- 1Department of Behavioral Sciences & Social Medicine, Florida State University College of Medicine, Tallahassee, FL, United States
- 2Center of Excellence for Perinatal Mood & Anxiety Disorders, Florida State University College of Medicine, Tallahassee, FL, United States
- 3Center for Medicine & Public Health Policy and Practice, Florida State University College of Medicine, Tallahassee, FL, United States
- 4Florida State University College of Medicine, Tallahassee, FL, United States
- 5Charlotte Edwards Maguire Medical Library, Florida State University College of Medicine, Tallahassee, FL, United States
- 6Department of Anesthesiology and Perioperative Medicine, Mayo Clinic, Rochester, MN, United States
- 7Department of Obstetrics & Gynecology, Mayo Clinic, Rochester, MN, United States
- 8Department of Psychiatry & Psychology, Mayo Clinic, Rochester, MN, United States
Background: Modified electroconvulsive therapy (mECT), the administration of ECT under general anesthesia with muscular relaxation, is indicated for perinatal depression complicated by high severity, psychosis, catatonia, or resistance to conventional therapeutics; however, knowledge gaps remain regarding its effectiveness and safety in depressed patients and its fetal/neonatal risk profile.
Materials and methods: We conducted a scoping review of the literature describing the effectiveness and safety (maternal, fetal, and neonatal) of mECT for perinatal depression. Online databases were searched (inception to December 31, 2024) to identify clinical trials, observational studies, case series, and case reports that were topically relevant. Information on key methodological details, clinical characteristics, interventions, and outcomes from each report was extracted by all investigators working in pairs, using an electronic abstraction form.
Results: A total of 82 reports (with information on >1,300 pregnancies/deliveries) were included, consisting mainly of case reports (n=57) and case series (n=14), with the remaining citations being non-randomized or retrospective studies. The reviewed reports collectively described a broad spectrum of effectiveness and safety outcomes associated with predominantly acute mECT across multiple forms of perinatal depression, multiple trimesters of pregnancy, and the postpartum. mECT conferred rapid benefit for depressive, psychotic, and catatonic symptoms in severely depressed perinatal patients when effectiveness outcomes were described. The most frequent adverse events were generally mild and transient. However, cases of placental abruption (n=1), premature delivery (n=21), congenital malformations (n=6), and stillbirth (n=4) were also reported across the reviewed reports. Due to limited information, causal links between mECT and many adverse events were difficult to establish and inferences about differential effectiveness and safety between important patient subgroups or variations in mECT technique could not be drawn.
Conclusion: mECT appears to be an effective acute phase treatment for severely ill perinatally depressed patients. Although the maternal safety profile of mECT appears reassuring, the available data are far from comprehensive. Moreover, fetal and neonatal safety risks are even less-well-characterized. mECT should be regarded as an important therapeutic option for severe cases of perinatal depression. Informed consent practices should reflect the knowledge gaps highlighted in this review in addition to the well-known side-effects of mECT and the substantial adverse consequences of untreated or undertreated maternal depression.
Systematic Review Registration: This project was registered on Open Science Forum, 10.17605/OSF.IO/KB67J.
1 Introduction
Depression is among the most common complications in the perinatal period, spanning pregnancy through the first postpartum year. A 2005 systematic review estimated prevalence rates of 8.5%-11% for antenatal depression and 6.5%-12.9% for postpartum depression (PPD) in the U.S., including cases of unipolar major depression and minor depression (1). Other reviews have documented even higher average prevalence rates of 17% for antenatal depression and 13% for PPD (2). Beyond high prevalence, the public health importance of perinatal depression is highlighted by its association with increased maternal, neonatal, and early childhood morbidity (including negative effects on language, motor, and emotional development), poor obstetric outcomes, economic loss, and early maternal mortality including death by suicide (3–6). Indeed, perinatal depression presents across a broad severity spectrum, ranging from mild symptoms to behavioral emergencies requiring psychiatric hospitalization (7, 8).
In non-perinatal patients, modified electroconvulsive therapy (ECT), the administration of ECT under general anesthesia with muscular relaxation, is a high-priority treatment for refractory unipolar or bipolar depression, psychosis, refractory catatonias, and other psychiatric conditions for which the customary lag times to therapeutic benefit with conventional antidepressive treatments would be unacceptable, including cases with high suicide risk, evidence of medical or nutritional compromise, and others (9, 10). mECT is also indicated for perinatal depression complicated by high severity, psychosis, catatonia, or resistance to conventional approaches (11).
Previous reviews on the effectiveness and safety of mECT during the perinatal period have broadly supported the utility of mECT for severe perinatal depression but have arrived at mixed conclusions regarding the interpretation of obstetric and fetal/neonatal risks (12–20). And with relatively few exceptions (11), adaptations to standard mECT technique for perinatal patients were often not summarized. We thus conducted a scoping review to provide an updated survey of the published literature on mECT for perinatal unipolar or bipolar depression and to identify important but underdeveloped areas in need of further study.
2 Materials and methods
2.1 Search strategy
We conducted a scoping review of the literature regarding mECT for perinatal depression, guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR) (21). A comprehensive literature search was conducted on December 31, 2024, by a research librarian (RR), in collaboration with the investigative team, using nine databases and registries (see Supplementary Table S1 and Appendix). The reference sections of reviewed papers and selected systematic and meta-analytic reviews were also be used to locate potentially relevant papers. The search strategy facilitated the retrieval of both published and grey literature references.
2.2 Inclusion and exclusion criteria
We selected relevant reports of the effects of mECT for human perinatal depression, published in English, based on the following population/problem, intervention, comparators/controls, and outcome(s) (PICO) elements:
● Population/problem: Perinatal depression was defined as clinically significant depression occurring during pregnancy and/or within the 12 months following the date of delivery. We included papers with unipolar or bipolar depressed patients, any subtype or severity level, with or without psychotic features. Perinatal catatonia or psychosis cases were considered if a mood disorder diagnosis was specified as an underlying cause, presumed or established. Cases where an underlying mood disorder diagnosis was unspecified could still be included if there was enough detail to suggest the presence of an acute episode of depression, based on agreement between two independent reviewers. Although we did not specify an age range, the inclusion of reproductive-aged persons was presumed given our focus on perinatal depression.
● Intervention: We required the use of mECT as a treatment intervention (with or without co-interventions), using any stimulus parameters or electrode placement montages. Reports describing acute-, continuation-, or maintenance-phase mECT treatments were included.
● Comparators/controls: We included both controlled and non-controlled studies. For controlled research, we did not define acceptable or non-acceptable comparator groups or conditions, as adequate control group design for randomized trials of ECT for depression is debated (22, 23).
● Efficacy or Effectiveness Outcome(s): Efficacy/effectiveness outcomes included acute-phase reduction (improvement) in the severity of depressive symptoms, acute-phase categorical treatment outcome (e.g., remission/full response, partial response, non-response, etc.), duration of clinical response, and maintenance phase effectiveness (e.g., time to relapse, recurrence, or loss of remission or response).
● Safety/Tolerability Outcome(s): Safety/tolerability outcomes included acceptability of mECT as an acute or maintenance treatment (estimated using all-cause dropout rates), cognitive effects based on neuropsychological tests or bedside measures, non-cognitive adverse maternal effects and obstetric safety endpoints (e.g., acute hyper or hypotension, placental abruption, uterine contractions, preterm labor or premature rupture of membranes, difficulty with airway management, aspiration, etc.), and fetal, neonatal, and childhood developmental complications (e.g., intrauterine fetal demise, fetal growth restriction, changes in fetal heart rate, congenital malformations, respiratory depression, low Apgar scores at birth, developmental delay, etc.).
2.3 Study selection
After excluding duplicate articles, five investigators (WVB, OM, KMM, AML, HKB) worked in pairs to screen the titles and abstracts to exclude irrelevant papers. The remaining articles were then subjected to full-text review by six investigators (WVB, OM, KMM, EES, AML, HKB) who worked in pairs to exclude reports that failed to meet inclusion/exclusion criteria. Discrepancies at each step were resolved by discussion and consensus.
2.4 Data extraction and analysis
Data extraction was performed by all investigators, who worked independently in pairs, using a standard electronic extraction form. Disagreements were resolved via discussion and consensus. When necessary, an additional team member with specific domain expertise served a tie-breaking role. In accordance with PRISMA reporting guidelines for scoping reviews (PRISMA-ScR) (21), methodological quality and risk of bias assessments were not reported.
The following information was extracted from the individual studies (see Supplementary Table S2): (1) Study characteristics including publication year, authors, study design/report type, and treatment setting; (2) Subject/enrollee details including mood disorder diagnoses, mECT indication(s), definitions of treatment resistance (if applicable), maternal age, multiple gestation status, obstetric and general medical morbidities, pre-ECT medications, and use of assisted reproductive technology; (3) Treatment details including ECT electrode placement, pulse width, frequency of mECT administration, and ECT dose; (4) Anesthesia technique including anesthetic induction agent(s), pharmacological adjuncts to anesthesia, and airway management approach; (5) Adaptations to standard mECT technique including maternal and fetal surveillance methods; and (6) Effectiveness and safety measures and outcomes. Selected characteristics were summarized as proportions and were presented in table or graphical form.
3 Results
3.1 Format and design characteristics of the included reports
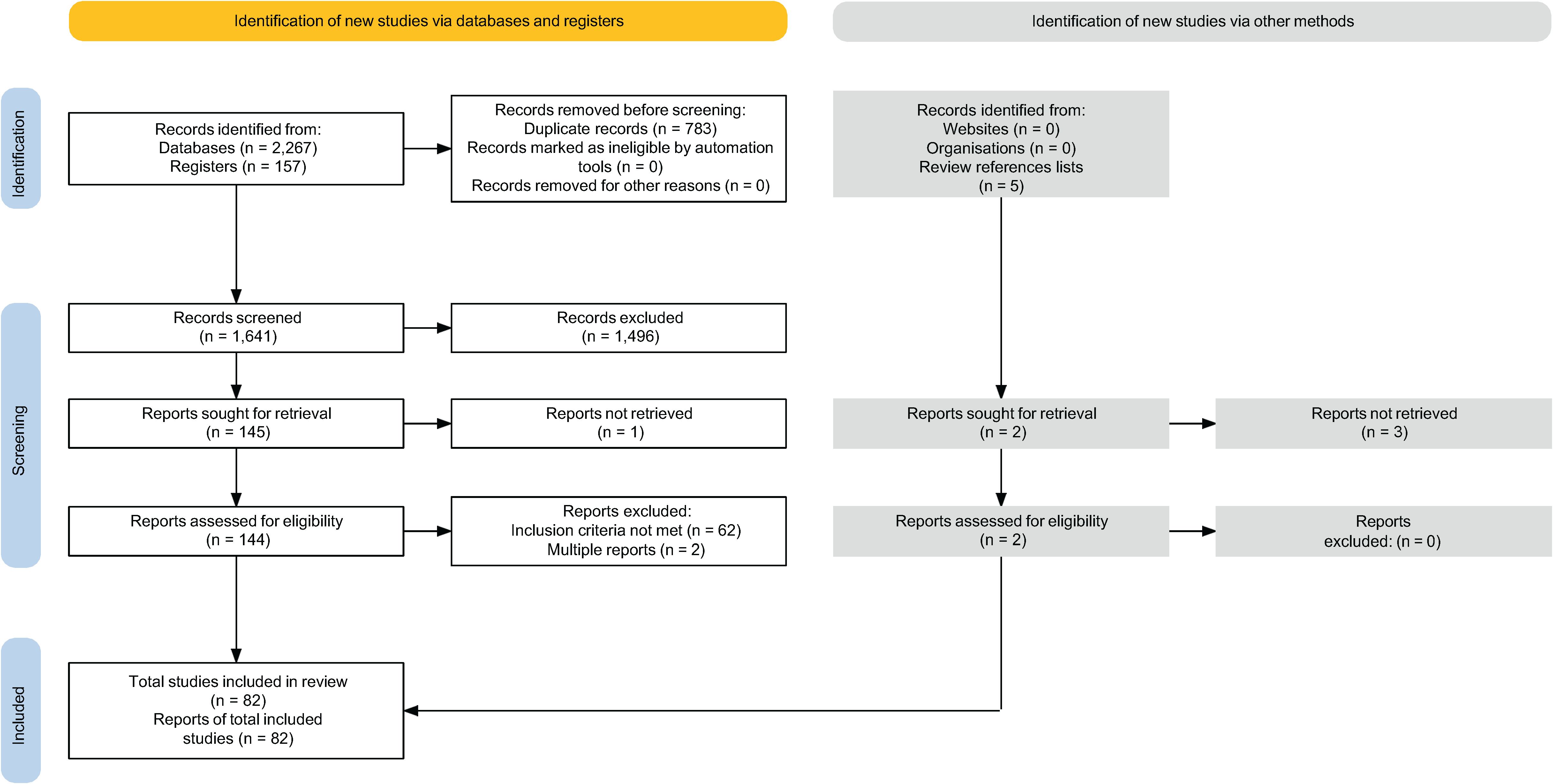
A total of 2,424 citations were identified from the initial literature searches across 14 registers and databases. After removing duplicates, 1,643 records underwent title/abstract screening, 146 of which were subjected to full-text review to determine eligibility for inclusion. On full text review, one case was found in both a published abstract and a published single case report, the former of which was excluded. We also included one of two full-length reports that described the same case. The remaining 82 reports (published between 1974 and 2024) met inclusion/exclusion criteria (Figure 1).
