- 1School of Integrated Chinese and Western Medicine, Nanjing University of Chinese Medicine, Nanjing, China
- 2School of Health Preservation and Rehabilitation, Nanjing University of Chinese Medicine, Nanjing, China
- 3Department of Neurology, Suzhou Ninth People’s Hospital, Suzhou Ninth Hospital Affiliated to Soochow University, Suzhou, China
Background: Depression and anxiety are significant global health concerns, with systemic inflammation playing a critical role in their pathophysiology. Recent studies have highlighted the C-reactive protein to lymphocyte ratio (CLR) as a potential biomarker of inflammation that may be associated with these mental health conditions. However, the relationship between CLR and depression and anxiety, especially within a diverse population, remains underexplored.
Methods: This study utilized data from the National Health and Nutrition Examination Survey (NHANES) (2015–2023) to examine the association between CLR and the prevalence of depression and anxiety. A total of 22,308 participants were included for depression analysis, and 16,138 participants were included for anxiety analysis. Depression was assessed using the PHQ-9, and anxiety was assessed through self-reported anxiety symptoms and medication use. CLR was calculated as the ratio of C-reactive protein to lymphocyte count, and logistic regression models were applied to analyze associations, adjusting for demographic and health-related variables.
Results: Higher CLR levels were significantly associated with increased odds of depression (OR: 1.49; 95% CI: 1.25–1.78) and anxiety (OR: 1.13; 95% CI: 1.02–1.26) after full adjustment for confounders. Non-linear relationships were observed, with specific inflection points for both depression (CLR = 0.96) and anxiety (CLR = 0.88), beyond which the risk of mental health disorders increased sharply. Subgroup analyses revealed that younger individuals and those without hypertension showed stronger associations between CLR and depression.
Conclusion: Elevated CLR is associated with an increased risk of depression and anxiety, suggesting the potential role of systemic inflammation in influencing mental health outcomes. CLR may serve as a useful biomarker for identifying populations at higher risk, underscoring the need for further research into early intervention strategies and targeted approaches to address systemic inflammation in mental health care.
1 Introduction
Depression and anxiety are among the most common mental disorders worldwide, affecting over 300 million people annually and contributing significantly to global disability and disease burden (1). These conditions are multifactorial, influenced by genetic, environmental, and biological factors, among which inflammation has emerged as a critical pathway linking physiological dysregulation to mental health disorders (2). Numerous studies have demonstrated that inflammatory biomarkers, such as C-reactive protein (CRP), are elevated in individuals with depression and anxiety, underscoring the role of systemic inflammation in the pathophysiology of these conditions (3, 4).
CRP, an acute-phase reactant produced by the liver in response to inflammation, has been widely utilized as a marker of systemic inflammation (5). Lymphocytes, a subset of white blood cells, are key mediators of immune responses, and their count often decreases during systemic inflammatory states (6). The C-reactive protein to lymphocyte ratio (CLR) has recently emerged as a novel inflammatory marker, reflecting both the pro-inflammatory and immunosuppressive aspects of systemic inflammation (7, 8). CLR has been linked to various chronic diseases, including cardiovascular disease, cancer, and autoimmune disorders (9, 10). However, its role in mental health conditions, particularly depression and anxiety, remains insufficiently explored. Emerging evidence suggests a bidirectional relationship between systemic inflammation and mental health. Pro-inflammatory cytokines can cross the blood-brain barrier, altering neurotransmitter metabolism, neural plasticity, and the