- 1Loyola University Chicago, Parkinson School of Health Sciences and Public Health, Maywood, IL, United States
- 2University of Utah Huntsman Mental Health Institute, Salt Lake City, UT, United States
Aims: To examine associations between psychedelic use and adverse health outcomes, including overdose, relapse, mental health crises, and hospitalizations, among individuals with substance use disorders (SUD), and to compare these outcomes across different treatment modalities including anesthetics and outpatient SUD services.
Design: Retrospective cohort study using propensity score-weighted quasi-Poisson regression models to estimate adjusted incidence rate ratios (aIRRs).
Setting: Data were drawn from Oracle EHR Real-World Data™ comprising 138 U.S. health systems restricted to those ≥12 years old from January 1, 2000, to August 31, 2023.
Participants: 3,209,798 patients with a documented SUD diagnosis from 2000 to 2023. Patients with a prior history of psychedelic use or hallucinogen-related diagnoses were excluded. The final cohort included 8,514 new psychedelic users and over 3.2 million non-users.
Measurements: Exposures were captured during a 3-month post-index period and included outpatient psychedelic prescriptions or procedures (primarily ketamine), general anesthetic outpatient prescriptions, and outpatient SUD services. Outcomes, assessed over 2 years, included SUD-related hospitalizations/emergency department (ED) visits, mental health crises, all-drug overdoses, and relapse. Propensity scores accounted for demographic, clinical, and behavioral confounders.
Findings: Psychedelic use was associated with significantly reduced rates of all adverse outcomes, including all-drug overdose (aIRR F;= F;0.48; 95% CI: 0.37-0.63), relapse (aIRR F;= F;0.68; 0.60-0.77), SUD hospitalizations/ED visits (aIRR F;= F;0.76; 0.69-0.82), and mental health crises (aIRR F;= F;0.82; 0.73-0.92), compared to no treatment. The combination of psychedelics, anesthetics, and outpatient services was associated with the strongest reduction in mental health crises (aIRR F;= F;0.21; 0.06-0.77). Trends were consistent in sensitivity analyses including patients with mental health conditions and comparisons to medication-assisted treatment.
Conclusions: In this large national cohort, psychedelic use, particularly when combined with anesthetic and outpatient care, was associated with reduced adverse health outcomes among people with SUD. These findings support further investigation into psychedelic-based interventions within integrated treatment frameworks.
Introduction
In 2023, 48.5 million Americans (17.1%) aged 12 and older had a substance use disorder (SUD) (1). These chronic conditions, characterized by cycles of abstinence, use, and relapse, contribute significantly to public health burdens, with drug overdose deaths rising from 8.2 per 100,000 in 2002 to 32.6 in 2022 (2). While individualized treatment, especially medication-assisted treatment (MAT), is effective (3, 4), pharmacologic options are currently limited to alcohol, opioid, and tobacco use disorders (OUD, AUD, and TUD), with no approved therapies for marijuana, cocaine, methamphetamine, or stimulant misuse (5). Barriers to accessing treatment persist, including provider limitations (6), referral gaps (7), stigma (8, 9), and insurance restrictions (10). Despite established medications, underutilization remains a critical issue (11–14). Among 54.2 million people needing treatment in 2023, only 23.6% received it (1). This highlights the need to explore alternative or adjunctive therapies for SUD.
Psychedelics (also called hallucinogens), including lysergic acid diethylamide (LSD), psilocybin, dimethyltryptamine (DMT), and mescaline (15, 16), are increasingly studied as therapeutic agents. Compounds such as ketamine and methylenedioxymethamphetamine (MDMA), while mechanistically distinct, are grouped with psychedelics in research due to similar consciousness-altering effects. Use of hallucinogens for nonmedical purposes has grown, with 8.8 million adults reporting use in 2023, up from 5.5 million in 2019 (1, 17). A longitudinal study found LSD use rose slightly from 3.7% in 2018 to 4.2% in 2021, while psilocybin or phencyclidine (PCP) use increased more notably from 3.4% to 6.6% (18). Most psychedelic users are poly-users (19). In contrast, clinical use remains limited to research trials and specialized health care settings, where safety profiles and therapeutic potential are being actively investigated. Though adverse effects such as mental health crises and emergency visits are reported (20–22), these are generally tied to unsupervised recreational use rather than clinical application.
Conversely, early clinical studies suggest psychedelics may offer benefits for addiction treatment and hence are gaining a resurgence in interest as a therapy option (23). Psilocybin has yielded smoking abstinence rates up to 80% at six months; far surpassing traditional interventions (24–26). Population-based studies have linked psychedelic use with reduced odds of opioid dependence (27, 28), cocaine use disorder (29), and emotional distress linked to substance use (30).
Ketamine, a dissociative agent with psychedelic properties, is already used for depression (31), and is being investigated for SUD (32). Electronic Health Record (EHR) and trial data show associations with greater remission in cocaine and stimulant use disorders (33, 34). Randomized trials report higher abstinence in ketamine-treated groups for both cocaine (35) and AUD (36). Proposed mechanisms include modulation of addiction-related neurocircuitry (32, 33, 37). Anesthetic combinations involving ketamine (e.g., with midazolam or propofol) also show promise in clinical outcomes (38–41). While anesthetics have been explored for pain and psychiatric conditions (42–45), their use in SUD remains under-researched.
In 2022, the National Institute on Drug Abuse (NIDA) launched an initiative to evaluate psychedelic therapies for SUD (46). Yet to date, no large-scale study has assessed psychedelic treatments, alone or in combination with anesthetics and established outpatient interventions, using EHR data in a general SUD population. This study addresses this gap by examining the real-world clinical use of these therapies, particularly ketamine, in structured healthcare settings. As the only currently legal and scalable psychedelic-like agent, ketamine offers a uniquely practical lens through which to evaluate system-level feasibility and outcomes. Moreover, by analyzing treatment exposures from EHRs, this study complements existing psychedelic literature that relies heavily on non-medical, self-reported, or unregulated use, and provides critical data on how psychedelic treatments function in real-world, clinically supervised settings. This study will be the first to provide a robust, large-scale estimate of the overall benefit or risk of psychedelic/anesthetic compounds used in conjunction with current SUD treatment efforts among a diverse U.S. population comprising all SUD types. Such findings would further encourage efforts to study psychedelic compounds as a potential alternative treatment option for SUD, helping to overcome the many barriers to current SUD treatment options.
Methods
Data source
This retrospective study used de-identified records from Oracle EHR Real-World Data™ (OERWD), comprising 138 U.S. health systems as of November 2024. The dataset includes over 111 million patients and approximately 2 billion encounters. Oracle EHR Real-World Data is extracted from the electronic medical records of hospitals in which Oracle has a data use agreement. Encounters may include pharmacy, clinical and microbiology laboratory, admission, and billing information from affiliated patient care locations. All admissions, medication orders and dispensing, laboratory orders and specimens are date and time stamped, providing a temporal relationship between treatment patterns and clinical information. Oracle has established Health Insurance Portability and Accountability Act-compliant operating policies to establish de-identification for Oracle EHR Real-World Data (47).