As shown in Figure 2A, 73 reports were published as full-length reports, while the remaining reports were published as abstracts (n=8) or other formats (n=1). Most reviewed citations were from case reports (n=57) and case series (n=14), with the remaining citations being from retrospective cohort, non-randomized prospective studies, or other designs. Sample sizes ranged from single case reports (n=1) to 793 individuals. Most of the included reports were from North America, followed by Europe, Western Pacific, and South-East Asian regions (Figure 2B).
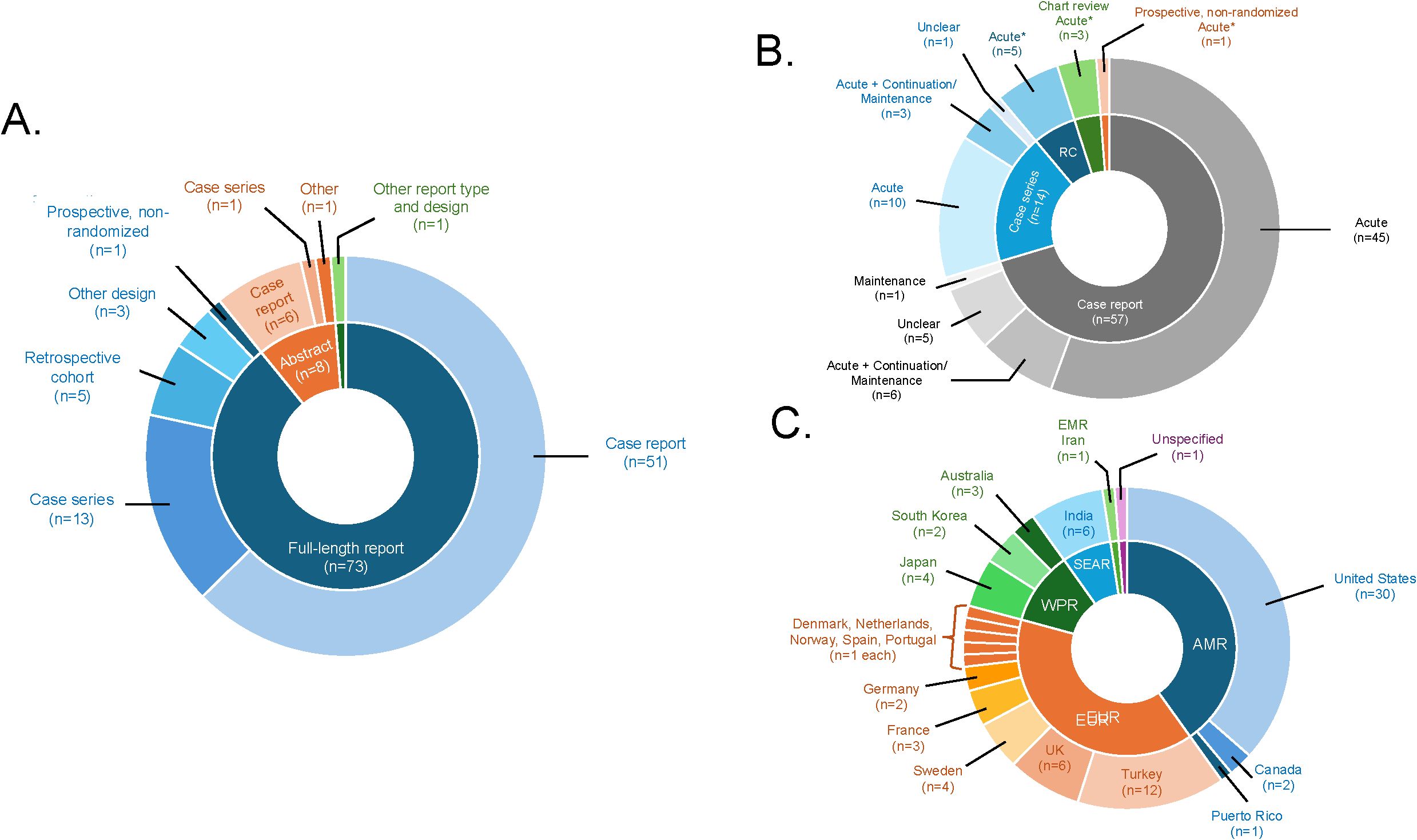
Figure 2. Graphical summary of reviewed studies. (A) displays the information on study or report design according to publication status (full-length report, conference abstract, other). (B) displays the information on phase(s) of modified ECT (mECT) treatment, by study or report design (including case reports, case series, chart review studies, prospective/non-randomized studies, and retrospective cohort studies [RC], absent one first-person account of postpartum mECT treatment and one qualitative mECT study). (C) displays the information on the countries in which individual studies or reports were conducted by World Health Organization region (including the Americas [AMR], the Eastern Mediterranean Region [EMR], the European Region [EUR], the South-East Asia Region [SEAR], and the Western Pacific Region [WPR]).
3.2 Age and clinical characteristics of mECT-treated patients
Mean or median ages from case series and observational studies ranged from 23.0 to 37.0 years. The age range of individual cases was 16.5 to 48 years. As shown in Tables 1 and 2, several reports included mixed samples of patients with severe mood disorders, psychotic disorders, unspecified postpartum psychoses, or unspecified catatonia. The most common perinatal mood disorder diagnoses were unspecified nonpsychotic unipolar depression (36 reports), unspecified psychotic unipolar depression (17 reports), nonpsychotic unipolar or bipolar major depression (12 reports each), unspecified bipolar disorder (11 reports), and unspecified unipolar depression with catatonic symptoms or bipolar mixed episodes with severe depression or suicidality (6 reports each).
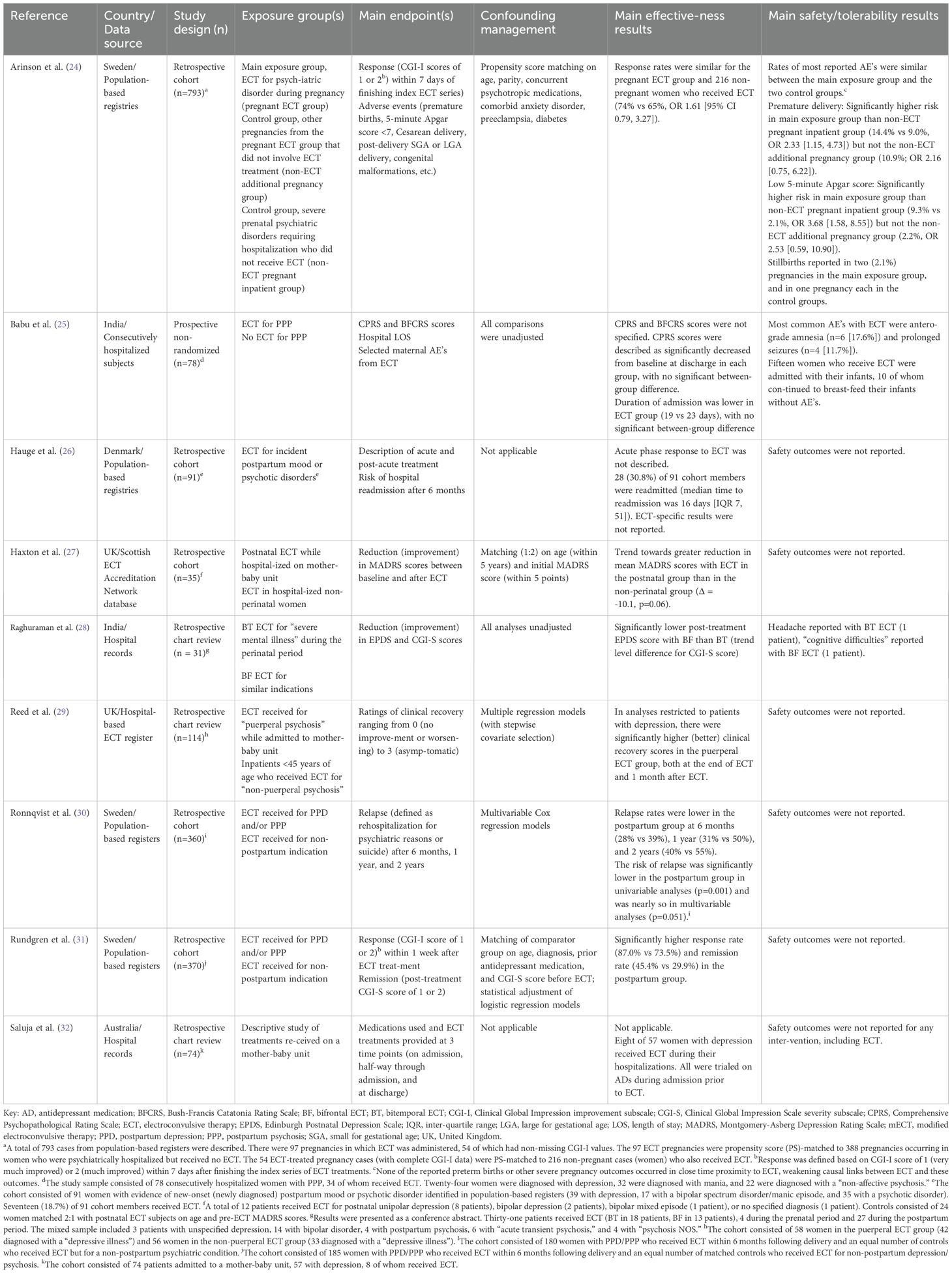
Table 1. Characteristics of observational and retrospective studies of modified electroconvulsive therapy (mECT) for perinatal depression.
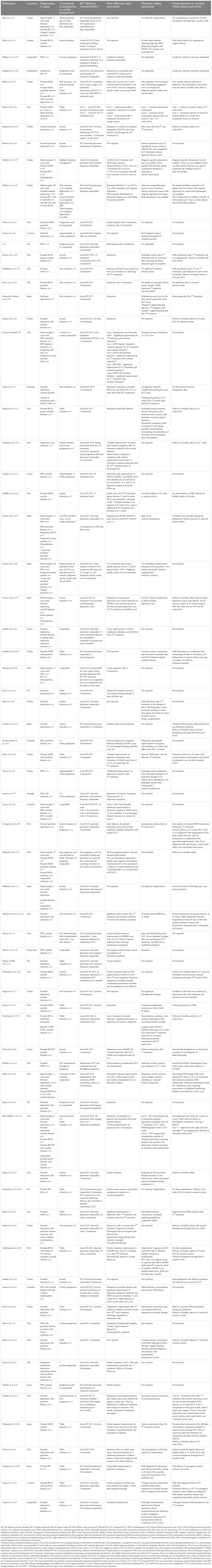
Table 2. Characteristics of case reports and case series describing modified electroconvulsive therapy (mECT) for perinatal depression.
