- 1Center for Clinical Psychiatry, Department of Neurosciences, KU Leuven, Leuven, Belgium
- 2Brain and Cognition, Laboratory for Biological Psychology, KU Leuven, Leuven, Belgium
- 3School of Psychology, Shandong Normal University, Ji’an, China
- 4Integrated Psychiatric Center Openbaar Psychiatrisch Zorgcentrum (OPZ), Geel, Belgium
- 5University Psychiatric Center, KU Leuven, Leuven-Kortenberg, Belgium
- 6Adapted Physical Activity and Psychomotor Rehabilitation, Department of Rehabilitation Sciences, KU Leuven, Leuven, Belgium
Background: Adolescence and early adulthood represent critical periods for the emergence of psychiatric symptoms, often spanning multiple symptom dimensions. Alterations in fear learning and generalization are implicated in anxiety-related disorders, yet research on these processes in youth with early-stage transdiagnostic psychiatric symptoms remains limited.
Methods: This study investigated fear learning and generalization in youth aged 16–24 years with transdiagnostic psychiatric symptoms (anxiety, depressive, and psychotic), as indexed by US expectancy ratings. Additionally, considering the modulatory impact of exercise on memory processes, we explored the effects of a 10-minute moderate-intensity exercise intervention using a randomized between-subject design.
Results: Contrary to hypotheses, the symptom group did not show impaired threat–safety discrimination or overgeneralization of fear. However, they exhibited elevated overall threat expectancy during generalization, suggesting that a bias in threat expectancy could represent an early vulnerability in threat processing. Dimensional analyses point to subtle symptom-specific differences in generalization patterns, underscoring the importance of modeling continuous symptom severity alongside group-based comparisons. No significant effects of acute exercise on fear acquisition or generalization were observed.
Conclusion: These findings highlight early alterations in threat processing in youth with early-stage psychiatric symptoms. Future research should investigate symptom-specific patterns in fear generalization, track their longitudinal development, and refine exercise interventions to effectively modulate fear processing.
1 Introduction
Mental health disorders typically arise in the context of complex genetic and environmental factors and are marked by multiple alterations at the level of cognitive processing. Among these are alterations in threat processing, such as fear learning and fear generalization (1). Fear learning, typically examined using a classical conditioning task, involves pairing a neutral stimulus with an aversive unconditioned stimulus (US) to create a conditioned stimulus (CS+) that signals danger, while another stimulus that is never paired with the US becomes the safety cue (CS-). Fear generalization occurs when fear extends from a conditioned stimulus to similar cues (2), which can be assessed by presenting generalization stimuli (GSs) that vary in similarity to the CS+. While this process is essential for survival, it can become maladaptive when fear overgeneralizes to similar neutral cues, potentially contributing to anxiety disorders and other forms of psychopathology (3).
Mental health symptoms often first emerge during adolescence and early adulthood (4). This period is characterized by early-stage, mixed symptom presentations that may not meet full diagnostic criteria (5), yet can signal increased risk for later psychopathology. Despite its clinical relevance, research on fear learning and generalization has left this important developmental window understudied. Investigating altered threat processing in this population is crucial for further understanding relevant mechanisms underlying the emergence of mental health disorders.
Previous research on threat processing has primarily focused on anxiety and stress-related disorders, such as generalized anxiety disorder and social anxiety disorder, where increased fear acquisition and overgeneralization of fear to ambiguous stimuli have been consistently demonstrated (2, 3, 6–8). Importantly, even at the subclinical level, anxiety symptoms have been linked to altered threat processing, with impaired discrimination and heightened generalization predicting more anxiety over time (9), and fear generalization correlating with anxious personality (10). However, alterations in threat processing may not be exclusive to the anxiety spectrum. For instance, reduced CS discrimination without clear generalization effects has been observed in individuals with major depressive disorder (11), while overgeneralization of fear has been associated with anhedonia, a core symptom of depression (12). Moreover, both depressive and psychotic symptoms have been linked to an attentional bias toward interpreting neutral stimuli as threatening (1), underscoring the potential transdiagnostic relevance of aberrant threat processing.
Early-stage psychiatric symptoms in young people represent a major contributor to health-related disability (13), underscoring the urgent need for accessible early intervention strategies. Exercise has gained increasing attention given its broad cognitive benefits and potential to influence affective learning processes (14, 15). Most exercise studies have focused on fear conditioning or fear extinction, but fear generalization has remained largely unexamined. Acute bouts of exercise can modulate neurobiological systems implicated in fear learning, including neurotransmitter function, HPA axis activity, and hippocampus-dependent memory processes (16, 17). Given the critical role of the hippocampus in regulating fear generalization (18), exercise may offer a promising means of modulating this process.
In this study, we used a classical fear conditioning paradigm to investigate fear learning and generalization in youth aged 16–24. To capture the heterogeneity and pluripotent nature typical of early-stage psychiatric symptom presentation (19, 20), we adopted a transdiagnostic approach focused on depressive, anxiety, and psychotic symptom dimensions rather than categorical diagnoses. We combined a group-level comparison with a dimensional analysis. First, a group with early-stage transdiagnostic symptoms was compared to a healthy control group. It was hypothesized that the symptom group would exhibit reduced threat-safety discrimination during acquisition, particularly reflected by increased fear responding to the safety cue, and increased fear generalization, defined as transfer of fear to the similar stimuli with trial-by-trial US expectancy ratings as our main outcome measure. Within the symptom group, a dimensional analysis was subsequently conducted to examine whether specific alterations in threat processing are related to individual symptom dimensions. This approach acknowledges the clinical relevance of identifying early-stage group differences while also aligning with recent advances towards dimensional models of psychopathology, which suggest that fear learning alterations vary continuously with symptom severity, rather than categorically (21). We hypothesized that anxiety symptoms would show the strongest association with alterations in threat processing, while depressive and psychotic symptom dimensions would show more subtle or distinct patterns of association.
Additionally, we explored whether a 10-minute bout of moderate-intensity exercise could influence fear acquisition and generalization, using a randomized controlled between-subject design. Exercise is well known to benefit mental health (22, 23) and can modulate fear memory processes (15). To date, most human research has focused on acute exercise in the context of fear extinction, generally enhancing consolidation and recall of extinction memories (24), and pointing to reduced threat expectancies following reinstatement (25). To our knowledge, no studies have directly examined whether acute exercise modulates fear generalization, despite its relevance to psychopathology (1, 3, 12). Based on exercise’s general memory enhancing effects, we hypothesized that acute exercise would improve fear learning, with stronger threat-safety discrimination and reduced fear generalization compared to the resting control condition.
