- 1School of Medicine, Ningbo University, Ningbo, Zhejiang, China
- 2The Affiliated Kangning Hospital of Ningbo University, Ningbo, Zhejiang, China
- 3Nursing Department, Ningbo College of Health Sciences, Ningbo, Zhejiang, China
- 4Lishui Second People’s Hospital, Lishui, Zhejiang, China
Background: Depression is a highly heterogeneous disorder with complex mechanisms. Given converging evidence implicating the amygdala in its pathophysiology, a systematic and quantitative synthesis is warranted to map the research landscape, hotspots, and emerging trends.
Objective: To systematically characterize the research landscape of depression–amygdala studies from 2015 to 2024 using bibliometric and visualization analyses, identify core hotspots and emerging themes, and track their evolution to inform subsequent mechanistic research and precision interventions.
Methods: Using data from the Web of Science Core Collection and Scopus (2015–2024), we conducted a bibliometric analysis of English-language publications on depression and the amygdala with Bibliometrix and VOSviewer. Publication trends, country and institutional contributions, highly cited papers, and keyword co-occurrence/clustering were analyzed to delineate the field’s structure and evolution.
Results: A total of 5,999 publications were included. Annual output increased steadily from 399 in 2015 to a peak of 831 in 2024. The United States (1,813, 30.2%), China (1,122, 18.7%), and Germany (357, 6.0%) were the top contributors. The ratio of multi-country publications (MCP) was highest for Germany (28.3%), followed by the United Kingdom (28.1%) and Canada (24.6%). At the institutional and journal levels, the University of California system, Harvard University, and Harvard Medical School ranked among the leading contributors. The international collaboration network exhibited a U.S.-centered structure, with frequent partnerships between the United States and China (n = 113), the United States and the United Kingdom (n = 86), and the United States and Germany (n = 81). Keyword co-occurrence and clustering analyses revealed four major thematic clusters: emotion regulation networks, biological signaling and regulation, developmental risk factors, and animal models. Overall, the field has evolved from region-specific studies toward network- and system-level integration, highlighting the amygdala’s pivotal role in the neurobiological mechanisms of depression and its potential as a candidate neuromarker.
Conclusion: Bibliometric evidence indicates that research on depression–amygdala relationships is moving toward multi-dimensional and cross-disciplinary integration. The amygdala’s involvement in emotion regulation and early-life stress is increasingly recognized; alterations in its functional connectivity show promise as a neuromarker of depression, though clinical translation requires multi-center validation and standardized analytic workflows.
1 Introduction
Mental disorders are a group of conditions characterized by persistent disturbances in emotion, cognition, or behavior that significantly impair functioning in learning, work, and interpersonal life. Major categories include depressive disorders, bipolar and related disorders, anxiety disorders, trauma- and stressor-related disorders, obsessive–compulsive and related disorders, psychotic disorders, and substance use disorders (1). Among these, depressive disorders are the most prevalent and represent major causes of disability and socioeconomic burden, thereby attracting sustained attention from both society and the scientific community (1–3). Depression is a common psychiatric disorder characterized by persistent low mood, loss of interest or pleasure, and reduced motivation, often accompanied by cognitive impairment, sleep disturbances, appetite changes, and other physiological or psychological symptoms (4). It severely affects emotional well-being, daily functioning, and overall quality of life, imposing substantial burdens on social and occupational performance (5). In recent years, the global prevalence of depression has continued to rise, with high relapse and disability rates making it one of the most pressing public health challenges worldwide (6). According to the World Health Organization (WHO), over 350 million people are affected by depression globally, with notably high incidence among adolescents, the elderly, and women (7). Forecasts suggest that by 2030, depression will rank among the top three causes of global disease burden, particularly in low- and middle-income countries where its societal and health impacts are more pronounced (8). Despite ongoing advances in research, clinical diagnosis still primarily relies on interviews and self-report scales, with a lack of objective and quantifiable biological markers (9). Consequently, identifying brain regions with potential as biomarkers—particularly those exhibiting functional abnormalities—has become a critical direction in elucidating the pathophysiology of depression.
Among these regions, the amygdala has garnered significant attention due to its pivotal role in emotion and cognitive processing. As a core structure of the limbic system, the amygdala is extensively connected to the cerebral cortex, subcortical areas, and brainstem, functioning as a hub in brain networks that support emotion regulation, learning, memory consolidation, attention allocation, and executive function (10, 11). In emotional processing, the amygdala is highly responsive to negative emotions such as fear and anger, rapidly activating upon exposure to threatening stimuli and initiating autonomic responses that modulate affective states (10, 12). In memory-related functions, the amygdala plays a key role in encoding and consolidating emotionally salient memories, particularly by interacting with the hippocampus and modulating neurotransmitter activity (13). While these functions support environmental adaptation, they may become dysregulated in pathological conditions. Neuroimaging studies indicate that, in response to negative emotional stimuli, patients with depression commonly exhibit amygdala hyperactivation (14–22), accompanied by reduced functional connectivity between the amygdala and prefrontal cortical regions (23–25). This neural phenotype is consistent with the negative information-processing bias, deficits in emotion regulation, and alterations in emotional memory observed in depression, and may contribute to the persistence and exacerbation of symptoms (26–29). As such, amygdala dysfunction is considered a central node in the neural mechanisms of depression and a potential target for intervention and neuroimaging-based biomarker development (30).
In recent years, bibliometric analysis—an approach based on publication, citation, and text-mining data—has been increasingly employed to assess research trends and scientific landscapes across disciplines (31). By quantifying relationships among authors, institutions, keywords, and publications, bibliometric methods reveal developmental trajectories, research hotspots, and the knowledge structure of a given field (31). This includes not only descriptive statistics such as publication volume and citation frequency but also advanced network analyses such as keyword co-occurrence, collaboration networks, and document clustering (32). Based on this, the present study focuses on literature addressing “amygdala” and “depression” from 2015 to 2024, employing bibliometric and visualization approaches using VOSviewer, ggplot2, and Bibliometrix. Through analyses of publication trends, country and institutional contributions, high-frequency keywords, co-occurrence networks, and literature clustering, this study aims to systematically map the central role of the amygdala in depression research, identify thematic hotspots, and elucidate the field’s evolutionary patterns. The findings are expected to provide theoretical and data-driven support for future mechanistic studies and the development of optimized intervention strategies. Meanwhile, advances in artificial medical intelligence and the Internet of Medical Things (IoMT) have opened avenues for multimodal integration of genetic and hormonal indicators with neural circuit–level readouts (33, 34). This technological convergence complements the amygdala-centered perspective of the present study and provides a foundation for hypothesis generation and translational exploration.
This study makes four key contributions. First, it merges and deduplicates Web of Science and Scopus records from 2015 to 2024 and applies a reproducible bibliometric workflow to map amygdala-related depression research. Second, it quantifies the global structure by characterizing a collaboration network centered on the United States, and by examining differences in the proportion of multi-country publications, thereby linking research output to patterns of knowledge flow. Third, it robustly identifies four major themes—emotion regulation networks, molecular and neural regulation, developmental risk factors, and animal models—demonstrating a shift toward system-level integration. Fourth, it highlights amygdala functional connectivity as a potential neuromarker for patient stratification and treatment prediction.
2 Methods
2.1 Data sources and literature search strategy
This bibliometric analysis was conducted using the Web of Science Core Collection (WoS) and Scopus databases—two of the most authoritative and widely used academic literature repositories (35). WoS indexes approximately 12,000 high-impact journals and is known for its precise citation tracking, standardized data, and broad subject coverage (36). Scopus includes over 24,000 journals and is recognized for its strong subject representation and robust search capabilities (37). Both databases adopt rigorous journal selection criteria, ensuring high data quality, credibility, and reproducibility. To enhance the comprehensiveness and robustness of the results, both WoS and Scopus were used as data sources in this study.
