- 1The Second School of Clinical Medicine, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China
- 2Department of Psychiatry, The Cancer Hospital of the University of Chinese Academy of Sciences (Zhejiang Cancer Hospital), Hangzhou, Zhejiang, China
With the aging global population, the prevalence of late-life depression (LLD) has shown a significant upward trend. The prolonged presence of a depressed mood can lead to feelings of hopelessness and helplessness, thereby enhancing suicidal impulses and behaviors, which seriously affect the physical and mental health of the older population. However, current research on suicide has mainly focused on adolescents and adults, resulting in a lack of data on the identification, assessment, and intervention of suicide risk in LLD. LLD treatments include psychotherapy, medication, physiotherapy, and Internet intervention techniques. First-line antidepressants, in addition to cognitive behavioral therapy and repetitive transcranial magnetic stimulation, have been shown to be effective in patients with LLD. In this study, we review the research progress in the following three aspects: symptomatological characteristics, assessment tools, and treatment methods of suicide in LLD, providing a reference for clinical workers and researchers in clinical practice.
1 Introduction
The world is facing rapid aging, with one out of every six people worldwide projected to be over the age of 64 by 2050 (1). As the population ages, 28.4% global older population experience depression (2). LLD is the occurrence of major depressive disorder in adults 60 years of age or older (3). Compared to younger patients with depression, late-life depression (LLD) is characterized by a lack of interest, somatic symptoms, sleep disturbances, and extensive cognitive deficits (3, 4). Social isolation, bereavement, and chronic illnesses are common risk factors for LLD (5). The symptoms of LLD are frequently masked by physical illness and cognitive decline, resulting in lower rates of diagnosis and treatment (6). Negative perceptions and behaviors are highly prevalent risk symptoms for LLD, and patients with LLD are more suicidal than individuals in other age groups (7). They are also more likely to use highly lethal methods, and suicide rates are higher among older men, with >50% of older adults who die by suicide meeting the criteria for major depression (8), which is far more than any other mental illness. Although a large body of research has been conducted on suicide among adolescents and adults, relatively little international research has been conducted on suicide among LLD. In this study, we review the symptomatological characteristics, assessment tools, and treatment advances for suicide in patients with LLD.
2 Symptomatological characteristics of suicide in LLD
2.1 Comorbidities
Multiple somatic diseases are common in the older population. Islam et al. found that 82% of the older population had at least one chronic disease, and more than 52% had at least two chronic diseases (9). Moreover, multiple somatic diseases have been associated with an increased risk of LLD (10). Among these, cardiovascular disease (CVD), type 2 diabetes, stroke, chronic pain, and cognitive dysfunction are the main diseases that commonly affect suicide in patients with LLD.
CVD is the most common comorbidity in the older population, and depressive symptoms are associated with an elevated risk of CVD (11). Simultaneously, patients with CVD are also more likely to have depressive symptoms (12), which may be related to the significant psychological burden of impaired cardiovascular function and the limitations in activity after the impairment. Studies have shown that 29% of patients with coronary heart disease (CHD) require antidepressant treatment within 1 year (13), which further improves their mental status and reduces the high levels of hopelessness, depression, and low self-esteem associated with CHD.
Type 2 diabetes mellitus (T2DM), another condition that requires long-term management, is associated with a 24% increased risk of developing depression (14), and the relative risk of suicide in patients with T2DM may be significantly increased following an episode of depressive disorder. The results of the meta-analysis further showed that patients with T2DM had higher rates of suicide and mortality than individuals without diabetes, regardless of whether major depressive disorder preceded the onset of T2DM (15), which may be related to the chronic nature of T2DM and long-term use of medication.
Older adults are at higher risk of stroke, and the prevalence of post-stroke depression ranges from 25% to 79% (16, 17). When organic brain lesions caused by stroke involve brain regions related to mood, including the frontal lobe, they can cause imbalances in neurotransmitters such as serotonin and dopamine (18), which can lead to depressive mood. After a stroke, the body initiates an inflammatory response, and the expression of inflammatory cytokines responsible for regulating the neuroendocrine stress system may ultimately inhibit neurotrophic factors in the brain (19), thereby increasing the risk of depression and suicide. Stroke survivors have a 73% higher risk of suicide than non-stroke survivors (20), which may be related to physical dysfunction and a lengthy rehabilitation process.
Chronic pain, such as arthritis and lower back pain, is prevalent in older adults. A retrospective study found that the prevalence of depression among patients with chronic pain in a chronic pain management clinic in South Africa was 32% (21). Increased feelings of pain frequently exacerbate depressive mood and suicidal risk, with approximately one-quarter of patients with chronic pain experiencing suicidal ideation in the past 2 weeks (22), which may be related to prolonged physical discomfort and social isolation. The interaction between various chronic diseases and LLD raises great concerns regarding the increased risk of death.
The older population usually faces cognitive decline, and studies have found that approximately 30% of people aged > 65 years have varying degrees of cognitive dysfunction (23). This not only affects their ability to deal with complex emotions and problems of daily living but also exacerbates their feelings of helplessness and hopelessness, which may accelerate the onset and progression of LLD. Individuals with LLD frequently exhibit cognitive impairments, including impaired executive functioning (24) and decision-making (25) etc. A large cohort study based on people aged >50 years in the United Kingdom with 16 years of follow-up found that linear changes in depressive symptoms were associated with accelerated memory decline. Conversely, memory decline over time was linked to changes in depressive symptoms (26). Notably, existing research confirms that executive function deficits (27) and memory impairment are more pronounced in suicide attempters (28). It follows that early identification of cognitive dysfunction in LLD is key to predicting and preventing suicidal behavior in clinical practice. Patients with LLD with lower cognitive function scores tend to be accompanied by higher suicide risk scores, which highlights the importance of implementing joint screening for cognitive function interventions and suicide risk in individuals with LLD.
2.2 Loneliness and social isolation
Socioenvironmental factors influence suicidal ideation in patients with LLD. Loneliness, despair, and depressive symptoms were found to be closely associated with suicide in older people, as demonstrated in a case-control study on suicide among rural Chinese older adults (29). Loneliness is a subjective emotional experience. On a psychological level, older adults can experience various negative emotions, including sadness and despair, due to loneliness. A longitudinal study in the United Kingdom found that after adjusting for all factors, for every 1-point increase in loneliness scores, depressive symptom severity scores increased by an average of 0.16 (30). Persistent loneliness leads to persistent depressive symptoms, which may form a vicious cycle between depression and loneliness, making suicidal thoughts more likely to develop and intensify. At a physiological level, loneliness triggers various alterations in neuroendocrine and immune functions. Chronically lonely older adults tend to have higher levels of stress hormones such as cortisol (31). Chronic elevation of these hormones affects the balance of neurotransmitters in the brain, particularly serotonin and dopamine, which are closely related to mood regulation and can lead to deeper depression and despair in older people, thereby increasing the risk of suicide. Concurrently, long-term social isolation will enhance the activation of the hypothalamic-pituitary-adrenocortical axis, with certain neuroendocrine components potentially reaching the immune organs through the nerve fibers of the sympathetic nervous system. This process may affect immune function, making older people more prone to various diseases (32). The interactions among physical discomfort, psychological loneliness, and depression will make them less tolerant of life, increasing their suicidal tendencies.
The main commonality of social isolation is the lack of social connectedness, particularly in older adults experiencing bereavement, declining physical health and independence, and estrangement from family or friends, which ultimately drives the development of LLD (33) and may lead to impairment of their cognitive functioning. A review showed that most Asian older adults are made to perceive themselves as a burden due to factors such as social isolation and rising healthcare costs (34). This sense of despair can lead them to feel that suicide is the ultimate means of escaping unbearable psychological pain. Evidence suggests a causal relationship between social isolation and suicide; in contrast, adequate social support has a significant protective effect against suicidal behavior (35). Although not all problems should be interpreted as symptoms of depression, early identification and treatment of affective disorders and improved social support remain key interventions for reducing the risk of suicide in older adults (36). Social and mental health problems should be considered synergistic factors to improve the reliability of suicidal ideation assessments.
