- 1Department of Endocrinology, The First Affiliated Hospital with Nanjing Medical University, Nanjing, China
- 2Dpartment of Endocrinology, Lianyungang Affiliated Hospital of Nanjing University of Chinese Medicine, Lianyungang, China
Persistent genital arousal disorder/genitopelvic dysesthesia (PGAD/GPD) is a rare clinical condition of uncertain etiology. It is characterized by involuntary genital arousal occurring in the absence of sexual interest or desire, and may be accompanied by abnormal sensations in the pelvic and reproductive regions. PGAD/GPD exerts a profound negative impact on patients’ physical and mental health, severely impairing daily functioning and, in some cases, leading to suicidal ideation. This case highlights the potential role of COVID-19 as a triggering factor in the development of PGAD/GPD. The marked improvement in symptoms following treatment with leuprolide suggests that dysregulation of gonadotropin/GnRH signaling may constitute a key pathogenic mechanism underlying this condition. We anticipate that this successful treatment case will provide valuable insights into the etiology and treatment strategies of PGAD/GPD.
Introduction
Persistent genital arousal disorder/genitopelvic dysesthesia (PGAD/GPD) is a rare condition affecting women’s sexual health, occurring at any age but most commonly after puberty (1). The condition is characterized by sudden and frequent spontaneous genital arousal, often accompanied by genitopelvic sensory abnormalities. Its nature differs from sexual arousal associated with sexual desire or subjective arousal. Symptoms of PGAD/GPD are typically not alleviated through masturbation or orgasm. It is fundamentally distinct from hypersexuality, which is defined by excessive sexual desire that may or may not include persistent genital arousal, whereas PGAD/GPD involves persistent genital arousal in the absence of sexual desire (2). This condition primarily affects women. Due to the unique nature of its symptoms, patients often experience psychological distress, including shame and guilt. Severe cases may be accompanied by anxiety, depression, or even suicidal ideation, significantly impairing quality of life.
However, there is currently limited global understanding of this condition, with only a few reports available. Existing treatment approaches are primarily exploratory in nature. Clinical efficacy remains poor, and follow-up data are scarce. Here, we report a case of PGAD/GPD treated with leuprolide, which demonstrated marked clinical improvement and was followed for nearly two years. The results are summarized below.
Case report
A 77-year-old female patient presented to our endocrinology outpatient clinic in February 2023 with a chief complaint of “recurrent spontaneous genital arousal for over 50 days.” At the time of presentation, she expressed shame and reluctance to speak, and her medical history was primarily provided by family members. Approximately 50 days after recovering from a COVID-19 infection, she developed spontaneous genital arousal characterized by sudden urges that were not alleviated by intercourse. These symptoms were accompanied by lower limb weakness, pelvic congestion, a burning sensation, and suicidal thoughts. There was no genital swelling or pain. Subsequently, the symptoms recurred intermittently with increasing frequency and duration, eventually becoming persistent without spontaneous resolution. Three days prior to presentation, hormonal tests conducted at another hospital confirmed postmenopausal status, and pelvic MRI revealed no significant abnormalities. Her medical history includes a modified radical mastectomy for right breast invasive ductal carcinoma performed seven years ago at another institution. Postoperatively, she has been maintained on 2.5 mg of letrozole daily. She denies any history of anxiety, depression, or hyperactive sexual desire. Menarche occurred at age 14, and menopause at age 50. She has had four pregnancies and has one son and one daughter. She has reported low spontaneous sexual desire over the past 20 years.