As shown in Figure 2C, most reviewed papers described acute-phase mECT while just 10 reports described continuation- or maintenance-phase treatment, with or without an acute phase (37, 39, 46, 50, 73, 76, 79, 81, 86, 97). The most common indication(s) for mECT, when specified, were treatment resistance (n=31) followed by psychotic symptoms/features (n=21) and high suicide risk (n=21). Indications for mECT were unspecified in 14 reports. Nearly all the 30 reports that addressed treatment-resistant depression defined treatment resistance as poor response to prior treatments (n=29), including one TMS-resistant case (51). One report identified intolerance of medications as the principal indication for mECT (35). Fourteen reports described mECT for catatonia, including 3 reports that explicitly identified benzodiazepine-resistant catatonia as the intended indication (67, 72, 95). ECT was the preferred treatment or treatment because of pregnancy in 10 reports (34, 37, 39, 52, 54–56, 66, 70, 97).
In terms of obstetric information, most reports described mECT in the setting of pregnancy (n=64) while 23 reports included cases of postnatal mECT delivery, with or without a prenatal treatment phase. When gestational information was provided, nearly all such reports involved singleton pregnancies/deliveries, whereas 2 reports included twin pregnancies/deliveries (69, 97). The pre-ECT use of in vitro fertilization was reported in one case (89).
General medical comorbidity was often not reported. For example, of the 9 observational studies, two provided information on the frequency of diabetes and one provided details on the frequency of underweight, overweight, and obesity based on BMI ranges (24, 26, 32). Only limited information on comorbid conditions was available from individual case reports and small case series. However, 15 such reports documented comorbid conditions including obesity, non-gestational hypertension, gestational and non-gestational diabetes, hyperthyroidism, chronic musculoskeletal pain syndromes, migraine headache, congenital neurological diseases, and others. Acute injuries (skeletal fractures) and intentional poisonings (acetaminophen toxicity) related to suicide attempts were described in three reports (13, 50, 61), while anorexia, weight loss, or other conditions related to nutritional compromise in severely depressed patients were documented five reports (45, 49, 62, 70, 99).
Medications taken on or around the time of ECT administration was more thoroughly documented than medical comorbidities. Summary data on the frequency of concurrently prescribed antidepressants, mood stabilizers, antipsychotic drugs, or benzodiazepines were provided for six of nine observational studies. Forty-nine of the 73 individual case reports and small case series included information on individual drugs falling within these same broad categories. In 8 reports, only past failed medication trials were reported (35, 60, 67, 69, 70, 75, 86, 103). The discontinuation of psychotropic medications during or in anticipation of pregnancy or the absence of psychotropic medications at the time of ECT was specified in 6 reports (44, 46, 47, 54, 66, 89).
3.3 ECT treatment characteristics
Of the 56 reports where electrode placement was clearly described, the most common types of ECT electrode placement were bitemporal (34 reports) and right unilateral (12 reports) using a brief pulse width. The use of right unilateral ultrabrief pulse ECT was described in 3 reports (35, 68, 82) and bifrontal ECT was described in 9 reports (13, 28, 30, 31, 41, 49, 66, 77, 81). The number of weekly sessions of acute ECT was often not provided in the reviewed reports. When the number of weekly sessions was specified, thrice-weekly ECT sessions were most described (25, 51, 52, 65, 68, 71, 78, 88, 89), although twice-weekly ECT sessions were also reported (49, 80). Information on ECT stimulus parameters in conjunction with electrode placement was provided in only 27 reports. For summary dose metrics, stimulus train energy values ranged from 29.6 J - 43.6 J, 124.7 mC - 436 mC, or 10% - 75% of maximal charge. Individual ECT dose elements from 23 individual reports included current amplitudes (170 V or 500–842 mA), pulse widths (0.5 msec - 1.6 msec), pulse or pulse-pair frequencies (20 Hz - 90 Hz or 125 pulses/sec), and stimulus train durations (1 sec - 8 sec). Seizure threshold titration or determination was mentioned in 6 reports; however, the exact parameters at each step were not specified (25, 26, 34, 68, 79, 80, 90).
3.4 Anesthesia technique
The most common anesthetic induction agents in the reviewed papers were propofol (21 reports), thiopental (16 reports), and methohexital (14 reports). One report described the use of ketamine augmentation of propofol anesthesia for ECT in the setting of third trimester pregnancy in hopes of enhancing antidepressive efficacy (78). Among the 9 observational studies, only one provided complete information on the anesthetic (thiopental 3–4 mg/kg) and neuromuscular blocking agent (succinylcholine 0.5-0.75 mg/kg) with doses (25). From case reports and small case series, 26 included complete information on anesthetic and neuromuscular blocking agents. Specifically reported anesthetic drugs (with dose ranges in total mg administered or mg/kg infused) included methohexital (50–170 mg or 1 mg/kg), propofol (140 mg or 1 mg/kg), thiopental (100–300 mg or 3–4 mg/kg), thiamylal (4 mg/kg), succinylcholine (40–120 mg or 1–2 mg/kg). However, anesthetic doses, neuromuscular blocking drugs, airway management techniques, pharmacological adjuncts to anesthetic induction agents, and modifications of anesthetic technique for perinatal safety were unspecified in most reports. In one case report, methohexital was switched to propofol to shorten seizure length after a prolonged ECT seizure that led to an episode of fetal bradycardia (46). Another case report referenced switching from thiopental to etomidate to increase seizure duration and based on “favorable experiences with the drug in obstetrical patients” (47). A third case report described adjusting the dose of methohexital in response to seizure duration and quality but not perinatal safety reasons (79).
3.5 Adaptations to standard ECT technique
Specific maternal and fetal monitoring procedures to accommodate pregnancy or postpartum status were described in fewer than half of the reviewed reports. Still, as shown in Supplementary Table S3, several adaptations to standard ECT technique were documented in case reports and case series. Twenty-three reports provided details on airway management techniques, including 12 that used endotracheal intubation during second- or third-trimester pregnancies and 10 reports of mask airway. One case report described the use of a supraglottic airway for subsequent ECT treatments due to difficult intubation under direct laryngoscopy during her first treatment at 20 week’s gestation (40). When described, maternal monitoring techniques included tocometry/tocodynamometry, ultrasound assessments, obstetrician attendance during the procedure, and ready access to emergent cesarean section capabilities in specific cases (Supplementary Table S3). Additional precautions applied in later stages of pregnancy (after the first trimester) included elevation of the right hip to prevent aortocaval compression, cricoid pressure to reduce the risk of regurgitation and aspiration of stomach contents, pre-hydration/pre-oxygenation, and endotracheal intubation. Fetal heart rate monitoring, non-stress tests/biophysical profiling, and ultrasonography for fetal morphology, fetal heart rate and uterine contractility monitoring were also described (Supplementary Table S3).
3.6 Effectiveness
Main effectiveness results from observational studies and from case series/reports are outlined in Table 1 and Table 2, respectively. Treatment effects were reported as categorical outcomes (e.g, remission, partial response, non-response, hospital readmission, etc.) in 35 (42.7%) reports, as continuous outcomes (absolute or relative change in rating scale scores) in 16 (19.5%) reports, and as narrative descriptions of outcomes (“clear,” “full,” or “complete” response, unspecified improvement in mood symptoms, etc.) in 17 (20.7%) reports. The most used rating scales were the Hamilton Depression Rating Scale (HAM-D, 12 reports), the Clinical Global Impression scale (-severity [CGI-S] or -improvement [CGI-I] subscales, 8 reports), the Montgomery Asberg Depression Rating Scale (MADRS, 5 reports) (104), the Bush Francis Catatonia Rating Scale (BFCRS, 3 reports) (105), and the Quick Inventory of Depressive Symptomatology (QIDS) (106) and the 9-item Public Health Questionnaire (PHQ-9, 1 report each) (107). Therapeutic outcomes were not assessed in 12 reports. One study each focused only on descriptions of interventions provided on a specialized mother-baby unit (32) and on qualitative outcomes (108).
3.6.1 Observational and chart review studies
3.6.1.1 ECT for perinatal vs non-perinatal mental health disorders
Controlled investigations of acute responses to mECT for perinatal depression collectively involved a variety of comparisons, mainly involving ECT for perinatal mental health conditions vs ECT for non-perinatal mental health disorders. For example, a retrospective cohort study of 793 pregnant patients identified in linked registers, including a population-based ECT registry, documented numerically higher positive response rates (CGI-I scores of 2 [much improved] or 1 [very much improved]) with ECT in patients with a perinatal psychiatric diagnosis than with ECT in non-pregnant patients with a psychiatric diagnosis (74% vs 65%) who were matched on propensity scores (24); however, between-group differences in response rates were not statistically significant. Using the same population-based ECT registry, significantly higher rates of response (using the definition above, assessed within 7 days after receiving ECT, 87.0% vs 73.5%) and remission (CGI-S scores of 2 [borderline ill] or 1 [not ill], 45.4% vs 29.9%) were also observed in patients who received ECT for PPD or postpartum psychosis than patients who received ECT for a non-postpartum indication (31).
In another retrospective cohort study, a population-based ECT database and records from an inpatient mother-baby unit were used to identify a cohort of severely depressed patients with postnatal depression/psychiatric disorders (baseline mean MADRS score 43.1, n=12) and patients who received ECT for non-perinatal psychiatric disorders (baseline mean MADRS score 41.3, n=23) (27). After ECT, the mean reduction in MADRS scores was 10.1 points greater for perinatal depression cases than non-perinatal controls (-30.8 vs. -20.7, p=0.06). At baseline, the proportion of individuals with severe depression (based on MADRS scores) was 83% in both the perinatal ECT group and the non-perinatal ECT group. After ECT, the proportion of severe depression cases was 8% in the perinatal ECT group and 22% in the non-perinatal ECT group.
In a university hospital register-based study, clinical recovery ratings (based on a 4-point Likert scale) were compared between patients who received ECT for puerperal mental health conditions and patients (<45 years of age) who received ECT for non-puerperal mental health conditions (29). Complete records were available for 114 of the 137 patients who received ECT, including 58 patients in the puerperal ECT group and 56 patients in the non-puerperal group. Analyses restricted to patients with depression showed a significantly higher proportion of patients rated as either “asymptomatic” or having achieved “marked improvement” in the puerperal depressed group than in the non-puerperal depressed group at the end of ECT (66.7% vs 27.3%, p<0.001) and at reassessment one month after ECT (61.9% vs 24.2%, p=0.003).
3.6.1.2 Comparisons of bitemporal and bifrontal ECT
One study retrospectively compared the effects of bitemporal (BT, n=18 patients) and bifrontal (BF, n=13 patients) ECT in a mixed cohort of 31 patients (28). Edinburgh Postnatal Depression Scale (EPDS) and CGI-S scores were compared between groups at hospital discharge. Mean numerical pre- and post-treatment EPDS and CGI-S scores were not provided; however, post-treatment EPDS scores were reported as being significantly lower with BF than BT ECT (p=0.004). CGI-S scores were reported to be lower with BF than BT ECT at the level of statistical trend (p=0.06).
3.6.1.3 ECT vs no ECT
Another study prospectively compared clinical responses in a mixed cohort of 78 patients with postpartum psychosis (24 with depression) who received acute ECT and those with similar indications who did not receive ECT (25). Data were analyzed for the entire cohort, without diagnosis-specific results. Psychopathology was assessed in all 78 patients, 34 of whom received ECT, using the Comprehensive Psychopathological Rating Scale (CPRS) (109). CPRS scores were similar between both groups at baseline (ECT 41.8 vs. no ECT, 39.5) and were similarly improved in each group at the end of follow-up (ECT, 4.5, no ECT, 4.2). Duration of admission was lower in the ECT group (19 days vs 23 days); however, there were no significant between-group differences in therapeutic outcomes or hospital lengths of stay.
