- 1Chung Shan Medical University Hospital, Department of Internal Medicine, Division of Endocrinology and Metabolism, Taichung, Taiwan
- 2Chung Shan Medical University Hospital, Department of Medical Research, Taichung, Taiwan
- 3Chung Shan Medical University, School of Medicine, Taichung, Taiwan
- 4Chung Shan Medical University, Institute of Medicine, Taichung, Taiwan
Background: Glucagon-like peptide-1 (GLP-1) receptor agonists, including tirzepatide, are increasingly prescribed for obesity and type 2 diabetes mellitus. Amid safety concerns raised by regulatory agencies regarding potential associations with suicidal ideation or behavior, real-world data on psychiatric risks are critically needed. This study aims to evaluate the association between tirzepatide and suicidal ideation or attempts compared to other anti-obesity medications in a real-world population.
Methods: A retrospective cohort study using data from the TriNetX US Collaborative Network, covering 66 healthcare organizations from May 2022 to April 2024. A total of 226,060 patients with overweight or obesity, including 29% with type 2 diabetes, were included. Propensity score matching was applied to compare tirzepatide (N=42,600) with non-GLP-1 anti-obesity medications (N=183,460).
Results: After matching, 16,321 pairs were analyzed. The mean age was 48.4 years, and 69.1% were female. Over a median follow-up of 365 days, 17 tirzepatide patients and 33 control patients experienced suicidal ideation or attempts. Tirzepatide was associated with a 48% lower risk of suicide ideation or attempts (aHR: 0.52, 95% CI: 0.28–0.91; P=0.001). Subgroup analyses by BMI, HbA1c, and GFR indicated consistent trends of lower risk. While these findings suggest a potential protective association, the observational nature of this study precludes definitive conclusions regarding causality.
Conclusions: Tirzepatide use was associated with a lower observed risk of suicidal ideation or suicide attempts compared with other anti-obesity medications. Long-term studies are needed to confirm these findings.
1 Introduction
Obesity is a prevalent and complex condition that significantly contributes to morbidity and mortality worldwide (1), with associated comorbidities including type 2 diabetes (T2D), cardiovascular disease, and a range of psychological disorders such as depression and anxiety (2–6). Despite advances in lifestyle and pharmacological interventions, effective long-term management of obesity remains challenging. Recently, tirzepatide, a novel dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist, has demonstrated considerable efficacy in improving glycemic control and promoting weight reduction in patients with obesity (7–9). Its dual action mechanism offers a promising approach for the treatment of obesity and related metabolic conditions.
However, the psychological impact of tirzepatide, particularly its potential association with mental health outcomes, remains underexplored. This is of particular concern given the European Medicines Agency’s (EMA) recent alert regarding a possible link between GLP-1 receptor agonists and an increased risk of suicidal ideation and behavior (10, 11). Although randomized controlled trials (RCTs) have not definitively established a psychiatric risk profile for other GLP-1 receptor agonists such as liraglutide and semaglutide, observational studies have largely indicated no increased risk of suicide with their use (12–15). Nevertheless, only a few studies to date have specifically evaluated the risk of suicidal ideation or attempts associated with tirzepatide, leaving a significant gap in the literature that warrants further investigation (11). Additionally, it remains uncertain whether weight loss alone, regardless of the specific intervention, exerts a protective or detrimental effect on suicide risk, as prior data on this topic are conflicting (6, 16, 17).
In light of these concerns, this study aims to investigate the potential association between tirzepatide use and the risk of suicide in patients with obesity. By addressing this gap, we seek to contribute to a more comprehensive understanding of the safety profile of tirzepatide and inform clinical decision-making.
2 Methods
2.1 Data source
This study utilized data from the TriNetX US Collaborative Network, a comprehensive real-world data platform that aggregates information from 66 healthcare organizations, including academic medical centers, community hospitals, and large integrated healthcare systems. The TriNetX platform collects clinical data directly from electronic health records (EHRs), billing systems, and disease registries, encompassing a wide range of demographics, diagnoses, treatments, and outcomes.
The credibility of the TriNetX platform is supported by its extensive use in peer-reviewed research and clinical studies, offering real-time access to a broad patient population across diverse geographic and healthcare settings (18, 19). The accuracy and completeness of its datasets are maintained through regular data quality assessments, leveraging the platform’s automated data cleaning algorithms and collaboration with healthcare institutions to ensure the reliability of the clinical information obtained.
2.2 Study population
The study population consisted of patients with overweight or obesity who were prescribed either tirzepatide or other anti-obesity medications, including bupropion, naltrexone, orlistat, topiramate, phentermine, or setmelanotide. Patients prescribed tirzepatide were designated as cases, while those receiving other anti-obesity medications served as active controls. This active comparator design was selected to ensure that all patients had similar motivations for weight management and shared comparable baseline characteristics, thus allowing for a more accurate comparison of outcomes.
The index date for the case group was defined as the date of the first tirzepatide prescription, while for the control group, it was the date of the first prescription for one of the aforementioned anti-obesity medications. Patients with a documented history of suicidal ideation or suicide attempts prior to the index date were excluded to minimize potential confounding, because the presence of prior suicidality can indicate a distinct clinical pathway and risk profile that might obscure new-onset events. Additionally, patients with prior prescriptions of other GLP-1 receptor agonist medications were excluded to eliminate potential confounding effects.
The study period began in May 2022 and concluded in April 2024, with a maximum follow-up period of one year per patient to ensure consistent comparisons between the tirzepatide and control groups. The median follow-up time was 365 days. We recognize that one year may be a relatively short period for assessing long-term psychiatric outcomes; however, this timeframe was selected to maintain uniform data availability in TriNetX and to reduce time-varying confounders.
