- 1Department of Mental Health, Asl Napoli 1 Centro, Naples, Italy
- 2Department of Mental Health, Asl Cuneo 2, Bra, Italy
- 3Department of Mental Health, Asl Biella, Biella, Italy
- 4Department of Biomedical and Clinical Sciences Luigi Sacco, Department of Psychiatry, ASST Fatebenefratelli-Sacco, University of Milan, Milan, Italy
- 5Department of Mental Health, Asl Teramo, Teramo, Italy
- 6Department of Psychiatry and Behavioral Sciences, Bipolar Disorders Clinic, Stanford Medical School, Stanford University, Stanford, CA, United States
- 7CRC ‘Aldo Ravelli’ for Neurotechnology & Experimental Brain Therapeutics, Milan, Italy
- 8Centro per lo Studio dei Meccanismi Molecolari alla base delle Patologie neuro-psico-geriatriche, University of Milan, Milan, Italy
Introduction: Treatment-resistant obsessive-compulsive disorder (OCD) remains a major clinical challenge, with a substantial proportion of patients failing to respond to standard treatment with selective serotonin reuptake inhibitors (SSRI). Vortioxetine, a multimodal antidepressant approved for major depressive disorder, has shown potential advantages in terms of tolerability and cognitive enhancement, but its efficacy in OCD has not been systematically explored.
Methods: This multicenter, retrospective, observational study analyzed the clinical records of 64 adult patients with a DSM-5 diagnosis of OCD who had failed to respond to at least one adequate SSRI trial and were treated with vortioxetine monotherapy (minimum dose: 20 mg/day; duration: ≥8 weeks). The primary outcome was reduction in total Y-BOCS score. Secondary outcomes included changes in HAM-D and HAM-A scores and frequency of adverse events.
Results: At week 8, 39.1% of patients met responder criteria (≥25% reduction in total Y-BOCS score). The mean Y-BOCS score decreased from 27.1 to 20.7 (p < 0.001). HAM-D and HAM-A scores showed significant improvements (HAM-D: from 21.0 to 12.6; HAM-A: from 26.9 to 16.1; both p < 0.001). The treatment was well tolerated, with nausea (29.7%) and sedation (18.8%) being the most common side effects; no serious adverse events occurred.
Conclusion: This study provides preliminary evidence of the efficacy and tolerability of vortioxetine monotherapy in SSRI-resistant OCD. The observed improvements in OCD, depressive and anxiety symptoms suggest that vortioxetine may represent a valuable therapeutic option. Further prospective controlled trials are needed to confirm these findings.
1 Introduction
Obsessive-compulsive disorder (OCD) is a chronic and distressing psychiatric disorder characterized by both intrusive thoughts (obsessions) and repetitive behaviors or mental actions (compulsions) (1). The global prevalence of the disorder is estimated at 2-3%, with a profound impact on individual functioning, quality of life and healthcare costs (2). Standard first-line treatments include selective serotonin reuptake inhibitors (SSRIs) and cognitive behavioral therapy, often with exposure and response prevention (3). However, despite these well-established interventions, a significant proportion of patients show only a partial response, requiring alternative pharmacological approaches to achieve adequate symptom control (4).
Furthermore, both pharmacological and placebo interventions are generally less effective in treating OCD than other anxiety disorders (5). Meta-analytic evidence suggests that the placebo effect in OCD trials is significantly lower than in generalized anxiety disorder, panic disorder and post-traumatic stress disorder, suggesting a higher threshold for symptom relief and a need for novel therapeutic strategies (5, 6). Treatments such as SSRIs, which are the most commonly used first-line pharmacological approach, usually show moderate responses, with approximately 40-60% of patients reporting residual symptoms and up to 30% considered treatment-resistant (4, 7). Current augmentation strategies, such as the addition of atypical antipsychotics, have shown some efficacy, but their side effects burden often limits long-term use (2, 8, 9). Among second generation antipsychotics risperidone and aripiprazole exhibit more robust evidences (10, 11), while paliperidone, quetiapine and olanzapine showed inconsistent results failing to outperform placebo in meta-analyses (12, 13). Recently, both cariprazine and brexpiprazole have shown preliminary promising safety and efficacy among resistant OCD augmentation strategies (14–16) but these results need confirmation on larger populations. Given these limitations, there is growing interest in vortioxetine as a potential monotherapy for treatment resistant OCD.
Vortioxetine, a multimodal serotonergic agent, is primarily approved for major depressive disorder (MDD), but its unique pharmacodynamic properties have attracted interest as a potential treatment for OCD (17, 18). Unlike conventional SSRIs, vortioxetine acts as a serotonin transporter inhibitor while modulating multiple serotonin receptors, including 5-HT1A agonism and 5-HT3, 5-HT7 and 5-HT1D antagonism. This pharmacological profile may improve cognitive flexibility and emotional regulation, both implicated in OCD (19). In addition, the pharmacokinetic profile of vortioxetine, characterized by a half-life of approximately 66 hours and minimal interactions with cytochrome P450 enzymes, may offer advantages in tolerability and dosing convenience compared to other serotonergic antidepressants (18).
Moreover, preliminary studies suggest that its multimodal action may provide benefits beyond those of conventional SSRIs, particularly in addressing cognitive rigidity, which is a core feature of OCD (20). In addition, its favorable tolerability profile and lower propensity for sexual dysfunction or weight gain compared with other antidepressant agents may improve adherence, overall treatment outcomes and satisfaction (18).
Despite its theoretical advantages, clinical data on the efficacy of vortioxetine in OCD remain scarce. To date, no study has systematically evaluated its impact on OCD symptomatology. This study aims to fill this gap by presenting real-world data on the efficacy and tolerability of vortioxetine monotherapy in a sample of 64 patients diagnosed with OCD who have failed to respond to SSRI treatment.