hypothalamic-pituitary-adrenal (HPA) axis, all of which are implicated in the development of depression and anxiety (11, 12). Conversely, chronic psychological stress and mental health disorders can exacerbate systemic inflammation, creating a vicious cycle that perpetuates both physical and mental health deterioration (13). A growing body of research has underscored the significant role of inflammatory markers, such as interleukins (e.g., IL-6), tumor necrosis factor-alpha (TNF-α), and trace elements including zinc and copper, in the pathophysiology of affective disorders (14, 15). Elevated concentrations of these biomarkers have been robustly linked to an increased risk of both depression and anxiety, suggesting that systemic inflammation plays a pivotal role in the dysregulation of mood and emotional homeostasis (16). These inflammatory mediators are thought to influence mood regulation through complex interactions with neurotransmitter systems, including serotonin, dopamine, and glutamate, thereby exacerbating or perpetuating affective symptoms (17). The dysregulation of these systems, in turn, may contribute to the onset and persistence of depressive and anxiety disorders, highlighting the intricate connection between immune responses and emotional regulation. Moreover, these markers may serve not only as indicators of the inflammatory burden but also as potential therapeutic targets for the management of mood disorders, warranting further investigation into their role in both the pathogenesis and treatment of these conditions.
Despite these advances, the interaction between demographic factors, comorbidities, and inflammatory markers such as CLR in depression and anxiety is not well understood. The National Health and Nutrition Examination Survey (NHANES) provides a valuable resource to investigate these associations in a diverse, representative sample of the U.S. population. Utilizing NHANES data from 2015 to 2023, this study aims to examine the relationship between CLR and the prevalence of depression and anxiety, while accounting for potential confounders such as age, sex, socioeconomic status, and lifestyle factors (18).Subgroup and interaction analyses aim to identify vulnerable populations where the association between CLR and mental health outcomes may be particularly pronounced, advancing our understanding of mental health heterogeneity and informing targeted prevention and intervention strategies (19).
This study represents the first comprehensive exploration of CLR as an inflammatory biomarker for depression and anxiety in a nationally representative cohort. By integrating robust statistical modeling with population-based data, we aim to shed light on the inflammatory underpinnings of mental health disorders and provide novel insights into their prevention and management.
2 Methods
2.1 Study design and population
This cross-sectional study was conducted using data from the National Health and Nutrition Examination Survey (NHANES) between 2015 and 2023. NHANES employs a complex, multistage probability sampling design to collect representative health and nutritional data from the U.S. population.
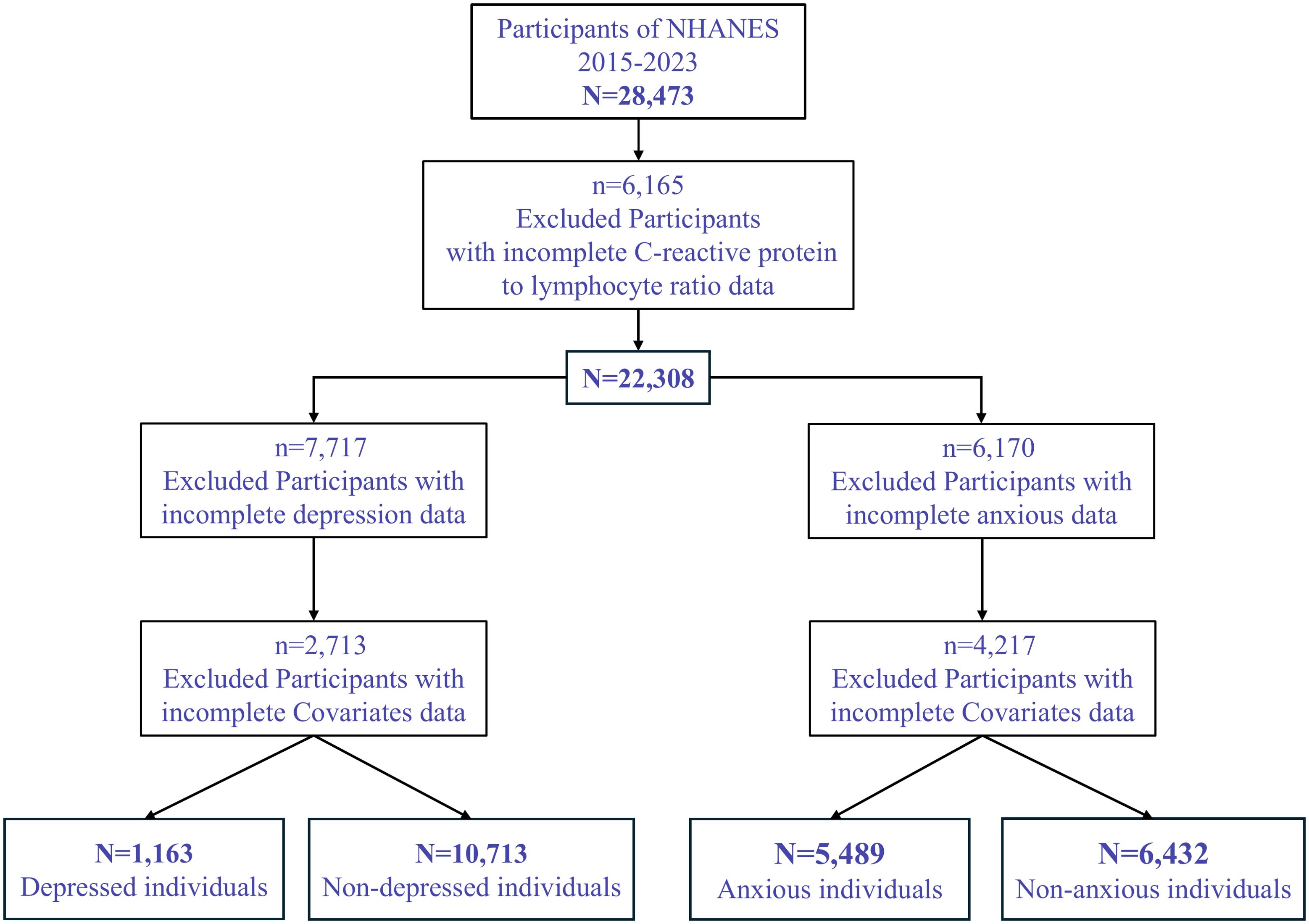
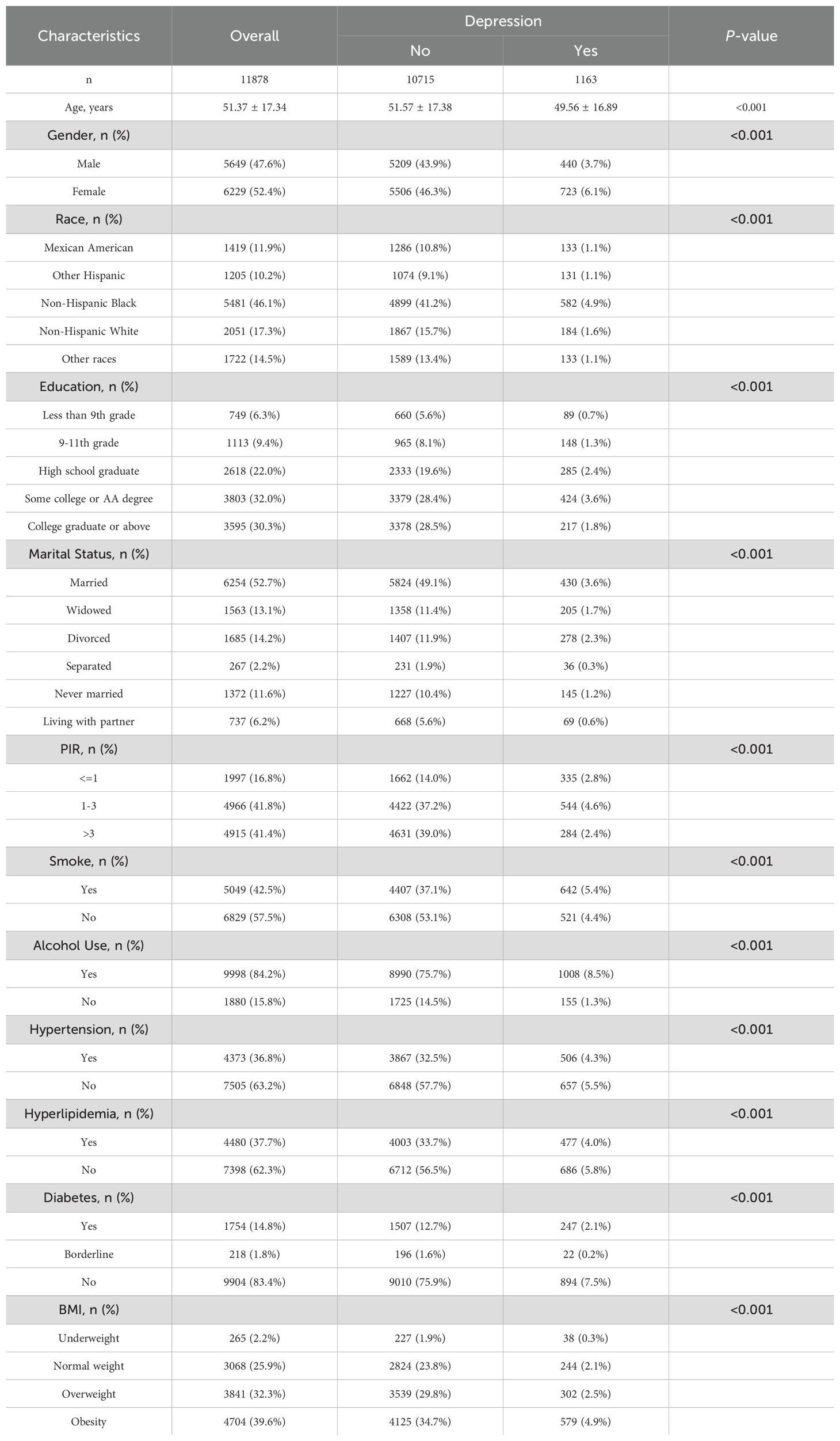
A total of 28,473 participants were initially included. After excluding 6,165 participants with missing data on C-reactive protein to lymphocyte ratio (CLR), 22,308 participants remained. For the depression analysis, 7,717 participants with missing depression data were further excluded, resulting in 14,591 individuals. An additional 2,713 participants with missing covariate data were excluded, yielding a final sample size of 11,878 individuals, including 1,163 participants with depression and 10,715 without (Figure 1).
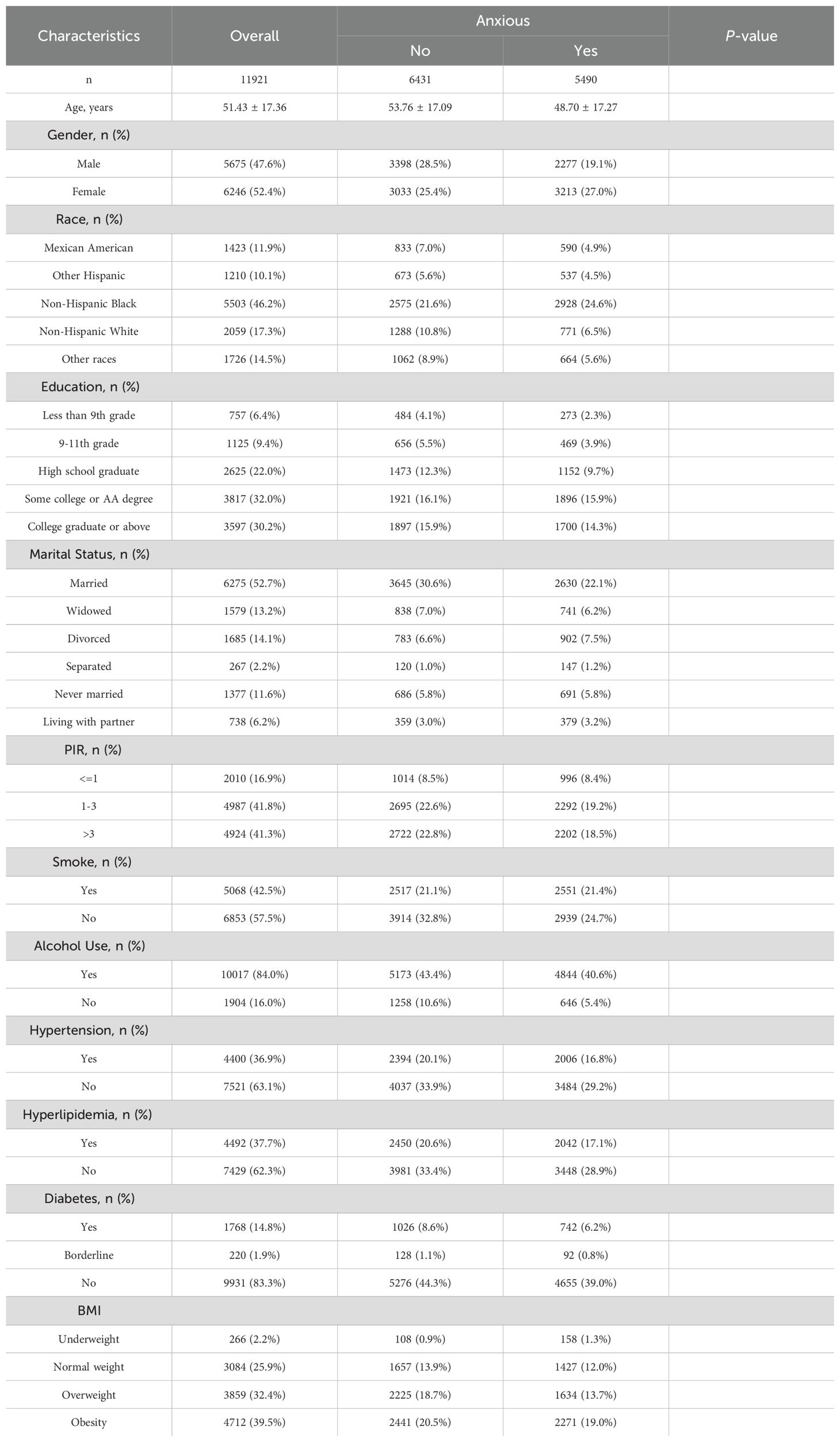
For the anxiety analysis, 6,170 participants with missing CLR data were excluded, resulting in 16,138 participants. After excluding 4,217 participants with missing covariate data, the final analytic sample comprised 11,921 participants, including 5,489 individuals with anxiety and 6,430 without.