Sample
Eligible patients had a qualifying SUD diagnosis (International Classification of Diseases, Ninth/Tenth Revision, Clinical Modification (ICD-9/10-CM) or Systemized Nomenclature of Medicine-Clinical Terms (SNOMED CT); Supplementary Table 1) between January 1, 2000, and August 31, 2023, and were ≥12 years old. The index date was defined as the first inpatient or emergency department (ED) encounter with a qualifying SUD code, or the first of ≥2 qualifying encounters within two years. Exclusions included prior hallucinogen use disorder, psychedelic-related overdose, or psychedelic use based on Current Procedural Terminology (CPT) procedure codes, National Drug Code (NDC) or Multum MediSource Lexicon (MMSL) medication prescriptions, or Logical Observation Identifiers Names and Codes (LOINC) positive urine drug tests (Supplementary Tables 1, 2). Patients required ≥3 months of post-index data.
Study design
This study used a retrospective cohort design that started enrollment of patients at index SUD encounter and assessed treatment usage over an ensuing three-month baseline period. After the baseline period, patients were followed for two years to assess outcomes of interest. Patients were allowed inclusion until August 31, 2023, to ensure those lastly recruited had at least three more months to assess baseline treatments, and then a following year of follow-up ending at data refresh in November 2024. Thus, those lastly recruited only had one year of possible follow-up, which was done to maximize the cohort sample. Sensitivity analyses assessed robustness among those with two full years of follow-up. Outcomes were measured using person-months from follow-up start to maximum discharge.
Outcomes
Primary outcomes included SUD-related hospitalizations or ED visits (Supplementary Table 1), mental health crises (e.g., anxiety, depression, suicidality (48); Supplementary Table 3), all-drug overdoses (e.g., drugs, alcohol, nicotine; Supplementary Table 4), and relapse (a composite of SUD visit, overdose, or detoxification; Supplementary Tables 1, 4, 5). Incident rates (IRs) were calculated per 10,000 person-months.
Exposures
Treatment exposures during baseline included outpatient SUD services (OS; Supplementary Table 6), general anesthetic prescriptions excluding ketamine (e.g., Propofol, Midazolam, Etomidate, Sevoflurane, Desflurane, Supplementary Table 7; from outpatient encounters not overlapping with any procedures, Supplementary Table 8) and psychedelic use (mainly ketamine; Supplementary Table 2). Psychedelics were classified based on outpatient procedures or prescriptions, with ketamine categorized solely under psychedelics. Exposures were analyzed as binary (yes/no), ordinal (number of treatments), and nominal (combinations of treatment types).
Additional measures
Covariates and factors included demographic (e.g., age, sex, race/ethnicity, insurance, geography), clinical (e.g., Charlson Comorbidity Index (49), index SUD type, chronic pain), behavioral (e.g., tobacco use, social determinants coded as ICD-10 Z55-Z65), and treatment history (e.g., anesthetic, benzodiazepine, medications for substance use disorder (MSUD) prescriptions; Supplementary Tables 1, 9-13) as determined by literature (32–34). Most were assessed any time before or during the baseline, with some restricted to specific periods.
Statistical analysis
Descriptive statistics and standardized mean differences (SMDs) evaluated covariate balance between treatment groups. To address covariate imbalance, propensity scores were calculated via logistic (binary) and multinomial (ordinal/nominal) regressions using the average treatment effect (ATE) estimand. Propensity score weighted covariate balance was visualized in Supplementary Figures 1A-L, and doubly robust adjustment was applied for residual imbalances. Adjusted incidence rate ratios (aIRRs) were estimated using mixed-effects quasi-Poisson regression, incorporating person-time as an offset, clustering by hospital, and weighted with propensity score weights. Models were further adjusted for covariates to mitigate residual confounding. Quasi-Poisson was chosen based on overdispersion confirmed via Cameron and Trivedi’s test (50). Model fit was assessed via residual deviance relative to degrees of freedom.
Sensitivity analyses
Supplemental analyses repeated all models among patients with a recent mental health diagnosis (within two years before or during baseline) and included adjustment for antidepressant use (e.g., selective serotonin reuptake inhibitors [SSRIs], serotonin-norepinephrine reuptake inhibitors [SNRIs], atypical antidepressants, tricyclic antidepressants, monoamine oxidase inhibitors [MAOIs]; Supplementary Table 14). A secondary analysis redefined OS to include MSUD, reflecting MAT. These were used to test treatment combinations involving MAT, anesthetics, and psychedelics. Due to warnings about combining sedating agents and MSUD medications (51–55), this analysis was exploratory. All tests were two-sided with α=0.05. Analyses were conducted using R version 4.0.2 (The R Foundation).
Results
Descriptive statistics
A total of 3,209,798 patients with SUD were analyzed. Of these, 0.3% (8,514) used psychedelic prescriptions, primarily ketamine, while 99.7% (3,201,284) did not. Baseline use of outpatient SUD services (OS) was observed in 3.7%, and general anesthetic prescriptions in 2.3%. Most patients were male (53.1%), non-Hispanic White (69.9%), and single (64.6%). Index diagnoses included 10% with OUD, 18.7% with AUD, 62.2% with TUD, and smaller proportions with cannabis (6%), stimulant (4.4%), or psychotropic medication (3.9%) use disorders; over 7% had multiple index SUDs (Table 1).
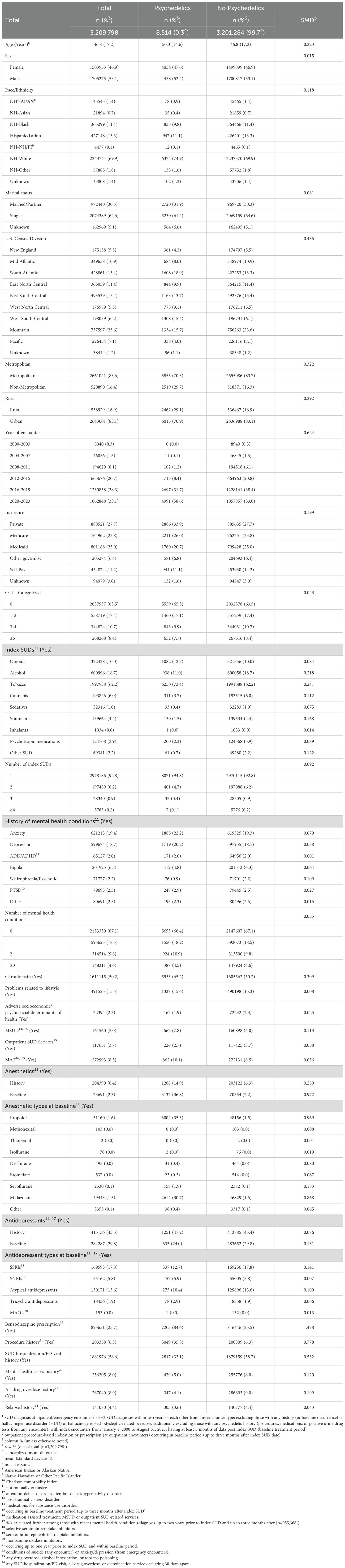
Table 1. Characteristics of patients diagnosed with SUD1, overall and by psychedelic use treatment2.
