- Sichuan Provincial Center for Mental Health, Sichuan Provincial People’s Hospital, School of Medicine, University of Electronic Science and Technology, Chengdu, China
Background: Generalized anxiety disorder (GAD) and hypertension (HTN) exhibit a clinically significant bidirectional relationship characterized by neuroendocrine dysregulation and autonomic dysfunction. Their comorbidity presents diagnostic and therapeutic challenges due to overlapping symptoms and fragmented care pathways.
Case presentation: We report a 61-year-old male with 26-year refractory HTN and new-onset GAD triggered post-thyroidectomy. Despite triple antihypertensive therapy (nifedipine, arotinolol, sacubitril/valsartan), blood pressure (BP) remained uncontrolled (176/105 mmHg) with severe anxiety (HAMA = 36). Secondary HTN investigations were negative. Multimodal management combining pharmacotherapy (escitalopram, tandospirone), transcranial magnetic stimulation (9 sessions), biofeedback (14 sessions), psychotherapy, and lifestyle interventions achieve: mean BP decreased significantly from 176/105 mmHg to 125/72 mmHg during hospitalization; significant anxiety reduction (HAMA = 3), mean BP stabilized at 131/77 mmHg with 50% reduction in antihypertensive dosages, normalization of elevated ACTH (99.9→normal pg/mL) and cortisol (18.7→normal μg/dL) and sustained improvement at 6-month follow-up.
Conclusion: This case demonstrates thyroidectomy-induced endocrine disruption as a novel trigger in the GAD-HT bidirectional loop. Multimodal therapy targeting shared neurobiological pathways (HPA axis, autonomic regulation, serotonin signaling) effectively breaks this cycle, underscoring the imperative for integrated mental-cardiovascular care in treatment-resistant cases.
Introduction
Hypertension (HTN) and generalized anxiety disorder (GAD) represent a significant bidirectional comorbidity with profound global health implications. HTN affects approximately 32% of adults worldwide, driven by genetic predisposition, lifestyle factors (e.g., high sodium intake, physical inactivity), and chronic stress exposure (1). Concurrently, GAD—characterized by persistent, excessive worry—has a global prevalence of 7.3% and demonstrates frequent comorbidity with cardiovascular conditions (2). Critically, these disorders engage in a self-perpetuating cycle: chronic anxiety exacerbates HTN through sympathetic nervous system hyperactivity and hypothalamic-pituitary-adrenal (HPA) axis dysregulation, while uncontrolled HTN amplifies psychological anxiety through physiological burden and illness-related worry (3). Notably, research has established a significant causal effect of anxiety on hypertension risk (4).
This pathophysiological interplay involves interconnected mechanisms. Neuroendocrine dysregulation—particularly involving the hypothalamic-pituitary-adrenal (HPA) axis—increases catecholamine release (5) and elevates cortisol levels thereby promoting vasoconstriction and endothelial dysfunction (6). Concurrent autonomic imbalance directly disrupts blood pressure (BP) regulation while exacerbating anxiety symptoms (7). Critically, autonomic dysfunction reduces heart rate variability (HRV) (8), which mediates 33–80% of anxiety’s effect on hypertension through altered baroreflex sensitivity (9). Furthermore, anxiety impairs self-care behaviors (e.g., medication adherence, dietary control) via maladaptive coping strategies (3, 10). Additionally, endocrine dysregulators such as thyroid hormone (TH) abnormalities can concurrently modulate vascular tone and anxiety pathways through adrenergic receptor upregulation and neurotransmitter alterations (11, 12). Collectively, these mechanisms drive persistent hypertension.
Clinically, this comorbidity presents substantial diagnostic and therapeutic challenges. Symptom overlap (e.g., palpitations, fatigue) obscures etiology, while pharmacotherapeutic interactions complicate management. Selective serotonin reuptake inhibitors (SSRIs), first-line GAD treatments, exert complex vascular effects through serotonin (5-HT) receptor modulation—where abrupt discontinuation may precipitate BP instability (13). Moreover, physiological stressors like thyroid dysfunction can trigger both conditions (14, 15). Current guidelines lack consensus on integrated approaches, often resulting in fragmented care between cardiovascular and mental health providers (16, 17).
This case report addresses this gap by examining a paradigmatic presentation of GAD and HTN exacerbated post-thyroidectomy. We elucidate three novel aspects:
1. The role of thyroidectomy-induced endocrine disruption as a bidirectional trigger;
2. Serotonin-mediated vascular modulation in SSRIs discontinuation phenomena;
3. Transcranial magnetic stimulation and biofeedback as a mechanism-specific intervention.
Through this lens, we demonstrate how multimodal management targeting shared neurobiological pathways can disrupt the GAD-HTN cycle, providing a framework for personalized comorbidity management.
