- 1Department of Psychiatry, Dalhousie University, Halifax, NS, Canada
- 2Research and Innovation, Nova Scotia Health Authority, Halifax, NS, Canada
- 3Department of Diagnostic Radiology, Dalhousie University, Halifax, NS, Canada
Introduction: Adolescence and young adulthood are simultaneously periods of significant brain development and the ages in which people often initiate cannabis use. This has led to significant interest in researching the effects that cannabis use in this period might have on the brains of users. This scoping review aims to summarize existing neuroimaging research on the effect of cannabis use in adolescence and/or young adulthood (ages 14-25) on brain structure, function, and metabolite concentrations.
Methods: Following scoping review methodology, databases containing neuroimaging studies assessing the effects of cannabis use between the ages of 14 and 25 on brain structure, function, and metabolite concentrations were searched.
Results: Our search yielded 3901 sources, of which 99 met inclusion criteria. The majority of included papers (84/99) found differences in the brain structure, function, and/or metabolite concentrations of adolescent/young adult cannabis users compared to non-using controls. Fewer studies explicitly assessed sex/gender differences, with 5 finding that sex/gender influenced the effect of cannabis use on the brain.
Conclusion: Based on the findings of this review, there is considerable evidence to suggest that cannabis use in adolescence/young adulthood causes changes in the brains of users, however, the low quality of relevant research and scarcity of long term follow up studies, in addition to the heterogeneity of the existing research suggests that more work needs to be done to understand this relationship.
1 Introduction
Cannabis use is common in adolescence and young adulthood (AYA), a period which ranges from approximately 10 years old to the mid-twenties (1). School-based studies have reported, for example, that approximately 1 in 5 students used cannabis in the past year, and among these students, 1 in 10 endorsed daily cannabis use (2). Of concern is that the rates of cannabis use among youth appear to be increasing over time (3). Within a 2023 survey of Canadians’ cannabis use, it was found that 43% of people between 16–19 years old and 48% of people between 20–24 years old reported past year cannabis use, which was almost twice the amount of those over the age of 25 (4). High rates of cannabis use in AYA are also seen internationally. A 2022 survey of 12th grade students in the United States found that 30.7% of respondents endorsed past-year cannabis use (5). Similarly, a European survey reported a past-year cannabis use prevalence of 18.6% among individuals aged 15 to 24 (6). The use of cannabis during AYA is of particular interest as this time frame is known to be significant for brain maturational processes underlying important adult cognitive functions (7), such as cognitive control (8), working memory (9), risk-taking (10), and reward (11). Past research on the effects of cannabis use during this life-stage has associated it with a number of possible negative outcomes, such as a variety of cognitive deficits (12), an increased likelihood of developing an anxiety disorder (13) or a depressive disorder (14), engaging in problematic substance use (15), and increased suicidality (14, 16). Cannabis use during AYA has also been associated with a greater likelihood of, and earlier onset of psychotic disorders (17), as well as worsened clinical outcomes for those young adults with current psychotic disorders (18). To understand the effect that cannabis use in AYA has on the brain, leading to changes in mental health and cognition, neuroimaging research has been applied.
Despite there being no consensus on the long-term effects of cannabis on the brain in AYA, there is a mixed body of research which suggests that such effects might exist. For example, previous research has reported that, compared to non-users, AYA cannabis users have abnormalities in brain morphology (19), resting-state brain activity (20), task-based brain activity (21), and metabolite concentrations (22). While fewer, some research has found no effect of AYA cannabis use on various brain measures (23). This body of potentially contradictory evidence, when examined more closely, has clearly differing definitions of “cannabis user,” small sample sizes, flawed study designs, and heterogenous imaging protocols severely limiting the usefulness of these findings. This makes it exceedingly difficult to meaningfully gauge the overall tenor of the results being published and as such, literature reviews are positioned to distill and synthesize these bodies of work to better inform this research area of interest.
Two recent and relevant reviews on AYA cannabis use and brain effects (24, 25) have been published. One of the reviews (24) was more a narrative review, with some lack of clarity in its methodology [and adherence to established guidelines; see (26)]. The next most recent publication (25), a systematic review (n = 90 studies), included sources derived from a search conducted in 2019, thus a more recent review which includes publications completed over the last 5 years, and with consideration of brain metabolite concentrations and more refined imaging protocols, is warranted.
To summarize existing knowledge regarding the effect that AYA cannabis use has or does not have on the structure, function, and metabolite concentrations of the user’s brain, we conducted a scoping review. Scoping reviews are designed to provide a broad overview of what is- and remains to be known in a research field, as opposed to a systematic review, which focuses on answering a very specific question (27). A scoping review methodology was also chosen for this reason and because they are most appropriate for situations in which the chosen literature is highly heterogeneous and/or in situations where resources are limited (28), both of which are true for the current review.
2 Method
This scoping review was conducted in accordance with the Joanna Briggs Institute (JBI) methodology for scoping reviews (29). The protocol for the current review was preregistered on Open Science Framework on May 23, 2024 (30, 31).
2.1 Inclusion criteria
This review included peer-reviewed studies published in English, theses/dissertations, and preprints using brain imaging and comparing cannabis users to non-users between the ages of 14 and 25 years old. This range was selected based on the WHO’s definition of AYA [10–24 years; (1)], the age ranges commonly used in research on AYA cannabis use, and the age by which puberty has typically started (32), indicating adolescence. Studies examining participants older than this age range were included if it was confirmed that participants regularly used cannabis between our age-range of interest. In addition, cannabis must have been the participants’ primary substance and its use by the participants must have been quantified. Participants were allowed to have preexisting psychiatric conditions (except substance use disorders related to a substance other than cannabis). Sex and gender effects were collected for this review when possible.
2.2 Exclusion criteria
This review did not include papers which assessed the acute effects of cannabinoid administration on the brain or animal studies.
2.3 Types of sources
This scoping review considered both experimental and quasi-experimental study designs including randomized controlled trials, non-randomized controlled trials, before and after observational studies and interrupted time-series studies. In addition, analytical observational studies including prospective and retrospective cohort studies, case-control studies and analytical cross-sectional studies were considered for inclusion. Also considered were descriptive observational study designs. Systematic reviews that meet the inclusion criteria were included with any relevant sources assessed and extracted.
2.4 Search strategy
A three-step search strategy was utilized in this review, with no limit on date of publication. First, an initial limited search of Ovid MEDLINE (NLM, Wolters Kluwer), CINAHL (EBSCO), PsycINFO (American Psychological Association), and Embase (Elsevier) was undertaken to identify relevant articles on the topic. The key words contained in the titles and abstracts of relevant articles, and the subheadings used to describe the articles were then used to develop a full search strategy for each database outlined in Appendix I. Using these terms, searches were conducted in all the listed databases as well as Google Scholar and MedRxiv.
2.5 Study selection
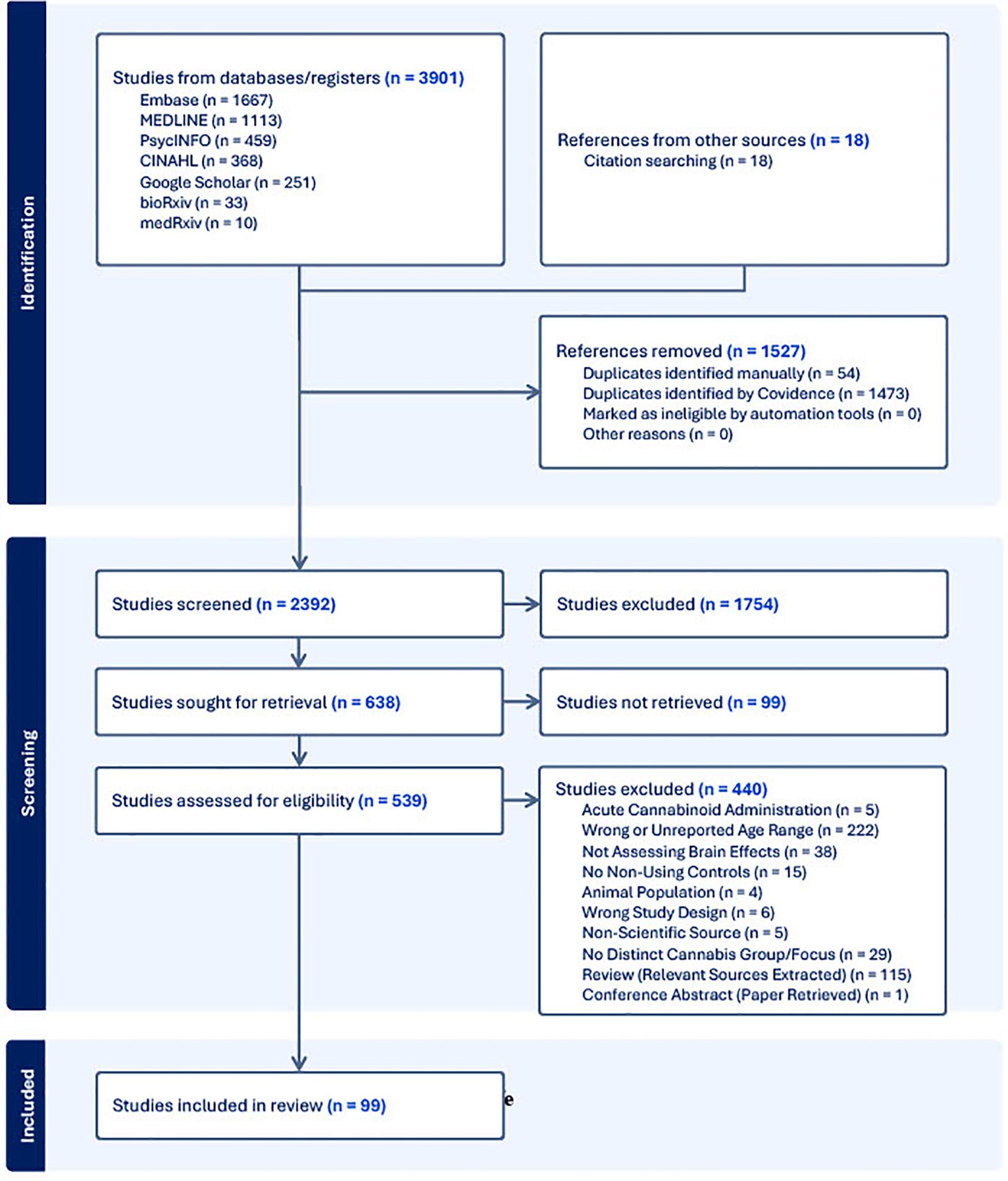
Following the search of each database, all identified citations were collected and uploaded into Covidence (33) where duplicates were removed. Following a pilot test, titles and abstracts were screened by two independent reviewers (L.N., C.H.L.). Potentially relevant sources were then retrieved in full and subsequently assessed in detail against the inclusion criteria. The reference list of all included sources were screened for additional relevant studies. Reasons for exclusion of sources were recorded and reported in the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews [PRISMA-ScR; (34)] flow diagram (Figure 1). Any disagreements that arose between the reviewers at each stage of the selection process was resolved either through consensus, or if that was not possible, then with input from an additional reviewer (C.E.C., P.G.T.).
2.6 Data extraction
Data were extracted by two independent reviewers using a data extraction tool developed by the study team and completed in Covidence. From each paper the following was extracted: authors, year of publication, study design, sample size, mean age and age range of sample (divided into cannabis users and non-users), and relevant findings. Any disagreements that arose between the reviewers were resolved through discussion, or with input from an additional reviewer.
2.7 Sex and gender considerations
As a secondary objective, we aimed to determine if and how sex and/or gender influences the effect of AYA cannabis use on the brain. The World Health Organization’s definitions of sex and gender were used, that is, sex being an individual’s sex assigned at birth (i.e., male or female), while gender referred to an individual’s identity and societal role (i.e., man or woman) (35)). Included papers were categorized as having assessed sex/gender differences or not, with relevant sex/gender data extracted where available. Many of the studies that stated they assessed sex/gender were not explicit on the constructs they were assessing, in these cases we categorized them based on the language used (i.e., sex or gender).
3 Results
3.1 Characteristics of included studies
Our search yielded 99 papers that met our inclusion criteria (See Figure 1; a number of papers included multiple imaging modalities). Out of these, 46 utilized structural neuroimaging protocols and 56 functional neuroimaging protocols. Out of the structural imaging studies, 29 used structural magnetic resonance imaging (sMRI), 12 used diffusion tensor imaging (DTI), 4 used magnetic resonance spectroscopy (MRS), and 1 used another imaging modality (CT) (See Table 1). Of the functional imaging studies, 39 used task-based functional magnetic resonance imaging (fMRI), 10 used resting-state fMRI, 5 used electroencephalography (EEG), and 2 used positron emission tomography (PET) (see Table 2).