Mental health comorbidities were common, with 19.4% having anxiety and 18.7% depression; nearly 32% had at least one mental health diagnosis. Over half experienced chronic pain or prior SUD-related hospitalization/ED use. Compared to non-users, psychedelic users were older, more often lived in non-metropolitan or rural areas, had more recent index encounters, private insurance, chronic pain, benzodiazepine prescriptions, procedures, anesthetic treatment, and lower baseline adverse outcomes (Table 1).
During follow-up, incidence rates per 10,000 person-months were 199.71 for SUD hospitalization/ED visits, 138.12 for mental health crises, 49.62 for all-drug overdoses, and 62.21 for relapse (Table 2).
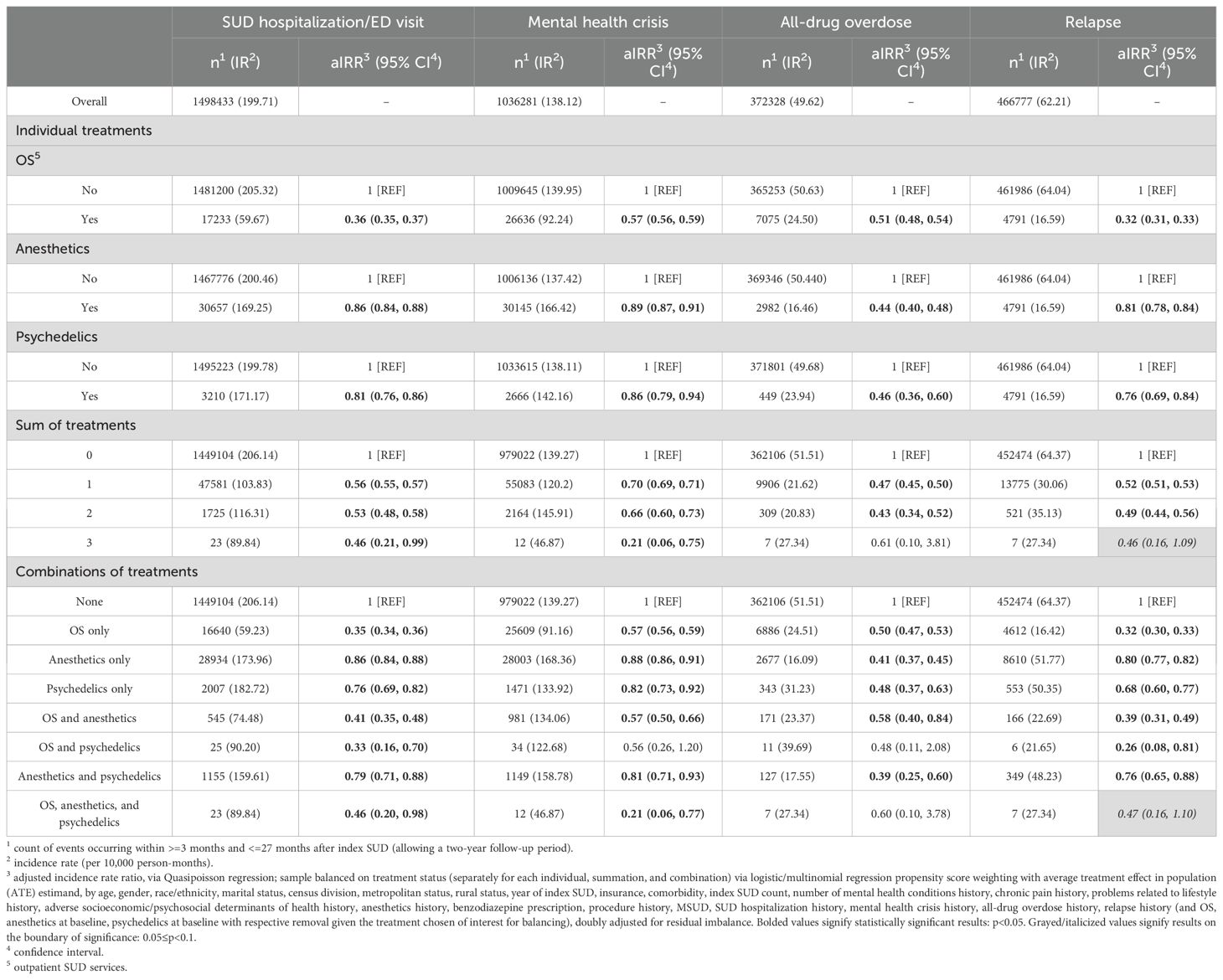
Table 2. Association between treatment and adverse healthcare outcomes (SUD-hospitalizations/ED visits, mental health crisis, all-drug overdose, relapse), among those with SUD.
Inferential statistics
After propensity score weighting, treatment groups showed lower rates of adverse outcomes. OS use was associated with the lowest rates of SUD hospitalization/ED visit (aIRR: 0.36; 95% confidence interval [CI]: 0.35-0.37), mental health crisis (aIRR: 0.57; 95% CI: 0.56-0.59), and relapse (aIRR: 0.32; 95% CI: 0.31-0.33) compared to those without OS. General anesthetics (aIRR: 0.44; 95% CI: 0.40-0.48) and psychedelics (aIRR: 0.46; 95% CI: 0.36-0.60) were associated with the lowest overdose rates (Table 2).
Patients receiving one or two treatments had lower rates of all outcomes compared to untreated patients. Those receiving all three treatments had a 54% lower rate of SUD hospitalization/ED visits (aIRR: 0.46; 95% CI: 0.21-0.99), 79% lower rate of mental health crises (aIRR: 0.21; 95% CI: 0.06-0.75), and 54% lower relapse (aIRR: 0.46; 95% CI: 0.16-1.09).
By treatment combinations, patients receiving only OS had significantly lower rates of all outcomes compared to those with no treatment: SUD hospitalization/ED visit (aIRR: 0.35; 95% CI: 0.34-0.36), mental health crisis (aIRR: 0.57; 95% CI: 0.56-0.59), overdose (aIRR: 0.50; 95% CI: 0.47-0.53), and relapse (aIRR: 0.32; 95% CI: 0.30-0.33). Anesthetic-only users had a 59% lower overdose rate (aIRR: 0.41; 95% CI: 0.37-0.45) and modest reductions across other outcomes. Psychedelic-only users had a 52% lower overdose rate (aIRR: 0.48; 95% CI: 0.37-0.63), along with reductions in hospitalization (aIRR: 0.76; 95% CI: 0.69-0.82), mental health crises (aIRR: 0.82; 95% CI: 0.73-0.92), and relapse (aIRR: 0.68; 95% CI: 0.60-0.77).
Patients receiving OS and anesthetics had significantly lower rates of all outcomes, including SUD hospitalization/ED visit (aIRR: 0.41; 95% CI: 0.35-0.48), mental health crisis (aIRR: 0.57; 95% CI: 0.50-0.66), overdose (aIRR: 0.58; 95% CI: 0.40-0.84), and relapse (aIRR: 0.39; 95% CI: 0.31-0.49). Those with OS and psychedelics experienced the lowest relapse rate (aIRR: 0.26; 95% CI: 0.08-0.81) and a 67% lower SUD hospitalization rate (aIRR: 0.33; 95% CI: 0.16-0.70). Anesthetics and psychedelics were associated with lower rates of all outcomes, including a 61% reduction in overdose (aIRR: 0.39; 95% CI: 0.25-0.60).