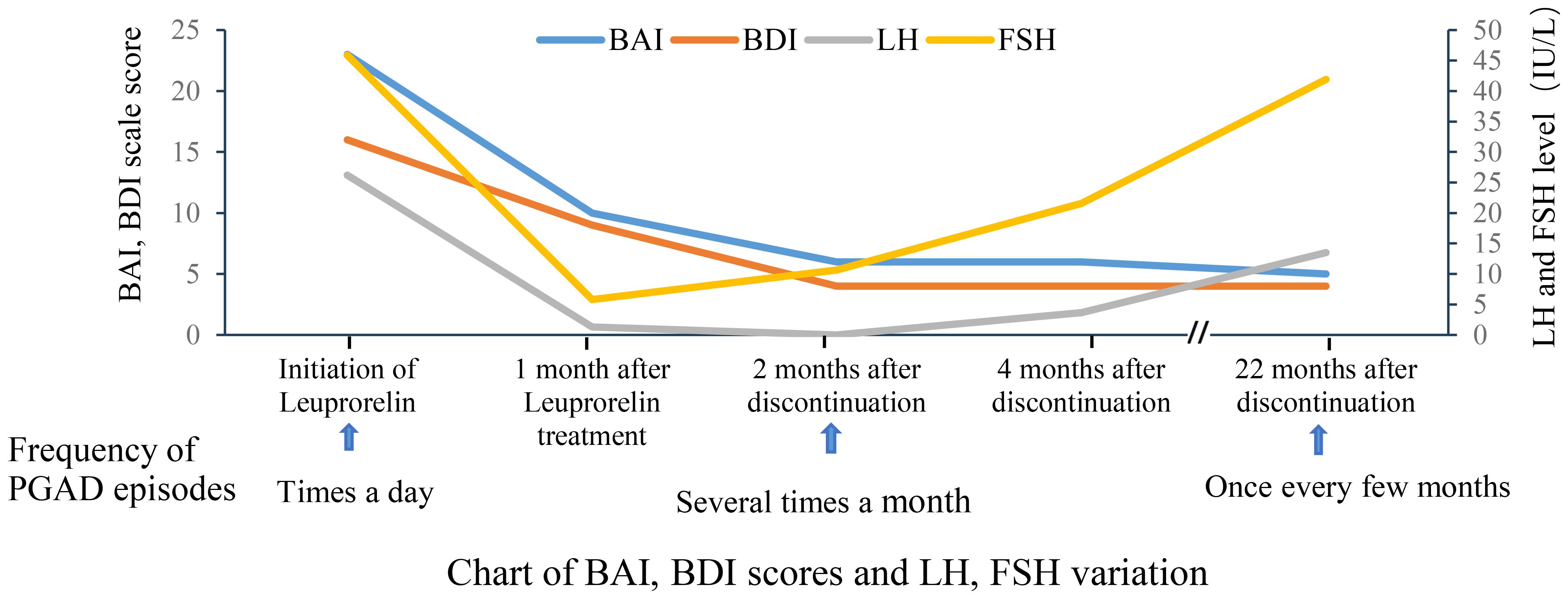
Based on a comprehensive analysis of the patient’s medical history and clinical presentation, a diagnosis of PGAD/GPD was made. The patient expressed recurrent suicidal ideation and demonstrated a strong desire for treatment. She was informed that no evidence-based treatment guidelines currently exist for PGAD/GPD, and that available therapeutic approaches are largely exploratory. Following approval from the Ethics Committee of the First Affiliated Hospital with Nanjing Medical University and written informed consent, a monthly subcutaneous injection of 3.75 mg leuprolide acetate sustained-release microspheres was initiated. Before treatment and during follow-up, the patient completed assessments using the Beck Anxiety Inventory (BAI) and the Beck Depression Inventory (BDI). Prior to treatment, the BAI score was 23 (15–25 indicates mild anxiety), and the BDI score was 16 (greater than 15 suggests possible depression), indicating mild anxiety and possible depression (Table 1, Figure 1).
