- 1Department of Pharmacy, The Affiliated Lianyungang Hospital of Xuzhou Medical University, Lianyungang, Jiangsu, China
- 2Jiangsu Key Laboratory of New Drug Research and Clinical Pharmacy & School of Pharmacy, Xuzhou Medical University, Xuzhou, Jiangsu, China
- 3Department of Pharmacy, Suzhou Research Center of Medical School, Suzhou Hospital, Affiliated Hospital of Medical School, Nanjing University, Suzhou, Jiangsu, China
- 4Department of Pharmacy, Xuzhou Oriental Hospital Affiliated to Xuzhou Medical University, Xuzhou, Jiangsu, China
- 5School of Nursing, Xuzhou Medical University, Xuzhou, Jiangsu, China
Introduction: Clozapine, as a core drug for the treatment of schizophrenia, is widely used in the drug treatment of schizophrenia patients. However, when multiple drugs are used in combination, it is not clear whether there are drug-drug interactions (DDI) of clozapine in patients with schizophrenia. This study aims to use population pharmacokinetics (PPK) modelling to predict DDI and individualized therapy of clozapine in schizophrenia patients.
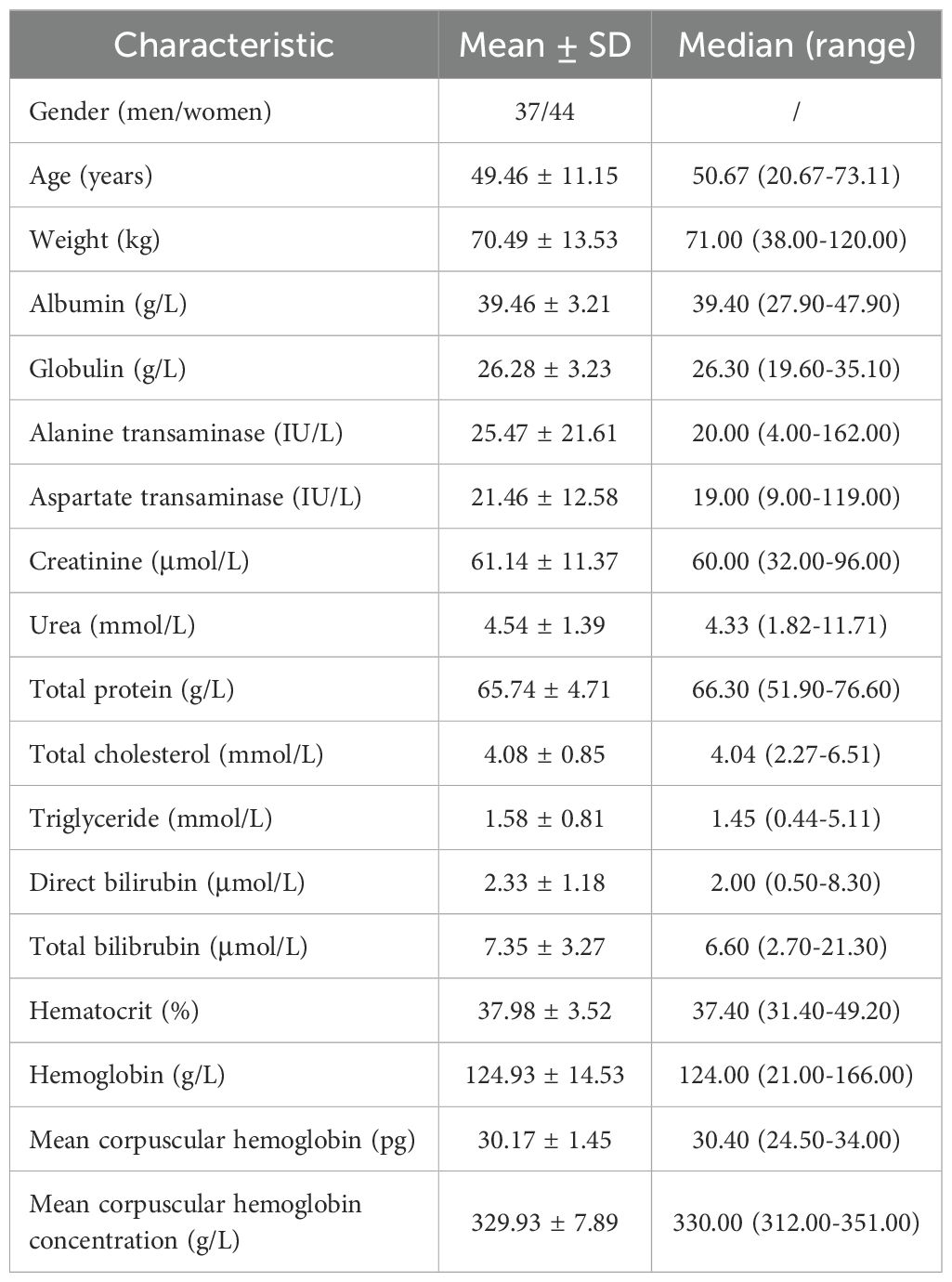
Methods: We collected 81 patients with schizophrenia and included their physiological data, biochemical data, treatment plans and information on combined medication during the clinical treatment process. Next, PPK modelling was used to analyze drugs with potential DDI when clozapine was used in schizophrenia patients, and dosage adjustments were recommended.
Results: Final analysis revealed that weight and coadministration of zopiclone affected clozapine clearance, and there was DDI with clozapine when zopiclone was used concurrently in schizophrenia patients. Further, for schizophrenia patients without zopiclone, 10 mg/kg/day, 9 mg/kg/day, 8 mg/kg/day and 7 mg/kg/day clozapine were recommended for 40–50 kg, 50–67 kg, 67–88 kg, and 88–120 kg patients, respectively. For schizophrenia patients with zopiclone, 6 mg/kg/day and 5 mg/kg/day clozapine were recommended for 40–70 kg and 70–120 kg patients, respectively. This study was the first to systematically analyze DDI when clozapine was used in schizophrenia patients and found DDI when zopiclone and clozapine were taken concurrently.
Conclusion: When zopiclone was taken concurrently, clozapine dosage need to be reduced. Based on this, schizophrenia patients individualized dosage adjustment was recommended.
Introduction
Schizophrenia is a psychotic disorder characterized by a combination of positive and negative symptoms, along with cognitive impairment, affective symptoms, and behavioral disturbances (1). In terms of epidemiology, schizophrenia has a lifetime prevalence of 1% (2, 3), and the incidence is high among young and middle-aged people, the sex ratio is close, the genetic factors are significant, and the risk of developing schizophrenia is significantly increased when the immediate family member is schizophrenia.
The mainstay of schizophrenia treatment is the use of atypical antipsychotic medications, and non-pharmacological treatment serves as a complementary therapy, where clozapine, as the core drug for treating schizophrenia, occupies an important position in the drug treatment of schizophrenia patients, especially suitable for refractory cases (4, 5). Clozapine is not only particularly useful in treatment-resistant schizophrenia as monotherapy, but it is also used in combination therapy, such as combination with aripiprazole, lurasidone, or cariprazine (6–13).
It is important that latest research indicating that the use of clozapine at recommended doses does not guarantee achieving therapeutic concentrations of clozapine and women and nonsmokers were at the highest risk of having toxic levels of clozapine (14). Drug-drug interactions (DDI) can significantly affect drug concentrations, and the occurrence of DDI is often accompanied during the treatment of mental disorders (15, 16). For example, zopiclone is metabolized by the CYP3A4 enzyme (17, 18), which may compete CYP3A4 metabolic enzymes with clozapine, influence clozapine clearance in schizophrenia patients. In the routine clinical practice, the dosage information for both clozapine and zopiclone are mainly based on the instructions.
Population pharmacokinetics (PPK), by integrating sparse clinical data with covariate modelling, addresses the limitations of traditional pharmacokinetics in real-world complex populations. Its core value lies in quantifying sources of variation (such as differences in liver and kidney functions), providing feasible pharmacokinetic research methods for special populations (children, the elderly), and identifying key influencing factors (especially DDI), supporting precision medical decision-making (19). At present, PPK has been widely used in the analysis of potential DDI in clinical practice. For example, Fujita et al. reported PPK Analysis of DDI between perampanel and carbamazepine using enzyme induction model in epileptic patients (20). Cleary et al. reported estimation of FMO3 ontogeny by mechanistic PPK modelling of risdiplam and its impact on DDI in children (21). Li et al. reported PPK of ruxolitinib in children with hemophagocytic lymphohistiocytosis: focus on the DDI (22). Courlet et al. reported PPK modelling to quantify the magnitude of DDI between amlodipine and antiretroviral drugs (23). Barcelo et al. reported PPK of dolutegravir: influence of DDI in a real-life setting (24).
Therefore, this study aims to using PPK modelling to predict DDI of clozapine in schizophrenia patients, and to recommend individualized dosage adjustments for these patients.
Methods
Data collection
This study was approved by the Research Ethics Committee of Xuzhou Oriental Hospital Affiliated to Xuzhou Medical University, which collected schizophrenia patients at Xuzhou Oriental Hospital Affiliated to Xuzhou Medical University from December 2023 to November 2024, including their physiological data, biochemical data, treatment plans and information on combined medication during the clinical treatment process, where the requirement for written informed consent could be waived since the data were collected retrospectively without patient identifiers. The dosage information for clozapine was mainly based on the instruction. The analytical technique used for the determination of clozapine was homogeneous enzyme immunoassay. The sample extraction times for plasma concentrations were before the next administration, which was the value of the trough concentration.
Model building
PPK model of clozapine in schizophrenia patients was set up, where CL/F, V/F, and Ka [fixed at 1.3/h (25, 26)] were the main pharmacokinetic parameters. In terms of individual variation, we chose to express it using Equation 1:
The abbreviation Zi denoted individual parameter, TV(Z) denoted typical individual parameter and ηi denoted symmetrical distribution.
