- 1Department of General Surgery, Xinxiang Central Hospital, Xinxiang, Henan, China
- 2Department of Psychiatry, Xinxiang Central Hospital, Xinxiang, Henan, China
Aim: To compare the incidence of AUD and correlation between metabolic changes and addictive behaviors in patients who underwent SG.
Methods: A retrospective study was conducted on 160 obese patients who underwent SG treatment at our hospital between February 2023 and April 2024 (SG group), and another 160 non-surgical obese patients admitted during the same period were selected as the control group. The Alcohol Use Disorders Identification Test (AUDIT)was used to assess the risk of AUD in both groups (AUDIT≥eight points defined as high risk) and to compare the differences in high-risk rates between the groups. Differences in impulsivity scores (Barratt Impulsivity Scale [BIS]-11), addictive behavior scores(Visual Analog Scale for Addictive Behaviors[VAS]),and glucose-fat metabolism indexes between the high-and low-risk AUD subgroups within the SG group were analyzed using Pearson correlation and multiple regression analyses to explore associations between metabolic indicators and addictive behavior scores.
Results: The SG group had a higher rate of alcohol use disorder (AUDIT ≥ 8 points) after surgery than the control group (26.88% vs. 8.125%) (χ² = 19.32, P < 0.001).The impulsivity score[BIS-11:(68.43 ± 9.35)points vs.(61.22 ± 8.71)points] and addictive behavior score[VAS:(6.42 ± 1.14)points vs.(3.88 ± 1.06)points]were significantly higher in the high-risk group than in the low-risk group(P<0.001).Fasting plasma glucose, glycated hemoglobin, and homeostasis model assessment of insulin resistance levels were significantly higher in the AUDIT high-risk group than in the low-risk group(P<0.001).lipoprotein cholesterol (TC, TG, LDL-C, HDL-C) did not differ significantly between high- and low-risk groups (P > 0.05).Glucose metabolism indices(fasting plasma glucose, glycated hemoglobin, and homeostasis model assessment of insulin resistance)were strongly and positively correlated with AUDIT and VAS scores(r=0.682–0.716,P<0.05).However, multivariate linear regression analysis indicated that impulsivity, addictive behavior propensity, and glucose metabolism abnormalities were not independently associated with statistical significance(P>0.05).The propensity for addictive behavior and abnormal glucose metabolism remained independent risk factors for AUD after SG(P<0.05),and the risk was significantly higher in men than in women. This age group had significantly higher AUDIT high-risk rates, BIS-11 impulsivity, and VAS addiction behavior scores vs. the >25 group (P<0.05).
Conclusion: Compared to nonsurgical patients with obesity, patients with obesity who underwent SG exhibited a significantly high incidence of AUD. Patients in the high-risk subgroup for AUD also showed high impulsivity scores, greater addictive behavior scores, and notable abnormalities in glucose metabolism indices.
Introduction
As obesity rates continue to increase globally, metabolic weight-loss surgery—particularly sleeve gastrectomy (SG)—is widely used to treat severe obesity and its associated metabolic disorders (1). This procedure significantly reduces gastric volume by removing approximately 80% of the gastric body from the greater curvature of the stomach and affects the secretion of gastrointestinal hormones, thereby improving metabolic status and reducing body weight (2). Despite the significant advantages of SG in improving type 2 diabetes mellitus, hypertension, and sleep apnea, long-term behavioral and psychological changes in patients after surgery, especially the transfer of addictive behaviors, have raised clinical concerns. In recent years, studies have noted (3, 4) that some patients experience an increased risk of developing alcohol use disorder (AUD) following SG. This phenomenon may be related to accelerated gastric emptying, changes in blood–brain barrier permeability, altered neurotransmitter sensitivity in the reward system, and postoperative fluctuations in hormone levels. Decreased satisfaction with food-related pleasure in some patients after surgery may prompt them to seek alternative forms of stimulation, such as alcohol, to satisfy the reward mechanisms of the dopamine system (5). In addition, patients after SG experience significant metabolic changes, including in glucose, lipid, and amino acid metabolism, which are closely linked to central nervous system function; alterations in these metabolites can affect emotional regulation, self-control, and susceptibility to addiction (6). However, few studies have examined the incidence of AUD, metabolic changes, and addictive behaviors in this population. Based on these considerations, the present study was conducted to compare the incidence of AUD between patients who underwent SG and nonsurgical patients with obesity, analyze the association between metabolic indicators and impulsivity and addictive behaviors within the SG group, and provide a scientific basis for behavioral interventions, presurgical risk assessment, and long-term follow-up management of patients after SG.
