Wang D Burschinski A Nomura N Takeuchi H et al . Antipsychotic dose, dopamine D2 receptor occupancy and extrapyramidal side-effects: a systematic review and dose-response meta-analysis. Mol Psychiatry. (2023) 28:3267–77. doi: 10.1038/s41380-023-02203-y
12
Pourhamzeh M Moravej FG Arabi M Shahriari E Mehrabi S Ward R et al . The roles of serotonin in neuropsychiatric disorders. Cell Mol Neurobiol. (2022) 42:1671–92. doi: 10.1007/s10571-021-01064-9
13
Stocken DD Mossop H Armstrong E Lewis S Dutton SJ Peckitt C et al . Good Statistical Practice-development of tailored Good Clinical Practice training for statisticians. Trials. (2024) 25:113. doi: 10.1186/s13063-024-07940-1
14
den Heijer JM Heuberger J Hijma H Kruithof AC van Smeden J Groeneveld GJ et al . Good Clinical Trials by removing defensive interpretation of Good Clinical Practice guidelines. Br J Clin Pharmacol. (2021) 87:4552–9. doi: 10.1111/bcp.14843
15
Jeon I Kim YK Song I Yoon DY Huh KY Jin X et al . The necessary conduct: Exploratory multiregional clinical trials in East Asia. Clin Transl Sci. (2021) 14:2399–407. doi: 10.1111/cts.13106
16
Kang C Yang J . Prevalence of mental disorders in China. Lancet Psychiatry. (2022) 9:13. doi: 10.1016/S2215-0366(21)00400-4
17
Collaborators GBDMD . Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. (2022) 9:137–50. doi: 10.1016/S2215-0366(21)00395-3
18
Brady LS Lisanby SH Gordon JA . New directions in psychiatric drug development: promising therapeutics in the pipeline. Expert Opin Drug Discov. (2023) 18:835–50. doi: 10.1080/17460441.2023.2224555
19
Eales B Helal NA Vattelana O Kronfol MM Fletcher EP Wang YM et al . Population pharmacokinetics (PopPK) support for pediatric dosing of biological products. J Clin Pharmacol. (2024) 64:1594–605. doi: 10.1002/jcph.6116
20
Rees E Owen MJ . Translating insights from neuropsychiatric genetics and genomics for precision psychiatry. Genome Med. (2020) 12:43. doi: 10.1186/s13073-020-00734-5
21
Dinnerstein E . The time for combination therapy research in Alzheimer's disease is now. J Alzheimers Dis. (2023) 93:925–6. doi: 10.3233/JAD-230254
22
Jiang Y Uhm H Ip FC Ouyang L Lo RMN Cheng EYL et al . A blood-based multi-pathway biomarker assay for early detection and staging of Alzheimer's disease across ethnic groups. Alzheimers Dement. (2024) 20:2000–15. doi: 10.1002/alz.13676
23
Knopman DS Amieva H Petersen RC Chetelat G Holtzman DM Hyman BT et al . Alzheimer disease. Nat Rev Dis Primers. (2021) 7:33. doi: 10.1038/s41572-021-00269-y
24
Kim HY Kim Y . Chemical-driven amyloid clearance for therapeutics and diagnostics of Alzheimer’s disease. Acc Chem Res. (2024) 57:3266–76. doi: 10.1021/acs.accounts.4c00458
25
Raulin AC Doss SV Trottier ZA Ikezu TC Bu G Liu CC . ApoE in Alzheimer's disease: pathophysiology and therapeutic strategies. Mol Neurodegener. (2022) 17:72. doi: 10.1186/s13024-022-00574-4
26
Koronyo Y Rentsendorj A Mirzaei N Regis GC Sheyn J Shi H et al . Retinal pathological features and proteome signatures of Alzheimer's disease. Acta Neuropathol. (2023) 145:409–38. doi: 10.1007/s00401-023-02548-2
27
Xu C Zhao L Dong C . A review of application of Aβ42/40 ratio in diagnosis and prognosis of Alzheimer’s disease. J Alzheimers Dis. (2022) 90:495–512. doi: 10.3233/JAD-220673
28
Jin Y Du Q Song M Kang R Zhou J Zhang H et al . Amyloid-beta-targeting immunotherapies for Alzheimer's disease. J Control Release. (2024) 375:346–65. doi: 10.1016/j.jconrel.2024.09.012
29
Dhillon S . Aducanumab: first approval. Drugs. (2021) 81:1437–43. doi: 10.1007/s40265-021-01569-z
30
Hoy SM . Lecanemab: first approval. Drugs. (2023) 83:359–65. doi: 10.1007/s40265-023-01851-2
