Abstract
Introduction:
Dementia and Major Depressive Disorder (MDD) are on the rise globally, with depression frequently observed throughout the progression of dementia, potentially accelerating cognitive decline and diminishing quality of life. This study aims to explore the interplay between cognitive impairment (CI) and depression in patients undergoing antidepressant treatment, emphasizing drug-related problems (DRPs) and the Rational Use of Medicines (RUM).
Materials and Methods:
Over a 6-year period, this cross-sectional study in Valencia, Spain, analyzed data from 777 patients aged over 50 concerned about their cognitive health. Cognitive status was assessed using three neuropsychological tests: Memory Impairment Screening (MIS), Verbal Semantic Fluency (VSF), and Pfeiffer’s Short Portable Mental State Questionnaire (SPMSQ). Various clinical and demographic variables associated with dementia were also evaluated.
Results:
The study identified a higher prevalence of CI among patients at risk of depression (GDS5 positive) compared to those without a depression risk. Patients with depression risk also demonstrated lower cognitive reserve, higher levels of loneliness, and increased use of antidepressants – notably tricyclic antidepressants (TCAs) – which are linked to anticholinergic burden and potential CI.
Conclusion:
Despite their widespread use, antidepressants raise concerns regarding their efficacy and safety, particularly due to the risk of exacerbating CI. This study underscores the need for careful management of antidepressant therapy and suggests exploring alternatives such as vortioxetine, which may offer cognitive benefits. Enhanced interprofessional collaboration and regular cognitive evaluations are recommended to improve patient outcomes and ensure the rational use of antidepressants.
1 Introduction
According to the World Alzheimer Report 2023, more than 55 million people currently live with dementia worldwide (1). Additionally, the World Health Organization (WHO) estimates that this number will increase to 78 million by 2030 and to 139 million by 2050 (2). Concurrently, the prevalence of Major Depressive Disorder (MDD), also known as clinical depression, has been rising in recent years. Approximately 280 million people worldwide suffer from depression, which represents about 3.8% of the global population (3).
Alzheimer’s Disease (AD) is the most prevalent form of dementia, accounting for 60% to 80% of all major cognitive disorders (4). It is now recognized as a biological continuum that starts with preclinical AD—an early stage where symptoms are minimal or absent—and advances to its more severe form: dementia (5). This progression involves a gradual accumulation of pathophysiological changes over many years, eventually resulting in clinically apparent disease and a subsequent decline in cognitive and functional abilities. This decline occurs without distinct boundaries between clinical stages (6). AD is believed to begin up to 20 years before the onset of noticeable symptoms, highlighting a lengthy preclinical phase that may offer opportunities for early intervention, especially given the current lack of curative pharmacological treatments (7).
The NIA-AA (National Institute on Aging – Alzheimer’s Association) categorizes the progression of AD into six stages. These range from the asymptomatic presence of abnormal biomarkers (Stage 1) to severe dementia (Stage 6). Intermediate stages include the emergence of mild symptoms (Stage 2), Mild Cognitive Impairment (MCI) without significant functional loss (Stage 3), and advancing to mild, moderate, and severe dementia (Stages 4-6), with increasingly impaired independence. This staging system is especially valuable in clinical trials, as it facilitates the classification of patients according to the severity of their disease (4).
Dementia and depression are closely associated conditions. Depression is a commonly observed manifestation throughout the clinical progression of dementia, occurring both at the onset of cognitive decline and in later stages as the disease progresses (8, 9). The exact etiopathogenic relationship between both conditions remains unclear, but it is widely accepted that they share some underlying neurological basis (10).
The coexistence of depression with dementia has been linked to a faster progression of cognitive impairment (CI) and lower quality of life (11, 12). Most studies conclude that patients with depression tend to exhibit more pronounced CI compared to those who are not depressed (13). Some theories propose that depression in older adults may stem from a psychological response to perceived cognitive decline, thereby suggesting a potential association between the onset of depression and CI (14). Other studies suggest that late-life depression is a risk factor for dementia, potentially heightening the probability of transitioning from MCI to full-blown dementia (15). However, the question of whether depression arises because of the condition or precedes dementia as a prodromal symptom is still under investigation (16).
Shared impairments, such as memory loss, sleep disturbances, and reduced social functioning, are prevalent in both depression and dementia. Their association may be further explained by genetics or common pathophysiological pathways, including neurodegeneration, inflammation, vascular risk factors, and hypothalamic-pituitary-adrenal axis dysregulation. Regardless of the exact nature of this link, the comorbidity of depressive symptoms and dementia is well-established and must be considered in the care of patients with CI or dementia. Therefore, it is crucial to determine whether treating depression can improve cognitive functioning (16, 17).
The main objective of WHO policy brief is to support successful implementation of the third WHO Global Patient Safety Challenge, “Medication Without Harm”, and to advocate for prioritizing medication safety within healthcare systems (18). The use of antidepressants in neurological diseases is very common in daily clinical practice, primarily due to the close relationship between psychiatric comorbidities and neurological conditions (14). All antidepressants work slightly differently, targeting specific neurotransmitters to modulate mood and behavior. Classical antidepressants primarily increase levels of serotonin, norepinephrine, or both in the synapse. However, newer (atypical) antidepressants also increase dopamine levels, act as antagonists of dopamine D2 receptors, and serve as 5-HT2A antagonists and 5-HT1A agonists. Additionally, they may antagonize α2 receptors or utilize a novel multimodal mechanism of serotonin modulation and stimulation, such as seen with vortioxetine. Vortioxetine’s pharmacodynamic profile is unique, combining serotonin transporter blockade with a range of modulatory effects on serotonin receptors. The 5-HT1A agonist and 5-HT3 antagonist activities are considered crucial for reducing the typical latency of action seen with most antidepressants and for improving cognitive symptoms (19). Furthermore, vortioxetine functions as a 5-HT1B partial agonist, 5-HT1D antagonist, and 5-HT7 antagonist (Table 1).
Table 1
| Antidepressant | ATC Code | NA | 5-HT | DA | Ach | α1 | α2 | 5-HT1 | 5-HT2 | H1 | NA selectivity versus 5-HT | Anticholinergic burden | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TCAs | Imipramine | N06AA02 | ++ | ++ | + | ++ | ++ | + | + | + | ++ | NA | 3 |
| Clomipramine | N06AA04 | ++ | +++ | + | +++ | ++ | + | + | ++ | ++ | 5-HT | 3 | |
| Amitriptyline | N06AA09 | ++ | ++ | + | +++ | +++ | ++ | + | ++ | +++ | NA | 3 | |
| Nortriptyline | N06AA10 | +++ | + | + | + | ++ | + | + | ++ | ++ | NA | 3 | |
| Doxepin | N06AA12 | ++ | + | + | ++ | +++ | + | + | ++ | +++ | NA | 3 | |
| Maprotiline | N06AA21 | +++ | + | + | + | +++ | + | 0 | + | +++ | NA | 2 | |
| SSRIs | Fluoxetine | N06AB03 | + | +++ | + | + | + | 0 | 0 | + | + | 5-HT | 1 |
| Citalopram | N06AB04 | + | +++ | + | 0 | + | 0 | 0 | + | + | 5-HT | 1 | |
| Paroxetine | N06AB05 | ++ | +++ | + | ++ | + | + | 0 | 0 | 0 | 5-HT | 2 | |
| Sertraline | N06AB06 | + | +++ | ++ | + | + | + | 0 | + | + | 5-HT | 1 | |
| Fluvoxamine | N06AB08 | + | +++ | + | 0 | + | + | 0 | + | 0 | 5-HT | 1 | |
| Escitalopram | N06AB10 | 0 | +++ | 0 | + | + | 0 | 0 | + | + | 5-HT | 1 | |
| SNRIs | Venlafaxine | N06AX16 | + | ++ | + | 0 | 0 | 0 | 0 | 0 | 0 | 5-HT | 1 |
| Duloxetine | N06AX21 | ++ | ++ | + | 0 | 0 | 0 | 0 | 0 | 0 | 5-HT | 0 | |
| Agomelatine | N06AX22 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ++ | 0 | 5-HT | 0 | |
| Desvenlafaxine | N06AX23 | ++ | +++ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | NA/5-HT | 1 | |
| SNRI | Reboxetine | N06AX18 | +++ | + | 0 | + | + | + | NA | 0 | |||
| NDRI | Bupropion | N06AX12 | + | 0 | ++ | 0 | + | 0 | 0 | 0 | + | NA | 1 |
| Others | Mianserin | N06AX03 | +++ | + | 0 | + | +++ | +++ | 0 | +++ | +++ | NA | 0 |
| Trazodone | N06AX05 | + | + | 0 | 0 | ++ | ++ | ++ | +++ | + | 5-HT | 1 | |
| Mirtazapine | N06AX11 | + | + | 0 | + | + | ++ | 0 | ++ | +++ | NA/5-HT | 1 | |
| Multimodal | Vortioxetine | N06AX26 | 0 | ++ | 0 | 0 | 0 | 0 | + | 0 | 0 | 5-HT | 0 |
Classification of antidepressants authorized and marketed in Spain according to their pharmacological profile and anticholinergic burden.