3.6.1.4 Relapse and rehospitalization after acute ECT
Other reports focused on relapse rates after an acute response to ECT, with relapse generally defined as rehospitalization for a psychiatric indication. For example, in a retrospective cohort study, rates of relapse (defined as rehospitalization for psychiatric reasons or suicide) were lower for patients who received ECT for PPD or postpartum psychosis (n=180) after 6 months (28% vs 39%), 1 year (31% vs 50%), and 2 years (40% vs 55%), as compared with control patients (n=180) who were <46 years of age and received ECT for a non-postpartum indication (30). The mean time to relapse (621 ± 548 days vs 440 ± 475 days) was numerically higher and the risk of relapse was significantly lower for patients who received ECT for PPD or postpartum psychosis (vs control patients) in unadjusted analyses (HR 0.61 [0.45, 0.83]). Statistical differences in relapse risk were nearly significant after adjusting for education level, unemployment, selected comorbidities, CGI-I score one week after treatment, drug treatment, and prior psychiatric admission history (HR 0.72 [0.52, 1.00]).
In a subsequent population-based study, linked registers were used to identify a mixed cohort of 91 patients with evidence of postpartum mood or psychotic disorders and no prior mood or psychotic disorder diagnoses and no history of ECT prior to giving birth (26). Cohort members were all psychiatrically hospitalized within the 6 weeks following delivery, 43% of whom were diagnosed with unipolar depression, 19% with bipolar disorder, and 38% with an unspecified psychotic disorder. A total of 17 (18.7%) patients received ECT. Rehospitalization (readmission to a psychiatric hospital within 6 months of the index hospital admission discharge date) occurred in 28 (30.8%) of the cohort members after a mean post-discharge interval of 16 days; however, ECT-specific results were not reported, nor were they compared with non-ECT treatment outcomes.
3.6.2 Case reports and case series
As shown in Table 2, all but 11 case reports or case series provided details on therapeutic outcomes of mECT. Most cases focused on acute-phase treatment, while 11 reports described continuation or maintenance ECT outcomes. Positive therapeutic responses to mECT were documented in 60 (84.5%) case reports or case series; however, as noted earlier, there were disparate methods for describing treatment outcome. For instance, positive treatment outcomes were narratively described (without the use of psychopathology measures) as “remission” (11 reports); “complete response,” “resolution” of symptoms,” or “recovery” (7 reports); “improvement” or “partial response” (20 reports); “good response” or “good results” (3 reports); or “well controlled” after ECT (1 report). Improvements in specific symptoms were narratively described in 2 reports. When psychopathology measures were used in single and double case reports, remission was documented in 6 reports (based on HAM-D score <7, QIDS-C <5, MADRS <6, PHQ-9 ≤4, Beck Depression Inventory <9, or CGI-S <2), response was documented in 1 report (based on a 50% improvement in 24-item HAM-D score), and non-response was documented in 2 reports (<50% improvement in 24- and 17-item HAM-D scores).
Patient follow-up was usually confined to the acute treatment phase, which often ended at hospital discharge. Several reports documented recurrences of psychiatric illness after an initial acute treatment series resulting in rehospitalization, with or without additional acute mECT treatments (54, 70, 82, 88, 92, 97, 101). When timelines were provided, symptom relapses were noted to occur within 2–3 weeks following the final ECT treatment or hospital discharge. The extension of a positive acute phase response with continuation or maintenance treatments was documented in 2 reports (73, 76). In one case, additional mECT treatments were provided to address two depressive recurrences following initial symptomatic remission in a patient who required prolonged hospitalization for high-risk pregnancy (99).
3.7 Adverse maternal, fetal, and neonatal events
3.7.1 Observational and chart review studies
Two observational and one chart review study reported safety or tolerability results in patients who received mECT for perinatal depression (and other indications in mixed cohorts). The largest of the three was a previously reviewed retrospective cohort study that used linked population-based registers to compare response rates in ECT-treated patients with a perinatal psychiatric diagnosis, non-ECT pregnancies from the same group of ECT-treated patients (non-ECT additional pregnancy group), and psychiatrically ill non-pregnant patients who received ECT (24). Control groups were matched with the main exposure group using propensity scores. Registry data included reports for specific complications including preeclampsia, diabetes, congenital malformations, stillbirth, Apgar scores (at 1-, 5-, and 10 minutes), birthweight, large/small for gestational age (LGA/SGA) status, and other maternal and neonatal complications. Most complications, including fetal malformations, LGA deliveries, and SGA deliveries, were comparable across exposure groups. As shown in Table 1, the risks of premature delivery and low 5-minute Apgar scores (< 7) were significantly higher for the pregnant ECT group than the non-ECT pregnant inpatient group, but not the non-ECT additional pregnancy group. Stillbirths occurred in 2 (2.1%) pregnancies in the pregnant ECT group, 1 (0.3%) pregnancy in the non-ECT pregnant inpatient group, and 1 (1.1%) pregnancy in the non-ECT additional pregnancy group.
A retrospective chart review study of 31 patients who received either BT or BF ECT during the prenatal (4 patients) or postpartum (27 patients) periods was presented as a conference abstract (28). Indications for ECT were described as “severe” cases with clinical diagnoses unspecified bipolar spectrum disorder (n=14), unspecified depression (n=3), unspecified psychotic disorder (n=4), unspecified postpartum psychosis (n=4), and “acute transient psychosis” (n=6). Maternal adverse effects of ECT included headache and cognitive difficulties. Fetal and neonatal safety outcomes, however, were not reported.
Preliminary maternal adverse effects and lactational safety data with ECT were provided in a prospective study 78 hospitalized patients with postpartum psychosis, 34 of whom received ECT (25). Clinical diagnoses assigned to cohort members included unspecified depression (n=24), acute manic episodes (n=32), and “non-affective” psychoses (n=22). The most common maternal adverse effects associated with ECT were memory disturbances (anterograde amnesia) in 6 (17.6%) patients and prolonged seizures in 4 (11.7%) patients, the latter managed with additional doses of sodium thiopental. Fifteen ECT-treated patients were hospitalized with their infants and continued breastfeeding without complications or apparent adverse effects.
3.7.2 Case reports and case series
3.7.2.1 Maternal and obstetric adverse events
As shown in Table 2, several reports documented common adverse effects known to be associated with ECT, including headache, nausea, myalgias, transient arrhythmias, hypertensive responses to ECT, cognitive disturbances, and post-ECT confusion. The most frequently reported perinatal adverse effects in patients were transient uterine contractions (14 reports), nearly all of which were transient (did not progress to preterm labor), although some required tocolytic therapy or prophylaxis. There were 5 reports of preterm labor with preterm deliveries occurring between 30+1 and 35+4 weeks EGA. Prolonged seizures were described in 4 reports, including one case of status epilepticus requiring aggressive doses of anesthetic medications to achieve seizure control, resulting in severe hypotension, the need for pressor support, and fetal demise (34). Other reported adverse events included pelvic pain, blood pressure reduction (owing to low intravascular volume), fetal growth restriction (presumed secondary to umbilical vein thrombosis), and prolonged neuromuscular blockade from administration of succinylcholine in a patient with pseudocholinesterase deficiency. Eleven reports specified no maternal complication with mECT, while information on adverse effects of mECT was not provided in 19 reports. There was one case of confirmed placental abruption diagnosed after Cesarean delivery at 37 weeks in a 35-year-old patient with severe depression and panic attacks who experienced the onset of regular uterine contractions, subsequent hypertonic-tetanic contractions, and blood pressure elevations followed by transient uterine bleeding during mECT at 34 weeks (94).
3.7.2.2 Fetal/neonatal adverse events
The most frequently reported fetal/neonatal adverse events occurring on or around the time of ECT administration were fetal heart rate decelerations (10 reports), the majority of which were transient and without long-term impact on fetal or delivery outcomes. There were 7 cases of premature deliveries occurring between 31- and 35-weeks’ gestation, 5 of which also included preterm labor occurring days (3 reports) to weeks (3 reports) following ECT treatment. In general, numerous risk factors for premature delivery were present including severe mood disorder symptoms (all cases), twin pregnancy (2 cases), infectious disease complication during pregnancy (1 case), substance exposures (1 case), food refusal or weight loss during pregnancy (2 cases), maternal age >35 years (1 case), and threatened abortion in the current pregnancy prior to ECT (1 case). In one case, preterm labor occurred 11 weeks after receiving just a single ECT administration (84). There were 6 reports of congenital malformations. Specific congenital defects included cardiovascular (atrial septal defect, coarctation of the aorta, transposition of the great vessels), musculoskeletal (equinovarus foot deformity, congenital hip dysplasia, hemivertebrae, fifth toe displacement), and gastrointestinal (anal atresia). The two reports documenting adverse events in ECT-treated patients with twin pregnancies involved multiple co-occurring congenital malformations and the neonatal demise of one twin, each, following subsequent surgeries (69, 97). There were two reports of fetal death/stillbirth (one occurring in the setting of severe drug-resistant status epilepticus and another from an undetermined cause) and one report of first trimester miscarriage occurring shortly after a third acute ECT treatment. Direct causal links between ECT and these adverse events were unclear. Normal deliveries or child development outcomes were specified in 21 reports, while no information on these outcomes was provided in 9 reports.
4 Discussion
The current report presents an updated scoping review of the literature describing the broad effectiveness and maternal, fetal, and neonatal safety of mECT for perinatal depression as well as details on mECT technique and technical adaptations that may bear on its safety or effectiveness in that population. We abstracted information from 82 reports that included information on over 1,300 pregnancies or deliveries. Our review was limited to mainly individual cases and case series, which accounted for 85% of the reviewed reports, with only a handful of controlled observational studies. The collected literature described a broad spectrum of effectiveness and safety outcomes with predominantly acute mECT for multiple forms of perinatal depression that presented across multiple trimesters of pregnancy and in the postpartum. As with non-puerperal depression, mECT appears to confer rapid benefit for depressive, psychotic, and catatonic symptoms in severely depressed perinatal patients including those with treatment-resistant illness. Reported adverse events, many with uncertain etiologic links to the procedure itself, are discussed further below.
To our knowledge, there have been 7 systematic reviews of the safety and/or effectiveness of ECT administered during the perinatal period (110, 111 12–15, 17). Most reviews focused on ECT effects during pregnancy and generally supported ECT as a rapidly beneficial alternative to conventional antidepressive medications, particularly for pharmaco-resistant cases or instances in which levels of clinical acuity are so high that therapeutic lag times associated with most antidepressive treatments would be unacceptable. Reports of postpartum mECT were fewer in number (about 25% of included papers in our review) than those addressing prenatal mECT, as was also the case with prior reviews. As such, the conclusions from this literature review, which addresses mECT for mainly the acute treatment of depression in pregnancy and postnatal samples, are broadly consistent with those of prior reviews in terms of efficacy as an acute-phase treatment modality.
Although there appears to be a reasonably consistent signal for rapid, acute phase antidepressive efficacy in perinatally depressed patients, several questions remain. The first pertains to who the reviewed evidence applies to. Although most reviewed papers included clearly defined cases of perinatal unipolar or bipolar depression, many of the reviewed reports (a little more than 25% of the total) involved mixed cohorts of individuals, not all of whom received ECT, with clinical diagnoses outside of unipolar or bipolar major depression. This included all 9 observational studies and 12 case series, with diagnoses ranging from unspecified catatonic syndromes to unspecified postpartum psychoses and diagnosed primary psychotic illnesses. In studies with mixed samples, diagnosis-specific outcomes were often not provided—an important limitation considering that, although ECT has robust broad spectrum efficacy across most mood and psychotic disorders (112, 113), general and obstetric adverse event risks from mECT may be disproportionately increased in patients with primary psychotic disorders due to especially high rates of general medical comorbidity (114); substance use (115); pregnancy and delivery complications (116, 117); smoking, obesity, and other negative lifestyle factors (118, 119); and lower rates of preventive or obstetric care seeking/medical follow-up (120, 121). Additionally, an essential core treatment for patients with chronic psychoses, antipsychotic drugs, may increase the risk of gestational diabetes (122), an independent risk factor for numerous pregnancy and neonatal complications (123). While the inclusion of papers with mixed samples and incomplete or disparate approaches to the reporting of diagnosis-specific outcomes are clear limitations, their inclusion in the paper is consistent with our objective of conducting a scoping literature review.
