- 1Medical Affairs, H. Lundbeck A/S, Copenhagen, Denmark
- 2Medical and Real-World Data Analytics, Otsuka Pharmaceutical Development & Commercialization Inc., Princeton, NJ, United States
- 3Medical Affairs, Otsuka Pharmaceutical Development & Commercialization Inc., Princeton, NJ, United States
Introduction: Sleep disturbances are common in major depressive disorder (MDD). This post hoc analysis aimed to evaluate the effects of adjunctive brexpiprazole in patients with MDD and sleep disturbance.
Methods: Data were pooled from three placebo-controlled trials of adjunctive brexpiprazole in patients with MDD and inadequate response to antidepressant treatments (ADTs) (ClinicalTrials.gov identifiers: NCT01360645, NCT01360632, NCT02196506). Using the Hamilton Depression Rating Scale Sleep Disturbance Factor (SDF) (sum of three insomnia items), patients were categorized by high (SDF ≥4) or low (SDF <4) baseline sleep disturbance. Change in Montgomery–Åsberg Depression Rating Scale (MADRS) Total, SDF, and other efficacy scores were evaluated for ADT + brexpiprazole 2 or 3 mg versus ADT + placebo. Safety was assessed by the incidence of treatment-emergent adverse events (TEAEs).
Results: At baseline, 689/1,160 (59.4%) patients had high sleep disturbance, and 471/1,160 (40.6%) had low sleep disturbance. At Week 6, ADT + brexpiprazole showed greater improvement in MADRS Total score versus ADT + placebo in both subgroups (high SDF: p<0.0001; low SDF: p=0.0058), and greater SDF score improvement in the high SDF subgroup (p=0.021). The incidence of TEAEs was higher with ADT + brexpiprazole than ADT + placebo in the high SDF subgroup (59.8%, 51.6%) and the low SDF subgroup (62.4%, 40.9%).
Conclusion: Over 6 weeks, adjunctive brexpiprazole was associated with improved depression severity versus adjunctive placebo, regardless of baseline sleep disturbance. In patients with high baseline sleep disturbance, improvement in sleep disturbance was greater with adjunctive brexpiprazole versus adjunctive placebo, and was generally not accompanied by daytime sedation. No new safety signals were observed within each subgroup.
1 Introduction
Sleep disturbances such as insomnia (difficulty sleeping) and hypersomnia (excessive sleeping) are frequently reported by patients with major depressive disorder (MDD) (1–3), and are included in MDD diagnostic criteria (4). As a pervasive feature of depression, sleep disturbances often precede depressive episodes and can persist as residual symptoms during remission in patients taking antidepressant treatment (ADT) (1, 5–7). Insomnia is associated with increased severity and duration of depressive episodes, and increased risk of relapse (8). Insomnia is also a risk factor for developing treatment-resistant depression, and is 1.6 times more common in patients with inadequate response to treatment (9). Sleep disturbances in MDD negatively impact patients’ quality of life and executive functioning (3, 10). Improving sleep in patients with MDD is therefore crucial for improving overall patient outcomes (8, 11).
Brexpiprazole is an atypical antipsychotic that is approved in the United States, Canada, and various other countries (but not in the European Union) as adjunctive therapy for patients with MDD and inadequate response to ADTs (12, 13). Whereas diverse treatment strategies may be used in clinical practice (14), recent guidelines from the Canadian Network for Mood and Anxiety Treatments (CANMAT) recommend brexpiprazole (or aripiprazole) as the first-line adjunctive treatment in difficult-to-treat depression (15). Real-world studies provide evidence for the efficacy of adjunctive brexpiprazole in MDD, and potentially extending to other treatment resistance scenarios (16–21).
A previous exploratory open-label study suggested that adjunctive brexpiprazole may improve various sleep parameters in patients with MDD and sleep disturbances, including total sleep time, sleep efficiency, wake time after sleep onset, sleep onset latency, latency to persistent sleep, and circadian rhythm (22, 23). Furthermore, in randomized controlled trials, adjunctive brexpiprazole has shown efficacy versus adjunctive placebo on the sleep item of a depression rating scale (24). Thus, it may be hypothesized that adjunctive brexpiprazole can help patients with MDD and sleep-related symptoms. The aim of this post hoc analysis was to evaluate the effects of adjunctive brexpiprazole versus adjunctive placebo in patients with MDD (and inadequate response to ADTs) and sleep disturbance, using pooled data from three randomized controlled trials.
2 Methods
2.1 Study design and patients
This post hoc analysis included pooled data from three similarly designed, randomized, double-blind, placebo-controlled, Phase 3 trials of adjunctive brexpiprazole in adults with MDD and inadequate response to ADTs: Pyxis (Trial 228; ClinicalTrials.gov identifier: NCT01360645) (25), Polaris (Trial 227; NCT01360632) (26), and Sirius (Trial 214; NCT02196506) (27). All three trials were conducted in compliance with the International Council for Harmonisation Good Clinical Practice guideline and local regulatory requirements, and with the principles laid out in the Declaration of Helsinki. The protocols were approved by independent ethics committees, and all patients provided written informed consent to participate.
Detailed trial designs have been previously published (25–27). In brief, the studies enrolled outpatients aged 18–65 years with MDD as per the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) criteria (28); a current depressive episode of ≥8 weeks; an inadequate response (defined as <50% improved) to 1–3 prior ADTs during the current episode; and a Hamilton Depression Rating Scale (HAM-D17) (29, 30) Total score of ≥18. Exclusion criteria included suicidal ideation or behavior, substance abuse or dependence, and specified DSM-IV-TR comorbidities. Comorbid DSM-IV-TR sleep disorders were not exclusionary.
In the trials, eligible patients received single-blind placebo together with an investigator-determined, open-label ADT (sertraline, escitalopram, duloxetine, fluoxetine, paroxetine controlled-release, or venlafaxine extended-release) for 8 weeks. The purpose of the 8-week prospective treatment phase was to identify patients with inadequate response to an additional ADT. Inadequate response to ADT was defined as <50% reduction in HAM-D17 Total score from the start to the end of the prospective treatment phase, HAM-D17 Total score of ≥14 at the end of the prospective treatment phase, <50% reduction in Montgomery–Åsberg Depression Rating Scale (MADRS) (31) Total score from the start of the prospective treatment phase to each scheduled visit, and a Clinical Global Impression – Improvement (CGI-I) (32) score of ≥3 at each scheduled visit during the prospective treatment phase.