3 Assessment tools for suicide risk in LLD
3.1 Common assessment scales
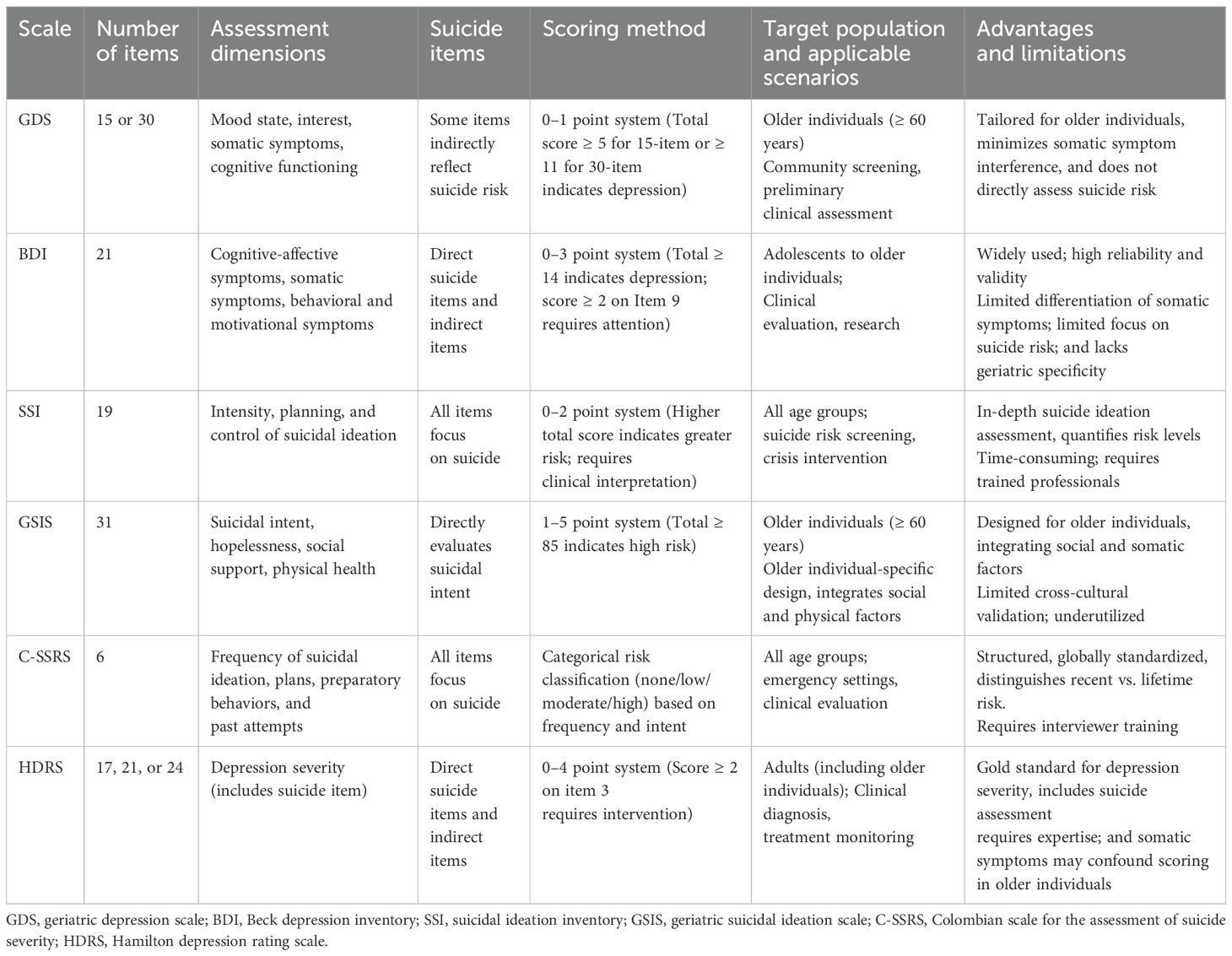
Various assessment tools have been widely used to assess suicide risk in patients with LLD, including examiner-rating and self-rating scales. The following are a few commonly used assessment scales, as shown in Table 1:
The geriatric depression scale (GDS), developed by Yesavage et al. in 1982 (37), is a depression assessment tool designed specifically for older adults with simple, easy-to-understand questions. The GDS-30 has been shown to be applicable to large-scale community-dwelling older Chinese adults with or without mild cognitive impairment (38).
The Beck depression inventory (BDI), developed by Beck et al. in 1961 (39), is a psychological assessment tool widely used to evaluate the degree of depression in individuals. The BDI-II has some modifications to the original scale to better meet the current diagnostic criteria for depression. Studies have applied it to clinical patients with LLD, and the results have shown high reliability (40, 41).
The scale for suicide ideation (SSI) was developed by Beck et al. in 1979 to measure the severity of suicidal ideation for various populations (42). In 1999, Beck et al. published a subscale for measuring suicidal ideation at the worst point in a patient’s life (suicide ideation at its worst point [SSI-W]), which was superior in predicting suicide (43).
The geriatric suicide ideation scale (GSIS), developed by Heisel et al. in 2006 (44), is a mental health assessment tool designed specifically for older adults. The GSIS has strong measurement properties for community-dwelling older adults, with implications for monitoring suicide risks (45).
The Columbia suicide severity rating scale (C-SSRS) is considered the gold standard for clinicians to assess suicide risk. The C-SSRS is highly predictive of suicide risk in older adults by capturing sensitivity regarding past suicidal behaviors and current suicidal intent, which informs some of its use in older adults (46).
The Hamilton depression rating scale (HDRS), published by Hamilton in 1960 (47), assesses patients’ depressive symptoms in multiple dimensions, including suicidal intent and behavior. Several modified versions of HDRS have been proposed. As noted by Carroll (48), the effectiveness of various versions is remarkable.
Based on the comparative analysis of suicide assessment instruments presented in the table, the following conclusions can be drawn regarding their characteristics and clinical applications: Overall, the above charts and comparisons of suicide entries suggest that the SSI shows particular strength in capturing detailed suicidal ideation, the GSIS excels in integrating social determinants, and the C-SSRS is the most immediate in assessing suicide risk, demonstrating behavioral strengths in clinical practice.
In clinical and research settings, these scales can be used in combination according to the specific situation to achieve the best assessment effect. In conclusion, there are few scales developed for the older population in the field of suicide assessment, and further research can be conducted on the suicide risk of older patients with depression and the establishment of norms in the older population to provide a tool for accurate measurement in the future.
3.2 Novel suicide risk assessment
Although accurate assessment of suicide risk in patients with LLD is the key to suicide prevention, it currently relies mainly on clinical interviews and scale assessments, which have limitations such as high subjectivity. Objective, quantifiable biomarkers and neuroimaging-based assessment methods and intelligent digital phenotype monitoring have become research hotspots, aiming to provide more accurate risk stratification and intervention targets.
Evidence suggests that elevated levels of inflammatory markers are associated with an increased risk of suicidal behavior in different populations (49). Recent studies have suggested that combinations of biomarkers such as CRP, IL-6, TNF-α and CXCL-2 may have predictive value for suicide risk (50). Of these, IL-6 is thought to be particularly relevant to suicidal behavior in older adults (51). In addition, comorbidity with co-morbid physical illnesses is common in patients with LLD, which significantly increases the inflammatory load and may further superimpose and amplify the risk of suicide. However, there is a relative lack of systematic studies on inflammatory markers in peripheral blood of patients with LLD, and further research is warranted to explore the role of these markers in the prediction of LLD-associated suicidal risk and the identification of intervention targets.
Neural patterns may provide important clinical value for early identification and intervention strategies for suicidal ideation in patients with LLD. The orbitofrontal cortex and dorsolateral prefrontal cortex (DLPFC) are particularly relevant to suicide because of their involvement in decision making, problem solving, and fluency (52). Another recent functional magnetic resonance imaging (fMRI) study found that functional connectivity and directionality of the ventral lateral prefrontal cortex to the caudate nucleus in patients with LLD differentiated between those with no suicidal risk and those with LLD with a history of suicidal ideation or attempts (53). Abnormal activation of the posterior cingulate cortex (PCC), a core node of the Default Mode Network (DMN), contributes to the recurrent negative memory regurgitation in elderly patients (54). In addition, the nucleus accumbens, among others, is a key brain region associated with suicidal behavior in LLD, and we can use machine learning to construct a high-precision prediction tool for suicidal tendency in patients with LLD on the basis of resting-state fMRI data from the relevant brain regions (55).