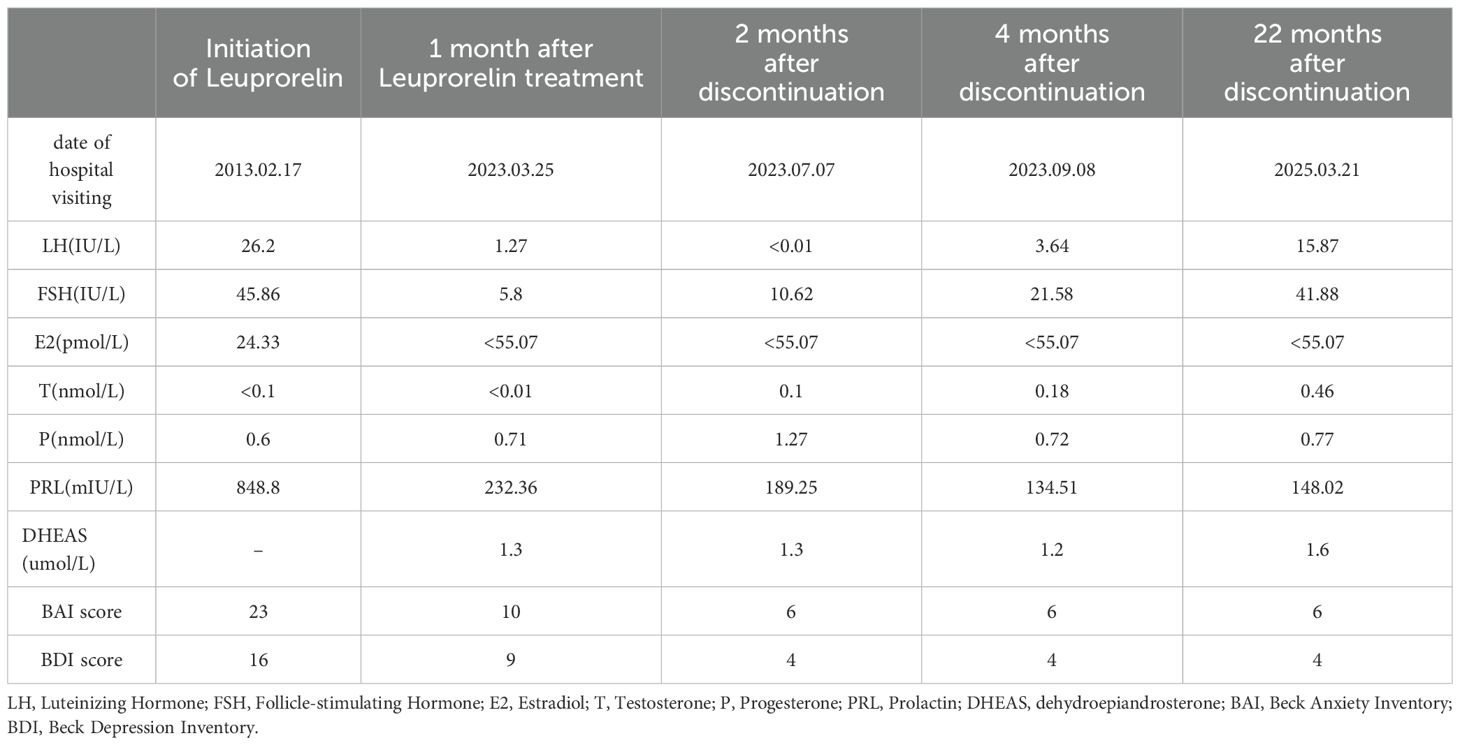
Table 1. Relevant laboratory examinations and BAI and BDI scores before, during and after treatment.
The patient reported a worsening of genital arousal symptoms within the first three days following leuprolide administration, which gradually improved after one week. One month later, symptoms showed marked improvement, with the BAI score reduced to 10 and the BDI score reduced to 9. Follow-up hormone levels were consistent with the physiological changes expected after Gonadotropin-Releasing Hormone Analog (GnRHa) therapy. Two months after treatment, the symptoms were largely absent, with only occasional brief recurrences that resolved spontaneously. At the three-month follow-up, the patient reported complete remission of symptoms, and leuprolide therapy was discontinued, with BAI and BDI scores of 5. Over the three-month treatment period, three injections of leuprolide acetate sustained-release microspheres were given.