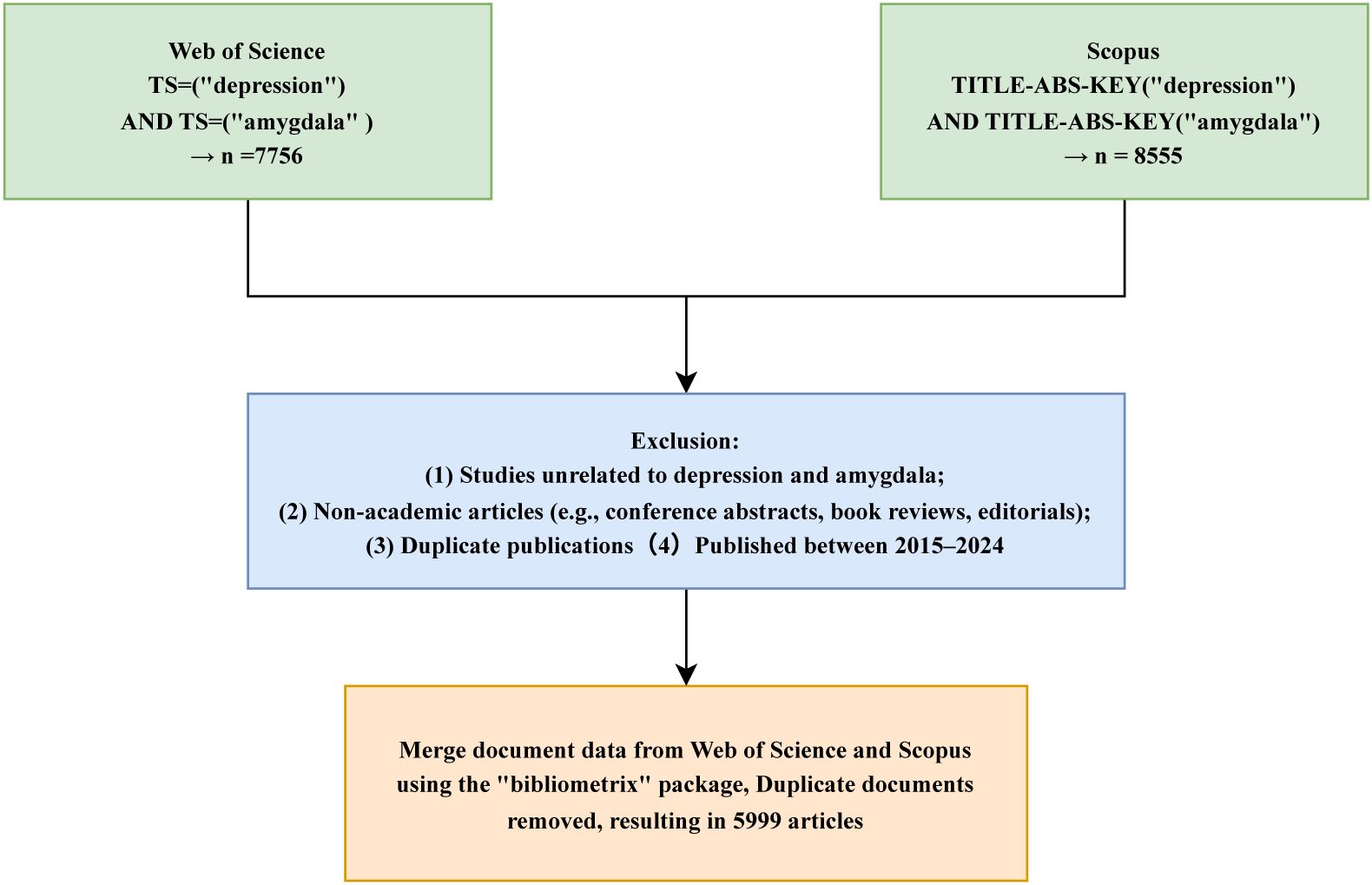
The search strategy focused on the keywords “depression” and “amygdala,” using the query TS = (“depression”) AND TS = (“amygdala”) for WoS and TITLE-ABS-KEY = (“depression”) AND TITLE-ABS-KEY = (“amygdala”) for Scopus. The inclusion criteria were as follows (1): English-language publications (2); document type limited to “Article”; and (3) publication years between 2015 and 2024. Exclusion criteria included conference abstracts, proceedings, book reviews, editorials, and other non-peer-reviewed materials. All searches and data exports were completed on April 27, 2025, to ensure consistency and reproducibility. Exported records were saved in.txt format, including core metadata fields such as article titles, authors, publication years, journal names, keywords, abstracts, and citation counts. The literature selection process is illustrated in Figure 1. The time window was restricted to 2015–2024 to enhance comparability within the DSM-5 diagnostic framework and to reflect the relative stability of MRI acquisition and preprocessing pipelines, while focusing on contemporary research trends.
2.2 Literature screening and data processing
After the initial data export, all records were manually reviewed and cleaned. Publications not meeting the inclusion criteria were removed, and duplicates were eliminated. Only peer-reviewed original research articles and review papers were retained. Records from the two databases were subsequently merged, and duplicate entries were systematically removed. The final dataset included information pertaining to key bibliometric indicators, such as publication volume, total citations, keyword distribution, author and collaboration networks, research institutions, countries/regions, journal sources, and cited references. In addition, cross-disciplinary studies have demonstrated the effectiveness of systems mapping combined with network visualization in identifying thematic structures and tracing knowledge evolution (38). Bibliometric research in the Internet of Medical Things (IoMT) domain has likewise demonstrated the feasibility of network-based hotspot detection and topic evolution tracking (39), providing methodological precedent and external validation for the visualization framework used in this study.
2.3 Data analysis and visualization
To comprehensively identify developmental trajectories, research hotspots, and emerging themes in the field of depression and the amygdala, this study employed a combination of bibliometric tools and visualization software, including Bibliometrix (v4.4.3), ggplot2 (v3.5.1), and VOSviewer (v1.6.19). Bibliometrix (v4.4.3) was used to perform fundamental bibliometric analyses, including annual publication trends, author and institutional productivity, citation frequencies, journal distributions, and country-level contributions. It also generated time-series plots, collaboration networks, and thematic evolution maps (31). ggplot2 (v3.5.1) was utilized to enhance the visual presentation of results, generating trend charts, heatmaps, and keyword distribution plots for improved clarity and aesthetic quality (40). VOSviewer (v1.6.19) was employed to construct keyword co-occurrence networks, co-citation networks, and cluster visualizations (41). In these network maps, node size represents keyword frequency or the number of publications, edge thickness indicates co-occurrence or co-citation strength, and node color differentiates between clusters or time periods.
3 Results
3.1 Annual publication trends
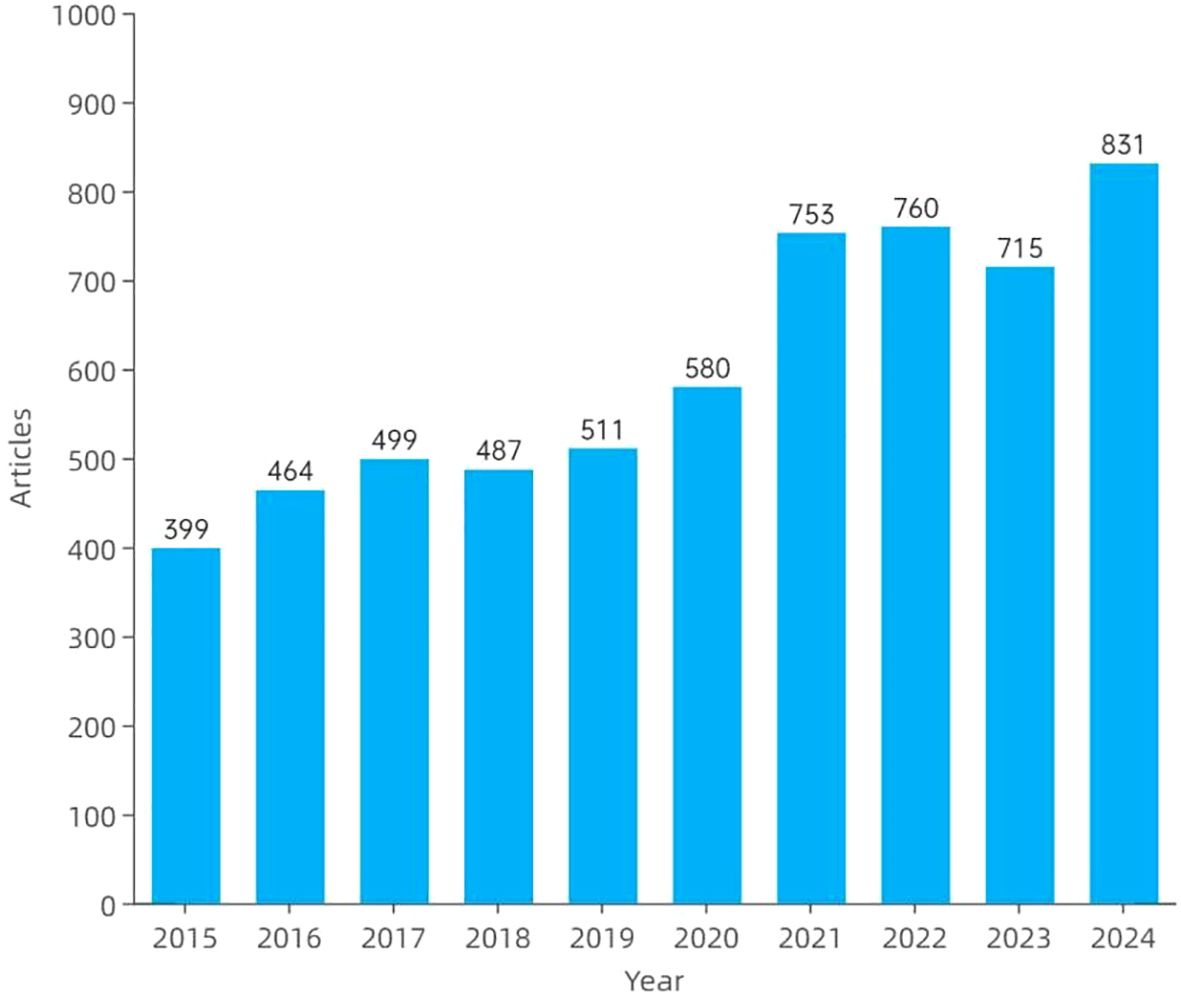
Figure 2 illustrates the annual publication trends in amygdala–depression research from 2015 to 2024. A total of 5,999 publications were included, consisting of 4,819 original articles and 1,180 review papers. Overall, the number of publications showed a consistent upward trajectory, increasing from 399 articles in 2015 to 831 in 2024. The growth was particularly evident from 2020 onwards, with 753 and 760 articles published in 2021 and 2022, respectively. Although a slight decline was observed in 2023, the number of publications rebounded in 2024, reaching an all-time high.
3.2 Analysis of country, institution, journal, and author distributions
In the field of amygdala–depression research, scientific output showed a marked concentration across countries, institutions, journals, and authors. Among 71 countries and regions, the United States ranked first in publication output (N = 1,813, 30.2%), followed by China (N = 1,122, 18.7%) and Germany (N = 357, 6.0%). Germany showed the highest rate of multiple-country publications (MCP = 28.3%), indicating stronger engagement in international collaboration, followed by the United Kingdom (28.1%) and Canada (24.6%) (Table 1).
