- 1Henan University of Chinese Medicine, Zhengzhou, China
- 2Third Affiliated Hospital of Henan University of Traditional Chinese Medicine, Zhengzhou, China
- 3Rehabilitation Medicine College, Henan University of Chinese Medicine, Zhengzhou, China
Introduction: Anxiety is closely related to sleep, and the two often interact with each other. Generalized anxiety disorder (GAD) is often accompanied by insomnia, but pharmacologic interventions are typically ineffective and cause safety concerns. Despite the potential of non-pharmacological interventions (NIPs), their relative efficacy has not been clarified. The network meta-analysis (NMA) aims to explore the impact of NIPs on alleviating insomnia symptoms in patients with GAD.
Methods and analysis: 24 randomized controlled trials (1,953 patients) evaluating 14 NIPs for GAD-related insomnia were utilized in this NMA. This study systematically searched the databases including PubMed, Web of Science, EMBASE, Cochran, CNKI, SinoMed, VIP, and Wanfang. Meanwhile, Bayesian method was employed in conjunction with Markov Chain Monte Carlo (MCMC) simulation. In addition, this study conducted statistical analyses by using R (version 4.4.1) and STATA (version 15.1). Interventions were ranked by standardized mean difference (SMD) and surface under the cumulative ranking curve (SUCRA); study quality was evaluated using the Cochrane Risk of Bias tool (ROB2.0). Sleep quality was assessed with Pittsburgh Sleep Quality Index (PSQI) as well as Insomnia Severity Index (ISI), and anxiety symptoms were measured using Hamilton Anxiety Scale (HAMA) and Self-rating Anxiety Scale (SAS).
Results: Acupuncture (AC) showed the best efficacy in improving sleep quality and alleviating anxiety symptoms. The combination of transcranial magnetic stimulation with psychotherapy (TMS+PT) significantly improved sleep quality and alleviating anxiety symptoms. Other interventions (e.g., relaxation therapy, exercise therapy, etc.) had limited efficacy.
Conclusion: AC and TMS+PT are the best NIPs to improve insomnia and relieve anxiety in GAD patients. In the future, conducting multicenter trials and in-depth mechanistic studies is expected to validate the efficacy and optimize the individualized treatment regimen.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, identifier, CRD420251010334.
1 Introduction
As the most common subtype of anxiety disorders, generalized anxiety disorder (GAD) is prevalent in adults, with an incidence rate of 4.1% to 6.6% (1). It is primarily manifested by excessive worry, persistent tension, attention-deficit disorder, panic attacks, easy fatigue and sleep disorders and others (2). As people’s pace of life accelerates and social pressure increases, the prevalence and co-morbidity rates of GAD are increasing year by year, making it a prevalent global health problem. Insomnia represents one of the prevalent symptoms in patients with GAD and is a component of the diagnostic criteria for GAD, showing a complex and interactive relationship between the two (3). A survey (4) indicated that about 74% of GAD patients were found with insomnia symptoms during periods of anxiety. Another study (5) demonstrated that anxiety-specific interventions were ineffective when insomnia problems were not addressed. The superimposed clinical effect produced by anxiety and insomnia problems therefore suggests the need to further explore therapeutic approaches targeted at improving insomnia symptoms in GAD patients.
The conventional clinical approach to managing GAD patients with insomnia is the combination of anxiolytic and hypnotic sedative medications. However, 50% of patients exhibit suboptimal responses to anxiolytics (6), while long-term use of hypnotic sedative drugs may induce drug dependence as well as tolerance (7). Thus, there is a pressing need to explore safer and more efficacious treatments. Non-pharmacological interventions (NIPs) for sleep, which do not involve the use of medications, are characterized by low risk, low cost and significant efficacy, representing a promising technique for improving insomnia symptoms in patients with GAD (8).
Currently, NIPs to alleviate insomnia include psychotherapy (PT), transcranial magnetic stimulation (TMS), relaxation therapy (RT), exercise therapy (ET), acupuncture (AC), and tuina (TN) (9). PT comprises cognitive behavioral therapy (CBT), mindfulness therapy (MT), and sleep hygiene education. A meta-analysis revealed that CBT significantly enhanced sleep quality in psychiatric patients with comorbid insomnia symptoms, and the treatment remained effective after 18 months of follow-up (10). Another randomized controlled trial (RCT) study demonstrated that MT proved to be effective in improving sleep quality in patients with anxiety disorders (11). TMS, a promising technique for the treatment of psychiatric disorders, is effective for both anxiety and insomnia symptoms in GAD patients when they receive 1 Hz low-frequency TMS in the parietal cortex (12). RT assists patients in reducing somatic tension and related symptoms of depression and anxiety, thereby facilitating better sleep (13). Other NIPs, such as AC, TN, Baduanjin, Taiji, and yoga, can alleviate patients’ anxiety and insomnia symptoms, thereby providing important implications for improving patients’ outcomes and life quality (14–17).