One month after discontinuing leuprolide, approximately 10 days following reinfection with COVID-19, a mild recurrence of sexual arousal symptoms was noted, though milder than previous episodes. The main symptom was a burning sensation in the perineal region, which typically occurred during leisure time and resolved with cognitive distraction. Two months after discontinuation, the patient returned for a follow-up visit and reported that symptoms had largely resolved, with occasional recurrence triggered by watching videos on a smartphone, which resolved with focused distraction. Re-evaluation of sex hormones showed that LH and FSH levels remained below postmenopausal baseline. The BAI score was 6, and the BDI score was 4. Four months after medication discontinuation, the patient reported two episodes over the preceding two months, both of which resolved quickly with mild intensity. Follow-up hormone levels indicated a gradual return of FSH and LH to postmenopausal levels. The BAI and BDI scores remained stable at 6 and 4 (Table 1, Figure 1), respectively. After seven months without medication, the patient experienced a resurgence of symptoms following attendance at a wedding banquet. These episodes lasted approximately 30 minutes and were accompanied by lower limb weakness and mild pelvic discomfort. Symptoms recurred intermittently every 2–3 days over a three-month period before resolving spontaneously. At the 22-month telephone follow-up, the patient reported mild and transient episodes of spontaneous genital arousal, which resolved without intervention. Hormone reassessment confirmed postmenopausal status with LH levels persistently lower than baseline. The BAI and BDI scores were 6 and 4, respectively.
Discussion
In 2001, Leiblum and Nathan first reported five cases of Persistent Sexual Arousal Syndrome (PSAS) in women, characterized by symptoms of unrelieved genital arousal (3). Considering that the condition was primarily genital in nature rather than sexual, Leiblum renamed it Persistent Genital Arousal Disorder (PGAD) in 2006 (4). In 2019, the International Society for the Study of Women’s Sexual Health (ISSWSH) convened a panel of experts to re-evaluate the terminology. The panel recognized that PGAD did not fully capture the range of symptoms experienced by patients with genitopelvic dysesthesia (GPD). As a result, the term PGAD was broadened to encompass GPD. The term PGAD/GPD maintains a primary focus on persistent arousal symptoms while acknowledging the associated physical manifestations (1).
Currently, the epidemiological characteristics and pathophysiological mechanisms of PGAD/GPD are not well understood. Due to its unique symptoms and privacy concerns, many patients experience self-shame or even guilt, which often leads to avoidance of medical consultation or delayed treatment. Additionally, clinicians frequently lack sufficient understanding of the condition, further contributing to the unclear epidemiological characteristics of this disorder to date. Epidemiological studies conducted abroad have reported prevalence rates ranging from 0.6% to approximately 3% (5, 6), suggesting that a considerable number of individuals may be affected globally. Limited information exists on the pathophysiology of PGAD/GPD, partly due to the absence of validated animal models for experimental study. Although a few international case reports have proposed various etiological hypotheses in recent years, these are generally unsupported by robust clinical or laboratory evidence due to the small number of cases. Current hypotheses include vascular changes, central or peripheral nerve dysfunction, Tarlov cysts, psychological, pharmacological, dietary, and hormonal factors, neurotransmitter imbalances, mechanical pressure on genital structures, or a combination of these (7–9). Given the limited understanding of disease mechanisms, clinical management remains largely exploratory, focusing on presumed etiologies. Systematic evaluations of treatment efficacy and safety through clinical trials are lacking. Most therapeutic approaches are drawn from case reports and may involve trigger removal, local anesthetics, cognitive-behavioral therapy, mindfulness techniques, hypnotherapy, pelvic floor physical therapy, electroconvulsive therapy, pharmacological therapies (such as antidepressants, anxiolytics, GnRHa, among others), or surgical intervention in cases involving Tarlov cysts (10–12). Reported outcomes for these treatments vary significantly. Deka et al. reported the successful treatment of a case of PGAD/GPD using leuprolide, but the treatment lacked psychological assessment and follow-up data after discontinuation of treatment (13). Our case report further supports the potential efficacy of GnRHa in PGAD/GPD management and contributes detailed symptom tracking and follow-up information across the treatment continuum.