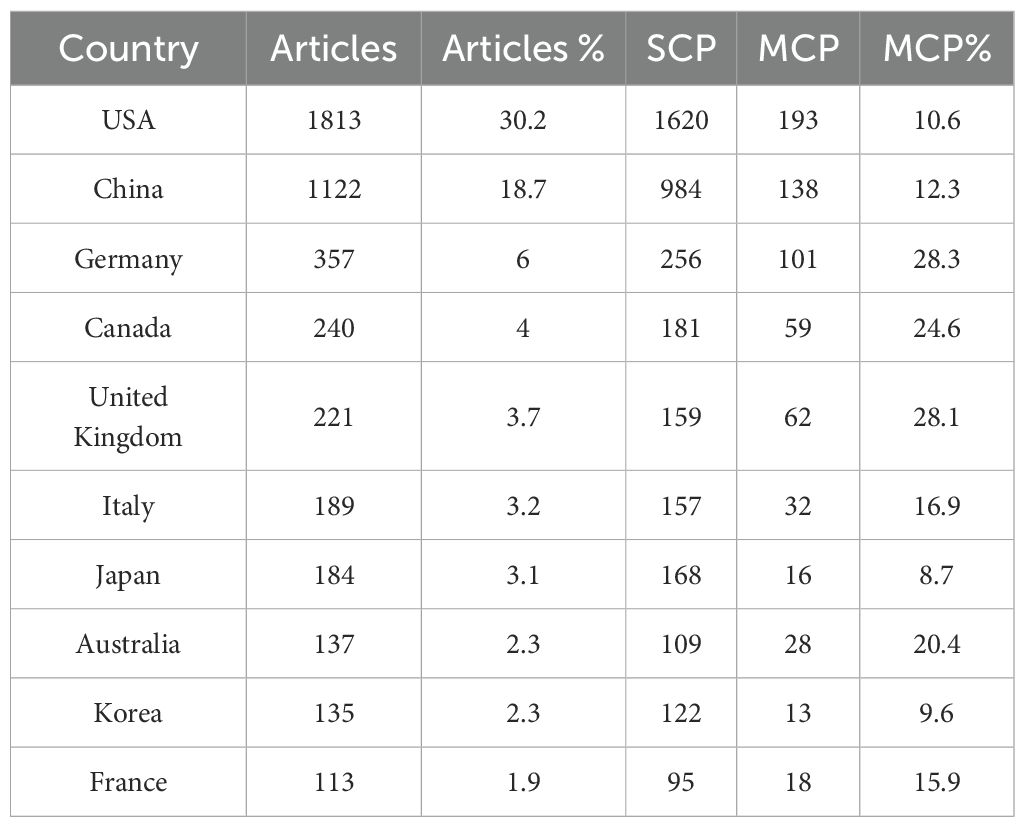
Table 1. Top 10 countries or regions by depression and amygdala publication volume from 2015 to 2024.
In terms of institutional output, the top three institutions were the University of California System (N = 435), Harvard University (N = 403), and Harvard Medical School (N = 376), as shown in Figure 3A. A total of 1,037 journals published literature in this field, with the top 10 journals contributing 1,103 papers. The Journal of Affective Disorders published the most articles (N = 210), followed by Translational Psychiatry (N = 133), Frontiers in Psychiatry (N = 122), and Behavioral Brain Research (N = 103) (Figure 3B). The most cited journal was Biological Psychiatry (9,638 citations), followed by NeuroImage (8,364) and the Journal of Neuroscience (6,724) (Figure 3C). A total of 27,564 authors contributed to this research domain. The top five most productive authors were Wang Y (N = 110), Zhang Y (N = 83), Li Y (N = 66), Wang J (N = 59), and Dannlowski U (N = 50), collectively contributing 368 publications, accounting for 6.13% of the total output (Figure 3D).
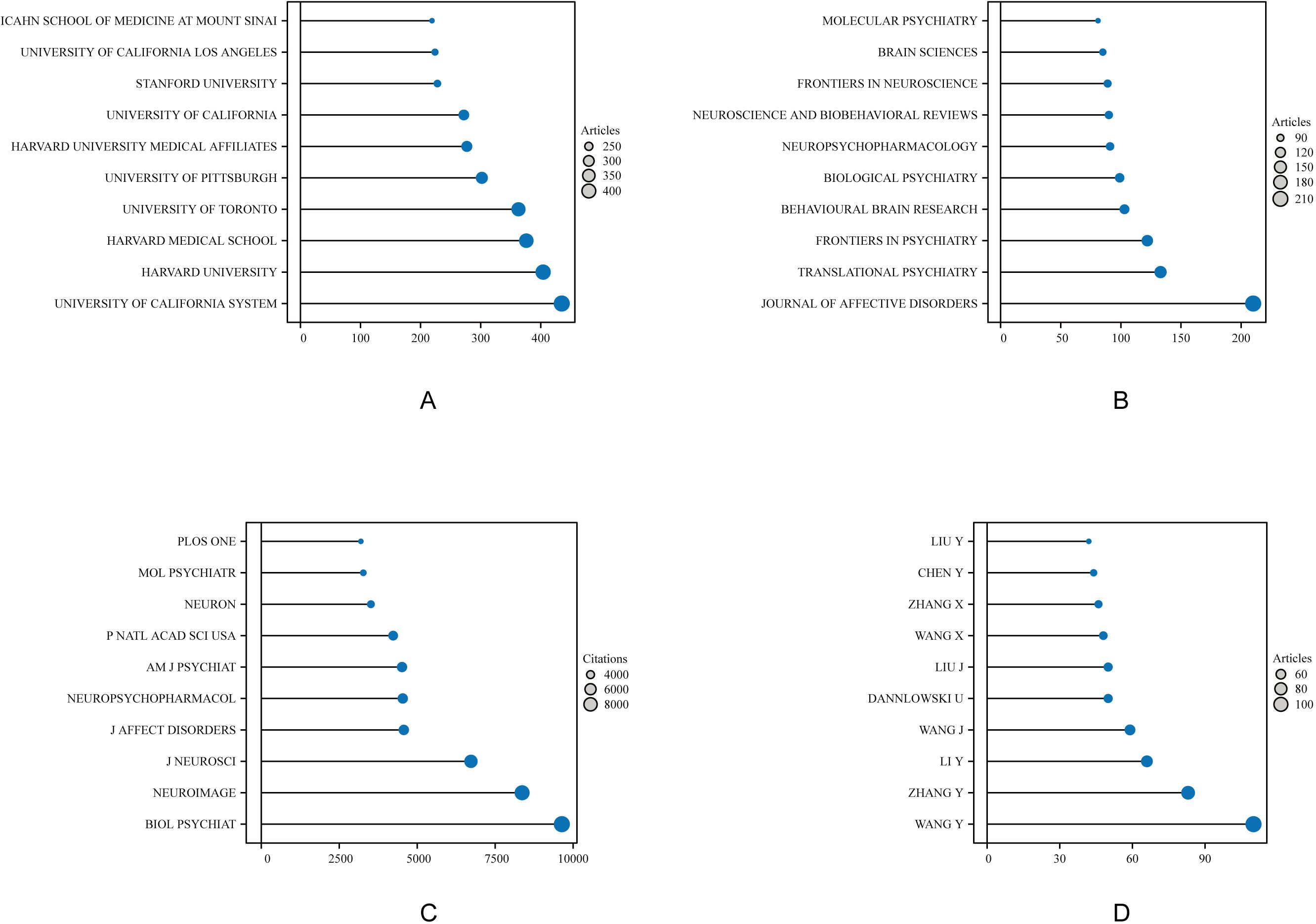
Figure 3. Top 10 institutions, journals, and authors in amygdala and depression research from 2015 to 2024. Each colored circle represents a distinct institution, journal, or author, with larger circles indicating higher counts. (A) Displays the top-ranking institutions by publication volume, reflecting their academic contribution and influence in this field. (B) Shows the journals with the highest number of publications, highlighting the core journals with strong academic impact. (C) Illustrates the top journals by total citation count, indicating their influence in knowledge dissemination and research recognition. (D) Depicts the top 10 most prolific authors, emphasizing their output and role in shaping this research domain.
3.3 Country and regional collaboration analysis
Figure 4 presents the global collaboration network in amygdala–depression research. The figure uses a geographical visualization to illustrate inter-country scientific cooperation, where the density and distribution of connecting lines reflect collaboration intensity. As shown in Figure 4; Table 2, the United States occupies a central position in the international collaboration network, maintaining strong research ties with multiple countries. The most frequent collaboration occurred between the United States and China (n = 113), followed by the United Kingdom (n = 86), Germany (n = 81), and Canada (n = 78). The United States also maintained active partnerships with Australia (n = 51) and the Netherlands (n = 44). Within Europe, Germany demonstrated notable regional collaboration, particularly with Switzerland (n = 49), the United Kingdom (n = 48), and the Netherlands (n = 39). Overall, the international collaboration network was primarily concentrated in North America, Western Europe, East Asia, and Oceania, forming a multilateral structure centered on the United States and radiating toward Germany, China, the United Kingdom, and other key countries. Frequent collaborations between China and the United States, and between the United States and European countries, highlighted a high degree of international integration in this research domain, facilitating global knowledge exchange and advancing the study of amygdala–depression relationships.
