- Department of Child Health Care, Wuhan Children’s Hospital (Wuhan Maternal and Child Healthcare Hospital), Tongji Medical College, Huazhong University of Science & Technology, Wuhan, China
Objective: To evaluate the levels of vitamin A (VA), vitamin D (VD) and vitamin E (VE) in children with Attention deficit hyperactivity disorder(ADHD) and analyze their association with ADHD symptoms.
Methods: A total of 657 children aged 4–10 years were collected, of whom 219 were diagnosed with ADHD (including 100 cases of attention deficit major, 14 cases of hyperactive impulsivity major, and 105 cases of mixed type) and 438 were used as healthy controls. High performance liquid chromatography (HPLC) was used to detect serum VA, VD (D2, D3 and total VD) and VE levels. The Weiss Functional Deficit Rating Scale (WFIRS) was used to evaluate the clinical symptoms of ADHD.
Results: The serum levels of VA, VD (D2, D3, total VD) and VE in the ADHD group were significantly lower than those in the control group. Among the different subtypes, the levels of VD3, total VD and VA in the attention deficit type and mixed type were significantly different from those in the control group, and the levels of total VD and VA in the hyperactive and impulsive type were significantly different. There was a correlation between the total score of ADHD symptoms and the scores of each dimension and vitamin levels.
Conclusion: The level of fat-soluble vitamins is significantly correlated with the prevalence, subtype symptoms and functional deficits of ADHD, suggesting that VA, VD and VE supplementation may be the adjuvant treatment for ADHD, but the specific causal relationship needs to be verified by further prospective studies.
1 Introduction
Attention deficit hyperactivity disorder (ADHD) is one of the most prevalent neurobehavioral disorders in childhood, characterized by age-inappropriate inattention and/or hyperactivity-impulsivity (1). It is classified into three clinical subtypes: predominantly inattentive type, predominantly hyperactive-impulsive type, and combined type. Although ADHD originates in childhood, its symptoms often persist into adolescence and adulthood, with some perspectives suggesting that ADHD may be a lifelong disorder. In recent years, the prevalence of ADHD has shown an upward trend. Global estimates indicate a prevalence rate of 7.6% (2), with some regions reporting rates as high as 10.3% (3). Notably, males exhibit a significantly higher prevalence than females. Research demonstrates that the onset of ADHD results from the combined action of genetic and environmental factors (4), yet its specific pathogenesis remains not fully understood. Existing studies have indicated that mutations in gene loci, vitamin and mineral supplementation, and gut microbiota levels are all associated with the development of ADHD and the severity of its symptoms (5–7).
In recent years, a substantial body of research has focused on the relationship between ADHD and nutritional elements, particularly fat-soluble vitamins such as vitamin A (VA), vitamin D (VD), and vitamin E (VE). VD, a neurosteroid hormone, is primarily synthesized from 7-dehydrocholesterol in the skin upon exposure to sunlight or obtained from dietary sources of VD2. By binding to VD receptors, VD exerts effects on various organs throughout the body. It regulates the expression of multiple genes, and its deficiency impacts the expression of synaptic proteins as well as the synthesis and metabolism of neurotransmitters. Furthermore, VD receptors are widely distributed in the brain, with particularly high concentrations in the substantia nigra, an area rich in dopaminergic neurons (8). Studies have demonstrated that VD functions as an enzyme involved in dopamine synthesis and enhances the expression of tyrosine kinase (9). Given that ADHD is closely associated with dysfunction of the dopamine transporter (DAT), which diminishes dopamine signaling by facilitating the reuptake of dopamine into presynaptic neurons, thereby influencing the cognitive function of ADHD patients (10), VD metabolism may play a role in the pathogenesis of ADHD.
On the other hand, VA is metabolized into retinoic acid (RA) within the body, where it is involved in hippocampal synaptic plasticity and plays a crucial role in memory that is dependent on the hippocampus (11). Animal studies have demonstrated that an increase in RA concentration is directly associated with improved memory performance (12). Additionally, human studies have indicated that lower circulating levels of retinol are predictive of a higher risk of cognitive function decline (13). Therefore, a deficiency in VA may impair hippocampal synaptic plasticity, leading to a decline in memory function, insufficient learning ability, and attention maintenance disorders, which may exacerbate cognitive deficits in patients with ADHD.
VE, primarily in the form of α-tocopherol, serves as a significant fat-soluble antioxidant and a crucial component of cellular membranes (14). VE effectively scavenges peroxyl radicals, halting the oxidation of polyunsaturated fatty acids (PUFAs) and thereby preventing oxidative damage. Furthermore, VE plays a role in modulating the immune system, mitigating inflammatory responses, and alleviating various types of stress (15). Research indicates that VE can reduce oxidative stress and neuroinflammation in patients with ADHD through its antioxidant, anti-inflammatory, and neuronal membrane-protective properties. This action contributes to improvements in hyperactivity, impulsivity, and overall functional status. Conversely, VE deficiency may worsen neuronal damage and inflammatory responses, potentially exacerbating ADHD symptoms.
Current research on the impact of fat-soluble vitamins on ADHD remains contentious and incomplete. Some preliminary studies have indicated a potential association between deficiencies in VD and VA and the occurrence of ADHD (16). However, other studies have found no significant correlation between VD levels and ADHD (17). To date, no research has established a relationship between VE levels and ADHD. In light of this background, the present study aims to evaluate the levels of VD, VA, and VE in children diagnosed with ADHD and to investigate their correlations with ADHD symptoms.