Materials and methods
General information
The sample size was calculated based on a comparison of the rates between the two groups using the following formula:
Where: Zα/2 is the normally distributed value when the significance level is α/2 (two-sided test); Zβ is the normally distributed value when the test efficacy is 1-β; p1 and p2 are the expected values of the high-risk rate of AUD for the SG and control groups, respectively; and Δ is the minimum clinically significant difference between the two groups. Based on the data from previous studies and pretests, the sample size required for each group was calculated to be approximately 150 cases, assuming a high-risk rate of AUD of 25% (3)in the SG group and 10% (4) in the control group, taking α = 0.05 (two-sided) and β = 0.20 (80% test efficacy). Considering possible non-response and data loss, and ensuring balanced sample sizes between the two groups, the final decision was made to include 160 cases in the SG group and 160 cases in the control group.
This study included 160 obese patients who underwent SG as the study subjects (SG group) and selected 160 non-surgical obese patients treated during the same period as the control group and the cases were selected from February 2023 to April 2024. In the SG group, there were 75 men and 85 women, with ages ranging from 18 to 64 years, and the preoperative body mass index (BMI) ranged from 31.57 to 40.53 kg/m². In the control group, there were 77 men and 83 women, aged 17 to 65 years, with preoperative BMI of 31.63 to 40.49 kg/m². General information on the two groups is shown in Table 1, which was statistically analyzed and showed no statistical difference (P > 0.05). This study was approved by our institutional ethics committee.

Table 1. Comparison of general information and high-risk rate of AUDIT between the two groups (n/%, ± s).
Inclusion criteria: (1) age 18 to 65 years, meeting the diagnostic criteria for severe obesity (7) (BMI ≥ 35 kg/m² or BMI ≥ 30 kg/m² combined with at least one obesity-related metabolic disease, such as type 2 diabetes mellitus and hypertension); (2) first-time treatment with SG, with no history of other bariatric surgeries; (3) no preoperative history of AUD (Alcohol Use Disorder Identification Test [AUDIT] < eight points) or history of substance abuse; (4) full postoperative follow-up for more than 6 months with good compliance; (5) the control group met the criteria for severe obesity but did not undergo any bariatric surgery and had no history of alcohol dependence.
Exclusion criteria were as follows: (1) patients with combined serious organic diseases; (2) previous history of mental illness or long-term use of psychotropic drugs; (3) alcohol dependence or other addictive behaviors already present before the operation; and (4) pregnant or lactating women.
Methods
Psychological and behavioral assessment
Demographic information (age and sex), BMI, and scores on psycho-behavioral scales [AUDIT (8), Barratt Impulsivity Scale (BIS-11) (9), and Visual Analog Scale for Addictive Behaviors (VAS) (10)] were collected through the hospital’s internal medical record system. The AUDIT scale contains 10 items, including three aspects: frequency of drinking, amount of drinking, symptoms of dependence, and drinking-related harm. Each entry is scored from zero to four points according to its severity (including “number of drinks per month”: zero = never, four = daily or almost daily), and the total score ranges from zero to 40 points. According to the international consensus: low risk: 0–7 points (no or mild drinking problem); high risk (AUD tendency): ≥ eight points. (ii) The BIS-11 contains 30 items divided into three dimensions: attentional impulsivity (cognitive), motor impulsivity (behavioral), and unplanned impulsivity (decision-making). A four-point Likert scale (one = “never” to four = “always”) was used, with a total score range of 30 to 120. High total scores indicate poor impulse control. (iii) The VAS quantifies the intensity of subjective craving by a self-report method using a 10 cm straight line (zero to 10 points) anchored at both ends as zero: no craving at all (including “don’t want to drink at all”) and 10: extremely strong craving (including “strongest desire to drink in life”). Participants marked the location of their current craving for alcohol, with a score of ≥ 4 indicating a significant tendency toward addictive behavior.Scale assessments were standardized to be conducted 6 months postoperatively.
Metabolic index testing
Five milliliters of elbow venous blood were collected from patients in the SG group at 6 months postoperatively, and the serum was separated by centrifugation (3000 rpm for 10 min) and tested. Glycated hemoglobin (HbA1c) levels were measured using a Bio-Rad Variant II Turbo Glycosylated Hemoglobin Analyzer. The test kit supported fasting blood glucose (FPG), total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) levels. A Roche Cobas c 501 automatic biochemistry analyzer was used for determination, and the kits were used as matching test kits. All steps of the assay strictly followed the operating specifications of the kits and laboratory quality control procedures. The insulin resistance index was calculated as follows: Homeostatic Model Assessment of Insulin Resistance(HOMA-IR) = fasting insulin × FPG/22.5.