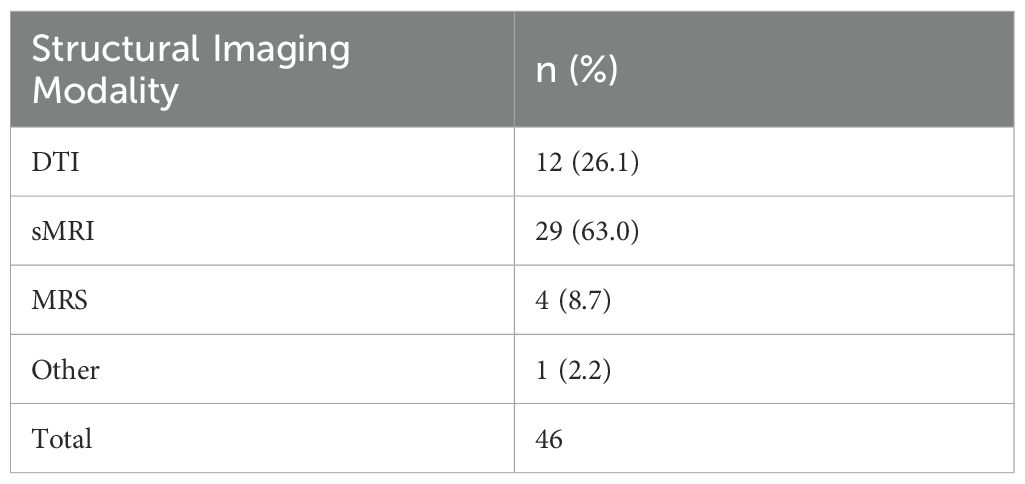
Table 1. Proportion of different structural imaging modalities utilized in studies included in the review.
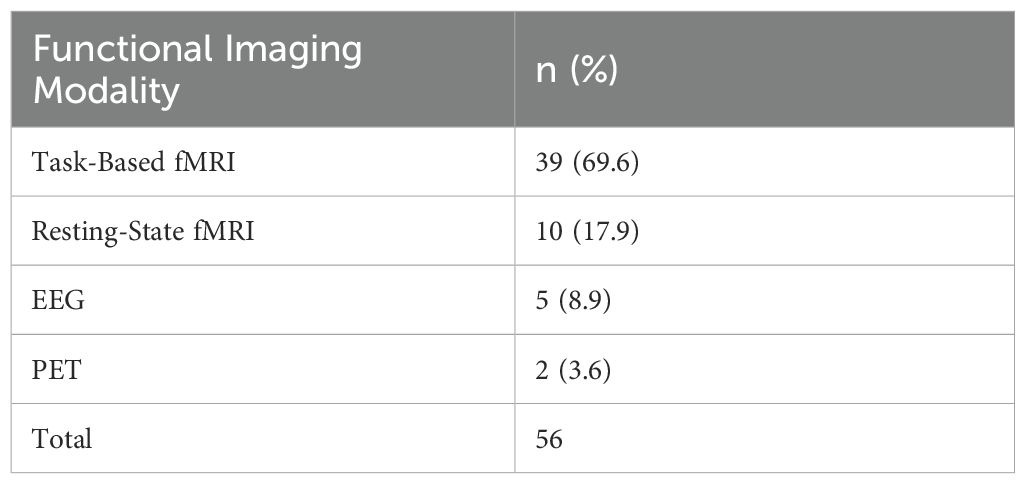
Table 2. Proportion of different functional imaging modalities utilized in studies included in the review.
Approximately 85% (84/99) of the included studies found at least one significant difference in various aspects of brain structure/function between individuals who used cannabis between the ages of 14 and 25 and those who did not. While all studies reported the gender and/or sex of the participants, only 17% (17/99) assessed sex/gender differences related to their reported outcome(s). Five (~5%) of these studies found a significant sex/gender difference. The aim of the remainder of this review is to discuss the results of the included papers (see Tables 3–10 for summaries of all included studies).
3.2 Structural neuroimaging results
3.2.1 sMRI
3.2.1.1 Cortical thickness
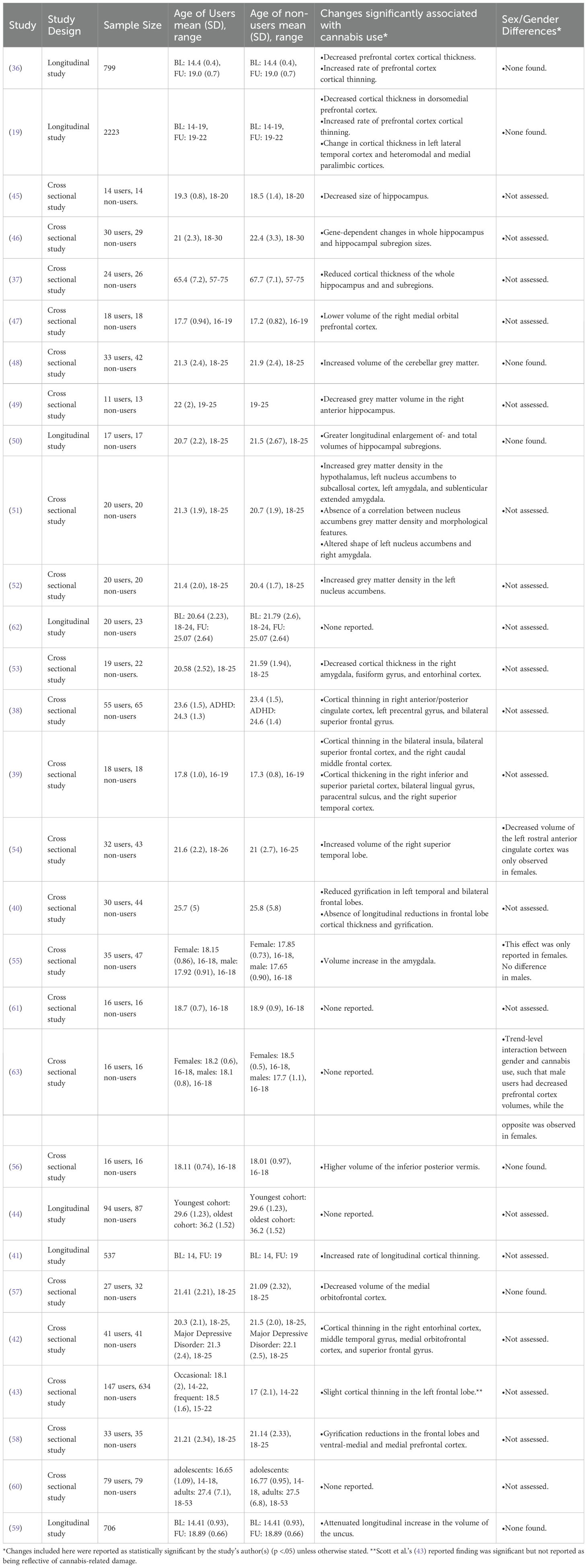
We categorized sMRI findings based on the morphological measure examined, here being cortical thickness or volume. Of the included sMRI papers, 38% (10/26) examined cortical thickness with 80% (8/10) reporting decreased cortical thickness in cannabis using AYA compared to non-using controls (19, 36–42). Cortical thinning was usually located within the frontal lobes (19, 36, 40, 42), the hippocampus (37) and the cingulate (38). Two studies found no evidence of cortical thinning related to cannabis use (43, 44).
3.2.1.2 Volume changes
Of the sMRI papers assessing brain volumes, 71% (15/21) found significant regional volume differences between users and non-users (45–59). The most common area of change reported was in the hippocampus, however, there was no consensus on change direction as both increases and decreases in volume were reported (45, 46, 49, 50). AYA cannabis use was only associated with decreasing volumes in the prefrontal cortex (47, 57, 58) with the amygdala showing evidence of volume increase in two studies (51, 55) but a decrease in another (53). Other brain areas reporting AYA cannabis related volume changes include the cerebellum [volume increase; (48, 56)], the nucleus accumbens [volume increase; (52)], and the uncus [absence of longitudinal volume growth; (59)]. The remaining 29% of the included studies found no significant effect of AYA cannabis use on brain volumes (43, 44, 60–63).
3.2.1.3 Abstinence in sMRI
Medina et al. (2010) reported that cerebellar volume increases related to cannabis use remained after a month of abstinence, Ashtari et al. (2011) reported on cannabis-related changes in hippocampal volumes which persisted after an average of 6.7 months of abstinence, and Burggren et al. (2018) found changes in hippocampal cortical thickness persisted after a minimum of 22 years of abstinence from regular use (37, 45, 56). However, Medina et al. (2009) found no abnormalities in prefrontal cortex volumes in cannabis users who had been abstinent for 1 month (63).
3.2.1.4 Sex/gender differences in sMRI
Possible sex/gender effects have been reported by McQueeny et al. (2011), who found cannabis associated increases in amygdala volume only in women (55). Maple (2016) had a similar finding, observing that only women cannabis users showed a significant decrease in the volume of the left rostral anterior cingulate cortex (54). Medina et al. (2009) reported that cannabis use tended to be associated with increased prefrontal cortex volume in women with the opposite being found in men, however this gender effect was not statistically significant (63).
3.2.1.5 Summary of sMRI findings
Taken together, the weight of the current evidence seems to suggest that AYA cannabis use is associated with cortical thinning, particularly in the frontal lobes. Volume changes related to AYA cannabis use were inconsistent in their direction, being commonly associated with both volume increases and decreases in the hippocampus and volume decreases in the prefrontal cortex. Additionally, the weight of this small body of literature seems to suggest that structural brain changes related to cannabis use in young adults are potentially persistent. However, more research and longer periods of abstinence are needed to bolster the strength of this claim. Due to the relatively few studies which explicitly assessed sex/gender effects, research regarding sex/gender differences in the impact of AYA cannabis use on sMRI measures should be pursued.
3.2.2 DTI
3.2.2.1 Fractional anisotropy changes
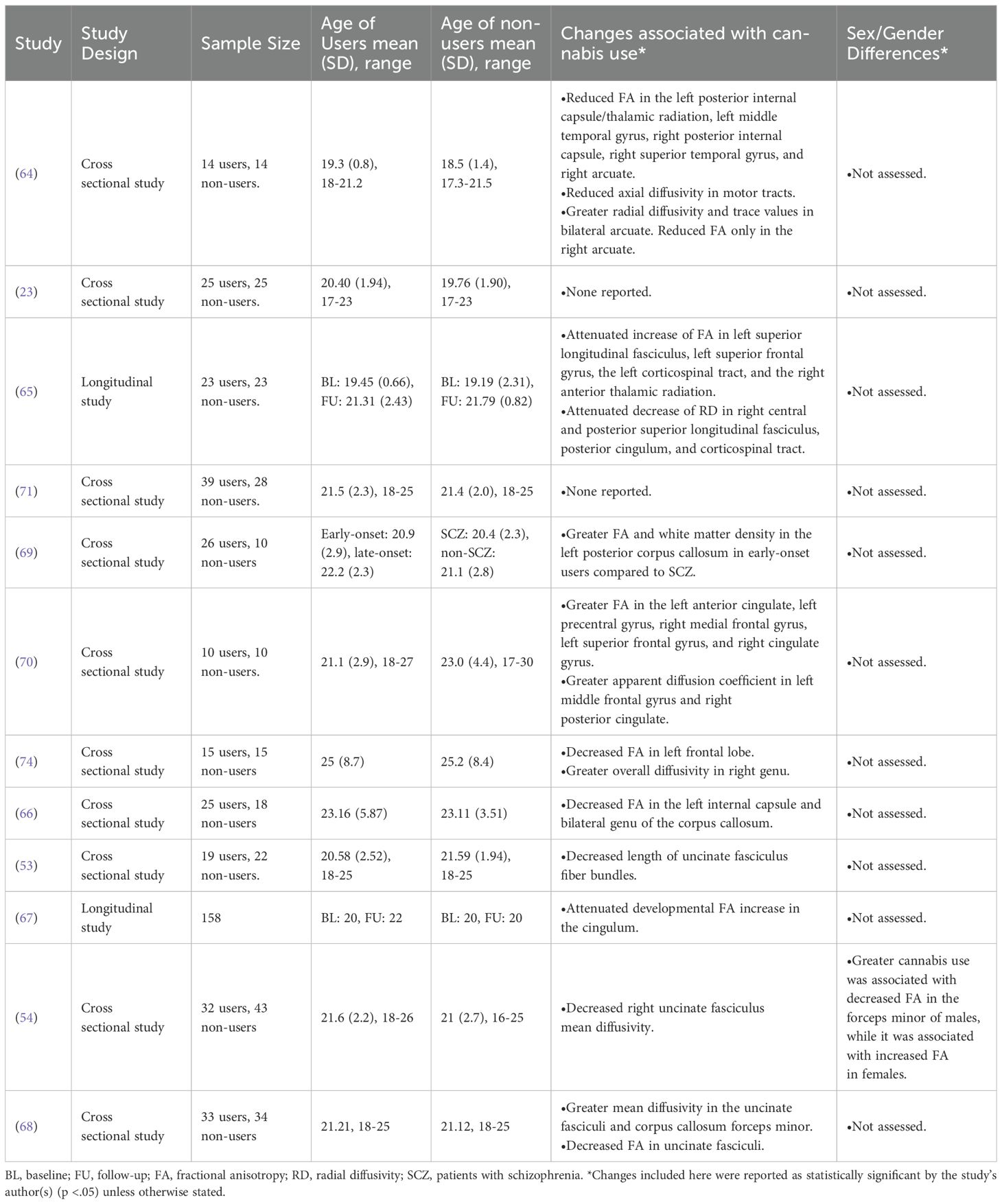
Of the DTI papers included in this review, 9/12 assessed fractional anisotropy (FA) as the primary DTI variable, a broad measure of the integrity of neuronal pathways. Approximately 56% (5/9) of these papers found lower FA values across the brain (64–68), indicating a loss of neuronal integrity from possible neuron/myelin abnormalities, or in axon packing/coherence. Other researchers have reported increased FA in multiple regions of the brain (69, 70)), hypothesized to indicate potential hyperconnectivity between brain regions. However, two studies have found no differences in FA between AYA users and non-users (23, 71). Furthermore, FA has been criticized to be too broad of a measure, limiting the interpretation of these findings (72).