2 Materials and methods
2.1 Participants and study design
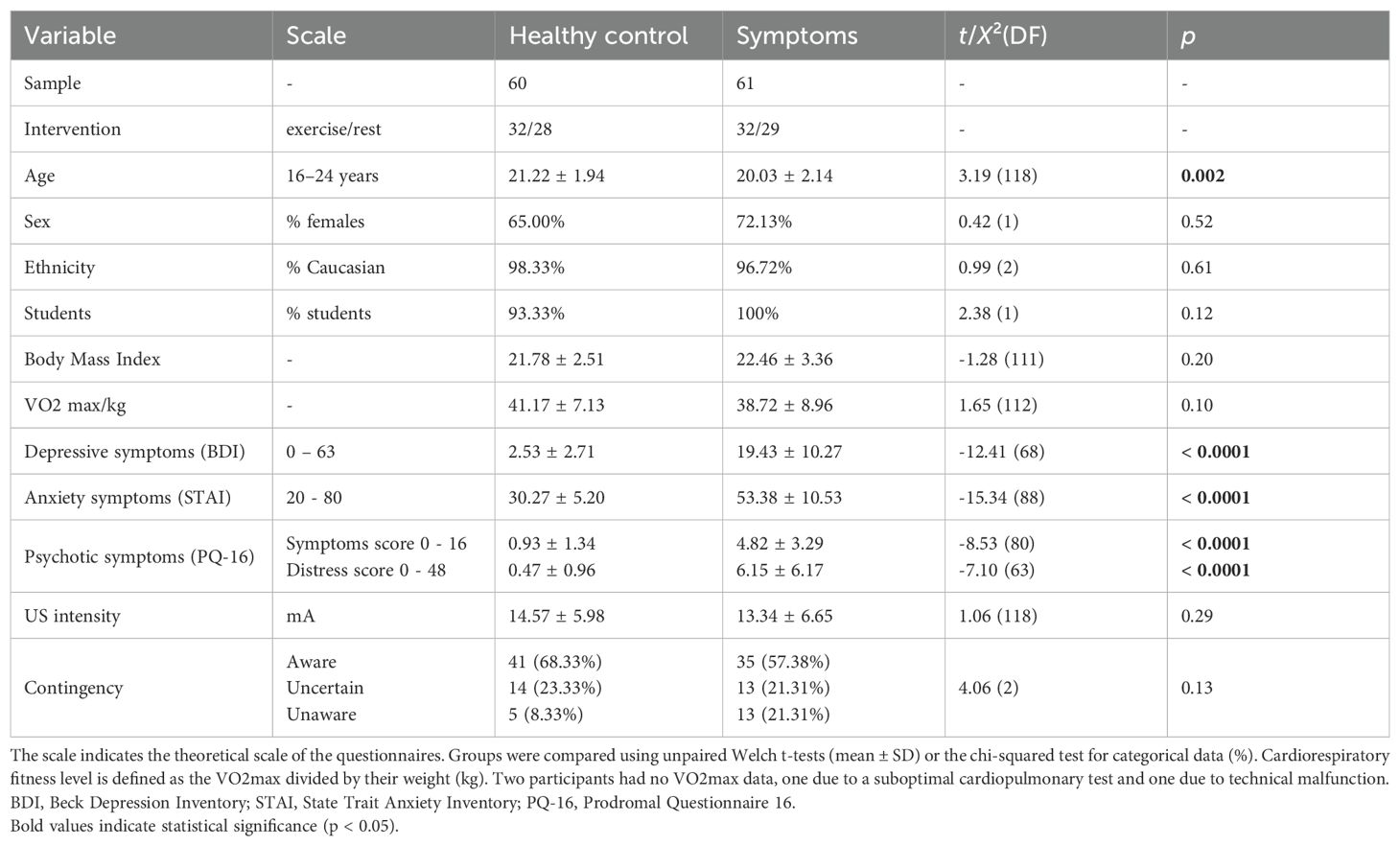
Adolescents and young adults aged 16–24 were recruited from the general population and mental health services. After a general screening (exclusion of self-reported major medical and neurological illness, current substance use, current psychiatric medication, autism spectrum disorder, intellectual disability and body mass index > 30), the presence of depressive, anxiety and psychotic symptoms was assessed for group allocation. The following pre-defined cut-offs were selected to capture both subclinical and early clinical symptom levels, consistent with prior studies: ≥ 11 on the Beck Depression Inventory (BDI) (26–28), ≥ 40 on the State Trait Anxiety Inventory (STAI) (29, 30), and ≥ 5 positive answers or ≥ 8 distress score on the Prodromal Questionnaire-16 (PQ-16) (31). Given that mental health symptoms in youth often span multiple symptom dimensions (5), we adopted a transdiagnostic approach to better capture the this early-stage symptom expression. Participants were allocated to the symptom group if they scored above the cut-offs for at least two symptom dimensions. Participants in the healthy control group scored below all cut-offs. The presence of childhood adversity was also assessed using a modified version of the Juvenile Victimization questionnaire (JVQ) (Supplementary Material).
The study consisted of two sessions. In session 1, participants performed a maximal cardiopulmonary exercise test (CPET, protocol in Supplementary Material) to measure the maximal oxygen consumption (VO2max). In addition, participants completed a reassessment of psychiatric symptoms to obtain recent scores. Session 2 took place within 10 days of session 1. In session 2, participants first completed either the acute exercise intervention or rest condition, which was immediately followed by the fear conditioning task. The study was approved by the Local Ethical Committee UZ/KU Leuven (S62702) and performed according to the Declaration of Helsinki. Participants or one of their legal guardians in case of minors provided written informed consent. Participants received monetary compensation for their participation.
2.2 Acute exercise intervention
Participants were randomized to either 10 minutes of moderate-intensity cycling (Kettler C8) or rest sitting on the ergometer, using RedCap (32) with permuted block randomization and stratified according to the presence of psychotic symptoms. The individual moderate-intensity exercise level was defined as 50% of their VO2max. Participants wore a Polar H10 heart rate monitor and the cycling load was adjusted to maintain heart rates between 45 to 60% of their VO2max. The mean intervention-to-task interval was 9min18s (SD = 3min19sec).
2.3 Fear conditioning paradigm
Fear learning and generalization were measured using a classical fear conditioning task programmed in Python (PsychoPy package) (33, 34). The task uses 10 rings of gradually increasing size (15% difference, diameter from 2 to 4.70 inches) (adapted from Lissek et al., 2008) (Figure 1). The third and eighth circles served as the conditioned stimuli, counterbalanced between participants to be the CS- or CS+ (condition 0 or 1), which allows investigation of both danger and safety learning, as well as generalization effects on both sides. A mild electrical shock was used as the unconditioned stimulus (US) and administered to the non-dominant wrist (DS7A electrical stimulator, Digitimer, Welwyn Garden City, UK). Before the experiment, the intensity of the US was individually calibrated. An initial brief 100 ms electrical shock of 2 mA was given and gradually increased with 2 mA. After each shock, participants reported the perceived intensity on a 5-point Likert scale. The final intensity was defined as ‘unpleasant, but not painful’ corresponding to a score of 4 out of 5, or the predetermined maximum current of 24 mA.
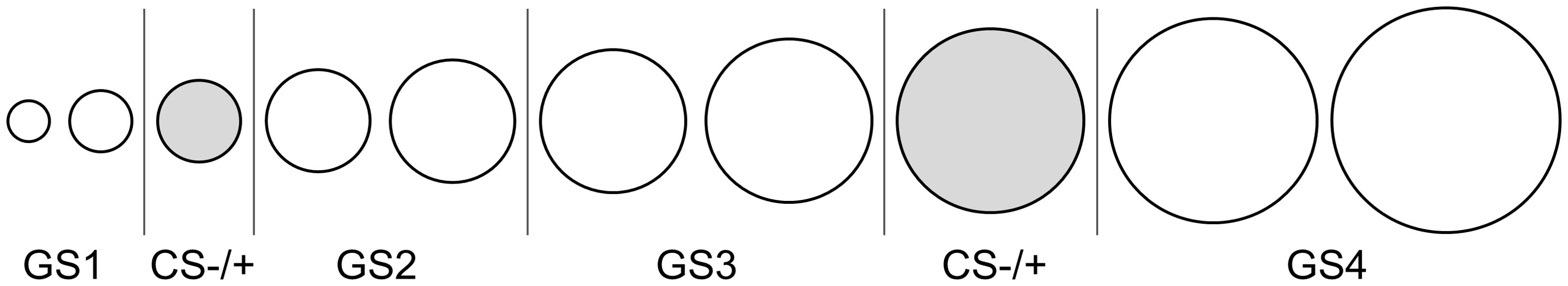
Figure 1. Conditioning and generalization stimuli. The third and eighth circles were counterbalanced to be the CS- or CS+. During acquisition, only the CS- and CS+ were shown. During generalization, all 10 circles were shown.
The task consisted of three phases (habituation, acquisition and generalization), during which circles were presented on a computer monitor (27-inch). Participants were instructed to learn to predict when they would receive an electrical shock. Fear learning was also assessed physiologically with electromyography (EMG), where a startle probe (40 ms, 95 dB, near-instantaneous rise time) was given, to evoke a fear-potentiated startle response, during half of the trials and a quarter of the intertrial intervals. Unfortunately, due to a technical malfunction in the trigger system, the recorded data could not be reliably analyzed and were therefore excluded. For every trial, participants were shown a fixation cross in the middle of the screen (1 sec), followed by a stimulus presentation (8 sec). Two seconds after the stimulus appeared, a rating scale was shown. Participants indicated their expectancy for receiving the US following that circle on a 10-point Likert scale (1 = definitely no shock, 10 = definitely a shock). The scale disappeared when an answer was given or at stimulus offset in case of a non-response. The intertrial interval was 2.2 sec or 3.2 sec for trials with an intertrial startle probe. During the habituation phase, the CS+ and CS- were shown each four times without the US. Secondly, during acquisition, CS+ and CS- were shown eight times each, with the CS+ paired with a shock at 75% reinforcement rate. In the generalization phase, the CS+ and CS- were shown eight times each, with in addition each of the GSs shown four times. CS+ reinforcement rate was 50%. All trials were semi-randomized with maximally two identical trials shown in a row and clustered within four blocks of 12 trials (2 CS+, 2 CS- and 1 of every GS). Afterwards, participants were asked about their CS-US contingency awareness. Their responses were classified as aware, uncertain or unaware by two researchers. Finally, participants completed post-experimental questions on US expectancy, valence, fear, and arousal level for all the CSs and GSs. A 10-point Likert scale was used to assess US expectancy and fear, while a self-assessment manikin (SAM) scale was used for valence and arousal (35).
