Abstract
Objective:
Cognitive impairment in schizophrenia (SCZ) is common, but the mechanism remains unclear. This study aimed to investigate whether brain activation during the functional near−infrared spectroscopy (fNIRS) verbal fluency test (VFT) task is associated with cognitive deficits and to evaluate the reliability of fNIRS as a clinical tool for diagnosing stable SCZ.
Methods:
A total of 45 stable SCZ patients and 30 healthy controls (HC) were included. Demographic information, Positive and Negative Symptom Scale (PANSS), and MATRICS Consensus Cognitive Battery (MCCB) were assessed. During VFT, hemodynamic responses in the frontotemporal cortex were monitored with fNIRS.
Results:
During VFT, individuals with SCZ demonstrated a reduced number of valid words, lower β value in channel 8, 25-26, 35–36 and 47-48, and decreased integral value (IV) in both the prefrontal lobe and bilateral temporal lobes. IV of the temporal lobes and the β value of channel 48 demonstrated sensitivity for diagnosis of SCZ, with an area under the receiver operating characteristic curve of 0.781 (95% CI: 0.667-0.896), and 0.762 (95% CI: 0.655-0.869), respectively. Moreover, IV of the temporal lobes correlated positively with multi-domain of cognition, including speed of processing, attention/vigilance, social cognition and MCCB total scores. The β value of channel 48 correlated positively with speed of processing.
Conclusion:
Our findings suggest that fNIRS may serve as a valuable clinical measure of cognition assessment, and IV of bilateral temporal lobes and β value of channel 48 can be used as candidate biomarkers to differentiate individuals with schizophrenia.
Introduction
Cognitive impairments have been widely recognized as core pathological features of schizophrenia (SCZ) that contribute significantly to functional disability and demonstrating limited responsiveness to interventions (1). Neuropsychological investigations have consistently identified a broad range of cognitive deficits in SCZ encompassing impairments in attention, processing speed, working memory and executive functions (2–4). Longitudinal studies have established that cognitive decline precedes the onset of psychotic symptoms by nearly a decade (5, 6). Emerging evidence suggests accelerated cognitive aging in specific domains among individuals with psychotic spectrum disorders compared to the general populations (7, 8). Nevertheless, the underlying neurobiological mechanisms remain poorly characterized.
Functional near-infrared spectroscopy (fNIRS), a non-invasive optical neuroimaging modality, quantifies cortical hemoglobin dynamics through infrared light absorption variations (9). It is particularly valuable for assessment of regional neuronal activity in the prefrontal and superior temporal cortices- brain regions that have been consistently implicated in the neuropathology of SCZ (10, 11). During verbal fluency tasks (VFT), SCZ patients exhibit attenuated hemodynamic responses in frontotemporal regions (channels 10, 11, 17, 19, 21, 23–32, 34, 36, 38–42, and 44–52) compared to controls (12). Similarly, both individuals at clinical high risk for psychosis (CHR) and patients with first-episode SCZ demonstrated significantly reduced neural activation during the verbal fluency task (VFT), particularly in the bilateral inferior prefrontal gyrus, right middle temporal gyrus, and left dorsolateral prefrontal cortex(lDLPFC) (13). These findings suggest that VFT may serve as an indicator of disrupted neural processing underlying the cognitive deficits associated with SCZ.
Recent studies have demonstrated the diagnostic sensitivity of fNIRS’s across neuropsychiatric disorders. A cross-sectional study examining psychosis-spectrum trajectories (healthy controls, clinical high-risk, first-episode psychosis, chronic schizophrenia) revealed distinct spatiotemporal activation patterns across clinical stages (14). In several studies, researchers found two indexes obtained from fNIRS measured VFT activity, the integral value (IV) and centroid value (CV), which can be used for diagnosis of major psychiatric disorders. In a Tawain cohort examining 192 patients with psychotic disorder, researchers found the frontal CV at 54 seconds enabled the accurate distinction between patients with major depressive disorder at a rate of 80.0% (sensitivity:78.9%, specificity: 72.5%) and those with bipolar MDD (15). Furthermore, a combined fNIRS index of IV and CV achieved sensitivity in classifying individuals with SCZ from healthy controls (16).
In the present study, we used 52-channel fNIRS to measure the prefrontal and temporal cortical hemodynamics during VFT performance, and assessed clinical symptoms and MATRICS Consensus Cognitive Battery (MCCB) in stable SCZ patients and matched controls. The main aim of the study was to validate and extend previous reports by testing the relationships between brain activation during the fNIRS-VFT task and cognitive deficits and to evaluate the reliability of fNIRS as a clinical aid for diagnostic tools in stable schizophrenia. This study may advance the clinical application of fNIRS in assisting the diagnosis of SCZ and provide an objective measure to facilitate research on neural mechanisms research and treatment.
Materials and methods
Participants
45 SCZ patients were enrolled in this study from February, 2022 to September, 2024 in the Changning Mental Health Center of Shanghai City. The inclusion criteria for this study were: (1) age 18–65 years; (2) meeting the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) for schizophrenia; (3) the Positive and Negative Syndrome Scale (PANSS) score ≤ 60 with a score of 3 or less on positive items (delusions, conceptual disorganization, hallucinatory behavior, grandiosity, or suspiciousness/persecution); (4) clinical stability, continuous antipsychotic treatment with stable dosage for at least 6 months before enrollment; (5) understand the experimental procedure; and (6) written informed consent. Exclusion criteria were (1) meeting DSM-5 criteria for other mental disorders; (2) exhibiting physical or mental unstable condition. During the same period, a sex- and age-matched control cohort (n=30) was recruited through community advertisements. Healthy controls underwent diagnostic interviews and evaluations to confirm the absence of any personal or family psychiatric history. The study was approved by the Ethics Review Committee of the Changning Mental Health Center of Shanghai City. In accordance with the Declaration of Helsinki, written informed consent was obtained from all participants before enrollment.
On the experimental day, SCZ patients were evaluated for psychopathology with the PANSS (17). Both patient and control participants completed the MATRICS Consensus Cognitive Battery to assess cognitive function across 7 domains: speed of processing, attention/vigilance, working memory, verbal learning, visual learning, reasoning and problem-solving, and social cognition (18). To ensure assessment validity, all assessments was evaluated by two well-trained psychiatrist who were blinded to the fNIRS study results.
