- 1Kiang Wu Nursing College of Macau, Macao, Macao SAR, China
- 2Department of Nursing, Hunan Normal University, Changsha, Hunan, China
Purpose: This study was conducted to appraise the comparative efficacy of single non-pharmacological methods on depression for cognitive dysfunction patients utilizing network meta-analysis (NMA) and resolve ambiguities in existing literature to help practitioners accurately determine the efficacy and formulate the optimal therapeutic models.
Design: Systematic Review and Network meta-analysis.
Methods: PubMed, Embase, Cochrane, and Web of Science were searched. The Cochrane Handbook for Systematic Reviews of Interventions was used to assess the risk of bias in the included studies. Two investigators independently undertook data extraction and quality evaluation.
Result: Overall, 26 articles incorporating 10 single non-pharmacological interventions were identified. Compared to control, GAME (SMD = −1.00, 95% CrI = −1.70 to −0.39) and mindfulness (SMD = −0.58, 95% CrI = −0.99 to −0.17) significantly alleviated depressive symptoms. RTBC (SMD = −0.49, 95% CrI = −0.88 to −0.09) and MUSIC (SMD = −0.47, 95% CrI = −0.84 to −0.08) showed moderate effects, and PE (SMD = −0.37, 95% CrI = −0.67 to −0.09) showed small effects.
Conclusion: In this network meta-analysis, we synthesized 26 trials to quantify the isolated impact of 10 single non-pharmacological interventions on depressive symptoms. Against usual care (basic medical support, sham stimulation, or wait-list), GAME and mindfulness produced the largest and statistically credible reductions. Reminiscence-therapy-based care (RTBC) and music therapy (MUSIC) generated medium benefits, whereas physical exercise (PE) yielded a small yet significant effect. These findings were robust across both direct and indirect evidence, underscoring GAME and mindfulness as the most effective stand-alone non-pharmacological options for mitigating depression.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42024517077.
1 Introduction
Dementia represents a serious global health challenge, affecting more than 55 million people worldwide—a figure projected to nearly triple by 2050 (1, 2). Mild cognitive impairment (MCI), a precursor to dementia, has a high prevalence of about 15.4% among older adults in countries such as China and increases the risk of developing Alzheimer’s disease (3, 4). The clinical manifestation is frequently complicated by depressive symptoms. Approximately 32% of patients with dementia also experience depressive symptoms, and another 16% present with comorbid major depressive disorder (5). This bidirectional and pernicious relationship, where depression accelerates cognitive decline and cognitive impairment exacerbates depression, significantly reduces quality of life (6) and elevates mortality rates while also increasing distress, burden, and depression in caregivers (7).
Current first-line pharmacological treatments for depression in dementia patients, such as selective serotonin reuptake inhibitors (SSRIs) and mirtazapine, show limited efficacy and substantial risks (8). A meta-analysis indicates that no single antidepressant (e.g., SSRIs, mirtazapine, venlafaxine) outperforms usual care in treating depression among older adults with cognitive impairment (5). Moreover, these agents carry substantial risks. For example, tricyclics are associated with orthostatic hypotension, anticholinergic effects, and fall risks, while SSRIs increase the risk of hyponatremia, gastrointestinal bleeding, and prolonged QT intervals (particularly with citalopram). These limitations highlight the pressing need to develop safer, more effective non-pharmacological alternatives (9).
Non-pharmacological interventions are recommended as the first-line approach by major clinical guidelines due to their favorable safety profiles and potential to address the multifaceted nature of the condition (9, 10). Such interventions, including RTBC (Reminiscence therapy-based care program) (11), rTMS (repetitive transcranial magnetic stimulation), EA (electro-acupuncture) (12), CE (creative expression) (13), PE (physical exercise), CT (cognitive therapy) (14), MUSIC (music therapy), GAME (game training) (15), AAA (animal-assisted interventions) (16), mindfulness, and other forms, aim to improve patients’ emotional state, enhance mental health, and restore cognitive function by improving brain neuroplasticity.
Despite a compelling theoretical foundation and recognition in clinical guidelines, the evidence for non-pharmacological interventions in alleviating depression among older adults with cognitive dysfunction remains fragmented and inconclusive. Most randomized controlled trials (RCTs) have evaluated non-pharmacological interventions, with few head-to-head comparisons to determine their relative efficacy (15, 17). Previous systematic reviews and meta-analyses have obscured, rather than clarified, the comparative efficacy of single non-pharmacological interventions for depression (5, 18). They have often mixed heterogeneous or multicomponent non-pharmacological interventions, conflating distinct psychological, social, and physical mechanisms, thus obscuring the efficacy of single modalities.
To address this gap, we performed a systematic review and network meta-analysis (NMA) of RCTs evaluating single interventions for depressive symptoms in older adults with cognitive impairment. NMA, an advanced statistical technique, enables indirect comparisons across interventions by integrating direct and indirect evidence to assess relative effects without integrating head-to-head trials (19), overcoming the limitations of prior reviews that lacked ranked efficacy estimates for distinct, single-component interventions. This study elucidates the comparative effectiveness of single non-pharmacological approaches in dementia-related depression, providing evidence-based guidance for precise, individualized clinical strategies.
2 Methods
This study followed the PRISMA-2020 guidelines and the extension statement for NMA (PRISMA-NMA) (20) and the Cochrane Handbook for the Systematic Review of Interventions (21). The NMA was preregistered at PROSPERO (CRD42024517077).
2.1 Data sources and searches
Publications were retrieved through PubMed, Embase, Cochrane, and WOS from 1 Jan 2010 to 4 Sep 2025, without language restriction, using MeSH words and free words. This timeframe was selected to mitigate potential methodological heterogeneity that could arise from earlier studies, as research approaches and standards in this field have evolved significantly over the past two decades. The search strings in keywords involved study population (elderly, Cognitive Impairment), outcome (Depression), and study types (randomized controlled trials [RCTs]). The search strategy was personalized for each database. Detailed search strategies in PubMed are shown in Supplementary Table 1.
2.2 Study selection
EndNote 20 was employed to remove duplicate records, followed by primary screening of titles and abstracts and review of full-text documents. Study design and setting, baseline demographics of participants, specific information about the intervention, and reported outcomes were independently extracted by two reviewers. Discrepancies were tackled via discussion. The reference lists of potentially eligible publications were also screened.
The trials were selected based on the PICOS principles: 1) Population: the older adults (average age ≥ 60 years old) diagnosed with cognitive dysfunction (i.e., MCI or dementia from mild to severe). Patients with severe physical or mental comorbidities were excluded. 2) Interventions: Single non-pharmacological interventions, defined as structured, therapeutic modalities (e.g., physical exercise [PE], music therapy, mindfulness) delivered as standalone treatments. 3) Comparisons: The controls received usual care, sham intervention, or did not receive any treatment to ensure a single mode of intervention. 4) Outcomes: Depression was measured using five validated scales: Cornell Scale for Depression in Dementia (CSDD), Beck Depression Inventory (BDI), Geriatric Depression Scale (GDS), Self-rating Depression Scale (SDS), and Neuropsychiatric Inventory–Clinician Rating Scale (NPI-CR). Each scale employed its own response format and scoring system, where higher scores indicated more pronounced depressive symptoms. To enable pooled analysis, we converted all total scores into standardized mean differences (SMD) using the Hedges g formula, with negative values indicating greater symptom reduction. 5) Study design: RCTs, regardless of blinding and publication status. Non-RCTs were excluded. Besides, animal experiments, case reports, individual cases, research advances, conference articles, expert experience, and duplicates were excluded. Studies were not eliminated based on the duration of intervention or follow-up, nor were they restricted by language.