Patients receiving all three treatments had a 54% lower rate of SUD hospitalization/ED visits (aIRR: 0.46; 95% CI: 0.20-0.98), a 53% lower relapse rate (aIRR: 0.47; 95% CI: 0.16-1.10), and a 79% lower mental health crisis rate (aIRR: 0.21; 95% CI: 0.06-0.77), though the association with overdose was not significant.
Supplemental analyses
In the subset with recent mental health conditions (n = 955,368; Supplementary Table 15), incidence rates were higher across all outcomes. Treatment associations remained directionally consistent, though statistical significance varied due to smaller subgroup sizes. Notably, trends for psychedelics, anesthetics, and OS persisted.
When redefining OS to include MSUD as MAT (Supplementary Table 16), results were similar. Patients with MAT had lower rates of SUD hospitalization (aIRR: 0.72; 95% CI: 0.71-0.73), mental health crises (aIRR: 0.74; 95% CI: 0.73-0.75), overdose (aIRR: 0.70; 95% CI: 0.68-0.73), and relapse (aIRR: 0.70; 95% CI: 0.69-0.71), though effects were slightly weaker than with OS alone. MAT in combination with anesthetics or psychedelics also showed protective trends, with the three-treatment model yielding reduced outcomes, though some estimates were non-significant.
Discussion
Among a large sample of patients with SUD, this study identified protective associations between outpatient SUD-related encounters, anesthetics, and psychedelics and adverse healthcare outcomes. These associations were found when treatments were used alone and were often stronger when used in combination. Several remained significant when restricted to those with recent mental health conditions. This study offers one of the first real-world, large-scale estimates of psychedelic and anesthetic use, primarily ketamine, in structured clinical settings, highlighting their potential integration within existing care systems. This study provides the first estimates of adverse outcomes associated with anesthetics and psychedelics used alongside outpatient care in a large, diverse SUD cohort.
Crucially, most published evidence on psychedelics stems from small trials or non-clinical surveys of naturalistic use. In contrast, this study utilizes clinical EHR data to assess psychedelic use in health system settings, making it one of the few pragmatic evaluations of these therapies as they are currently implemented. This distinction is essential, as real-world outcomes are influenced not only by pharmacology but also by clinical supervision, patient selection, and healthcare context.
Psychedelic use was rare, with only 0.3% of patients having a qualifying exposure. Several factors likely explain this. First, psychedelic exposure was limited to outpatient prescriptions or procedures among patients without prior use. Most qualifying indications involved ketamine, which remains uncommon (292 per million Medicaid enrollees in 2020) (56). Psychedelic treatments are often reserved for patients unresponsive to conventional therapy and were only recently included in clinical practice for SUD or mental health (15). Our dataset, which captures structured care, may underreport usage that occurs in informal settings. In a survey of over 2,000 Canadian patients, 33.7% reported using psychedelics to self-treat a health condition, but only 4.4% did so with a therapist and 3.6% in clinical settings (57). Thus, true prevalence may be higher. Second, although research highlights benefits of psychedelics in treating substance use (24, 27–30, 35, 36), adverse effects are still possible (21, 22, 58). In a survey of over 300 U.S. psychologists, many expressed cautious interest, citing psychiatric risks (59). Finally, there was also a large overlap between psychedelic use and anesthetic use found in this patient sample and is likely explained by the compound ketamine which has known psychedelic properties and is more often used as a form of anesthesia (60).
This study assessed associations between three treatment categories, OS, anesthetics, and psychedelics, and four adverse outcomes: SUD-related hospitalizations or ED visits, mental health crises, all-drug overdose, and relapse. Across outcomes, each treatment was associated with significantly lower rates relative to no treatment. These effects were also observed among those who received only one treatment. OS was most protective for hospitalizations, mental health crises, and relapse. This aligns with research showing that outpatient treatment improves abstinence, reduces use, and decreases rehospitalization (61–64). In contrast, the results indicate that anesthetics and psychedelics were most strongly protective of overdose compared to OS treatment. The capacity of anesthetic drugs to impact substance use outcomes has not been thoroughly explored in research likely due to the addictive potential of non-opioid anesthetics (65). However, a recent animal study did conclude that isoflurane had a strong inhibitory effect on cocaine-reinforced behavior in rats (66). The protective association found between anesthetics and all-drug overdose is a novel discovery that should be further explored in future research. Ample research has shown evidence of psychedelic compounds reducing substance use tendencies. Multiple surveys have revealed individuals reporting reductions in, alcohol, cannabis, and opioid cravings following naturalistic psychedelic use (67–69). Congruent with these findings, psychedelic use among the patient sample likely resulted in a substance use reduction that was illustrated by the strong protective association for all-drug overdose.
Patients receiving multiple treatments experienced lower rates of all outcomes compared to those without any treatment. This supports recommendations to combine pharmacotherapy and behavioral therapy for optimal outcomes (70, 71). The combined use of all three treatments (OS, anesthetics, and psychedelics) was significantly protective against hospitalization and mental health crises. Notably, the greatest protective association in the primary analysis, 79% reduction, was observed between all three treatments and mental health crises. Anesthetics and ketamine are used to treat mental illness (31, 39, 72), and outpatient care is known to reduce mental health symptom severity (61, 73). Therefore, the strongest mental health protection seen with all three treatments is plausible. Additionally, three treatment strategies did result in a protective association with relapse being on the boundary of significance but all-drug overdose being insignificant. The sample subset of patients that used a sum of three treatment strategies was very small, which likely influenced the statistical power of the analysis explaining the lack of statistical significance seen in this group. The consistent protective effect, though insignificant, is still notable and warrants further efforts to study the impacts of these treatments used together.
We also examined outcomes by explicit treatment combinations. Those receiving both OS and anesthetics or both anesthetics and psychedelics had significantly lower rates across all outcomes. These combinations have precedent in procedural and psychiatric contexts (39, 74, 75). For example, ketamine combined with anesthetics is widely used in procedural sedation and may confer mental health benefits. Our findings suggest these pairings could improve outcomes in SUD as well. The OS and psychedelic group showed the lowest relapse and hospitalization rates in the primary analysis. This supports prior findings that psychological interventions enhance psychedelic treatment. A trial combining psilocybin with motivational enhancement therapy reported reduced alcohol cravings and increased abstinence (76). A review of ketamine for SUD suggested its effects may be amplified when combined with cognitive-behavioral therapy (77). While the combination showed protective trends for mental health crises and overdose, results were not statistically significant, likely due to small sample size. Still, these findings support further evaluation of integrated psychedelic therapies for SUD.
In the subset of patients with a recent mental health condition, trends were consistent, though statistical significance was attenuated in several comparisons due to reduced sample size. Incidence rates were higher across all outcomes, consistent with literature showing that SUD with psychiatric comorbidity results in worse health (78–83). Despite this, treatment effects remained directionally protective, suggesting these approaches may benefit complex patients.