Ecological momentary assessment (EMA), which allows for the assessment of participants’ behavioral, emotional, and perceptual experiences in both real-time and real-life environments (56), may capture signals of fluctuations in suicidal ideation that cannot be detected by traditional scales. Several studies have piloted ecological transient assessment and digital phenotyping approaches to assess suicidal risk factors in older adults (57, 58). The application of artificial intelligence (AI) to EMA data is an emerging field, with AI technology showing significant potential for analyzing EMA data to identify dynamic patterns and early warning signs related to suicide risk. However, research evidence on the effectiveness of EMA in suicide prevention is scarce, and research in this area faces challenges such as data heterogeneity and lack of algorithmic interpretability (59). Future longitudinal studies should be designed to coordinate data organization and combine the insights of traditional methods with the power of data-driven technology.
Studies have even shown that AI models can achieve high accuracy in suicide risk prediction by analyzing features such as voice frequency and pause patterns, and that voice biomarkers have demonstrated their feasibility for automated screening for suicide risk in real telemedicine scenarios (60). Voice biomarkers extracted from raw speech signals combined with AI show significant potential for the early diagnosis of LLD (61). Gender-related acoustic and rhythmic-related characteristics may serve as valid identifiers of LLD (62). Future studies should explore in depth the mechanisms linking age-specific phonological patterns and suicide risk in LLD to establish more accurate risk stratification models across ages.
Recent advances in suicide risk assessment for late-life depression have increasingly focused on innovative, technology-driven approaches that complement traditional scales. Digital phenotyping, which collects continuous behavioral and physiological data via smartphones and wearable devices, offers real-time, personalized monitoring of mood and risk factors. Machine learning models applied to electronic health records enable the identification of complex risk patterns often missed in clinical interviews. Natural language processing techniques analyze clinical notes to detect subtle indicators of suicidal ideation, while voice biomarker analysis provides a non-invasive method to assess emotional state through speech characteristics. These emerging tools hold promise for improving early detection and intervention; however, challenges, such as digital literacy and cognitive decline in older adults, must be addressed to ensure effective implementation.
4 Treatment of suicide in LLD
Although research suggests that older adults’ emotional processing improves with age (63), and emotional stability is maintained through autonomic compensatory mechanisms (64), it is worth noting that age-related structural and functional brain changes specific to patients with LLD may affect emotion regulation in a maladaptive manner; e.g., LLD appears to be associated with a more severe burden of white matter disease with reduced integrity of the frontal-striatal-limbic network, as well as reduced volume and/or cortical thickness in areas such as the PFC, orbitofrontal cortex, anterior and posterior cingulate gyrus, and hippocampus (65), reward processing, and disconnections between regions associated with subjective positive affect (66), all of which may make it impossible to suppress despair-driven impulsive behavior. Because the neurobiological factors underlying depressive disorders and suicidal risk behaviors are intricately linked (67), LLD-focused treatment may mitigate suicide risk through common mechanisms that undermine the drivers of suicide risk at its source. The majority of elderly suicide deaths can be attributed to depression, and Beautrais found that the significantly reduced suicide rates in the elderly following if LLD intervention (68). Therefore, preventing and treating depression can constitute a powerful suicide prevention strategy for this age group.
The treatment of LLD with suicide risk requires a comprehensive intervention that combines pharmacotherapy, psychotherapy, social support, and other multifaceted means. Owing to the complexity of physical conditions and cognitive functions in older patients, treatment plans should be individualized and early intervention should be emphasized. The following are several commonly used therapeutic approaches:
4.1 Pharmacotherapy
Medication is currently one of the basic treatments for LLD, and commonly used drugs include antidepressants and antipsychotics. The use of medications helps reduce the suicidal ideation in patients with depression (69), which is essential for LLD. Antidepressants are the most well-studied treatment options, selective 5-hydroxytryptamine reuptake inhibitors are widely recognized as first-line therapeutic agents for LLD (70). Among them, sertraline is a well-tolerated and safe antidepressant suitable for older patients, particularly for those with comorbidities of physical diseases (71). Consensus Statement of the Spanish Psychogeriatric Association states that dual-acting antidepressants are considered more effective than SSRIs for LLD (72). Norepinephrine-5-hydroxytryptamine reuptake inhibitors (SNRIs) are suitable for patients with LLD who have comorbid chronic pain, including duloxetine (73) and venlafaxine (74), which help to alleviate depressed mood and somatic symptoms. Additionally, it should be noted that SNRIs, particularly venlafaxine, may cause an increase in blood pressure in patients with LLD (75), and blood pressure should be monitored when using this class of drugs. Noradrenergic and specific 5-hydroxytryptaminergic antidepressants, such as mirtazapine, are recommended for older patients with anxiety, insomnia, anorexia, and weight loss (76).
Other antidepressants, including tricyclic antidepressants (TCAs) and monoamine oxidase inhibitors (MAOIs), are of limited use in older adults because of their more frequent side effects, particularly on the heart and blood pressure (77, 78). Vortioxetine significantly improves depressive symptoms and cognitive functioning in patients with depression by modulating 5-HT receptors and multimodal action and is usually well tolerated in older adults (79). The National Disease Management Guideline states that in cases of suicidal ideation in the hospitalized setting, nasal administration of eketamine may be considered in addition to antidepressants (80). As a novel antidepressant, esketamine, has been shown to rapidly and powerfully reduce suicidal ideation in patients with major depression (81). Additionally, for refractory LLD, second-generation antipsychotics, including aripiprazole (82) and quetiapine (83), may have potentiating effects. Owing to the presence of multiple comorbidities and susceptibility to drug interactions in older adults, it is important to pay close attention to the adverse effects of drugs when using appropriate medications.
Cognitive dysfunction and depressive symptoms are also closely linked to LLD. Although antidepressant medications may improve cognitive function in patients with LLD, particularly memory and learning, by ameliorating depressive symptoms (84), this effect is relatively limited. In contrast, cognition-promoting medications have a more direct mechanism of action to improve cognition, which, in turn, improves patient compliance with antidepressant treatment and enhances self-efficacy, both of which are important for alleviating depression and preventing suicide. Cholinesterase inhibitors such as donepezil, which are primarily used to improve cognitive function in patients with Alzheimer’s disease, may be considered for the cognitive decline commonly observed in patients with LLD and may have a positive effect on their cognitive function. However, donepezil can have a temporary positive effect on cognitive function when added to antidepressant maintenance therapy for LLD (85).
Another cognition-improving drug, N-methyl-D-aspartate receptor antagonists such as memantine, has been shown to be well tolerated in combination with escitalopram for treating depressive symptoms and cognitive improvement in LLD (86). Additionally, medications should be adjusted according to the physiological changes in older adults to avoid adverse drug reactions. The risk of relapse in patients with LLD is high, and maintenance therapy is necessary to minimize this risk. Hospitalization for monitoring and treatment is necessary for patients at a high risk of suicide.
4.2 Psychotherapy
Expert Consensus Guidelines recommend antidepressant medication combined with psychological intervention as the treatment of choice for LLD (70). Psychotherapy is an important component of suicide interventions for patients with LLD, particularly those with mild-to-moderate LLD. Cognitive behavioral therapy (CBT) can help patients identify and adjust to negative thinking patterns, and for those with LLD, it can effectively alleviate depressive symptoms and reduce suicide risk (87). Additionally, certain treatment gaps exist in the older population due to limited mobility, stigma, or geographic barriers, which may be effectively addressed by Internet-based CBT (iCBT). Meta-analysis reinforces the effectiveness of iCBT in alleviating depressive symptoms in older adults (88). iCBT interventions can prevent suicidal-related thoughts and behaviors (89). Virtual Reality-Enhanced Cognitive Behavioral Therapy (VR-CBT) is a new type of psychological intervention that combines traditional Cognitive Behavioral Therapy (CBT) with Virtual Reality (VR) technology. VR-CBT can stimulate multisensory channels, promote emotional regulation and cognitive flexibility in older adults, and compensate for the monotony of traditional talk therapy. The immersive nature of VR-CBT makes it a potential alternative treatment for patients who are intolerant of, or who do not respond well to, traditional antidepressant medications for the treatment of depression and suicidal ideation (90). Mindfulness-Based Cognitive Therapy (MBCT) is a novel treatment for depression that combines mindfulness meditation with elements of CBT to improve rumination in older patients by modulating overactivation of the Default Mode Network (DMN) (91). MBCT may ameliorate cognitive deficits specific to suicide ideators and attempters (92), enabling individuals to regulate negative emotions. MBCT is increasingly being used in the elderly population, with age-related modifications including more sedentary meditation, shorter duration of each session, etc., and age-modified MBCT is more helpful in controlling depressive symptoms in elderly patients (93). Another common psychotherapeutic approach, interpersonal therapy (IPT), can alleviate depressive symptoms by helping patients improve their relationships with others, IPT is an effective treatment option for LLD and may ultimately help reduce the risk of suicide in this high-risk population (94). Problem-solving therapy (PST) is also an effective treatment for LLD (95), and participants with LLD and executive dysfunction who receive PST are more likely to have reduced suicidal ideation (96).