31
Kang C . Donanemab: first approval. Drugs. (2024) 84:1313–8. doi: 10.1007/s40265-024-02087-4
32
Harris A . Approach to schizophrenia. Intern Med J. (2023) 53:473–80. doi: 10.1111/imj.16068
33
Paul SM Yohn SE Brannan SK Neugebauer NM Breier A . Muscarinic receptor activators as novel treatments for schizophrenia. Biol Psychiatry. (2024) 96:627–37. doi: 10.1016/j.biopsych.2024.03.014
34
Brown TJ Shipley LA . Determination of xanomeline (LY246708 tartrate), an investigational agent for the treatment of Alzheimer's disease, in rat and monkey plasma by capillary gas chromatography with nitrogen-phosphorus detection. J Chromatogr B BioMed Appl. (1995) 665:337–44. doi: 10.1016/0378-4347(94)00538-g
35
Shannon HE Rasmussen K Bymaster FP Hart JC Peters SC Swedberg MD et al . Xanomeline, an M(1)/M(4) preferring muscarinic cholinergic receptor agonist, produces antipsychotic-like activity in rats and mice. Schizophr Res. (2000) 42:249–59. doi: 10.1016/s0920-9964(99)00138-3
36
Yohn SE Harvey PD Brannan SK Horan WP . The potential of muscarinic M(1) and M(4) receptor activators for the treatment of cognitive impairment associated with schizophrenia. Front Psychiatry. (2024) 15:1421554. doi: 10.3389/fpsyt.2024.1421554
37
Alt A Pendri A Bertekap RL Jr. Li G Benitex Y Nophsker M et al . Evidence for classical cholinergic toxicity associated with selective activation of M1 muscarinic receptors. J Pharmacol Exp Ther. (2016) 356:293–304. doi: 10.1124/jpet.115.226910
38
Bender AM Jones CK Lindsley CW . Classics in chemical neuroscience: xanomeline. ACS Chem Neurosci. (2017) 8:435–43. doi: 10.1021/acschemneuro.7b00001
39
Yohn SE Weiden PJ Felder CC Stahl SM . Muscarinic acetylcholine receptors for psychotic disorders: bench-side to clinic. Trends Pharmacol Sci. (2022) 43:1098–112. doi: 10.1016/j.tips.2022.09.006
40
Kaul I Sawchak S Walling DP Tamminga CA Breier A Zhu H et al . Efficacy and safety of xanomeline-trospium chloride in schizophrenia: A randomized clinical trial. JAMA Psychiatry. (2024) 81:749–56. doi: 10.1001/jamapsychiatry.2024.0785
41
Kaul I Sawchak S Correll CU Kakar R Breier A Zhu H et al . Efficacy and safety of the muscarinic receptor agonist KarXT (xanomeline-trospium) in schizophrenia (EMERGENT-2) in the USA: results from a randomised, double-blind, placebo-controlled, flexible-dose phase 3 trial. Lancet. (2024) 403:160–70. doi: 10.1016/S0140-6736(23)02190-6
42
Syed YY . Xanomeline/trospium chloride: first approval. Drugs. (2025) 85:103–9. doi: 10.1007/s40265-024-02126-0
43
Kim JJ Hibbs RE . Direct structural insights into GABA(A) receptor pharmacology. Trends Biochem Sci. (2021) 46:502–17. doi: 10.1016/j.tibs.2021.01.011
44
Thompson SM . Modulators of GABA(A) receptor-mediated inhibition in the treatment of neuropsychiatric disorders: past, present, and future. Neuropsychopharmacology. (2024) 49:83–95. doi: 10.1038/s41386-023-01728-8
45
Scott LJ . Brexanolone: first global approval. Drugs. (2019) 79:779–83. doi: 10.1007/s40265-019-01121-0
46
Edinoff AN Odisho AS Lewis K Kaskas A Hunt G Cornett EM et al . Brexanolone, a GABA(A) modulator, in the treatment of postpartum depression in adults: A comprehensive review. Front Psychiatry. (2021) 12:699740. doi: 10.3389/fpsyt.2021.699740
47
Palasz E Wysocka A Gasiorowska A Chalimoniuk M Niewiadomski W Niewiadomska G . BDNF as a promising therapeutic agent in Parkinson's disease. Int J Mol Sci. (2020) 21:1170. doi: 10.3390/ijms21031170
48
Wang CS Kavalali ET Monteggia LM . BDNF signaling in context: From synaptic regulation to psychiatric disorders. Cell. (2022) 185:62–76. doi: 10.1016/j.cell.2021.12.003