(NA: noradrenaline reuptake blockade; 5-HT: serotonin reuptake blockade; DA: dopamine reuptake blockade; Ach: cholinergic receptors; α1: α1 receptors; α2: α2 receptors; 5-HT: serotonin receptors; H1: histamine receptors; 0: no effect; +: minimal effect; ++: moderate effect; +++: pronounced effect. Groups include tricyclics (TCA), selective serotonin re-uptake inhibitors (SSRI), serotonin and noradrenaline re-uptake inhibitors (SNRI), selective noradrenaline re-uptake inhibitors antidepressants (NSRI), noradrenaline and dopamine re-uptake inhibitors (NDRI), multimodal and others) Elaborated based on: Brunton L, Knollmann B, editors. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 14th ed. New York: McGraw-Hill Education; 2022.
The use of antidepressant medication has been implicated in the acceleration of CI symptoms, potentially through mechanisms such as increased anticholinergic burden and adverse vascular effects. These pharmacological impacts may contribute to an elevated risk of developing dementia (14, 20). Additionally, older adults may be more susceptible to these adverse effects due to age-related changes in pharmacokinetics and pharmacodynamics, including decreased acetylcholine-mediated transmission in the brain and increased permeability of the blood-brain barrier (21).
This underscores the need for more specific knowledge about which drugs would provide the maximum benefit for patients (22). However, this research includes challenges in accurately assessing and categorizing all present alterations, as well as the diversity of pharmacological treatments available.
The Rational Use of Medicines (RUM) is defined as the process through which “patients receive medications appropriate to their clinical needs, in doses that meet their own individual requirements, for an adequate period of time, and at the lowest cost to them and their community” (23). In accordance with Pharmaceutical Care Network of Europe (PCNE), a Drug-Related Problem (DRP) is defined as, “an event or circumstance involving drug therapy that actually or potentially interferes with desired health outcomes” (24). DRPs are classified based on where the failure occurs: need, safety, or effectiveness (25). Necessity refers to instances in which a medication is unnecessary, such as when a patient receives treatment without a valid clinical indication or when there is an unclear problem or complaint requiring further clarification before pharmacological intervention. Effectiveness pertains to problems related to the absence or potential absence of the desired therapeutic effect, which may arise from inappropriate drug selection, dosing, or adherence issues that hinder achieving optimal clinical outcomes. Finally, safety involves situations where the patient experiences or is at risk of experiencing adverse drug events, including side effects or toxicities, that compromise patient health (26).
Despite the risk of anticholinergic burden, antidepressants are prescribed to treat and prevent depression because they are considered safe, effective, and necessary. However, anticholinergic drugs are often non-selective, and their prolonged use can lead to severe adverse events such as CI (27).
Through this study, we aim to investigate the association between the risk of CI and depression in patients undergoing antidepressant treatment with potential DRP.
2 Materials and methods
2.1 Study design
This cross-sectional study was conducted in health centers, pharmacies, and various patients’ associations across the province of Valencia (Spain), utilizing simple random sampling.
The different clinical and demographic data were collected contemporaneously with the screenings carried out from 2018 to 2024 through patient interviews. These data are part of the Cathedra DeCo project.
The study of human subjects has ethical implications. This study was reviewed and approved by the Ethical Committee for Clinical Research with Medications of the Arnau de Vilanova Health Department (MOR-ROY-2018–013, date of approval: 18 July 2018). All participants signed informed consent to participate in the study.
2.2 Cognitive status
To detect those patients with possible CI, three neuropsychological tests were performed, following the recommendations of the local government through the Conselleria de Sanitat de la Comunitat Valenciana (28). Thus, patients were assessed with the following tests: Memory Impairment Screening (MIS), Verbal Semantic Fluency (VSF) and Pfeiffer’s Short Portable Mental State Questionnaire (SPMSQ). The MIS was validated in Spanish by Böhm et al., with a maximum score of 8 and a cut-off point of 4 or less. In the initial phase, participants are required to read aloud four related words from different categories. Following an unstructured distraction period, a free recall test is conducted, with semantic cues provided for words or categories that the participant cannot recall. Freely recalled items are awarded 2 points, while those recalled with a cue receive 1 point, resulting in a scoring range from 0 to 8 (29). The VSF was validated in Spanish by López Pérez-Díaz et al., with a cut-off point of 10. This test measures the number of items within a category that a subject can recall in one minute (30). The SPMSQ was validated in Spanish by Martínez de la Iglesia et al, with a maximum score of 10 and a cut-off point of 3 errors (4 errors for illiterate individuals). This test evaluates various aspects of intellectual functioning, including short-term memory, long-term memory, current event information, orientation, and the ability to perform serial mathematical tasks (31). The sensitivity, specificity and test duration of the above are shown in Table 2. The complementary use of the three tests aims to increase the likelihood of detecting cases of CI, since, in some instances, the combination of multiple questionnaires may represent the most appropriate strategy for a comprehensive evaluation (28). A positive screening result is considered when any of the tests exceeds the validated clinical threshold, in line with our objective of promoting early identification.
Table 2
| Screening test | Sensibility | Specificity | Duration (minutes) | Cut-off points | Test score (mean ± sd) |
|---|---|---|---|---|---|
| MIS | 0,74 | 0,96 | 2 | ≤ 4 | 6.66 ± 1.84 |
| SPMSQ | 0,85 | 0,79 | 3 | ≥ 3 | 0.99 ± 1.22 |
| VSF | 0,74 | 0,80 | 1 | 10 | 18.53 ± 7.09 |
Sensitivity, specificity, and time duration of the short test used in cognitive impairment detection.
(MIS, Memory Impairment Screening; SPMSPQ, Pfeiffer’s Short Portable Mental State Questionnaire; VSF, Verbal Semantic Fluency).
Patients with at least one positive cognitive test were classified as individuals with CI and those who did not fail any test as patients without CI. Consequently, subjects with a score compatible with the presence of CI in any of the three tests were referred to Primary Care for medical diagnosis.
2.3 Variables
In addition to the different neuropsychological tests, different clinical and demographic variables were collected, including age, sex, marital status, study level, hearing loss, group activities, number of friends seen in the last week, and subjective memory complaint (SMC).
We also included the Cognitive Reserve Questionnaire (CRQ) (32), where values less than or equal to 6 indicate low cognitive reserve, values between 7 and 9 indicate medium/low cognitive reserve, values between 10 and 14 indicate medium/high cognitive reserve, and values greater than or equal to 15 indicate high cognitive reserve; the Sense Of Coherence (SOC) (33); the Purpose In Life (PIL) (34); the Engaged Living Scale (ELS) (35); a Brief Resilient Coping Scale (BRCS) (36), where values below 13 indicate low resilience, values between 13 and 17 indicate intermediate resilience, and values above 17 indicate high resilience; the Loneliness Scale (UCLA) (37), where values higher or equal to 6 are associated with loneliness; and the Yesavage Scale For Geriatric Depression (GDS5) (38), where values greater than or equal to 2 are associated with a risk of depression. Prior validation studies in older Spanish populations demonstrated that this threshold balances sensitivity and specificity effectively for screening purposes (39). Finally, antidepressant drug (N05 and N06 ATC codes) prescription was also recorded. Information on current medication prescriptions was extracted from patients’ electronic health records at the time of cognitive assessment. The list of antidepressants considered, along with their dosages and frequencies, is provided in Appendix I.
2.4 Study subjects
The initial population consisted of 1,086 patients over the age of 50 who were concerned about their cognitive health and interested in undergoing screening for CI, provided they met the selection criteria. These criteria included: age ≥50 years, presence of subjective memory complaints, and provision of informed consent. Patients were excluded if they had a diagnosis of Alzheimer’s disease (AD) or other dementias, mental illnesses, or significant sensory deficits. A diagnosis of depression and/or the use of antidepressants at the time of the surveys was not part of the inclusion or exclusion criteria for this study.
Participants were recruited through three main pathways (1): the service was offered by community pharmacists directly to patients at the pharmacy (2); referral from primary care physicians; or (3) patients proactively sought participation after learning about the project. Data collection was carried out through structured interviews with the patient, conducted at the community pharmacy, the primary care center, or a patient association facility.
As shown in Figure 1, for this specific study, only the 777 patients with available GDS5 data were included. To calculate whether we had sufficient statistical power, we used the G*Power program [REF] to determine the minimum sample size for the study, comparing two independent groups and assuming a medium effect size with a significance level (Alpha) of 0.05. Finally, the statistical power achieved is about 0.99 (40).