4.3 Neuromodulation therapy
Neuromodulation therapy is particularly important for patients with LLD who are receiving antidepressant medications and may encounter adverse reactions, drug interactions, or unresponsiveness to treatment.
The Canadian Network for Mood and Anxiety Treatment (CANMAT) guidelines recommend ECT as the treatment of choice for patients with major depressive episodes who are at high risk for suicide (97). The rate of remission is significantly higher with ECT for severe LLD, and it should be given a more prominent place in managing these patients (98); however, it can lead to cognitive deficits and adverse effects on the cardiovascular system, among others (99).
Modified electroconvulsive therapy (MECT) is a type of ECT performed under general anesthesia, using inotropes and anesthetics to minimize side effects and improve safety. MECT is an effective, well-tolerated, and safe method for treating patients with refractory LLD (100). MECT is preferred for older patients because it reduces discomfort and anxiety during treatment. MECT can be combined with antidepressant medications to improve therapeutic efficacy, particularly during the acute phase of treatment (101).
Repetitive transcranial magnetic stimulation (rTMS) is a noninvasive brain stimulation technique that stimulates specific brain areas using pulsed magnetic fields. The rTMS has been shown to be effective in young adults, and new data support its use in LLD (102), particularly in those who do not respond well to traditional medication. rTMS targeting dorsal and ventral subregions of the lateral prefrontal cortex (LPFC) in LLD reduces depression scores and improves remission rates (103). Efficacy studies have shown that rTMS is effective and well-tolerated in reducing suicidal ideation and depression severity (104). The rTMS is considered a safe treatment with relatively few side effects and potential cognition-enhancing effects (105). Most patients experience mild discomfort (e.g., scalp tingling or mild headache) during treatment; however, these symptoms are usually transient (106). rTMS has fewer side effects and is particularly suitable for older patients compared with medication.
4.4 Social support and interventions
A possible explanation for the limited effectiveness of depression-focused interventions in reducing the incidence of suicidal behaviors among older adults is the multifactorial etiology of these behaviors. Although loneliness, limited social support, and financial problems, may drive the association between depression and suicidal behaviors in the elderly (107, 108), there is a lack of clear evidence-based interventions to reduce loneliness in older adults (109). Studies confirm that, social support could help strengthen coping skills to deal with loneliness to prevent and treat LLD (110, 111). Maintaining quality social contact should be recommended as part of depression treatment in addition to medication and other treatments. While there are no studies testing whether improved social connectedness is a mechanism for reducing suicide, we do not yet know what treatments are recommended. Social prescription activities such as soccer, gardening, and art, are accessible and low-cost interventions that not only provide direct health benefits, but also effectively promote social connectedness among older adults by increasing social support and reducing isolation (112, 113), which have been implemented in a variety of settings with promising results currently. In addition, sustained and standardized “warm call” interventions can enhance the sense of social connectedness and autonomy of older persons living alone in the community and counteract their negative perceptions (114). A previous study showed that LLD patients’ experience of being listened to in a trusting relationship can reconstruct their self-esteem and self-help motivation, which provides a clinical rationale for personalized suicide prevention strategies, with the subsequent need to deepen the contextualized validation of crisis interventions in cross-cultural contexts (115).
It is important to note that cultural continuity and community protection should be key factors in suicide prevention (116). For example, in the context of traditional Chinese culture, depression can be alleviated through intergenerational relationships (referring to the patterns of interaction, distribution of responsibilities, and emotional ties between different age groups within a family or social structure) that enhance older people’s self-assessed health and sense of well-being (117), and ultimately prevent suicide. The National Institute for Health and Clinical Excellence similarly emphasized the important role of community services and family support for people with LLD who have suicidal ideation (118).
In addition, the use of some new technologies has been welcomed by older people. PARO for long-term care has been described diversely as a companion, social, and seal robot (119), which has been variously described as companion robot, social robot, and seal robot. It can provide alternative social connections for older adults through multi-sensor interactions that mimic the responses of living organisms, which can have a significant positive impact on reducing depression and loneliness in older adults (120, 121). Technology-based interventions such as PARO can form a buffer for suicide risk protection by alleviating the negative emotions of emotional numbing, social disconnection, and feelings of worthlessness of geriatric depression.
Primary care is a strategic setting for the treatment and prevention of LLD suicide, drawing on the Prevention of Suicide in Primary Care Elderly: Collaborative Trial (PROSPECT) of interdisciplinary interventions, which comprise the collaboration of primary care GPs, psychiatrists, and care managers for the management of depression, with primary care GPs being responsible for day-to-day health management and initial medication, psychiatrists focusing on the development of individualized treatment strategies and supervising the overall process. The core innovative role of the care manager is to coordinate the communication between doctors, patients, and family members through dynamic assessment, adherence management, and standardized treatment, with the aim of achieving standardized downstreaming and efficient collaboration in the provision of mental health services (122). Studies have demonstrated that this intervention leads to a remission of depressive symptoms in 49.7% of the patients with LLD and is effective in reducing the risk of suicide (123).
There are still many gaps in current research. In the future, we need to examine the mechanism of social contact intervention for suicide and its direct effect on suicidal behavior in high-risk populations, and to accumulate contextualized experience in exploring suicidal crisis intervention in different cultural contexts. Future studies need more long-term longitudinal follow-up to explore the trajectory of depression in old age and the effects of different interventions over a longer time horizon.
5 Discussion
Suicide risk remains high in patients with LLD. However, a large variability exists in the presentation of LLD and adult depression, and many aspects of its diagnosis, assessment, and treatment remain unknown. Structured rating scales and novel suicide risk assessment enable clinicians and researchers to more precisely identify and quantify suicidality, thereby facilitating early intervention. This could be further explored in the future to identify measures suitable for the early screening and assessment of suicide risk in patients with LLD. Through interdisciplinary collaborative innovation, a paradigm shift from reactive management to proactive prevention of suicide risk in LLD may be achievable. Current therapeutic approaches encompass psychotherapy, pharmacotherapy, neuromodulation therapy, and internet-based intervention technologies. Emerging evidence suggests that antidepressant medications and non-pharmacological adjunctive therapies, such as transcranial magnetic stimulation, can efficiently mitigate suicide risk among patients with LLD. Notably, the generalizability of assessment tools and interventions for suicide risk in patients with LLD may have limitations due to cultural and geographic biases, such as conflicting values, differences in healthcare infrastructure, etc., and thus culturally adapted assessment tools should be developed in the future.
Author contributions
TX: Conceptualization, Writing – original draft, Writing – review & editing. YM: Supervision, Writing – review & editing. YW: Conceptualization, Writing – review & editing. WZ: Validation, Writing – review & editing. HC: Investigation, Writing – review & editing. EY: Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the Science and Technology Plan Project of Zhejiang Province (Project number: 2021C03106).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. United Nations Department of Economic and Social Affairs. World social report 2023: leaving no one behind in an ageing world. (2023). Available online at: https://desapublications.un.org/publications/world-social-report-2023-leaving-no-one-behind-ageing-world.