2 Subjects and methods
This is a multicenter, retrospective, observational study analyzing the clinical records of inpatients and outpatients diagnosed with OCD according to DSM-5 criteria (1). Patients were treated between January 2023 and December 2024 at the Mental Health Departments of Alba-Bra, Biella, Naples, and Teramo, as well as at the Department of Biomedical and Clinical Sciences Luigi Sacco, University of Milan, Italy. To be included in the analysis patients had to meet the following criteria: age ≥18 years; a total score of ≥16 on the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) (21); documented failure to respond to at least one adequate trial of a SSRI, defined as insufficient response after a minimum of 12 weeks at a therapeutic dosage; completion of at least 8 weeks of treatment with vortioxetine monotherapy; availability of complete sociodemographic and clinical data, including the Y-BOCS, the Hamilton Depression Rating Scale (HAM-D) (22), and the Hamilton Anxiety Rating Scale (HAM-A) (23) scores at baseline, week 2, week 4, week 6 and week 8. Insufficient response was defined as less than 25% reduction in Y-BOCS score, based on clinical assessments. Although some guidelines recommend defining treatment resistance as failure of two or more SSRI trials, we adopted a more inclusive criterion to reflect real-world prescribing practices and maximize generalizability. The most frequently used SSRIs before vortioxetine initiation were sertraline (100–200 mg/day), fluoxetine (40–60 mg/day), and escitalopram (15–20 mg/day). All were administered at therapeutic dosages for a minimum of 12 weeks. Due to the retrospective design, individual medication histories were not fully available for all patients. All patients received vortioxetine at a minimum target dose of 20 mg/day, which was reached within the first 7–14 days of treatment. Initial dosing and titration were determined by clinical judgement. The use of other psychotropic medications, such as SSRIs, tricyclic antidepressants and antipsychotics, was not allowed. Concomitant medications used prior to vortioxetine initiation (e.g., for sleep or anxiety management) were allowed and recorded, but no additional psychotropic drugs were introduced during the 8-week observation period. This study was conducted in accordance with the principles of the Declaration of Helsinki. Ethical approval was not required, as this was a retrospective analysis of anonymized clinical data collected during routine care. All patients provided written informed consent for the use of their anonymized clinical data for research and educational purposes. Specific informed consent was also obtained for the off-label use of vortioxetine, which is approved in Italy solely for the treatment of MDD (maximum licensed dose: 20 mg/day). Sociodemographic, clinical, and safety data were extracted from medical records. Patients were assessed in accordance with routine clinical practice at baseline and at weeks 2, 4, 6, and 8 of treatment. All diagnoses and assessments were carried out by psychiatrists with extensive clinical experience. Treatment response was defined as a reduction of ≥25% in the total Y-BOCS score from baseline to week 8. Depressive and anxiety symptoms were assessed using the HAM-D and the HAM-A. All adverse events, whether spontaneously reported by the patients or observed by clinicians, were documented using the UKU Side Effect Rating Scale (24). Statistical analyses were conducted using IBM SPSS Statistics Software, version 19. Paired t-tests were used to assess differences in Y-BOCS, HAM-D, and HAM-A scores between baseline and week 8. Repeated measures ANOVA was also performed to evaluate changes in these scores over time. Statistical significance was set at p-value < 0.05.
3 Results
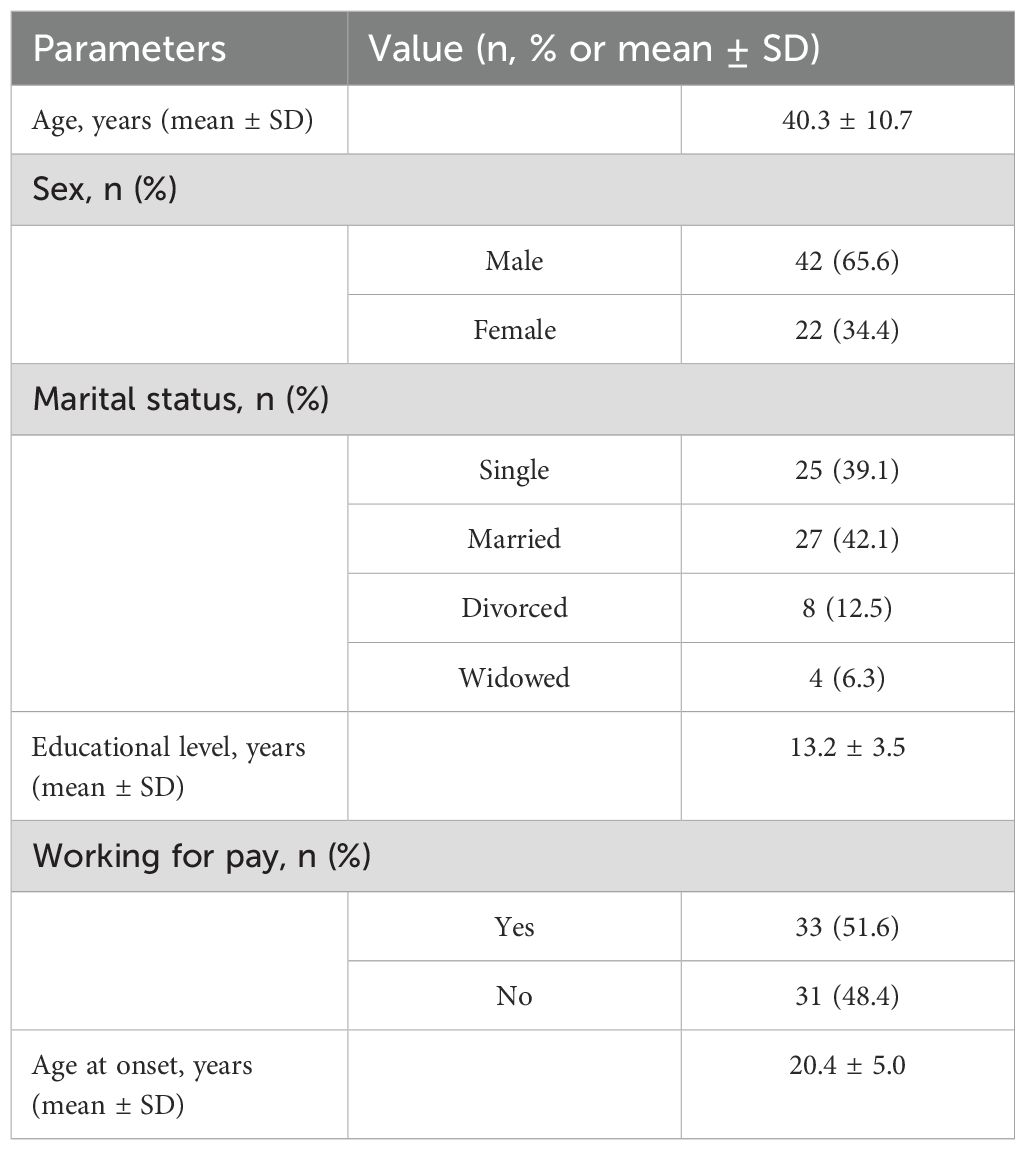
A total of 64 patients met inclusion criteria and were included in the analysis. Tables 1, 2, 3 report the sociodemographic and clinical characteristics of the sample. The mean daily dose of vortioxetine was 25.9 mg (± 6.2); 6 patients (9.4%) received the dose of 40 mg/day.