Figure 1
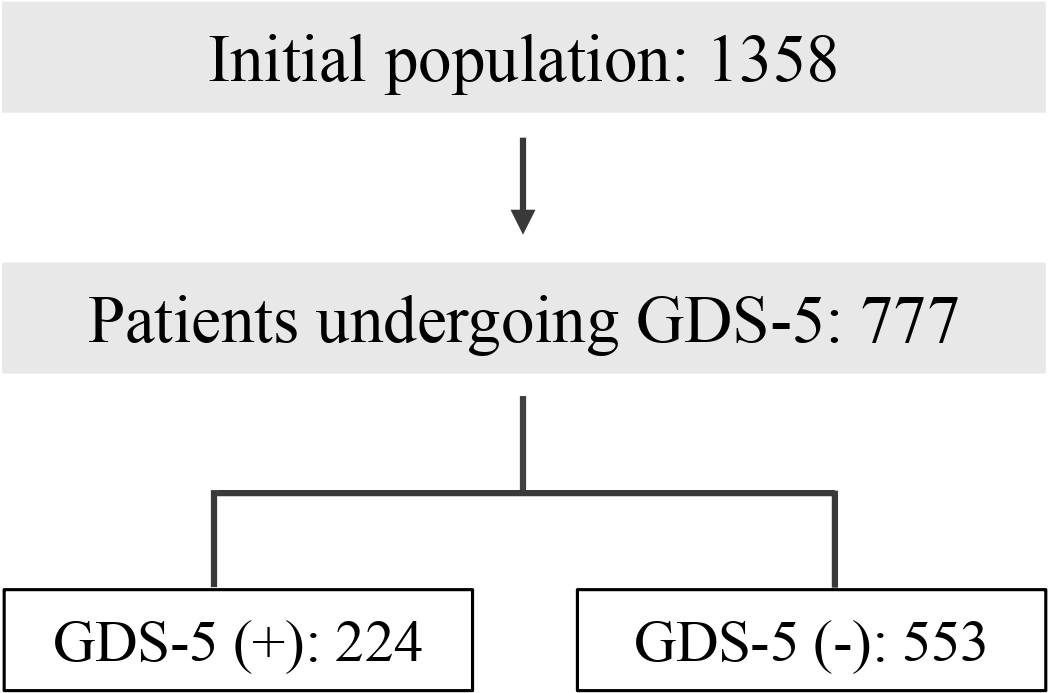
Study population (GDS5: 5 Point Geriatric Depression Scale).
2.5 Statistical analysis
A descriptive analysis was carried out to compare the different variables between patients with GDS5 risk and patients without GDS5 risk. Categorical variables were compared by the Pearson’s Chi-squared test or the nonparametric alternative through Fisher’s exact test when appropriate. They were depicted by percentages. Numerical variables were represented through mean and standard deviation and compared with two-sample t-tests after testing compliance with the assumptions of normality (Shapiro-Wilk test) and homogeneity of variance (Levene’s test). An equivalent non-parametric alternative to the t-test was employed if such assumptions were not met. In that sense, the Wilcoxon rank-sum method was used. All tests were two-tailed, and a p-value < 0.05 indicated statistical significance. In Appendix II, a logistic regression model adjusted by age and sex has been calculated to reinforce the discoveries of the statistical tests. Moreover, this study is an exploratory rather than a confirmatory study that aims to discover new working hypotheses on the relationship between antidepressant treatment and dementia. Then, we did not adjust p-values for multiple comparisons given the descriptive nature of this epidemiologic study, in line with existing recommendations (41).
Data analyses were performed using R (version 4.3.1) with R studio (version 2023.12.0.369) (42).
3 Results
Among the 777 patients who underwent the GDS5 test, 224 obtained a score within the range indicating a risk of depression, while 553 did not.
The distribution of scores for each cognitive test is summarized in Table 2. The average MIS score was (6.66 ± 1.84), with 113 (14.54%) participants scoring below the cut-off (≤4). On the SPMSQ, participants had a median of 0.99 ± 1.22, with 85 (10.94%) exceeding the cut-off for CI. The VSF test showed a median score (18.53 ± 7.09), and 48 individuals (6.18%) scored below the threshold of 10 words. These results show variability in cognitive performance among the study population (Table 2).
3.1 Dementia-related factors
As shown in Table 3 and Figure 2, the percentage of patients with CI in the group of GDS5 risk was significantly higher than in patients without GDS5 risk [n = 59 (26.46%), n = 102 (18.61%); p-value = 0.0151]. Additionally, individuals with CI had higher odds of having GDS5 risk [ORs (CI 95%): 1.64 (1.12, 2.39); p-value = 0.0098] (LR model in Appendix II).
Table 3
| Dementia related factors | GDS5 risk (n = 224) | GDS5 without risk (n = 553) | P-value | |
|---|---|---|---|---|
| Age, mean(std) a | 70.45 (12.49) | 70.28 (11.03) | 0.8516 | |
| Sex (Female) n(%) b | 165 (73.66) | 340 (61.48) | 0.0013 * | |
| SMC, n(%) b | 119 (53.12) | 206 (37.32) | 0.0001 * | |
| CI, n(%) b | 59 (26.46) | 102 (18.61) | 0.0151 * | |
| MIS | Mean(std) a | 6.53 (1.80) | 6.71 (1.85) | 0.2161 |
| No risk, n(%) b | 193 (86.16) | 468 (84.63) | 0.6483 | |
| Risk, n(%) b | 30 (13.39) | 83 (15.01) | ||
| SPMSQ | Mean(std) a | 1.21 (1.35) | 0.90 (1.15) | 0.0013 * |
| No risk, n(%) b | 192 (85.71) | 500 (90.42) | 0.0759 | |
| Risk, n(%) b | 32 (14.29) | 53 (9.58) | ||
| VSF | Mean(std) a | 16.84 (6.84) | 19.21 (7.08) | <0.0001 * |
| No risk, n(%) b | 201 (89.73) | 522 (94.39) | 0.0123 * | |
| Risk, n(%) b | 22 (9.82) | 26 (4.70) | ||
| CRQ b | High, n(%) | 9 (20.97) | 171 (32.51) | 0.0292 * |
| Low, n(%) | 43 (23.12) | 98 (18.63) | ||
| Medium/high, n(%) | 63 (33.87) | 159 (30.23) | ||
| Medium/low, n(%) | 41 (22.04) | 98 (18.63) | ||
| Study level c | Illiterate, n(%) | 3 (5.45) | 1 (0.75) | 0.0009 * |
| Read and write, n(%) | 2 (3.64) | 0 (0.00) | ||
| Primary, n(%) | 16 (29.09) | 19 (14.18) | ||
| Secondary, n(%) | 16 (29.09) | 38 (28.363) | ||
| Superior studies, n(%) | 18 (32.73) | 76 (56.72) | ||
| Group activities, n(%) b | 57 (30.48) | 241 (45.9) | 0.0002 * | |
| Marital status c | Married, n(%) | 111 (49.55) | 377 (68.55) | 0.0001 * |
| Couple, n(%) | 1 (0.45) | 11 (2.00) | ||
| Divorced, n(%) | 20 (8.93) | 33 (6.00) | ||
| Single, n(%) | 16 (7.14) | 25 (4.55) | ||
| Widower, n(%) | 76 (33.93) | 104 (18.91) | ||
| Hearing loss (yes), n(%) b | n = 103 (45.98) | n = 208 (37.61) | 0.0310 * | |
| Friends, mean(std) a | 6.70 (7.20) | 8.20 (8.30) | 0.3897 | |
| SOC, mean(std) d | 61.20 (12.80) | 72.30 (10.40) | <0.0001 * | |
| PIL, mean(std) a | 27.10 (6.10) | 31.10 (4.60) | <0.0001 * | |
| ELS, mean(std) d | 66.50 (15.80) | 77.30 (13.10) | <0.0001 * | |
| UCLA Loneliness Scale b | Solitud, n(%) | 43 (28.29) | 17 (4.93) | <0.0001 * |
| Non-solitud, n(%) | 109 (71.71) | 328 (95.07) | ||
| BRCS b | High, n(%) | 40 (21.39) | 203 (38.59) | <0.0001 * |
| Intermedia, n(%) | 97 (51.87) | 279 (53.04) | ||
| Low, n(%) | 50 (26.74) | 44 (8.37) | ||
| Antidepressant medication | Antidepressants, n(%) b | 95 (42.41) | 118 (21.34) | <0.0001 * |
| SSRIs, n(%) b | 54 (24.11) | 56 (10.13) | <0.0001 * | |
| Vortioxetine, n(%) c | 5 (2.23) | 5 (0.90) | 0.1620 | |
| TCAs, n(%) b | 13 (5.80) | 8 (1.45) | 0.0007 * | |
| Benzodiacepins, n(%) b | 60 (26.79) | 75 (13.56) | <0.0001 * | |
| Other, n(%) b | 15 (6.70) | 18 (3.25) | 0.0312 * | |
| No medication, n(%) a | 17 (7.59) | 64 (11.57) | 0.0997 | |
| Anticholinergic burden a | Burden, mean(std) | 1.6 (1.9) | 0.8 (1.3) | <0.0001 * |
| Antidepressants, mean(std) | 0.9 (1.3) | 0.4 (0.9) | <0.0001 * | |
| Other drugs, mean(std) | 0.7 (1.2) | 0.5 (0.9) | 0.0175 * | |
Association of dementia-related factors with depression risk estimated by the GDS5.
