- 1Macao Observatory for Social Development, University of Saint Joseph, Macao, Macao SAR, China
- 2Unit of Psychiatry, Department of Public Health and Medicinal Administration, Institute of Translational Medicine, Faculty of Health Sciences, University of Macau, Macao, Macao SAR, China
- 3Centre for Cognitive and Brain Sciences, University of Macau, Macao, Macao SAR, China
- 4Department of Epidemiology and Biostatistics, School of Public Health, Jilin University, Changchun, China
- 5Section of Psychiatry, University of Notre Dame Australia, Fremantle, WA, Australia
- 6Division of Psychiatry, School of Medicine, University of Western Australia, Perth, WA, Australia
- 7School of Public Health, Southeast University, Nanjing, China
- 8School of Nursing, Hong Kong Polytechnic University, Hong Kong, Hong Kong SAR, China
- 9Department of Psychiatry, The Melbourne Clinic and St Vincent’s Hospital, University of Melbourne, Richmond, VIC, Australia
- 10Beijing Key Laboratory of Mental Disorders, National Clinical Research Center for Mental Disorders & National Center for Mental Disorders, Beijing Anding Hospital, Capital Medical University, Beijing, China
Objective: Poor sleep quality is common among patients with Parkinson’s disease (PD), although the reported prevalence rates vary between studies. This meta-analysis examined the overall prevalence of poor sleep quality in patients with PD and identified potential factors contributing to the differences in prevalence across studies.
Methods: Both PRISMA and MOOSE guidelines were applied in this meta-analysis. A systematic search was conducted in PubMed, EMBASE, PsycINFO, Web of Science, CNKI and Wangfang from their inception to November 4, 2023. Studies were selected based on predefined PICOS criteria (i.e., PD patients, prevalence of poor sleep quality, cross-sectional/cohort designs). Study quality/risk of bias was assessed using a standardized 8-item tool. Pooled prevalence was calculated sources of heterogeneity (e.g., age, sex, depression, anxiety, cognition scores, disease severity, and medication dose) were explored via subgroup and meta-regression analyses. A random-effects model was utilized to calculate the overall prevalence and corresponding 95% confidence intervals (CIs).
Results: In total, 63 studies involving 9,382 PD patients were included. The overall prevalence of poor sleep quality was 58.07% (95% CI: 54.26–61.88%). Higher rates were related to various factors including studies from Europe & Central Asia, Upper middle income countries, mixed patient sources, lower diagnostic cutoffs, and use of Movement Disorder Society PD criteria. Meta-regression analysis showed that late onset PD was associated with poorer sleep quality in patients with PD.
Conclusion: Poor sleep quality is common in PD patients. Regular monitoring of sleep quality and promotion of sleep hygiene should be prioritized in the management of patients with PD. Additionally, further research on sleep and PD is warranted in low- and middle-income countries to ensure the applicability of findings across diverse populations.
Systematic Review Registration: https://inplasy.com/inplasy-2023-10-0022/, identifier INPLASY2023100022.
1 Introduction
Parkinson’s disease (PD) is a progressive neurological disorder that interferes with the initiation and execution of voluntary movement, resulting in difficulty performing basic activities of daily living (1). PD is an age-related condition as its prevalence steadily increases with age (2). Although the cause is still unknown, many researchers believe that the disease is determined by the interaction between genetic and environmental factors, leading to a gradual degeneration of neurons in susceptible areas of the brain (2). Apart from the typical motor symptoms, non-motor symptoms in patients with PD, including sleep problems, autonomic dysfunction and sensory abnormalities (3), also have a significant impact on quality of life and overall health (4, 5).
Sleep problems in PD patients, such as poor sleep quality, are among the most prominent non-motor symptoms that considerably impact on the quality of life in both patients and caregivers (6, 7). The causes of sleep problems in PD patients involve disease-related factors such as dopaminergic medications, co-morbid mood disorders, and other related factors (8), as well as the associated symptoms and treatments of PD (9).
Poor sleep quality is one of the major sleep problems experienced by PD patients (10). Sleep quality is defined as one’s satisfaction with sleep experience, encompassing aspects such as sleep onset, sleep maintenance, subjective sleep quality, and mental state upon awakening (11). Both subjective and objective methods can be employed to assess sleep quality. Objective techniques usually have a high reliability in collecting sleep parameter data, such as using actigraphy and polysomnography (12). However, these objective sleep quality tests are costly, time-consuming, not easily accessible by most practitioners, and unsuitable for use in epidemiological studies and clinical practice (13). As such, subjective measures on sleep quality have been developed for such purposes, for instance, the Pittsburgh Sleep Quality Index (PSQI). The PSQI is the most utilized subjective measurement of sleep quality across different populations, providing scores that allow for categorization as “good” or “poor” (14, 15). In addition, the Parkinson’s Disease Sleep Scale (PDSS) is another widely used visual analog scale that addresses several symptoms associated with sleep disorders in PD, including sleep quality (16).
Previous studies on the prevalence of poor sleep quality in patients with PD have reported rates ranging from 20% to 88% (17–19). In addition, poor sleep quality in PD patients has been found to be associated with more severe comorbid depressive and anxiety symptoms, reduced cognitive performance, and more severe PD symptoms (20). Therefore, to address the negative impact of poor sleep quality on PD patients, it is important to understand the epidemiological patterns of poor sleep quality and its associated factors in this population.
To date, no systematic review and meta-analysis have been published on the prevalence estimates of poor sleep quality in PD patients. To address this gap, this meta-analysis aimed to assess the global prevalence of poor sleep quality in PD patients and identify potential factors contributing to the differences in prevalence in this population.
2 Method
2.1 Search strategy
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and Meta-analyses Of Observational Studies in Epidemiology (MOOSE) checklist were followed in conducting this meta-analysis (21). Three investigators (TLS, YYW and JXL) systematically and independently searched literature in the PubMed, EMBASE, PsycINFO, Web of Science, China National Knowledge Infrastructure (CNKI) and Wangfang databases from their inception date until November 4, 2023, using the following search items: “Parkinson disease “ AND (“Sleep Quality” OR “Qualities, Sleep” OR “Quality, Sleep” OR “Sleep Qualities” OR “quality of sleeping” OR “sleeping quality” OR “Pittsburgh sleep quality index” OR “PSQI”) AND (“prevalence” OR “epidemiology” OR “rate”). The study protocol was registered with the International Platform of Registered Systematic Review and Meta-analysis Protocols (INPLASY; registration number: INPLASY2023100022).
2.2 Inclusion and exclusion criteria
The same three investigators (TLS, YYW and JXL) independently reviewed the titles and abstracts of relevant publications and then read the full texts of to assess eligibility. The inclusion criteria were developed using the PICOS acronym: Participants (P): patients with PD according to study-defined diagnostic criteria, such as the UK PD Society Brain Bank criteria (22) and the Movement Disorder Society (MDS) clinical diagnostic criteria for PD (23); Intervention (I): not applicable; Comparison (C): NR; Outcome (O): prevalence of poor sleep quality or having information that could generate an estimation of that prevalence, using standard tools, such as the PSQI, to measure the quality of the sleep; Study design (S): cross-sectional and cohort studies (only baseline data were analyzed in cohort studies) with accessible data published in English or Chinese journals. Exclusion criteria included reviews, systematic reviews, meta-analyses, case studies, and commentaries. Further, studies conducted specifically on PD samples with a primary sleep disorder (e.g., PD patients recruited from sleep clinics who suffered from insomnia disorder or obstructive sleep apnea (OSA)) were excluded to avoid the risk of overrepresenting sleep disturbances and selection bias in PD samples (24, 25). Only the study with the most detailed information was included in the meta-analysis if a dataset was utilized in more than one study (26).
2.3 Data extraction and quality assessment
Participant and study data, such as the first author, publication year, sampling method, sleep quality measures, number of PD patients, cut-off value, illness duration, number of participants with poor sleep quality, and sleep quality scores, mean age and male proportion of study sample, diagnostic criteria, were extracted. Additionally, to characterize the multidimensional aspects of sleep, we extracted specific components from standardized sleep tools (e.g., the PSQI domains: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbance, use of sleep medications, and daytime dysfunction).
Such components including sleep medication use could enable the evaluation of naturally occurring sleep within broadly representative PD cohorts, rather than selective studies that focused on sleep comorbidities and effects of treatment.
Study quality was assessed using a standardized instrument for epidemiological studies (27, 28) with the following eight items: (1) target population was defined clearly; (2) probability sampling or entire population surveyed; (3) response rate was ≥80%; (4) non-responders were clearly described; (5) sample was representative of the target population; (6) data collection methods were standardized; (7) validated criteria were used to diagnose PD; and (8) prevalence estimates were given with confidence intervals (CIs) and detailed by subgroups. The total score ranged from 0 to 8. Studies with a total score of “7–8” were considered as “high quality,” “4–6” as “moderate quality,” and “0–3” as “low quality” based on previous studies (25, 29, 30).
2.4 Statistical analyses
All the statistical analyses were conducted using R program (31). A random-effects model was used to synthesis the pooled prevalence of poor sleep quality and its 95% confidence intervals (95% CI) (32). I2 statistics were used to determine the degree of study heterogeneity, with 25%, 50%, and 75% tentatively indicating low, moderate, and high heterogeneity, respectively (33). Subgroup analyses for categorical variables (e.g., study regions, countries by economic status according to the World Bank’s criteria (34), study design, cut-off value signifying poor sleep quality, and patients resources), and meta-regression analysis for continuous variables (mean age, proportions of male, depression, anxiety and cognitive dysfunction respectively, mean scores of MoCA, mean scores of MMSE and mean scores of Hoehn and Yahr Staging, mean levodopa equivalent daily dose and quality assessment score) were conducted to explore the sources of potential heterogeneity. The Egger’s test was used to evaluate publication bias, and a visual funnel plot for asymmetry was also provided. If there was publication bias (P<0.05), a trim-and-fill analysis was used. Sensitivity analyses were carried out to assess the stability of results by individually eliminating each study. The significance level was set at 0.05 (two-tailed).
