- 1Department of Psychology, Jiangsu University Medical School, Zhenjiang, Jiangsu, China
- 2Department of Psychology, Sichuan Normal University, Chengdu, Sichuan, China
- 3Department of Neurology, Nanjing University of Chinese Medicine Affiliated Hospital of Lianyungang (Lianyungang Hospital of Traditional Medicine), Lianyungang, China
- 4Department of Neurosurgery, Baylor Scott and White Health Center, Temple, TX, United States
- 5Department of Surgery, Texas A and M University, Temple, TX, United States
Major depressive disorder (MDD) is a kind of mental disorder with high mortality, suicide and relapse rates, and might be the world's leading cause of health burden by 2030. Growing evidence suggests that neuroinflammation is closely linked to depressive pathogenesis and suggests that MDD can be called a microglia disease. And activation of the P2X7R/NLRP3 signaling pathway in microglia is a key mechanism causing nerve damage. In addition, it is recently found that gut microbiota might initiate neuroinflammatory processes underlying MDD, and gut microbiota dysbiosis can be affected by sleep to ameliorate neuroinflammatory processes. In this paper, we reviewed recent advances about gut-brain axis interactions with neuroinflammation, which might shed light on the mechanisms and treatment of depression.
1 Introduction
Major Depressive Disorders (MDD) is manifested primarily as enduring depressed mood, psychomotion retardation, cognitive impairment, with some somatic symptoms such as sleep disturbances, fatigue, and appetite changes (e.g., weight loss in most cases, though increased appetite and weight gain may occur in some patients), as well as loss of libido. Global statistics have indicated approximately half of annual 800,000 suicide deaths are due to individuals diagnosed with MDD. Notably, depressed individuals exhibit a nearly 20-fold higher risk of suicide mortality compared with the general population (1). The World Health Organization (WHO) predicts that MDD will become the leading cause of global disease burden by 2030 (2). Even though the incidence rate of MDD is high, the precise etiology and pathogenesis of MDD remain elusive.
The monoamine hypothesis has dominated MDD research, and the first-line treatment for MDD are still conventional antidepressants which primarily target monoamine neurotransmitter modulation (3–5). However, these conventional medications demonstrate limited efficacy, with only 30-50% of the cases having been fully recovered, but frequently causing adverse effects such as sexual dysfunction and weight gain (6). It is important to elucidate novel pathogenic mechanisms and identify alternative treatment targets. Recent studies has been increasingly focused on the immune-inflammatory hypothesis, possibly due to the high comorbidity rates between MDD and chronic inflammatory conditions such as rheumatoid arthritis, cardiovascular disease, metabolic syndrome, and inflammatory bowel disease (7), which points to potential pathophysiological mechanisms.
In addition, emerging evidence highlights the critical role of the gut-brain axis (GBA) in neuropsychiatric disorders, including MDD, anxiety, and Alzheimer’s disease. Promisingly, the probiotic Bifidobacterium adolescentis has demonstrated therapeutic effects by reducing hippocampal pro-inflammatory cytokine levels (e.g., IL-1β, TNF-α) and ameliorating depression-like behaviors in chronically stressed mouse models (8), suggesting microbiota modulation might be a possible treatment strategy. Gut microbiota dysbiosis has been shown to induce neuroinflammation in the ventral hippocampus, a key brain region implicated in mood and cognition functions of brain (9). However, most of the tradition studies only discussed the effects of gut microbiota on MDD or neuroinflammation on MDD. Some recent studies found that gut microbiota might affect MDD via modulating neuroinflammation (10, 11), and based on this viewpoint, this paper aims to critically examine recent evidence on the effects of gut microbiota that influence the neuroinflammation and thus MDD.
2 Gut-brain axis
The brain regulates gastrointestinal sensory, motor, and secretory functions via neuroendocrine pathways including the hypothalamic-pituitary-adrenal (HPA) axis and autonomic nervous system. The gut in turn influences the central nervous system (CNS) through different pathways such as gut microbiota-derived metabolites, neurotransmitters, hormones, etc., which influence the enteric nervous system (ENS) and the immune system. This complex bidirectional pathway can be called the brain-gut axis (GBA). The gut microbiota (GM), comprising over 1014 microorganisms across four dominant phyla (Bacteroidetes, Firmicutes, Actinobacteria, Proteobacteria), plays a vital role in GBA dynamics. While maintaining relative stability throughout adulthood, microbial composition demonstrates plasticity in response to physiological dysfunctions and psychological stress. The changes of gut microbiota not only leads to increased local inflammatory responses within the gut but also induces neuro- inflammation in the brain, especially in the stress-related brain regions (12).
Many substances are involved in the gut-brain axis, such as carbohydrates, proteins, and peptides that are not absorbed by the host. These substances can be metabolized by intestinal microorganisms to produce a variety of biologically active substances, including amino acids, short-chain fatty acids (SCFAs), organic acids, phenolic compounds, phenylalanine derivatives, and indoles (12). These microbial metabolites might serve two functions: as major energy substrates for the colonic epithelium and as key signaling molecules for gut-brain communication. During stressful situations, such as gut microbiota dysbiosis, the intestinal barrier integrity can be impaired via structural and functional alterations. This barrier dysfunction facilitates pathogens and pro-inflammatory molecules into systemic circulation to initiate oxidative stress cascades and neuroinflammation in the brain. By integrating neuroimmune and endocrine pathways, they link gut microbial ecology to brain inflammatory states and mood regulation dynamics.
2.1 Gut-brain axis and sleep
Emerging research showed that sleep disorder is not only an important process that accompany MDD, but also showed significant cross-talk with the gut microbiota. Many recent studies have proved that microbiota can affect sleep through some specific pathways, and gut microbiota might be the reason for sleep changes at ageing, thus both the gut microbiota and sleep changes are suggested to be significant signatures that are associated with health and disease in the last decades of life (13). A number of studies have shown that the composition of the gut microbiota undergoes significant changes with age (14, 15); and it was shown that general age-related changes in the intestinal microflora include the reduced number of species and quantitative composition of Bifidobacteria in the elderly people is the decrease in (16). One of the possible explanations for their adhesion to the intestinal wall due to changes in the chemical composition and structure of the colon mucous membrane. The changes in the bacteria, in turn, can affect the permeability of the intestinal wall, and also the release of some neurotransmitters, such as aminobutyric acid (γ-Aminobutyric acid, GABA) (17, 18) and serotonin, which are important neurotransmitters that is involved in sleep neurotransmitter and metabolic processes in the brain. Very interestingly, it is found that the bacteria Bifidobacterium and Lactobacillus are actively involved in the production of aminobutyric acid (γ-Aminobutyric acid, GABA) (17, 18).
In addition, it is of our interest that chronic sleep disruption impacts gut microbiota. A study in animals has revealed that chronic sleep fragmentation alters taxonomic profiles of fecal microbiota and induces systemic and adipose tissue inflammation and insulin resistance (19). In another research, better sleep quality in healthy older adults was associated with better neuropsychological test performance and higher abundance of microbial phyla Verrucomicrobia and Lentisphaerae in the stool samples (20). In addition, many studies have confirmed the causal relationship between gut microbiota and sleep disorders, and some studies really found that changes in gut microbiota composition might be the reasons for age-related changes in sleep. Furthermore many sleep disorders are due to instable gut microbiota, and changes with dietary composition can achieve sound sleep via maintaining microbiota homeostasis (21). Thus microbiota-targeted interventions might be a good way to improve sleep, and sleep in turn can affect microbiota composition to improve emotional disorders, particularly for MDD patients experiencing high stress or with sleep complaints (22).
2.2 Gut-brain axis and neuroinflammation
Neuroinflammation is an inflammatory response caused by dysregulation of the synthesis and release of various pro-inflammatory and anti-inflammatory cytokines in the brain (23). The inflammatory response may be caused by infection, autoimmune disease, trauma, or other factors. Recent studies found that dysbiosis of intestinal flora is an important factor for neuroinflammation by changing the inflammatory factors. For example, it is found that disruption of the gut microbiota reduces short chain fatty acids (SCFAs) levels, hindering their anti-inflammatory effects. Dysbiosis of intestinal flora can damage the intestinal barrier, allowing pathogen-associated molecular patterns (PAMPs) such as bacterial lipopolysaccharides (LPS) to activate innate immune cells (e.g., macrophages, dendritic cells) and Toll-like receptors (e.g., TLR4) on intestinal epithelial cells, triggering the NF-κb pathway and releasing pro-inflammatory factors and chemokines. This further leads to the transmission of immune stimulation signals from the periphery to the central nervous system, resulting in an increase in Th17 cells (pro-inflammatory) and a decrease in Treg cells (anti-inflammatory). Peripheral monocytes can migrate into the brain, and differentiate into M1-type macrophages to release high levels of IL-1β, IL-6, TNF-α, etc., which in turn disrupt the integrity of the blood-brain barrier (BBB) and induce neuroinflammation (24).
Clinical studies have discovered the presence of various inflammatory mediators such as IL-1β, IL-6, tumor necrosis factor α (TNF-α), Toll-like receptor 3 (TLR3), and Toll-like receptor 4 (TLR4) in brains of patients with MDD patients who committed suicide, and the levels of these inflammatory factors were significantly higher than those of normal individuals (25). And it is suggested that activation of microglia and astrocytes is a characteristic change in neuroinflammation, and their activation increases the expression of pro-inflammatory cytokines and/or the production of reactive oxygen species (ROS), which results in neuronal damage, characterized by diminished neurogenesis, reduced dendritic spine density, and impaired synaptic plasticity (26–28). These alterations might affect mood and cognitive function, thereby increasing the risk of MDD (26).
2.3 The GBA and neuroinfllamation in MDD
In recent years, with the rapid advancement of molecular biology, the gut microbiota has become a prominent topic in scientific research. Gut microbiota are composed of several species of microorganisms, including bacteria, yeast, and viruses. The dominant gut microbial phyla are Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, Fusobacteria, and Verrucomicrobia, with the two phyla Firmicutes and Bacteroidetes representing 90% of gut microbiota. The Firmicutes phylum is composed of more than 200 different genera such as Lactobacillus, Bacillus, Clostridium, Enterococcus, and Ruminicoccus. Clostridium genera represent 95% of the Firmicutes phyla. Bacteroidetes consists of predominant genera such as Bacteroides and Prevotella. The Actinobacteria phylum is proportionally less abundant and mainly represented by the Bifidobacterium genus (29). Gut microbiota composition is highly variable and the variation itself is considered as physiological in the context of healthy gut microbiota, according to age, ethnicity, lifestyle, and dietary habits. However, these physiological gut microbiota variations have huge implications in intestinal and extra-intestinal disorders. Indeed, dysbiosis is often defined as an alteration of gut microbiota composition and a cause or a consequence of disorders. It is often difficult to ascertain whether the change is beneficial or detrimental (30), even though Lactobacillus reuteri, Blautia producta, Lactobacillus and Bifidobacterium strains shows beneficial effects.