(GDS5: 5 Point Geriatric Depression Scale; CI: Cognitive Impairment; SMC: Subjective Memory Complaint; CRQ: Cognitive Reserve Questionnaire; SOC: Sense of Coherence; PIL: Purpose in Life; ELS: Engaged Living Scale; BRCS: Brief Resilience Coping Scale; UCLA: University of California, Los Angeles Loneliness Scale; SSRIs: Serotonin Reuptake Inhibitors; TCAs: Tricyclic Antidepressants) The variables marked with an asterisk (*) are statistically significant. a Wilcoxon rank sum test (p-value < 0.05). b Chi-squared test (p-value < 0.05). c Fisher’s Exact Test (p-value < 0.05). d Two Sample t-test (p-value < 0.05).
Figure 2
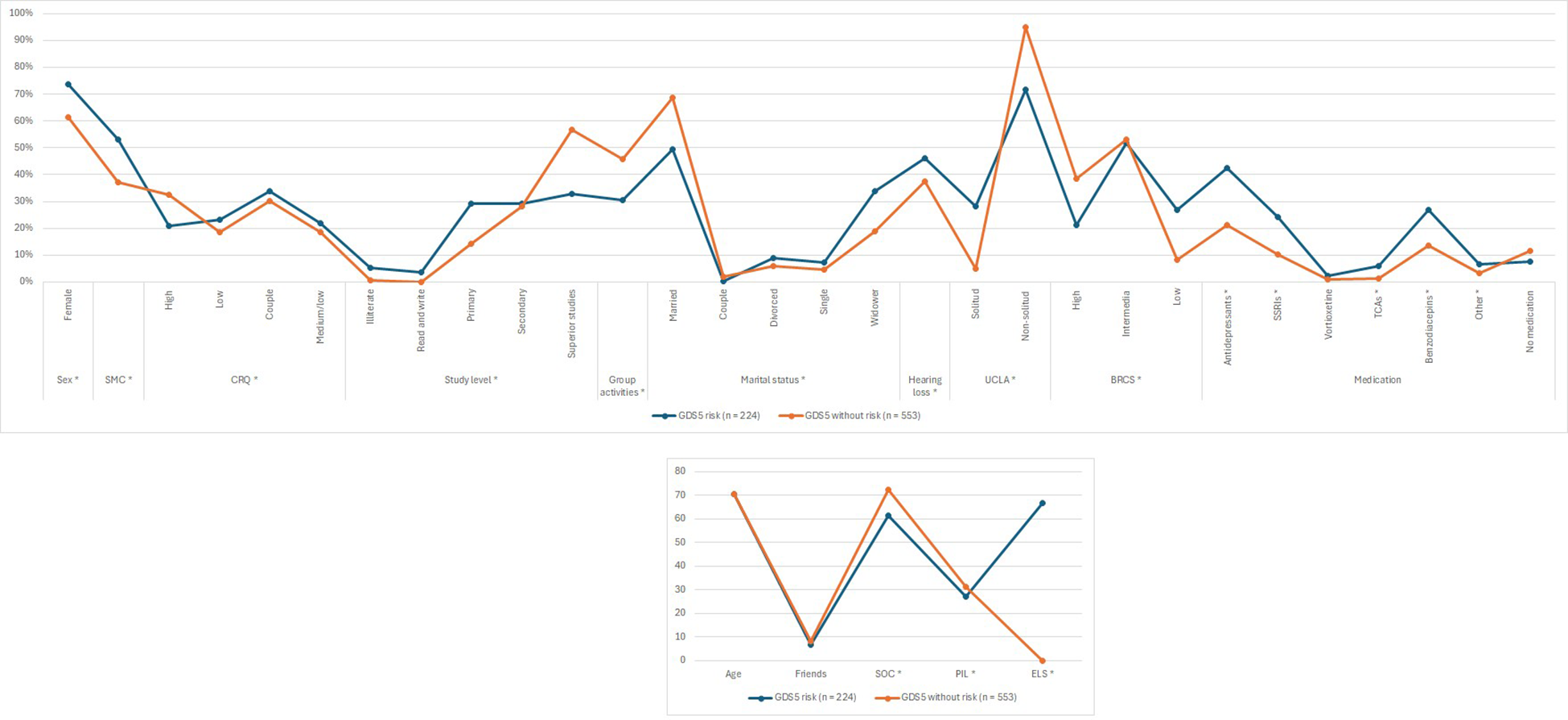
Association of dementia-related factors with depression risk estimated by the GDS5 (GDS5, 5 Point Geriatric Depression Scale; CI, Cognitive Impairment; SMC, Subjective Memory Complaint; CRQ, Cognitive Reserve Questionnaire; SOC, Sense of Coherence; PIL, Purpose in Life; ELS, Engaged Living Scale; BRCS, Brief Resilience Coping Scale; UCLA, University of California, Los Angeles Loneliness Scale; SSRIs, Serotonin Reuptake Inhibitors; TCAs, Tricyclic Antidepressants) The variables marked with an asterisk (*) are statistically significant.
Most patients with GDS5 risk were female [n = 165 (73.66%), n = 340 (61.48%); p-value = 0.0013]. Similarly, there were significantly more individuals with SMC in the GDS5 risk group than in the non-risk group [n = 119 (53.12%), n = 206 (37.32%); p-value = 0.0001]. Consistently, results presented in Appendix II show that participants with SMC had significantly higher odds of having GDS5(+) [Ors (CI 95%): 1.84 (1.34, 2.53); p-value = 0.0002]. In addition, participants with GDS5 risk had significantly higher punctuation in SPMSQ [1.21 (1.35), 0.90 (1.15); p-value = 0.0013] and lower punctuation in VSF [16.84 (6.84), 19.21 (7.08); p-value < 0.0001].
When talking about cognitive reserve, there were more patients with CRQ high marks in the group without risk [n = 39 (20.97%), n = 171 (32.51%); p-value = 0.0292]. Nevertheless, the percentage of patients with low [n = 43 (23.12%), n = 98 (18.63%); p-value = 0.0292], medium/low [n = 41 (22.04%), n = 98 (18.63%); p-value = 0.0292] and medium/high [n = 63 (33.87%), n = 159 (30.23%); p-value = 0.0292] marks was higher in the GDS5 risk group. Furthermore, there were significantly more patients with studies such as primary [n = 16 (29.09%), n = 19 (14.18%); p-value = 0.0009], secondary [n = 16 (29.09%), n = 38 (28.36%); p-value = 0.0009], or superior [n = 18 (32.73%), n = 76 (56.72%); p-value = 0.0009] in the group without risk than in the group with risk. Likewise, there were more patients participating in group activities in the group without risk [n = 57 (30.48%), n = 241 (45.90%); p-value = 0.0002].
Regarding the marital status, there were more patients married [n = 111 (49.55%), n = 377 (68.55%); p-value = 0.0001] and as a couple [n = 1 (0.45%), n = 11 (2.00%); p-value = 0.0001] in the group without risk of depression. In contrast, there were more patients divorced [n = 20 (8.93%), n = 33 (6.00%); p-value = 0.0001], single [n = 16 (7.14%), n = 25 (4.55%); p-value = 0.0001] and widowed [n = 76 (33.93%), n = 104 (18.91%); p-value = 0.0001] in the group with risk of depression.
The percentage of patients with GDS5 risk with hearing loss was significantly higher than patients without risk [n = 103 (45.98%), n = 208 (37.61%); p-value = 0.0310]. Moreover, individuals with GDS5 risk were significantly lonelier than those without risk. This is shown in the UCLA test [n = 43 (28.29%), n = 17 (4.93%); p-value < 0.0001], in the number of friends [6.70 (7.20), 8.20 (8.30); p-value = 0.3897] and in the percentage of patients that practice any group activity [n = 57 (30.48%), n = 241 (45.90%); p-value = 0.0002].