2.2 Definition of depression and anxiety
The 9-item PHQ-9 was used to assess depressive symptoms. PHQ-9 is well accepted as an accurate and reliable tool for screening depression. The total PHQ-9 score varies from 0 to 27, with higher scores indicating more severe depression. Compared with the clinical interview criteria, a PHQ score of ≥10 had a sensitivity of 85% and a specificity of 89% for major depressive disorder (MDD) (20). The PHQ-9 is a widely used tool for screening depression, but it is not a diagnostic tool and may not fully capture the complexity of depression. It is important to note that a score of ≥10 indicates potential major depressive disorder (MDD), but further clinical evaluation is required for a formal diagnosis. The limitations of self-reported data in assessing mental health symptoms should also be acknowledged.
Anxiety was assessed using self-reported frequency of anxiety-related feelings and anxiety medication use, as recorded in the NHANES mental health questionnaire. Participants were asked, “How often do you feel worried, nervous, or anxious? Would you say daily, weekly, monthly, a few times a year, or never?” Those who reported experiencing anxiety daily or weekly or who reported current use of anxiety medication were classified as having significant anxiety symptoms (21). This approach aligns with methodologies employed in prior studies and captures both self-reported emotional experiences and clinical management of anxiety.
2.3 Calculation of C-reactive protein to lymphocyte ratio
C-reactive protein (CRP) levels were measured via a high-sensitivity immunoturbidimetric assay, while lymphocyte counts were obtained from complete blood count analyses using automated hematology analyzers. CLR was calculated as the ratio of CRP (mg/L) to lymphocyte count (×10^9/L) and categorized into quartiles for comparative analyses.
2.4 Covariates
Demographic, socioeconomic, and health-related variables were included as covariates to adjust for potential confounding. These included age, gender, race/ethnicity, education level, marital status, body mass index (BMI), poverty-to-income ratio (PIR), smoking status, alcohol consumption, diabetes, hypertension, and hyperlipidemia.
2.5 Statistical analysis
All statistical analyses were performed using SPSS 27.0 and R version 4.4.2. Continuous variables were expressed as mean ± standard deviation (SD) and compared using t-tests, while categorical variables were expressed as frequencies (%) and analyzed using chi-square tests. A two-tailed P-value <0.05 was considered statistically significant. To explore the relationship between CLR and the risk of depression and anxiety, weighted logistic regression models were used, incorporating NHANES sample weights to account for the survey’s complex design. Three models were constructed: Model 1: Unadjusted. Model 2: Adjusted for age and gender. Model 3: Fully adjusted for all covariates. Non-linear associations between CLR and mental health outcomes were evaluated using restricted cubic spline (RCS) regression analyses. These models assessed dose-response relationships and identified potential thresholds for risk. RCS allows for the identification of threshold effects or inflection points where the risk of mental health outcomes changes more sharply. This method is particularly useful when the relationship between independent and dependent variables is not linear, as in the case of CLR and mental health outcomes. Subgroup analyses and interaction tests were conducted to identify effect modifiers, including age, gender, and comorbid conditions. Interaction terms were incorporated into logistic regression models to determine whether the association between CLR and mental health outcomes varied across subgroups.
2.6 Ethical considerations
The NHANES protocol was approved by the National Center for Health Statistics Research Ethics Review Board, and all participants provided informed consent. This study utilized publicly available, de-identified data, exempting it from further ethical approval in accordance with the Declaration of Helsinki.
3 Results
3.1 Baseline characteristics of participants
The study cohort consisted of 11,878 participants for the depression analysis and 11,921 participants for the anxiety analysis, as detailed in Tables 1, 2. The mean age of participants was approximately 51 years, with a slightly higher representation of females (52.4%) than males (47.6%). Significant differences were observed in demographic and clinical characteristics between participants with and without depression or anxiety (P < 0.001 for all variables). For instance, individuals with depression had a younger mean age (49.56 ± 16.89 years) compared to those without (51.57 ± 17.38 years), and a similar trend was observed in the anxiety cohort.
Educational attainment also varied significantly; participants with depression or anxiety were less likely to have completed higher education. Marital status revealed that never-married individuals and those separated or divorced exhibited higher rates of depression and anxiety. Additionally, comorbid conditions such as hypertension, hyperlipidemia, and diabetes were more prevalent among individuals with mental health symptoms.
3.2 Relationship between CLR and depression
The association between the C-reactive protein to lymphocyte ratio (CLR) and depression was assessed through weighted logistic regression models (Table 3). In the unadjusted model (Model 1), participants in the highest quartile of CLR (Q4) had significantly higher odds of depression compared to those in the lowest quartile (Q1) (odds ratio [OR]: 1.82; 95% confidence interval [CI]: 1.54–2.16; P < 0.001). After adjusting for age, sex, and race (Model 2), the association remained significant (OR: 1.82; 95% CI: 1.53–2.16; P < 0.001). In the fully adjusted model (Model 3), which accounted for all covariates, the odds ratio was slightly attenuated but remained statistically significant (OR: 1.49; 95% CI: 1.25–1.78; P < 0.001).
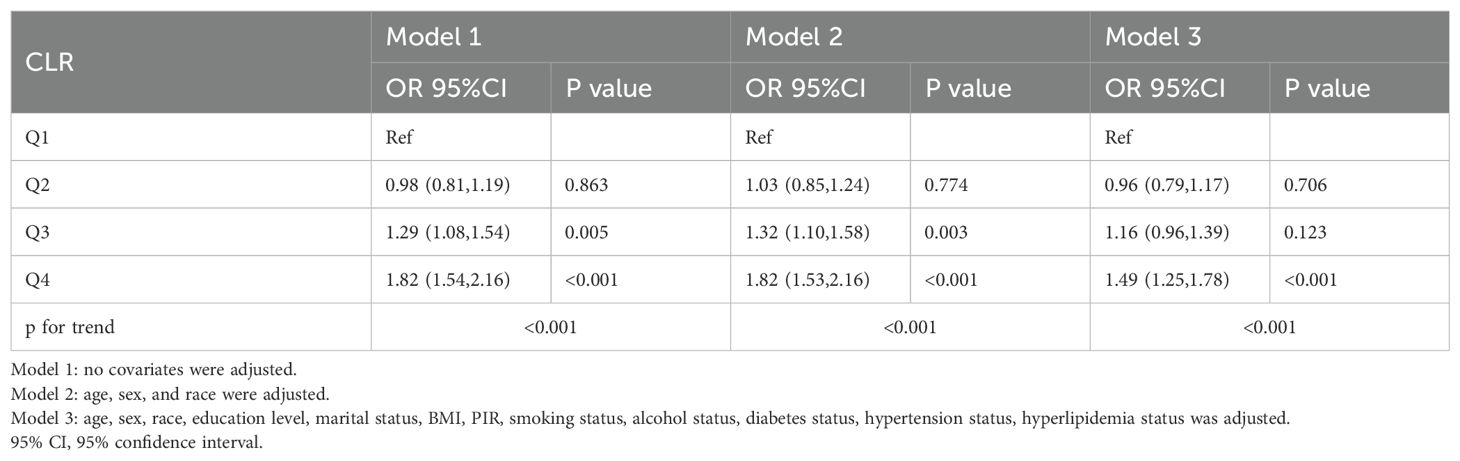
Table 3. Weighted logistic regression analyses of association between the C-reactive protein to lymphocyte ratio and depression.
Restricted cubic spline (RCS) regression analysis (Figure 2) indicated a non-linear dose-response relationship between CLR and depression risk, with a notable inflection point at CLR = 0.96. Beyond this threshold, the risk of depression increased sharply, suggesting a critical level of systemic inflammation associated with elevated mental health risks.
