- 1School of Social Science, Arts and Humanities, Lincoln University College, Petaling Jaya, Malaysia
- 2Department of Communication and Media Studies, Marian College Kuttikkanam, Kuttikkanam, India
- 3Department of Emergency Medicine, Jaya Matha Hospital Koothattukulam, Ernakulam, India
1 Introduction: Media stress as an emerging neuroimmune challenge
Over the past decade, the pervasive influence of digital media has restructured not just our information environment, but potentially our biological systems. Social media, 24-hour news cycles, and algorithmically amplified crisis content now form a persistent backdrop to modern life. Recent studies show that repeated exposure to emotionally charged news—whether about war, pandemics, or climate disasters—can provoke prolonged psychological stress, which in turn may impact immune function (1). While media psychology has studied the behavioral consequences of such exposure, its immunological ramifications remain underexplored.
This opinion article argues that chronic media-induced psychological stress constitutes a distinct immunological stressor, with measurable effects on neuroinflammatory signaling, blood–brain barrier (BBB) integrity, and central nervous system (CNS) immune surveillance. We propose that this form of stress exposure deserves attention from immunologists not merely as a psychosocial factor, but as a potential driver of low-grade systemic inflammation and neuroimmune dysregulation, especially in vulnerable populations. While this opinion focuses on general neuroimmune impacts, we emphasize the relevance of media-induced stress in the context of multiple sclerosis (MS), a chronic neuroinflammatory disease. Emerging research suggests that stress and immune dysregulation may influence disease onset and flare-ups, making MS a relevant disease model for this discussion (2).
2 Brain–immune crosstalk under stress: The biological logic
The brain and immune system operate as a bidirectional network, engaging in a tightly regulated dialogue through neuroendocrine and cytokine pathways. The hypothalamic–pituitary–adrenal (HPA) axis and sympathetic nervous system (SNS) play critical roles in initiating immune responses to psychological stimuli. When the brain perceives a threat—whether physical or symbolic—glucocorticoids and catecholamines are released, modulating cytokine production, lymphocyte trafficking, and microglial activity (3).
However, persistent stress—particularly from unresolved or ambiguous threats as commonly portrayed in media—leads to HPA axis dysregulation (4). Cortisol feedback inhibition becomes impaired, resulting in prolonged elevation of inflammatory mediators, including IL-6, TNF-α, and C-reactive protein (CRP) (5). This biochemical milieu primes the innate immune system while also disrupting adaptive responses, increasing susceptibility to viral infections and autoimmunity.
Importantly, the blood–brain barrier, normally a shield for the CNS, becomes compromised under chronic stress conditions. Animal models show increased permeability and elevated expression of endothelial adhesion molecules following repeated social defeat or immobilization stress. This facilitates peripheral cytokines and immune cells crossing into the CNS, where they interact with microglia, the resident immune sentinels of the brain (6). In autoimmune disorders such as MS, these mechanisms are particularly relevant: stress-induced blood–brain barrier (BBB) permeability and peripheral immune infiltration are hypothesized to trigger demyelinating episodes (7).
3 Microglial priming and the stress–inflammation loop
Microglia, the innate immune cells of the CNS, typically remain in a surveillant state, dynamically sampling their environment. Under pathological or stress conditions, they can polarize into M1 (pro-inflammatory) or M2 (anti-inflammatory) states, depending on the context (8). In stress-induced scenarios, however, microglia exhibit a “primed” phenotype—hypersensitive to secondary stimuli and prone to exaggerated cytokine release, even in response to mild challenges.
In rodent models, exposure to chronic unpredictable stress increases Iba1+ microglial density and upregulates expression of TNF-α and IL-1β in hippocampal and prefrontal regions. These alterations correlate with behavioral symptoms analogous to depression and cognitive inflexibility (9). The neuroinflammatory cascade, once triggered, further reinforces HPA axis activation—creating a self-sustaining stress–inflammation loop.