Resilience (BRCS), sense of coherence (SOC) [61.20 (12.80), 72.30 (10.40); p-value < 0.0001], purpose in life (PIL) [27.10 (6.10), 31.10 (4.60); p-value < 0.0001] and the engaged living scale (ELS) [66.50 (15.80), 77.30 (13.10); p-value < 0.0001] were also significantly higher in patients without risk of depression than in those with risk.
Finally, the percentage of individuals taking antidepressants [n = 95 (42.41%), n = 118 (21.34%); p-value < 0.0001] was higher in the GDS5 risk group. Specifically, SSRI [n = 54 (24.11%), n = 56 (10.13%); p-value < 0.0001], TCAs [n = 13 (5.80%), n = 8 (1.45%); p-value = 0.0007] and other antidepressants [n = 15 (6.70%), n = 18 (3.25%); p-value = 0.0312] (Table 3).
3.2 Rational use of antidepressant treatment
3.2.1 Necessity of antidepressant treatment
This section analyzes the necessity of antidepressant treatment in the group of patients classified as high-risk according to the GDS5 scale. The results show that a significantly higher proportion of patients (57.59%, total: n = 129) were not receiving antidepressant treatment compared to those who were (42.41%, total: n = 95), with a statistically significant difference [X2 (1) = 10.30; p-value = 0.0001]. However, as shown in Appendix II, individuals using antidepressants had higher likelihood of having GDS5(+).
3.2.2 Efficacy of antidepressant treatment
Regarding the efficacy of antidepressant treatment, in the group of patients classified as without risk according to the GDS5 scale, the results show that a significant majority of patients (78.66%, total: n = 435) were not receiving antidepressant treatment, compared to a smaller percentage who were (21.34%, total n: = 118), with a statistically significant difference [X2 (1) = 363.06; p-value < 0.0001].
3.2.3 Safety of antidepressant treatment
Concerning the safety of antidepressant treatment, as shown in Table 4, within the GDS5-risk group, significantly more patients with CI (34.04%, total: n = 32) and SMC (65.26%, total: n = 62) were taking antidepressants than those who were not (CI: 20.93%, total: n = 27 and, SMC: 44.19%, total: n = 57) [CI: X2 (1) = 9.64; p-value = 0.0284 and SMC: X2 (1) = 20.02; p-value = 0.0018]. Similarly, within the GDS5-without-risk group, a significantly higher number of patients with SMC were also receiving antidepressant treatment (51.69%, total: n = 61). However, no statistically significant differences were observed regarding CI in this group.
Table 4
| GDS5 (+) | |||
|---|---|---|---|
| Non-antidepressants | Antidepressants | P-value a | |
| CI, n(%) | n = 27 (20.93%) | n = 32 (34.04%) | 0.0284 * |
| SMC, n(%) | n = 57 (44.19%) | n = 62 (65.26%) | 0.0018 * |
| GDS5 (–) | |||
|---|---|---|---|
| Non-antidepressants | Antidepressants | P-value a | |
| CI, n(%) | n = 76 (17.63%) | n = 26 (22.22%) | 0.2581 |
| SMC, n(%) | n = 145 (33.41%) | n = 61 (51.69%) | 0.0003 * |
Association of antidepressants usage with depression risk estimated by the GDS5.
(GDS5: 5 Point Geriatric Depression Scale; CI: cognitive impairment; SMC: subjective memory complaint) The variables marked with an asterisk (*) are statistically significant. a Chi-squared test (p-value < 0.05).
Furthermore, Figure 3A illustrates the estimated risk of depression, as measured by the GDS5, in relation to overall antidepressant consumption. In contrast, Figure 3B shows the risk of depression stratified by the type of antidepressant used. The data indicate that antidepressant treatment is associated with 42% ineffectiveness, 58% necessity, and 21% efficacy. Moreover, TCAs are consumed twice as frequently when the GDS5 result is positive compared to when it is negative.
Figure 3
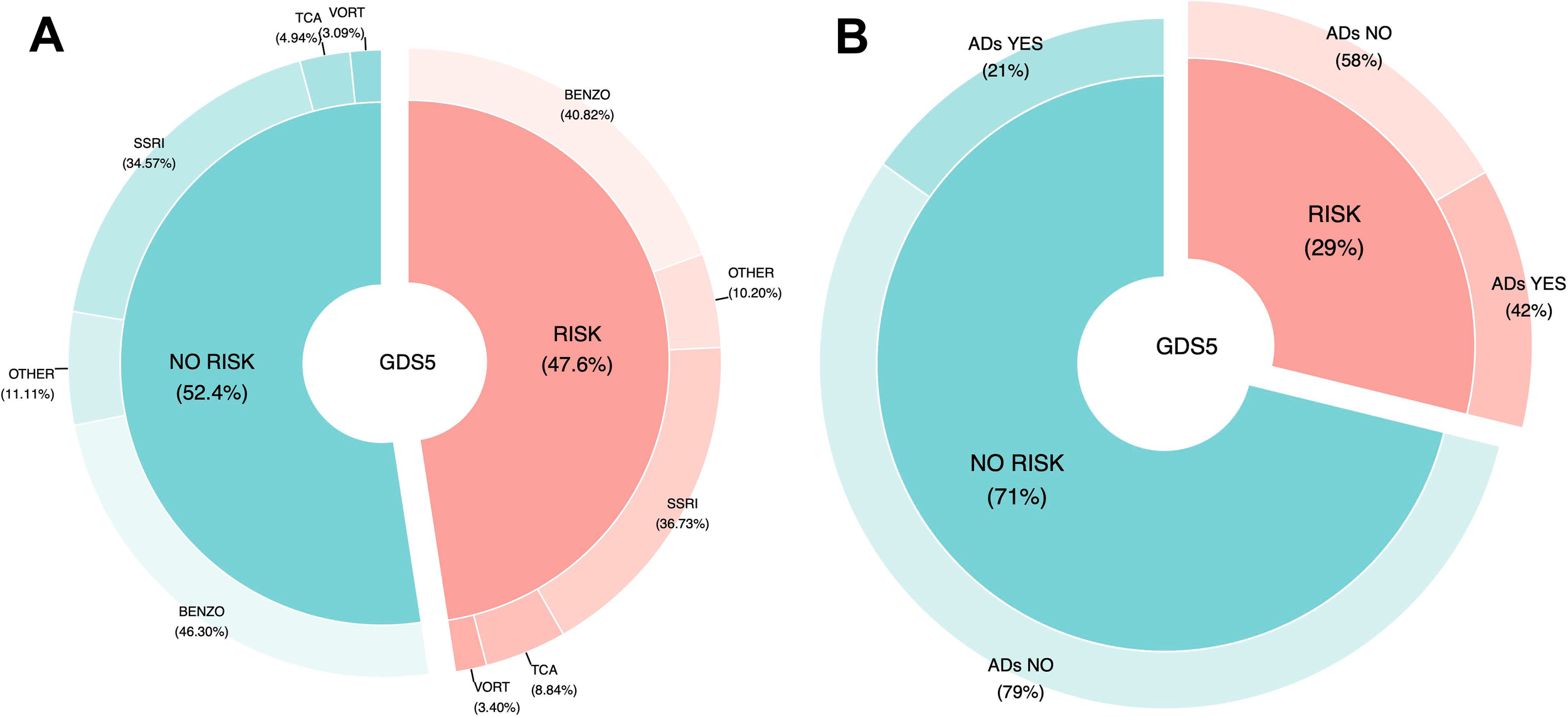
Association of antidepressants usage with depression risk estimated by the GDS5. (A) Comparison between participants using any antidepressant and those not using them, within the GDS-5 “risk” and “no-risk” groups. (B) Comparison among specific antidepressant classes in participants with and without depression risk according to the GDS-5. (GDS5, 5 Point Geriatric Depression Scale; ADs, Antidepressants; SSRIs, Serotonin Reuptake Inhibitors; TCAs, Tricyclic Antidepressants; VORT, Vortioxetine; BENZO, Benzodiazepines).
4 Discussion
This study’s primary contribution is the examination of the association between depression and cognitive status, and how this relationship relates to the use of antidepressant treatment. In this cross-sectional study, conducted with a sample of 777 patients, we investigated the necessity, effectiveness, and safety of antidepressant treatment, as well as its association with cognitive status.
Despite its undefined etiology, SMC should not be considered a trivial symptom in the elderly population, as it may indicate current alterations in mood or cognition and predict the future onset of dementia (43). SMC has been associated with depressive symptoms in older adults with MCI, making early detection and management of these symptoms highly important (44). This is in line with our results, as CI and SMC were associated with depression risk, according to GDS5.