2.3 Study outcome
The primary endpoint of this study was the first occurrence of a composite outcome, including suicidal ideation or suicide attempts, after the index date. These diagnoses were obtained from clinical visits documented within the EHRs, ensuring a high degree of reliability, as they were made by qualified healthcare professionals during clinical assessments.
2.4 Study covariates
We carefully selected a comprehensive set of covariates for analysis, ensuring that these variables were obtained either on or within one year prior to the index date. The covariates included a range of demographic factors such as age, sex, ethnicity, race, and marital status. Socioeconomic status, family history of mental and behavioral disorders, and lifestyle-related factors were also considered to account for potential influences on suicide risk.
Baseline clinical characteristics were incorporated into the analysis, including body mass index (BMI), serum creatinine, and HbA1c levels. Pre-existing medical conditions associated with an increased risk of suicide, such as mood disorders, anxiety, schizophrenia, alcohol use disorder, and chronic pain, were thoroughly assessed. In addition, the analysis considered patients’ previous medical interventions, including a history of bariatric surgery and prescriptions for antidepressants, antidiabetic medications, and other relevant therapies. Detailed coding for this study is provided in Supplementary Table 1.
2.5 Statistical analysis
We applied 1:1 propensity score matching (PSM) using greedy nearest neighbor matching with a 0.1 caliper to balance baseline characteristics between the tirzepatide and control groups. This caliper width is the default PSM setting in TriNetX, and is commonly used to optimize balance while retaining a sufficiently large sample. However, we acknowledge that such a caliper may exclude certain patients, potentially introducing selection bias and reducing generalizability. Matching was performed for all covariates to minimize selection bias.
Kaplan-Meier analysis was used to estimate the probability of the primary outcome over time, with censoring applied for patients exiting the cohort. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated to compare time-to-event rates between groups. Cox proportional hazards models were employed for this comparison. A p-value of<0.05 as statistically significant. All analyses were performed within the TriNetX platform using its default analytical software.
For subgroup analyses by sex, ethnicity, race, age, BMI, HbA1c, and eGFR, we did not apply formal multiple-comparison corrections due to the low event rate and the exploratory nature of these analyses. Additionally, we did not perform more advanced sensitivity analyses (e.g., Bayesian modeling or inverse probability weighting [IPW]) due to TriNetX constraints and the rarity of events, which may limit statistical power and generalizability. No imputation was performed for missing data. PSM was conducted using available values, and patients with missing covariates were excluded from that variable-specific analysis within the TriNetX platform.
3 Results
3.1 Baseline demographic
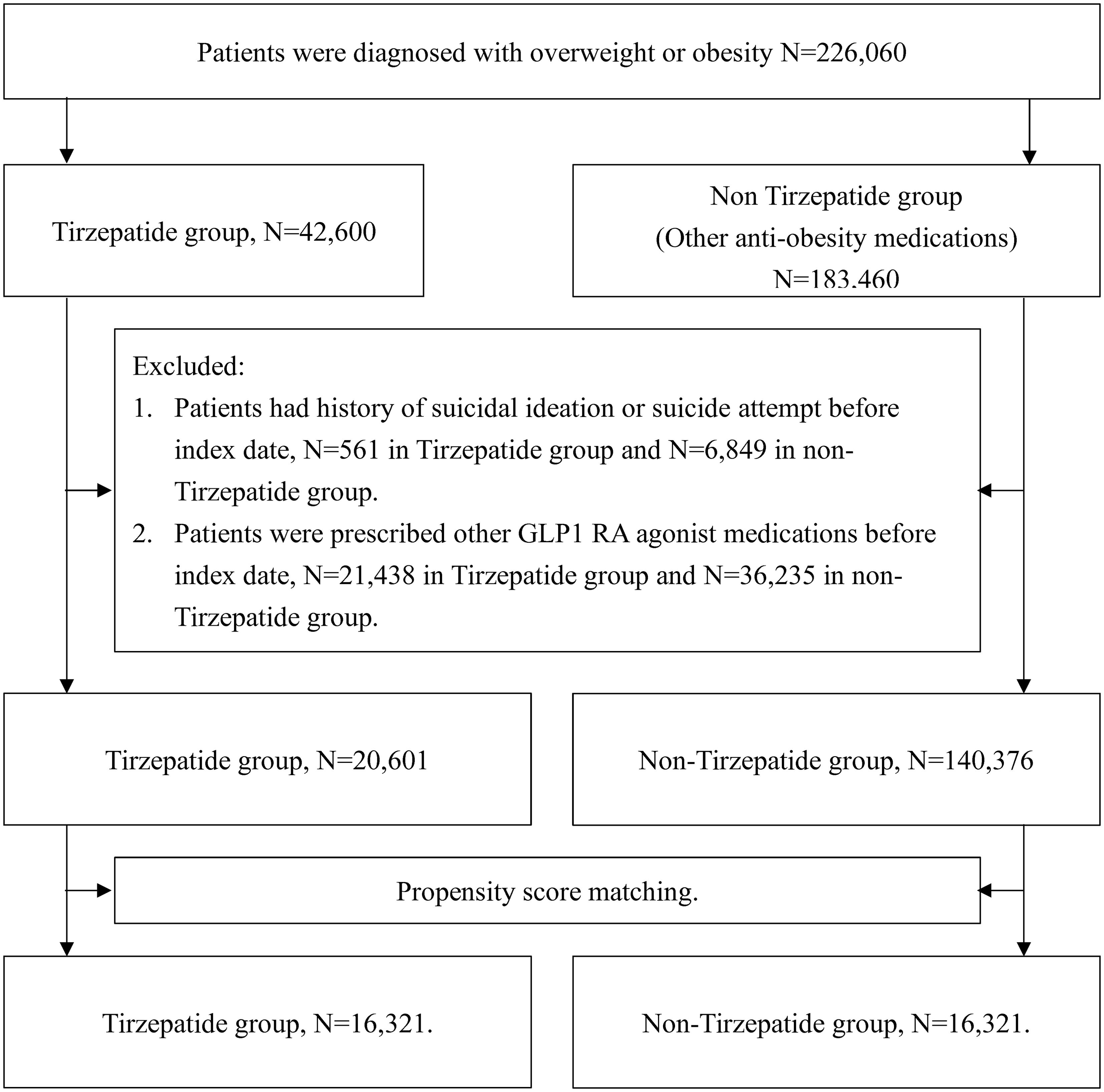
A total of 226,060 patients with overweight or obesity were identified from the TriNetX database, comprising 42,600 patients prescribed tirzepatide and 183,460 patients prescribed non-tirzepatide anti-obesity medications. Following the application of exclusion criteria, including the removing patients with a history of suicidal ideation, suicide attempts, or prior use of any GLP-1 receptor agonists, 20,601 patients in the tirzepatide group and 140,376 patients in the control group were eligible for analysis (Figure 1).