This mechanism has implications for psychiatric and neurodegenerative conditions. Primed microglia have been implicated in neuropsychiatric conditions such as major depressive disorder (MDD) and generalized anxiety disorder (GAD), and they may also play a role in neuroinflammatory conditions like multiple sclerosis (MS), where microglial overactivation contributes to demyelination. (10). In this light, repeated media stress exposure may function as an environmental primer, particularly in individuals with genetic or epigenetic vulnerabilities. This priming process may be particularly harmful in MS, where pro-inflammatory microglial activation is associated with demyelination and neurodegeneration. Stress-induced microglial shifts could potentially exacerbate inflammatory responses in individuals predisposed to or living with MS (11).
4 Media-induced stress as an immunological risk factor
The idea that psychological stress can “get under the skin” is not new. However, with the rise of pervasive media, we now face a stressor that is ubiquitous, persistent, and individually targeted. Unlike acute traumas, media-induced stress is chronic, symbolic, and often vicarious—which paradoxically amplifies uncertainty and emotional engagement (12). This creates conditions conducive to low-grade systemic inflammation.
For conceptual clarity, stressors can be broadly classified into acute stressors—short-term, discrete events such as accidents, natural disasters, or examinations—and chronic stressors—long-term, ongoing pressures such as caregiving burden, job insecurity, or financial hardship. While acute stressors often provoke a rapid but transient neuroendocrine and immune response, chronic stressors tend to produce prolonged HPA axis activation and low-grade inflammation. Media-related stress can operate in both domains: sudden distressing news or online harassment may act as acute triggers, whereas continuous exposure to algorithmically reinforced negative content may function as a chronic background stressor (13). The three major stressor categories—acute, chronic, and media-related—are depicted in Figure 1, showing both unique and overlapping pathways relevant to neuroimmune dysregulation.
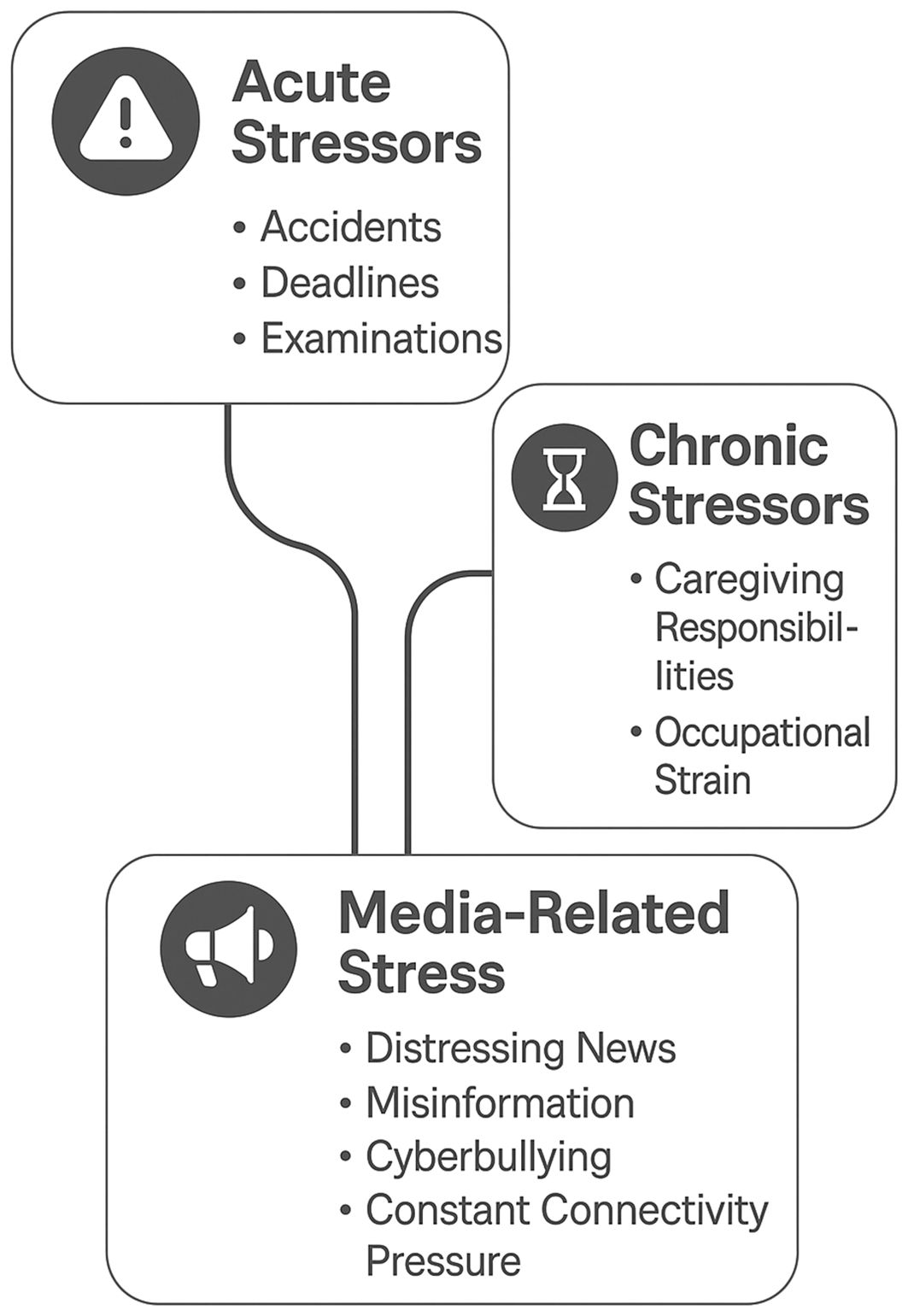
Figure 1. Conceptual model showing acute, chronic, and media-related stressors, with examples and overlaps indicating potential interactions and cumulative effects on neuroimmune function.
