- 1Fran and Earl Ziegler College of Nursing, University of Oklahoma Health Sciences, Oklahoma, OK, United States
- 2Hudson College of Public Health, University of Oklahoma Health Sciences, Tulsa Schusterman Center, Tulsa, OK, United States
Background: Exposure to Adverse Childhood Experiences (ACEs) is associated with increased risk of substance use in women, including cannabis use during pregnancy. Less is known, however, about how resilience factors moderate the association of ACEs on cannabis use in early motherhood.
Methods: We used survey data from 126 predominately low-income and diverse mothers enrolled in a longitudinal study in the South Central U.S. Multiple logistic regression models evaluated associations between ACEs and cannabis use through three years postpartum, stratified by resilience scores (median split). Adjusted models controlled for sociodemographic factors, postnatal depression, and prenatal substance use. Average predicted probabilities were estimated from fully adjusted models.
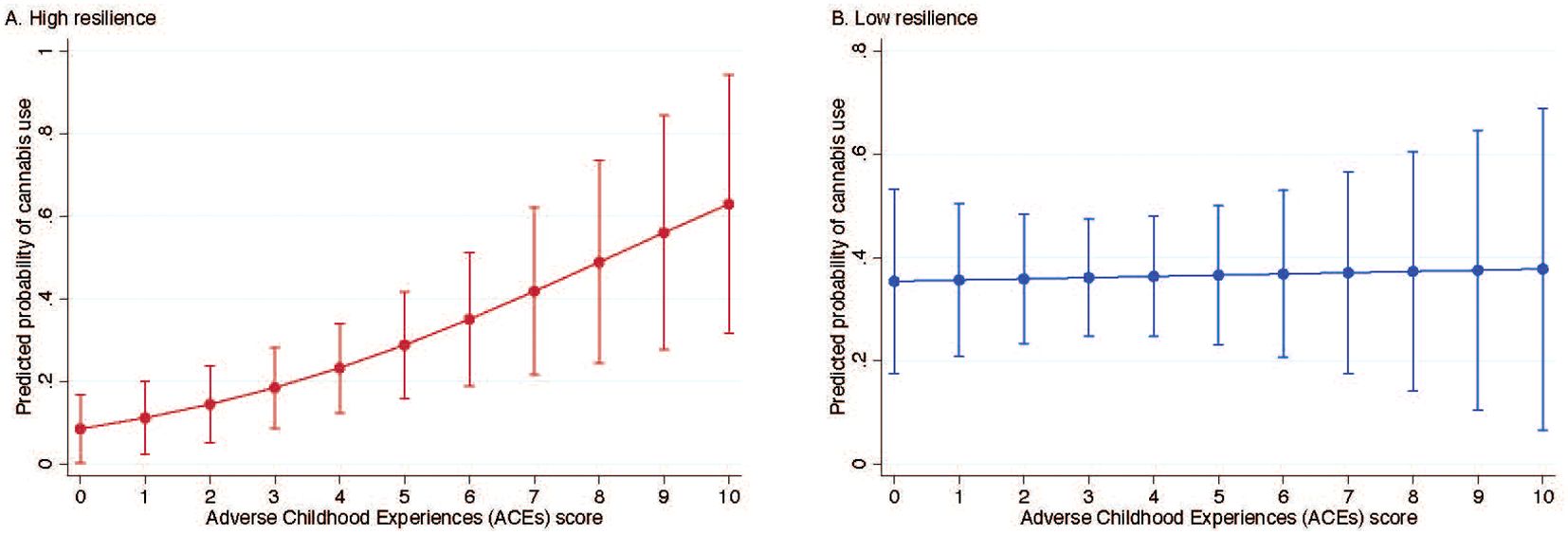
Results: Among individuals with high resilience, each unit increase in ACEs was associated with significantly higher odds of cannabis use in early motherhood (adjusted OR = 1.38; 95% CI: 1.07–1.78). No significant association was observed among those with low resilience (adjusted OR = 1.02; 95% CI: 0.77–1.34). In the high resilience group, the average predicted probability of cannabis use increased from 8.5% at 0 ACEs to 62.9% at 10 ACEs; in the low resilience group, average predicted probabilities of cannabis use was high (~36%) regardless of ACE score.
Conclusions: The findings suggest that although ACEs are a social determinant of cannabis use in early motherhood, resilience may be protective, particularly among those with low and moderate ACE exposure. However, its protective effect diminishes with higher ACE exposure.
1 Introduction
Adverse childhood experiences (ACEs) continue to be a public health concern, with nearly 70% of the U.S. population experiencing one or more ACEs in 2022, and 23% experiencing at least four or more (1). Strong evidence exists for the link between ACEs and poorer health and wellbeing during adulthood, including negative impacts on physical and mental health (2, 3), disruption in interpersonal relations (4), and increased participation in health-harming behaviors like substance use (5). Inequities persist for women and minoritized people who are more likely to experience severe ACEs than other groups (6). Recent evidence suggests that resilience may protect against the adverse mental health effects of ACEs during the perinatal period (7, 8), but less is known about its role to moderate the behavioral consequences of ACEs, which could have important implications for health behavior policy and practice.
Cannabis use is common and increasing among women of childbearing ages, in large part due to recent legislation for recreational and/or medical use in the majority of U.S. states (9). Between 1992 and 2022, cannabis consumption in the U.S. increased 15-fold, and daily or near-daily cannabis use is now more common than daily or near-daily alcohol use (17.7 million daily or near-daily cannabis users as compared to 14.7 million daily or near-daily alcohol users) (10). Cannabis use in pregnancy is associated with numerous adverse maternal and child health outcomes, including 50% greater risk for perinatal mortality, 40% higher risk for preterm birth, and twice the risk of developmental delay at 12 months of age (11). Given the widespread prevalence of cannabis use during pregnancy (16.0% nationally before 2020) and recent dramatic increases in use (12), cannabis associated developmental delays are the second most common cause of developmental disabilities, after alcohol (11).