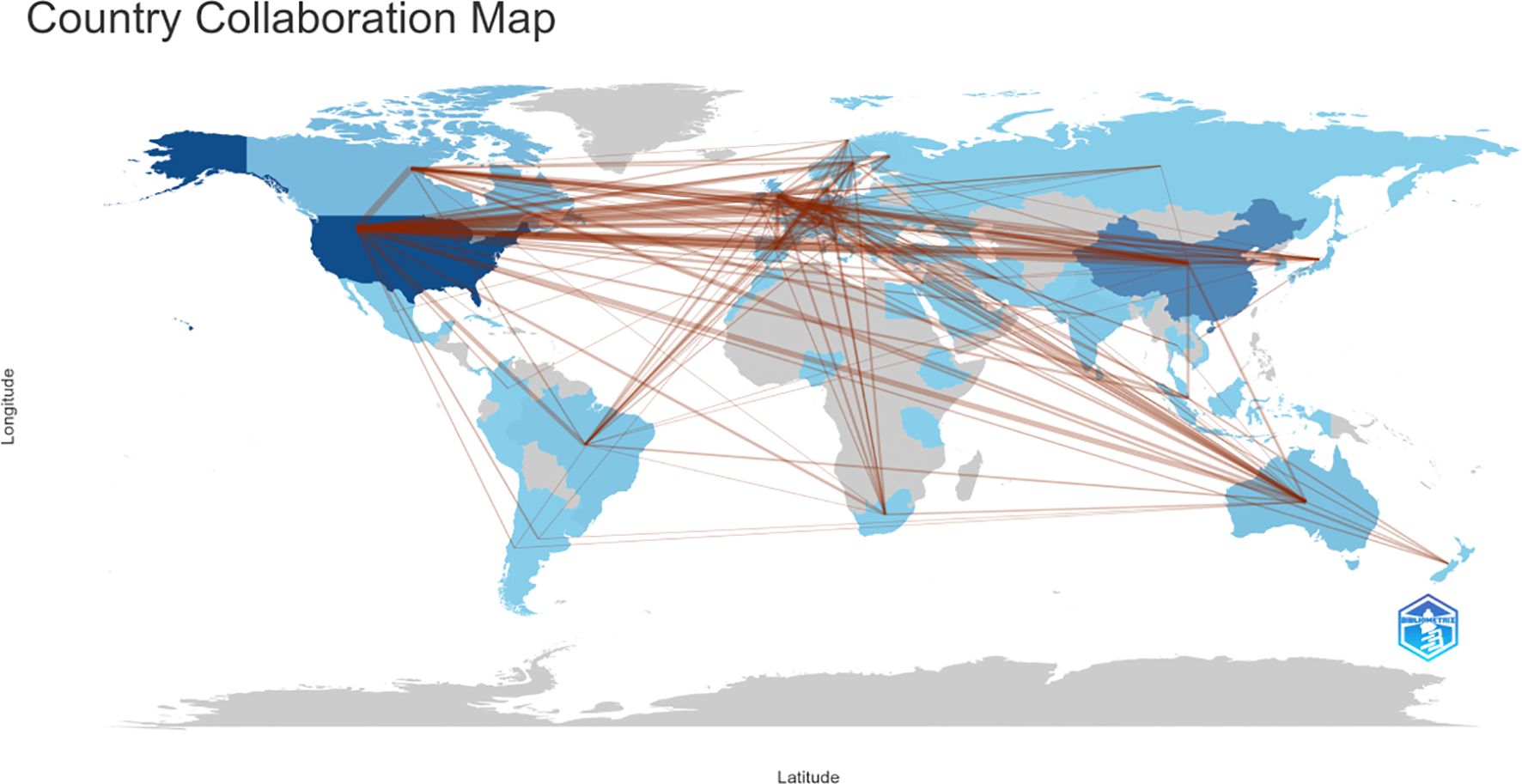
Figure 4. Country and regional collaboration in amygdala and depression research (2015–2024). The color segmentation includes blue (with publications) and grey (without publications). The thickness of the red lines indicates the number of co-published papers. The color intensity corresponds to the number of publications).
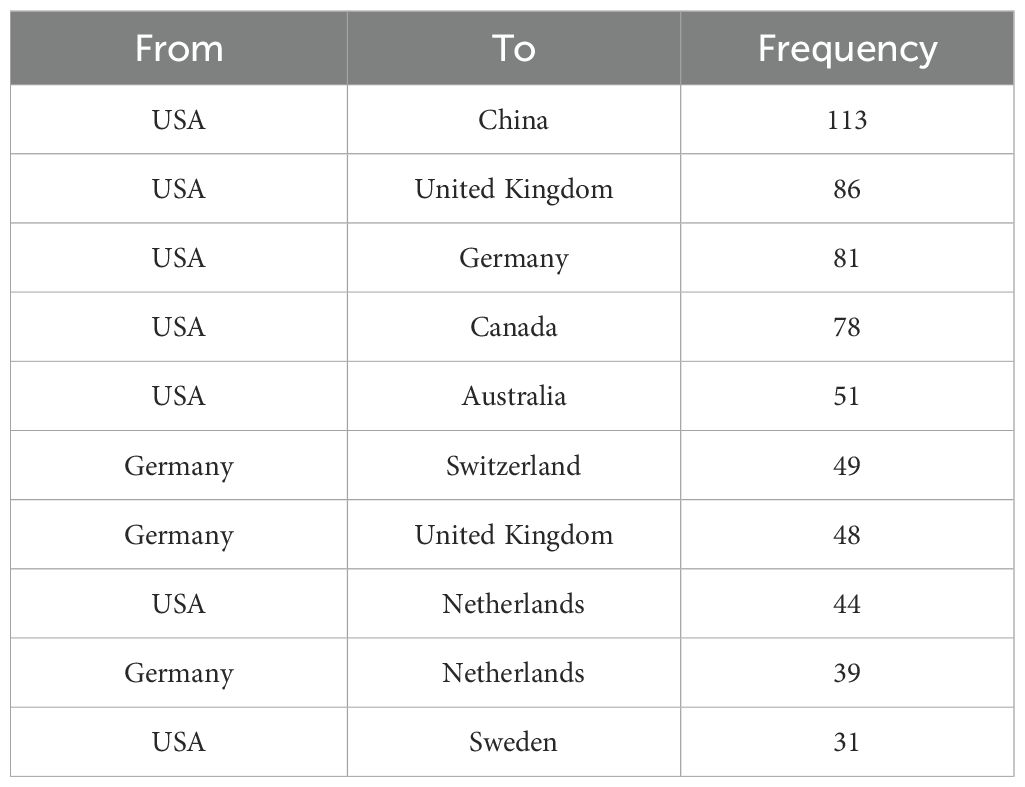
Table 2. Top 10 collaboration pairs among countries in amygdala and depression research from 2015 to 2024.
3.4 Global literature co-citation analysis
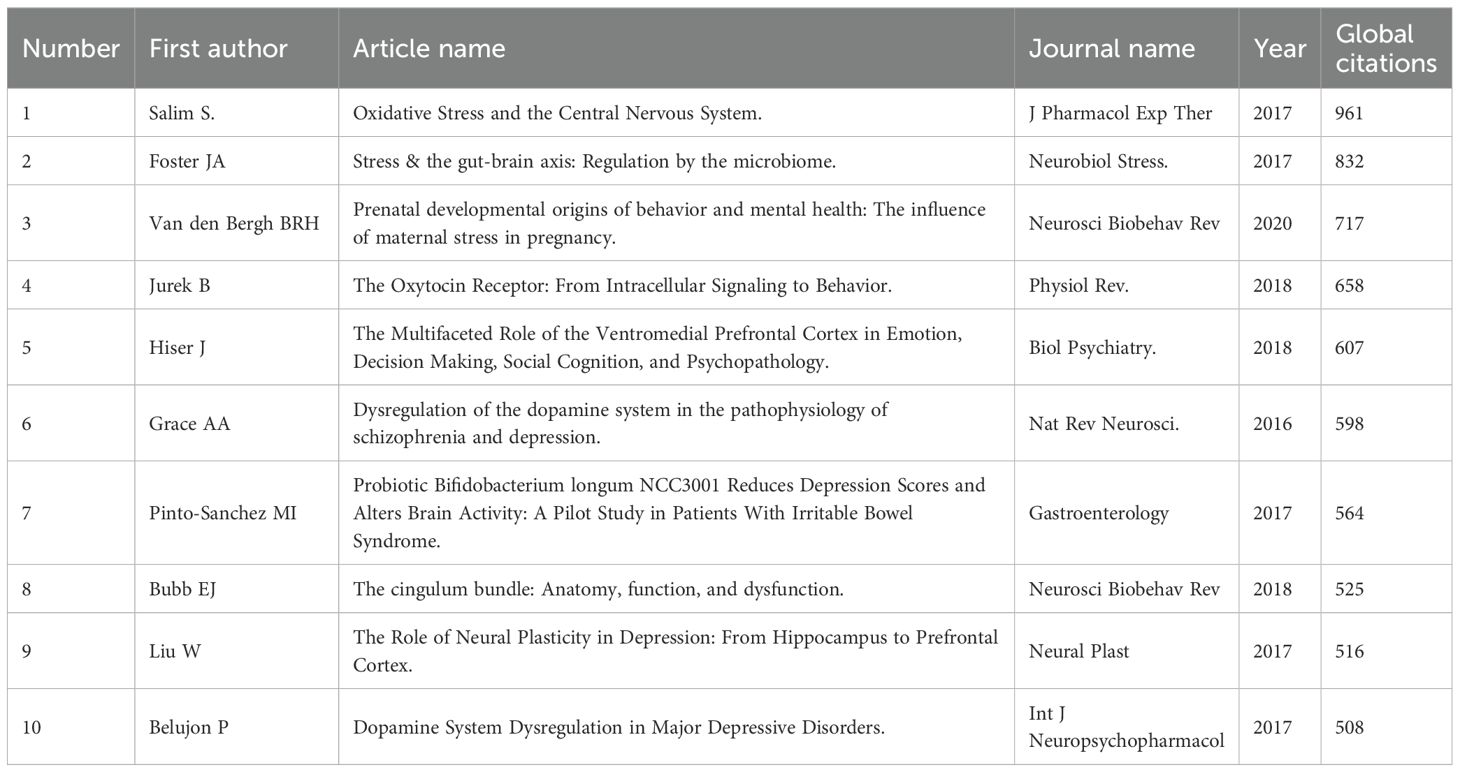
Global citations are a key bibliometric indicator, reflecting the total number of times a document has been cited across databases. They serve as a measure of a publication’s overall influence within the academic literature. In amygdala–depression research, the most globally cited publication was a 2017 review by Salim S., titled “Oxidative Stress and the Central Nervous System,” published in Journal of Pharmacology and Experimental Therapeutics, with 961 citations. This article emphasized the critical role of oxidative stress in central nervous system dysfunction. The second most cited work was by Foster J.A., also from 2017, titled “Stress and the Gut–Brain Axis: Regulation by the Microbiome,” published in Neurobiology of Stress, with 832 citations. It highlighted the role of gut microbiota in modulating stress responses through the gut–brain axis. Ranked third was a 2020 review by Van den Bergh B.R.H., titled “Prenatal Developmental Origins of Behavior and Mental Health: The Influence of Maternal Stress in Pregnancy,” published in Neuroscience & Biobehavioral Reviews, with 717 citations. This work systematically explored the long-term effects of prenatal stress on neurodevelopment and mental health outcomes (Table 3).