Patients with an inadequate response to ADT were randomized to 6 weeks of double-blind treatment with fixed-dose brexpiprazole (1, 2, or 3 mg/day, depending on the study) or placebo, adjunctive to their continued ADT. Brexpiprazole was initiated at a dose of 0.5 mg/day, titrated to 1 mg/day after 1 week, and titrated to the allocated dose after 2 weeks. Concomitant benzodiazepines and non-benzodiazepine sleep aids were prohibited except for the short-term management of treatment-emergent agitation/anxiety and insomnia, respectively; these drugs could not be taken in the 12 hours before a scheduled efficacy or safety assessment.
2.2 Sleep disturbance subgroups
In this post hoc analysis, sleep disturbance was measured by the Sleep Disturbance Factor (SDF), which is the sum of three HAM-D17 item scores: item 4 “insomnia – early”, item 5 “insomnia – middle”, and item 6 “insomnia – late” (33, 34). Each item is rated on a 3-point scale: 0 (absent), 1 (occasional), and 2 (frequent). Scores for the three insomnia items are summed to form the SDF score, which ranges from 0 (no sleep disturbance) to 6 (maximum sleep disturbance) (29, 30, 34). Patients were categorized by baseline (the randomization visit, prior to dosing) level of sleep disturbance, defined as in previous literature as high (SDF score ≥4), or low (SDF score <4) (34). Patients with an SDF score of 0 (indicating no sleep disturbance) were also included in the low SDF subgroup.
2.3 Outcome measures
The primary efficacy endpoint in each of the three trials was change in MADRS (31) Total score from baseline to Week 6 of the randomized treatment phase. The MADRS, a measure of depression severity, was administered at baseline and at weekly intervals throughout the randomized phase, and was the main depression outcome of this post hoc analysis.
Change in depression severity was also assessed using the clinician-reported HAM-D17 (29, 30) Total score and Clinical Global Impression – Severity (CGI-S) (32) score. MADRS response rates (defined as a ≥50% reduction in MADRS Total score from baseline to Week 6) were also assessed. Change in functioning was assessed using the patient-reported Sheehan Disability Scale (SDS) (35, 36) score. In this post hoc analysis, change in sleep disturbance was assessed using the SDF score, and the MADRS “reduced sleep” item score. Rating scales were administered by trained and experienced clinicians, who were certified for the trials to administer the MADRS and HAM-D17. The number of raters within each trial center was kept to a minimum.
In this post hoc analysis, safety was assessed by the incidence of treatment-emergent adverse events (TEAEs), with a focus on sedating TEAEs (somnolence, fatigue, sedation, lethargy) and activating TEAEs (akathisia, restlessness, insomnia, initial insomnia, middle insomnia, terminal insomnia).
2.4 Data analysis
In this post hoc analysis, data were pooled for adjunctive brexpiprazole doses of 2 or 3 mg/day, reflecting the recommended-to-maximum brexpiprazole doses for the adjunctive treatment of MDD in the United States (12). Separately, data were pooled for adjunctive placebo.
Efficacy analyses were conducted for all patients randomized per final protocols who received at least one dose of double-blind medication and had both a baseline and at least one post-baseline MADRS Total evaluation in the randomized treatment phase. Safety analyses were conducted for all patients who received at least one dose of double-blind medication in the randomized treatment phase. Patients without a baseline HAM-D17 measurement could not be categorized into SDF subgroups, and were therefore excluded. Change in MADRS Total score, change in HAM-D17 Total score, change in CGI-S score, MADRS response rates, and change in SDS score were assessed in the high SDF and low SDF subgroups. Change in sleep endpoints – SDF score, and MADRS “reduced sleep” item score – were assessed in the high SDF subgroup, only.
Patient baseline demographic and clinical characteristics were summarized using descriptive statistics. For MADRS Total score, CGI-S score, SDS score, and MADRS “reduced sleep” item score, least squares (LS) mean changes from baseline were calculated using a mixed model for repeated measures (MMRM) method with model terms of study (to account for potential heterogeneity across studies), treatment, visit, treatment-by-visit and baseline-by-visit interaction. An unstructured covariance was used by default; normality and other covariance structures were examined by fitting the MMRM with alternative assumptions (such as t-distributed residuals/random effect, homogeneity or heterogeneity of variance, or autocorrelation over visits). For HAM-D17 Total score and SDF score, LS mean changes from baseline were calculated using an analysis of covariance (ANCOVA) model on the last observation carried forward (LOCF) dataset, with treatment and study center as the main effects and baseline value as the covariate. For MADRS response rates, the Cochran–Mantel–Haenszel association test, controlling for study site, was conducted using LOCF. All p-values were tested at a nominal 0.05 level (two-sided) with no adjustment for multiplicity. Cohen’s d effect sizes (37) were also calculated. The incidence of TEAEs were summarized using descriptive statistics.
Analyses were performed using SAS version 9.4 (SAS Institute Inc; Cary, NC).
3 Results
3.1 Patients
Data were analyzed for a total of 1,160 patients (efficacy and safety samples), of whom 689 (59.4%) were in the high SDF subgroup (ADT + brexpiprazole 2 or 3 mg/day, n=348; ADT + placebo, n=341) and 471 (40.6%) were in the low SDF subgroup (ADT + brexpiprazole 2 or 3 mg/day, n=229; ADT + placebo, n=242).
Baseline demographics and clinical characteristics were similar across the three trials (25–27). Within each pooled subgroup, baseline demographic and clinical characteristics were generally similar between ADT + brexpiprazole and ADT + placebo treatment groups (Table 1). Baseline depression severity was higher in the high SDF subgroup than in the low SDF subgroup, but was similar between treatment arms within each subgroup.