3 Results
3.1 Search results and study characteristics
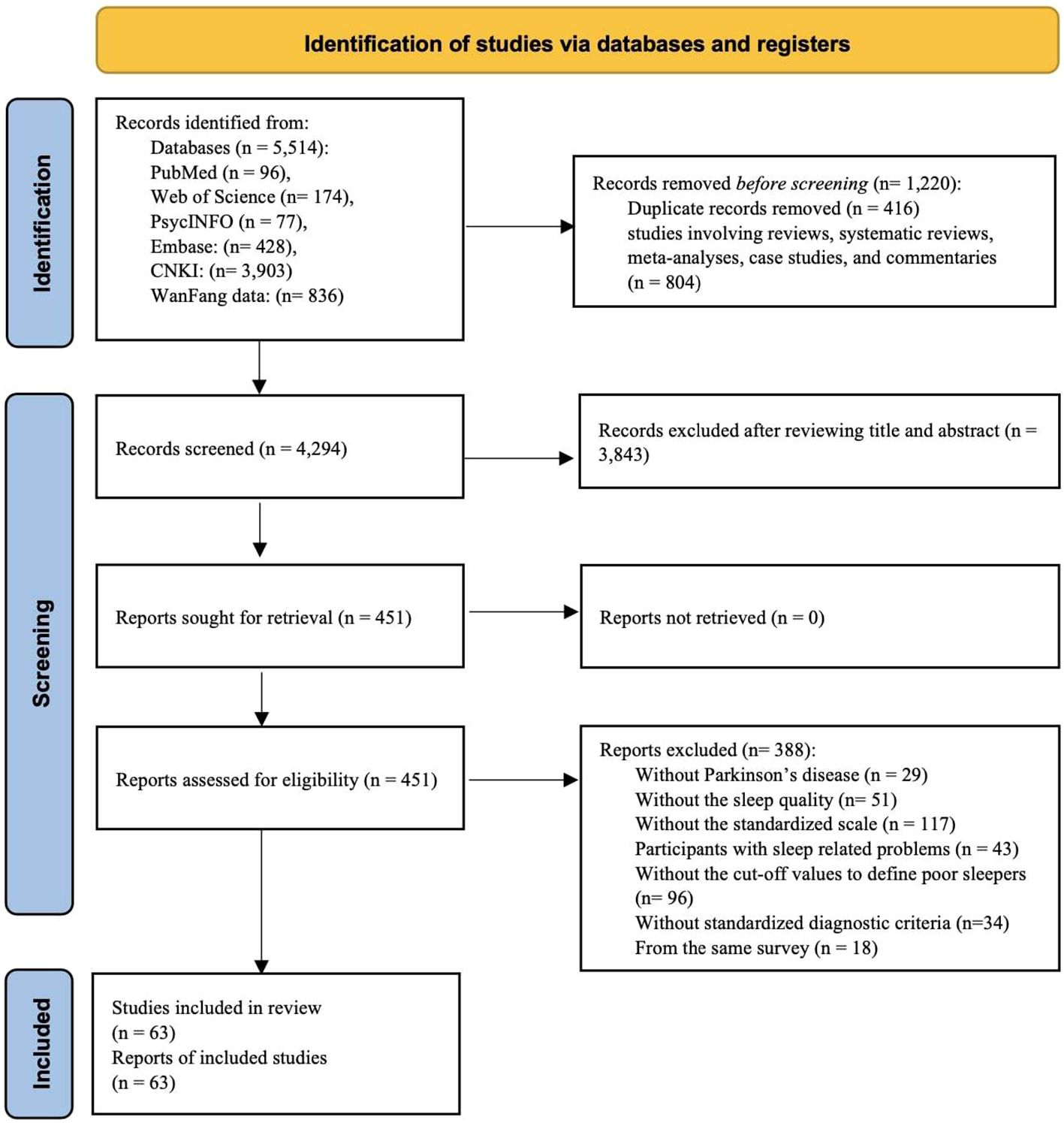
Out of a total of 5,514 publications initially retrieved, 1,220 were excluded. The remaining 4,294 studies were screened for eligibility. By screening the titles and abstracts, we identified 451 potentially eligible studies. After screening the full text of these studies, we ultimately included 63 studies in this meta-analysis (see Figure 1).
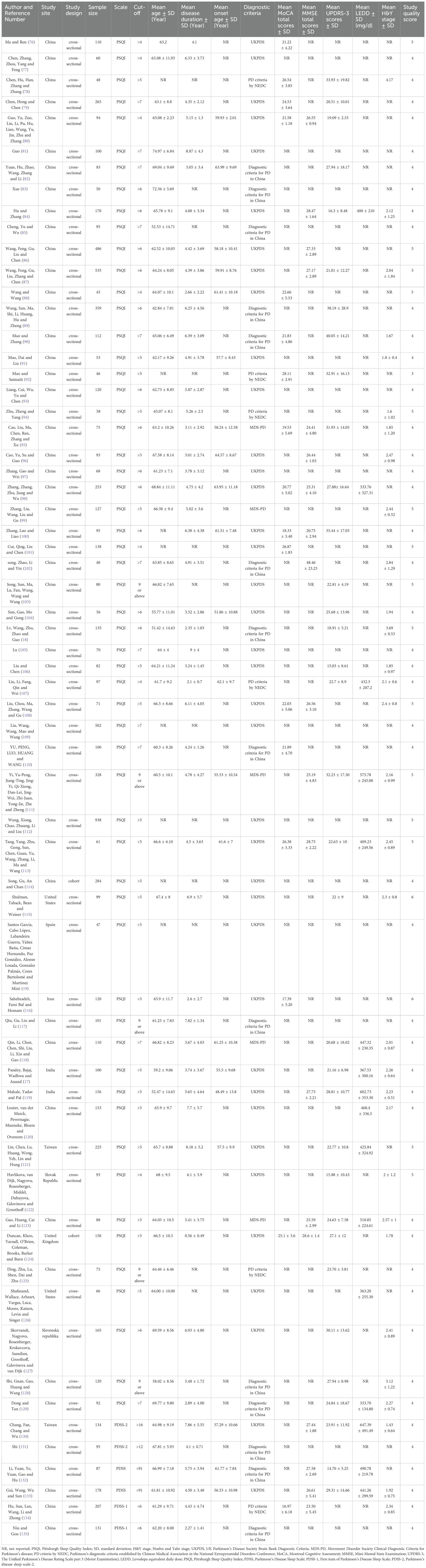
As shown in Table 1, 63 studies covering a total of 9,382 participants were included, with the sample sizes ranging from 40 to 938. The mean age of participants ranged from 51.42 to 74.97 years, the mean disease duration ranged from 0.56 to 9 years, and the mean age at onset ranged from 48.49 to 64.57 years. Regarding the use of scales for sleep quality in the PD patients, 58 studies used the PSQI, 2 studies used the PDSS, 2 studies used the PDSS-1 (the first entry in the Parkinson’s Disease Sleep Scale), and 2 studies used the PDSS-2 (Parkinson’s Disease Sleep Scale-2). We applied quality assessment on all 63 studies, where one was rated “low quality” (1.89%), while 62 studies were rated as “moderate quality” (98.41%), with study quality assessment scores ranging from 3 to 6 (See Supplementary Table S1).