As expected, our study found that a lower cognitive reserve (measured by the CRQ, low levels of education, or lack of participation in group activities) was associated with an increased risk of depression. A previous study concluded that higher cognitive and brain reserve was associated with a reduced risk of experiencing depressive episodes in old age. However, this protective effect was lessened when individuals with clinically relevant depressive symptoms at the start of the study were excluded, indicating that the advantage of a greater cognitive and brain reserve in preventing depressive episodes in old age partly depends on the presence of existing depressive symptoms (45). Additionally, it has been proposed that while high cognitive reserve generally provides cognitive protection, depression may undermine this advantage, leading to greater cognitive difficulties. This underscores the complex interplay between cognitive reserve, mental health, and cognition (46).
We also observed an association between the risk of depression and loneliness (interpreted from the UCLA scale, divorced marital status and hearing loss). Although being divorced does not necessarily imply social isolation, it has been proposed that people who have a partner and live with someone may be more cognitively and socially active (15). On the other hand, multiple studies report associations between hearing loss, depression, and brain changes in middle-aged and older adults. While the direct relationship between depression arising from hearing loss and cognitive performance is still being investigated, the cumulative findings from current studies support the hypothesis that depression acts as a mediator between these factors (47). Meaning in Life (MiL) (according to SOC, PIL and ELS) and resilience (according to BRCS) were also associated with the risk of depression. Studies have shown that MiL may influence several risk factors for cognitive decline (48), as individuals who report higher levels of meaning engage in more physical activity and are less likely to have diabetes or high blood pressure, both of which are risk factors for dementia (49). Additionally, emerging research strongly supports the idea that a sense of purpose in life is linked to favorable cognitive outcomes in older adults, including enhanced cognitive performance and resilience against dementia-related neuropathology (50). Moreover, a higher sense of purpose in life appeared to mitigate some of the negative effects of depressive symptoms on memory performance (51).
According to the PCNE and the WHO, pharmaceutical care is based on the concept of the responsible use of medicines, which involves optimizing their effectiveness, efficiency, and safety (52). Regarding the rational use of antidepressant treatment in our study sample, the association between an increased risk of depression and lower antidepressant use may highlight the need for initiating treatment in certain cases. Conversely, the observed link between a higher risk of depression and increased antidepressant use suggests a potential need to reassess the current therapeutic strategies. Additionally, the observed association between lower depression risk and reduced use of antidepressants may reflect that individuals with fewer depressive symptoms do not require pharmacological treatment. This analysis highlights a potential deficiency in access to appropriate treatment or a lack of early diagnosis, which is crucial for early pharmaceutical intervention. These findings emphasize the importance of closely follow up in the management of antidepressant therapy to optimize treatment outcomes (53, 54).
Several studies have examined the risk of cognitive decline with anticholinergic medication use in elderly patients, consistently finding an association with poor cognitive performance in various settings (55–58). They recognize the critical need to optimize its use among elderly adults and agree that it should be discouraged when suitable alternatives are available. Our results indicated that the consumption of TCAs doubled when the GDS5 score was positive. This finding is particularly notable given that TCAs have a high anticholinergic burden (59), as shown in Table 1.
Although a recent study showed that elderly users of SSRIs and other antidepressants have a higher risk of developing dementia than elderly users of TCAs (60), a previous meta-analysis indicated that the use of antidepressants, particularly TCAs and SSRIs, is associated with an increased risk of CI in older adults, regardless of the presence of dementia (61). Additionally, TCAs and Monoamine Oxidase Inhibitors (MAOIs) may have a higher association with executive function impairment compared to other classes of antidepressants, such as Serotonin-Norepinephrine Reuptake Inhibitors (SNRIs) (62). For instance, TCAs like nortriptyline have been linked to lower baseline cognitive performance in areas such as verbal functions, visual memory, and psychomotor speed. These medications can exacerbate cognitive deficits due to their anticholinergic effects, which interfere with acetylcholine neurotransmission, a key factor for memory and learning. Furthermore, TCAs carry a higher risk of cardiovascular side effects and overdose toxicity, making them less advisable for older adults (63, 64).
While the anticholinergic action of TCAs is the primary factor contributing to CI, significantly affecting cholinergic receptors (57), their impact on other receptors, such as histaminergic and adrenergic, also plays a role in their overall side effect profile, though to a lesser extent. These combined effects likely account for the heightened risk of CI associated with the use of TCAs (65) TCAs are known to cause CI through several mechanisms, including their interaction with histaminergic and adrenergic receptors. Specifically, TCAs block H1 histamine receptors, leading to sedation and drowsiness, which can impair attention and memory. This prolonged sedation interferes with cognitive functions, especially in tasks requiring focus and memory retention. Additionally, TCAs block α1-adrenergic receptors, which contributes to orthostatic hypotension and dizziness, further complicating cognitive processing and concentration abilities. This combination of receptor interactions can lead to both cognitive and mood disturbances, particularly affecting elderly or sensitive patients (60).
For those patients diagnosed with depression who remain untreated or do not improve with their current antidepressants, alternatives should be considered. Vortioxetine (66), a multimodal antidepressant with potential procognitive effects, could enhance the safety of antidepressant treatment (67), quality of life and health outcomes (68) related to depression. Unlike other antidepressants, Vortioxetine (69) acts as a modulator of multiple serotonergic receptors, including serotonergic receptors (5-HT1A, 5-HT1B, 5-HT1D, 5-HT3, and 5-HT7), as well as a serotonin reuptake inhibitor and 5-HT1A receptor partial agonist. These mechanisms uniquely modulate serotonergic neurotransmission, offering broader effects than simply elevating serotonin levels.
4.1 Strengths and Limitations
Data collection was based on a comprehensive face-to-face interview lasting approximately 90 minutes, ensuring the acquisition of detailed and reliable information on dietary intake and lifestyle. Cognitive function was assessed using three complementary tests (MIS, SPMSQ, and SVF), each capturing different cognitive domains with varying degrees of sensitivity and specificity, thereby allowing for a broader and more nuanced evaluation of cognitive performance. Integrating all three assessments strengthens the ability to correctly identify individuals with CI, ultimately leading to greater diagnostic precision.
Our study has certain limitations, primarily due to the low percentage of patients diagnosed with depression currently receiving treatment with vortioxetine (3.40%). During the period from 2020 to 2024, vortioxetine has not been widely adopted as a primary treatment option in clinical practice, especially compared to other medication groups such as SSRIs. Furthermore, an inherent limitation of cross-sectional studies is their inability to establish causal inference. However, they enable the identification of associations that can generate new hypothesis for future research.
Another limitation of the present study is the use of the GDS-5, a self-reported screening instrument, rather than a clinician-rated diagnostic tool for depression. While the GDS-5 is practical and validated for use in large-scale studies, it may not fully capture clinically diagnosed depression and can be subject to self-reporting bias.
Although CI risk was estimated using validated screening questionnaires, they are widely employed in Primary Care settings. This approach aligns with routine clinical practice for early detection. Importantly, participants who screened positive were subsequently referred for comprehensive clinical evaluation and diagnostic confirmation. This two-step process ensures both practical applicability in primary care and reliability in identifying true cases of CI.
Finally, while the sample size was sufficient to detect small-to-moderate associations, the exploratory nature of the study calls for confirmation through longitudinal or interventional research.
5 Conclusion
This study underscores the importance of cognitive assessments and regular monitoring in patients undergoing antidepressant treatment, particularly in relation to the risk of CI and its association with depression. Strengthening interprofessional collaboration, especially between pharmacists, primary care providers, and neurology specialists, can significantly improve the safety, appropriateness, and effectiveness of antidepressant therapy. Such collaboration allows for the identification of patients receiving antidepressant treatment from community pharmacies who show limited improvement, enabling timely referrals to primary care or neurology units. This approach is crucial for addressing early CI, its link with depression, and the potential side effects associated with anticholinergic burden.
Additionally, further research is needed to explore the cognitive effects of vortioxetine. If its cognitive benefits are confirmed, updating prescription guidelines would be essential to optimize treatment outcomes for patients at risk of CI.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethical Committee for Clinical Research with Medications of the Arnau de Vilanova Health Department (MOR-ROY-2018–013, date of approval: 18 July 2018). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MG-P: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. JP: Data curation, Formal Analysis, Methodology, Software, Writing – review & editing. MG-Z: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Writing – original draft. GA-M: Data curation, Writing – original draft. JS-L: Funding acquisition, Validation, Writing – review & editing. ML: Conceptualization, Funding acquisition, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. We also wish to acknowledge Cathedra DeCo MICOF-CEU UCH for their partial funding and support of this research. This work was supported by Cátedra DeCo MICOF – UCH and Ayudas a la Formación de Jóvenes Investigadores CEU - Santander.
Acknowledgments
We extend our sincere gratitude to all the healthcare professionals and patients who participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Long S . World Alzheimer Report 2023: Reducing dementia risk: never too early, never too late. WW. London, England: Alzheimer’s Disease International (2023).