3.2.2.2 Other DTI metrics
DTI can generate other indices of neuronal integrity. However, within the included DTI studies, there was a great deal of heterogeneity regarding the measures used and the areas assessed. Ashtari et al. (2009) found decreased axial diffusivity in the motor pathways and increased radial diffusivity and apparent diffusion coefficient in the arcuate fasciculus (64). Becker et al. (2015) found changes in radial diffusion in multiple subcortical areas, reflecting potential cannabis-related changes to neuronal microstructure (65, 73). Gruber et al. (2011) found greater overall diffusivity in the right genu, which also potentially reflects neuronal degradation (73, 74). Both Maple (2016) and Shollenbarger et al. (2015b) reported that the uncinate fasciculus had decreased mean diffusivity in AYA cannabis users (54, 68) and Levar et al. (2018) found that fiber bundles in the uncincate fasciculus were significantly shorter in AYA cannabis users than in non-using controls (53). Interestingly, DeLisi et al. (2006) found a greater apparent diffusion coefficient in the frontal and subcortical areas of cannabis users compared to controls, which contrasts others findings (70).
3.2.2.3 Sex/gender differences in DTI
Of the included DTI studies, only Maple (2016) examined sex/gender differences, reporting decreased FA in the forceps minor of AYA cannabis using men, while finding increased FA in AYA cannabis using women (54).
3.2.2.4 Abstinence
Ashtari et al.’s (2009) finding of cannabis use-related changes in FA were documented in users who were abstinent for at least 3 months (M = 6.7 months) (64). Shollenbarger et al. (2015b) also found that the cannabis-related change in mean diffusivity of the uncinate fasciculus persisted after a week of abstinence (68).
3.2.2.5 Summary of DTI findings
Despite the wide-ranging results included in this section, collectively they indicate that cannabis use during AYA is associated with changes to the microstructure of the brain. Many of the included studies found that AYA cannabis use was associated with changes in the FA of multiple brain areas, loosely suggesting cannabis-related neuronal hyper- and hypoconnectivity. Other, more robust measures of neuronal microstructure were also used by the included studies to support the notion that AYA cannabis use is associated with changes to neuronal microstructure. Regarding sex/gender differences and abstinence effects in the DTI literature, very little can be concluded overall due to the paucity of relevant reporting on the topic, highlighting the need for future researchers to consider these constructs in their studies of AYA cannabis use and neuronal microstructure.
3.2.3 MRS
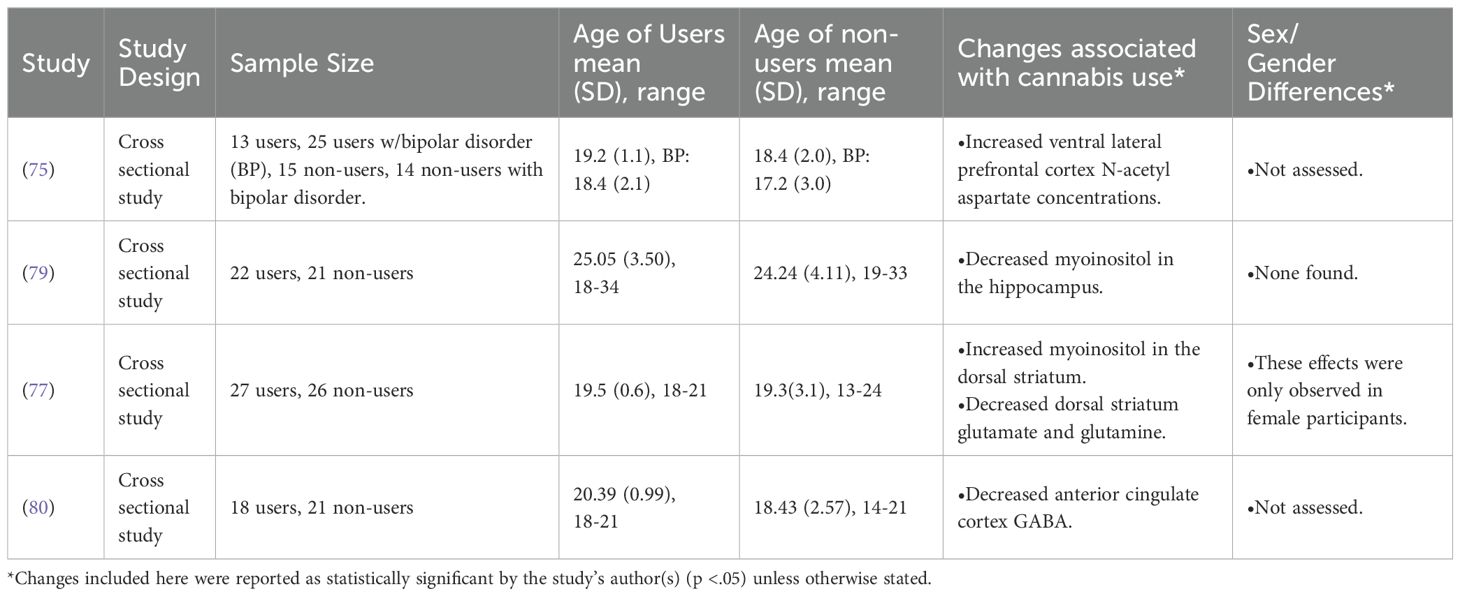
Of the relatively few (n = 4) MRS studies included, there were no significant themes or overlapping results. Bitter et al. (2014) found that cannabis users had an increased concentration of N-acetyl aspartate (NAA) in the ventral lateral prefrontal cortex, a marker of neuronal integrity and potentially increased neuronal density (75, 76). Muetzel et al. (2013) found increased concentrations of myoinositol in the dorsal striatum in cannabis users, potentially indicating neuroinflammation (76, 77). In the same study, Muetzel et al. (2013) also found lower concentrations of glutamine and glutamate in the dorsal striatum, potentially indicating decreased energy metabolism (78) and deficits in excitatory neurotransmission (76) in AYA cannabis users compared to non-users (77). Interestingly, Blest-Hopley et al. (2020) found decreased myoinositol in the hippocampus, again potentially indicating a neuroinflammation process in this region (76, 79). Finally, Subramaniam et al. (2022) found decreased GABA in the anterior cingulate cortex, indicating a deficit in inhibitory neurotransmission (78, 80).
3.2.3.1 Sex/gender differences in MRS
Only 2 of the included MRS studies explicitly assessed sex and/or gender differences related to the impact of AYA cannabis use on the brain (77, 79). Of these, only Muetzel et al. (2013) reported a significant effect. Specifically, their finding of increased myoinositol and decreased glutamate and glutamine in the dorsal striatum was only present in female participants (77).
3.2.3.2 Summary of MRS findings
Again, due to the few MRS studies included in this review and their lack of uniformity regarding brain areas and metabolites of interest, there is no way to meaningfully abstract across these results. The limited number of studies assessing sex/gender and the complete lack of included studies explicitly assessing abstinence also make these constructs an important area of future research.
3.2.4 Other structural imaging modalities
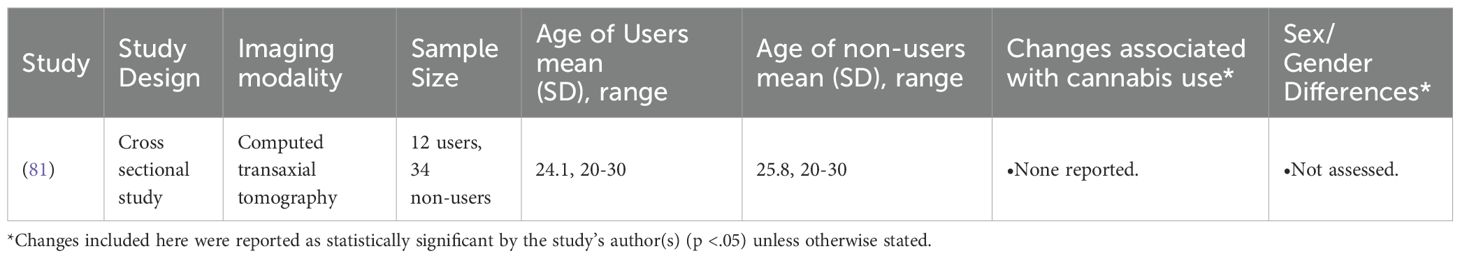
The remaining structural imaging study utilized computed transaxial tomography (CT). In this early study, Co et al. (1977) reported no effect of cannabis use on any morphological brain measure in this cohort (81).
3.3 Functional neuroimaging
3.3.1 Task-based fMRI
3.3.1.1 Cognitive control
Of the included task-based fMRI studies, 31% (12/39) found that cannabis users’ brains responded significantly differently from non-users during tasks requiring cognitive control or decision making. During tasks requiring greater than normal levels of cognitive control (e.g., incongruent trials in the Stroop task or “no-go” trials in a go/no-go task), 10% of the included studies found that AYA cannabis use was associated with heightened activation in a number of brain regions compared to non-using controls (21, 82–84). Similarly, with increasing task difficulty, Harding et al. (2012) found greater functional connectivity between multiple brain areas (85). However, other studies found that trials requiring cognitive control elicited less activity throughout the brains of AYA cannabis users compared to non-users. (86–88). Cyr et al. (2019) found that multiple brain regions which showed elevated activation in controls during trials requiring cognitive control showed no changes in cannabis users and Gruber et al. (2012) found that a smaller area of the anterior cingulate cortex (ACC) was activated in cannabis users compared to controls on trials of this type (89, 90). Adding to this, Kroon et al. (2022) found that task difficulty increases were not associated with increased activation of the superior temporal gyrus in cannabis users (91)). Only one study found an effect of cannabis use on brain activity during trials which did not require above-average cognitive exertion (i.e., congruent trials in the Stroop task or “go” trials in a go/no-go task). In this study, Ames et al. (2013) found that cannabis users had greater activity in an array of brain areas during compatible trials of an implicit association task (86). However, both Claus et al. (2018) and Smith et al. (2011) found no differences in the activation patterns of the brains of cannabis users and non-users during tasks of cognitive control (92, 93).
3.3.1.2 Learning and memory
The next most common fMRI task type was those which assessed learning and/or memory in some capacity (11/39). In a variety of memory tasks, the included studies found that AYA cannabis users displayed patterns of activation that significantly differed from the brains of non-users (94–97), with no particular pattern predominating. With respect to specific stages of memory, cannabis use was found to be associated with an absence of hippocampal activation during the encoding of new memories in two studies (98, 99). In addition, these studies found that cannabis use was associated significantly with altered patterns of activity across other areas of the brain during encoding, such as the frontal lobes (98, 99). During the recall of previously encoded information, Blest-Hopley et al. (2021) found no related activity change in the left posterior cingulate gyrus and right precuneus alongside alterations in the patterns of activation in midbrain structures (100). Further, Dager et al. (2018) found decreased recall-related activity in cannabis users (98). Regarding learning, Hubbard et al. (2023) observed a delayed increase in functional connectivity between frontoparietal and striatal regions as learning took place in cannabis users (101). In opposition, as part of a single longitudinal study, Cousijn et al. (2014a) and Cousijn et al. (2014b) reported no cannabis-related changes in brain function during memory tasks (102, 103). Considered together, these results suggest that there likely exists changes to memory functioning attributable to AYA cannabis use.
3.3.1.3 Reward
The third most common type (8/39) of task-based fMRI studies assessed risk and reward systems. The first observable trend among these studies was that cannabis users displayed significantly increased activation in response to rewarding stimuli compared to non-users (104–106). Similarly, Nestor et al. (2020) found that cannabis users had increased functional connectivity across the brain when expecting reward (107). These studies suggest that the brains of AYA cannabis users are more sensitive to reward. Unlike these studies, Martz et al. (2016) observed that cannabis users had decreased activity in the nucleus accumbens when expecting reward (108). Lichenstein et al. (2017) found that cannabis users who increased their use throughout adolescence exhibited a pattern of brain activity opposite to non/low users and consistently high users when experiencing reward (109). Specifically, cannabis users who increased their use between the ages of 14–19 displayed inversely correlated patterns of activity between the nucleus accumbens and prefrontal cortex when winning in a card guessing game at age 20, as opposed to non/low users and consistently high users which displayed a direct relationship between nucleus accumbens and prefrontal cortex activation (109). Contrary to these studies, Karoly et al. (2015) and Macedo et al. (2024) found that AYA cannabis use was not associated with any changes in reward processing (110, 111).