Verbal fluency task
Participants performed a standardized Chinese-language VFT consisting of a total of 160 seconds (19). Each trial consisted: (1) a 10-s pre-task baseline period, (2) a 30-s first repeated counting period, (3) a 60-s task period subdivided into three 20-s blocks and (4) a 70-s post-task baseline period (20). During the task period, participants were asked to form as many valid words as possible from three commonly used characters: “tian”(sky; Block 1), “da”(big; Block 2), and “bai”(while; Block 3), and 20 s for each character. The total number of word phrases generated by each participant will be recorded. During the test, participants were instructed to keep eyes open, sit upright and minimize their movements.
fNIRS measurements
We employed a 52-channel fNIRS system (ETG-4100, Hitachi Medical Co., Japan) to record evoked cortical activity during the VFT task (21). The ETG-4100 comprised 17 emitter locations and 16 light detectors arranged in a 3×11 matrix with a 3.0 cm inter-optode distance. This configuration was positioned according to the international 10–20 EEG system to cover the prefrontal and bilateral temporal cortex. Specifically, detector No. 26 was aligned over the glabella, ensuring the bottom edge of the fNIRS probe holder remained parallel to the eyebrows and coincident with the Fp1-Fp2 line. Each adjacent emitter-detector pair formed one of the 52 measurement channels, with the system operating at a sampling rate of 10 Hz. Each emitter delivered two wavelengths of near-infrared light (695 nm and 830 nm), which penetrated the scalp and skull to interact with cortical tissue. The detectors captured the optical signals, and using the modified Beer-Lambert law, the system calculated changes in the relative concentrations of oxygenated hemoglobin (oxy-Hb) and deoxygenated hemoglobin (deoxy-Hb).
VFT data processing
The ETG-4100 system automatically performs post-task integral analysis following the VFT. In this procedure, the pre-task baseline was set as the last 10 seconds of the preceding 30-second interval, while the post-task baseline was defined as the first 55 seconds of the following 70-second interval. There are two key indexes reflecting the temporal midpoint of the fNIRS signal: the IV and the CV. They were calculated with 5-second moving average method (22). The IV measures the cumulative magnitude of the hemodynamic response across the entire 60-s task activation phase, while the CV indicates the temporal midpoint of the fNIRS signal change, covering both the task and post-task intervals. In line with prior research, the converted hemoglobin concentration data was utilized instead of raw optical intensity data to maintain the relationship between IV and CV in post-processing stage (21). Subsequently, the converted hemoglobin concentration changes were analyzed utilizing the NIRS-KIT software package within MATLAB R2013b (23). VFT data were directly obtained from Hitachi ETG4100 systems.
The original fNIRS data were preprocessed by preprocessed using the NIRS-KIT package. To eliminate slow time drifts, a first-order detrending procedure was applied. Next, motion correction was conducted by the correlation-based signal improvement method. Subsequently, artifacts were removed by applying a band-pass filter to restrict the frequency range of the data to 0.01–0.08 Hz (24). Finally, a general linear regression model (GLM) analysis was conducted to model the oxyhemoglobin response during the stimulus condition, with the aim of estimating the β coefficient as an indicator of individual task-related neural activity (23).
Statistical analysis
Statistical analyses were performed using SPSS 22.0 (SPSS Inc., Chicago, IL, USA) and R Studio 4.1(RStudio Team, 2022). Continuous variables, including age, education(years), IV, CV, and β values for each channel, underwent Shapiro-Wilk tests to confirm normality. Normally distributed data were expressed as mean ± standard deviation (mean ± SD), while non-normally distributions were reported with interquartile range (IQR) as [M (Q1, Q3)]. Chi-square test was used to analyze the inter-group differences of gender. Internal channel activation was determined through one-sample analyses: parametric t-tests (H0: β=0). Intergroup channel activation comparisons (SCZ vs HC) employed independent t-tests. Forward stepwise logistic regression was adopted to identify potential features associated with diagnosis of SCZ. Model performance was assessed using receiver operating characteristic (ROC) curve analysis, with area under the curve (AUC). Pearson correlation analysis was employed to explore the associations between fNIRS features and cognitive performance.
Results
Demographic and clinical characteristics
No significant group differences were observed in age, sex and education (p>0.05; Table 1). Compared with controls, the stable SCZ group exhibited significant lower scores in MCCB total and four subdomains: speed of processing, attention/vigilance, reasoning and problem-solving, and social cognition (p<0.05).
Table 1
| Variable | SCZ N=45 | HC N=30 | t/χ2(p) |
|---|---|---|---|
| Age (years) | 42.78 ± 6.88 | 40.57 ± 8.47 | 1.242 (0.218) |
| Sex (M/F) | 21/24 | 15/15 | 0.800 (0.777) |
| Education (years) | 14.53 ± 2.62 | 15.23 ± 2.92 | -1.083 (0.282) |
| PANSS-total | 47.12 ± 9.33 | NA | NA |
| PANSS-P | 10.71 ± 3.34 | NA | NA |
| PANSS-N | 14.33 ± 4.64 | NA | NA |
| PANSS-G | 22.24 ± 3.90 | NA | NA |
| MCCB (T-score) | 40.44 ± 11.48 | 49.62 ± 9.53 | 3.579 (0.001) |
| Speed of processing | 39.36 ± 13.09 | 53.83 ± 9.32 | 5.592 (<0.001) |
| Attention/vigilance | 41.44 ± 10.73 | 49.55 ± 10.76 | 3.169 (0.002) |
| Working memory | 47.69 ± 10.39 | 46.41 ± 8.33 | -0.555 (0.580) |
| Verbal learning | 37.33 ± 10.41 | 39.38 ± 9.00 | 0.869 (0.388) |
| Visual learning | 51.29 ± 12.70 | 52.21 ± 9.65 | 0.332 (0.741) |
| Reasoning and problem-solving | 46.24 ± 8.40 | 55.45 ± 8.84 | 4.507 (<0.001) |
| Social cognition | 45.04 ± 12.37 | 51.00 ± 10.72 | 2.195 (0.032) |
Demographic and clinical characteristics.
SCZ, schizophrenia; HC, healthy control; PANSS, Positive and Negative Syndrome Scale, which includes positive symptoms, negative symptoms and general psychopathology subscales; MCCB, Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery.