Statistical methods
The data in this study were confirmed to be normally distributed by a normality test, and all statistical analyses were performed using SPSS 26.0. For categorical variables, the number of cases (n) and their corresponding percentages (%) were used for description, and the chi-square test (χ²) was utilized to compare different groups. Measured data that conformed to a normal distribution were expressed as ( ± s), and an independent samples t-test was used to assess differences between groups. Pearson correlation was used to analyze the correlation between metabolic changes and addictive behaviors in patients who underwent SG. A multiple linear regression model was developed using the backward method in stepwise regression to explore potential influences on the risk of postoperative AUD in patients with SG. The goodness of fit of the model was evaluated by the adjusted R² value, and statistical significance was verified using ANOVA. P < 0.05 was considered statistically significant.
Results
General information and AUDIT high-risk rate across groups
The high-risk rate of postoperative AUD (AUDIT ≥ eight points) was 26.88% in the SG group and 8.13% in the control group (P < 0.001). There were no statistically significant differences (P > 0.05) in baseline data, including age, BMI, sex, or glycolipid metabolism indices, between the two groups (Table 1).
Impulsivity and addictive behavior scores in AUDIT subgroups
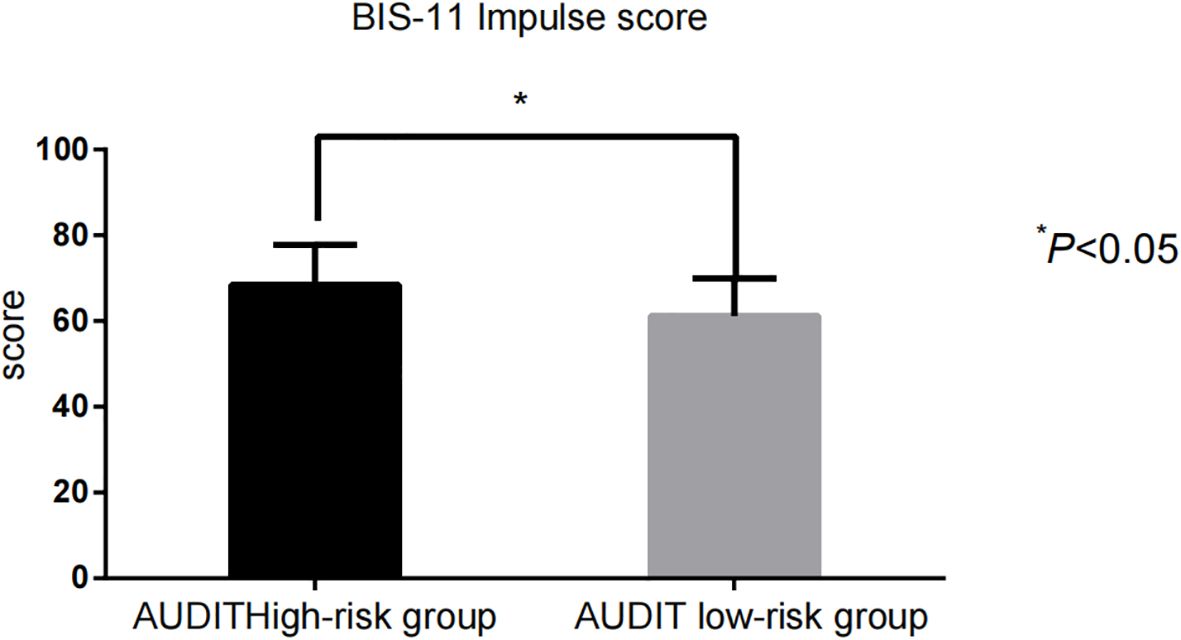
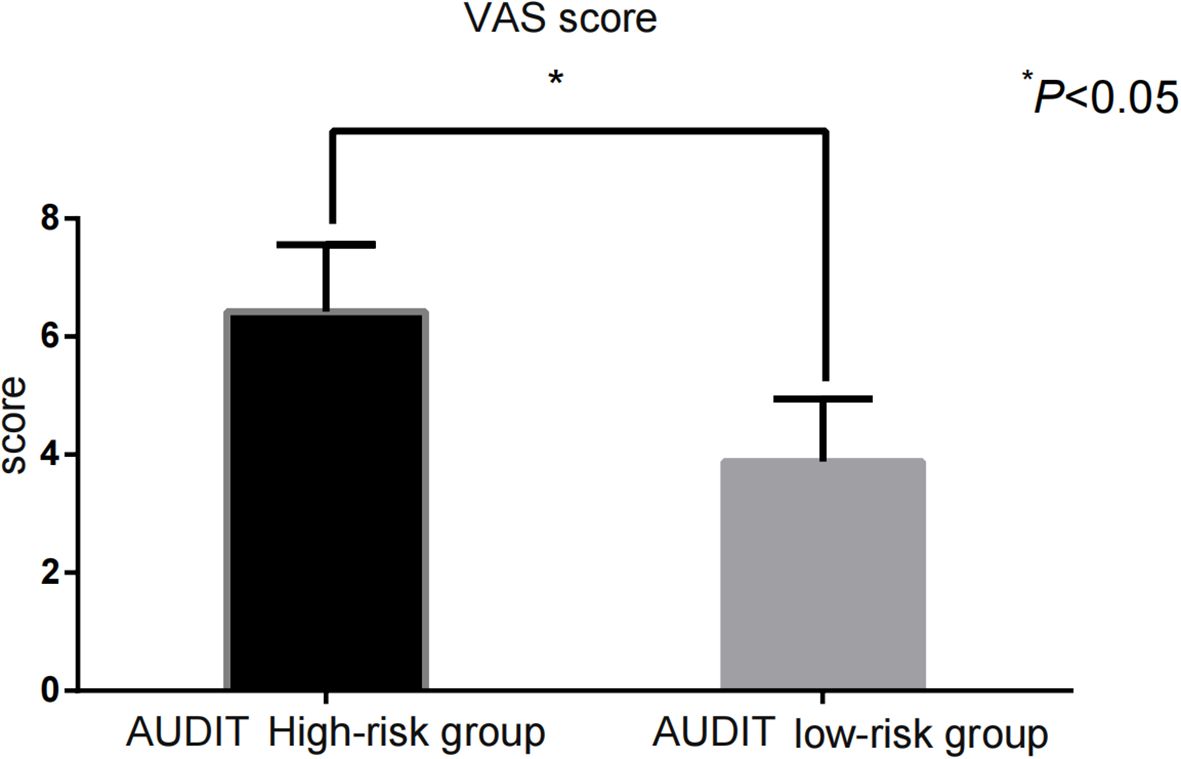
The impulsivity scores [BIS-11: (68.43 ± 9.35) vs. (61.22 ± 8.71)] and addictive behavior scores [VAS: (6.42 ± 1.14) vs. (3.88 ± 1.06)] in the AUDIT high-risk group were significantly higher than those in the low-risk group (P < 0.001) (Figure 1, Figure 2).
Postoperative glucose metabolism in AUDIT subgroups
Postoperative levels of FPG, HbA1c, and Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) were significantly higher in the AUDIT high-risk group than in the low-risk group (P < 0.001) (Table 2).

Table 2. Comparison of postoperative glucose metabolism indexes between AUDIT high-risk and low-risk groups ( ± s).
Postoperative lipid metabolism in AUDIT subgroups
There were no statistically significant differences in lipid metabolism indices—including TC, TG, LDL-C, and HDL-C—between patients in the AUDIT high-risk and low-risk groups (P > 0.05) (Table 3).

Table 3. Comparison of postoperative lipid metabolism indexes between AUDIT high-risk and low-risk groups ( ± s).
Correlations between metabolic indices, AUDIT, and VAS scores