Cannabis use during early motherhood is also not without risk. When lactating women use cannabis, Delta-9-tetrahydrocannabinol (THC) and other cannabis metabolites can accumulate in breastmilk for up to 6 days after last use, exposing breastfed infants (13, 14). Additionally, cannabis use alters breastmilk composition including macronutrient makeup and immunoglobin levels (14, 15), which has unknown impacts on infant development. Substance use in the home also poses risks for secondhand exposure and accidental ingestion (16), intergenerational transfer of ACEs (17, 18), and increased negative parenting behaviors (19). For example, cannabis use may impair working memory (20), which is an essential function when caring for infants and young children (21).
Although most women who use cannabis before pregnancy discontinue use during pregnancy, as many as two-thirds reinitiate use during the postpartum period (22, 23). This period can be a particularly high-risk time for return to cannabis use due to increased stress, ongoing mental health symptoms, and prior health conditions (24, 25). Postpartum women commonly report using cannabis to relax, reduce stress, improve mood, and manage both mental and physical health symptoms (23, 26, 27). While the postpartum period is often defined as ending between 6 weeks and 1 year after delivery (28, 29), the motives that drive cannabis use often persist beyond this timeframe (30) and underscores the need to examine potential protective factors across the early motherhood period.
Resilience generally refers to the ability to bounce back or recover from threats following adversity (31) and is associated with favorable maternal and child health outcomes (32, 33). Resilience can be enhanced through positive relationships and strong connections with others (34) and is a modifiable target for intervention (35). Recent evidence suggests that resilience buffers adverse maternal mental health consequences for those with a history of ACEs. Young-Wolff and colleagues (8) found that in pregnancy, higher ACE scores are associated with higher depression and anxiety among those with low levels of resilience, and Armans and colleagues (7) found that high levels of resilience are protective against pregnancy stress for those with low to moderate ACE scores.
While prior research has established a link between ACEs and cannabis use in pregnancy (36), less is known about the relationship between ACEs and cannabis use in early motherhood. Specifically, no studies to date have examined the moderating role of resilience in this relationship, which could identify important targets for interventions to support maternal health and reduce substance use after pregnancy. This study draws on the Health Belief Model (37) to conceptualize how resilience may influence health-risk behaviors like cannabis use, given experiences of early adversity. Although the effects of ACEs may increase an individual’s risk for stress-related coping, resilience may increase behavioral control, reduce reliance on maladaptive coping, and alter beliefs about risks versus benefits of use. The aim of this study was to examine the association between ACEs and cannabis use in early motherhood and to explore whether resilience moderates this relationship. We anticipated that higher ACEs would be associated with cannabis use, and that higher resilience would buffer the association between ACEs and cannabis use.
2 Methods
2.1 Data source and study population
The current study is a secondary analysis of survey data collected as part of a longitudinal study conducted in the South Central U.S. that examined the effects of maternal stressors on maternal and child health and well-being outcomes. The study population was recruited from a clinical population of racially diverse pregnant and postpartum women seeking prenatal care at one of two university-affiliated women’s health clinics. Potential participants were assessed for eligibility at their first prenatal care visit. Individuals were eligible to participate if they were less than 16 weeks’ gestation at enrollment, planning to continue their pregnancy, and planning to be a primary caretaker of the offspring. Those unable to speak and read English or under the age of 15 or over the age of 45 were ineligible for participation. Participants gave informed consent, and those under 18 gave informed assent and had parental consent for participation. Study enrollment occurred between 2017 and 2018 and participants were followed through December 2020. Enrolled participants completed online self-report questionnaires across nine timepoints during pregnancy, the postpartum period, and early motherhood through two years after delivery. Cannabis legalization for medical reasons occurred during the postpartum phase of the study. For the current analysis, we included only participants with complete data for the outcome of interest (i.e., cannabis use in early motherhood) (n=126). The study was approved by the authors’ university Institutional Review Board.
2.2 Variables
2.2.1 Exposure
The Adverse Childhood Experiences (ACEs) Questionnaire (2) assesses 10 categories of adverse experiences before the age of 18, including abuse (physical, emotional, sexual), neglect (physical, emotional), and household dysfunction (e.g., parental separation/divorce, household substance abuse, mental illness, domestic violence, incarceration). Each “Yes” response is scored as 1 point, yielding a total score ranging from 0 to 10, with higher scores indicating greater exposure to ACEs. In this study, the ACE score was treated as a continuous variable. For descriptive purposes only, we also categorized ACE scores into mild (0–1), moderate (2–3), and severe (≥4) exposure groups based on established cutpoints, with a score of 4 or higher being commonly used to define severe exposure (2, 7, 38).
2.2.2 Outcome
The outcome of interest was cannabis use during early motherhood, defined as any self-reported use as measured with the question, “Do you currently use marijuana?” at three separate timepoints: approximately six months postpartum (assessment 6), approximately 15 months postpartum (assessment 8), and approximately 22 months postpartum (assessment 9). We created a binary variable indicating cannabis use if participants endorsed use at any of the three timepoints (0 = no, 1 = yes); those who responded “no” at all timepoints were categorized as not using cannabis in early motherhood.
2.2.3 Potential moderator
Resilience was assessed using the Brief Resilience Scale (BRS), a 6-item validated instrument designed to measure the ability to recover from stress (39). Participants responded to each item using a five-point Likert scale ranging from 1 (strongly disagree) to 5 (strongly agree). Items include: “I tend to bounce back quickly after hard times,” “I have a hard time making it through stressful events,” “It does not take me long to recover from a stressful event,” “It is hard for me to snap back when something bad happens,” “I usually come through difficult times with little trouble,” and “I tend to take a long time to get over setbacks in my life.” Negatively worded items (“I have a hard time making it through stressful events,” “It is hard for me to snap back when something bad happens,” “I tend to take a long time to get over setbacks in my life”) were reverse coded before calculating scores. The final score was calculated by summing responses to the six items, giving a total score ranging from 6-30, with higher scores indicating greater resilience.