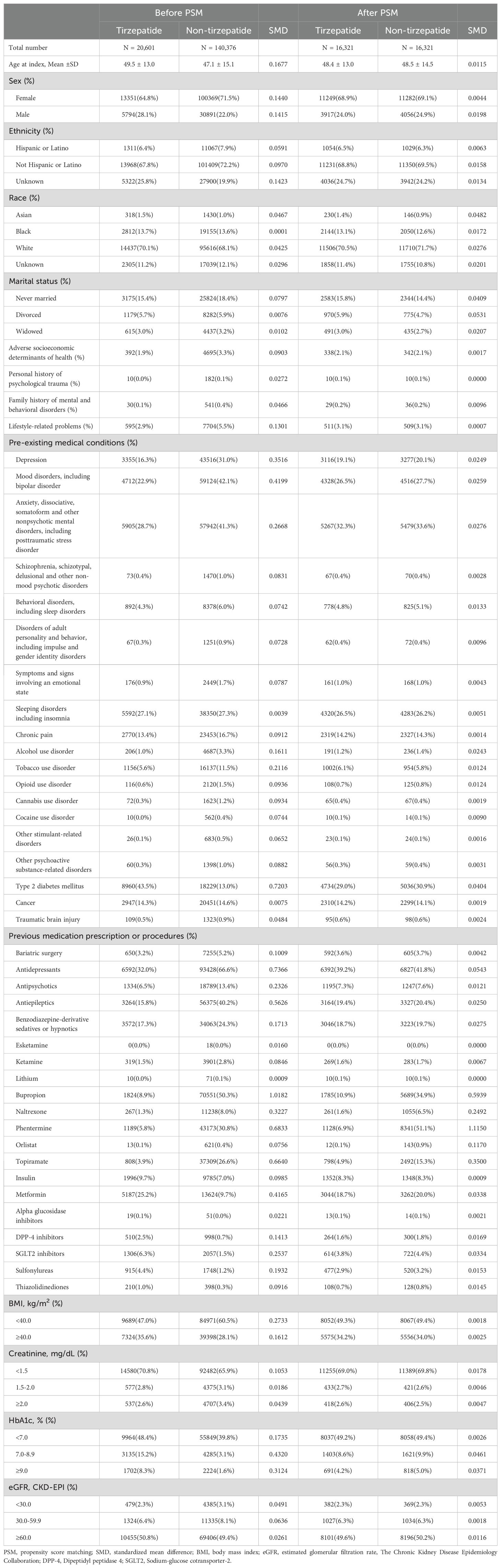
Before PSM, significant differences were observed between the tirzepatide and control groups in terms of age, BMI, and comorbid conditions. To address these imbalances, 1:1 PSM was performed, resulting in 16,321 matched pairs based on all pre-specified covariates. After matching, baseline characteristics, including age, sex, ethnicity, race, BMI, HbA1c, and pre-existing mental health conditions, were well balanced between the two groups, with no statistically significant differences (standardized mean difference [SMD]< 0.1 for all comparisons; Table 1).
The mean age of the study population was 48.4 years, with females comprising 69.1% of the cohort. The racial distribution was well-balanced between groups, with 71% identifying as White, 13% as Black or African American, and 1.4% as Asian. Regarding lifestyle-related factors, 3.1% of patients in both groups had documented issues such as smoking (6%) or alcohol use (1.2%). Pre-existing mental health conditions were prevalent, with 19.1% diagnosed with depression, 26.5% with mood disorders, and 32.3% with anxiety disorders. In terms of metabolic health, 49.2% of patients had baseline HbA1c levels<7%, and 50% had an estimated glomerular filtration rate (eGFR) >60 mL/min/1.73 m². Detailed baseline characteristics before and after matching are presented in Table 1.