2.3 Risk of bias (quality) assessment
The Cochrane Collaboration risk-of-bias 2 (RoB 2) tool was used to assess the risk of bias in the included RCTs (22). The tool comprised five domains through which bias can be introduced (1): bias arising from the randomization process; (2) bias from deviations from intended interventions; (3) bias from missing outcome data; (4) bias in measurement of the outcome; and (5) bias in selection of the reported result. Each domain was evaluated with response options of “yes,” “probably yes,” “probably no,” “no,” or “no information,” and domain-level judgments subsequently informed an overall risk-of-bias rating of “low risk,” “some concerns,” or “high risk of bias”.
Study quality was assessed independently by two authors; and disagreements were resolved through discussion, with a third author consulted when consensus could not be reached.
2.4 Data extraction
The author, country, publication year, basic features of participants (stage of dementia, severity of depression), interventions (type, frequency, duration, and total sessions), and outcome measurement tools were extracted from each included RCT. A standardized, pre-piloted form was developed. Before formal use, two independent reviewers tested the form on three randomly selected trials; ambiguous items were discussed, reworded, and consolidated to improve clarity and completeness. The final version was then applied to all included studies.
2.5 Statistical analysis
2.5.1 Data synthesis and heterogeneity assessment
All analyses were performed in R (version 4.3.2) using the gemtc package for Bayesian NMA. Due to varying depression assessment scales across studies, SMDs with 95% confidence intervals (CIs) were calculated as the pooled effect size, interpreted as small (0.2), moderate (0.5), or large (0.8) effects (23). Heterogeneity was quantified using the I² statistic, categorized as none (0%), low (25%), moderate (50%), or high (75%). A sensitivity analysis based on pairwise meta-analysis was conducted to further explore the sources and impacts of heterogeneity.
2.5.2 NMA
A Bayesian random-effects NMA was conducted to compare the efficacy of single non-pharmacological interventions, integrating direct and indirect evidence. Interventions were ranked by surface under the cumulative ranking curve (SUCRA) values (24). Model convergence was verified using Gelman-Rubin diagnostics, with simulations employing four chains, 25,000 iterations (first 5,000 discarded), and a deviance information criterion (DIC) for model fit. Local inconsistency was assessed via node-splitting, with DIC differences <5 indicating consistency. To obtain more robust results, we adopted the random-effects model for NMA. The Bayesian model used default vague priors in the gemtc R package: relative treatment effects followed a normal distribution with a mean of 0 and a variance of (15 × 1.04)^2. The standard deviation of between-study heterogeneity followed a uniform distribution of 0 to 1.04, where 1.04 was automatically determined from the data and represented a significant difference on the outcome scale.
2.5.3 Subgroup and sensitivity analyses
Subgroup analyses were performed to explore the sources of heterogeneity, stratified by depression assessment instrument, baseline depression severity, and dementia stage; between-subgroup differences were tested using χ² (p > 0.05 indicating minimal explanatory variance). Scales with limited trials (e.g., SDS [k=1]; NPI-C [k=1]) were excluded from subgrouping. Sensitivity analyses were stratified by primary instrument and excluded high-risk-of-bias studies to evaluate robustness.
2.5.4 Publication bias and small-study effects
Publication bias was evaluated using comparison-adjusted funnel plots, plotting study effect sizes against standard errors for each intervention versus control. Asymmetry was tested with network-specific Egger’s regression (multi-level structure; p > 0.10 indicating no small-study effects). Standard funnel plots supplemented pairwise meta-analyses of interventions versus usual care.
3 Result
3.1 Identification of relevant studies
Following PRISMA guidelines (20), our search yielded 4687 records. After removal of duplicates and screening of titles and abstracts, 36 full-text articles were assessed for eligibility, among which 18 were excluded for not meeting the inclusion criteria, and 8 were excluded due to inaccessible full texts. In addition, 8 studies were identified through the reference lists of relevant reviews. Ultimately, 26 RCTs were included in the NMA, encompassing 1893 participants and 10 distinct single non-pharmacological interventions (Figure 1). Of these, 25 RCTs were published in English and one in Spanish.
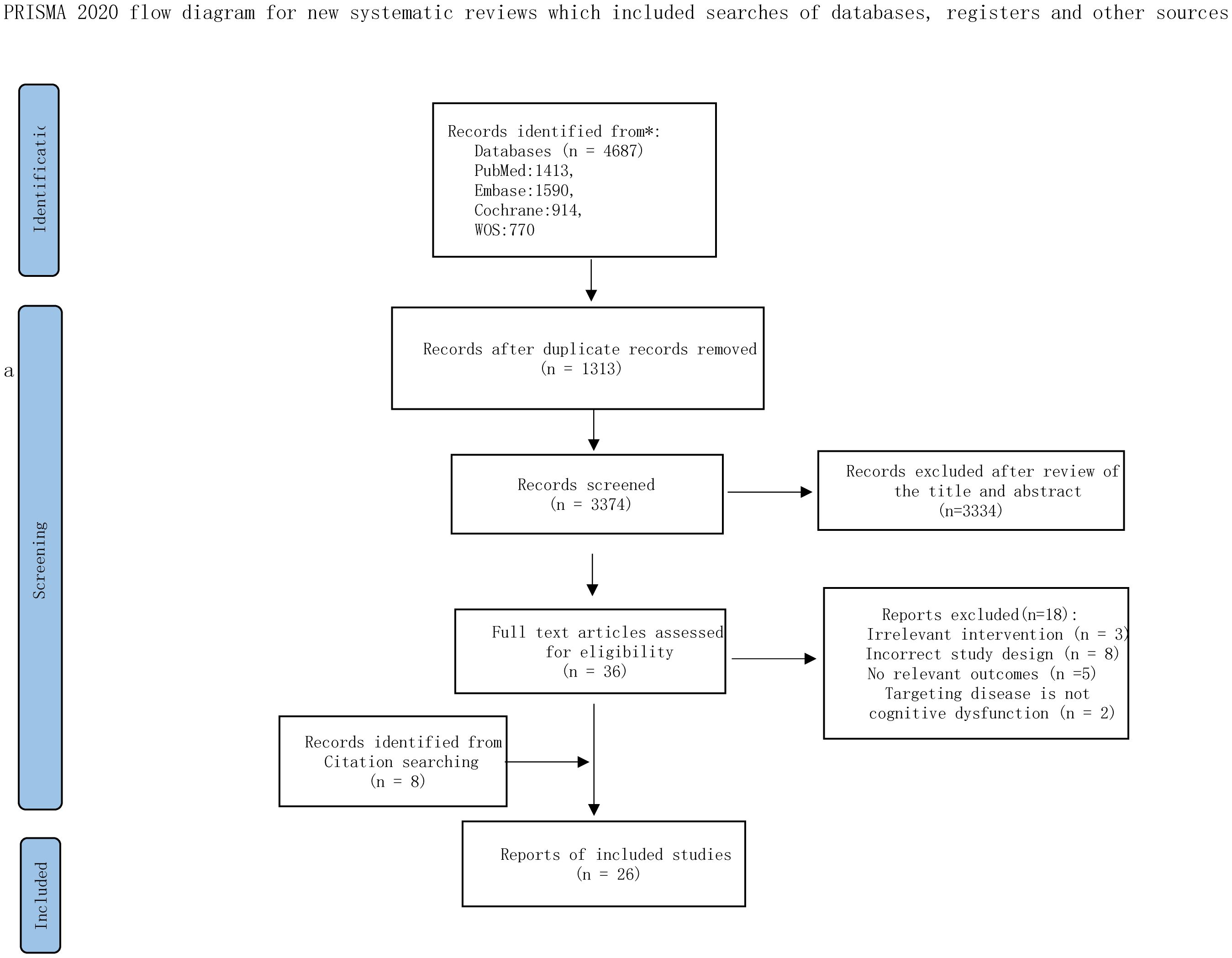
Figure 1. The PRISMA flowchart of selection process. Adapted from Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi:10.1136/bmj.n71. Licensed under CC BY 4.0.