In terms of random residual variation, we chose to express it using Equation 2:
The abbreviation Yi denoted observed concentration, Xi denoted individual predicted concentration, and ϵ1 denoted symmetrical distribution.
In terms of relationship between parameter and weight, we chose to express it using Equation 3:
The abbreviation Ui denoted individual parameter, Vi denoted individual weight, Vstd denoted standard weight of 70 kg, and Ustd denoted typical individual parameter. W denoted allometric coefficients: 0.75 and 1 for CL/F and V/F, respectively (27).
In terms of continuous or categorical covariate parameter, we chose to express it using Equation 4 or 5, respectively:
The abbreviation Ri denoted individual parameter, TV(R) denoted typical individual parameter, Q denoted the parameter needed to be fitted, Si denoted covariate of the i-th individual, and Sm denoted population median for the covariate. To construct covariate model, we used two-step method.
Model evaluation
We used visual diagram and bootstrap methods to evaluate the final clozapine PPK model of schizophrenia patients.
Dosage simulation
We used Monte Carlo simulation to simulate the clozapine concentrations of schizophrenia patients under different simulated clozapine dosages, including 1 mg/kg/day, 2 mg/kg/day, 3 mg/kg/day, 4 mg/kg/day, 5 mg/kg/day, 6 mg/kg/day, 7 mg/kg/day, 8 mg/kg/day, 9 mg/kg/day, 10 mg/kg/day. Additionally, the simulated patients were divided into two parts: (a) schizophrenia patients not taking zopiclone, and (b) schizophrenia patients taking zopiclone, where simulated weight groups contained 40 kg, 60 kg, 80 kg, 100 kg, 120 kg. We simulated each scenario 1000 times, and the therapeutic range was 350–800 ng/ml along with 1000 ng/ml toxicity threshold (28–31).
Results
Patient’s data
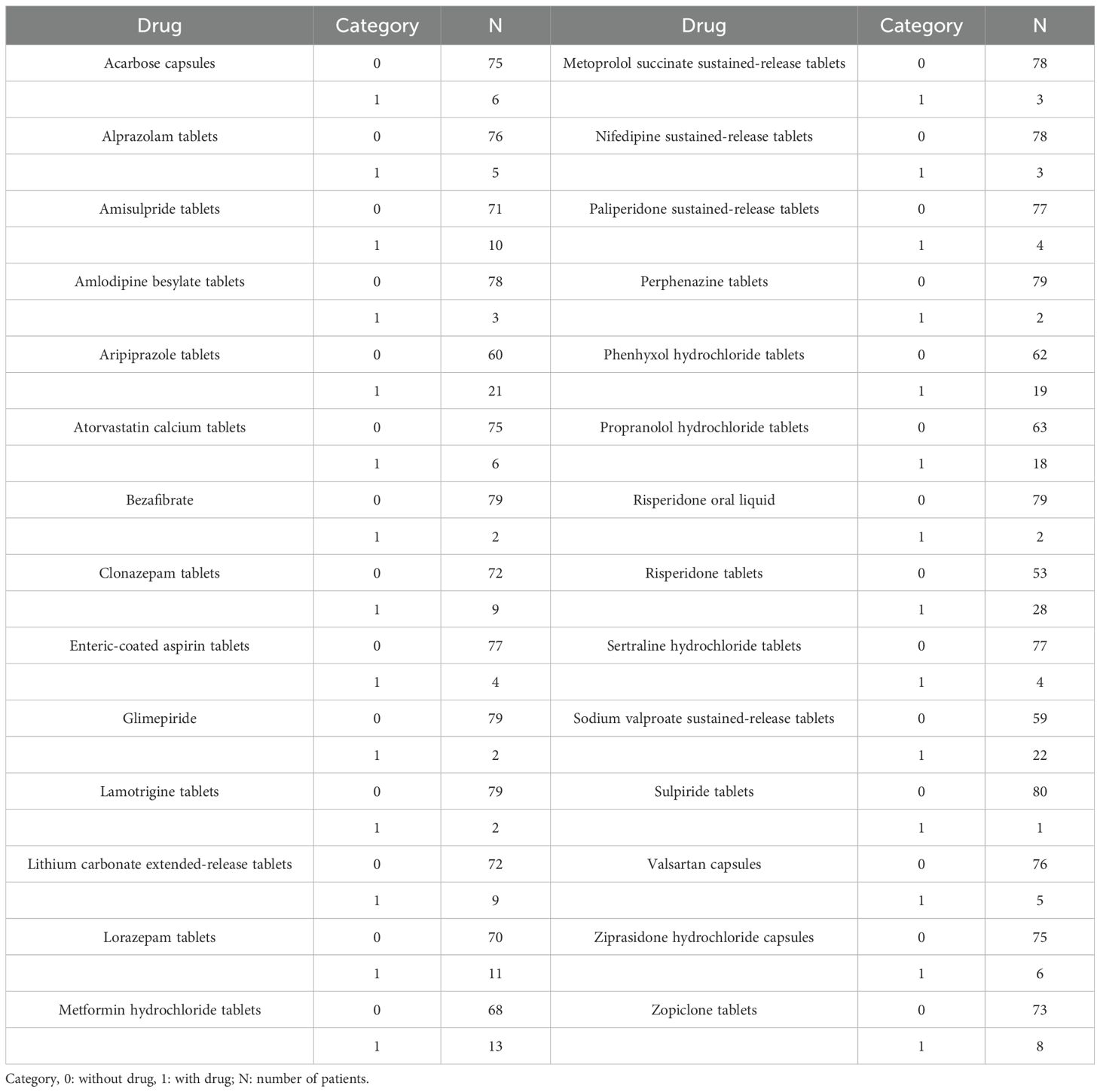
We collected 81 patients with schizophrenia. 37 schizophrenia patients were men and 44 schizophrenia patients were women. The age ranges were from 20.67 to 73.11 years old, and weight ranges were from 38.00 to 120.00 kg. Tables 1 and 2 denoted demographic data of schizophrenia patients and drug combination, respectively.
PPK modelling
Equations 6 and 7 were the final clozapine PPK model of schizophrenia patients:
ZOP denoted zopiclone and when schizophrenia patients took ZOP, ZOP denoted 1, otherwise ZOP denoted 0.
Model evaluation
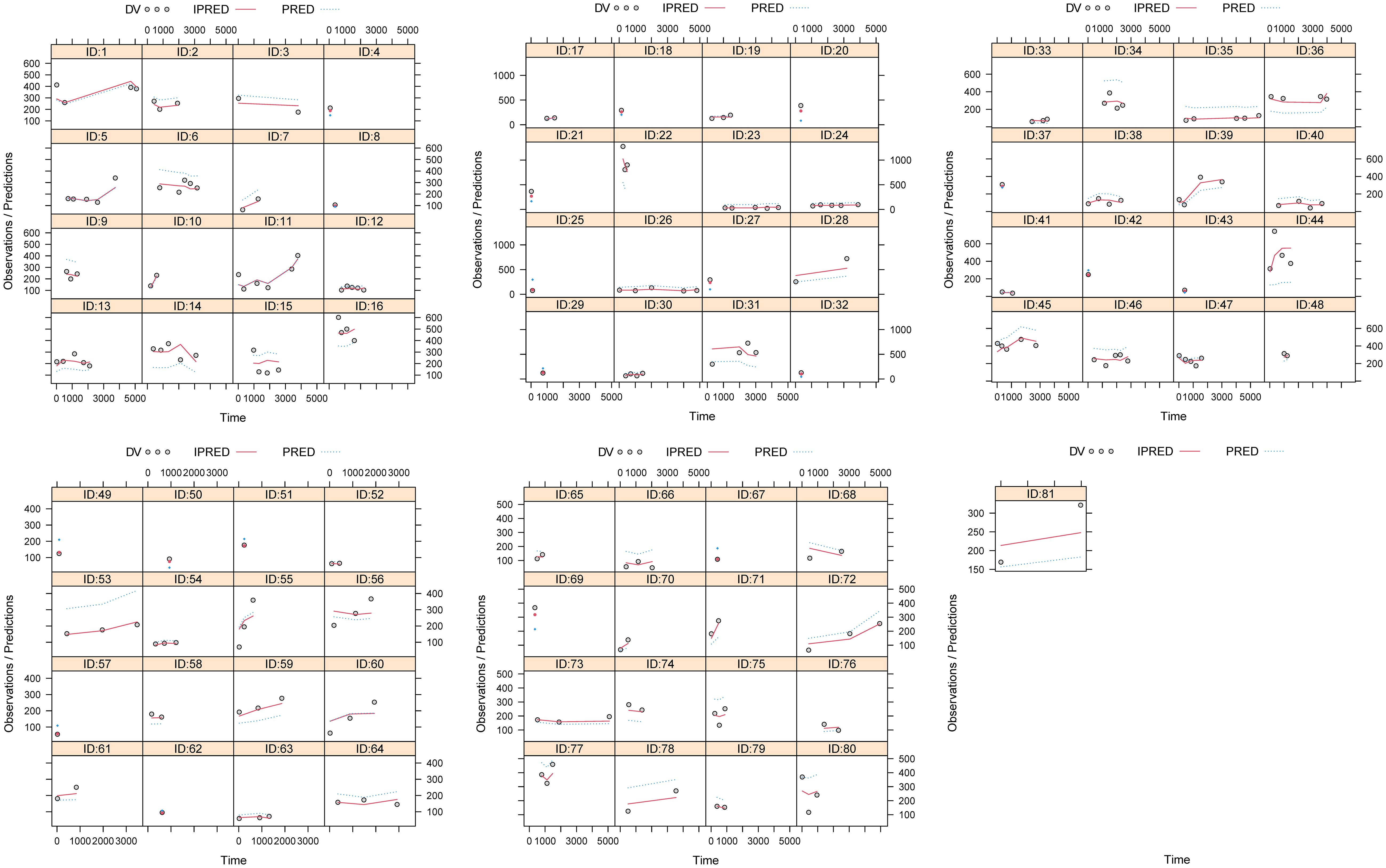
Figures 1, 2 and Table 3 denoted visual diagram, individual plots and bootstrap, showing clozapine PPK model of schizophrenia patients was credible. When schizophrenia patients taking zopiclone, the clozapine clearance of schizophrenia patients was reduced by 25.4%, which was shown in Figure 3.