Media-induced stress shares features with other chronic symbolic stressors, such as job insecurity, social exclusion, or caregiving burden. These ambiguous, persistent stressors do not involve physical danger, but elicit long-term physiological arousal and immune activation. Including media within this stress category helps contextualize its unique intensity and reach (14). Media-induced stress can be defined as the chronic psychological and physiological arousal resulting from repeated exposure to emotionally charged or threatening digital content— often algorithmically reinforced across digital platforms. Key dimensions include exposure frequency, emotional valence, content type (e.g., violence, health threat), and perceived helplessness (15). This study measured digital stress through self-reported media exposure and emotional response scales, which were correlated with immune assays. It highlights that digital environments may biologically affect immune competence, particularly in young populations.
Recent population studies have linked high media consumption during COVID-19 to elevated IL-6 and CRP levels, poor sleep, and increased autoimmune flares. Digital stress scores correlate with peripheral monocyte activation and impaired Natural Killer (NK) cell cytotoxicity in adolescents and young adults (16). These findings underscore that media exposure is not a neutral behavioral choice, but a potential immunomodulator. In this study, digital stress was assessed via self-reported media exposure, perceived emotional distress, and algorithmic content tracking, which were correlated with blood markers for immune function. Monocyte activity and NK cell assays were performed using peripheral blood samples.
Moreover, emerging data suggest that algorithmically tailored content may worsen immune dysregulation by reinforcing threat perception. Platforms that continuously deliver personalized health fears, geopolitical instability, or climate anxiety contribute to a tonic arousal state, mimicking chronic threat vigilance observed in PTSD (17). This ‘neuroimmune hyperarousal’ may contribute to flare-ups in chronic inflammatory conditions—including neuroimmune disorders such as MS— especially in individuals who are predisposed to or already living with MS.