Case presentation
History of illness
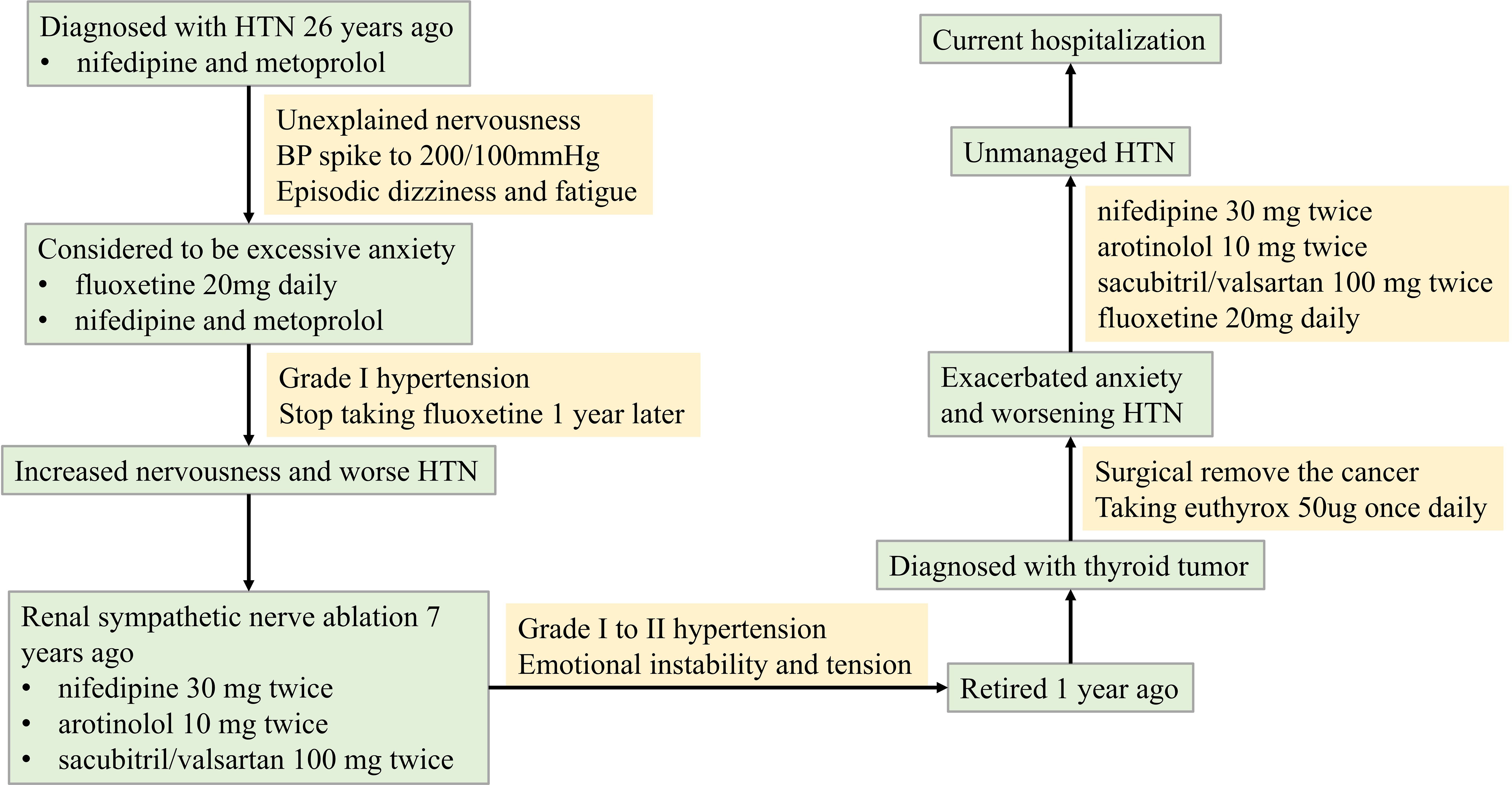
A 61-year-old male was diagnosed with recurrent HTN 26 years ago. He was prescribed nifedipine and metoprolol, which stabilized his BP at around 130/80mmHg. Despite taking his antihypertensive medication regularly, his BP would spike to over 200/100mmHg when he felt nervousness, leading to symptoms of dizziness and fatigue. Upon the advice of a psychiatrist, he started taking fluoxetine 20mg daily, which gradually helped lower his BP in conjunction with his antihypertensive drugs. However, he abruptly stopped taking fluoxetine a year later without any apparent reason. This led to increased nervousness and ineffective control of his BP with his antihypertensive medication.
The patient had renal sympathetic nerve ablation 7 years ago and is currently taking nifedipine 30 mg twice daily, arotinolol 10 mg twice daily, and sacubitril/valsartan 100 mg twice daily. He reported that his BP ranged from 140-170/80–100 mmHg when he was emotionally unstable and nervousness, and 140-150/80–90 mmHg when he felt relaxed during treatment with these antihypertensive medications.
This patient, who had retired one year prior, was diagnosed with a thyroid tumor and subsequently underwent thyroidectomy. Postoperatively, levothyroxine replacement therapy (50 μg daily) was initiated. He subsequently developed significant health-related anxiety, manifesting as severe nervousness, dizziness, insomnia, fatigue, and appetite loss. Consequently, fluoxetine (20 mg daily) was prescribed. Despite this intervention, his HTN became increasingly refractory to control. After one month of fluoxetine treatment without adequate HTN management, he required hospitalization. A timeline of the illness course is provided in Figure 1.
Medical history
He has type 2 diabetes. Both parents and younger brother had HTN, and his father died of a stroke while his mother died of cerebral hemorrhage.