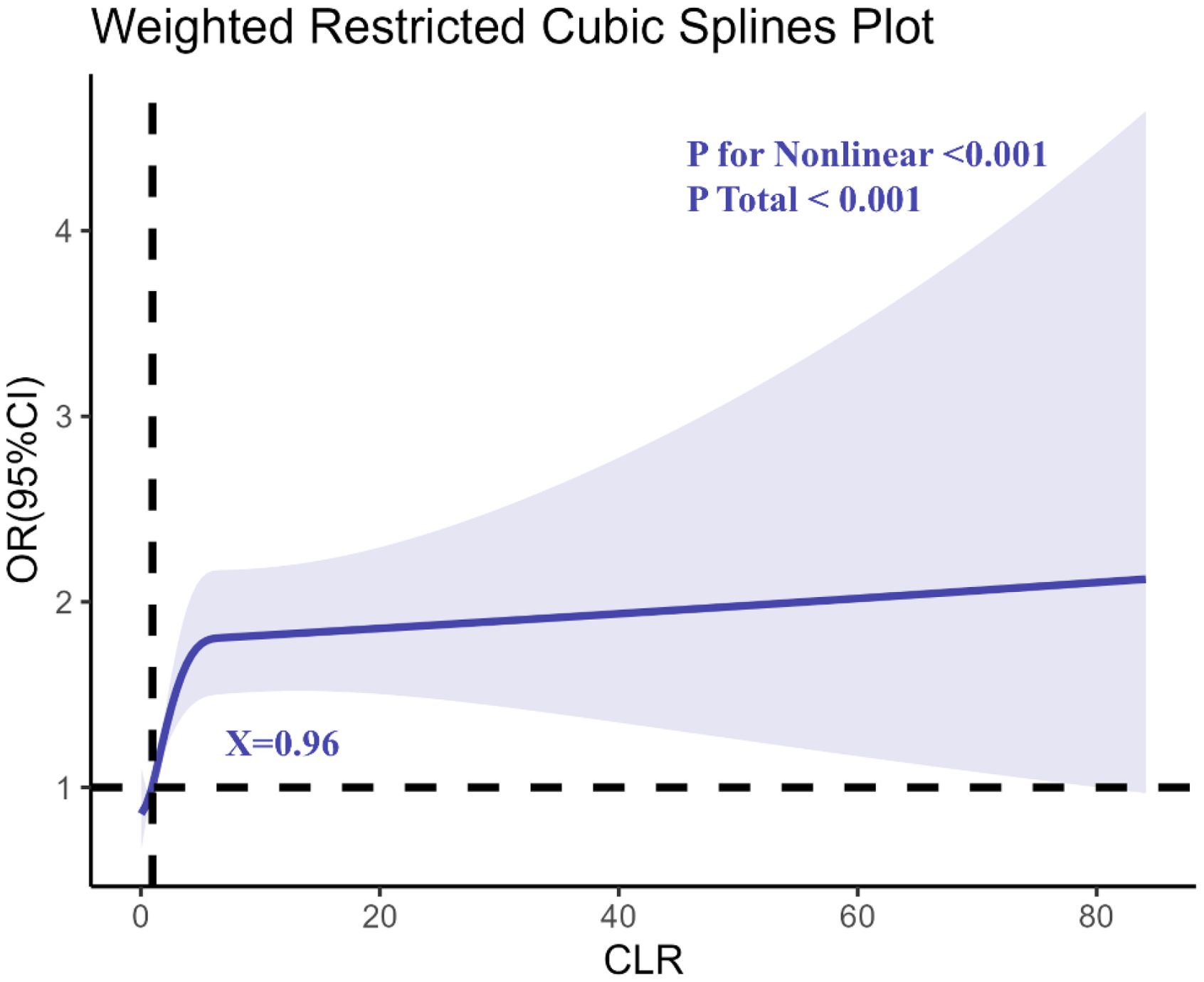
Figure 2. Determination of the association between C-reactive protein to lymphocyte ratio (CLR) and depression by restricted cubic spline (RCS) regression analysis.
3.3 Relationship between CLR and anxiety
Similarly, the weighted logistic regression analysis demonstrated a significant association between CLR and anxiety (Table 4). In Model 1, participants in Q4 had a 15% higher likelihood of anxiety compared to Q1 (OR: 1.15; 95% CI: 1.04–1.28; P = 0.006). This association was strengthened after adjusting for age, sex, and race in Model 2 (OR: 1.22; 95% CI: 1.10–1.35; P < 0.001) and persisted in the fully adjusted Model 3 (OR: 1.13; 95% CI: 1.02–1.26; P = 0.023).
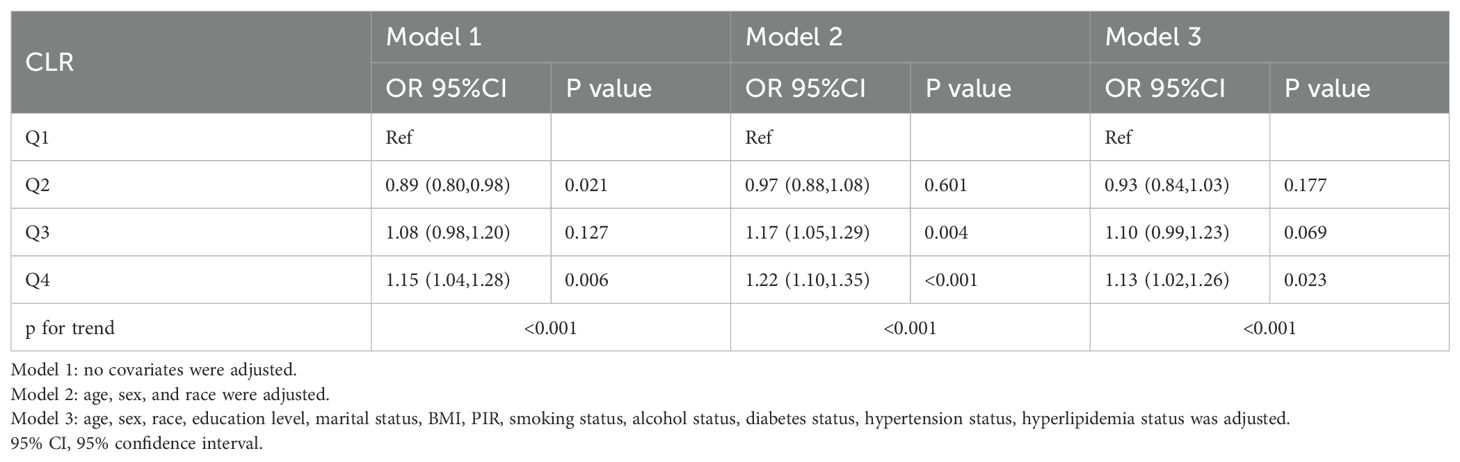
Table 4. Weighted logistic regression analyses of association between the C-reactive protein to lymphocyte ratio and anxious.
The RCS regression analysis (Figure 3) revealed a non-linear relationship between CLR and anxiety, with an inflection point at CLR = 0.88. This indicates that the risk of anxiety begins to rise steeply beyond this level, highlighting a potential threshold for systemic inflammation contributing to anxiety disorders.
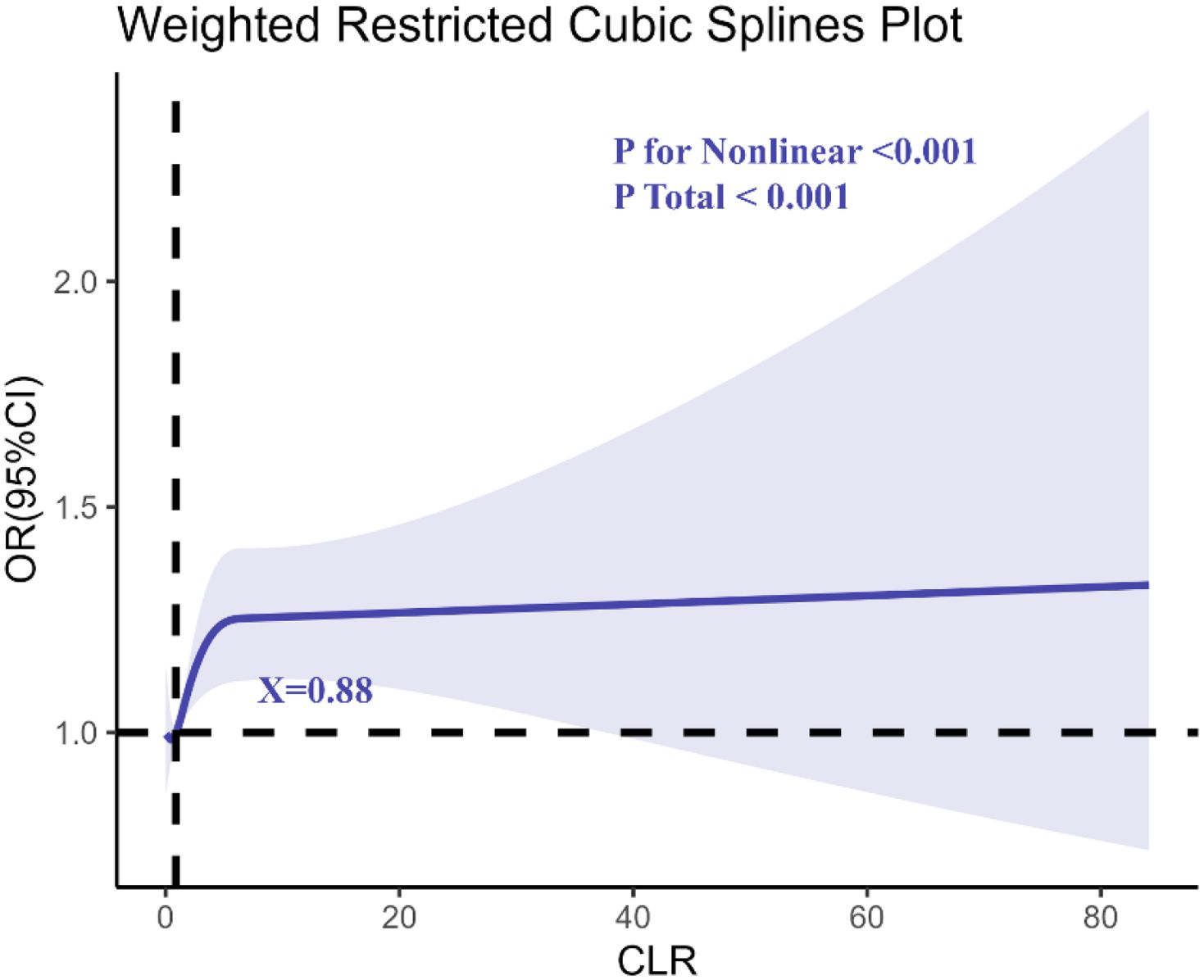
Figure 3. Determination of the association between C-reactive protein to lymphocyte ratio (CLR) and anxiety by restricted cubic spline (RCS) regression analysis.