2 Participants and methods
2.1 Participants
We collected patient information from 657 children aged 4 to 10 years who visited Wuhan Children’s Hospital due to “inattention or hyperactivity” between June 2023 and June 2024. All children suspected of having ADHD underwent a comprehensive evaluation, which included a detailed review of their current health status, developmental history, and family medical history. Developmental pediatricians conducted thorough physical examinations and in-depth interviews with parents in accordance with the diagnostic criteria outlined in the Diagnostic and Statistical Manual of Mental Disorders (DSM-5). This approach aimed to gain a comprehensive understanding of the children’s daily performance and behavioral characteristics, thereby facilitating accurate ADHD diagnoses. Ultimately, 219 children met the diagnostic criteria for ADHD, comprising 171 boys and 48 girls, with an average age of 8.15 years. Among these, 100 cases were classified as predominantly inattentive type, primarily characterized by difficulties in maintaining attention, easy distraction by external stimuli, and frequent lapses in focus during academic tasks and other activities requiring concentration. Additionally, 14 cases were identified as predominantly hyperactive-impulsive type, exhibiting behavioral characteristics such as excessive limb movements, an inability to remain seated quietly, challenges in participating in activities quietly, and often accompanied by excessive running and noisy behaviors. Furthermore, 105 cases were classified as combined type, exhibiting core symptoms of both inattention and hyperactivity-impulsivity, with more complex and diverse clinical manifestations. Simultaneously, 438 healthy children without any physical or behavioral abnormalities were selected from the same outpatient clinic to serve as the healthy control (HC) group, which included 340 boys and 98 girls, with an average age of 7.93 years.This study was approved by the Medical Ethics Committee of Wuhan Children’s Hospital Affiliated with Huazhong University of Science and Technology (No. 2025592).
2.2 Exclusion criteria
The exclusion criteria were children: (1) Children with other genetic, neuropsychiatric and physical diseases, such as cerebral palsy, epilepsy, and schizophrenia; (2) Have not taken nutritional supplements containing vitamins such as VA, VD, VE and so on in the past 3 months; (3) Children suffering from malnutrition, picky eating, digestive diseases, etc., which may affect the absorption and metabolism of micronutrients. (4) Taking drugs that may affect the metabolism of VA, VD or VE.
2.3 Behavioral assessments
The Weiss Functional Impairment Rating Scales (WFIRS) was developed by Margaret D Weiss in 2007 to assess the impact of symptoms and behavioral or emotional problems on clinically relevant functional domains in children with ADHD. WFIRS is divided into self-report and parent versions. The Weiss Functional Impairment Rating Scale-Parent Report (WFIRS-P) was assessed by parents or caregivers, and the Chinese version was revised in 2001 by the team of Professor Wang Yufeng, Institute of Mental Health, Peking University. WFIRS – P consists of a total of 50 items divided into 6 functional areas, namely family, learning and school, life skills, children’s self-concept, social activities and risk-taking activities. Each item is scored on a four-point Likert scale: 0 (never), 1 (sometimes), 2 (often), and 3 (always or often). This study used the Chinese version of the WFIRS-P scale.
2.4 Laboratory measurements
High-performance liquid chromatography was employed to conduct blood tests on all enrolled children, assessing various detection indices, including the levels of vitamin A (VA), vitamin D2 (VD2), vitamin D3 (VD3), total vitamin D (VD), and vitamin E (VE). Serum VA levels < 300 ng/ml and 300–800 ng/ml were judged to be VA deficient and normal, respectively. Serum VD levels < 30 ng/ml and 30–100 ng/ml were judged to be VD insufficiency/deficiency and normal, respectively. SerumVE levels < 5168.6 ng/ml, 5168.6–20000 ng/ml and >20000 ng/ml were judged to be VE deficiency, normal and overdose, respectively.
2.5 Statistical analysis
All data analyses were conducted using SPSS version 27.0. Prior to analysis, the Kolmogorov-Smirnov test was utilized to evaluate the normality of continuous variables. Continuous data are presented as mean ± standard deviation, while categorical data are expressed as frequencies and percentages. Based on the distribution of the variables, independent samples t-tests were employed to compare continuous data between the two groups. Chi-square tests were utilized to identify differences in categorical variables between the groups. The Spearman correlation coefficient test was applied to assess the significance of factors influencing serum concentrations of fat-soluble vitamins. A p-value of less than 0.05 was considered statistically significant.
3 Results
3.1 Comparison of sociodemographic features between the HC group and ADHD group
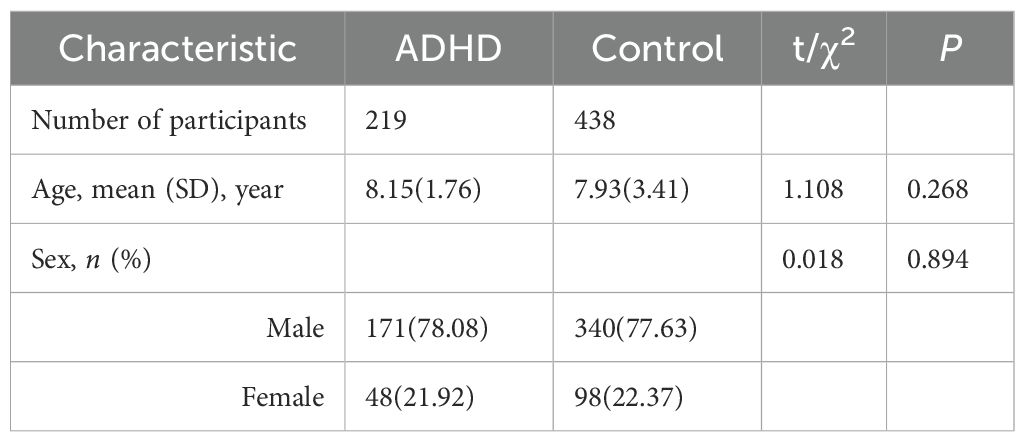
Table 1 shows the sociodemographic data of the two groups. There were no significant differences in gender or age between children with ADHD and those in the HC (healthy control) group.
