- 1Department of Graduate School, Heilongjiang University of Chinese Medicine, Harbin, China
- 2Department of Medicine IV, The Tumour Hospital of Harbin Medical University, Harbin, China
- 3Department of Acupuncture and Moxibustion, The First Affiliated Hospital of Heilongjiang University of Chinese Medicine, Harbin, China
Background: Depression or depressive symptoms exacerbate the burden in patients with chronic fatigue syndrome (CFS). The therapeutic effects of various non-pharmacological interventions remain unclear.
Objective: This paper aims to evaluate the effectiveness of different non-pharmacological measures in alleviating depression or depressive symptoms in patients with CFS through network meta-analysis.
Methods: PubMed, Cochrane Library, Web of Science, Embase, CNKI, Wanfang, CBM, VIP, and Sinomed databases were searched for randomized controlled trials (RCTs) until March 26, 2025. The Cochrane Risk of Bias Assessment Tool 2.0 was utilized to appraise the risk of bias. A network meta-analysis was conducted using the GeMTC package in R (4.4.2). This protocol has been registered in PROSPERO (CRD420251020737).
Results: 47 RCTs involving 4,028 participants were included. Compared with control measures, diet therapy was most effective in improving depression or depressive symptoms in patients with CFS (SMD = -5.64, 95% CI: -8.98 to -2.29), followed by moxibustion (Mox) (SMD = -2.91, 95% CI: -4.61 to -1.22), acupuncture (Ap) + Mox + acupoint embedding (SMD = -3.16, 95% CI: -0.39 to -5.98), and Ap + Mox (SMD = -2.53, 95% CI: -1.17 to -3.91).
Conclusion: Diet therapy is the most effective in improving depression or depressive symptoms in patients with CFS, followed by Mox. Further carefully designed RCTs are warranted to substantiate these findings.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD420251020737.
1 Introduction
Chronic fatigue syndrome (CFS) is a chronic disease that affects multiple systems and has complex and diverse symptoms, usually manifested as persistent fatigue and pain that cannot be relieved by rest and worsens after exercise (1). The prevalence of CFS is approximately 0.3%-3.3%. Due to the diversity of diagnoses, the actual number of affected people in the United States may be much higher. Emotional problems such as anxiety and depression are widespread among CFS patients (2). Depression is an affective disorder characterized primarily by pronounced and persistent low mood, with symptoms coexisting across emotional, physical, and cognitive dimensions (3). Depression and CFS have many similarities in symptoms and have only been clearly divided into two diseases in recent years, with a distinct bidirectional relationship (4). Michael et al. (5) found that CFS patients had greatly elevated plasma levels of pro-inflammatory cytokines, which may induce or exacerbate depressive symptoms. Compared with CFS without depression, CFS with depression may cause more severe endocrine disorders (6). In terms of psychological cognition, maladaptive perfectionism is associated with depression levels in CFS patients (7). Doctors’ disregard for fatigue in CFS patients and illegal diagnosis and treatment are also factors contributing to comorbid depression (8). CFS patients suffer from severe physical and social functional impairment, face unemployment and economic crisis, and have an enhanced risk of depression (9). Depression is one of the independent risk factors for CFS, exacerbating the symptoms and functional impairment of chronic diseases. For every one-point increase in the depression scale score, the overall risk of CFS increases by 49% (10). CFS with concomitant depression impairs patients’ social functioning, significantly undermining their quality of life and increasing disability and suicide rates, imposing a substantial burden on individuals, the economy, and the healthcare system. It represents an urgent issue that requires resolution (11).
There is no specific drug for CFS, and symptomatic treatment is the main approach. When depressive symptoms appear, analgesics and antidepressants are often used. However, the effectiveness of these drugs is controversial, and their adverse reactions and addiction are obvious (12). Even clinical and animal studies have indicated that certain types of antidepressants and analgesics enhance CFS risk (13, 14). A large body of evidence suggests that non-pharmacological therapies can effectively alleviate depressive symptoms of CFS. For example, cognitive behavioral therapy (CBT) can alleviate fatigue symptoms and improve depression in CFS patients (15, 16). Acupuncture (Ap) and moxibustion (Mox) can improve physical and mental fatigue symptoms and psychological status in CFS patients (17). B. A. Gordon et al. (18) found that graded exercise considerably improved physical function, quality of life, and depressive symptoms in CFS patients. In this study, we only considered non-pharmacological therapies.
In addition to efficacy, non-pharmacological therapies have several advantages over pharmacological therapies, including fewer side effects, high tolerability, low cost, increased patient compliance, favorable doctor-patient interaction, personalized regimens, and no restrictions on medical resources (19). The European consensus on diagnosis and treatment released in 2024 recommends that when CFS is secondary to depression, pacing therapy and psychotherapy should be the priority for treatment (20). The guidelines issued by the American College of Physicians also recommend that CBT be given priority in the treatment of depression (21), which suggests that non-pharmacological therapies may have obvious advantages in the treatment of CFS patients with depressive symptoms. However, in this field, the evaluation of many non-pharmacological therapies such as Ap and exercise lacks high-quality evidence, and the effectiveness of some therapies is controversial. For example, Internet-based CBT relieved symptoms in CFS patients with comorbid depressive symptoms by about 20% compared with non-depressed patients (22). Exercise therapy may exceed the patient’s energy limit, causing characteristic post-exertional malaise (PEM) and affecting efficacy (23). Nevertheless, there is currently a lack of comparative studies on the efficacy of non-pharmacological therapies in improving depressive symptoms in CSF, This paper employs a network meta-analysis (NMA) method to systematically search Chinese and English databases for studies related to non-pharmacological therapies for depressive symptoms in CFS patients, to comprehensively analyze current non-pharmacological therapies in this field. It has the advantage of integrating direct and indirect comparisons using existing research while comparing multiple interventions, to investigate the efficacy of non-pharmacological treatments for depressive symptoms in CFS patients. This, to a certain extent, fills the gap in clinical research, provides a basis for non-pharmacological treatment options for clinical treatment of depressive symptoms in CSF patients, and offers references and suggestions for public health decisions and clinical guidelines.
2 Methods
This study followed the PRISMA guidelines and their requirements for NMA (24). The study protocol was registered in the PROSPERO (CRD420251020737).
2.1 Search strategy
PubMed, Cochrane Library, Embase, Web of Science, CNKI, VIP, Wanfang, and Sinomed databases were searched from establishment date to March 26, 2025, and the language was restricted to Chinese and English. The search was conducted using a combination of subject terms and free terms. The medical subject terms included “Fatigue Syndrome”, “Chronic Fatigue Syndrome”; “Depression”, “Depressive Disorder”, “Central Depression”; and “randomized controlled trial”. References in other relevant articles and gray literature were manually searched to pinpoint studies that met the criteria. The specific search strategy used is listed in Supplementary Table 1.
2.2 Inclusion and exclusion criteria
Literature meeting the criteria were included: (1) Study subjects: CSF patients; (2) Interventions: any non-pharmacological treatment; with conventional care and sham AP as control interventions; (3) Study type: randomized controlled trial (RCT); (4) Outcome measures: depression or depressive symptoms, assessed using scales, including PROMIS Depression Short Form (PROMIS Depression-SF), Hospital anxiety and depression scale (HADS), Beck Depression Inventory (BDI), Patient Health Questionnaire-9 (PHQ-9), SPHERE, Self-rating depression scale (SDS), Symptom Checklist-90 (SCL-90), Brief Symptom Inventory (BSI), and Hamilton Depression Scale (HAMD), and Depression Status Inventory (DSI).
The following types of literature were ruled out: (1) animal or cell experiments, case reports, reviews, scientific experimental plans, letters, editorials, and conference papers; (2) literature with missing data or serious errors; (3) duplicated publications; (4) literature without full text; (5) literature with overlapping study participants; (6) literature with no data or data that cannot be extracted; (7) literature with irrelevant intervention measures; (8) Non-English or non-Chinese language.
2.3 Data extraction
The retrieved literature was imported into EndNote, and two researchers (Baiyi Jiang and Xue Xia) independently screened the titles and abstracts and then read the full text for a secondary screening. For literature with discrepancies, re-evaluation was conducted after discussion or consultation with a third researcher (Long Wang). Data extraction was performed independently by two researchers using a pre-designed electronic form, including first author, publication year, country, randomization and blinding design, intervention and control measures, treatment duration, basic information of study subjects, and outcome measures.
2.4 Quality assessment
Two authors (Baiyi Jiang and Xue Xia) independently used the Cochrane risk of bias tool (ROB 2.0) to appraise the quality of eligible studies (25) in five aspects: random sequence generation, allocation concealment, blinding implementation, missing data, and selective reporting. Each aspect was rated as “high risk”, “some concerns”, or “low risk”. If all five items were low risk or only one item was rated as some risk and the rest were rated as low risk, the article was considered low risk. If four or more of the five items were rated as some risk or any one item was high risk, the article was considered high-risk. In all other cases, the article was considered moderate risk. The two authors independently completed the literature quality assessment, and any discrepancies were reviewed by a third author (Long Wang).