3.5 Keyword co-occurrence and research hotspots
To visually demonstrate the distribution of research themes in the field of amygdala and depression, word cloud and pie charts were generated based on author keywords. Additionally, the “KeyWords Plus” field was extracted using Bibliometrix, yielding a total of 20,355 keywords. High-frequency terms were primarily related to brain structure, psychiatric disorders, population characteristics, and research methodologies. The most frequently occurring keywords were “amygdala” (3,778 occurrences, 7%) and “depression” (3,125 occurrences, 6%), underscoring their central role in this research area. Other frequently occurring terms included “male” (2,679), “female” (2,197), “brain” (1,918), “hippocampus” (1,716), “anxiety” (1,556), “controlled study” (1,485), and “prefrontal cortex” (1,262), reflecting a strong research focus on sex differences, emotional disorders, and the relationship between brain structure and function (Figure 5A).
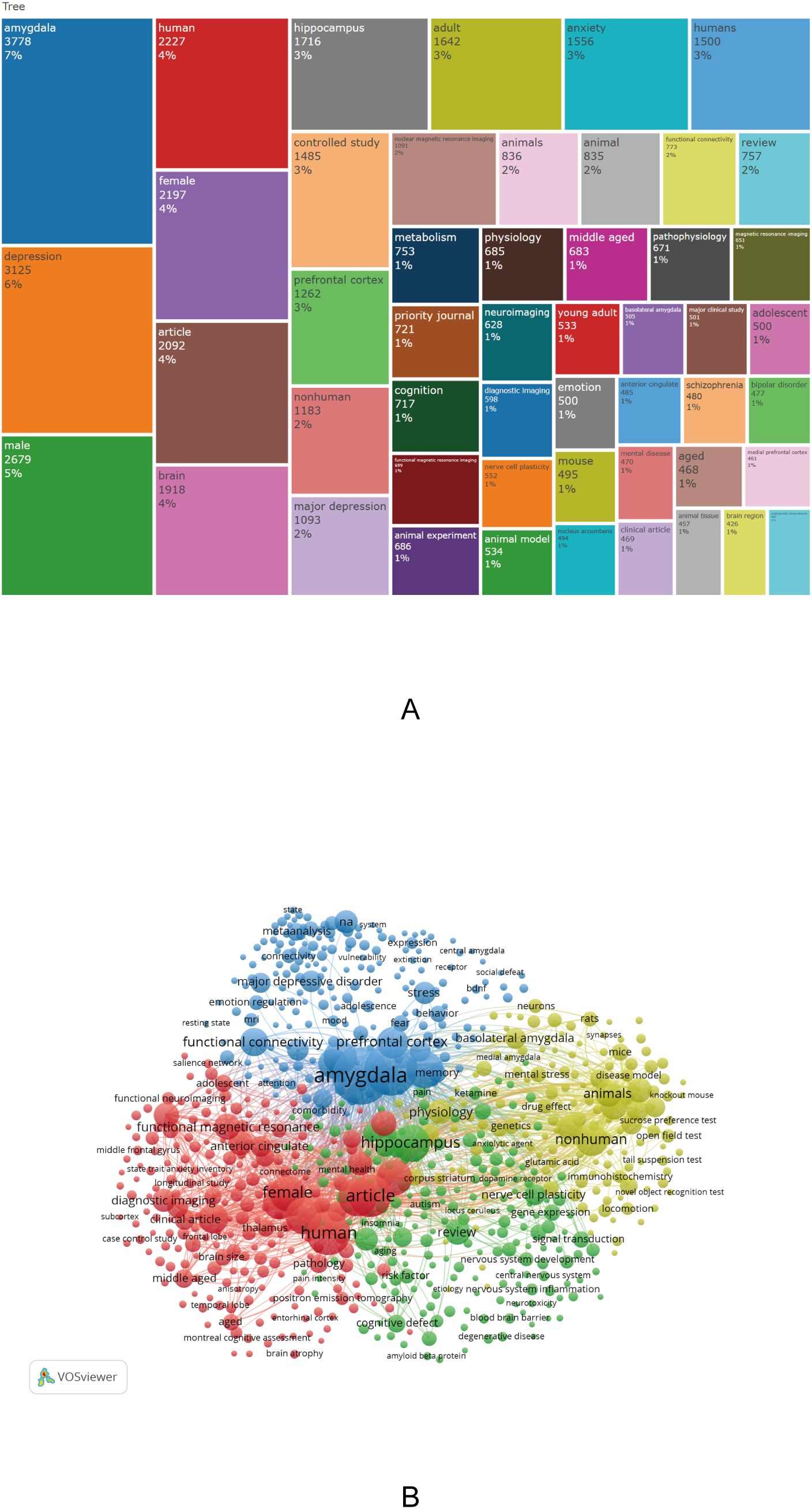
Figure 5. Keyword clustering analysis in depression–amygdala research. (A) Dendrogram of the top 50 keywords in the field. (B) Keyword co-occurrence cluster map (2015–2024) generated by Bibliometrix.
In addition, a comprehensive keyword co-occurrence analysis was conducted using VOSviewer, with the parameters set to “All keywords” and “Full counting.” A total of 750 keywords with a minimum occurrence of 40 were identified and grouped into four thematic clusters, visualized in red, green, blue, and yellow, reflecting the multidimensional thematic structure of the field (Figure 5B). Cluster 1 (Red) focuses on neuroimaging features, abnormal brain function, and cognitive impairment; Cluster 2 (Green) emphasizes neurotransmitter regulation, gene expression, and pharmacological mechanisms, reflecting the biological underpinnings of depression; Cluster 3 (Blue) highlights emotional regulation and developmental risk factors, particularly the impact of early-life adversity on emotion processing and amygdala function; and Cluster 4 (Yellow) comprises animal model–based experimental research, focusing on neural circuitry, endocrine regulation, and behavioral mechanisms. Collectively, these results indicate that research on amygdala–depression spans multiple domains, including neuroimaging, biological mechanisms, psychological development, and experimental neuroscience.
4 Discussion
In recent years, research on the amygdala and depression has shown a marked increase and conceptual shift. The bibliometric results of this study reveal that global publications have risen sharply since 2020, with research focus gradually moving from single region functional analyses toward multilevel explorations of neural networks and systemic mechanisms. This trend reflects not only advances in neuroimaging and data analytics but also a growing consensus within the scientific community that the pathophysiology of depression has shifted from a “localized abnormality” model to a “systems integration” framework. Close collaboration among research institutions in the United States, China, and Europe has further accelerated this transition, propelling the field from fragmented studies toward a globally connected and mechanistically integrated stage of development.
4.1 Global landscape and research evolution: from regional abnormalities to systemic integration
The continuous expansion of research on depression and the amygdala reflects an increasingly sophisticated understanding of the core emotional circuitry underlying mood disorders. Early investigations primarily focused on structural alterations or activation differences within the amygdala, whereas more recent high-impact studies have revealed broader system-level mechanisms. Salim (2017) identified oxidative stress as a key contributor to central nervous system dysfunction (42); Foster (2017) and Pinto-Sanchez (2017) extended this concept to the gut–brain axis, emphasizing that microbiota can influence amygdala activity through immune and metabolic pathways, thereby modulating emotional regulation (43, 44). Van den Bergh (2020) demonstrated that prenatal stress disrupts the development of amygdala–prefrontal circuits, providing a neurodevelopmental basis for early preventive interventions (45).