3.3.1.4 Social cognition
Only a small number (3/39) of the included studies assessed how cannabis use impacts social cognition. Gilman et al. (2016a) found that cannabis users lacked the usual elevated activation of the insula when being socially excluded (112). Further research has found the opposite, namely that tasks of social cognition elicit greater brain activity in cannabis users: Gilman et al. (2016b) found that a greater area of the striatum was activated by cannabis users when they were being socially influenced and that cannabis users had an increase in nucleus accumbens activity when succumbing to social influence relative to non-users (52). Finally, Gilman et al. (2016c) observed increased caudate activation in cannabis users during social influence generally and greater inferior frontal gyrus activity when rejecting social influence (113).
3.3.1.5 Emotion processing
Another small portion of included fMRI studies (3/39) investigated how cannabis use impacts the processing of emotion. Heitzeg et al. (2015) found that cannabis users displayed decreased activity in many areas when viewing negatively valanced words while also displaying increased activity when viewing positively valanced words (114). Nichols et al. (2021) made a similar observation, finding that cannabis users had greater activity in the left superior temporal gyrus in response to positive stimuli and less activity in response to negative stimuli (115). Spechler et al. (2020) found that cannabis use was associated with increased activity in the right amygdala when presented with angry faces at a baseline assessment and that cannabis users displayed no longitudinal increase in this response compared to non-users (116).
3.3.1.6 Other tasks
In a cue-reactivity task, Cousijn et al. (2013a) presented cannabis users and non-users with cannabis-related images while undergoing a fMRI, finding that cannabis users displayed greater brain activity in multiple regions when viewing cannabis-related images (117). Lopez-Larson et al. (2012) had cannabis users and non-users do a finger-tapping task and found that it elicited decreased activity in the cerebellum and right cingulate gyrus of cannabis users compared to non-users (118).
3.3.1.7 Abstinence
Regarding the effects of abstinence on cannabis-related task based functional changes, Macedo et al. (2024) found no abnormalities in brain activity in <1-month abstinent users undergoing a monetary incentive delay task, but also found no effect in current users, so the effect of abstinence on changes in reward processing in this case is unclear (111). Within the included studies, abnormalities in brain activity during tasks of spatial working memory (94, 97) and response inhibition (84) were still observable after 28 days of abstinence. Finally, Sullivan et al. (2022) found blunted activation in the anterior cingulate cortex in response to fearful faces in cannabis users who were abstinent for 2 weeks (88).
3.3.1.8 Sex/gender differences in task-based fMRI
Dager et al. (2018) reported a sex effect in that only AYA cannabis using males displayed reduced activity in the bilateral inferior frontal gyrus and left hippocampus when correctly responding to a figural memory task (98). Sullivan et al. (2022) found, compared to women, AYA cannabis users who were men had significantly greater connectivity between the cerebellum and right rostral anterior cingulate cortex during a go/no-go task with emotional faces, which made their brain activity more in-line with non-users (88).
3.3.1.9 Summary of fMRI findings
Abstracting across the included fMRI studies makes it clear that AYA cannabis use is likely associated with a vast array of changes in brain activity during cognitive tasks. Significant increases and decreases in activity in a variety of brain areas were found during tasks of cognitive control, learning and memory, reward, social cognition, emotion processing, cue reactivity, and motor control. The fMRI studies with explicitly abstinent AYA cannabis users suggest that cannabis-associated changes in brain activity persist after 2 weeks to a month of non-use. Regarding sex/gender differences, due to the lack of studies explicitly assessing sex/gender and the heterogenous results of those which did, very little can be concluded until further evidence comes forward.
3.3.2 Resting-state fMRI
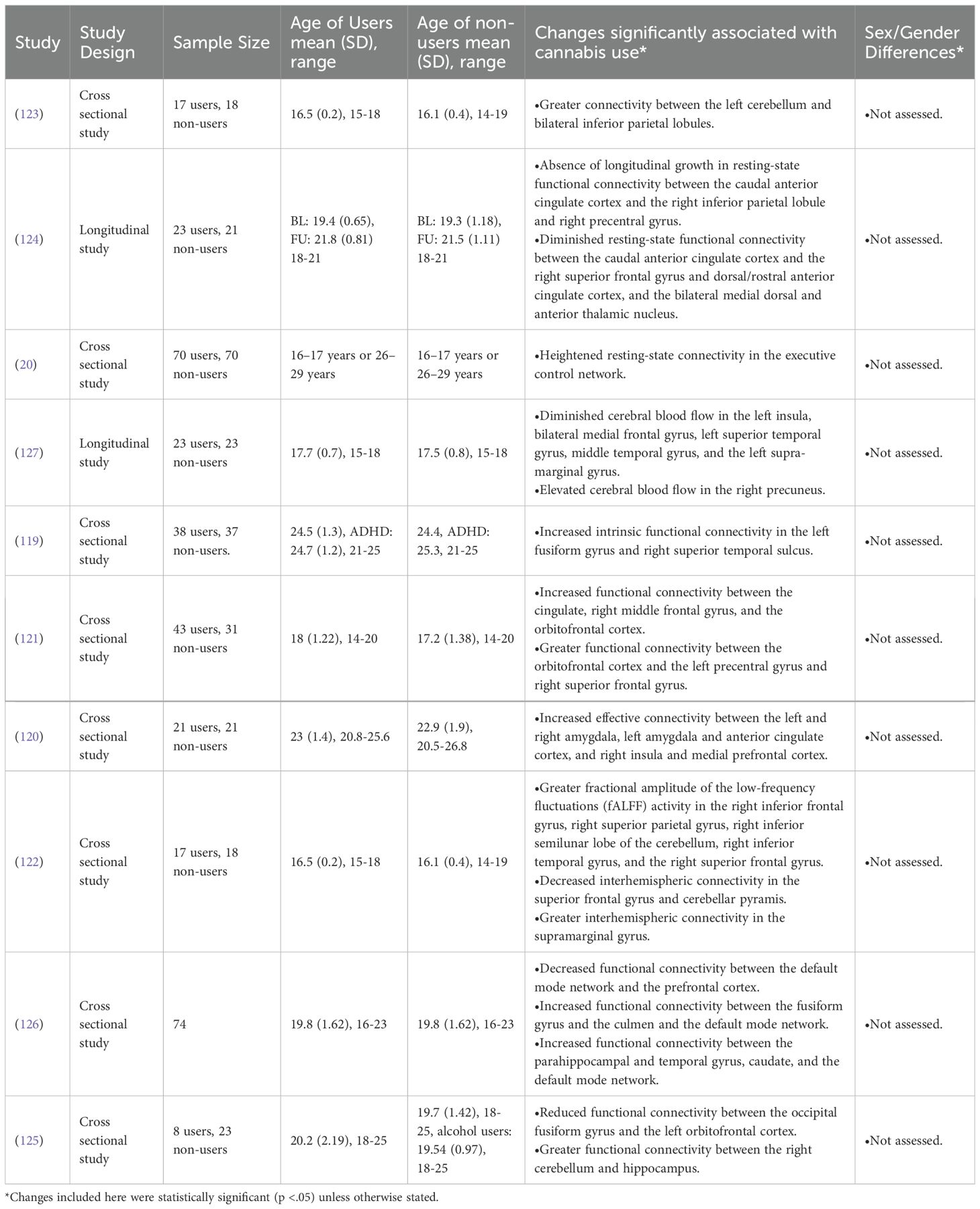
60% (6/10) of the included rs-fMRI studies found increased connectivity between many regions of the brain at rest in cannabis users (20, 119–123). Other researchers have found that cannabis users exhibit decreased connectivity between a variety of regions of the brain (124–126). Common areas of change include the default mode network and (119, 126), and the orbitofrontal cortex (121, 125).
3.3.2.1 Abstinence in rs-fMRI
Jacobus et al. (2012) found significant differences in cerebral blood flow at baseline in AYA cannabis users compared to non-users, but this difference disappeared after the users underwent 4 weeks of abstinence (127).
3.3.2.2 Summary of rs-fMRI findings
Across the included studies, it appears clear that AYA cannabis use is associated with changes to the resting-state activity of the brain, particularly the default mode network and orbitofrontal cortex where both increases and decreases in activity have been observed. Following a trend present in other imaging modalities, very little re-fMRI evidence regarding the effects of sex/gender or abstinence exists, meaning these constructs should be the focus of future research.
3.3.3 EEG
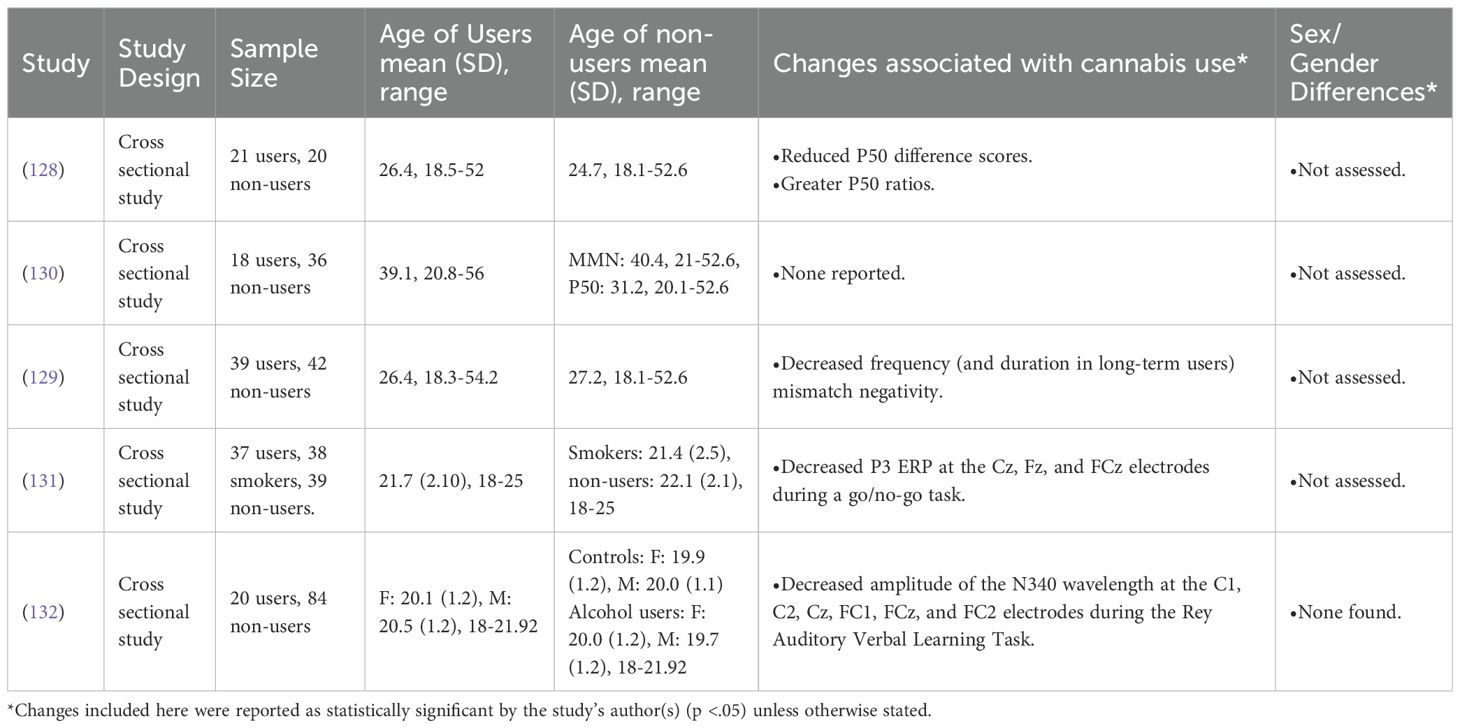
Broyd et al. (2013) found that AYA cannabis users displayed diminished sensory gating and Greenwood et al. (2014) found significant abnormalities in EEG signals related to mismatch negativity (MMN) in AYA cannabis users (128, 129). Broyd et al. (2016) replicated their sensory gating study, although findings were only different at a trend level (p>.05) (130). Apart from these findings, Maij et al. (2017) found that AYA cannabis users exhibited a significantly lower average amplitude of the P3 ERP at the Cz, Fz, and FCz electrodes during a go/no-go task, which the authors interpret as indicating deficient response inhibition (131). Finally, regarding memory, Smith et al. (2017) reported that AYA cannabis users had significantly lower N340 amplitude at electrodes C1, C2, Cz, FC1, FCz, and FC2 during the Rey Auditory Verbal Learning Task, indicating deficient memory (132).
3.3.3.1 Abstinence studies with EEG
The only EEG study to explicitly examine abstinent users was Broyd et al. (2016), reporting no significant abnormalities in ERPs relating to sensory processing in AYA cannabis users who had been abstinent for >1 month [M = 3.5 years; (130)]. Since Broyd et al. (2016) did not also make these measurements prior to abstinence nor compare these users to current cannabis users, the strength of this finding alone is questionable. However, when viewed in the context of Broyd et al.’s (2013) finding of deficiencies in sensory gating in current cannabis users, Broyd et al.’s (2016) findings more strongly suggest that a period of >1 month may remediate some of the sensory deficits related to AYA cannabis use (128, 130).
3.3.3.2 Summary of EEG findings
The included EEG studies found that AYA cannabis use was associated with brain activity changes linked to sensory gating, executive function, and memory. Due to the small number of these studies overall as well as studies assessing sex/gender or abstinence, more research on the brain effects of AYA cannabis use using EEG is warranted. However, when comparing the included studies which assessed abstinence to each other, their results suggest that abstinence may reverse AYA cannabis use-related changes to brain activity detected by EEG.