2.5 Statistical analysis
All outcome measures included were continuous variables. Since the scales used for outcome measurements varied across studies, the standardized mean difference (SMD) was adopted as the effect size measure. This study employed a Markov chain Monte Carlo method to construct a Bayesian NMA model and iterated the model to estimate the relative efficacy of different treatment regimens (26). When using non-informative priors, it is assumed that all values within the confidence range of the result are equally likely to occur, and only data from the included studies are used in the analysis (27, 28). Using noninformative priors avoids introducing subjectivity and/or nonrandomized data into the analysis models. During the verification process, four model chains, annealing of 10,000, iteration of 50,000, detection step size of 10, and initial value of 2.5 were set to obtain posterior distribution. The NMA was implemented based on r transitivity, homogeneity, and consistency assumptions. Heterogeneity was examined using the mtc:anohe function in the GeMTC package. When the overall I2 < 50%, the heterogeneity among the included studies in the same comparison was viewed as acceptable, satisfying the homogeneity assumption. The node splitting method was used to test the inconsistency between direct and indirect comparisons using the mtc.nodesplit function in the GeMTC package. When P > 0.05, it indicated no inconsistency between the direct and indirect comparisons, satisfying the consistency assumption. The convergence of the results was judged by calculating the potential scale reduction factor (PSRF), with 1 as the standard, and 1 ≤ PSRF < 1.05 indicated successful convergence. A network structure was constructed with SMDs between intervention measures as the line and each intervention measure as the node. The lines represent head-to-head comparisons among interventions. The surface area under the cumulative ranking curve (SUCRA) was estimated. Funnel plots were drawn to assess possible publication bias. All statistical analyses were made using R (version 4.4.2) and STATA (version 17) software.
3 Results
3.1 Literature search and screening process
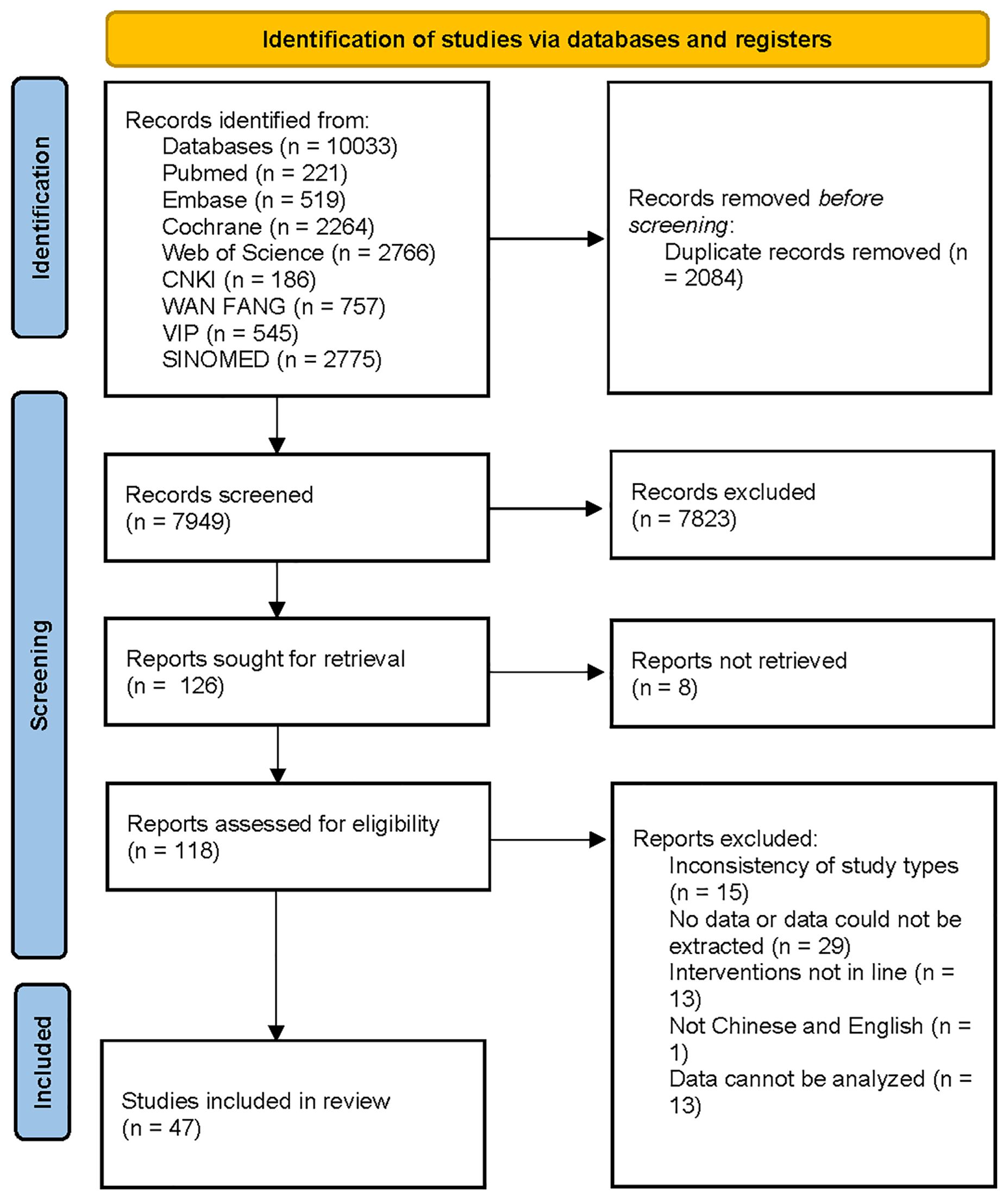
10,033 documents were retrieved, of which 2,084 duplicates were eliminated. After preliminary reading of the titles and abstracts, 7,823 documents were excluded. Based on the secondary screening of the full text, documents were included or excluded strictly. Finally, 47 RCTs were included. The screening process is displayed in Figure 1.
3.2 Basic characteristics and quality assessment
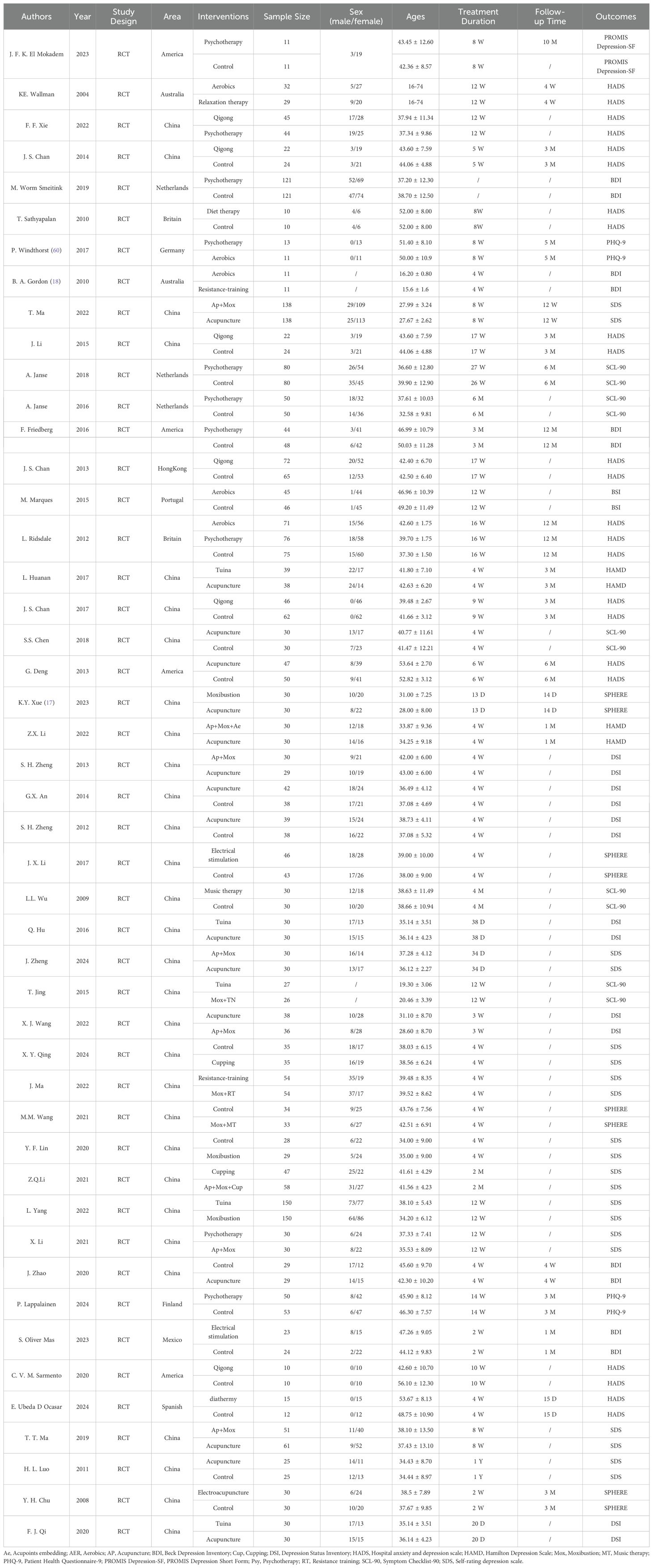
The 47 included RCTs were from 10 countries (America, Australia, China, Netherlands, Britain, Germany, Portugal, Finland, Mexico, and Spain), involving 4,028 patients (1,246 males and 2,707 females) (sex data were sourced from 45 studies, and 2 studies did not report sex), aged 16–74 years. Interventions included AP, Mox, Cupping (Cup), Tuina, music therapy (MT), electroacupuncture, relaxation therapy, psychotherapy (Psy), aerobics (AER), diet therapy, resistance training (RT), Qigong, electrical stimulation, physiotherapy, Ap + Mox, Ap + Mox + Acupoints embedding (Ae), Psy + AER, Mox + RT, Mox + MT, and Ap + Mox + Cup. The control interventions included standard care, sham AP, sham transcranial direct current stimulation (tDCS), waiting list, no treatment, sham diathermy, health education, and sham chocolate. Basic characteristics of the included studies are summarized in Table 1.