3.4 Relationship between CLR and co-occurring depression and anxiety
Supplementary Table S1 presents the results of the weighted logistic regression analyses examining the association between the C-reactive protein to lymphocyte ratio (CLR) and co-occurring depression and anxiety. The findings from the three models are as follows:
● Model 1 Q4: OR 1.67 (95% CI: 1.39, 2.00), p < 0.001
● Model 2 Q4: OR 1.67 (95% CI: 1.39, 2.01), p < 0.001
● Model 3 Q4: OR 1.31 (95% CI: 1.08, 1.58), p = 0.006
These results indicate a significant association between CLR and the risk of co-occurring depression and anxiety, with the association remaining robust after adjusting for demographic and health-related covariates. Specifically, the fully adjusted model (Model 3) suggests that higher CLR levels are independently associated with an increased risk of co-occurring depression and anxiety, highlighting the potential of CLR as a biomarker for identifying at-risk populations.
Supplementary Figure S1 illustrates the results of the restricted cubic spline (RCS) regression analysis examining the relationship between the C-reactive protein to lymphocyte ratio (CLR) and co-occurring depression and anxiety. The analysis revealed a critical inflection point at CLR = 0.90, beyond which the relationship between CLR and mental health outcomes shifted from protective to detrimental. Specifically, when CLR was below 0.90, it appeared to serve as a protective factor, whereas CLR values exceeding 0.90 were associated with an increased risk of co-occurring depression and anxiety. This finding emphasizes the non-linear nature of the relationship between systemic inflammation and mental health, suggesting potential clinical implications for identifying at-risk populations based on CLR levels.
3.5 Subgroup and interaction analyses
3.5.1 CLR and depression
Subgroup analyses demonstrated significant variations in the relationship between CLR and depression across different demographic and clinical categories (Figure 4). Gender-stratified analysis revealed that the association was stronger in females (OR: 1.62; 95% CI: 1.34–1.97; P < 0.001) compared to males (OR: 1.50; 95% CI: 1.23–1.85; P < 0.001), though the interaction test was not statistically significant (P for interaction = 0.595).
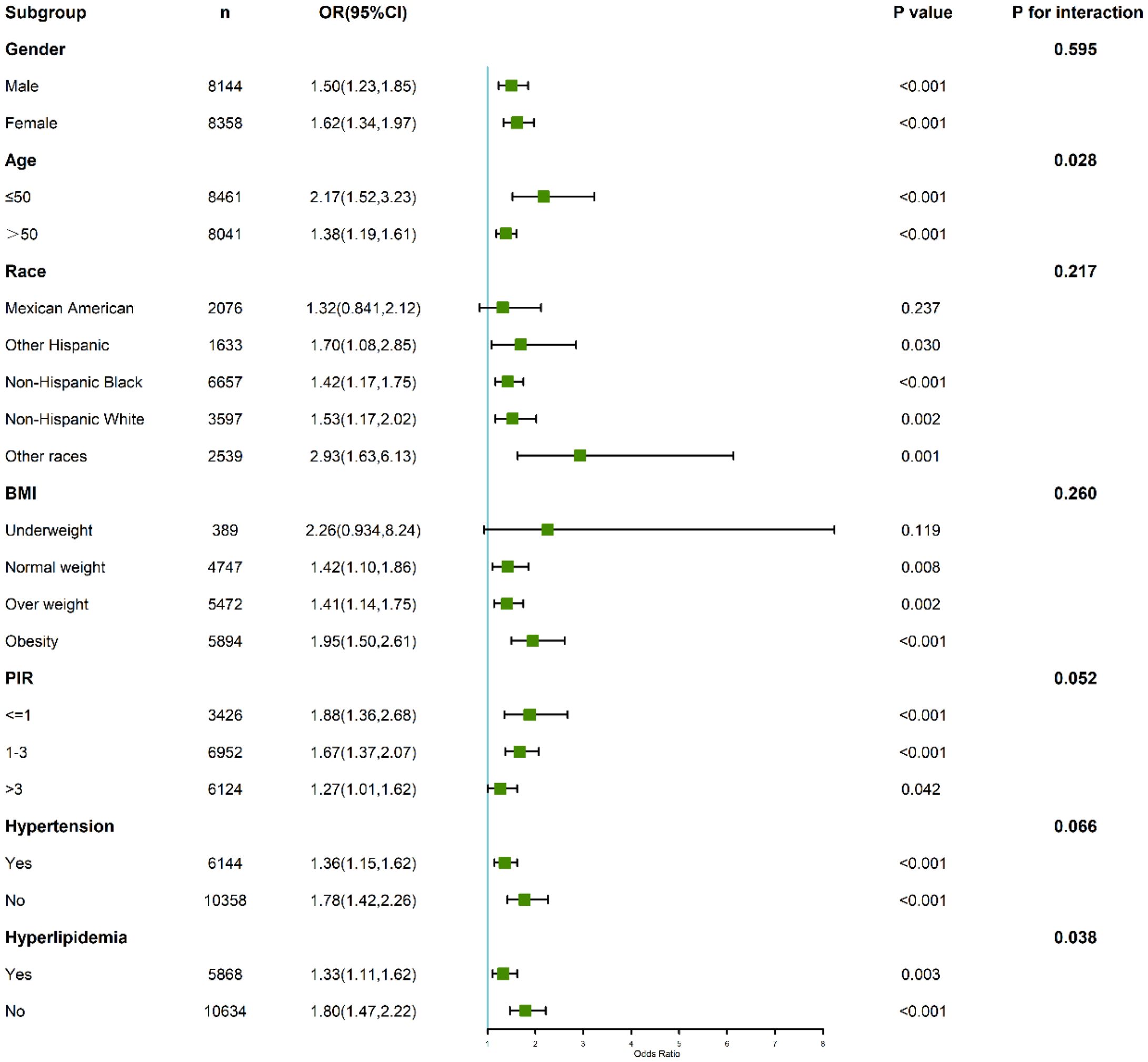
Figure 4. Subgroup and interaction analyses of the association between C-reactive protein to lymphocyte ratio (CLR) and depression.
Age also modified the relationship, with participants aged ≤50 years showing a heightened association (OR: 2.17; 95% CI: 1.53–3.23; P < 0.001) compared to those older than 50 years (OR: 1.38; 95% CI: 1.19–1.61; P < 0.001). The interaction test confirmed statistical significance (P for interaction = 0.028), indicating that age plays a significant role in modulating the relationship between CLR and depression.
Among individuals with hypertension, the highest CLR quartile was associated with an increased risk of depression (OR: 1.36; 95% CI: 1.15–1.62). Notably, this association was even stronger among those without hypertension (OR: 1.78; 95% CI: 1.42–2.26). The interaction test approached but did not reach statistical significance (P for interaction = 0.066), suggesting a potential modifying effect of hypertension status on the relationship between CLR and depression.
3.5.2 CLR and anxiety
For anxiety, the interaction analysis revealed subtle yet important variations (Figure 5). Gender-stratified results showed that males (OR: 1.05; 95% CI: 0.999–1.10; P = 0.057) and females (OR: 1.04; 95% CI: 0.996–1.09; P = 0.075) had similar risk patterns, with no significant interaction (P for interaction = 0.830).