2.2.4 Covariates
Covariates included age, years of education, race/ethnicity (White, Black, Hispanic, American Indian/Alaska Native), union status (married/cohabiting vs. not cohabiting/single/divorced/widowed/separated), parity (number of previous births), postnatal depression, and prenatal substance use, including alcohol, tobacco, opioids, and cannabis. Participants were asked, “Since getting pregnant, how often having you been … using [alcohol/tobacco/opioids/marijuana]?” Never responses were coded as “0”, and any use was coded as a “1” for each type of substance use. Depression was assessed using the 20-item Center for Epidemiologic Studies Depression Scale (CES-D), a widely used and validated measure of depressive symptoms (40). Scores range from 0 to 60, with a score of 16 or higher indicative of clinical depression.
2.3 Statistical analysis
Descriptive statistics were used to compare characteristics of the sample and ACEs scores stratified by cannabis use during early motherhood. Group comparisons were conducted using Student’s t tests, chi-squared tests, or Mann-Whitney U tests, as appropriate. We evaluated dose-response relationships between the number of adverse childhood experiences (ACEs) and cannabis use using unadjusted and adjusted logistic regression models, with results presented as odds ratios (ORs) and 95% confidence intervals (CIs). Adjusted models controlled for age, educational attainment, race/ethnicity, union status, parity, postnatal depression, and prenatal use of alcohol, tobacco, opioids, and cannabis. All covariates, except race/ethnicity, were modeled as continuous or dichotomous variables due to sample size constraints. To assess whether the association between ACEs and cannabis use varied by resilience, we included resilience as a continuous variable and specified an interaction term for continuous ACEs × continuous resilience in the fully adjusted model. Given the statistically significant interaction, we present regression models stratified by resilience level to illustrate the modifying effect of resilience on the association between ACEs and cannabis use. To facilitate interpretation, resilience was dichotomized at the sample median (BRS score = 22), which is a commonly used data-driven approach in the absence of a validated cut-point (41). We report stratified ORs and 95% CIs comparing individuals with high (above median) versus low (at or below median) resilience. As a final step, we estimated and plotted average predicted probabilities of cannabis use across ACE scores, stratified by resilience level, using marginal effects from the adjusted models.
Missing data >5% were present for several covariates, including education (10.3%), depression (19.8%), and prenatal substance use (6.4%). We examined patterns of missing data and found no significant differences in characteristics between participants with and without missing values, suggesting data were likely missing completely at random. To address missingness, we used multiple imputation via the Markov Chain Monte Carlo (MCMC) method under the assumption of multivariate normality (42). Multivariable logistic regression models were conducted across five imputed datasets, and results were pooled using standard Rubin’s rules (43). As a sensitivity analysis, we performed a complete case analysis data to ensure that imputation did not affect our results. We additionally conducted a sensitivity analysis without adjusting for prenatal cannabis use to assess whether results changed when this covariate was excluded. All tests were two-sided, and statistical significance was defined as p < 0.05. Data management and analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC) and Stata version 17 (StataCorp LLC, College Station, TX).
3 Results
Among the 126 participants, 17.5% (n = 31) reported cannabis use during early motherhood (Table 1). Cannabis use was reported by 9 participants (7.1%) at assessment six, 18 (14.3%) at assessment eight, and 18 (14.3%) at assessment nine, with 10 participants (7.8%) reporting use at multiple timepoints and 4 (3.2%) at all three timepoints. Self-reported prenatal alcohol use was significantly more common among individuals who reported cannabis use compared to those who did not (20.0% vs. 6.8%; p = 0.04). Similarly, self-reported prenatal cannabis use was more frequent among those who used cannabis during early motherhood (25.8% vs. 6.3%; p = 0.002). The mean ACEs score was significantly higher among individuals who reported cannabis use compared to non-users (mean [SD]: 4.2 [3.4] vs. 2.9 [2.8]; p = 0.045). When ACEs were categorized, a greater proportion of cannabis users reported severe ACEs (scores 4–10) compared to non-users (54.8% vs. 31.6%; p = 0.04). No statistically significant differences were observed between groups with respect to age, education, race/ethnicity, union status, parity, postnatal depression, prenatal use of tobacco or opioids, or resilience.