β value comparison between stable SCZ and HC groups in VFT
Examining the activation of each channel during the VFT showed different neural activation between the stable SCZ and HC groups. The HC group exhibited significant hemodynamic activation (β>0, pFDR<0.05) in 27 channels: 7-9, 14, 16-21, 24-29, 31, 35-39, and 46-50. In contrast, the stable SCZ group demonstrated activation in only 8 channels (16, 27-29, 38-39, and 40-50). Between-group comparisons identified significant β-value reductions in SCZ patients across key prefrontal regions (channels 8, 25–26, 35–36, and 47–48; pFDR<0.05) (Table 2).
Table 2
| Channel | Region | SCZ | HC | t (p) | Cohen’s d |
|---|---|---|---|---|---|
| 8 | lDLPFC | 0.003 ± 0.013 | 0.012 ± 0.014 | 2.810 (0.006) | 0.665 |
| 25 | rDLPFC | 0.002 ± 0.015 | 0.014 ± 0.020 | 3.001 (0.004) | 0.665 |
| 26 | mPFC | 0.004 ± 0.016 | 0.014 ± 0.016 | 2.785 (0.007) | 0.707 |
| 35 | rVLPFC | 0.001 ± 0.013 | 0.016 ± 0.022 | 3.425 (0.001) | 0.891 |
| 36 | mPFC | 0.004 ± 0.015 | 0.014 ± 0.017 | 2.812 (0.006) | 0.663 |
| 38 | mPFC | 0.008 ± 0.017 | 0.023 ± 0.022 | 3.332 (0.001) | 0.785 |
| 47 | mPFC | 0.003 ± 0.015 | 0.017 ± 0.020 | 3.317 (0.001) | 0.782 |
| 48 | mPFC | 0.004 ± 0.013 | 0.020 ± 0.018 | 4.019 (<0.001) | 1.008 |
Comparison of β value between stable SCZ and HC groups in VFT.
SCZ, schizophrenia; HC, healthy control; lDLPFC, left dorsolateral prefrontal cortex; rDLPFC, right dorsolateral prefrontal cortex; mPFC, medial prefrontal cortex; rVLPFC, right ventrolateral prefrontal cortex.
Comparison of VFT performance, IV and CV between groups
The HC group demonstrated superior VFT performance in terms of total number of valid words (Table 3). Regarding the IV, significant differences were observed in both the prefrontal lobe and bilateral temporal lobes (p<0.001) when comparing the HC and SCZ groups, with the SCZ group exhibiting lower scores than the HC group (Table 3). In terms of CV, no notable differences were found in either the frontal lobe or the bilateral temporal lobes between the two groups (all p>0.05).
Table 3
| Indicators | SCZ | HC | t/Z(p) | Effect size |
|---|---|---|---|---|
| VFT performance | 8.98 ± 3.22 | 11.97 ± 3.44 | 3.817 (<0.001) | 0.761a |
| IV of the prefrontal lobe | 25.40 (-3.00,66.90) | 57.70 (35.30,77.00) | -2.742 (0.006) | 0.316b |
| CV of the prefrontal lobe | 59.59 ± 15.33 | 56.43 ± 8.80 | -1.022 (0.310) | -0.241a |
| IV of the Bilateral temporal lobes | 28.00 (-1.55, 65.20) | 110.10 (49.98, 157.50) | -4.110 (<0.001) | 0.475b |
| CV of the Bilateral temporal lobes | 58.66 ± 15.21 | 59.67 ± 7.65 | 0.340 (0.735) | 0.080a |
Differences in VFT performance, IV and CV between groups.
SCZ, schizophrenia; HC, healthy control; IV, integral value; CV, centroid value; a Cohen’s d is calculated for the effect size; b r is calculated for the effect size.
Predictive value of fNIRS index on classification of HC and SCZ
Binary logistic regression analysis revealed that IV from bilateral temporal regions and β-values from channel 48 served as significant neurophysiological predictors for schizophrenia diagnosis (Table 4). Diagnostic performance evaluation demonstrated distinct discriminatory capacities: IV of the bilateral temporal lobes achieved an AUC of 0.781, and the β value of channel 48 yielded an AUC of 0.762 (Table 5, Figure 1). Combined implementation of these biomarkers provided an AUC of 0.820 (95%CI:0.720-0.920). Using the optimal feature thresholds, scatter plots for IV of the bilateral temporal lobes and β value of channel 48 for each group are shown in Figure 2.
Figure 1
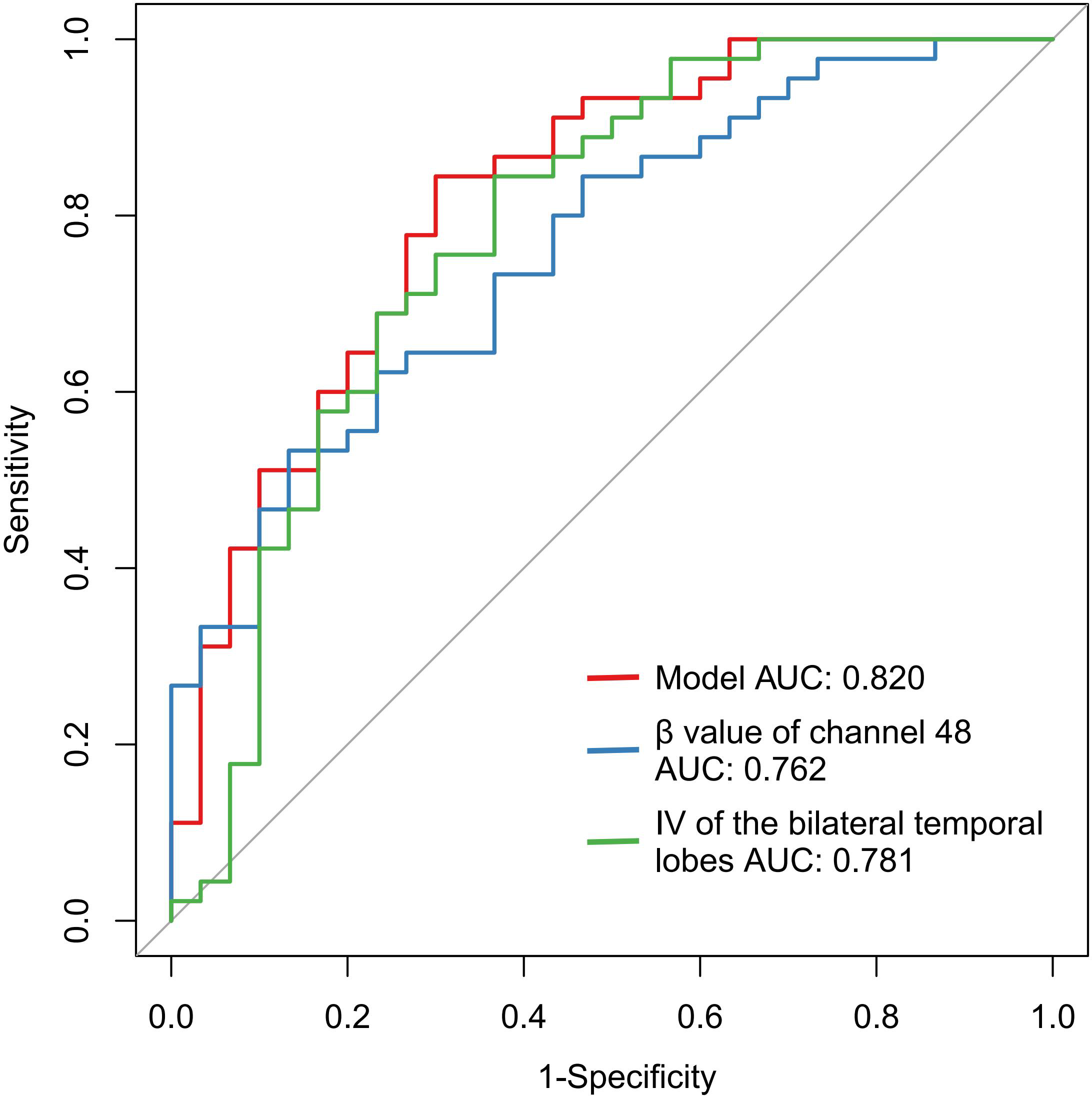
ROC for fNIRS features and combined index for the classification of SCZ.
