- 1The Fourth People’s Hospital of Chengdu, Chengdu, Sichuan, China
- 2The Clinical Hospital of Chengdu Brain Science Institute, MOE Key Lab for Neuroinformation, University of Electronic Science and Technology of China, Chengdu, Sichuan, China
Objective: To investigate the severity and influencing factors of hyperprolactinemia (HPRL) in hospitalized schizophrenia patients.
Methods: This retrospective study enrolled schizophrenia inpatients from a tertiary psychiatric hospital (2022-2023) with monitored prolactin (PRL) levels. Participants were categorized into normal PRL, mild HPRL, moderate HPRL, and severe HPRL groups. Laboratory indices and medication information were collected, and an ordered logistic regression modeling was conducted to analyze the influence of HPRL severity.
Results: Among 3,641 hospitalized schizophrenia patients, 2,519 (69.18%) underwent PRL monitoring during hospitalization. A total of 1,425 patients were included for HPRL severity analysis, with 903 (63.40%) exhibiting HPRL (mild: 52.05%, moderate: 30.01%, severe: 17.94%). The mean PRL level was 983.66 ± 1001.98 mIU/L, with severe HPRL reaching 3233.66 ± 1001.98 mIU/L. The ordered multivariate logistic regression model showed that HPRL severity was negatively correlated with aripiprazole use, male sex, fasting glucose, aspartate aminotransferase (AST), and follicle-stimulating hormone (FSH), but positively correlated with the use of sulpiride, paliperidone, amisulpride, risperidone, blonanserin, trihexyphenidyl, and anxiolytics.
Conclusion: HPRL is highly prevalent in schizophrenia patients, with distinct clinical profiles across severity levels. HPRL severity is associated with specific antipsychotics, anxiolytics, trihexyphenidyl, and metabolic indicators, underscoring the need for risk stratification and individualized management.
1 Introduction
Hyperprolactinemia (HPRL) refers to a state of persistently elevated prolactin (PRL) levels in peripheral blood (1). HPRL may be asymptomatic despite high PRL levels, or it may present with manifestations such as amenorrhea, galactorrhea, gynecomastia, and sexual dysfunction (2). Growing evidence suggests associations between HPRL and disorders involving multiple target systems, including the digestive, reproductive, immune, nervous, endocrine, and integumentary systems (3–5). Notably, long-term HPRL increases the risk of osteoporosis, gynecological tumors, cardiovascular diseases, and cognitive dysfunction (6–9). Studies indicate that higher PRL levels correlate with a broader spectrum of HPRL-related adverse effects, necessitating prompt intervention for symptomatic or severe HPRL (10, 11).
Schizophrenia is recognized as one of the most severe mental disorders, with studies demonstrated that HPRL affects up to 70% of patients overall (12, 13). HPRL in schizophrenia patients was often overlooked due to the lack of overt symptoms; however, given its long-term consequences, an increasing number of guidelines and expert consensus statements recommend tailored interventions based on HPRL severity (2, 14–16). The unequivocal and extensive negative impacts of HPRL demand proactive intervention and meticulous management (17).
HPRL in schizophrenia is typically attributed to antipsychotic medications (APDs) (2, 9, 18). However, APDs use may not be the only contributing factor, as increased PRL levels have been observed in many treatment-naïve first-episode schizophrenia patients (19, 20). Furthermore, few studies have focused on the severity of HPRL in this population, and the specific factors influencing HPRL severity remain systematically unelucidated. Therefore, this study analyzes the clinical characteristics and determinants of varying HPRL severity levels in hospitalized schizophrenia patients, aiming to provide a basis for early identification and stratified management of HPRL by severity.
2 Materials and methods
2.1 Study design and ethics statement
This retrospective cohort study was conducted at the Fourth People’s Hospital of Chengdu, a tertiary psychiatric hospital in China. Inpatients diagnosed with schizophrenia (aged 18–75 years) between January 2022 and December 2023 were included. Inclusion criteria:(1) Diagnosis of schizophrenia according to the International Classification of Diseases, 10th Revision (ICD-10); (2) Available prolactin (PRL) measurement data during hospitalization; (3) For patients with multiple PRL tests, the highest value was used for analysis. Exclusion criteria: (1) History of Parkinson’s disease, hypothalamic-pituitary disorders, or other organic brain diseases; (2) Pregnancy or lactation; (3) Ongoing intensive care unit admission (ICU); (4) Incomplete clinically relevant data.
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Committee of the Fourth People’s Hospital of Chengdu. All patients were informed that their clinical data might be used for research purposes prior to data collection.