Glucose metabolism indices (FPG, HbA1c, and HOMA-IR) were strongly and positively correlated with both the AUDIT and VAS scores (r = 0.682–0.716, P < 0.05). The BIS-11 impulsivity score was also significantly correlated with the AUDIT score (r = 0.669, P < 0.001) (Table 4).

Table 4. Results of correlation analysis of postoperative metabolic index changes with postoperative AUDIT score and VAS score.
Multiple regression analysis of factors influencing the risk of AUD after SG
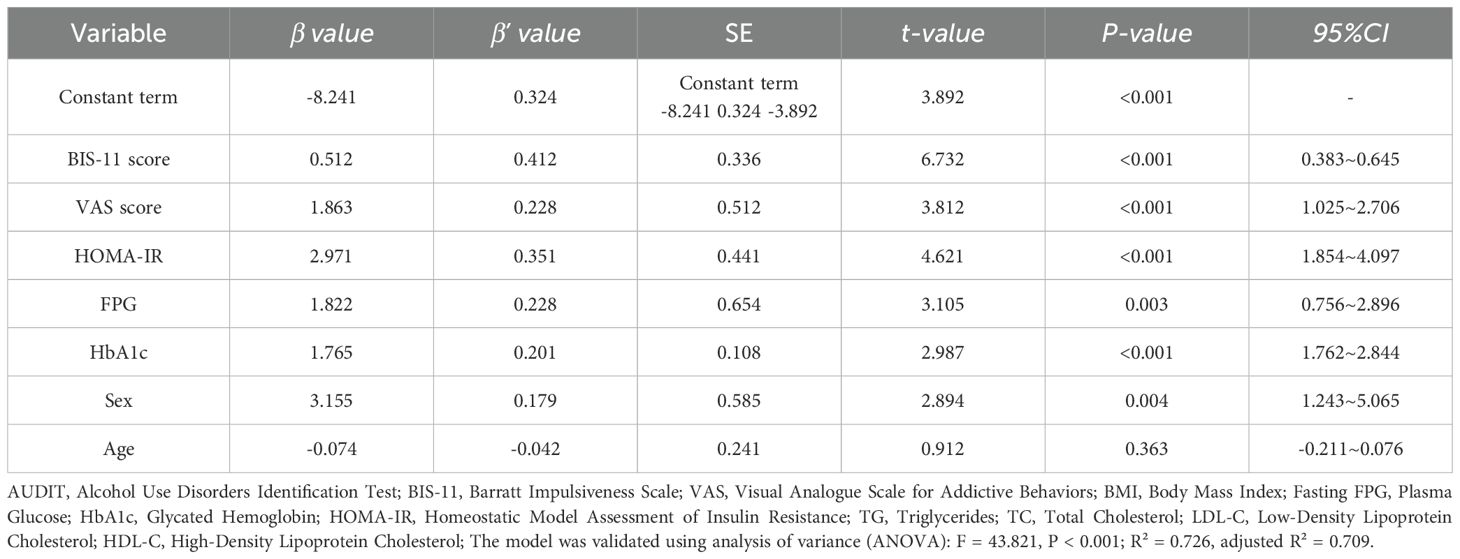
In this study, the presence or absence of AUD after surgery in patients who underwent SG was the dependent variable (yes = one, no = zero), and glucose metabolism indices (FPG, HbA1c, HOMA-IR), BIS-11 impulsivity scores, and VAS scores were included as continuous variables in the multiple linear regression model. Confounders included age (continuous variable) and sex (men vs. women). Impulsivity, propensity for addictive behaviors, and abnormal glucose metabolism remained independent risk factors for AUD after SG (P < 0.05), and the risk was significantly higher in men than in women (Table 5).
Subgroup analysis of SG patients aged 18–25 years
To further investigate the impact of age on the risk of AUD postoperatively, a subgroup analysis was conducted on patients aged 18–25 years in the SG group (n = 28, accounting for 17.5%). The results showed that the high-risk rate for AUDIT, BIS-11 impulsivity scores, and VAS addiction behavior scores were significantly higher in this age group than in the >25-year-old group (P<0.05) (Table 6).

Table 6. Comparison of AUD risk and related behavioral indicators between patients aged 16–25 and those aged >25 in the SG group.
Discussion
In recent years, with the widespread use of metabolic weight-loss surgery, behavioral and psychological changes in postoperative patients have gradually received attention. SG, as a mainstream weight-loss surgical modality, is effective in reducing body weight and ameliorating metabolic disorders, while potentially triggering certain behavioral problems, such as AUD. Studies have shown (11–13) that the incidence of AUD is higher in patients after SG than in the general population, which may be related to the physiological and psychological changes caused by surgery. On one hand, the rapid weight loss and improved metabolic status of patients after SG may temporarily improve their self-esteem and quality of life, thereby increasing the risk of substance abuse, such as alcohol (14). On the other hand, the stress response during surgery and discomfort experienced during postoperative recovery may also motivate some patients to alleviate their anxiety and depression through substances, such as alcohol (15, 16). Therefore, alcohol use behavior in patients undergoing postoperative SG should be closely monitored, and timely interventions and guidance should be provided to prevent the occurrence of AUD.