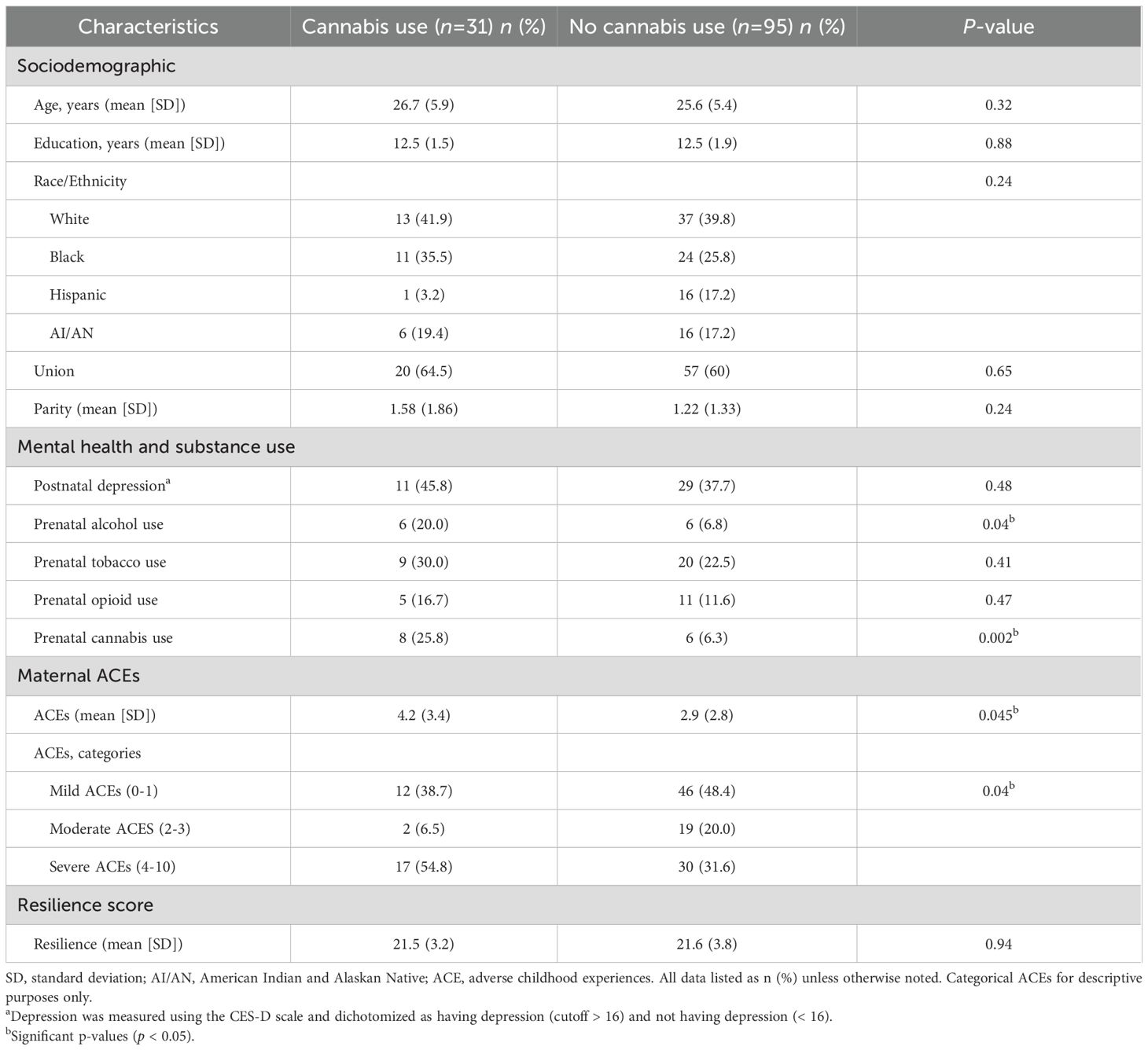
Table 1. Characteristics of individuals reporting cannabis use and no cannabis use in early motherhood.
In the unadjusted and adjusted models, each unit increase in ACEs was significantly associated with higher odds of cannabis use in early motherhood (unadjusted OR, 1.15; 95% CI, 1.00-1.31; adjusted OR, 1.20; 95% CI, 1.01-1.43) (data not shown). In the fully adjusted model, the interaction between ACEs score and resilience was statistically significant (p = 0.04), indicating that the effect of ACEs score on cannabis use in early motherhood varied by level of resilience (supplemental material, Supplementary Table S1). Among individuals with high resilience, each unit increase in ACEs score was associated with higher odds of cannabis use in early motherhood in both the unadjusted (OR = 1.26, 95% CI: 1.04–1.52) and adjusted models (OR = 1.38, 95% CI: 1.07–1.78) (Table 2). No significant association between ACEs score and cannabis use was observed among those with low resilience in either the unadjusted (OR = 1.01, 95% CI: 0.81–1.24) or adjusted models (OR = 1.02, 95% CI: 0.77–1.34). Sensitivity analyses produced similar results to the main findings (supplemental material, Supplementary Tables S2, S3). Among those with high resilience, the probability of cannabis use increased from 8.5% (95% CI: 1.9–16.8) at 0 ACEs to 62.9% (95% CI: 31.7–94.1) at 10 ACEs (Figure 1A). Among those with low resilience, predicted probabilities were relatively stable, ranging from 35.4% (95% CI: 17.5–53.2) at 0 ACEs to 37.8% (95% CI: 6.6–68.9) at 10 ACEs (Figure 1B).
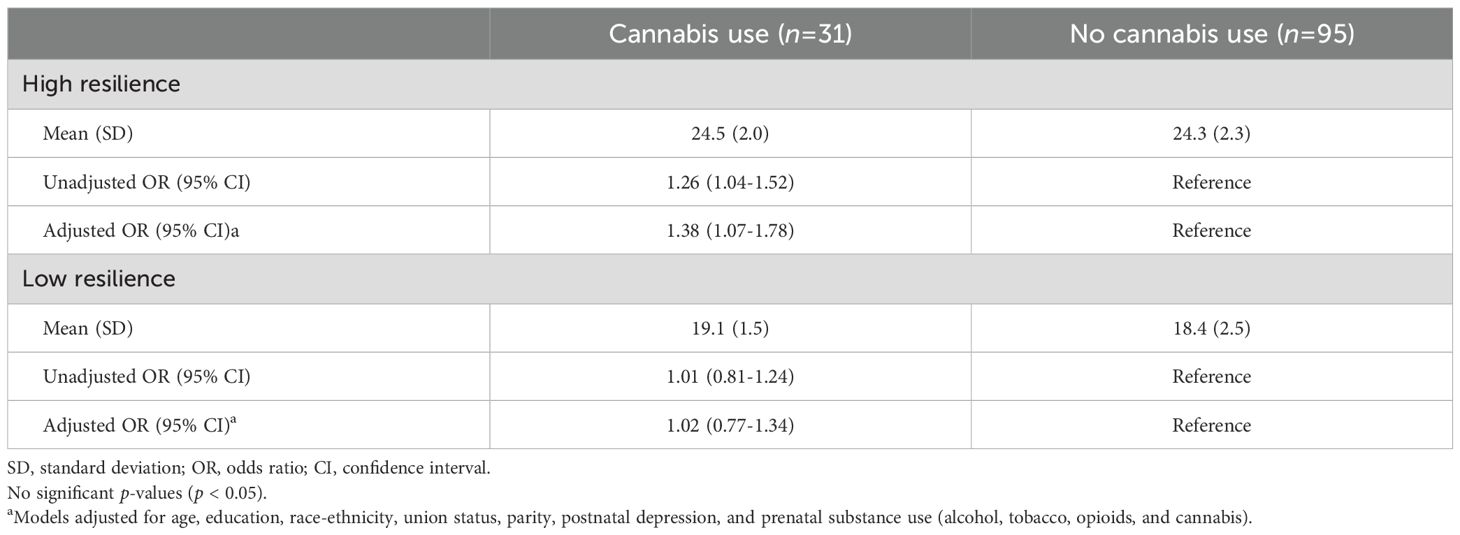
Table 2. Associations between adverse childhood experiences and cannabis use in early motherhood, stratified by resilience level.