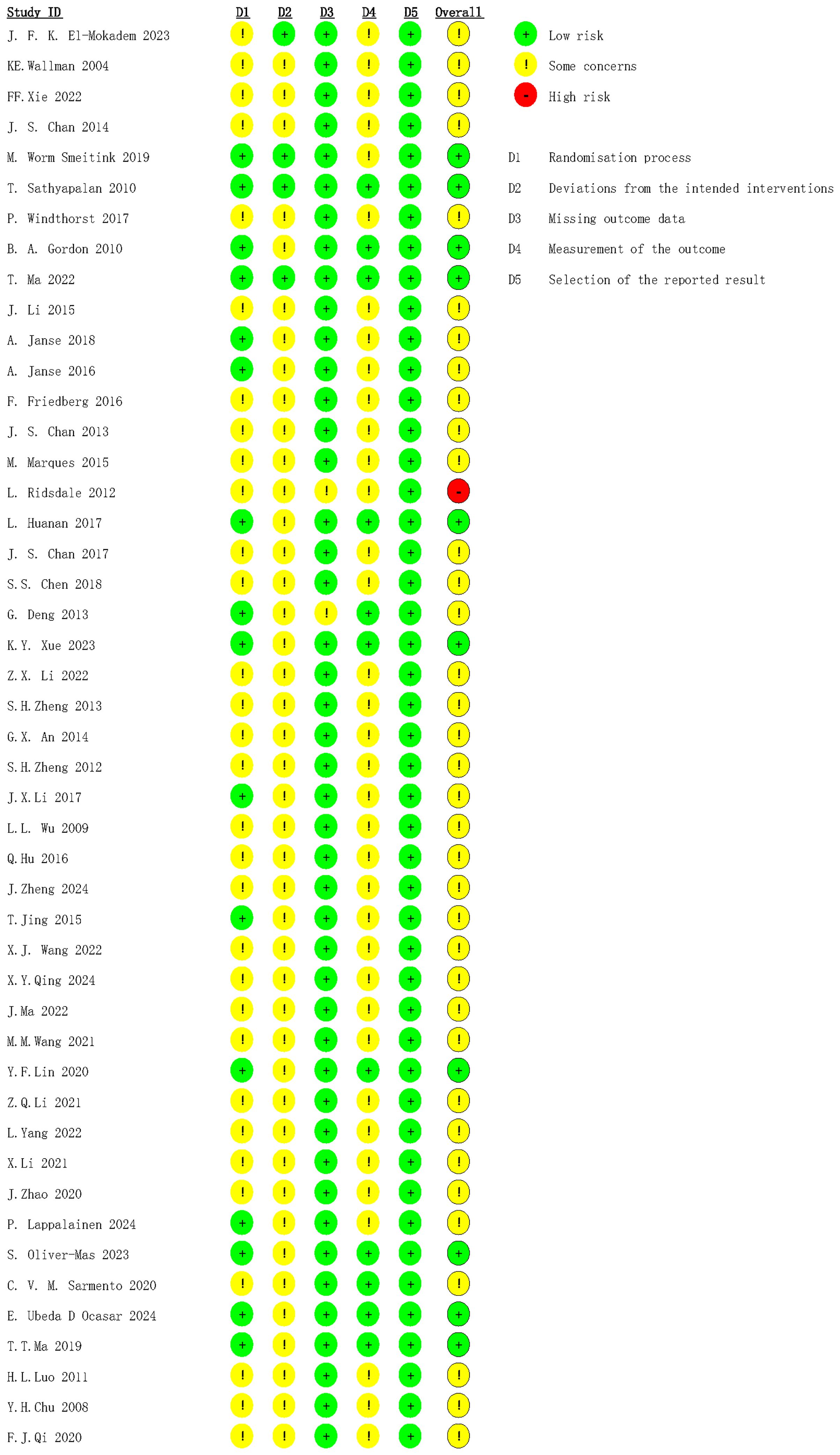
The bias risk assessment showed that 10 RCTs were rated as low risk, and most studies had some concerns. The reasons for moderate risk were the absence of blinding and intention-to-treat analysis. One study was rated as high risk because baseline data of patients included differed greatly, and there were deviations from conventional medical care during the study (Figure 2).
3.3 NMA results
3.3.1 Network diagram
In the network diagram, each dot symbolizes an intervention measure, and the size of the dot is positively correlated with the number of RCTs involved in each intervention measure; the larger the dot, the more studies are included. The lines connecting two dots indicate direct comparisons between these two interventions, and the thickness of the lines symbolizes the number of RCTs between the two treatment regimens; the thicker line indicates more comparative studies (Figure 3). The node splitting method was employed to analyze the results of each closed loop. P values of outcome measures were all greater than 0.05, suggesting no local inconsistency (Supplementary Figure 1). PSRF was equal to 1, indicating that the model was completely convergent. The specific convergence results are shown in Supplementary Figures 2, 3.
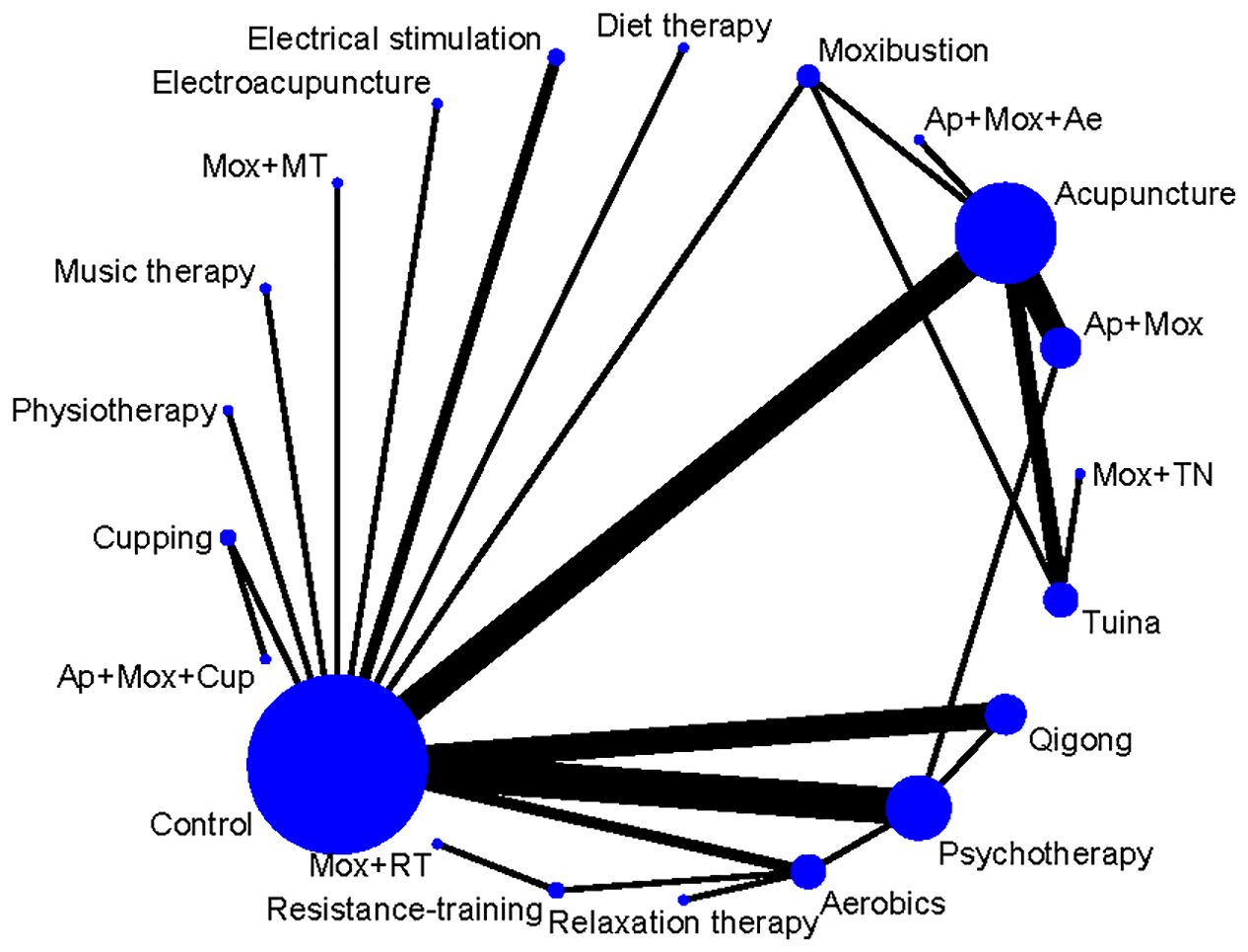
Figure 3. Network diagram of non-pharmacological therapies for treating depressive symptoms in patients with CFS. Ae, Acupoints embedding; AER, Aerobics; AP, Acupuncture; Cup, Cupping; Mox, Moxibustion; MT, Music therapy; Psy, Psychotherapy; RT, Resistance training.