Hospitalization treatment and follow-up
Despite undergoing comprehensive evaluation for secondary HTN during hospitalization, including renal artery ultrasound, aldosterone/renin and sleep apnea screening, all test results were negative. Moreover, the patient’s HTN remained refractory to antihypertensive therapy with a triple oral regimen consisting of nifedipine, arotinolol, and sacubitril-valsartan. Episodes of severe hypertension necessitated intravenous nitroglycerin to lower blood pressure, which further heightened the patient’s nervousness and anxiety. Subsequent psychiatric evaluation diagnosed GAD.
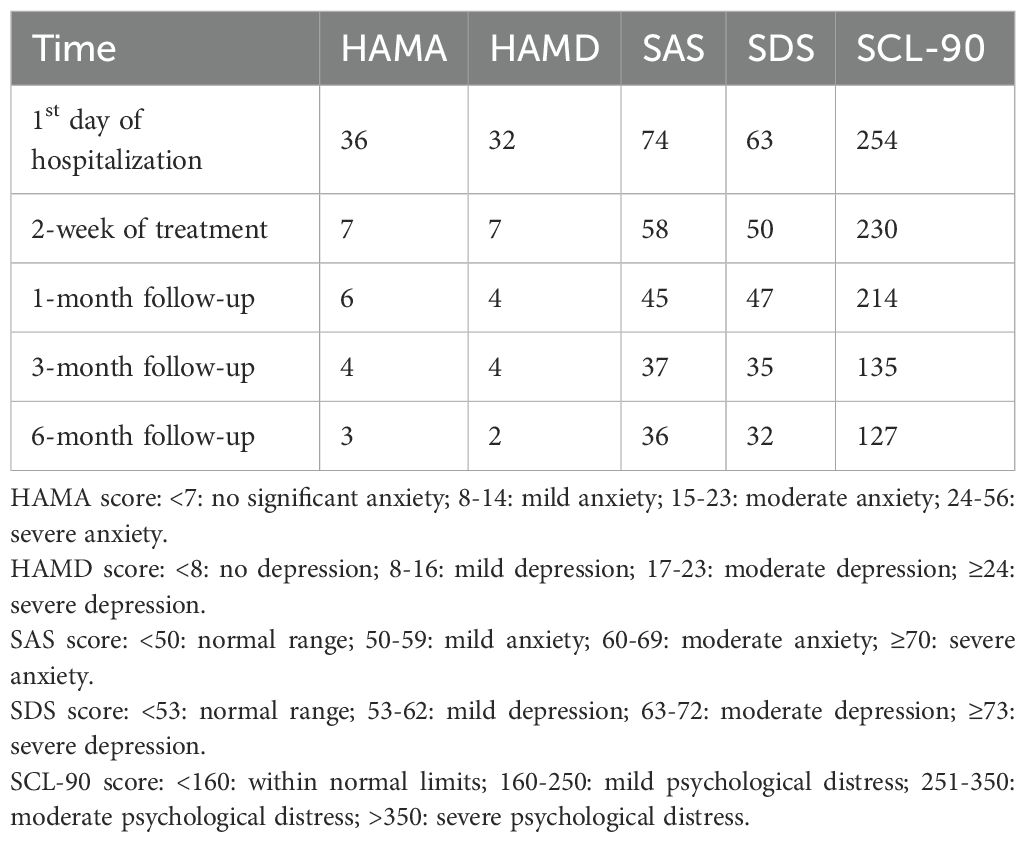
Initial psychiatric assessment indicated severe anxiety and moderate-to-severe depression. The patient scored 36 on the Hamilton Anxiety Rating Scale (HAMA), 32 on the Hamilton Depression Rating Scale (HAMD), 74 on the Self-Rating Anxiety Scale (SAS), and 63 on the Self-Rating Depression Scale (SDS) (Table 1). Endocrine laboratory findings included: Adrenocorticotropic hormone (ACTH) level of 99.9 pg/mL (normal range: 7.2–63.3 pg/mL), cortisol level of 18.7 µg/dL (normal range: 6.02–18.4 µg/dL), and glycated hemoglobin (HbA1c) of 6.36% (normal range: 4–6%). Thyroid function tests revealed an elevated free thyroxine (FT4) level of 26.2 pmol/L (normal range: 12.0–22.0 pmol/L) and a suppressed thyroid-stimulating hormone (TSH) level of 0.07 mIU/L (normal range: 0.27–4.20 mIU/L). HRV analysis demonstrated reduced overall autonomic nervous system activity, relative parasympathetic predominance, and impaired sympathovagal balance (LF/HF=0.458).