2.2 Study group stratification and data collection
Fasting venous blood samples were collected in the morning, and serum PRL levels were measured using chemiluminescent immunoassay. HPRL was defined as serum PRL ≥530 mIU/L for females and ≥424 mIU/L for males (2). Severity stratification was as follows: (1) Mild HPRL: HPRL and PRL <1060 mIU/L; (2) Moderate HPRL: PRL ≥1060 mIU/L and <2120 mIU/L; (3) Severe HPRL: PRL ≥2120 mIU/L (2, 18).
Patients were categorized into normal PRL, mild HPRL, moderate HPRL, and severe HPRL groups. Clinical data were retrospectively extracted from electronic medical records, including demographics (age, sex), medical history, comorbidities, family history, laboratory tests (serum chemistry, hormonal profiles), medication use, and repetitive transcranial magnetic stimulation (rTMS) treatments at the time of PRL testing. Variables with significant intergroup differences (P<0.05) were included in the ordinal logistic regression model.
2.3 Statistical analysis
In this study, continuous variables were summarized as means ± standard deviations, and compared using one-way analysis of variance (ANOVA). Categorical variables were summarized as frequencies or percentages (%) and analyzed by chi-square test. The Bonferroni correction was applied for pairwise comparisons, with the adjusted significance level set as α=0.05/56 = 0.00089. An ordinal logistic regression model was used to explore risk factors for HPRL severity. Statistical analysis was performed using SPSS version 25.0 for Windows (SPSS Inc, Chicago, IL) and a two-sided P < 0.05 was considered statistically significant.
3 Results
3.1 Severity distribution of HPRL in hospitalized schizophrenia patients
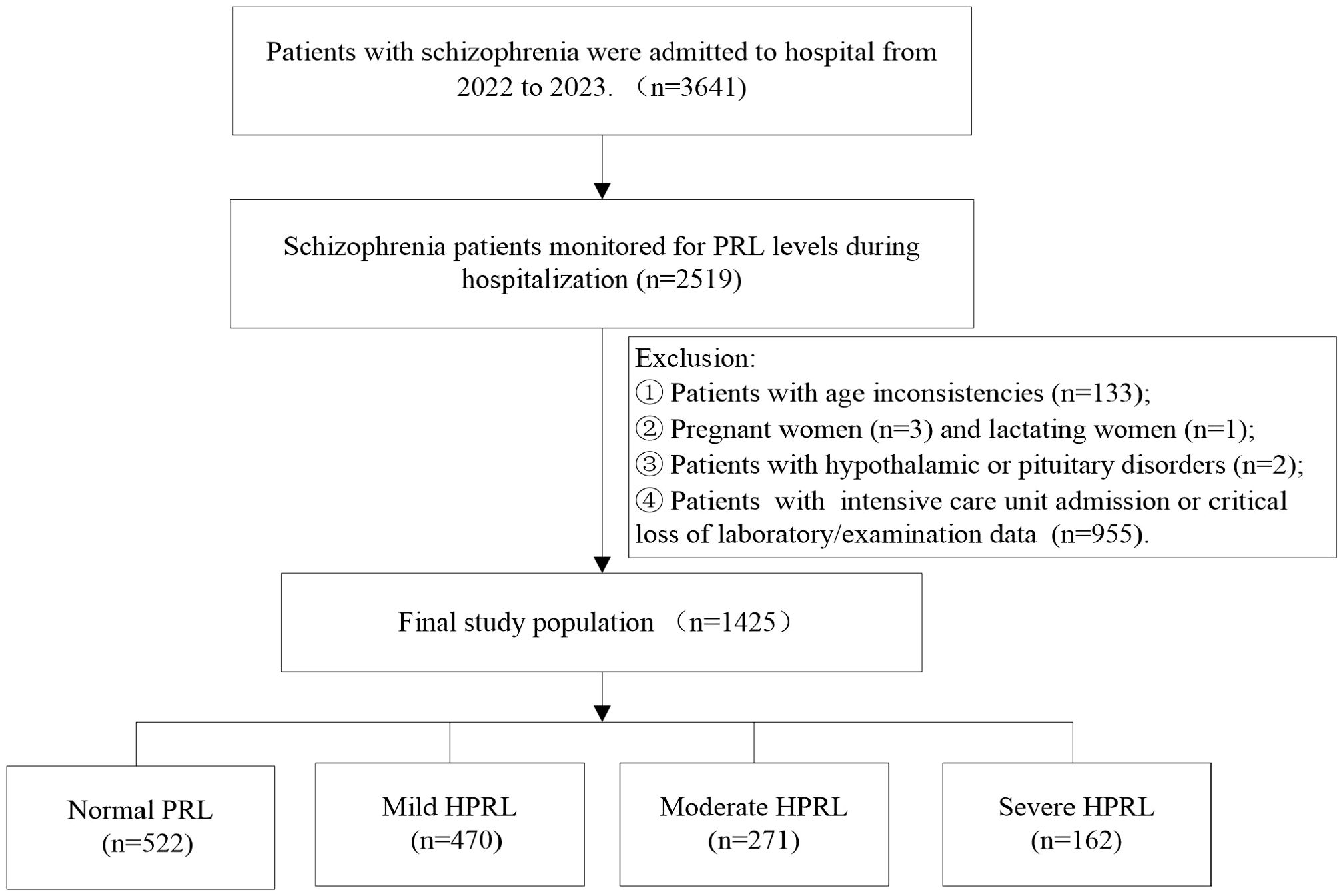
During 2022-2023, a total of 3,641 inpatient schizophrenia patients were enrolled, among whom 2,519 (69.18%) had their PRL levels monitored. HPRL occurred in 1,612 cases, with an incidence rate of 63.99% (1,612/2,519).