3.2 Characteristics of the included RCTs
The 26 RCTs were conducted between 2010 and 2022, with 1893 participants in 11.
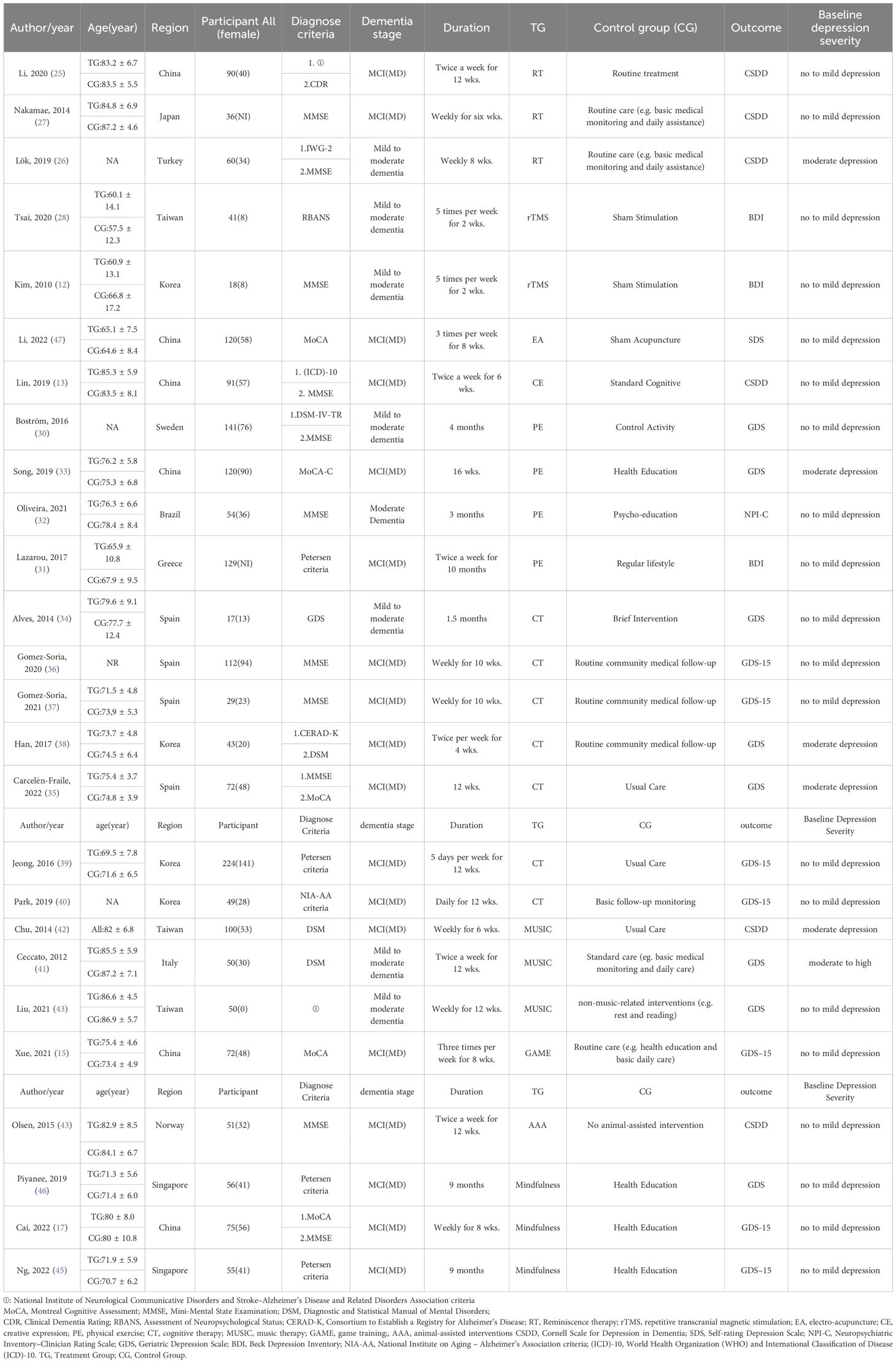
countries (Spain, China, Korea, Singapore, Sweden, Japan, Turkey, Italy, Brazil, Norway, and Greece). The participants were mostly aged 60–80 years old, and the commonly applied diagnostic criteria encompassed the Montreal Cognitive Assessment, Mini-Mental State Examination, and the DSM. Different countries and regions may follow revised guidelines or standards that they have recognized. The severity of cognitive dysfunction ranged from mild to severe.
Interventions included RTBC (25–27), rTMS (12, 28), EA (29), CE (13), PE (30–33), CT (34–40), MUSIC (41–43), GAME (15), AAA (44), and Mindfulness (17, 45, 46). Controls received usual care, health education, sham interventions, placebo, or no treatment. Intervention durations ranged from 1.5 to 10 months (e.g., twice weekly for 6–12 weeks for RTBC, MUSIC, and AAA; 5 times weekly for 2 weeks for rTMS). Depression was assessed using GDS/GDS-15 (k=15), CSDD (k=6), BDI (k=3), SDS (k=1), and NPI-CR (k=1). Detailed characteristics are listed in Table 1.
3.3 Risk of bias assessment
Risk of bias was evaluated using the RoB 2 tool (22).
Overall, 53.8% of studies (n = 14) had low risk, 30.8% (n = 8) raised some concerns, and 15.4% (n = 4) had high risk (Figure 2).