3.1 Efficacy
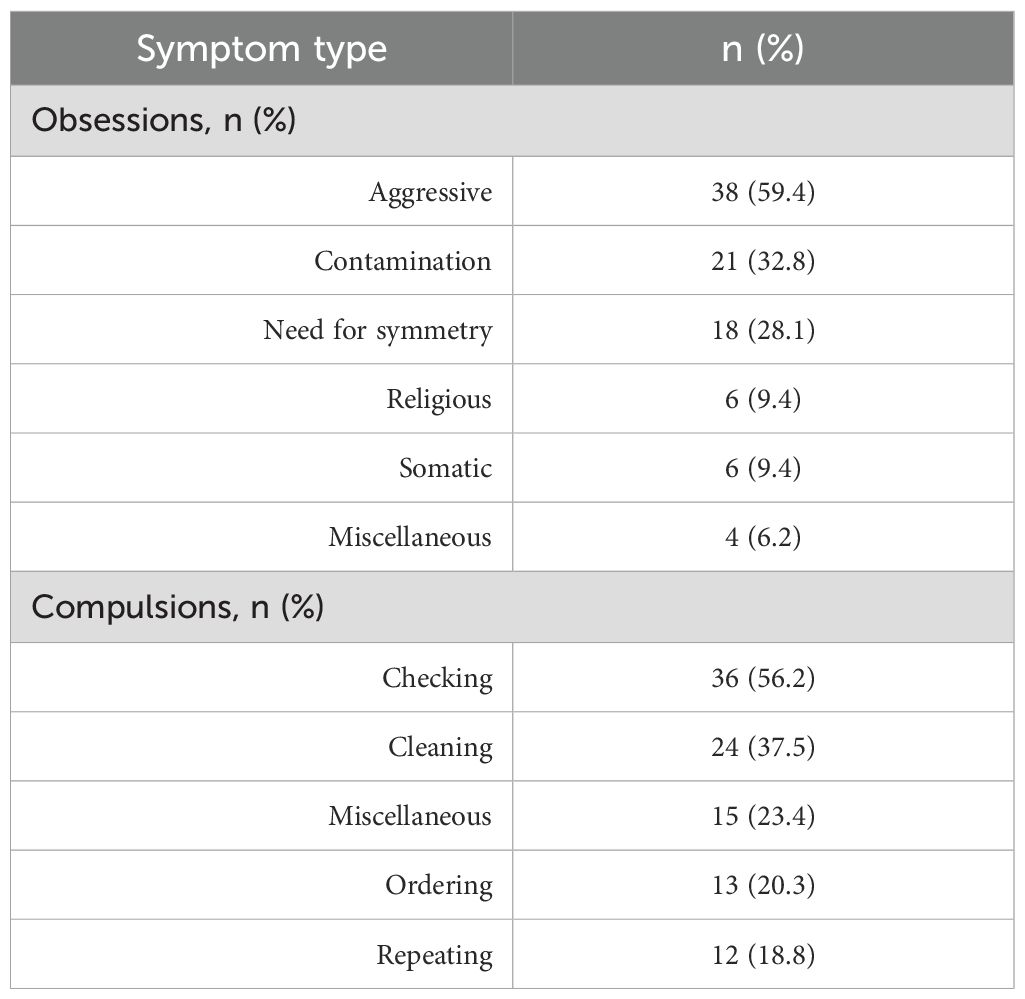
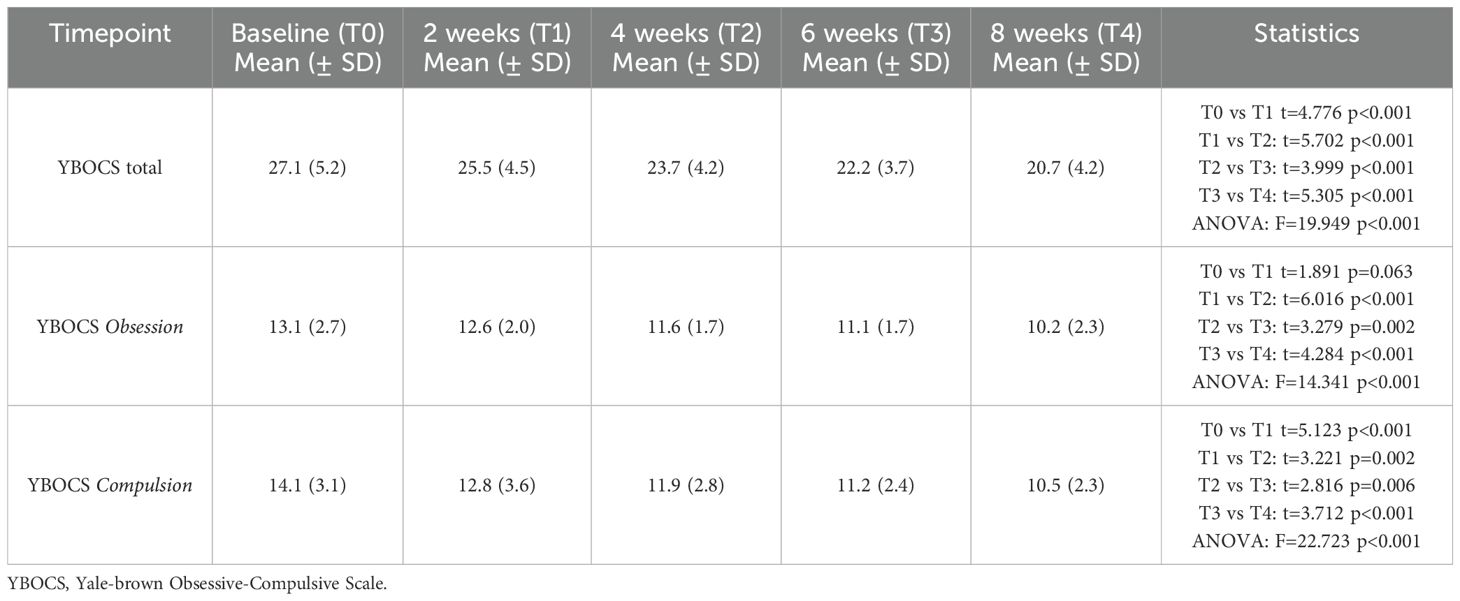
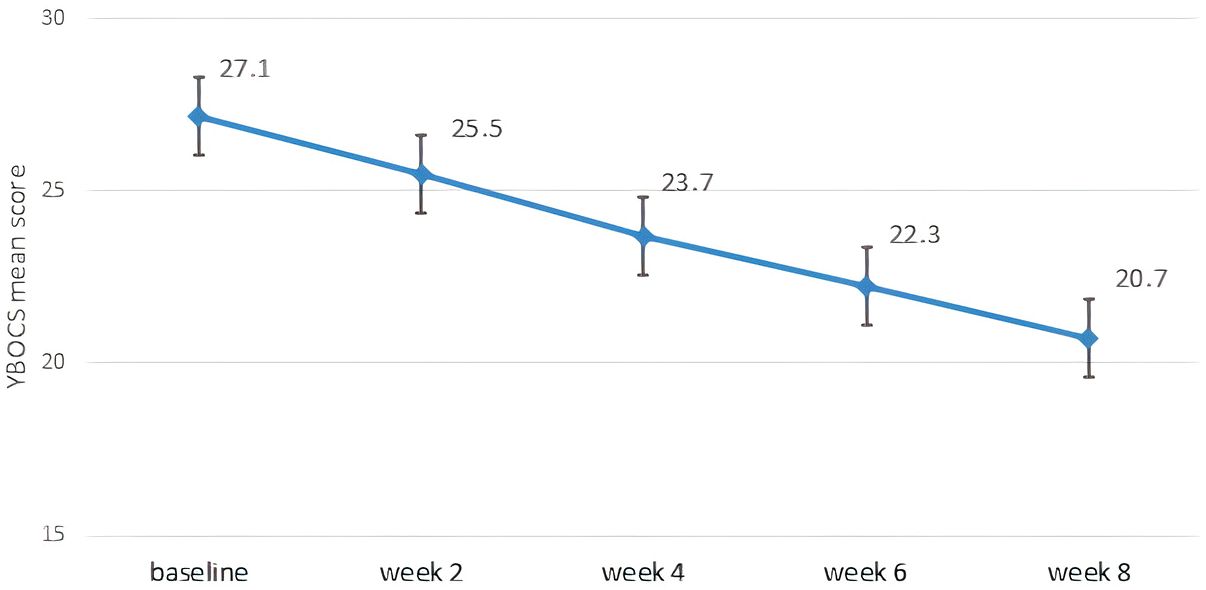
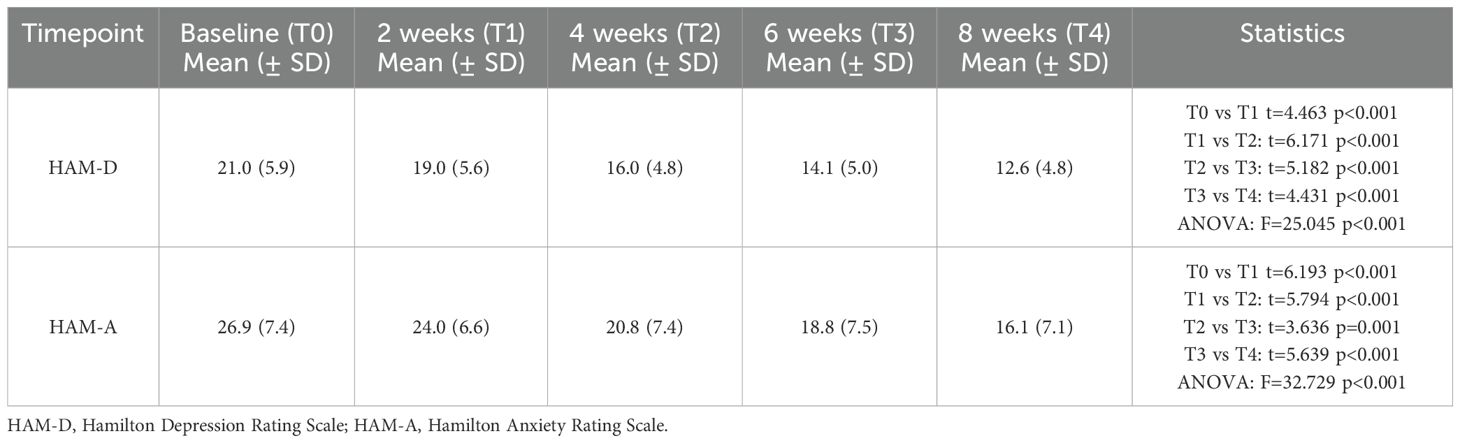
A significant reduction in obsessive-compulsive symptoms was observed over the 8-week study period. The total Y-BOCS score decreased from a baseline mean of 27.1 (± 5.2) to 20.7 (± 4.2) at week 8 (p<0.001, paired t-test). Improvements were also observed in both the obsessions and compulsions subscales (Table 4). Figure 1 illustrates the progressive reduction in total Y-BOCS scores across the study time points. The mean obsession score declined from 13.1 to 10.2, while the mean compulsion score declined from 14.1 to 10.5. Repeated measures ANOVA confirmed significant reductions over time for all Y-BOCS domains (total score: F=19.949, p<0.001). At endpoint, 25 patients (39.1%) met responder criteria, defined as a ≥25% reduction in total Y-BOCS score from baseline. Significant reductions in depressive and anxiety symptoms were also observed over time (Table 5). The mean HAM-D score decreased from 21.0 (± 5.9) at baseline to 12.6 (± 4.8) at week 8 (F=25.045, p<0.001), while the mean HAM-A score decreased from 26.9 (± 7.4) to 16.1 (± 7.1) (F= 32.729, p<0.001).