4 Discussion
The aim of this study was to examine the association between ACEs and cannabis use in early motherhood and to explore whether resilience moderates this relationship. ACEs have been associated with prenatal substance use including alcohol (44), cannabinoids (45), cannabis (36, 46) and other substance use (45–47). Kendall-Tackett et al. (48) also found that ACEs were associated with frequency of cannabis use among pregnant women but when the number of health problems were accounted for, ACEs were no longer a significant predictor of cannabis use behavior. In our sample, higher ACEs were associated with an increased likelihood of cannabis use during early motherhood among those with high resilience, but no association was observed among those with low resilience. These findings suggest that resilience may influence how early life adversity relates to cannabis use behaviors in the postpartum period.
Together, our findings suggest that resilience influences the extent to which adversity affects cannabis use behavior. Specifically, individuals with low resilience may have an elevated risk of cannabis use that is not influenced by ACE exposure. That is, individuals with low resilience may engage in higher rates of cannabis use overall, so the marginal effect of increasing ACEs on the probability of cannabis use is small because the behavior is already elevated. Prior research has shown that when positive coping mechanisms are unavailable, individuals are likely to use substances to manage distress (49). In individuals with low resilience, mental health conditions and maladaptive coping strategies may be more common (8, 50), contributing to sustained or elevated rates of cannabis use, especially in early motherhood when stress levels are high (51). Coping is also a commonly reported motivation for cannabis use (52, 53) and may contribute to worsening stress responses, creating a cycle of repeated substance use and distress (54), even in the absence of additional trauma exposure.
On the other hand, although higher resilience has been found to mitigate negative outcomes in various contexts (7, 8, 32, 33), we found that resilience did not appear to protect against cannabis use among individuals with high ACE exposure, especially among those who reported experiencing 6–10 ACEs. Among individuals with high resilience, there may be limits to its protective effects under conditions of high or chronic adversity, such as exposure to numerous ACEs. Prior research has shown that exposure to multiple ACEs has a detrimental impact on healthy development (2–5) and adaptation (55, 56) to the degree that resilience may not protect against the influence from adversity. Individuals may engage in high-risk behaviors like substance use to regulate or alleviate symptoms related to adversity experiences including developmental effects (17), lower self-esteem (57) and depression (58).
Although the findings seem contradictory, other researchers have also found that resilience is less effective at high levels of ACEs. For example, Armans et al. (7) found that high resilience moderated the association between ACEs and pregnancy-specific stress at low and moderate levels of ACEs but not severe ACEs. A similar pattern has been described in studies examining the effects of resilience on the development of personality disorders and engagement in health risk behaviors in young adults exposed to childhood adversity, where resilience was less protective at higher levels of adversity (56). In contrast, Young-Wolf (8) reported that high resilience was protective against mental health conditions at high levels of ACEs. Together with extant literature, our findings indicate that the cumulative impact of multiple experiences of childhood adversity may be profound enough such that resilience is inadequate to mitigate some outcomes–particularly for behavioral outcomes such as cannabis use.
Providers caring for women and children should remain aware of the relationship between low resilience, adversity, and cannabis use and consider providing brief intervention to increase positive coping behaviors among mothers (59). Although high individual resilience was not protective in our sample for those with high ACE scores, providers can work with families to increase family resilience, which has been shown to improve outcomes among families impacted by ACEs (60).
4.1 Strength and limitations
Our findings should be interpreted in light of several strengths and limitations. A major strength of the study is its prospective, longitudinal design, which allowed for the collection of data across pregnancy and into early motherhood. We also used validated measures for key constructs, including ACEs, resilience, and depressive symptoms, which strengthens the reliability of the findings. First, however, all measures for the present study, including cannabis use and resilience, were collected through participant self-report. We recognize that including more objective measures, in particular for cannabis use, would have strengthened the validity of the assessments. However, prior research suggests that self-reported substance use is often underreported (61), and such underreporting would likely influence prevalence estimates rather than bias the direction of observed associations. Second, self-reported measures capture static responses to constructs that may fluctuate over time, such as resilience or patterns of cannabis use, limiting the ability to capture dynamic changes. Third, some of the covariates in our model (e.g., age, education, tobacco use) were not significant predictors of cannabis use despite their well-supported documentation in prior research (62–64). These findings may demonstrate that cannabis use during early motherhood is becoming more common across demographic groups, or that our study was underpowered to detect small effect sizes. Fourth, although we adjusted for a range of conceptually important covariates, the possibility of residual confounding from unmeasured variables cannot be ruled out. Finally, the modest sample size and focus on a specific regional clinical population may limit the generalizability of the findings to broader or more diverse populations. Future research can expand this work with representative data and by examining additional factors that buffer the effects of ACEs on health behaviors and can be modified through intervention.