In this study, the incidence of a high risk of postoperative AUD was significantly higher in patients who underwent SG than in obese controls who did not undergo surgery (P < 0.001), suggesting a trend toward an increased risk of addictive behaviors after SG. This finding is consistent with those of previous studies (17–19), suggesting that SG may increase the risk of postoperative AUD. This mechanism may be related to the following factors: physiological structural changes that accelerate alcohol absorption, gastrointestinal hormonal changes that interfere with the brain’s stress response and reward mechanisms, and changes in the neurotransmitter system that increase the craving for alcohol. Collectively, these factors are believed to contribute to an elevated risk of AUD in postoperative patients (20).
The mechanisms linking SG to an elevated AUD risk are multifactorial and extend beyond behavioral shifts. A critical factor is the alteration of alcohol pharmacokinetics. Post-surgery anatomical changes lead to faster gastric emptying and reduced first-pass metabolism, resulting in a quicker and higher peak in blood alcohol concentration (BAC) from the same amount of alcohol (21, 22). This heightened level of intoxication could reinforce drinking behavior. Furthermore, the interplay with gut hormones is complex. While metabolic surgery is known to increase endogenous levels of glucagon-like peptide-1 (GLP-1), a hormone associated with satiety, recent placebo-controlled studies show that acute alcohol consumption paradoxically decreases GLP-1 concentrations in both post-surgery and non-surgical individuals (21, 22). This reduction in a key satiation signal might contribute to alcohol’s “apéritif effect,” potentially undermining appetite control and promoting consumption. These hormonal and pharmacokinetic changes, combined with a heightened risk for alcohol-induced hypoglycemia observed in the post-surgical population (21, 22), create a complex physiological environment that could lower the threshold for developing AUD. It has also been shown that a higher Body Mass Index (BMI) is associated with a lower level of response to alcohol, a relationship largely accounted for by total body water, which influences alcohol concentration (23).