Following an acute phase response to mECT, the continuation (extension) of antidepressive effects becomes most relevant, given the possibility of depressive symptom relapse once a course of ECT has ended. In a meta-analysis of 32 studies of non-puerperal depressive patients who responded to an acute course of ECT, estimated relapse rates were 37% at 6 months and 51% at one year (124), highlighting the need for continuation and maintenance ECT for a significant number of acutely treated patients. The estimated reduction in relapse risk with maintenance ECT in non-puerperal depressed patients may be substantial, even when compared to pharmacologically treated controls (RR for relapse, 0.8 to 0.5) (125); however, to our knowledge, no such estimates have been established for perinatally depressed patients. Very few reports included in this scoping review provided detailed information on the duration of acute responses and only 9 case reports (39, 46, 50, 73, 76, 79, 81, 86, 97) and one small case series (37) described the delivery of continuation of maintenance ECT. A smaller number of reports described early relapses of severe depressive symptoms after an initial acute ECT series (82, 88, 92, 101), some of which required additional ECT treatment sessions (54, 70, 97, 99). Although the case literature reviewed here and existing literature from non-puerperal samples of depressed patients raises the strong possibility of benefit, the effectiveness of continuation and maintenance mECT for perinatal depression remains understudied, constituting an important and persisting clinical knowledge gap.
For decades, ketamine has been used as an alternative anesthesia induction agent when ECT seizures become too short or too difficult to elicit when using first- and second-line agents such as methohexital, propofol, or etomidate (126). Ketamine is not an anesthetic of choice for mECT owing to the potential for increasing seizure duration and for enhanced hemodynamic responses with associated elevations in intracranial pressure (127). However, ketamine and its S-enantiomer, esketamine, have since become established, rapidly acting antidepressive agents in their own right (128), which has raised interest in the therapeutic potential of ketamine-augmented ECT. Only one of the reviewed reports described the clinical effects of ketamine-augmented ECT for an acutely suicidal depressed patient during pregnancy (78). In this case, remission occurred after 8 treatments. However, time course to remission was not described, and there was no viable means of determining if ketamine augmentation was required to achieve that outcome. As of this writing, the evidence base for non-puerperal depression does not show an advantage of prioritizing ketamine over other anesthesia induction agents for the purposes of maximizing therapeutic efficacy (129). Furthermore, ketamine is contraindicated in pregnancy given a lack of sufficient reproductive and safety data (130), as well as potential associations of ketamine exposure with neurotoxicity during fetal development in preclinical studies and adverse neurodevelopmental outcomes in neonates with repeated exposure to ketamine anesthesia (131–133). The drug label for esketamine indicates that it is also not recommended during pregnancy owing to insufficient reproductive and neonatal safety data (134). As such, augmentation with ketamine or esketamine for the specific purpose of enhancing the efficacy of mECT or accelerating the time to remission with mECT in depressed pregnant patients cannot be recommended at this time.
Interestingly, eight of the reviewed reports described the indication for ECT as the preferred treatment for the specific context of pregnancy, motivated in some cases by the desire to minimize potentially teratogenic medication exposures and other adverse effects (34, 37, 39, 52, 54–56, 66, 70). However, the safety of ECT in pregnant patients has been the subject of debate. In prior reviews and for most cases in this review, ECT appeared to be relatively well tolerated. Frequently reported maternal and fetal adverse events from both prior reviews and ours included transient fetal arrhythmias/fetal bradycardia, uterine contractions, abdominal or pelvic pain, premature deliveries, placental abruption, threatened abortion, and vaginal bleeding. In most cases where adverse events were reported, a healthy term delivery was the ultimate outcome. On the other hand, only one observational study investigated a limited range of neonatal adverse events associated with ECT during 97 pregnancies (24), reporting 2 cases of stillbirth, 14 cases of premature delivery, and 9 cases of 5-minute Apgar scores <7 (with none in the range of 0-3). Individual case reports and case series also documented a variety of adverse fetal/neonatal outcomes including 2 cases of fetal death/stillbirth (33, 42) and one case of miscarriage (48); 7 cases of premature delivery between 31 and 35+4 weeks gestation due to pregnancy induced hypertension (57), preterm labor (65, 69), preterm premature rupture of membranes (72), fetal growth restriction (100), and recurrence of depressive symptoms (101); 6 reports of congenital malformations (37, 41, 69, 84, 97, 101), and one report of confirmed placental abruption (94). However, none of these reports can be considered comprehensive in terms of adverse event surveillance. And in the absence of valid denominator data, adverse events from individual case reports (e.g., congenital malformations) cannot be used to estimate event rates for comparisons against background rates [e.g., 3% background congenital malformations rate (135), even if all reported cases were true cases.
Prior reviews of safety data for ECT during pregnancy have documented similar adverse events in ECT-treated pregnancies but differed in their main conclusions. Four reviews supported the safety of ECT during pregnancy (12, 15, 17, 110, 111). However, two reviews suggested that ECT should be considered only when other treatment options are ineffective or infeasible based on low data quality (16) or high reported frequencies of adverse events in general (29%) and child fatalities in particular (7.1%) (14). The review by Leiknes and colleagues (14) has been criticized for including studies dating back to the early 1940s, decades before modified ECT was standard-of-care practice, and for counting adverse events that were not likely to be related to ECT (19, 136). In our review, only a limited number of papers included sufficient details on confounding factors such as maternal psychiatric and general medical comorbidities, pre-ECT obstetric complications, and medications taken prior to or during index courses of mECT, making it difficult in many instances to draw firm causal links between ECT and reported adverse events (137).
The ethical considerations pertaining to the inclusion of pregnant patients in clinical therapeutics research continue to be debated (138). Therefore, it is highly unlikely that clinical decisions pertaining to the effectiveness and safety of mECT for perinatal depression will be guided by evidence from randomized trials any time soon, especially when taking into account the added complexities of addressing issues of capacity and valid informed consent for research participation in highly-vulnerable patients who may require mECT for severe and often psychotic or catatonic illnesses (139). For the time being, we anticipate increasing use of data from linked national registers, such as the Swedish National Quality Registry for ECT, which includes information on patient diagnoses, symptom severities, ECT indications and treatment characteristics, treatment course, side-effects, concomitant medications, and other data elements (140). Linkages with other registries, including birth registries, enabled the construction of retrospective cohorts that include perinatally depressed patients in two of the larger-scale studies included in this review (24, 30). Similarly, we anticipate that electronic medical records-linkage systems will become increasingly important data sources for future studies of mECT for perinatal depression, especially with the continued development of integrated networks between multiple healthcare institutions and linkages with additional sources of structured and unstructured data, such as vital records and clinical symptom measures (141).
There are several limitations for this review in addition to those outlined above. First, the results of this review are current only through 12/31/2024, when the literature search was conducted. Second, a specific definition of the perinatal period or perinatal onset was seldom provided in the reviewed reports, particularly for the postpartum. Third, even though treatment resistance was a specific indication for mECT in several reports, detailed information was often not available regarding the quality of therapeutic trials preceding ECT, including information on previously used therapeutic interventions, their respective doses or frequencies of use, previous treatment durations, or estimated levels of adherence. When information was provided about pre-ECT treatments, it was often unclear if they were utilized during the current episode of depression, making it difficult to define the levels of therapeutic resistance ECT was being administered for. Fourth, even a relatively brief acute series mECT involves a complex set of interventions, co-interventions, and procedures that may each act as confounders or effect modifiers for questions related to its overall effectiveness or safety for perinatal depression. Regarding confounding, it was not possible to draw clear conclusions regarding causal links between ECT and several of the reported adverse events, as discussed earlier. Concerning effect modification, it was not possible to assess therapeutic or safety outcomes between subgroups defined by concomitant psychotropic or other types of medications, anesthetic agents, obstetric history, primary psychiatric diagnosis, psychiatric or general medical comorbidity, or ECT electrode placement, although controlled evidence has shown generally comparable response rates for ECT with BT, RUL, and BF lead placements in people with non-puerperal unipolar or bipolar depression (142–144). And finally, post-ECT follow-up periods were generally brief across the reviewed reports; thus, longer-term outcomes were unknown for most individual reports.
5 Conclusions
The current report presents an updated scoping review of the literature describing the broad effectiveness and key safety issues relevant to mECT for perinatal depression. Although registry studies have contributed meaningfully to our understanding of mECT effectiveness and safety in recent years, over 89% of the reviewed literature was still comprised of case reports and case series. No randomized trials were available for review. Although the maternal safety profile of mECT appears reassuring thus far, the available data are far from comprehensive. Fetal and neonatal safety risks are less-well-understood, and lactation information was often not included in reports of postpartum ECT. Therefore, many efficacy and safety questions remain, including for continuation or maintenance mECT and for important patient subgroups discussed in this review. Future reports of mECT for perinatal depression should focus on thoroughly describing treatment parameters, including frequencies of treatment sessions, initial doses and titration methods, and complete information on subsequent stimulation parameters (including changes), as has been recommended by others outside of the perinatal context (145).