Dysbiosis of the gut microbiota has been increasingly linked to a variety of chronic diseases, including metabolic, immune, and neurological disorders (30). Animal studies found that rodents exposed to the chronic unpredictable mild stimulus (CUMS) do undergo significant changes in gut microbiota structure, manifested as significant increase in the relative abundance of Odoribacter, Rikenella, Streptococcus, Anaerotruncus, Clostridium, and Helicobacter (31). Parallel findings in chronic social defeat stress (CSDS) models reveal simultaneous emergence of depressive-like behaviors and gut dysbiosis (32). The causal role of gut microbiota in MDD pathophysiology is further substantiated by fecal microbiota transplantation (FMT) studies. Transplantation of MDD patient-derived microbiota into germ-free mice recapitulates core behavioral deficits, including anhedonia, locomotor hypoactivity, and weight loss, with behavioral severity correlating strongly with systemic IL-6 levels (33). Paradoxically, transplantation of healthy individuals microbiota into germ-free rodents induces transient depressive-like states, suggesting bidirectional microbiota-brain communication may involve neuroimmune priming mechanisms.
In addition, antibiotics can clear the gut of bacteria, thereby reducing bacterial translocation, decreasing the liver burden, and alleviating neuroinflammation, thus providing therapeutic effects. For example, rifaximin, an unabsorbable, broad-spectrum, gastrointestinal-specific antibiotic, lowers serum ammonia and endotoxin levels, potentially alleviating symptoms (34). Antibiotic administration attenuated behavioral impairments, mitigated gut dysbiosis, reduced intestinal inflammation, and partially rescued MDD. The results highlight the antibiotic treatment as a potential therapy for opioid withdrawal sequelae (35). In addition to combating dysbiosis, certain antibiotics, such as minocycline and doxycycline, have been shown to inhibit matrix metalloproteinase activity and prevent mitochondrial dysfunction, microglial activation, offering therapeutic benefits (36). Another study has demonstrated that Prebiotics specifically Fructooligosaccharides (FOS) and Galactooligosaccharides (GOS) can affect neuroinflammation, depression, and anxiety-like behavior in a mouse model fed a high-fat diet (HFD) (37). Despite these beneficial effects, the use of antibiotics remains controversial, with evidence indicating that long-term use can eliminate beneficial bacteria, leading to dysbiosis and disease exacerbation.
Compared with healthy populations, patients with MDD exhibit significant alterations in gut microbiota diversity and taxonomic composition. Metagenomic analyses found that MDD patients demonstrate elevated relative abundances of Eggerthella(Actinobacteria), Subdoligranulum, and Coprococcus (Lachnospiraceae), alongside marked reductions in SCFA-producing bacteria such as Ruminococcaceae. This dysbiosis is particularly pronounced in the inflammatory MDD subtype, with an increase in Bacteroidetes phylum abundance and a decrease in Clostridiales order abundance. Notably, the Sellimonas genus shows dose-dependent positive correlation with MDD severity, exhibiting pro-inflammatory effects potentially mediated via the TLR4/NF-κB pathway (38). Conversely, change in the bacteria can shape local and systemic immune responses, and it is found that microbial metabolites, including short-chain and branched-chain fatty acids, bile acids, tryptophan derivatives, and others, influence local and systematic immune cells (39, 40), including resident activated T lymphocyte, including IL-10 producing, T-bet expressing CD4+ Tr1 T cells (41).
GBA-targeted interventions has demonstrated potential antidepressant therapy effects, and it is found that matrine can reverse CUMS-induced Firmicutes/Bacteroidetes ratio imbalance in mice, concurrently upregulating hippocampal BDNF expression to ameliorate depressive behaviors (42). Similar study in Wistar-Kyoto depressive model rats, found that 1-month Lactobacillus helveticus NS8 intervention increased fecal bacterial abundance to 8.7-fold and reversed anhedonia (43). In contrast, chronic stress induced MDD in mice, and led to significant reduction in indole-3-lactic acid (ILA) in both the gut and brain, and supplementation with the psychobiotic Bifidobacterium breve CCFM1025 (Bre1025) restored ILA concentrations to normal levels (44). In addition, traditional Chinese formula Jiannao Wan alleviates chronic restraint stress (CRS)-induced anxiety-like behaviors through Akkermansia muciniphila enrichment and modulation of tryptophan-serotonin metabolism (45). Furthermore, gut-selective antibiotic rifaximin prevents CUMS-induced firmicutes reduction and suppresses hippocampal IL-1β overexpression, with the therapeutic efficacy correlating with restored microbiota-derived SCFA levels (27).
Recent studies have found that gut microbiota-derived metabolites play a pivotal regulatory role in the pathogenesis of MDD (Figure 1) (12). SCFAs significantly improve brain function through multiple pathways, including modulating blood brain barrier (BBB) permeability, suppressing neuroinflammatory responses, and promoting hippocampal neurogenesis. Notably, butyrate, acting as a histone deacetylase (HDAC) inhibitor, exerts neuroprotective effects via epigenetic regulatory mechanisms. Importantly, alterations in gut microbiota composition can simultaneously influence both SCFAs biosynthesis and neurotransmitter production (e.g.5-HT), and this dual regulatory mechanism holds significant implications in depressive pathology. Wu et al. (46) demonstrated that depressive mice exhibited significantly reduced abundance of Akkermansia genus, which showed strong positive correlations with acetate and 5-HT levels (46). Emerging research has unveiled interactions between environmental factors and gut metabolites: Ethanol exposure can induce intestinal structural/functional abnormalities and trigger neuroinflammation, ultimately leading to depressive-like behaviors, while exogenous supplementation of SCFAs can effectively reverse these pathological alterations (47). Similarly adenosine supplementation increased serum SCFA levels in mice, thereby alleviating MDD-like behaviors in CSDS mice (48). Beyond protective metabolites, certain microbiota-derived metabolites may exhibit neurotoxicity, for example, lipopolysaccharide (LPS) not only inhibits hippocampal neural progenitor cell (NPCs), it can also induce neuronal apoptosis through activation of the TLR4 signaling pathway, which is a pro-inflammatory process particularly prominent in neurodegenerative diseases. Similarly, quinovic acid glycosides derived from gut microbiota have been proposed to be activators of neuroinflammatory pathways. These gut microbiota-derived compounds might work as activators for brain-resident immune cells (e.g., microglia) and the release of pro-inflammatory cytokines, which may induce cytotoxicity toward neurons (38, 49). Gut microbiota such as Bacteroidetes and Firmicutes phyla might participate in producing precursor molecules of trimethylamine N-oxide (TMAO), which has been closely linked to neurological disorders, including MDD (50). Collectively, these findings shed light on the effects of gut microbiota, which might play a pivotal role in the pathogenesis of MDD through direct or indirect mechanisms (Figure 1).
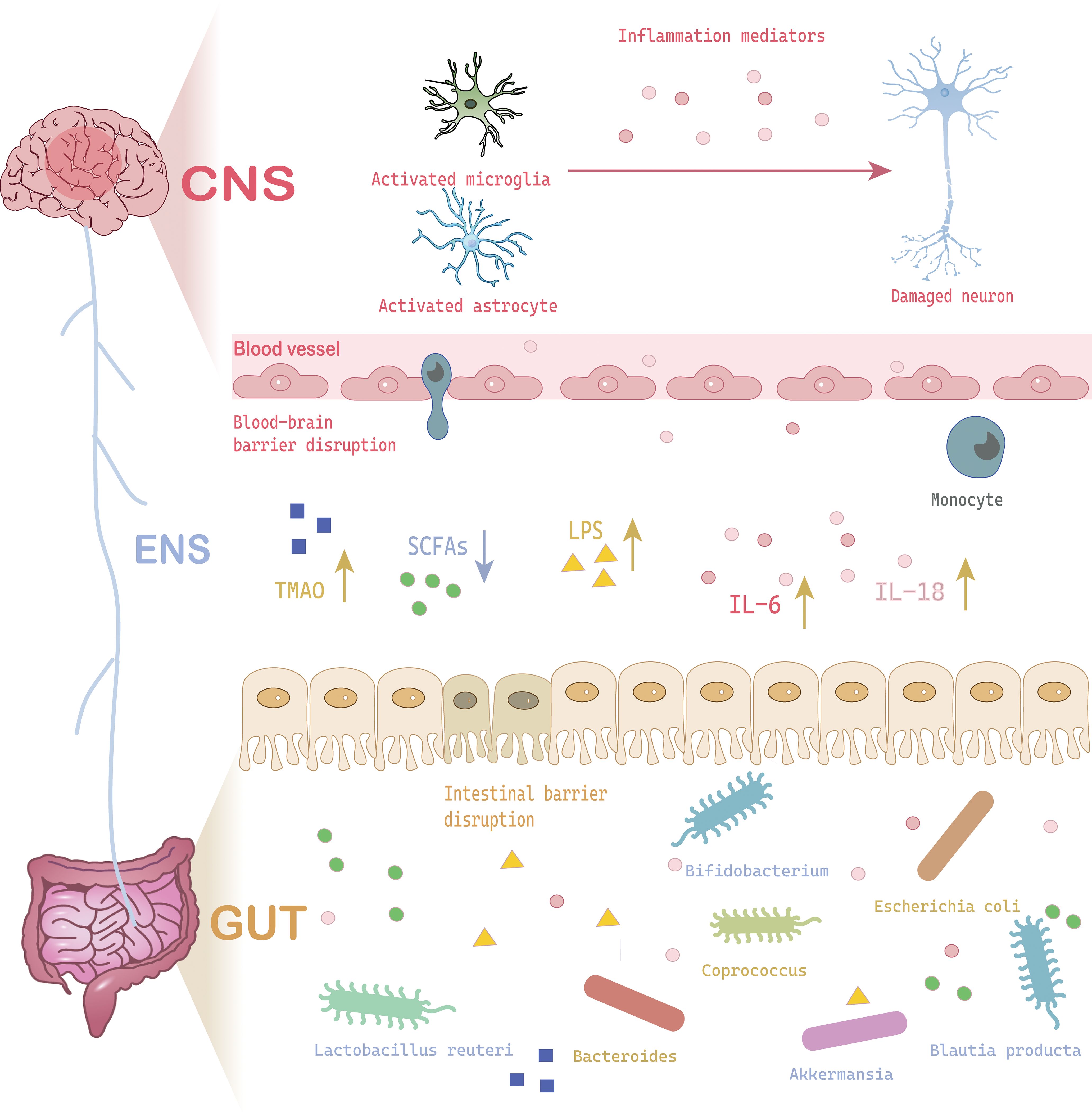
Figure 1. A schematic cartoon shows the interaction between gut microbiota and neuroinflammation to induce MDD. The brain-gut-microbiota axis interacts through neural signaling, immune signaling, and chemical signaling. When gut microbiota dysbiosis occurs due to stress or other factors, the released metabolites (SCFAs, LPS, TMAO) can cause damage to the CNS (increased BBB permeability, glial cell activation, neuronal damage). The stress response to CNS damage can further alter the gut microbiota, stimulating inflammatory responses, creating a vicious cycle that leads to MDD. CNS, Central Nervous System; IL-6, Interleukin 6; IL-18, Interleukin 18; LPS, Lipopolysaccharide; SCFA, Short-Chain Fatty Acids; TMAO, Trimethylamine N-Oxide.