Regarding metabolic indices, this study found that glucose metabolic indices (FPG, HbA1c, and HOMA-IR) were significantly higher in the AUD high-risk group than in the low-risk group and were strongly and positively correlated with AUDIT and VAS scores (r = 0.682–0.716, P < 0.001), suggesting that abnormal glucose metabolism in the postoperative period may be closely related to addictive behaviors. This suggests that abnormal glucose metabolism may enhance sensitivity to alcohol-related reward stimuli by affecting brain insulin signaling, dopamine metabolism, and other pathways, which, in turn, increase the risk of addiction. In addition, abnormal insulin signaling in the nucleus ambiguous, a central neuromodulator, may be involved in the development of addictive behaviors (24, 25). By contrast, lipid metabolism indices (TC, TG, LDL-C, and HDL-C) did not differ significantly between the high- and low-risk groups and were not significantly correlated with AUDIT or VAS scores, suggesting that lipid metabolism does not have a significant effect on addictive behavior. This is because lipid metabolism primarily functions in energy storage and cellular structure maintenance, rather than influencing the central reward pathway (26).
The results of this study showed that the high-risk group for AUD had significantly higher BIS-11 impulsivity scores and VAS addiction behavior scores than the low-risk group. After adjusting for confounding factors such as age and gender, impulsivity, addiction behavior tendencies, and abnormal glucose metabolism remained independent risk factors for AUD risk after SG surgery (P<0.05), and male patients had a significantly higher risk than female patients (27). The risk was significantly higher in men than in women. This indicates that the presence of impulsivity, a tendency toward addictive behavior, abnormal glucose metabolism, and being a man not only correlate with a greater tendency to use alcohol but are also associated with more pronounced behavioral impulsivity and subjective craving. Individuals with high impulsivity lack self-control and are more likely to make impulsive drinking decisions when exposed to alcohol-related cues (25). Increased psychological stress in patients after SG, owing to various factors, such as altered body image, lifestyle adjustments, and metabolic changes, may further exacerbate impulsive tendencies, thereby increasing the risk of AUD (28). It is worth noting that alcohol consumption itself significantly impacts glycemic control by affecting hepatic glucose production, insulin secretion, and sensitivity. Therefore, the observed association between postoperative glycemic abnormalities and a high AUD risk may be bidirectional; drinking behavior could directly influence metabolic status, rather than the relationship being strictly causal in one direction. Although our correlation and regression analyses revealed a significant link, this study’s cross-sectional design cannot rule out this reverse causality. Future studies could better clarify the directionality between these factors by using more precise quantification of alcohol intake (e.g., daily alcohol intake in grams), controlling for metabolic baseline, or applying a longitudinal follow-up design.
A subgroup analysis was conducted on patients aged 18–25 years in the SG group. The results showed that the high-risk rate for AUDIT in this age group reached 42.86%, significantly higher than that in older patients; simultaneously, their BIS-11 impulsivity scores and VAS addiction behavior scores were also significantly elevated. This finding supports previous research suggesting that adolescents and young adults are more susceptible to addiction-related behaviors. Although the age variable did not emerge as an independent risk factor in the multivariate regression analysis, this may be because its effects were overshadowed by stronger predictive factors such as impulsivity and metabolic variables in the overall sample. Therefore, in preoperative assessment and postoperative intervention, the younger patient population (especially those aged 18–25) should be prioritized as a focus group. It is recommended to strengthen behavioral intervention and impulse control training for this population in postoperative psychological management.
The limitations of this study include its relatively small sample size and its single-center retrospective design, which introduces potential selection bias. Particular caution is warranted in interpreting the high-risk rate (42.86%) observed in the young subgroup (n=28) due to this small sample size. Therefore, this finding should be viewed as a preliminary hypothesis requiring validation in larger studies. To address these limitations and validate the current findings, future multicenter, prospective cohort studies are needed. Furthermore, building on existing knowledge that ghrelin hormone not only regulates appetite but also participates in alcohol reward behavior (29), future research could combine ghrelin level detection with neuroimaging techniques to further analyze its role in postoperative addictive behavior. Regarding our subgroup analysis, it focused exclusively on SG patients to elucidate surgery-specific pathways. Future studies with larger non-surgical cohorts are necessary to clarify whether similar metabolic-addiction relationships exist in obesity without surgical intervention.