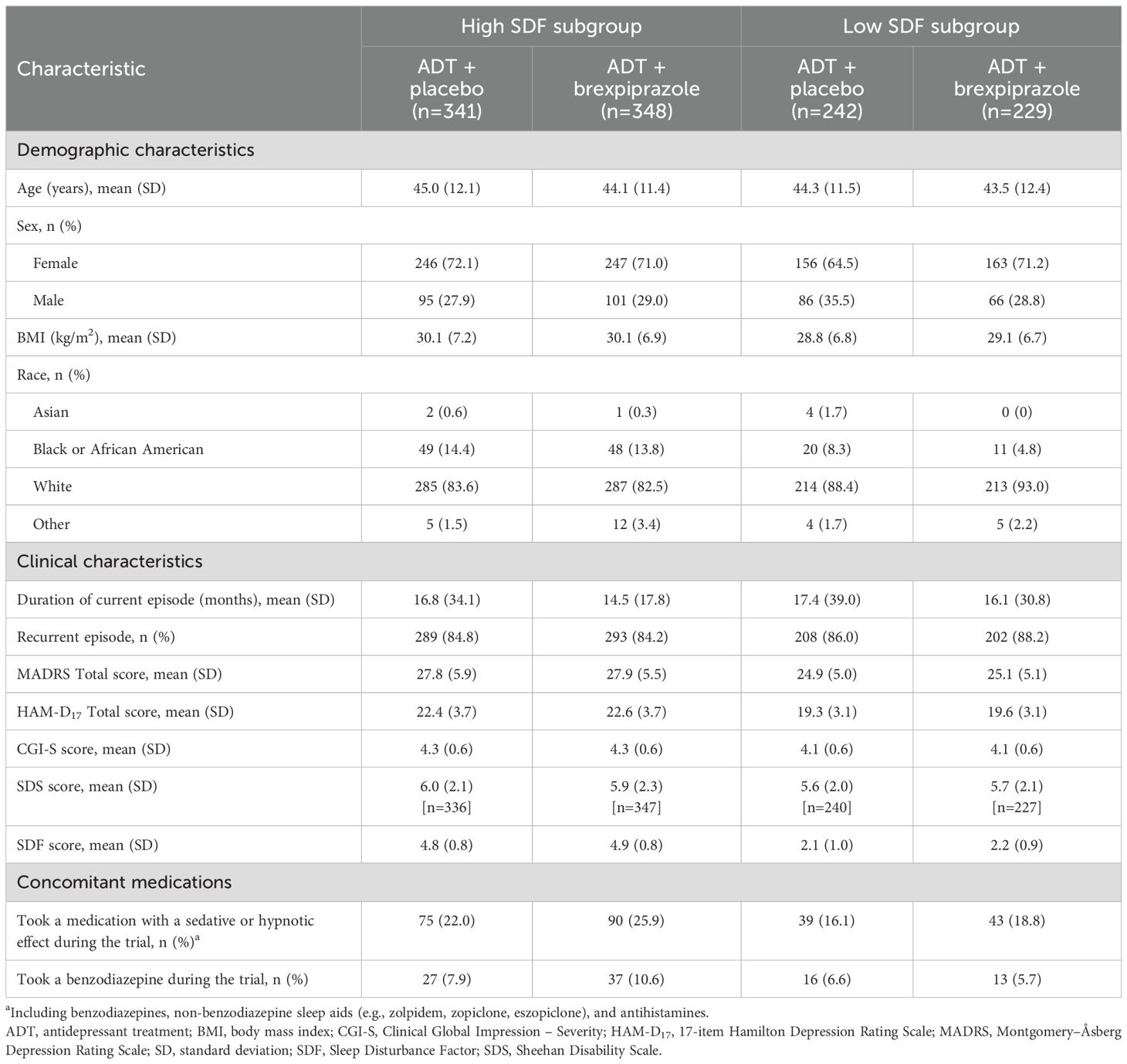
Table 1. Baseline demographic and clinical characteristics and concomitant medications in high SDF (≥4) and low SDF (<4) subgroups.
3.2 Efficacy
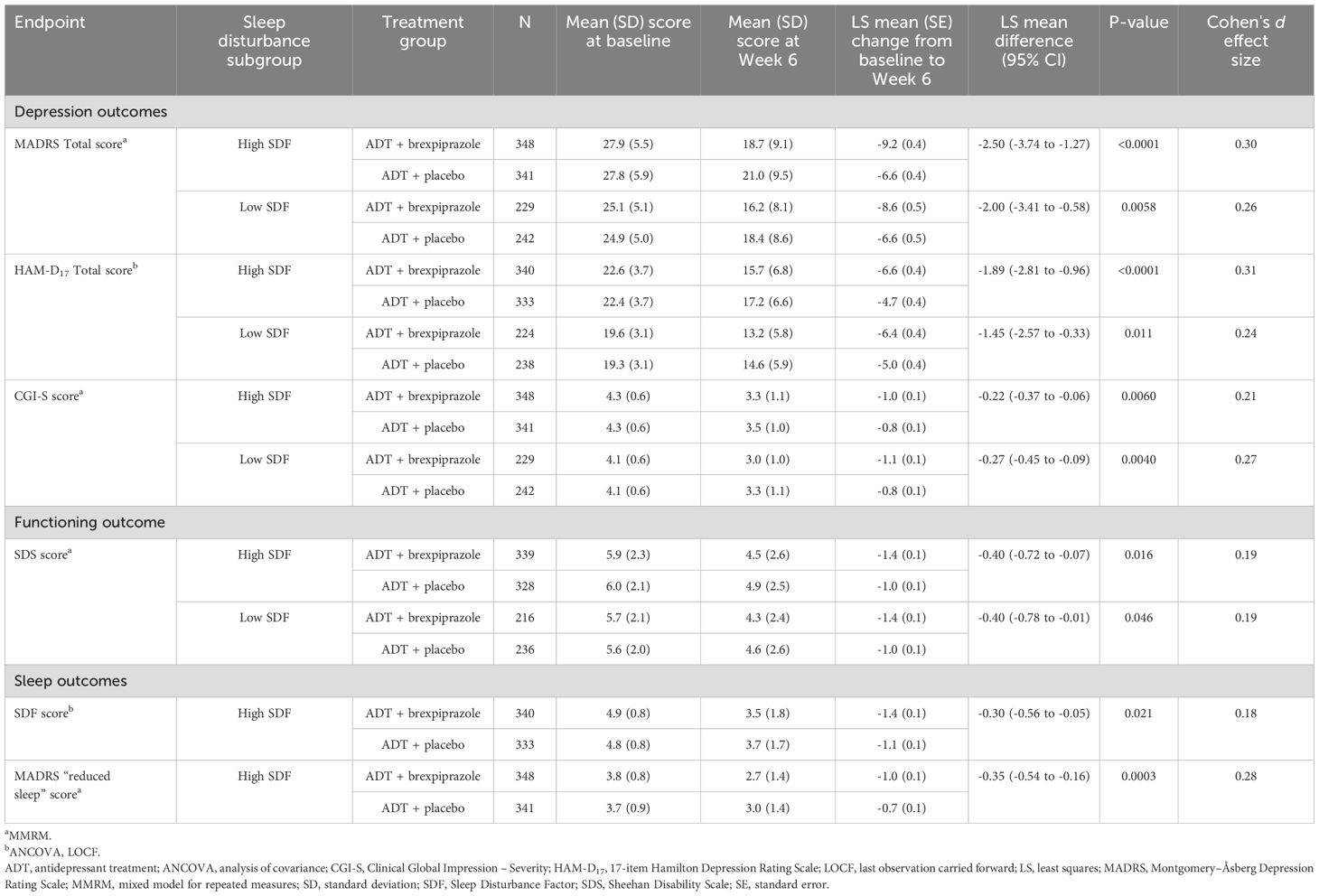
On depression outcomes, the LS mean change from baseline to Week 6 in MADRS Total score was greater with ADT + brexpiprazole than ADT + placebo (p<0.0001) in the high SDF subgroup. Greater improvement between treatment groups was observed from Week 2 (p<0.01) onwards (Table 2, Figure 1A). In the low SDF subgroup, greater improvement with ADT + brexpiprazole versus ADT + placebo was observed at Week 6 (p=0.0058) and at some earlier visits (Table 2, Figure 1B). LS mean changes from baseline to Week 6 in HAM-D17 Total score and CGI-S score were also greater with ADT + brexpiprazole versus ADT + placebo (p<0.05) in the high SDF subgroup and