3.2 Safety and tolerability
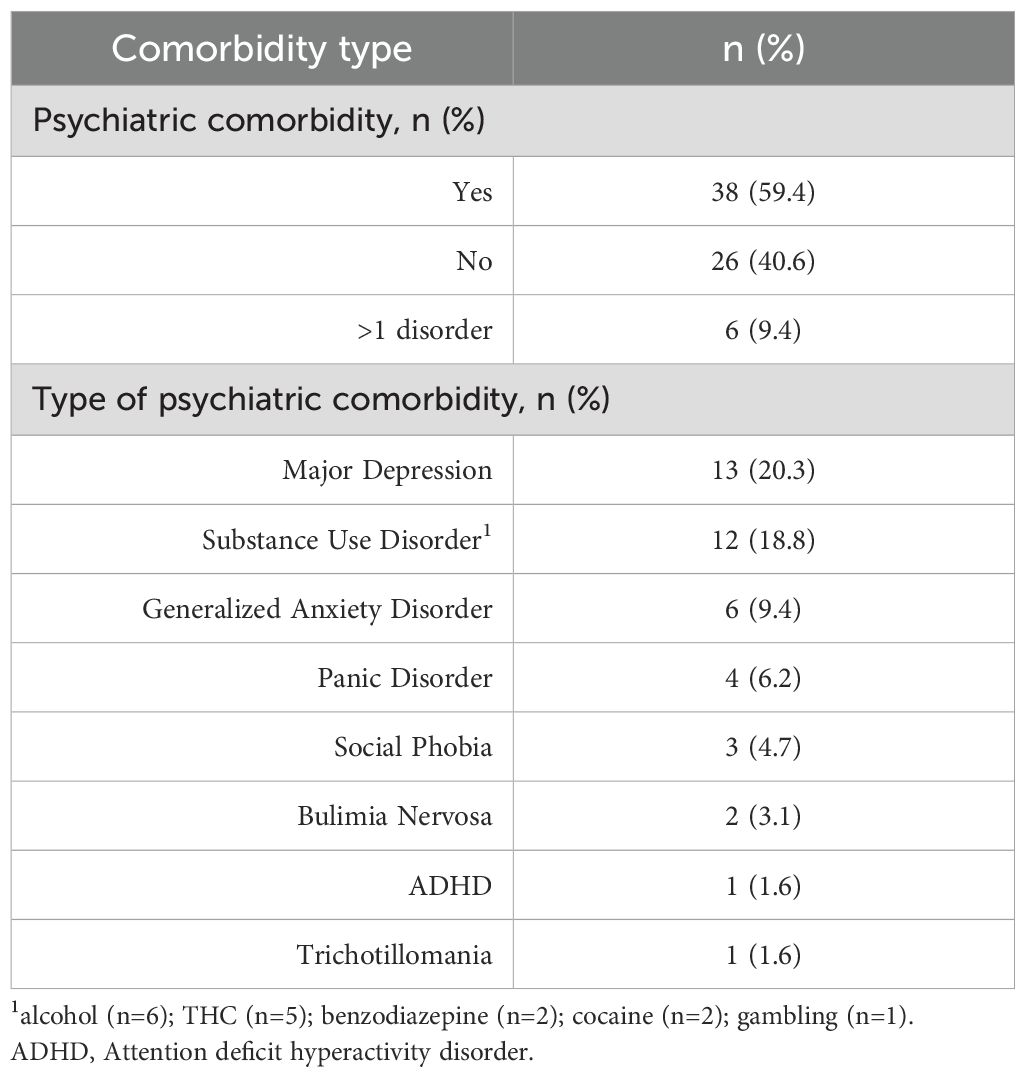
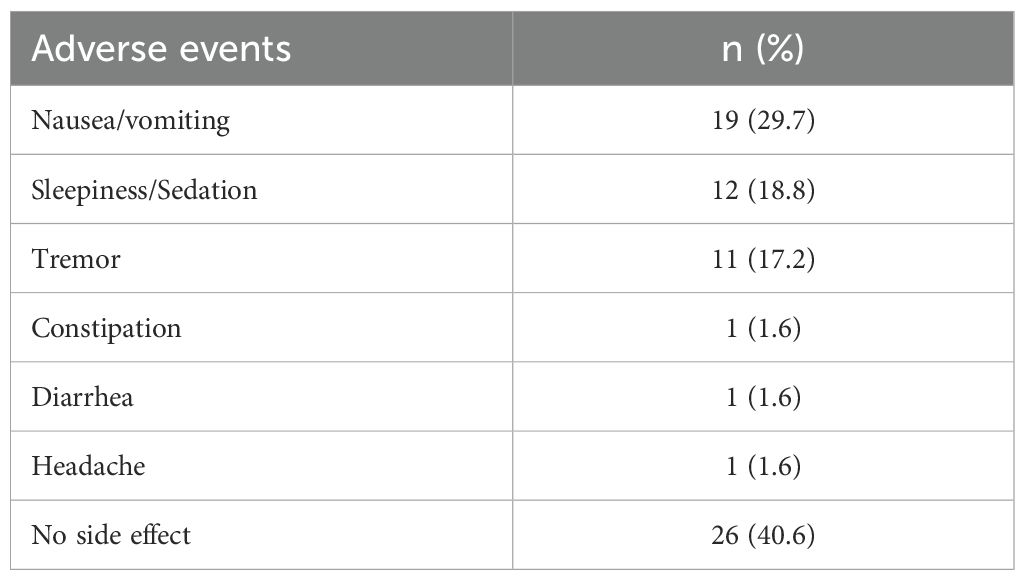
Overall, 38 patients (59.4%) reported at least one adverse event. All side effects were rated as mild. The most common was nausea or vomiting, reported by 19 patients (29.7%), followed by sleepiness or sedation (18.8%) and tremor (17.2%). A full list of reported adverse events is presented in Table 6. No safety issue or serious adverse events were reported.