Pragmatically, while mECT offers the prospect of rapid antidepressive benefit, patients with lower-acuity or less-impairing depression will not require–or even desire—a therapeutic procedure as intensive as mECT (146). For cases of perinatal depression with severe psychosis, catatonia (malignant and benzodiazepine-resistant catatonia, in particular), suicidality, other direct and serious threats to physical integrity (e.g., nutritional compromise, etc.), and marked treatment resistance, ECT is an indispensable therapeutic option that should not be withheld (11, 147). When these severe indications are absent, but ECT is still a therapeutic preference, the tilting of benefits over risks will usually be far less steep. In such cases, we recommend supportive, measured communication that considers the risks of untreated or undertreated depression, the possibility (though not a guarantee) of rapid benefit from mECT, a relatively reassuring maternal safety profile based on information to date, and potential fetal/neonatal risks–including from relatively rare but potentially severe complications such as prolonged seizures, prolonged uterine contractions, decreased uterine blood flow, and hypoxic damage—and precautions and strategies used to manage those risks. Given the high levels of acuity that are likely to be encountered in perinatally depressed patients being considered for mECT, the capacity for providing valid informed consent will require rigorous evaluation, including an assessment of patients’ ability to consider both their own needs and risks as well as those of the fetus or infant (148). Whenever possible, the assessment of ECT suitability and risk stratification should be initially conducted and periodically reviewed by a multidisciplinary team including psychiatry, obstetrics, anesthesiology, and pediatrics.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
WB: Writing – original draft, Formal analysis, Project administration, Conceptualization, Supervision, Investigation, Writing – review & editing. OM: Investigation, Writing – review & editing. CH: Writing – review & editing, Investigation. RR: Conceptualization, Writing – review & editing, Formal analysis, Writing – original draft. ES: Investigation, Writing – review & editing. AL: Writing – review & editing, Investigation. KM: Writing – review & editing, Investigation. HB: Conceptualization, Writing – review & editing, Investigation.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2025.1619098/full#supplementary-material
References
1. Gaynes BN, Gavin N, Meltzer-Brody S, Lohr KN, Swinson T, Gartlehner G, et al. Perinatal depression: Prevalence, screening accuracy, and screening outcomes. Evid Rep Technol Assess (Summ). (2005) 119:1–8. doi: 10.1037/e439372005-001
2. Underwood L, Waldie K, D’Souza S, Peterson ER, and Morton S. A review of longitudinal studies on antenatal and postnatal depression. Arch Womens Ment Health. (2016) . 19:711–20. doi: 10.1007/s00737-016-0629-1
3. Dagher RK, Bruckheim HE, Colpe LJ, Edwards E, and White DB. Perinatal depression: challenges and opportunities. J Women’s Health. (2021) . 30:154–9. doi: 10.1089/jwh.2020.8862
4. Grogoriadis S, VanderPorten EH, Mamisashvili L, Tolminson G, Dennis C-L, Koren G, et al. The impact of maternal depression during pregnancy on perinatal outcomes: a systematic review and meta-analysis. J Clin Psychiatry. (2013) . 74:e321–41. doi: 10.4088/JCP.12r07968
5. Lommerse K, Knight M, Nair M, Deneux-Tharaux C, and van den Akker T. The impact of reclassifying suicides in pregnancy and in the postnatal period on maternal mortality ratios. Int J Obstet Gynaecol. (2019) . 126:1088–92. doi: 10.1111/1471-0528
6. Lommerse KM, Merelle S, Rietveld AL, Berkelmans G, and van den Akker T. Netherlands Audit Committee Maternal Mortality and Morbidity. The contribution of suicide to maternal mortality: A nationwide population-based cohort study. BJOG. (2024) . 131:1392–8. doi: 10.1111/1471-0528.17784
7. Rodriguez-Cabezas L and Clark C. Psychiatric emergencies in pregnancy and postpartum. Clin Obstet Gynecol. (2018) . 61:615–27. doi: 10.1097/GRF.0000000000000377
8. Waqas A, Nadeem M, and Rahman A. Exploring heterogeneity in perinatal depression: a comprehensive review. BMC Psychiatry. (2023) . 23:643. doi: 10.1186/s12888-023-05121-z
9. Bobo WV. The diagnosis and management of bipolar I and II disorders: clinical practice update. Mayo Clin Proc. (2017) . 92:1532–51. doi: 10.1016/j.mayocp.2017.06.022
10. Kirov G, Jauhar S, Sienaert P, Kellner CH, and McLoughlin DM. Electroconvulsive therapy for depression: 80 years of progress. Br J Psychiatry. (2021) . 219:594–7. doi: 10.1192/bjp.2021.37
11. Ward HB, Fromson JA, Cooper JJ, De Oliveira G, and Almeida M. Recommendations for the use of ECT in pregnancy: literature review and proposed clinical protocol. Arch Women’s Ment Health. (2018) . 21:715–22. doi: 10.1007/s00737-018-0851-0
12. Cipolla S, Catapano P, Messina M, Pezzella P, and Giordano GM. Safety of electroconvulsive therapy (ECT) in pregnancy: a systematic review of case reports and case series. Arch Women’s Ment Health. (2024) . 27:157–78. doi: 10.1007/s00737-023-01394-1
13. Gressier F, Rotenberg S, Cazas O, and Hardy P. Postpartum electroconvulsive therapy: a systematic review and case report. Gen Hosp Psychiatry. (2015) . 37:310–4. doi: 10.1016/j.genhosppsych.2015.04.009
14. Leiknes KA, Cooke MJ, Jarosch-von Schweder L, Harboe I, and Hoie B. Electroconvulsive therapy during pregnancy: a systematic review of case studies. Arch Women’s Ment Health. (2015) . 18:1–39. doi: 10.1007/s00737-013-0389-0
15. Miller LJ. Use of electroconvulsive therapy during pregnancy. Hosp Comm Psychiatry. (1994) . 45:444–50. doi: 10.1176/ps.45.5.444
16. Pacheco F, Guiomar R, Brunoni AR, Buhagiar R, Evagorou O, Roca-LOecumberri A, et al. Efficacy of non-invasive brain stimulation in decreasing depression symptoms during the peripartum period: a systematic review. J Psychiatr Res. (2021) . 140:443–60. doi: 10.1016/j.jpsychires.2021.06.005
17. Pompili M, Dominici G, Giordano G, Longo L, Serafini G, Lester D, et al. Electroconvulsive treatment during pregnancy: a systematic review. Expert Rev Neurother. (2014) . 14:1377–90. doi: 10.1586/14737175.2014.972373
18. Saatcioglu O and Tomruk NB. The use of electroconvulsive therapy in pregnancy: a review. Isr J Psychiatry Relat Sci. (2011) . 48:6–11.
19. Sinha P, Goyal P, and Andrade C. A meta-review of the safety of electroconvulsive therapy in pregnancy. J ECT. (2017) . 33:81–8. doi: 10.1097/YCT.0000000000000362
20. Spodniaková B, Halmo M, and Nosáľová P. Electroconvulsive therapy in pregnancy—a review. J Obstet Gynaecol. (2015) . 35:659–62. doi: 10.3109/01443615.2014.990427
21. Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. (2018) . 169:467–73. doi: 10.7326/M18-0850
22. Andrade C. Active placebo, the parachute meta-analysis, the Nobel Prize, and the efficacy of electroconvulsive therapy. J Clin Psychiatry. (2021) 82:21f13992. doi: 10.4088/JCP.21f13992
23. Rasmussen KG. Sham electroconvulsive therapy studies in depressive illness: a review of the literature and consideration of the placebo phenomenon in electroconvulsive therapy practice. J ECT. (2009) 25:54–9. doi: 10.1097/YCT.0b013e3181719b23
24. Arnison T, Rask O, Nordenskjöld A, and Movahed Rad P. Safety of and response to electroconvulsive therapy during pregnancy: Results from population-based nationwide registries. Acta Psychiatr Scand. (2024) . 150:360–71. doi: 10.1111/acps.13623
25. Babu GN, Thippeswamy H, and Chandra PS. Use of electroconvulsive therapy (ECT) in postpartum psychosis–a naturalistic prospective study. Arch Womens Ment Health. (2013) . 16:247–51. doi: 10.1007/s00737-013-0342-2
26. Hauge C, Rohde C, and Østergaard SD. Treatment of postpartum psychotic- or mood disorder requiring admission: A nationwide study from Denmark. Acta Psychiatr Scand. (2024) .150:395–403. doi: 10.1111/acps.13585
27. Haxton C, Kelly S, Young D, and Cantwell R. The efficacy of electroconvulsive therapy in a perinatal population: A comparative pilot study. J ECT. (2016) . 32:113–5. doi: 10.1097/YCT.0000000000000278
28. Raghuraman BS, Varshney P, Sinha HTP, Ganjekar S, Desai G, and Chandra PS. Electroconvulsive therapy (ECT) for severe mental illness (SMI) during perinatal period: The role of bifrontal (BF) ECT. Brain Stimul. (2019) .12:388. doi: 10.1016/j.brs.2018.12.242
29. Reed P, Sermin N, Appleby L, and Faragher B. A comparison of clinical response to electroconvulsive therapy in puerperal and non-puerperal psychoses. J Affect Disord. (1999) . 54:255–60. doi: 10.1016/s0165-0327(99)00012-9
30. Ronnqvist I, Brus O, Hammar A, Landen M, Lundberg J, Nordanskog P, et al. Rehospitalization of postpartum depression and psychosis after electroconvulsive therapy: A population-based study with a matched control group. J ECT. (2019) . 35:264–71. doi: 10.1097/YCT.0000000000000578
31. Rundgren S, Brus O, Bave U, Landen M, Lundberg J, Nordanskog P, et al. Improvement of postpartum depression and psychosis after electroconvulsive therapy: A population-based study with a matched comparison group. J Affect Disord. (2018) 235:258–64. doi: 10.1016/j.jad.2018.04.043
32. Saluja S, Cooter A, Roberts S, and Branjerdporn G. Pharmacotherapy and electroconvulsive therapy prescription for women with depressive and anxiety disorders in a psychiatric mother-baby unit. Australas Psychiatry. (2024) . 32:573–81. doi: 10.1177/10398562241278856
33. Baki ED, Akici OC, Guzel HI, Kokulu S, Ela Y, and Sivaci RG. Our anesthesia experience during electroconvulsive therapy in pregnant patients. Braz J Anesth. (2016) . 66:555. doi: 10.1016/j.bjane.2014.07.001
34. Balki M, Castro C, and Ananthanarayan C. Status epilepticus after electroconvulsive therapy in a pregnant patient. Int J Obstet Anesth. (2006) . 15:325–8. doi: 10.1016/j.ijoa.2006.01.005
35. Ballone NT. Clinical considerations for electroconvulsive therapy in a breastfeeding mother for postpartum depression without psychosis. J ECT. (2023) 39:e17.
36. Bergink V and Koorengevel KM. Treatment of postpartum depression with psychotic features. Arch Womens Ment Health. (2011) 14:S60.
37. Bulut M, Bez Y, Kaya MC, Copoglu US, Bulbul F, and Savas HA. Electroconvulsive therapy for mood disorders in pregnancy. J ECT. (2013) . 29:e19–20. doi: 10.1097/YCT.0b013e318277cce2
38. Bhatia SC, Baldwin SA, and Bhatia SK. Electroconvulsive therapy during the third trimester of pregnancy. J ECT. (1999) 15:270–4.
39. Bozkurt A, Karlidere T, Isintas M, Ozmenler NK, Ozsahin A, and Yanarates O. Acute and maintenance electroconvulsive therapy for treatment of psychotic depression in a pregnant patient. J ECT. (2007) . 23:185–7. doi: 10.1097/YCT.0b013e31806db4dd
40. Brown NI, Mack PF, Mitera DM, and Dhar P. Use of the ProSeal laryngeal mask airway in a pregnant patient with a difficult airway during electroconvulsive therapy. Br J Anaesth. (2003) . 91:752–4. doi: 10.1093/bja/aeg227
41. Bulbul F, Copoglu US, Alpak G, Unal A, Demir B, Tastan MF, et al. Electroconvulsive therapy in pregnant patients. Gen Hosp Psychiatry. (2013) . 35:636–9. doi: 10.1016/j.genhosppsych.2013.06.008
42. Bulbul F, Copoglu US, Demir B, Bulut M, Alpak G, Unal A, et al. Sociodemographic characteristics and clinical follow-up results of pregnant patients hospitalized for psychiatric disorders. Dusunen Adam J Psychiatry Neurol Sci. (2014) . 27:21–6. doi: 10.5350/DAJPN2014270103
43. Chase T, Shah A, Maines J, and Fusick A. Psychotic pregnancy denial: a review of the literature and its clinical considerations. J Psychosom Obstet Gynecol. (2012) 42:253–7. doi: 10.1080/0167482X.2020.1789584
44. Choi B-S, Kim J-M, and Lee H-Y. A young woman who suffered a fractured vertebra during electroconvulsive therapy. Psychiatr Ann. (2018) 48:532–5. doi: 10.3928/00485713-20181010-01
45. Cutajar P, Wilson D, and Mukherjee T. ECT used in depression following childbirth, in a woman with learning disabilities. Br J Learn Disabil. (1998) 26:115–7. doi: 10.1111/j.1468-3156.1998.tb00062.x
46. De Asis SJ, Helgeson L, and Ostroff R. The use of propofol to prevent fetal deceleration during electroconvulsive therapy treatment. J ECT. (2013) . 29:e57–8. doi: 10.1097/YCT.0b013e318290f9e7
47. DeBattista C, Cochran M, Barry JJ, and Brock-Utne JG. Fetal heart rate decelerations during ECT-induced seizures: is it important?. Acta Anaesthesiol Scand. (2003) 47:101–3. doi: 10.1034/j.1399-6576.2003.470119.x
48. Echevarría Moreno M, Martin Muñoz J, Sanchez Valderrabanos J, and Vázquez Gutierrez T. Electroconvulsive therapy in the first trimester of pregnancy. J ECT. (1998) . 14:251–4. doi: 10.1097/00124509-199812000-00006
49. Erturk A, Aktoz F, Orgul G, Mutlu E, Demir B, and Tuncer ZS. Administration of electroconvulsive therapy for major depression during pregnancy: a case report. J Obstet Gynaecol. (2020) . 40:277–8. doi: 10.1080/01443615.2019.1628727
50. Forray A and Ostroff RB. The use of electroconvulsive therapy in postpartum affective disorders. J ECT. (2007) . 23:188–93. doi: 10.1097/yct.0b013e318074e4b1
51. Gahr M, Blacha C, Connemann BJ, Freudenmann RW, and Schönfeldt-Lecuona C. Successful treatment of major depression with electroconvulsive therapy in a pregnant patient with previous non-response to prefrontal rTMS. Pharmacopsychiatry. (2012) . 45:79–80. doi: 10.1055/s-0031-1297936
52. Gannon JM, Gopalan P, Solai LK, Lim G, Phillips JM, Beck S, et al. ECT for a pregnant patient with bipolar disorder in the COVID-19 Era: A clinical conundrum. Bipolar Disord. (2021) . 23:524–7. doi: 10.1111/bdi.13061
53. Gonzales N, Quinn DK, and Rayburn W. Perinatal catatonia: a case report and literature review. Psychosomatics. (2014) 55:708–14. doi: 10.1016/j.psym.2014.01.009
54. Griffiths EJ, Lorenz RP, Baxter S, and Talon NS. Acute neurohumoral response to electroconvulsive therapy during pregnancy. A Case Rep J Reprod Med. (1989) . 34:907–11.