2
World Health Organization . Dementia (2023). Available online at: https://www.who.int/news-room/fact-sheets/detail/dementia (Accessed May 2, 2025).
3
World Health Organization . Depression (2023). Available online at: https://www.who.int/news-room/fact-sheets/detail/depression (Accessed May 3, 2025).
4
Alzheimer's Association . Alzheimer’s disease facts and figures. Alzheimer’s & Dementia, Vol. 17 (2021). pp. 327–406.
5
Jack CR Bennett DA Blennow K Carrillo MC Dunn B Haeberlein SB et al . NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dementia. (2018) 14:535–62. doi: 10.1016/j.jalz.2018.02.018
6
Aisen PS Cummings J Jack CR Morris JC Sperling R Frölich L et al . On the path to 2025: understanding the Alzheimer’s disease continuum. Alzheimers Res Ther. (2017) 9:60. doi: 10.1186/s13195-017-0283-5
7
Livingston G Huntley J Sommerlad A Ames D Ballard C Banerjee S et al . Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. (2020) 396:413–46. doi: 10.1016/S0140-6736(20)30367-6
8
Chemerinski E Petracca G Sabe L Kremer J Starkstein SE . The specificity of depressive symptoms in patients with alzheimer’s disease. Am J Psychiatry. (2001) 158:68–72. doi: 10.1176/appi.ajp.158.1.68
9
Lyketsos CG DelCampo L Steinberg M Miles Q Steele CD Munro C et al . Treating depression in alzheimer disease. Arch Gen Psychiatry. (2003) 60:737. doi: 10.1001/archpsyc.60.7.737
10
Botto R Callai N Cermelli A Causarano L Rainero I . Anxiety and depression in Alzheimer’s disease: a systematic review of pathogenetic mechanisms and relation to cognitive decline. Neurological Sci. (2022) 43:4107–24. doi: 10.1007/s10072-022-06068-x
11
Tatsumi H Nakaaki S Torii K Shinagawa Y Watanabe N Murata Y et al . Neuropsychiatric symptoms predict change in quality of life of Alzheimer disease patients: A two-year follow-up study. Psychiatry Clin Neurosci. (2009) 63:374–84. doi: 10.1111/j.1440-1819.2009.01955.x
12
Shim YS Yang DW . Depression as prognostic factor: 6 months follow-up in a geriatric institution. Arch Gerontol Geriatr. (2006) 43:277–83. doi: 10.1016/j.archger.2005.11.002
13
Milwain EJ Nagy Z . Depressive symptoms increase the likelihood of cognitive impairment in elderly people with subclinical alzheimer pathology. Dement Geriatr Cognit Disord. (2005) 19:46–50. doi: 10.1159/000080971
14
Wang GHM Li P Wang Y Guo J Wilson DL Lo-Ciganic WH . Association between antidepressants and dementia risk in older adults with depression: A systematic review and meta-analysis. J Clin Med. (2023) 12:6342. doi: 10.3390/jcm12196342
15
Sjöberg L Fratiglioni L Lövdén M Wang HX . Low mood and risk of dementia: the role of marital status and living situation. Am J Geriatric Psychiatry. (2020) 28:33–44. doi: 10.1016/j.jagp.2019.08.014
16
Singh-Manoux A Dugravot A Fournier A Abell J Ebmeier K Kivimäki M et al . Trajectories of depressive symptoms before diagnosis of dementia. JAMA Psychiatry. (2017) 74:712. doi: 10.1001/jamapsychiatry.2017.0660
17
Harerimana NV Liu Y Gerasimov ES Duong D Beach TG Reiman EM et al . Genetic evidence supporting a causal role of depression in alzheimer’s disease. Biol Psychiatry. (2022) 92:25–33. doi: 10.1016/j.biopsych.2021.11.025
18
World Health Organization . Medication wothout harm:policy brief (2024). Available online at: https://www.who.int/publications/i/item/9789240062764 (Accessed May 2, 2025).
19
Weiss F Pacciardi B D’Alessandro G Caruso V Maremmani I Pini S et al . The role of vortioxetine in the treatment of depressive symptoms in general hospital psychiatry: A case-series and PRISMA-compliant systematic review of the literature. J Clin Med. (2024) 13:531. doi: 10.3390/jcm13020531
20
Wang HM Chen WH Chang SH Zhang T Shao H Guo J et al . Association between first-line antidepressant use and risk of dementia in older adults: a retrospective cohort study. (2023).
21
Shi S Klotz U . Age-related changes in pharmacokinetics. Curr Drug Metab. (2011) 12:601–10. doi: 10.2174/138920011796504527
22
Price A Rayner L Okon-Rocha E Evans A Valsraj K Higginson IJ et al . Antidepressants for the treatment of depression in neurological disorders: a systematic review and meta-analysis of randomised controlled trials. J Neurol Neurosurg Psychiatry. (2011) 82:914–23. doi: 10.1136/jnnp.2010.230862
23
World Health Organization . Conference of experts on the rational use of drugs (Nairobi, Kenya, 25–29 november 1985): report by the director-general. (1985).
24
Pharmaceutical Care Network Europe (PCNE) . The PCNE classification for drug-related problems V8.01. (2019).
25
Espejo J . Drug related problems: Definition and proposal for its inclusion in the International Classification of Primary Care (ICPC) from WONCA. Pharm Care España. (2002) 4:122–7.
26
Pharmaceutical Care Network Europe . PCNE classification for drug-related problems V9.1 (2020). Available online at: https://www.pcne.org/working-groups/2/drug-related-problem-classification/11 (Accessed May 2, 2025).
27
Aziz R Steffens D . Overlay of late-life depression and cognitive impairment. Focus (Madison). (2017) 15:35–41. doi: 10.1176/appi.focus.20160036
28
Ramos H Pardo J Sánchez R PuChades E Pérez-Tur J Navarro A et al . Pharmacist-physician interprofessional collaboration to promote early detection of cognitive impairment: increasing diagnosis rate. Front Pharmacol. (2021) 12. doi: 10.3389/fphar.2021.579489
29
Böhm P Peña-Casanova J Gramunt N Manero RM Terrón C Quiñones-Ubeda S . Spanish version of the Memory Impairment Screen (MIS): normative data and discriminant validity. Neurologia. (2005) 20:402–11.
30
López Pérez-Díaz AG Calero MD Navarro-González E . Prediction of cognitive impairment in the elderly by analysing their performance in verbal fluency and in sustained attention. Rev Neurol. (2013) 56:1–7.
31
Martínez de la Iglesia J DueñasHerrero R Carmen Onís Vilches M Aguado Taberné C Albert Colomer C Luque Luque R . Adaptación y validación al castellano del cuestionario de Pfeiffer (SPMSQ) para detectar la existencia de deterioro cognitivo en personas mayores de 65 años. Med Clin (Barc). (2001) 117:129–34.
32
Rami González L Valls Pedret C Bartrés Faz D Caprile Elola-Olaso C Solé Padullés C Castellví Sampol M et al . Cuestionario de reserva cognitiva. Valores obtenidos en población anciana sana y con enfermedad de Alzheimer. Rev Neurol. (2011) 52:195.
33
Antonovsky A . The structure and properties of the sense of coherence scale. Soc Sci Med. (1993) 36:725–33. doi: 10.1016/0277-9536(93)90033-Z
34
Ryff CD Keyes CLM . The structure of psychological well-being revisited. J Pers Soc Psychol. (1995) 69:719–27. doi: 10.1037/0022-3514.69.4.719
35
Trompetter HR ten Klooster PM Schreurs KMG Fledderus M Westerhof GJ Bohlmeijer ET . Measuring values and committed action with the Engaged Living Scale (ELS): Psychometric evaluation in a nonclinical sample and a chronic pain sample. Psychol Assess. (2013) 25:1235–46. doi: 10.1037/a0033813
36
Sinclair VG Wallston KA . The development and psychometric evaluation of the brief resilient coping scale. Assessment. (2004) 11:94–101.
37
Russell DW . UCLA loneliness scale (Version 3): reliability, validity, and factor structure. J Pers Assess. (1996) 66:20–40. doi: 10.1207/s15327752jpa6601_2
38
Hoyl MT Alessi CA Harker JO Josephson KR Pietruszka FM Koelfgen M et al . Development and testing of a five-item version of the geriatric depression scale. J Am Geriatr Soc. (1999) 47:873–8. doi: 10.1111/j.1532-5415.1999.tb03848.x
39
Martínez de la Iglesia J Onis Vilches MC Dueñas Herrero R Albert Colomer C Aguado Taberné C Luque Luque R . Versión española del cuestionario de Yesavage abreviado (GDS) para el despistaje de depresión en mayores de 65 años: adaptación y validación. Rev Med Familiar y Comunitaria. (2002) 12:620–30.