4 Discussion
This is, to our knowledge, the first clinical study to systematically evaluate vortioxetine monotherapy in patients with SSRI-resistant OCD. In our 8-week retrospective observational analysis, approximately 39% of patients achieved a ≥25% improvement in total Y-BOCS score. This finding supports the pharmacodynamic rationale suggesting that vortioxetine’s multimodal serotonergic action may benefit patients who have failed to respond to previous SSRI treatment (18, 25). While this response rate may appear modest, it is clinically meaningful in a treatment-resistant population where even partial improvement can represent a significant therapeutic gain. This response rate is broadly consistent with those observed in SSRI augmentation trials using atypical antipsychotics such as risperidone or aripiprazole, which typically show response rates around 35–40% in treatment-resistant OCD populations (10, 11). Other agents like quetiapine or olanzapine have demonstrated less robust or inconsistent efficacy (12, 13), and switching strategies—whether to a different SSRI or to clomipramine—may be limited by tolerability concerns or variable outcomes (24). In this context, vortioxetine may represent a viable monotherapy alternative, warranting further investigation. Furthermore, no patient experienced a significant worsening of OCD symptoms, highlighting the tolerability and overall safety of the drug in this difficult-to-treat population.
Given the lack of prior studies evaluating vortioxetine in OCD, direct comparisons are limited. However, findings from the TRUE study (26), a recent similar 8-week observational study in patients with comorbid MDD and generalized anxiety disorder (GAD) showed significant improvement in depressive symptoms, as measured by the Montgomery–Åsberg Depression Rating Scale (MADRS) and a marked reduction in HAM-A scores, with most patients moving from moderate/severe to mild anxiety over the observation period. Similarly, our study documented meaningful reductions in HAM-D and HAM-A scores, suggesting that vortioxetine’s antidepressant and anxiolytic properties may extend to patients with SSRI-resistant OCD. This is particularly relevant given that depressive and anxiety symptoms are common in OCD and may negatively impact global functioning and treatment response (27). A pharmacological agent capable of addressing both OCD and affective symptoms may thus offer a valuable treatment option in complex and comorbid clinical presentations. Although Badr et al. enrolled patients with MDD and GAD, the parallel findings of mood and anxiety relief, coupled with good tolerability, underscore a consistent therapeutic signal that merits further, larger confirmatory investigations.
In contrast to the TRUE study, which enrolled patients predominantly from the United Arab Emirates, the RECONNECT trial (28) was carried out in European and Asian countries which are often considered more similar to our local healthcare context. This study involved adult outpatients diagnosed with severe MDD with comorbid severe GAD. Notably, RECONNECT mandated a forced up-titration from 10 mg to 20 mg of vortioxetine after the first week, resulting in almost all patients reaching and maintaining the 20 mg dose for 8 weeks. This protocol highlighted both the feasibility and tolerability of early dose escalation in a difficult-to-treat group. In our study, the average dose of vortioxetine was 26 mg/day, with titration up to 40 mg/day in 10% of patients. Although only a subset of our patients received higher dosages, the results still support the tolerability of vortioxetine at higher than usual doses in complex clinical settings, even in a shorter time frame. This dosing strategy reflects clinical decisions made in a treatment-resistant population and is supported by vortioxetine’s favorable safety profile. Previous studies and real-world data suggest that upward titration may be feasible and well-tolerated, even beyond the approved range for depression, particularly when standard doses prove insufficient in severe or refractory cases. A recently published umbrella review by Wang et al. (29) indicates that the recommended range of vortioxetine doses for adults with MDD is generally 5–20 mg/day, with 20 mg/day emerging as the dose most consistently linked to robust improvements in depressive symptoms, cognition, anxiety, and quality of life (30, 31). Importantly, the review suggests that while 10 mg/day is often effective for depression, a clear dose-response pattern supports further benefit at 20 mg/day, without a concomitant, clinically prohibitive increase in side effects. However, the dose range reported by Wang et al. (29) may not fully account for the characteristics of OCD. Given that OCD often proves more resistant and may require more aggressive antidepressant dosing strategies, our use of vortioxetine up to 40 mg/day aligns with the broader principle that 20 mg/day is typically the upper standard dose for MDD and related conditions, yet individual patients with refractory features may benefit from a cautious exploration of higher doses. Our study’s retrospective design and real-world setting did not allow for the systematic evaluation of higher doses of vortioxetine in all patients. As a result, we were unable to draw firm conclusions about the relative benefits or risks of escalating vortioxetine beyond 20 mg/day in this population. Future prospective, dose-ranging research in SSRI-resistant OCD will be essential to clarify the potential added value of doses above the conventional day limit.
The tolerability profile of vortioxetine observed in our study aligns with findings from previous clinical trials and meta-analyses. In our sample, the most frequently reported adverse event was nausea, consistent with the RECONNECT and TRUE studies, which also identified it as the most common side effect. Similarly, the network meta-analysis by Kishi et al. (32) confirmed an increased incidence of nausea and vomiting associated with vortioxetine compared with placebo. However, no serious adverse events leading to discontinuation were observed in our study, in contrast to the RECONNECT study, in which two patients discontinued treatment due to dysgeusia and hypersensitivity, and the TRUE study, in which one serious adverse event (0.8%) was reported. Notably, the mean daily dose in our study (25.9 mg/day, with 10% of patients reaching 40 mg/day) was higher than the standard 10–20 mg/day used in the two other studies, but the tolerability remained comparable. The tolerability profile of vortioxetine contrasts with second-line strategies such as high-dose SSRI or augmentation with antipsychotics, which may carry a greater risk of side effects (3, 13). Further research is needed to clarify whether individual susceptibility to adverse effects of vortioxetine varies with dose and patient characteristics. Although SSRIs remain the first-line therapy (4), approximately 40–60% of patients do not attain complete remission (13), highlighting the need for alternative approaches. The combination of 5-HT1A agonism and 5-HT3/5-HT7 antagonism inherent in vortioxetine has been proposed to improve cognitive flexibility and reduce obsessive thoughts (19). Our data, showing significant changes in the Y-BOCS alongside tolerable side effects, suggest that this multimodal mechanism may translate into clinically meaningful benefits for at least a subset of patients with SSRI-resistant OCD. From a clinical perspective, vortioxetine may be considered as a monotherapy option in SSRI-resistant OCD, particularly in patients who have experienced poor tolerability or limited benefit from augmentation strategies. When using doses above 20 mg/day, we recommend close monitoring of tolerability and symptom progression during the first 2–4 weeks. Informed consent should include discussion of the off-label nature of this indication. In our sample, no serious adverse events were reported, and even higher doses were well tolerated, which supports its safety in real-world settings when appropriately monitored.