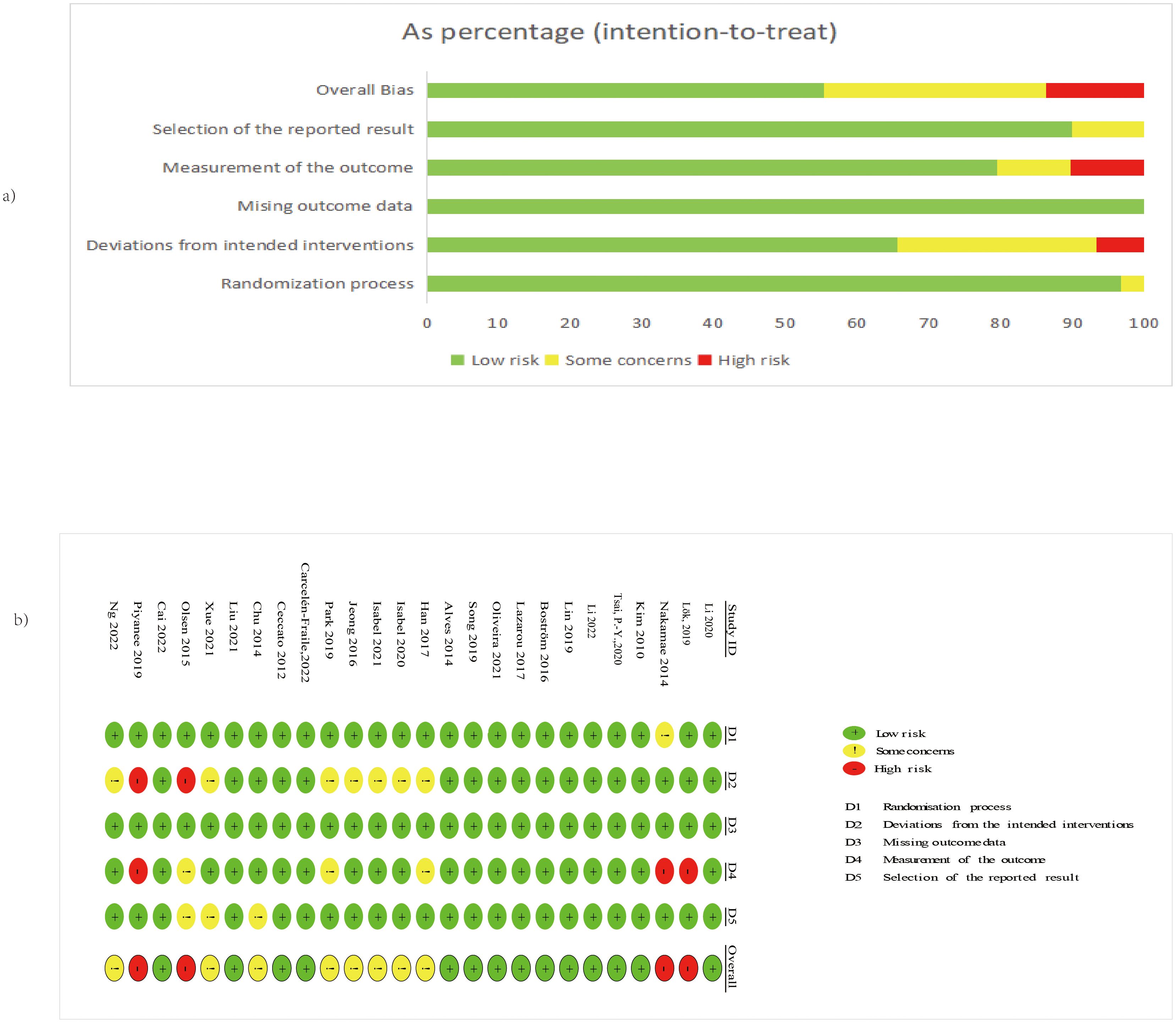
Figure 2. (A) The overall risk of bias for all included studies; (B) The risk of bias for each study. Adapted from Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi:10.1136/bmj.n71. Licensed under CC BY 4.0.
Domain-specific risks were predominantly low: randomization process (96.2% low), deviations from intended interventions (65.4% low), missing outcome data (100% low), measurement of the outcome (76.9% low), and selection of the reported result (88.5% low). High-risk studies were identified in the domains of deviations from interventions and outcome measurement. High-risk studies were Olsen et al. (2016) (domains D2) (27), (domains D4), Lök et al. (26) (domains D4), and (46) (domains D2 and D4); concerns primarily involved blinding and reporting (Figure 2) As shown in Supplementary Figure 1, the exclusion of high-risk studies did not materially alter the pooled effect estimates.
3.4 Results of NMA
The NMA included 26 RCTs comparing 10 interventions against the control. Global heterogeneity was low (I² = 2%) (Supplementary Figure 2).
Compared to control, GAME (SMD = −1.00, 95% CrI = −1.70 to −0.39), and mindfulness (SMD = −0.58, 95% CrI = −0.99 to −0.17) significantly alleviated depressive symptoms. RTBC (SMD = −0.49, 95% CrI = −0.88 to −0.09) and MUSIC (SMD = −0.47, 95% CrI = −0.84 to −0.08) showed moderate effects, and PE (SMD = −0.37, 95% CrI = −0.67 to −0.09) showed small effects. AAA (SMD = −0.66, 95% CrI = −1.40 to 0.07), rTMS (SMD = −0.16, 95% CrI = −0.78 to 0.48), EA (SMD = −0.20, 95% CrI = −0.76 to 0.36), CE (SMD = 0.02, 95% CrI = −0.58 to 0.62), and CT (SMD = −0.23, 95% CrI = −0.50 to 0.01) showed non-significant effects (Figure 3 and Supplementary Figure 3-9).
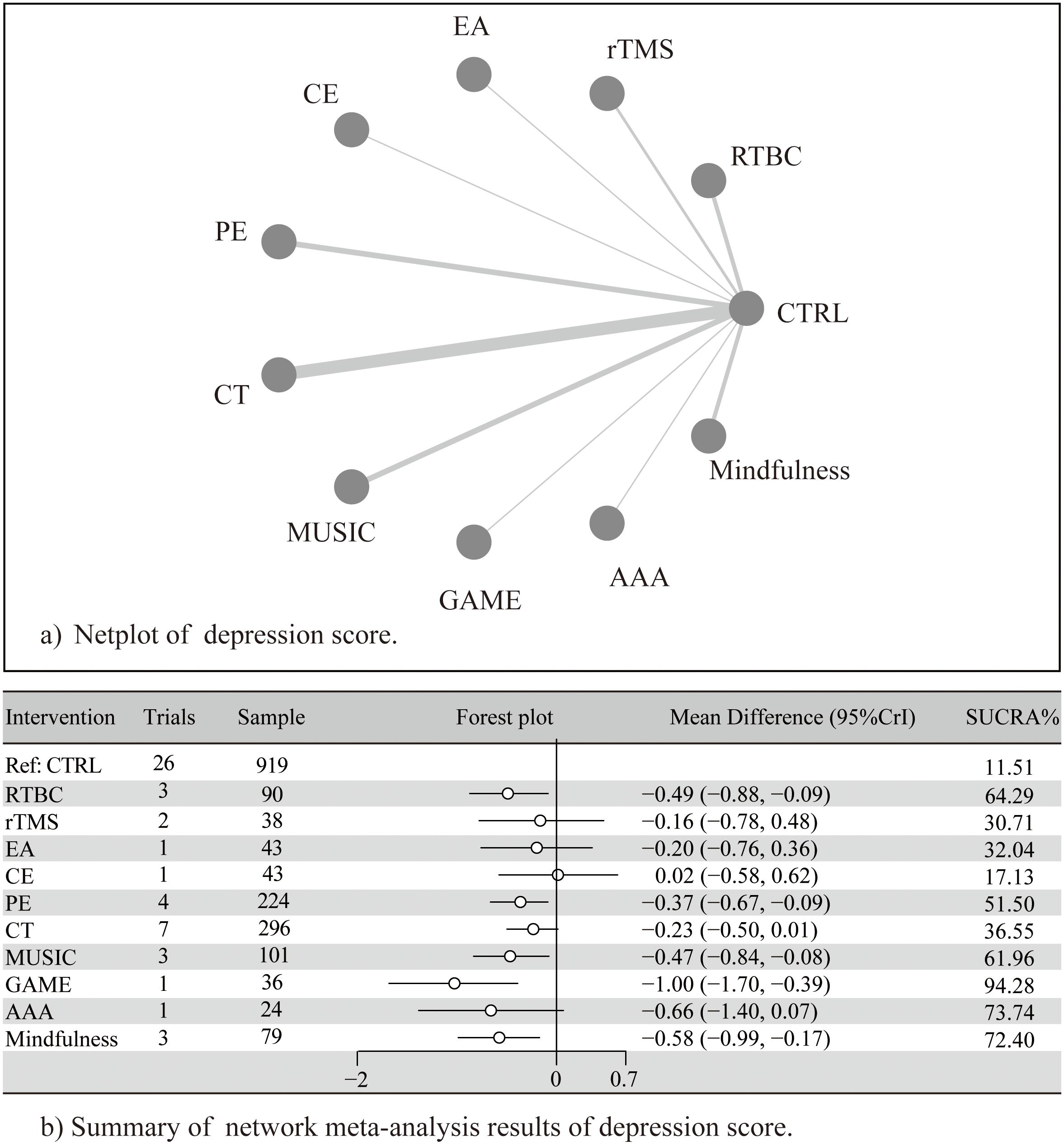
Figure 3. (A) Netplot of depression score; (B) Summary of network meta-analysis of depression score. Adapted from Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi:10.1136/bmj.n71. Licensed under CC BY 4.0.