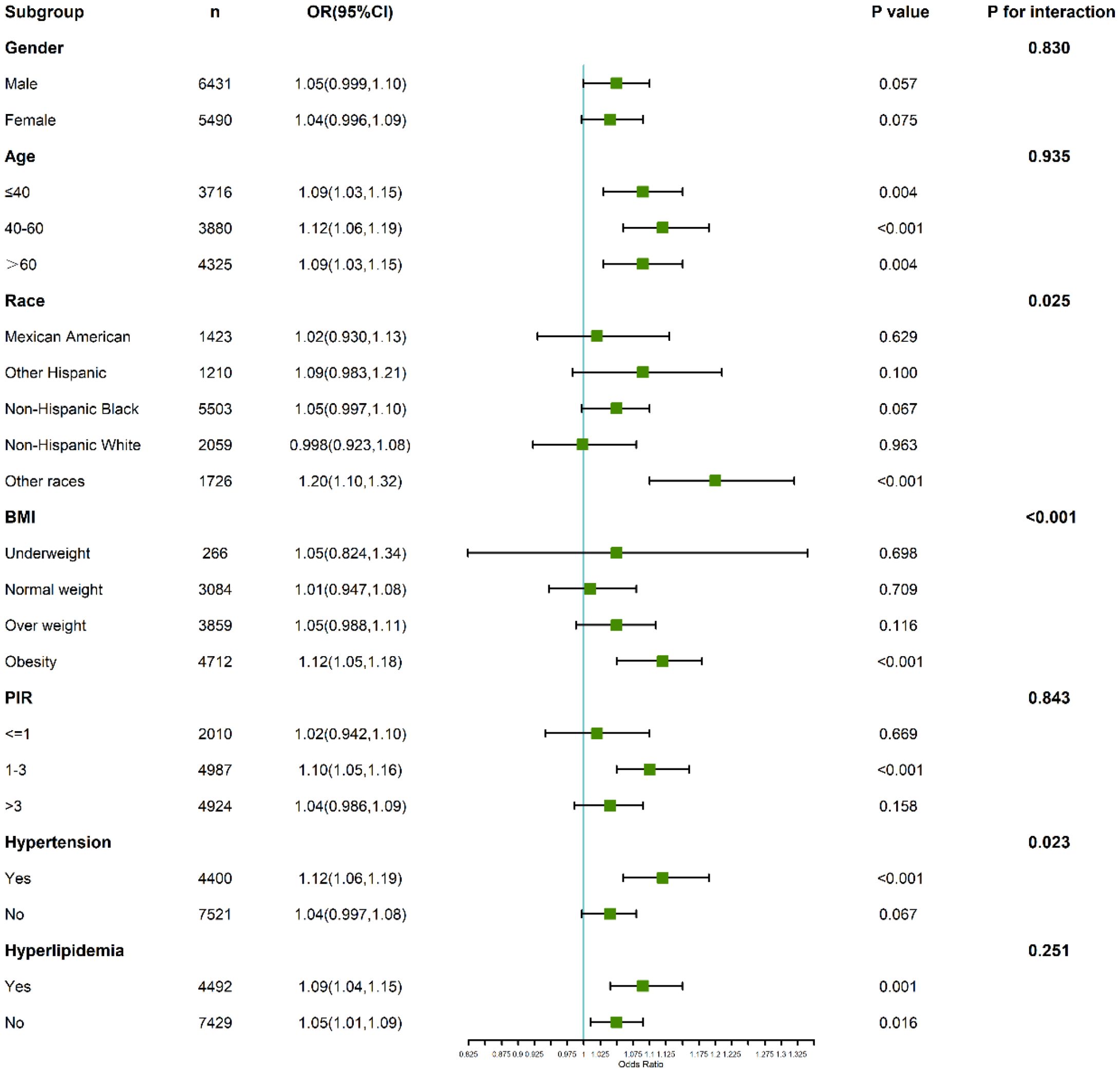
Figure 5. Subgroup and interaction analyses of the association between C-reactive protein to lymphocyte ratio (CLR) and anxiety.
Participants aged ≤40 years demonstrated a stronger association between CLR and anxiety (OR: 1.09; 95% CI: 1.03–1.15; P = 0.004) compared to those above 40 years, where the relationship was attenuated. The interaction test, however, did not reach statistical significance (P for interaction = 0.935).
In individuals with hyperlipidemia, those in the highest CLR quartile exhibited an elevated anxiety risk (OR: 1.22; 95% CI: 1.10–1.35), suggesting a synergistic effect of inflammatory and lipid-related pathways in anxiety pathogenesis. Interaction tests emphasized the need to consider metabolic conditions when interpreting these associations.
3.5.3 CLR and co-occurring depression and anxiety
Supplementary Figure S2 presents the results of subgroup and interaction analyses exploring the association between the C-reactive protein to lymphocyte ratio (CLR) and co-occurring depression and anxiety. The subgroup analysis revealed that certain groups, including females under the age of 40, Mexican American individuals, obese individuals, and those with hypertension or hyperlipidemia, were more vulnerable to the effects of CLR.
Interaction analysis revealed significant interactions between CLR and body mass index (BMI), as well as poverty-to-income ratio (PIR) (p < 0.05). However, no significant interactions were found between CLR and gender, age, race, hypertension, or hyperlipidemia (p > 0.05). These findings suggest that metabolic factors, such as BMI, and socioeconomic factors, such as PIR, may moderate the relationship between CLR and co-occurring depression and anxiety, highlighting the importance of considering these factors when interpreting the role of inflammation in mental health.
4 Discussion
This study provides compelling evidence linking elevated CLR with increased risks of depression and anxiety. The identification of inflection points at CLR = 0.96 for depression and CLR = 0.88 for anxiety emphasizes the critical thresholds of systemic inflammatory burden that disproportionately elevate mental health risks. These findings align with previous research suggesting that systemic inflammation plays a central role in the etiology of mental health disorders (22). Biomarkers like CLR, which capture the balance of pro-inflammatory and immunosuppressive processes, offer novel insights into the complex interplay between physiological and psychological health (23, 24).
Mechanistically, systemic inflammation may disrupt the central nervous system through several pathways (25). Pro-inflammatory cytokines, including interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), can cross the blood-brain barrier, where they interfere with neurotransmitter metabolism, impair neuroplasticity, and activate microglial cells (26, 27). These processes contribute to structural and functional alterations in brain regions implicated in mood regulation, such as the prefrontal cortex and hippocampus (28, 29). Chronic stress further exacerbates this cycle by activating the hypothalamic-pituitary-adrenal (HPA) axis, leading to sustained glucocorticoid secretion and heightened systemic inflammation (30, 31). The kynurenine pathway is increasingly recognized as a pivotal mechanism linking systemic inflammation to depression. Pro-inflammatory cytokines, such as interleukin-6 (IL-6), have been shown to activate this pathway, leading to the conversion of tryptophan into kynurenine, which subsequently produces neurotoxic metabolites, including quinolinic acid (32). Quinolinic acid, an excitotoxin, can disrupt neurotransmitter systems, particularly by impairing serotonin and glutamate metabolism, which are critical for mood regulation. This metabolic disturbance in the central nervous system is thought to contribute to the development of depressive symptoms (33). Recent studies have provided substantial evidence supporting the role of the kynurenine pathway in the pathophysiology of depression, suggesting that elevated levels of kynurenine and quinolinic acid may serve as biomarkers for inflammation-associated mood disorders (34). Future research could further elucidate how this pathway interacts with other systemic inflammation markers, such as the C-reactive protein to lymphocyte ratio (CLR), to deepen our understanding of the inflammatory underpinnings of depression and potentially guide new therapeutic interventions. These neuroinflammatory pathways have been consistently implicated in both depression and anxiety.
Subgroup analyses in this study revealed important population-specific variations in the CLR-mental health relationship. Younger individuals exhibited a stronger association between CLR and depression, potentially due to a more robust immune response in early adulthood (35). This heightened inflammatory sensitivity may amplify the neurotoxic effects of cytokines, accelerating the onset or progression of mood disorders (36). Similarly, the amplified risk observed in individuals without hypertension suggests that baseline cardiovascular health may modulate the impact of systemic inflammation on mental health (37). These findings highlight the importance of considering demographic and clinical contexts when interpreting the role of inflammatory biomarkers.
For anxiety, the heightened association observed in individuals with hyperlipidemia underscores the synergistic interaction between metabolic and inflammatory dysregulation (38–40). Hyperlipidemia is characterized by the accumulation of pro-inflammatory lipids and cytokines, which may exacerbate systemic inflammation and its downstream effects on the brain (41, 42). This is consistent with prior studies linking metabolic syndrome to increased risks of anxiety disorders, suggesting that targeted interventions addressing metabolic health could mitigate the burden of anxiety (43).