In the second supplemental analysis, MAT was defined as a combination of MSUD and OS. While all treatments showed protective trends, OS alone was more protective than MAT across all outcomes. This may reflect differences in illness severity, with MAT patients representing more chronic or complex cases. Alternatively, lower adherence or greater side effect burden could reduce effectiveness. Despite this, MAT still showed significant protective effects, consistent with its known benefits in reducing withdrawal symptoms and substance use (84, 85).
When MAT was used in combination with anesthetics or psychedelics, results remained protective for most outcomes. Some associations did not reach statistical significance, which could reflect lower power or pharmacological interactions. While combining sedatives with medications for opioid, alcohol, or tobacco use disorders carries known risks (51–55), patients may require complex regimens. Encouragingly, these patients still experienced reduced adverse outcomes relative to those without treatment, suggesting benefits may outweigh risks in appropriate cases.
Taken together, these findings underscore the clinical relevance of ketamine as a pragmatic psychedelic treatment option within U.S. healthcare systems. By leveraging structured clinical data rather than relying solely on self-report or experimental trials, this study contributes meaningfully to the real-world evidence base guiding future SUD treatment innovation. While outpatient therapy remains a critical foundation, adjunctive treatments, particularly ketamine and other anesthetics, could further reduce relapse, overdose, and mental health burden. Given the novelty of psychedelic therapies and the complexity of their use alongside other medications, additional research is warranted to determine optimal timing, combinations, and patient selection.
Limitations
There are several limitations of this study design to consider when interpreting the results of these analyses. For one, the retrospective study design only produces correlational estimates between SUD treatment methods and adverse healthcare outcomes limiting the ability to assume causality of these variables. Furthermore, the data examined in this study is limited to health indications within OERWD-affiliated centers and may not be completely generalizable to health centers outside of this specific database. Additionally, it is important to note that the psychedelic treatment cohort in our analyses is largely made up of prescription ketamine indications. The true number of psychedelic users (primarily self-medicating) is likely larger than the number that could be captured with the OERWD. Moreover, the analysis lacked details on dosing, frequency, administration route, and treatment setting, which limited the ability to explore potential dose-response effects or distinguish structured therapeutic use from general medical administration. In reference to SUD treatment combinations, patients using a combination of treatment methods had an indication of a particular treatment method within the span of three months as opposed to explicit simultaneous indications. This methodology could have potentially included patients that were switching from one treatment to another rather than intentionally using a combination of treatment strategies. Further, specific use contexts (e.g., the combination of ketamine and anesthetics being used specifically for SUD treatment) was not explicitly captured in this study; rather analyses used longitudinal context (i.e., identifying patients with SUD that had following indications of both psychedelics and anesthetics within a three-month period) to determine treatment assignments. Finally, although extensive propensity score weighting was used to reduce bias, the possibility of unmeasured confounding remains. For example, providers’ prescribing behavior, patient motivation, treatment adherence, and socioeconomic context may all influence treatment selection and outcomes but were not fully considered. Despite the given limitations, our study’s results fill a gap in the current literature of the potential impacts of psychedelic compounds on SUD treatment-related healthcare outcomes when used with and without traditional treatment strategies. Most importantly, these results stem from an entire patient population of overall SUD from a large, diverse, nationwide sample.
Conclusion
In a diverse national cohort of patients with SUD, psychedelic, anesthetic, and outpatient treatment, alone and in combination, were associated with significantly reduced risks of relapse, overdose, psychiatric crisis, and hospitalization. These findings support the growing investigation of psychedelic-based and anesthetic-supported interventions as part of an integrated treatment model for SUD. Further research should examine treatment timing, setting, and interactions with standard therapies to optimize outcomes and safety.
Data availability statement
The datasets generated during and/or analyzed during the current study are not publicly available because of restrictions by Oracle Cerner, the owner of the data. Data could be accessed by signing a data sharing agreement with Oracle Cerner and covering any costs that may be involved (contact Kendra Stillwell: a2VuZHJhLnN0aWxsd2VsbEBjZXJuZXJlbnZpemEuY29t). Further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by the institutional review board at Loyola University Chicago. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
FQ: Conceptualization, Investigation, Methodology, Project administration, Supervision, Writing – review & editing. AM: Investigation, Writing – original draft, Writing – review & editing. BT: Data curation, Formal Analysis, Investigation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. PT: Investigation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We acknowledge Oracle Cerner for its national data access and computation capabilities. We thank Rona Bern for working on the initial literature review.
Conflict of interest
Paul Thielking serves as the Chief Scientific Officer at Numinus Wellness, Inc Cedar Clinical Research. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2025.1648104/full#supplementary-material
Abbreviations
SUD, substance use disorder; U.S., United States; ED, emergency department; MAT, medication-assisted treatment; OUD, opioid use disorder; AUD, alcohol use disorder; TUD, tobacco use disorder; LSD, lysergic acid diethylamide; DMT, dimethyltryptamine; MDMA, methylenedioxymethamphetamine; PCP, phencyclidine; EHR, electronic health records; NIDA, National Institute on Drug Abuse; OERWD, Oracle EHR Real-World Data™; ICD-9/10-CM, International Classification of Diseases, Ninth/Tenth Revision, Clinical Modification; SNOMED CT, Systemized Nomenclature of Medicine-Clinical Terms; CPT, Current Procedural Terminology; NDC, National Drug Code; MMSL, Multum MediSource Lexicon; LOINC, Logical Observation Identifiers Names and Codes; aIRR, adjusted incident rate ratio; IR, incident rate; OS, outpatient SUD services; MSUD, medication for substance use disorder; SMD, standardized mean differences; ATE, average treatment effect; SSRI, selective serotonin reuptake inhibitors; SNRIs, serotonin-norepinephrine reuptake inhibitors; MAOIs, monoamine oxidase inhibitors CI, confidence interval.
References
1. Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: Results from the 2023 National Survey on Drug Use and Health. Rockville, MD: Center for Behavioral Health Statistics and Quality Substance Abuse and Mental Health Services Administration. (2024). Available online at: https://www.samhsa.gov/data/report/2023-nsduh-annual-national-report.
2. Spencer MR, Garnett MF, and Miniño AM. Drug Overdose Deaths in the United States, 2002-2022 (2023). Available online at: https://stacks.cdc.gov/view/cdc/135849 (Accessed June 16, 2025).
3. Volkow ND. Principles of drug addiction treatment: A research-based guide. North Bethesda, MD: DIANE Publishing (2011).
4. Gollinge A and Ploussiou H. Substance Use Disorders Treatment Options. Rockville, MD: SAMHSA Blog blog. (2025). Available online at: https://www.samhsa.gov/blog/substance-use-disorders-treatment-options (Accessed June 16, 2025).
5. US Food and Drug Administration. Focus Area: Substance Use Disorders. US Food and Drug Administration. (2022). Available online at: https://www.fda.gov/science-research/focus-areas-regulatory-science-report/focus-area-substance-use-disorders.