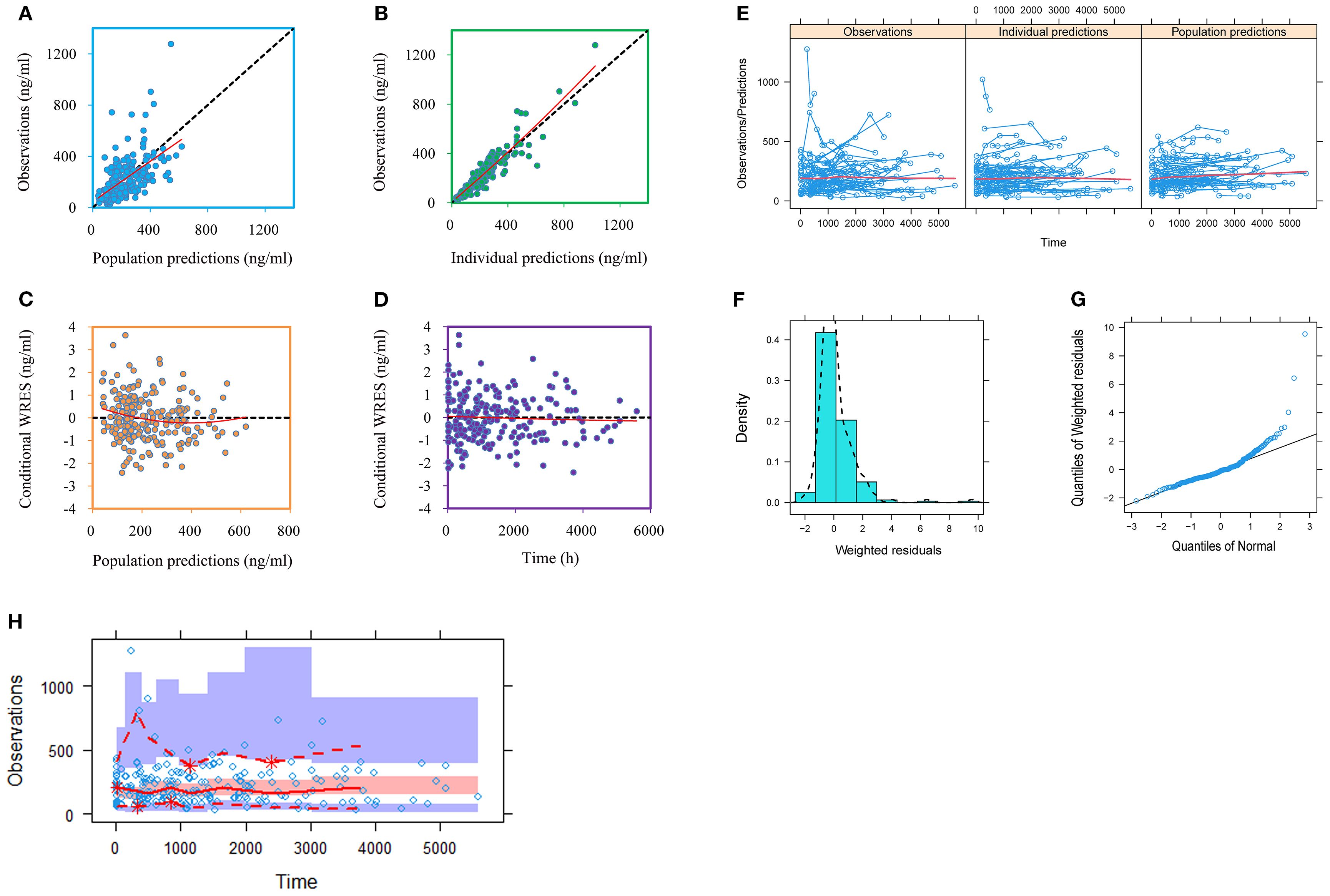
Figure 1. Model evaluation. (A) Observations vs. population predictions. (B) Observations vs. individual predictions. (C) Conditional weighted residuals (WRES) vs. population predictions. (D) Conditional WRES vs. time. (E) Observations/Predictions vs. time. (F) Density vs. weighted residuals. (G) Quantilies of weighted residuals vs. quantilies of normal. (H) Visual predictive check (VPC) of model.
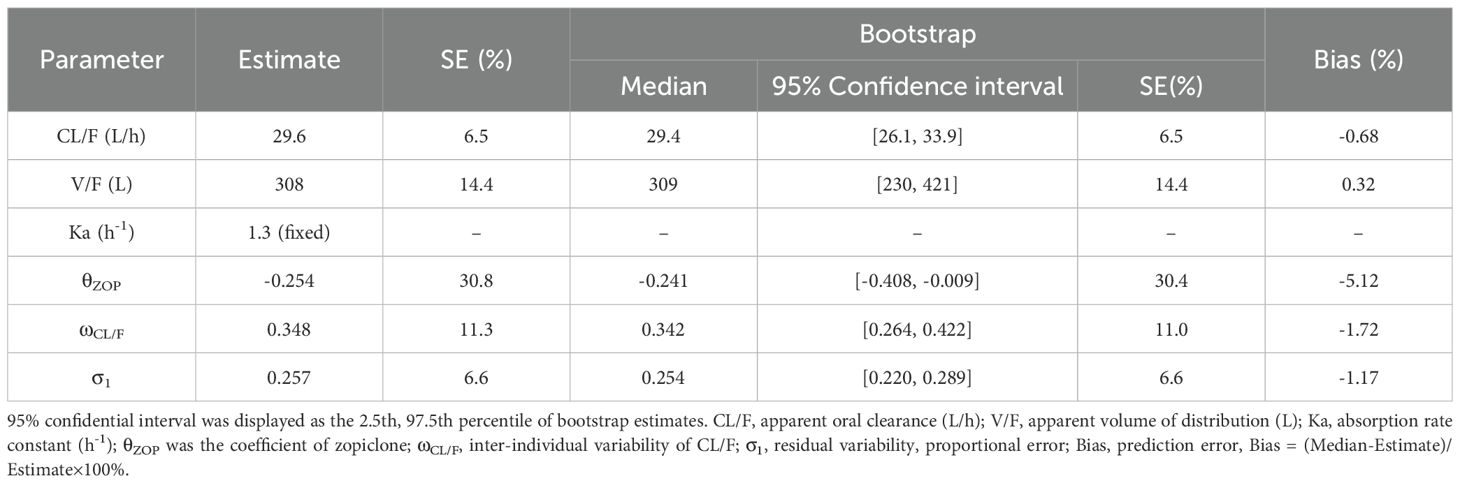
Table 3. Parameter estimates of clozapine final model and bootstrap validation in schizophrenia patients.
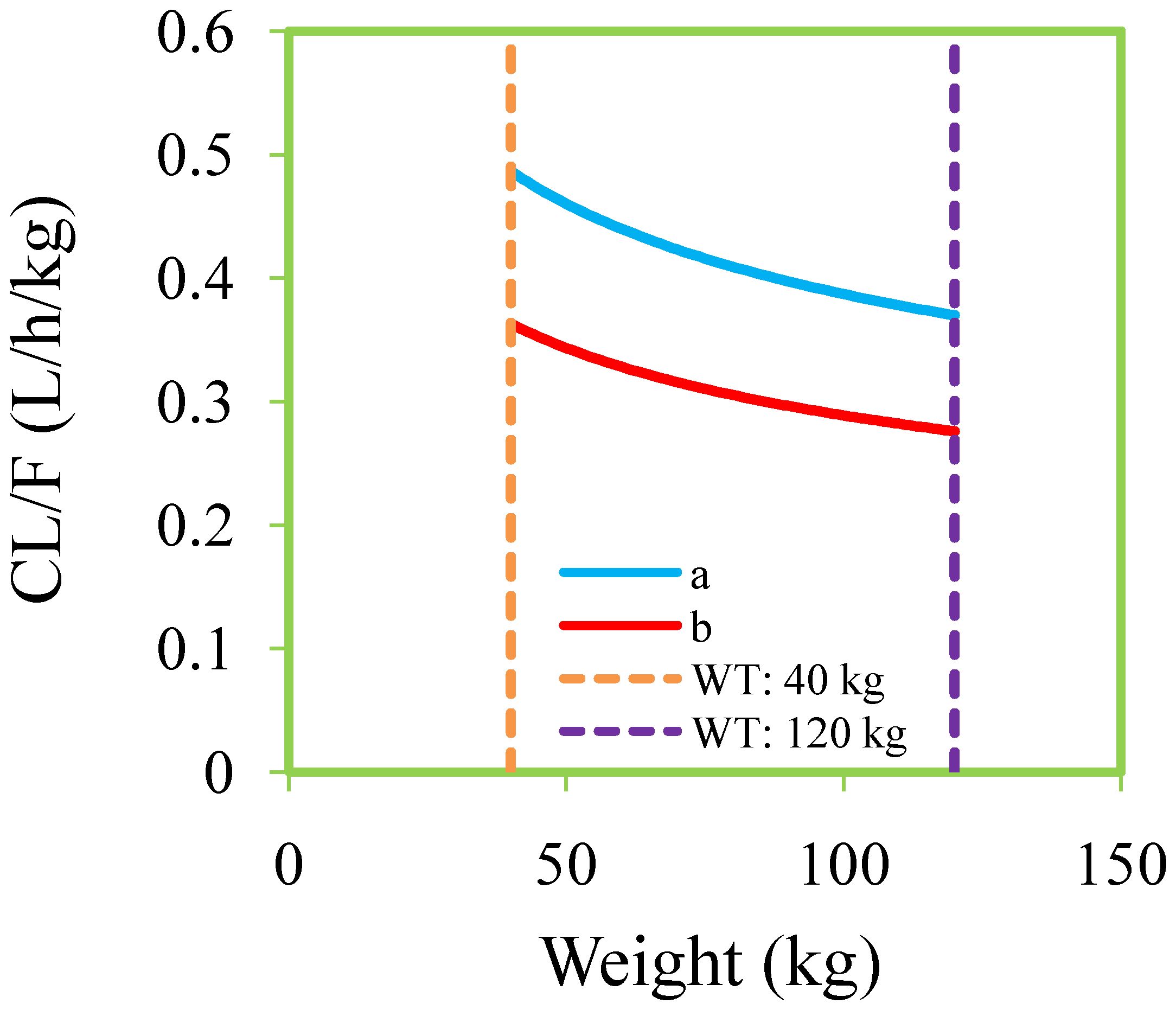
Figure 3. Clozapine clearance rates of schizophrenia patients. (A) Schizophrenia patients not taking zopiclone. (B) Schizophrenia patients taking zopiclone.