3 Core pathways of gut microbiota in regulating neuroinflammation
3.1 Gut microbiota-glia axis in neuroinflammation
The results of a growing number of studies (51, 52) now support the presence of the characteristic alterations of neuroinflammation in MDD, which are mainly manifested as microglia activation and/or accompanied by astrocyte activation, etc (Figure 1). The gut microbiota and glial cells play an important interplay in driving neuroinflammation that leads to MDD. Microglia (MG) are resident immune cells of the CNS, working as resting and activated states. Microglia activation is commonly seen in response to injury, ischemia, and other stimuli. Activated microglia showed enhanced phagocytosis and can secrete various inflammatory factors, complement system, ROS, and other mediators (53). Based on their phenotypic expression, activated microglia can be categorized into two distinct polarization states, M1 and M2, which can be further identified by recognizing markers and morphology under different conditions. The morphological changes of microglia are diverse, roughly including six types: Ramified type, Amoeboid type, Bulbous endings of microglial processes type, Ball-and-chain structures type, Hyper-ramified type, Jellyfish type, and Rod type (54). Commonly used microglial markers for the M1 phenotype include MHC-II, CD16, CD32, CD80, CD86, CD40, etc. And the surface markers for the M2 phenotype are Ym1, CD206, CD68 and Arg1. The abnormal expression of translocator protein (TSPO) is considered a marker of microglia activation, anyway (55). PET imaging studies have found that compared with non-depressed patients, depressed patients have an increased density of TSPO ligands in the PFC, ACC, and hippocampal structures, showing increased uptake of TSPO ligands.
Pathogen-associated molecular patterns (PAMPS) or damage-associated molecular patterns (DAMPS) can stimulate resting-state microglia through Toll-like receptors (TLRs) or ATP receptors, respectively, and convert them to the M1 type under the action of IFN-γ (56). M1 microglia stimulation can lead to inflammatory cascades and neuronal degeneration by secretion of pro-inflammatory cytokines such as TNF-α, IL, IFN, NO, ROS, etc. Anti-inflammatory transmitters, such as IL-4 and IL-3, can induce the transformation of microglia to the M2 phenotype (57). M2 glial cells, on the other hand, inhibit inflammation and provide neuroprotection through the secretion of transforming growth factor-β (TGF-β), IL-10, and neurotrophic factors. M1 and M2 regulate the development and regression of neuroinflammation in MDD. For instance, exercise was shown to improve MDD-like behavior in CUMS mice by restoring this balance via the Lipocalin/AdipoR1 pathway (33). Since the development of MDD is closely related to microglia, it has been suggested that MDD is a “microglia disease” (26). Consistently, this idea has been supported by many studies, including one study by Steiner et al. (58), which showed that suicidal depressive patients had increased microglia density in the dorsolateral prefrontal cortex (dlPFC), anterior cingulate cortex (ACC), and the mid-thalamus compared with a healthy population. In a non-inflammatory rodent model, researchers exposed mice to chronic stress conditions, and found that the mice exhibited depressive-like behaviors with significant activation of microglia in their hippocampal structures (59). These studies have proved that microglia activation is an important process that leads to neuroinflammation and induces MDD.
Interestingly, it is recently found that gut microbiota is a critical regulator of microglial function and stress susceptibility. Some studies reported that germ-free mice exhibited reduced expression of genes associated with inflammation and immune surveillance in microglia, alongside higher cell density and distinct morphological features—including a more segmented morphology, longer processes, and increased branching complexity (60). Conversely, fecal microbiota transplantation from mice subjected to CUMS induced microglial priming in the hippocampal dentate gyrus (DG), characterized by hyper-ramified morphology. It is suggested that gut microbiota modulates microglial development and function through three primary mechanisms (28): (a) Microbial metabolites directly influence microglial maturation and activation states; (b) Immune-mediated regulation: The microbiota indirectly modulates microglial morphology, abundance, and function via cytokines produced by peripheral immune cells; (c) Neural signaling: Vagal nerve stimulation by the microbiota suppresses pro-inflammatory cytokine production (e.g., IL-1β, IL-6) in microglia (28). Thus targeted modulation of gut microbiota or inhibition of microglial activation represents a promising therapeutic strategy. For instance, Lactobacillus reuteri has been shown to ameliorate anxiety- and MDD-like behaviors in mice by reducing microglial hyperactivation (61, 62). Beyond Lactobacillus reuteri, the administration of beneficial bacterial strains such as Blautia producta, some of Lactobacillus and Bifidobacterium strains, restores homeostatic microglial function and attenuates neuroinflammatory responses (63). However, inconsistencies exist regarding how specific microbial metabolites directly mediate neuroprotection, highlighting the need for standardized methodologies and longitudinal studies (64).
3.2 Astrocytes, microglia and neuroinflammation
Astrocytes are also involved in neuroinflammation in MDD via interaction synergistically with microglia, for example, upon microglial activation, astrocytes can amplify inflammatory signals and produce neurotoxic factors (65–67). An examination of the brains of deceased individuals who had experienced severe MDD showed a reduction in both the quantity and concentration of astrocytes in their brain tissue (68). In animal models of MDD, astrocyte activation and increased expression of IL-1β, TNF-α, and IFN-γ can be found in the brains of MDD animals (69). Mice exposed to chronic unpredictable mild stress (CUMS) were shown to have increased GFAP expression in brain regions associated with depressive behaviors, and this was accompanied by the destruction of gut microbiota (altered ratio of Lactobacillus to Clostridium (70). In addition, repetitive transcranial magnetic stimulation (rTMS) and fluoxetine treatment was effective in alleviating depression-like behaviors by weakening the activity of astrocytes (71). Similarly, sodium butyrate therapy significantly increases the abundance of beneficial bacteria, such as Christensenellaceae, Blautia, and Lactobacillus (72).
Astrocytes are the most sensitive cells in the brain, that can sense the environmental changes such as cytokines, metabolites (73), and astrocytes respond to local signals within the brain but are also modulated by the gut microbiota. As an environmental factor, the gut microbiota can directly or indirectly influences astrocyte development, maturation, and functionality via changing intestinal permeability to increase/reduce inflammatory spread, and thus astrocyte hyperactivation (74). This regulation by increasing/decreasing gut permeability, limiting the entry of toxic substances into the bloodstream, thereby changing inflammation spread and astrocyte overactivation, leading to central neuro-inflammatory effects, and thus MDD and anxiety-like behavior in mice (75).
Broadly, gut microbiota can regulate astrocyte through immune, vagal, neuroendocrine, and microbial metabolite-mediated pathways. Immune pathways play a pivotal role in preserving the homeostasis between the gut microbiota and astrocytes. Gut dysbiosis generates pro-inflammatory cytokines that cross the BBB, activating astrocytes and microgila to drive their transition to an inflammatory phenotype. Under steady-state conditions, TRAIL (TNF-related apoptosis-inducing ligand) expression in astrocytes is driven by interferon-γ (IFNγ) produced by meningeal natural killer (NK) cells. LAMP1+/TRAIL+ astrocytes restrict neuroinflammation by inducing T-cell apoptosis. In contrast, vagal signaling mediates microbiota-astrocyte crosstalk (76), by stimulation anti-inflammatory cytokines to suppress neuroinflammation, and results in therapeutic potential for treatment-resistant MDD (77, 78). Selective serotonin reuptake inhibitors (SSRIs) can enhance vagal nerve activity, thereby increasing sleep and astrocyte reactivity. Paroxetine, a selective serotonin reuptake inhibitor (SSRI), suppresses neuroinflammation through inhibition of the nuclear transcription factor-κβ (NF-κβ) signaling pathway. Importantly, subdiaphragmatic vagotomy has been demonstrated to abolish the antidepressant effects of SSRIs, indicating that vagus nerve-mediated communication between the gut microbiota and astrocytes is essential for their therapeutic efficacy. In addition, gut microbial dysbiosis increases BBB permeability, allowing metabolites such as SCFAs and LPS to traverse the BBB and activate astrocytes (56).
3.3 Microglia in neuroinflammation in MDD
There are two steps that activate inflammation in microglia: Toll-like receptors (TLRs) or ATP receptors. In response to chronic stress, PAMPs or DAMPs activate pattern-recognition, neuroinflammation in microglia can be activated by damage-associated molecular pattern (DAMP) or pathogen- associated molecular pattern (PAMP) to activate NFκB, and TLR-mediated activation of the NF-κβ pathway serves as the first signal and promotes the up-regulation of pro-IL-1β and pro-IL-18. As a second step, ATP activation of P2X7R encourages the binding of NLRP3 to apoptosis-associated speck-like protein containing a CARD (ASC) and activates caspase-1, which converts and releases IL-1β and IL-18 precursors into mature forms (Figure 2). The activation process of IL-1β and IL-18 precursors requires various exogenous and endogenous molecules (such as ATP, etc.) and other signaling events, such as K+ efflux, reactive oxygen species (ROS) generation, and mitochondrial dysfunction, which promote NLRP3 inflammasome assembly. Caspase-1 can self-activate and cleaves pro-IL-1β and pro-IL-18 into mature forms IL-1β and IL-18, which are released into the extracellular space to amplify the inflammatory response. Many current studies focus on the ATP-P2X7R/NLRP3 signaling pathway to study the process of microglial cell inflammatory in MDD.