3.2 Comparison of vitamin content between the case and HC groups
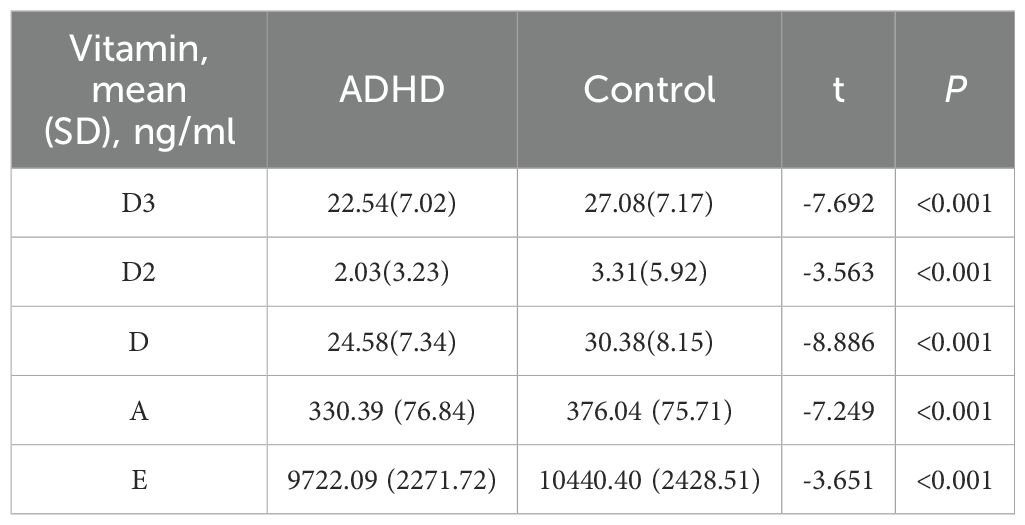
Serum concentrations of VA, VD, and VE in the two groups are shown in Table 2. The data show that in terms of VD3, VD2, VD, VA, and VE. The mean contents in the ADHD group were 22.54 ng/ml, 2.03 ng/ml, 24.58 ng/ml, 330.39 ng/ml, and 9722.09 ng/ml, respectively, all lower than the control group’s corresponding values of 27.08 ng/ml, 3.31 ng/ml, 30.38 ng/ml, 376.04 ng/ml, and 10440.40 ng/ml. The mean contents of VD3, VD2, VD, VA, and VE in the ADHD group were all lower than those in the control group. Results of independent samples t-tests showed t-values of -7.692, -3.563, -8.886, -7.249, and -3.651, respectively, with all P < 0.001, indicating extremely significant differences.
3.3 Comparison of vitamins between different ADHD types and the control group
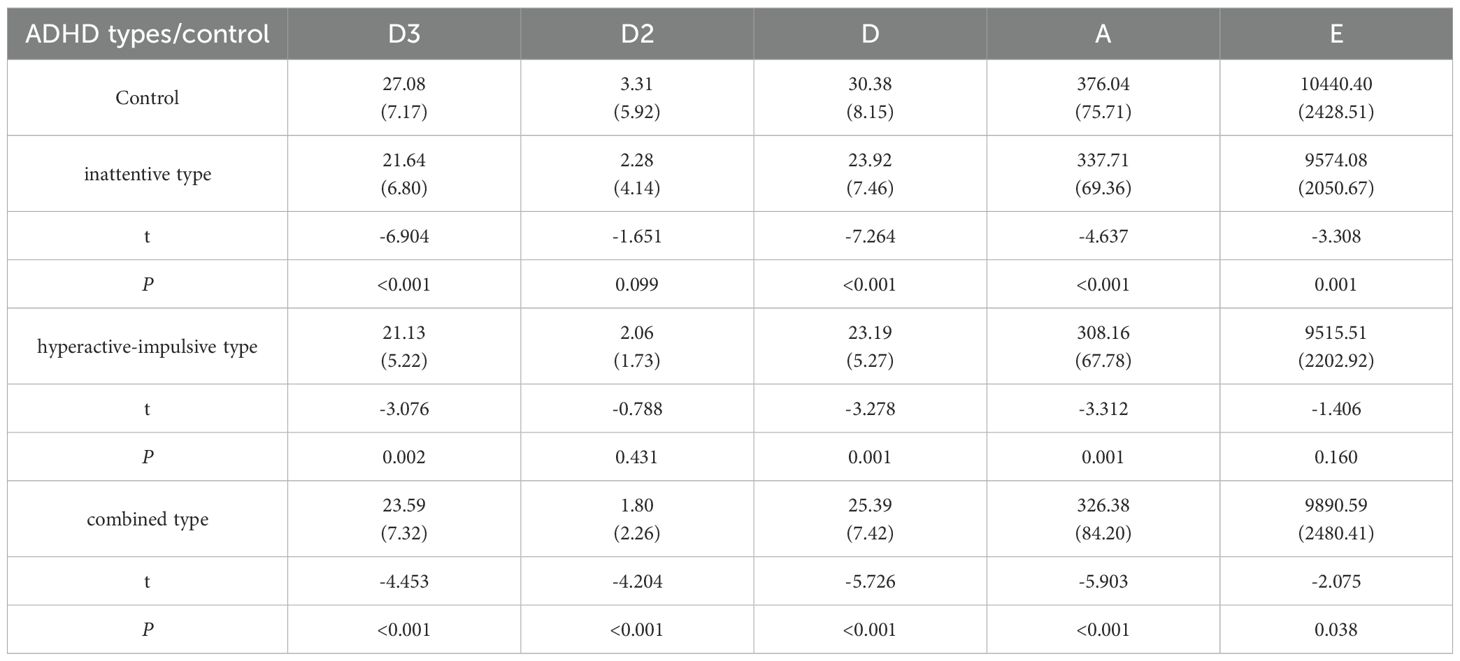
Table 3 compares the vitamin contents of different ADHD subtypes (inattentive type, hyperactive-impulsive type, combined type) with those of the control group: In terms of VD3, the contents in all ADHD subtypes were significantly lower than those in the control group (P < 0.001 for the inattentive and combined types, and P = 0.002 for the hyperactive-impulsive type). For VD2, only the combined type showed an extremely significant decrease compared to the control group (P < 0.001), with no significant differences in the other subtypes. VD levels in all subtypes were significantly lower than those in the control group (P < 0.001 for the inattentive and combined types, and P = 0.001 for the hyperactive-impulsive type). VA contents in all subtypes were significantly lower than those in the control group (P < 0.001). For VE, the inattentive and combined types were significantly lower than the control group (P = 0.001 and P = 0.038, respectively), while there was no significant difference in the hyperactive-impulsive type (P = 0.160).