49
Nieto RR Carrasco A Corral S Castillo R Gaspar PA Bustamante ML et al . BDNF as a biomarker of cognition in schizophrenia/psychosis: an updated review. Front Psychiatry. (2021) 12:662407. doi: 10.3389/fpsyt.2021.662407
50
Wolf D Ayon-Olivas M Sendtner M . BDNF-regulated modulation of striatal circuits and implications for Parkinson's disease and dystonia. Biomedicines. (2024) 12:1761. doi: 10.3390/biomedicines12081761
51
Kang SS Wu Z Liu X Edgington-Mitchell L Ye K . Treating Parkinson’s disease via activation of BDNF/TrkB signaling pathways and inhibition of delta-secretase. Neurotherapeutics. (2022) 19:1283–97. doi: 10.1007/s13311-022-01248-1
52
Wong S Le GH Vasudeva S Teopiz KM Phan L Meshkat S et al . Preclinical and clinical efficacy of kappa opioid receptor antagonists for depression: A systematic review. J Affect Disord. (2024) 362:816–27. doi: 10.1016/j.jad.2024.07.030
53
Han Y Yuan K Zheng Y Lu L . Orexin receptor antagonists as emerging treatments for psychiatric disorders. Neurosci Bull. (2020) 36:432–48. doi: 10.1007/s12264-019-00447-9
54
Mony L Paoletti P . Mechanisms of NMDA receptor regulation. Curr Opin Neurobiol. (2023) 83:102815. doi: 10.1016/j.conb.2023.102815
55
Bose M Farias Quipildor G Ehrlich ME Salton SR . Intranasal peptide therapeutics: A promising avenue for overcoming the challenges of traditional CNS drug development. Cells. (2022) 11:3629. doi: 10.3390/cells11223629
56
Iqbal I Saqib F Mubarak Z Latif MF Wahid M Nasir B et al . Alzheimer's disease and drug delivery across the blood-brain barrier: approaches and challenges. Eur J Med Res. (2024) 29:313. doi: 10.1186/s40001-024-01915-3
57
Fonseca LC Lopes JA Vieira J Viegas C Oliveira CS Hartmann RP et al . Intranasal drug delivery for treatment of Alzheimer's disease. Drug Delivery Transl Res. (2021) 11:411–25. doi: 10.1007/s13346-021-00940-7
58
Lofts A Abu-Hijleh F Rigg N Mishra RK Hoare T . Using the intranasal route to administer drugs to treat neurological and psychiatric illnesses: rationale, successes, and future needs. CNS Drugs. (2022) 36:739–70. doi: 10.1007/s40263-022-00930-4
59
Drath I Richter F Feja M . Nose-to-brain drug delivery: from bench to bedside. Transl Neurodegener. (2025) 14:23. doi: 10.1186/s40035-025-00481-w
60
Subramanian S Haroutounian S Palanca BJA Lenze EJ . Ketamine as a therapeutic agent for depression and pain: mechanisms and evidence. J Neurol Sci. (2022) 434:120152. doi: 10.1016/j.jns.2022.120152
61
Swainson J Thomas RK Archer S Chrenek C MacKay MA Baker G et al . Esketamine for treatment resistant depression. Expert Rev Neurother. (2019) 19:899–911. doi: 10.1080/14737175.2019.1640604
62
Perez-Ruixo C Rossenu S Zannikos P Nandy P Singh J Drevets WC et al . Population pharmacokinetics of esketamine nasal spray and its metabolite noresketamine in healthy subjects and patients with treatment-resistant depression. Clin Pharmacokinet. (2021) 60:501–16. doi: 10.1007/s40262-020-00953-4
63
Upadhyay R Ghosh P Desavathu M . Advancement in the Nose-to-Brain Drug delivery of FDA-approved drugs for the better management of Depression and Psychiatric disorders. Int J Pharm. (2024) 667:124866. doi: 10.1016/j.ijpharm.2024.124866
64
Bulbul EO Karantas ID Okur ME Siafaka PI Okur NU . Schizophrenia; A review on promising drug delivery systems. Curr Pharm Des. (2020) 26:3871–83. doi: 10.2174/1381612826666200523173102
65
Abruzzo A Cerchiara T Luppi B Bigucci F . Transdermal delivery of antipsychotics: rationale and current status. CNS Drugs. (2019) 33:849–65. doi: 10.1007/s40263-019-00659-7
66