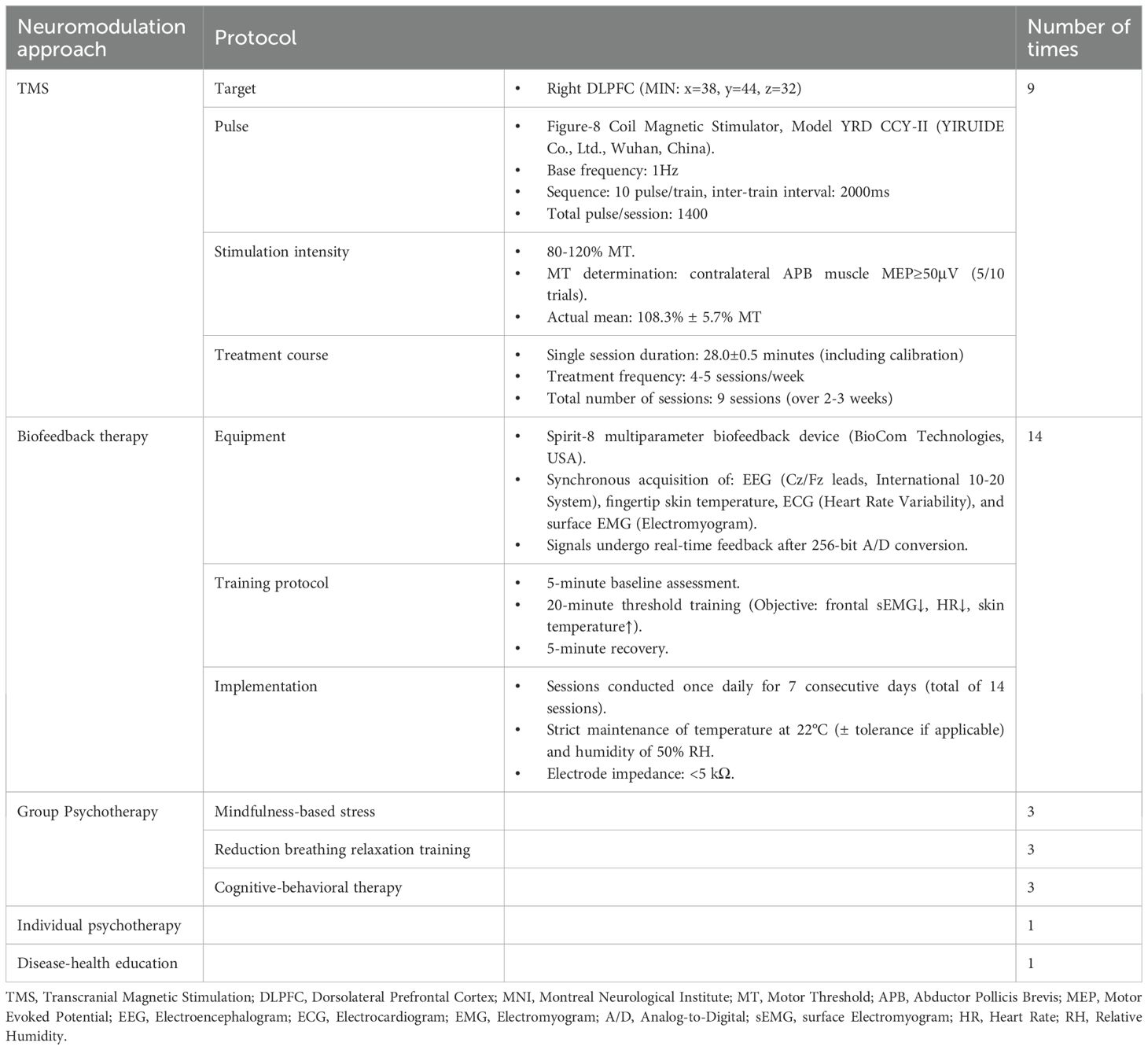
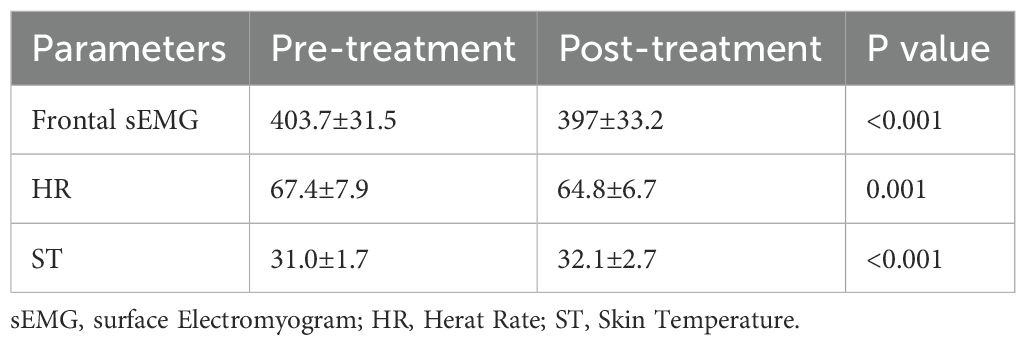
During the two-week hospitalization, the patient achieved significant improvement in anxiety and depressive symptoms through combined pharmacotherapy (escitalopram, tandospirone, alprazolam) and multimodal neuromodulation which comprised transcranial magnetic stimulation (TMS), biofeedback, group psychotherapy, individual psychotherapy, and disease-specific health education (Table 2). The dosages of oral medication and ambulatory mean BP during hospitalization was in Supplementary Table S1. Physiological parameters monitored during biofeedback sessions including frontal surface electromyogram (sEMG), heart rate (HR) and skin temperature progressively normalized (Table 3). Notably, HR stabilized after seven biofeedback session (Figure 2A). Post-treatment psychometric scores decreased to HAMA = 7, HAMD = 7, SAS = 58 and SDS = 50 (Table 1). Concurrently, hypertension control substantially improved despite maintain the same oral antihypertension regimen. Mean BP decreased significantly from 176/105 mmHg to 125/72 mmHg (Figure 2B). discharge psychiatric evaluation indicated minimal residual anxiety and resolution of depressive symptoms to a mild level (Table 1). By discharge, ACTH and cortisol levels had normalized.