Table 4
| Predictors in the model | Beta | S.E | P | OR | 95%CI | |
|---|---|---|---|---|---|---|
| IV of the bilateral temporal lobes | -0.014 | 0.005 | 0.004 | 0.986 | 0.976 | 0.995 |
| β value of channel 48a | -0.051 | -0.022 | 0.020 | 0.951 | 0.911 | 0.992 |
Logistic regression for predicting the patients with SCZ from HC.
SCZ, schizophrenia; HC, healthy control; IV, integral value; a Expand by 10^4 times.
Table 5
| Indicators | AUC | p | 95%CI | Cutoff | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| IV of the bilateral temporal lobes | 0.781 | <0.001 | [0.667, 0.896] | 72.60 | 0.633 | 0.844 |
| β value of channel 48 | 0.762 | <0.001 | [0.655, 0.869] | 0.0042 | 0.867 | 0.533 |
| Model | 0.820 | <0.001 | [0.720-0.920] | 0.589 | 0.700 | 0.844 |
The diagnostic performance in distinguishing the patients with SCZ from HC by ROC analysis.
SCZ, schizophrenia; HC, healthy control; IV, integral value.
Figure 2
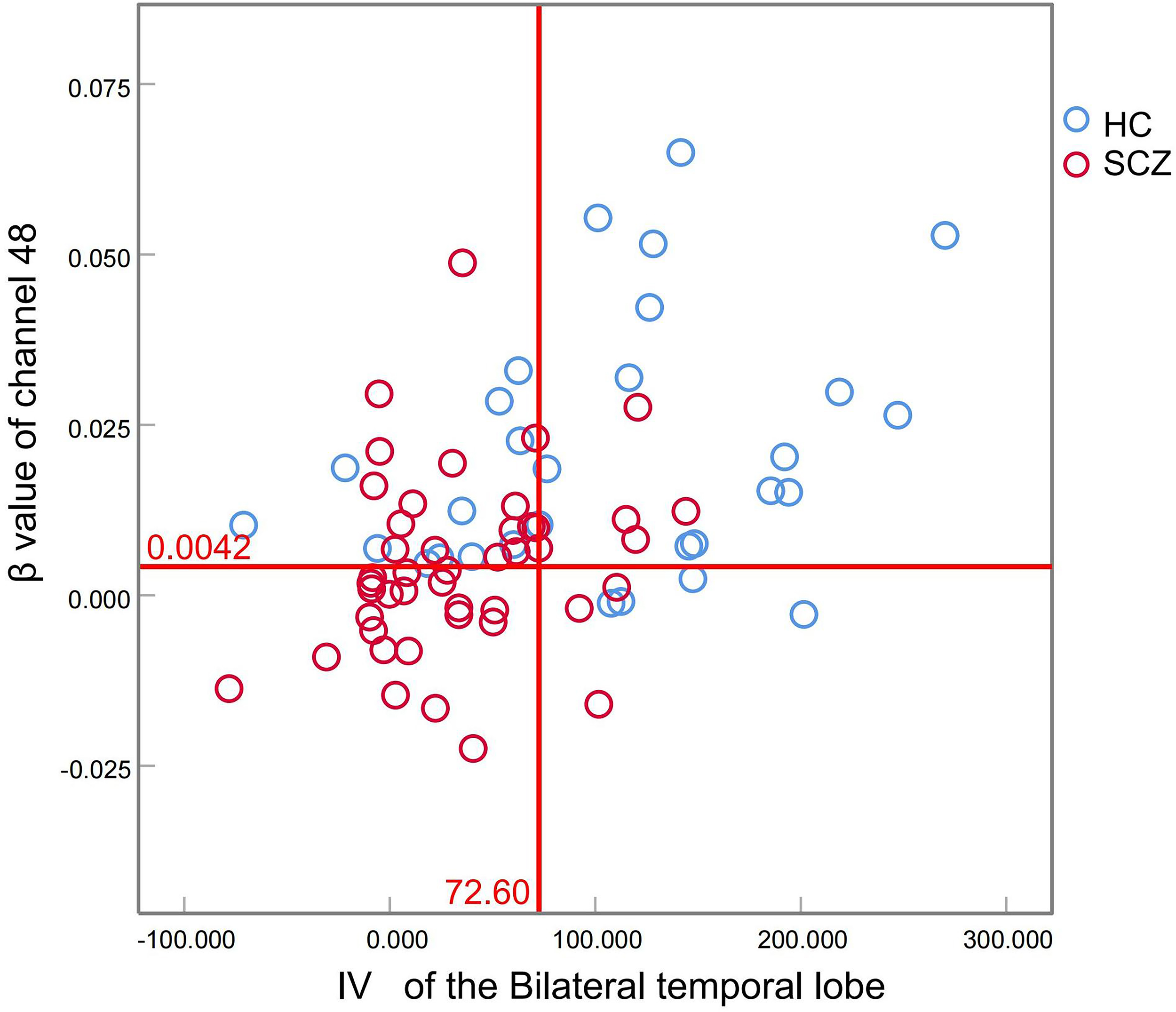
Scatter plots of IV of the bilateral temporal lobes and β value of channel 48 for each group.