3.3.2 Summary results for each outcome measure
47 studies reported depression scale scores. Overall heterogeneity was 90%, indicating high heterogeneity (Supplementary Figure 4), so a random-effects model was selected. NMA results revealed that compared with the control group, CFS patients who received Ap, Mox, Tuina, diet therapy, electrical stimulation, Ap + Mox, or Ap + Mox + Ae had lowered depression scale scores (Control vs Ap: SMD = -1.91, 95% CI: -2.9 to -0.94; Control vs. Mox: SMD = -2.91, 95% CI: -4.61 to -1.22; Control vs. Tuina: SMD = -2, 95% CI: -3.64 to -0.38; Control vs Diet therapy: SMD = -5.64, 95% CI: -8.98 to -2.29; Electrical stimulation vs Control: SMD = 1.9, 95% CI: 0.03 to 3.78; Ap + Mox vs Control: SMD = 2.53, 95% CI: 1.17 to 3.91; Ap + Mox + Ae vs Control: SMD = 3.16, 95% CI: 0.39 to 5.98). Compared with the control group, the four exercise therapies (relaxation therapy, AER, RT, and Qigong) did not markedly improve depressive symptoms in CFS patients (Control vs. Relaxation therapy: SMD = 0.73, 95% CI: -1.23 to 2.71; Control vs. AER: SMD = -0.38, 95% CI: -1.81 to 1.05; Control vs. RT: SMD = -0.73, 95% CI: -3.8 to 2.33; Control vs. Qigong: SMD = -0.43, 95% CI: -1.52 to 0.66).
Compared with Psy, CFS patients who received Ap and Mox treatment showed notable decreases in depression scale scores (Psy vs Ap: SMD = -1.37, 95% CI: -2.64 to -0.11; Psy vs Mox: SMD = -2.37, 95% CI: -4.27 to -0.48).
Compared with AER, CFS patients who received Mox, diet therapy, or Ap + Mox treatment showed significant reductions in depression scale scores (Mox vs AER: SMD = -2.54, 95% CI: -4.75 to -0.31; AER vs. diet therapy: SMD = 5.26, 95% CI: 1.61 to 8.9; AER vs. Ap + Mox: SMD = 2.15, 95% CI: 0.2 to 4.12) (Table 2).
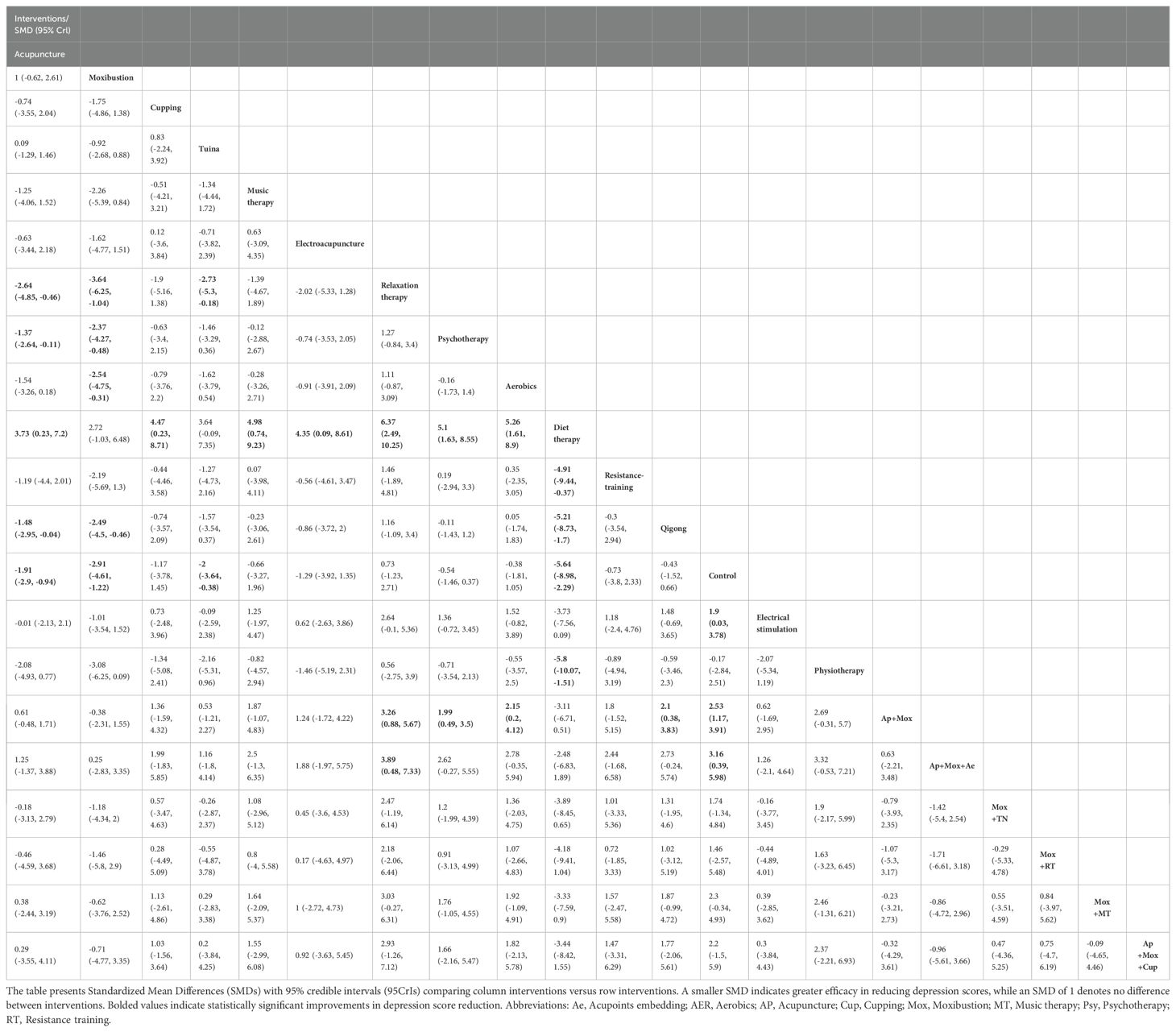
Table 2. Pairwise comparisons of the effect of non-exercise therapies on the reduction of depression scale scores in CFS patients.
SUCRA probability ranking results showed diet therapy (96.72%) > Mox (79.99%) > Ap + Mox + Ae (79.59%) > Ap + Mox (74.27%) > Mox + MT (66.38%). Diet therapy alone had the greatest effect on reducing depression scores in CFS patients (Figure 4).