3.3.4 PET
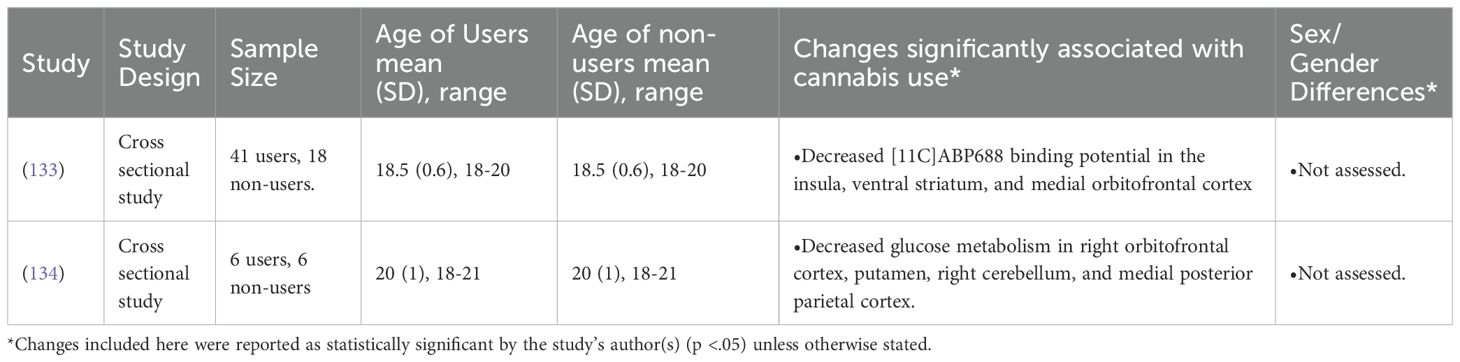
Of all studies included in the current review, only 2 used PET. Cox et al. (2020) reported an interaction between adolescent cannabis use and level of externalizing traits, such that participants who scored highly on their measure of externalizing traits and used cannabis regularly had a significantly decreased availability of glutamate receptors in their orbitofrontal cortex, striatum, and insula (133). Sevy et al. (2008) found that male adolescents who were former cannabis users had decreased glutamate metabolism in the putamen, right cerebellum, precuneus, right orbitofrontal cortex, and the right medial posterior parietal cortex (134).
3.3.4.1 Abstinence and PET
Sevy et al.’s (2008) finding of deficient glutamate metabolism in cannabis users was found in participants who were abstinent for at least one month [M = 15 weeks; (134)].
3.3.4.2 Summary of PET findings
Due to only 2 studies in the current review using PET to assess the brains of AYA cannabis users, no sweeping conclusions can be made generally or regarding sex/gender and abstinence.
4 Discussion
The purpose of this scoping review was to summarize the existing evidence regarding the effect that cannabis use during AYA (ages 14-25) may have on the structure, function, and metabolic function of the brain. Our summary of existing literature suggests that AYA cannabis use is associated with changes in cortical thickness, brain volumes, neuronal white matter microstructure, metabolite concentrations, task-based and resting-state functional brain activity, and neurotransmitter metabolism/receptor density. However, these results must be considered in light of the limitations of a scoping review, namely being the absence of evidence quality assessments and meta-analyses. These findings should also be considered alongside the goal of the current review, which was not to provide an answer to the question of AYA cannabis use’s effect on the brain, but to collate and summarize relevant research.
Regarding the effects of abstinence from cannabis use on the brain, we found a limited number of conflicting findings. The majority of included studies which assessed abstinent AYA cannabis users found that cannabis-related changes persisted over time (45, 56, 64, 68, 84, 88, 94, 97, 128, 134), with one study finding that changes in the thickness of the layers of the hippocampus persisted for decades (37). The remaining studies instead found that abstinent AYA cannabis users did not differ from non-users on their particular neuroimaging measure (63, 111, 127, 130). However, only one of these latter studies assessed the participants pre- and post-abstinence (127), limiting the usefulness of these results. Overall, these findings suggest that abstinence does not necessarily reverse cannabis-related brain changes when use is initiated in adolescence or emerging adulthood, which is concerning for this age group considering the brain development that occurs during this time (7).
Regarding sex and/or gender differences related to the brain effects of cannabis in the included studies, only 17/99 explicitly assessed how sex/gender impacted their results. Of these 17, only 5 found a significant sex or gender difference (54, 55, 77, 88, 98). McQueeny et al. (2011), Maple (2016), and Sullivan et al. (2022) assessed gender, while Muetzel et al. (2013) and Dager et al. (2018) assessed sex. In addition to these significant findings, Medina et al. (2009) found a trend-level effect of gender on AYA cannabis use-related brain volume changes (63). These findings are salient enough to elicit AYA sex/gender considerations as well as concern over how rare the practice of assessing sex/gender differences in conjunction with cannabis use in neuroimaging studies is. The potential interaction between sex/gender and cannabis brain effects with this AYA cohort is important in our further biological understanding of these interactions, as well as any sex/gender specific knowledge translation activity. It is thus important that future neuroimaging research investigating the effects of AYA cannabis use on the brain incorporates sex/gender analyses into their designs.
The current review has multiple strengths: we had our searches developed with the help of evidence synthesis experts and topic experts, we had two independent reviewers screen and extract data from all included studies, and we searched through multiple databases and preprint servers. However, the aforementioned results need to be considered in light of the limitations of scoping review methodology (34), We did not include an assessment of bias or assessment of the quality of the included studies. While a systematic review and meta-analysis could evaluate the quality of the evidence, a scoping review aggregates the results to a more easily actionable state. Systematic reviews and meta-analyses necessitate a more specific question and more restrictive inclusion criteria which would not allow us to properly assess the range of evidence that exists regarding the effect of adolescent/young adult cannabis use on the brain (27). In short, although a systematic review and meta-analysis would provide more rigor than a scoping review, it would require far more resources and limit the amount of evidence available for review. Another limitation of our review is the fact that it included mostly cross-sectional studies, which does not allow the establishment of temporal precedence, meaning that it is just as likely that brain abnormalities reported in this review could have existed prior to- or even led to AYA cannabis use. Finally, with this scoping review, cannabis use patterns, as well as preferred cannabis product used, were not a primary focus. Further research should consider these and other variables to inform the literature.
There are many potential implications of this review, including at the policy level. A prudent review and assessment of age of access to cannabis products, in those jurisdictions where cannabis is legalized, is warranted. Governments and local authorities should also invest in continued age-specific education around the potential brain effects resulting from AYA cannabis use and AYA specific interventions/harm reduction techniques. Finally, research needs to not only continue in this area, but it needs to do so with more consistency and rigor.
5 Conclusion
Here we show that there is evidence that cannabis use during AYA is associated with changes to brain structure and function, that these effects can still be seen after varying periods of abstinence, and that the sex/gender of the user might influence the relationship between AYA cannabis use and brain changes. Even though it is not the goal of the current review, it is worth noting that coming to a strong conclusion about the relationship between AYA cannabis use and brain changes would be exceedingly difficult due to the heterogeneity of the existing literature. Despite this, there are some consistent signals that are coming out of the literature showing, on balance, a negative impact on brain structure and function with cannabis use in this age group. What is difficult to comment on at this time, is the potential varying effects of amounts of cannabis used and potency of product on these brain imaging variables, and effects of longer period of abstinence. With few studies examining sex/gender, little can be said on these specific effects as well, so we recommend that future research meaningfully consider detailed cannabis use patterns/products used and sex/gender in their designs and analyses.
Author contributions
LN: Conceptualization, Investigation, Methodology, Writing – original draft, Writing – review & editing. CC: Methodology, Conceptualization, Writing – review & editing, Supervision. PT: Supervision, Writing – review & editing, Funding acquisition, Methodology, Conceptualization.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This review was funded by a grant provided by the Government of Alberta, Canada.
Acknowledgments
We would like to thank the reference librarians of Dalhousie University for their assistance in developing our search terms and protocol. We would also like to thank Chloe Hambly Lapointe for her work in the review process and data extraction.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. World Health Organization. Adolescent and young adult health (2024). Available online at: https://www.who.int/news-room/fact-sheets/detail/adolescents-health-risks-and-solutions (Accessed March 03, 2025).
2. Sampasa-Kanyinga H, Hamilton HA, LeBlanc AG, and Chaput J-P. Cannabis use among middle and high school students in Ontario: a school-based cross-sectional study. CMAJ Open. (2018) 6:E50–6. doi: 10.9778/cmajo.20170159
3. Hamilton AD, Jang JB, Patrick ME, Schulenberg JE, and Keyes KM. Age, period and cohort effects in frequent cannabis use among US students: 1991–2018. Addiction. (2019) 114:1763–72. doi: 10.1111/add.14665
4. Government of Canada. Canadian cannabis survey 2023: Summary (2024). Canadian Cannabis Survey 2023: Summary - Canada.ca. Available online at: https://www.canada.ca/en/health-canada/services/drugs-medication/cannabis/research-data/canadian-cannabis-survey-2023-summary.html#s2-2 (Accessed March 05, 2025).
5. Miech RA, Johnston LD, Patrick ME, O’Malley PM, Bachman JG, and Schulenberg JE. Monitoring the Future national survey results on drug use, 1975-2022: Secondary school students. Ann Arbor (MI: Institute for Social Research, University of Michigan (2023). Available online at: https://monitoringthefuture.org/results/publications/ (Accessed July 18, 2025).
6. European Union Drugs Agency. Cannabis – the current situation in Europe (European Drug Report 2025) (2025). Available online at: www.euda.europa.eu (Accessed July 17, 2025).
7. Konrad K, Firk C, and Uhlhaas PJ. Brain development during adolescence. Dtsch Arztebl Int. (2013) 110(25):425–431. doi: 10.3238/arztebl.2013.0425
8. Veroude K, Jolles J, Croiset G, and Krabbendam L. Changes in neural mechanisms of cognitive control during the transition from late adolescence to young adulthood. Dev Cognit Neurosci. (2013) 5:63–70. doi: 10.1016/j.dcn.2012.12.002
9. Gómez CM, Barriga-Paulino CI, Rodríguez-Martínez EI, Rojas-Benjumea MÁ, Arjona A, and Gómez-González J. The neurophysiology of working memory development: from childhood to adolescence and young adulthood. Rev Neurosci. (2018) 29:261–82. doi: 10.1515/revneuro-2017-0073
10. Steinberg L. A social neuroscience perspective on adolescent risk-taking. Dev Rev. (2008) 28:78–106. doi: 10.1016/j.dr.2007.08.002
11. Van Leijenhorst L, Zanolie K, Van Meel CS, Westenberg PM, Rombouts SARB, and Crone EA. What motivates the adolescent? brain regions mediating reward sensitivity across adolescence. Cereb Cortex. (2010) 20:61–9. doi: 10.1093/cercor/bhp078
12. Dellazizzo L, Potvin S, Giguère S, and Dumais A. Evidence on the acute and residual neurocognitive effects of cannabis use in adolescents and adults: a systematic meta-review of meta-analyses. Addiction. (2022) 117:1857–70. doi: 10.1111/add.15764
13. Degenhardt L, Coffey C, Romaniuk H, Swift W, Carlin JB, Hall WD, et al. The persistence of the association between adolescent cannabis use and common mental disorders into young adulthood. Addiction. (2013) 108:124–33. doi: 10.1111/j.1360-0443.2012.04015.x
14. Gobbi G, Atkin T, Zytynski T, Wang S, Askari S, Boruff J, et al. Association of cannabis use in adolescence and risk of depression, anxiety, and suicidality in young adulthood: a systematic review and meta-analysis. JAMA Psychiatry. (2019) 76:426–34. doi: 10.1001/jamapsychiatry.2018.4500
15. Taylor M, Collin SM, Munafò MR, MacLeod J, Hickman M, and Heron J. Patterns of cannabis use during adolescence and their association with harmful substance use behaviour: findings from a UK birth cohort. J Epidemiol Community Health. (1978) 2017) 71:764–70. doi: 10.1136/jech-2016-208503
16. Schmidt K, Tseng I, Phan A, Fong T, and Tsuang J. A systematic review: adolescent cannabis use and suicide. Addict Disord Their Treat. (2020) 19:146–51. doi: 10.1097/ADT.0000000000000196
17. Myran DT, Pugliese M, Harrison LD, Solmi M, Anderson KK, Fiedorowicz JG, et al. changes in incident schizophrenia diagnoses associated with cannabis use disorder after cannabis legalization. JAMA Netw Open. (2025) 8:e2457868. doi: 10.1001/jamanetworkopen.2024.57868
18. Athanassiou M, Dumais A, Gnanhoue G, Abdel-Baki A, Jutras-Aswad D, and Potvin S. A systematic review of longitudinal studies investigating the impact of cannabis use in patients with psychotic disorders. Expert Rev Neurother. (2021) 21:779–91. doi: 10.1080/14737175.2021.1942845
19. Albaugh MD, Owens MM, Juliano A, Ottino-Gonzalez J, Cupertino R, Cao Z, et al. Differential associations of adolescent versus young adult cannabis initiation with longitudinal brain change and behavior. Mol Psychiatry. (2023) 28:5173–82. doi: 10.1038/s41380-023-02148-2
20. Ertl N, Lawn W, Mokrysz C, Freeman TP, Alnagger N, Borissova A, et al. Associations between regular cannabis use and brain resting-state functional connectivity in adolescents and adults. J Psychopharmacol. (2023) 37:904–19. doi: 10.1177/02698811231189441
21. Hatchard T, Fried PA, Hogan MJ, Cameron I, and Smith AM. Marijuana use impacts cognitive interference: an fMRI investigation in young adults performing the counting Stroop task. J Addict Res Ther. (2014) 5:197–203. doi: 10.4172/2155-6105.1000197
22. Prescot AP, Locatelli AE, Renshaw PF, and Yurgelun-Todd DA. Neurochemical alterations in adolescent chronic marijuana smokers: a proton MRS study. Neuroimage. (2011) 57:69–75. doi: 10.1016/j.neuroimage.2011.02.044
23. Atmaca HE. Effects of recreational cannabis use and subclinical psychosis risk on brain white matter integrity and structural connectivity in adolescence. Ankara, Turkey, Bilkent University (2022).