Watchorn J Clasky AJ Prakash G Johnston IAE Chen PZ Gu FX . Untangling mucosal drug delivery: engineering, designing, and testing nanoparticles to overcome the mucus barrier. ACS Biomater Sci Eng. (2022) 8:1396–426. doi: 10.1021/acsbiomaterials.2c00047
67
Sabbagh F Kim BS . Recent advances in polymeric transdermal drug delivery systems. J Control Release. (2022) 341:132–46. doi: 10.1016/j.jconrel.2021.11.025
68
Ramadon D McCrudden MTC Courtenay AJ Donnelly RF . Enhancement strategies for transdermal drug delivery systems: current trends and applications. Drug Delivery Transl Res. (2022) 12:758–91. doi: 10.1007/s13346-021-00909-6
69
Hatta K Usui C Nakamura H . Acceptability of transdermal antipsychotic patches by patients who refuse oral medication and their effectiveness in preventing recurrence of delirium: a retrospective observational study. Int Clin Psychopharmacol. (2023) 38:23–7. doi: 10.1097/YIC.0000000000000428
70
Zhou M Derakhshanian S Rath A Bertrand S DeGraw C Barlow R et al . Asenapine transdermal patch for the management of schizophrenia. Psychopharmacol Bull. (2020) 50:60–82. doi: https://pubmed.ncbi.nlm.nih.gov/33012873/
71
Wong WF Ang KP Sethi G Looi CY . Recent advancement of medical patch for transdermal drug delivery. Medicina (Kaunas). (2023) 59:778. doi: 10.3390/medicina59040778
72
Lima LM Alves MA do Amaral DN . Homologation: A versatile molecular modification strategy to drug discovery. Curr Top Med Chem. (2019) 19:1734–50. doi: 10.2174/1568026619666190808145235
73
Pandey M Choudhury H Fern JLC Kee ATK Kou J Jing JLJ et al . 3D printing for oral drug delivery: a new tool to customize drug delivery. Drug Delivery Transl Res. (2020) 10:986–1001. doi: 10.1007/s13346-020-00737-0
74
Paul D Sanap G Shenoy S Kalyane D Kalia K Tekade RK . Artificial intelligence in drug discovery and development. Drug Discov Today. (2021) 26:80–93. doi: 10.1016/j.drudis.2020.10.010
75
Bousman CA Maruf AA Marques DF Brown LC Muller DJ . The emergence, implementation, and future growth of pharmacogenomics in psychiatry: a narrative review. Psychol Med. (2023) 53:7983–93. doi: 10.1017/S0033291723002817
76
Teuscher N . The history and future of population pharmacokinetic analysis in drug development. Xenobiotica. (2024) 54:394–400. doi: 10.1080/00498254.2023.2291792
77
Kim DD Barr AM Rafizadeh R Procyshyn RM . Population pharmacokinetics and dosing of long-acting injectable antipsychotics. J Psychiatry Neurosci. (2021) 46:E516–E7. doi: 10.1503/jpn.210079
78
Brocks DR Hamdy DA . Bayesian estimation of pharmacokinetic parameters: an important component to include in the teaching of clinical pharmacokinetics and therapeutic drug monitoring. Res Pharm Sci. (2020) 15:503–14. doi: 10.4103/1735-5362.301335
79
Hayato S Takenaka O Sreerama Reddy SH Landry I Reyderman L Koyama A et al . Population pharmacokinetic-pharmacodynamic analyses of amyloid positron emission tomography and plasma biomarkers for lecanemab in subjects with early Alzheimer's disease. CPT Pharmacometrics Syst Pharmacol. (2022) 11:1578–91. doi: 10.1002/psp4.12862
80
Vasiliu O . Efficacy, tolerability, and safety of toludesvenlafaxine for the treatment of major depressive disorder-A narrative review. Pharm (Basel). (2023) 16:411. doi: 10.3390/ph16030411
81
Daniel WA Bromek E Danek PJ Haduch A . The mechanisms of interactions of psychotropic drugs with liver and brain cytochrome P450 and their significance for drug effect and drug-drug interactions. Biochem Pharmacol. (2022) 199:115006. doi: 10.1016/j.bcp.2022.115006
82
Kane JM Agid O Baldwin ML Howes O Lindenmayer JP Marder S et al . Clinical guidance on the identification and management of treatment-resistant schizophrenia. J Clin Psychiatry. (2019) 80:18com12123. doi: 10.4088/JCP.18com12123