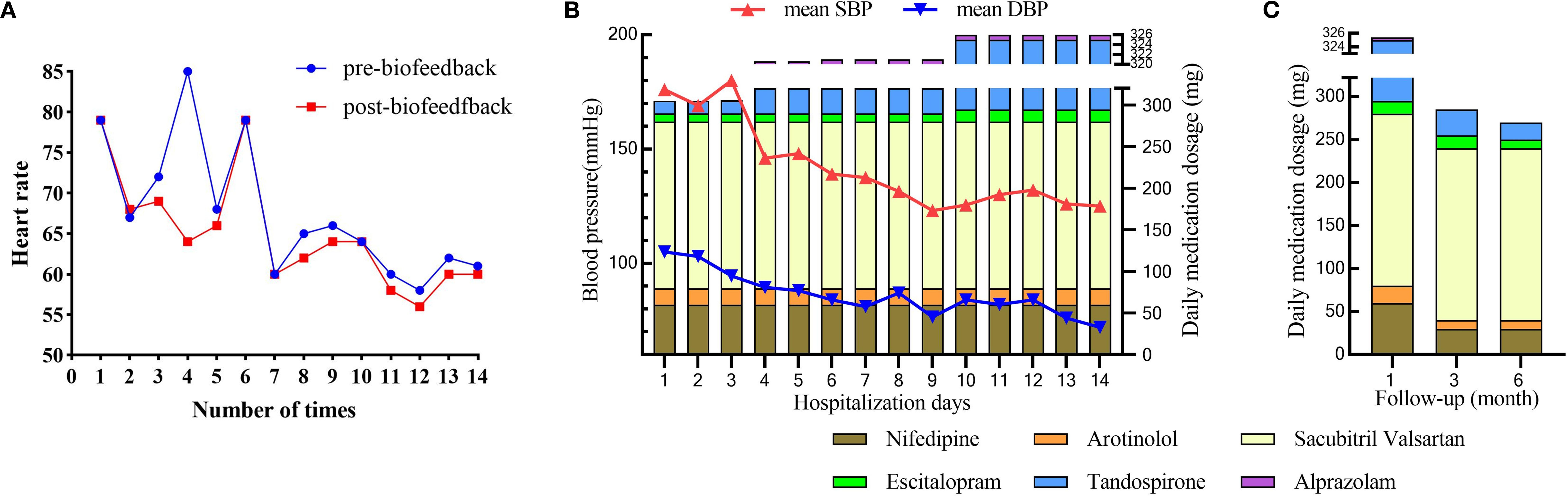
Figure 2. The outcome after multimodal therapy during hospitalization and follow-up. (A) Changes in heart rate during the period of biofeedback therapy; (B) Changes in mean systolic blood pressure (SBP) and diastolic blood pressure (DBP) alongside daily medication dosages over 14 days of hospitalization; (C) Changes in daily medication dosage over a 6-month follow-up period.
Over the six-month follow-up period, the patient consistently adhered to a heart-healthy lifestyle, including a low-sodium, low-fat diet and regular aerobic exercise (cycling or swimming). His anti-anxiety medications were tapered, with only escitalopram and tandospirone continued (Supplementary Table S2). Thyroid function remained stable under levothyroxine supplementation (50 μg daily), FT4 levels between 16 and 19 pmol/L and TSH levels between 0.28 and 2.6 mIU/L. Psychiatric assessments indicated progressive improvement in anxiety and depression, with corresponding scores HAMA = 3, HAMD = 2, SAS = 36, and SDS = 32 (Table 1). Concurrently, hypertension control substantially improved; mean blood pressure (BP) stabilized at 131/77 mmHg, enabling a 50% dosage reduction in both nifedipine and arotinolol (Figure 2C).
Discussion
This paradigmatic case elucidates the complex bidirectional pathophysiology linking GAD and HTN, demonstrating the critical importance of integrated management. Our patient’s 26-year history of recurrent HTN, exacerbated acutely post-thyroidectomy and accompanied by newly diagnosed severe GAD, presented a therapeutic challenge that was only resolved through combined psychiatric and cardiovascular intervention. The significant improvement in both anxiety symptoms (HAMA reduced from 36 to 7 during hospitalization; stabilized at 3 at 6 months) and HTN control (mean BP decreased from 176/105 mmHg to 125/72 mmHg during hospitalization; stabilized at 131/77 mmHg on reduced antihypertensive doses long-term) following targeted neuromodulation and pharmacotherapy underscores the interplay between these conditions.
The abrupt discontinuation of fluoxetine (20mg daily) one year prior highlights a critical clinical consideration. SSRIs exert complex vascular effects through serotonin (5-HT) receptor modulation, particularly involving vasoconstrictive 5-HT2A receptors. Abrupt cessation likely contributed to anxiety relapse and BP instability via dual mechanisms: peripheral withdrawal directly altering vascular reactivity and central serotonin depletion exacerbating anxiety, thereby increasing sympathetic tone (18, 19). This observation aligns with evidence linking SSRIs discontinuation syndrome to both psychological symptom rebound and cardiovascular dysregulation (20).