3.2 Primary outcome
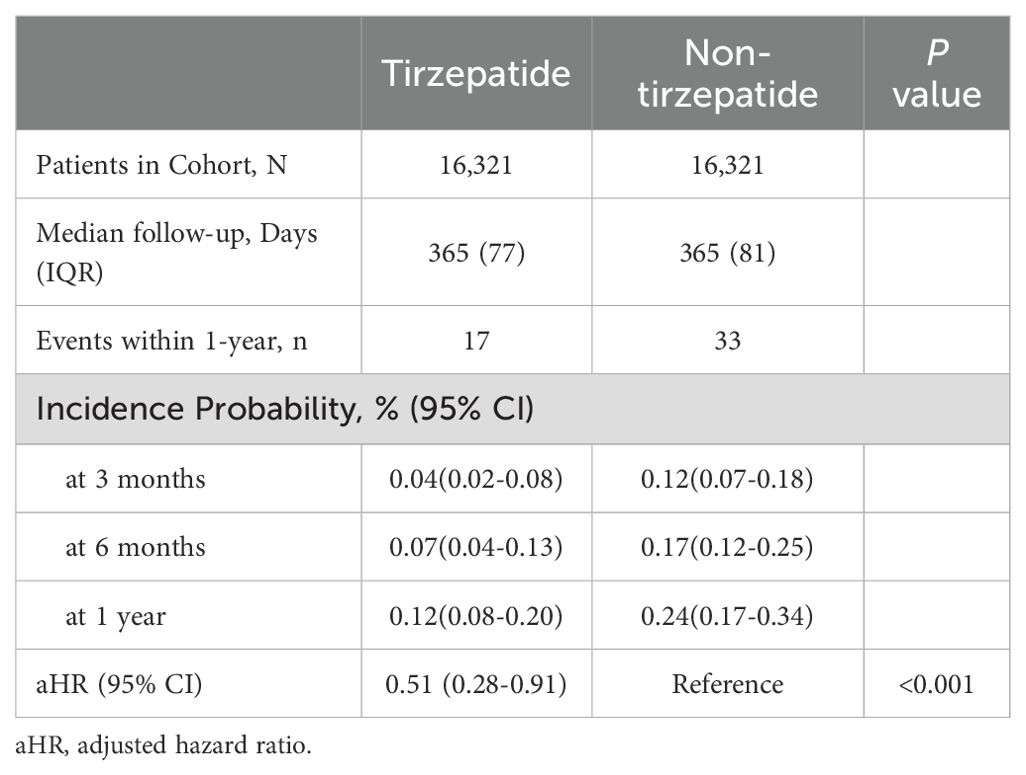
During a median follow-up of 365 days, the composite primary endpoint of suicidal ideation or suicide attempts occurred in 17 patients in the tirzepatide group and 33 patients in the control group. This corresponds to an absolute rate of 1.04 cases per 1000 individuals in the tirzepatide group and 2.02 per 1000 in the control group, yielding an absolute risk reduction (ARR) of 0.98 per 1000 (NNT=1020). The adjusted hazard ratio (aHR) for the tirzepatide group compared to the control group was 0.52 (95% CI: 0.28–0.91, p = 0.001), indicating a significantly decreased risk of suicidal ideation or attempts in patients treated with tirzepatide. Figure 2 shows that Kaplan-Meier analysis demonstrated a significant reduction in the risk of suicidal ideation or attempts in the tirzepatide group compared to the control group (log-rank p< 0.001).
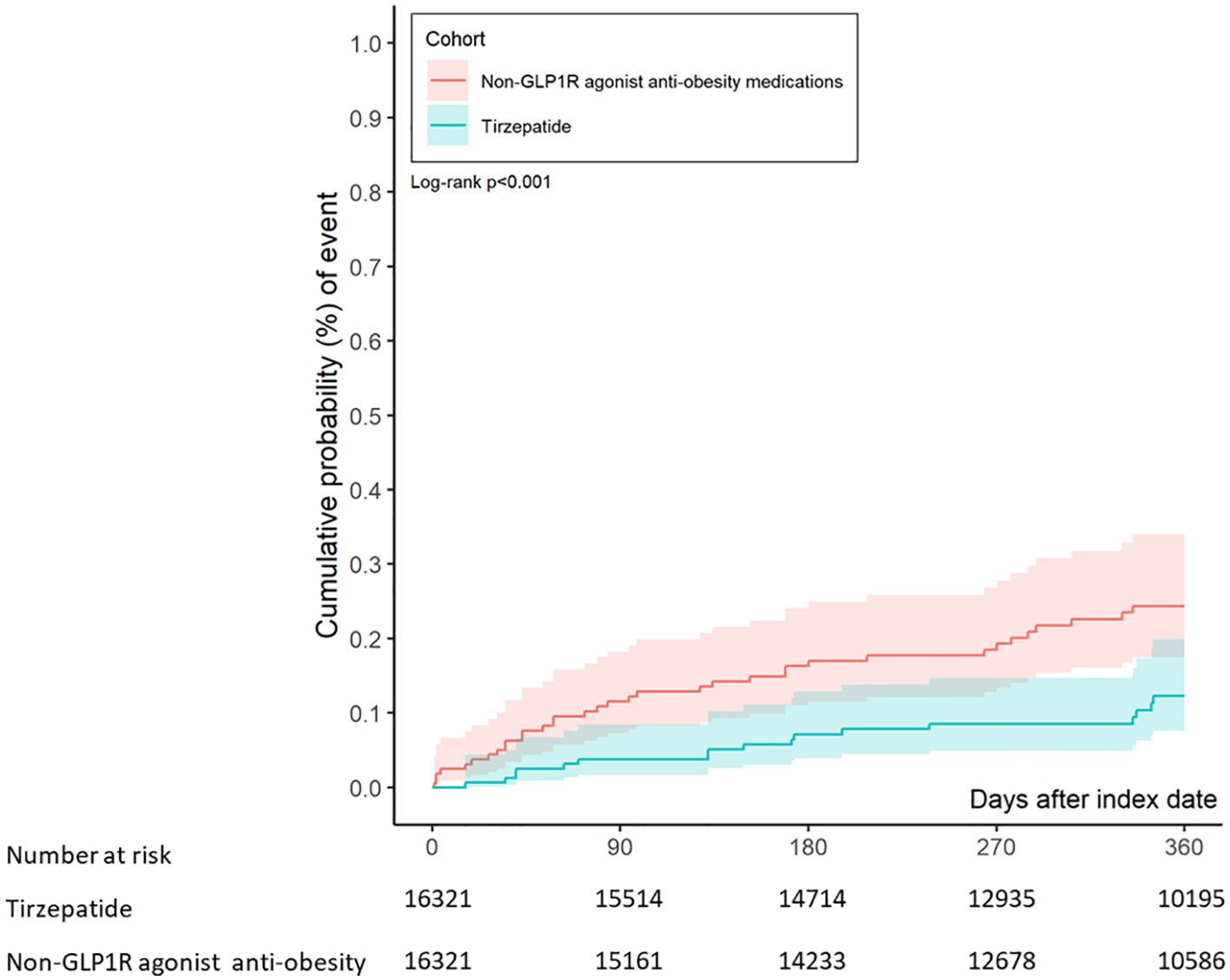
Figure 2. Kaplan-Meier chart evaluating the risk of suicidal ideation or suicide attempts in patients treated with tirzepatide and other anti-obesity medications.