The clinical implications of these findings are substantial. CLR, as an easily measurable and cost-effective biomarker, could serve as a valuable tool for early identification of individuals at risk of depression and anxiety. Integrating CLR measurement into routine health assessments may facilitate targeted interventions, particularly in high-risk groups such as younger adults and individuals with metabolic dysregulation. Anti-inflammatory therapies, including nonsteroidal anti-inflammatory drugs (NSAIDs), and lifestyle modifications, such as increased physical activity and dietary improvements, hold promise in mitigating the inflammatory burden associated with mental health disorders (44–46). In addition to nonsteroidal anti-inflammatory drugs(NSAIDs) and lifestyle modifications, targeting specific cytokines through receptor antagonists, such as IL-6 inhibitors and TNF-α inhibitors, has shown promise in reducing inflammation and improving symptoms of depression and anxiety in some clinical trials. These therapies may offer more targeted approaches for managing inflammation-related mood disorders, although further studies are needed to assess their long-term efficacy and safety in mental health populations (47). Future clinical trials should explore the efficacy of these interventions in reducing CLR levels and improving mental health outcomes.
Despite the strengths of this study, including its large sample size and representative population, certain limitations must be acknowledged. The cross-sectional design precludes causal inferences, and unmeasured confounding factors may have influenced the observed associations (48). Additionally, the use of CLR as a standalone biomarker does not capture the full complexity of systemic inflammation. Combining CLR with other inflammatory markers, such as high-sensitivity CRP or fibrinogen, may enhance its predictive utility (49). While we have adjusted for a range of demographic and health-related variables as covariates, the cross-sectional design of the study prevents us from fully accounting for potential unmeasured confounders. Future longitudinal studies could help further clarify the causal relationship between CLR and mental health outcomes.
5 Conclusion
In conclusion, this study highlights the potential of CLR as a biomarker linking systemic inflammation to depression and anxiety. By identifying critical thresholds and subgroup-specific variations, our findings contribute to a deeper understanding of the inflammatory mechanisms underlying mental health disorders. These insights pave the way for targeted prevention and intervention strategies, underscoring the need for integrated approaches to address the systemic nature of mental health challenges.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://wwwn.cdc.gov/nchs/nhanes/default.aspx.
Author contributions
YS: Conceptualization, Writing – original draft, Methodology. YG: Data curation, Writing – original draft. HY: Formal Analysis, Writing – original draft. GZ: Writing – review & editing. XC: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the National Natural Science Foundation of China (No.82274218), the Foundation of Jiangsu CM Clinical Innovation Center of Degenerative Bone & Joint Disease (Jiangsu science and education of traditional Chinese medicine (2021)No. 6) and Innovation Project for Postgraduate Training in Jiangsu Province (KYCX25_2356).
Acknowledgments
We also sincerely thank all the projects (National Health and Nutrition Examination Survey) who participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2025.1607982/full#supplementary-material
Abbreviations
CRP, C-reactive protein; CLR, C-reactive protein to lymphocyte ratio; HPA, Hypothalamic-pituitary-adrenal; NHANES, National Health and Nutrition Examination Survey; PHQ-9, Patient Health Questionnaire-9; MDD, Major depressive disorder; NSAIDs, Nonsteroidal anti-inflammatory drugs; BMI, Body mass index; PIR, Poverty-to-income ratio; IL-6, Interleukin-6; TNF-α, Tumor necrosis factor-alpha; RCS, Restricted cubic spline; OR, Odds ratio; CI, Confidence interval; SD, Standard deviation; SPSS, Statistical Package for the Social Sciences.
References
1. Kris-Etherton PM, Petersen KS, Hibbeln JR, Hurley D, Kolick V, Peoples S, et al. Nutrition and behavioral health disorders: depression and anxiety. Nutr Rev. (2021) 79:247–60. doi: 10.1093/nutrit/nuaa025
2. Hartmann TE, Robertson CV, Miller TD, Hunter JR, and Skein M. Associations between exercise, inflammation and symptom severity in those with mental health disorders. Cytokine. (2021) 146:155648. doi: 10.1016/j.cyto.2021.155648
3. Milaneschi Y, Kappelmann N, Ye Z, Lamers F, Moser S, Jones PB, et al. Association of inflammation with depression and anxiety: evidence for symptom-specificity and potential causality from UK Biobank and NESDA cohorts. Mol Psychiatry. (2021) 26:7393–402. doi: 10.1038/s41380-021-01188-w
4. von Känel R, Rosselet K, Gessler K, Haeussler A, Aschmann J, Rodriguez H, et al. Preoperative depression and anxiety as predictors of postoperative C-reactive protein levels in patients undergoing cardiac surgery: a prospective observational study. Swiss Med Wkly. (2022) 152:40018. doi: 10.57187/smw.2022.40018
5. Sproston NR and Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. (2018) 9:754. doi: 10.3389/fimmu.2018.00754
6. Moro-García MA, Mayo JC, Sainz RM, and Alonso-Arias R. Influence of inflammation in the process of T lymphocyte differentiation: proliferative, metabolic, and oxidative changes. Front Immunol. (2018) 9:339. doi: 10.3389/fimmu.2018.00339
7. Chen X, Lin Z, Chen Y, and Lin C. C-reactive protein/lymphocyte ratio as a prognostic biomarker in acute pancreatitis: a cross-sectional study assessing disease severity. Int J Surg. (2024) 110:3223–9. doi: 10.1097/JS9.0000000000001273
8. Maina G, Adami M, Ascione G, Bondi E, De Berardis D, Delmonte D, et al. Nationwide consensus on the clinical management of treatment-resistant depression in Italy: a Delphi panel. Ann Gen Psychiatry. (2023) 22:48. doi: 10.1186/s12991-023-00478-7
9. Demirkol ME, Bilgin S, Kahveci G, Kurtkulagi O, Atak Tel BM, Duman TT, et al. C-reactive protein-to-lymphocyte ratio is a reliable marker in patients with COVID-19 infection: the CLEAR COVID study. Cir Cir. (2022) 90:596–601. doi: 10.24875/CIRU.22000124
10. Zhao T and Lv T. Correlation between serum bilirubin, blood uric acid, and C-reactive protein and the severity of chronic obstructive pulmonary disease. J Health Popul Nutr. (2024) 43:105. doi: 10.1186/s41043-024-00593-5
11. Palmer ER, Morales-Muñoz I, Perry BI, Marwaha S, Warwick E, Rogers JC, et al. Trajectories of inflammation in youth and risk of mental and cardiometabolic disorders in adulthood. JAMA Psychiatry. (2024) 81:1130–7. doi: 10.1001/jamapsychiatry.2024.2193
12. Chang J, Jiang T, Shan X, Zhang M, Li Y, Qi X, et al. Pro-inflammatory cytokines in stress-induced depression: Novel insights into mechanisms and promising therapeutic strategies. Prog Neuropsychopharmacol Biol Psychiatry. (2024) 131:110931. doi: 10.1016/j.pnpbp.2023.110931
13. Morrow AL, Boero G, and Balan I. Emerging evidence for endogenous neurosteroid modulation of pro-inflammatory and anti-inflammatory pathways that impact neuropsychiatric disease. Neurosci Biobehav Rev. (2024) 158:105558. doi: 10.1016/j.neubiorev.2024.105558
14. Huang D, Lai S, Zhong S, and Jia Y. Association between serum copper, zinc, and selenium concentrations and depressive symptoms in the US adult population, NHANES (2011-2016). BMC Psychiatry. (2023) 23:498. doi: 10.1186/s12888-023-04953-z
15. Li Z, Wang W, Xin X, Song X, and Zhang D. Association of total zinc, iron, copper and selenium intakes with depression in the US adults. J Affect Disord. (2018) 228:68–74. doi: 10.1016/j.jad.2017.12.004
16. Russo AJ. Decreased zinc and increased copper in individuals with anxiety. Nutr Metab Insights. (2011) 4:1–5. doi: 10.4137/NMI.S6349
17. Liu L, Jia P, Liu T, Liang J, Dang Y, Rastegar-Kashkooli Y, et al. Metabolic dysfunction contributes to mood disorders after traumatic brain injury. Ageing Res Rev. (2025) 104:102652. doi: 10.1016/j.arr.2024.102652