The patient exhibited marked spontaneous genital arousal symptoms following two laboratory-confirmed COVID-19 infections. The underlying mechanism is hypothesized to involve COVID-19-mediated activation of the hypothalamic-pituitary-gonadal (HPG) axis, leading to increased pulsatile secretion of gonadotropin-releasing hormone (GnRH) and subsequent symptom development and exacerbation of PGAD/GPD. During the initial phase of leuprolide treatment (within the first three days post-injection), the patient’s symptoms worsened, which may be attributed to the “flare-up effect” induced by GnRH agonists. Subsequently, symptoms rapidly improved, likely due to suppression of the HPG axis. Based on laboratory test results and clinical progression, we speculate that leuprolide may suppress pulsatile GnRH secretion, thereby inhibiting pituitary luteinizing hormone (LH) levels and improving the patient’s symptomatology. Following the discontinuation of leuprolide acetate, both LH and FSH levels gradually returned to pre-treatment values; however, the patient’s symptoms did not worsen significantly. Potential underlying mechanisms include the following: (1) GnRHa may induce a “resetting” effect on the HPG axis, thereby suppressing hormonal signaling pathways associated with PGAD/GPD (14); (2) GnRHa may modulate relevant neuroendocrine pathways through epigenetic mechanisms (15). Furthermore, 22 months after treatment cessation, FSH returned to pre-treatment levels, while LH remained relatively suppressed. This may be attributable to the more potent and sustained inhibitory effect of GnRHa on the LH secretion pathway (16). The dissociation between LH suppression and the recovery of FSH to pre-treatment levels may indicate a stronger correlation between LH dynamics and clinical manifestations. Notably, a significant positive correlation between LH and sexual arousal has been well-documented, as demonstrated by studies on male and female pre-ovulatory libido peaks (17, 18). Additionally, BAI and BDI scores showed significant improvement (Figure 1, Table 1), suggesting that anxiety and depressive symptoms may be secondary to the underlying physical condition. This may partially account for the limited efficacy of current international treatment regimens that rely solely on psychotropic medications for managing PGAD/GPD.
Notably, the incidence of central precocious puberty among adolescents has significantly increased during the COVID-19 pandemic. For example, in a cohort study of school-aged girls in Shanghai, the incidence of precocious puberty in 2020 was significantly higher than in 2016–2019, accompanied by elevated GnRH levels (19). GnRH neurons originate from the olfactory bulb, develop in the olfactory cortex, and eventually mature in the hypothalamus (20). COVID-19 infection is frequently associated with olfactory dysfunction, and live viruses have been isolated from hypothalamic tissue, supporting the hypothesis of direct viral effects on hypothalamic regulation (21). To date, no studies have specifically investigated the impact of COVID-19 on LH levels in postmenopausal women. A recent meta-analysis reported that, compared to the control group, COVID-19 patients exhibited significantly elevated LH levels, a marked reduction in the FSH/LH ratio, and no significant changes in estradiol concentrations (22). Additionally, post-COVID-19 infection has been linked to increased expression of Kiss1 and its receptor GPR54, which is believed to enhance GnRH pulse frequency (23). GnRH and its receptors are not only localized in the hypothalamus but are also widely distributed throughout the limbic system and cerebral cortex, particularly the prefrontal cortex, where they play roles in regulating sexual desire and impulse control via modulation of the dopaminergic (DA) pathway (24, 25). Although the mechanism by which COVID-19 infection triggers PGAD/GPD by interfering with the HPGA axis remains unclear, COVID-19 infection and its associated physiological and psychological changes may disrupt the GnRH pulse rhythm and amplitude, potentially contributing to its onset.