6. Farhoudian A, Razaghi E, Hooshyari Z, Noroozi A, Pilevari A, Mokri A, et al. Barriers and facilitators to substance use disorder treatment: an overview of systematic reviews. Subst Abuse: Res Treat. (2022) 16:11782218221118462. doi: 10.1177/11782218221118462
7. Blevins CE, Rawat N, and Stein MD. Gaps in the substance use disorder treatment referral process: provider perceptions. J Addict Med. (2018) 12:273–7. doi: 10.1097/adm.0000000000000400
8. Barnett ER, Knight E, Herman RJ, Amarakaran K, and Jankowski MK. Difficult binds: A systematic review of facilitators and barriers to treatment among mothers with substance use disorders. J Subst Abuse Treat. (2021) 126:108341. doi: 10.1016/j.jsat.2021.108341
9. Mancher M, Leshner AI, and National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Sciences Policy; Committee on Medication-Assisted Treatment for Opioid Use Disorder. Barriers to broader use of medications to treat opioid use disorder. Medications for opioid use disorder save lives. Washington, DC: National Academies Press (US) (2019).
10. Cantor JH, DeYoreo M, Hanson R, Kofner A, Kravitz D, Salas A, et al. Patterns in geographic distribution of substance use disorder treatment facilities in the US and accepted forms of payment from 2010 to 2021. JAMA Network Open. (2022) 5:e2241128–e2241128. doi: 10.1001/jamanetworkopen.2022.41128
11. Bhardwaj A, Sousa JL, Huskamp HA, Kofner A, Kravitz D, Salas A, et al. Prescribing medications for alcohol use disorder: A qualitative study of primary care physician decision making. Ann Fam Med Jul-Aug. (2023) 21:332–7. doi: 10.1370/afm.2997
12. Jones CM, Han B, Baldwin GT, Einstein EB, and Compton WM. Use of medication for opioid use disorder among adults with past-year opioid use disorder in the US, 2021. JAMA Network Open. (2023) 6:e2327488–e2327488. doi: 10.1001/jamanetworkopen.2023.27488
13. King C, Beetham T, Smith N, Englander H, Hadland SE, Bagley SM, et al. Treatments used among adolescent residential addiction treatment facilities in the US, 2022. JAMA. (2023) 329:1983–5. doi: 10.1001/jama.2023.6266
14. Vestal C. In fighting an opioid epidemic, medication-assisted treatment is effective but underused. Health Affairs. (2016) 35:1052–7. doi: 10.1377/hlthaff.2016.0504
16. Johnson MW, Hendricks PS, Barrett FS, and Griffiths RR. Classic psychedelics: An integrative review of epidemiology, therapeutics, mystical experience, and brain network function. Pharmacol Ther. (2019) 197:83–102. doi: 10.1016/j.pharmthera.2018.11.010
17. Livne O, Shmulewitz D, Walsh C, and Hasin DS. Adolescent and adult time trends in US hallucinogen use, 2002–19: any use, and use of ecstasy, LSD and PCP. Addiction. (2022) 117:3099–109. doi: 10.1111/add.15987
18. Keyes KM and Patrick ME. Hallucinogen use among young adults ages 19–30 in the United States: Changes from 2018 to 2021. Addiction. (2023) 118:2449–54. doi: 10.1111/add.16259
19. Mallaroni P, Mason NL, Vinckenbosch FRJ, and Ramaekers JG. The use patterns of novel psychedelics: experiential fingerprints of substituted phenethylamines, tryptamines and lysergamides. Psychopharmacology. (2022) 239:1783–96. doi: 10.1007/s00213-022-06142-4
20. Garel N, Tate S, Nash K, and Lembke A. Trends in hallucinogen-associated emergency department visits and hospitalizations in California, USA, from 2016 to 2022. Addiction. (2024) 119:960–4. doi: 10.1111/add.16432
21. Hinkle JT, Graziosi M, Nayak SM, and Yaden DB. Adverse events in studies of classic psychedelics: A systematic review and meta-analysis. JAMA Psychiatry. (2024) 81:1225–35. doi: 10.1001/jamapsychiatry.2024.2546
22. Marrocu A, Kettner H, Weiss B, Zeifman RJ, Erritzoe D, and Carhart-Harris RL. Psychiatric risks for worsened mental health after psychedelic use. J Psychopharmacology. (2024) 38:225–35. doi: 10.1177/02698811241232548
23. Zafar R, Siegel M, Harding R, Barba T, Agnorelli C, Suseelan S, et al. Psychedelic therapy in the treatment of addiction: the past, present and future. Review. Front Psychiatry. (2023) 14:1183740. doi: 10.3389/fpsyt.2023.1183740
24. Johnson MW, Garcia-Romeu A, Cosimano MP, and Griffiths RR. Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. J Psychopharmacology. (2014) 28:983–92. doi: 10.1177/0269881114548296
25. Cahill K, Stevens S, and Lancaster T. Pharmacological treatments for smoking cessation. JAMA. (2014) 311:193–4. doi: 10.1001/jama.2013.283787
26. Mottillo S, Filion KB, Bélisle P, Joseph L, Gervais A, O'Loughlin J, et al. Behavioural interventions for smoking cessation: a meta-analysis of randomized controlled trials. Eur Heart J. (2008) 30:718–30. doi: 10.1093/eurheartj/ehn552
27. Pisano VD, Putnam NP, Kramer HM, Franciotti KJ, Halpern JH, and Holden SC. The association of psychedelic use and opioid use disorders among illicit users in the United States. J Psychopharmacology. (2017) 31:606–13. doi: 10.1177/0269881117691453
28. Jones G, Ricard JA, Lipson J, and Nock MK. Associations between classic psychedelics and opioid use disorder in a nationally-representative U.S. adult sample. Sci Rep. (2022) 12:4099. doi: 10.1038/s41598-022-08085-4
29. Jones GM and Nock MK. Exploring protective associations between the use of classic psychedelics and cocaine use disorder: a population-based survey study. Sci Rep. (2022) 12:2574. doi: 10.1038/s41598-022-06580-2
30. de Veen BTH, Schellekens AFA, Verheij MMM, and Homberg JR. Psilocybin for treating substance use disorders? Expert Rev Neurotherapeutics. (2017) 17:203–12. doi: 10.1080/14737175.2016.1220834
31. Gao M, Rejaei D, and Liu H. Ketamine use in current clinical practice. Acta Pharmacologica Sinica. (2016) 37:865–72. doi: 10.1038/aps.2016.5
32. Famuła A, Radoszewski J, Czerwiec T, Sobiś J, and Więckiewicz G. Ketamine in substance use disorder treatment: A narrative review. Alpha Psychiatry Mar. (2024) 25:206–11. doi: 10.5152/alphapsychiatry.2024.241522
33. Gao Z, Winhusen TJ, Gorenflo M, Ghitza UE, Davis PB, Kaelber DC, et al. Repurposing ketamine to treat cocaine use disorder: integration of artificial intelligence-based prediction, expert evaluation, clinical corroboration and mechanism of action analyses. Addiction 2023/07/01. (2023) 118:1307–19. doi: 10.1111/add.16168
34. Gao Z, Winhusen TJ, Gorenflo MP, Dorney I, Ghitza UE, Kaelber DC, et al. Artificial intelligence-based drug repurposing with electronic health record clinical corroboration: A case for ketamine as a potential treatment for amphetamine-type stimulant use disorder. Addiction. (2025) 120:732–44. doi: 10.1111/add.16715
35. Dakwar E, Nunes EV, Hart CL, Dorney I, Ghitza UE, Kaelber DC, et al. A single ketamine infusion combined with mindfulness-based behavioral modification to treat cocaine dependence: A randomized clinical trial. Am J Psychiatry. (2019) 176:923–30. doi: 10.1176/appi.ajp.2019.18101123
36. Dakwar E, Levin F, Hart CL, Basaraba C, Choi J, Pavlicova M, et al. A single ketamine infusion combined with motivational enhancement therapy for alcohol use disorder: A randomized midazolam-controlled pilot trial. Am J Psychiatry. (2019) 177:125–33. doi: 10.1176/appi.ajp.2019.19070684
37. Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. (2010) 329:959–64. doi: 10.1126/science.1190287
38. Khajavi M, Emami A, Etezadi F, Safari S, Sharifi A, and Shariat Moharari R. Conscious sedation and analgesia in colonoscopy: ketamine/propofol combination has superior patient satisfaction versus fentanyl/propofol. Anesth Pain Med. (2013) 3:208–13. doi: 10.5812/aapm.9653
39. Kurdi MS, Theerth KA, and Deva RS. Ketamine: Current applications in anesthesia, pain, and critical care. Anesth Essays Res. (2014) 8:283–90. doi: 10.4103/0259-1162.143110
40. Gündüz M, Sakalli S, Güneş Y, Kesiktaş E, Ozcengiz D, and Işik G. Comparison of effects of ketamine, ketamine-dexmedetomidine and ketamine-midazolam on dressing changes of burn patients. J Anaesthesiol Clin Pharmacol. (2011) 27:220–4. doi: 10.4103/0970-9185.81823
41. Butterworth IV JF, Mackey DC, and Wasnick JD. “Morgan & Mikhail's Clinical Anesthesiology, 7e,” In: Chapter 9: Intravenous Anesthetics. New York, NY: McGraw-Hill Education. (2022). Available online at: https://accessanesthesiology.mhmedical.com/content.aspx?aid=1190604104.