According to inclusion and exclusion criteria, 1,425 inpatient schizophrenia patients were finally included for analysis of influencing factors of HPRL severity: The mean age was 44.57 ± 15.11 years, including 616 males (43.23%) and 809 females (56.77%). Among them, 522 cases were in the normal group, and 903 cases had HPRL. The numbers of mild, moderate, and severe HPRL were 470 (52.05%), 271 (30.01%), and 162 (17.94%), respectively (Figure 1). The mean PRL level was 983.66 ± 1,001.98 mIU/L. The PRL levels in the normal group, mild, moderate, and severe HPRL groups were 264.70 ± 125.4 mIU/L, 771.63 ± 171.73 mIU/L, 1,487.22 ± 289.69 mIU/L, and 3,233.66 ± 1,001.98 mIU·L-1, respectively.
3.2 Influencing factors of HPRL severity in hospitalized schizophrenia patients
Intergroup comparisons showed significant differences in thyroid function panel, testosterone, progesterone, follicle-stimulating hormone (FSH), high-density lipoprotein (HDL), lactate dehydrogenase (LDH), alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), alanine aminotransferase (ALT), bilirubin parameters (indirect, direct, total), uric acid, blood urea nitrogen (BUN), fasting glucose, and frequency of repetitive transcranial magnetic stimulation (rTMS) (P < 0.05). Demographic/clinical factors with statistical significance included sex, diabetes status, use of anxiolytics, trihexyphenidyl, sulpiride, perphenazine, amisulpride, aripiprazole, olanzapine, risperidone, paliperidone, blonanserin, antipsychotic combination therapy, and rTMS exposure (P< 0.05; Table 1).
Ordinal logistic regression analysis revealed that HPRL severity was negatively associated with aripiprazole use [OR=0.637, 95%CI (0.487, 0.823)], male sex [OR=0.171, 95%CI (0.017, 0.854)], glucose levels [OR=0.866, 95%CI (0.803, 0.934)], aspartate aminotransferase (AST) [OR=0.978, 95%CI (0.969, 0.987)], and FSH [OR=0.987, 95%CI (0.983, 0.992)]. Positive associations were observed with sulpiride [OR=10.281, 95%CI (1.892, 55.634)], paliperidone [OR=7.735, 95%CI (4.285, 13.932)], amisulpride [OR=6.746, 95%CI (3.245, 14.021)], risperidone [OR=4.621, 95%CI (3.135, 6.827)], blonanserin [OR=3.826, 95%CI (1.495, 9.798)], trihexyphenidyl [OR=3.006, 95%CI (2.035, 4.465)], and anxiolytic use [OR=1.863, 95%CI (1.095, 3.164; Table 2)].