24. Scott JC. Impact of adolescent cannabis use on neurocognitive and brain development. Child Adolesc Psychiatr Clin N Am. (2023) 32:21–42. doi: 10.1016/j.chc.2022.06.002
25. Lichenstein SD, Manco N, Cope LM, Egbo L, Garrison KA, Hardee J, et al. Systematic review of structural and functional neuroimaging studies of cannabis use in adolescence and emerging adulthood: evidence from 90 studies and 9441 participants. Neuropsychopharmacology. (2022) 47:1000–28. doi: 10.1038/s41386-021-01226-9
26. Tricco AC, Lillie E, Zarin W, O’Brien K, Colquhoun H, Kastner M, et al. A scoping review on the conduct and reporting of scoping reviews. BMC Med Res Methodol. (2016) 16:15. doi: 10.1186/s12874-016-0116-4
27. Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, and Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. (2018) 18:143. doi: 10.1186/s12874-018-0611-x
28. Grimshaw J. A knowledge synthesis chapter . Available online at: https://www.cihr-irsc.gc.ca/e/41382.html (Accessed April 28, 2025).
29. Peters MD, Godfrey C, McInerney P, Munn Z, Tricco AC, and Khalil H. Scoping reviews. In: Aromataris E, Lockwood C, Porritt K, Pila B, and Jordan Z, editors. JBI Manual for Evidence Synthesis. Adelaide, Australia, JBI (2024). doi: 10.46658/JBIMES-24-09
31. Nosko JA, Crocker CE, Hambly Lapointe C, and Tibbo PG. Cannabis use in adolescence and young adulthood and its effects on brain structure and function: a scoping review. (2024). doi: 10.17605/OSF.IO/EK9BF
32. The National Health Service. Early or delayed puberty. NHS. Available online at: https://www.nhs.uk/conditions/early-or-delayed-puberty/ (Accessed April 29, 2025).
33. Veritas Health Innovation. Covidence Systematic Review Software. Available online at: www.covidence.org (Accessed May 27, 2024).
34. Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. (2018) 169:467–73. doi: 10.7326/M18-0850
35. World Health Organization. Gender and health. Available online at: https://www.who.int/health-topics/gender#tab=tab_1 (Accessed March 03, 2025).
36. Albaugh MD, Ottino-Gonzalez J, Sidwell A, Lepage C, Juliano A, Owens MM, et al. Association of cannabis use during adolescence with neurodevelopment. JAMA Psychiatry. (2021) 78:1031–40. doi: 10.1001/jamapsychiatry.2021.1258
37. Burggren AC, Siddarth P, Mahmood Z, London ED, Harrison TM, Merrill DA, et al. Subregional hippocampal thickness abnormalities in older adults with a history of heavy cannabis use. Cannabis Cannabinoid Res. (2018) 3:242–51. doi: 10.1089/can.2018.0035
38. Lisdahl KM, Tamm L, Epstein JN, Jernigan T, Molina BSG, Hinshaw SP, et al. The impact of ADHD persistence, recent cannabis use, and age of regular cannabis use onset on subcortical volume and cortical thickness in young adults. Drug Alcohol Depend. (2016) 161:135–46. doi: 10.1016/j.drugalcdep.2016.01.032
39. Lopez-Larson MP, Bogorodzki P, Rogowska J, McGlade E, King JB, Terry J, et al. Altered prefrontal and insular cortical thickness in adolescent marijuana users. Behav Brain Res. (2011) 220:164–72. doi: 10.1016/j.bbr.2011.02.001
40. Mata I, Perez-Iglesias R, Roiz-Santianez R, Tordesillas-Gutierrez D, Pazos A, Gutierrez A, et al. Gyrification brain abnormalities associated with adolescence and early-adulthood cannabis use. Brain Res. (2010) 1317:297–304. doi: 10.1016/j.brainres.2009.12.069
41. Owens MM, Albaugh MD, Allgaier N, Yuan D, Robert G, Cupertino RB, et al. Bayesian causal network modeling suggests adolescent cannabis use accelerates prefrontal cortical thinning. Transl Psychiatry. (2022) 12. doi: 10.1038/s41398-022-01956-4
42. Radoman M, Hoeppner SS, Schuster RM, Evins AE, and Gilman JM. Marijuana use and major depressive disorder are additively associated with reduced verbal learning and altered cortical thickness. Cognit Affect Behav Neurosci. (2019) 19:1047–58. doi: 10.3758/s13415-019-00704-4
43. Scott JC, Rosen AFG, Moore TM, Roalf DR, Satterthwaite TD, Calkins ME, et al. Cannabis use in youth is associated with limited alterations in brain structure. Neuropsychopharmacology. (2019) 44:1362–9. doi: 10.1038/s41386-019-0347-2
44. Meier MH, Schriber RA, Beardslee J, Hanson J, and Pardini D. Associations between adolescent cannabis use frequency and adult brain structure: a prospective study of boys followed to adulthood. Drug Alcohol Depend. (2019) 202:191–9. doi: 10.1016/j.drugalcdep.2019.05.012
45. Ashtari M, Avants B, Cyckowski L, Cervellione KL, Roofeh D, Cook P, et al. Medial temporal structures and memory functions in adolescents with heavy cannabis use. J Psychiatr Res. (2011) 45:1055–66. doi: 10.1016/j.jpsychires.2011.01.004
46. Batalla A, Lorenzetti V, Chye Y, Yücel M, Soriano-Mas C, Bhattacharyya S, et al. The Influence of DAT1, COMT, and BDNF genetic polymorphisms on total and subregional hippocampal volumes in early onset heavy cannabis users. Cannabis Cannabinoid Res. (2018) 3:1–10. doi: 10.1089/can.2017.0021
47. Churchwell JC, Lopez-Larson M, and Yurgelun-Todd DA. Altered frontal cortical volume and decision making in adolescent cannabis users. Front Psychol. (2010) 1:225. doi: 10.3389/fpsyg.2010.00225
48. Cousijn J, Wiers RW, Ridderinkhof KR, van den Brink W, Veltman DJ, and Goudriaan AE. Grey matter alterations associated with cannabis use: results of a VBM study in heavy cannabis users and healthy controls. Neuroimage. (2012) 59:3845–51. doi: 10.1016/j.neuroimage.2011.09.046
49. Demirakca T, Sartorius A, Ende G, Meyer N, Welzel H, Skopp G, et al. Diminished gray matter in the hippocampus of cannabis users: possible protective effects of cannabidiol. Drug Alcohol Depend. (2011) 114:242–5. doi: 10.1016/j.drugalcdep.2010.09.020
50. Garimella A, Rajguru S, Singla UK, and Alluri V. Marijuana and the hippocampus: a longitudinal study on the effects of marijuana on hippocampal subfields. Prog Neuropsychopharmacol Biol Psychiatry. (2020) 101. doi: 10.1016/j.pnpbp.2020.109897
51. Gilman JM, Kuster JK, Lee S, Lee MJ, Kim BW, Makris N, et al. Cannabis use is quantitatively associated with nucleus accumbens and amygdala abnormalities in young adult recreational users. J Neurosci. (2014) 34:5529–38. doi: 10.1523/JNEUROSCI.4745-13.2014
52. Gilman JM, Lee S, Kuster JK, Lee MJ, Kim BW, van der Kouwe A, et al. Variable activation in striatal subregions across components of a social influence task in young adult cannabis users. Brain Behav. (2016) 6. doi: 10.1002/brb3.459
53. Levar N, Francis AN, Smith MJ, Ho WC, and Gilman JM. Verbal memory performance and reduced cortical thickness of brain regions along the uncinate fasciculus in young adult cannabis users. Cannabis Cannabinoid Res. (2018) 3:56–65. doi: 10.1089/can.2017.0030
54. Maple KE. Cannabis use and affective processing: a brain structure analysis. Milwaukee, Wisconsin, USA, University of Wisconsin-Milwaukee (2016). Available online at: http://digital.library.wisc.edu/1793/90978 (Accessed July 5, 2024).
55. McQueeny T, Padula CB, Price J, Medina KL, Logan P, and Tapert SF. Gender effects on amygdala morphometry in adolescent marijuana users. Behav Brain Res. (2011) 224:128–34. doi: 10.1016/j.bbr.2011.05.031
56. Medina KL, Nagel BJ, and Tapert SF. Abnormal cerebellar morphometry in abstinent adolescent marijuana users. Psychiatry Res Neuroimaging. (2010) 182:152–9. doi: 10.1016/j.pscychresns.2009.12.004
57. Price JS, McQueeny T, Shollenbarger S, Browning EL, Wieser J, and Lisdahl KM. Effects of marijuana use on prefrontal and parietal volumes and cognition in emerging adults. Psychopharmacol (Berl). (2015) 232:2939–50. doi: 10.1007/s00213-015-3931-0
58. Shollenbarger SG, Price J, Wieser J, and Lisdahl K. Impact of cannabis use on prefrontal and parietal cortex gyrification and surface area in adolescents and emerging adults. Dev Cognit Neurosci. (2015) 16:46–53. doi: 10.1016/j.dcn.2015.07.004
59. Yu T, Jia T, Zhu L, Desrivières S, Macare C, Bi Y, et al. Cannabis-associated psychotic-like experiences are mediated by developmental changes in the parahippocampal gyrus. J Am Acad Child Adolesc Psychiatry. (2020) 59:642–9. doi: 10.1016/j.jaac.2019.05.034
60. Weiland BJ, Thayer RE, Depue BE, Sabbineni A, Bryan AD, and Hutchison KE. Daily marijuana use is not associated with brain morphometric measures in adolescents or adults. J Neurosci. (2015) 35:1505–12. doi: 10.1523/JNEUROSCI.2946-14.2015
61. Medina KL, Nagel BJ, Park A, McQueeny T, and Tapert SF. Depressive symptoms in adolescents: associations with white matter volume and marijuana use. J Child Psychol Psychiatry. (2007) 48:592–600. doi: 10.1111/j.1469-7610.2007.01728.x. ISSN 00219630.