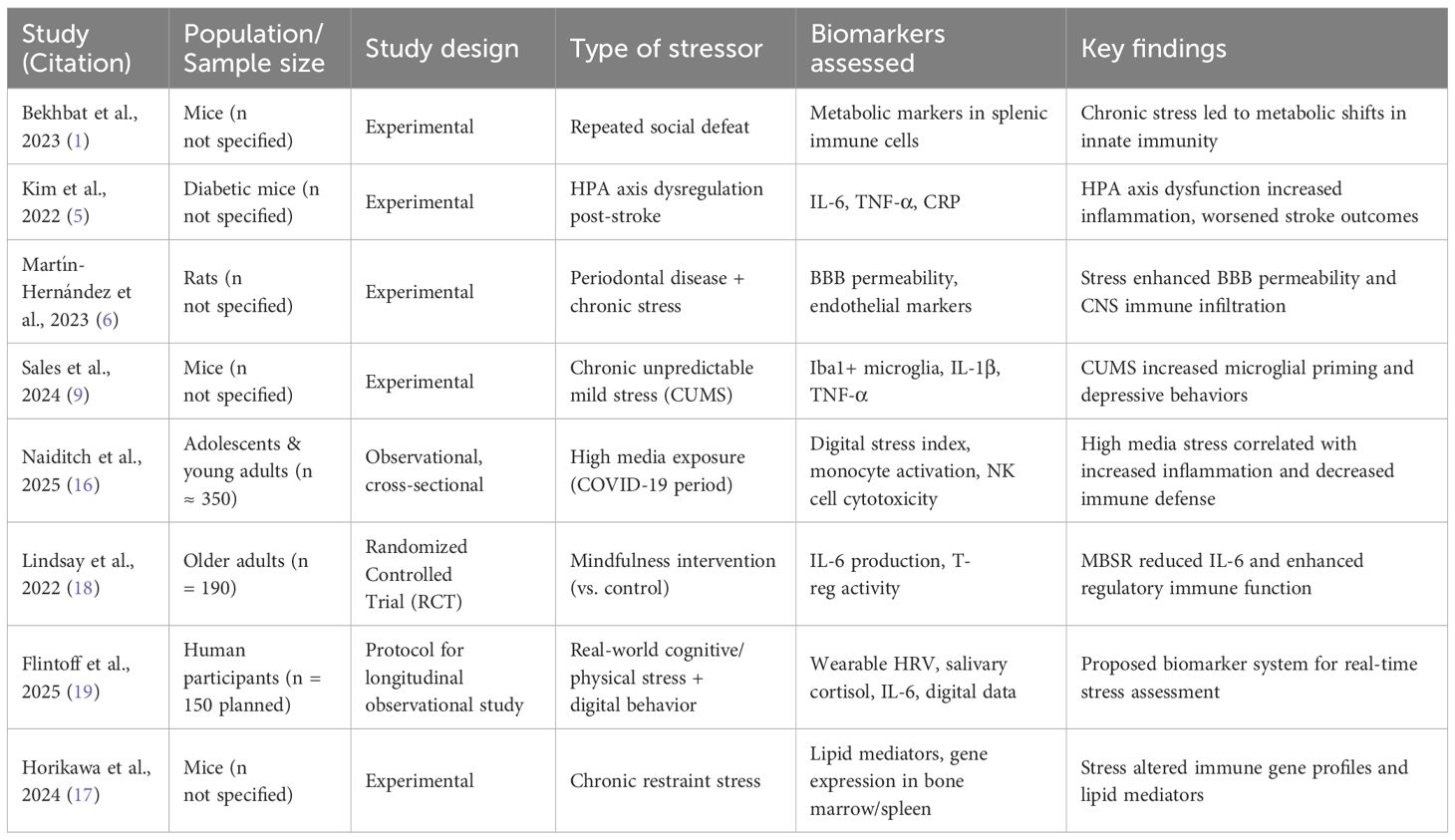
Table 1 provides a summary of representative animal and human studies supporting the link between psychological stressors—including media exposure—and immune dysregulation.
5 Toward a framework for biomarker-guided monitoring
Given the accumulating evidence, it is imperative to develop a translational framework to identify and monitor the immunological footprint of media-induced stress. We propose three promising biomarker domains:
1. Circulating cytokines: Longitudinal monitoring of IL-6, IL-1β, TNF-α, and CRP levels can reveal chronic inflammatory tone.
2. Neuroimaging markers: fMRI and Positron Emission Tomography (PET) imaging of microglial activation (e.g., via translocator protein – TSPO - tracers) can track neuroinflammation in vivo.