3.4 Relationship between total ADHD deficit scores and vitamin levels
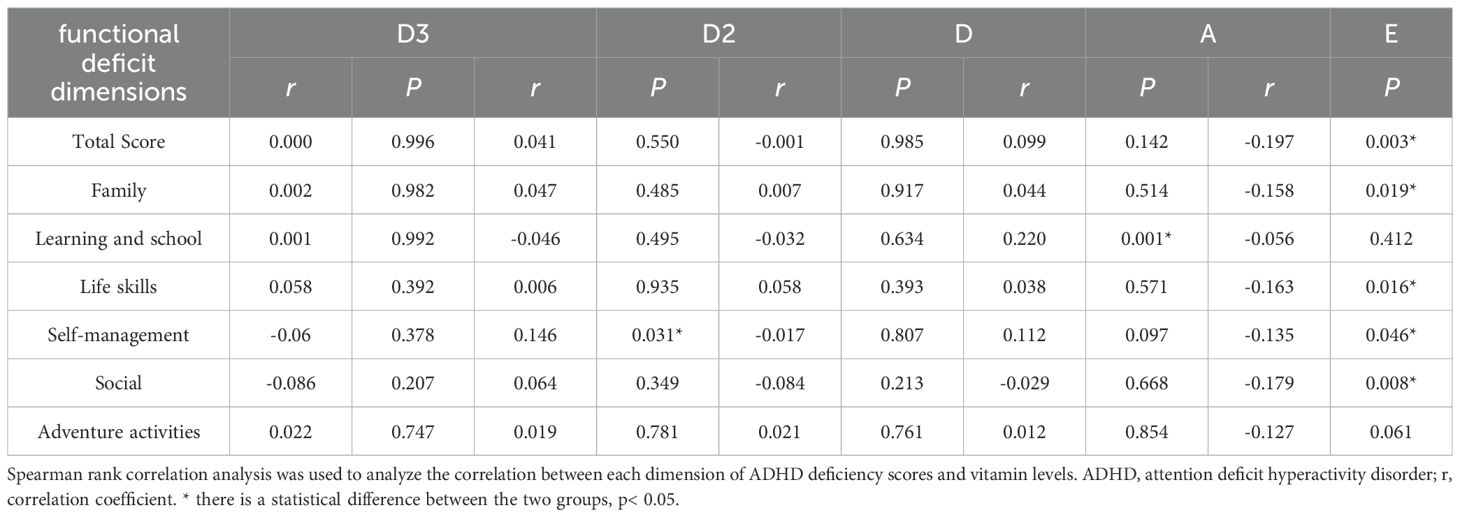
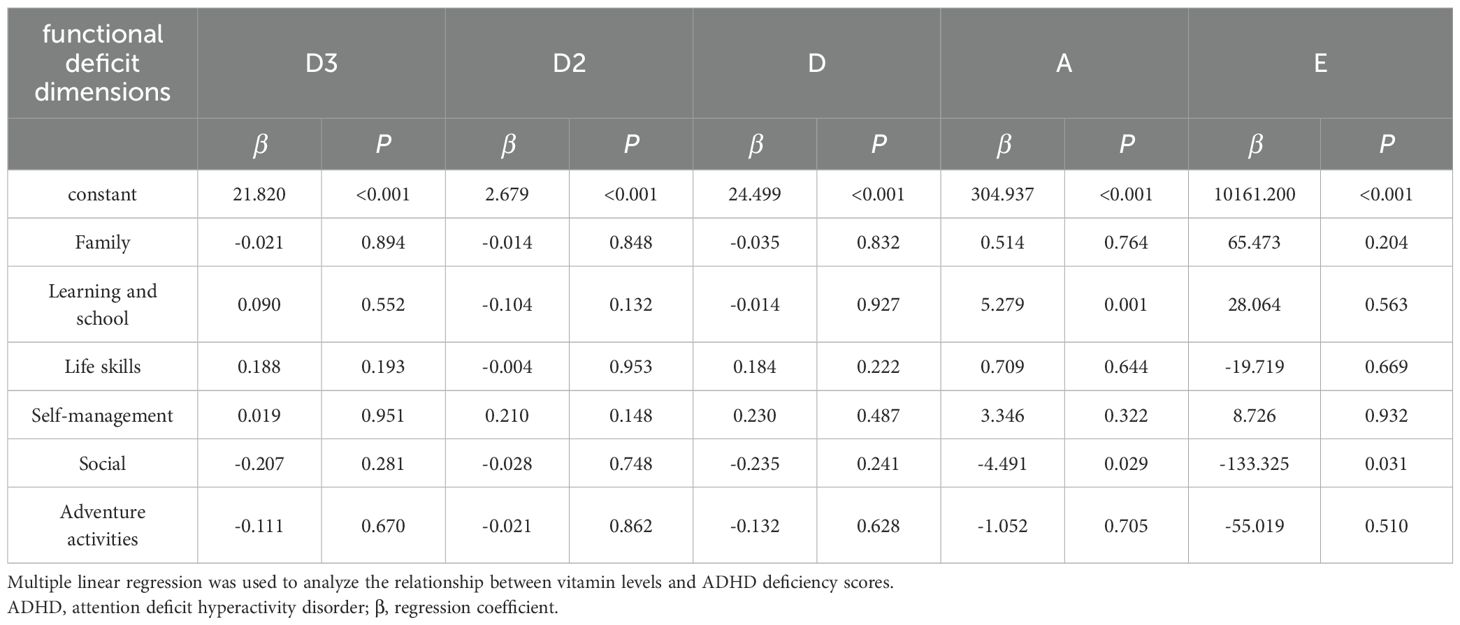
Table 4 uses Spearman correlation analysis to explore the relationship between total ADHD deficit scores and vitamin levels. The results show VD3 was significantly negatively correlated with the total score, family dimension, and learning and school dimension (P < 0.05). VD2 was only significantly positively correlated with the self-management dimension (P < 0.05). VD had no significant correlation with any dimension (P > 0.05). VA was only significantly positively correlated with the learning and school dimension (P < 0.05). VE was significantly negatively correlated with the total score, family dimension, life skills dimension, self-management dimension, and social activities dimension (P < 0.05). To further clarify the predictive effect of each vitamin level on ADHD functional deficit scores, we conducted a multiple linear regression analysis, the results of which are presented in Table 5. The results showed that among all vitamin indices and functional deficit dimensions: VA exhibited a significant positive predictive effect on the learning and school dimension score (β=5.279, P=0.001) and a significant negative predictive effect on the social dimension score (β=-4.491, P=0.029), suggesting that it may simultaneously affect learning-related functional impairments and social function improvement. VE showed a significant negative predictive effect on the social dimension score (β=-133.325, P=0.031), supporting its potential role in alleviating social functional deficits.VD3, VD2, and VD levels had no significant predictive effects on any dimension scores (all P>0.05); VE also had no significant predictive effects on the total score, family, life skills, and other dimensions, while VA showed no significant predictive effects on non-learning and non-social dimensions (all P>0.05).
4 Discussion
This study yielded three significant findings. First, there were notable differences in vitamin content between the ADHD group and the control group, with levels of vitamins A, D (including D3 and D2), and E being significantly lower in the ADHD group. This suggests a potential association between vitamin levels and ADHD. Second, specific ADHD subtypes exhibited distinct relationships with vitamin levels. The findings indicated that, relative to the control group, various ADHD subtypes demonstrated significant differences in multiple vitamin contents. Notably, the inattentive type and the combined type showed significant differences in levels of VD3, D, and A, while the hyperactive-impulsive type exhibited significant differences in VD and A levels. These results imply that certain ADHD subtypes may correlate with vitamin levels; however, the causal relationship remains to be confirmed through further research. Third, ADHD deficit scores displayed multidimensional correlations with vitamin levels. The study revealed that total ADHD deficit scores were correlated with vitamin levels to varying extents. Specifically, VD2 was positively correlated with the self-management dimension, VA was positively correlated with the learning and school dimension, and VE was negatively correlated with the total score and dimensions such as family, learning and school, life skills, self-management, social activities, and risk-taking activities. These findings highlight the varying associations between different vitamins and the dimensions of ADHD, illuminating the complex relationship between vitamin levels and ADHD deficit scores, and providing a crucial direction for future research on their association.