62. Koenders L, Lorenzetti V, de Haan L, Suo C, Vingerhoets WAM, van den Brink W, et al. Longitudinal study of hippocampal volumes in heavy cannabis users. J Psychopharmacol. (2017) 31:1027–34. doi: 10.1177/0269881117718380
63. Medina KL, McQueeny T, Nagel BJ, Hanson KL, Yang TT, Tapert SF, et al. Prefrontal cortex morphometry in abstinent adolescent marijuana users: subtle gender effects. Addict Biol. (2009) 14:457–68. doi: 10.1111/j.1369-1600.2009.00166.x
64. Ashtari M, Cervellione K, Cottone J, Ardekani BA, and Kumra S. Diffusion abnormalities in adolescents and young adults with a history of heavy cannabis use. J Psychiatr Res. (2009) 43:189–204. doi: 10.1016/j.jpsychires.2008.12.002
65. Becker MP, Collins PF, Lim KO, Muetzel RL, and Luciana M. Longitudinal changes in white matter microstructure after heavy cannabis use. Dev Cognit Neurosci. (2015) 16:23–35. doi: 10.1016/j.dcn.2015.10.004
66. Gruber SA, Dahlgren MK, Sagar KA, Gonenc A, and Lukas SE. Worth the wait: effects of age of onset of marijuana use on white matter and impulsivity. Psychopharmacol (Berl). (2014) 231:1455–65. doi: 10.1007/s00213-013-3326-z
67. Lichenstein SD, Shaw DS, and Forbes EE. Cannabis, connectivity, and coming of age: Associations between cannabis use and anterior cingulate cortex connectivity during the transition to adulthood. Front Hum Neurosci. (2022) 16:951204. doi: 10.3389/fnhum.2022.951204
68. Shollenbarger SG, Price J, Wieser J, and Lisdahl K. Poorer frontolimbic white matter integrity is associated with chronic cannabis use, FAAH genotype, and increased depressive and apathy symptoms in adolescents and young adults. NeuroImage Clin. (2015) 8:117–25. doi: 10.1016/j.nicl.2015.03.024
69. Dekker N, Schmitz N, Peters BD, van Amelsvoort TA, Linszen DH, and de Haan L. Cannabis use and callosal white matter structure and integrity in recent-onset schizophrenia. Psychiatry Res Neuroimaging. (2010) 181:51–6. doi: 10.1016/j.pscychresns.2009.06.003
70. DeLisi LE, Bertisch HC, Szulc KU, Majcher M, Brown K, Bappal A, et al. A preliminary DTI study showing no brain structural change associated with adolescent cannabis use. Harm Reduct J. (2006) 3. doi: 10.1186/1477-7517-3-17
71. Cousijn J, Toenders YJ, Velzen LS, and Kaag AM. The relation between cannabis use, dependence severity and white matter microstructure: a diffusion tensor imaging study. Addict Biol. (2022) 27. doi: 10.1111/adb.13081
72. Figley CR, Uddin MN, Wong K, Kornelsen J, Puig J, and Figley TD. Potential pitfalls of using fractional anisotropy, axial diffusivity, and radial diffusivity as biomarkers of cerebral white matter microstructure. Front Neurosci. (2022) 15:799576. doi: 10.3389/fnins.2021.799576
73. Solowij N, Zalesky A, Lorenzetti V, and Yücel M. Chapter 40 - chronic cannabis use and axonal fiber connectivity. In: Preedy VR, editor. Handbook of Cannabis and Related Pathologies. Amsterdam, Netherlands, Elsevier Inc (2017). doi: 10.1016/B978-0-12-800756-3.00046-6
74. Gruber SA, Silveri MM, Dahlgren MK, and Yurgelun-Todd D. Why so impulsive? White matter alterations are associated with impulsivity in chronic marijuana smokers. Exp Clin Psychopharmacol. (2011) 19:231–42. doi: 10.1037/a0023034
75. Bitter SM, Weber WA, Chu W-J, Adler CM, Eliassen JC, Strakowski SM, et al. N-acetyl aspartate levels in adolescents with bipolar and/or cannabis use disorders. J Dual Diagn. (2014) 10:39–43. doi: 10.1080/15504263.2013.869077
76. Kulich M, Fisher LM, and Voelker C. Imaging findings in mild traumatic brain injury. In: Hoffer ME and Balaban CD, editors. Neurosensory Disorders in Mild Traumatic Brain Injury. Amsterdam, Netherlands, Elsevier (2019). p. 23–47. doi: 10.1016/B978-0-12-812344-7.00003-0
77. Muetzel RL, Marjańska M, Collins PF, Becker MP, Valabrègue R, Auerbach EJ, et al. In vivo 1H magnetic resonance spectroscopy in young-adult daily marijuana users. NeuroImage Clin. (2013) 2:581–9. doi: 10.1016/j.nicl.2013.04.011
78. Licata SC and Renshaw PF. Neurochemistry of drug action: insights from proton magnetic resonance spectroscopic imaging and their relevance to addiction. Ann N Y Acad Sci. (2010) 1187:148–71. doi: 10.1111/j.1749-6632.2009.05143.x
79. Blest-Hopley G, O’Neill A, Wilson R, Giampietro V, Lythgoe D, Egerton A, et al. Adolescent-onset heavy cannabis use associated with significantly reduced glial but not neuronal markers and glutamate levels in the hippocampus. Addict Biol. (2020) 25:1–11. doi: 10.1111/adb.12827
80. Subramaniam P, Prescot A, McGlade E, Renshaw P, and Yurgelun-Todd D. Examination of gamma-aminobutyric acid and glutamate-glutamine levels in association with impulsive behavior in adolescent marijuana users. Drug Alcohol Depend. (2022) 233. doi: 10.1016/j.drugalcdep.2022.109326
81. Co BT, Goodwin DW, Gado M, Mikhael M, and Hill SY. Absence of cerebral atrophy in chronic cannabis users. Evaluation by computerized transaxial tomography. J Am Med Assoc. (1977) 237:1229–30. doi: 10.1001/jama.1977.03270390045024
82. Aloi J, Blair KS, Crum KI, Meffert H, White SF, Tyler PM, et al. Adolescents show differential dysfunctions related to Alcohol and Cannabis Use Disorder severity in emotion and executive attention neuro-circuitries. NeuroImage Clin. (2018) 19:782–92. doi: 10.1016/j.nicl.2018.06.005
83. Sagar KA, Dahlgren MK, Gonenc A, Racine MT, Dreman MW, and Gruber SA. The impact of initiation: Early onset marijuana smokers demonstrate altered Stroop performance and brain activation. Dev Cognit Neurosci. (2015) 16:84–92. doi: 10.1016/j.dcn.2015.03.003
84. Tapert SF, Schweinsburg AD, Drummond SPA, Paulus MP, Brown SA, Yang TT, et al. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacol (Berl). (2007) 194:173–83. doi: 10.1007/s00213-007-0823-y
85. Harding IH, Solowij N, Harrison BJ, Takagi M, Lorenzetti V, Lubman DI, et al. Functional connectivity in brain networks underlying cognitive control in chronic cannabis users. Neuropsychopharmacology. (2012) 37:1923–33. doi: 10.1038/npp.2012.39
86. Ames SL, Grenard JL, Stacy AW, Xiao L, He Q, Wong SW, et al. Functional imaging of implicit marijuana associations during performance on an Implicit Association Test (IAT). Behav Brain Res. (2013) 256:494–502. doi: 10.1016/j.bbr.2013.09.013
87. Maple KE. Cannabis-using youth demonstrated blunted rostral anterior cingulate cortex activation, but normal functional connectivity, during an emotional go/no-go task. Milwaukee, Wisconsin, USA, University of Wisconsin-Milwaukee (2019). Available online at: http://digital.library.wisc.edu/1793/92140 (Accessed June 24, 2024).
88. Sullivan RM, Maple KE, Wallace AL, Thomas AM, and Lisdahl KM. Examining inhibitory affective processing within the rostral anterior cingulate cortex among abstinent cannabis-using adolescents and young adults. Front Psychiatry. (2022) 13:851118. doi: 10.3389/fpsyt.2022.851118
89. Cyr M, Tau GZ, Fontaine M, Levin FR, and Marsh R. Deficient functioning of frontostriatal circuits during the resolution of cognitive conflict in cannabis-using youth. J Am Acad Child Adolesc Psychiatry. (2019) 58:702–11. doi: 10.1016/j.jaac.2018.09.436
90. Gruber SA, Dahlgren MK, Sagar KA, Gönenc A, and Killgore WDS. Age of onset of marijuana use impacts inhibitory processing. Neurosci Lett. (2012) 511:89–94. doi: 10.1016/j.neulet.2012.01.039
91. Kroon E, Kuhns L, and Cousijn J. Context dependent differences in working memory related brain activity in heavy cannabis users. Psychopharmacol (Berl). (2022) 239:1373–85. doi: 10.1007/s00213-021-05956-y
92. Claus ED, Feldstein Ewing SW, Magnan RE, Montanaro E, Hutchison KE, and Bryan AD. Neural mechanisms of risky decision making in adolescents reporting frequent alcohol and/or marijuana use. Brain Imaging Behav. (2018) 12:564–76. doi: 10.1007/s11682-017-9723-x
93. Smith AM, Zunini RAL, Anderson CD, Longo CA, Cameron I, Hogan MJ, et al. Impact of marijuana on response inhibition: an fMRI study in young adults. J Behav Brain Sci. (2011) 1:124–33. doi: 10.4236/jbbs.2011.13017
94. Schweinsburg AD, Nagel BJ, Schweinsburg BC, Park A, Theilmann RJ, and Tapert SF. Abstinent adolescent marijuana users show altered fMRI response during spatial working memory. Psychiatry Res Neuroimaging. (2008) 163:40–51. doi: 10.1016/j.pscychresns.2007.04.018
95. Smith AM, Longo CA, Fried PA, Hogan MJ, and Cameron I. Effects of marijuana on visuospatial working memory: an fMRI study in young adults. Psychopharmacol (Berl). (2010) 210:429–38. doi: 10.1007/s00213-010-1841-8
96. Tervo-Clemmens B, Simmonds D, Calabro FJ, Montez DF, Lekht JA, Day NL, et al. Early cannabis use and neurocognitive risk: a prospective functional neuroimaging study. Biol Psychiatry Cognit Neurosci Neuroimaging. (2018) 3:713–25. doi: 10.1016/j.bpsc.2018.05.004
97. Padula CB, Schweinsburg AD, and Tapert SF. Spatial working memory performance and fmri activation interactions in abstinent adolescent marijuana users. Psychol Addict Behav. (2007) 21:478–87. doi: 10.1037/0893-164X.21.4.478
98. Dager AD, Tice MR, Book GA, Tennen H, Raskin SA, Austad CS, et al. Relationship between fMRI response during a nonverbal memory task and marijuana use in college students. Drug Alcohol Depend. (2018) 188:71–8. doi: 10.1016/j.drugalcdep.2018.03.025
99. Schweinsburg AD, Schweinsburg BC, Nagel BJ, Eyler LT, and Tapert SF. Neural correlates of verbal learning in adolescent alcohol and marijuana users. Addiction. (2011) 106:564–73. doi: 10.1111/j.1360-0443.2010.03197.x
100. Blest-Hopley G, O’Neill A, Wilson R, Giampietro V, and Bhattacharyya S. Disrupted parahippocampal and midbrain function underlie slower verbal learning in adolescent-onset regular cannabis use. Psychopharmacol (Berl). (2021) 238:1315–31. doi: 10.1007/s00213-019-05407-9
101. Hubbard NA, Miller KB, Aloi J, Bajaj S, Wakabayashi KT, and Blair RJR. Evaluating instrumental learning and striatal–cortical functional connectivity in adolescent alcohol and cannabis use. Addict Biol. (2023) 28:1–13. doi: 10.1111/adb.13258
102. Cousijn J, Wiers RW, Ridderinkhof KR, van den Brink W, Veltman DJ, and Goudriaan AE. Effect of baseline cannabis use and working-memory network function on changes in cannabis use in heavy cannabis users: a prospective fMRI study. Hum Brain Mapp. (2014) 35:2470–82. doi: 10.1002/hbm.22342
103. Cousijn J, Vingerhoets WAM, Koenders L, de Haan L, van den Brink W, Wiers RW, et al. Relationship between working-memory network function and substance use: a 3-year longitudinal fMRI study in heavy cannabis users and controls. Addict Biol. (2014) 19:282–93. doi: 10.1111/adb.12111
104. Acheson A, Ray KL, Hines CS, Li K, Dawes MA, Mathias CW, et al. Functional activation and effective connectivity differences in adolescent marijuana users performing a simulated gambling task. J Addict. (2015) 2015. doi: 10.1155/2015/783106
105. Skumlien M, Mokrysz C, Freeman TP, Wall MB, Bloomfield M, Lees R, et al. Neural responses to reward anticipation and feedback in adult and adolescent cannabis users and controls. Neuropsychopharmacology. (2022) 47:1976–83. doi: 10.1038/s41386-022-01316-2
106. Cousijn J, Wiers RW, Ridderinkhof KR, van den Brink W, Veltman DJ, Porrino LJ, et al. Individual differences in decision making and reward processing predict changes in cannabis use: a prospective functional magnetic resonance imaging study. Addict Biol. (2013) 18:1013–23. doi: 10.1111/j.1369-1600.2012.00498.x
107. Nestor LJ, Behan B, Suckling J, and Garavan H. Cannabis-dependent adolescents show differences in global reward-associated network topology: a functional connectomics approach. Addict Biol. (2020) 25. doi: 10.1111/adb.12752
108. Martz ME, Trucco EM, Cope LM, Hardee JE, Jester JM, Zucker RA, et al. Association of marijuana use with blunted nucleus accumbens response to reward anticipation. JAMA Psychiatry. (2016) 73:838–44. doi: 10.1001/jamapsychiatry.2016.1161
109. Lichenstein SD, Musselman S, Shaw DS, Sitnick S, and Forbes EE. Nucleus accumbens functional connectivity at age 20 is associated with trajectory of adolescent cannabis use and predicts psychosocial functioning in young adulthood. Addiction. (2017) 112:1961–70. doi: 10.1111/add.13882
110. Karoly HC, Bryan AD, Weiland BJ, Mayer A, Dodd A, and Feldstein Ewing SW. Does incentive-elicited nucleus accumbens activation differ by substance of abuse? An examination with adolescents. Dev Cognit Neurosci. (2015) 16:5–15. doi: 10.1016/j.dcn.2015.05.005