83
Maruf AA Bousman CA . Approaches and hurdles of implementing pharmacogenetic testing in the psychiatric clinic. PCN Rep. (2022) 1:e26. doi: 10.1002/pcn5.26
84
Hu X Yu C Dong T Yang Z Fang Y Jiang Z . Biomarkers and detection methods of bipolar disorder. Biosens Bioelectron. (2023) 220:114842. doi: 10.1016/j.bios.2022.114842
85
Zeng Y Chourpiliadis C Hammar N Seitz C Valdimarsdottir UA Fang F et al . Inflammatory biomarkers and risk of psychiatric disorders. JAMA Psychiatry. (2024) 81:1118–29. doi: 10.1001/jamapsychiatry.2024.2185
86
Maheshwari S Singh A Ansari VA Mahmood T Wasim R Akhtar J et al . Navigating the dementia landscape: Biomarkers and emerging therapies. Ageing Res Rev. (2024) 94:102193. doi: 10.1016/j.arr.2024.102193
87
Schmidt ME Kezic I Popova V Melkote R van der Ark P Pemberton DJ et al . Efficacy and safety of aticaprant, a kappa receptor antagonist, adjunctive to oral SSRI/SNRI antidepressant in major depressive disorder: results of a phase 2 randomized, double-blind, placebo-controlled study. Neuropsychopharmacology. (2024) 49:1437–47. doi: 10.1038/s41386-024-01862-x
88
Jiang Y Zou D Li Y Gu S Dong J Ma X et al . Monoamine neurotransmitters control basic emotions and affect major depressive disorders. Pharm (Basel). (2022) 15:1203. doi: 10.3390/ph15101203
89
Pastis I Santos MG Paruchuri A . Exploring the role of inflammation in major depressive disorder: beyond the monoamine hypothesis. Front Behav Neurosci. (2023) 17:1282242. doi: 10.3389/fnbeh.2023.1282242
90
Micale V Di Bartolomeo M Di Martino S Stark T Dell'Osso B Drago F et al . Are the epigenetic changes predictive of therapeutic efficacy for psychiatric disorders? A translational approach towards novel drug targets. Pharmacol Ther. (2023) 241:108279. doi: 10.1016/j.pharmthera.2022.108279
91
Yuan M Yang B Rothschild G Mann JJ Sanford LD Tang X et al . Epigenetic regulation in major depression and other stress-related disorders: molecular mechanisms, clinical relevance and therapeutic potential. Signal Transduct Target Ther. (2023) 8:309. doi: 10.1038/s41392-023-01519-z
92
Herrera-Luis E Benke K Volk H Ladd-Acosta C Wojcik GL . Gene-environment interactions in human health. Nat Rev Genet. (2024) 25:768–84. doi: 10.1038/s41576-024-00731-z
93
Panariello F Fanelli G Fabbri C Atti AR De Ronchi D Serretti A . Epigenetic basis of psychiatric disorders: A narrative review. CNS Neurol Disord Drug Targets. (2022) 21:302–15. doi: 10.2174/1871527320666210825101915
94
Sherje AP Londhe V . Development and Evaluation of pH-Responsive Cyclodextrin-Based in situ Gel of Paliperidone for Intranasal Delivery. AAPS PharmSciTech. (2018) 19:384–94. doi: 10.1208/s12249-017-0844-8
95
Pereira M Cruz MT Fortuna A Bicker J . Restoring the epigenome in Alzheimer's disease: advancing HDAC inhibitors as therapeutic agents. Drug Discov Today. (2024) 29:104052. doi: 10.1016/j.drudis.2024.104052
96
Davis N Taylor B Abelleira-Hervas L Karimian-Marnani N Aleksynas R Syed N et al . Histone deacetylase-3 regulates the expression of the amyloid precursor protein and its inhibition promotes neuroregenerative pathways in Alzheimer's disease models. FASEB J. (2024) 38:e23659. doi: 10.1096/fj.202301762RR
97
Onishi T Maeda R Terada M Sato S Fujii T Ito M et al . A novel orally active HDAC6 inhibitor T-518 shows a therapeutic potential for Alzheimer's disease and tauopathy in mice. Sci Rep. (2021) 11:15423. doi: 10.1038/s41598-021-94923-w
98