in the low SDF subgroup (Table 2). In the high SDF subgroup, MADRS response rates at Week 6 were 27.0% (94/348) with ADT + brexpiprazole and 20.8% (71/341) with ADT + placebo (p=0.032). In the low SDF subgroup, MADRS response rates were 29.3% (67/229) with ADT + brexpiprazole and 21.5% (52/242) with ADT + placebo (p=0.052).
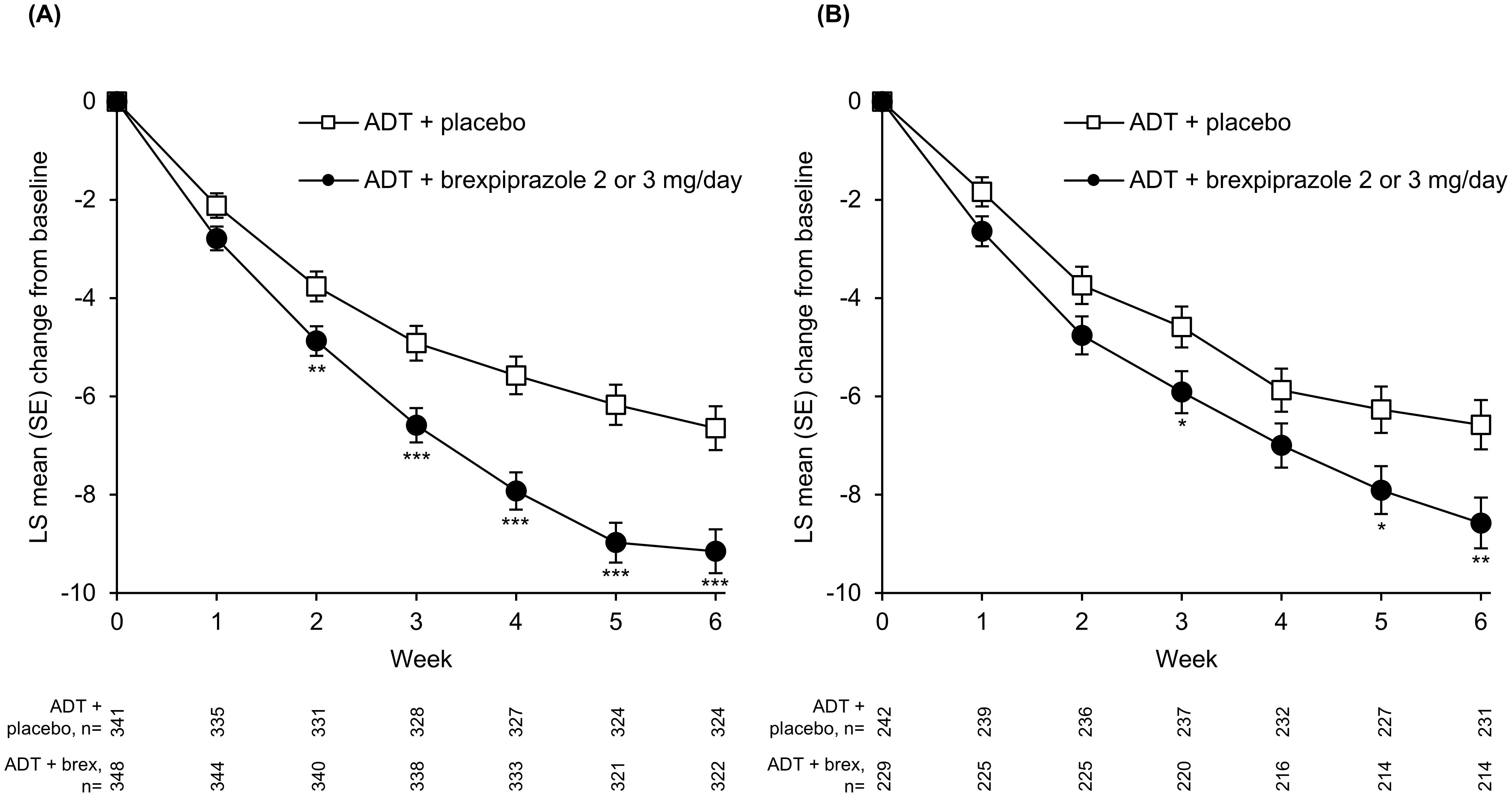
Figure 1. Mean change in MADRS Total score in (A) high SDF (≥4) and (B) low SDF (<4) subgroups. *p<0.05, **p<0.01, ***p<0.001 versus ADT + placebo; MMRM. Mean MADRS baseline score: high SDF subgroup: ADT + placebo, 27.8; ADT + brexpiprazole, 27.9; low SDF subgroup: ADT + placebo, 24.9; ADT + brexpiprazole, 25.1. ADT, antidepressant treatment; LS, least squares; MADRS, Montgomery–Åsberg Depression Rating Scale; MMRM, mixed model for repeated measures; SDF, Sleep Disturbance Factor; SE, standard error.