2. Hu T, Zhao X, Wu M, Li Z, Luo L, Yang C, et al. Prevalence of depression in older adults: a systematic review and meta-analysis. Psychiatry Res. (2022) 311:114511. doi: 10.1016/j.psychres.2022.114511
3. Taylor WD. Clinical practice. Depression in the elderly. N Engl J Med. (2014) 371:1228–36. doi: 10.1056/NEJMcp1402180
4. Husain-Krautter S and Ellison JM. Late-life depression: essentials and essential distinctions. Focus. (2012) 19:282–93. doi: 10.1176/appi.focus.20210006
5. Alexopoulos GS. Depression in the elderly. Lancet. (2005) 365:1961–70. doi: 10.1016/S0140-6736(05)66665-2
6. Paun O. Older adults and late-life depression. J Psychosoc Nurs Ment Health Serv. (2023) 61:8–9. doi: 10.3928/02793695-20230307-02
7. Fernandez-Rodrigues V, Sanchez-Carro Y, Lagunas LN, Rico-Uribe LA, Pemau A, Diaz-Carracedo P, et al. Risk factors for suicidal behaviour in late-life depression: a systematic review. World J Psychiatry. (2022) 12:187–203. doi: 10.5498/wjp.v12.i1.187
8. Conwell Y and Thompson C. Suicidal behaviors in elders. Psychiatr Clin North Am. (2008) 31:333–56. doi: 10.1016/j.psc.2008.01.004
9. Islam MM, Valderas JM, Yen L, Dawda P, Jowsey T, and McRae IS. Multimorbidity and comorbidity of chronic diseases among the senior Australians: prevalence and patterns. PloS One. (2014) 9:e83783. doi: 10.1371/journal.pone.0083783
10. Triolo F, Harber-Aschan L, Belvederi Murri M, Calderón-Larrañaga A, Vetrano DL, Sjöberg L, et al. The complex interplay between depression and multimorbidity in late life: risks and pathways. Mech Ageing Dev. (2020) 192:111383. doi: 10.1016/j.mad.2020.111383
11. Li H, Zheng D, Li Z, Wu Z, Feng W, Cao X, et al. Association of depressive symptoms with incident cardiovascular diseases in middle-aged and older Chinese adults. JAMA Netw Open. (2019) 2:e1916591. doi: 10.1001/jamanetworkopen.2019.16591
12. Hare DL, Toukhsati SR, Johansson P, and Jaarsma T. Depression and cardiovascular disease: a clinical review. Eur Heart J. (2014) 35:1365–72. doi: 10.1093/eurheartj/eht462
13. Kuhlmann SL, Arolt V, Haverkamp W, Martus P, Ströhle A, Waltenberger J, et al. Prevalence, 12-month prognosis, and clinical management need of depression in patients with coronary heart disease patients: a prospective cohort study. Psychother Psychosom. (2019) 88:300–11. doi: 10.1159/000501502
14. Nouwen A, Winkley K, Twisk J, Lloyd CE, Peyrot M, Ismail K, et al. Type 2 diabetes mellitus as a risk factor for the onset of depression: a systematic review and meta-analysis. Diabetologia. (2010) 53:2480–6. doi: 10.1007/s00125-010-1874-x
15. Huang CJ, Huang YT, Lin PC, Hsieh HM, and Yang YH. Mortality and suicide related to major depressive disorder before and after type 2 diabetes mellitus. J Clin Psychiatry. (2022) 83:20m13692. doi: 10.4088/JCP.20m13692
16. De Ryck A, Fransen E, Brouns R, Geurden M, Peij D, Mariën P, et al. Poststroke depression and its multifactorial nature: results from a prospective longitudinal study. J Neurol Sci. (2014) 347:159–66. doi: 10.1016/j.jns.2014.09.038
17. Thambirajah N, Senanayake S, Gooneratne K, Suraweera C, Ranasinghe L, and Kumbukage M. Post-stroke depression: prevalence, associated factors, and relationship to disability in a Tertiary Care Center in Sri Lanka. J Neurosci Rural Pract. (2022) 13:73–9. doi: 10.1055/s-0041-1741504
18. Frank D, Gruenbaum BF, Zlotnik A, Semyonov M, Frenkel A, and Boyko M. Pathophysiology and current drug treatments for post-stroke depression: a review. Int J Mol Sci. (2022) 23:15114. doi: 10.3390/ijms232315114
19. Jiao JT, Cheng C, Ma YJ, Huang J, Dai MC, Jiang C, et al. Association between inflammatory cytokines and the risk of post-stroke depression, and the effect of depression on the outcomes of patients with ischemic stroke in a 2-year prospective study. Exp Ther Med. (2016) 12:1591–8. doi: 10.3892/etm.2016.3494
20. Vyas MV, Wang JZ, Gao MM, and Hackam DG. Association between stroke and the subsequent risk of suicide: a systematic review and meta-analysis. Stroke. (2021) 52:1460–4. doi: 10.1161/STROKEAHA.120.032692
21. van Vreede JJ, Parker R, and van Nugteren J. A History of depression in patients attending a chronic pain management clinic in South Africa: a retrospective chart review. S Afr J Psychiatr. (2022) 28:1673. doi: 10.4102/sajpsychiatry.v28i0.1673
22. Kwon CY and Lee B. Prevalence of suicidal behavior in patients with chronic pain: a systematic review and meta-analysis of observational studies. Front Psychol. (2023) 14:1217299. doi: 10.3389/fpsyg.2023.1217299
23. Manly JJ, Jones RN, Langa KM, Ryan LH, Levine DA, McCammon R, et al. Estimating the prevalence of dementia and mild cognitive impairment in the US: the 2016 Health and Retirement Study harmonized the Cognitive Assessment Protocol Project. JAMA Neurol. (2022) 79:1242–9. doi: 10.1001/jamaneurol.2022.3543
24. da Silva AG, Malloy-Diniz LF, Garcia MS, Figueiredo CGS, Figueiredo RN, Diaz AP, et al. Cognition as a therapeutic target in the suicidal patient approach. Front Psychiatry. (2018) 9:31. doi: 10.3389/fpsyt.2018.00031
25. Alacreu-Crespo A, Guillaume S, Sénèque M, Olié E, and Courtet P. Cognitive modelling to assess decision-making impairments in patients with current depression and with/without suicide history. Eur Neuropsychopharmacol. (2020) 36:50–9. doi: 10.1016/j.euroneuro.2020.04.006
26. Yin J, John A, and Cadar D. Bidirectional associations of depressive symptoms and cognitive function over time. JAMA Netw Open. (2024) 7:e2416305. doi: 10.1001/jamanetworkopen.2024.16305
27. Olsson P, Wiktorsson S, Sacuiu S, Marlow T, Östling S, Fässberg MM, et al. Cognitive function in older suicide attempters and a population-based comparison group. J Geriatr Psychiatry Neurol. (2016) 29:133–41. doi: 10.1177/0891988715627015
28. Richard-Devantoy S, Berlim MT, and Jollant F. Suicidal behaviour and memory: a systematic review and meta-analysis. World J Biol Psychiatry. (2015) 16:544–66. doi: 10.3109/15622975.2014.925584
29. Niu L, Jia C, Ma Z, Wang G, Sun B, Zhang D, et al. Loneliness, hopelessness and suicide in later life: a case-control psychological autopsy study in rural China. Epidemiol Psychiatr Sci. (2020) 29:e119. doi: 10.1017/S2045796020000335
30. Lee SL, Pearce E, Ajnakina O, Johnson S, Lewis G, Mann F, et al. The Association between loneliness and depressive symptoms among adults aged 50 years and older: a 12-year population-based cohort study. Lancet Psychiatry. (2021) 8:48–57. doi: 10.1016/S2215-0366(20)30383-7
31. Pourriyahi H, Yazdanpanah N, Saghazadeh A, and Rezaei N. Loneliness: an immunometabolic syndrome. Int J Environ Res Public Health. (2021) 18:12162. doi: 10.3390/ijerph182212162
32. Cacioppo JT, Cacioppo S, Capitanio JP, and Cole SW. The neuroendocrinology of social isolation. Annu Rev Psychol. (2015) 66:733–67. doi: 10.1146/annurev-psych-010814-015240
33. De Leo D. Late-life suicide in an aging world. Nat Aging. (2022) 2:7–12. doi: 10.1038/s43587-021-00160-1
34. Christensen M, Chan HY, Chan YY, Cheng KY, Cheung TY, Li TY, et al. Suicide ideation in older people: a qualitative review and meta-aggregation of Asian studies. Front Psychiatry. (2023) 14:1169820. doi: 10.3389/fpsyt.2023.1169820
35. Motillon-Toudic C, Walter M, Séguin M, Carrier JD, Berrouiguet S, and Lemey C. Social isolation and suicide risk: literature review and perspectives. Eur Psychiatry. (2022) 65:e65. doi: 10.1192/j.eurpsy.2022.2320