40
Faul F Erdfelder E Buchner A Lang AG . Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav Res Methods. (2009) 41:1149–60. doi: 10.3758/BRM.41.4.1149
41
Bender R Lange S . Adjusting for multiple testing—when and how? J Clin Epidemiol. (2001) 54:343–9. doi: 10.1016/s0895-4356(00)00314-0
42
Posit PBC . RStudio: Integrated development environment for R [Computer software]. Posit Software, PBC (2025). Available online at: https://posit.co/products/open-source (Accessed April 30, 2025).
43
Brigola AG Manzini CSS Oliveira GBS Ottaviani AC Sako MP Vale FAC . Subjective memory complaints associated with depression and cognitive impairment in the elderly: A systematic review. Dement Neuropsychol. (2015) 9:51–7. doi: 10.1590/S1980-57642015DN91000009
44
Song D Yu DSF Li PWC He G Shen C Chen G et al . Role of depressive symptoms in subjective memory complaint in older adults with mild cognitive impairment. Int J Older People Nurs. (2020) 15. doi: 10.1111/opn.12279
45
Zijlmans JL Vernooij MW Ikram MA Luik AI . The role of cognitive and brain reserve in late-life depressive events: The Rotterdam Study. J Affect Disord. (2023) 320:211–7. doi: 10.1016/j.jad.2022.09.145
46
Lara E Martín-María N Miret M Olaya B Haro JM Ayuso-Mateos JL . Is there a combined effect of depression and cognitive reserve on cognitive function? Findings from a population-based study. Psychol Health. (2022) 37:1132–47. doi: 10.1080/08870446.2021.1927030
47
Huber M Reuter L Weitgasser L Pletzer B Rösch S Illg A . Hearing loss, depression, and cognition in younger and older adult CI candidates. Front Neurol. (2023) 14. doi: 10.3389/fneur.2023.1272210
48
García C Moreno L Alacreu M Muñoz FJ Martínez LA . Addressing psychosocial factors in cognitive impairment screening from a holistic perspective: the deCo-booklet methodology design and pilot study. Int J Environ Res Public Health. (2022) 19:12911. doi: 10.3390/ijerph191912911
49
Sutin AR Luchetti M Stephan Y Terracciano A . Meaning in life and risk of cognitive impairment: A 9-Year prospective study in 14 countries. Arch Gerontol Geriatr. (2020) 88:104033. doi: 10.1016/j.archger.2020.104033
50
Buchman AS . Effect of purpose in life on the relation between alzheimer disease pathologic changes on cognitive function in advanced age. Arch Gen Psychiatry. (2012) 69:499. doi: 10.1001/archgenpsychiatry.2011.1487
51
Lewis NA Hill PL . Sense of purpose promotes resilience to cognitive deficits attributable to depressive symptoms. Front Psychol. (2021) 12. doi: 10.3389/fpsyg.2021.698109
52
Griese-Mammen N Hersberger KE Messerli M Leikola S Horvat N van Mil JWF et al . PCNE definition of medication review: reaching agreement. Int J Clin Pharm. (2018) 40:1199–208. doi: 10.1007/s11096-018-0696-7
53
Fava GA . Rational use of antidepressant drugs. Psychother Psychosom. (2014) 83:197–204. doi: 10.1159/000362803
54
von Knorring J Baryshnikov I Jylhä P Talaslahti T Heikkinen M Isometsä E . Prospective study of antidepressant treatment of psychiatric patients with depressive disorders: treatment adequacy and outcomes. BMC Psychiatry. (2023) 23:888. doi: 10.1186/s12888-023-05390-8
55
Gray SL Anderson ML Dublin S Hanlon JT Hubbard R Walker R et al . Cumulative use of strong anticholinergics and incident dementia. JAMA Intern Med. (2015) 175:401. doi: 10.1001/jamainternmed.2014.7663
56
Risacher SL McDonald BC Tallman EF West JD Farlow MR Unverzagt FW et al . Association between anticholinergic medication use and cognition, brain metabolism, and brain atrophy in cognitively normal older adults. JAMA Neurol. (2016) 73:721. doi: 10.1001/jamaneurol.2016.0580
57
Chatterjee S Bali V Carnahan RM Johnson ML Chen H Aparasu RR . Anticholinergic medication use and risk of dementia among elderly nursing home residents with depression. Am J Geriatric Psychiatry. (2016) 24:485–95. doi: 10.1016/j.jagp.2015.12.011
58
Fox C Richardson K Maidment ID Savva GM Matthews FE Smithard D et al . Anticholinergic medication use and cognitive impairment in the older population: the medical research council cognitive function and ageing study. J Am Geriatr Soc. (2011) 59:1477–83. doi: 10.1111/j.1532-5415.2011.03491.x
59
Ramos H Moreno L Pérez-Tur J Cháfer-Pericás C García-Lluch G Pardo J . CRIDECO anticholinergic load scale: an updated anticholinergic burden scale. Comparison with the ACB scale in spanish individuals with subjective memory complaints. J Pers Med. (2022) 12:207. doi: 10.3390/jpm12020207
60
Santandreu J Caballero FF Gómez-Serranillos MP González-Burgos E . Risk of dementia among antidepressant elderly users: A population-based cohort analysis in Spain. J Affect Disord. (2024) 349:54–61. doi: 10.1016/j.jad.2024.01.002
61
Han L McCusker J Cole M Čapek R Abrahamowicz M . Antidepressant use and cognitive functioning in older medical patients with major or minor depression. J Clin Psychopharmacol. (2011) 31:429–35. doi: 10.1097/JCP.0b013e318221b2f8
62
Masdrakis VG Markianos M Baldwin DS . Apathy associated with antidepressant drugs: a systematic review. Acta Neuropsychiatr. (2023) 35:189–204. doi: 10.1017/neu.2023.6
63
Pruckner N Holthoff-Detto V . Antidepressant pharmacotherapy in old-age depression—a review and clinical approach. Eur J Clin Pharmacol. (2017) 73:661–7. doi: 10.1007/s00228-017-2219-1
64
Allan CL Ebmeier KP . Review of treatment for late-life depression. Adv Psychiatr Treat. (2013) 19:302–9. doi: 10.1192/apt.bp.112.010835
65
Vos CF Aarnoutse RE Op de Coul MJM Spijker J Groothedde-Kuyvenhoven MM Mihaescu R et al . Tricyclic antidepressants for major depressive disorder: a comprehensive evaluation of current practice in the Netherlands. BMC Psychiatry. (2021) 21:481. doi: 10.1186/s12888-021-03490-x
66
Huang IC Chang TS Chen C Sung JY . Effect of vortioxetine on cognitive impairment in patients with major depressive disorder: A systematic review and meta-analysis of randomized controlled trials. Int J Neuropsychopharmacol. (2022) 25:969–78. doi: 10.1093/ijnp/pyac054
67
Moon SW Kim JW Kim DH Lee KY Reines EH Lee M et al . Safety and effectiveness of vortioxetine for major depressive disorder: Real-world evidence from a population-based study in South Korea. Front Psychiatry. (2023) 14. doi: 10.3389/fpsyt.2023.1075939
68
Santos García D Alonso Losada MG Cimas Hernando I Cabo López I Yáñez Baña R Alonso Redondo R et al . Vortioxetine improves depressive symptoms and cognition in parkinson’s disease patients with major depression: an open-label prospective study. Brain Sci. (2022) 12:1466. doi: 10.3390/brainsci12111466
69
Bishop MM Fixen DR Linnebur SA Pearson SM . Cognitive effects of vortioxetine in older adults: a systematic review. Ther Adv Psychopharmacol. (2021) 11. doi: 10.1177/20451253211026796
Summary
Keywords
cognitive impairment, depression, antidepressive agents, pharmacological therapy, rational use of medication
Citation
Gil-Peinado M, Pardo J, García-Zamora M, Adsuar-Meseguer GM, Sendra-Lillo J and Moreno L (2025) Evaluating the rational use of antidepressant in older patients: a comprehensive analysis of its association with cognitive impairment. Front. Psychiatry 16:1624989. doi: 10.3389/fpsyt.2025.1624989
Received
11 May 2025
Accepted
23 September 2025
Published
20 October 2025
Volume
16 - 2025
Edited by
Francesco Panza, University of Bari Aldo Moro, Italy
Reviewed by
BoruJ Jin, The First Affiliated Hospital of China Medical University, China
Lingfeng Xue, King’s College London, United Kingdom
Updates
Copyright
© 2025 Gil-Peinado, Pardo, García-Zamora, Adsuar-Meseguer, Sendra-Lillo and Moreno.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lucrecia Moreno, lmoreno@uchceu.es
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.