Further analysis of suicidal ideation or attempts at 3, 6, and 12 months revealed adjusted hazard ratios of 0.04 (95% CI: 0.02–0.08), 0.07 (95% CI: 0.04–0.13), and 0.12 (95% CI: 0.08–0.20), respectively, demonstrating a consistent reduction in risk over time in the tirzepatide group compared to the control group (Table 2).
For subgroup analysis, the number of events was too small to conduct robust analyses, with most subgroups having fewer than 10 events, as shown in Figure 3. Stratified analyses by sex, ethnicity, race, age, BMI, HbA1c, and eGFR yielded consistent findings across all subgroups. Notably, female patients and those aged 45–64 years demonstrated directionally lower risks compared with their counterparts, although these findings did not reach statistical significance. In accordance with privacy protection protocols, when the number of outcomes in any subgroup was less than 10, the result was rounded up to 10 (Figure 3). Because of the limited statistical power, these subgroup results should be interpreted with caution.
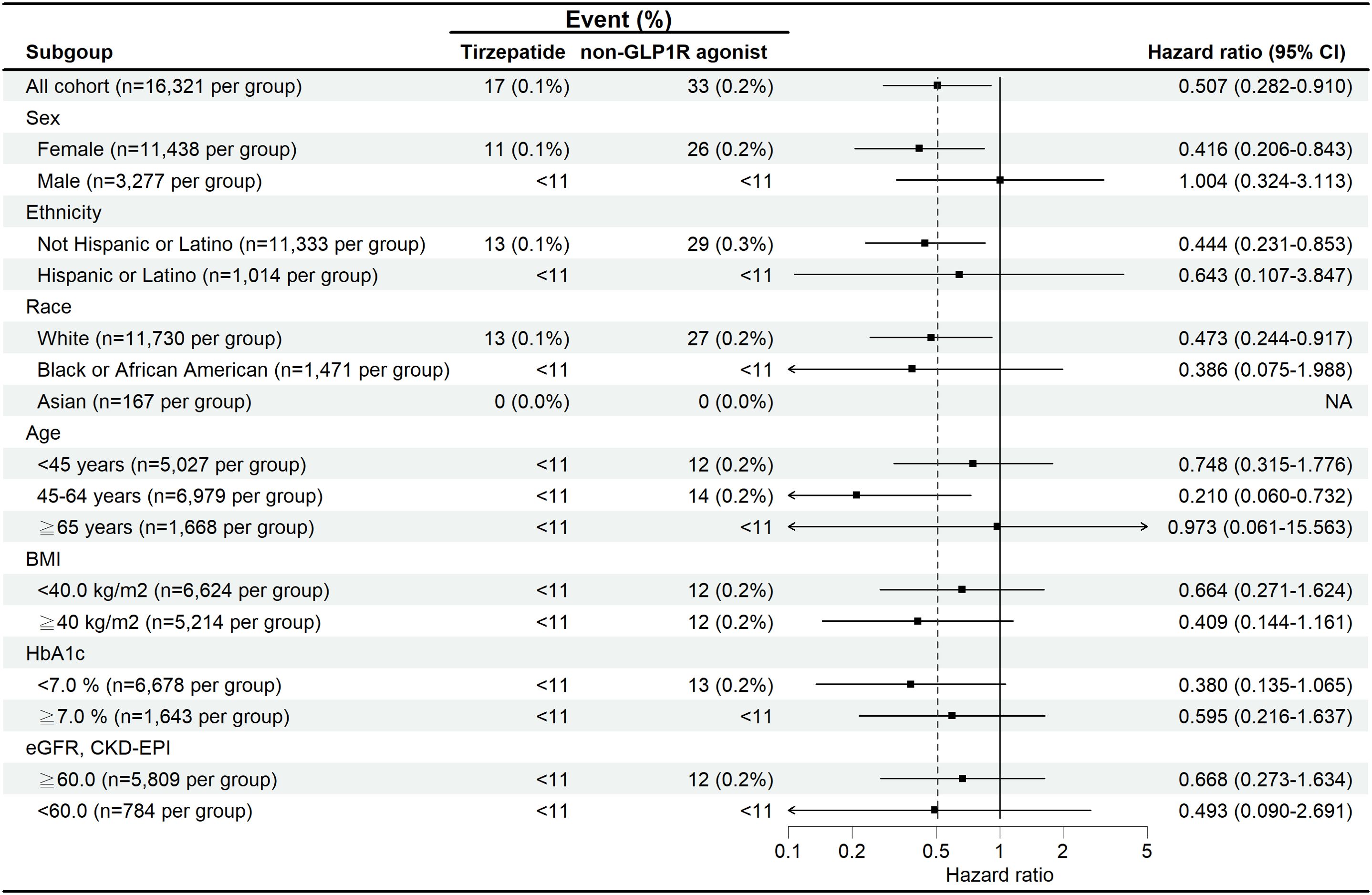
Figure 3. Subgroup analysis of the risk of suicidal ideation and suicide attempts in patients treated with tirzepatide compared with non-GLP-1 receptor agonist anti-obesity medications.