Dosage recommendation and safety evaluation
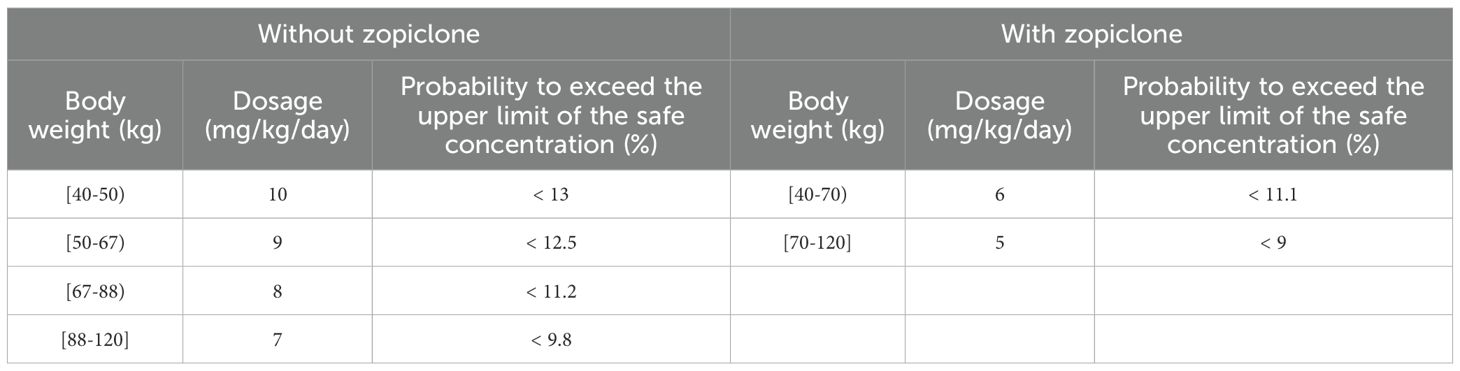
Figures 4 and 5 denoted simulated clozapine concentrations of schizophrenia patients not taking zopiclone and simulated clozapine concentrations of schizophrenia patients taking zopiclone, respectively. Figure 6 denoted the probabilities to attain the target clozapine concentrations of schizophrenia patients. For schizophrenia patients without zopiclone, 10 mg/kg/day, 9 mg/kg/day, 8 mg/kg/day and 7 mg/kg/day clozapine were recommended for 40–50 kg, 50–67 kg, 67–88 kg, and 88–120 kg patients, respectively. For schizophrenia patients with zopiclone, 6 mg/kg/day and 5 mg/kg/day clozapine were recommended for 40–70 kg and 70–120 kg patients, respectively. Figure 7 denoted the probabilities to exceed the upper limit of safe concentrations of schizophrenia patients. The detailed dosage recommendation and safety evaluation were shown in Table 4.
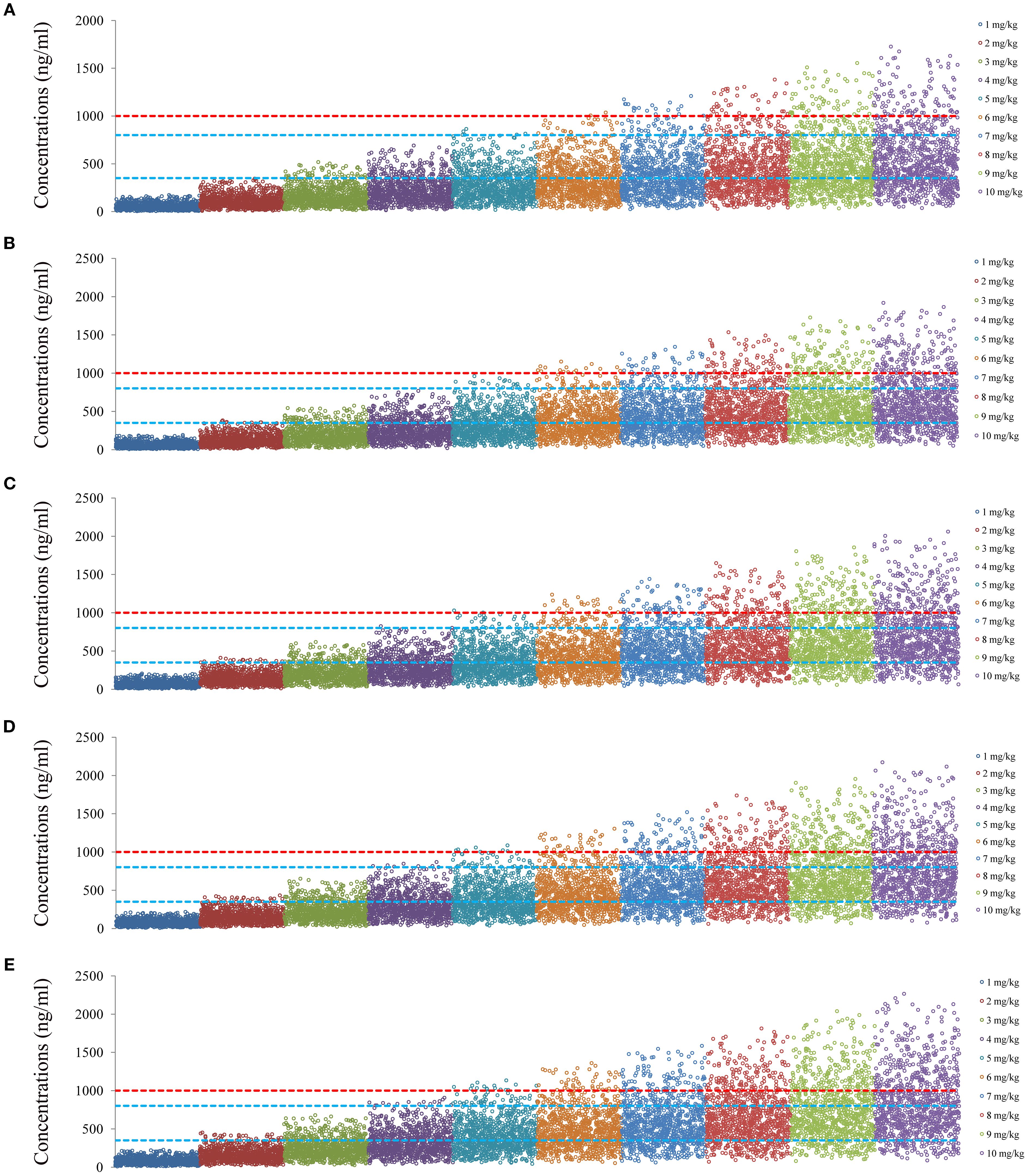
Figure 4. Simulated clozapine concentrations of schizophrenia patients not taking zopiclone. (A) 40 kg schizophrenia patients. (B) 60 kg schizophrenia patients. (C) 80 kg schizophrenia patients. (D) 100 kg schizophrenia patients. (E) 120 kg schizophrenia patients.