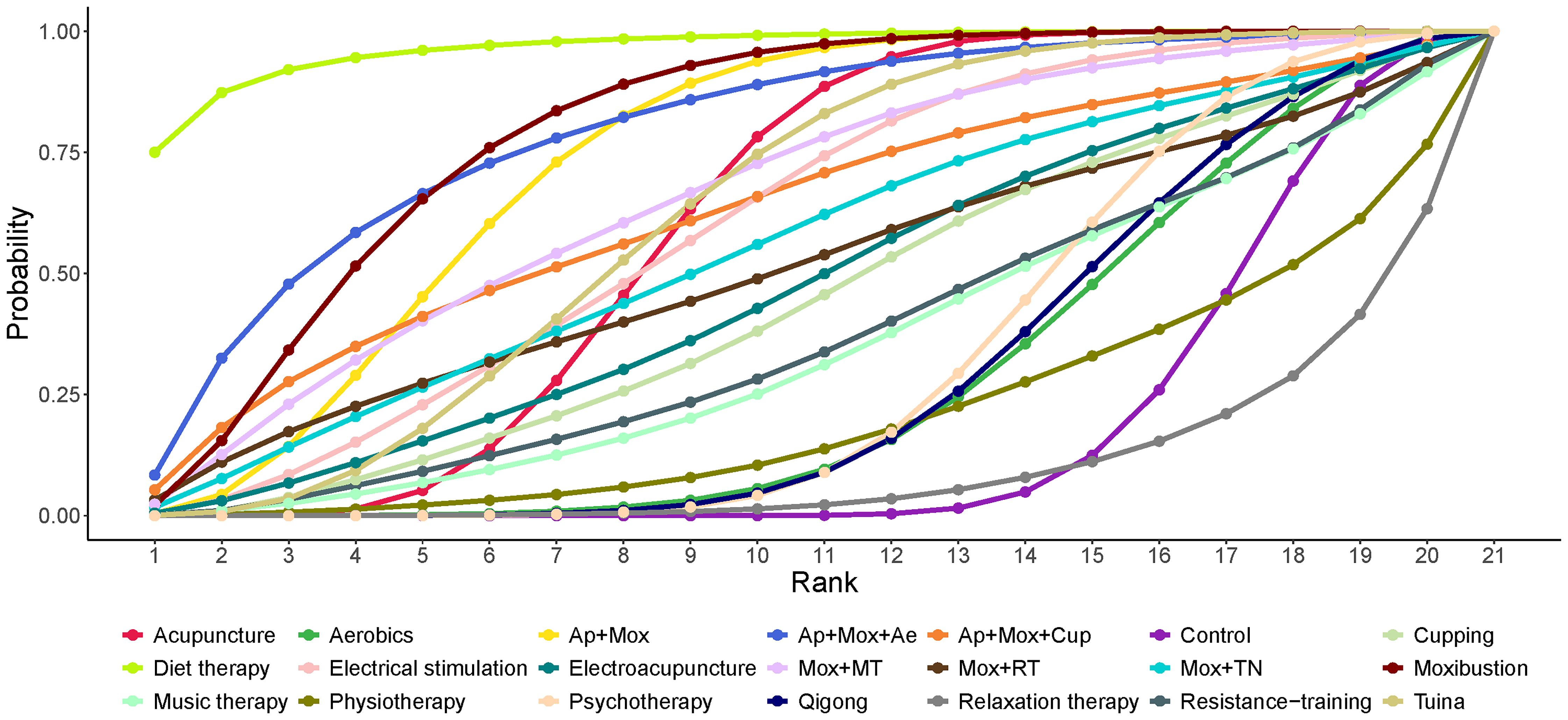
Figure 4. Area under the SUCRA curve for the effect of different non-pharmacological therapies on reducing depression scale scores in patients with CFS. Ae, Acupoints embedding; AER, Aerobics; AP, Acupuncture; Cup, Cupping; Mox, Moxibustion; MT, Music therapy; Psy, Psychotherapy; RT, Resistance training.
3.4 Publication bias
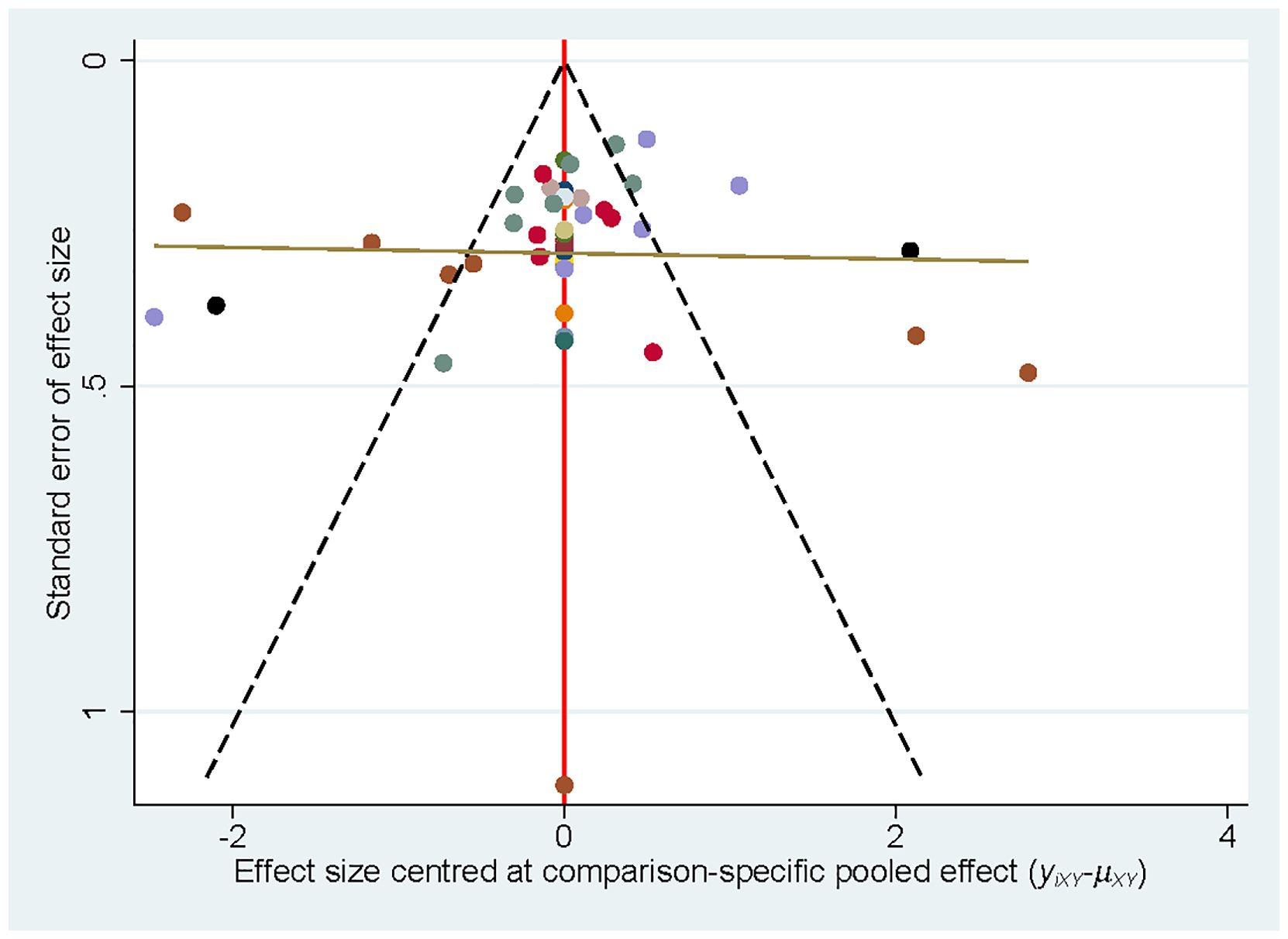
Publication bias was examined by plotting a correction-comparison funnel plot. The results showed symmetrical funnel plots, indicating no publication bias (Figure 5).
3.5 Meta-regression
A meta-regression analysis was performed on the sample size to explore the possible sources of heterogeneity and the stability of results. The results showed that the regression results were significant in pairwise comparisons of some intervention methods, indicating that sample size is a possible source of heterogeneity.
4 Discussion
In our results, Ap, Mox, Tuina, diet therapy, electrical stimulation, Ap + Mox, and Ap + Mox + Ae significantly reduced depression scores in CFS patients compared to the control intervention, demonstrating reliable efficacy in treating depression.
The high comorbidity rate between CFS and depression depends on the shared pathological mechanisms between the two conditions. Nakatomi et al. (29) found that high expression of activated microglia translocator protein in the hippocampus of CFS patients was positively correlated with depression scores. CFS patients with comorbid major depression exhibit mixed immune responses and neuroinflammation in widespread brain regions, which is associated with the severity of neuropsychological symptoms (30). CFS patients exhibit reduced tricarboxylic acid cycle activity, mitochondrial energy metabolism dysfunction, and systemic metabolic dysfunction (31). Mitochondrial energy metabolism dysfunction is also a pathological mechanism of depression (32). CFS and depression patients show abnormalities in hypothalamic-pituitary-adrenal (HPA) axis regulation and disrupted mechanisms of cortisol secretion feedback (33). Depressed and physically inactive CFS patients may present with hypocortisolemia (34). In CFS patients, the binding potential values of central 5-HT1A receptors, as well as the levels of serotonin (5-HT) transporters and receptors, are significantly reduced (35, 36). Meanwhile, 5-HT dysfunction is already recognized as a contributing factor to depression (37). Additionally, both CFS and depression patients exhibit a significant reduction in gray matter volume in the hippocampus and prefrontal cortex (38, 39). CFS patients exhibit reduced heart rate variability and cerebral blood flow (CBF) (40). A decrease in whole-brain average CBF is notably associated with experiencing more depressive episodes in adulthood (41). In summary, CFS and depression share mechanistic changes in immune-inflammatory responses, neuroendocrine regulation, and brain structure and function. These changes explain the high comorbidity rate between the two conditions and provide potential therapeutic targets for treating depressive symptoms in CFS patients.