4 Discussion
This study provides the first evidence supporting the safety of tirzepatide in relation to suicidal ideation and attempts. Over a median follow-up of 365 days, tirzepatide was associated with a 48% lower risk of suicidal ideation or attempts (aHR: 0.52, 95% CI: 0.28–0.91, p = 0.001). The consistent risk reduction observed at 3, 6, and 12 months further indicates a potential protective effect of tirzepatide over time.
Although the primary focus of our study was on patients with overweight or obesity, approximately 29% of the cohort also had T2D, a significant subgroup due to the well-established link between T2D, psychiatric disorders, and obesity. Our study demonstrated a consistently lower risk of suicide attempts in these patients. Subgroup analyses exploring the association between BMI, HbA1c, and GFR indicated that lower suicide ideation and attempt risks were observed across these metabolic variables. Despite the overall low incidence of suicide-related events, patients with better-controlled HbA1c levels and higher GFR appeared to have a reduced risk, highlighting the potential mental health benefits of improved glycemic control and kidney function in this population. Additionally, subgroup analyses (Figure 3) suggested directionally lower risks of suicidal ideation or attempts among female patients and those aged 45–64 years, whereas findings among individuals aged ≥65 years and those<45 years were neutral. These observations may be explained by limited statistical power due to very low event numbers, as well as competing mental health risk factors in older adults. Metabolic subgroups demonstrated similar patterns, with patients having HbA1c<7% or eGFR ≥60 mL/min/1.73 m² showing trends toward lower risk. These associations are biologically plausible given the links between metabolic health, quality of life, and psychological well-being; however, all subgroup findings should be regarded as exploratory.
Our findings align with previous observational studies on GLP-1 RAs, which generally report no significant association between these medications and increased suicide risk (12–15). Interestingly, Almeida et al. found that individuals exposed to GLP-1 RAs were at a greater risk of being prescribed antidepressants, suggesting a potential influence of GLP-1 RAs on mood. While this might seem contradictory to our findings, it is important to note that increased antidepressant use does not necessarily equate to increased suicidal ideation or attempts. In fact, antidepressant prescriptions may reflect proactive management of underlying depression, potentially reducing the risk of severe outcomes like suicide (21). Given the high prevalence of pre-existing depression and anxiety in our cohort, concomitant antidepressant or other psychotropic medication use is likely common. However, incomplete capture of medication adherence, dosage changes, and polypharmacy within TriNetX may introduce residual confounding.
The psychiatric safety observed in our study is consistent with findings from major RCTs involving tirzepatide, such as the SURMOUNT trials (7, 8, 22). These studies primarily focused on weight reduction and metabolic outcomes, but they also reported improvements in overall quality of life. While the primary outcomes of these trials did not specifically assess suicidal ideation or behaviors, the significant weight loss achieved with tirzepatide could have positively impacted patients’ psychological well-being, indirectly reducing the risk of mental health-related adverse outcomes. The substantial weight reduction observed in the SURMOUNT trials, up to 20%, is well-established to improve self-esteem, body image, and physical functioning, which are key factors in mitigating suicide risk. Our findings, combined with data from the SURMOUNT studies, suggest that tirzepatide’s comprehensive benefits on metabolic and psychological health make it a safe and effective option for patients with obesity, including those at risk for mental health issues.
The reduction in suicidal ideation and attempts observed in patients treated with tirzepatide could be explained by several biological mechanisms. GLP-1 receptor agonists, including tirzepatide, are thought to exert neuroprotective effects through the modulation of neuroinflammation, the hypothalamic-pituitary-adrenal (HPA) axis, and neurotransmitter pathways, which play key roles in mood regulation and stress response (23). Tirzepatide’s dual agonism, targeting both GLP-1 and GIP receptors, may provide additional benefits (24). While GLP-1 receptor agonists have shown potential in mood stabilization, the GIP pathway may further enhance emotional and cognitive outcomes, potentially contributing to the observed reduction in suicide risk (25). GIP signaling, though less studied in psychiatric contexts, has been linked to improved cognitive processes in animal models, and this dual pathway activation could offer unique neuropsychiatric benefits (26, 27).
The study has several strengths, including the use of a large, real-world database from the TriNetX network, which enhances the generalizability of our findings. We applied rigorous methodology, including PSM, and compared tirzepatide with other active anti-obesity medications to ensure balanced and robust comparisons. Moreover, the inclusion of a wide range of covariates, such as socioeconomic status and multiple comorbidities (e.g., depression, anxiety), enabled a comprehensive analysis. Finally, we evaluated suicide risk across multiple time frames, 3, 6, and 12 months, offering valuable insights into both the short- and intermediate-term effects of tirzepatide.