Post-thyroidectomy endocrine disruption emerged as a significant bidirectional trigger. The patient developed profound health-related anxiety alongside recurrent HTN following surgery. Laboratory findings revealed transient iatrogenic hyperthyroidism (TSH 0.07 mIU/L, FT4 26.2 pmol/L at admission), subsequently stabilized with levothyroxine (TSH 0.28-2.6 mIU/L, FT4 16–19 pmol/L at follow-up). Thyroid hormone (TH) dysregulation likely exacerbated both conditions: TH excess can upregulate β-adrenergic receptors, increasing sympathetic tone and vascular resistance (21), while simultaneously disrupting neurotransmitter systems (e.g., serotonin, dopamine) critical for mood regulation (22, 23). This TH instability, interacting with the underlying GAD-HTN pathophysiology, created a self-perpetuating cycle of physiological stress and psychological distress.
Concurrent HPA axis hyperactivity, evidenced by markedly elevated admission ACTH (99.9 pg/mL) and cortisol (18.7 μg/dL) levels (normalizing after integrated treatment), represents a core neuroendocrine mechanism in GAD-HTN comorbidity (24). The HRV profile observed in this patient—characterized by globally reduced variability, paradoxical relative parasympathetic predominance, and a markedly low LF/HF ratio (0.458)—presents a critical neurobiological signature of the GAD-HTN bidirectional loop (9). While autonomic dysfunction typically manifests as sympathetic hyperactivity in HTN or GAD individually (25, 26), this specific HRV pattern suggests a maladaptive compensatory response: chronic sympathetic overdrive may have exhausted autonomic reserves, leading to blunted overall HRV and ineffective parasympathetic counter-regulation. To simultaneously address neuroendocrine dysregulation and autonomic nervous system (ANS) imbalance, we developed a multimodal therapeutic protocol for this patient.
First, the biofeedback can directly address the identified ANS imbalance (27). Using synchronous multi-parameter feedback (sEMG, HR, skin temperature), we trained the patient to consciously modulate autonomic output, increasing skin temperature while reducing frontal muscle tension and HR. This precision targeting of the specific ANS dysfunction revealed by admission HRV—conducted over 14 intensive sessions—likely contributed significantly to the rapid stabilization of both BP and anxiety symptoms during hospitalization and the sustained improvement at follow-up.
Second, our neuropharmacological strategy demonstrates rational synergy with neuromodulation to restore autonomic homeostasis. The medication regimen—escitalopram (a SSRIs) and tandospirone (a 5-HT1A receptor partial agonist)—were carefully selected not only for anxiolytic efficacy but also for their favorable autonomic profiles. Escitalopram minimally impacts cardiac conduction and may improve HRV over time through reduced central anxiety drive (28, 29). Tandospiron’s action on presynaptic 5-HT1A autoreceptors in the dorsal raphe nucleus modulates downstream projections to brainstem autonomic centers, potentially dampening excessive sympathetic outflow (30). Crucially, this pharmacotherapy complemented rather than replaced neuromodulation. The TMS protocol targeted the right dorsolateral prefrontal cortex (DLPFC), a key node in the cortical inhibition pathway over limbic and autonomic hyperactivity (31). Low-frequency (1Hz) stimulation to the right DLPFC likely enhanced inhibitory control, thereby reducing amygdala-driven sympathetic activation and facilitating the ANS retraining achieved through biofeedback.
This integrated “top-down” (TMS modulating cortical control) and “bottom-up” (biofeedback training peripheral ANS responses) approach, pharmacologically supported, represents a significant advance over isolated pharmacological or device-based interventions commonly reported. The resolution of HPA axis markers alongside clinical improvement supports their role in sustaining the comorbid cycle.
Finally, we believe that this multimodal strategy—TMS modulates cortical excitability in anxiety-relevant prefrontal circuits (32), sacubitril-valsartan concurrently enhances natriuretic peptides and inhibits the renin-angiotensin-aldosterone system (RAAS) (33), complemented by pharmacotherapy (escitalopram, tandospirone), biofeedback, psychotherapy, lifestyle modification, and endocrine management—successfully disrupted the GAD-HTN feedback loop. The progressive improvement across all metrics (anxiety scales, BP, endocrine markers, HRV) during hospitalization and sustained at 6-month follow-up validates this integrated approach.
Conclusion
This case demonstrates a successful multimodal, mechanism-targeted approach for managing treatment-resistant GAD-HTN comorbidity. Key elements include vigilance regarding SSRI discontinuation effects, recognition of endocrine triggers (e.g., post-surgical thyroid dysfunction), biomarker-guided therapy (HPA axis hormones, HRV, thyroid function), and synergistic neuromodulation-biofeedback interventions.
Current literature predominantly documents ANS improvements with single-modality interventions (e.g., SSRIs or cognitive-behavioral therapy or exercise (17, 34, 35), while internationally endorsed guidelines for treatment-resistant anxiety disorders remain lacking (16). Critically, this case establishes the therapeutic efficacy of multimodal intervention—combining pharmacotherapy, targeted TMS, biofeedback, psychotherapy, and lifestyle modification—for severe autonomic dysfunction in treatment-resistant GAD-HT comorbidity. This integrated multimodal approach—simultaneously addresses all tiers of the “neuro-cardio-endocrine axis” —disrupts the pathological cycle typically resistant to conventional fragmented treatment modalities.