Correlation between fNIRS features and cognition
Pearson correlation analyses revealed significant positive associations between IV of the bilateral temporal lobes and multiple cognitive domains: processing speed (r = 0.380, p = 0.001), attention/vigilance (r = 0.234, p = 0.045), social cognition (r = 0.267, p = 0.021), and MCCB total scores (r = 0.260, p = 0.027) (Figure 3). The β value of channel 48 correlated positively with speed of processing (r=0.258, p=0.027).
Figure 3
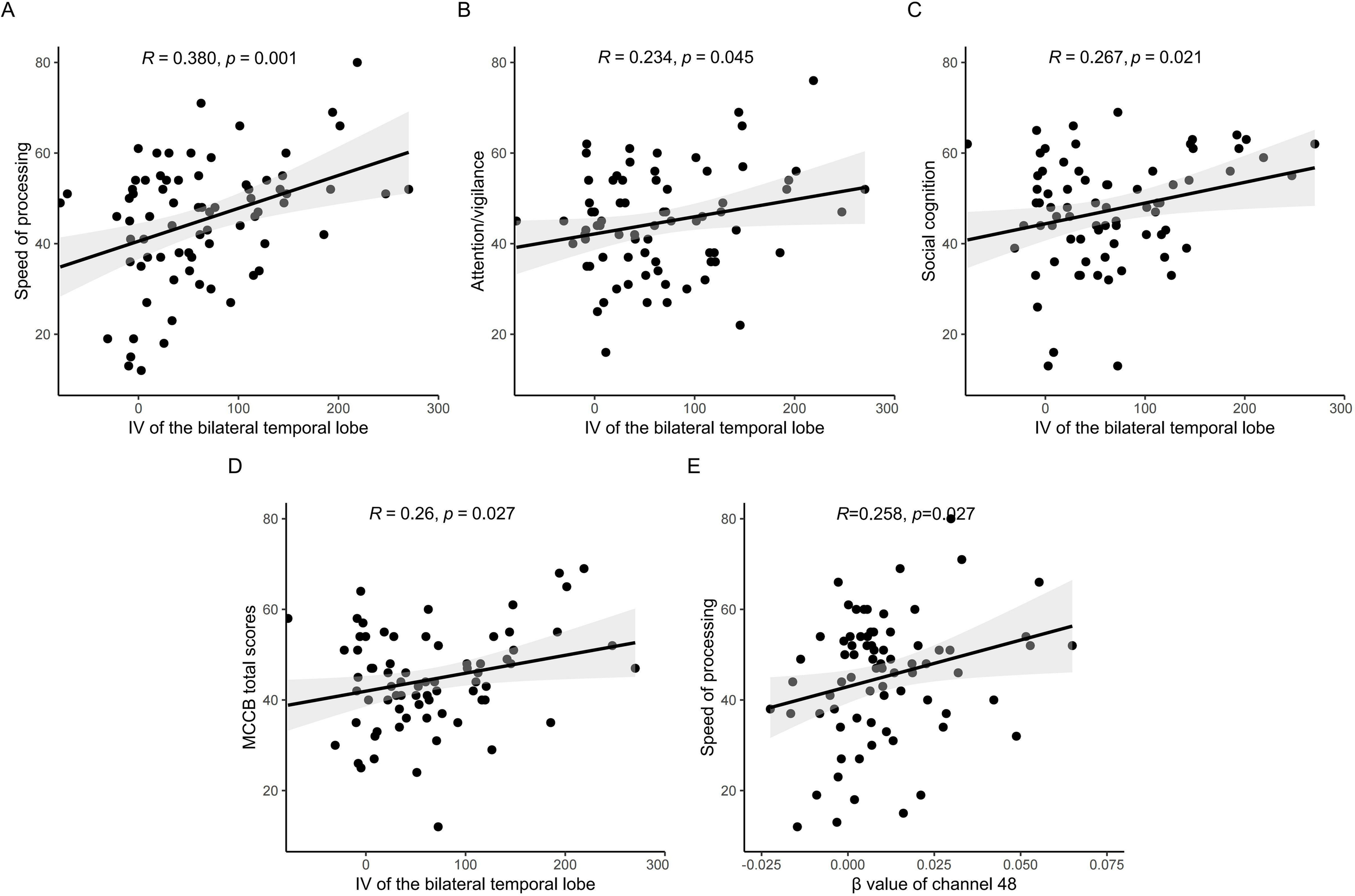
Correlation analysis between fNIRS features and cognition. IV of the bilateral temporal lobes correlated positively with processing speed (A), attention/vigilance (B), social cognition (C), and MCCB total scores (D), The β value of channel 48 correlated positively with speed of processing (E).
Discussion
For many years, electroencephalography (EEG), fNIRS, and functional magnetic resonance imaging (fMRI) have been employed to investigate the functional activity of the cerebral cortex. EEG and fNIRS offer several advantages over fMRI in the study of dynamic brain activity: they are portable, cost-effective, and characterized by higher temporal resolution compared to fMRI (25). EEG exhibits high temporal resolution, and it is particularly susceptible to motion artifacts. In contrast, fNIRS provides distinct advantages including tolerance to motion artifacts, and user-friendly operational requirements (26). fNIRS, a state-of-the-art non-invasive brain functional imaging technology to detect the cortical oxygenation activity in real-time, is widely used in psychiatric populations (27, 28).
The VFT task, considered the gold-standard cognitive probe for fNIRS studies, effectively engages in the ability to retrieve and produce verbal material from the lexico-semantic memory, and to test executive control ability, such as processing speed, working memory, response inhibition and tasks requiring cognitive flexibility (29). The VFT performance activates and crucially relies on the superior medial frontal cortex, ventrolateral prefrontal cortex, and anterior temporal lobe (30). Poor performance on the VFT has been consistently linked to impairments in verbal and working memory, as well as executive functioning and problem-solving abilities in mental illness (31). Many studies have reported the brain dysfunction in patients with mental illness during fNIRS-VFT task. In patients with SCZ, reduced brain activation in the prefrontal cortex and superior temporal cortex was reported (32). Individuals with clinical high risk for psychosis demonstrate abnormal brain activation of rSTG (16). Crucially, differences in the performance of the fNIRS-VFT has been used to distinguish various psychiatric disorders. The present study investigated brain activation deficits during the Chinese version VFT using a 52-channel fNIRS device in stable SCZ and healthy controls, as well as evaluated the potential of these brain activation deficits as a clinical aid for diagnostic tools in SCZ.