2.4 Perceptual discrimination paradigm
To control for differences in perceptual discrimination ability (36), participants completed a perceptual discrimination task. This was completed after the fear conditioning task to ensure that the exercise manipulation was always performed directly before the fear conditioning. Participants were presented with two sequential circles and asked to indicate whether these were identical or different. During each trial, a fixation cross was presented in the center of the screen (2 sec), then the first circle (1 sec), followed by a blank screen (2 sec) and the second circle (1 sec). The task consisted of 10 pairs of identical circles, four pairs of CS- and CS+, and 32 pairs comparing the GSs to the CS- and CS+.
2.5 Statistical analysis
Statistical analyses were conducted in RStudio (version 4.1.2) (37). Data were analyzed with linear multilevel models with a random intercept (R package lmerTest, version 3.1.3) (38). Post hoc tests were performed using the R package emmeans (version 1.8.2) with FDR correction. Three participants (one healthy control, two with symptoms) were excluded from analyses (two due to pressing wrong buttons, one due to technical error). For both the habituation and generalization phase, one extra participant from the symptom group was excluded due to a technical error. The GSs were clustered into four categories (GS1 – GS4) (39). The a priori defined covariates age, sex and VO2max, and their interactions with stimulus were included in all models. Primary analyses focused on US expectancy ratings as outcomes. Although physiological fear-potentiated startle data were collected, a technical malfunction resulted in substantial data loss, preventing reliable analysis.
Primary analyses focused on US expectancy as an outcome. Separate linear multilevel models were fitted for each phase of the task and included the fixed effects group, intervention, stimulus, trial, condition and contingency, as well as the interactions group x stimulus, intervention x stimulus and contingency awareness x stimulus. Details on the habituation phase can be found in Supplementary Material. In the acquisition model, the interaction trial x stimulus was additionally included to account for the learning effect. To investigate the shape of the generalization gradient, we conducted a trend analysis using a linear multilevel model including variables to test for linear, quadratic and cubic trends across stimuli. Besides group differences, we conducted dimensional analyses to examine how continuous variation in symptom severity was associated with threat-safety discrimination and fear generalization. These analyses were restricted to the symptom group, as the healthy control group was selected to have low levels of psychiatric symptoms and childhood adversity, resulting in limited variability. In these models, the group variable was replaced with continuous scores for depressive (BDI), anxiety (STAI), and psychotic (PQ-16) symptoms. Additionally, we accounted for childhood adversity by including the interaction stimulus x childhood adversity severity, which was derived from the Juvenile Victimization Questionnaire.
Perceptual discrimination accuracy was assessed using linear regression models with general perceptual discrimination accuracy and accuracy per stimulus type (CSs and GSs) as outcome variables. Independent variables included group, intervention, contingency, and a priori defined covariates (age, sex and VO2max). The influence of perceptual discrimination on generalization was assessed by adding mean CS+ discrimination and general accuracy, both interacted with stimulus, to the generalization model.
Participant’s response times and post-experimental ratings were evaluated in separate models (Supplementary Material). A sensitivity analysis for US expectancy was conducted excluding 18 participants who did not reach the threshold for the electrical shock before reaching the maximum amount (Supplementary Material).
3 Results
3.1 Sample
A total of 124 adolescents and young adults took part in the study, of whom 121 (60 healthy controls and 61 with symptoms) could be included for analysis (Table 1). The symptom group was on average slightly younger (t(118) = 3.19, p = 0.002). Most participants in the symptom group scored above the cut-offs on all three symptom dimensions or had a combination of anxiety and depressive symptoms (Supplementary Table 1). The selected US intensity was on average 13.95 mA (SD = 6.33). The majority of participants were aware of the CS-US contingency (62.81% aware, 22.31% uncertain, 14.88% unaware) and there was no significant group difference (t(2) = 4.06, p = 0.13). The sample characteristics of the intervention groups can be found in Supplementary Table 2.
3.2 US expectancy
3.2.1 Acquisition
In acquisition, we observed significant interactions between stimulus x group (β = -0.52, SE = 0.19, p = 0.007) and stimulus x intervention (β = -0.52, SE = 0.29, p = 0.005). We observed adequate threat-safety discrimination with significantly lower US expectancy values for the CS- compared to the CS+ across groups and interventions (all p < 0.001), but no significant differences between the groups or interventions (Figure 2A). The trial x stimulus interaction was significant (β = 0.33, SE = 0.02, p < 0.0001), showing a learning effect (Figure 2B). Furthermore, older participants gave significantly lower US expectancy ratings to the CS- (β = 0.27, SE = 0.10, p = 0.005, 95% CI [-0.44, -0.08]), but not for the CS+. We observed a significant interaction of stimulus with VO2max (β = 0.20, SE = 0.09, p = 0.03), but this effect did not survive FDR-corrected post hoc tests ([-0.38, 0.03]).
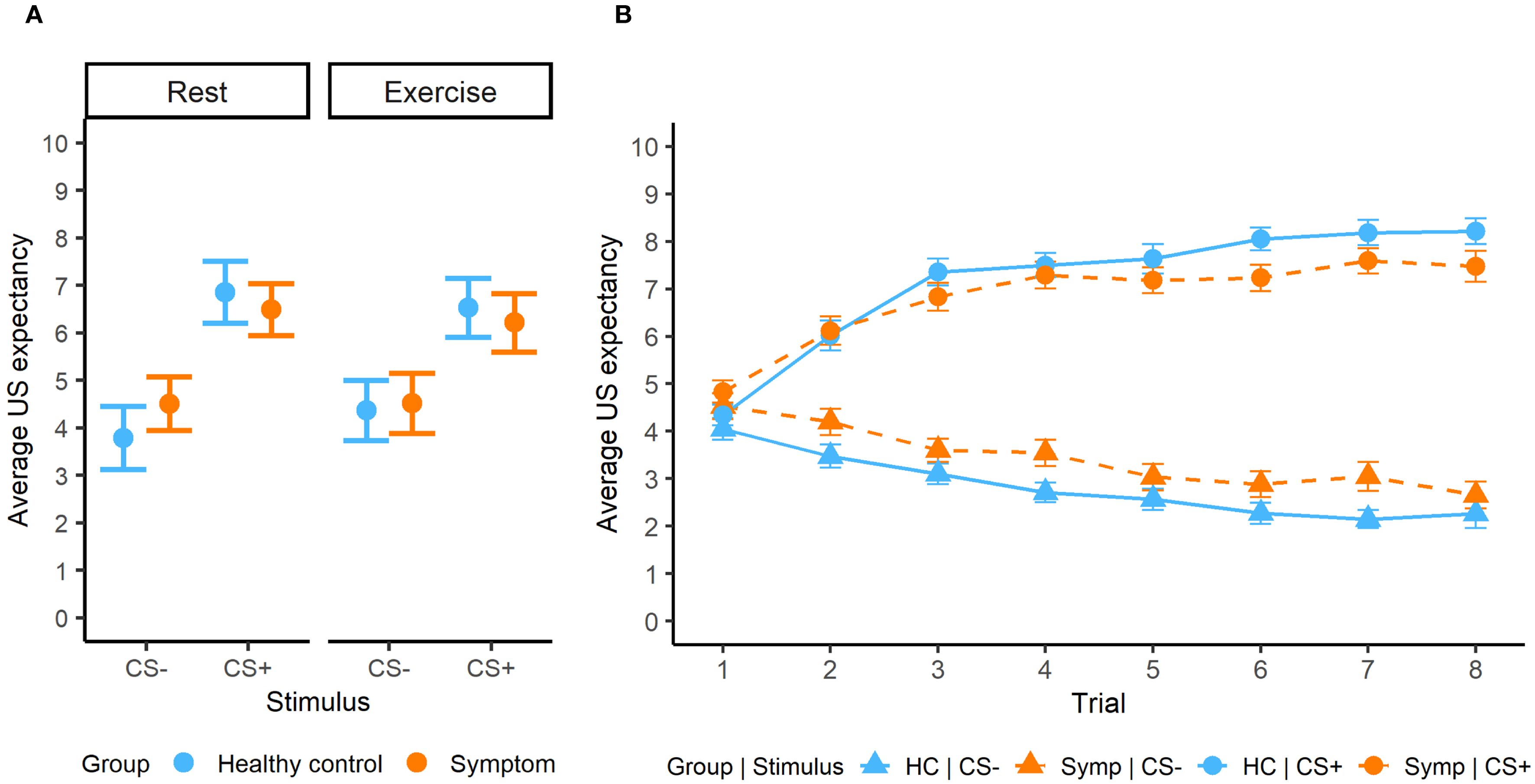
Figure 2. (A) Average US expectancy ratings during the acquisition phase for both groups and interventions. (B) Learning effect during acquisition phase.