42. Yu L, Zhu X, Peng K, Qin H, Yang K, Cai F, et al. Propofol alleviates anxiety-like behaviors associated with pain by inhibiting the hyperactivity of PVNCRH neurons via GABAA receptor β3 subunits. Advanced Sci. (2024) 11:2309059. doi: 10.1002/advs.202309059
43. Simmonds MK, Rashiq S, Sobolev IA, Dick BD, Gray DP, Stewart BJ, et al. The effect of single-dose propofol injection on pain and quality of life in chronic daily headache: a randomized, double-blind, controlled trial. Anesth Analg. (2009) 109:1972–80. doi: 10.1213/ANE.0b013e3181be3f86
44. Hsiao H-T, Liu Y-Y, Wang JC-F, Lin Y-C, and Liu Y-C. The analgesic effect of propofol associated with the inhibition of hypoxia inducible factor and inflammasome in complex regional pain syndrome. J Biomed Sci. (2019) 26:74. doi: 10.1186/s12929-019-0576-z
45. Krusz JC. IV propofol for treatment of chronic intractable cluster headache: A case series. Pract Pain Management. (2017) 17. Available online at: https://www.medcentral.com/pain/chronic/iv-propofol-treatment-chronic-intractable-cluster-headache-case.
46. National Institute on Drug Abuse. Psychedelic and Dissociative Drugs. US Department of Health and Human Services, National Institutes of Health. (2023). Available online at: https://nida.nih.gov/research-topics/psychedelic-dissociative-drugs.
47. Ehwerhemuepha L, Carlson K, Moog R, Bondurant B, Akridge C, Moreno T, et al. Cerner real-world data (CRWD) - A de-identified multicenter electronic health records database. Data Brief. (2022) 42:108120. doi: 10.1016/j.dib.2022.108120
48. Agnoli A, Xing G, Tancredi DJ, Magnan E, Jerant A, and Fenton JJ. Association of dose tapering with overdose or mental health crisis among patients prescribed long-term opioids. JAMA. (2021) 326:411–9. doi: 10.1001/jama.2021.11013
49. Charlson ME, Pompei P, Ales KL, and MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. (1987) 40:373–83. doi: 10.1016/0021-9681(87)90171-8
50. Cameron AC and Trivedi PK. Regression-based tests for overdispersion in the Poisson model. J Econometrics. (1990) 46:347–64. doi: 10.1016/0304-4076(90)90014-K
51. Dowell D, Ragan KR, Jones CM, Baldwin GT, and Chou R. CDC clinical practice guideline for prescribing opioids for pain-United States, 2022. MMWR: Recommendations reports: Morbidity mortality weekly Rep Recommendations reports/Centers Dis Control. (2022) 71:1–95. doi: 10.15585/mmwr.rr7103a1
52. Silva A, Costa B, Castro I, Mourão J, and Vale N. New perspective for drug-drug interaction in perioperative period. J Clin Med. (2023) 12:14. doi: 10.3390/jcm12144810
53. Petri CR and Richards JB. Management of sedation and analgesia in critically ill patients receiving long-acting naltrexone therapy for opioid use disorder. Ann Am Thorac Soc. (2020) 17:1352–7. doi: 10.1513/AnnalsATS.202005-554CME
54. Bausch Health Companies Inc. Medication Guide Wellbutrin XL (bupropion hydrochloride). Silver Spring, MD: U.S. Food and Drug Administration (2024). Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/021515s046lbl.pdf.
56. Aguilar AG, Beauregard BA, Conroy CP, Khatiwoda YT, Horsford SME, Nichols SD, et al. Pronounced regional variation in esketamine and ketamine prescribing to US medicaid patients. J Psychoactive Drugs Jan-Mar. (2024) 56:33–9. doi: 10.1080/02791072.2023.2178558
57. Glynos NG, Nicholas K, Kevin B, and Lucas P. The relationship between naturalistic psychedelic use and clinical care in Canada. J Psychoactive Drugs. (2023) 55:660–71. doi: 10.1080/02791072.2023.2242353. J. KD.