3. Neuroendocrine metrics: Cortisol awakening response and diurnal slope can indicate HPA axis dysregulation.
These measures could be combined into a composite “neuroimmune stress index,” capable of stratifying individuals based on physiological reactivity to media stress. Integrating wearable stress sensors and passive digital behavior data (e.g., doomscrolling patterns, screen time analytics) with immune biomarkers may further refine risk prediction models (19). While the concept of a “neuroimmune stress index” is promising, its widespread implementation is limited by cost and invasiveness. In non-clinical settings, scalable alternatives may include salivary cortisol tracking, digital behavior monitoring, and validated stress questionnaires. Future studies should pilot such models in populations with chronic conditions like MS, where immune modulation is clinically relevant (20).
6 Future directions: Toward preventive neuroimmunology
To mitigate the neuroimmunological burden of media-induced stress, several translational pathways can be explored:
● Cognitive immunomodulation: Behavioral interventions such as mindfulness-based stress reduction (MBSR) and cognitive behavioral therapy (CBT) have shown promise in lowering IL-6 and improving regulatory T cell function in stressed individuals (18). Incorporating media hygiene into these protocols—e.g., setting exposure limits or applying algorithmic filters—could optimize outcomes. Examples of media hygiene practices include setting daily screen time limits, muting high-intensity news channels during vulnerable periods, disabling autoplay features, and using content filters to reduce algorithmic amplification of distressing content (21).
● Anti-inflammatory psychopharmacology: Trials using low-dose anti-inflammatory agents (e.g., NSAIDs, minocycline, NLRP3 inhibitors) in depression offer a precedent for targeting inflammation in stress-linked psychiatric disorders. These could be repurposed in high-risk, media-exposed populations pending biomarker validation.
● Precision public health messaging: Immunologists and public health professionals must collaborate with media platforms to develop immune-informed communication strategies—ones that convey risk without triggering excessive alarm, and that reinforce resilience over fear (22).
● AI-assisted immune monitoring: Machine learning models trained on multimodal data (wearable stress signals, social media usage, cytokine trends) can help flag early signs of neuroimmune dysregulation, enabling timely intervention (23).
In addition, physical activity has been shown to exert anti-inflammatory effects and modulate HPA axis function. As a low-cost, accessible intervention, it may buffer against neuroimmune consequences of chronic stress. Integrating media hygiene and physical activity into personalized health strategies could provide effective, non-pharmacological modulation of stress-induced immune activation (24).
7 Conclusion
The immunological impact of media-induced stress represents a neglected frontier in neuroimmunology. In a world where digital interfaces shape psychological states, and where stress is increasingly symbolic and chronic, it is no longer sufficient to regard media as merely behavioral or societal (25). It must be seen as a biological exposure pathway—one capable of modifying immune trajectories across the lifespan.
This article calls for a rethinking of brain–immune crosstalk in light of 21st-century stressors. By integrating biomarker discovery, imaging technologies, and digital behavior analytics, we can illuminate the molecular consequences of mediated anxiety and develop precision interventions. Just as we have begun to stratify traumatic brain injury or cancer immunotherapy using spatial and temporal immune data, so too must we map the invisible toll of digital stress.
The future of immunology will not be immune to media. It must embrace its influence—with rigor, ethics, and translational clarity. This is especially relevant in neuroimmune conditions such as multiple sclerosis, where systemic inflammation and BBB disruption can have disease-modifying consequences.