111. Macedo I, Paiva TO, Pasion R, Daedelow L, Heinz A, Magalhaes A, et al. Light cannabis use and the adolescent brain: an 8-years longitudinal assessment of mental health, cognition, and reward processing. Psychopharmacol (Berl). (2024) 241:1447–61. doi: 10.1007/s00213-024-06575-z
112. Gilman JM, Curran MT, Calderon V, Schuster RM, and Evins AE. Altered neural processing to social exclusion in young adult marijuana users. Biol Psychiatry Cognit Neurosci Neuroimaging. (2016) 1:152–9. doi: 10.1016/j.bpsc.2015.11.002
113. Gilman JM, Schuster RM, Curran MT, Calderon V, van der Kouwe A, and Evins AE. Neural mechanisms of sensitivity to peer information in young adult cannabis users. Cognit Affect Behav Neurosci. (2016) 16:646–61. doi: 10.3758/s13415-016-0421-8
114. Heitzeg MM, Cope LM, Martz ME, Hardee JE, and Zucker RA. Brain activation to negative stimuli mediates a relationship between adolescent marijuana use and later emotional functioning. Dev Cognit Neurosci. (2015) 16:71–83. doi: 10.1016/j.dcn.2015.09.003
115. Nichols ES, Penner J, Ford KA, Wammes M, Neufeld RWJ, Mitchell DGV, et al. Emotion regulation in emerging adults with major depressive disorder and frequent cannabis use. NeuroImage Clin. (2021) 30. doi: 10.1016/j.nicl.2021.102575
116. Spechler PA, Chaarani B, Orr C, Albaugh MD, Fontaine NR, Higgins ST, et al. Longitudinal associations between amygdala reactivity and cannabis use in a large sample of adolescents. Psychopharmacol (Berl). (2020) 237:3447–58. doi: 10.1007/s00213-020-05624-7
117. Cousijn J, Goudriaan AE, Ridderinkhof KR, van den Brink W, Veltman DJ, and Wiers RW. Neural responses associated with cue-reactivity in frequent cannabis users. Addict Biol. (2013) 18:570–80. doi: 10.1111/j.1369-1600.2011.00417.x
118. Lopez-Larson MP, Rogowska J, Bogorodzki P, Bueler CE, McGlade EC, and Yurgelun-Todd DA. Cortico-cerebellar abnormalities in adolescents with heavy marijuana use. Psychiatry Res Neuroimaging. (2012) 202:224–32. doi: 10.1016/j.pscychresns.2011.11.005
119. Kelly C, Castellanos FX, Tomaselli O, Lisdahl K, Tamm L, Jernigan T, et al. Distinct effects of childhood ADHD and cannabis use on brain functional architecture in young adults. NeuroImage Clin. (2017) 13:188–200. doi: 10.1016/j.nicl.2016.09.012
120. Ma L, Hettema JM, Cousijn J, Bjork JM, Steinberg JL, Keyser-Marcus L, et al. Resting-state directional connectivity and anxiety and depression symptoms in adult cannabis users. Biol Psychiatry Cognit Neurosci Neuroimaging. (2021) 6:545–55. doi: 10.1016/j.bpsc.2020.09.015
121. Lopez-Larson MP, Rogowska J, and Yurgelun-Todd D. Aberrant orbitofrontal connectivity in marijuana smoking adolescents. Dev Cognit Neurosci. (2015) 16:54–62. doi: 10.1016/j.dcn.2015.08.002
122. Orr C, Morioka R, Behan B, Datwani S, Doucet M, Ivanovic J, et al. Altered resting-state connectivity in adolescent cannabis users. Am J Drug Alcohol Abuse. (2013) 39:372–81. doi: 10.3109/00952990.2013.848213
123. Behan B, Connolly CG, Datwani S, Doucet M, Ivanovic J, Morioka R, et al. Response inhibition and elevated parietal-cerebellar correlations in chronic adolescent cannabis users. Neuropharmacology. (2014) 84:131–7. doi: 10.1016/j.neuropharm.2013.05.027
124. Camchong J, Collins PF, Becker MP, Lim KO, and Luciana M. Longitudinal alterations in prefrontal resting brain connectivity in non-treatment-seeking young adults with cannabis use disorder. Front Psychiatry. (2019) 10:514. doi: 10.3389/fpsyt.2019.00514
125. Wilson A, Gicas K, Stevens WD, Sergio L, and Wojtowicz M. Substance use is associated with worse mental health and altered resting state functional connectivity in female university athletes at baseline: a pilot study. PloS One. (2021) 16. doi: 10.1371/journal.pone.0253261
126. Osuch EA, Manning K, Hegele RA, Theberge J, Neufeld R, Mitchell D, et al. Depression, marijuana use and early-onset marijuana use conferred unique effects on neural connectivity and cognition. Acta Psychiatr Scand. (2016) 134:399–409. doi: 10.1111/acps.12629
127. Jacobus J, Goldenberg D, Wierenga CE, Tolentino NJ, Liu TT, and Tapert SF. Altered cerebral blood flow and neurocognitive correlates in adolescent cannabis users. Psychopharmacol (Berl). (2012) 222:675–84. doi: 10.1007/s00213-012-2674-4
128. Broyd SJ, Greenwood L-M, Croft RJ, Dalecki A, Todd J, Michie PT, et al. Chronic effects of cannabis on sensory gating. Int J Psychophysiol. (2013) 89:381–9. doi: 10.1016/j.ijpsycho.2013.04.015
129. Greenwood L-M, Broyd SJ, Croft R, Todd J, Michie PT, Johnstone S, et al. Chronic effects of cannabis use on the auditory mismatch negativity. Biol Psychiatry. (2014) 75:449–58. doi: 10.1016/j.biopsych.2013.05.035
130. Broyd SJ, Greenwood L, van Hell HH, Croft RJ, Coyle H, Lee-Bates B, et al. Mismatch negativity and P50 sensory gating in abstinent former cannabis users. Neural Plast. (2016) 2016. doi: 10.1155/2016/6526437
131. Maij DLR, van de Wetering BJM, Franken IHA, Maij DL, van de Wetering BJ, and Franken IH. Cognitive control in young adults with cannabis use disorder: An event-related brain potential study. J Psychopharmacol. (2017) 31:1015–26. doi: 10.1177/0269881117719262
132. Smith JL, De Blasio FM, Iredale JM, Matthews AJ, Bruno R, Dwyer M, et al. Verbal learning and memory in cannabis and alcohol users: an event-related potential investigation. Front Psychol. (2017) 8:2129. doi: 10.3389/fpsyg.2017.02129
133. Cox SML, Tippler M, Jaworska N, Smart K, Castellanos-Ryan N, Durand F, et al. mGlu5 receptor availability in youth at risk for addictions: effects of vulnerability traits and cannabis use. Neuropsychopharmacology. (2020) 45:1817–25. doi: 10.1038/s41386-020-0708-x
134. Sevy S, Smith GS, Ma Y, Dhawan V, Chaly T, Kingsley PB, et al. Cerebral glucose metabolism and D2/D3 receptor availability in young adults with cannabis dependence measured with positron emission tomography. Psychopharmacol (Berl). (2008) 197:549–56. doi: 10.1007/s00213-008-1075-1
Appendix I: database search terms
CINAHL (EBSCO)
(MH “Cannabis”) OR (MH “Cannabinoids”) OR (MH “Cannabidiol”) OR TI ((cannabis or marijuana or “thc” or pot or weed or “cbd” or tetrahydrocannabinol or cannabidiol or Cannabinoid*)) OR AB ((cannabis or marijuana or “thc” or pot or weed or “cbd” or tetrahydrocannabinol or cannabidiol or Cannabinoid*)).
AND
(MH “Adolescence”) OR (MH “Young Adult”) OR TI (adolescen* or “young adult*” or teen* or youth* or “Young person” OR “young people” OR “emerging adult*” or juvenile) OR AB (adolescen* or “young adult*” or teen* or youth* or “Young person” OR “young people” OR “emerging adult*” or juvenile).
AND
(MH “Neuroradiography”) OR (MH “Brain Cortical Thickness”) OR (MH “Dopaminergic Imaging”) OR (MH “Echoencephalography”) OR TI (“Neuroradiography” OR “Brain Cortical Thickness” OR “Dopaminergic Imaging” OR “Echoencephalography” OR brain or “white matter” OR “grey matter” OR “gray matter” OR “functional imaging” OR “structural imaging” OR spectroscop* OR “MRI” “Magnetic resonance imaging” OR”FMRI” OR “DTI” OR “Diffusion tensor imaging” OR MRS OR “Magnetic resonance spectroscopy” OR “prefrontal cortex” OR “pre-frontal cortex” OR “neural pathways” OR “connectivity” OR “neuroimaging” OR “fractional anisotropy” OR FA OR “resting state” OR DWI OR “diffusion weighted imaging”) OR AB (“Neuroradiography” OR “Brain Cortical Thickness” OR “Dopaminergic Imaging” OR “Echoencephalography” OR brain or “white matter” OR “grey matter” OR “gray matter” OR “functional imaging” OR “structural imaging” OR spectroscop* OR “MRI” “Magnetic resonance imaging” OR”FMRI” OR “DTI” OR “Diffusion tensor imaging” OR MRS OR “Magnetic resonance spectroscopy” OR “prefrontal cortex” OR “pre-frontal cortex” OR “neural pathways” OR “connectivity” OR “neuroimaging” OR “fractional anisotropy” OR FA OR “resting state” OR DWI OR “diffusion weighted imaging”).
Ovid MEDLINE (NLM, Wolters Kluwer)
Cannabis/OR (cannabis or cannabinoid* or cannabidiol or marijuana or “THC” or pot or weed or “CBD” or tetrahydrocannabinol).ti,ab.
AND
Adolescent/OR Young Adult/OR (adolescen* or “young adult*” or teen* or youth* or “Young person” or “young people” or “emerging adult*” or juvenile).ti,ab.
AND
positron emission tomography computed tomography/or single photon emission computed tomography computed tomography/or neuroimaging/or brain cortical thickness/or diffusion tensor imaging/or functional neuroimaging/or brain mapping/or connectome/or dopaminergic imaging/or exp magnetic resonance imaging/OR (“positron emission tomography” or “computed tomography” or “single photon emission computed tomography” or brain or “white matter” or “grey matter” or “gray matter” or “functional imaging” or “structural imaging” or spectroscop* or “MRI Magnetic resonance imaging” or “FMRI” or “DTI” or “diffusion tensor imaging” or “MRS” or “Magnetic resonance spectroscopy” or “prefrontal cortex” or “pre-frontal cortex” or “neural pathways” or “connectivity” or “neuroimaging” or “fractional anisotropy” or “FA” or “resting state” or “DWI” or “diffusion weighted imaging”).ti,ab.
PsycINFO (American Psychological Association).
cannabis/or cannabinoids/or marijuana/OR “cannabis use”/OR “cannabis use disorder”/OR (cannabis or cannabinoid* or cannabidiol or marijuana or “THC” or pot or weed or “CBD” or tetrahydrocannabinol).ti,ab.
AND
exp Late Adolescence/or exp Early Adolescence/OR emerging adulthood/OR (adolescen* or “young adult*” or teen* or youth* or “Young person” or “young people” or “emerging adult*” or juvenile).ti,ab.
AND
neuroimaging/or encephalography/or magnetic resonance imaging/or brain connectivity/or diffusion tensor imaging/OR functional magnetic resonance imaging/OR white matter/OR gray matter/OR spectroscopy/OR (brain or “white matter” or “grey matter” or “gray matter” or “functional imaging” or “structural imaging” or spectroscop* or “MRI Magnetic resonance imaging” or “FMRI” or “DTI” or “Diffusion tensor imaging” or “MRS” or “Magnetic resonance spectroscopy” or “prefrontal cortex” or “pre-frontal cortex” or “neural pathways” or “connectivity” or “neuroimaging” or “fractional anisotropy” or “FA” or “resting state” or “DWI” or “diffusion weighted imaging” or encephalography).ti,ab.
Embase (Elsevier)
cannabis/or cannabinoid/OR cannabis addiction/OR cannabis smoking/or “cannabis use”/OR (cannabis or cannabinoid* or cannabidiol or marijuana or “THC” or pot or weed or “CBD” or tetrahydrocannabinol).ti,ab.
AND
adolescent/or juvenile/OR young adult/OR (adolescen* or “young adult*” or teen* or youth* or “Young person” or “young people” or “emerging adult*” or juvenile).ti,ab.
AND
neuroimaging/OR functional neuroimaging/OR nuclear magnetic resonance spectroscopy/OR diffusion tensor imaging/OR diffusion weighted imaging/OR white matter/OR gray matter/OR (brain or “white matter” or “grey matter” or “gray matter” or “functional imaging” or “structural imaging” or spectroscop* or “MRI Magnetic resonance imaging” or “FMRI” or “DTI” or “Diffusion tensor imaging” or “MRS” or “Magnetic resonance spectroscopy” or “prefrontal cortex” or “pre-frontal cortex” or “neural pathways” or “connectivity” or “neuroimaging” or “fractional anisotropy” or “FA” or “resting state” or “DWI” or “diffusion weighted imaging”).ti,ab.
MedRxiv and Google Scholar.
cannabis OR marijuana.
AND
adolescen* OR teen* OR youth* OR young.
AND
neuroimaging OR MRI OR MRS OR brain.
Keywords: cannabis, adolescence, young adulthood, brain, neuroimaging
Citation: Nosko L, Crocker CE and Tibbo PG (2025) Cannabis use in adolescence and young adulthood and its effects on brain structure and function: a scoping review. Front. Psychiatry 16:1644105. doi: 10.3389/fpsyt.2025.1644105
Received: 09 June 2025; Accepted: 14 August 2025;
Published: 03 September 2025.
Edited by:
Toine Pieters, Utrecht University, NetherlandsReviewed by:
Stefanie Hassel, University of Calgary, CanadaChristian Thurstone, Denver Health Medical Center, United States
Copyright © 2025 Nosko, Crocker and Tibbo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lilith Nosko, TGlsaXRoLk5vc2tvQGRhbC5jYQ==
 Lilith Nosko
Lilith Nosko Candice E. Crocker
Candice E. Crocker Phil G. Tibbo
Phil G. Tibbo