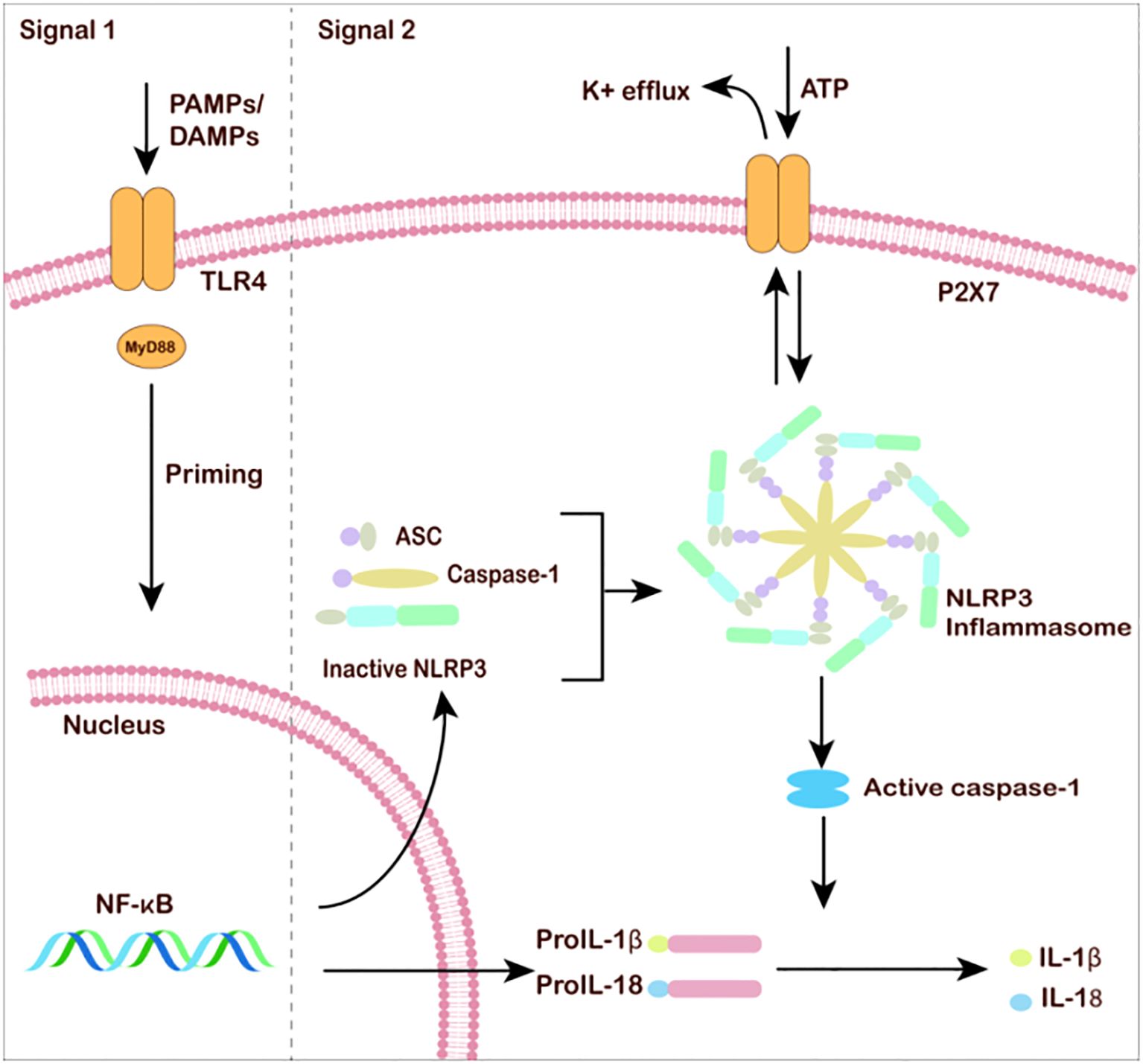
Figure 2. Microglia and neuroinflammation under MDD. The classic NLRP3 inflammasome activation requires two steps: the priming process involves pathogen-associated molecular patterns (PAMPs)/damage-associated molecular patterns (DAMPs) or cytokine-induced NF-κB activation. After NF-κB enters the cell nucleus, the transcription of inflammasome-related genes is unregulated. The activation process requires various exogenous and endogenous molecules (such as ATP, etc.). Other signaling events, such as K+ efflux, reactive oxygen species (ROS) generation, and mitochondrial dysfunction, promote NLRP3 inflammasome assembly. Caspase-1 self-activates and cleaves pro-IL-1β and pro-IL-18. Mature IL-1β and IL-18 are released into the extracellular space to amplify the inflammatory response.
3.4 Molecular mechanism of neuroinflammation in MDD
3.4.1 TLRs, NF-κB, and MDD
TLRs belong to a family of pattern recognition receptors, the first line of immune defense in the human body. Even though there are a diverse repertoire of TLRs (e.g., TLR2 for bacterial lipoproteins, TLR3 for dsRNA, TLR7/8 for ssRNA, TLR9 for CpG DNA), TLR4 is arguably the most critical member in the context of sensing specific danger signals associated with neuroinflammation macrophages and microglia express. TLR4 recognizes PAMPs and DAMPs, as well as heat shock proteins, high mobility group proteins, LPS, and microbial-associated pattern molecules in macrophages and microglia. It is believed that TLR4 receptors can signal through both MyD88 and non-MyD88 pathways to activate NF-κB, thereby promoting the production and release of pro-inflammatory cytokines and chemokines such as IL-1β and TNF-α.
There is an increasing body of evidence suggesting that TLR4 activation plays a vital role in neuroinflammation and is an independent risk factor for MDD severity. Some studies found that experimental animals given lipopolysaccharide (LPS), an agonist of the TLR4 receptor, can exhibit depressive-like behaviors such as decreased interest, pleasure, appetite, and increased despair time (79). Elevated levels of TLR4 expression can be found in the hippocampus in chronic stress animal model, and TLR4 signaling is responsible for sex differences in persistent depressive behaviors in mice (8). Many studies showed a positive correlation between depressive-like behavior and TLR4 levels, and similar studies found that the expression levels of TLR-4 and NF-κB in peripheral blood mononuclear cells of depressed patients were higher than those of non-depressed patients (73), and the previously higher TLR4 mRNA levels were decreased after antidepressant treatment (80).
3.4.2 ATP/P2X7R and MDD
Stress induced ATP release plays a pivot role in neuroinflammation in MDD patients, and P2X7 receptor (P2X7R), an ATP-gated non-selective cation channel, that is widely expressed in glial cells and neurons, is involved in the regulation of cell proliferation and apoptosis, sensory pathways, and immune response. P2X7R not only promotes microglia migration and phagocytosis but also regulates microglia secretion of pro-inflammatory factors and chemokines. Studies have shown that extracellular ATP can combine with P2X7R to promote the release of pro-inflammatory factors, such as IL-1β and IL-18, which enhance neuroinflammation (81). P2X7R is also expressed in astrocytes and is involved in a variety of their physiological functions, such as glutamate release and glutamate excitotoxicity inflammation (82).
Several studies have also confirmed the involvement of P2X7R in the development of neuroinflammation which triggers MDD, and it is found that the expression of P2X7 is positively correlated with MDD. For example, Ren et al. (83) have reported that injection of the P2X7R agonist, adenosine 5’-triphosphate, or its structural analog, dibenzoyl-ATP, aggravated depressive-like behavior in mice. In an acute restraint stress model, rats showed increased inflammation by increasing large amounts of ATP and activating P2X7R (84). Xie et al. (85) demonstrated that the P2X7R receptor antagonist, Kaumas Brilliant Blue G (BBG), down-regulated the expression of P2X7 and NLRP3, limiting pro-inflammatory factors and helping to alleviate the central inflammatory environment. P2X7R knockout mice no longer showed depressive or anxious behaviors after exposure to chronic psychological stress (86). Li (Li et al. (87) found that Na+/K+-ATPase α1 (NKAα1) can form a complex with the P2X7R receptor under pathological conditions to promote the activation of microglial cell inflammation, and applying a monoclonal antibody to stabilize the membrane NKAα1 to block the inflammation can alleviate MDD symptoms, suggesting a new therapy target.
3.4.3 NLRP3/Caspase-1 and MDD
The relationship between the NLRPs (NOD-, LRR, and pyrin domain containing) family and MDD has attracted much attention in recent studies, particularly the NLR family pyrin structural domain 3 (NLRP3). NLRP3 is activated enzymatically and binds to apoptosis-associated speck-like protein (ASC), which recruits pro-Caspase1 protein, to form NLRP3 inflammatory vesicles. NLRP3 inflammatory vesicles mediate the proteolytic cleavage of pro-Caspase1, activating caspase-1 and ultimately inducing the release of pro-inflammatory cytokines. The activity of NLRP3 inflammatory vesicles and its moderate regulation determine the morphology of microglia and the intensity of neuroinflammatory responses (Figure 2) (88).
It has been shown that patients diagnosed with MDD have significantly elevated mRNA levels of NLRP3 and caspase-1 in their blood (89). Knockout of either NLRP3 or caspase attenuates depressive-like behaviors in mice after chronic stress (90, 91). Under physiological conditions, IL-1β induced by NLRP3 inflammatory vesicles is essential for emotional responses and learning (92). However, high levels of IL-1β induce abnormal structural functioning of synapses, which can lead to MDD (93). IL-18 is considered a predictor of MDD risk, and Wu et al. (46) found that IL-18 injected into the amygdala increased depression-like behavior in mice. The inhibitor MCC950 reduced NLRP3, IL-1β, and IL-18 levels in the hippocampus and improved depression behavior (94). These studies suggest that NLRP3 inflammatory vesicles and downstream signaling pathways are essential in neuroinflammation and MDD.
3.5 Gut microbiota modulates the inflammation pathways
Gut microbiota collaborates with the molecular mechanisms described above to regulate neuroinflammation, forming an intricate gut-brain axis network. For example, LPS and butyrate are two important microbial metabolites in neuroinflammation. Butyrate produced by the Lachnospiraceae family inhibits histone deacetylase (HDAC), thereby blocking the nuclear translocation of the NF-κB p65 subunit. Meanwhile, LPS binds to the TLR4/MD2 complex, triggering MyD88/TRIF-dependent IKK phosphorylation, thereby releasing IκB’s inhibition of NF-κB and driving the transcription of TNF-α and IL-6. And improving LPS levels and pro-inflammatory cytokines (IL-1β, IL-6, TNF-α) and inhibiting the TLR4/MyD88 pathway with herbal formula Zuogui Jiangtang Jieyu (ZJJ) by downregulating Gram-negative bacteria abundance alleviated diabetes-associated MDD (95). Another example, chicoric acid (CA) attenuated neuroinflammation by suppressing the TLR4/MyD88 pathway and restoring gut microbiota balance (96). In addition, Histicola and Bifico have been shown to increase the abundance of gut microbiota (Lactobacillus, Desulfovibrio, Akkermansia), downregulate LTR4/NF-κB, and improve estrogen-induced MDD (97, 98).
P2X7R is expressed in intestinal cells and plays a role in both health and disease in the gastrointestinal system. Under pathological conditions, the intestinal barrier is disrupted, leading to decreased levels of ZO-1 and claudin-1, increased ATP release by the microbiota, and activation of P2X7R. When the concentration of extracellular ATP produced by the intestinal microbiota is high, the sIgA response induced by intestinal lymphoid tissue is suppressed, thereby facilitating the colonization of pathogenic bacteria (99). However, BBG(P2X7R antagonist) reversed the relative abundance of Bacteroidetes and Akkermansia in alcohol-fed mice. alcohol-fed mice (100).
The assembly and activation of NLRP3 inflammasomes are key steps in the process by which gut microbiota dysbiosis drives neuroinflammation, which in turn promotes the onset and progression of MDD. Caspase-1 inhibition via genetic knockout or pharmacological intervention reduces depression and anxiety-like behaviors in mice. Depressive behavior was improved in NLRP3-/- mice, and the types of gut bacteria (Firmicutes, Proteobacteria, Ruminococcus, Prevotella, Bacteroidetes) were also altered (101). Under steady-state conditions, SCFAs exert a dual regulatory effect on NLRP3, primarily exerting anti-inflammatory effects. Studies have shown that under TLR-activated conditions, SCFAs can shift from anti-inflammatory to pro-inflammatory effects by inhibiting HDAC activity, activating the NLRP3 inflammasome, and promoting the release of IL-1β (102). Fecal microbiota transplantation (FMT) from healthy mice to postpartum MDD (PPD) mice alleviated depression/anxiety-like behaviors, reduced NLRP3/caspase-1-mediated inflammation in the gut and hippocampus, increased SCFA levels. In contrast, enriching beneficial bacteria (e.g.,Lactobacillus) restored gut dysbiosis and reducing Akkermansia abundance (103), and exercise intervention increased Lactobacillus abundance while suppressing NLRP3/caspase-1 pathway activation (104).