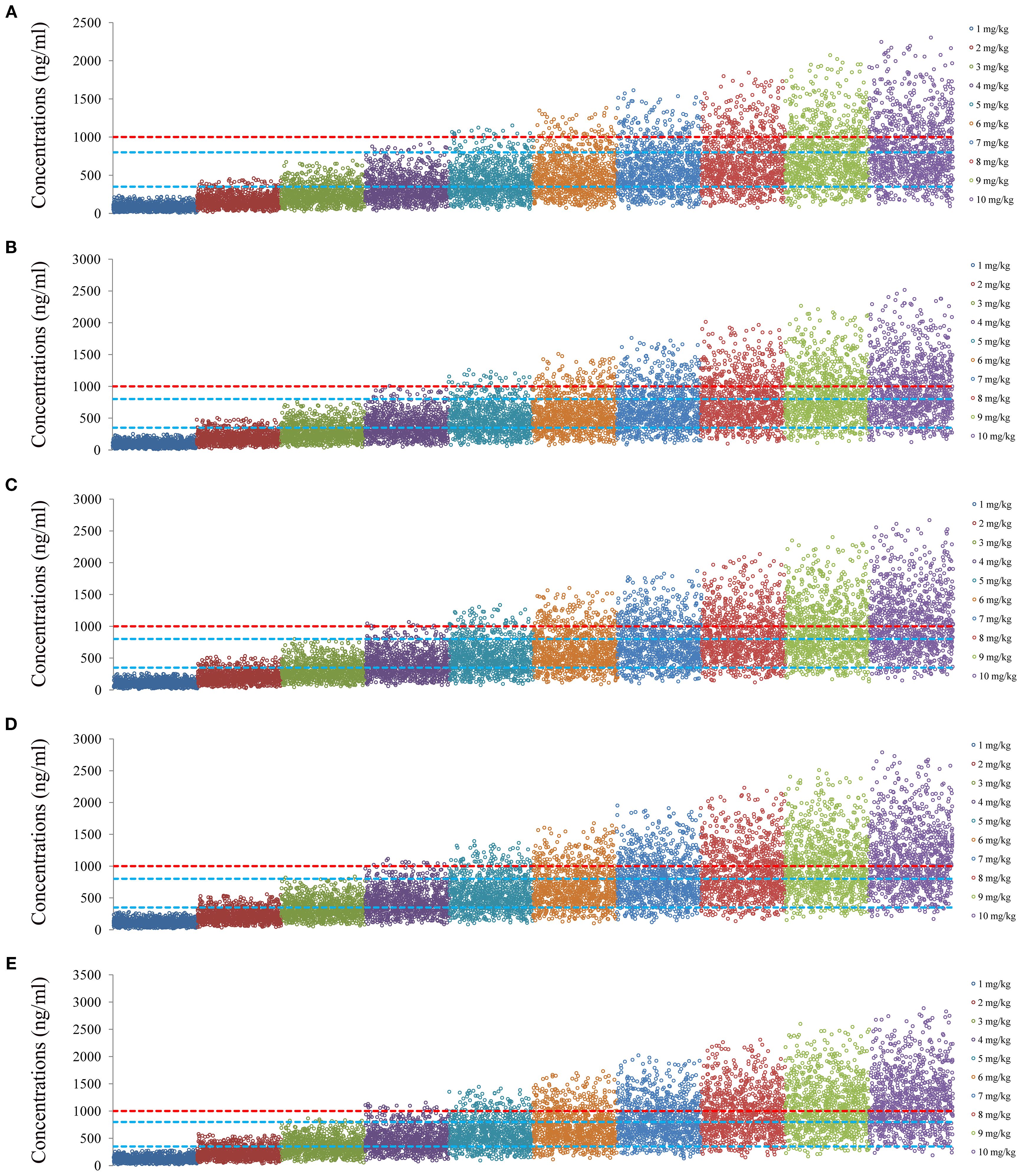
Figure 5. Simulated clozapine concentrations of schizophrenia patients taking zopiclone. (A) 40 kg schizophrenia patients. (B) 60 kg schizophrenia patients. (C) 80 kg schizophrenia patients. (D) 100 kg schizophrenia patients. (E) 120 kg schizophrenia patients.
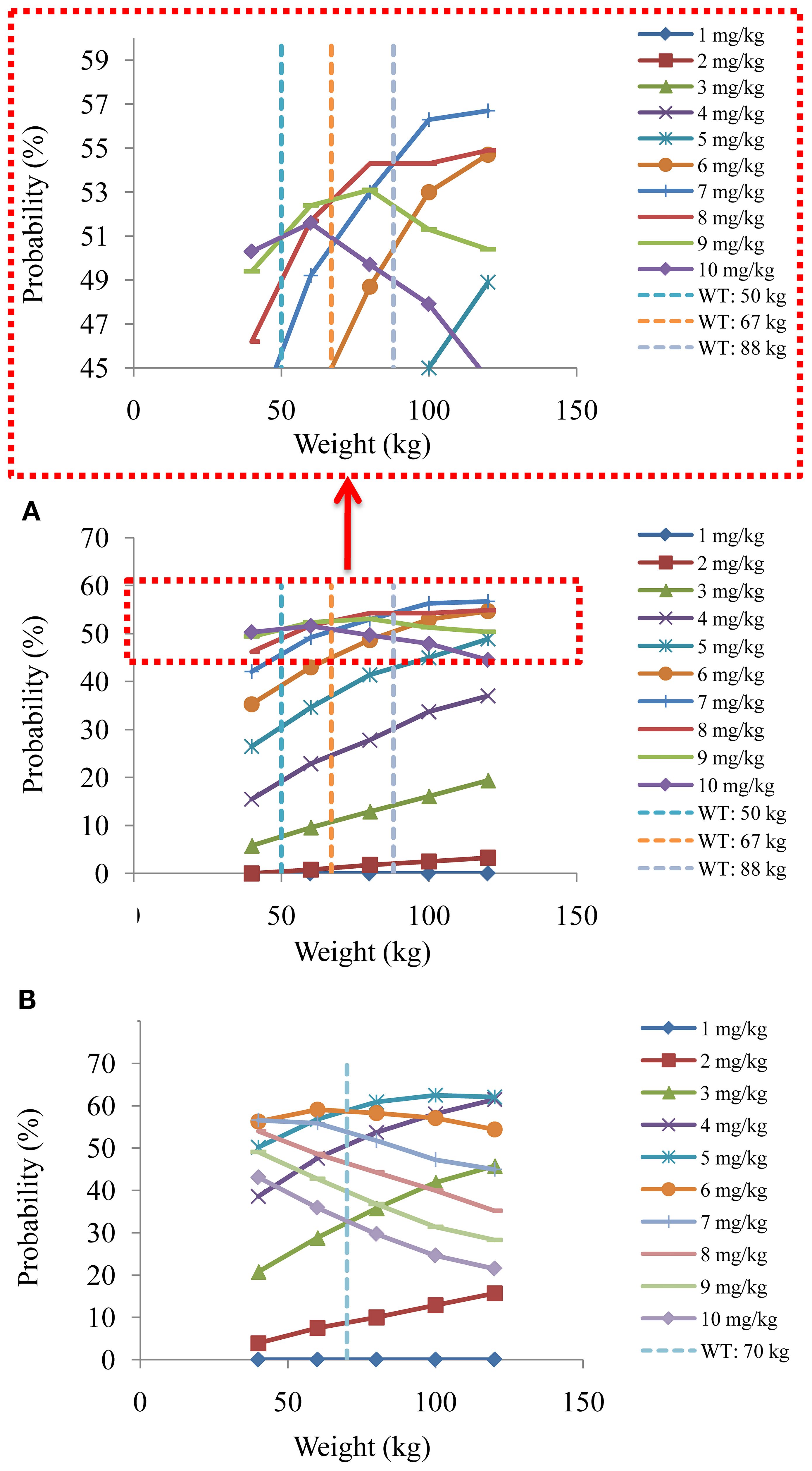
Figure 6. The probabilities to attain the target clozapine concentrations of schizophrenia patients. (A) Schizophrenia patients not taking zopiclone. (B) Schizophrenia patients taking zopiclone.
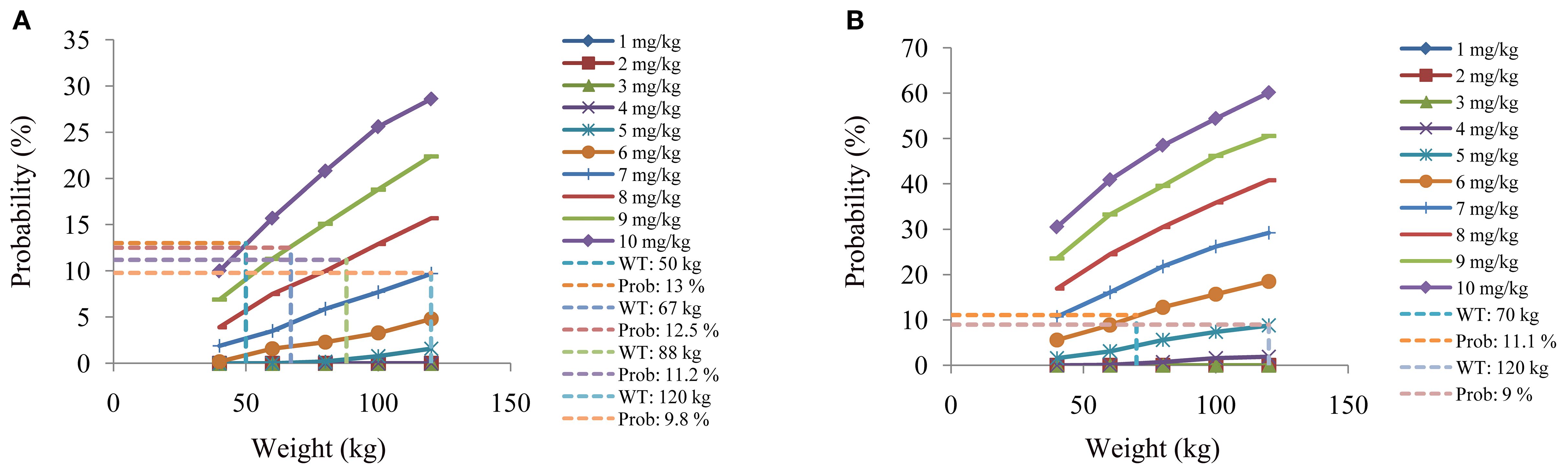
Figure 7. The probabilities to exceed the upper limit of safe concentrations of schizophrenia patients. (A) Schizophrenia patients not taking zopiclone. (B) Schizophrenia patients taking zopiclone.