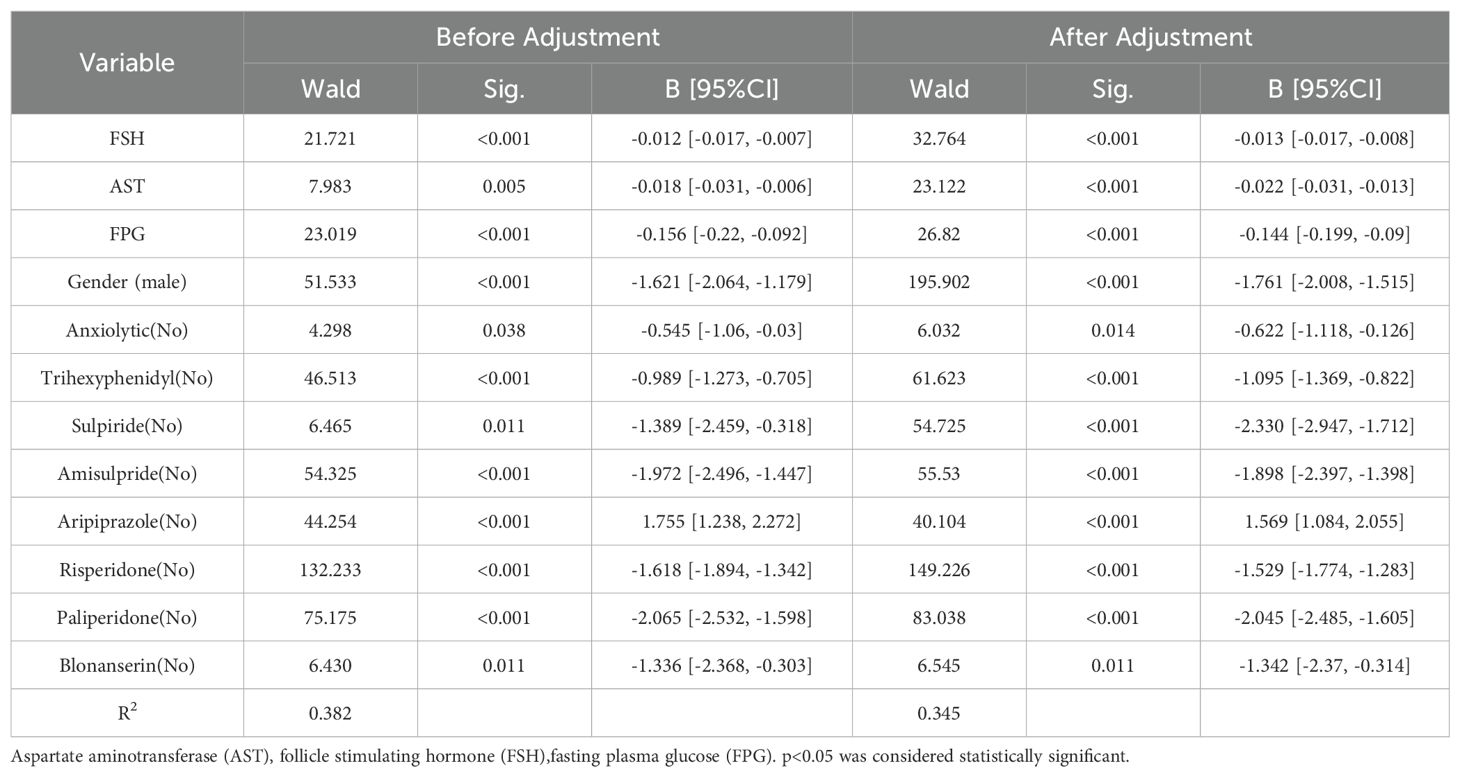
Table 2. Ordered logistic regression analysis of factors influencing HPRL severity in schizophrenia patients.
4 Discussion
HPRL is frequently overlooked in clinical practice due to the absence of obvious outward symptoms and patients’ reluctance to report symptoms they perceive as shameful (17, 21). This study revealed that only 69.18% of hospitalized schizophrenia patients underwent PRL monitoring. However, given the long-term effects of HPRL and the fact that schizophrenia patients usually need to maintain the treatment plan during hospitalization after discharge, it is necessary to monitor PRL levels during hospitalization and choose treatment plans with less impact on PRL (2, 22). It is recommended that psychiatrists pay more attention to PRL monitoring.
Among monitored patients, the HPRL incidence was 63.40%, marginally lower than previous reports, which might be due to selection bias in PRL monitoring. Notably, while HPRL severity was predominantly mild-to-moderate, a substantial proportion (17.94%) exhibited severe HPRL (mean PRL = 3233.66 ± 1001.98 mIU/L), exceeding the upper normal limit by 6-7-fold. This finding is clinically significant, as PRL levels correlate directly with the risk and extent of HPRL-related adverse effects. Severe HPRL necessitates intervention regardless of symptomatology due to established associations with accelerated osteoporosis and cardiovascular morbidity, and studies have also suggested that there may be a correlation between PRL levels and the occurrence of breast cancer (6, 8, 14). Consequently, identifying determinants of HPRL severity warrants focused attention.
Our analysis revealed significant differences across HPRL severity strata in demographics, thyroid/sex hormones, hepatic/renal function and glucose metabolism. Except for thyroid hormones and sex hormones, the lower the values of liver function indicators (AST, ALT, IB, TB), uric acid, and urea nitrogen, the higher the PRL level. These variations may be linked to PRL’s broad metabolic effects, encompassing its roles in glucose-insulin homeostasis, lipid metabolism, and hepatic/renal regulation (23–25). Beyond the well-documented influences of antipsychotic drugs and female sex, the use of anxiolytics and trihexyphenidyl also varied significantly with severity—a finding not widely recognized in clinical practice. Furthermore, the more rTMS treatments received, the higher the PRL level. rTMS, a non-invasive brain stimulation technique, has demonstrated efficacy in alleviating negative symptoms and cognitive dysfunction in schizophrenia. However, current evidence regarding rTMS’s effect on PRL remains controversial, with studies reporting both increases and decreases in PRL levels (26, 27). Collectively, these findings underscore the complex, multifactorial nature of PRL regulation and the broad physiological roles of PRL, necessitating further research for validation.