36. Lapierre S, Erlangsen A, Waern M, De Leo D, Oyama H, Scocco P, et al. A systematic review of elderly suicide prevention programs. Crisis. (2011) 32:88–98. doi: 10.1027/0227-5910/a000076
37. Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. (1982) 17:37–49. doi: 10.1016/0022-3956(82)90033-4
38. Huang F, Wang H, Wang Z, Zhang J, Du W, Jia X, et al. Is geriatric depression scale a valid instrument to screen depression in Chinese community-dwelling elderly? BMC Geriatr. (2021) 21:310. doi: 10.1186/s12877-021-02266-y
39. Beck AT, Ward CH, Mendelson M, Mock J, and Erbaugh J. An Inventory for measuring depression. Arch Gen Psychiatry. (1961) 4:561–71. doi: 10.1001/archpsyc.1961.01710120031004
40. Steer RA, Rissmiller DJ, and Beck AT. Use of the Beck Depression Inventory – II with depressed geriatric inpatients. Behav Res Ther. (2000) 38:311–8. doi: 10.1016/s0005-7967(99)00068-6
41. Segal DL, Coolidge FL, Cahill BS, and O’Riley AA. Psychometric properties of the Beck Depression Inventory – II (BDI-II) among community-dwelling older adults. Behav Modif. (2008) 32:3–20. doi: 10.1177/0145445507303833
42. Beck AT, Kovacs M, and Weissman A. Assessment of suicidal intention: the scale for suicide ideation. J Consult Clin Psychol. (1979) 47:343–52. doi: 10.1037//0022-006x.47.2.343
43. Beck AT, Brown GK, Steer RA, Dahlsgaard KK, and Grisham JR. Suicide ideation at its worst point: a predictor of eventual suicide in psychiatric outpatients. Suicide Life Threat Behav. (1999) 29:1–9. doi: 10.1111/j.1943-278X.1999.tb00758.x
44. Heisel MJ and Flett GL. The Development and initial validation of the geriatric suicide ideation scale. Am J Geriatr Psychiatry. (2006) 14:742–51. doi: 10.1097/01.JGP.0000218699.27899.f9
45. Heisel MJ and Flett GL. Investigating the psychometric properties of the Geriatric Suicide Ideation Scale (GSIS) among community-residing older adults. Aging Ment Health. (2016) 20:208–21. doi: 10.1080/13607863.2015.1072798
46. Mai J, Bower E, and Van Orden K. Evaluating the validity of the Columbia-suicide severity rating scale for lonely older adults. Innov Aging. (2021) 5:917–8. doi: 10.1093/geroni/igab046.3325
47. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. (1960) 23:56–62. doi: 10.1136/jnnp.23.1.56
48. Carroll BJ. Whythe hamilton depression rating scale endures? Am J Psychiatry. (2005) 162:2395–6; author reply 2397. doi: 10.1176/appi.ajp.162.12.2395-a
49. Brundin L, Bryleva EY, and Thirtamara Rajamani K. Role of inflammation in suicide: from mechanisms to treatment. Neuropsychopharmacology. (2017) 42:271–83. doi: 10.1038/npp.2016.116
50. Yang Y, Gu K, and Li J. Relationship between serum inflammatory cytokines and suicide risk in patients with major depressive disorder. Front Psychiatry. (2024) 15:1422511. doi: 10.3389/fpsyt.2024.1422511
51. Lozupone M, Donghia R, Sardone R, Mollica A, Berardino G, Lampignano L, et al. Apolipoprotein E genotype, inflammatory biomarkers, and non-psychiatric multimorbidity contribute to the suicidal ideation phenotype in older age. The Salus in Apulia study. J Affect Disord. (2022) 319:202–12. doi: 10.1016/j.jad.2022.09.046
52. Van Heeringen C, Bijttebier S, and Godfrin K. Suicidal brains: a review of functional and structural brain studies in association with suicidal behaviour. Neurosci Biobehav Rev. (2011) 35:688–98. doi: 10.1016/j.neubiorev.2010.08.007
53. Shao R, Gao M, Lin C, Huang CM, Liu HL, Toh CH, et al. Multimodal neural evidence on the corticostriatal underpinning of suicidality in late-life depression. Biol Psychiatry Cognit Neurosci Neuroimaging. (2022) 7:905–15. doi: 10.1016/j.bpsc.2021.11.011
54. Gerlach AR, Karim HT, Kolobaric A, Boyd BD, Kahru K, Krafty RT, et al. Network homeostasis: functional brain network alterations and relapse in remitted late-life depression. Neuropsychopharmacology. (2025) 1–9. doi: 10.1038/s41386-025-02138-8
55. Lin C, Huang CM, Chang W, Chang YX, Liu HL, Ng SH, et al. Predicting suicidality in late-life depression by 3D convolutional neural network and cross-sample entropy analysis of resting-state fMRI. Brain Behav. (2024) 14:e3348. doi: 10.1002/brb3.3348
56. Burke LE, Shiffman S, Music E, Styn MA, Kriska A, Smailagic A, et al. Ecological momentary assessment in behavioral research: addressing technological and human participant challenges. J Med Internet Res. (2017) 19:e77. doi: 10.2196/jmir.7138
57. Kim H, Lee S, Lee S, Hong S, Kang H, and Kim N. Depression prediction by using ecological momentary assessment, actiwatch data, and machine learning: observational study on older adults living alone. JMIR MHealth UHealth. (2019) 7:e14149. doi: 10.2196/14149
58. Badal VD, Lee EE, Daly R, Parrish EM, Kim HC, Jeste DV, et al. Dynamics of loneliness among older adults during the COVID-19 pandemic: pilot study of ecological momentary assessment with network Analysis. Front Digit Health. (2022) 4:814179. doi: 10.3389/fdgth.2022.814179
59. Melia R, Musacchio Schafer K, Rogers ML, Wilson-Lemoine E, and Joiner TE. The application of AI to ecological momentary assessment data in suicide research: systematic review. J Med Internet Res. (2025) 27:e63192. doi: 10.2196/63192
60. Iyer R, Nedeljkovic M, and Meyer D. Using voice biomarkers to classify suicide risk in adult telehealth callers: a retrospective observational study. JMIR Ment Health. (2022) 9:e39807. doi: 10.2196/39807
61. Lin Y, Liyanage BN, Sun Y, Lu T, Zhu Z, Liao Y, et al. A deep learning-based model for detecting depression in senior population. Front Psychiatry. (2022) 13:1016676. doi: 10.3389/fpsyt.2022.1016676
62. Lee S, Suh SW, Kim T, Kim K, Lee KH, Lee JR, et al. Screening major depressive disorder using vocal acoustic features in the elderly by sex. J Affect Disord. (2021) 291:15–23. doi: 10.1016/j.jad.2021.04.098
63. Scheibe S and Carstensen LL. Emotional aging: recent findings and future trends. J Gerontol B Psychol Sci Soc Sci. (2010) 65B:135–44. doi: 10.1093/geronb/gbp132
64. Mather M. The emotion paradox in the aging body and brain. Ann N Y Acad Sci. (2024) 1536:13–41. doi: 10.1111/nyas.15138
65. Kim YK and Han KM. Neural substrates for late-life depression: A selective review of structural neuroimaging studies. Prog Neuropsychopharmacol Biol Psychiatry. (2021) 104:110010. doi: 10.1016/j.pnpbp.2020.110010
66. Dombrovski AY, Szanto K, Clark L, Reynolds CF, and Siegle GJ. Reward signals, attempted suicide, and impulsivity in late-life depression. JAMA Psychiatry. (2013) 70:1. doi: 10.1001/jamapsychiatry.2013.75
67. Jackson NA and Jabbi MM. Integrating biobehavioral information to predict mood disorder suicide risk. Brain Behav Immun Health. (2022) 24:100495. doi: 10.1016/j.bbih.2022.100495
68. Beautrais AL. A case control study of suicide and attempted suicide in older adults. Suicide Life Threat Behav. (2002) 32:1–9. doi: 10.1521/suli.32.1.1.22184
69. Weitz E, Hollon SD, Kerkhof A, and Cuijpers P. Do depression treatments reduce suicidal ideation? The effects of CBT, IPT, pharmacotherapy, and placebo on suicidality. J Affect Disord. (2014) 167:98–103. doi: 10.1016/j.jad.2014.05.036
70. Alexopoulos GS, Katz IR, Reynolds CF, Carpenter D, Docherty JP, and Expert Consensus Panel for Pharmacotherapy of Depressive Disorders in Older Patients. The expert consensus guideline series. Pharmacotherapy of depressive disorders in older patients. The Expert Consensus Guideline Series. Postgrad Med. (2001), 1–86. Available online at: https://pubmed.ncbi.nlm.nih.gov/17205639/.