Conclusions
In conclusion, PGAD/GPD is a complex and under-recognized disorder with a potentially multifactorial etiology. Treatment should be individualized to target the most likely underlying cause, as hormone-suppressive therapy may not be suitable for all patients. The present report describes a unique clinical scenario (a surgical history of breast cancer), thereby providing a formal indication for GnRHa use. However, given that the application of GnRHa for PGAD/GPD is off-label and the lack of randomized controlled trial data supporting its application, the generalizability of this approach remains limited. Nevertheless, it offers novel insights into the pathophysiology of the disease and potential strategies for clinical management. Given women’s relatively conservative attitudes toward “sex,” as exemplified by the patient in this case who was hesitant to speak during her initial outpatient visit, feelings of shame and embarrassment may prevent patients from actively seeking medical help. In addition, clinical physicians generally lack sufficient understanding of PGAD/GPD. Through this case, we aim to raise awareness among both patients and clinicians—including but not limited to endocrinologists, urologists, gynecologists, psychiatrists, and neurologists—to improve recognition and treatment of PGAD/GPD in future clinical practice.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the First Affiliated Hospital with Nanjing Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
WR: Formal Analysis, Writing – original draft, Data curation, Investigation. HZ: Writing – review & editing, Supervision. HF: Validation, Supervision, Visualization, Writing – review & editing, Methodology.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We acknowledge the patient for participating and supporting this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Goldstein I, Komisaruk BR, Pukall CF, Kim NN, Goldstein AT, Goldstein SW, et al. International society for the study of women’s sexual health (ISSWSH) review of epidemiology and pathophysiology, and a consensus nomenclature and process of care for the management of persistent genital arousal disorder/genito-pelvic dysesthesia (PGAD/GPD). J sexual Med. (2021) 184:665–97. doi: 10.1016/j.jsxm.2021.01.172
2. Pukall CF. Persistent genital arousal disorder/genitopelvic dysesthesia. Clin obstetrics gynecology. (2025) 681:32–6. doi: 10.1097/grf.0000000000000908
3. Leiblum SR and Nathan SG. Persistent sexual arousal syndrome: a newly discovered pattern of female sexuality. J sex marital Ther. (2001) 274:365–80. doi: 10.1080/009262301317081115
4. Leiblum S, Seehuus M, and Brown C. Persistent genital arousal: disordered or normative aspect of female sexual response? J sexual Med. (2007) 43:680–9. doi: 10.1111/j.1743-6109.2007.00495.x
5. Dèttore D and Pagnini G. Persistent genital arousal disorder: A study on an italian group of female university students. J sex marital Ther. (2021) 471:60–79. doi: 10.1080/0092623x.2020.1804022
6. Jackowich RA and Pukall CF. Prevalence of persistent genital arousal disorder in 2 north american samples. J sexual Med. (2020) 1712:2408–16. doi: 10.1016/j.jsxm.2020.09.004
7. Jackowich RA, Pink L, Gordon A, and Pukall CF. Persistent genital arousal disorder: A review of its conceptualizations, potential origins, impact, and treatment. Sexual Med Rev. (2016) 44:329–42. doi: 10.1016/j.sxmr.2016.06.003
8. Feigenbaum F and Boone K. Persistent genital arousal disorder caused by spinal meningeal cysts in the sacrum: successful neurosurgical treatment. Obstetrics gynecology. (2015) 1264:839–43. doi: 10.1097/aog.0000000000001060
9. Vale FBC, Lopes GP, and Peixoto Silva A. Persistent genital arousal disorder/genito-pelvic dysesthesia (PGAD/GPD): from diagnostic approach to treatment - A narrative review. J sex marital Ther. (2025) 512:163–74. doi: 10.1080/0092623x.2025.2455132