3.2 Pooled prevalence of poor sleep quality, PSQI global scores and PSQI component scores in Parkinson’s disease patients
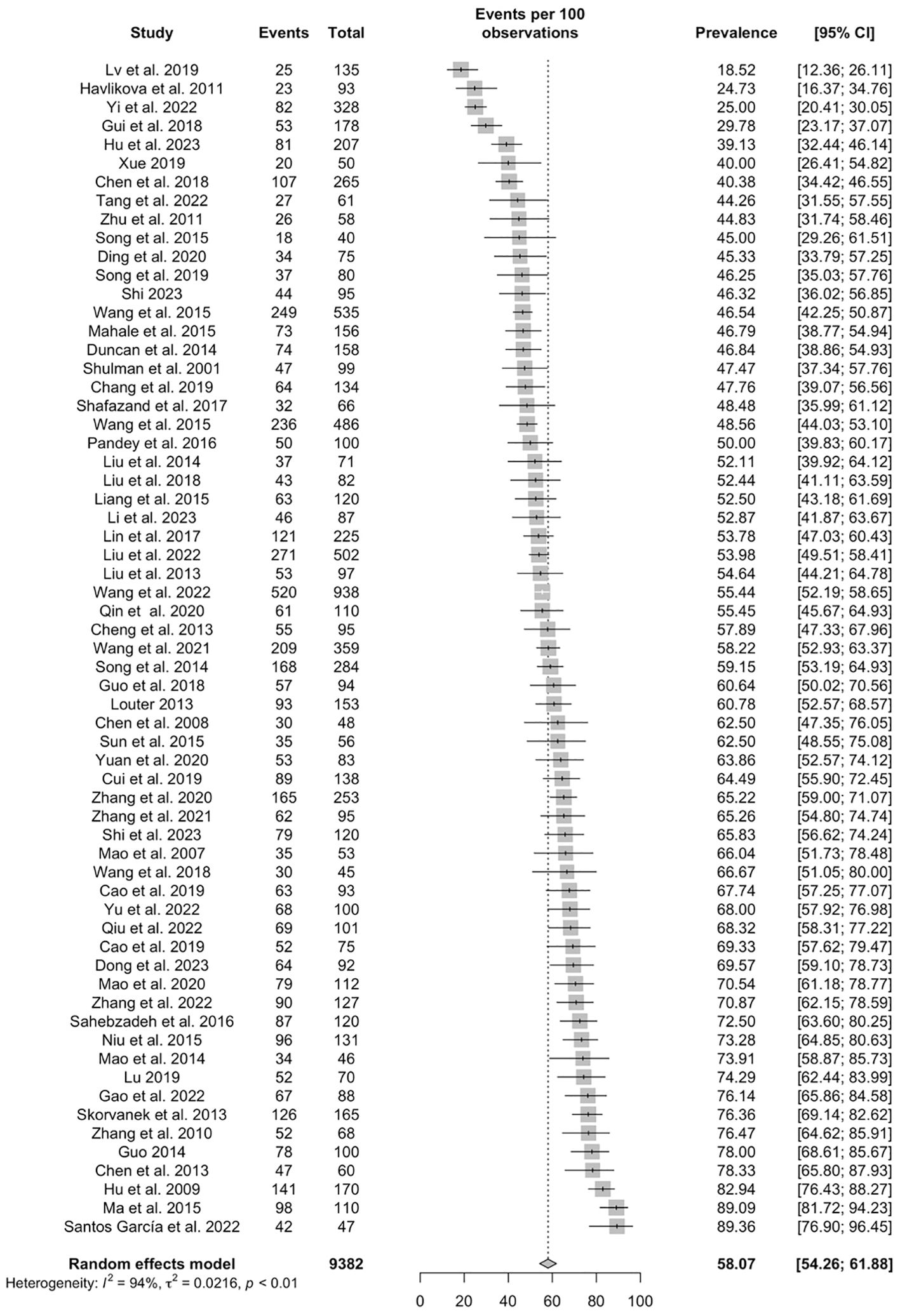
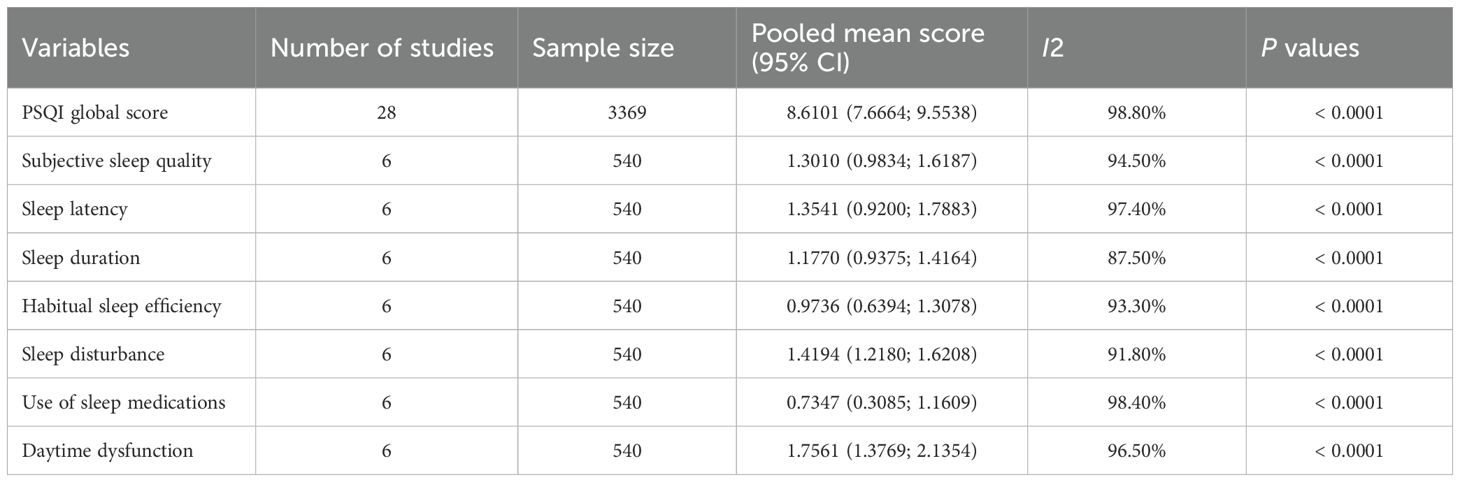
Based on the 63 studies, the pooled prevalence of poor sleep quality in PD patients was 58.07% (95% CI: 54.26–61.88%) (Figure 2). As shown in Table 2, 28 studies with 3,369 participants reported PSQI global scores among PD patients, with a pooled PSQI total score of 8.6 (95% CI: 7.66-9.55). The seven PSQI sleep component scores were reported in 6 studies (N=540). The pooled PSQI component scores ranged from 0.73 (95% CI: 0.31-1.16) for “sleep medication use” to 1.76 (95% CI: 1.38-2.14) for “daytime dysfunction” (see Table 2).
3.3 Subgroup and meta-regression analyses
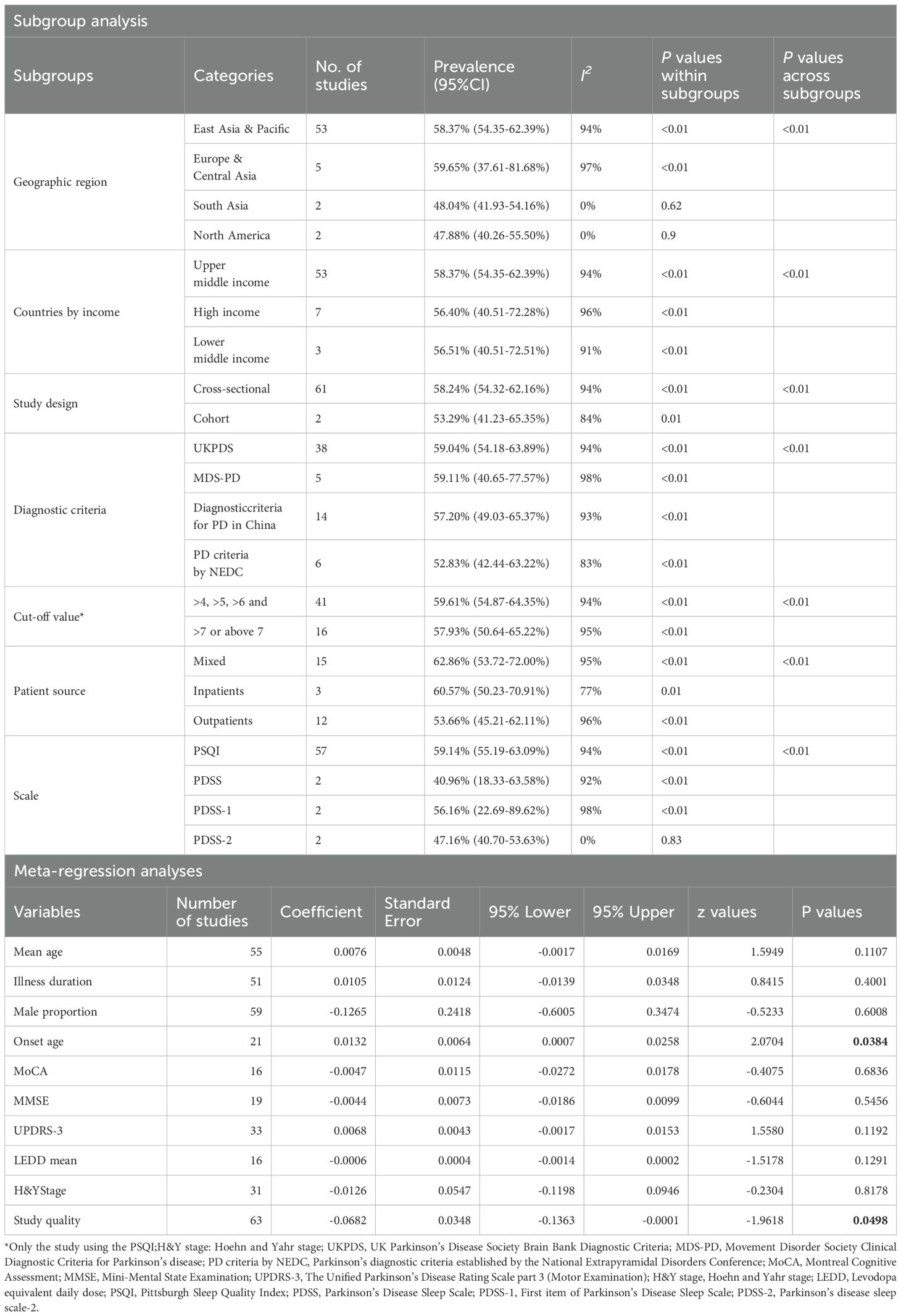
Subgroup analysis by comparing means showed that study site was significantly associated with the prevalence of poor sleep quality (p < 0.01). Specifically, the highest prevalence of poor sleep quality in PD patients was reported in studies conducted in Europe & Central Asia (59.65% 95%CI: 37.63-81.68%), while the lowest figure was reported in North America (47.88%, 95%CI: 40.26–55.50%). There was also a significant subgroup difference in prevalence of poor sleep quality between countries by income (P <0.01): the highest prevalence was reported in upper-middle income countries (58.37%, 95%CI: 54.35–62.39%), while the lowest prevalence was reported in high income countries (56.40%, 95%CI: 40.51–72.28%). There were significant subgroup differences between PD diagnostic criteria (P < 0.01); the pooled prevalence of poor sleep quality was 59.04% (95% CI: 54.18-63.89%) in studies using the UK Parkinson’s Disease Society Brain Bank Diagnostic Criteria (UKPDS), 59.11% (95% CI: 40.65-77.57%) in studies using the Movement Disorder Society Clinical Diagnostic Criteria for Parkinson’s disease (MDS-PD), 57.20% (95% CI: 49.03-65.37%) in studies using the Diagnostic criteria of Chinese Parkinson’s Disease & Movement Disorders Society, Society for Neurology, Chinese Medical Association (Diagnostic criteria for PD in China) and 52.83% (95% CI: 42.44-63.22%) in studies using the diagnostic criteria for Parkinson’s disease established by the National Extrapyramidal Disorders Conference (PD criteria by NEDC). Furthermore, the pooled prevalence rates of poor sleep quality were 60.57% (95% CI: 50.23-70.91%) in inpatients, 53.66% (95% CI: 45.21-62.11%) in outpatients, and 62.86% (95% CI: 53.72-72.00%) in the mixed group. Moreover, there were significant differences (p < 0.01) between studies using different measures on sleep quality; the prevalence of poor sleep quality was 59.14% (95% CI: 55.19-63.09%) in studies using the PSQI, 40.96% (95% CI: 18.33-63.58%) in studies using the PDSS, 56.16% (95% CI: 22.69-89.62%) in studies using PDSS-1, and 47.16% (95% CI: 40.70-53.63%) in the studies using PDSS-2 (Table 3). In meta-regression analyses, onset age (β=0.0132, z=2.0704, p=0.0384) was positively associated with the prevalence of poor sleep quality. However, mean age, illness duration, levodopa equivalent daily dose, and The Unified Parkinson’s Disease Rating Scale part 3 (Motor Examination) score were not found to be statistically significant in the meta-regression (Table 3).