Nonetheless, our study has several limitations. First, as a retrospective observational analysis using EHR data, residual confounding is possible, even though we employed PSM and adjusted for known psychiatric and metabolic covariates, as well as socioeconomic status and lifestyle-related factors (smoking and alcohol use). However, we were unable to account for the severity of psychiatric illness or healthcare access, which may influence both treatment allocation and suicidality risk. Second, we excluded patients with prior suicidal ideation or suicide attempts to reduce confounding, but this prevented us from evaluating whether tirzepatide could reduce suicidality in that high-risk subset. Third, the low event rate, 17 cases in the tirzepatide group and 33 in the control group, limits statistical power and magnifies the impact of potential misclassification. In addition, reliance on diagnostic coding may underestimate the true incidence of suicidal ideation, as sensitivity and specificity of such codes are uncertain. Fourth, our follow-up period was only one year, which may not capture longer-term or delayed psychiatric events. In addition, we did not separately analyze censoring due to treatment discontinuation, switching, or death, and no competing-risks approach was applied. Given the low number of events and platform constraints, such analyses were not feasible. Fifth, while TriNetX spans multiple healthcare organizations, its data primarily derive from U.S. health systems and may not fully generalize to underserved or non-U.S. populations. Sixth, we did not perform imputation for missing data. Analyses were conducted using available values within the TriNetX platform, and patients with missing covariates were excluded from the relevant variable-specific analyses. This may introduce bias if missingness was not random. Seventh, consistent weight-loss data and patient-reported measures of mood or treatment satisfaction were not available in the TriNetX platform. This may limit our ability to evaluate whether the observed protective association is mediated by improvements in psychological well-being. Finally, our subgroup analyses did not use multiple-comparison corrections and should be regarded as exploratory, particularly given the small number of events. In addition, TriNetX’s privacy policy required rounding of event counts<10 to 10, which may slightly distort reported incidence rates but does not materially affect HR estimates derived from Cox models.
In conclusion, our results indicate that tirzepatide was not associated with an increased risk of suicidal ideation or suicide attempts. Although a HR below unity suggests a possible protective association, the absolute risk reduction was very small, and this finding should be considered tentative and hypothesis-generating. The key clinical message is that no safety signal for increased suicidality was observed. Longer-term prospective studies or randomized clinical trials with psychiatric endpoints are warranted to validate this association and to explore potential biological mechanisms in greater detail.
Data availability statement
This population-based study obtained data from the TrinetX platform (accessible at https://trinetx.com/), for which third-party restrictions apply to the availability of this data. The data were used under license for this study with restrictions that do not allow for data to be redistributed or made publicly available. To gain access to the data, a request can be made to TriNetX (join@trinetx.com), but costs might be incurred, and a data-sharing agreement would be necessary.
Ethics statement
To ensure patient confidentiality, all datasets within TriNetX are fully de-identified in compliance with the Health Insurance Portability and Accountability Act (HIPAA) and the General Data Protection Regulation (GDPR) (20). The de-identification process involves the removal of personal identifiers such as names, addresses, and any direct patient identifiers, with data aggregated at the cohort level to prevent re-identification. Additionally, the use of TriNetX in this research was approved by the Institutional Review Board of Chung Shan Medical University Hospital (CSMUH), under the approval number CS1-24047. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
WY: Conceptualization, Investigation, Writing – original draft, Data curation. JH: Formal analysis, Writing – review & editing, Data curation, Methodology, Conceptualization, Visualization, Investigation, Software. SL: Conceptualization, Writing – review & editing, Methodology, Investigation, Data curation, Validation. CH: Supervision, Methodology, Conceptualization, Investigation, Funding acquisition, Resources, Project administration, Writing – review & editing. YY: Conceptualization, Writing – review & editing, Investigation, Validation, Methodology, Formal analysis, Supervision. EK: Investigation, Conceptualization, Resources, Writing – review & editing, Funding acquisition, Software, Writing – original draft, Data curation, Supervision, Project administration, Visualization, Formal analysis, Validation, Methodology.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by grants from the Chung Shan Medical University Hospital (CSH-2022-A-004).
Acknowledgments
Special thanks to all study team for their dedication and support in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2025.1626103/full#supplementary-material
References
1. Koliaki C, Dalamaga M, and Liatis S. Update on the obesity epidemic: after the sudden rise, is the upward trajectory beginning to flatten? Curr Obes Rep. (2023) 12:514–27. doi: 10.1007/s13679-023-00527-y
2. Flegal KM, Kit BK, Orpana H, and Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: A systematic review and meta-analysis. JAMA. (2013) 309:71. doi: 10.1001/jama.2012.113905
3. Apovian CM. Obesity: definition, comorbidities, causes, and burden. Am J Manag Care. (2016) 22:s176–185.
4. Luppino FS, De Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BW, et al. Overweight, obesity, and depression: A systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. (2010) 67:220. doi: 10.1001/archgenpsychiatry.2010.2
5. Graham C and Frisco M. The relationship between obesity and suicide ideation among young adults in the United States. SSM - Popul Health. (2022) 18:101106. doi: 10.1016/j.ssmph.2022.101106
6. Klinitzke G, Steinig J, Blüher M, Kersting A, and Wagner B. Obesity and suicide risk in adults—A systematic review. J Affect Disord. (2013) 145:277–84. doi: 10.1016/j.jad.2012.07.010
7. Jastreboff AM, Aronne LJ, Ahmad NN, Wharton S, Connery L, Alves B, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. (2022) 387:205–16. doi: 10.1056/NEJMoa2206038
8. Zhao L, Cheng Z, Lu Y, Liu M, Chen H, Zhang M, et al. Tirzepatide for weight reduction in Chinese adults with obesity: the SURMOUNT-CN randomized clinical trial. JAMA. (2024) 332:551–60. doi: 10.1001/jama.2024.9217
9. Pan X, Tan B, Chin YH, Lee ECZ, Kong G, Chong B, et al. Efficacy and safety of tirzepatide, GLP -1 receptor agonists, and other weight loss drugs in overweight and obesity: a network meta-analysis. Obesity. (2024) 32:840–56. doi: 10.1002/oby.24002
10. European Medicines Agency. EMA statement on ongoing review of GLP-1 receptor agonists(2023). Available online at: www.ema.europa.eu/en/news/ema-statement-ongoing-review-glp-1receptor-agonists (Accessed September 1, 2025).