In recent years, numerous studies have demonstrated that VD plays a significant role in brain development and function, influencing neurotransmission, neuroimmunomodulation, and exhibiting antioxidant effects. Furthermore, VD is implicated in behavioral and neuropsychiatric disorders (18, 19). Epidemiological evidence from previous meta-analyses indicates that insufficient perinatal VD levels substantially increase the risk of developing attention-deficit/hyperactivity disorder (ADHD) later in life (20). Additionally, VD supplementation has been shown to alleviate ADHD symptoms without causing severe adverse reactions (21). Our study found that VD levels were significantly lower in children with ADHD, aligning with the conclusions of the aforementioned studies. From a neurobiological perspective, dopaminergic dysfunction in the prefrontal cortex represents a critical link in the pathogenesis of ADHD (22), and VD is essential for the development and functional maintenance of dopaminergic neurons. Animal studies have demonstrated that VD deficiency during developmental stages leads to alterations in brain morphology and structure, disrupts the normal development of dopaminergic pathways, and results in dysfunction across multiple neurotransmitter systems (22). VD regulates the genetic expression of tyrosine hydroxylase, a rate-limiting enzyme in dopamine synthesis, thereby modulating dopamine biosynthesis (9). Furthermore, VD influences the synthesis of glial cell line-derived neurotrophic factor (GDNF) and its receptor, which modulates the survival and differentiation of dopaminergic neurons and impacts the GDNF/Ret signaling pathway, thereby maintaining the normal function of dopaminergic neurons (23). These neurodevelopmental abnormalities may constitute the neurobiological basis for the occurrence of ADHD. As an immunomodulatory molecule, VD is associated with ADHD and its related immune-mediated diseases. Multiple studies have focused on the relationship between cytokines and ADHD, revealing the potential role of inflammatory responses in the neurodevelopmental mechanisms underlying ADHD. Two independent studies have demonstrated that interleukin-6 (IL-6) is the only cytokine exhibiting a significant upward trend in ADHD patients (24, 25). Additionally, research on children with ADHD has indicated that plasma levels of IL-16 and IL-13 correlate with executive function performance (26). Our study, along with previous data, suggests that VD levels may influence the risk of developing ADHD and executive function by affecting brain development and immune-inflammatory responses. Therefore, monitoring and supplementation of VD should be considered fundamental interventions for children with ADHD.