4 The effects of neuroinflammation on the nervous system
4.1 Neuroinflammation affects synaptic plasticity
Synaptic plasticity underpins both structural and functional plasticity of the nervous system, with structural plasticity denoting alterations in the quantity, morphology, and architecture of the pre- and post-synaptic membranes and their interstices resulting from internal and external stimuli. Conversely, functional plasticity pertains to alterations in the intensity or efficacy of synaptic transmission, primarily long-term synaptic plasticity, encompassing long-term potentiation (LTP) and long-term MDD (LTD). Impaired synaptic plasticity can readily result in cognitive and emotional learning and adaptions, and finally emotional related problems, including MDD.
In the resting state, microglia govern synaptic pruning to enhance synaptic functional stabilization. When glial cells are activated due to neuroinflammation, too much secreted pro-inflammatory cytokines influence synaptic plasticity. Nguyen et al. (105) have shown that IL-33 signaling drives experience-dependent synaptic plasticity in the hippocampus with IL-33 receptor depletion from microglia, resulting in decreased dendritic spines and newborn neurons. In addition, the concentration of tumor necrosis factor α (TNF-α) varies in terms of its regulation of synaptic plasticity, with low concentrations of TNF-α promoting synaptic plasticity and high concentrations, particularly under inflammatory conditions, inducing dysregulation of synaptic transmission and plasticity. TNF-α production also encourages the release of glutamate from astrocytes, which enhances excitotoxicity in neurons (106). Thus, it is clear that inflammation is extensively involved in neuronal damage, which is closely related to MDD.
4.2 Neuroinflammation inhibits hippocampal neurogenesis
Chronic stress can lead to atrophy, apoptosis, and reduction in the number of hippocampal neurons; thus, neuronal regeneration is a crucial mechanism for recovery from MDD (2). Neurogenesis, the mechanism through which neural stem cells (NSCs) generate new neurons, enhances the brain’s structural plasticity. While some previous views suggested that this phenomenon is limited to childhood or early adolescence, some recent studies (107) reveal that neurogenesis continues throughout adulthood, for example, the hippocampus, situated in the medial temporal lobe, is a vital anatomical area within the limbic nervous system. It governs advanced neural functions, including mood and cognition, and is one of the brain regions recognized for neurogenesis. It is suggested that adult neurogenesis is limited to the subventricular zone (SVZ) of the lateral ventricles and the subgranular zone (SGZ) of the dentate gyrus (DG) of the hippocampus under physiological conditions. However the neurogenesis might involve all hippocampal regions under pathological condition, and this study has garnered significant focus in contemporary MDD research, and has evolved into a new field of study, namely adult hippocampal neurogenesis (AHN),.
Adult hippocampal neurogenesis (AHN) refers to the neuronal proliferation, differentiation, and maturation processes in the adult hippocampus. The AHN comprises a sequence of neurogenic cascade reactions wherein the DG undergoes asymmetric divisions to produce neural progenitor cells (NPCs), which subsequently differentiate into adult neuronal cells. The surviving adult neuronal cells evolve into immature neurons, ultimately maturing into DG granule neurons that integrate into the established hippocampal circuitry. The heightened expression of pro-inflammatory genes in mice subjected to Repeated social defeat (RSD) indicated a notable decrease in early neuronal markers DCX on day ten post-RSD. At the same time, impaired differentiation of NPC neurons proliferating during RSD was observed after 28 days, coinciding with the activation of neuroinflammation (108). Administration of anti-inflammatory medications, such as NSAIDs, cytokine inhibitors, statins, and minocycline, has been demonstrated to reduce the hippocampus neurogenesis (109–111). Neuroinflammation diminishes hippocampus neurogenesis, thereby leading to depression-like behaviors.
Indeed, it is found that the number of NCSs in the DG region of the hippocampus is reduced in MDD patients, and vascularization is decreased. In addition, neuroimaging studies have shown (112) that hippocampal volume is reduced in the brains of depressed patients compared with healthy persons. Studies in animals demonstrated that prolonged chronic stress stimulates the proliferation of hippocampal neurons. At the same time, antidepressant administration enhances the quantity of hippocampus NPCs and the expression of trophic factors, among other effects (113). Zhang et al. (114) reported that chronic stress diminishes the expression of Nuclear Receptor Binding Factor 2 (NRBF2), leading to the disruption of neurogenesis-related protein networks, a reduction of NCSs and that the overexpression of NRBF2 mitigates MDD-like behavior induced by CSDS.
4.3 Neurotrophic factors
Briain derived neurotropic factor (BDNF) is one of the most well-studied neurotrophic factors, a key player in psychiatric disorders, and one of the reliable biomarkers used to monitor MDD. BDNF can initiate multiple signaling pathways by activating distinct receptor types, primarily the TrkB and the p75 neurotrophic factor receptor (p75NTR) pathways, which can activate phosphatidylinositol 3-kinase (PI3K)-AKT and extracellular signal-regulated kinase (ERK) by binding to TrkB, etc (115), and chronic exposure to stress or inflammation leads to decreased BDNF levels (116), which can also be found in animal models of MDD.
Cytokines released during glial cell activation at neuroinflammation are posited to obstruct neurotrophic signaling in neurons, thereby impeding BDNF-induced activation of PI3K and Erk1/2, which subsequently hinder axon growth and neuronal marker expression, culminating in neuronal apoptosis (117). For example, the flavonoid leucovorin has been shown to reduce pro-inflammatory cytokine levels (118) and has recently been shown to enhance the TrkB/BDNF pathway, and stimulate FGF/FGFR1 signaling, which helps up-regulate the expression of BDNF, promote the differentiation of NCSs and neuronal growth, and also provide a new therapeutic strategy for MDD (119). Other neural growth factors (NGF) have also been demonstrated to influence neuroinflammation, with NGF blocking TLR4-mediated activation of the NF-κB and JNK pathways and attenuating pro-inflammatory responses in glial cells (120). NGF has been known to accelerate macrophage polarization of the M2 phenotype and to increase secretion of pro-regenerative factors (GAP-43, NF-200), thereby facilitating neuroinflammatory responses (121).
5 Conclusion and limitations
With the advances of research on MDD, there has been a better understanding of the specific mechanisms and signaling pathways involved in MDD. The brain-gut axis, which is a complex interactive system, plays a significant role in the pathophysiology of MDD. Dysbiosis of the gut microbiota can produce metabolites that compromise barrier functions and trigger the release of inflammatory factors. Additionally, these metabolites activate glial cells, leading to neuronal damage, synaptic dysfunction, and inhibition of hippocampal neurogenesis, ultimately contributing to neuroinflammation. Therefore, targeting the brain-gut axis to suppress glial cell activity and reduce their inflammatory activation may represent a potential therapeutic strategy for MDD. In addition, sleep disorders, which is related with both the etiology of MDD and also affects the gut microbiota, can modulate the inter-play among the gut microbiota and neuroinflammation.
However, many concerns about precise mechanisms of the brain-gut axis in MDD remain to be elucidated. Further elucidation of these mechanisms will lay the foundation for developing novel antidepressant drugs, breaking through the limitations of traditional monoamine-based treatments. For example, traditional monoamine hypothesis suggested that MDD is due to limitation of the three monoamines (dopamine, norepinephrine and 5-HT), and antidepressants that can increase in these three monoamines can alleviate MDD. The role of brain-gut axis in modulation of the monoamines are still need to be explained, indeed, 90–95% of serotonin in the whole body is produced in the gastrointestinal tract, thus the interplay between brain-gut axis with monoamines might shed more light on the mechanisms of MDD.
Author contributions
JL: Writing – original draft. BW: Data curation, Writing – review & editing. LZ: Software, Writing – review & editing. XQ: Writing – review & editing. FW: Writing – original draft. SG: Writing – review & editing. XM: Writing – review & editing. JH: Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The work was partly supported by National Nature Science Foundation in China (82171392 FW), a grant from Nanjing University of Chinese Medicine to XM (XZR 20200091), a grant from Jiangsu Province to JL, Postgraduate Research and Practice Innovation Program (SJCX24-2451), and Jiangsu University Student Research Project (23A856).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chesney E, Goodwin GM, and Fazel S. Risks of all-cause and suicide mortality in mental disorders: A meta-review. World Psychiatry. (2014) 13:153–60. doi: 10.1002/wps.20128
2. Han S, Zheng R, Li S, Liu L, Wang C, Jiang Y, et al. Progressive brain structural abnormality in MDD assessed with MR imaging by using causal network analysis. psychol Med. (2023) 53:2146–55. doi: 10.1017/S0033291721003986
3. Gu S, Wang F, Patel NP, Bourgeois JA, and Huang JH. A model for basic emotions using observations of behavior in drosophila. Front Psychol. (2019) 10:781. doi: 10.3389/fpsyg.2019.00781
4. Gu S, He Z, Xu Q, Dong J, Xiao T, Liang F, et al. The relationship between 5-hydroxytryptamine and its metabolite changes with post-stroke MDD. Front Psychiatry. (2022) 13:871754. doi: 10.3389/fpsyt.2022.871754
5. Jiang Y, Zou D, Li Y, Gu S, Dong J, Ma X, et al. Monoamine neurotransmitters control basic emotions and affect major depressive disorders. Pharm (Basel Switzerland). (2022) 15:1203. doi: 10.3390/ph15101203
6. Dean OM, Maes M, Ashton M, Berk L, Kanchanatawan B, Sughondhabirom A, et al. Protocol and rationale-the efficacy of minocycline as an adjunctive treatment for major depressive disorder: A double blind, randomised, placebo controlled trial. Clin Psychopharmacol Neurosci. (2014) 12:180–8. doi: 10.9758/cpn.2014.12.3.180
7. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet (London England). (2018) 392:1789–858. doi: 10.1016/S0140-6736(18)32279-7
8. Yang E-J, Frolinger T, Iqbal U, Estill M, Shen L, Trageser KJ, et al. The role of the Toll like receptor 4 signaling in sex-specific persistency of MDD-like behavior in response to chronic stress. Brain Behavior Immun. (2024) 115:169–78. doi: 10.1016/j.bbi.2023.10.006
9. Pearson-Leary J, Zhao C, Bittinger K, Eacret D, Luz S, Vigderman AS, et al. The gut microbiome regulates the increases in depressive-type behaviors and in inflammatory processes in the ventral hippocampus of stress vulnerable rats. Mol Psychiatry. (2020) 25:1068–79. doi: 10.1038/s41380-019-0380-x
10. Dabboussi N, Debs E, Bouji M, Rafei R, and Fares N. Balancing the mind: Toward a complete picture of the interplay between gut microbiota, inflammation and major depressive disorder. Brain Res Bull. (2024) 216:111056. doi: 10.1016/j.brainresbull.2024.111056
11. Godzien J, Kalaska B, Rudzki L, Barbas-Bernardos C, Swieton J, Lopez-Gonzalvez A, et al. Probiotic lactobacillus plantarum 299v supplementation in patients with major depression in a double-blind, randomized, placebo-controlled trial: A metabolomics study. J Affect Disord. (2025) 368:180–90. doi: 10.1016/j.jad.2024.09.058.?