Author contributions
ST: Conceptualization, Supervision, Visualization, Writing – original draft, Writing – review & editing. KM: Writing – original draft, Writing – review & editing, Investigation, Validation. MV: Writing – original draft, Writing – review & editing. AT: Writing – original draft, Writing – review & editing. AG: Writing – original draft, Writing – review & editing. CJ: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bekhbat M, Drake J, Reed EC, Lauten TH, Natour T, Vladimirov VI, et al. Repeated social defeat stress leads to immunometabolic shifts in innate immune cells of the spleen. Brain Behav Immun - Health. (2023) 34:100690. doi: 10.1016/j.bbih.2023.100690
2. Lebel Y, Milo T, Bar A, Mayo A, and Alon U. Excitable dynamics of flares and relapses in autoimmune diseases. iScience. (2023) 26:108084. doi: 10.1016/j.isci.2023.108084
3. Zhang Y, Dong Y, Zhu Y, Sun D, Wang S, Weng J, et al. Microglia-specific transcriptional repression of interferon-regulated genes after prolonged stress in mice. Neurobiol Stress. (2022) 21:100495. doi: 10.1016/j.ynstr.2022.100495
4. Malta MB, Martins J, Novaes LS, Dos Santos NB, Sita L, Camarini R, et al. Norepinephrine and glucocorticoids modulate chronic unpredictable stress-induced increase in the type 2 CRF and glucocorticoid receptors in brain structures related to the HPA axis activation. Mol Neurobiol. (2021) 58:4871–85. doi: 10.1007/s12035-021-02470-2
5. Kim S, Park ES, Chen PR, and Kim E. Dysregulated hypothalamic–pituitary–adrenal axis is associated with increased inflammation and worse outcomes after ischemic stroke in diabetic mice. Front Immunol. (2022) 13:864858. doi: 10.3389/fimmu.2022.864858
6. Martín-Hernández D, Martínez M, Robledo-Montaña J, Muñoz-López M, Virto L, Ambrosio N, et al. Neuroinflammation related to the blood–brain barrier and sphingosine-1-phosphate in a pre-clinical model of periodontal diseases and depression in rats. J Clin Periodontol. (2023) 50:642–56. doi: 10.1111/jcpe.13780
7. Uher T, McComb M, Galkin S, Srpova B, Oechtering J, Barro C, et al. Neurofilament levels are associated with blood–brain barrier integrity, lymphocyte extravasation, and risk factors following the first demyelinating event in multiple sclerosis. Multiple Sclerosis J. (2021) 27:220–31. doi: 10.1177/1352458520912379
8. Liu X, Ma J, Ding G, Gong Q, Wang Y, Yu H, et al. Microglia Polarization from M1 toward M2 Phenotype Is Promoted by Astragalus Polysaccharides Mediated through Inhibition of miR-155 in Experimental Autoimmune Encephalomyelitis. Oxid Med Cell Longevity. (2021) 2021:5753452. doi: 10.1155/2021/5753452
9. Sales ISL, De Souza AG, Chaves Filho AJM, Sampaio TL, Da Silva DMA, Valentim JT, et al. Antidepressant-like effect of riparin I and riparin II against CUMS-induced neuroinflammation via astrocytes and microglia modulation in mice. Behav Pharmacol. (2024) 35:314–26. doi: 10.1097/FBP.0000000000000788
10. Su Q, Ren Y-H, Liu G-W, Gao Y-P, Zhang J-X, Zhang J-N, et al. Trichostatin A relieves anxiety-and depression-like symptoms in APP/PS1 mice. Front Pharmacol. (2024) 15:1333235. doi: 10.3389/fphar.2024.1333235
11. Distéfano-Gagné F, Bitarafan S, Lacroix S, and Gosselin D. Roles and regulation of microglia activity in multiple sclerosis: Insights from animal models. Nat Rev Neurosci. (2023) 24:397–415. doi: 10.1038/s41583-023-00709-6
12. Teng W, Zhi H, Wang R, and Zhou M. Feeling the pain of others”: examining the framing effects of cancer metaphors on media vicarious traumatization. Health Commun. (2024), 1–9. doi: 10.1080/10410236.2024.2444351