While no significant associations with continuous psychiatric symptom scores could be detected in the dimensional analysis, we observed a significant stimulus-dependent association with childhood adversity (β = 0.13, SE = 0.05, p = 0.02), with increased expectancy for the CS+ in association with increased adversity severity ([0.01, 0.20]). The slope was not significant for the CS- ([-0.11, 0.07]).
3.2.2 Generalization
During generalization, a significant interaction between group and stimulus was observed (F5,5454 = 3.55, p = 0.003). Post hoc tests showed significantly higher US expectancy ratings in the symptom group compared to the healthy control group for the GS1 (β = -0.64, SE = 0.26, p = 0.01), CS+ (β = -1.06, SE = 0.26, p = 0.0001), GS2 (β = -0.68, SE = 0.26, p = 0.01) and CS- (β = -0.52, SE = 0.26, p = 0.048) (Figure 3). While there was an interaction between intervention and stimulus (p = 0.02), post hoc tests were not significant. Generalization results were not influenced by differences in perceptual discrimination ability (Supplementary Material). Trend analysis showed a cubic trend for stimulus (β = -3.35, SE = 0.46, p < 0.0001), which did not differ between the groups, indicating a similar generalization gradient.
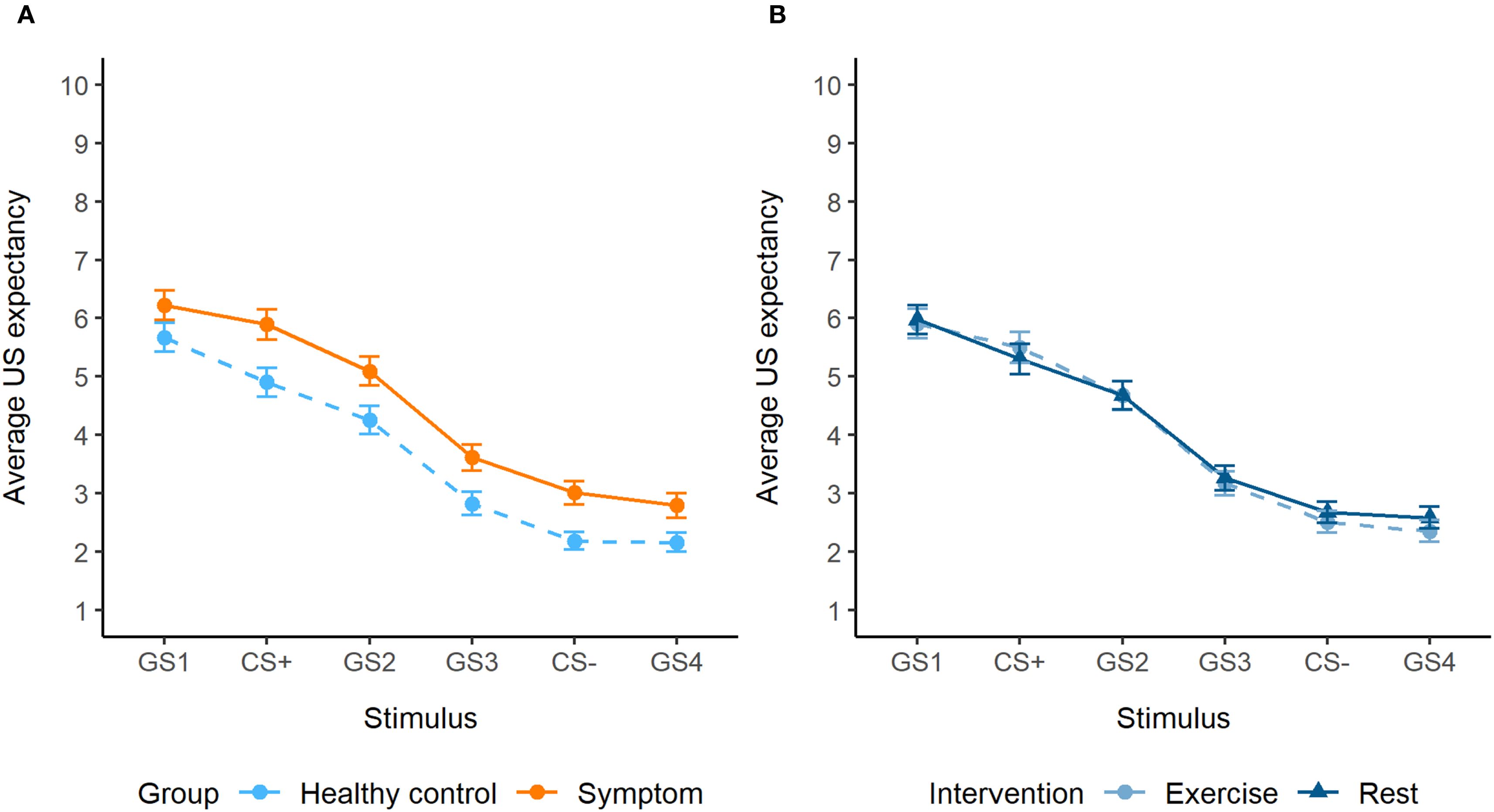
Figure 3. (A) Generalization gradients of the symptom group and healthy control group, with overall increased expectancy ratings in the symptom group (significant for the GS1, CS+, GS2 and CS-). (B) Generalization gradients of the exercise and rest intervention, showing no significant differences.
Furthermore, older participants displayed lower US expectancy values for GS3 (95% CI [-0.99, -0.19]) and GS4 ([-0.84, -0.04]). There was a stimulus-dependent effect of sex (F5,5454 = 5.15, p < 0.0001), but this did not remain significant in post hoc tests. Overall, US expectancy decreased across trials (β = -0.033, SE = 0.002, p < 0.0001).
The dimensional analysis showed significant positive, stimulus-dependent associations of US expectancy with depressive (F5,2697 = 3.64, p = 0.003), anxiety (F5,2697 = 8.12, p < 0.0001) and psychotic symptoms (F5,2697 = 3.06, p = 0.009), but not with childhood adversity (p = 0.2). Post hoc tests detected significant positive slopes for the GS1 ([0.006, 0.13]) and GS3 ([0.003, 0.07]) in association with depressive symptoms, and for the GS2 ([0.001, 0.28]) in association with psychotic symptoms. The slopes in association with anxiety symptoms were not significantly different from zero. For visualization purposes, we plotted US expectancy as a function of high and low symptom scores based on a median split (Figure 4). This categorical grouping was not part of the statistical analysis plan and should be interpreted as exploratory, serving only to aid visual interpretation of the generalization patterns. The plots suggest that higher anxiety and psychotic symptoms are particularly associated with a reduced peak at GS1 compared to lower symptom levels. In contrast, higher depressive symptoms were primarily associated with elevated US expectancy on the safety side of the gradient.