71. Ishtiak-Ahmed K, Rohde C, Otte C, Gasse C, and Köhler-Forsberg O. Comparative effectiveness of selective serotonin reuptake inhibitors (SSRIs) for depression in 43,061 older adults with chronic somatic diseases: a Danish target trial emulation study. Gen Hosp Psychiatry. (2024) 87:83–91. doi: 10.1016/j.genhosppsych.2024.02.002
72. Agüera-Ortiz L, Claver-Martín MD, Franco-Fernández MD, López-Álvarez J, Martín-Carrasco M, Ramos-García MI, et al. Depression in the elderly. Consensus statement of the Spanish psychogeriatric association. Front Psychiatry. (2020) 11:380. doi: 10.3389/fpsyt.2020.00380
73. Romera I, Pérez V, Menchón JM, Schacht A, Papen R, Neuhauser D, et al. Early vs. conventional switching of antidepressants in patients with MDD and moderate to severe pain: a double-blind randomized study. J Affect Disord. (2012) 143:47–55. doi: 10.1016/j.jad.2012.05.024
74. Rej S, Dew MA, and Karp JF. Treating concurrent chronic low back pain and depression with low-dose venlafaxine: an initial identification of “easy-to-use” clinical predictors of early response. Pain Med. (2014) 15:1154–62. doi: 10.1111/pme.12456
75. Calvi A, Fischetti I, Verzicco I, Belvederi Murri M, Zanetidou S, Volpi R, et al. Antidepressant drug affects on blood pressure. Front Cardiovasc Med. (2021) 8:704281. doi: 10.3389/fcvm.2021.704281
76. Kato M, Baba H, Takekita Y, Naito M, Koshikawa Y, Bandou H, et al. Usefulness of mirtazapine and SSRIs in late-life depression: post hoc analysis of the GUNDAM study. European Journal of Clinical Pharmacology. (2003) 79:1515–24. doi: 10.1007/s00228-023-03563-8
77. Shulman KI, Fischer HD, Herrmann N, Huo CY, Anderson GM, and Rochon PA. Current prescription patterns and safety profile of irreversible monoamine oxidase inhibitors: a population-based cohort study of older adults. J Clin Psychiatry. (2009) 70:1681–6. doi: 10.4088/JCP.08m05041blu
78. Santandreu J, Caballero FF, Gómez-Serranillos MP, and González-Burgos E. Association between tricyclic antidepressants and health outcomes among older people: a systematic review and meta-analysis. Maturitas. (2024) 188:108083. doi: 10.1016/j.maturitas.2024.108083
79. Di Nicola M, Adair M, Rieckmann A, and Christensen MC. Effectiveness of vortioxetine in elderly patients with major depressive disorder in real-world clinical practice: results from the RELIEVE study. J Psychopharmacol. (2024) 38:615–23. doi: 10.1177/02698811241260996
80. Härter M, Prien P, and NVL Guideline Group. Clinical practice guideline. Clinical practice guideline: the diagnosis and treatment of unipolar depression—National disease management guideline. Dtsch Ärztebl Int. (2023) 120:355–61. doi: 10.3238/arztebl.m2023.0074
81. Ionescu DF, Fu DJ, Qiu X, Lane R, Lim P, Kasper S, et al. Esketamine nasal spray rapid reduction of depressive symptoms in patients with major depressive disorder who have active suicide ideation with intent: results of a Phase 3, double-blind, randomized study (Aspire II). Int J Neuropsychopharmacol. (2021) 24:22–31. doi: 10.1093/ijnp/pyaa068
82. Lewis G and Lewis G. Aripiprazole augmentation in older persons with treatment-resistant depression. N Engl J Med. (2023) 388:1137–8. doi: 10.1056/NEJMe2301045
83. Katila H, Mezhebovsky I, Mulroy A, Berggren L, Eriksson H, Earley W, et al. Randomized, double-blind study of the efficacy and tolerability of extended release quetiapine fumarate (quetiapine XR) monotherapy in elderly patients with major depressive disorder. Am J Geriatr Psychiatry. (2013) 21:769–84. doi: 10.1016/j.jagp.2013.01.010
84. Ainsworth NJ, Marawi T, Maslej MM, Blumberger DM, McAndrews MP, Perivolaris A, et al. Cognitive outcomes after antidepressant pharmacotherapy for late-life depression: a systematic review and meta-analysis. Am J Psychiatry. (2024) 181:234–45. doi: 10.1176/appi.ajp.20230392
85. Reynolds CF 3rd, Butters MA, Lopez O, Pollock BG, Dew MA, Mulsant BH, et al. Maintenance treatment of depression in old age: a randomized, double-blind, placebo-controlled evaluation of the efficacy and safety of donepezil combined with antidepressant pharmacotherapy. Arch Gen Psychiatry. (2011) 68:51–60. doi: 10.1001/archgenpsychiatry.2010.184
86. Lavretsky H, Laird KT, Krause-Sorio B, Heimberg BF, Yeargin J, Grzenda A, et al. A randomized double-blind placebo-controlled trial of combined escitalopram and memantine for older adults with major depression and subjective memory complaints. Am J Geriatr Psychiatry. (2020) 28:178–90. doi: 10.1016/j.jagp.2019.08.011
87. Khazanov GK, Xu C, Hollon SD, DeRubeis RJ, and Thase ME. Adding cognitive therapy to antidepressant medications decreases suicidal ideation. J Affect Disord. (2021) 281:183–91. doi: 10.1016/j.jad.2020.12.032
88. Qiu Y, Wu M, Liu J, Li C, Yu Y, Zeng L, et al. Effectiveness of information technology-based cognitive behavioral therapy on depression and anxiety symptoms among older adults: systematic review and meta-analysis. Gen Hosp Psychiatry. (2025) 93:9–19. doi: 10.1016/j.genhosppsych.2024.12.022
89. Mühlmann C, Madsen T, Hjorthøj C, Forman JL, Kerkhof AJFM, Nordentoft M, et al. Effectiveness of an Internet-based self-help therapy program for suicidal ideation with follow-up at 6 months: results of a randomized controlled trial. J Clin Psychiatry. (2021) 82:20m13803. doi: 10.4088/JCP.20m13803
90. Lee M, Jang S, Shin HK, Choi SW, Kim HT, Oh J, et al. Virtual reality-based cognitive behavior therapy for major depressive disorder: an alternative to pharmacotherapy for reducing suicidality. Yonsei Med J. (2025) 66:25–36. doi: 10.3349/ymj.2024.0002
91. Prakash RS, De Leon AA, Patterson B, Schirda BL, and Janssen AL. Mindfulness and the aging brain: a proposed paradigm shift. Front Aging Neurosci. (2014) 6:120. doi: 10.3389/fnagi.2014.00120
92. Chesin MS, Benjamin-Phillips CA, Keilp J, Fertuck EA, Brodsky BS, and Stanley B. Improvements in executive attention, rumination, cognitive reactivity, and mindfulness among high-suicide risk patients participating in adjunct mindfulness-based cognitive therapy: preliminary findings. J Altern Complement Med. (2016) 22:642–9. doi: 10.1089/acm.2015.0351
93. Wang YH, Wang YL, Leung DKY, Ng ZLY, Chan OLH, Wong SMY, et al. Effectiveness of an age-modified mindfulness-based cognitive therapy (MBCT) in improving mental health in older people with depressive symptoms: a non-randomised controlled trial. BMC Complement Med Ther. (2025) 25:81. doi: 10.1186/s12906-025-04781-6
94. Heisel MJ, Talbot NL, King DA, Tu XM, and Duberstein PR. Adapting interpersonal psychotherapy for older adults at risk for suicide. Am J Geriatr Psychiatry. (2015) 23:87–98. doi: 10.1016/j.jagp.2014.03.010
95. Kirkham JG, Choi N, and Seitz DP. Meta-analysis of problem solving therapy for the treatment of major depressive disorder in older adults. Int J Geriatr Psychiatry. (2016) 31:526–35. doi: 10.1002/gps.4358
96. Gustavson KA, Alexopoulos GS, Niu GC, McCulloch C, Meade T, and Areán PA. Problem-solving therapy reduces suicidal ideation in depressed older adults with executive dysfunction. Am J Geriatr Psychiatry. (2016) 24:11–7. doi: 10.1016/j.jagp.2015.07.010
97. Corrigendum to Canadian Network for Mood and Anxiety Treatments (CANMAT). Update on Clinical Guidelines for Management of Major Depressive Disorder in Adults: réseau canadien pour les traitements de l’humeur et de l’anxiété (CANMAT) 2023: mise à jour des lignes directrices cliniques pour la prise en charge du trouble dépressif majeur chez les adultes. Can J Psychiatry. (2023) 70:652. doi: 10.1177/07067437251349087