The league table also revealed significant differences (Table 2). GAME outperformed CE (SMD = 1.05, CrI =0.17 to 1.95), and CT SMD = 0.79, CrI =0.1 to 1.5); SUCRA rankings indicated the following probability order for intervention efficacy: GAME (94.3%), AAA (73.7%), Mindfulness (72.4%), RTBC (64.3%), MUSIC (62.0%), PE (51.5%), CT (36.6%), EA (34.6%), rTMS (32.0%), CE (17.1%), and CTRL (11.5%). Notably, although AAA ranked second according to SUCRA, its effect estimate crossed the null line, suggesting uncertainty in its true efficacy and highlighting the need for cautious interpretation of rankings based on limited direct evidence. The ranking of effects of different non-pharmacological interventions and the description of corresponding treatments are detailed in Table 3 and Supplementary Figure 10.
3.5 Publication bias, subgroup analyses, and sensitivity analyses
3.5.1 Publication bias and small-study effects
Funnel plots showed symmetry (Supplementary Figure 11), supported by Egger’s test (p=0.43), indicating no evidence of publication bias or small-study effects. Outlying points may reflect study heterogeneity rather than bias.
3.5.2 Subgroup analyses by depression scale
Stratification by primary assessment scales (e.g., GDS, CSDD, BDI) yielded intervention-favoring effects (all 95% CIs excluded zero), with negligible between-subgroup heterogeneity (χ²=0.42, df=2, p=0.81), indicating that different scales minimally affected the results (Supplementary Figure 1).
3.5.3 Sensitivity analyses
In the NMA, pairwise comparisons revealed high heterogeneity for MUSIC (I² = 62%) and PE (I² = 68%). To evaluate the robustness of this finding, we conducted leave-one-out sensitivity analyses, sequentially omitting each study from the pairwise SMD estimate. Results indicated that exclusion of any single study did not substantially alter the pooled SMD, suggesting that the observed heterogeneity was acceptable and unlikely driven by a single study. These findings support the stability of the network estimates (Supplementary Figures S12-14).
4 Discussion
This NMA confirms the efficacy of non-pharmacological interventions in alleviating depressive symptoms in older adults with dementia. Four interventions (RTBC, PE, MUSIC, and mindfulness) outperformed the control, with GAME demonstrating superior efficacy across multiple outcome measures, positioning it as a potential first-line therapy. Compared with previous meta-analyses that identified effective combinations of non-pharmacological and pharmacological interventions, such as cognitive stimulation combined with rehabilitation (48) and cognitive stimulation combined with cholinesterase inhibitors (5), for alleviating depressive symptoms in individuals with dementia (predominantly without major depressive disorder), our findings highlight GAME and mindfulness as the most promising single-modality interventions. These differences may be attributed to variations in study populations (our analysis included participants with MCI and mild-to-severe dementia, whereas earlier studies focused exclusively on dementia), intervention scope (single versus combined modalities), and outcome assessment (we incorporated multiple validated scales without prioritizing a specific tool, while Watt et al. emphasized the Cornell Scale for depression in dementia). Such methodological distinctions, while enabling broader evidence synthesis in our study, also contributed to increased variability in comparative results.
4.1 Mechanisms of non-pharmacological interventions
For depression in older adults with cognitive impairment, such as MCI and dementia, non-pharmacological interventions exert their effects through multifaceted mechanisms that target cognitive, emotional, and social domains. These approaches often synergistically address neurodegeneration, inflammation, and psychosocial stressors inherent to cognitive impairment, offering safer alternatives to pharmacotherapy, which has shown limited efficacy and notable adverse effects (5, 9).
GAME represents a promising non-pharmacological strategy for mitigating the multifaceted challenges of dementia, particularly by fostering social engagement and emotional resilience. It promotes social interaction, emotional expression, and interpersonal communication among individuals with cognitive impairments, providing opportunities for releasing emotional stress and achieving a sense of accomplishment. In turn, it enhances self-efficacy and subjective well-being, critical psychological buffers that impede dementia-related cognitive decline and alleviate depressive symptoms. By creating structured and enjoyable collaborative environments, games enable older adults to re-establish interpersonal connections, thereby reducing isolation, fostering a renewed sense of agency, and indirectly mitigating the emotional toll caused by cognitive decline (15, 49).
MUSIC, mindfulness, and RTBC cultivate supportive environments that acknowledge personal experiences and emotional narratives, which foster emotional resilience and regulation and produce antidepressant benefits. For instance, MUSIC promotes well-being and reduces depressive symptoms by evoking pleasant memories, fostering peer support, and enhancing self-confidence and a sense of belonging. Empirical evidence confirms its value as a nursing intervention to enhance cognition, quality of life, and mood in Alzheimer’s patients (26, 50–52). Similarly, mindfulness alleviates psychological distress BY promoting present-moment awareness, while RTBC reconstructs positive self-narratives and strengthens interpersonal bonds via reminiscence.
PE, in contrast, exerts its antidepressant effects through robust neurobiological adaptations. It enhances hippocampal neuroplasticity, strengthens antioxidant defenses, and maintains cognitive-emotional homeostasis while suppressing neurodegeneration and inflammation, hallmarks of comorbid dementia and depression. At the molecular level, PE modulates the hypothalamic-pituitary-adrenal (HPA) axis, elevates neurotrophic factors, such as brain-derived neurotrophic factor (BDNF), and attenuates neuroinflammation, collectively improving mood (48, 53, 54).
4.2 The superiority of GAME therapy
The superior performance of GAME in this NMA, as evidenced by its large effect size (SMD = −1.00) compared to other interventions, may be attributed to its unique integration of cognitive, physical, and social elements in an engaging gamification design. Unlike single-targeted approaches, such as PE (primarily targets neurobiological pathways) or psychosocial therapies (focuses on emotional validation), GAME incorporates three elements: mental stimulation (e.g., memory and problem-solving tasks), motor activities (e.g., interactive movements in video or board games), and social collaboration (e.g., group games that promote interpersonal bonds). This multifaceted nature may enhance adherence through intrinsic motivation and enjoyment, leading to broader impacts on behavioral and psychological symptoms of dementia, including depression (49). A previous study has demonstrated that incorporating memory games (e.g., remembering the sequence and color of balls), coordination games, and solitaire or board games (e.g., poker, puzzles, Chinese letter games, and number guess games) into game training interventions can significantly reduce depressive symptoms, improve cognition, and enhance subjective well-being. This therapy outperforms usual care due to synergistic effects on brain plasticity, social relationships, and participant engagement 15. The entertainment value and adaptability of the GAME therapy make it a versatile tool, which may explain why it can promote continued engagement and holistic symptom relief in dementia populations, thus outperforming other therapies.
4.3 Strengths
This NMA advances evidence synthesis on non-pharmacological interventions for depressive symptoms in older adults with cognitive impairment, including MCI and dementia. Integrating 26 RCTs across multiple countries and over 2,000 participants, it comprehensively evaluates 10 single interventions, resolving fragmentation in prior reviews that conflate heterogeneous approaches. Methodological strengths include adherence to PRISMA guidelines, low heterogeneity, symmetrical funnel plots (no publication bias), and high consensus in RoB 2 assessments, with most studies showing low risk in key domains.