Compared with HC, our study revealed that stable SCZ patients demonstrated poorer performance in MCCB total scores and cognitive subdomains (speed of processing, attention/vigilance, reasoning and problem-solving, and social cognition). These results may reflect impaired brain inefficiency in SCZ. Our findings align with previous studies demonstrating widespread cognitive dysfunction in SCZ patients across MCCB domains (3, 8). In this study, the SCZ group showed reduced activation values primarily in the mPFC and other regions including rVLPFC, rDLPFC, and lDLPFC relative to controls. Moreover, task-related β values were positive correlated with speed of processing. Our results are in line with previous studies that reduced resting cerebral blood flow and regional glucose metabolism in the PFC of SCZ patients (33). Findings from TMS/hd‐EEG measurements also indicate that intrinsic defects in both activity and connectivity of PFC in SCZ, and that these defects are specifically associated with cognitive impairment (34). Moreover, the PFC plays a critical role in regulating information processing speed and working memory (35). Hypofrontality in DLPFC has been linked to cognitive deficits in SCZ, and neuromodulation using rTMS over DLPFC has shown potential for improving cognitive function (36). Similar results were also found in EGG studies. Compared with controls, distinct functional connectivity patterns involving a wide range of brain regions- including the inferior, superior, and middle temporal gyri, the fusiform gyrus, the superior as well as the middle frontal gyri -were observed in EEG study of patients with chronic SCZ (37). In addition, previous studies have demonstrated that patients with schizophrenia exhibit reduced cortical inhibition in the motor cortex and DLPFC, with these deficits frequently correlating with symptom severity (38, 39).
Specially, a pronounced reduction in IVs in the frontal and bilateral temporal lobes observed during the VFT in the SCZ group, while no significant inter-group differences were noticed in CV values in our study. The IV of the bilateral temporal lobes exhibited positive correlations with processing speed, attention/vigilance, social cognition, and MCCB total scores. In a global ENIGMA study, convergent functional and structural epicenters across all stages of SCZ were predominantly localized within transmodal regions, including the parietal, temporal, and frontal lobes (10). Hypo-connectivity of the default-mode network (DMN) may reflect the impaired integration of inner activity, thereby offering a potential mechanistic explanation for the reduced IV in SCZ (40). Consistent with previous studies, the above results indicate that fNIRS may serve as a valuable clinical tool for assessing cognition in schizophrenia. The fNIRS technology has been widely utilized to detect biomarkers and evaluate cognitive impairments in various mental disorders. For instance, in major depressive disorder, both verbal learning and working memory positively correlated with functional connectivity of the bilateral frontotemporal cortex (41). During the Trial Making Test the Mazes Test, the mean changes in relative oxyhemoglobin concentration demonstrated negative correlations with PFC activation in the SCZ patients (31).
The IV measures the total hemodynamic response during the 60-s activation phase, and this biomarker has demonstrated diagnostic utility in mood disorders, particularly for differentiating depression subtypes (22). In a study involving ultra-high risk for psychosis individuals, first-episode psychosis, chronic schizophrenia, and healthy controls, IV was a significant biomarker for differentiating psychosis spectrum in various clinical stages with AUC range from 0.511 to 0.633 (p<0.05) (14). In a Chinese clinical population, temporal lobe IV was used to assist the diagnosis of major psychiatric disorders with an AUC of an 0.275, and the AUC improved to 0.923 with combined IV and CV (20). The former two studies indicated the combined use of IV and CV could improve the diagnostic sensitivity. In our results, IV of the bilateral temporal lobes alone distinguished patients from healthy controls with an AUC of 0.781(sensitivity 0.633, specificity 0.844). No significant reduction in CV was observed in stable SCZ patients in this study, suggesting preserved cortical homeostasis following task completion in our SCZ cohort. Notably, task-related β value of channel 48 is valuable in diagnosing SCZ from HC with an AUC of 0.762(sensitivity 0.867, specificity 0.533). Integration of the bilateral temporal IV and β value of channel 48 provided an AUC of 0.820. This may preliminarily suggest the high accuracy and reliability of using fNIRS as a clinical aid for diagnostic tools in SCZ.
Our findings not only highlight the distinct neurofunctional signatures in frontotemporal circuitry in SCZ but also validate fNIRS as a non-invasive and practical clinical tool for cognitive assessments. Nevertheless, all findings must be interpreted with caution as this study has several limitations. Deep learning techniques have demonstrated substantial value in fNIRS research aimed at diagnosis of SCZ, particularly in studies involving limited sample sizes (42, 43). In the current study, the SCZ group only included 45 individuals and further studies with deep learning techniques are required to confirm our results. Second, we only observed the cross-sectional outcome between the groups; the longitudinal changes in cognitive function and brain functional connectivity of SCZ are still not fully understood. Third, potential pharmacological confounders persist as patients maintained heterogeneous antipsychotic regimens, disease stages, highlighting the need for subgroup analyses.
Conclusion
In conclusion, our findings suggest that fNIRS may serve as a valuable clinical measure of cognition assessment, and IV of bilateral temporal lobes and β value of channel 48 can be used as candidate biomarkers to differentiate individuals with schizophrenia.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Review Committee of the Changning Mental Health Center of Shanghai. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
SZ: Conceptualization, Investigation, Writing – review & editing, Funding acquisition, Writing – original draft, Methodology, Formal Analysis. TL: Resources, Investigation, Data curation, Writing – review & editing. LC: Data curation, Writing – review & editing, Investigation, Resources. TC: Investigation, Data curation, Resources, Writing – review & editing. XK: Methodology, Writing – review & editing, Supervision. YM: Data curation, Resources, Project administration, Conceptualization, Writing – review & editing, Funding acquisition, Methodology, Investigation.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the Multidisciplinary Cross Research Foundation of Shanghai Jiao Tong University (YG2024QNA55); Research Project of Shanghai Changning District Health Commission (20214Y029); Key Specialty Support Project of Shanghai Changning District Health Commission (20233003) and the Youth Project of Shanghai Changning District Health Commission (2024QN16).