Our findings showed that VA levels in the patient group were significantly lower than in the control group, and that VA levels were positively correlated with school-related behavioral problems. VA and its active metabolite retinoic acid (RA) may be linked with ADHD and retinal development through an association with the nervous system. Retinoic acid is believed to maintain the function of the central nervous system and hippocampal synaptic plasticity. VA deficiency impairs hippocampal synaptic plasticity, diminishing learning and attention (27). Any additional deficiency of VA will lead to abnormal development of neural pathways of the Central Nervous System thereby increasing the risk of attention deficit hyperactivity. Studies on animals have shown that prenatal models of ADHD in mice have synaptic abnormalities in the hippocampus (28). Neuroimaging of hyperactivity disorder patients showed reduced intracranial and hippocampal volumes (29). The behavioral issues of individuals with ADHD may correspond with an inability of their synapses to change. As reported by researchers, RA regulates various actions in the nervous system, dopaminergic signalling pathways and also helps in neurogenesis and differentiation of striatal neurons which may cause disordered behavior (30). Prior research has suggested that VA and RA supplementation is able to improve motor-related problems (31, 32), which is consistent with our findings. Moreover, a study has shown that VA supplementation reduces the serotonin level (33). Additionally, both dopamine and serotonin are involved in the pathophysiology of ADHD (34) To conclude, VA and its metabolites have different effects on ADHD pathogenesis and behavioral functions.
In this study, we found that VE levels were associated with the prevalence of ADHD and various behavioral deficits. As a fat-soluble organic antioxidant, VE protects cell membranes and nucleic acids, reduces reactive oxygen species (ROS), and lipid peroxidation products such as malondialdehyde (MDA), thereby mitigating oxidative stress damage to cells (35). Furthermore, VE serves as an effective immunomodulator present in the cell membranes of all cells, safeguarding them from oxidative damage, preserving the oxidation of polyunsaturated fatty acids in immune cell membranes, and decreasing the production of inflammatory markers such as tumor necrosis factor (TNF)-α and interleukin-6 (IL-6) (36). Existing studies have identified a significant positive correlation between IL-6, tumor necrosis factor, and hyperactive-impulsive ADHD in children and adolescents (37), which aligns with our findings. Additionally, the immunomodulatory role of VD is also linked to IL-6, leading us to speculate that VD and VE may have a certain association in their immunological effects on ADHD. Multiple prior animal studies have demonstrated a clear connection between VE deficiency and impaired immune function, with VE supplementation capable of reversing this impairment (38, 39). ADHD is associated with immunoregulatory defects, and abnormal immune function may play a role in the pathological process of ADHD. Thus, immune dysfunction resulting from VE deficiency may increase the risk of developing ADHD, while maintaining adequate VE levels could be crucial for sustaining normal immune function and preventing ADHD.
5 Conclusion
In conclusion, the levels of fat-soluble vitamins are significantly associated with the prevalence of ADHD, its subtype symptoms, and functional deficits. This finding opens new avenues for investigating the pathological mechanisms underlying ADHD and for developing clinical interventions. For ADHD patients, particularly those with vitamin deficiencies, supplementation with vitamins A, D, and E may serve as an effective adjuvant therapy. Furthermore, the establishment of tailored vitamin monitoring and supplementation protocols based on ADHD subtypes merits further investigation. However, the specific causal relationships remain unclear, necessitating additional prospective studies and intervention trials to confirm the impact of vitamin supplementation on ADHD.
5.1 Limitations
This study has several limitations. Firstly, it employs a cross-sectional survey design with a case-control analysis, which restricts the ability to infer the duration of fat-soluble vitamin deficiencies from the collected data. Consequently, we can only establish correlations rather than infer causal relationships. Secondly, the levels of fat-soluble vitamins are influenced by various factors, including familial influences, dietary behaviors, sunlight exposure duration, and the timing of blood sampling. Unfortunately, we were unable to analyze the impacts of these factors.Third, this study is a cross-sectional study, and the effect on long-term vitamin deficiency cannot be assessed. In future studies, longitudinal studies can be designed to track the relationship between vitamin deficiency and the development of ADHD symptoms, and to investigate and control factors that may affect vitamin levels, such as diet and lifestyle habits, in more detail when collecting samples.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Medical Ethics Committee of Wuhan Children’s Hospital Affiliated, Wuhan Children’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the research content does not involve identifying information such as the children’s names or medical record IDs, and no interventional experiments are conducted.
Author contributions