Future research should investigate the biomarkers like HRV parameters and HPA axis markers (ACTH/cortisol) for predicting treatment response in this comorbid population. Prospective randomized controlled trials should also be implemented to definitively compare the efficacy of our specific regimen (SSRIs + targeted TMS + biofeedback) against standard care or less intensive interventions. Addressing these key areas is essential to refine targeted interventions and improve outcomes for this challenging patient population.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Bioethics Committee of Sichuan Provincial People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JC: Visualization, Conceptualization, Validation, Resources, Writing – review & editing, Data curation, Methodology, Formal Analysis, Software, Supervision, Writing – original draft, Investigation. YY: Data curation, Methodology, Formal Analysis, Investigation, Visualization, Conceptualization, Writing – review & editing. FJ: Methodology, Conceptualization, Resources, Project administration, Validation, Supervision, Writing – review & editing, Data curation.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2025.1600910/full#supplementary-material
References
1. Wang J and Wang X. Study on hypertension and risk of hypertension and cardiovascular disease. J Clin Nurs Res. (2022) 6:79–83. doi: 10.26689/jcnr.v6i5.4171
2. Petelin DS, Sorokina OY, Troshina DV, Siginevich YA, Efimochkina SM, and Volel BA. Anxiety disorders in general medical practice-clinical picture, diagnostics, optimized approaches to therapy. Med Council. (2023) 0:110–8. doi: 10.21518/ms2023-053
3. Versteeg SJ and Stefanatou A. Hypertension and anxiety: comorbidity, treatment, and access to resources. Cardiol Cardiovasc Res. (2023) 1:1–6. doi: 10.52106/2996-3885.1003
4. Fan C, Wang J, Guo B, Wang S, Kong D, Liu M, et al. Exploring the causal relationships between anxiety, obesity, and hypertension: A two-sample mendelian randomization study. Am J Hypertens. (2025) 38:852–8. doi: 10.1093/ajh/hpaf072
5. Kinlein SA, Wilson CD, and Karatsoreos IN. Dysregulated Hypothalamic–Pituitary–Adrenal Axis Function Contributesto Altered Endocrine and Neurobehavioral Responses to Acute Stress. Front Psychiatry. (2015) 6:1–9. doi: 10.3389/fpsyt.2015.00031
6. Toda N and Nakanishi-Toda M. How mental stress affects endothelial function. Pflugers Arch - Eur J Physiol. (2011) 462:779–94. doi: 10.1007/s00424-011-1022-6
7. Hering D, Lachowska K, and Schlaich M. Role of the sympathetic nervous system in stress-mediated cardiovascular disease. Curr Hypertens Rep. (2015) 17:80. doi: 10.1007/s11906-015-0594-5
8. Agorastos A, Mansueto AC, Hager T, Pappi E, Gardikioti A, and Stiedl O. Heart rate variability as a translational dynamic biomarker of altered autonomic function in health and psychiatric disease. Biomedicines. (2023) 11:1591. doi: 10.3390/biomedicines11061591
9. Chen T-Y, Kao C-W, Cheng S-M, and Liu C-Y. Mediating effect of heart rate variability on the relationship between anxiety symptoms and blood pressure in patients with primary hypertension. Appl Psychophysiol Biofeedback. (2024) 49:473–82. doi: 10.1007/s10484-024-09641-6
10. Celano CM, Daunis DJ, Lokko HN, Campbell KA, and Huffman JC. Anxiety disorders and cardiovascular disease. Curr Psychiatry Rep. (2016) 18:101. doi: 10.1007/s11920-016-0739-5
11. Hafe MV, Neves JS, Vale C, Borges-Canha M, and Leite-Moreira A. The impact of thyroid hormone dysfunction on ischemic heart disease. Endocr Connect. (2019) 8:R76–90. doi: 10.1530/EC-19-0096
12. Bernal J. Thyroid Hormones and Mood Disorders. In: Thyroid and Brain: Understanding the Actions of Thyroid Hormones in Brain Development and Function. UAE: BENTHAM SCIENCE PUBLISHERS (2024). p. 266–75. doi: 10.2174/9789815274226124010018
13. Mortensen JK and Andersen G. Safety considerations for prescribing SSRI antidepressants to patients at increased cardiovascular risk. Expert Opin Drug Saf. (2022) 21:467–75. doi: 10.1080/14740338.2022.1986001
14. Anwar U, Arshad J, Naeem UH, Zahid A, Jehan AS, Ramzan S, et al. The impact of thyroid hormone imbalance on cardiovascular health: A population-based study. Cureus. (2024) 16:e76457. doi: 10.7759/cureus.76457
15. Ginsberg JP. Editorial: dysregulation of autonomic cardiac control by traumatic stress and anxiety. Front Psychol. (2016) 7:945. doi: 10.3389/fpsyg.2016.00945
16. Domschke K, Seuling PD, Schiele MA, Bandelow B, Batelaan NM, Bokma WA, et al. The definition of treatment resistance in anxiety disorders: a Delphi method-based consensus guideline. World Psychiatry. (2024) 23:113–23. doi: 10.1002/wps.21177
17. Bereza BG, MaChado M, Ravindran AV, and Einarson TR. Evidence-based review of clinical outcomes of guideline-recommended pharmacotherapies for generalized anxiety disorder. Can J Psychiatry. (2012) 57:470–8. doi: 10.1177/070674371205700805