The results outline clear efficacy ratings, prioritizing game therapy, music therapy, and mindfulness, which provide person-centered guidance for clinicians, caregivers, and policymakers to tailor strategies based on dementia stage and depression severity. They may be safer alternatives to pharmacotherapy, which have limited efficacy and risks (e.g., SSRIs, tricyclics), providing guideline support for non-pharmacological options.
Future research should emphasize real-world feasibility, including caregiver training, long-term evaluations, and cost-benefit analysis. Personalized interventions for dementia patients with comorbid depression are essential, where funding policies and integration into standard care are critical. Overall, this NMA highlights the potential of single non-pharmacological modalities in enhancing outcomes, easing caregiver burden, and tackling the global dementia crisis through accessible, community-based care.
4.4 Limitations
This meta-analysis has several limitations. First, the included studies exhibited heterogeneity in participant cognitive dysfunction levels, ranging from MCI due to Alzheimer’s disease to mild-to-moderate and moderate dementia. This variability may affect the efficacy of non-pharmacological interventions, as cognitive and functional capacities differ across stages. For instance, interventions, like mindfulness and cognitive training, may be more effective for MCI or mild dementia, where cognitive reserve is relatively preserved, but they may require adaptations for moderate dementia. Due to the limited number of studies in each intervention and dementia stage, we could not stratify results by cognitive impairment level, restricting conclusions about optimal interventions for specific stages. Second, variability in intervention protocols (e.g., duration, frequency) and participant characteristics, including dementia stages, limits the generalizability of findings. Additionally, the network geometry was characterized by sparse data for some interventions and heavy reliance on indirect comparisons, which increases uncertainty in efficacy rankings. Furthermore, although the SUCRA values helped to summarize the relative ranking of the interventions, these rankings should be interpreted cautiously, especially for interventions (such as GAME and AAA) that were used by only one small trial. A high SUCRA value does not necessarily indicate confirmed efficacy, particularly when the associated credible intervals are wide or overlap with the null. The restricted scope of interventions focused on structured therapeutic modalities and did not encompass broader lifestyle domains, such as integrated management of physical activity, sedentary behavior, and sleep—areas that are critically linked to mental health in older adults (55–57). Future research should standardize intervention protocols and investigate their efficacy across distinct cognitive impairment stages to enhance clinical applicability. While no evidence of publication bias was detected through funnel plots and statistical tests, the small number of included RCTs constrains the robustness of these assessments. Future research should standardize intervention protocols and investigate their efficacy across distinct cognitive impairment stages to enhance clinical applicability.
5 Conclusions
Game therapy, music therapy, and mindfulness are the most effective single non-pharmacological interventions for reducing depressive symptoms relative to controls. Reminiscence therapy-based care provides moderate benefits, and physical exercise offers modest improvements. Methodological limitations call for caution in interpreting the findings, and high-quality RCTs are needed for validation. Future research should validate these findings through larger, direct-comparison trials and expand the comparative framework to include a broader range of lifestyle-oriented interventions.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
ZT: Conceptualization, Data curation, Writing – review & editing, Methodology, Resources, Writing – original draft, Investigation, Formal Analysis, Software. BL: Writing – review & editing, Resources, Methodology, Formal Analysis. LS: Writing – review & editing, Methodology. LL: Supervision, Writing – review & editing, Funding acquisition. Y-CC: Supervision, Writing – review & editing, Project administration.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by Hunan Provincial Natural Science Foundation (No. 2025JJ50667) and the Kiang Wu Nursing College of Macau Fund (No. 2025APR01). The funders had no role in study design, data collection and analysis, decision to publish, and manuscript preparation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2025.1608616/full#supplementary-material
Supplementary Table 1 | PubMed Search strategy
Supplementary Figure 1 | Subgroup analyses by depression scale. (A) Subgroup CSDD; (B) Subgroup GDS; (C) Subgroup BDI
Supplementary Figure 2 | Results of network meta-analysis
Supplementary Figure 3 | Comparative Analysis between trt 2 versus CTRL
Supplementary Figure 4 | Comparative Analysis between trt 3, 4 versus CTRL
Supplementary Figure 5 | Comparative Analysis between trt 5 versus CTRL
Supplementary Figure 6 | Comparative Analysis between trt 6 versus CTRL
Supplementary Figure 7 | Comparative Analysis between trt 7 versus CTRL
Supplementary Figure 8 | Comparative Analysis between trt 8 and 9 versus CTRL
Supplementary Figure 9 | Comparative Analysis between trt 11 and 12 versus CTRL
Supplementary Figure 10 | The figure of ranking probability of reduction of depression scores
Supplementary Figure 11 | Funnel plots
Supplementary Figure 12 | MUSIC Sensitivity Analyses. (A) Intervention position regression; (B) Instrument regression; (C) Duration meta-regression; (D) Dementia stage regression
Supplementary Figure 13 | PE sensitivity analyses. (A) Duration meta-regression; (B) Dementia stage regression; (C) Depression severity regression
Supplementary Figure 14 | Before and after removing high-risk articles
Abbreviations
Standard mean differences, (SMD); World Health Organization, (WHO); Mild cognitive impairment, (MCI); Alzheimer’s disease, (AD); Reminiscence therapy-based care program, (RTBC); repetitive transcranial magnetic stimulation ,(rTMS); electro-acupuncture, (EA); creative expression, (CE); physical exercise (PE); cognitive therapy, (CT); music therapy, (MUSIC); game training, (GAME); animal-assisted interventions, (AAA).