12. Xu Q, Jiang M, Gu S, Zhang X, Feng G, Ma X, et al. Metabolomics changes in brain-gut axis after unpredictable chronic mild stress. Psychopharmacology. (2022) 239:729–43. doi: 10.1007/s00213-021-05958-w
13. Zhang Y, Zhou S, Han H, and Du X. Bridging the gap between gut microbiota and sleep disorders through intermediary metabolites. J Affect Disord. (2025) 374:350–5. doi: 10.1016/j.jad.2024.12.104
14. Salazar N, Valdes-Varela L, Gonzalez S, Gueimonde M, and de Los Reyes-Gavilan CG. Nutrition and the gut microbiome in the elderly. Gut Microbes. (2017) 8:82–97. doi: 10.1080/19490976.2016.1256525
15. Nagpal R, Mainali R, Ahmadi S, Wang S, Singh R, Kavanagh K, et al. Gut microbiome and aging: physiological and mechanistic insights. Nutr Healthy Aging. (2018) 4:267–85. doi: 10.3233/NHA-170030
16. Askarova S, Umbayev B, Masoud AR, Kaiyrlykyzy A, Safarova Y, Tsoy A, et al. The links between the gut microbiome, aging, modern lifestyle and alzheimer’s disease. Front Cell Infect Microbiol. (2020) 10:104. doi: 10.3389/fcimb.2020.00104
17. Junges VM, Closs VE, Nogueira GM, and Gottlieb MGV. Crosstalk between gut microbiota and the central nervous system: a focus for Alzheimer’s disease. Curr Alzheimer Res. (2018) 15:1179–90. doi: 10.2174/1567205015666180904155908
18. Strandwitz P and Strandwitz P. Neurotransmitter modulation by the gut microbiota. Brain Res. (2018) 1693(Pt B):128–33. doi: 10.1016/j.brainres.2018.03.015
19. Poroyko VA, Carreras A, Khalyfa A, Khalyfa AA, Leone V, Peris E, et al. Chronic sleep disruption alters gut microbiota, induces systemic and adipose tissue inflammation and insulin resistance in mice. Sci Rep. (2016) 6:35405. doi: 10.1038/srep35405
20. Anderson JR, Carroll I, Azcarate-Peril MA, Rochette AD, Heinberg LJ, Peat C, et al. A preliminary examination of gut microbiota, sleep, and cognitive flexibility in healthy older adults. Sleep Med. (2017) 38:104–7. doi: 10.1016/j.sleep.2017.07.018
21. Li Y, Pan F, Shen X, Li Y, Pan F, and Shen X. Association of the dietary index for gut microbiota with sleep disorder among US adults: the mediation effect of dietary inflammation index. Front Nutr. (2025) 12:1528677. doi: 10.3389/fnut.2025.1528677
22. Cavon J, Basso M, Kadosh KC, and Gibbons SM. The human gut microbiome and sleep across adulthood: associations and therapeutic potential. Lett Appl Microbiol. (2025) 78(4):ovaf043. doi: 10.1093/lambio/ovaf043
23. Won E, Na K-S, and Kim Y-K. Associations between melatonin, neuroinflammation, and brain alterations in MDD. Int J Mol Sci. (2022) 23. doi: 10.3390/ijms23010305
24. Cruz-Pereira JS, Rea K, Nolan YM, O’Leary OF, Dinan TG, Cryan JF, et al. Depression’s Unholy Trinity: dysregulated stress, immunity, and the Microbiome. Annu Rev Psychol. (2020) 71(1):49–78. doi: 10.1146/annurev-psych-122216-011613
25. Miller AH, Maletic V, and Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major MDD. Biol Psychiatry. (2009) 65:732–41. doi: 10.1016/j.biopsych.2008.11.029
26. Yirmiya R, Rimmerman N, and Reshef R. MDD as a microglial disease. Trends Neurosci. (2015) 38:637–58. doi: 10.1016/j.tins.2015.08.001
27. Li H, Xiang Y, Zhu Z, Wang W, Jiang Z, Zhao M, et al. Rifaximin-mediated gut microbiota regulation modulates the function of microglia and protects against CUMS-induced MDD-like behaviors in adolescent rat. J Neuroinflamm. (2021) 18:254. doi: 10.1186/s12974-021-02303-y
28. Lukens JR and Eyo UB. Microglia and neurodevelopmental disorders. Annu Rev Neurosci. (2022) 45:425–45. doi: 10.1146/annurev-neuro-110920-023056
29. Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, et al. Enterotypes of the human gut microbiome. Nature. (2011) 473:174–80. doi: 10.1038/nature09944
30. Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano GAD, Gasbarrini A, et al. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. (2019) 7:14. doi: 10.3390/microorganisms7010014
31. Zhang M, Li A, Yang Q, Li J, Zheng L, Wang G, et al. Matrine alleviates depressive-like behaviors via modulating microbiota–gut–brain axis in CUMS-induced mice. J Trans Med. (2023) 21:145. doi: 10.1186/s12967-023-03993-z
32. Wang J, Zhou T, Liu F, Huang Y, Xiao Z, Qian Y, et al. Influence of gut microbiota on resilience and its possible mechanisms. Int J Biol Sci. (2023) 19:2588–98. doi: 10.7150/ijbs.82362
33. Liu J, Lv X, Ye T, Zhao M, Chen Z, Zhang Y, et al. Microbiota-microglia crosstalk between blautia producta and neuroinflammation of parkinson’s disease: A bench-to-bedside translational approach. Brain Behavior Immun. (2024) 117:270–82. doi: 10.1016/J.BBI.2024.01.010
34. Torre A, Cordova-Gallardo J, and Frati Munari AC. Rifaximin alfa and liver diseases: more than a treatment for encephalopathy, a disease modifier. Ther Clin Risk Manag. (2023) 19:839–51. doi: 10.2147/TCRM.S425292
35. Yu DY, Gao JQ, Yang XX, Gao FF, Liu JL, Shen MQ, et al. Antibiotic treatment improves gut dysbiosis and depression-like behavior induced by morphine withdrawal. Neuropharmacology. (2025) 278:110579. doi: 10.1016/j.neuropharm.2025.110579
36. Yadav N, Thakur AK, Shekhar N, and Ayushi. Potential of antibiotics for the treatment and Management of Parkinson’s disease: an overview. Curr Drug Res Rev. (2021) 13:166–71. doi: 10.2174/2589977513666210315095133
37. Paiva IHR, Maciel LM, Silva RSD, Mendonça IP, Souza JRB, and Peixoto CA. Prebiotics modulate the microbiota-gut-brain axis and ameliorate anxiety and depression-like behavior in HFD-fed mice. Food Res Int. (2024) 182:114153. doi: 10.1016/j.foodres.2024.114153
38. Park KJ and Gao Y. Gut-brain axis and neurodegeneration: Mechanisms and therapeutic potentials. Front Neurosci. (2024) 18:1481390. doi: 10.3389/fnins.2024.1481390
39. Perl M, Fante MA, Herfeld K, Scherer JN, Poeck H, and Thiele Orberg E. Microbiota-derived metabolites: Key modulators of cancer immunotherapies. Med. (2025) 15:100773. doi: 10.1016/j.medj.2025.100773
40. Singh P and Mohanty B. Microbiota and neuropeptides in dysbiosis-driven inflammation: emerging therapeutic perspectives. Endocr Res. (2025) 23:1–18. doi: 10.1080/07435800.2025.2520252
41. Ansaldo E, Yong D, Carrillo N, McFadden T, Abid M, Corral D, et al. T-bet expressing Tr1 cells driven by dietary signals dominate the small intestinal immune landscape. bioRxiv. (2025) 4. doi: 10.1101/2025.06.30.662190
42. Zhang M, Li A, Yang Q, Li J, Zheng L, Wang G, et al. Matrine alleviates depressive-like behaviors via modulating microbiota-gut-brain axis in CUMS-induced mice. J Trans Med. (2023) 21:145. doi: 10.1186/s12967-023-03993-z
43. Alatan H, Liang S, Shimodaira Y, Wu X, Hu X, Wang T, et al. Supplementation with Lactobacillus helveticus NS8 alleviated behavioral, neural, endocrine, and microbiota abnormalities in an endogenous rat model of MDD. Front Immunol. (2024) 15:1407620. doi: 10.3389/fimmu.2024.1407620
44. Qian X, Li Q, Zhu H, Chen Y, Lin G, Zhang H, et al, Qian X, Li Q, Zhu H, Chen Y, Lin G, Zhang H, et al. Bifidobacteria with indole-3-lactic acid-producing capacity exhibit psychobiotic potential via reducing neuroinflammation. Cell Reports Med. (2024) 5(11):101798. doi: 10.1016/j.xcrm.2024.101798
45. Pan J, Jin J, Ge H, Yin K, Chen X, Han L, et al, Pan J, Jin J, Ge H, Yin K, Chen X, Han L, et al. Malibatol A regulates microglia M1/M2 polarization in experimental stroke in a PPARγ-dependent manner. J Neuroinflammation. (2015) 12(1):51. doi: 10.1186/s12974-015-0270-3
46. Wu M, Tian T, Mao Q, Zou T, Zhou C, Xie J, et al. Associations between disordered gut microbiota and changes of neurotransmitters and short-chain fatty acids in depressed mice. Trans Psychiatry. (2020) 10:1–10. doi: 10.1038/s41398-020-01038-3
47. Shen H, Zhang C, Zhang Q, Lv Q, Liu H, Yuan H, et al. Gut microbiota modulates depressive-like behaviors induced by chronic ethanol exposure through short-chain fatty acids. J Neuroinflamm. (2024) 21:290. doi: 10.1186/s12974-024-03282-6
48. Huang Y, You Y, Wang W, Chen Y-H, Zhang H, Li Q-P, et al. Adenosine regulates depressive behavior in mice with chronic social defeat stress through gut microbiota. Neuropharmacology. (2025) 262:110209. doi: 10.1016/j.neuropharm.2024.110209
49. Grasselli J, Elez E, Caratù G, Matito J, Santos C, Macarulla T, et al. Concordance of blood- and tumor-based detection of RAS mutations to guide anti-EGFR therapy in metastatic colorectal cancer. Ann Oncol. (2017) 28:1294–301. doi: 10.1093/annonc/mdx112
50. Praveenraj SS, Sonali S, Anand N, Tousif HA, Vichitra C, Kalyan M, et al. The role of a gut microbial-derived metabolite, trimethylamine N-oxide (TMAO), in neurological disorders. Mol Neurobiol. (2022) 59:6684–700. doi: 10.1007/s12035-022-02990-5