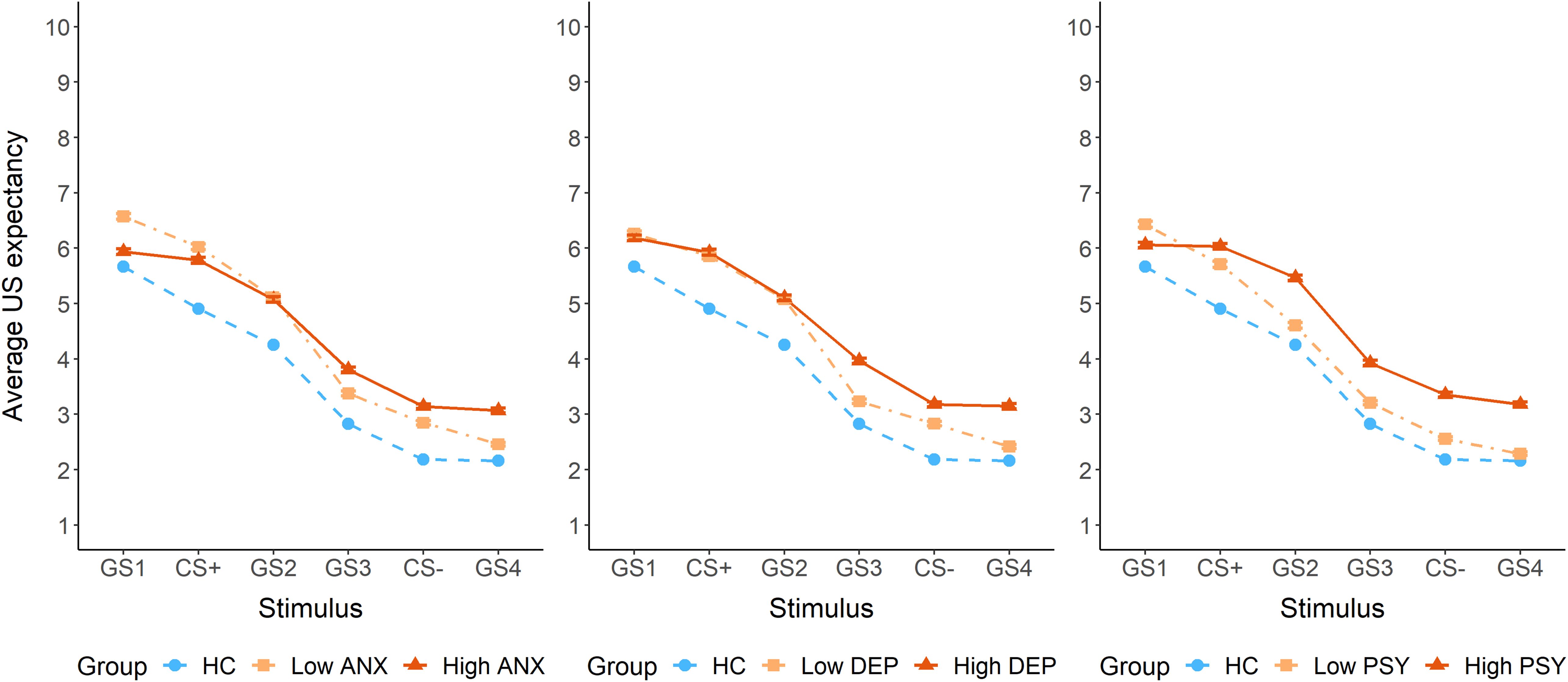
Figure 4. US expectancy ratings during the generalization phase, plotted separately for high and low symptom groups based on a median split for anxiety symptoms (State Trait Anxiety Inventory – STAI), depressive symptoms (Beck Depression Inventory – BDI), and psychotic symptoms (Prodromal Questionnaire – PQ-16). ANX = anxiety symptoms, DEP = depressive symptoms, PSY = psychotic symptoms.
3.2.3 Contingency awareness
During acquisition, a significant interaction between contingency awareness and stimulus was found (F2,1745 = 32.78, p < 0.0001), with less extreme values for CS- and CS+ in the unaware participants compared to the uncertain and aware participants (all p < 0.01), but no significant difference between uncertain and aware participants (Figure 5A). Nevertheless, the unaware participants acquired threat-safety learning with significantly different US expectancy ratings for the CS+ and CS- (β = -2.09, SE = 0.25, p < 0.0001). Also in the generalization phase there was a significant stimulus-dependent effect of contingency awareness (F10,5454 = 8.72, p < 0.0001), with significant differences for the GS3 (unaware vs aware β = 0.89, SE = 0.36, p = 0.047), the CS- (unaware vs uncertain β = 1.38, SE = 0.42, p = 0.003; unaware vs aware β = 1.12, SE = 0.36, p = 0.003) and the GS4 (unaware vs uncertain β = 1.68, SE = 0.42, p = 0.0001; unaware vs aware β = 1.48, SE = 0.36, p = 0.0001) (Figure 5B). A sensitivity analysis was conducted excluding the unaware participants (n = 18), but this did not affect the results (Supplementary Material).

Figure 5. (A) Average US expectancy ratings during the acquisition phase for each contingency awareness group (B) Generalization gradient for each contingency awareness group.
4 Discussion
4.1 Increased overall fear instead of overgeneralization in the symptom group
Adequate threat-safety discrimination was observed during acquisition across groups, based on subjective US expectancy ratings. Although the symptom group showed slightly less distinct US expectancy values (e.g., higher for CS−, lower for CS+), these differences were not statistically significant, suggesting intact threat-safety learning. Both groups exhibited similar generalization gradients, and we found no evidence of fear overgeneralization. Controlling for perceptual discrimination ability did not change these results.
These findings contrast with our hypotheses and prior literature linking anxiety and stress-related disorders to increased fear to safety cues and overgeneralization of fear (3, 6, 8). Several factors may explain why we did not observe the expected group differences. First, our sample comprised youth with subclinical or early-stage psychiatric symptoms rather than a clinical population; stronger effects may emerge in individuals with more severe symptomatology. Nevertheless, fear generalization has been proposed as a dimensional phenotype (3), and a meta-analysis showed increased generalization even in healthy individuals high in anxious traits (10). Reduced threat-safety discrimination has similarly been linked to elevated trait anxiety (40), and longitudinal work suggests that impaired CS-/CS+ discrimination can predict future anxiety (9). However, findings are mixed; other studies have failed to observe associations between trait anxiety and either acquisition or generalization processes (41, 42), underscoring the complexity of these processes. Notably, anxiety-related increases in threat responding may reflect differences in intolerance of uncertainty, which may shape threat-safety discrimination as shown in prior work using skin conductance (43).
Another consideration is the heterogeneity of our transdiagnostic sample, which included young people with anxiety, depressive and psychotic symptoms. While reduced discrimination and fear overgeneralization have been most consistently linked to anxiety, aberrant threat processing may not be exclusive to anxiety symptomatology. For instance, enhanced generalization was observed in youth with subclinical depressive and psychotic symptoms, but only in those with high levels of childhood maltreatment (1). It is therefore possible that the symptom overlap, although typical at this developmental stage, may have obscured more specific alterations in threat processing at the group level.
Importantly, the symptom group displayed overall elevated US expectancy ratings (significant for GS1, CS+, GS2, and CS-) compared to the healthy controls during generalization. This occurred despite intact discrimination abilities during acquisition, suggesting a cognitive-affective bias in interpreting ambiguous stimuli as threatening rather than a learning impairment. This points to a general threat expectancy bias in the symptom group, aligning with prior work linking a cognitive threat bias to not only anxiety, but depression and psychosis as well (1). Notably, this increased US expectancy emerged only during generalization and not during acquisition. The generalization phase introduces greater ambiguity by means of a new context, novel GSs and a lower reinforcement rate, reducing predictability and increasing uncertainty. This may be particularly distressing for youth with mental health symptoms due to heightened intolerance of uncertainty, leading to amplified fear. This pattern underscores the importance of also considering overall levels of fear responding, as this may reveal early threat-related biases not captured by gradient shape alone. Indeed, Stegmann et al. emphasized the importance of examining mean fear responses alongside generalization gradients (44), and a recent study observed similar overall increased US expectancy ratings in adolescents with anxiety disorders (45).
Although more research is needed, elevated US expectancy may represent an early-stage vulnerability marker in threat processing during the transitional period between late adolescence and young adulthood, possibly preceding more specific alterations in discrimination or generalization. While our transdiagnostic approach fits with the characteristics of our population, its heterogeneity may mask similarities or distinctions in threat processing patterns tied to individual symptom dimensions – a question we explore further in our dimensional analysis.
4.2 Symptom-specific alterations in fear generalization patterns
The dimensional analysis examined whether specific psychiatric symptoms were differentially associated with alterations in fear learning and generalization patterns. Although no significant associations emerged during acquisition, anxiety, depressive and psychotic symptoms significantly influenced US expectancy during generalization. Post hoc analyses showed increased US expectancy only for GS1 and GS3 in association with depressive symptoms, and for GS2 in association with psychotic symptoms. While statistical effects were modest, likely reflecting the smaller sample size of the symptom group and applied FDR correction, these analyses suggest subtle symptom-specific variations in US expectancy during generalization.
Notably, childhood adversity was unrelated to US expectancy during generalization but was associated with heightened CS+ responding during acquisition. This finding contrasts with the majority of previous literature finding reduced threat-safety discrimination in individuals with childhood adversity (46). Although not the focus of this study, this distinction does suggest that adversity may primarily impact initial fear learning, whereas psychiatric symptoms may affect how threat generalizes.