Acknowledgments
The authors thank all the subjects who participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Guo JY Ragland JD Carter CS . Memory and cognition in schizophrenia. Mol Psychiatry. (2019) 24:633–42. doi: 10.1038/s41380-018-0231-1
2
Maas DA Martens MB Priovoulos N Zuure WA Homberg JR Nait-Oumesmar B et al . Key role for lipids in cognitive symptoms of schizophrenia. Transl Psychiatry. (2020) 10:399. doi: 10.1038/s41398-020-01084-x
3
Barch DM Ceaser A . Cognition in schizophrenia: core psychological and neural mechanisms. Trends Cognit Sci. (2012) 16:27–34. doi: 10.1016/j.tics.2011.11.015
4
Zhao Y Xiao W Chen K Zhan Q Ye F Tang X et al . Neurocognition and social cognition in remitted first-episode schizophrenia: correlation with VEGF serum levels. BMC Psychiatry. (2019) 19:403. doi: 10.1186/s12888-019-2397-8
5
Mollon J Reichenberg A . Cognitive development prior to onset of psychosis. Psychol Med. (2018) 48:392–403. doi: 10.1017/S0033291717001970
6
Schulz J Sundin J Leask S Done DJ . Risk of adult schizophrenia and its relationship to childhood IQ in the 1958 British birth cohort. Schizophr Bull. (2014) 40:143–51. doi: 10.1093/schbul/sbs157
7
Fett AJ Velthorst E Reichenberg A Ruggero CJ Callahan JL Fochtmann LJ et al . Long-term changes in cognitive functioning in individuals with psychotic disorders: findings from the Suffolk county mental health project. JAMA Psychiatry. (2020) 77:387–96. doi: 10.1001/jamapsychiatry.2019.3993
8
Zanelli J Mollon J Sandin S Morgan C Dazzan P Pilecka I et al . Cognitive change in schizophrenia and other psychoses in the decade following the first episode. Am J Psychiatry. (2019) 176:811–9. doi: 10.1176/appi.ajp.2019.18091088
9
Kumar V Shivakumar V Chhabra H Bose A Venkatasubramanian G Gangadhar BN . Functional near infra-red spectroscopy (fNIRS) in schizophrenia: A review. Asian J Psychiatr. (2017) 27:18–31. doi: 10.1016/j.ajp.2017.02.009
10
Georgiadis F Lariviere S Glahn D Hong LE Kochunov P Mowry B et al . Connectome architecture shapes large-scale cortical alterations in schizophrenia: a worldwide ENIGMA study. Mol Psychiatry. (2024) 29:1869–81. doi: 10.1038/s41380-024-02442-7
11
Mo F Zhao H Li Y Cai H Song Y Wang R et al . Network localization of state and trait of auditory verbal hallucinations in schizophrenia. Schizophr Bull. (2024) 50:1326–36. doi: 10.1093/schbul/sbae020
12
Chou PH Koike S Nishimura Y Satomura Y Kinoshita A Takizawa R et al . Similar age-related decline in cortical activity over frontotemporal regions in schizophrenia: a multichannel near-infrared spectroscopy study. Schizophr Bull. (2015) 41:268–79. doi: 10.1093/schbul/sbu086
13
Wei Y Su W Zhang T Webler R Tang X Zheng Y et al . Structural and functional abnormalities across clinical stages of psychosis: A multimodal neuroimaging investigation. Asian J Psychiatr. (2024) 99:104153. doi: 10.1016/j.ajp.2024.104153
14
Koike S Satomura Y Kawasaki S Nishimura Y Kinoshita A Sakurada H et al . Application of functional near infrared spectroscopy as supplementary examination for diagnosis of clinical stages of psychosis spectrum. Psychiatry Clin Neurosci. (2017) 71:794–806. doi: 10.1111/pcn.12551
15
Chou PH Liu WC Lin WH Hsu CW Wang SC Su KP . NIRS-aided differential diagnosis among patients with major depressive disorder, bipolar disorder, and schizophrenia. J Affect Disord. (2023) 15(341):366–73. doi: 10.1016/j.jad.2023.08.101
16
Wei Y Tang X Zhang T Su W Xu L Cui H et al . Reduced temporal activation during a verbal fluency test in clinical high risk of psychosis: a functional near-infrared spectroscopy-based study. Gen Psychiatr. (2022) 35:e100702. doi: 10.1136/gpsych-2021-100702
17
Kay SR Fiszbein A Opler LA . The positive and negative syndrome scale (Panss) for schizophrenia. Schizophr Bull. (1987) 13:261–76. doi: 10.1093/schbul/13.2.261
18
Rowland LM Pradhan S Korenic S Wijtenburg SA Hong LE Edden RA et al . Elevated brain lactate in schizophrenia: a 7 T magnetic resonance spectroscopy study. Transl Psychiatry. (2016) 6:e967. doi: 10.1038/tp.2016.239
19
Ren Y Cui G Feng K Zhang X Yu C Liu P . A scoping review of utilization of the verbal fluency task in Chinese and Japanese clinical settings with near-infrared spectroscopy. Front Psychiatry. (2024) 15:1282546. doi: 10.3389/fpsyt.2024.1282546
20
Wei Y Chen Q Curtin A Tu L Tang X Tang Y et al . Functional near-infrared spectroscopy (fNIRS) as a tool to assist the diagnosis of major psychiatric disorders in a Chinese population. Eur Arch Psychiatry Clin Neurosci. (2021) 271:745–57. doi: 10.1007/s00406-020-01125-y
21
Qiao Y Song X Yan J Pan W Chia C Zhao D et al . Neurological activation during verbal fluency task and resting-state functional connectivity abnormalities in obsessive-compulsive disorder: a functional near-infrared spectroscopy study. Front Psychiatry. (2024) 15:1416810. doi: 10.3389/fpsyt.2024.1416810
22
Takizawa R Fukuda M Kawasaki S Kasai K Mimura M Pu S et al . Neuroimaging-aided differential diagnosis of the depressive state. Neuroimage. (2014) 85 Pt 1:498–507. doi: 10.1016/j.neuroimage.2013.05.126
23
Hou X Zhang Z Zhao C Duan L Gong Y Li Z et al . NIRS-KIT: a MATLAB toolbox for both resting-state and task fNIRS data analysis. Neurophotonics. (2021) 8(1):010802. doi: 10.1117/1.NPh.8.1.010802
24