Despite the positive effects of NIPs on sleep quality in GAD patients demonstrated in many studies, these investigations exclusively focus on a single intervention for improving sleep and lack direct or indirect comparisons across different interventions. Consequently, it is impossible to rank these NIPs based on their efficacy. Network meta-analysis (NMA), a statistical methodology, facilitates the synthesis of direct and indirect comparisons within a multi-therapy analytical framework and allows the assessment of the prioritization and ranking of multiple interventions (18). Therefore, this NMA was implemented to ascertain the efficacy of currently available NIPs in alleviating insomnia and anxiety symptoms in GAD patients, thus providing evidence-based support for the optimization of individualized therapeutic strategies in clinical practice.
2 Methods
This study adhered to the Preferred Reporting Items for Systematic Evaluation and Meta-Analysis (PRISMA) guidelines and its requirements for NMA (19). We have completed the PRISMA 2020 Checklist and the PRISMA 2020 for Abstract Checklist, available in Supplementary Files S1 and S2, respectively. The study protocol has been registered in the PROSPERO (CRD420251010334). In accordance with the PRISMA 2020 statement, we report the following amendments made to the study protocol after registration. First, the target condition was refined from “anxiety disorders” to generalized anxiety disorder (GAD) to enhance clinical specificity, as insomnia represents one of the most prevalent symptoms in GAD patients, and the relationship between the two is complex and interactive. Second, the search strategy was expanded to include additional databases to improve retrieval comprehensiveness. All amendments were finalized prior to conducting the quantitative synthesis and did not alter the predetermined outcomes or eligibility criteria.
2.1 Search strategy
The publications in eight databases were searched in the study (PubMed, Web of Science, EMBASE, Cochran, CNKI, SinoMed, VIP, Wanfang). The time frame ranged from the construction of the database to March 11, 2025, and the language was confined to Chinese and English. The search was conducted employing a synthesis of subject terms and free words. The former one included Anxiety, Anxiety Disorder*, Insomnia, Sleep Quality, Sleep Initiation and Maintenance Disorders, RCT and Randomized Controlled Trial. Meanwhile, we manually searched for ClinicalTrials.gov (2020-2025) and WHO ICTRP platforms to screen for unpublished RCTS, and we did not identify unpublished studies. The specific search strategy used is described in Supplementary File S3.
2.2 Inclusion and exclusion criteria
According to the PICOS framework, literature conforming to the following criteria was included in this NMA: (i) P (Population): GAD patients with insomnia symptoms, where GAD meets the diagnostic criteria of the Chinese Classification and Diagnostic Criteria Chinese Classification of Mental Disorders, Third Edition (CCMD-III) (20), the International Classification of Diseases, Eleventh Edition (ICD-11) (21), or the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) (3), and who are not comorbid with other psychiatric disorders. (ii) I (Intervention): NIPs were administered to at least one group of subjects. NIPs refer to therapeutic methods to achieve a therapeutic effect with no use of medications (22), including PT, TMS, RT, ET, AC, TN, and others. (iii)C (Comparison): Treatment as usual (TAU), placebo, sham surgery, waitlist, and other NIPs. Since placebo, sham surgery, and waitlist interventions showed no significant effect on the outcome measures of the subjects, the two authors considered combining them into a “Control group”. (iv) O (Outcome): The primary outcome indicator was sleep quality assessed by overall scores from self-rating sleep scales, encompassing the Pittsburgh Sleep Quality Index (PSQI) (23), Insomnia Severity Index (ISI) (24), PROMIS Sleep Disturbance short form (25), and Self-rating Scale of Sleep (SRSS) (26). Secondary outcome indicators referred to the severity of anxiety measured using self-rating measures such as the Hamilton Anxiety Scale (HAMA) (27), Self-rating Anxiety Scale (SAS) (28), Beck Anxiety Inventory (BAI) (29), and Generalized Anxiety Disorder 7 - item Scale (GAD-7) (30). (v) S (Study): Randomized controlled trial (RCT), with or without blinding.
Literature meeting the following criteria was excluded: (i) Those involving animal or cellular experiments, case reports, scientific experiment protocols, commentaries, letters, editorials, conference papers; (ii) Studies with missing data or significant errors; (iii) Duplicate publications; (iv) Unavailable full text; (v) Literature with duplicate participants included in the study; (vi) Insomnia induced by other psychiatric disorders, including depression, schizophrenia, post-traumatic stress disorder, or drug side effects; (vii) Patients taking sleeping pills or other sleep aids.