In addition, several pivotal studies have expanded this mechanistic framework by linking the amygdala to neurotransmitter and structural pathways. Jurek (2018) and Hiser (2018) revealed that oxytocin and its receptor modulate connectivity between the amygdala and ventromedial prefrontal cortex (vmPFC), offering potential therapeutic targets (46, 47). Grace (2016) and Belujon (2017) reported that anhedonia in depression may stem from dysregulation of dopaminergic input from the amygdala and ventral hippocampus (48, 49). Structurally, Bubb et al. (2018) highlighted the cingulum bundle as a crucial tract linking the amygdala to the frontal lobe, suggesting that its disruption may underlie impaired emotion–cognition integration (50). Collectively, these findings converge on the amygdala as a multimodal hub integrating oxidative, immune, endocrine, and neurocircuit mechanisms—laying the groundwork for emerging systems-level frameworks of depression.
Consequently, the neurobiological model of depression has evolved from a single-region abnormality paradigm to a cross-system dysregulation framework, in which oxidative stress, inflammation, hormones, and neurotransmitters converge on the amygdala to jointly modulate emotion and cognition. This mechanistic transition parallels the bibliometric trends observed in this study, with a marked increase in interdisciplinary collaborations and multimodal imaging projects since 2020. These initiatives emphasize the interplay among functional connectivity, inflammatory signaling, and behavioral phenotypes, reflecting a collective shift in the field—from identifying isolated abnormalities to elucidating integrated mechanisms and constructing multilevel explanatory models.
4.2 Thematic clusters and scientific implications: integrating multi-level mechanisms
Keyword clustering analysis revealed four major thematic clusters within the depression–amygdala research field. These clusters are not independent but together form a continuum that bridges neural network dynamics, molecular regulation, developmental processes, and behavioral manifestations.
Cluster 1 focuses on resting-state functional connectivity and emotion-regulation networks, highlighting dysregulation within the prefrontal–amygdala circuitry. Numerous studies have demonstrated reduced amygdala–prefrontal and amygdala–limbic connectivity in patients with depression, indicating impaired top-down emotional control (47, 51–54). Such “prefrontal inhibition failure” corresponds closely to the negative emotional bias characteristic of depression and represents one of the most consistent neuroimaging signatures across studies. Cluster 2 encompasses molecular and neuroregulatory mechanisms, emphasizing the coupling between emotional circuits and neurotransmitter systems. Abnormal amygdala–ventral hippocampal connectivity has been linked to dopaminergic imbalance, providing a neural basis for anhedonia (46, 49). Oxytocin signaling enhances amygdala–prefrontal coupling, suggesting a potential neurobiological target for emotion regulation (46, 47). Meanwhile, chronic stress–induced microglial activation within the amygdala supports an inflammatory mechanism by which impaired synaptic plasticity contributes to mood dysregulation (55, 56). Cluster 3 addresses early-life stress and developmental emotion regulation, underscoring how environmental adversity shapes neural development. Prenatal and childhood stress have been shown to alter amygdala maturation and functional connectivity, establishing early emotional vulnerability (45, 57–59). Maternal depression, prenatal stress, and early abuse are all associated with disrupted amygdala–prefrontal connectivity in offspring, providing a neural basis for the intergenerational transmission of depression risk (45, 57, 60). Cluster 4 concerns animal models and systemic regulation, emphasizing the integration of physiological systems and neural circuitry. Animal experiments have demonstrated that oxidative stress, gut microbiota, glutamatergic transmission, and oxytocin signaling can modulate amygdala activity, providing direct evidence for neuro-immune-metabolic integration in depression pathophysiology (61–63). Taken together, these four thematic clusters delineate a hierarchical framework spanning molecular, neural, and behavioral levels. They underscore the amygdala’s pivotal role as an integrative hub within multidimensional regulatory networks and provide empirical foundations for the unified theoretical model proposed in the next section.
4.3 Theoretical integration: the amygdala as a cross-level emotional hub
Integrating bibliometric findings with mechanistic evidence, this study proposes a unified framework positioning the amygdala as a key intermediary node linking molecular biology, neural circuitry, and emotional behavior. Abnormalities in inflammation, stress hormones, and neurotransmission may alter the balance among the frontoparietal network (FPN), default mode network (DMN), and salience network (SN) through amygdala-centered modulation, leading to impaired emotional regulation and core depressive symptoms such as anhedonia.
Within this framework, the neuropathological features of depression can be understood as an imbalance between heightened emotional reactivity and weakened cognitive regulation. The amygdala and related limbic structures exhibit excessive sensitivity to negative emotional stimuli, persistently transmitting amplified negative signals to cortical regions. Meanwhile, the prefrontal cortex—particularly the dorsolateral prefrontal cortex (DLPFC) and ventromedial prefrontal cortex (vmPFC)—shows diminished regulatory and inhibitory control, failing to constrain amygdala hyperactivation. This “enhanced emotion generation–limited regulation” pattern of circuit-level dysfunction has been validated by multiple neuroimaging studies (15, 64, 65). Functional MRI research has demonstrated heightened amygdala responses to negative stimuli in patients with depression (15, 64), along with reduced prefrontal activation during cognitive reappraisal or inhibitory tasks (64). Meta-analyses and resting-state studies further reveal decreased amygdala–prefrontal connectivity, reflecting impaired emotional control (66, 67). Comprehensive reviews support this framework, suggesting that hyperactive emotion-processing networks maintain persistent negative affect, while inefficient prefrontal regulation amplifies sustained negative thinking and cognitive bias (65, 68).
Therefore, amygdala-based functional dysconnectivity represents more than regional hyperactivation—it reflects a breakdown in the coordination between emotional generation and cognitive control systems. This circuit imbalance provides a coherent neurobiological explanation for the heterogeneity and symptom persistence observed in depression. In summary, the amygdala has evolved from a passive marker of emotional response to an active hub of emotional regulation and therapeutic intervention. This integrative model offers new insights into the multidimensional neuropathology of depression and establishes a theoretical foundation for precision diagnosis and circuit-targeted interventions in affective disorders.
4.4 Clinical and translational implications: from neural biomarkers to therapeutic targets
Current evidence indicates that amygdala functional connectivity has three advantages as a candidate biomarker for depression: it is quantifiable, interpretable, and amenable to intervention. First, in diagnosis and patient stratification, individual differences in resting-state amygdala connectivity can help identify age- and sex-specific neural signatures across different disease stages (52, 53). When combined with peripheral inflammatory markers or neurotransmitter profiles, these connectivity alterations may serve as cross-modal biomarkers, bridging brain imaging and molecular indices to refine diagnostic precision.
Second, in prognosis and treatment prediction, baseline amygdala–insula connectivity strength has been shown to predict subsequent symptom remission and treatment responsiveness (54). This suggests that circuit-level metrics could be used as dynamic indicators for tracking therapeutic outcomes and guiding personalized interventions.
Third, in intervention and neuroplasticity modulation, noninvasive neuromodulation techniques—such as repetitive transcranial magnetic stimulation (rTMS), transcranial direct current stimulation (tDCS), theta-burst stimulation (TBS), and real-time fMRI neurofeedback—can effectively restore the functional integrity of the prefrontal–amygdala circuitry. Preclinical findings further support this translational potential: animal studies have demonstrated that oxytocin and glutamatergic pathways modulate amygdala excitability, providing mechanistic targets for circuit-specific therapies (46, 47, 61, 62).
In parallel, advances in artificial intelligence (AI) and the Internet of Medical Things (IoMT) are paving the way for the digitization of amygdala-based biomarkers (34, 39). Functional connectivity features may soon be transformed into computational indicators for multimodal prediction, remote monitoring, and relapse prevention. Establishing standardized protocols for imaging acquisition, preprocessing, and data sharing across centers will be essential for ensuring reproducibility and scalability. Collectively, these developments highlight the translational promise of amygdala-centered neural markers in achieving precision prevention, personalized intervention, and long-term management of depressive disorders.
4.5 Limitations and future directions
Several limitations should be acknowledged in this study. First, the use of only the Web of Science Core Collection (WoS) and Scopus databases may have introduced coverage bias. Second, the restriction to English-language publications and a fixed search time window may have excluded recent or non-English studies. Third, differences in cross-database deduplication procedures and parameter settings may affect the precision of clustering results. Moreover, this study did not perform country-level thematic comparisons to avoid attribution bias caused by multi-institutional and multi-country authorship. Finally, variations in citation definitions and threshold criteria across databases prevented the listing of highly cited papers within each cluster.
Despite these limitations, this study integrated global bibliometric evidence with mechanistic research findings to reveal the systematic evolution and knowledge structure of depression–amygdala research. Future research should focus on three key directions: (i) longitudinal and multimodal validation of the amygdala’s causal role as a neural regulatory hub; (ii) construction of multicenter imaging–molecular–behavioral databases to identify replicable and generalizable neural biomarker systems; and (iii) development of individualized intervention models based on amygdala functional connectivity, achieving a closed loop from mechanistic understanding to precision treatment.
Overall, abnormalities in amygdala functional connectivity reflect a system-level disequilibrium spanning from molecular to network levels in depression. The amygdala has thus evolved from a passive structure of emotional reactivity to an active integrative hub in affective regulation. The findings of this study not only map the intellectual evolution of the field but also provide a theoretical foundation for future research on multidimensional mechanisms and circuit-guided interventions in affective disorders.