Regarding functioning, the LS mean change from baseline to Week 6 in SDS score was greater with ADT + brexpiprazole versus ADT + placebo (p<0.05) in the high SDF subgroup and in the low SDF subgroup (Table 2).
In the high SDF subgroup, on sleep outcomes, the LS mean change from baseline to Week 6 in SDF score and in MADRS “reduced sleep” item score was greater with ADT + brexpiprazole versus ADT + placebo (p<0.05 for both measures) (Table 2).
3.3 Safety
The overall incidence of TEAEs was higher with ADT + brexpiprazole than ADT + placebo in the high SDF subgroup (59.8% compared with 51.6%) and in the low SDF subgroup (62.4% compared with 40.9%) (Table 3).
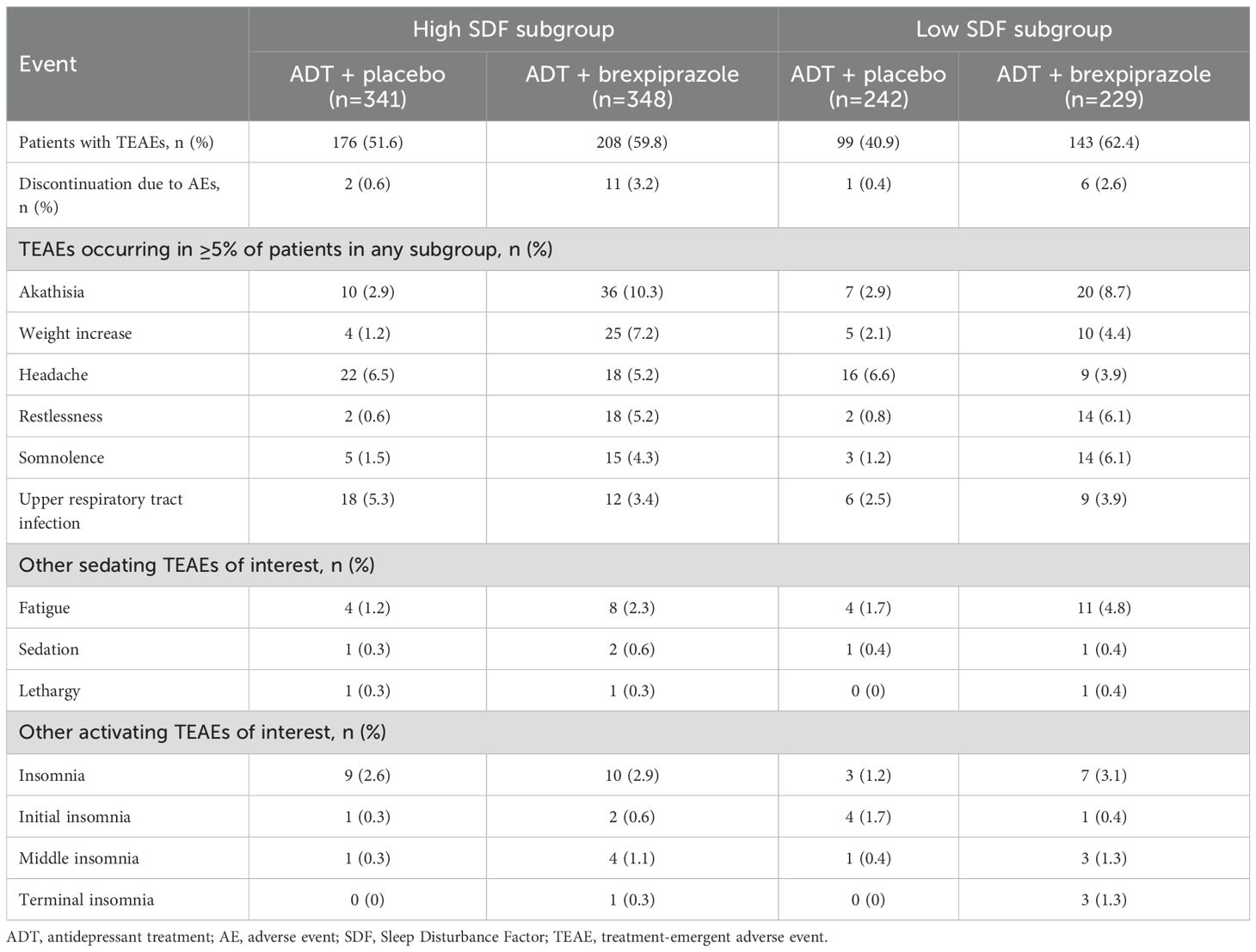
Table 3. Summary of treatment-emergent adverse events (TEAEs) in high SDF (≥4) and low SDF (<4) subgroups.
In the high SDF subgroup, the TEAEs with an incidence ≥5% in the ADT + brexpiprazole group and greater than ADT + placebo were akathisia, weight increase, and restlessness (Table 3). In the low SDF subgroup, the TEAEs with an incidence ≥5% in the ADT + brexpiprazole group and greater than ADT + placebo were akathisia, restlessness, and somnolence (Table 3).
4 Discussion
In this pooled analysis of three randomized trials in MDD, adjunctive brexpiprazole was associated with greater improvements in depression symptoms (MADRS Total score, HAM-D17 Total score, CGI-S score) and functioning (SDS score) compared with adjunctive placebo, in patients with high and low baseline sleep disturbance. Additionally, in patients with high baseline sleep disturbance, adjunctive brexpiprazole was associated with greater improvement in sleep disturbance (SDF score, MADRS “reduced sleep” item score) compared with adjunctive placebo. These results support previous findings from an 8-week exploratory, flexible-dose, open-label study, in which sleep disturbances and depressive symptoms improved with adjunctive brexpiprazole in patients with MDD who had sleep disturbances (22, 23).