ML: Writing – original draft, Investigation, Resources, Data curation, Conceptualization. HG: Formal Analysis, Writing – original draft, Data curation, Methodology. YC: Supervision, Writing – original draft, Resources, Data curation.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Acknowledgments
The phenomenal contribution of patients (children) and their parents are acknowledged by all authors of this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wolraich ML, Hagan JF, Allan C, Chan E, Davison D, Earls M, et al. Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. (2019) 144:e20192528. doi: 10.1542/peds.2019-2528, PMID: 31570648
2. Salari N, Ghasemi H, Abdoli N, Rahmani A, Shiri MH, Hashemian AH, et al. The global prevalence of adhd in children and adolescents: a systematic review and meta-analysis. Ital J Pediatr. (2023) 49. doi: 10.1186/s13052-023-01456-1, PMID: 37081447
3. Al-Wardat M, Etoom M, Almhdawi KA, Hawamdeh Z, and Khader Y. Prevalence of attention-deficit hyperactivity disorder in children, adolescents and adults in the middle east and north africa region: a systematic review and meta-analysis. BMJ Open. (2024) 14:e078849. doi: 10.1136/bmjopen-2023-078849, PMID: 38238059
4. Cecil CAM and Nigg JT. Epigenetics and adhd: reflections on current knowledge, research priorities and translational potential. Mol Diagn Ther. (2022) 26:581–606. doi: 10.1007/s40291-022-00609-y, PMID: 35933504
5. Demontis D, Walters RK, Martin J, Mattheisen M, Als TD, Agerbo EJ, et al, ADHD Working Group of the Psychiatric Genomics Consortium (PGC), Early Lifecourse & Genetic Epidemiology (EAGLE) Consortium, 23andMe Research Team, Demontis D, Walters RK, Martin J, et al. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat Genet. (2019) 51:63–75. doi: 10.1038/s41588-018-0269-7, PMID: 30478444
6. Rucklidge JJ, Frampton CM, Gorman B, and Boggis A. Vitamin–mineral treatment of attention-deficit hyperactivity disorder in adults: double-blind randomised placebo-controlled trial. Br J Psychiatry. (2014) 204:306–15. doi: 10.1192/bjp.bp.113.132126, PMID: 24482441
7. Aarts E, Ederveen THA, Naaijen J, Zwiers MP, Boekhorst J, Timmerman HM, et al. Gut microbiome in adhd and its relation to neural reward anticipation. PloS One. (2017) 12:e0183509. doi: 10.1371/journal.pone.0183509, PMID: 28863139
8. Pertile RAN, Cui X, and Eyles DW. Vitamin d signaling and the differentiation of developing dopamine systems. Neuroscience. (2016) 333:193–203. doi: 10.1016/j.neuroscience.2016.07.020, PMID: 27450565
9. Cui X, Pertile R, Liu P, and Eyles DW. Vitamin d regulates tyrosine hydroxylase expression: n-cadherin a possible mediator. Neuroscience. (2015) 304:90–100. doi: 10.1016/j.neuroscience.2015.07.048, PMID: 26210580
10. Miskowiak KW, Obel ZK, Guglielmo R, Bonnin C del M, Bowie CR, Balanzá-Martínez V, et al. Efficacy and safety of established and off-label adhd drug therapies for cognitive impairment or attention-deficit hyperactivity disorder symptoms in bipolar disorder: a systematic review by the isbd targeting cognition task force. Bipolar Disord. (2024) 26:216–39. doi: 10.1111/bdi.13414, PMID: 38433530
11. Dumetz F, Ginieis R, Bure C, Marie A, Alfos S, Pallet V, et al. Neuronal morphology and synaptic plasticity in the hippocampus of vitamin a deficient rats. Nutr Neurosci. (2022) 25:779–90. doi: 10.1080/1028415X.2020.1809877, PMID: 32924835
12. Dumetz F, Buré C, Alfos S, Bonneu M, Richard E, Touyarot K, et al. Normalization of hippocampal retinoic acid level corrects age-related memory deficits in rats. Neurobiol Aging. (2020) 85:1–10. doi: 10.1016/j.neurobiolaging.2019.09.016, PMID: 31689598
13. Huang X, Zhang H, Zhen J, Dong S, Guo Y, Van Halm-Lutterodt N, et al. Diminished circulating retinol and elevated α-toh/retinol ratio predict an increased risk of cognitive decline in aging chinese adults, especially in subjects with apoe2 or apoe4 genotype. Aging (Albany NY). (2018) 10:4066–83. doi: 10.18632/aging.101694, PMID: 30573705
14. Sauvé B, Chorfi Y, Létourneau-Montminy M-P, and Guay F. Vitamin 25(oh)d3, e, and c supplementation impact the inflammatory and antioxidant responses in piglets fed a deoxynivalenol-contaminated diet and challenged with lipopolysaccharides. Toxins. (2024) 16:297. doi: 10.3390/toxins16070297, PMID: 39057937
15. Ralla T, Kluenter A-M, Litta G, Müller M-A, Bonrath W, and Schäfer C. Over 100 years of vitamin e: an overview from synthesis and formulation to application in animal nutrition. J Anim Physiol Anim Nutr. (2024) 108:646–63. doi: 10.1111/jpn.13919, PMID: 38205908
16. Li H-H, Yue X-J, Wang C-X, Feng J-Y, Wang B, and Jia F-Y. Serum levels of vitamin a and vitamin d and their association with symptoms in children with attention deficit hyperactivity disorder. Front Psychiatry. (2020) 11:599958. doi: 10.3389/fpsyt.2020.599958, PMID: 33329153
17. Reinehr T, Langrock C, Hamelmann E, Lücke T, Koerner-Rettberg C, Holtmann M, et al. 25-hydroxvitamin d concentrations are not lower in children with bronchial asthma, atopic dermatitis, obesity, or attention-deficient/hyperactivity disorder than in healthy children. Nutr Res. (2018) 52:39–47. doi: 10.1016/j.nutres.2018.01.002, PMID: 29764626
18. Groves NJ and Burne THJ. The impact of vitamin d deficiency on neurogenesis in the adult brain. Neural Regenerat Res. (2017) 12:393–94. doi: 10.4103/1673-5374.202936, PMID: 28469647
19. Cui X, Pelekanos M, Liu P-Y, Burne THJ, McGrath JJ, and Eyles DW. The vitamin d receptor in dopamine neurons; its presence in human substantia nigra and its ontogenesis in rat midbrain. Neuroscience. (2013) 236:77–87. doi: 10.1016/j.neuroscience.2013.01.035, PMID: 23352937