5 Conclusion
This study documents the moderating role of resilience on the association between ACEs and cannabis use in early motherhood. Among those with lower levels of resilience, cannabis use was high regardless of ACEs. Among those with higher levels of resilience, cannabis use was low among those with low and moderate levels of childhood adversity. Among those with high ACE scores (particularly among those with more than 6 adverse childhood experiences), cannabis use was high. This suggests that resilience influences the extent to which adversity affects cannabis use behavior, and while it is protective against cannabis use at lower levels of adversity, it does not appear to reduce cannabis use among those with high levels of adversity.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the University of Oklahoma Health Sciences Center Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
AR: Conceptualization, Visualization, Writing – original draft, Writing – review & editing. EC: Formal Analysis, Investigation, Methodology, Software, Supervision, Writing – original draft, Writing – review & editing. KS: Conceptualization, Data curation, Funding acquisition, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported in part by the National Institutes of Health (P20GM109097 and U54HD113173). The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health. Portions of these findings were presented as a poster at the 47 th annual research conference of the Midwest Nursing Research Society, Des Moines, Iowa. Financial support for open access publishing was provided by the Fran and Earl Ziegler Endowed Chair in Nursing Research, held by Karina Shreffler at the Fran and Earl Ziegler College of Nursing, University of Oklahoma Health Sciences Center.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2025.1621161/full#supplementary-material
References
1. Kumar S, Campbell JA, Wang X, Xu Y, Nagavally S, and Egede LE. Trends in prevalence of adverse childhood experiences by sociodemographic factors in the United States: Behavioral Risk Factor Surveillance System 2009–2022. BMC Public Health. (2024) 24:2615. doi: 10.1186/s12889-024-20125-4
2. Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The Adverse Childhood Experiences (ACE) study. Am J Prev Med. (1998) 14:245–58. doi: 10.1016/S0749-3797(98)00017-8
3. Rod NH, Bengtsson J, Elsenburg LK, Taylor-Robinson D, and Rieckmann A. Hospitalisation patterns among children exposed to childhood adversity: a population-based cohort study of half a million children. Lancet Public Health. (2021) 6:e826–35. doi: 10.1016/S2468-2667(21)00158-4
4. Maloney E, Dowling C, and O’Reilly G. Adverse childhood experiences and interpersonal functioning in adulthood: A systemic review. Eur J Trauma Dissociation. (2025) 9:100534. doi: 10.1016/j.ejtd.2025.100534
5. Racine N, McDonald S, Chaput K, Tough S, and Madigan S. Pathways from maternal adverse childhood experiences to substance use in pregnancy: findings from the all our families cohort. J Women’s Health. (2021) 30:1795–803. doi: 10.1089/jwh.2020.8632
6. Martin EL, Neelon B, Brady KT, Guille C, Baker NL, Ramakrishnan V, et al. Differential prevalence of Adverse Childhood Experiences (ACEs) by gender and substance used in individuals with cannabis, cocaine, opioid, and tobacco use disorders. Am J Drug Alcohol Abuse. (2023) 49:190–8. doi: 10.1080/00952990.2023.2171301
7. Armans M, Addante S, Ciciolla L, Anderson M, and Shreffler K. Resilience during pregnancy: How early life experiences are associated with pregnancy-specific stress. Advers Resilience Sci. (2020) 1:295–305. doi: 10.1007/s42844-020-00017-3
8. Young-Wolff KC, Alabaster A, Mccaw B, Stoller N, Watson C, Sterling S, et al. Adverse childhood experiences and mental and behavioral health conditions during pregnancy: The role of resilience. J Women’s Health. (2018) 28. doi: 10.1089/jwh.2018.7108
9. Alshaarawy O and Anthony JC. Cannabis use among women of reproductive age in the United States: 2002–2017. Addict Behav. (2019) 99:106082. doi: 10.1016/j.addbeh.2019.106082
10. Caulkins JP. Changes in self-reported cannabis use in the United States from 1979 to 2022. Addiction. (2024) 119:1648–52. doi: 10.1111/add.16519
11. Lo JO, Shaw B, Robalino S, Ayers CK, Durbin S, Rushkin MC, et al. Cannabis use in pregnancy and neonatal outcomes: A systematic review and meta-analysis. Cannabis Cannabinoid Res. (2024) 9:470–85. doi: 10.1089/can.2022.0262
12. Hayes S, Delker E, and Bandoli G. The prevalence of cannabis use reported among pregnant individuals in the United States is increasing, 2002-2020. J Perinatol. (2023) 43:387–9. doi: 10.1038/s41372-022-01550-y
13. Bertrand KA, Hanan NJ, Honerkamp-Smith G, Best BM, and Chambers CD. Marijuana use by breastfeeding mothers and cannabinoid concentrations in breast milk. Pediatrics. (2018) 142:e20181076. doi: 10.1542/peds.2018-1076
14. Josan C, Shiplo S, Fusch G, Raha S, and Shea AK. Cannabis use during lactation may alter the composition of human breast milk. Pediatr Res. (2023) 93:1959–68. doi: 10.1038/s41390-022-02315-1
15. Narayanan P, Bertrand K, Waalen J, Chambers C, Ferran K, and Bandoli G. The effect of cannabis consumption during lactation on the macronutrient concentrations in breast milk. Breastfeeding Med. (2025) 20:33–41. doi: 10.1089/bfm.2024.0083
16. Tweet MS, Nemanich A, and Wahl M. Pediatric edible cannabis exposures and acute toxicity: 2017–2021. Pediatrics. (2023) 151:e2022057761. doi: 10.1542/peds.2022-057761