3.4 Publication bias and sensitivity analyses
The funnel plot assessment and Egger’s test value suggested no significant publication bias (Egger’s test t = 1.42, P= 0.1608) (See Supplementary Figure S1). Sensitivity analyses did not identify any outlying studies that could significantly change primary results (See Supplementary Figure S2). To avoid selection bias, no studies were excluded based solely on quality assessment. Sensitivity analysis confirmed that low-quality studies did not significantly alter the pooled prevalence estimates.
4 Discussion
To the best of our knowledge, this was the first systematic review and meta-analysis to examine the prevalence of poor sleep quality in patients with PD. Based on 63 studies, comprising 9,382 PD patients, the prevalence of poor sleep quality in patients with PD was 58.07% (95% CI: 54.26-61.88%). The factors associated with the prevalence of poor sleep quality included study region, income, diagnostic criteria, patient source and PD age of onset.
The pooled PSQI global score in this study was 8.61 (95% CI: 7.67-9.55), which was higher than the findings reported in most other population studies. For example, the global PSQI scores in a general population sample were 3.18 ± 2.28 in Japan (35), 5.00 ± 3.37 in Germany (36), 5.14 ± 3.90 in Spain (37), and 5.5 ± 2.8 in Singapore (38). The overall prevalence of poor sleep quality in PD patients was 58.07% (95% CI: 54.26-61.88%), which is substantially higher than most figures reported in other populations, including Singaporean working population (42.5%; 95% CI: 37.9-47.1%) (38), Chinese elderly population (35.9%; 95% CI: 30.6%-41.2%) (39), perinatal and postpartum women (54.2%; 95% CI: 47.9-60.5%) (24), medical students (55%; 95% CI 48.0%-62.0%) (40), patients with tinnitus (53.5%; 95% CI: 40.2-66.8%) (41), and patients with irritable bowel syndrome (37.6%; 95% CI: 31.4-44.3%) (42).
The high prevalence of poor sleep quality in PD patients may be attributed to the disease characteristics. Degenerative changes in the brain associated with the disease can directly impact sleep/wake mechanisms, leading to sleep disruptions (43). Additionally, movement difficulties, such as the inability to move around in bed, involuntary movements, dystonia, and pain caused by leg spasms, all of which can interfere with sleep maintenance (44). However, the overall prevalence of poor sleep quality in PD patients is lower than the rates reported in patients with chronic non-cancer pain (75.3%, 95% CI: 62.8-87.8%) (45), and hemodialysis patients (75.30%, 95% CI: 70.08-82.50%) (46). The prevalence is also lower than that in certain occupational groups with high work demands, such as nurses (61.00%, 95% CI: 55.8-66.1%) (47) and military personnel (69.00%, 95% CI: 62.33-75.30%) (25), who often experience high stress, shift work, long work hours, high burnout, and exposure to trauma (48).
In subgroup analyses, we found that the overall prevalence of poor sleep quality in PD patients was significantly lower in North America (47.88%, 95% CI: 40.26-55.50%) and high-income countries (56.40%, 95% CI: 40.51-72.28%) compared to other regions and countries. The burden of PD appears to be greater in low-income areas, which may contribute to poorer sleep quality (49, 50). On the other hand, higher-income countries may have access to better healthcare facilities, resources, and support, which can indirectly lower the risk of poor sleep quality PD patients (51). Greater healthcare resources in high-income countries may lead to more effective management of symptoms in PD patients in these countries, and thereby improving their sleep quality.
The overall prevalence of poor sleep quality was significantly higher among inpatients (60.57%, 95% CI: 50.23-70.91%) compared to outpatients (53.66%, 95% CI: 45.21-62.11%), which may be attributed to the severity of PD. Hospitalized patients usually suffer from a more advanced stage of the disease, necessitating closer medical monitoring and treatment (52). Certain symptoms, such as tremors, muscle stiffness, and difficulties with motor control, can all contribute to disrupted sleep (53, 54). Furthermore, changes in the environment such as being hospitalized or separated from their families and regular living environment can also contribute to a decline in sleep quality (55, 56). Consequently, hospitalized PD patients are more likely to encounter sleep problems.
In the subgroup analyses, the pooled prevalence of poor sleep quality in studies using the MDS-PD (59.11%, 95% CI: 40.65-77.57%) was significantly higher than those using the diagnostic PD criteria by NEDC (52.83%, 95% CI: 42.44-63.22%). Notably, certain studies had applied the UK Parkinson’s Disease Society Brain Bank (UKPDS) criteria and the Parkinson’s diagnostic criteria established by Chinese Medical Association (CMA) in the National Extrapyramidal Disorders Conference (NEDC), both of which differ from the Movement Disorder Society Clinical Diagnostic Criteria for Parkinson’s disease(MDS-PD) in terms of diagnostic strictness and clinical application. Such diagnostic variations represent a key source of heterogeneity: the MDS-PD incorporates advanced biomarkers and updated clinical features to maximize specificity (23), whereas the UKPDS relies primarily on classic motor symptoms with established high sensitivity but lower specificity for early PD. The CMA guidelines adapt international standards to regional practices, potentially introducing operational variability, and NEDC (1984) lacks contemporary validation with possible under-identification of non-motor phenotypes (57, 58). Such methodological heterogeneity can directly influence prevalence estimates, as stricter criteria (e.g., MDS-PD) may capture patients with more advanced pathology and higher comorbidity burden, while broader criteria (e.g., NEDC/UKPDS) can include borderline cases with milder sleep disturbances. Consequently, the observed prevalence differences likely reflect both true biological variability and diagnostic threshold effects, necessitating cautious interpretation of cross-criteria study comparisons.
Subgroup analyses also revealed significantly higher pooled prevalence rates in cross-sectional studies (58.24%, 95% CI: 54.32-62.16%) compared to cohort studies (53.29%, 95% CI: 41.23-65.35%). It should be noted that only a small number of cohort studies (n = 2) were included, which might have resulted in less reliable estimates. Similarly, the higher prevalence observed in studies using the PSQI might partially reflect selection bias, due to the small number of studies using the PDSS (n = 2), PDSS-1 (n = 2), and PDSS-2 (n=2). Therefore, the findings of these subgroup analysis are tentative.
In meta-regression analyses, the age of onset of PD was positively associated with prevalence of poor sleep quality. Previous studies found that gender and age of onset are significant factors influencing the clinical phenotype of PD, affecting both motor symptoms and various non-motor symptoms (59). Patients with late-onset PD tend to experience more frequent non-motor symptoms, including cognitive dysfunction, autonomic dysfunction, and sleep disturbances (60, 61). A study conducted in China on non-motor symptoms in PD found positive correlations between age, daily levodopa dose, and Non-Motor Symptoms Scale (NMSS) scores in patients with late-onset PD. However, these correlations were not observed in those with early-onset PD, suggesting that age and daily levodopa dose may play a more significant role in the severity of non-motor symptoms in patients with late-onset PD compared to those with early-onset PD (62). Several brainstem nuclei are potentially involved in the mechanisms underlying non-motor symptoms such as gastrointestinal regulation, pain perception, emotion control, and sleep-wake cycles (63, 64). The presence of Lewy bodies, which are composed of abnormal α-synuclein and are found outside the dopaminergic neurons of the midbrain, probably contribute to the pathology of non-motor manifestations in PD (63, 65, 66). Different distribution and severity of central nervous system lesions and pathophysiological course of PD between patients with early-onset and late-onset PD may be crucial factors influencing the non-motor features (e.g., sleep quality) associated with the disease (62).
Overall, our meta-analysis indicates that patients with PD have a high prevalence of poor sleep quality. Sleep problems are closely associated with mental health (67), and patients with PD often experience psychological stress (68), anxiety and depression (69, 70). Therefore, maintaining good sleep quality plays a crucial role in sustaining emotional and mental stability and enhancing the mental well-being and quality of life for patients (71). Cognitive decline and difficulties with concentration are common challenges faced by PD patients (72, 73). Prioritizing sleep quality in patients with PD is essential for managing symptoms, enhancing mental health, and preserving cognitive functioning. By optimizing the sleep environment, establishing healthy sleep habits, and utilizing medications or other treatments, when necessary, the sleep quality of patients with PD can be improved. Moreover, meta-regression showed that study quality was negatively associated with sleep quality in PD patients. Thus, lower study quality may lead to underestimation of sleep problems in PD patients. Improving the methodological rigor of studies may help to more accurately assess the severity of sleep quality problems (74).