55. Grover S, Sahoo S, Chakrabarti S, Basu D, Singh SM, and Avasthi A. ECT in the postpartum period: A retrospective case series from a tertiary health care center in India. Indian J Psychol Med. (2018) . 40:562–7. doi: 10.4103/IJPSYM.IJPSYM_105_18
56. Grover S, Sharma P, and Chakrabarti S. Use of electroconvulsive therapy during postpartum: A retrospective chart review. Indian J Psychiatry. (2024) . 66:572–5. doi: 10.4103/Indianjpsychiatry.Indianjpsychiatry_165_24
57. Grover S, Sikka P, Saini SS, Shni N, Chakrabarti S, Dua D, et al. Use of modified bilateral electroconvulsive therapy during pregnancy: A case series. Indian J Psychiatry. (2017) . 59:487–92. doi: 10.4103/psychiatry.IndianJPsychiatry_50_17
58. Guillet C, Didi Roy R, Hussami A, and Girod JC. Electroconvulsive therapy and dopa-responsive dystonia: Improvements in neurological symptoms after electroconvulsive therapy treatment. J ECT. (2020) 36:E53–4. doi: 10.1097/YCT.0000000000000696
59. Gunduz T, Yucel E, Tan D, Gercek A, Ilter E, Celik A, Haliloglu B, and Ozekici U. Induction of preterm uterine contractions with electroconvulsive therapy in a 32 week pregnant woman: A case report. Turk Jinekoloji ve Obstetrik Dernegi Dergisi. (2010) 7:96.
61. Howe GB and Srinivasan M. A case study on the successful management of Cotard's syndrome in pregnancy. Int J Psychiatry Clin Pract. (1999) 3:293–5. doi: 10.3109/13651509909068399
62. Isik M and Esin G. Temporomandibular dislocation secondary to modified electroconvulsive therapy. Anadolu Psikiyatri Derg. (2019) 20:336.
63. Iwasaki K, Sakamoto A, Hoshino T, and Ogawa R. Electroconvulsive therapy with thiamylal or propofol during pregnancy. Can J Anesth. (2002) 49:324–5. doi: 10.1007/BF03020541
64. Reveles Jensen KH, Pedersen ST, Vinther Hansen M, and Jørgensen MB. Shocking colours - ECT temporarily improves colour perception in a colour-blind patient. Brain Stimul. (2020) 13:957–8. doi: 10.1016/j.brs.2020.04.018
65. Kasar M, Saatcioglu O, and Kutlar T. Electroconvulsive therapy use in pregnancy. J ECT. (2007) 23:183–4. doi: 10.1097/yct.0b013e318065b12f
66. Kisa C, Yildirim SG, Aydemir C, Cebeci S, and Goka E. Prolonged electroconvulsive therapy seizure in a patient taking ciprofloxacin. J ECT. (2005) 21:43–4. doi: 10.1097/00124509-200503000-00012
67. Leite D and Antunes AF. Postpartum depression, catatonia and COVID-19 infection: One case, different clinical presentations. Eur Psychiatry. (2022) 65:S566. doi: 10.1192/j.eurpsy.2022.1450
68. Levy Y, Austin M-P, and Halliday G. Use of ultra-brief pulse electroconvulsive therapy to treat severe postnatal mood disorder. Australas Psychiatry. (2012) . 20:429–32. doi: 10.1177/1039856212458979
69. Livingston J, Johnstone W, and Hadi H. Electroconvulsive therapy in a twin pregnancy: A case report. Am J Perinatol. (1994) .11:116–8. doi: 10.1055/s-2007-994569
70. Maletzky BM. The first-line use of electroconvulsive therapy in major affective disorders. J ECT. (2004) . 20:112–7. doi: 10.1097/00124509-200406000-00007
71. Malhotra N, Vani, Malhotra P, and Bhardwaj R. Modified electroconvulsive therapy during pregnancy. J Anaesth Clin Pharmacol. (2008) 24:351–2.
72. Martinez-Sosa N, Delaney J, and McLeod-Bryant S. A challenging case of catatonia during pregnancy. Pers Med Psychiatry. (2020) 23-24:100064. doi: 10.1016/j.pmip.2020.100064
73. May MH and Reynolds-May MF. Postpartum depression treated in private practice. In Taylor CB (Ed.), How to Practice Evidence-Based Psychiatry: Basic Principles and Case Studies. (2010), 287–305. Washington, DC: American Psychiatric Publishing, Inc.
76. O’Reardon JP, Cristancho MA, von Andreae CV, Cristancho P, and Weiss D. Acute and maintenance electroconvulsive therapy for treatment of severe major depression during the second and third trimesters of pregnancy with infant follow-up to 18 months. J ECT. (2011) 27:e2–e26. doi: 10.1097/yct.0b013e3181e63160
77. Ozgul U, Erdogan MA, Sanli M, Erdil F, Begec Z, and Durmus M. Anaesthetic management in electroconvulsive therapy during early pregnancy. Turkish J Anesth Reanimation. (2014) . 42:145–7. doi: 10.5152/tjar.2014.73645
78. Patel A, Saucier AC, Hobday C, and Chacko R. Safety and efficacy of ketamine-augmented electroconvulsive therapy in third trimester pregnancy complicated by covid-19. Baylor Univ Med Center Proc. (2022) . 35:874–5. doi: 10.1080/08998280.2022.2106415
79. Pesiridou A, Baquero G, Cristancho P, Wakil L, Altinay M, Kim D, et al. A case of delayed onset of threatened premature labor in association with electroconvulsive therapy in the third trimester of pregnancy. J ECT. (2010) . 26:228–30. doi: 10.1097/yct.0b013e3181c3aef3
80. Pierre D, Pericaud A, Guerby P, Castel A, Schmitt L, and Yrondi A. Bitemporal electroconvulsive therapy during the second trimester of pregnancy in bipolar disroders: a case report. J ECT. (2020) 36:E14–5. doi: 10.1097/YCT.0000000000000634
81. Pinette MG, Santarpio C, Wax JR, and Blackstone J. Electroconvulsive therapy in pregnancy. Obstet Gynecol. (2007) . 110:465–6. doi: 10.1097/01.aog.0000265588.79929.98
82. Rabie N, Shah R, Ray-Griffith S, Coker JL, Magann EF, and Stowe ZN. Continuous fetal monitoring during electroconvulsive therapy: A prospective observation study. Int J Women’s Health. (2021) . 13:1–7. doi: 10.2147/ijwh.s290934
83. Ratan DA and Friedman T. Capgras syndrome in postpartum depression. Ir J Psychol Med. (1997) 14:117–8.
84. Ray-Griffith SL, Coker JL, Rabie N, Eads LA, Golden KJ, and Stowe ZN. Pregnancy and electroconvulsive therapy. J ECT. (2016) . 32:104–12. doi: 10.1097/yct.0000000000000297
85. Repke JT and Berger NG. Electroconvulsive therapy in pregnancy. Obstet Gynecol. (1984) 63:39S–41S.
86. Richardson AL, Russai R, Queenan K, Murtagh J, Whelan M, and Lucas DN. Electroconvulsive therapy for symptomatic bipolar disorder in the third trimester of pregnancy. Int J Obstetric Anesth. (2018) S61:s60.
87. Rineh HM, Khoshrang H, Alavi CE, Rimaz S, Biazar G, Rad RS, and Sani MK. Anesthesia management of electroconvulsive therapy at the late of pregnancy: A case report. Int J Womens Health Reprod Sci. (2020) 8:239–42. doi: 10.15296/ijwhr.2020.39
88. Sahan E and Zengin-Eroglu M. Negativism associated urinary bladder overdistension: A case report. Dusunen Adam: J Psychiatry Neurol Sci. (2017) 30:262–5. doi: 10.5350/dajpn2017300311
89. Salzbrenner S, Breeden A, Jarvis S, and Rodriguez W. A 48-year-old woman primigravid via in vitro fertilization with severe bipolar depression and preeclampsia treated successfully with electroconvulsive therapy. J ECT. (2011) 27:e1–e3. doi: 10.1097/yct.0b013e3181ca4d22
90. Sandal G and Cetin H. Electroconvulsive therapy during pregnancy as a possible cause of mobius syndrome: Additional clinical observation. Genet Couns. (2014) 25:357–61.
91. Sarma S, Quinn E, and Branjerdporn G. Safe delivery of electroconvulsive therapy in postpartum depression patient with type 1 Chari malformation: a case study. J ECT. (2024) 40:e10–11. doi: 10.1097/YCT.0000000000000999
92. Serim B, Ulaş H, Özerdem A, and Alkın T. Electroconvulsive therapy in an adolescent pregnant patient. Progr Neuropsychopharmacol Biol Psychiatry. (2010) . 34:546–7. doi: 10.1016/j.pnpbp.2009.11.014
93. Shea AK and Wolfman W. The role of hormone therapy in the management of severe postpartum depression in patients with Turner syndrome. Menopause. (2017) 24:1309–12. doi: 10.1097/GME.0000000000000915
94. Sherer DM, D’Amico ML, Warshal DP, Stern RA, Grunert HF, and Abramowicz JS. Recurrent mild abruptio placentae occurring immediately after repeated electroconvulsive therapy in pregnancy. Am J Obstet Gynecol. (1991) . 65:652–3. doi: 10.1016/0002-9378(91)90302-8
95. Strain AK, Meltzer-Brody S, Bullard E, and Gaynes BN. Postpartum catatonia treated with electroconvulsive therapy: A case report. Gen Hosp Psychiatry. (2012) 34:436.e3–436.e4. doi: 10.1016/j.genhosppsych.2011.11.010
96. Takubo Y, Nemoto T, Obata Y, Baba Y, Yamaguchi T, Katagiri N, et al. Effectiveness of kangaroo care for a patient with postpartum depression and comorbid mother-infant bonding disorder. Case Rep Psychiatry. (2019) 2019:9157214. doi: 10.1155/2019/9157214
98. Watanabe A, Ayani N, Waratani M, Hasegawa T, Ishii M, Matsuoka T, et al. A case of fetal tachycardia after electroconvulsive therapy a possible effect of maternal hypoxia and uterine contractions. Case Rep Psychiatry. (2019) 2019:3709612. doi: 10.1155/2019/3709612
99. Wise MG, Ward SC, and Townsend Parchman W. Case report of ECT during high-risk pregnancy. Am J Psychiatry. (1984) .141:99–10. doi: 10.1176/ajp.141.1.99
100. Yamada T, Sawa R, Abe H, Kikuti F, Mine K, Ishikawa G, et al. Serious bipolar depression amalgamation pregnancy with severe IUGR would be caused by placenta thrombus formation after ECT repeating itself. Placenta. (2007) . 28:A4–4. doi: 10.1016/j.placenta.2007.08.001
101. Yang HS, Seo HJ, and Lee YK. Anesthetic care for electroconvulsive therapy during pregnancy. Korean J Anesthesiol. (2011) . 60:217–20. doi: 10.4097/kjae.2011.60.3.217
102. Ying P, Carlisle G, and Reubins G. Pseudocholinesterase deficiency in a pregnant woman receiving ECT resulting in prolonged intubation and possible fetal distress. J ECT. (2022) . 38:e46.