Discussion
Clozapine is a classic old drug that was synthesized over 65 years ago, which is the only psychotherapeutic drug been used to treat treatment-resistant schizophrenia. up to now, the actual mechanism why clozapine has a remarkable therapeutic effect in the treatment of treatment-resistant schizophrenia (32). Regarding when to initiate clozapine therapy for patients with schizophrenia remains contentious. According to current guidelines, clozapine is recommended for initiation when patients show no response after two adequate courses of treatment with standard antipsychotic drugs (32). However, recent research indicates that in patients with first-episode schizophrenia experiencing their first psychotic relapse, neither continuing the same non-clozapine oral antipsychotic nor switching to another non-clozapine oral antipsychotic demonstrated beneficial effects for relapse prevention (33). These findings, combined with existing knowledge regarding clozapine’s association with reduced mortality, challenge current treatment guidelines recommending clozapine as a third-line therapy (33). That is to say, the earlier clozapine is used among patients with schizophrenia, the more benefits they may gain.
However, patients with schizophrenia often have more concomitant medications. In the clinical application process, combined medication often needs to be viewed dialectically. It is a double-edged sword. Reasonable application can significantly enhance the therapeutic effect, but unreasonable use may cause serious risks. Especially when combined medication causes bad DDI, it may pose a serious threat to the efficacy of the drug and even the health of the patient. When the harmful drug significantly increases or decreases the clearance rate of the victimized drug, and thereby significantly reduces or increases the blood concentration of the target drug, it is necessary to focus on pharmaceutical care, and even a new administration plan for the target drug needs to be reformulated.
These DDI clinical cases include the use of immunosuppressants after organ transplantation (34–38), clinical use of hypoglycemic drugs (39–41), individualized administration of anti-hepatitis virus drugs (42, 43), clinical use of anticoagulant drugs (44–46), individualized drug administration for patients with dementia (47), clinical medication for patients with asthma (48), individualized drug administration for patients with liver and kidney damage (49, 50), rational drug use for patients with heart failure (51), individualized drug administration for patients with atrial fibrillation (52), clinical medication for patients with HIV (53–56), precise medication for special pediatric populations (57, 58), individualized drug administration for the special population of pregnant women (59, 60), use of antibiotics for critically infected patients in the ICU (61), clinical medication for COVID-19 patients (62), clinical use of anti-tumor drugs in cancer patients (63–71), clinical use of pain treatment drugs for cancer patients (72), which have been widely carried out.
Based on this, in the current research, we used PPK modelling to predict DDI of clozapine in schizophrenia patients. This study included the drugs used in combination in the schizophrenia population, including acarbose capsules, alprazolam tablets, amisulpride tablets, amlodipine besylate tablets, aripiprazole tablets, atorvastatin calcium tablets, bezafibrate, clonazepam tablets, enteric-coated aspirin tablets, glimepiride, lamotrigine tablets, lithium carbonate extended-release tablets, lorazepam tablets, metformin hydrochloride tablets, metoprolol succinate sustained-release tablets, nifedipine sustained-release tablets, paliperidone sustained-release tablets, perphenazine tablets, phenhyxol hydrochloride tablets, propranolol hydrochloride tablets, risperidone oral liquid, risperidone tablets, sertraline hydrochloride tablets, sodium valproate sustained-release tablets, sulpiride tablets, valsartan capsules, ziprasidone hydrochloride capsules, zopiclone tablets.
Finally, the PPK model found that weight and coadministration of zopiclone affected the clearance rate of clozapine, and there was DDI with clozapine when zopiclone was used concurrently in schizophrenia patients. When schizophrenia patients took zopiclone simultaneously, the clozapine clearance rate of the patients decreased by 25.4%. This is mainly because clozapine is mainly metabolized in the liver through CYP3A4 and CYP1A2 (4, 73, 74), and zopiclone is also metabolized by the CYP3A4 enzyme (17, 18), which may compete CYP3A4 metabolic enzymes with clozapine, influence clozapine clearance in schizophrenia patients. Furthermore, we optimized the optimal dosage adjustment of clozapine in schizophrenia patients with or without zopiclone through Monte Carlo simulation. For schizophrenia patients without zopiclone, 10 mg/kg/day, 9 mg/kg/day, 8 mg/kg/day and 7 mg/kg/day clozapine were recommended for 40–50 kg, 50–67 kg, 67–88 kg, and 88–120 kg patients, respectively. For schizophrenia patients with zopiclone, 6 mg/kg/day and 5 mg/kg/day clozapine were recommended for 40–70 kg and 70–120 kg patients, respectively.
Conclusion
This study was the first to systematically analyze DDI when clozapine was used in schizophrenia patients and found DDI when zopiclone and clozapine were taken concurrently. Furthermore, when zopiclone was taken concurrently, the requirement of clozapine dosage needed to reduce. Based on this, schizophrenia patients individualized dosage adjustment was recommended.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by the Research Ethics Committee of Xuzhou Oriental Hospital Affiliated to Xuzhou Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the data were collected retrospectively without patient identifiers.
Author contributions
H-HH: Data curation, Formal Analysis, Methodology, Project administration, Software, Supervision, Visualization, Writing – original draft. YZ: Data curation, Formal Analysis, Investigation, Methodology, Software, Writing – original draft. JW: Investigation, Methodology, Software, Writing – original draft. XT: Data curation, Formal Analysis, Methodology, Software, Writing – original draft. YL: Data curation, Formal Analysis, Investigation, Writing – original draft. S-MH: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. CZ: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. XC: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. D-DW: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the grant from Jiangsu Key Laboratory of New Drug Research and Clinical Pharmacy, The Xuzhou Special Fund for Promoting Scientific and Technological Innovation (No. KC23217, No. KC23254), The Medical Research Project of Jiangsu Provincial Health Commission (No. Z2023010), Jiangsu Province Education Science Planning Project (No. C/2022/01/36), Xuzhou Medical University Labor Education Special Project (No. X1d202209), Jiangsu Province Higher Education Informatization Research Topic (No. 2023JSETKT136), Xuzhou Medical University Research Topic of Higher Education Teaching Reform (No. Xjyzrd202304). Xuzhou Medical University Research Project on Reform of Postgraduate Education and Teaching (No. XYJGKT202506). Xuzhou Medical University Teaching Academic Research Topics (No. 2024ZDKT02-Y03). Suzhou Applied Basic Research Science and Technology Innovation Project (No. SYWD2024258).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. McCutcheon RA, Reis Marques T, and Howes OD. Schizophrenia-an overview. JAMA Psychiatry. (2020) 77:201–10. doi: 10.1001/jamapsychiatry.2019.3360
2. Saha S, Chant D, Welham J, and McGrath J. A systematic review of the prevalence of schizophrenia. PloS Med. (2005) 2:e141. doi: 10.1371/journal.pmed.0020141
3. Yoo S, Montazeri A, McNulty H, Potvin Kent M, Bennett D, and Little J. Global evaluation of the impact of food fortification with folic acid on rates of schizophrenia. Schizophr Res. (2025) 280:39–47. doi: 10.1016/j.schres.2025.04.007
4. Wysokinski A and Dreczka J. Clozapine Toxicity Predictor: Deep neural network model predicting clozapine toxicity and its therapeutic dose range. Psychiatry Res. (2024) 342:116256. doi: 10.1016/j.psychres.2024.116256
5. Tiihonen J, Tanskanen A, Mittendorfer-Rutz E, Howes OD, Correll CU, Siskind D, et al. Effectiveness of clozapine augmentation with specific doses of other antipsychotics in schizophrenia: a meta-analysis from two nationwide cohorts. World Psychiatry. (2025) 24:250–9. doi: 10.1002/wps.21316
6. Sepede G, Di Iorio G, Spano MC, Lorusso M, Sarchione F, Santacroce R, et al. A case of resistant schizophrenia successfully treated with clozapine/long-acting injectable aripiprazole combination. Clin Neuropharmacol. (2016) 39:322–4. doi: 10.1097/WNF.0000000000000191
7. Huh L and Lee BJ. Efficacy of low-dose aripiprazole to treat clozapine-associated tardive dystonia in a patient with schizophrenia. Turk Psikiyatri Derg. (2017) 28:208–11.