4.1 Factors positively associated with HPRL severity
Our study found that the severity of HPRL was positively correlated with the use of APDs (sulpiride, paliperidone, amisulpride, risperidone, blonanserin), anxiolytics and trihexyphenidyl. PRL is released from the anterior pituitary and is inhibited by dopamine (3). Most APDs can block the D2 receptor in the brain, leading to de-inhibition of prolactin secretion and an increase in PRL (18, 28, 29). Among APDs, there are prolactin-sparing drugs (such as aripiprazole) and prolactin-raising drugs (such as risperidone and amisulpride) (30). The ability of antipsychotic drugs to cross the blood-brain barrier, their affinity for DRD2, and their selectivity are related to the severity of HPRL (2). Previous studies have reported that sulpiride, paliperidone, amisulpride, risperidone, and blonanserin increase PRL, and we further found that their severity was positively correlated with HPRL.
Furthermore, we also found that the use of anxiolytics was positively correlated with the severity of HPRL. In clinical treatment, schizophrenia patients may use drugs such as buspirone and tandospirone to treat anxiety. Studies have shown that the anti-anxiety drug buspirone increases PRL secretion through a dopaminergic mechanism (31, 32). Therefore, we should also pay attention to the effects of anti-anxiety drugs and anxiety itself on PRL.
Interestingly, we found that the use of the anticholinergic drug trihexyphenidyl was positively correlated with HPRL, which may be related to the need for trihexyphenidyl in combination with antipsychotic drugs to treat extrapyramidal symptoms (EPS), and the latter may indirectly affect dopaminergic regulation. It is also possible that the severity of EPS is positively correlated with the severity of HPRL. However, some studies have shown that patients with Parkinson’s disease have higher PRL levels, possibly because of the degeneration of dopaminergic neurons in Parkinson’s disease, and trihexyphenidyl, as an oral anticholinergic drug, does not cause an increase in PRL levels (33, 34). The precise role of trihexyphenidyl in HPRL severity merits dedicated study.
4.2 Factors negatively associated with HPRL severity
We found that the severity of HPRL in patients with schizophrenia was negatively correlated with the use of aripiprazole, male gender, glucose levels, follicle-stimulating hormone (FSH), and aspartate aminotransferase (AST). Multiple studies have confirmed that aripiprazole can reduce PRL levels and is recommended for the treatment of HPRL caused by antipsychotic drugs (14, 35, 36). Aripiprazole is a potent partial dopamine D2 agonist, which means that in the case of other antipsychotic drugs blocking D2 receptors and causing low dopamine activity, aripiprazole acts as a dopamine agonist, restoring the tonic inhibition of prolactin cells in the anterior pituitary, thereby reducing PRL (14).
Males are less likely to develop HPRL than females, and this difference may be due to the fact that female estrogen can directly stimulate the proliferation and hypertrophy of PRL cells and promote PRL release (37, 38). This study found that the fasting blood glucose in the PRL normal group was higher than that in the PRL elevated group, but both were within the normal blood glucose range. Studies have found that patients with schizophrenia and type 2 diabetes mellitus have lower PRL levels (17, 39). PRL has complex effects on glucose metabolism. Studies have shown that hypoglycemic stress leads to a transient increase in PRL, and low levels of PRL are associated with insulin resistance (39, 40). The mutual regulation relationship between PRL and blood glucose needs further study.
The severity of HPRL demonstrated a negative correlation with FSH levels. This association may stem from HPRL impairing the pulsatile secretion of hypothalamic gonadotropin-releasing hormone (GnRH), consequently suppressing FSH secretion (3). HPRL is negatively correlated with AST, possibly because PRL is metabolized in the liver. Similarly, an inverse correlation was observed between HPRL severity and AST levels, potentially reflecting hepatic metabolism of PRL. Conversely, emerging evidence suggests that elevated PRL may represent an endogenous protective mechanism, mitigating liver injury through currently undefined pathways (24). This compensatory role is further supported by observations that prolactin levels increase proportionally with the severity of liver cirrhosis (41, 42). While these inverse relationships offer valuable insights for modulating PRL levels, their underlying mechanisms require further elucidation.
5 Limitations
Our study also had some limitations. The retrospective design limited our ability to account for confounders like obesity and smoking, potentially affecting result accuracy. We also lacked data on medication duration, switching history, and specific dosages, hindering a complete interpretation of medication-HPRL severity associations. Secondly, although PRL testing is part of the clinical pathway for treating schizophrenia in China, there were still a few patients who did not have their PRL levels tested during hospitalization, which may result in incomplete or biased data. Thirdly, findings from this single-center study may not generalize to other settings. Future prospective, multi-center studies with detailed medication records and control for confounders are needed.