In our results, diet therapy ranked first in terms of efficacy. The intervention measure in this study was chocolate rich in polyphenols. The active ingredient in cocoa, polyphenols, is a common antioxidant in food. Research has shown that the flavonoids in cocoa polyphenols have protective effects on neurons, shielding them from damage caused by oxidative stress (42). In addition, polyphenols regulate the transmission of monoamine neurotransmitters, reduce the circulation and brain concentration of pro-inflammatory mediators, regulate the HPA axis, promote hippocampal neurogenesis, enhance brain-derived neurotrophic factor (BDNF), and show antidepressant effects by regulating the composition of the intestinal microbiota via supporting the growth of beneficial bacteria and inhibiting pathogenic bacteria (43, 44). Polyphenols have also shown positive results in alleviating CFS symptoms (45). The study we included excluded the sweetness of chocolate and the potential pleasure derived from its energy-boosting effects. The study had high quality and low risk of bias. However, the sample size was small, and only one study was included. Therefore, the best efficacy of diet therapy (high-cocoa liquid/polyphenol-rich chocolate) in improving depressive symptoms in CFS patients should be interpreted with caution. Further studies are warranted to substantiate the efficacy of cocoa or diet therapy.
Ap, Mox, and Ap + Mox also showed favorable therapeutic effects. Ap and Mox are widely used traditional Chinese medicine methods. Both can dredge the meridians, promote the movement of qi and blood, and modulate the balance of qi and blood. Mox has a warming effect and is widely applied for diseases with weak symptoms, including CFS, which may explain why Mox is more effective than Ap. Laboratory evidence suggests that Mox effectively regulates the behavior, immune function, and HPA axis of rats with CFS, thereby alleviating fatigue symptoms (46, 47). Mox can also repair the intestinal barrier by regulating the intestinal flora structure of patients, significantly improving the fatigue status of CFS patients (48). Ap can suppress glial cell activation, mitigate neuroinflammation (49), repair damaged neural tissue structure in the hippocampus (50), regulate HPA axis dysfunction, inhibit HPA axis hyperactivity (51), and adjust gut microbiota abundance, thereby alleviating depressive symptoms in CFS.
Tuina therapy is a type of external treatment in traditional Chinese medicine, which has the functions of dredging meridians, regulating internal organs, and relaxing the mind and body. It also has the advantages of being simple, safe, and having no adverse reactions (52). Abdominal massage can reduce the organ indices of the hypothalamus, pituitary gland, and adrenal glands, key organs in the HPA axis in CFS rats, alleviate damage to hippocampal tissue, inhibit hippocampal cell apoptosis, and increase hippocampal cell viability, thereby helping to maintain the normal physiological functions of hippocampal neurons (53). In addition, in improving depressive behavior in rats with chronic stress, it can upregulate ERK phosphorylation in the hippocampus and prefrontal cortex, activate the ERK pathway, and promote the expression of the effector protein BDNF (54).
Electrical stimulation therapy includes tDCS and transcutaneous electrical nerve stimulation (TENS), which use weak electrical stimulation to regulate neural activity or relieve pain. Transcranial electrical stimulation is divided into anodal (positive) and cathodal (negative). Positive stimulation enhances cortical excitability, while negative stimulation reduces cortical excitability, primarily regulating brain neuronal activity and widely applied in the field of psychiatry. TEAS, on the other hand, acts on the skin surface. TENS can enhance the learning and memory abilities of CFS rats, possibly by improving tissue structure of the hippocampal CA1 region and upregulating ERK/CREB/BDNF expression (55). TENS can reduce serum IL-1β and IL-6 concentrations in patients with late-pregnancy depression (56). Through functional magnetic resonance imaging, Ma et al. (57) discovered that transcutaneous cranial-auricular acupoint electrical stimulation can modulate the function of the abnormal emotion-related brain network ‘insula-frontal lobe-limbic system’ and exert an antidepressant effect. Research has found that patients with depression exhibit reduced CBF and slowed metabolism in the left dorsolateral prefrontal cortex (DLPFC), while the right DLPFC shows accelerated metabolism. tDCS can enhance the excitability of the left DLPFC while inhibiting the right DLPFC, thereby regulating the activity of the brain’s emotional circuitry and alleviating depressive symptoms by stimulating the prefrontal cortex (58). A systematic review on fibromyalgia suggests that when tDCS is applied to the DLPFC, it improves patients’ fatigue symptoms (59).
Additionally, our results suggested that four types of exercise therapy, relaxation therapy, AER, RT, and Qigong, were less effective. P. Windthorst (60) et al. failed to observe any significant therapeutic effects of graded exercise therapy in improving depressive symptoms in CFS patients. Graded exercise therapy may exacerbate symptoms in certain CFS patients, possibly related to the characteristic PEM of CFS (61). Psychotherapy also did not show significant effects, which may be related to several factors, including the fact that CBT was not designed to target depressive symptoms in the included studies and the diverse etiology of CFS.
According to the SUCRA probability ranking results, we should give priority to recommending diet therapy as an intervention for depressive symptoms in CFS patients. However, due to the limited number of articles included in the diet therapy intervention, its therapeutic effect may be exaggerated. Therefore, we are currently more inclined to recommend Mox, which ranks second or third in the SUCRA probability ranking, and the combined treatment of Ap + Mox + Ae.
To our knowledge, this is the first study comparing non-pharmacological therapies for depression or depressive symptoms in patients with CFS. We conducted an extensive literature review and included the most comprehensive studies to date on non-pharmacological therapies for treating depressive symptoms in CFS. These strengths enable our findings to support clinicians in selecting appropriate treatment options based on patient tolerance, thereby facilitating personalized treatment approaches. Additionally, our findings provide evidence for the development of clinical guidelines.
However, this study has some limitations. The included studies exhibit a certain degree of heterogeneity. First, the scales used for depression outcomes were inconsistent. Although there was no significant heterogeneity in the outcomes when the SMD was used to merge the effect size, it could only reflect the aggravation or relief of the patient’s depressive symptoms through numerical changes, and there were still limitations in the interpretation of the results. The risk of bias in most included studies was medium; some interventions were included in a few studies with small sample sizes, so the results should be viewed with caution. Clinical studies with large sample sizes should be supplemented in future studies to reduce heterogeneity. Therefore, we recommend that future high-quality RCTs focus more on non-pharmacological therapies for treating depressive symptoms in CFS patients to enhance the reliability of research results. These results strengthen the existing evidence and provide valuable insights for patients, healthcare providers, and policymakers.
5 Conclusion
This study evaluated the efficacy of various non-pharmacological therapies in alleviating depression or depressive symptoms in patients with CFS through network analysis. The results showed that Ap, Mox, Tuina, diet therapy, electrical stimulation, Ap + Mox, and Ap + Mox + Ae were significantly more effective than the control intervention in reducing depression scale scores in CFS patients, demonstrating reliable efficacy in antidepressant effects. Diet therapy was the most effective, followed by moxibustion, Ap + Mox + Ae, Ap + Mox, and Mox + MT. Further high-quality RCTs are warranted to enrich this field of research.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
BJ: Formal Analysis, Investigation, Writing – original draft, Writing – review & editing. MC: Conceptualization, Writing – original draft, Supervision. XX: Writing – review & editing, Investigation, Formal Analysis. LW: Writing – review & editing, Supervision, Methodology, Resources, Funding acquisition.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was supported by the National Natural Science Foundation of China (grant numbers 8130 3044); the Natural Science Foundation of Heilongjiang Province (grant number LH2022H082); and the Heilongjiang Provincial Administration of Traditional Chinese Medicine Project (grant number ZHY2020-120).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2025.1657615/full#supplementary-material
Supplementary Figure 1 | Local inconsistency test for non-pharmacological therapies for depressive symptoms in CFS patients.
Supplementary Figure 2 | Density plot for convergence results.
Supplementary Figure 3 | Trajectory plot for PSRF convergence.
Supplementary Figure 4 | Local heterogeneity test results.
Abbreviations
Ae, Acupoints embedding; AER, Aerobics; AP, Acupuncture; BDI, Beck Depression Inventory; BDNF, Brain-derived neurotrophic factor; CBF, cerebral blood flow; CFS, chronic fatigue syndrome; Cup, Cupping; DSI, Depression Status Inventory; HPA, Hypothalamic-pituitary-adrenal axis; 5-HT, Serotonin; HADS, Hospital anxiety and depression scale; HAMD, Hamilton Depression Scale; Mox, Moxibustion; MT, Music therapy; PHQ-9, Patient Health Questionnaire-9; PROMIS Depression-SF, PROMIS Depression Short Form; Psy, Psychotherapy; RT, Resistance training; SCL-90, Symptom Checklist-90; SDS, Self-rating depression scale; tDCS, Transcranial direct current stimulation; TENS, Transcutaneous Electrical Nerve Stimulation.