Effective management of sleep disturbances in MDD requires a balance between improving nighttime sleep quality and minimizing excessive daytime sedation (38). Many commonly used antidepressants, such as selective serotonin reuptake inhibitors, have been associated with worsening sleep disturbances, particularly insomnia (1). Additionally, while some atypical antipsychotics, such as quetiapine, may improve symptoms of sleep disturbances (measured by the SDF score) in patients with MDD (39, 40), their benefits may be a result of sedative effects (41). In the present analysis, the most common sedating TEAE with adjunctive brexpiprazole was somnolence in the low SDF subgroup (6.1%); all other sedating TEAEs occurred at an incidence of <5%. Prior analyses suggest that brexpiprazole is not a sedating (or activating) drug (42). Overall, augmentation strategies should be individualized depending on the requirements and preferences of each patient (15).
In the present analysis, there were no notable differences in TEAEs between the high SDF and low SDF subgroups. Regardless of SDF status, the most common TEAE with adjunctive brexpiprazole was akathisia (8.7–10.3%), as noted for the total sample in prior analyses (24). Weight increase was reported by 4.4–7.2% of patients on adjunctive brexpiprazole; prior analyses indicate that adjunctive brexpiprazole is associated with moderate weight gain (1.5 kg) over 6 weeks (43). Although akathisia and weight gain can potentially impact treatment adherence and tolerability (44, 45), the proportion of patients who discontinued adjunctive brexpiprazole due to adverse events was low (2.6–3.2%), indicating that the majority of patients tolerated treatment. Overall, no new safety signals were observed with adjunctive brexpiprazole in the present analysis (24).
The efficacy of adjunctive brexpiprazole in patients with sleep disturbances may be attributed to its receptor binding profile. Brexpiprazole has antagonist properties at 5-HT2A receptors (46), which may promote slow-wave sleep, and be linked to cognitive performance and improved daytime functioning (47, 48). Additionally, brexpiprazole’s α1-adrenoceptor antagonism may enhance sleep quality (46, 49, 50) by reducing excessive noradrenergic activity, which has been associated with hyperarousal, disruptions in sleep and wakefulness, insomnia, and heightened states of alertness (51, 52).
Strengths of this analysis include the large dataset derived from three Phase 3, placebo-controlled, randomized trials. Limitations include its post hoc nature, meaning that results should be considered hypothesis generating, and the lack of adjustment for multiple comparisons, which may increase the risk of Type I error. The SDF, used as a proxy for sleep disturbances, includes insomnia-related items only (34), and cannot assess other aspects of sleep disturbance such as sleep architecture, hypersomnia, and circadian rhythm alterations. Additionally, clinical trial inclusion and exclusion criteria may limit generalizability of the results to broader patient populations. Further research is needed to validate these results in broader patient populations and to assess long-term effects.
In conclusion, in patients with MDD and an inadequate response to ADTs, adjunctive brexpiprazole was associated with improvements in depression and functioning regardless of baseline sleep disturbance, and improvement in sleep disturbances in patients with high baseline sleep disturbance. Improvement in sleep disturbance was generally not accompanied by TEAEs of daytime sedation, and no new safety signals were observed within each subgroup. Further prospective and long-term studies are needed to confirm these exploratory findings and to assess their generalizability to real-world settings. Nonetheless, given the challenges in managing sleep disturbances in MDD (1), these findings suggest that adjunctive brexpiprazole may be a valuable treatment option for patients with MDD and sleep disturbances.
Data availability statement
To submit inquiries related to Otsuka clinical research, or to request access to individual participant data (IPD) associated with any Otsuka clinical trial, please visit https://clinical-trials.otsuka.com/. For all approved IPD access requests, Otsuka will share anonymized IPD on a remotely accessible data sharing platform.
Ethics statement
The studies involving humans were approved by The International Council For Harmonisation Good Clinical Practice Guidelines. Trial protocols were approved by the governing institutional review board or independent ethics committee for each investigational site or country. This was a post hoc analysis of previously published trials. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
FA: Conceptualization, Investigation, Methodology, Supervision, Writing – review & editing. ZZ: Data curation, Formal analysis, Validation, Writing – review & editing. MH: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by Otsuka Pharmaceutical Development & Commercialization Inc. (Princeton, NJ, USA) and H. Lundbeck A/S (Valby, Denmark). The sponsors were involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; and decision to submit the manuscript for publication.
Acknowledgments
Writing support was provided by Hazel Ramwi, MSc, and colleagues of Cambridge (a division of Prime, Knutsford, UK), funded by Otsuka Pharmaceutical Development & Commercialization Inc. and H. Lundbeck A/S. Parts of this work were presented as a poster at the 34th CINP World Congress of Neuropsychopharmacology; 7–10 May 2023; Montreal, Canada.
Conflict of interest
Author FA is a full-time employee of H. Lundbeck A/S. Authors ZZ and MH are full-time employees of Otsuka Pharmaceutical Development & Commercialization Inc.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Murphy MJ and Peterson MJ. Sleep disturbances in depression. Sleep Med Clin. (2015) 10:17–23. doi: 10.1016/j.jsmc.2014.11.009
2. Geoffroy PA, Hoertel N, Etain B, Bellivier F, Delorme R, Limosin F, et al. Insomnia and hypersomnia in major depressive episode: prevalence, sociodemographic characteristics and psychiatric comorbidity in a population-based study. J Affect Disord. (2018) 226:132–41. doi: 10.1016/j.jad.2017.09.032
3. Jermann F, Perroud N, Favre S, Aubry JM, and Richard-Lepouriel H. Quality of life and subjective sleep-related measures in bipolar disorder and major depressive disorder. Qual Life Res. (2022) 31:117–24. doi: 10.1007/s11136-021-02929-8
4. American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 5th ed., text rev. Washington, DC: American Psychiatric Association (2022) p. 177–214.