Strengths of this meta-analysis include high number of included studies and large sample size, and the utilization of comprehensive analytical methods, such as subgroup and meta-regression analyses, to identify factors associated with poor sleep quality. Nonetheless, several limitations should be noted. First, despite use of sophisticated statistical methods, heterogeneity still remained in the subgroup analyses, as is expected in meta-analyses of epidemiological surveys, as pointed out in previous studies (25, 47). Second, certain factors related to poor sleep quality, such as living environment, psychiatric comorbidities, and educational background, were not included in most studies. Third, wide confidence intervals for subgroups might be related to small sample sizes or the use of different PD diagnostic criteria in some studies. Fourth, the overrepresentation of Chinese studies might introduce bias that could affect the generalizability of the findings to other populations (75). Fifth, while our analysis identified key correlates of poor sleep quality, the preponderance of cross-sectional data precluded any causal conclusions. Longitudinal studies are warranted to explore the temporal relationships, particularly between sleep deterioration and PD progression trajectories. Furthermore, significant heterogeneity in PD diagnostic criteria (e.g., MDS-PD vs. UKPDS vs. CMA vs. NEDC) could result in methodological variability. Although this reflects real-world clinical practice, differences in diagnostic sensitivity/specificity might influence pooled prevalence estimates and subgroup comparability. Future meta-analyses would benefit from standardized application of contemporary diagnostic frameworks of PD.
In conclusion, poor sleep quality is common among patients with PD, particularly in lower middle-income countries and Europe & Central Asia regions. To mitigate the adverse effects of poor sleep quality in PD patients, regular monitoring of sleep quality and sleep hygiene is crucial, particularly in high-risk subgroups. Additionally, further studies of sleep quality and PD patients in low- and middle-income countries are warranted, as well as prospective cohort studies to clarify the causality between poor sleep quality and other factors.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
TS: Conceptualization, Data curation, Formal Analysis, Methodology, Writing – original draft, Writing – review & editing. YW: Data curation, Formal Analysis, Methodology, Writing – review & editing. JL: Data curation, Formal Analysis, Methodology, Writing – review & editing. WB: Data curation, Formal Analysis, Methodology, Writing – review & editing. HS: Writing – review & editing. SR: Data curation, Writing – review & editing. HZ: Data curation, Writing – review & editing. GU: Writing – review & editing. ZS: Data curation, Writing – review & editing. TC: Data curation, Writing – review & editing. CN: Conceptualization, Writing – review & editing. YX: Conceptualization, Writing – original draft, Writing – review & editing. GW: Data curation, Methodology, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was supported by Beijing High Level Public Health Technology Talent Construction Project (Discipline Backbone-01-028), the Beijing Municipal Science & Technology Commission (No. Z181100001518005), and the Beijing Hospitals Authority Clinical Medicine Development of Special Funding Support (XMLX202128) and the University of Macau (MYRG2019-00066-FHS; MYRG2022-00187-FHS).
Acknowledgments
The authors are grateful to all participants and clinicians involved in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2025.1606743/full#supplementary-material
Supplementary Table 1 | Quality assessment of included studies.
Supplementary Figure 1 | Funnel plot of pooled prevalence of poor sleep quality in Parkinson’s disease patients.
Supplementary Figure 2 | Sensitivity analysis of pooled prevalence of poor sleep quality in Parkinson’s disease patients.
References
1. Triegaardt J, Han TS, Sada C, Sharma S, and Sharma P. The role of virtual reality on outcomes in rehabilitation of Parkinson’s disease: meta-analysis and systematic review in 1031 participants. Neurol Sci. (2020) 41:529–36. doi: 10.1007/s10072-019-04144-3
2. Pringsheim T, Jette N, Frolkis A, and Steeves TDL. The prevalence of Parkinson’s disease: A systematic review and meta-analysis. Movement Disord. (2014) 29:1583–90. doi: 10.1002/mds.25945
3. Pfeiffer RF. Non-motor symptoms in Parkinson’s disease. Parkinsonism Related Disord. (2016) 22:S119–22. doi: 10.1016/j.parkreldis.2015.09.004
4. Martinez-Martin P, Rodriguez-Blazquez C, Kurtis MM, Chaudhuri KR, and Group oBotNV. The impact of non-motor symptoms on health-related quality of life of patients with Parkinson’s disease. Movement Disord. (2011) 26:399–406. doi: 10.1002/mds.23462
5. Prakash KM, Nadkarni NV, Lye W-K, Yong M-H, and Tan E-K. The impact of non-motor symptoms on the quality of life of Parkinson’s disease patients: a longitudinal study. Eur J Neurol. (2016) 23:854–60. doi: 10.1111/ene.12950
6. Stavitsky K and Cronin-Golomb A. Sleep quality in Parkinson disease: an examination of clinical variables. Cognit Behav Neurol. (2011) 24:43–9. doi: 10.1097/WNN.0b013e31821a4a95
7. Comella CL. Sleep disturbances and excessive daytime sleepiness in Parkinson disease: an overview. J Neural Transm Suppl. (2006) 70):349–55. doi: 10.1007/978-3-211-45295-0_53
8. Najafi MR, Chitsaz A, Askarian Z, and Najafi MA. Quality of sleep in patients with Parkinson’s disease. Int J Prev Med. (2013) 4:S229–33. doi: 10.1186/s12883-024-03548-9
9. Schrempf W, Brandt MD, Storch A, and Reichmann H. Sleep disorders in parkinson’s disease. J Parkinson’s Dis. (2014) 4:211–21. doi: 10.3233/JPD-130301
10. Santos-García D, Castro ES, de Deus Fonticoba T, Panceiras MF, Enriquez JM, González JP, et al. Sleep problems are related to a worse quality of life and a greater non-motor symptoms burden in parkinson’s disease. J Geriatric Psychiatry Neurol. (2021) 34:642–58. doi: 10.1177/0891988720964250
11. Kline C. Sleep quality. In: Gellman MD and Turner JR, editors.Encyclopedia of Behavioral Medicine. Springer, New York (2013). p. 1811–3.
12. Krystal AD and Edinger JD. Measuring sleep quality. Sleep Med. (2008) 9:S10–7. doi: 10.1016/S1389-9457(08)70011-X
13. Fabbri M, Beracci A, Martoni M, Meneo D, Tonetti L, and Natale V. Measuring subjective sleep quality: A review. Int J Environ Res Public Health. (2021) 18:1082. doi: 10.3390/ijerph18031082
14. Buysse DJ, Reynolds CF, Monk TH, Berman SR, and Kupfer DJ. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. (1989) 28:193–213. doi: 10.1016/0165-1781(89)90047-4
15. Landry GJ, Best JR, and Liu-Ambrose T. Measuring sleep quality in older adults: a comparison using subjective and objective methods. Front Aging Neurosci. (2015) 7:166. doi: 10.3389/fnagi.2015.00166
16. Tse Winona, Liu Yiming, Barthlen Gabriele M, Hälbig Thomas D, Tolgyesi Sonia V, and Gracies Jean-Michel. Clinical usefulness of the Parkinson’s disease sleep scale. Parkinsonism Related Disord. (2005) 11:317–21. doi: 10.1016/j.parkreldis.2005.02.006
17. Pandey S, Bajaj BK, Wadhwa A, and Anand KS. Impact of sleep quality on the quality of life of patients with Parkinson’s disease: a questionnaire based study. Clin Neurol Neurosurg. (2016) 148:29–34. doi: 10.1016/j.clineuro.2016.06.014
18. Lv D, Wang X, Zhu Y, Zhao L, and Guo Q. Clinical analysis of parkinson’s disease with anxiety, depression and sleep disorders. Clin Misdiagn Misther. (2019) 32:83–8. doi: 10.3969/j.issn.1002-3429.2019.02.019
19. Santos García D, Cabo López I, Labandeira Guerra C, Yáñez Baña R, Cimas Hernando MI, Paz González JM, et al. Safinamide improves sleep and daytime sleepiness in Parkinson’s disease: results from the SAFINONMOTOR study. Neurol Sci. (2022) 43:2537–44. doi: 10.1007/s10072-021-05607-2
20. Junho BT, Kummer A, Cardoso FE, Teixeira AL, and Rocha NP. Sleep quality is associated with the severity of clinical symptoms in Parkinson’s disease. Acta Neurol Belgica. (2018) 118:85–91. doi: 10.1007/s13760-017-0868-6
21. Page Matthew J, McKenzie Joanne E, Bossuyt Patrick M, Boutron Isabelle, Hoffmann Tammy C, Mulrow Cynthia D, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PloS Med. (2021) 18:e1003583. doi: 10.1371/journal.pmed.1003583
22. Daniel S and Lees A. Parkinson’s Disease Society Brain Bank, London: overview and research. J Neural Transm Supplementum. (1993) 39:165–72.
23. Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Movement Disord. (2015) 30:1591–601. doi: 10.1002/mds.26424
24. Yang Y, Li W, Ma TJ, Zhang L, Hall BJ, Ungvari GS, et al. Prevalence of poor sleep quality in perinatal and postnatal women: A comprehensive meta-analysis of observational studies. Front Psychiatry. (2020) 11:161. doi: 10.3389/fpsyt.2020.00161
25. Bai W, Gui Z, Chen MY, Zhang Q, Lam MI, Si TL, et al. Global prevalence of poor sleep quality in military personnel and veterans: A systematic review and meta-analysis of epidemiological studies. Sleep Med Rev. (2023) 71:101840. doi: 10.1016/j.smrv.2023.101840
26. Dong M, Wang SB, Li Y, Xu DD, Ungvari GS, Ng CH, et al. Prevalence of suicidal behaviors in patients with major depressive disorder in China: A comprehensive meta-analysis. J Affect Disord. (2018) 225:32–9. doi: 10.1016/j.jad.2017.07.043
27. Boyle MH. Guidelines for evaluating prevalence studies. Evid Based Ment Health. (1998) 1:37–9. doi: 10.1136/ebmh.1.2.37
28. Loney PL, Chambers LW, Bennett KJ, Roberts JG, and Stratford PW. Critical appraisal of the health research literature: prevalence or incidence of a health problem. Chronic Dis Canada. (1998) 19:170–6.