Despite these promising results, several limitations warrant caution. First, the lack of a control group precludes definitive conclusions about the efficacy of vortioxetine compared with other treatments. Second, the observational design and the relatively short follow-up period of 8 weeks limit the assessment of long-term outcomes and relapse rates. In addition, the study did not include functional or patient-reported outcome measures that could have provided further insight into the clinical relevance of symptom changes. Third, our sample size, although adequate for a preliminary assessment, limits the statistical power to detect small differences or to conduct meaningful subgroup analyses (e.g., by specific OCD symptom dimensions). Fourth, we did not systematically control for concomitant psychosocial interventions or for previous SSRIs, doses and treatment duration, which may have influenced response. Finally, the non-randomized selection of patients, based on clinician judgment for vortioxetine initiation, may have introduced bias that could limit the generalizability of our findings. In addition, the limited sample size may have reduced the statistical power to detect small or moderate effects and precluded subgroup analyses based on clinical or demographic variables. As a result, the findings should be interpreted as preliminary and exploratory. Given the retrospective design and absence of a control group, causal interpretations must be made with caution. Observed improvements could partially reflect factors unrelated to vortioxetine itself, including spontaneous symptom fluctuation, regression to the mean, or contextual therapeutic effects. Future randomized controlled trials will be necessary to isolate the drug’s specific contribution. Although we adopted a ≥25% Y-BOCS reduction as the threshold for treatment response, more stringent criteria such as a 35% reduction have been proposed (33), which may limit direct comparability with other recent studies. Moreover, we were unable to systematically retrieve data on the time elapsed between OCD onset and the initiation of first-line treatment, which may have influenced illness course and treatment responsiveness.
5 Conclusion
This retrospective study provides preliminary evidence supporting the potential efficacy and tolerability of vortioxetine in patients with SSRI-resistant OCD. The observed reduction in obsessive-compulsive, depressive, and anxiety symptoms highlights the potential of multimodal serotonergic modulation for refractory cases. Future studies should include randomized controlled trials comparing vortioxetine not only to placebo but also to established pharmacological strategies such as SSRI augmentation with antipsychotics. Trials should adopt standardized responder criteria (e.g., ≥35% Y-BOCS reduction) and incorporate multidimensional outcome measures, including functional status, quality of life, and cognitive flexibility. Stratification by prior treatment history and symptom dimensions may also help identify patient subgroups most likely to benefit from vortioxetine monotherapy. Longitudinal follow-up and multidimensional outcome measures would help clarify the durability and magnitude of therapeutic effects. If confirmed, vortioxetine could become part of a broader pharmacological toolkit aimed at improving outcomes in this challenging patient population.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
VM: Conceptualization, Writing – original draft, Writing – review & editing. EP: Conceptualization, Writing – review & editing, Formal Analysis. CC: Methodology, Data curation, Writing – review & editing. AM: Writing – review & editing, Data curation. FR: Data curation, Writing – review & editing. TP: Writing – review & editing, Data curation. MO: Writing – review & editing, Data curation. DD: Writing – review & editing, Data curation. BB: Writing – review & editing, Methodology. BD’O: Methodology, Supervision, Conceptualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: APA (2013).
2. Fineberg NA, Chamberlain SR, and Roberts A. Multidisciplinary approaches to the treatment of obsessive–compulsive disorder: Current perspectives. Neuropsychiatr Dis Treat. (2019) 15:2337–51. doi: 10.2147/NDT.S187048
3. Benatti B, Camuri G, Dell’Osso B, Cremaschi L, Sembira E, Palazzo C, et al. Which factors influence onset and latency to treatment in generalized anxiety disorder, panic disorder, and obsessive–compulsive disorder? Int Clin Psychopharmacol. (2016) 31:347–52. doi: 10.1097/YIC.0000000000000137
4. Pittenger C and Bloch MH. Pharmacological treatment of obsessive-compulsive disorder. Psychiatr Clin North Am. (2014) 37:375–91. doi: 10.1016/j.psc.2014.05.006
5. Sugarman MA, Kirsch I, and Huppert JD. Obsessive-compulsive disorder has a reduced placebo (and antidepressant) response compared to other anxiety disorders: A meta-analysis. J Affect Disord. (2017) 218:217–26. doi: 10.1016/j.jad.2017.04.068
6. Loerinc AG, Meuret AE, Twohig MP, Rosenfield D, Bluett EJ, and Craske MG. Response rates for CBT for anxiety disorders: Need for standardized criteria. Clin Psychol Rev. (2015) 42:72–82. doi: 10.1016/j.cpr.2015.08.004
7. Pallanti S and Quercioli L. Treatment-refractory obsessive-compulsive disorder: methodological issues, operational definitions and therapeutic lines. Prog Neuropsychopharmacol Biol Psychiatry. (2006) 30:400–12. doi: 10.1016/j.pnpbp.2005.11.028
8. Maina G, Albert U, Pessina E, Salvi V, and Bogetto F. Antipsychotics in obsessive-compulsive disorder. Curr Psychiatry Rev. (2000) 1:293–301. doi: 10.2174/157340005774575028
9. Van Roessel PJ, Grassi G, Aboujaoude EN, Menchón JM, Van Ameringen M, and Rodríguez CI. Treatment-resistant OCD: pharmacotherapies in adults. Compr Psychiatry. (2023) 120:152352. doi: 10.1016/j.comppsych.2022.152352
10. Suhas S, Malo PK, Kumar V, Issac TG, Chithra NK, Bhaskarapillai B, et al. Treatment strategies for serotonin reuptake inhibitor-resistant obsessive-compulsive disorder: a network meta-analysis of randomized controlled trials. World J Biol Psychiatry. (2023) 24:162–77. doi: 10.1080/15622975.2022.2082525
11. Zhou DD, Zhou XX, Li Y, Zhang KF, Lv Z, Chen XR, et al. Augmentation agents to serotonin reuptake inhibitors for treatment-resistant obsessive-compulsive disorder: a network meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. (2019) 90:277–87. doi: 10.1016/j.pnpbp.2018.12.009
12. Komossa K, Depping AM, Meyer M, Kissling W, and Leucht S. Second-generation antipsychotics for obsessive compulsive disorder. Cochrane Database Syst Rev. (2010) 12:CD008141. doi: 10.1002/14651858.CD008141.pub2
13. Dold M, Aigner M, Lanzenberger R, and Kasper S. Antipsychotic augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder: a meta-analysis of double-blind, randomized, placebo-controlled trials. Int J Neuropsychopharmacol. (2013) 16:557–74. doi: 10.1017/S1461145712000740
14. Martiadis V, Pessina E, Martini A, Raffone F, Cattaneo CI, De Berardis D, et al. Serotonin reuptake inhibitors augmentation with cariprazine in patients with treatment-resistant obsessive-compulsive disorder: a retrospective observational study. CNS Spectr. (2024) 22:1–4. doi: 10.1017/S1092852924000348
15. Martiadis V, Pessina E, Martini A, Raffone F, Besana F, Olivola M, et al. Brexpiprazole augmentation in treatment resistant OCD: safety and efficacy in an Italian sample. Psychiatr Danub. (2024) 36:396–401.