51. Cazareth J, Guyon A, Heurteaux C, Chabry J, and Petit-Paitel A. Molecular and cellular neuroinflammatory status of mouse brain after systemic lipopolysaccharide challenge: Importance of CCR2/CCL2 signaling. J Neuroinflamm. (2014) 11:132. doi: 10.1186/1742-2094-11-132
52. Graeber MB, Streit WJ, and Kreutzberg GW. The microglial cytoskeleton: Vimentin is localized within activated cells in situ. J Neurocytol. (1988) 17:573–80. doi: 10.1007/BF01189811
53. Troubat R, Barone P, Leman S, Desmidt T, Cressant A, Atanasova B, et al. Neuroinflammation and MDD: A review. Eur J Neurosci. (2021) 53:151–71. doi: 10.1111/ejn.14720
54. Vidal-Itriago A, Radford RAW, Aramideh JA, Maurel C, Scherer NM, Don EK, et al. Microglia morphophysiological diversity and its implications for the CNS. Front Immunol. (2022) 13:997786. doi: 10.3389/fimmu.2022.997786
55. Godeanu S and Cătălin B. The complementary role of morphology in understanding microglial functional heterogeneity. Int J Mol Sci. (2025) 26:3811. doi: 10.3390/ijms26083811
56. Wang H, Liu C, Han M, Cheng C, and Zhang D. TRAM1 promotes microglia M1 polarization. J Mol Neurosci: MN. (2016) 58:287–96. doi: 10.1007/s12031-015-0678-3
57. Pan J, Jin J, Ge H, Yin K, Chen X, Han L, et al. Malibatol A regulates microglia M1/M2 polarization in experimental stroke in a PPARγ-dependent manner. J Neuroinflamm. (2015) 12:51. doi: 10.1186/s12974-015-0270-3
58. Brisch R, Wojtylak S, Saniotis A, Steiner J, Gos T, Kumaratilake J, et al. The role of microglia in neuropsychiatric disorders and suicide. Eur Arch Psychiatry Clin Neurosci. (2022) 272:929–45. doi: 10.1007/s00406-021-01334-z
59. Wang Y-L, Han Q-Q, Gong W-Q, Pan D-H, Wang L-Z, Hu W, et al. Microglial activation mediates chronic mild stress-induced depressive- and anxiety-like behavior in adult rats. J Neuroinflamm. (2018) 15:21. doi: 10.1186/s12974-018-1054-3
60. Matcovitch-Natan O, Winter DR, Giladi A, Vargas Aguilar S, Spinrad A, Sarrazin S, et al. Microglia development follows a stepwise program to regulate brain homeostasis. Science. (2016) 353:aad8670. doi: 10.1126/science.aad8670
61. Duan C, Huang L, Zhang C, Zhang L, Xia X, Zhong Z, et al. Gut commensal-derived butyrate reverses obesity-induced social deficits and anxiety-like behaviors via regulation of microglial homeostasis. Eur J Pharmacol. (2021) 908:174338. doi: 10.1016/j.ejphar.2021.174338
62. Jang H-M, Lee K-E, and Kim D-H. The Preventive and Curative Effects of Lactobacillus reuteri NK33 and Bifidobacterium adolescentis NK98 on Immobilization Stress-Induced Anxiety/MDD and Colitis in Mice. Nutrients. (2019) 11:819. doi: 10.3390/nu11040819
63. Liu P, Liu Z, Wang J, Wang J, Gao M, Zhang Y, et al. Immunoregulatory role of the gut microbiota in inflammatory MDD. Nat Commun. (2024) 15:3003. doi: 10.1038/s41467-024-47273-w
64. Owusu Kyei-Baffour V, Kumar Vijaya A, and Burokas A. Psychobiotics and the gut-brain axis: advances in metabolite quantification and their implications for mental health. Crit Rev Food Sci Nutr. (2025) 43:1–20. doi: 10.1080/10408398.2025.2459341
65. Saijo K, Winner B, Carson CT, Collier JG, Boyer L, Rosenfeld MG, et al, Saijo K, Winner B, Carson CT, Collier JG, Boyer L, Rosenfeld MG, et al. A Nurr1/CoREST transrepression pathway attenuates neurotoxic inflammation in activated microglia and astrocytes. Cell. (2009) 137(1):47–59. doi: 10.1016/j.cell.2009.01.038
66. Wang F, Qi X, Zhang J, Huang JH, Wang F, Qi X, Zhang J, and Huang JH. Astrocytic modulation of potassium under seizures. Neural Regen Res. (2020) 15(6):980–7. doi: 10.4103/1673-5374.270295
67. Bélanger M, Magistretti PJ, Bélanger M, and Magistretti PJ. The role of astroglia in neuroprotection. Dialogues Clin Neurosci. (2009) 11(3):281–95. doi: 10.31887/DCNS.2009.11.3/mbelanger
68. Enache D, Pariante CM, and Mondelli V. Markers of central inflammation in major depressive disorder: A systematic review and meta-analysis of studies examining cerebrospinal fluid, positron emission tomography and post-mortem brain tissue. Brain Behavior Immun. (2019) 81:24–40. doi: 10.1016/j.bbi.2019.06.015
69. Fan C, Song Q, Wang P, Li Y, Yang M, and Yu SY. Neuroprotective effects of ginsenoside-rg1 against MDD-like behaviors via suppressing glial activation, synaptic deficits, and neuronal apoptosis in rats. Front Immunol. (2018) 9:2889. doi: 10.3389/fimmu.2018.02889
70. Lv W, Wu X, Chen W, Li Y, Zhang G, Chao L, et al. The gut microbiome modulates the changes in liver metabolism and in inflammatory processes in the brain of chronic unpredictable mild stress rats. Oxid Med Cell Longevity. (2019) 2019:1–14. doi: 10.1155/2019/7902874
71. Yuan Q, Lei Y, Yu K, Wu J, Xu Z, Wen C, et al. Repetitive transcranial magnetic stimulation and fluoxetine attenuate astroglial activation and benefit behaviours in a chronic unpredictable mild stress mouse model of MDD. World J Biol Psychiatry. (2024) 25:82–94. doi: 10.1080/15622975.2023.2279958
72. Zhou D, Pan Q, Xin F-Z, Zhang R-N, He C-X, Chen G-Y, et al. Sodium butyrate attenuates high-fat diet-induced steatohepatitis in mice by improving gut microbiota and gastrointestinal barrier. World J Gastroenterol. (2017) 23:60–75. doi: 10.3748/wjg.v23.i1.60
73. Hassamal S. Chronic stress, neuroinflammation, and MDD: An overview of pathophysiological mechanisms and emerging anti-inflammatories. Front Psychiatry. (2023) 14:1130989. doi: 10.3389/fpsyt.2023.1130989
74. Xu Q, Sun L, Chen Q, Jiao C, Wang Y, Li H, et al. Gut microbiota dysbiosis contributes to MDD-like behaviors via hippocampal NLRP3-mediated neuroinflammation in a postpartum MDD mouse model. Brain Behavior Immun. (2024) 119:220–35. doi: 10.1016/j.bbi.2024.04.002
75. Cristiano C, Cuozzo M, Coretti L, Liguori FM, Cimmino F, Turco L, et al. Oral sodium butyrate supplementation ameliorates paclitaxel-induced behavioral and intestinal dysfunction. Biomed Pharmacother = Biomed Pharmacotherapie. (2022) 153:113528. doi: 10.1016/j.biopha.2022.113528
76. Sanmarco LM, Wheeler MA, Gutiérrez-Vázquez C, Polonio CM, Linnerbauer M, Pinho-Ribeiro FA, et al. Gut-licensed IFNγ+ NK cells drive LAMP1+TRAIL+ anti-inflammatory astrocytes. Nature. (2021) 590:473–9. doi: 10.1038/s41586-020-03116-4
77. Bottomley JM, LeReun C, Diamantopoulos A, Mitchell S, and Gaynes BN. Vagus nerve stimulation (VNS) therapy in patients with treatment resistant MDD: A systematic review and meta-analysis. Compr Psychiatry. (2019) 98:152156. doi: 10.1016/j.comppsych.2019.152156
78. Matteoli G, Gomez-Pinilla PJ, Nemethova A, Di Giovangiulio M, Cailotto C, van Bree SH, et al. A distinct vagal anti-inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen. Gut. (2014) 63:938–48. doi: 10.1136/gutjnl-2013-304676
79. Guo L-T, Wang S-Q, Su J, Xu L-X, Ji Z-Y, Zhang R-Y, et al. Baicalin ameliorates neuroinflammation-induced depressive-like behavior through inhibition of toll-like receptor 4 expression via the PI3K/AKT/FoxO1 pathway. J Neuroinflamm. (2019) 16:95. doi: 10.1186/s12974-019-1474-8
80. Pastis I, Santos MG, and Paruchuri A. Exploring the role of inflammation in major depressive disorder: Beyond the monoamine hypothesis. Front Behav Neurosci. (2023) 17:1282242. doi: 10.3389/fnbeh.2023.1282242
81. Moreira PI, Cardoso SM, Santos MS, and Oliveira CR. The key role of mitochondria in Alzheimer’s disease. J Alzheimer’s Disease: JAD. (2006) 9:101–10. doi: 10.3233/jad-2006-9202
82. Chisari M, Barraco M, Bucolo C, Ciranna L, and Sortino MA. Purinergic ionotropic P2X7 and metabotropic glutamate mGlu5 receptors crosstalk influences pro-inflammatory conditions in microglia. Eur J Pharmacol. (2023) 938:175389. doi: 10.1016/j.ejphar.2022.175389
83. Ren W-J, Zhao Y-F, Li J, Rubini P, Yuan Z-Q, Tang Y, et al. P2X7 receptor-mediated MDD-like reactions arising in the mouse medial prefrontal cortex. Cereb Cortex (New York N.Y.: 1991). (2023) 33:8858–75. doi: 10.1093/cercor/bhad166
84. Haapakoski R, Ebmeier KP, Alenius H, and Kivimäki M. Innate and adaptive immunity in the development of MDD: An update on current knowledge and technological advances. Prog Neuropsychopharmacol Biol Psychiatry. (2016) 66:63–72. doi: 10.1016/j.pnpbp.2015.11.012
85. Xie Y, Han R, Li Y, Li W, Zhang S, Wu Y, et al. P2X7 receptor antagonists modulate experimental autoimmune neuritis via regulation of NLRP3 inflammasome activation and Th17 and Th1 cell differentiation. J Neuroinflamm. (2024) 21:73. doi: 10.1186/s12974-024-03057-z
86. Yue N, Huang H, Zhu X, Han Q, Wang Y, Li B, et al. Activation of P2X7 receptor and NLRP3 inflammasome assembly in hippocampal glial cells mediates chronic stress-induced depressive-like behaviors. J Neuroinflamm. (2017) 14:102. doi: 10.1186/s12974-017-0865-y
87. Li T, He J, Cao H, Zhang Y, Chen J, Xiao Y, et al. All-atom RNA structure determination from cryo-EM maps. Nat Biotechnol. (2024) 43:97–105. doi: 10.1038/s41587-024-02149-8