98. Spaans HP, Sienaert P, Bouckaert F, van den Berg JF, Verwijk E, Kho KH, et al. Speed of remission in elderly patients with depression: electroconvulsive therapy v. medication Br J Psychiatry. (2015) 206:67–71. doi: 10.1192/bjp.bp.114.148213
99. Dai X, Zhang R, Deng N, Tang L, and Zhao B. Anesthetic influence on electroconvulsive therapy: a comprehensive review. Neuropsychiatr Dis Treat. (2024) 20:1491–502. doi: 10.2147/NDT.S467695
100. Jiang X, Xie Q, Liu LZ, Zhong BL, Si L, and Fan F. Efficacy and safety of modified electroconvulsive therapy for the refractory depression in elderly patients. Asia Pac Psychiatry. (2020) 12:e12411. doi: 10.1111/appy.12411
101. Yin BW and Yang L. Comparative efficacy of augmenting escitalopram with modified electroconvulsive therapy or high-frequency repetitive transcranial magnetic stimulation on depressive symptoms, quality of life, and cognitive function in treatment-resistant depression. Tohoku J Exp Med. (2024) 262:191–9. doi: 10.1620/tjem.2023.J103
102. Valiengo L, Maia A, Cotovio G, Gordon PC, Brunoni AR, Forlenza OV, et al. Repetitive transcranial magnetic stimulation for major depressive disorder in older adults: a systematic review and meta-analysis. J Gerontol A Biol Sci Med Sci. (2022) 77:851–60. doi: 10.1093/gerona/glab235
103. Kaster TS, Daskalakis ZJ, Noda Y, Knyahnytska Y, Downar J, Rajji TK, et al. Efficacy, tolerability, and cognitive effects of deep transcranial magnetic stimulation for late-life depression: a prospective randomized controlled trial. Neuropsychopharmacology. (2018) 43:2231–8. doi: 10.1038/s41386-018-0121-x
104. Chen GW, Hsu TW, Ching PY, Pan CC, Chou PH, and Chu CS. Efficacy and tolerability of repetitive transcranial magnetic stimulation on suicidal ideation: a systemic review and meta-analysis. Front Psychiatry. (2022) 13:884390. doi: 10.3389/fpsyt.2022.884390
105. Martin DM, McClintock SM, Forster JJ, Lo TY, and Loo CK. Cognitive enhancing effects of rTMS administered to the prefrontal cortex in patients with depression: a systematic review and meta-analysis of individual task effects. Depress Anxiety. (2017) 34:1029–39. doi: 10.1002/da.22658
106. O’Connell NE, Marston L, Spencer S, DeSouza LH, and Wand BM. Non-invasive brain stimulation techniques for chronic pain. Cochrane Database Syst Rev. (2018) 3:CD008208. doi: 10.1002/14651858.CD008208.pub4
107. Hassan F, Liu L, and Feng C. Double burden of distress: exploring the joint associations of loneliness and financial strain with suicidal ideation during the COVID-19 pandemic in Canada. Int J Environ Res Public Health. (2025) 22:682. doi: 10.3390/ijerph22050682
108. Cacioppo JT, Hawkley LC, and Thisted RA. Perceived social isolation makes me sad: 5-year cross-lagged analyses of loneliness and depressive symptomatology in the Chicago Health, Aging, and Social Relations Study. Psychol Aging. (2010) 25:453–63. doi: 10.1037/a0017216
109. Berg-Weger M and Morley J. Editorial: loneliness in Old Age: an unaddressed Health Problem. J Nutr Health Aging. (2020) 24:243–5. doi: 10.1007/s12603-020-1323-6
110. Wen Z, Wang H, Liang Q, Liu L, Zhang W, and Zhang X. Mediating effect of social support and resilience between loneliness and depression in older adults: a systematic review and meta-analytic structural equation modeling. J Affect Disord. (2024) 365:246–57. doi: 10.1016/j.jad.2024.08.062
111. Woods A, Solomonov N, Liles B, Guillod A, Kales HC, and Sirey JA. Perceived social support and interpersonal functioning as predictors of treatment response among depressed older adults. Am J Geriatr Psychiatry. (2021) 29:843–52. doi: 10.1016/j.jagp.2020.12.021
112. Paquet C, Whitehead J, Shah R, Adams AM, Dooley D, Spreng RN, et al. Social prescription interventions addressing social isolation and loneliness in older adults: meta-review integrating on-the-ground resources. J Med Internet Res. (2023) 25:e40213. doi: 10.2196/40213
113. Sadio R, Henriques A, Nogueira P, and Costa A. Social prescription for the elderly: a community-based scoping review. Prim Health Care Res Dev. (2024) 25:e46. doi: 10.1017/S1463423624000410
114. Houston K, Shannonhouse L, Kelleher K, LeBlanc E, Dailey A, Fullen MC, et al. ‘My life is not over’: an evaluation of a standardized and manualized eight week warm calling phone intervention for community dwelling older adults. Aging Ment Health. (2025), 1–10. doi: 10.1080/13607863.2025.2515181
115. Sjöberg M, Hed S, Wiktorsson S, Berg AI, Strand J, Doering S, et al. Experiences of healthcare interactions before and after suicidal behaviour among older adults attending geropsychiatric services: an interpretative phenomenological analysis. BMJ Open. (2025) 15:e100636. doi: 10.1136/bmjopen-2025-100636
116. Allen J, Wexler L, Apok CA, Black J, Chaliak JA, Cueva K, et al. Indigenous community-level protective factors in the prevention of suicide: enlarging a definition of cultural continuity in rural Alaska Native communities. Prev Sci. (2025) 26:246–57. doi: 10.1007/s11121-025-01782-2
117. Feng W, Geng P GE, Ge H, Gao Q, Cai W, Jing Q, et al. The influence of intergenerational relationships on depressive symptoms in elderly patients with multiple chronic conditions: the mediating roles of self-rated health and well-being. BMC Public Health. (2025) 25:1478. doi: 10.1186/s12889-025-22759-4
118. National Institute for Health and Care Excellence. Guidelines. In: Depression in adults: treatment and management. National Institute for Health and Care Excellence (NICE) copyright © NICE, London (2022).
119. Valentí Soler M, Agüera-Ortiz L, Olazarán Rodríguez J, Mendoza Rebolledo C, Pérez Muñoz A, Rodríguez Pérez I, et al. Social robots in advanced dementia. Front Aging Neurosci. (2015) 7:133. doi: 10.3389/fnagi.2015.00133
120. Yen HY, Huang CW, Chiu HL, and Jin G. The effect of social robots on depression and loneliness for older residents in long-term care facilities: A meta-analysis of randomized controlled trials. J Am Med Dir Assoc. (2024) 25:104979. doi: 10.1016/j.jamda.2024.02.017
121. Yen HY, Huang CW, Chiu HL, and Jin G. Social companion robots for alleviating depression and loneliness in institutional older adults. Psychiatry Res. (2023) 328:115425. doi: 10.1016/j.psychres.2023.115425
122. Bruce ML, Ten Have TR, Reynolds CF 3rd, Katz II, Schulberg HC, Mulsant BH, et al. Reducing suicidal ideation and depressive symptoms in depressed older primary care patients: a randomized controlled trial. JAMA. (2004) 291:1081–91. doi: 10.1001/jama.291.9.1081
Keywords: older adults, depression, late-life depression, suicide, assessment tools, treatment methods
Citation: Xu T, Mao Y, Wang Y, Zhang W, Cao H and Yu E (2025) Advances in the assessment and study of suicide in late-life depression. Front. Psychiatry 16:1610730. doi: 10.3389/fpsyt.2025.1610730
Received: 12 April 2025; Accepted: 17 July 2025;
Published: 30 July 2025.
Edited by:
Allyson Kelley, Allyson Kelley & Associates PLLC, United StatesReviewed by:
Noboru Fujise, Kumamoto University Hospital, JapanEmily Bower, Pacific University, United States
Copyright © 2025 Xu, Mao, Wang, Zhang, Cao and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Enyan Yu, eXVlbnlhbkBhbGl5dW4uY29t
 Tianmei Xu1
Tianmei Xu1 Yanping Mao
Yanping Mao Ye Wang
Ye Wang Wenxuan Zhang
Wenxuan Zhang Enyan Yu
Enyan Yu