17. Anda RF, Whitfield CL, Felitti VJ, Chapman D, Edwards VJ, Dube SR, et al. Adverse childhood experiences, alcoholic parents, and later risk of alcoholism and depression. PS. (2002) 53:1001–9. doi: 10.1176/appi.ps.53.8.1001
18. Morgan MH, Coe JL, Kranzler EC, Rehberg K, Ingersoll R, Namrow N, et al. Implementation of family centered substance use treatment for pregnant and postpartum people to prevent the intergenerational transmission of adverse childhood experiences. Child Abuse Negl. (2024) 157:107066. doi: 10.1016/j.chiabu.2024.107066
19. Wesemann DG, Wilson AC, and Riley AR. Parental cannabis use, negative parenting, and behavior problems of young children. Subst Use Misuse. (2022) 57:2015–9. doi: 10.1080/10826084.2022.2130001
20. Gowin JL, Ellingson JM, Karoly HC, Manza P, Ross JM, Sloan ME, et al. Brain function outcomes of recent and lifetime cannabis use. JAMA Netw Open. (2025) 8:e2457069. doi: 10.1001/jamanetworkopen.2024.57069
21. Martin RCB, Bridgett DJ, Mayes LC, and Rutherford HJV. Maternal working memory, emotion regulation, and responsivity to infant distress. J Appl Dev Psychol. (2020) 71:101202. doi: 10.1016/j.appdev.2020.101202
22. Allen AM, Jung AM, Alexander AC, Allen SS, Ward KD, and al’Absi M. Cannabis use and stressful life events during the perinatal period: cross-sectional results from Pregnancy Risk Assessment Monitoring System (PRAMS) data, 2016. Addiction. (2020) 115:1707–16. doi: 10.1111/add.15003
23. Eitel AE, Witcraft SM, McRae-Clark AL, Brady K, King C, and Guille C. Exploration into patterns of cannabis use across pregnancy and postpartum. J Addict Med. (2024) 18:327–30. doi: 10.1097/ADM.0000000000001270
24. Forray A, Merry B, Lin H, Ruger JP, and Yonkers KA. Perinatal substance use: a prospective evaluation of abstinence and relapse. Drug Alcohol Depend. (2015) 150:147–55. doi: 10.1016/j.drugalcdep.2015.02.027
25. Young-Wolff KC, Green A, Iturralde E, Altschuler A, Does MB, Jackson-Morris M, et al. Intentions to use cannabis postpartum: A qualitative study of pregnant individuals who used cannabis during early pregnancy. J Women’s Health. (2024) 33:435–45. doi: 10.1089/jwh.2023.0066
26. Withanarachchie V, Rychert M, and Wilkins C. Motherhood and medicinal cannabis. Drug Alcohol Rev. (2025) 44:1024–135. doi: 10.1111/dar.14027
27. Brabete AC, Greaves L, Poole N, Huber E, and Stinson J. Women’s experiences with nicotine and cannabis vaping during pregnancy and postpartum. Healthc (Basel Switzerland). (2025) 13:223. doi: 10.3390/healthcare13030223
28. Chauhan G and Tadi P. Physiology, postpartum changes. In: StatPearls. StatPearls Publishing, Treasure Island (FL (2025). Available online at: http://www.ncbi.nlm.nih.gov/books/NBK555904/.
29. Saldanha IJ, Adam GP, Kanaan G, Zahradnik ML, Steele DW, Danilack VA, et al. Postpartum care up to 1 year after pregnancy: a systematic review and meta-analysis. Agency for healthcare research and quality (AHRQ) (2023). Available online at: https://effectivehealthcare.ahrq.gov/products/postpartum-care-one-year/research (Accessed June 20, 2025).
30. Colorado Department of Public Health and Environment. Postpartum behavioral health in Colorado. (2021). Available online at: https://drive.google.com/file/d/1Kp2ixDIAiGPty7MN8j4XHJF9G439AGmb/view?usp=embed_facebook (Accessed June 17, 2025).
31. Masten AS. Ordinary magic: Resilience processes in development. Am Psychol. (2001) 56:227–38. doi: 10.1037/0003-066X.56.3.227
32. Cheadle ACD, Ramos IF, and Schetter CD. Stress and resilience in pregnancy. In: Sweeny K, Robbins ML, and Cohen LM, editors. The Wiley Encyclopedia of Health Psychology, 1st. Hoboken, New Jersey, USA: Wiley (2020). p. 717–23. doi: 10.1002/9781119057840.ch124
33. Sexton MB, Hamilton L, McGinnis EW, Rosenblum KL, and Muzik M. The roles of resilience and childhood trauma history: Main and moderating effects on postpartum maternal mental health and functioning. J Affect Disord. (2015) 174:562–8. doi: 10.1016/j.jad.2014.12.036
34. Burt KB and Paysnick AA. Resilience in the transition to adulthood. Dev Psychopathol. (2012) 24:493–505. doi: 10.1017/S0954579412000119
35. Liu JJW, Ein N, Gervasio J, Battaion M, Reed M, and Vickers K. Comprehensive meta-analysis of resilience interventions. Clin Psychol Review. (2020) 82:101919. doi: 10.1016/j.cpr.2020.101919
36. Testa A, Jackson DB, Boccio C, Ganson KT, and Nagata JM. Adverse childhood experiences and marijuana use during pregnancy: Findings from the North Dakota and South Dakota PRAMS, 2017–2019. Drug Alcohol Dependence. (2022) 230:109197. doi: 10.1016/j.drugalcdep.2021.109197
37. Rosenstock IM. Historical origins of the health belief model. Health Educ Monographs. (1974) 2:328–35. doi: 10.1177/109019817400200403
38. Thomas JC, Magel C, Tomfohr-Madsen L, Madigan S, Letourneau N, Campbell TS, et al. Adverse childhood experiences and HPA axis function in pregnant women. Hormones Behav. (2018) 102:10–22. doi: 10.1016/j.yhbeh.2018.04.004
39. Smith BW, Dalen J, Wiggins K, Tooley E, Christopher P, and Bernard J. The brief resilience scale: Assessing the ability to bounce back. Int J Behav Med. (2008) 15:194–200. doi: 10.1080/10705500802222972
40. Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl psychol Measurement. (1977) 1:385–401. doi: 10.1177/014662167700100306
41. Tustumi F. Choosing the most appropriate cut-point for continuous variables. Rev Col Bras Cir. (2022) 49:e20223346. doi: 10.1590/0100-6991e-20223346-en
42. Schunk D. A Markov chain Monte Carlo algorithm for multiple imputation in large surveys. AStA. (2008) 92:101–14. doi: 10.1007/s10182-008-0053-6