88. Lamkanfi M and Kanneganti T-D. Nlrp3: An immune sensor of cellular stress and infection. Int J Biochem Cell Biol. (2010) 42:792–5. doi: 10.1016/j.biocel.2010.01.008
89. Alcocer-Gómez E, de Miguel M, Casas-Barquero N, Núñez-Vasco J, Sánchez-Alcazar JA, Fernández-Rodríguez A, et al. NLRP3 inflammasome is activated in mononuclear blood cells from patients with major depressive disorder. Brain Behavior Immun. (2014) 36:111–7. doi: 10.1016/j.bbi.2013.10.017
90. Alcocer-Gómez E, Ulecia-Morón C, Marín-Aguilar F, Rybkina T, Casas-Barquero N, Ruiz-Cabello J, et al. Stress-induced depressive behaviors require a functional NLRP3 inflammasome. Mol Neurobiol. (2016) 53:4874–82. doi: 10.1007/s12035-015-9408-7
91. Feng Y, Zhang C, Wei Z, Li G, Gan Y, Liu C, et al. Gene variations of glutamate metabolism pathway and epilepsy. Acta Epileptologica. (2022) 4:31. doi: 10.1186/s42494-022-00103-2
92. Gu S, Li Y, Jiang Y, Huang JH, and Wang F. Glymphatic dysfunction induced oxidative stress and neuro-inflammation in major MDD disorders. Antioxidants (Basel Switzerland). (2022) 11:2296. doi: 10.3390/antiox11112296
93. Zhang C, Yang Y, Gao Y, and Sun D. NaF-induced neurotoxicity via activation of the IL-1β/JNK signaling pathway. Toxicology. (2022) 469:153132. doi: 10.1016/j.tox.2022.153132
94. Zhang L, Jiang Y-H, Fan C, Zhang Q, Jiang Y-H, Li Y, et al. MCC950 attenuates doxorubicin-induced myocardial injury in vivo and in vitro by inhibiting NLRP3-mediated pyroptosis. Biomed Pharmacother. (2021) 143:112133. doi: 10.1016/j.biopha.2021.112133
95. Wei L, Hui Y, Jinxi W, Shihui L, Hongping L, Jian L, et al. Zuogui Jiangtang Jieyu prescription improves diabetes-related MDD by modulation of gut microbiota and neuroinflammation in hippocampus. Heliyon. (2024) 10:e39291. doi: 10.1016/j.heliyon.2024.e39291
96. Wang N, Feng B-N, Hu B, Cheng Y-L, Guo Y-H, and Qian H. Neuroprotection of chicoric acid in a mouse model of Parkinson’s disease involves gut microbiota and TLR4 signaling pathway. Food Funct. (2022) 13:2019–32. doi: 10.1039/D1FO02216D
97. Huang F, Liu X, Xu S, Hu S, Wang S, Shi D, et al. Prevotella histicola Mitigated Estrogen Deficiency-Induced MDD via Gut Microbiota-Dependent Modulation of Inflammation in Ovariectomized Mice. Front Nutr. (2022) 8:805465. doi: 10.3389/fnut.2021.805465
98. Yu X, Yu X, Yang Y, Cheng W, Shi M, Chen L, et al. Probiotic bifico ameliorates MDD- and anxiety-like behaviors induced by estrogen deficiency via NLRP3 inflammasome inhibition. J Inflammation Res. (2025) 18:8153–71. doi: 10.2147/JIR.S511931
99. Proietti M, Perruzza L, Scribano D, Pellegrini G, D’Antuono R, Strati F, et al. ATP released by intestinal bacteria limits the generation of protective IgA against enteropathogens. Nat Commun. (2019) 10:250. doi: 10.1038/s41467-018-08156-z
100. Su Q, Tian Y, Liu Z, Ci L, and Lv X. Purinergic P2X7 receptor blockade mitigates alcohol-induced steatohepatitis and intestinal injury by regulating MEK1/2-ERK1/2 signaling and egr-1 activity. Int Immunopharmacol. (2019) 66:52–61. doi: 10.1016/j.intimp.2018.11.012
101. Zhang Y, Huang R, Cheng M, Wang L, Chao J, Li J, et al. Gut microbiota from NLRP3-deficient mice ameliorates depressive-like behaviors by regulating astrocyte dysfunction via circHIPK2. Microbiome. (2019) 7:116. doi: 10.1186/s40168-019-0733-3
102. Wang W, Dernst A, Martin B, Lorenzi L, Cadefau-Fabregat M, Phulphagar K, et al. Butyrate and propionate are microbial danger signals that activate the NLRP3 inflammasome in human macrophages upon TLR stimulation. Cell Rep. (2024) 43:114736. doi: 10.1016/j.celrep.2024.114736
103. Xu F, Chen H, Gao Y, Yang X, Zhang C, and Ni X. Sodium butyrate ameliorates postoperative delirium by regulating gut microbiota dysbiosis to inhibit astrocyte activation in aged mice. Neurochemical Res. (2024) 49:3342–55. doi: 10.1007/s11064-024-04245-2
104. Lv H, Wang S, Tian M, Wang L, Gao J, Zhao Q, et al. Exercise preconditioning ameliorates cognitive impairment in mice with ischemic stroke by alleviating inflammation and modulating gut microbiota. Mediators Inflammation. (2022) 2022:2124230. doi: 10.1155/2022/2124230
105. Nguyen PT, Dorman LC, Pan S, Vainchtein ID, Han RT, Nakao-Inoue H, et al. Microglial remodeling of the extracellular matrix promotes synapse plasticity. Cell. (2020) 182:388–403.e15. doi: 10.1016/j.cell.2020.05.050
106. Linnerbauer M, Wheeler MA, and Quintana FJ. Astrocyte crosstalk in CNS inflammation. Neuron. (2020) 108:608–22. doi: 10.1016/j.neuron.2020.08.012
107. Fang S, Wu Z, Guo Y, Zhu W, Wan C, Yuan N, et al. Roles of microglia in adult hippocampal neurogenesis in MDD and their therapeutics. Front Immunol. (2023) 14:1193053. doi: 10.3389/fimmu.2023.1193053
108. McKim DB, Niraula A, Tarr AJ, Wohleb ES, Sheridan JF, and Godbout JP. Neuroinflammatory dynamics underlie memory impairments after repeated social defeat. J Neurosci. (2016) 36:2590–604. doi: 10.1523/JNEUROSCI.2394-15.2016
109. Liu Z, Fan Y, Won SJ, Neumann M, Hu D, Zhou L, et al. Chronic treatment with minocycline preserves adult new neurons and reduces functional impairment after focal cerebral ischemia. Stroke. (2007) 38:146–52. doi: 10.1161/01.STR.0000251791.64910.cd
110. Vergil Andrews JF, Selvaraj DB, Kumar A, Roshan SA, Anusuyadevi M, and Kandasamy M. A mild dose of aspirin promotes hippocampal neurogenesis and working memory in experimental ageing mice. Brain Sci. (2023) 13. doi: 10.3390/brainsci13071108
111. Wu A and Zhang J. Neuroinflammation, memory, and MDD: New approaches to hippocampal neurogenesis. J Neuroinflamm. (2023) 20:283. doi: 10.1186/s12974-023-02964-x
112. Chen F, Bertelsen AB, Holm IE, Nyengaard JR, Rosenberg R, and Dorph-Petersen K-A. Hippocampal volume and cell number in MDD, schizophrenia, and suicide subjects. Brain Res. (2020) 1727:146546. doi: 10.1016/j.brainres.2019.146546
113. Boldrini M, Hen R, Underwood MD, Rosoklija GB, Dwork AJ, Mann JJ, et al. Hippocampal angiogenesis and progenitor cell proliferation are increased with antidepressant use in major MDD. Biol Psychiatry. (2012) 72:562–71. doi: 10.1016/j.biopsych.2012.04.024
114. Zhang S-Q, Deng Q, Zhu Q, Hu Z-L, Long L-H, Wu P-F, et al. Cell type-specific NRBF2 orchestrates autophagic flux and adult hippocampal neurogenesis in chronic stress-induced MDD. Cell Discov. (2023) 9:1–23. doi: 10.1038/s41421-023-00583-7
115. Ali NH, Al-kuraishy HM, Al-Gareeb AI, Alnaaim SA, Saad HM, and Batiha GE-S. The molecular pathway of p75 neurotrophin receptor (p75NTR) in parkinson’s disease: the way of new inroads. Mol Neurobiol. (2024) 61:2469–80. doi: 10.1007/s12035-023-03727-8
116. Lima Giacobbo B, Doorduin J, Klein HC, Dierckx RAJO, Bromberg E, and de Vries EFJ. Brain-derived neurotrophic factor in brain disorders: focus on neuroinflammation. Mol Neurobiol. (2019) 56:3295–312. doi: 10.1007/s12035-018-1283-6
117. Sominsky L, De Luca S, and Spencer SJ. Microglia: Key players in neurodevelopment and neuronal plasticity. Int J Biochem Cell Biol. (2018) 94:56–60. doi: 10.1016/j.biocel.2017.11.012
118. Rodríguez-Landa JF, German-Ponciano LJ, Puga-Olguín A, and Olmos-Vázquez OJ. Pharmacological, neurochemical, and behavioral mechanisms underlying the anxiolytic- and antidepressant-like effects of flavonoid chrysin. Molecules. (2022) 27. doi: 10.3390/molecules27113551
119. Dong X, Pei G, Yang Z, and Huang S. Flavonoid chrysin activates both TrkB and FGFR1 receptors while upregulates their endogenous ligands such as brain derived neurotrophic factor to promote human neurogenesis. Cell Proliferation. (2024) 58:e13732. doi: 10.1111/cpr.13732
120. Fodelianaki G, Lansing F, Bhattarai P, Troullinaki M, Zeballos MA, Charalampopoulos I, et al. ). Nerve Growth Factor modulates LPS - induced microglial glycolysis and inflammatory responses. Exp Cell Res. (2019) 377:10–6. doi: 10.1016/j.yexcr.2019.02.023
Keywords: neuroinflammation, MDD, gut-brain axis, microglia, astrocytes
Citation: Li J, Wan B, Zhou L, Qian X, Wang F, Gu S, Ma X and Huang JH (2025) Gut microbiota dysbiosis induces neuroinflammation in major depressive disorders: mechanisms targeting the gut-brain axis. Front. Psychiatry 16:1629182. doi: 10.3389/fpsyt.2025.1629182
Received: 16 May 2025; Accepted: 25 August 2025;
Published: 18 September 2025.
Edited by:
Gábor Gazdag, Jahn Ferenc Dél-Pesti Kórház és Rendelőintézet, HungaryReviewed by:
Rohan Gupta, University of South Carolina, United StatesPanida Sittipo, Burapha University, Thailand
Copyright © 2025 Li, Wan, Zhou, Qian, Wang, Gu, Ma and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Simeng Gu, Z3NtXzIwMDdAMTI2LmNvbQ==; Fushun Wang, MTM4MTQ1NDExMzhAMTYzLmNvbQ==; Xianjun Ma, bWF4aWFuanVuQDEyNi5jb20=
 Jiayi Li
Jiayi Li Bei Wan2
Bei Wan2 Fushun Wang
Fushun Wang Simeng Gu
Simeng Gu Jason H. Huang
Jason H. Huang