Luo R Liao L . Real-time changes in brain activity during sacral neuromodulation for overactive bladder: evidence from functional near-infrared spectroscopy. Front Neurosci. (2025) 19:1436172. doi: 10.3389/fnins.2025.1436172
25
Li RH Yang DL Fang F Hong KS Reiss AL Zhang YC . Concurrent fNIRS and EEG for brain function investigation: A systematic, methodology-focused review. Sensors-Basel. (2022) 22:5865. doi: 10.3390/s22155865
26
AL-Quraishi MS Elamvazuthi I Tang TB Al-Qurishi M Adil SH Ebrahim M . Bimodal Data Fusion of Simultaneous Measurements of EEG and fNIRS during Lower Limb Movements. Brain Sci. (2021) 11:713. doi: 10.3390/brainsci11060713
27
Akin A . fNIRS-derived neurocognitive ratio as a biomarker for neuropsychiatric diseases. Neurophotonics. (2021) 8:35008. doi: 10.1117/1.NPh.8.3.035008
28
Xia D Quan W Wu T . Optimizing functional near-infrared spectroscopy (fNIRS) channels for schizophrenic identification during a verbal fluency task using metaheuristic algorithms. Front Psychiatry. (2022) 13:939411. doi: 10.3389/fpsyt.2022.939411
29
Tassi E Boscutti A Mandolini GM Moltrasio C Delvecchio G Brambilla P . A scoping review of near infrared spectroscopy studies employing a verbal fluency task in bipolar disorder. J Affect Disord. (2022) 298:604–17. doi: 10.1016/j.jad.2021.11.019
30
Yang J Ji X Quan W Liu Y Wei B Wu T . Classification of schizophrenia by functional connectivity strength using functional near infrared spectroscopy. Front Neuroinform. (2020) 14:40. doi: 10.3389/fninf.2020.00040
31
Zhu G Zhang H Wei X Jing H Zhang H Zhao S et al . Increased and sex-differentiated medial prefrontal cortex activation during the MATRICS Consensus Cognitive Battery in schizophrenia: A fNIRS study. Asian J Psychiatr. (2024) 99:104137. doi: 10.1016/j.ajp.2024.104137
32
Quan WX Wu TN Li ZH Wang YD Dong WT Lv B . Reduced prefrontal activation during a verbal fluency task in Chinese-speaking patients with schizophrenia as measured by near-infrared spectroscopy. Prog Neuropsychopharmacol Biol Psychiatry. (2015) 58:51–8. doi: 10.1016/j.pnpbp.2014.12.005
33
Molina V Lubeiro A Soto O Rodriguez M Alvarez A Hernandez R et al . Alterations in prefrontal connectivity in schizophrenia assessed using diffusion magnetic resonance imaging. Prog Neuropsychopharmacol Biol Psychiatry. (2017) 76:107–15. doi: 10.1016/j.pnpbp.2017.03.001
34
Ferrarelli F Riedner BA Peterson MJ Tononi G . Altered prefrontal activity and connectivity predict different cognitive deficits in schizophrenia. Hum Brain Mapp. (2015) 36:4539–52. doi: 10.1002/hbm.22935
35
Hernandez-Sauret A Martin de la Torre O Redolar-Ripoll D . Use of transcranial magnetic stimulation (TMS) for studying cognitive control in depressed patients: A systematic review. Cognit Affect Behav Neurosci. (2024) 24:972–1007. doi: 10.3758/s13415-024-01193-w
36
Francis MM Hummer TA Vohs JL Yung MG Visco AC Mehdiyoun NF et al . Cognitive effects of bilateral high frequency repetitive transcranial magnetic stimulation in early phase psychosis: a pilot study. Brain Imaging Behav. (2019) 13:852–61. doi: 10.1007/s11682-018-9902-4
37
Chang Q Li CC Tian Q Bo QJ Zhang JC Xiong YB et al . Classification of first-episode schizophrenia, chronic schizophrenia and healthy control based on brain network of mismatch negativity by graph neural network. IEEE T Neur Sys Reh. (2021) 29:1784–94. doi: 10.1109/Tnsre.2021.3105669
38
Rogasch NC Daskalakis ZJ Fitzgerald PB . Cortical inhibition, excitation, and connectivity in schizophrenia: A review of insights from transcranial magnetic stimulation. Schizophr Bulletin. (2014) 40:685–96. doi: 10.1093/schbul/sbt078
39
Radhu N Dominguez LG Farzan F Richter MA Semeralul MO Chen R et al . Evidence for inhibitory deficits in the prefrontal cortex in schizophrenia. Brain. (2015) 138:483–97. doi: 10.1093/brain/awu360
40
Zhang S Li W Xiang Q Kuai X Zhuo K Wang J et al . Longitudinal alterations of modular functional-metabolic coupling in first-episode schizophrenia. J Psychiatr Res. (2022) 156:705–12. doi: 10.1016/j.jpsychires.2022.10.067
41
Lu X Zhang Y Zhong S Lai S Yan S Song X et al . Cognitive impairment in major depressive disorder with non-suicidal self-injury: Association with the functional connectivity of frontotemporal cortex. J Psychiatr Res. (2024) 177:219–27. doi: 10.1016/j.jpsychires.2024.07.008
42
Eastmond C Subedi A De S Intes X . Deep learning in fNIRS: a review. Neurophotonics. (2022) 9:41411. doi: 10.1117/1.NPh.9.4.041411
43
Li Z Wang Y Quan W Wu T Lv B . Evaluation of different classification methods for the diagnosis of schizophrenia based on functional near-infrared spectroscopy. J Neurosci Methods. (2015) 241:101–10. doi: 10.1016/j.jneumeth.2014.12.020
Summary
Keywords
schizophrenia, cognition, diagnosis, fNIRS, VFT
Citation
Zhang S, Li T, Chen L, Cong T, Kuai X and Mu Y (2025) Functional near-infrared spectroscopy as a diagnostic aid for stable schizophrenia. Front. Psychiatry 16:1635854. doi: 10.3389/fpsyt.2025.1635854
Received
27 May 2025
Accepted
18 August 2025
Published
22 September 2025
Volume
16 - 2025
Edited by
Rihui Li, University of Macau, China
Reviewed by
Yuxi Luo, Sun Yat-sen University, China
Tongning Wu, China Academy of Information and Communications Technology, China
Updates
Copyright
© 2025 Zhang, Li, Chen, Cong, Kuai and Mu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yonggang Mu, myg613@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.