Conclusion
In summary, the elevated risk of postoperative AUD following SG is not only closely related to psych behavioral traits, such as increased impulsivity, but may also be influenced by glucose metabolism disorders. Therefore, glucose metabolism levels and psych behavioral characteristics should be key components of preoperative risk assessment. Based on the findings, it is recommended to establish a surgery-led multidisciplinary collaborative mechanism, involving referrals from surgery to endocrinology and psychology/psychiatry at 6, 12, and 24 months postoperatively; endocrinology would monitor glucose metabolism indices, such as FPG and HbA1c, every 3 months and dynamically adjust lifestyle interventions or medication regimens (including metformin) according to HOMA-IR; and psychological or psychiatric services would conduct quarterly assessments of impulsivity (BIS-11) and addictive behavior tendencies (VAS), implementing targeted cognitive behavioral therapy and positive thinking training. Simultaneously, the nutrition department would provide dietary support during periods of abstinence, thereby forming a closed loop of metabolic regulation and behavioral interventions for comprehensive postoperative management.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Xinxiang Central Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
FW: Conceptualization, Data curation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. YF: Data curation, Formal Analysis, Software, Writing – original draft. JL: Investigation, Methodology, Writing – original draft. JY: Conceptualization, Formal Analysis, Writing – original draft. ZL: Software, Validation, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhao S, Li R, Zhou J, et al. Sleeve gastrectomy with transit bipartition: a review of the literature. Expert Rev Gastroenterol Hepatol. (2023) 17:451–9. doi: 10.1080/17474124.2023.2206563
2. Ma R, Jiang PQ, Liu SY, et al. Obesity-Surgery is not the end. World J Gastrointest Surg. (2024) 16:3643–6. doi: 10.4240/wjgs.v16.i12.3643
3. Şen O, Ünübol H, Gökhan Türkçapar A, et al. Risk of alcohol use disorder after sleeve gastrectomy. J Laparoendosc Adv Surg Tech A. (2021) 31:24–8. doi: 10.1089/lap.2020.0306
4. Wong E, Fleishman A, Brem A, et al. High-risk alcohol use and disordered eating behavior before and 1 year after sleeve gastrectomy. Obes Surg. (2022) 32:593–8. doi: 10.1007/s11695-021-05847-3
5. Kenkre JS, Gesell S, Keller A, et al. Alcohol Misuse post-Metabolic and Bariatric Surgery: A Systematic Review of Longer-term Studies with Focus on new Onset Alcohol use Disorder and Differences Between Surgery Types. Curr Obes Rep. (2024) 13:596–616. doi: 10.1007/s13679-024-00577-w
6. McPheeters M, O’Connor EA, Riley S, et al. Pharmacotherapy for alcohol use disorder: a systematic review and meta-analysis. JAMA. (2023) 330:1653–65. doi: 10.1001/jama.2023.19761
7. Stegenga H, Haines A, et al. Diagnosis, assessment and management of overweight and obesity: summary of updated NICE guidance. Br Med J Chin Edition. (2015) 18:531–5. doi: 10.1136/bmj.g6608
8. Neufeld M, Bunova A, Ferreira-Borges C, et al. The Alcohol Use Disorders Identification Test (AUDIT) in the Russian language - a systematic review of validation efforts and application challenges. Subst Abuse Treat Prev Policy. (2021) 16:76. doi: 10.1186/s13011-021-00404-8
9. Tsatali M, Moraitou D, Papantoniou G, et al. Measuring impulsivity in greek adults: psychometric properties of the barratt impulsiveness scale (BIS -11) and impulsive behavior scale (Short version of UPPS-P). Brain Sci. (2021) 11:1007. doi: 10.3390/brainsci11081007
10. He S, Renne A, Argandykov D, et al. Comparison of an emoji-based visual analog scale with a numeric rating scale for pain assessment. JAMA. (2022) 328:208–9. doi: 10.1001/jama.2022.7489
11. Danpanichkul P, Chen VL, Chaiyakunapruk N, et al. Socio-economic association of alcohol use disorder and cardiovascular and alcohol-associated liver disease from 2010 to 2019. Aliment Pharmacol Ther. (2024) 60:340–9. doi: 10.1111/apt.18095
12. Iossa A, Ciccioriccio MC, Zerbinati C, et al. Alcohol ingestion symptoms after sleeve gastrectomy: intoxication or drunkenness? a prospective study from a Bariatric Center of Excellence. Eat Weight Disord. (2020) 25:1719–25. doi: 10.1007/s40519-019-00813-6