References
1. Cahill S. WHO’s global action plan on the public health response to dementia: some challenges and opportunities. Aging Ment Health. (2020) 24:197–9. doi: 10.1080/13607863.2018.1544213
2. Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. (2020) 396:413–46. doi: 10.1016/S0140-6736(20)30367-6
3. Deng Y, Zhao S, Cheng G, Yang J, Li B, Xu K, et al. The prevalence of mild cognitive impairment among chinese people: A meta-analysis. Neuroepidemiology. (2021) 55:79–91. doi: 10.1159/000512597
4. Xu H, Yang R, Dintica C, Qi X, Song R, Bennett DA, et al. Association of lifespan cognitive reserve indicator with the risk of mild cognitive impairment and its progression to dementia. Alzheimers Dement. (2020) 16:873–82. doi: 10.1002/alz.12085
5. Watt JA, Goodarzi Z, Veroniki AA, Nincic V, Khan PA, Ghassemi M, et al. Comparative efficacy of interventions for reducing symptoms of depression in people with dementia: systematic review and network meta-analysis. Bmj. (2021) 372:n532. doi: 10.1136/bmj.n532
6. Agüera-Ortiz L, García-Ramos R, Grandas Pérez FJ, López-Álvarez J, Montes Rodríguez JM, Olazarán Rodríguez FJ, et al. Depression in alzheimer’s disease: A delphi consensus on etiology, risk factors, and clinical management. Front Psychiatry. (2021) 12:638651. doi: 10.3389/fpsyt.2021.638651
7. Baharudin AD, Din NC, Subramaniam P, and Razali R. The associations between behavioral-psychological symptoms of dementia (BPSD) and coping strategy, burden of care and personality style among low-income caregivers of patients with dementia. BMC Public Health. (2019) 19:447. doi: 10.1186/s12889-019-6868-0
8. An H, Choi B, Park KW, Kim D-H, Yang D-W, Hong CH, et al. The effect of escitalopram on mood and cognition in depressive alzheimer’s disease subjects. J Alzheimers Dis. (2017) 55:727–35. doi: 10.3233/JAD-160225
9. Kales HC, Gitlin LN, and Lyketsos CG. Assessment and management of behavioral and psychological symptoms of dementia. Bmj. (2015) 350:h369. doi: 10.1136/bmj.h369
10. Bessey LJ and Walaszek A. Management of behavioral and psychological symptoms of dementia. Curr Psychiatry Rep. (2019) 21:66. doi: 10.1007/s11920-019-1049-5
11. Cheng C, Fan W, Liu C, Liu Y, and Liu X. Reminiscence therapy-based care program relieves post-stroke cognitive impairment, anxiety, and depression in acute ischemic stroke patients: a randomized, controlled study. Ir J Med Sci. (2021) 190:345–55. doi: 10.1007/s11845-020-02273-9
12. Kim BR, Kim DY, Chun MH, Yi JH, and Kwon JS. Effect of repetitive transcranial magnetic stimulation on cognition and mood in stroke patients: a double-blind, sham-controlled trial. Am J Phys Med Rehabil. (2010) 89:362–8. doi: 10.1097/PHM.0b013e3181d8a5b1
13. Lin R, Chen HY, Li H, and Li J. Effects of creative expression therapy on Chinese elderly patients with dementia: an exploratory randomized controlled trial. Neuropsychiatr Dis Treat. (2019) 15:2171–80. doi: 10.2147/NDT.S200045
14. Bahar-Fuchs A, Martyr A, Goh AM, Sabates J, and Clare L. Cognitive training for people with mild to moderate dementia. Cochrane Database Syst Rev. (2019) 3:Cd013069. doi: 10.1002/14651858.CD013069.pub2
15. Xue B, Xiao A, Luo X, and Li R. The effect of a game training intervention on cognitive functioning and depression symptoms in the elderly with mild cognitive impairment: A randomized controlled trial. Int J Methods Psychiatr Res. (2021) 30:e1887. doi: 10.1002/mpr.1887
16. Hu M, Zhang P, Leng M, Li C, and Chen L. Animal-assisted intervention for individuals with cognitive impairment: A meta-analysis of randomized controlled trials and quasi-randomized controlled trials. Psychiatry Res. (2018) 260:418–27. doi: 10.1016/j.psychres.2017.12.016
17. Cai ZZ, Lin R, Wang XX, Yan YJ, and Li H. Effects of mindfulness in patients with mild cognitive impairment with insomnia: A double-blind randomized controlled trial. Geriatr Nurs. (2022) 47:239–46. doi: 10.1016/j.gerinurse.2022.08.001
18. Sun Y, Ji M, Leng M, Li X, Zhang X, and Wang Z. Comparative efficacy of 11 non-pharmacological interventions on depression, anxiety, quality of life, and caregiver burden for informal caregivers of people with dementia: A systematic review and network meta-analysis. Int J Nurs Stud. (2022) 129:104204. doi: 10.1016/j.ijnurstu.2022.104204
19. Hoaglin DC, Hawkins N, Jansen JP, Scott DA, Itzler R, Cappelleri JC, et al. Conducting indirect-treatment-comparison and network-meta-analysis studies: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 2. Value Health. (2011) 14:429–37. doi: 10.1016/j.jval.2011.01.011
20. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. (2015) 162:777–84. doi: 10.7326/M14-2385
21. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. (2019) 10:Ed000142. doi: 10.1002/14651858.ED000142
22. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. Bmj. (2019) 366:l4898. doi: 10.1136/bmj.l4898
23. Higgins JP, Thompson SG, Deeks JJ, and Altman DG. Measuring inconsistency in meta-analyses. Bmj. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
24. Salanti G, Ades AE, and Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. (2011) 64:163–71. doi: 10.1016/j.jclinepi.2010.03.016
25. Li M, Lyu JH, Zhang Y, Gao M, Li R, Mao P, et al. Efficacy of group reminiscence therapy on cognition, depression, neuropsychiatric symptoms, and activities of daily living for patients with alzheimer disease. J Geriatr Psychiatry Neurol. (2020) 33:272–81. doi: 10.1177/0891988719882099
26. Lök N, Bademli K, and Selçuk-Tosun A. The effect of reminiscence therapy on cognitive functions, depression, and quality of life in Alzheimer patients: Randomized controlled trial. Int J Geriatr Psychiatry. (2019) 34:47–53. doi: 10.1002/gps.4980
27. Nakamae T, Yotsumoto K, Tatsumi E, and Hashimoto T. Effects of productive activities with reminiscence in occupational therapy for people with dementia: A pilot randomized controlled study*. Hong Kong J Occup Ther. (2014) 24:13–9. doi: 10.1016/j.hkjot.2014.01.003
28. Tsai PY, Lin WS, Tsai KT, Kuo CY, and Lin PH. High-frequency versus theta burst transcranial magnetic stimulation for the treatment of poststroke cognitive impairment in humans. J Psychiatry Neurosci. (2020) 45:262–70. doi: 10.1503/jpn.190060
29. Huang L, Yin X, Li W, Cao Y, Chen Y, Lao L, et al. Effects of acupuncture on vascular cognitive impairment with no dementia: A randomized controlled trial. J Alzheimers Dis. (2021) 81:1391–401. doi: 10.3233/JAD-201353
30. Boström G, Conradsson M, Hörnsten C, Rosendahl E, Lindelöf N, Holmberg H, et al. Effects of a high-intensity functional exercise program on depressive symptoms among people with dementia in residential care: a randomized controlled trial. Int J Geriatr Psychiatry. (2016) 31:868–78. doi: 10.1002/gps.4401
31. Lazarou I, Parastatidis T, Tsolaki A, Gkioka M, Karakostas A, Douka S, et al. International ballroom dancing against neurodegeneration: A randomized controlled trial in greek community-dwelling elders with mild cognitive impairment. Am J Alzheimers Dis Other Demen. (2017) 32:489–99. doi: 10.1177/1533317517725813
32. Oliveira AM, Radanovic M, Mello PCH, Buchain PC, Vizzotto ADB, Harder J, et al. Adjunctive therapy to manage neuropsychiatric symptoms in moderate and severe dementia: randomized clinical trial using an outpatient version of tailored activity program. J Alzheimers Dis. (2021) 83:475–86. doi: 10.3233/JAD-210142
33. Song D and Yu DSF. Effects of a moderate-intensity aerobic exercise programme on the cognitive function and quality of life of community-dwelling elderly people with mild cognitive impairment: A randomised controlled trial. Int J Nurs Stud. (2019) 93:97–105. doi: 10.1016/j.ijnurstu.2019.02.019