6 Conclusions
This cross-sectional study analyzed the severity distribution and risk factors of HPRL in hospitalized patients with schizophrenia. The results revealed a high prevalence of HPRL in this patient population, with its severity being significantly associated with specific antipsychotics, anxiolytics, trihexyphenidyl, and metabolic indicators. These findings thus highlight the importance of implementing risk stratification and personalized management approaches in this patient cohort.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the ethics committee of the Institutional Review Committee of the Fourth People’s Hospital of Chengdu. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YY: Data curation, Methodology, Writing – original draft, Formal analysis, Funding acquisition. LL: Writing – review & editing, Funding acquisition. MY: Methodology, Writing – review & editing, Funding acquisition.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was funded by National Natural Science Foundation of China (grant number 62373079), Science and Technology Department of Sichuan Province (grant number 2025ZNSFSC0734), Health Commission of Sichuan Province (grant number 24CXTD11), Health Commission of Chengdu (grant number 2024141, grant number 2024254, grant number 2025495).
Acknowledgments
Address all correspondence and requests for reprints No. 8, Hulixi Lane 1, Chengdu Fourth People’s Hospital, Sichuan Province, China; Telephone number: +86- 18919570065; Fax number: +86-028-69515859; Email:NzE0NzU0NTQ4QHFxLmNvbQ==.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Melmed S, Casanueva FF, Hoffman AR, Kleinberg DL, Montori VM, Schlechte JA, et al. Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. (2011) 96:273–88. doi: 10.1210/jc.2010-1692, PMID: 21296991
2. Montejo ÁL, Arango C, Bernardo M, Carrasco JL, Crespo-Facorro B, Cruz JJ, et al. Multidisciplinary consensus on the therapeutic recommendations for iatrogenic hyperprolactinemia secondary to antipsychotics. Front Neuroendocrinol. (2017) 45:25–34. doi: 10.1016/j.yfrne.2017.02.003, PMID: 28235557
3. Freeman ME, Kanyicska B, Lerant A, and Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev. (2000) 80:1523–631. doi: 10.1152/physrev.2000.80.4.1523, PMID: 11015620
4. Wang M, Wu X, Chai F, Zhang Y, and Jiang J. Plasma prolactin and breast cancer risk: a meta- analysis. Sci Rep. (2016) 6:25998. doi: 10.1038/srep25998, PMID: 27184120
5. Samperi I, Lithgow K, and Karavitaki N. Hyperprolactinaemia. J Clin Med. (2019) 8:2203. doi: 10.3390/jcm8122203, PMID: 31847209
6. Solmi M, Lähteenvuo M, Correll CU, Tanskanen A, Tiihonen J, and Taipale H. Antipsychotic use and risk of low-energy fractures in people with schizophrenia: A nationwide nested case-control study in Finland. Schizophr Bull. (2023) 49:78–89. doi: 10.1093/schbul/sbac152, PMID: 36334051
7. González-Rodríguez A, Labad J, and Seeman MV. Antipsychotic-induced Hyperprolactinemia in aging populations: Prevalence, implications, prevention and management. Prog Neuropsychopharmacol Biol Psychiatry. (2020) 101:109941. doi: 10.1016/j.pnpbp.2020.109941, PMID: 32243999
8. Taipale H, Solmi M ML, Tanskanen A, Correll CU, and Tiihonen J. Antipsychotic use and risk of breast cancer in women with schizophrenia: a nationwide nested case-control study in Finland. Lancet Psychiatry. (2021) 8:883–91. doi: 10.1016/s2215-0366(21)00241-8, PMID: 34474013
9. Inder WJ and Castle D. Antipsychotic-induced hyperprolactinaemia. Aust N Z J Psychiatry. (2011) 45:830–7. doi: 10.3109/00048674.2011.589044, PMID: 21714721
10. Stiles CE, Steeds RP, and Drake WM. Monitoring patients receiving dopamine agonist therapy for hyperprolactinaemia. Ann Endocrinol (Paris). (2021) 82:182–86. doi: 10.1016/j.ando.2020.02.007, PMID: 32178837
11. Meaney AM and O’Keane V. Prolactin and schizophrenia: clinical consequences of hyperprolactinaemia. Life Sci. (2002) 71:979–92. doi: 10.1016/s0024-3205(02)01775-7, PMID: 12088758