To explore symptom-specific generalization patterns, we visually examined median-split plots. Importantly, these exploratory visualizations were not part of the main statistical analysis and should be interpreted cautiously. For anxiety and psychotic symptoms, we observed increased US expectancy on the safety side, alongside decreased discrimination between the CS+ and GS1 (i.e. perceptually most extreme but unreinforced stimulus). While healthy controls displayed an extremity bias or peak shift, with highest expectancy at GS1, this pattern was lost in those with high anxiety and psychotic symptoms. This loss of differentiation may reflect a diminished capacity to discriminate subtle ambiguities. Alternatively, intolerance of uncertainty may prompt avoidance of nuanced threat evaluation, resulting in a more uniform response across stimuli (47, 48). Depressive symptoms appeared to affect only the safety side of the gradient, with higher symptom severity linked to increased US expectancy for safety cues. This pattern may reflect impairments in reward processing, leading to a pessimistic bias or deficits in fear inhibition. This aligns with prior work showing increased US expectancy of safe stimuli in young people with greater anhedonia-apprehension (12).
Together, these findings suggest that elevated US expectancy and altered generalization gradients may reflect distinct processes. While group comparisons capture broad vulnerability, dimensional analyses point to symptom-specific variations that may signal early differentiation within a shared risk profile. However, statistical effects were limited, and interpretation should be cautious due to the reduced power from sample size and stratification. Exploratory visual trends nonetheless suggest symptom-related differences that merit further investigation. Future studies with larger samples could apply more sensitive analytical techniques (e.g., multivariate pattern analysis, latent profile modelling) to better characterize these patterns, which could possibly inform early detection and targeted interventions.
4.3 No acute exercise effects on fear acquisition or generalization
While prior studies have primarily examined exercise effects on fear conditioning and extinction, this study is, to our knowledge, the first to investigate whether acute exercise influences fear generalization. Contrary to our hypotheses, the intervention did not affect threat–safety learning or fear generalization, based on subjective US expectancy ratings, in either healthy individuals or those with elevated psychiatric symptoms. Although animal studies link acute exercise to memory enhancement (15, 49, 50), humans studies have mostly focused on extinction paradigms, where exercise post-extinction reduced fear (25, 51, 52). Fear generalization, however, depends not only on memory strength but also on the specificity of learned threat associations. Interestingly, a recent study found that 20 minutes of vigorous exercise after extinction enhanced generalization to perceptually similar stimuli (53). Exercise might enhance the salience or consolidation of the original threat memory, resulting in more generalization. Our null findings may reflect that the 10-minute moderate-intensity intervention was insufficient in duration or intensity (54). Future research should investigate how exercise duration, intensity and timing affect fear generalization.
4.4 Decreased threat-safety discrimination and overgeneralization of fear in contingency unaware participants
Participants unaware of the CS–US contingency showed significantly reduced threat–safety discrimination compared to uncertain and aware participants. This likely contributed to their flatter generalization gradient, characterized by significantly higher US expectancy ratings for the safety cue and surrounding stimuli during generalization. The existence of contingency-unaware fear conditioning remains debated, with limited supporting evidence (55). Nonetheless, unaware participants in our study still exhibited a significant CS+/CS– differentiation, albeit reduced, suggesting either implicit learning or difficulty articulating the association. Of note, in our sample, 62.81% of participants were classified as aware, which aligns with the complexity of the task, including the number of stimuli and the reduced reinforcement rate during generalization. Variability in how contingency awareness is assessed hinders strong conclusions and highlights the need for standardized procedures in future work.
4.5 Fear learning in adolescents and young adults
Prior research on age-related differences in fear learning is mixed, but often shows decreased threat-safety discrimination and increased fear generalization in younger children (56, 57). However, existing studies predominantly focus on either adults or young children, leaving a gap in adolescent to young adult populations. We selected a 16-24-year-old sample to capture a developmental period of heightened vulnerability for the emergence of mental health problems. Within this range, older participants showed decreased US expectancy ratings for the CS- during acquisition and for the GS3 and GS4 during generalization, suggesting enhanced safety learning. Post-experimental ratings supported this, indicating increased valence but decreased fear, arousal and US expectancy, as age increased. This pattern of results aligns with the idea that older individuals in this age range may have developed more effective safety learning or a better ability to differentiate between threat and safety. While this age span enables the examination of threat processing across a clinically relevant transition period, ongoing brain maturation beyond 18 years warrants caution when comparing these findings to studies restricted to adolescents or adults. These findings contribute to the growing literature on age-related differences, emphasizing the importance of examining fear conditioning across developmental stages.
4.6 Limitations
This study has several limitations. First, the heterogeneity of the sample with mixed psychiatric symptoms poses challenges when comparing with other clinical samples. Additionally, participants were predominantly highly educated and Caucasian, limiting generalizability. Second, using individually selected shock intensities for the US led to 18 participants not reaching the predefined threshold, resulting in a milder subjective experience of the shock. Although a sensitivity analysis was conducted, this may still have influenced results. While sex was included as a covariate, further exploration of sex differences and hormonal influences is necessary considering their relevance in affective disorders. Third, due to design constraints because of the intervention, the fear conditioning and perceptual discrimination tasks were not counterbalanced, which may have increased fatigue or reduced engagement during the latter task. This could have limited variability and contributed to the null association between perceptual discrimination and fear conditioning performance. An additional limitation is that, although US-expectancy ratings provide a validated measure of subjective fear learning (58), we were unable to analyze physiological indices of fear learning (fear-potentiated startle) due to a technical malfunction in the trigger system. While we prioritized the accuracy and reliability of the data focusing on US expectancy outcomes, which captures conscious threat appraisal important in translational and clinical contexts, objective measures would have complemented these findings and strengthened conclusions regarding implicit fear responses. Furthermore, while dimensional analyses were pre-specified and informative, the smaller sample size limited statistical power to detect stimulus-specific effects. Despite trends suggesting symptom-specific variations in generalization patterns, these results should therefore be interpreted with caution and further research is necessary. Lastly, longitudinal studies, using larger sample sizes and incorporating psychophysiological measures, are needed to better understand causality and directionality of the association between fear generalization and psychiatric symptoms.
5 Conclusion and future perspectives
In conclusion, youth with early-stage psychiatric symptoms did not show expected deficits in discrimination or overgeneralization but exhibited overall elevated threat expectancy during generalization, as indexed by US expectancy ratings. This may reflect an early-stage vulnerability in threat processing. Dimensional analysis point to modest symptom-specific differences in generalization patterns, underscoring the importance of examining continuous symptom severity, alongside group-based comparisons in understanding fear learning during the critical transition from late adolescence to early adulthood. Future research should use larger samples to validate these effects and explore longitudinal trajectories on how these patterns evolve with symptom progression. Additionally, further work is needed to identify exercise characteristics that may influence fear learning, to better assess its utility as an intervention.
Data availability statement
The datasets presented in this article are not readily available because the data that support the findings of this study are available on request from the corresponding author (LJ). The pseudo-anonymized data are not publicly available due to the sensitive nature of the clinical data and privacy of research participants. Data are located in controlled access data storage at KU Leuven. Data sharing is possible after submitting a formal project outline, having a formal data sharing agreement and when the request does not interfere with in-house research plans. Co-authorship for data collection and processing is required. Requests to access the datasets should be directed to Lise Jennen, bGlzZS5qZW5uZW5Aa3VsZXV2ZW4uYmU=.
Ethics statement
The studies involving humans were approved by Local Ethical Committee UZ/KU Leuven. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from all participants. For minors, written informed consent was provided by the participants’ legal guardians/next of kin.
Author contributions
LJ: Formal Analysis, Project administration, Visualization, Methodology, Writing – original draft, Conceptualization, Investigation. CS: Writing – review & editing, Methodology, Software, Conceptualization. ZQ: Investigation, Writing – review & editing. VM: Project administration, Investigation, Writing – review & editing. KV: Methodology, Writing – review & editing. DV: Conceptualization, Supervision, Funding acquisition, Writing – review & editing, Methodology. RV: Supervision, Writing – review & editing, Funding acquisition, Conceptualization, Methodology.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by a FWO Senior Clinical Investigator (1803616N) grant awarded to RV and by the Funds Julie Renson, Queen Fabiola and King Baudoin Foundation (Chair for Transition Psychiatry). LJ was awarded a PhD Fellowship from Research Foundation Flanders (FWO -11M4722N), ZQ received a scholarship from the China Scholarship Council (CSC 202009110102), and DV is funded by an internal KU Leuven grant (3M190683).