29. Cai H, Chen P, Zhang Q, Lam MI, Si TL, Liu YF, et al. Global prevalence of major depressive disorder in LGBTQ+ samples: A systematic review and meta-analysis of epidemiological studies. J Affect Disord. (2024) 360:249–58. doi: 10.1016/j.jad.2024.05.115
30. Chen P, Lam MI, Si TL, Zhang L, Balbuena L, Su Z, et al. The prevalence of poor sleep quality in thegeneral population in China: a meta-analysis of epidemiological studies. Eur Arch Psychiatry Clin Neurosci. (2024) 274:1–14. doi: 10.1007/s00406-024-01764-5
31. R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, Vienna, Austria (2013). Available online at: http://www.R-project.org/ (Accessed December 1, 2023).
32. Harris RJ, Deeks JJ, Altman DG, Bradburn MJ, Harbord RM, and Sterne JAC. Metan: fixed- and random-effects meta-analysis. Stata J. (2008) 8:3–28. doi: 10.1177/1536867x0800800102
33. Higgins JPT, Thompson SG, Deeks JJ, and Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
34. The World Bank Group.Countries and Economies. (2023). https://data.worldbank.org (Accessed December 5, 2023).
35. Okubo N, Matsuzaka M, Takahashi I, Sawada K, Sato S, Akimoto N, et al. Relationship between self-reported sleep quality and metabolic syndrome in general population. BMC Public Health. (2014) 14:562. doi: 10.1186/1471-2458-14-562
36. Hinz A, Glaesmer H, Brähler E, Löffler M, Engel C, Enzenbach C, et al. Sleep quality in the general population: psychometric properties of the Pittsburgh Sleep Quality Index, derived from a German community sample of 9284 people. Sleep Med. (2017) 30:57–63. doi: 10.1016/j.sleep.2016.03.008
37. Madrid-Valero JJ, Martínez-Selva JM, Couto B, Sánchez-Romera JF, and Ordoñana JR. Age and gender effects on the prevalence of poor sleep quality in the adult population. Gaceta Sanitaria. (2017) 31:18–22. doi: 10.1016/j.gaceta.2016.05.013
38. Visvalingam N, Sathish T, Soljak M, Chua AP, Dunleavy G, Divakar U, et al. Prevalence of and factors associated with poor sleep quality and short sleep in a working population in Singapore. Sleep Health. (2020) 6:277–87. doi: 10.1016/j.sleh.2019.10.008
39. Lu L, Wang SB, Rao W, Zhang Q, Ungvari GS, Ng CH, et al. The prevalence of sleep disturbances and sleep quality in older chinese adults: A comprehensive meta-analysis. Behav Sleep Med. (2019) 17:683–97. doi: 10.1080/15402002.2018.1469492
40. Jahrami H, Dewald-Kaufmann J, Faris M-I, AlAnsari AMS, Taha M, and AlAnsari N. Prevalence of sleep problems among medical students: a systematic review and meta-analysis. J Public Health. (2020) 28:605–22. doi: 10.1007/s10389-019-01064-6
41. Gu H, Kong W, Yin H, and Zheng Y. Prevalence of sleep impairment in patients with tinnitus: a systematic review and single-arm meta-analysis. Eur Arch Oto-Rhino-Laryngol. (2022) 279:2211–21. doi: 10.1007/s00405-021-07092-x
42. Wang B, Duan R, and Duan L. Prevalence of sleep disorder in irritable bowel syndrome: A systematic review with meta-analysis. Saudi J Gastroenterol. (2018) 24:141–50. doi: 10.4103/sjg.SJG_603_17
43. Högl BE, Gómez-Arévalo G, García S, Scipioni O, Rubio M, Blanco M, et al. A clinical, pharmacologic, and polysomnographic study of sleep benefit in Parkinson’s disease. Neurology. (1998) 50:1332–9. doi: 10.1212/wnl.50.5.1332
44. Schütz L, Sixel-Döring F, and Hermann W. Management of sleep disturbances in parkinson’s disease. J Parkinson’s Dis. (2022) 12:2029–58. doi: 10.3233/JPD-212749
45. Sun Y, Laksono I, Selvanathan J, Saripella A, Nagappa M, Pham C, et al. Prevalence of sleep disturbances in patients with chronic non-cancer pain: A systematic review and meta-analysis. Sleep Med Rev. (2021) 57:101467. doi: 10.1016/j.smrv.2021.101467
46. Mirghaed MT, Sepehrian R, Rakhshan A, and Gorji H. Sleep quality in Iranian hemodialysis patients: A systematic review and meta-analysis. Iran J Nurs Midwifery Res. (2019) 24:403–9. doi: 10.4103/ijnmr.IJNMR_184_18
47. Zeng LN, Yang Y, Wang C, Li XH, Xiang YF, Hall BJ, et al. Prevalence of poor sleep quality in nursing staff: A meta-analysis of observational studies. Behav Sleep Med. (2020) 18:746–59. doi: 10.1080/15402002.2019.1677233
48. Vidotti V, Ribeiro RP, Galdino MJQ, and Martins JT. Burnout Syndrome and shift work among the nursing staff. Rev Latino-Americana Enfermagem. (2018) 26:e3022. doi: 10.1590/1518-8345.2550.3022
49. Lix LM, Hobson DE, Azimaee M, Leslie WD, Burchill C, and Hobson S. Socioeconomic variations in the prevalence and incidence of Parkinson’s disease: a population-based analysis. J Epidemiol Community Health. (2010) 64:335–40. doi: 10.1136/jech.2008.084954
50. Perales F and Plage S. Losing ground, losing sleep: Local economic conditions, economic vulnerability, and sleep. Soc Sci Res. (2017) 62:189–203. doi: 10.1016/j.ssresearch.2016.08.006
51. Jourdain VA and Schechtmann G. Health economics and surgical treatment for parkinson’s disease in a world perspective: results from an international survey. Stereotactic Funct Neurosurg. (2014) 92:71–9. doi: 10.1159/000355215
52. Oguh O and Videnovic A. Inpatient management of parkinson disease: current challenges and future directions. Neurohospitalist. (2012) 2:28–35. doi: 10.1177/1941874411427734
53. Merlino G and Gigli GL. Sleep-related movement disorders. Neurol Sci. (2012) 33:491–513. doi: 10.1007/s10072-011-0905-9
54. De Cock VC, Vidailhet M, and Arnulf I. Sleep disturbances in patients with parkinsonism. Nat Clin Pract Neurol. (2008) 4:254–66. doi: 10.1038/ncpneuro0775
55. Dobing S, Frolova N, McAlister F, and Ringrose J. Sleep quality and factors influencing self-reported sleep duration and quality in the general internal medicine inpatient population. PloS One. (2016) 11:e0156735. doi: 10.1371/journal.pone.0156735
56. Parsapour K, Kon AA, Dharmar M, McCarthy AK, Yang HH, Smith AC, et al. Connecting hospitalized patients with their families: case series and commentary. Int J Telemed Appl. (2011) 2011:804254. doi: 10.1155/2011/804254
57. Lihua Y. National symposium on extrapyramidal diseases held in Shanghai. Shanghai Med. (1985) 02):105.
58. Postuma RB and Berg D. The New Diagnostic Criteria for Parkinson's Disease. Int Rev Neurobiol. (2017). 132:55–78. doi: 10.1016/bs.irn.2017.01.008
59. Liu M, Luo YJ, Gu HY, Wang YM, Liu MH, Li K, et al. Sex and onset-age-related features of excessive daytime sleepiness and night-time sleep in patients with Parkinson’s disease. BMC Neurol. (2021) 21:165. doi: 10.1186/s12883-021-02192-x
60. Hu T, Ou R, Liu H, Hou Y, Wei Q, Song W, et al. Gender and onset age related-differences of non-motor symptoms and quality of life in drug-naïve Parkinson’s disease. Clin Neurol Neurosurg. (2018) 175:124–9. doi: 10.1016/j.clineuro.2018.11.001
61. Kägi G, Klein C, Wood NW, Schneider SA, Pramstaller PP, Tadic V, et al. Nonmotor symptoms in Parkin gene-related parkinsonism. Movement Disord. (2010) 25:1279–84. doi: 10.1002/mds.22897
62. Guo X, Song W, Chen K, Chen X, Zheng Z, Cao B, et al. Gender and onset age-related features of non-motor symptoms of patients with Parkinson’s disease – A study from Southwest China. Parkinsonism Related Disord. (2013) 19:961–5. doi: 10.1016/j.parkreldis.2013.06.009
63. Braak H, Tredici KD, Rüb U, de Vos RAI, Jansen Steur ENH, and Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. (2003) 24:197–211. doi: 10.1016/S0197-4580(02)00065-9
64. Grinberg LT, Rueb U, ATdL A, and Heinsen H. Brainstem pathology and non-motor symptoms in PD. J Neurol Sci. (2010) 289:81–8. doi: 10.1016/j.jns.2009.08.021
65. Ferrer I, López-Gonzalez I, Carmona M, Dalfó E, Pujol A, and Martínez A. Neurochemistry and the non-motor aspects of PD. Neurobiol Dis. (2012) 46:508–26. doi: 10.1016/j.nbd.2011.10.019
66. Blesa J, Foffani G, Dehay B, Bezard E, and Obeso JA. Motor and non-motor circuit disturbances in early Parkinson disease: which happens first?Nat Rev Neurosci. (2022) 23:115–28. doi: 10.1038/s41583-021-00542-9
67. Chen P, Zhang L, Sha S, Lam MI, Lok KI, Chow IHI, et al. Prevalence of insomnia and its association with quality of life among Macau residents shortly after the summer 2022 COVID-19 outbreak: A network analysis perspective. Original Research. Front Psychiatry. (2023) 14:1113122. doi: 10.3389/fpsyt.2023.1113122
68. Austin KW, Ameringer SW, and Cloud LJ. An integrated review of psychological stress in parkinson’s disease: biological mechanisms and symptom and health outcomes. Parkinson’s Dis. (2016) 2016:9869712. doi: 10.1155/2016/9869712
69. Dissanayaka NN, Sellbach A, Matheson S, O'Sullivan JD, Silburn PA, Byrne GJ, et al. Anxiety disorders in Parkinson’s disease: Prevalence and risk factors. Movement Disord. (2010) 25:838–45. doi: 10.1002/mds.22833
70. Schrag A. Quality of life and depression in Parkinson’s disease. J Neurol Sci. (2006) 248:151–7. doi: 10.1016/j.jns.2006.05.030
71. Leger D. Sleep and Quality of Life in Insomnia, In: Sleep and Quality of Life in Clinical Medicine, Verster JC, Pandi-Perumal SR, and Streiner DL, Editors. (2008) Totowa, NJ: Humana Press p. 47–51.