16. Giacovelli L, Piccoli E, Landi P, Vismara M, Benatti B, and Dell’Osso B. Brexpiprazole augmentation in treatment-resistant obsessive-compulsive disorder: a preliminary retrospective observational study. Int Clin Psychopharmacol. (2025). doi: 10.1097/YIC.0000000000000583
17. Pizarro M, Fontenelle LF, Paravidino DC, Yücel M, Miguel EC, and de Menezes GB. An updated review of antidepressants with marked serotonergic effects in obsessive-compulsive disorder. Expert Opin Pharmacother. (2014) 15:1391–401. doi: 10.1517/14656566.2014.914493
18. Huang IC, Chang TS, Chen C, and Sung JY. Effect of vortioxetine on cognitive impairment in patients with major depressive disorder: a systematic review and meta-analysis of randomized controlled trials. Int J Neuropsychopharmacol. (2022) 25:969–78. doi: 10.1093/ijnp/pyac054
19. Millan MJ, Goodwin GM, and Meyer-Lindenberg A. The bioRx model of vortioxetine. Int J Neuropsychopharmacol. (2017) 20:1–12. doi: 10.1093/ijnp/pyw081
20. Fineberg NA, Day GA, de Koenigswarter N, Reghunandanan S, Kolli S, Jefferies-Sewell K, et al. The neuropsychology of obsessive-compulsive personality disorder: a new analysis. CNS Spectr. (2015) 20:490–9. doi: 10.1017/S1092852914000662
21. Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, et al. The Yale-Brown Obsessive Compulsive Scale: Development, use, and reliability. Arch Gen Psychiatry. (1989) 46:1006–11. doi: 10.1001/archpsyc.1989.01810110048007
22. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. (1960) 23:56–62. doi: 10.1136/jnnp.23.1.56
23. Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. (1959) 32:50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x
24. Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K, et al. The UKU side effect rating scale. Acta Psychiatr Scand Suppl. (1987) 334:1–100. doi: 10.1111/j.1600-0447.1987.tb10566.x
25. Fineberg NA, Reghunandanan S, Simpson HB, Phillips KA, Richter MA, Matthews K, et al. Obsessive-compulsive disorder (OCD): Practical strategies for pharmacological and somatic treatment in adults. Psychiatry Res. (2015) 227:114–25. doi: 10.1016/j.psychres.2014.12.003
26. Badr B, Al Gailani H, Alkhoori S, Butt H, Daher M, Dheyaa B, et al. Effectiveness of 8-week treatment with vortioxetine on depressive symptoms in major depressive disorder patients with comorbid generalized anxiety disorder in UAE (TRUE). Ann Gen Psychiatry. (2024) 23:41. doi: 10.1186/s12991-024-00526-w
27. Ruscio AM, Stein DJ, Chiu WT, and Kessler RC. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry. (2010) 15:53–63. doi: 10.1038/mp.2008.94
28. Christensen MC, Schmidt S, and Grande I. Effectiveness of vortioxetine in patients with major depressive disorder comorbid with generalized anxiety disorder: Results of the RECONNECT study. J Psychopharmacol. (2022) 36:566–77. doi: 10.1177/02698811221090627
29. Wang P, Wang WW, Liu YQ, Li WQ, Hu JX, Su YA, et al. The dose-response relationship of vortioxetine on major depressive disorder: an umbrella review. Psychiatry Res. (2024) 340:116118. doi: 10.1016/j.psychres.2024.116118
30. Adair M, Christensen MC, Florea I, Loft H, and Fagiolini A Vortioxetine in patients with major depressive disorder and high levels of anxiety symptoms: An updated analysis of efficacy and tolerability. J Affect Disord. (2023) 328:345–54. doi: 10.1016/j.jad.2023.01.074
31. Baldwin DS, Anderson IM, Nutt DJ, Allgulander C, Bandelow B, den Boer JA, et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: A revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol. (2014) 28:403–39. doi: 10.1177/0269881114525674
32. Kishi T, Ikuta T, Sakuma K, Okuya M, Hatano M, Matsuda Y, et al. Antidepressants for the treatment of adults with major depressive disorder in the maintenance phase: a systematic review and network meta-analysis. Mol Psychiatry. (2023) 28:402–9. doi: 10.1038/s41380-022-01824-z
Keywords: vortioxetine, obsessive-compulsive disorder, treatment-resistant, efficacy, tolerability, SSRI-resistant
Citation: Martiadis V, Pessina E, Cattaneo CI, Martini A, Raffone F, Prodi T, Olivola M, De Berardis D, Benatti B and Dell’Osso BM (2025) Efficacy and tolerability of vortioxetine monotherapy in SSRI-resistant OCD: a retrospective multicenter study. Front. Psychiatry 16:1617345. doi: 10.3389/fpsyt.2025.1617345
Received: 24 April 2025; Accepted: 20 May 2025;
Published: 13 June 2025.
Edited by:
Amir Garakani, Yale University, United StatesReviewed by:
Valerio Ricci, San Luigi Gonzaga University Hospital, ItalyDamien Doolub, Henri Laborit Hospital Center, France
Copyright © 2025 Martiadis, Pessina, Cattaneo, Martini, Raffone, Prodi, Olivola, De Berardis, Benatti and Dell’Osso. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vassilis Martiadis, dmFzc2lsaXMubWFydGlhZGlzQGdtYWlsLmNvbQ==
 Vassilis Martiadis
Vassilis Martiadis Enrico Pessina2
Enrico Pessina2 Fabiola Raffone
Fabiola Raffone Domenico De Berardis
Domenico De Berardis Bernardo Maria Dell’Osso
Bernardo Maria Dell’Osso