Acknowledgments
We sincerely thank all study participants for their enthusiasm and commitment, as well as the organizations that supported our recruitment efforts. We are also grateful to Maarten Jackers for his assistance with data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. During the preparation of this work the author (LJ) used ChatGPT (OpenAI) in order to improve readability and language. After using this tool, the author reviewed and edited the content as needed and takes full responsibility for the content of the publication.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2025.1657470/full#supplementary-material
References
1. Lange I, Goossens L, Bakker J, Michielse S, van Winkel R, Lissek S, et al. Neurobehavioral mechanisms of threat generalization moderate the link between childhood maltreatment and psychopathology in emerging adulthood. J Psychiatry Neurosci. (2018) 44:1–10. doi: 10.1503/jpn.180053
2. Fraunfelter L, Gerdes ABM, and Alpers GW. Fear one, fear them all: A systematic review and meta-analysis of fear generalization in pathological anxiety. Neurosci Biobehav Rev. (2022) 139:104707. doi: 10.1016/j.neubiorev.2022.104707
3. Cooper SE, van Dis EAM, Hagenaars MA, Krypotos AM, Nemeroff CB, Lissek S, et al. A meta-analysis of conditioned fear generalization in anxiety-related disorders. Neuropsychopharmacology. (2022) 47:1652–61. doi: 10.1038/s41386-022-01332-2
4. Solmi M, Radua J, Olivola M, Croce E, Soardo L, Salazar de Pablo G, et al. Age at onset of mental disorders worldwide: large-scale meta-analysis of 192 epidemiological studies. Mol Psychiatry. (2021) 17:22. doi: 10.1038/s41380-021-01161-7
5. Carpenter JS, Iorfino F, Cross SP, Davenport TA, Hermens DF, Rohleder C, et al. Combining clinical stage and pathophysiological mechanisms to understand illness trajectories in young people with emerging mood and psychotic syndromes. Med J Australia. (2019) 211:12–22. doi: 10.5694/mja2.50383
6. Lissek S, Kaczkurkin AN, Rabin S, Geraci M, Pine DS, and Grillon C. Generalized anxiety disorder is associated with overgeneralization of classically conditioned fear. Biol Psychiatry. (2014) 75:909–15. doi: 10.1016/j.biopsych.2013.07.025
7. Duits P, Cath DC, Lissek S, Hox JJ, Hamm AO, Engelhard IM, et al. Updated meta-analysis of classical fear conditioning in the anxiety disorders. Depress Anxiety. (2015) 32:239–53. doi: 10.1002/da.22353
8. Dymond S, Dunsmoor JE, Vervliet B, Roche B, and Hermans D. Fear Generalization in Humans: Systematic Review and Implications for Anxiety Disorder Research. Behav Ther. (2015) 46:561–82. doi: 10.1016/J.BETH.2014.10.001
9. Lenaert B, Boddez Y, Griffith JW, Vervliet B, Schruers K, and Hermans D. Aversive learning and generalization predict subclinical levels of anxiety: A six-month longitudinal study. J Anxiety Disord. (2014) 28:747–53. doi: 10.1016/j.janxdis.2014.09.006
10. Sep MSC, Steenmeijer A, and Kennis M. The relation between anxious personality traits and fear generalization in healthy subjects: A systematic review and meta-analysis. Neurosci Biobehav Rev. (2019) 107:320–8. doi: 10.1016/j.neubiorev.2019.09.029
11. Wurst C, Schiele MA, Stonawski S, Weiß C, Nitschke F, Hommers L, et al. Impaired fear learning and extinction, but not generalization, in anxious and non-anxious depression. J Psychiatr Res. (2021) 135:294–301. doi: 10.1016/j.jpsychires.2021.01.034
12. Rosenberg BM, Young KS, Nusslock R, Zinbarg RE, and Craske MG. Anhedonia is associated with overgeneralization of conditioned fear during late adolescence and early adulthood. J Anxiety Disord. (2024) 105:102880. doi: 10.1016/j.janxdis.2024.102880
13. Erskine HE, Moffitt TE, Copeland WE, Costello EJ, Ferrari AJ, Patton G, et al. A heavy burden on young minds: The global burden of mental and substance use disorders in children and youth. Psychol Med. (2015) 45:1561–3. doi: 10.1017/S0033291714002888
14. Ashdown-Franks G, Firth J, Carney R, Carvalho AF, Hallgren M, Koyanagi A, et al. Exercise as Medicine for Mental and Substance Use Disorders: A Meta-review of the Benefits for Neuropsychiatric and Cognitive Outcomes. Sport Med. (2020) 50:151–70. doi: 10.1007/s40279-019-01187-6
15. Keyan D and Bryant RA. The capacity for acute exercise to modulate emotional memories: A review of findings and mechanisms. Neurosci Biobehav Rev. (2019) 107:438–49. doi: 10.1016/j.neubiorev.2019.09.033
16. Jennen L, Mazereel V, Lecei A, Samaey C, Vancampfort D, and van Winkel R. Exercise to spot the differences: a framework for the effect of exercise on hippocampal pattern separation in humans. Rev Neurosci. (2022) 0:1–28. doi: 10.1515/revneuro-2021-0156
17. St-Pierre DH and Richard D. The Effect of Exercise on the Hypothalamic-Pituitary-Adrenal Axis. In: Hackney A and Constantini N, editors. Contemporary Endocrinology. Humana Press Inc, Humana, Cham (2020). p. 41–54.
18. Lissek S, Bradford DE, Alvarez RP, Burton P, Espensen-Sturges T, Reynolds RC, et al. Neural substrates of classically conditioned fear-generalization in humans: A parametric fMRI study. Soc Cognit Affect Neurosci. (2014) 9:1134–42. doi: 10.1093/scan/nst096
19. Scott J, Iorfino F, Capon W, Crouse J, Nelson B, Chanen AM, et al. Staging 2.0: refining transdiagnostic clinical staging frameworks to enhance reliability and utility for youth mental health. Lancet Psychiatry. (2024) 11:461–71. doi: 10.1016/S2215-0366(24)00060-9
20. Shah JL, Scott J, McGorry PD, Cross SPM, Keshavan MS, Nelson B, et al. Transdiagnostic clinical staging in youth mental health: a first international consensus statement. World Psychiatry. (2020) 19:233–42. Available online at: https://onlinelibrary.wiley.com/doi/abs/10.1002/wps.20745 (Accessed December 1, 2020).
21. Cooper SE, Perkins ER, Webler RD, Dunsmoor JE, and Krueger RF. Integrating threat conditioning and the hierarchical taxonomy of psychopathology (HiTOP) to advance the study of anxiety-related psychopathology. J Psychopathol Clin Sci. (2024) 133(8):716–32. doi: 10.1037/abn0000945
22. Schuch FB, Vancampfort D, Richards J, Rosenbaum S, Ward PB, and Stubbs B. Exercise as a treatment for depression: A meta-analysis adjusting for publication bias. J Psychiatr Res. (2016) 77:42–51. doi: 10.1016/j.jpsychires.2016.02.023
23. Rebar AL, Stanton R, Geard D, Short C, Duncan MJ, and Vandelanotte C. A meta-meta-analysis of the effect of physical activity on depression and anxiety in non-clinical adult populations. Health Psychol Rev. (2015) 9:366–78. doi: 10.1080/17437199.2015.1022901
24. Azar A, Hubert T, Adams TG, Cisler JM, and Crombie KM. Exercise and Fear and Safety Learning. In: Current Topics in Behavioral Neurosciences Heidelberg: Springer (2024). p. 125–40.
25. Crombie KM, Sartin-Tarm A, Sellnow K, Ahrenholtz R, Lee S, Matalamaki M, et al. Aerobic exercise and consolidation of fear extinction learning among women with posttraumatic stress disorder. Behav Res Ther. (2021) 142:103867. doi: 10.1016/j.brat.2021.103867
26. Beck AT, Steer RA, and Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation (1996).
27. Whisman MA and Richardson ED. Normative Data on the Beck Depression Inventory-Second Edition (BDI-II) in College Students. J Clin Psychol. (2015) 71:898–907. doi: 10.1002/jclp.22188
28. Leal SL, Tighe SK, Jones CK, and Yassa MA. Pattern separation of emotional information in hippocampal dentate and CA3. Hippocampus. (2014) 24:1146–55. doi: 10.1002/hipo.22298
30. Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, and Jacobs GA. State-Trait Anxiety Inventory for Adults: Manual, Instrument and Scoring. Mountain View, California: Consult Psychol Press Inc (1987).