72. Maquet P. The role of sleep in learning and memory. Science. (2001) 294:1048–52. doi: 10.1126/science.1062856
73. Hobson JA and Pace-Schott EF. The cognitive neuroscience of sleep: neuronal systems, consciousness and learning. Nat Rev Neurosci. (2002) 3:679–93. doi: 10.1038/nrn915
74. Brown CA, Kuo M, Phillips L, Berry R, and Tan M. Non-pharmacological sleep interventions for youth with chronic health conditions: A critical review of the methodological quality of the evidence. Disability Rehabilitation. (2013) 35:1221–55. doi: 10.3109/09638288.2012.723788
75. Henrich J, Heine SJ, and Norenzayan A. The weirdest people in the world?Behav Brain Sci. (2010) 33:61–83. doi: 10.1017/S0140525X0999152X
76. Ma H and Ren Y. Correlation analysis of sleep disorder and cognitive dysfunction in patients with Parkinson disease. Chin J Clinicians(Electronic Ed). (2015) 9:39–45. doi: 10.3877/cma.j.issn.1674-0785.2015.23.011
77. Chen J, Zhang Q, Zhou S, Yang J, and Feng Y. Analysis of sleep disorders in patients with Parkinson’s disease. Zhejiang Med. (2013) 35:1275–7.
78. Chen Q, Hu G, Han Y, Zhang H, and Zhang Y. Clinical study on non-motor symptoms of Parkinson’s disease. Chin J Gerontol. (2008) 28:1829–30. doi: 10.3969/j.issn.1005-9202.2008.18.028
79. Chen Z, Hong X, and Chen ZH. Corrlation between sleep disturbance and motor, non-motor symptoms in patients with parkinson’s disease. J Clin Res. (2018) 35:445–448,452. doi: 10.3969/j.issn.1671-7171.2018.03.009
80. Guo P, Yu S, Zuo L, Liu L, Pu Y, Hu Y, et al. The clinical features, related factors and the changes of video polysomnography in patients with Parkinson disease accompanied with sleep disorders. Chin J Neuroimmunol Neurol. (2018) 25:26–31.
81. Guo J. Analysis and nursing care of factors related to sleep disorders in patients with Parkinson’s disease. Chin J Pratical Nervous Dis. (2014) 17:139–40.
82. Yuan D, Hu J, Zhao Q, Wang X, Zhang X, and Li JF. Correlation between progression of Parkinson disease and depression and sleep disorders. J China Med Univ. (2020) 49:326–30. doi: 10.12007/j.issn.0258-4646.2020.04.008
83. Xue D. Investigation on the current status of sleep disorders in patients with Parkinson’s disease and nursing methods. Electronic J Pract Clin Nurs Sci. (2019) 4:103,105.
84. Hu X and Zhang B. Clinical analysis of sleep disorders in patients with Parkinson’s disease. J Int Neurol Neurosurg. (2009) 36:201–5. doi: 10.16636/j.cnki.jinn.2009.03.013
85. Cheng L, Yu J, and Wu D. Analysis on related factors of the sleep disorder to patients with Parkinson disease. Chin J Pratical Nervous Dis. (2013) 16:17–8.
86. Wang X, Feng T, Gu Z, Liu P, and Chen B. Assessment of sleep disturbance based on the clinical heterogeneity of early-stage idiopathic Parkinson’s disease. Clin Med China. (2015) 2):103–6. doi: 10.3760/cma.j.issn.1008-6315.2015.02.003
87. Wang X, Feng T, Gu Z, Liu P, Zhang X, and Chen B. Relationship between sleep disturbance and non-motor symptoms in Parkinson’s disease patients. Chin J Geriatric Heart Brain Vessel Dis. (2015) 17:507–10. doi: 10.3969/j.issn.1009-0126.2015.05.019
88. Wang H and Wang Y. Eletroencephalogram changes of patients with parkinson’s disease, sleep disorder and cognition hypofuntion. Syst Med. (2018) 3:20–2. doi: 10.19368/j.cnki.2096-1782.2018.11.020
89. Wang Z, Sun W, Ma J, Shi X, Li M, Huang S, et al. The analysis of the relationship between sleep disorders and mental symptoms in patients with Parkinson disease. Chin J Nerv Ment Dis. (2021) 47:78–82. doi: 10.3969/j.issn.1002-0152.2021.02.003
90. Mao J and Zhang M. Effects of sleep quality on prospective memory and neuropsychological related indicators in patients with Parkinson’s disease. Zhejiang Clin Med J. (2020) 22:259–260,265.
91. Mao C, Dai Y, and Liu C. Analysis of sleep disorder in patients with early parkinson’s disease. Jiangsu Med J. (2007) 33:897–9. doi: 10.3969/j.issn.0253-3685.2007.09.013
92. Mao Y and Saimaiti Y. Clinical observation of non-motor symptoms in patients with Parkinson’s disease. Med Inf Chin. (2014) 20):124–5. doi: 10.3969/j.issn.1006-1959.2014.20.136
93. Liang P, Cui L, Wu Z, Yu J, and Chen M. A survey of’Sleep related cognitions in pafients with pakinson”s disease and related nursing. Clin Med Engineering. (2015) 22:928–9. doi: 10.3969/j.issn.1674—4659.2015.07.0928
94. Zhu Y, Zheng H, and Yang Q. Incidence and related factors of psychosis in parkinson disease. Chin J Med Guide. (2011) 13:1682–3. doi: 10.3969/j.issn.1009-0959.2011.10.017
95. Cao W, Liu X, Ma C, Chen Y, Ren X, Zhang H, et al. Study on related factors of non ⁃ motor symptoms in patients with different motor phenotypes of Parkinson’s disease. J Nanjing Med University(Natural Sci). (2019) 39:1764–8. doi: 10.7655/NYDXBNS20191213
96. Cao S, Yu D, Su R, and Guo Y. Analysis of the incidence of sleep disorders and related influencing factors in patients with Parkinson’s disease. Chin J Integr Med On Cardio-/Cerebrovascuiar Dis. (2019) 17:1257–9. doi: 10.12102/j.issn.1672-1349.2019.08.041
97. Zhang J, Gao P, and Wei J. Investigation on the current status of sleep disorders in patients with Parkinson’s disease and nursing strategies. Chin Community Physician (Med Professional). (2010) 12:175–6.
98. Zhang H, Zhang L, Zhu J, Jiang X, and Wu Z. Analysis of the incidence and clinical features of sleep disorders in patients with Parkinson’s disease. Pract Geritr. (2020) 34:674–8. doi: 10.3969/j.issn.1003-9198.2020.07.011
99. Zhang Y, Liu Y, Wang W, Liu H, and Gu P. Influential factors of autonomic nervous dysfunction in patients with Parkinson’s disease. J Clin Internal Med. (2022) 39:386–90. doi: 10.3969/j.issn.1001-9057.2022.06.008
100. Zhang Y, Luo C, and Liao C. Clinical analysis of sleep disorders and cognitive impairment in Parkinson’s disease. Front Med (Chinese). (2021) 11:96–7.