8. Balcioglu YH, Gokcay H, and Yesilkaya UH. One plus one sometimes equals more than two: long-acting injectable aripiprazole adjunction in clozapine-resistant schizophrenia. Clin Neuropharmacol. (2020) 43:166–8. doi: 10.1097/WNF.0000000000000404
9. Olivola M, Arienti V, Bassetti N, Giovanna G, and Brondino N. Lurasidone augmentation of clozapine in refractory schizophrenia: A case series. J Clin Psychopharmacol. (2023) 43:157–60. doi: 10.1097/JCP.0000000000001662
10. Siwek M, Chrobak AA, Gorostowicz A, Krol P, and Dudek D. Lurasidone augmentation of clozapine in schizophrenia-retrospective chart review. Brain Sci. (2023) 13:445. doi: 10.3390/brainsci13030445
11. Wang YH, Liu CY, Her YN, Wu HH, Yao CY, and Chen TY. Lurasidone successfully reversed clozapine-induced type 2 diabetes mellitus and hypertriglyceridemia in a patient with schizophrenia. Am J Ther. (2023) 30:e490–1. doi: 10.1097/MJT.0000000000001566
12. Dmuhovskis A and Taube M. Cariprazine and clozapine combination for the treatment of psychosis in a young, female patient with schizophrenia: a case report. Front Psychiatry. (2024) 15:1452980. doi: 10.3389/fpsyt.2024.1452980
13. Pappa S, Csehi R, Caldwell-Dunn E, Dombi ZB, and Hjorth S. Cariprazine & Clozapine: A systematic review of a promising antipsychotic combination for treatment-resistant schizophrenia. Int J Neuropsychopharmacol. (2025) 28:pyaf053. doi: 10.1093/ijnp/pyaf053
14. Mach A, Wnorowska A, Siwek M, Wojnar M, and Radziwon-Zaleska M. Clinical and pharmacological factors influencing serum clozapine and norclozapine levels. Front Pharmacol. (2024) 15:1356813. doi: 10.3389/fphar.2024.1356813
15. Vermeulen A, Piotrovsky V, and Ludwig EA. Population pharmacokinetics of risperidone and 9-hydroxyrisperidone in patients with acute episodes associated with bipolar I disorder. J Pharmacokinet Pharmacodyn. (2007) 34:183–206. doi: 10.1007/s10928-006-9040-2
16. Zhang C, Jiang L, Hu K, Chen L, Zhang YJ, Shi HZ, et al. Effects of aripiprazole on olanzapine population pharmacokinetics and initial dosage optimization in schizophrenia patients. Neuropsychiatr Dis Treat. (2024) 20:479–90. doi: 10.2147/NDT.S455183
17. Alderman CP, Gebauer MG, Gilbert AL, and Condon JT. Possible interaction of zopiclone and nefazodone. Ann Pharmacother. (2001) 35:1378–80. doi: 10.1345/aph.1A074
18. Tornio A, Neuvonen PJ, and Backman JT. The CYP2C8 inhibitor gemfibrozil does not increase the plasma concentrations of zopiclone. Eur J Clin Pharmacol. (2006) 62:645–51. doi: 10.1007/s00228-006-0155-6
19. Hu K, Fu M, Huang X, He S, Jiao Z, and Wang D. Editorial: Model-informed drug development and precision dosing in clinical pharmacology practice. Front Pharmacol. (2023) 14:1224980. doi: 10.3389/fphar.2023.1224980
20. Fujita Y, Murai M, Muraki S, Suetsugu K, Tsuchiya Y, Hirota T, et al. Population pharmacokinetic analysis of drug-drug interactions between perampanel and carbamazepine using enzyme induction model in epileptic patients. Ther Drug Monit. (2023) 45:653–9. doi: 10.1097/FTD.0000000000001055
21. Cleary Y, Kletzl H, Grimsey P, Heinig K, Ogungbenro K, Silber Baumann HE, et al. Estimation of FMO3 ontogeny by mechanistic population pharmacokinetic modelling of risdiplam and its impact on drug-drug interactions in children. Clin Pharmacokinet. (2023) 62:891–904. doi: 10.1007/s40262-023-01241-7
22. Li Z, Zhang Q, He H, Sun N, Zhang R, Yang CQ, et al. Population pharmacokinetics of ruxolitinib in children with hemophagocytic lymphohistiocytosis: focus on the drug-drug interactions. Cancer Chemother Pharmacol. (2023) 91:121–32. doi: 10.1007/s00280-022-04494-4
23. Courlet P, Guidi M, Alves Saldanha S, Cavassini M, Stoeckle M, Buclin T, et al. Population pharmacokinetic modelling to quantify the magnitude of drug-drug interactions between amlodipine and antiretroviral drugs. Eur J Clin Pharmacol. (2021) 77:979–87. doi: 10.1007/s00228-020-03060-2
24. Barcelo C, Aouri M, Courlet P, Guidi M, Braun DL, Gunthard HF, et al. Population pharmacokinetics of dolutegravir: influence of drug-drug interactions in a real-life setting. J Antimicrob Chemother. (2019) 74:2690–7. doi: 10.1093/jac/dkz217
25. Li LJ, Shang DW, Li WB, Guo W, Wang XP, Ren YP, et al. Population pharmacokinetics of clozapine and its primary metabolite norclozapine in Chinese patients with schizophrenia. Acta Pharmacol Sin. (2012) 33:1409–16. doi: 10.1038/aps.2012.71
26. Shang DW, Li LJ, Wang XP, Wen YG, Ren YP, Guo W, et al. Population pharmacokinetic/pharmacodynamic model of clozapine for characterizing the relationship between accumulated exposure and PANSS scores in patients with schizophrenia. Ther Drug Monit. (2014) 36:378–86. doi: 10.1097/FTD.0000000000000014
27. Anderson BJ and Holford NH. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol. (2008) 48:303–32. doi: 10.1146/annurev.pharmtox.48.113006.094708
28. Liu HC, Chang WH, Wei FC, Lin SK, Lin SK, and Jann MW. Monitoring of plasma clozapine levels and its metabolites in refractory schizophrenic patients. Ther Drug Monit. (1996) 18:200–7. doi: 10.1097/00007691-199604000-00015
29. Schulte P. What is an adequate trial with clozapine?: therapeutic drug monitoring and time to response in treatment-refractory schizophrenia. Clin Pharmacokinet. (2003) 42:607–18. doi: 10.2165/00003088-200342070-00001
30. Stark A and Scott J. A review of the use of clozapine levels to guide treatment and determine cause of death. Aust N Z J Psychiatry. (2012) 46:816–25. doi: 10.1177/0004867412438871
31. Wills KH, Behan SJ, Nance MJ, Dawson JL, Polasek TM, Hopkins AM, et al. Combining therapeutic drug monitoring and pharmacokinetic modelling deconvolutes physiological and environmental sources of variability in clozapine exposure. Pharmaceutics. (2021) 14:47. doi: 10.3390/pharmaceutics14010047
32. Correll CU. Clozapine: past, present and future. World Psychiatry. (2025) 24:153–4. doi: 10.1002/wps.21335
33. Taipale H, Tanskanen A, Howes O, Correll CU, Kane JM, and Tiihonen J. Comparative effectiveness of antipsychotic treatment strategies for relapse prevention in first-episode schizophrenia in Finland: a population-based cohort study. Lancet Psychiatry. (2025) 12:122–30. doi: 10.1016/S2215-0366(24)00366-3
34. Maruyama T, Kasai H, Fukaya Y, Shiokawa M, Kimura T, and Hamada Y. Drug-drug interactions between letermovir and tacrolimus in Japanese renal transplant recipients simulated using a physiologically based pharmacokinetic model. Front Microbiol. (2024) 15:1480874. doi: 10.3389/fmicb.2024.1480874
35. Coppinger C and Anderson PL. Considerations for drug-drug interactions between long-acting antiretrovirals and immunosuppressants for solid organ transplantation. Expert Opin Drug Metab Toxicol. (2025) 21:343–6. doi: 10.1080/17425255.2024.2448970
36. Pagan Santini RA, Nair V, Berlinrut I, Nair G, and Bhaskaran M. Drug-drug interactions leading to tacrolimus toxicity in a renal transplant patient with COVID-19: the role of paxlovid and the mitigating use of phenytoin. Cureus. (2025) 17:e80902. doi: 10.7759/cureus.80902
37. Wang P, Lu J, and Yang J. Physiologically based pharmacokinetic modeling supports investigation of potential drug-drug interactions in the pre- and early post-hematopoietic stem cell transplantation stages. Front Pharmacol. (2025) 16:1578643. doi: 10.3389/fphar.2025.1578643
38. Zhao YC, Zhang YK, Gao W, Liu HY, Xiao CL, Hou JJ, et al. A preliminary exploration of liver microsomes and PBPK to uncover the impact of CYP3A4/5 and CYP2C19 on tacrolimus and voriconazole drug-drug interactions. Sci Rep. (2025) 15:6389. doi: 10.1038/s41598-025-91356-7
39. Kha QH, Nguyen NTK, Le NQK, and Kang JH. Development and validation of a machine learning model for predicting drug-drug interactions with oral diabetes medications. Methods. (2024) 232:81–8. doi: 10.1016/j.ymeth.2024.10.012
40. Dwivedi J, Kaushal S, Wal P, Darshan JC, Sharma A, Nathiya D, et al. A data mining approach on polypharmacy and drug-drug interactions of common diabetes medications. Curr Drug Metab. (2025). doi: 10.2174/0113892002358291250401190533
41. Min JS, Jo SJ, Lee S, Kim DY, Kim DH, Lee CB, et al. A comprehensive review on the pharmacokinetics and drug-drug interactions of approved GLP-1 receptor agonists and a dual GLP-1/GIP receptor agonist. Drug Des Devel Ther. (2025) 19:3509–37. doi: 10.2147/DDDT.S506957
42. Billi M, Soloperto S, Bonora S, D’Avolio A, and De Nicolo A. Clinical pharmacology of bulevirtide: focus on known and potential drug-drug interactions. Pharmaceutics. (2025) 17:250. doi: 10.3390/pharmaceutics17020250
43. Nakamoto D, Piao Y, Mizutani H, Wakabayashi R, Otokita S, Stead A, et al. Prescription patterns of comedications associated with drug-drug interactions risk in HCV-infected patients undergoing direct-acting antiviral treatment: an analysis of an administrative claims database in Japan. J Pharm Health Care Sci. (2025) 11:33. doi: 10.1186/s40780-025-00442-5
44. Li M, Xiao J, Yu T, Huang L, Cai R, Yu H, et al. Analysis of hemorrhagic drug-drug interactions between P-gp inhibitors and direct oral anticoagulants from the FDA Adverse Event Reporting System. Expert Opin Drug Saf. (2024) 23:1453–61. doi: 10.1080/14740338.2024.2376693
45. Grymonprez M, Capiau A, Steurbaut S, Boussery K, Mehuys E, Somers A, et al. Pharmacodynamic drug-drug interactions and bleeding outcomes in patients with atrial fibrillation using non-vitamin K antagonist oral anticoagulants: a nationwide cohort study. Cardiovasc Drugs Ther. (2025) 39:133–43. doi: 10.1007/s10557-023-07521-5
46. Tombolini E, Squizzato A, Podda GM, Aghemo A, Ferri N, Segato S, et al. Drug-drug interactions between DAAs and anticoagulants or antiplatelets: A position paper of the Italian anticoagulation clinics. Liver Int. (2025) 45:e16177. doi: 10.1111/liv.16177
47. Munoz-Contreras MC, Cerda B, Lopez-Roman FJ, and Segarra I. Patients with dementia: prevalence and type of drug-drug interactions. Front Pharmacol. (2024) 15:1472932. doi: 10.3389/fphar.2024.1472932
48. Momcilovic M, Turcic P, Butkovic F, and Grle SP. A retrospective study on potential drug–drug interactions in patients with severe asthma receiving biological therapy: a single-center experience. BMC Pulm Med. (2025) 25:23. doi: 10.1186/s12890-025-03495-2
49. Chen S, Shen C, Tian Y, Peng Y, Hu J, Xie H, et al. Physiologically based pharmacokinetic modeling and simulation of topiramate in populations with renal and hepatic impairment and considerations for drug-drug interactions. CPT Pharmacometrics Syst Pharmacol. (2025) 14:510–22. doi: 10.1002/psp4.13292
50. Ren J, Shan D, Yue G, and He Q. Optimizing zanubrutinib dosing in patients: A PBPK-BO model approach to drug-drug interactions and patients with hepatic impairment. J Clin Pharmacol. (2025). doi: 10.1002/jcph.70042
51. Hvarchanova N, Radeva-Ilieva M, and Georgiev KD. Using physiologically based pharmacokinetic models for assessing pharmacokinetic drug-drug interactions in patients with chronic heart failure taking narrow therapeutic window drugs. Pharm (Basel). (2025) 18:477. doi: 10.3390/ph18040477
52. Bischof T, Nagele F, Kalkofen MM, Blechschmidt MEO, Domanovits H, Zeitlinger M, et al. Drug-drug-interactions in patients with atrial fibrillation admitted to the emergency department. Front Pharmacol. (2024) 15:1432713. doi: 10.3389/fphar.2024.1432713
53. De Bellis E, Donnarumma D, Zarrella A, Mazzeo SM, Pagano A, Manzo V, et al. Drug-drug interactions between HIV antivirals and concomitant drugs in HIV patients: what we know and what we need to know. Pharmaceutics. (2024) 17:31. doi: 10.3390/pharmaceutics17010031
54. Senneker T. Drug-drug interactions between gender-affirming hormone therapy and antiretrovirals for treatment/prevention of HIV. Br J Clin Pharmacol. (2024) 90:2366–82. doi: 10.1111/bcp.16097
55. Branch C, Parson-Martinez J, and Cory TJ. Drug-drug interactions in HIV-infected patients receiving chemotherapy. Expert Opin Drug Metab Toxicol. (2025) 21:15–27. doi: 10.1080/17425255.2024.2408004
56. Calza L, Giglia M, Zuppiroli A, Cretella S, Vitale S, Appolloni L, et al. Decrease in drug-drug interactions between antiretroviral drugs and concomitant therapies in older people living with HIV-1 switching to bictegravir/emtricitabine/tenofovir alafenamide. New Microbiol. (2025) 48:70–7.