31. Savill M, D’Ambrosio J, Cannon TD, and Loewy RL. Psychosis risk screening in different populations using the Prodromal Questionnaire: A systematic review. Early Interv Psychiatry. (2018) 12:3–14. doi: 10.1111/eip.12446
32. Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: Building an international community of software platform partners. J BioMed Inform. (2019) 95. doi: 10.1016/j.jbi.2019.103208
33. Peirce JW. PsychoPy-Psychophysics software in Python. J Neurosci Methods. (2007) 162:8–13. doi: 10.1016/j.jneumeth.2006.11.017
34. Samaey C, Lecei A, Jackers M, Jennen L, Schruers K, Vervliet B, et al. Childhood adversity is associated with reduced threat-safety discrimination and increased fear generalization in 12- to 16-year-olds. J Child Psychol Psychiatry Allied Discip. (2025) 66:821–33. doi: 10.1111/jcpp.14092
35. Bradley MM and Lang PJ. Measuring emotion: The self-assessment manikin and the semantic differential. J Behav Ther Exp Psychiatry. (1994) 25:49–59. doi: 10.1016/0005-7916(94)90063-9
36. Zaman J, Chalkia A, Zenses AK, Bilgin AS, Beckers T, Vervliet B, et al. Perceptual variability: Implications for learning and generalization. Psychon Bull Rev. (2021) 28:1–19. doi: 10.3758/s13423-020-01780-1
37. R Core Team. RStudio: A language and environment for statistical computing. Vienna, Austria: RStudio (2021). Available online at: https://www.r-project.org/ (Accessed May 6, 2022).
38. Kuznetsova A, Brockhoff PB, and Christensen RHB. lmerTest Package: Tests in Linear Mixed Effects Models. J Stat Software. (2017) 82:1–26. doi: 10.18637/jss.v082.i13
39. Lissek S, Biggs AL, Rabin SJ, Cornwell BR, Alvarez RP, Pine DS, et al. Generalization of conditioned fear-potentiated startle in humans: Experimental validation and clinical relevance. Behav Res Ther. (2008) 46:678–87. doi: 10.1016/j.brat.2008.02.005
40. Haddad ADM, Pritchett D, Lissek S, and Lau JYF. Trait anxiety and fear responses to safety cues: Stimulus generalization or sensitization? J Psychopathol Behav Assess. (2012) 34:323–31. doi: 10.1007/s10862-012-9284-7
41. Mertens G, Bouwman V, and Engelhard IM. Conceptual fear generalization gradients and their relationship with anxious traits: Results from a Registered Report. Int J Psychophysiol. (2021) 170:43–50. doi: 10.1016/j.ijpsycho.2021.09.007
42. Torrents-Rodas D, Fullana MA, Bonillo A, Caseras X, Andión O, and Torrubia R. No effect of trait anxiety on differential fear conditioning or fear generalization. Biol Psychol. (2013) 92:185–90. doi: 10.1016/j.biopsycho.2012.10.006
43. Johnson D, Ho W, Uddin B, Tetteh-Quarshie S, and Morriss J. Evidence for Different Roles of Inhibitory and Prospective Intolerance of Uncertainty During Threat Discrimination Learning. Collabra Psychol. (2023) 9:1198–208. doi: 10.1525/collabra.74822
44. Stegmann Y, Schiele MA, Schümann D, Lonsdorf TB, Zwanzger P, Romanos M, et al. Individual differences in human fear generalization—pattern identification and implications for anxiety disorders. Transl Psychiatry. (2019) 9. doi: 10.1038/s41398-019-0646-8
45. Reinhard J, Mittermeier A, Brandstetter L, Mowat K, Slyschak A, Reiter AMF, et al. Fear conditioning and fear generalization in children and adolescents with anxiety disorders. Eur Child Adolesc Psychiatry. (2023) 33:2163–72. doi: 10.1007/s00787-023-02304-7
46. Ruge J, Ehlers MR, Kastrinogiannis A, Klingelhöfer-Jens M, Koppold A, Abend R, et al. How adverse childhood experiences get under the skin: A systematic review, integration and methodological discussion on threat and reward learning mechanisms. Elife. (2024) 13:1–35. doi: 10.7554/eLife.92700
47. Morriss J, Macdonald B, and Van Reekum CM. What is going on around here? Intolerance of uncertainty predicts threat generalization. PloS One. (2016) 11. doi: 10.1371/journal.pone.0154494
48. Morriss J, Butler D, and Ellett L. Intolerance of uncertainty and psychosis: A systematic review. Br J Clin Psychol. (2025) 64:344–54. doi: 10.1111/bjc.12509
49. Baruch DE, Swain RA, and Helmstetter FJ. Effects of exercise on pavlovian fear conditioning. Behav Neurosci. (2004) 118:1123–7. doi: 10.1037/0735-7044.118.5.1123
50. Falls WA, Fox JH, and MacAulay CM. Voluntary exercise improves both learning and consolidation of cued conditioned fear in C57 mice. Behav Brain Res. (2010) 207:321–31. doi: 10.1016/j.bbr.2009.10.016
51. Crombie KM, Azar A, Botsford C, Heilicher M, Moughrabi N, Gruichich TS, et al. Aerobic exercise after extinction learning reduces return of fear and enhances memory of items encoded during extinction learning. Ment Health Phys Act. (2023) 24:100510. doi: 10.1016/j.mhpa.2023.100510
52. Keyan D and Bryant RA. Acute exercise-induced enhancement of fear inhibition is moderated by BDNF Val66Met polymorphism. Transl Psychiatry. (2019) 9. doi: 10.1038/s41398-019-0464-z
53. Jentsch VL, Wolf OT, Otto T, and Merz CJ. The impact of physical exercise on the consolidation of fear extinction memories. Psychophysiology. (2023) 60:1–19. doi: 10.1111/psyp.14373
54. Tanner MK, Hake HS, Bouchet CA, and Greenwood BN. Running from fear: Exercise modulation of fear extinction. In: Neurobiology of Learning and Memory, vol. 151. NIH Public Access: Amsterdam (2018). p. 28–34. Available online at: from:/pmc/articles/PMC6557445/ (Accessed March 3, 2023).
55. Mertens G and Engelhard IM. A systematic review and meta-analysis of the evidence for unaware fear conditioning. Neurosci Biobehav Rev. (2020) 108:254–68. doi: 10.1016/j.neubiorev.2019.11.012
56. Lonsdorf TB and Merz CJ. More than just noise: Inter-individual differences in fear acquisition, extinction and return of fear in humans - Biological, experiential, temperamental factors, and methodological pitfalls. Neurosci Biobehav Rev. (2017) 80:703–28. doi: 10.1016/j.neubiorev.2017.07.007
57. Lau JY, Britton JC, Nelson EE, Angold A, Ernst M, Goldwin M, et al. Distinct neural signatures of threat learning in adolescents and adults. Proc Natl Acad Sci U S A. (2011) 108:4500–5. doi: 10.1073/pnas.1005494108
Keywords: fear conditioning, depression, anxiety, psychosis, adolescents, young adults, acute exercise intervention
Citation: Jennen L, Samaey C, Qiao Z, Mazereel V, Vansteelandt K, Vancampfort D and van Winkel R (2025) Fear learning and generalization in youth with early-stage transdiagnostic psychiatric symptoms and the impact of acute exercise. Front. Psychiatry 16:1657470. doi: 10.3389/fpsyt.2025.1657470
Received: 01 July 2025; Accepted: 27 August 2025;
Published: 19 September 2025.
Edited by:
Jayne Morriss, University of Southampton, United KingdomReviewed by:
Dharani Keyan, University of New South Wales, AustraliaDavid Johnson, The College of Staten Island (CUNY), United States
Copyright © 2025 Jennen, Samaey, Qiao, Mazereel, Vansteelandt, Vancampfort and van Winkel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lise Jennen, bGlzZS5qZW5uZW5Aa3VsZXV2ZW4uYmU=
 Lise Jennen
Lise Jennen Celine Samaey
Celine Samaey Zhiling Qiao1,3
Zhiling Qiao1,3 Victor Mazereel
Victor Mazereel Davy Vancampfort
Davy Vancampfort Ruud van Winkel
Ruud van Winkel