5. Li SX, Lam SP, Chan JWY, Yu MWM, and Wing YK. Residual sleep disturbances in patients remitted from major depressive disorder: a 4-year naturalistic follow-up study. Sleep. (2012) 35:1153–61. doi: 10.5665/sleep.2008
6. Pandi-Perumal SR, Monti JM, Burman D, Karthikeyan R, BaHammam AS, Spence DW, et al. Clarifying the role of sleep in depression: a narrative review. Psychiatry Res. (2020) 291:113239. doi: 10.1016/j.psychres.2020.113239
7. Nierenberg AA, Husain MM, Trivedi MH, Fava M, Warden D, Wisniewski SR, et al. Residual symptoms after remission of major depressive disorder with citalopram and risk of relapse: a STAR*D report. Psychol Med. (2010) 40:41–50. doi: 10.1017/S0033291709006011
8. Franzen PL and Buysse DJ. Sleep disturbances and depression: risk relationships for subsequent depression and therapeutic implications. Dialogues Clin Neurosci. (2008) 10:473–81. doi: 10.31887/DCNS.2008.10.4/plfranzen
9. Cepeda MS, Reps J, and Ryan P. Finding factors that predict treatment-resistant depression: results of a cohort study. Depress Anxiety. (2018) 35:668–73. doi: 10.1002/da.22774
10. Cui F, Liu Q, Lv X, Leonhart R, Tian H, Wei J, et al. Severe sleep disturbance is associated with executive function impairment in patients with first-episode, treatment-naïve major depressive disorders. BMC Psychiatry. (2021) 21:198. doi: 10.1186/s12888-021-03194-2
11. Drake CL, Kalmbach DA, Cheng P, Ahmedani BK, Peterson EL, Joseph CLM, et al. Sleep to Reduce Incident Depression Effectively (STRIDE): study protocol for a randomized controlled trial comparing stepped-care cognitive-behavioral therapy for insomnia versus sleep education control to prevent major depression. Trials. (2022) 23:967. doi: 10.1186/s13063-022-06850-4
12. Otsuka Pharmaceutical Co., Ltd. Rexulti® (brexpiprazole) tablets, for oral use. Prescribing information. United States (2025). Available at: https://www.otsuka-us.com/media/document/Rexulti-PI.pdf.
13. Otsuka Pharmaceutical Co., Ltd. Rexulti® (brexpiprazole tablets). Product monograph. Canada (2024). Available at: https://rexultimonograph.ca/.
14. Maina G, Adami M, Ascione G, Bondi E, De Berardis D, Delmonte D, et al. Nationwide consensus on the clinical management of treatment-resistant depression in Italy: a Delphi panel. Ann Gen Psychiatry. (2023) 22:48. doi: 10.1186/s12991-023-00478-7
15. Lam RW, Kennedy SH, Adams C, Bahji A, Beaulieu S, Bhat V, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2023 update on clinical guidelines for management of major depressive disorder in adults. Can J Psychiatry. (2024) 69:641–87. doi: 10.1177/07067437241245384
16. Seetasith A, Greene M, Hartry A, and Burudpakdee C. Real-world economic outcomes of brexpiprazole and extended-release quetiapine adjunctive use in major depressive disorder. Clinicoecon Outcomes Res. (2019) 11:741–55. doi: 10.2147/CEOR.S220007
17. Wee SN, Liman C, Waters HC, Houle CR, Renteria M, Mukherjee SS, et al. Life engagement improvement following initiation of brexpiprazole treatment in patients with MDD: a naturalistic, retrospective real-world study. Clinicoecon Outcomes Res. (2023) 15:195–208. doi: 10.2147/CEOR.S395255
18. Severtson SG, Hadzi Boskovic D, Huang D, Talon B, Eisenberg D, Kapadia S, et al. Reductions in depressive symptoms after brexpiprazole augmentation among patients with major depressive disorder receiving antidepressant therapy in real-world settings. Poster presented at the 37th Annual Psych Congress, 29 October–2 November, Boston, MA, USA (2024).
19. Mok YM, Tan PLL, Bose R, Herr KJ, and Ung KEK. A real-life study of brexpiprazole as an adjunctive treatment for major depressive disorder in Asian patients in Singapore (BADA). Clin Psychopharmacol Neurosci. (2024) 22:531–6. doi: 10.9758/cpn.23.1143
20. Martiadis V, Pessina E, Martini A, Raffone F, Besana F, Olivola M, et al. Brexpiprazole augmentation in treatment resistant OCD: safety and efficacy in an Italian sample. Psychiatr Danub. (2024) 36:396–401.
21. Giacovelli L, Piccoli E, Landi P, Vismara M, Benatti B, and Dell’Osso B. Brexpiprazole augmentation in treatment-resistant obsessive-compulsive disorder: a preliminary retrospective observational study. Int Clin Psychopharmacol. (2025). doi: 10.1097/YIC.0000000000000583
22. Krystal AD, Mittoux A, Meisels P, and Baker RA. Effects of adjunctive brexpiprazole on sleep disturbances in patients with major depressive disorder: an open-label, flexible-dose, exploratory study. Prim Care Companion CNS Disord. (2016) 18(5). doi: 10.4088/PCC.15m01914
23. Krystal AD, Mittoux A, Lindsten A, and Baker RA. Chronobiologic parameter changes in patients with major depressive disorder and sleep disturbance treated with adjunctive brexpiprazole: an open-label, flexible-dose, exploratory substudy. J Affect Disord. (2021) 278:288–95. doi: 10.1016/j.jad.2020.09.026
24. Thase ME, Zhang P, Weiss C, Meehan SR, and Hobart M. Efficacy and safety of brexpiprazole as adjunctive treatment in major depressive disorder: overview of four short-term studies. Expert Opin Pharmacother. (2019) 20:1907–16. doi: 10.1080/14656566.2019.1638913
25. Thase ME, Youakim JM, Skuban A, Hobart M, Augustine C, Zhang P, et al. Efficacy and safety of adjunctive brexpiprazole 2 mg in major depressive disorder: a Phase 3, randomized, placebo-controlled study in patients with inadequate response to antidepressants. J Clin Psychiatry. (2015) 76:1224–31. doi: 10.4088/JCP.14m09688
26. Thase ME, Youakim JM, Skuban A, Hobart M, Zhang P, McQuade RD, et al. Adjunctive brexpiprazole 1 and 3 mg for patients with major depressive disorder following inadequate response to antidepressants: a Phase 3, randomized, double-blind study. J Clin Psychiatry. (2015) 76:1232–40. doi: 10.4088/JCP.14m09689
27. Hobart M, Skuban A, Zhang P, Augustine C, Brewer C, Hefting N, et al. A randomized, placebo-controlled study of the efficacy and safety of fixed-dose brexpiprazole 2 mg/d as adjunctive treatment of adults with major depressive disorder. J Clin Psychiatry. (2018) 79:17m12058. doi: 10.4088/JCP.17m12058
28. American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th ed., text rev. 4th ed. Washington, DC: American Psychiatric Association (2000) p. 155–88.
29. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. (1960) 23:56–62. doi: 10.1136/jnnp.23.1.56
30. Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. (1967) 6:278–96. doi: 10.1111/j.2044-8260.1967.tb00530.x
31. Montgomery SA and Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. (1979) 134:382–9. doi: 10.1192/bjp.134.4.382
32. Guy W. ECDEU assessment manual for psychopharmacology, revised. Rockville, MD: National Institute of Mental Health (1976) p. 218–22.
33. O’Brien KP and Glaudin V. Factorial structure and factor reliability of the Hamilton Rating Scale for Depression. Acta Psychiatr Scand. (1988) 78:113–20. doi: 10.1111/j.1600-0447.1988.tb06311.x
34. Fava M, Hoog SL, Judge RA, Kopp JB, Nilsson ME, and Gonzales JS. Acute efficacy of fluoxetine versus sertraline and paroxetine in major depressive disorder including effects of baseline insomnia. J Clin Psychopharmacol. (2002) 22:137–47. doi: 10.1097/00004714-200204000-00006
35. Sheehan KH and Sheehan DV. Assessing treatment effects in clinical trials with the discan metric of the Sheehan Disability Scale. Int Clin Psychopharmacol. (2008) 23:70–83. doi: 10.1097/YIC.0b013e3282f2b4d6
36. Sheehan DV, Harnett-Sheehan K, and Raj BA. The measurement of disability. Int Clin Psychopharmacol. (1996) 11:89–95. doi: 10.1097/00004850-199606003-00015
38. Thase ME, Murck H, and Post A. Clinical relevance of disturbances of sleep and vigilance in major depressive disorder: a review. Prim Care Companion J Clin Psychiatry. (2010) 12:e1–10. doi: 10.4088/PCC.08m00676gry
39. Bauer M, McIntyre RS, Szamosi J, and Eriksson H. Evaluation of adjunct extended-release quetiapine fumarate on sleep disturbance and quality in patients with major depressive disorder and an inadequate response to on-going antidepressant therapy. Int J Neuropsychopharmacol. (2013) 16:1755–65. doi: 10.1017/S146114571300031X
40. Trivedi MH, Bandelow B, Demyttenaere K, Papakostas GI, Szamosi J, Earley W, et al. Evaluation of the effects of extended release quetiapine fumarate monotherapy on sleep disturbance in patients with major depressive disorder: a pooled analysis of four randomized acute studies. Int J Neuropsychopharmacol. (2013) 16:1733–44. doi: 10.1017/S146114571300028X
41. Citrome L, McIntyre RS, Manning JS, and McIntosh D. Activating and sedating properties of medications used for the treatment of major depressive disorder and their effect on patient functioning. J Clin Psychiatry. (2019) 80:lu18052ah1. doi: 10.4088/JCP.lu18052ah1
42. Citrome L. Activating and sedating adverse effects of second-generation antipsychotics in the treatment of schizophrenia and major depressive disorder: absolute risk increase and number needed to harm. J Clin Psychopharmacol. (2017) 37:138–47. doi: 10.1097/JCP.0000000000000665
43. Newcomer JW, Eriksson H, Zhang P, Meehan SR, and Weiss C. Changes in metabolic parameters and body weight in patients with major depressive disorder treated with adjunctive brexpiprazole: pooled analysis of Phase 3 clinical studies. J Clin Psychiatry. (2019) 80:18m12680. doi: 10.4088/JCP.18m12680
44. Kim JH and Byun HJ. The relationship between akathisia and subjective tolerability in patients with schizophrenia. Int J Neurosci. (2010) 120:507–11. doi: 10.3109/00207451003760106
45. Pierce A, Carr B, and Keener A. Tolerability of weight gain from psychotropic medications in depressed patients. J Mood Disord Ther. (2024) 3:40–4. doi: 10.36959/418/583
46. Maeda K, Sugino H, Akazawa H, Amada N, Shimada J, Futamura T, et al. Brexpiprazole I: in vitro and in vivo characterization of a novel serotonin–dopamine activity modulator. J Pharmacol Exp Ther. (2014) 350:589–604. doi: 10.1124/jpet.114.213793
47. Landolt HP and Wehrle R. Antagonism of serotonergic 5-HT2A/2C receptors: mutual improvement of sleep, cognition and mood? Eur J Neurosci. (2009) 29:1795–809. doi: 10.1111/j.1460-9568.2009.06718.x
48. Vanover KE and Davis RE. Role of 5-HT2A receptor antagonists in the treatment of insomnia. Nat Sci Sleep. (2010) 2:139–50. doi: 10.2147/nss.s6849
49. Taylor F and Raskind MA. The alpha1-adrenergic antagonist prazosin improves sleep and nightmares in civilian trauma posttraumatic stress disorder. J Clin Psychopharmacol. (2002) 22:82–5. doi: 10.1097/00004714-200202000-00013
50. Paiva HS, Filho IJZ, and Cais CFDS. Using prazosin to treat posttraumatic stress disorder and associations: a systematic review. Psychiatry Investig. (2021) 18:365–72. doi: 10.30773/pi.2020.0411
51. Jain R, Chepke C, Davis LL, McIntyre RS, and Raskind MA. Dysregulation of noradrenergic activity: its role in conceptualizing and treating major depressive disorder, schizophrenia, agitation in Alzheimer’s disease, and posttraumatic stress disorder. J Clin Psychiatry. (2024) 85:plunaro2417ah. doi: 10.4088/JCP.plunaro2417ah
Keywords: brexpiprazole, major depressive disorder, sleep disturbance, adjunctive, antidepressant, sleep disturbance factor
Citation: Ardic F, Zhang Z and Hogan M (2025) Effects of adjunctive brexpiprazole in patients with major depressive disorder and sleep disturbance: a post hoc analysis of three randomized trials. Front. Psychiatry 16:1618176. doi: 10.3389/fpsyt.2025.1618176
Received: 25 April 2025; Accepted: 14 July 2025;
Published: 07 August 2025.
Edited by:
Nicolas A Nuñez, Mayo Clinic, United StatesReviewed by:
Axel Steiger, Ludwig Maximilian University of Munich, GermanyVassilis Martiadis, Department of Mental Health, Italy
Xinhua Shen, Third People’s Hospital of Huzhou, China
Copyright © 2025 Ardic, Zhang and Hogan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ferhat Ardic, RkVBUkBsdW5kYmVjay5jb20=
 Ferhat Ardic
Ferhat Ardic Zhen Zhang
Zhen Zhang Michael Hogan3
Michael Hogan3