58. Carbonaro TM, Bradstreet MP, Barrett FS, MacLean KA, Jesse R, Johnson MW, et al. Survey study of challenging experiences after ingesting psilocybin mushrooms: Acute and enduring positive and negative consequences. J Psychopharmacol. (2016) 30:1268–78. doi: 10.1177/0269881116662634
59. Davis AK, Gabrielle A-L, Megan E, Brian P, and Luoma J. Attitudes and Beliefs about the Therapeutic Use of Psychedelic Drugs among Psychologists in the United States. J Psychoactive Drugs. (2022) 54:309–18. doi: 10.1080/02791072.2021.1971343
60. Rosenbaum SB, Gupta V, Patel P, and Palacios JL. Ketamine. Treasure Island, FL: StatPearls Publishing (2024).
61. Watkins LE, Patton SC, Drexler K, Rauch SAM, and Rothbaum BO. Clinical effectiveness of an intensive outpatient program for integrated treatment of comorbid substance abuse and mental health disorders. Cogn Behav Practice. (2023) 30:354–66. doi: 10.1016/j.cbpra.2022.05.005
62. McCarty D, Braude L, Lyman DR, Dougherty RH, Daniels AS, Ghose SS, et al. Substance abuse intensive outpatient programs: assessing the evidence. Psychiatr Serv. (2014) 65:718–26. doi: 10.1176/appi.ps.201300249
63. Gryczynski J, Nordeck CD, Welsh C, Mitchell SG, O’Grady KE, and Schwartz RP. Preventing hospital readmission for patients with comorbid substance use disorder. Ann Internal Med. (2021) 174:899–909. doi: 10.7326/M20-5475
64. Worley MJ, Trim RS, Tate SR, Hall JE, and Brown SA. Service utilization during and after outpatient treatment for comorbid substance use disorder and depression. J Subst Abuse Treat. (2010) 39:124–31. doi: 10.1016/j.jsat.2010.05.009
65. Deng L, Wu L, Gao R, Xu X, Chen C, and Liu J. Non-opioid anesthetics addiction: A review of current situation and mechanism. Brain Sci. (2023) 13:9. doi: 10.3390/brainsci13091259
66. Yoon SS, Lee BH, Lee SH, Choi SH, Jeong S-J, Kim SC, et al. Effects of isoflurane anesthesia on addictive behaviors in rats. Psychopharmacology. (2022) 239:3621–32. doi: 10.1007/s00213-022-06236-z
67. Johnson MW, Garcia-Romeu A, and Griffiths RR. Long-term follow-up of psilocybin-facilitated smoking cessation. Am J Drug Alcohol Abuse. (2017) 43:55–60. doi: 10.3109/00952990.2016.1170135
68. Garcia-Romeu A, Davis AK, Erowid F, Erowid E, Griffiths RR, and Johnson MW. Cessation and reduction in alcohol consumption and misuse after psychedelic use. J Psychopharmacology. (2019) 33:1088–101. doi: 10.1177/0269881119845793
69. Garcia-Romeu A, Davis AK, Erowid E, Erowid F, Griffiths RR, and Johnson MW. Persisting reductions in cannabis, opioid, and stimulant misuse after naturalistic psychedelic use: an online survey. Front Psychiatry. (2019) 10:955. doi: 10.3389/fpsyt.2019.00955
70. Ray LA, Meredith LR, Kiluk BD, Walthers J, Carroll KM, and Magill M. Combined pharmacotherapy and cognitive behavioral therapy for adults with alcohol or substance use disorders: A systematic review and meta-analysis. JAMA Network Open. (2020) 3:e208279–e208279. doi: 10.1001/jamanetworkopen.2020.8279
71. National Institute on Drug Abuse. Treatment and Recovery. U.S. Department of Health and Human Services. (2020). Available online at: https://nida.nih.gov/publications/drugs-brains-behavior-science-addiction/treatment-recovery.
72. Vutskits L. General anesthetics to treat major depressive disorder: clinical relevance and underlying mechanisms. Anesth Analgesia. (2018) 126:208–16. doi: 10.1213/ANE.0000000000002594
73. Glover-Wright C, Coupe K, Campbell AC, Keen C, Lawrence P, Kinner SA, et al. Health outcomes and service use patterns associated with co-located outpatient mental health care and alcohol and other drug specialist treatment: A systematic review. Drug Alcohol Review. (2023) 42:1195–219. doi: 10.1111/dar.13651
74. Sepulveda Ramos C, Thornburg M, Long K, Sharma K, Roth J, Lacatusu D, et al. The therapeutic effects of ketamine in mental health disorders: A narrative review. Cureus. (2022) 14:e23647. doi: 10.7759/cureus.23647
75. Brenna CTA, Goldstein BI, Zarate CA Jr., and Orser BA. Repurposing general anesthetic drugs to treat depression: A new frontier for anesthesiologists in neuropsychiatric care. Anesthesiology. (2024) 141:222–37. doi: 10.1097/ALN.0000000000005037
76. Bogenschutz MP, Forcehimes AA, Pommy JA, Wilcox CE, Barbosa P, and Strassman RJ. Psilocybin-assisted treatment for alcohol dependence: A proof-of-concept study. J Psychopharmacology. (2015) 29:289–99. doi: 10.1177/0269881114565144
77. Jones JL, Mateus CF, Malcolm RJ, Brady KT, and Back SE. Efficacy of ketamine in the treatment of substance use disorders: A systematic review. Front Psychiatry. (2018) 9:277. doi: 10.3389/fpsyt.2018.00277
78. Watkins KE, Paddock SM, Hudson TJ, Ounpraseuth S, Schrader AM, Hepner KA, et al. Association between quality measures and mortality in individuals with co-occurring mental health and substance use disorders. J Subst Abuse Treat. (2016) 69:1–8. doi: 10.1016/j.jsat.2016.06.001
79. Prince JD and Wald C. Risk of criminal justice system involvement among people with co-occurring severe mental illness and substance use disorder. Int J Law Psychiatry. (2018) 58:1–8. doi: 10.1016/j.ijlp.2018.02.002
80. Najt P, Fusar-Poli P, and Brambilla P. Co-occurring mental and substance abuse disorders: A review on the potential predictors and clinical outcomes. Psychiatry Res. (2011) 186:159–64. doi: 10.1016/j.psychres.2010.07.042
81. Fazel S, Geddes JR, and Kushel M. The health of homeless people in high-income countries: descriptive epidemiology, health consequences, and clinical and policy recommendations. Lancet. (2014) 384:1529–40. doi: 10.1016/s0140-6736(14)61132-6
82. Carrà G, Bartoli F, Crocamo C, Brady KT, and Clerici M. Attempted suicide in people with co-occurring bipolar and substance use disorders: Systematic review and meta-analysis. J Affect Disord. (2014) 167:125–35. doi: 10.1016/j.jad.2014.05.066
83. Lai HMX, Cleary M, Sitharthan T, and Hunt GE. Prevalence of comorbid substance use, anxiety and mood disorders in epidemiological surveys, 1990–2014: A systematic review and meta-analysis. Drug Alcohol Dependence. (2015) 154:1–13. doi: 10.1016/j.drugalcdep.2015.05.031
84. Swimmer KR and Sandelich S. Substance use disorder. Emergency Med Clinics North America. (2024) 42:53–67. doi: 10.1016/j.emc.2023.06.023
Keywords: psychedelics, ketamine, substance use disorder, real-world evidence, electronic health records, drug overdose, mental health, relapse
Citation: Qeadan F, McCunn A, Tingey B and Thielking P (2025) Associations between psychedelic use and adverse outcomes in substance use disorders: a real-world EHR-based cohort study. Front. Psychiatry 16:1648104. doi: 10.3389/fpsyt.2025.1648104
Received: 16 June 2025; Accepted: 29 September 2025;
Published: 24 October 2025.
Edited by:
Ilya Blokhin, Harvard University, United StatesCopyright © 2025 Qeadan, McCunn, Tingey and Thielking. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fares Qeadan, ZnFlYWRhbkBsdWMuZWR1
 Fares Qeadan
Fares Qeadan Ashlie McCunn
Ashlie McCunn Benjamin Tingey1
Benjamin Tingey1