20. Khoshbakht Y, Bidaki R, and Salehi-Abargouei A. Vitamin d status and attention deficit hyperactivity disorder: a systematic review and meta-analysis of observational studies. Adv Nutr. (2018) 9:9–20. doi: 10.1093/advances/nmx002, PMID: 29438455
21. Gan J, Galer P, Ma D, Chen C, and Xiong T. The effect of vitamin d supplementation on attention-deficit/hyperactivity disorder: a systematic review and meta-analysis of randomized controlled trials. J Child Adolesc Psychopharmacol. (2019) 29:670–87. doi: 10.1089/cap.2019.0059, PMID: 31368773
22. Kesby JP, Turner KM, Alexander S, Eyles DW, McGrath JJ, and Burne THJ. Developmental vitamin d deficiency alters multiple neurotransmitter systems in the neonatal rat brain. Int J Dev Neurosci. (2017) 62:1–7. doi: 10.1016/j.ijdevneu.2017.07.002, PMID: 28716540
23. Pertile RAN, Cui X, Hammond L, and Eyles DW. Vitamin d regulation of gdnf/ret signaling in dopaminergic neurons. FASEB J. (2018) 32:819–28. doi: 10.1096/fj.201700713R, PMID: 29018141
24. DonFrancesco R, Nativio P, Borrelli E, Giua E, Andriola E, Villa MP, et al. Serum cytokines in pediatric neuropsychiatric syndromes: focus on attention deficit hyperactivity disorder. Minerva Pediatr. (2021) 73:398–404. doi: 10.23736/S2724-5276.16.04642-9, PMID: 28006890
25. Darwish AH, Elgohary TM, and Nosair NA. Serum interleukin-6 level in children with attention-deficit hyperactivity disorder (adhd). J Child Neurol. (2019) 34:61–7. doi: 10.1177/0883073818809831, PMID: 30430896
26. Oades RD, Myint A-M, Dauvermann MR, Schimmelmann BG, and Schwarz MJ. Attention-deficit hyperactivity disorder (adhd) and glial integrity: an exploration of associations of cytokines and kynurenine metabolites with symptoms and attention. Behav Brain Functions. (2010) 6:32. doi: 10.1186/1744-9081-6-32, PMID: 20534153
27. De Hoog E, Lukewich MK, and Spencer GE. Retinoid receptor-based signaling plays a role in voltage-dependent inhibition of invertebrate voltage-gated ca2+ channels. J Biol Chem. (2019) 294:10076–93. doi: 10.1074/jbc.RA118.006444, PMID: 31048374
28. Piña R, Rozas C, Contreras D, Hardy P, Ugarte G, Zeise ML, et al. Atomoxetine reestablishes long term potentiation in a mouse model of attention deficit/hyperactivity disorder. Neuroscience. (2020) 439:268–74. doi: 10.1016/j.neuroscience.2019.10.040, PMID: 31809728
29. Boedhoe PSW, van Rooij D, Hoogman M, Twisk JWR, Schmaal L, Abe Y, et al. Subcortical brain volume, regional cortical thickness and cortical surface area across attention-deficit/hyperactivity disorder (adhd), autism spectrum disorder (asd), and obsessive-compulsive disorder (ocd). Am J Psychiatry. (2020) 177:834–43. doi: 10.1176/appi.ajp.2020.19030331, PMID: 32539527
30. Steeves TDL, Ko JH, Kideckel DM, Rusjan P, Houle S, Sandor P, et al. Extrastriatal dopaminergic dysfunction in tourette syndrome. Ann Neurol. (2010) 67:170–81. doi: 10.1002/ana.21809, PMID: 20225192
31. Pan J, Yu J, Sun L, Xie C, Chang L, Wu J, et al. ALDH1A1 regulates postsynaptic μ-opioid receptor expression in dorsal striatal projection neurons and mitigates dyskinesia through transsynaptic retinoic acid signaling. Sci Rep. (2019) 9:3602. doi: 10.1038/s41598-019-40326-x, PMID: 30837649
32. Marie A, Leroy J, Darricau M, Alfos S, De Smedt-Peyrusse V, Richard E, et al. Preventive vitamin a supplementation improves striatal function in 6-hydroxydopamine hemiparkinsonian rats. Front Nutr. (2022) 9:811843. doi: 10.3389/fnut.2022.811843, PMID: 35178422
33. Guo M, Zhu J, Yang T, Lai X, Liu X, Liu J, et al. Vitamin a improves the symptoms of autism spectrum disorders and decreases 5-hydroxytryptamine (5-ht): a pilot study. Brain Res Bull. (2018) 137:35–40. doi: 10.1016/j.brainresbull.2017.11.001, PMID: 29122693
34. Oades RD. Dopamine-serotonin interactions in attention-deficit hyperactivity disorder (adhd). Prog Brain Res. (2008) 172:543–65. doi: 10.1016/S0079-6123(08)00926-6, PMID: 18772050
35. Espinosa-Diez C, Miguel V, Mennerich D, Kietzmann T, Sánchez-Pérez P, Cadenas S, et al. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. (2015) 6:183–97. doi: 10.1016/j.redox.2015.07.008, PMID: 26233704
36. Salinthone S, Kerns AR, Tsang V, and Carr DW. α-tocopherol (vitamin e) stimulates cyclic amp production in human peripheral mononuclear cells and alters immune function. Mol Immunol. (2013) 53:173–78. doi: 10.1016/j.molimm.2012.08.005, PMID: 22947771
37. Cortese S, Angriman M, Comencini E, Vincenzi B, and Maffeis C. Association between inflammatory cytokines and adhd symptoms in children and adolescents with obesity: a pilot study. Psychiatry Res. (2019) 278:7–11. doi: 10.1016/j.psychres.2019.05.030, PMID: 31129493
38. Chang WP, Hom JS, Dietert RR, Combs GF, and Marsh JA. Effect of dietary vitamin e and selenium deficiency on chicken splenocyte proliferation and cell surface marker expression. Immunopharmacol Immunotoxicol. (1994) 16:203–23. doi: 10.3109/08923979409007091, PMID: 8077607
Keywords: attention deficit hyperactivity disorder, fat-soluble vitamins, vitamin A, vitamin D, vitamin E
Citation: Li M, Gu H and Chen Y (2025) Serum levels of fat-soluble vitamins in children with attention deficit hyperactivity disorder and relationship with symptom subtypes. Front. Psychiatry 16:1646885. doi: 10.3389/fpsyt.2025.1646885
Received: 14 June 2025; Accepted: 09 October 2025;
Published: 28 October 2025.
Edited by:
Ayhan Bilgiç, İzmir University of Economics, TürkiyeReviewed by:
Say How Ong, Institute of Mental Health, SingaporeFatemeh Razavinia, Ahvaz Jundishapur University of Medical Sciences, Iran
Copyright © 2025 Li, Gu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Chen, Zmx5eWljaGVuQDEyNi5jb20=
 Mengqi Li
Mengqi Li Hailin Gu
Hailin Gu Yan Chen
Yan Chen