57. Adamiszak A, Drobinska J, Niewiadomska-Wojnalowicz I, Derwich K, Grzeskowiak E, and Bienert A. Potential drug-drug interactions analysis in Polish pediatric hemato-oncologic unit, including acute lymphoblastic leukemia patients. Pharmacol Rep. (2025) 77:751–60. doi: 10.1007/s43440-025-00719-4
58. Soliman A, Rodriguez-Vera L, Alarcia-Lacalle A, Pippa LF, Subhani S, Lukacova V, et al. Leveraging omeprazole PBPK/PD modeling to inform drug-drug interactions and specific recommendations for pediatric labeling. Pharmaceutics. (2025) 17:373. doi: 10.3390/pharmaceutics17030373
59. Atoyebi S, Montanha MC, Nakijoba R, Orrell C, Mugerwa H, Siccardi M, et al. Physiologically based pharmacokinetic modeling of drug-drug interactions between ritonavir-boosted atazanavir and rifampicin in pregnancy. CPT Pharmacometrics Syst Pharmacol. (2024) 13:1967–77. doi: 10.1002/psp4.13268
60. Kiiza D, Rostami-Hochaghan D, Alhassan Y, Seden K, Reynolds H, Kaboggoza JP, et al. Clinical, pharmacological, and qualitative characterization of drug-drug interactions in pregnant women initiating HIV therapy in Sub-Saharan Africa. J Antimicrob Chemother. (2024) 79:2334–42. doi: 10.1093/jac/dkae232
61. Xu S, Song Z, Bai J, and Wang J. Prevalence and clinical significance of potential drug-drug interactions of antimicrobials in Intensive Care Unit patients: a retrospective study. BMC Pharmacol Toxicol. (2025) 26:104. doi: 10.1186/s40360-025-00925-z
62. Alfehaid LS, Farah S, Omer A, Weber BN, Alkhezi O, Tawfik YMK, et al. Drug-drug interactions and the clinical tolerability of colchicine among patients with COVID-19: A secondary analysis of the COLCORONA randomized clinical trial. JAMA Netw Open. (2024) 7:e2431309. doi: 10.1001/jamanetworkopen.2024.31309
63. Bolek H, Yazgan SC, Yekeduz E, Kaymakcalan MD, McKay RR, Gillessen S, et al. Androgen receptor pathway inhibitors and drug-drug interactions in prostate cancer. ESMO Open. (2024) 9:103736. doi: 10.1016/j.esmoop.2024.103736
64. Bu F, Cho YS, He Q, Wang X, Howlader S, Kim DH, et al. Prediction of pharmacokinetic drug-drug interactions involving anlotinib as a victim by using physiologically based pharmacokinetic modeling. Drug Des Devel Ther. (2024) 18:4585–600. doi: 10.2147/DDDT.S480402
65. Kapagan T, Bulut N, and Erdem GU. Polypharmacy and drug-drug interactions in metastatic breast cancer patients receiving cyclin-dependent kinase (CDK) 4/6 inhibitors. J Oncol Pharm Pract. (2024) 30:1403–10. doi: 10.1177/10781552231218959
66. Zarrabi S, Hosseini E, Sadeghi K, Vaezi M, and Shahrami B. Assessment of drug-drug interactions among patients with hematologic Malignancy: A clinical pharmacist-led study. J Oncol Pharm Pract. (2024), 10781552241281664. doi: 10.1177/10781552241281664
67. Agostinetto E, Pfeiler G, Hlauschek D, Mayer EL, Lambertini M, de Azambuja E, et al. Drug-drug interactions between palbociclib and proton pump inhibitors in early breast cancer: an exploratory analysis of PALLAS (ABCSG-42/AFT-05/BIG-14-13/PrE0109). ESMO Open. (2025) 10:104096. doi: 10.1016/j.esmoop.2024.104096
68. Alkathiri MA, Bamogaddam RF, Alhabshi HA, AlAjmi MN, Alashgaai AA, Assiri GA, et al. Potential drug-drug interactions among geriatric oncology patients: a retrospective study in Saudi Arabia. BMC Geriatr. (2025) 25:300. doi: 10.1186/s12877-025-05965-y
69. Patil PH, Desai MP, Baburaj G, Rao M, Kunhikatta V, Udupa K, et al. A single-centre retrospective evaluation of potential drug-drug interactions in breast cancer patients undergoing CDK 4/6 inhibitors chemotherapy. J Oncol Pharm Pract. (2025) 10781552251314811. doi: 10.1177/10781552251314811
70. Ratan C, Rajeev M, Krishnan K, Jayamohanan H, Kartha N, Vijayan M, et al. Assessment of potential drug-drug interactions in hospitalized cancer patients. J Oncol Pharm Pract. (2025) 31:256–65. doi: 10.1177/10781552241235573
71. Sahin TK, Kavgaci G, Guven DC, and Aksoy S. Drug-Drug interactions and special considerations in breast cancer patients treated with CDK4/6 inhibitors: A comprehensive review. Cancer Treat Rev. (2025) 137:102956. doi: 10.1016/j.ctrv.2025.102956
72. Kotlinska-Lemieszek A, Klepstad P, and Haugen DF. Clinically significant drug-drug interactions involving opioid analgesics used for pain treatment in patients with cancer: update of a systematic review. Expert Opin Drug Metab Toxicol. (2025) 21:703–15. doi: 10.1080/17425255.2025.2491743
73. Kuhn AK, Determan ML, Wright JA, Matey E, and Leung JG. The potential influence of estrogen-containing oral contraception on clozapine metabolism in a patient with known pharmacogenomic status. Ment Health Clin. (2024) 14:220–3. doi: 10.9740/mhc.2024.06.220
Keywords: population pharmacokinetics modelling, drug-drug interactions, individualized therapy, clozapine, schizophrenia
Citation: Han H-H, Zhang Y, Wang J, Tian X, Li Y, He S-M, Zhang C, Chen X and Wang D-D (2025) Population pharmacokinetics modelling to predict DDI from zopiclone on clozapine in schizophrenia patients. Front. Psychiatry 16:1664678. doi: 10.3389/fpsyt.2025.1664678
Received: 12 July 2025; Accepted: 01 September 2025;
Published: 22 September 2025.
Edited by:
Massimo Tusconi, University of Cagliari, ItalyReviewed by:
Marcin Siwek, Medical College, Krakow, PolandAmelia Ramón López, Miguel Hernández University of Elche, Spain
Copyright © 2025 Han, Zhang, Wang, Tian, Li, He, Zhang, Chen and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Su-Mei He, aGVoZTgyMDRAMTYzLmNvbQ==; Cun Zhang, MTc1MTI2NTYzNjVAMTYzLmNvbQ==; Xiao Chen, Y2hlbnhpYW8xMTI3MzNAMTYzLmNvbQ==; Dong-Dong Wang, MTM4NTIwMjk1OTFAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Huan-Huan Han1†
Huan-Huan Han1† Su-Mei He
Su-Mei He Cun Zhang
Cun Zhang Xiao Chen
Xiao Chen Dong-Dong Wang
Dong-Dong Wang