34. Alves J, Alves-Costa F, Magalhães R, Gonçalves OF, and Sampaio A. Cognitive stimulation for Portuguese older adults with cognitive impairment: a randomized controlled trial of efficacy, comparative duration, feasibility, and experiential relevance. Am J Alzheimers Dis Other Demen. (2014) 29:503–12. doi: 10.1177/1533317514522541
35. Carcelén-Fraile MDC, Llera-DelaTorre AM, Aibar-Almazán A, Afanador-Restrepo DF, Baena-Marín M, Hita-Contreras F, et al. Cognitive stimulation as alternative treatment to improve psychological disorders in patients with mild cognitive impairment. J Clin Med. (2022) 11:39–47. doi: 10.3390/jcm11143947
36. Gomez-Soria I, Peralta-Marrupe P, and Plo F. Cognitive stimulation program in mild cognitive impairment A randomized controlled trial. Dement Neuropsychol. (2020) 14:110–7. doi: 10.1590/1980-57642020dn14-020003
37. Gómez-Soria I, Andrés Esteban EM, Gómez Bruton A, and Peralta-Marrupe P. Análisis del efecto a largo plazo de un programa de estimulación cognitiva en mayores con deterioro cognitivo leve en Atención Primaria: ensayo controlado aleatorizado. Atención Primaria. (2021) 53:102053. doi: 10.1016/j.aprim.2021.102053
38. Han JW, Son KL, Byun HJ, Ko JW, Kim K, Hong JW, et al. Efficacy of the Ubiquitous Spaced Retrieval-based Memory Advancement and Rehabilitation Training (USMART) program among patients with mild cognitive impairment: a randomized controlled crossover trial. Alzheimers Res Ther. (2017) 9:39. doi: 10.1186/s13195-017-0264-8
39. Jeong JH, Na HR, Choi SH, Kim J, Na DL, Seo SW, et al. Group- and home-based cognitive intervention for patients with mild cognitive impairment: A randomized controlled trial. Psychother Psychosom. (2016) 85:198–207. doi: 10.1159/000442261
40. Park J, Kim SE, Kim EJ, Lee BI, Jeong JH, Na HR, et al. Effect of 12-week home-based cognitive training on cognitive function and brain metabolism in patients with amnestic mild cognitive impairment. Clin Interv Aging. (2019) 14:1167–75. doi: 10.2147/CIA.S200269
41. Ceccato E, Vigato G, Bonetto C, Bevilacqua A, Pizziolo P, Crociani S, et al. STAM protocol in dementia: a multicenter, single-blind, randomized, and controlled trial. Am J Alzheimers Dis Other Demen. (2012) 27:301–10. doi: 10.1177/1533317512452038
42. Chu H, Yang CY, Lin Y, Ou K-L, Lee T-Y, O’Brien AP, et al. The impact of group music therapy on depression and cognition in elderly persons with dementia: a randomized controlled study. Biol Res Nurs. (2014) 16:209–17. doi: 10.1177/1099800413485410
43. Liu MN, Liou YJ, Wang WC, Su K-C, Yeh H-L, Lau C, et al. Group music intervention using percussion instruments to reduce anxiety among elderly male veterans with alzheimer disease. Med Sci Monit. (2021) 27:e928714. doi: 10.12659/MSM.928714
44. Olsen C, Pedersen I, Bergland A, Enders-Slegers MJ, Patil G, and Ihlebaek C. Effect of animal-assisted interventions on depression, agitation and quality of life in nursing home residents suffering from cognitive impairment or dementia: a cluster randomized controlled trial. Int J Geriatr Psychiatry. (2016) 31:1312–21. doi: 10.1002/gps.4436
45. Ng TKS, Tan XR, Todd M, Chen AC-C, Feng L, Lu Y, et al. Effects of mindful awareness practice (MAP) on subclinical depressive and anxiety symptoms and general cognitive function in older adults with mild cognitive impairment: A 5-year follow-up of the MAP-randomized controlled trial. J Alzheimers Dis. (2022) 90:1677–88. doi: 10.3233/JAD-220641
46. Piyanee P, Kowitlawakul Y, Lopez V, Tang CT, Hoek KE, Gan GL, et al. The effects of mindfulness and health education programs on the emotional state and cognitive function of elderly individuals with mild cognitive impairment: A randomized controlled trial. J Clin Neurosci. (2019) 68:211–7. doi: 10.1016/j.jocn.2019.05.031
47. Li X, La L, Lu L, Yan L, Deng K, Li Z, et al. Comparative efficacy of acupuncture-related techniques for mild cognitive impairment: A Bayesian network analysis. Front Neurol. (2022) 13:942682. doi: 10.3389/fneur.2022.942682
48. Burley CV, Burns K, Lam BCP, and Brodaty H. Nonpharmacological approaches reduce symptoms of depression in dementia: A systematic review and meta-analysis. Ageing Res Rev. (2022) 79:101669. doi: 10.1016/j.arr.2022.101669
49. Zheng J, Chen X, and Yu P. Game-based interventions and their impact on dementia: a narrative review. Australas Psychiatry. (2017) 25:562–5. doi: 10.1177/1039856217726686
50. Lin TH, Liao YC, Tam KW, Chan L, and Hsu TH. Effects of music therapy on cognition, quality of life, and neuropsychiatric symptoms of patients with dementia: A systematic review and meta-analysis of randomized controlled trials. Psychiatry Res. (2023) 329:115498. doi: 10.1016/j.psychres.2023.115498
51. Gonzalez J, Mayordomo T, Torres M, Sales A, and Meléndez JC. Reminiscence and dementia: a therapeutic intervention. Int Psychogeriatr. (2015) 27:1731–7. doi: 10.1017/S1041610215000344
52. Ito E, Nouchi R, Dinet J, Cheng CH, and Husebø BS. The effect of music-based intervention on general cognitive and executive functions, and episodic memory in people with mild cognitive impairment and dementia: A systematic review and meta-analysis of recent randomized controlled trials. Healthcare (Basel). (2022) 10:1462. doi: 10.3390/healthcare10081462
53. Heissel A, Heinen D, Brokmeier LL, Skarabis N, Kangas M, Vancampfort D, et al. Exercise as medicine for depressive symptoms? A systematic review and meta-analysis with meta-regression. Br J Sports Med. (2023) 57:1049–57. doi: 10.1136/bjsports-2022-106282
54. Huang X, Zhao X, Li B, Cai Y, Zhang S, Wan Q, et al. Comparative efficacy of various exercise interventions on cognitive function in patients with mild cognitive impairment or dementia: A systematic review and network meta-analysis. J Sport Health Sci. (2022) 11:212–23. doi: 10.1016/j.jshs.2021.05.003
55. Liang W, Wang Y, Huang Q, Shang B, Su N, Zhou L, et al. Adherence to 24-hour movement guidelines among chinese older adults: prevalence, correlates, and associations with physical and mental health outcomes. JMIR Public Health Surveill. (2024) 10:e46072. doi: 10.2196/46072
56. Wang X, Ye X, and Chen Y. Association between socioeconomic status and healthy lifestyle with depressive symptoms in older adults: Evidence from five prospective cohort studies. Am J Prev Med. (2026) 2025:108138. doi: 10.1016/j.amepre.2025.108138
Keywords: cognitive dysfunction, depression, aging, non-pharmacological intervention, network meta-analysis
Citation: Tan Z, Li B, Su L, Liu L and Chuang Y-C (2025) Single non-pharmacological intervention of depression in the elderly with cognitive dysfunction: a systematic review and network meta-analysis. Front. Psychiatry 16:1608616. doi: 10.3389/fpsyt.2025.1608616
Received: 09 April 2025; Accepted: 10 November 2025; Revised: 28 October 2025;
Published: 25 November 2025.
Edited by:
Taolin Chen, Sichuan University, ChinaReviewed by:
Chunchen Xiang, Capital Medical University, ChinaEjdane Coşkun, Osmaniye Korkut Ata University, Türkiye
Copyright © 2025 Tan, Li, Su, Liu and Chuang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yao-Chen Chuang, amFzb25jaHVhbmdAa3duYy5lZHUubW8=; Lihua Liu, TGxoMTM5NUAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
‡ORCID: Zhengyi Tan, orcid.org.0009-0004-9704-0830
 Zhengyi Tan
Zhengyi Tan Baiyun Li1,2†
Baiyun Li1,2† Lanxin Su
Lanxin Su Yao-Chen Chuang
Yao-Chen Chuang