5 Conclusion
Using data from the Web of Science Core Collection and Scopus, this bibliometric study mapped the 2015–2024 landscape of amygdala-related depression research. Publication output rose markedly after 2020, and the field has shifted from single-region accounts to systems-level frameworks. Keyword and clustering results converge on four themes: emotion-regulation networks, molecular and neural regulation, developmental risk factors, and animal models. Across these themes, amygdala functional connectivity emerges as a promising candidate neuromarker for stratification and treatment prediction. Despite limitations (database scope, language/time window, cross-database harmonization), the overall trajectory points to mechanistic precision and system integration. Future work should adopt harmonized, multicenter, multimodal designs with age/sex stratification, and integrate peripheral biomarkers and AI-enabled analytics to translate candidate markers into scalable clinical readouts.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s. The data supporting this study are available from the Web of Science and Scopus databases.
Author contributions
YL: Writing – review & editing, Writing – original draft. DY: Writing – review & editing, Formal Analysis, Writing – original draft. MC: Writing – review & editing, Writing – original draft. LD: Writing – review & editing, Writing – original draft. LJ: Writing – review & editing, Writing – original draft. SG: Writing – review & editing, Writing – original draft.
Ethics statement
This study is a literature review and did not directly involve actual participants, experiments, or surveys. Therefore, ethical approval and participant consent were not applicable.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2025.1642936/full#supplementary-material
References
1. Reed GM, First MB, Kogan CS, Hyman SE, Gureje O, Gaebel W, et al. Innovations and changes in the ICD-11 classification of mental, behavioural and neurodevelopmental disorders. World Psychiatry. (2019) 18:3–19. doi: 10.1002/wps.20611
2. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. (2022) 9:137–50. doi: 10.1016/S2215-0366(21)00395-3
3. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet (London England). (2021) 398:1700–12. doi: 10.1016/S0140-6736(21)02143-7
4. Li S, Guo B, Yang Q, Yin J, Jiang Y, Tian L, et al. Factors associated with depression in residents in the post-epidemic era. QJM. (2022) 115:605–9. doi: 10.1093/qjmed/hcac181
5. Monroe SM and Harkness KL. Major depression and its recurrences: life course matters. Annu Rev Clin Psychol. (2022) 18:329–57. doi: 10.1146/annurev-clinpsy-072220-021440
6. Shorey S, Ng ED, and Wong CHJ. Global prevalence of depression and elevated depressive symptoms among adolescents: A systematic review and meta-analysis. Br J Clin Psychol. (2022) 61:287–305. doi: 10.1111/bjc.12333
7. Malhi GS and Mann JJ. Depression. Lancet (London England). (2018) 392:2299–312. doi: 10.1016/S0140-6736(18)31948-2
8. Wang Y, Jiang G, Wang L, Chen M, Yang K, Wen K, et al. Association of the depressive scores, depressive symptoms, and conversion patterns of depressive symptoms with the risk of new-onset chronic diseases and multimorbidity in the middle-aged and elderly Chinese population. EClinicalMedicine. (2022) 52:101603. doi: 10.1016/j.eclinm.2022.101603
9. Dibeklioglu H, Hammal Z, and Cohn JF. Dynamic multimodal measurement of depression severity using deep autoencoding. IEEE J Biomed Health Inf. (2018) 22:525–36. doi: 10.1109/JBHI.2017.2676878
10. Phelps EA and LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. (2005) 48:175–87. doi: 10.1016/j.neuron.2005.09.025
11. Šimić G, Tkalčić M, Vukić V, Mulc D, Španić E, Šagud M, et al. Understanding emotions: origins and roles of the amygdala. Biomolecules. (2021) 11:823. doi: 10.3390/biom11060823
12. Ghaemi Kerahrodi J and Michal M. The fear-defense system, emotions, and oxidative stress. Redox Biol. (2020) 37:101588. doi: 10.1016/j.redox.2020.101588
13. Paz R and Pare D. Physiological basis for emotional modulation of memory circuits by the amygdala. Curr Opin Neurobiol. (2013) 23:381–6. doi: 10.1016/j.conb.2013.01.008
14. Zhong M, Wang X, Xiao J, Yi J, Zhu X, Liao J, et al. Amygdala hyperactivation and prefrontal hypoactivation in subjects with cognitive vulnerability to depression. Biol Psychol. (2011) 88:233–42. doi: 10.1016/j.biopsycho.2011.08.007
15. Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, and Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. (2001) 50:651–8. doi: 10.1016/S0006-3223(01)01263-X
16. Fu CH, Williams SC, Cleare AJ, Scott J, Mitterschiffthaler MT, Walsh ND, et al. Neural responses to sad facial expressions in major depression following cognitive behavioral therapy. Biol Psychiatry. (2008) 64:505–12. doi: 10.1016/j.biopsych.2008.04.033
17. Fu CH, Williams SC, Cleare AJ, Brammer MJ, Walsh ND, Kim J, et al. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Arch Gen Psychiatry. (2004) 61:877–89. doi: 10.1001/archpsyc.61.9.877
18. Suslow T, Konrad C, Kugel H, Rumstadt D, Zwitserlood P, Schöning S, et al. Automatic mood-congruent amygdala responses to masked facial expressions in major depression. Biol Psychiatry. (2010) 67:155–60. doi: 10.1016/j.biopsych.2009.07.023
19. Victor TA, Furey ML, Fromm SJ, Ohman A, and Drevets WC. Relationship between amygdala responses to masked faces and mood state and treatment in major depressive disorder. Arch Gen Psychiatry. (2010) 67:1128–38. doi: 10.1001/archgenpsychiatry.2010.144
20. Abler B, Erk S, Herwig U, and Walter H. Anticipation of aversive stimuli activates extended amygdala in unipolar depression. J Psychiatr Res. (2007) 41:511–22. doi: 10.1016/j.jpsychires.2006.07.020
21. Kessler H, Taubner S, Buchheim A, Münte TF, Stasch M, Kächele H, et al. Individualized and clinically derived stimuli activate limbic structures in depression: an fMRI study. PloS One. (2011) 6:e15712. doi: 10.1371/journal.pone.0015712
22. Siegle GJ, Thompson W, Carter CS, Steinhauer SR, and Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biol Psychiatry. (2007) 61:198–209. doi: 10.1016/j.biopsych.2006.05.048
23. Carballedo A, Scheuerecker J, Meisenzahl E, Schoepf V, Bokde A, Möller HJ, et al. Functional connectivity of emotional processing in depression. J Affect Disord. (2011) 134:272–9. doi: 10.1016/j.jad.2011.06.021
24. Chen CH, Suckling J, Ooi C, Fu CH, Williams SC, Walsh ND, et al. Functional coupling of the amygdala in depressed patients treated with antidepressant medication. Neuropsychopharmacology. (2008) 33:1909–18. doi: 10.1038/sj.npp.1301593
25. Dannlowski U, Ohrmann P, Konrad C, Domschke K, Bauer J, Kugel H, et al. Reduced amygdala-prefrontal coupling in major depression: association with MAOA genotype and illness severity. Int J Neuropsychopharmacol. (2009) 12:11–22. doi: 10.1017/S1461145708008973
26. Hakamata Y, Mizukami S, Izawa S, Hori H, Matsui M, Moriguchi Y, et al. Contextual memory bias in emotional events: Neurobiological correlates and depression risk. Psychoneuroendocrinology. (2025) 171:107218. doi: 10.1016/j.psyneuen.2024.107218
27. Tottenham N, Weissman MM, Wang Z, Warner V, Gameroff MJ, Semanek DP, et al. Depression risk is associated with weakened synchrony between the amygdala and experienced emotion. Biol Psychiatry Cogn Neurosci Neuroimaging. (2021) 6:343–51. doi: 10.1016/j.bpsc.2020.10.011
28. Hakamata Y, Mizukami S, Izawa S, Okamura H, Mihara K, Marusak H, et al. Implicit and explicit emotional memory recall in anxiety and depression: Role of basolateral amygdala and cortisol-norepinephrine interaction. Psychoneuroendocrinology. (2022) 136:105598. doi: 10.1016/j.psyneuen.2021.105598
29. Tani Y, Koyama Y, Doi S, Sugihara G, Machida M, Amagasa S, et al. Association between gratitude, the brain and cognitive function in older adults: Results from the NEIGE study. Arch Gerontology Geriatrics. (2022) 100:104645. doi: 10.1016/j.archger.2022.104645
30. Erdmann T, Berwian IM, Stephan KE, Seifritz E, Walter H, and Huys QJM. Amygdala reactivity, antidepressant discontinuation, and relapse. JAMA Psychiatry. (2024) 81:1081–9. doi: 10.1001/jamapsychiatry.2024.2136
31. Jin L, Wu L, Zhang J, Jia W, Zhou H, Jiang S, et al. Quantitative analysis of literature on diagnostic biomarkers of Schizophrenia: revealing research hotspots and future prospects. BMC Psychiatry. (2025) 25:186. doi: 10.1186/s12888-025-06644-3
32. He T, Wang D, Wu L, and Jin L. The role of glial cells in neuralgia: a bibliometric exploration. Front Neurol. (2025) 16:1496526. doi: 10.3389/fneur.2025.1496526
33. Akhtar F, Belal Bin Heyat M, Sultana A, Parveen S, Muhammad Zeeshan H, Merlin SF, et al. Medical intelligence for anxiety research: Insights from genetics, hormones, implant science, and smart devices with future strategies. WIREs Data Min Knowledge Discov. (2024) 14:e1552. doi: 10.1002/widm.1552
34. Heyat MBB, Akhtar F, Munir F, Sultana A, Muaad AY, Gul I, et al. Unravelling the complexities of depression with medical intelligence: exploring the interplay of genetics, hormones, and brain function. Complex Intelligent Syst. (2024) 10:5883–915. doi: 10.1007/s40747-024-01346-x
35. Falagas ME, Pitsouni EI, Malietzis GA, and Pappas G. Comparison of PubMed, Scopus, Web of Science, and Google Scholar: strengths and weaknesses. FASEB J. (2008) 22:338–42. doi: 10.1096/fj.07-9492LSF
36. Gusenbauer M. Beyond Google Scholar, Scopus, and Web of Science: An evaluation of the backward and forward citation coverage of 59 databases' citation indices. Res Synthesis Methods. (2024) 15:802–17. doi: 10.1002/jrsm.1729
37. Martín-Martín A, Thelwall M, Orduna-Malea E, and Delgado López-Cózar E. Google Scholar, Microsoft Academic, Scopus, Dimensions, Web of Science, and OpenCitations' COCI: a multidisciplinary comparison of coverage via citations. Scientometrics. (2021) 126:871–906. doi: 10.1007/s11192-020-03690-4
38. Heyat MBB, Akhtar F, Khan MH, Ullah N, Gul I, Khan H, et al. Detection, treatment planning, and genetic predisposition of bruxism: A systematic mapping process and network visualization technique. CNS Neurological Disord Drug Targets. (2021) 20:755–75. doi: 10.2174/19963181MTExyMzM33
39. Zeeshan HM, Heyat MBB, Hayat MAB, Parveen S, Akhtar F, Sayeed E, et al. (2023). Worldwide research trends and hotspot on IOMT based on bibliometric analysis, in: 2023 20th International Computer Conference on Wavelet Active Media Technology and Information Processing (ICCWAMTIP), Chengdu, China: IEEE, 2023 15–17 Dec.