References
1. Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue S, Board on the Health of Select P, and Institute of M. The National Academies Collection: Reports funded by National Institutes of Health. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness. Washington (DC: National Academies Press (2015).
2. Chen R, Zhang Z, and Wang Z. A study on the clinical characteristics of cognitive impairment in chronic fatigue syndrome. Liaoning J Traditional Chin Med. (2019) 46:1222–7. doi: 10.13192/j.issn.1000-1719.2019.06.033
3. Cui L, Li S, Wang S, Wu X, Liu Y, Yu W, et al. Major depressive disorder: hypothesis, mechanism, prevention and treatment. Signal Transduct Target Ther. (2024) 9:30. doi: 10.1038/s41392-024-01738-y
4. Noor N, Urits I, Degueure A, Rando L, Kata V, Cornett EM, et al. A comprehensive update of the current understanding of chronic fatigue syndrome. Anesth Pain Med. (2021) 11:e113629. doi: 10.5812/aapm.113629
5. Maes M, Twisk FN, and Ringel K. Inflammatory and cell-mediated immune biomarkers in myalgic encephalomyelitis/chronic fatigue syndrome and depression: inflammatory markers are higher in myalgic encephalomyelitis/chronic fatigue syndrome than in depression. Psychother Psychosom. (2012) 81:286–95. doi: 10.1159/000336803
6. Papadopoulos A, Ebrecht M, Roberts AD, Poon L, Rohleder N, and Cleare AJ. Glucocorticoid receptor mediated negative feedback in chronic fatigue syndrome using the low dose (0.5 mg) dexamethasone suppression test. J Affect Disord. (2009) 112:289–94. doi: 10.1016/j.jad.2008.05.001
7. Wright A, Fisher PL, Baker N, O'Rourke L, and Cherry MG. Perfectionism, depression and anxiety in chronic fatigue syndrome: A systematic review. J Psychosom Res. (2021) 140:110322. doi: 10.1016/j.jpsychores.2020.110322
8. Lehman AM, Lehman DR, Hemphill KJ, Mandel DR, and Cooper LM. Illness experience, depression, and anxiety in chronic fatigue syndrome. J Psychosom Res. (2002) 52:461–5. doi: 10.1016/s0022-3999(02)00318-5
9. Larkin D and Martin CR. The interface between chronic fatigue syndrome and depression: A psychobiological and neurophysiological conundrum. Neurophysiol Clin. (2017) 47:123–9. doi: 10.1016/j.neucli.2017.01.012
10. Bhui KS, Dinos S, Ashby D, Nazroo J, Wessely S, and White PD. Chronic fatigue syndrome in an ethnically diverse population: the influence of psychosocial adversity and physical inactivity. BMC Med. (2011) 9:26. doi: 10.1186/1741-7015-9-26
11. Daniels J, Parker H, and Salkovskis PM. Prevalence and treatment of Chronic Fatigue Syndrome/Myalgic Encephalomyelitis and co-morbid severe health anxiety. Int J Clin Health Psychol. (2020) 20:10–9. doi: 10.1016/j.ijchp.2019.11.003
12. Richman S, Morris MC, Broderick G, Craddock TJA, Klimas NG, and Fletcher MA. Pharmaceutical interventions in chronic fatigue syndrome: A literature-based commentary. Clin Ther. (2019) 41:798–805. doi: 10.1016/j.clinthera.2019.02.011
13. Chen C, Yip HT, Leong KH, Yao WC, Hung CL, Su CH, et al. Presence of depression and anxiety with distinct patterns of pharmacological treatments before the diagnosis of chronic fatigue syndrome: a population-based study in Taiwan. J Transl Med. (2023) 21:98. doi: 10.1186/s12967-023-03886-1
14. Lee JS, Kang JY, Park SY, Hwang SJ, Bae SJ, and Son CG. Central 5-HTergic hyperactivity induces myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)-like pathophysiology. J Transl Med. (2024) 22:34. doi: 10.1186/s12967-023-04808-x
15. Maas Genannt Bermpohl F, Kucharczyk-Bodenburg AC, and Martin A. Efficacy and acceptance of cognitive behavioral therapy in adults with chronic fatigue syndrome: A meta-analysis. Int J Behav Med. (2024) 31:895–910. doi: 10.1007/s12529-023-10254-2
16. López-López JA, Davies SR, Caldwell DM, Churchill R, Peters TJ, Tallon D, et al. The process and delivery of CBT for depression in adults: a systematic review and network meta-analysis. Psychol Med. (2019) 49:1937–47. doi: 10.1017/s003329171900120x
17. Xue K, Quan F, Tang J, Xiao C, Lu C, and Cui J. Bamboo moxibustion therapy for chronic fatigue syndrome: a randomized controlled trial. Chin Acupuncture. (2023) 43:493–8. doi: 10.13703/j.0255-2930.20220818-k0001
18. Gordon BA, Knapman LM, and Lubitz L. Graduated exercise training and progressive resistance training in adolescents with chronic fatigue syndrome: a randomized controlled pilot study. Clin Rehabil. (2010) 24:1072–9. doi: 10.1177/0269215510371429
19. Watt JA, Goodarzi Z, Veroniki AA, Nincic V, Khan PA, Ghassemi M, et al. Comparative efficacy of interventions for reducing symptoms of depression in people with dementia: systematic review and network meta-analysis. Bmj. (2021) 372:n532. doi: 10.1136/bmj.n532
20. Hoffmann K, Hainzl A, Stingl M, Kurz K, Biesenbach B, Bammer C, et al. Interdisciplinary, collaborative D-A-CH (Germany, Austria and Switzerland) consensus statement concerning the diagnostic and treatment of myalgic encephalomyelitis/chronic fatigue syndrome. Wien Klin Wochenschr. (2024) 136:103–23. doi: 10.1007/s00508-024-02372-y
21. Qaseem A, Barry MJ, and Kansagara D. Nonpharmacologic versus pharmacologic treatment of adult patients with major depressive disorder: A clinical practice guideline from the american college of physicians. Ann Intern Med. (2016) 164:350–9. doi: 10.7326/m15-2570
22. Kuut TA, Buffart LM, Braamse AMJ, Müller F, and Knoop H. Is the effect of cognitive behaviour therapy for chronic fatigue syndrome (ME/CFS) moderated by the presence of comorbid depressive symptoms? A meta-analysis of three treatment delivery formats. J Psychosom Res. (2024) 184:111850. doi: 10.1016/j.jpsychores.2024.111850
23. Geraghty K, Hann M, and Kurtev S. Myalgic encephalomyelitis/chronic fatigue syndrome patients' reports of symptom changes following cognitive behavioural therapy, graded exercise therapy and pacing treatments: Analysis of a primary survey compared with secondary surveys. J Health Psychol. (2019) 24:1318–33. doi: 10.1177/1359105317726152
24. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
25. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. Bmj. (2019) 366:l4898. doi: 10.1136/bmj.l4898
26. van Valkenhoef G, Lu G, de Brock B, Hillege H, Ades AE, and Welton NJ. Automating network meta-analysis. Res Synth Methods. (2012) 3:285–99. doi: 10.1002/jrsm.1054
27. Jansen JP, Crawford B, Bergman G, and Stam W. Bayesian meta-analysis of multiple treatment comparisons: an introduction to mixed treatment comparisons. Value Health. (2008) 11:956–64. doi: 10.1111/j.1524-4733.2008.00347.x
28. van de Schoot R, Broere JJ, Perryck KH, Zondervan-Zwijnenburg M, and van Loey NE. Analyzing small data sets using Bayesian estimation: the case of posttraumatic stress symptoms following mechanical ventilation in burn survivors. Eur J Psychotraumatol. (2015) 6:25216. doi: 10.3402/ejpt.v6.25216
29. Nakatomi Y, Mizuno K, Ishii A, Wada Y, Tanaka M, Tazawa S, et al. Neuroinflammation in patients with chronic fatigue syndrome/myalgic encephalomyelitis: an ¹¹C-(R)-PK11195 PET study. J Nucl Med. (2014) 55:945–50. doi: 10.2967/jnumed.113.131045
30. Maes M and Carvalho AF. The compensatory immune-regulatory reflex system (CIRS) in depression and bipolar disorder. Mol Neurobiol. (2018) 55:8885–903. doi: 10.1007/s12035-018-1016-x
31. Fluge Ø, Mella O, Bruland O, Risa K, Dyrstad SE, Alme K, et al. Metabolic profiling indicates impaired pyruvate dehydrogenase function in myalgic encephalopathy/chronic fatigue syndrome. JCI Insight. (2016) 1:e89376. doi: 10.1172/jci.insight.89376
32. Chen H, Ma X, Wang Y, Xue S, Zhou C, Peng Z, et al. Recent advances in research on mitochondrial dysfunction and the pathogenesis of depression. J Neuroanatomy. (2023) 39:476–80. doi: 10.16557/j.cnki.1000-7547.2023.04.015
33. Wang Y. Neuroendocrine mechanisms of the HPA axis and 5-HT system in patients with chronic fatigue syndrome and advances in Chinese and Western medical treatment. Modern Distance Educ Traditional Chin Med China. (2016) 14:143–6. doi: 10.3969/j.issn.1672-2779.2016.19.062
34. Papadopoulos AS and Cleare AJ. Hypothalamic-pituitary-adrenal axis dysfunction in chronic fatigue syndrome. Nat Rev Endocrinol. (2011) 8:22–32. doi: 10.1038/nrendo.2011.153
35. Cleare AJ, Messa C, Rabiner EA, and Grasby PM. Brain 5-HT1A receptor binding in chronic fatigue syndrome measured using positron emission tomography and [11C]WAY-100635. Biol Psychiatry. (2005) 57:239–46. doi: 10.1016/j.biopsych.2004.10.031
36. Badawy AA, Morgan CJ, Llewelyn MB, Albuquerque SR, and Farmer A. Heterogeneity of serum tryptophan concentration and availability to the brain in patients with the chronic fatigue syndrome. J Psychopharmacol. (2005) 19:385–91. doi: 10.1177/0269881105053293
37. Kraus C, Castrén E, Kasper S, and Lanzenberger R. Serotonin and neuroplasticity - Links between molecular, functional and structural pathophysiology in depression. Neurosci Biobehav Rev. (2017) 77:317–26. doi: 10.1016/j.neubiorev.2017.03.007
38. Okada T, Tanaka M, Kuratsune H, Watanabe Y, and Sadato N. Mechanisms underlying fatigue: a voxel-based morphometric study of chronic fatigue syndrome. BMC Neurol. (2004) 4:14. doi: 10.1186/1471-2377-4-14
39. Jia Y, Chen J, Liu T, and Zhong S. Recent advances in research on working memory and brain imaging in patients with major depressive disorder. J Clin Psychiatry. (2016) 26:55–7.