Soltan OM Abdelrahman KS Bass AKA Takizawa K Narumi A Konno H . Design of Multi-Target drugs of HDACs and other Anti-Alzheimer related Targets: Current strategies and future prospects in Alzheimer's diseases therapy. Bioorg Chem. (2024) 151:107651. doi: 10.1016/j.bioorg.2024.107651
99
Lee S Kim TK Choi JE Choi Y You M Ryu J et al . Dysfunction of striatal MeCP2 is associated with cognitive decline in a mouse model of Alzheimer's disease. Theranostics. (2022) 12:1404–18. doi: 10.7150/thno.68439
100
Guo F Fan J Liu JM Kong PL Ren J Mo JW et al . Astrocytic ALKBH5 in stress response contributes to depressive-like behaviors in mice. Nat Commun. (2024) 15:4347. doi: 10.1038/s41467-024-48730-2
101
Popescu BO Batzu L Ruiz PJG Tulba D Moro E Santens P . Neuroplasticity in parkinson's disease. J Neural Transm (Vienna). (2024) 131:1329–39. doi: 10.1007/s00702-024-02813-y
102
Tartt AN Mariani MB Hen R Mann JJ Boldrini M . Dysregulation of adult hippocampal neuroplasticity in major depression: pathogenesis and therapeutic implications. Mol Psychiatry. (2022) 27:2689–99. doi: 10.1038/s41380-022-01520-y
103
Choquet D Opazo P Zhang H . AMPA receptor diffusional trapping machinery as an early therapeutic target in neurodegenerative and neuropsychiatric disorders. Transl Neurodegener. (2025) 14:8. doi: 10.1186/s40035-025-00470-z
104
Qin Z Shi DD Li W Cheng D Zhang YD Zhang S et al . Berberine ameliorates depression-like behaviors in mice via inhibiting NLRP3 inflammasome-mediated neuroinflammation and preventing neuroplasticity disruption. J Neuroinflammation. (2023) 20:54. doi: 10.1186/s12974-023-02744-7
105
Raza A Rasheed T Nabeel F Hayat U Bilal M Iqbal HMN . Endogenous and exogenous stimuli-responsive drug delivery systems for programmed site-specific release. Molecules. (2019) 24:1117. doi: 10.3390/molecules24061117
106
Fang H Xu S Wang Y Yang H Su D . Endogenous stimuli-responsive drug delivery nanoplatforms for kidney disease therapy. Colloids Surf B Biointerfaces. (2023) 232:113598. doi: 10.1016/j.colsurfb.2023.113598
107
Ding H Tan P Fu S Tian X Zhang H Ma X et al . Preparation and application of pH-responsive drug delivery systems. J Control Release. (2022) 348:206–38. doi: 10.1016/j.jconrel.2022.05.056
108
Pokharel M Park K . Light mediated drug delivery systems: a review. J Drug Targeting. (2022) 30:368–80. doi: 10.1080/1061186X.2021.2005610
109
Wei P Cornel EJ Du J . Ultrasound-responsive polymer-based drug delivery systems. Drug Delivery Transl Res. (2021) 11:1323–39. doi: 10.1007/s13346-021-00963-0
110
Lopez Ruiz A Ramirez A McEnnis K . Single and multiple stimuli-responsive polymer particles for controlled drug delivery. Pharmaceutics. (2022) 14:421. doi: 10.3390/pharmaceutics14020421
111
Brewster PR Mohammad Ishraq Bari S Walker GM Werfel TA . Current and future directions of drug delivery for the treatment of mental illnesses. Adv Drug Delivery Rev. (2023) 197:114824. doi: 10.1016/j.addr.2023.114824
Summary
Keywords
psychiatric disorders, pharmacotherapy, clinical trials, innovative drugs, drug optimization, trial design
Citation
Xu C, Zhao Y, Yang X, Zheng J and Tang Q (2025) Exploration of current situation of psychotropic drugs research and development in China based on drug clinical trials. Front. Psychiatry 16:1599038. doi: 10.3389/fpsyt.2025.1599038
Received
17 April 2025
Accepted
25 June 2025
Published
15 July 2025
Volume
16 - 2025
Edited by
Marijn Lijffijt, IonTX, Inc, United States
Reviewed by
Yuhao Xie, St. John’s University, United States
Ming-Rui Li, Anyang Tumor Hospital, China
Updates
Copyright
© 2025 Xu, Zhao, Yang, Zheng and Tang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chen Xu, 545782884@qq.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.