40. Gustavsson EK, Zhang D, Reynolds RH, Garcia-Ruiz S, and Ryten M. ggtranscript: an R package for the visualization and interpretation of transcript isoforms using ggplot2. Bioinf (Oxford England). (2022) 38:3844–6. doi: 10.1101/2022.03.28.486050
41. Van Eck NJ and Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. (2010) 84:523–38. doi: 10.1007/s11192-009-0146-3
42. Salim S. Oxidative stress and the central nervous system. J Pharmacol Exp Ther. (2017) 360:201–5. doi: 10.1124/jpet.116.237503
43. Foster JA, Rinaman L, and Cryan JF. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol Stress. (2017) 7:124–36. doi: 10.1016/j.ynstr.2017.03.001
44. Pinto-Sanchez MI, Hall GB, Ghajar K, Nardelli A, Bolino C, Lau JT, et al. Probiotic bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: A pilot study in patients with irritable bowel syndrome. Gastroenterology. (2017) 153:448–59.e8. doi: 10.1053/j.gastro.2017.05.003
45. Van Den Bergh BRH, Van Den Heuvel MI, Lahti M, Braeken M, De Rooij SR, Entringer S, et al. Prenatal developmental origins of behavior and mental health: The influence of maternal stress in pregnancy. Neurosci Biobehav Rev. (2020) 117:26–64. doi: 10.1016/j.neubiorev.2017.07.003
46. Jurek B and Neumann ID. The oxytocin receptor: from intracellular signaling to behavior. Physiol Rev. (2018) 98:1805–908. doi: 10.1152/physrev.00031.2017
47. Hiser J and Koenigs M. The multifaceted role of the ventromedial prefrontal cortex in emotion, decision making, social cognition, and psychopathology. Biol Psychiatry. (2018) 83:638–47. doi: 10.1016/j.biopsych.2017.10.030
48. Grace AA. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat Rev Neurosci. (2016) 17:524–32. doi: 10.1038/nrn.2016.57
49. Belujon P and Grace AA. Dopamine system dysregulation in major depressive disorders. Int J Neuropsychopharmacol. (2017) 20:1036–46. doi: 10.1093/ijnp/pyx056
50. Bubb EJ, Metzler-Baddeley C, and Aggleton JP. The cingulum bundle: Anatomy, function, and dysfunction. Neurosci Biobehav Rev. (2018) 92:104–27. doi: 10.1016/j.neubiorev.2018.05.008
51. Ramasubbu R, Konduru N, Cortese F, Bray S, Gaxiola-Valdez I, and Goodyear B. Reduced intrinsic connectivity of amygdala in adults with major depressive disorder. Front Psychiatry. (2014) 5:17. doi: 10.3389/fpsyt.2014.00017
52. Guan L, Liu R, Wang C, Fan Q, Zhou J, Wang Y, et al. Abnormal resting-state functional connectivity in subregions of amygdala in adults and adolescents with major depressive disorder. BMC Psychiatry. (2024) 24:540. doi: 10.1186/s12888-024-05977-9
53. Tang S, Lu L, Zhang L, Hu X, Bu X, Li H, et al. Abnormal amygdala resting-state functional connectivity in adults and adolescents with major depressive disorder: A comparative meta-analysis. EBioMedicine. (2018) 36:436–45. doi: 10.1016/j.ebiom.2018.09.010
54. Connolly CG, Ho TC, Blom EH, LeWinn KZ, Sacchet MD, Tymofiyeva O, et al. Resting-state functional connectivity of the amygdala and longitudinal changes in depression severity in adolescent depression. J Affect Disord. (2017) 207:86–94. doi: 10.1016/j.jad.2016.09.026
55. Calcia MA, Bonsall DR, Bloomfield PS, Selvaraj S, Barichello T, and Howes OD. Stress and neuroinflammation: a systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology. (2016) 233:1637–50. doi: 10.1007/s00213-016-4218-9
56. Zheng ZH, Tu JL, Li XH, Hua Q, Liu WZ, Liu Y, et al. Neuroinflammation induces anxiety- and depressive-like behavior by modulating neuronal plasticity in the basolateral amygdala. Brain Behavior Immun. (2021) 91:505–18. doi: 10.1016/j.bbi.2020.11.007
57. Uy JP, Tan AP, Broeckman B, Gluckman PD, Chong YS, Chen H, et al. Effects of maternal childhood trauma on child emotional health: maternal mental health and frontoamygdala pathways. J Child Psychol Psychiatry Allied Disciplines. (2023) 64:426–36. doi: 10.1111/jcpp.13721
58. Teicher MH and Samson JA. Annual Research Review: Enduring neurobiological effects of childhood abuse and neglect. J Child Psychol Psychiatry Allied Disciplines. (2016) 57:241–66. doi: 10.1111/jcpp.12507
59. Nogovitsyn N, Addington J, Souza R, Placsko TJ, Stowkowy J, Wang J, et al. Childhood trauma and amygdala nuclei volumes in youth at risk for mental illness. psychol Med. (2022) 52:1192–9. doi: 10.1017/S0033291720003177
60. Qiu A, Anh TT, Li Y, Chen H, Rifkin-Graboi A, Broekman BF, et al. Prenatal maternal depression alters amygdala functional connectivity in 6-month-old infants. Trans Psychiatry. (2015) 5:e508. doi: 10.1038/tp.2015.3
61. Althammer F, Roy RK, Kirchner MK, Lira EC, Schimmer S, Charlet A, et al. Impaired oxytocin signalling in the central amygdala in rats with chronic heart failure. J Physiol. (2024) 602:6259–80. doi: 10.1113/JP286297
62. Fazmiya MJA, Sultana A, Rahman K, Heyat MBB, Sumbul, Akhtar F, et al. Current insights on bioactive molecules, antioxidant, anti-inflammatory, and other pharmacological activities of cinnamomum camphora linn. Oxid Med Cell Longevity. (2022) 2022:9354555. doi: 10.1155/2022/9354555
63. He S, Huang X, Zheng J, Zhang Y, and Ruan X. An NTS-CeA projection modulates depression-like behaviors in a mouse model of chronic pain. Neurobiol Dis. (2022) 174:105893. doi: 10.1016/j.nbd.2022.105893
64. Johnstone T, Van Reekum CM, Urry HL, Kalin NH, and Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. (2007) 27:8877–84. doi: 10.1523/JNEUROSCI.2063-07.2007
65. Disner SG, Beevers CG, Haigh EA, and Beck AT. Neural mechanisms of the cognitive model of depression. Nat Rev Neurosci. (2011) 12:467–77. doi: 10.1038/nrn3027
66. Etkin A and Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. (2007) 164:1476–88. doi: 10.1176/appi.ajp.2007.07030504
67. Zeng LL, Shen H, Liu L, Wang L, Li B, Fang P, et al. Identifying major depression using whole-brain functional connectivity: a multivariate pattern analysis. Brain: J Neurol. (2012) 135:1498–507. doi: 10.1093/brain/aws059
Keywords: depression, amygdala, bibliometrics, emotion regulation, resting-state functional connectivity, neural mechanisms
Citation: Li Y, Yang D, Cao M, Dong L, Jin L and Gao S (2025) The role of the amygdala in depression: a bibliometric analysis (2015–2024). Front. Psychiatry 16:1642936. doi: 10.3389/fpsyt.2025.1642936
Received: 20 August 2025; Accepted: 27 October 2025;
Published: 13 November 2025.
Edited by:
Julian Macoveanu, Copenhagen University Hospital, DenmarkReviewed by:
Md Belal Bin Heyat, Westlake University, ChinaElizabeth Haris, The University of Melbourne, Australia
Copyright © 2025 Li, Yang, Cao, Dong, Jin and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liuyin Jin, MjQwNzU4MTUzNkBxcS5jb20=; Shugui Gao, Z2Fvc2h1Z3VpQHNpbmEuY29t
 Yuanyuan Li
Yuanyuan Li Dengxian Yang2
Dengxian Yang2 Mengye Cao
Mengye Cao Liuyin Jin
Liuyin Jin