43. Honaker J, King G, and Blackwell M. Amelia II: a program for missing data. J Stat Software. (2011) 45:1–47. doi: 10.18637/jss.v045.i07
44. Frankenberger D, Clements-Nolle K, and Yang W. The association between adverse childhood experiences and alcohol use during pregnancy in a representative sample of adult women. Womens Health Issues. (2015) 25:688–95. doi: 10.1016/j.whi.2015.06.007
45. Duka S, Rahman S, Hansen SE, and Esernio-Jenssen D. The effect of maternal adverse childhood experiences (ACEs) on substance use during pregnancy. Matern Child Health J. (2023) 27:153–65. doi: 10.1007/s10995-023-03768-4
46. Osofsky JD, Osofsky HJ, Frazer AL, Fields-Olivieri MA, Many M, Selby M, et al. The importance of adverse childhood experiences during the perinatal period. Am Psychol. (2021) 76:350–63. doi: 10.1037/amp0000770
47. Chung EK, Nurmohamed L, Mathew L, Elo IT, Coyne JC, and Culhane JF. Risky health behaviors among mothers-to-be: the impact of adverse childhood experiences. Acad Pediatr. (2010) 10:245–51. doi: 10.1016/j.acap.2010.04.003
48. Kendall-Tackett K, Poulin SR, and Garner C. Health problems mediate the effects of adverse childhood experiences on the frequency of cannabis use in a sample of pregnant and breastfeeding women. J Interpers Violence. (2025) 40:2518–37. doi: 10.1177/08862605241270084
49. Hyman SM and Sinha R. Stress-related factors in cannabis use and misuse: Implications for prevention and treatment. J Subst Abuse Treat. (2009) 36:400–13. doi: 10.1016/j.jsat.2008.08.005
50. Wong MCS, Huang J, Wang HHX, Yuan J, Xu W, Zheng ZJ, et al. Resilience level and its association with maladaptive coping behaviours in the COVID-19 pandemic: a global survey of the general populations. Global Health. (2023) 19:1. doi: 10.1186/s12992-022-00903-8
51. American Psychological Association. Parental burnout and stress (2024). Available online at: https://www.apa.org/topics/stress/parental-burnout (Accessed April 25, 2025).
52. Ko JY, Tong VT, Bombard JM, Hayes DK, Davy J, and Perham-Hester KA. Marijuana use during and after pregnancy and association of prenatal use on birth outcomes: A population-based study. Drug Alcohol Depend. (2018) 187:72–8. doi: 10.1016/j.drugalcdep.2018.02.017
53. Patrick ME, Bray BC, and Berglund PA. Reasons for marijuana use among young adults and long-term associations with marijuana use and problems. J Stud Alcohol Drugs. (2016) 77:881–8. doi: 10.15288/jsad.2016.77.881
54. al’Absi M and Allen AM. Impact of acute and chronic cannabis use on stress response regulation: challenging the belief that cannabis is an effective method for coping. Front Psychol. (2021) 12:687106. doi: 10.3389/fpsyg.2021.687106
55. Hughes K, Bellis MA, Hardcastle KA, Sethi D, Butchart A, Mikton C, et al. The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. Lancet Public Health. (2017) 2:e356–66. doi: 10.1016/S2468-2667(17)30118-4
56. Flynn TP, Parnes JE, and Conner BT. Personality disorders, risky behaviors, and adversity: the moderating role of resilience. Psychol Rep. (2022) 125:2936–55. doi: 10.1177/00332941211028998
57. Taillieu TL and Brownridge DA. Aggressive parental discipline experienced in childhood and internalizing problems in early adulthood. J Fam Violence. (2013) 28:445. doi: 10.1007/s10896-013-9513-1
58. Nelson DB, Uscher-Pines L, Staples SR, and Ann Grisso J. Childhood violence and behavioral effects among urban pregnant women. J Women’s Health. (2010) 19:1177–83. doi: 10.1089/jwh.2009.1539
59. Kurtz SP, Pagano ME, Buttram ME, and Ungar M. Brief interventions for young adults who use drugs: The moderating effects of resilience and trauma. J Subst Abuse Treat. (2019) 101:18–24. doi: 10.1016/j.jsat.2019.03.009
60. Thompson EM, Corcoran F, and Hodges HR. Adverse childhood experiences and resilience: family resilience as a promotive factor in young children’s flourishing. SAGE Open. (2024) 14:21582440241302899. doi: 10.1177/21582440241302899
61. Macleod J, Hickman M, and Smith GD. Reporting bias and self-reported drug use. Addiction. (2005) 100:562–3. doi: 10.1111/j.1360-0443.2005.01099.x
62. Haight SC, King BA, Bombard JM, Coy KC, Ferré CD, Grant AM, et al. Frequency of cannabis use during pregnancy and adverse infant outcomes, by cigarette smoking status – 8 PRAMS states, 2017. Drug Alcohol Dependence. (2021) 220:108507. doi: 10.1016/j.drugalcdep.2021.108507
63. Grywacheski V, Ali J, Baker MM, Gheorghe M, Wong SL, and Orpana HM. Opioid and cannabis use during pregnancy and breastfeeding in relation to sociodemographics and mental health status: a descriptive study. J Obstetr Gynaecol Canada. (2021) 43:329–36. doi: 10.1016/j.jogc.2020.09.017
Keywords: adverse childhood experiences, cannabis, resilience, postpartum, maternal
Citation: Roland A, Charron E and Shreffler KM (2025) Adverse childhood experiences, resilience, and cannabis use in early motherhood. Front. Psychiatry 16:1621161. doi: 10.3389/fpsyt.2025.1621161
Received: 30 April 2025; Accepted: 14 July 2025;
Published: 07 August 2025.
Edited by:
Deepthi S Varma, University of Florida, United StatesReviewed by:
Chaela Nutor, Emory University, United StatesMadison Kelm, The Pennsylvania State University, United States
Copyright © 2025 Roland, Charron and Shreffler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Karina M. Shreffler, S2FyaW5hLVNocmVmZmxlckBvdWhzYy5lZHU=
 Alysa Roland
Alysa Roland Elizabeth Charron
Elizabeth Charron Karina M. Shreffler
Karina M. Shreffler