12. Jauhar S, Johnstone M, and McKenna PJ. Schizophrenia. Lancet. (2022) 399:473–86. doi: 10.1016/s0140-6736(21)01730-x, PMID: 35093231
13. An FR, Yang R, Wang ZM, Ungvari GS, Ng CH, Chiu HF, et al. Hyperprolactinemia, prolactin-related side effects and quality of life in Chinese psychiatric patients. Compr Psychiatry. (2016) 71:71–6. doi: 10.1016/j.comppsych.2016.08.009, PMID: 27639124
14. Lu Z, Sun Y, Zhang Y, Chen Y, Guo L, Liao Y, et al. Pharmacological treatment strategies for antipsychotic-induced hyperprolactinemia: a systematic review and network meta-analysis. Transl Psychiatry. (2022) 12:267. doi: 10.1038/s41398-022-02027-4, PMID: 35790713
15. Grigg J, Worsley R, Thew C, Gurvich C, Thomas N, and Kulkarni J. Antipsychotic-induced hyperprolactinemia: synthesis of world-wide guidelines and integrated recommendations for assessment, management and future research. Psychopharmacol (Berl). (2017) 234:3279–97. doi: 10.1007/s00213-017-4730-6, PMID: 28889207
16. Montejo ÁL, Arango C, Bernardo M, Carrasco JL, Crespo-Facorro B, Cruz JJ, et al. Spanish consensus on the risks and detection of antipsychotic drug-related hyperprolactinaemia. Rev Psiquiatr Salud Ment. (2016) 9:158–73. doi: 10.1016/j.rpsm.2015.11.003, PMID: 26927534
17. Zhu J, Wang H, Huang S, Zhang Y, Liu X, Li Y, et al. Factors influencing prolactin levels in chronic long-term hospitalized schizophrenic patients with co-morbid type 2 diabetes mellitus. Front Psychiatry. (2022) 13:1034004. doi: 10.3389/fpsyt.2022.1034004, PMID: 36329924
18. Haddad PM and Wieck A. Antipsychotic-induced hyperprolactinaemia: mechanisms, clinical features and management. Drugs. (2004) 64:2291–314. doi: 10.2165/00003495-200464200-00003, PMID: 15456328
19. Riecher-Rössler A, Rybakowski JK, Pflueger MO, Beyrau R, Kahn RS, Malik P, et al. Hyperprolactinemia in antipsychotic-naive patients with first-episode psychosis. Psychol Med. (2013) 43:2571–82. doi: 10.1017/s0033291713000226, PMID: 23590895
20. Riecher-Rössler A. Oestrogens, prolactin, hypothalamic-pituitary-gonadal axis, and schizophrenic psychoses. Lancet Psychiatry. (2017) 4:63–72. doi: 10.1016/s2215-0366(16)30379-0, PMID: 27856396
21. Shettima FB, Wakil MA, Sheikh TL, Abdulaziz M, Wakawa IA, and Beida O. Antipsychotics-related hyperprolactinaemia among patients with schizophrenia in Maiduguri. S Afr J Psychiatr. (2024) 30:2133. doi: 10.4102/sajpsychiatry.v30i0.2133, PMID: 38444408
22. Keepers GA, Fochtmann LJ, Anzia JM, Benjamin S, Lyness JM, Mojtabai R, et al. The american psychiatric association practice guideline for the treatment of patients with schizophrenia. Focus (Am Psychiatr Publ). (2020) 18:493–97. doi: 10.1176/appi.focus.18402, PMID: 33343262
23. Kirsch P, Kunadia J, Shah S, and Agrawal N. Metabolic effects of prolactin and the role of dopamine agonists: A review. Front Endocrinol (Lausanne). (2022) 13:1002320. doi: 10.3389/fendo.2022.1002320, PMID: 36246929
24. Xu L, Yuan Y, Che Z, Tan X, Wu B, Wang C, et al. The hepatoprotective and hepatotoxic roles of sex and sex-related hormones. Front Immunol. (2022) 13:939631. doi: 10.3389/fimmu.2022.939631, PMID: 35860276
25. Auriemma RS, De Alcubierre D, Pirchio R, Pivonello R, and Colao A. Glucose abnormalities associated to prolactin secreting pituitary adenomas. Front Endocrinol (Lausanne). (2019) 10:327. doi: 10.3389/fendo.2019.00327, PMID: 31191454
26. Hedges DW, Salyer DL, Higginbotham BJ, Lund TD, Hellewell JL, Ferguson D, et al. Transcranial magnetic stimulation (TMS) effects on testosterone, prolactin, and corticosterone in adult male rats. Biol Psychiatry. (2002) 51:417–21. doi: 10.1016/s0006-3223(01)01266-5, PMID: 11904136
27. Jannati A, Oberman LM, Rotenberg A, and Pascual-Leone A. Assessing the mechanisms of brain plasticity by transcranial magnetic stimulation. Neuropsychopharmacology. (2023) 48:191–208. doi: 10.1038/s41386-022-01453-8, PMID: 36198876