103. Dorn JB. Electroconvulsive therapy with fetal monitoring in a bipolar pregnant patient. Convuls Ther. (1985) 1:217–21.
104. Montgomery SA and Asberg M. A new depression scale designed to be sensitivie to change. Br J Psychiatry. (1979) . 134:382–9. doi: 10.1192/bjp.134.4.382
105. Bush G, Fink M, Petrides G, Dowling F, and Francis A. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. (1996) . 93:129–36. doi: 10.1111/j.1600-0447.1996.tb09814.x
106. Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, et al. The 16-item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. (2003) . 54:573–83. doi: 10.1016/s0006-3223(02)01866-8
107. Kroenke K, Spitzer RL, and Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. (2001) . 16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x
108. Coman A and Bondevik H. The ethical imperative of trauma-sensitive care for electroconvulsive therapy (ECT): Recipients’ experiences with care. J Ment Health. (2024) 33:177–84. doi: 10.1080/09638237.2023.2210650
109. Asberg M, Montgomery SA, Perris C, Schalling D, and Sedvall G. A comprehensive psychopathological rating scale. Acta Psychiatr Scand. (1978) . 57:5–27. doi: 10.1111/j.1600-0447.1978.tb02357.x
110. Anderson EL and Reti IM. ECT in pregnancy: a review of the literature from 1941 to 2007. Psychosom Med. (2009) . 71:235–42. doi: 10.1097/PSY.0b013e318190d7ca
111. Calaway K, Coshal S, Jones K, Coverdale J, and Livingston R. A systematic review of the safety of electroconvulsive therapy use during the first trimester of pregnancy. J ECT. (2016) 32:230–5. doi: 10.1097/YCT.0000000000000330
112. Sinclair DJM, Zhao S, Qi F, Yakyoma K, Kwong JSW, and Adams DE. Electroconvulsive therapy for treatment-resistant schizophrenia. Schizophr Bull. (2019) . 45:730–2. doi: 10.1093/schbul/sbz037
113. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet. (2003) . 361:799–808. doi: 10.1016/S0140-6736(03)12705-5
114. Jones DR, Macias C, Barreira PJ, Fisher WH, Hargreaves WA, and Harding CM. Prevalence, severity, and co-occurrence of chronic physical health problems of persons with serious mental illness. Psychiatr Serv. (2004) . 55:1250–7. doi: 10.1176/appi.ps.55.11.1250
115. Nesvag R, Knudsen GP, Bakken IJ, Hoye A, Ystrom E, Suren P, et al. Substance use disorders in schizophrenia, bipolar disorder, and depressive illness: a registry-based study. Soc Psychiatry Psychiatr Epidemiol. (2015) . 50:1267–76. doi: 10.1007/s00127-015-1025-2
116. Fabre C, Pauly V, Baumstarck K, Etchecopar-Etchart D, Orleans V, Llorca P-M, et al. Pregnancy, delivery and neonatal complications in women with schizophrenia: a national population-based cohort study. Lancet Regional Health Europe. (2021) . 10:100209. doi: 10.1016/j.lanepe.2021.100209
117. Judd F, Komiti A, Sheehan P, Newman L, Castle D, and Everall I. Adverse obstetric and neonatal outcomes in women with severe mental illness: To what extent can they be prevented? Schizophr Res. (2014) . 157:305–9. doi: 10.1016/j.schres.2014.05.030
118. Brown S, Birtwistle J, Roe L, and Thompson C. The unhealthy lifestyle of people with schizophrenia. Psychol Med. (1999) . 29:697–701. doi: 10.1017/s0033291798008186
119. Brown S, Inskip H, and Barraclough B. Causes of the excess mortality of schizophrenia. Br J Psychiatry. (2000) . 177:212–7. doi: 10.1192/bjp.177.3.212
120. Fleischhacker WW, Cetkovich-Bakmas M, De Hert M, Hennekens CH, Lambert M, Leucht S, et al. Comorbid somatic illnesses in patients with severe mental disorders: clinical, policy, and research challenges. J Clin Psychiatry. (2008) . 69:514–9. doi: 10.4088/jcp.v69n0401
121. Seeman MV. Clinical interventions for women with schizophrenia: pregnancy. Acta Psychiatr Scand. (2012) . 127:12–22. doi: 10.1111/j.1600-0447.2012.01897.x
122. Betcher HK, Montiel C, and Clark CT. Use of antipsychotic drugs during pregnancy. Curr Treat Options Psych. (2019) . 6:17–31. doi: 10.1007/s40501-019-0165-5
123. Ye W, Luo C, Huang J, Li C, Liu Z, and Liu F. Gestational diabetes mellitus and adverse pregnancy outcomes: systematic review and meta-analysis. BMJ. (2022) . 377:e067946. doi: 10.1136/bmj-2021-067946
124. Jelovac A, Kolshus E, and McLoughlin DM. Relapse following successful electroconvulsive therapy for major depression: a meta-analysis. Neuropsychopharmacol. (2013) . 38:2467–74. doi: 10.1038/npp.2013.149
125. Rowland T, Mann R, and Azeem S. The efficacy and tolerability of continuation and maintenance electroconvulsive therapy for depression: a systematic review of randomized and observational studies. J ECT. (2023) . 39:141–50. doi: 10.1097/YCT.0000000000000914
126. Kellner CH and Iosifescu DV. Ketamine and ECT: better alone than together? Lancet Psychiatry. (2017) . 4:348–9. doi: 10.1016/S2215-0366(17)30099-8
127. Ding Z and White PF. Anesthesia for electroconvulsive therapy. Anesth Analgesia. (2002) . 94:1351–64. doi: 10.1097/00000539-200205000-00057
128. McIntyre RM, Rosenblat JD, Nemeroff CB, Sanacora G, Murrough JW, Berk M, et al. Synthesizing the evidence for ketamine and esketamine in treatment-resistant depression: an international expert opinion on the available evidence and implementation. Am J Psychiatry. (2021) . 178:383–99. doi: 10.1176/appi.ajp.2020.20081251
129. McGirr A, Berlim MT, Bond DJ, Chan PY, Yatham LN, and Lam RW. Adjunctive ketamine in electroconvulsive therapy: Updated systematic review and meta-analysis. Br J Psychiatry. (2017) . 210:403–7. doi: 10.1192/bjp.bp.116.195826
130. Pacilio RM, Lopez JF, Parikh SV, Patel PD, and Geller JA. Safe ketamine use in pregnancy: a nationwide survey and retrospective review of informed consent, counseling, and testing practices. J Clin Psychiatry. (2024) 85:24m15293. doi: 10.4088/JCP.24m15293
131. Dong C, Rovnaghi CR, and Anand KJ. Ketamine exposure during embryogenesis inhibits cellular proliferation in rat fetal cortical neurogenic regions. Acta Anaesthesiol Scand. (2016) . 60:579–87. doi: 10.1111/aas.12689
132. Yan J, Li T-R, Zhang Y, Lu Y, and Jiang H. Repeated exposure to anesthetic ketamine can negatively impact neurodevelopment in infants: a prospective preliminary clinical study. J Child Neurol. (2014) . 29:1333–8. doi: 10.1177/0883073813517508
133. Zhao T, Li Y, Wei W, Savage S, Zhou L, and Ma D. Ketamine administered to pregnant rats in the second trimester causes long-lasting behavioral disorders in offspring. Neurobiol Dis. (2014) . 68:145–55. doi: 10.1016/j.nbd.2014.02.009
134. Spravato package label. (2024). Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/211243s015lbl.pdf (Accessed April 25, 2025).
135. Centers for Disease Control and Prevention (CDC). Update on overall prevalence of major birth defects--Atlanta, Georgia, 1978–2005. MMWR Morb Mortal Wkly Rep. (2008) 57:1–5. Available online at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5701a2.htm (Accessed August 6, 2025).
136. Coshal S, Jones K, Coverdale J, and Livingston R. An overview of reviews on the safety of electroconvulsive therapy administered during pregnancy. J Psychiatr Prac. (2019) . 25:2–6. doi: 10.1097/PRA.0000000000000359
137. Joseph KS, Mehrabadi A, and Lisonkova S. Confounding by indication and related concepts. Curr Epidemol Rep. (2014) . 1:1–8. doi: 10.1007/s40471-013-0004-y
138. Rubin R. Addressing barriers to inclusion of pregnant women in clinical trials. JAMA. (2018) . 320:742–4. doi: 10.1001/jama.2018.9989
139. Biros M. Capacity, vulnerability, and informed consent for research. J Law Med Ethics. (2018) . 46:72–8. doi: 10.1177/1073110518766021
140. Nordanskog P, Hulten M, Landen M, Lundberg J, von Knorring L, and Nordenskjold A. Electroconvulsive therapy in Sweden 2013: data from the National Quality Register for ECT. J ECT. (2015) . 31:263–7. doi: 10.1097/YCT.0000000000000243
141. Casey JA, Schwartz BS, Stewart WF, and Adler NE. Using electronic health records for population health research: a review of methods and applications. Annu Rev Public Health. (2016) . 37:61–81. doi: 10.1146/annurev-publhealth-032315-021353
142. Bailine SH, Rifkin A, Kayne E, Selzer JA, Vital-Herne J, Blieka M, et al. Comparison of bifrontal and bitemporal ECT for major depression. Am J Psychiatry. (2000) . 157:121–3. doi: 10.1176/ajp.157.1.121
143. Kellner CH, Knapp R, Husain MM, Rasmussen K, Sampson S, Cullum M, et al. Bifrontal, bitemporal and right unilateral electrode placement in ECT: randomised trial. Br J Psychiatry. (2010) . 196:226–34. doi: 10.1192/bjp.bp.109.066183
144. Semkovska M, Landau S, Dunne R, Kolshus E, Kavanagh A, Jelovac A, et al. Bitemporal versus high-dose unilateral twice-weekly electroconvulsive therapy for depression (EFFECT-Dep): a pragmatic, randomized, non-inferiority trial. Am J Psychiatry. (2016) . 173:408–17. doi: 10.1176/appi.ajp.2015.15030372
145. Peterchev AV, Rosa MA, Deng Z-D, Preudic J, and Lisanby SH. ECT stimulus parameters: rethinking dosage. J ECT. (2010) . 26:159–74. doi: 10.1097/YCT.0b013e3181e48165
146. Rosenquist PB, Dunn A, Rapp S, Gaba A, and McCall WV. What predicts patients’ expressed likelihood of choosing electroconvulsive therapy as a future treatment? J ECT. (2006) . 22:33–7. doi: 10.1097/00124509-200603000-00007
147. Xiao H, Meng Y, Liu S, Cao Y, Sun H, Deng G, et al. Non-invasive brain stimulation for treating catatonia: a systematic review. Front Psychiatry. (2023) . 14:1135583. doi: 10.3389/fpsyt.2023.1135583
Keywords: perinatal depression, peripartum depression, postpartum depression, prenatal depression, electroconvulsive therapy, non-invasive brain stimulation
Citation: Bobo WV, Moore O, Hurley CB, Rosasco R, Sharpe EE, Larish AM, Moore KM and Betcher HK (2025) Modified electroconvulsive therapy for perinatal depression: scoping review. Front. Psychiatry 16:1619098. doi: 10.3389/fpsyt.2025.1619098
Received: 27 April 2025; Accepted: 16 July 2025;
Published: 18 August 2025.
Edited by:
Laith Alexander, King’s College London, United KingdomReviewed by:
Else Schneider, University Psychiatric Clinic Basel, SwitzerlandEmily Beydler, University of Pennsylvania, United States
Copyright © 2025 Bobo, Moore, Hurley, Rosasco, Sharpe, Larish, Moore and Betcher. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: William V. Bobo, d2lsbGlhbS5ib2JvQG1lZC5mc3UuZWR1
 William V. Bobo
William V. Bobo Owen Moore4
Owen Moore4 Catherine B. Hurley
Catherine B. Hurley Katherine M. Moore
Katherine M. Moore