10. Kapuśniak NE and Piegza M. Persistent genital arousal disorder - the present knowledge. Psychiatria polska. (2022) 566:1203–19. doi: 10.12740/PP/OnlineFirst/135253
11. Martín-Vivar M, Villena-Moya A, Mestre-Bach G, Hurtado-Murillo F, and Chiclana-Actis C. Treatments for persistent genital arousal disorder in women: A scoping review. J sexual Med. (2022) 196:961–74. doi: 10.1016/j.jsxm.2022.03.220
12. Kruger THC, Köhne S, and Kümpers F. Pharmacotherapy of persistent genital arousal disorder/genito-pelvic dysesthesia: an updated review and data from a registry. Expert Opin pharmacotherapy. (2024) 2515:2005–13. doi: 10.1080/14656566.2024.2415696
13. Deka K, Dua N, Kakoty M, and Ahmed R. Persistent genital arousal disorder: Successful treatment with leuprolide (antiandrogen). Indian J Psychiatry. (2015) 573:326–8. doi: 10.4103/0019-5545.166633
14. Horvath JE, Bajo AM, Schally AV, Kovacs M, Herbert F, and Groot K. Effects of long-term treatment with the luteinizing hormone-releasing hormone (LHRH) agonist Decapeptyl and the LHRH antagonist Cetrorelix on the levels of pituitary LHRH receptors and their mRNA expression in rats. Proc Natl Acad Sci United States America. (2002) 9923:15048–53. doi: 10.1073/pnas.232579499
15. Li F, Zhang M, Zhang Y, Liu T, and Qu X. GnRH analogues may increase endometrial Hoxa10 promoter methylation and affect endometrial receptivity. Mol Med Rep. (2015) 111:509–14. doi: 10.3892/mmr.2014.2680
16. Huang C, Shen X, Mei J, Sun Y, Sun H, and Xing J. Effect of recombinant LH supplementation timing on clinical pregnancy outcome in long-acting GnRHa downregulated cycles. BMC pregnancy childbirth. (2022) 221:632. doi: 10.1186/s12884-022-04963-x
17. LaFerla JJ, Anderson DL, and Schalch DS. Psychoendocrine response to sexual arousal in human males. Psychosomatic Med. (1978) 402:166–72. doi: 10.1097/00006842-197803000-00007
18. Bullivant SB, Sellergren SA, Stern K, Spencer NA, Jacob S, Mennella JA, et al. Women’s sexual experience during the menstrual cycle: identification of the sexual phase by noninvasive measurement of luteinizing hormone. J sex Res. (2004) 411:82–93. doi: 10.1080/00224490409552216
19. Chen Y, Chen J, Tang Y, Zhang Q, Wang Y, Li Q, et al. Difference of precocious puberty between before and during the COVID-19 pandemic: A cross-sectional study among shanghai school-aged girls. Front endocrinology. (2022) 13:839895. doi: 10.3389/fendo.2022.839895
20. Naulé L, Maione L, and Kaiser UB. Puberty, A sensitive window of hypothalamic development and plasticity. Endocrinology. (2021) 162. doi: 10.1210/endocr/bqaa209
21. Stein SR, Ramelli SC, Grazioli A, Chung JY, Singh M, Yinda CK, et al. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature. (2022) 612:758–63. doi: 10.1038/s41586-022-05542-y
22. Cai Z, Zhong J, Jiang Y, and Zhang J. Associations between COVID-19 infection and sex steroid hormones. Front endocrinology. (2022) 13:940675. doi: 10.3389/fendo.2022.940675
23. Mohamed MS, Johansson A, Jonsson J, and Schiöth HB. Dissecting the molecular mechanisms surrounding post-COVID-19 syndrome and neurological features. Int J Mol Sci. (2022) 23. doi: 10.3390/ijms23084275
24. Manfredi-Lozano M, Leysen V, Adamo M, Paiva I, Rovera R, Pignat JM, et al. GnRH replacement rescues cognition in Down syndrome. Sci (New York NY). (2022) 3776610:eabq4515. doi: 10.1126/science.abq4515
Keywords: leuprolide, case report, treatment, PGAD, generalized pareto distribution (GPD)
Citation: Rong W, Zhou H and Fan H (2025) Successful treatment of PGAD/GPD with leuprolide: a case report. Front. Psychiatry 16:1623256. doi: 10.3389/fpsyt.2025.1623256
Received: 10 May 2025; Accepted: 10 July 2025;
Published: 05 August 2025.
Edited by:
Mirko Manchia, University of Cagliari, ItalyReviewed by:
Craig Atwood, University of Wisconsin-Madison, United StatesJunping Wen, Fujian Provincial Hospital, China
Copyright © 2025 Rong, Zhou and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongqi Fan, aHFmYW5fMjAwNEAxNjMuY29t
 Wang Rong
Wang Rong Hongwen Zhou
Hongwen Zhou Hongqi Fan
Hongqi Fan