13. Nazmin F, Gunturu S, Jaka S, et al. Alcohol use disorder following bariatric surgery: a narrative review. Actas Esp Psiquiatr. (2025) 53:605–15. doi: 10.62641/aep.v53i3.1830
14. White GE, Boles RE, Courcoulas AP, et al. Predictors of alcohol use, alcohol-related problems, and substance use following adolescent metabolic and bariatric surgery. Ann Surg Open. (2024) 5:e461. doi: 10.1097/AS9.0000000000000461
15. White GE, Boles RE, Courcoulas AP, et al. A prospective cohort of alcohol use and alcohol-related problems before and after metabolic and bariatric surgery in adolescents. Ann Surg. (2023) 278:e519–25. doi: 10.1097/SLA.0000000000005759
16. Oliveira KC, Santa-Cruz F, Leão LMS, et al. Alcohol use disorder and depression in patients after undergoing bariatric surgery. Arq Bras Cir Dig. (2025) 38:e1871–9. doi: 10.1590/0102-6720202500002e1871
17. Riedel O, Braitmaier M, Dankhoff M, et al. Alcohol use disorders after bariatric surgery: a study using linked health claims and survey data. Int J Obes (Lond). (2024) 48:1656–63. doi: 10.1038/s41366-024-01606-3
18. Mousavi M, Tabesh MR, Moghadami SM, et al. Determining the importance of lifestyle risk factors in predicting binge eating disorder after bariatric surgery using machine learning models and lifestyle scores. Obes Surg. (2025) 35:1396–406. doi: 10.1007/s11695-025-07765-0
19. Köhne S, Hillemacher T, Glahn A, et al. Emerging drugs in phase II and III clinical development for the treatment of alcohol use disorder. Expert Opin Emerg Drugs. (2024) 29:219–32. doi: 10.1080/14728214.2024.2342951
20. Witkiewitz K, Fernandez AC, Green EW, et al. Diagnosis of alcohol use disorder and alcohol-associated liver disease. Clin Liver Dis. (2024) 28:699–713. doi: 10.1016/j.cld.2024.06.009
21. Molina-Castro M, Seyedsadjadi N, Nieto D, et al. The glucagon-like peptide-1 and other endocrine responses to alcohol ingestion in women with versus without metabolic surgery. Addict Biol. (2024) 29:e13441. doi: 10.1111/adb.13441
22. Molina-Castro M, Rowitz B, and Pepino MY. Glucagon-like peptide-1, fibroblast growth factor 21, and other endocrine responses to alcohol ingestion in women before and after metabolic surgery. Front Pharmacol. (2025) 16:1575156. doi: 10.3389/fphar.2025.1575156
23. Vatsalya V, Gorka S, Stangl BL, et al. Body mass index is inversely associated with level of response to alcohol: role of total body water. J Stud Alcohol Drugs. (2024) 85:227–36. doi: 10.15288/jsad.23-00134
24. Tareen K, Clifton EG, Perumalswami P, et al. Treatment of alcohol use disorder: behavioral and pharmacologic therapies. Clin Liver Dis. (2024) 28:761–78. doi: 10.1016/j.cld.2024.06.011
25. Jogendran M, Huynh L, and Flemming JA. Alcohol use disorder pharmacotherapy in patients with alcohol-related liver disease: a scoping review. Can J Gastroenterol Hepatol. (2025) 31:645–9. doi: 10.1155/cjgh/6455092
26. Díaz LA, König D, Weber S, et al. Management of alcohol use disorder: a gastroenterology and hepatology-focused perspective. Lancet Gastroenterol Hepatol. (2025) 10:475–90. doi: 10.1016/S2468-1253(24)00380-7
27. Zhou H and Gelernter J. Human genetics and epigenetics of alcohol use disorder. J Clin Invest. (2024) 134:e172–8. doi: 10.1172/JCI172885
28. Lékó AH and Leggio L. Barriers to alcohol use disorder treatment in patients with alcohol-associated liver disease. Clin Liver Dis. (2024) 28:779–791. doi: 10.1016/j.cld.2024.06.012
Keywords: sleeve gastrectomy, alcohol use disorder, metabolic changes, addictive behavior, correlation analysis
Citation: Wang F, Fan Y, Li J, Yan J and Li Z (2025) Incidence rate of alcohol use disorder and correlation between metabolic changes and addictive behavior in patients after sleeve gastrectomy. Front. Psychiatry 16:1652020. doi: 10.3389/fpsyt.2025.1652020
Received: 23 June 2025; Accepted: 30 September 2025;
Published: 21 October 2025.
Edited by:
Ramón Sotomayor-Zárate, Universidad de Valparaiso, ChileReviewed by:
Mario Rivera-Meza, University of Chile, ChileMiriam Beatriz Virgolini, Universidad Nacional de Córdoba, Argentina
Copyright © 2025 Wang, Fan, Li, Yan and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fuqin Wang, d2ZxMTIzNDU2MDYxM0B5ZWFoLm5ldA==
 Fuqin Wang
Fuqin Wang Youjie Fan
Youjie Fan Jianhua Li1
Jianhua Li1