101. Cui W, Qing S, Liu L, and Chen Q. Clinical characteristics and prognosis of cognitive impairment in patients with Parkin⁃ son’s disease. J Guangxi Med Univ. (2019) 36:1923–6. doi: 10.16190/j.cnki.45-1211/r.2019.12.010
102. Song Y, Zhao Z, Li Y, and Yin Q. Influence factors and nursing of sleep disorder parkinson’s disease. Chin Foreign Med Res. (2015) 13:95–7. doi: 10.14033/j.cnki.cfmr.2015.23.050
103. Song J, Sun X, Ma L, Lu L, Fan K, Wang Z, et al. Analysis of cognitive impairment and its related factors in Parkinson’s disease. Chin J Gerontol. (2019) 39:858–62. doi: 10.3969/j.issn.1005-9202.2019.04.031
104. Sun L, Gao X, Mo D, and Gong D. Clinical study on sleep disorders and related factors in patients with Parkinson’s disease. Nerve Injury Funct Reconstruction. (2015) 2):159–61. doi: 10.3870/sjsscj.2015.02.021
105. Lu Y. Investigation on the current status of sleep disorders in patients with Parkinson’s disease and research on nursing strategies. Shanxi Med J. (2019) 48:388–90. doi: 10.3969/j.issn.0253-9926.2019.03.050
106. Liu H and Chen K. Relationship study between Glu and GABA and sleep disorders in patients with Parkinson’s disease. Proceeding Clin Med. (2018) 27:27–9. doi: 10.16047/j.cnki.cn14-1300/r.2018.01.008
107. Liu Y, Li J, Fang Q, Qin Y, and Wei Y. Study of health related quality of life in patients with Parkinson’s disease. World Latest Med Inform. (2013) 16):58–59,31. doi: 10.3969/j.issn.1671-3141.2013.16.031
108. Liu H, Chou F, Ma L, Zhang Y, Wang M, and Gu P. Effect of sleep quality on cognitive function in mild-o-moderate Parkinson patients. J Neurosci Ment Health. (2014) 14:502–5. doi: 10.3969/j.issn.1009-6574.2014.05.021
109. Liu D, Wang Y, Wang Q, Mao H, and Wang S. Study on the correlation between discharge readiness, family resilience and alexithymia in patients with Parkinson’s disease. Chin Gen Pract Nursing. (2022) 20:4723–6. doi: 10.12104/j.issn.1674-4748.2022.33.029
110. Yu L, Peng L, Luo T, Huang C, and Wang Z. Relationship study between sleep disorders and EEG activity, neuropsychological indicators and health-related quality of life in patients with parkinson’s disease. Prog Modern Biomed. (2022) 22:3863–7. doi: 10.13241/j.cnki.pmb.2022.20.012
111. Yi Q, Chen Y-P, Li J-T, Li J-Y, Qiu Q-X, Wang D-L, et al. Worse sleep quality aggravates the motor and non-motor symptoms in parkinson’s disease. Article. Front Aging Neurosci. (2022) 14:887094. doi: 10.3389/fnagi.2022.887094
112. Wang J, Xiong K, Chao J, Zhuang S, Li J, and Liu C. Seasonal variations of nonmotor symptoms in patients with Parkinson’s disease in Southeast China. Article in Press. Chin Med J. (2022). doi: 10.1097/CM9.0000000000002276
113. Tang X, Yang J, Zhu Y, Gong H, Sun H, Chen F, et al. High PSQI score is associated with the development of dyskinesia in Parkinson’s disease. Article. NPJ Parkinson’s Dis. (2022) 8:124. doi: 10.1038/s41531-022-00391-y
114. Song Y, Gu Z, An J, and Chan P. Gender differences on motor and non-motor symptoms of de novo patients with early Parkinson’s disease. Neurol Sci. (2014) 35:1991–6. doi: 10.1007/s10072-014-1879-1
115. Shulman LM, Taback RL, Bean J, and Weiner WJ. Comorbity of the nonmotor symptoms of Parkinson’s disease. Article. Movement Disord. (2001) 16:507–10. doi: 10.1002/mds.1099
116. Sahebzadeh N, Farsi Baf MM, and Homam SM. Sleep quality and cognitive impairment in patients with Parkinson’s disease in Iran. Article. Turk Norol Dergisi. (2016) 22:121–6. doi: 10.4274/tnd.84755
117. Qiu F, Gu P, Liu W, and Li D. The spectrum characteristics of Parkinson’s disease (PD) patients with sleep disorders. Neurol Sci. (2022) 43:327–33. doi: 10.1007/s10072-021-05240-z
118. Qin X, Li X, Chen G, Chen X, Shi M, Liu XK, et al. Clinical features and correlates of poor nighttime sleepiness in patients with parkinson’s disease. Parkinsons Dis. (2020) 2020:6378673. doi: 10.1155/2020/6378673
119. Mahale R, Yadav R, and Pal PK. Quality of sleep in young onset Parkinson’s disease: Any difference from older onset Parkinson’s disease. Parkinsonism Relat Disord. (2015) 21:461–4. doi: 10.1016/j.parkreldis.2015.02.007
120. Louter M, van der Marck MA, Pevernagie DAA, Munneke M, Bloem BR, and Overeem S. Sleep matters in Parkinson’s disease: Use of a priority list to assess the presence of sleep disturbances. Article. Eur J Neurol. (2013) 20:259–65. doi: 10.1111/j.1468-1331.2012.03836.x
121. Lin YY, Chen RS, Lu CS, Huang YZ, Weng YH, Yeh TH, et al. Sleep disturbances in Taiwanese patients with Parkinson’s disease. Brain Behav. (2017) 7:e00806. doi: 10.1002/brb3.806
122. Havlikova E, van Dijk JP, Nagyova I, Rosenberger J, Middel B, Dubayova T, et al. The impact of sleep and mood disorders on quality of life in Parkinson’s disease patients. J Neurol. (2011) 258:2222–9. doi: 10.1007/s00415-011-6098-6
123. Gao L, Huang W, Cai L, and Li H. Association between sleep disturbances and pain subtypes in Parkinson’s disease. Neurol Sci. (2022) 43:4785–90. doi: 10.1007/s10072-022-06030-x
124. Duncan GW, Khoo TK, Yarnall AJ, O'Brien JT, Coleman SY, Brooks DJ, et al. Health-related quality of life in early Parkinson’s disease: The impact of nonmotor symptoms. Movement Disord. (2014) 29:195–202. doi: 10.1002/mds.25664
125. Ding M, Zhu Y, Lu Z, Shen H, Dai J, and Zhu X. Analysis of independent risk factors affecting cognitive function in patients with Parkinson’s disease. Chin J Pract Nervous Dis. (2020) 23:1590–5. doi: 10.12083/sysj.2020.17.006
126. Shafazand S, Wallace DM, Arheart KL, Vargas S, Luca CC, Moore H, et al. Insomnia, sleep quality, and quality of life in mild to moderate parkinson’s disease. Ann Am Thorac Soc. (2017) 14:412–9. doi: 10.1513/AnnalsATS.201608-625OC
127. Skorvanek M, Nagyova I, Rosenberger J, Krokavcova M, Ghorbani Saeedian R, Groothoff JW, et al. Clinical determinants of primary and secondary fatigue in patients with Parkinson’s disease. J Neurol. (2013) 260:1554–61. doi: 10.1007/s00415-012-6828-4
128. Shi F, Guan H, Gao Z, Huang Y, and Wang D. Clinical characteristics of parkinson’S disease patients with sleep disorder and effect of anti resistance exercise intervention. J Int Psychiatry. (2023) 50:799–801,805. doi: 10.13479/j.cnki.jip.2023.04.011
129. Dong R and Tan H. Correlation of sleep disorders with emotional apathy and executive function in Parkinson disease. J Apoplexy Nervous Dis. (2023) 40:807–12. doi: 10.19845/j.cnki.zfysjjbzz.2023.0180
130. Chang CW, Fan JY, Chang BL, and Wu YR. Anxiety and levodopa equivalent daily dose are potential predictors of sleep quality in patients with parkinson disease in Taiwan. Front Neurol. (2019) 10:340. doi: 10.3389/fneur.2019.00340
131. Shi Y. Incidence of sleep disturbances in patients with Parkinson’s disease and its relationship to negative Emotions and qualiy of life. J Int Psychiatry. (2023) 50:796–8. doi: 10.13479/j.cnki.jip.2023.04.009
132. Li H, Yuan X, Ye Q, Yuan C, Gao C, and Hu Y. Analysis of factors influencing sleep disorders and the distribution characteristics of traditional Chinese medicine syndromes in people with early⁃or mid⁃stage Parkinson’s disease. Shanghai J Tradit Chin Med. (2023) 57:21–5. doi: 10.16305/j.1007-1334.2023.2208083
133. Gui X, Wang L, Wu C, and Sun X. Characteristics of sleep disturbances in Parkinson’ s disease patients with young onset and late onset. Zhejiang Med. (2018) 40:1698–701. doi: 10.12056/j.issn.1006-2785.2018.40.15.2017-3203
134. Hu Y, Sun Y, Lan Z, Wang H, Li X, and Zhong Y. Analysis of the relationships betwen changes in polysomnographic EEG map features and cogni- tive dysfunction in patients with Parkinson’s disease. Chin J Stereotact Funct Neurosurg. (2023) 36:76–81. doi: 10.19854/j.cnki.1008-2425.2023.02.0003
Keywords: Parkinson’s disease, sleep quality, meta-analysis, prevalence, epidemiology
Citation: Si TL, Wang Y-Y, Li J-X, Bai W, Sun H-L, Rao S-Y, Zhu H-Y, Ungvari GS, Su Z, Cheung T, Ng CH, Xiang Y-T and Wang G (2025) Poor sleep quality among patients with Parkinson’s disease: a meta-analysis and systematic review. Front. Psychiatry 16:1606743. doi: 10.3389/fpsyt.2025.1606743
Received: 16 May 2025; Accepted: 26 June 2025;
Published: 16 July 2025.
Edited by:
Luigi De Gennaro, Sapienza University of Rome, ItalyReviewed by:
Caterina Leitner, Vita-Salute San Raffaele University, ItalyLoida Camargo, University of Cartagena, Colombia
Copyright © 2025 Si, Wang, Li, Bai, Sun, Rao, Zhu, Ungvari, Su, Cheung, Ng, Xiang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chee H. Ng, Y25nQHVuaW1lbGIuZWR1LmF1; Yu-Tao Xiang, eHl1dGx5QGdtYWlsLmNvbQ==; Gang Wang, Z2FuZ3dhbmdkb2NAY2NtdS5lZHUuY24=
†These authors have contributed equally to this work
 Tong Leong Si
Tong Leong Si Yue-Ying Wang2,3†
Yue-Ying Wang2,3† Wei Bai
Wei Bai Shu-Ying Rao
Shu-Ying Rao Gabor S. Ungvari
Gabor S. Ungvari Zhaohui Su
Zhaohui Su Teris Cheung
Teris Cheung Chee H. Ng
Chee H. Ng Yu-Tao Xiang
Yu-Tao Xiang Gang Wang
Gang Wang