28. Petty RG. Prolactin and antipsychotic medications: mechanism of action. Schizophr Res. (1999) 35:S67–73. doi: 10.1016/s0920-9964(98)00158-3, PMID: 10190227
29. Wieck A and Haddad PM. Antipsychotic-induced hyperprolactinaemia in women: pathophysiology, severity and consequences. Selective Literat Rev Br J Psychiatry. (2003) 182:199–204. doi: 10.1192/bjp.182.3.199, PMID: 12611781
30. Andrade C. Prolactin-raising and prolactin-sparing antipsychotic drugs and the risk of fracture and fragility fracture in patients with schizophrenia, dementia, and other disorders. J Clin Psychiatry. (2023) 84:23f14790. doi: 10.4088/JCP.23f14790, PMID: 36724110
31. Maskall DD, Zis AP, Lam RW, Clark CM, and Kuan AJ. Prolactin response to buspirone challenge in the presence of dopaminergic blockade. Biol Psychiatry. (1995) 38:235–9. doi: 10.1016/0006-3223(94)00264-4, PMID: 8547445
32. Sharpe M, Clements A, Hawton K, Young AH, Sargent P, and Cowen PJ. Increased prolactin response to buspirone in chronic fatigue syndrome. J Affect Disord. (1996) 41:71–6. doi: 10.1016/0165-0327(96)00075-4, PMID: 8938208
33. Rinieris P, Hatzimanolis J, Markianos M, and Stefanis C. Effects of 4 weeks treatment with chlorpromazine and/or trihexyphenidyl on the pituitary-gonadal axis in male paranoid schizophrenics. Eur Arch Psychiatry Neurol Sci. (1988) 237:189–93. doi: 10.1007/bf00449905, PMID: 3203697
34. Nitkowska M, Tomasiuk R, Czyżyk M, and Friedman A. Prolactin and sex hormones levels in males with Parkinson’s disease. Acta Neurol Scand. (2015) 131:411–6. doi: 10.1111/ane.12334, PMID: 25399742
35. Qiao Y, Yang F, Li C, Guo Q, Wen H, Zhu S, et al. Add-on effects of a low-dose aripiprazole in resolving hyperprolactinemia induced by risperidone or paliperidone. Psychiatry Res. (2016) 237:83–9. doi: 10.1016/j.psychres.2015.12.033, PMID: 26921057
36. Besag FMC, Vasey MJ, and Salim I. Is adjunct aripiprazole effective in treating hyperprolactinemia induced by psychotropic medication? A narrative review. CNS Drugs. (2021) 35:507–26. doi: 10.1007/s40263-021-00812-1, PMID: 33880739
37. Schoretsanitis G, de Leon J, and Diaz FJ. Prolactin levels: sex differences in the effects of risperidone, 9-hydroxyrisperidone levels, CYP2D6 and ABCB1 variants. Pharmacogenomics. (2018) 19:815–23. doi: 10.2217/pgs-2018-0053, PMID: 29914302
38. Borba VV, Zandman-Goddard G, and Shoenfeld Y. Prolactin and autoimmunity. Front Immunol. (2018) 9:73. doi: 10.3389/fimmu.2018.00073, PMID: 29483903
39. Ken-Dror G, Fluck D, Lean MEJ, Casanueva FF, and Han TS. The relationship between low prolactin and type 2 diabetes. Rev Endocr Metab Disord. (2024) 25:1087–95. doi: 10.1007/s11154-024-09886-w, PMID: 38760578
40. Gierach M, Bruska-Sikorska M, Rojek M, and Junik R. Hyperprolactinemia and insulin resistance. Endokrynol Pol. (2022) 73:959–67. doi: 10.5603/EP.a2022.0075, PMID: 36621922
41. Jha SK and Kannan S. Serum prolactin in patients with liver disease in comparison with healthy adults: A preliminary cross-sectional study. Int J Appl Basic Med Res. (2016) 6:8–10. doi: 10.4103/2229-516x.173984, PMID: 26958514
Keywords: schizophrenia, hyperprolactinemia (HPRL), antipsychotics, severity factors, prolactin
Citation: Yang Y, Li L and Yang M (2025) Severity and influencing factors of hyperprolactinemia in hospitalized schizophrenia patients: a cross-sectional study. Front. Psychiatry 16:1658334. doi: 10.3389/fpsyt.2025.1658334
Received: 02 July 2025; Accepted: 12 August 2025;
Published: 02 September 2025.
Edited by:
Michael J. Marino, Research Laboratories Merck, United StatesReviewed by:
Joanne Mathiasen, Drexel University, United StatesYuqian Pan, The Third Affiliated Hospital of Southern Medical University, China
Copyright © 2025 Yang, Li and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mi Yang, NTY1MTM2MTcwQHFxLmNvbQ==
 Yan Yang
Yan Yang Li Li1,2
Li Li1,2