13. Denq B, Denq W, and Hsu W. Stress and its impact on social media usage. J Tech Writing Commun. (2019) 49:232–45. doi: 10.1177/0047281618772076
14. Montgomery RM. Molecular mechanisms of chronic stress in immune dysregulation: from cytokine networks to clinical manifestations. Wired Neurosci. (2024) 1:24–53. doi: 10.62162/WNSC10609.2
15. Kesner L, Juríčková V, Grygarová D, and Horáček J. Impact of media-induced uncertainty on mental health: narrative-based perspective. JMIR Ment Health. (2025) 12:e68640. doi: 10.2196/68640
16. Naiditch H, Betts MR, Larman HB, Levi M, and Rosenberg AZ. Immunologic and inflammatory consequences of SARS-CoV-2 infection and its implications in renal disease. Front Immunol. (2025) 15:1376654. doi: 10.3389/fimmu.2024.1376654
17. Horikawa I, Nagai H, Taniguchi M, Chen G, Shinohara M, Suzuki T, et al. Chronic stress alters lipid mediator profiles associated with immune-related gene expressions and cell compositions in mouse bone marrow and spleen. J Pharmacol Sci. (2024) 154:279–93. doi: 10.1016/j.jphs.2024.02.010
18. Lindsay EK, Creswell JD, Stern HJ, Greco CM, Walko TD, Dutcher JM, et al. Mindfulness-based stress reduction increases stimulated IL-6 production among lonely older adults: A randomized controlled trial. Brain Behav Immun. (2022) 104:6–15. doi: 10.1016/j.bbi.2022.05.001
19. Flintoff JM, Pattinson C, Ahamed S, Ali S, Bagley A, Broszczak D, et al. Predictive biomarkers of performance under stress: A two-phase study protocol to develop a wearable monitoring system. BMJ Open Sport Exercise Med. (2025) 11:e002410. doi: 10.1136/bmjsem-2024-002410
20. Maes M, Almulla AF, You Z, and Zhang Y. Neuroimmune, metabolic and oxidative stress pathways in major depressive disorder. Nat Rev Neurol. (2025) 21:473–489. doi: 10.1038/s41582-025-01116-4
21. Kyianytsia I. INSTRUMENTS FOR PREVENTING MEDIA DEPENDENCY AND FAKE NEWS USING AI. Baltic J Legal Soc Sci. (2024) 3:179–84. doi: 10.30525/2592-8813-2024-3-18
22. Acconito C, Angioletti L, and Balconi M. Impact of public health communication for prevention and personal resilience at the time of crisis. A pilot study with psychophysiological and self-report measures. J Health Psychol. (2025) 30:498–511. doi: 10.1177/13591053241247599
23. Onim Md.SH and Thapliyal H. CASD-OA: context-aware stress detection for older adults with machine learning and cortisol biomarker. Proc Great Lakes Symposium VLSI. (2023) 2023:103–8. doi: 10.1145/3583781.3590218
24. Mueller B, Figueroa A, and Robinson-Papp J. Structural and functional connections between the autonomic nervous system, hypothalamic–pituitary–adrenal axis, and the immune system: A context and time dependent stress response network. Neurol Sci. (2022) 43:951–60. doi: 10.1007/s10072-021-05810-1
Keywords: media-induced stress, neuroinflammation, HPA axis, brain-immune interaction, microglial priming, cytokines, mental health immunology
Citation: Thomas S, Marykutty KM, Vijayakumar M, Thomas A, Gilbert AR and Jose CM (2025) Neuroimmunological impact of media-induced stress: rethinking inflammation and brain-immune crosstalk. Front. Psychiatry 16:1652541. doi: 10.3389/fpsyt.2025.1652541
Received: 24 June 2025; Accepted: 18 August 2025;
Published: 28 August 2025.
Edited by:
Rahul Mallick, University of Eastern Finland, FinlandReviewed by:
Laura Bellingacci, University of Perugia, ItalyInês Pereira- Figueiredo, ThekidsFellows, Portugal
Mariya Ivanovska, Plovdiv Medical University, Bulgaria
Copyright © 2025 Thomas, Marykutty, Vijayakumar, Thomas, Gilbert and Jose. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sobi Thomas, ZnIuc29iaUBtYXJpYW5jb2xsZWdlLm9yZw==
 Sobi Thomas
Sobi Thomas K. M. Marykutty3
K. M. Marykutty3 Anson Thomas
Anson Thomas