18. Watts SW, Morrison SF, Davis RP, and Barman SM. Serotonin and blood pressure regulation. Pharmacol Rev. (2012) 64:359–88. doi: 10.1124/pr.111.004697
19. Figari WJA, Herrmann S, Akogyeram C, and Qian Q. New onset hypertension following abrupt discontinuation of citalopram. Clin Nephrol. (2014) 82:202–4. doi: 10.5414/CN107731
20. Sharp T and Collins H. Mechanisms of SSRI Therapy and Discontinuation. In: Browning M, Cowen PJ, and Sharp T, editors. Emerging Neurobiology of Antidepressant Treatments. Springer International Publishing, Cham (2024). p. 21–47. doi: 10.1007/7854_2023_452
21. Prakash K, Hamid P, Prakash K, and Hamid P. Thyroid hormone resistance syndrome: from molecular mechanisms to its potential contribution to hypertension. Cureus. (2023) 15:e49913. doi: 10.7759/cureus.49913
22. Singh B and Sundaresh V. Thyroid hormone use in mood disorders: revisiting the evidence. J Clin Psychiatry. (2022) 83:69–71. doi: 10.4088/JCP.22ac14590
23. Maddaloni G, Barsotti N, Migliarini S, Giordano M, Nazzi S, Picchi M, et al. Impact of serotonin deficiency on circadian dopaminergic rhythms. Int J Mol Sci. (2024) 25:6475. doi: 10.3390/ijms25126475
24. Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, et al. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr Physiol. (2016) 6:603–21. doi: 10.1002/j.2040-4603.2016.tb00694.x
25. Mancia G and Grassi G. The Autonomic nervous system and hypertension. Circ Res. (2014) 114:1804–14. doi: 10.1161/CIRCRESAHA.114.302524
26. Teed AR, Feinstein JS, Puhl M, Lapidus RC, Upshaw V, Kuplicki RT, et al. Association of generalized anxiety disorder with autonomic hypersensitivity and blunted ventromedial prefrontal cortex activity during peripheral adrenergic stimulation: A randomized clinical trial. JAMA Psychiatry. (2022) 79:323–32. doi: 10.1001/jamapsychiatry.2021.4225
27. Rice KM, Blanchard EB, and Purcell M. Biofeedback treatments of generalized anxiety disorder: Preliminary results. Biofeedback Self-Regulation. (1993) 18:93–105. doi: 10.1007/BF01848110
28. Almeman A, Altwijri A, Alsaif E, Alahmad R, Alyahya A, Alruwais N, et al. Investigation of the clinical significance of escitalopram-induced electrocardiographic changes in men: A pilot observational study. Int J Pharm Pharm Sci. (2019) 11:23–5. doi: 10.22159/ijpps.2019v11i4.31449
29. Machhi TR, Patel CR, Pandya SK, and Kantharia ND. Effect of fluoxetine and escitalopram on heart rate variability (HRV) and serum potassium level in patients of depression and anxiety disorders. J Pharmacol Pharmacotherapeutics. (2023) 14:231–7. doi: 10.1177/0976500X231213153
30. Huang X, Yang J, Yang S, Cao S, Qin D, Zhou Y, et al. Role of tandospirone, a 5-HT1A receptor partial agonist, in the treatment of central nervous system disorders and the underlying mechanisms. Oncotarget. (2017) 8:102705–20. doi: 10.18632/oncotarget.22170
31. Li Q, Fu Y, Liu C, and Meng Z. Transcranial direct current stimulation of the dorsolateral prefrontal cortex for treatment of neuropsychiatric disorders. Front Behav Neurosci. (2022) 16:893955. doi: 10.3389/fnbeh.2022.893955
32. Cilli SL, Goldberg MA, Cosmo C, Arulpragasam AR, Zand Vakili A, Berlow YA, et al. Transcranial magnetic stimulation for posttraumatic stress disorder and generalized anxiety disorder. Berlin, Heidelberg: Springer. (2024). 1–28. doi: 10.1007/7854_2024_540
33. Scott NJA, Charles CJ, Prickett TCR, Frampton CM, Richards AM, and Rademaker MT. Phosphodiesterase 9 inhibition combined with valsartan and with sacubitril/valsartan in experimental ovine heart failure. J Am Heart Assoc. (2024) 13:e036689. doi: 10.1161/JAHA.124.036689
34. Charlesworth G, Sadek S, Schepers A, and Spector A. Cognitive behavior therapy for anxiety in people with dementia: a clinician guideline for a person-centered approach. Behav Modif. (2015) 39:390–412. doi: 10.1177/0145445514561317
Keywords: generalized anxiety disorder, hypertension, bidirectional relationship, multimodal therapy approach, neurobiological pathway
Citation: Chen J, Yang Y and Jiang F (2025) Case Report: Generalized anxiety disorder and hypertension: a bidirectional loop unraveled by integrated management. Front. Psychiatry 16:1600910. doi: 10.3389/fpsyt.2025.1600910
Received: 27 March 2025; Accepted: 17 September 2025;
Published: 02 October 2025.
Edited by:
Liliane Lins-Kusterer, Universidade Federal da Bahia, BrazilReviewed by:
Mohsen Khosravi, Zahedan University of Medical Sciences, IranAnqi Duan, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Copyright © 2025 Chen, Yang and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fugui Jiang, amlhbmdmdWd1aWpmZ0AxNjMuY29t
 Junming Chen
Junming Chen Fugui Jiang
Fugui Jiang