18. Liu B, Sun X, Li X, Lu F, Xing G, Ma G, et al. Associations of C-reactive protein to lymphocyte ratio and metabolic-dysfunction-associated steatotic liver disease: evidence from NHANES 2017-2018. BMC Gastroenterol. (2024) 24:475. doi: 10.1186/s12876-024-03458-7
19. Sæther LS, Szabo A, Akkouh IA, Haatveit B, Mohn C, Vaskinn A, et al. Cognitive and inflammatory heterogeneity in severe mental illness: Translating findings from blood to brain. Brain Behav Immun. (2024) 118:287–99. doi: 10.1016/j.bbi.2024.03.014
20. Zhou X, Tao XL, Zhang L, Yang QK, Li ZJ, Dai L, et al. Association between cardiometabolic index and depression: National Health and Nutrition Examination Survey (NHANES) 2011-2014. J Affect Disord. (2024) 351:939–47. doi: 10.1016/j.jad.2024.02.024
21. Bai L, Wen Z, Zhu Y, Jama HA, Sawmadal JD, and Chen J. Association of blood cadmium, lead, and mercury with anxiety: a cross-sectional study from NHANES 2007-2012. Front Public Health. (2024) 12:1402715. doi: 10.3389/fpubh.2024.1402715
22. Schrock JM, McDade TW, Carrico AW, D’Aquila RT, and Mustanski B. Traumatic events and mental health: The amplifying effects of pre-trauma systemic inflammation. Brain Behav Immun. (2021) 98:173–84. doi: 10.1016/j.bbi.2021.08.208
23. Zhao Y, Ghaedi A, Azami P, Nabipoorashrafi SA, Drissi HB, Dezfouli MA, et al. Inflammatory biomarkers in cardiac syndrome X: a systematic review and meta-analysis. BMC Cardiovasc Disord. (2024) 24:276. doi: 10.1186/s12872-024-03939-3
24. Fiorillo A, Demyttenaere K, Martiadis V, and Martinotti G. Editorial: Treatment resistant depression (TRD): epidemiology, clinic, burden and treatment. Front Psychiatry. (2025) 16:1588902.PMID: 40171309. doi: 10.3389/fpsyt.2025.1588902
25. Evans AK, Saw NL, Woods CE, Vidano LM, Blumenfeld SE, Lam RK, et al. Impact of high-fat diet on cognitive behavior and central and systemic inflammation with aging and sex differences in mice. Brain Behav Immun. (2024) 118:334–54. doi: 10.1016/j.bbi.2024.02.025
26. Liang ZK, Xiong W, Wang C, Chen L, Zou X, Mai JW, et al. Resolving neuroinflammatory and social deficits in ASD model mice: Dexmedetomidine downregulates NF-κB/IL-6 pathway via α2AR. Brain Behav Immun. (2024) 119:84–95. doi: 10.1016/j.bbi.2024.03.040
27. Okamoto N, Hoshikawa T, Honma Y, Chibaatar E, Ikenouchi A, Harada M, et al. Effect modification of tumor necrosis factor-α on the kynurenine and serotonin pathways in major depressive disorder on type 2 diabetes mellitus. Eur Arch Psychiatry Clin Neurosci. (2024) 274:1697–707. doi: 10.1007/s00406-023-01713-8
28. Sarrazin DH, Gardner W, Marchese C, Balzinger M, Ramanathan C, Schott M, et al. Prefrontal cortex molecular clock modulates development of depression-like phenotype and rapid antidepressant response in mice. Nat Commun. (2024) 15:7257. doi: 10.1038/s41467-024-51716-9
29. Sancho-Balsells A, Borràs-Pernas S, Flotta F, Chen W, Del Toro D, Rodríguez MJ, et al. Brain-gut photobiomodulation restores cognitive alterations in chronically stressed mice through the regulation of Sirt1 and neuroinflammation. J Affect Disord. (2024) 354:574–88. doi: 10.1016/j.jad.2024.03.075
30. Mbiydzenyuy NE and Qulu LA. Stress, hypothalamic-pituitary-adrenal axis, hypothalamic-pituitary-gonadal axis, and aggression. Metab Brain Dis. (2024) 39:1613–36. doi: 10.1007/s11011-024-01393-w
31. Zuloaga DG, Lafrican JJ, and Zuloaga KL. Androgen regulation of behavioral stress responses and the hypothalamic-pituitary-adrenal axis. Horm Behav. (2024) 162:105528. doi: 10.1016/j.yhbeh.2024.105528
32. Correia AS and Vale N. Tryptophan metabolism in depression: A narrative review with a focus on serotonin and kynurenine pathways. Int J Mol Sci. (2022) 23:8493. doi: 10.3390/ijms23158493
33. Dell’Osso L, Carmassi C, Mucci F, and Marazziti D. Depression, serotonin and tryptophan. Curr Pharm Des. (2016) 22:949–54. doi: 10.2174/1381612822666151214104826
34. Chen LM, Bao CH, Wu Y, Liang SH, Wang D, Wu LY, et al. Tryptophan-kynurenine metabolism: a link between the gut and brain for depression in inflammatory bowel disease. J Neuroinflammation. (2021) 18:135. doi: 10.1186/s12974-021-02175-2
35. Yang CH, Lv JJ, Kong XM, Chu F, Li ZB, Lu W, et al. Global, regional and national burdens of depression in adolescents and young adults aged 10–24 years, from 1990 to 2019: findings from the 2019 Global Burden of Disease study. Br J Psychiatry. (2024) 225:311–20. doi: 10.1192/bjp.2024.69
36. Cohen J, Mathew A, Dourvetakis KD, Sanchez-Guerrero E, Pangeni RP, Gurusamy N, et al. Recent research trends in neuroinflammatory and neurodegenerative disorders. Cells. (2024) 13:511. doi: 10.3390/cells13060511
37. Xu Y, Ning W, Zhang Y, Ba Y, Liu H, Liu L, et al. Associations between cardiovascular health (Life’s essential 8) and mental disorders. Clin Cardiol. (2024) 47:e70019. doi: 10.1002/clc.70019
38. Mehdi SMA, Costa AP, Svob C, Pan L, Dartora WJ, Talati A, et al. Depression and cognition are associated with lipid dysregulation in both a multigenerational study of depression and the National Health and Nutrition Examination Survey. Transl Psychiatry. (2024) 14:142. doi: 10.1038/s41398-024-02847-6
39. Sanchez C, Colson C, Gautier N, Noser P, Salvi J, Villet M, et al. Dietary fatty acid composition drives neuroinflammation and impaired behavior in obesity. Brain Behav Immun. (2024) 117:330–46. doi: 10.1016/j.bbi.2024.01.216
40. Favaretto E, Bedani F, Brancati GE, De Berardis D, Giovannini S, Scarcella L, et al. Synthesising 30 years of clinical experience and scientific insight on affective temperaments in psychiatric disorders: State of the art. J Affect Disord. (2024) 362:406–15. doi: 10.1016/j.jad.2024.07.011
41. Chen PH, Hsiao CY, Chiang SJ, Chung KH, and Tsai SY. Association of lipids and inflammatory markers with left ventricular wall thickness in patients with bipolar disorder. J Affect Disord. (2024) 358:12–8. doi: 10.1016/j.jad.2024.05.020
42. Shaikh SR, Beck MA, Alwarawrah Y, and MacIver NJ. Emerging mechanisms of obesity-associated immune dysfunction. Nat Rev Endocrinol. (2024) 20:136–48. doi: 10.1038/s41574-023-00932-2
43. Lee IT, Rees J, King S, Kim A, Cherlin T, Hinkle S, et al. Depression, anxiety, and risk of metabolic syndrome in women with polycystic ovary syndrome: A longitudinal study. J Clin Endocrinol Metab. (2024) 110(3):e750–e756. doi: 10.1210/clinem/dgae256
44. Alizadeh Pahlavani H. Possible role of exercise therapy on depression: Effector neurotransmitters as key players. Behav Brain Res. (2024) 459:114791. doi: 10.1016/j.bbr.2023.114791
45. Lehrer S and Rheinstein PH. Nonsteroidal anti-inflammatory drugs (NSAIDs) reduce suicidal ideation and depression. Discov Med. (2019) 28:205–12. doi: 10.14294/DM.2019.1549
46. Bringmann HC, Berghöfer A, Jeitler M, Michalsen A, Brunnhuber S, and Haller H. Meditation-based lifestyle modification in mild-to-moderate depression: outcomes and moderation effects of spirituality. J Integr Complement Med. (2024) 30:532–42. doi: 10.1089/jicm.2023.0179
47. Roman M and Irwin MR. Novel neuroimmunologic therapeutics in depression: A clinical perspective on what we know so far. Brain Behav Immun. (2020) 83:7–21. doi: 10.1016/j.bbi.2019.09.016
48. VanderWeele TJ. Principles of confounder selection. Eur J Epidemiol. (2019) 34:211–9. doi: 10.1007/s10654-019-00494-6
Keywords: C-reactive protein to lymphocyte ratio, depression, anxiety, inflammation, mental health, NHANES
Citation: Su Y, Ge Y, Yang H, Zhai G and Cheng X (2025) The link between systemic inflammation and mental disorders: a study on CLR, depression, and anxiety in a US cohort. Front. Psychiatry 16:1607982. doi: 10.3389/fpsyt.2025.1607982
Received: 08 April 2025; Accepted: 29 July 2025;
Published: 18 August 2025.
Edited by:
Vassilis Martiadis, Asl Napoli 1 Centro, ItalyReviewed by:
Marcin Siwek, Jagiellonian University, PolandFabiola Raffone, Asl Napoli 1 Centro, Italy
Copyright © 2025 Su, Ge, Yang, Zhai and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guojie Zhai, Z2p6aGFpXzE5ODFAMTYzLmNvbQ==; Xiaolan Cheng, Y2hlbmd4aWFvbGFuMzdAbmp1Y20uZWR1LmNu
 Yue Su
Yue Su Yuhan Ge2
Yuhan Ge2 Xiaolan Cheng
Xiaolan Cheng