40. Boissoneault J, Letzen J, Robinson M, and Staud R. Cerebral blood flow and heart rate variability predict fatigue severity in patients with chronic fatigue syndrome. Brain Imaging Behav. (2019) 13:789–97. doi: 10.1007/s11682-018-9897-x
41. Chiappelli J, Adhikari BM, Kvarta MD, Bruce HA, Goldwaser EL, Ma Y, et al. Depression, stress and regional cerebral blood flow. J Cereb Blood Flow Metab. (2023) 43:791–800. doi: 10.1177/0271678x221148979
42. Ishige K, Schubert D, and Sagara Y. Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free Radic Biol Med. (2001) 30:433–46. doi: 10.1016/s0891-5849(00)00498-6
43. Park M, Choi J, and Lee HJ. Flavonoid-rich orange juice intake and altered gut microbiome in young adults with depressive symptom: A randomized controlled study. Nutrients. (2020) 12(6):1815–30. doi: 10.3390/nu12061815
44. Gamage E, Orr R, Travica N, Lane MM, Dissanayaka T, Kim JH, et al. Polyphenols as novel interventions for depression: Exploring the efficacy, mechanisms of action, and implications for future research. Neurosci Biobehav Rev. (2023) 151:105225. doi: 10.1016/j.neubiorev.2023.105225
45. Jones K and Probst Y. Role of dietary modification in alleviating chronic fatigue syndrome symptoms: a systematic review. Aust N Z J Public Health. (2017) 41:338–44. doi: 10.1111/1753-6405.12670
46. Zhao L and Jiang G. The effects of moxibustion on the behavior and hypothalamic-pituitary-adrenal axis hormone levels of rats with chronic fatigue syndrome model. Shandong J Traditional Chin Med. (2014) 33:301–3. doi: 10.16295/j.cnki.0257-358x.2014.04.023
47. Lin Y, Jiang G, Li Y, and Cai J. The effects of moxibustion on the behavioral and immune systems of rats with chronic fatigue syndrome. Shanghai J Traditional Chin Med. (2017) 51:93–6. doi: 10.16305/j.1007-1334.2017.06.027
48. Lin Y, Jin X, Zhu J, Chen Y, Sheng J, He J, et al. The effect of ginger moxibustion therapy on chronic fatigue syndrome and its impact on patients' Gut microbiota. Chin Acupuncture. (2021) 41:269–74. doi: 10.13703/j.0255-2930.20200210-k0001
49. Lin S, Zhu M, Chen W, Zhang Y, Lin J, Pu L, et al. Acupuncture stimulation of Yamen (GV 15), Fengfu (GV 16), Baihui (GV 20), Shuigou (GV 26) and Hegu (LI 4) reduces brain microglia activation in a traumatic brain injury rat model. J Tradit Chin Med. (2020) 40:267–74.
50. Liu C, Lei B, and Zhang P. The effects of acupuncture on cognitive function and hippocampal neurons in rats with chronic fatigue syndrome. Chin J Rehabil Med. (2021) 36:1009–11. doi: 10.1097/CM9.0000000000001498
51. Qu C, Xie K, Qu J, and Liu B. Experimental study on the effects of acupuncture on HPA axis function in rats with chronic fatigue caused by multiple factors. Liaoning J Traditional Chin Med. (2010) 37:2055–7. doi: 10.13192/j.ljtcm.2010.10.203.quzhj.076
52. Hou R, Wang S, Li Y, Gao J, Sang J, and Zhao M. Clinical research progress on tuina intervention for patients with depression. World Traditional Chin Med. (2024) 19:887–92. doi: 10.3969/j.issn.1673-7202.2024.06.022
53. An C, Zhang X, Ning J, Tan T, and Li H. The effects of abdominal massage on organ indices, hippocampal cell morphology, and cell apoptosis in rats with chronic fatigue syndrome model. J Beijing Univ Chin Med. (2024) 47:845–52. doi: 10.3969/j.issn.1006-2157.2024.06.016
54. Du J, Li J, Sun P, Wang Y, and Zhang J. The effect of massage on depressive behavior in chronically stressed rats and its mechanism. Chin J Appl Physiol. (2021) 37:327–31. doi: 10.12047/j.cjap.6088.2021.025
55. Zhong X, Tong B, Yang Y, Zeng H, Lin C, Jing Y, et al. The effect of transcutaneous electrical nerve stimulation on learning and memory abilities in rats with chronic fatigue syndrome and discussion of its mechanism. Acupuncture Res. (2023) 48:317–24. doi: 10.13702/j.1000-0607.20221032
56. Chen W, Li L, Wang H, and Jiang N. The effect of transcutaneous electrical nerve stimulation on depression and serum inflammatory cytokines in late pregnancy. Acupuncture Res. (2018) 43:43–7. doi: 10.13702/j.1000-0607.160781
57. Ma Y, He J, Lu X, Sun J, Guo C, Luo Y, et al. Transcutaneous cranial-auricular acupoint electrical stimulation “regulating the hub and awakening the spirit” anti-depressant brain effect rs-f MRI study. Chin J Traditional Chin Med. (2023) 38:2048–54.
58. Zhang L, Guo D, Liu S, Liu X, Sheng Y, and Ming D. Research progress on transcranial direct current stimulation (tDCS) for the treatment of depression. Chin J Biomed Engineering. (2018) 37:616–24. doi: 10.3969/j.issn.0258-8021.2018.05.013
59. Conde-Antón Á, Hernando-Garijo I, Jiménez-Del-Barrio S, Mingo-Gómez MT, Medrano-de-la-Fuente R, and Ceballos-Laita L. Effects of transcranial direct current stimulation and transcranial magnetic stimulation in patients with fibromyalgia. A systematic review Neurologia (Engl Ed). (2023) 38:427–39. doi: 10.1016/j.nrleng.2020.07.025
60. Windthorst P, Mazurak N, Kuske M, Hipp A, Giel KE, Enck P, et al. Heart rate variability biofeedback therapy and graded exercise training in management of chronic fatigue syndrome: An exploratory pilot study. J Psychosom Res. (2017) 93:6–13. doi: 10.1016/j.jpsychores.2016.11.014
Keywords: depression, non-pharmacological therapy, network meta-analysis, systematic review, chronic fatigue syndrome
Citation: Jiang B, Cao M, Xia X and Wang L (2025) Effect of nonpharmacologic therapies on depressive symptoms in patients with chronic fatigue syndrome: a network meta-analysis. Front. Psychiatry 16:1657615. doi: 10.3389/fpsyt.2025.1657615
Received: 01 July 2025; Accepted: 31 July 2025;
Published: 19 August 2025.
Edited by:
Guang Chen, The University of Hong Kong, Hong Kong SAR, ChinaReviewed by:
Jiayan Zhou, Stanford University, United StatesGuanghui Zhu, China Academy of Chinese Medical Sciences, China
Copyright © 2025 Jiang, Cao, Xia and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Long Wang, d2xrZXlhbkAxNjMuY29t
 Baiyi Jiang
Baiyi Jiang Mengru Cao
Mengru Cao Xue Xia
Xue Xia Long Wang
Long Wang