11. Tobaiqy M and Elkout H. Psychiatric adverse events associated with semaglutide, liraglutide and tirzepatide: a pharmacovigilance analysis of individual case safety reports submitted to the EudraVigilance database. Int J Clin Pharm. (2024) 46:488–95. doi: 10.1007/s11096-023-01694-7
12. Wang W, Volkow ND, Berger NA, Davis PB, Kaelber DC, and Xu R. Association of semaglutide with risk of suicidal ideation in a real-world cohort. Nat Med. (2024) 30:168–76. doi: 10.1038/s41591-023-02672-2
13. Ueda P, Söderling J, Wintzell V, Svanström H, Pazzagli L, Eliasson B, et al. GLP-1 receptor agonist use and risk of suicide death. JAMA Intern Med. (2024) 184:e244369. doi: 10.1001/jamainternmed.2024.4369
14. Tang H, Lu Y, Donahoo WT, Shao H, Shi L, Fonseca VA, et al. Glucagon-like peptide-1 receptor agonists and risk for suicidal ideation and behaviors in U.S. Older adults with type 2 diabetes: A target trial emulation study. Ann Intern Med. (2024) 177:1004–15. doi: 10.7326/M24-0329
15. Hurtado I, Robles C, Peiró S, García-Sempere A, and Sanfélix-Gimeno G. Association of glucagon-like peptide-1 receptor agonists with suicidal ideation and self-injury in individuals with diabetes and obesity: a propensity-weighted, population-based cohort study. Diabetologia. (2024) 67:2471–80. doi: 10.1007/s00125-024-06243-z
16. Hung A, Maciejewski ML, Berkowitz TSZ, Arterburn DE, Mitchell JE, Bradley KA, et al. Bariatric surgery and suicide risk in patients with obesity. Ann Surg. (2023) 278:e760–5. doi: 10.1097/SLA.0000000000005825
17. Cabanas-Sánchez V, Yu T, Rodríguez-Artalejo F, and Martínez-Gómez D. Weight loss as a risk factor for suicide. A prospective cohort study in more than 200,000 adults. Obes Res Clin Pract. (2023) 17:269–70. doi: 10.1016/j.orcp.2023.04.002
18. Taquet M, Dercon Q, Todd JA, and Harrison PJ. The recombinant shingles vaccine is associated with lower risk of dementia. Nat Med. (2024) 30:2777–81. doi: 10.1038/s41591-024-03201-5
19. Huang LA, Lo SC, Yang YS, Huang CN, Wang CC, Wang YH, et al. Association of COVID-19 infection with subsequent thyroid dysfunction: an international population-based propensity score matched analysis. Thyroid Off J Am Thyroid Assoc. (2024) 34:442–9. doi: 10.1089/thy.2023.0626
20. TrinetX. Trinetx-publication-guidelines. Available online at: https://trinetx.com/real-world-resources/case-studies-publications/trinetx-publication-guidelines/ (Accessed September 1, 2025).
21. Almeida OP, Fong Z, Hill Almeida LM, Sanfilippo FM, Page A, and Etherton-Beer C. Cross-sectional, case-control and longitudinal associations between exposure to glucagon-like peptide-1 receptor agonists and the dispensing of antidepressants. Diabetes Obes Metab. (2024) 26:2925–32. doi: 10.1111/dom.15616
22. Malhotra A, Grunstein RR, Fietze I, Weaver TE, Redline S, Azarbarzin A, et al. Tirzepatide for the treatment of obstructive sleep apnea and obesity. N Engl J Med. (2024) 391:1193–205. doi: 10.1056/NEJMoa2404881
23. Hölscher C. Central effects of GLP-1: new opportunities for treatments of neurodegenerative diseases. J Endocrinol. (2014) 221:T31–41. doi: 10.1530/JOE-13-0221
24. Seino Y, Fukushima M, and Yabe D. GIP and GLP-1, the two incretin hormones: Similarities and differences. J Diabetes Investig. (2010) 1:8–23. doi: 10.1111/j.2040-1124.2010.00022.x
25. Zheng Z, Zong Y, Ma Y, Tian Y, Pang Y, Zhang C, et al. Glucagon-like peptide-1 receptor: mechanisms and advances in therapy. Signal Transduct Target Ther. (2024) 9:234. doi: 10.1038/s41392-024-01931-z
26. Nyberg J, Jacobsson C, Anderson MF, and Eriksson PS. Immunohistochemical distribution of glucose-dependent insulinotropic polypeptide in the adult rat brain. J Neurosci Res. (2007) 85:2099–119. doi: 10.1002/jnr.21349
Keywords: tirzepatide, suicide, obesity, psychiatry, diabets mellitus
Citation: Yu W-S, Huang J-Y, Lo S-C, Huang C-N, Yang Y-S and Kornelius E (2025) Association of tirzepatide and the risk of suicide in a real-world cohort. Front. Psychiatry 16:1626103. doi: 10.3389/fpsyt.2025.1626103
Received: 13 May 2025; Accepted: 27 October 2025;
Published: 17 November 2025.
Edited by:
Luciane Cruz Lopes, University of Sorocaba, BrazilReviewed by:
Raffaele Carraro, La Princesa University Hospital, SpainKyle Valentino, University of Toronto, Canada
Copyright © 2025 Yu, Huang, Lo, Huang, Yang and Kornelius. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Edy Kornelius, a29ybjNsaXVzODJAZ21haWwuY29t
 Wei-Shin Yu1
Wei-Shin Yu1 Jing-Yang Huang
Jing-Yang Huang Chien-Ning Huang
Chien-Ning Huang Yi-Sun Yang
Yi-Sun Yang Edy Kornelius
Edy Kornelius