2.3 Study selection and data extraction
The retrieved documents were imported into EndNote 21.2. Two researchers (Yun Li and Jing Zhang) independently checked the titles and abstracts as per the inclusion and exclusion criteria, and then read the full texts. Subsequently, both researchers independently performed data extraction using Microsoft Excel 2023. The extracted data included: (a) basic information (first author, publication year, country, study design); (b) participant characteristics (sample size, sex ratio); (c) intervention and control measures (type, duration, follow-up period); and (d) outcomes and measurement tools. During both study selection and data extraction processes, the researchers remained blinded to each other’s entries. Any disagreements were resolved through consultation with a senior researcher (Yan Chen) for final adjudication.
2.4 Quality assessment
Two researchers (Zhenhao Lv and Yiwen Li) appraised the quality of eligible studies independently by applying the Cochrane Risk of Bias tool (ROB2.0) (31). The ROB2.0 criteria included five domains: generation of random sequences, allocation concealment, blinding, missing data, and selective reporting, each of which was rated as “high risk,” “some risk,” or “low risk”. For evaluating the overall quality of the articles, if all five domains were rated as low risk or only one domain was rated as medium risk with the rest being low risk, the overall risk of bias in the study was low; if four or more of the five domains were rated as medium risk or any one was rated as high risk, the overall risk of bias in the article was high; and the overall risk of bias in the study was medium for the rest of the scenarios. Two authors assessed the literature quality independently, and a third author (Yun Li) addressed dissents between the two researchers.
2.5 Statistical analysis
The included studies assessed outcomes with different scales, and the outcome indicators were continuous variables. Therefore, the standardized mean difference (SMD) was adopted as the effect size. In this study, a Markov Chain Monte Carlo (MCMC) method was leveraged to construct a Bayesian NMA model, with iterations of the model to evaluate the relative efficacy of various treatment regimens (18). The testing process was performed with a model chain value of 4, an annealing value of 10,000, an iteration value of 50,000, a step size of 10 for each test, and a baseline value of 2.5. This process aimed to get the posterior distribution (32). The NMA required three basic assumptions to be met, namely the assumption of transmissibility, homogeneity, and consistency. Heterogeneity analysis was carried out with the mtc:anohe function of the GeMTC package. Heterogeneity across included studies between the same comparisons was considered acceptable when the overall I2 was <50%, satisfying the homogeneity assumption. Inconsistency between direct and indirect comparisons was tested by node splitting using the mtc.nodesplit function in the GeMTC.package. When p > 0.05, it was considered that there was no inconsistency between direct and indirect comparisons, meeting the assumption of consistency (33). The consistency model was compared with the inconsistency model using the Deviance Information Criterion (DIC). A DIC difference of ≥5 was considered to indicate a significant improvement in model fit (suggesting potential inconsistency), whereas a smaller difference was interpreted as the absence of global inconsistency. The results were judged for convergence by computing the potential scale reduction factor (PSRF). With 1 as the standard, 1 ≤ PSRF < 1.05 was considered as successful convergence. A network structure was constructed, with the relationship of SMD between the interventions as lines and the interventions as nodes. Nodes denoted different interventions, and connecting lines indicated head-to-head comparisons across interventions. Cumulative probability ranking plots were leveraged to estimate all cumulative ranking probabilities and report them as the SUCRA, i.e., cumulative probability ranking; funnel plots and egger’s test were utilized to evaluate possible publication bias. All included studies had complete data with no missing values; therefore, no imputation or other missing-data procedures were required. All statistical analyses described above were performed using R (version 4.4.1) and STATA (version 15.1).
Among the included studies, one was a three-arm RCT comparing control group, scalp acupuncture and body acupuncture (34). Scalp acupuncture and Body acupuncture are both types of acupuncture; they are distinguished by differences in the selection of acupuncture points. This study divided the single three-arm RCT into two two-arm RCTs to preserve the two-by-two comparative analytic framework, i.e., this multi-group trial was divided into two distinct comparisons: (A) control group vs. scalp acupuncture and (B) control group vs. body acupuncture. This approach enabled consistency in the assumption of independent treatment effects across studies in the NMA (35).
3 Results
3.1 Literature search and screening process
A total of 35,481 records were searched from databases. After removing 12,560 duplicates, 22,761 records were excluded through preliminary screening of titles and abstracts. Two additional records were excluded because the full text could not be retrieved. We assessed 158 full-text articles for eligibility. Following strict application of the inclusion and exclusion criteria, 137 articles were excluded for not meeting the criteria, resulting in 23 articles being included in the final analysis. These 23 articles reported on 24 two-arm RCTs (including 2 studies derived from one three-arm RCT). The detailed screening process is shown in Figure 1.
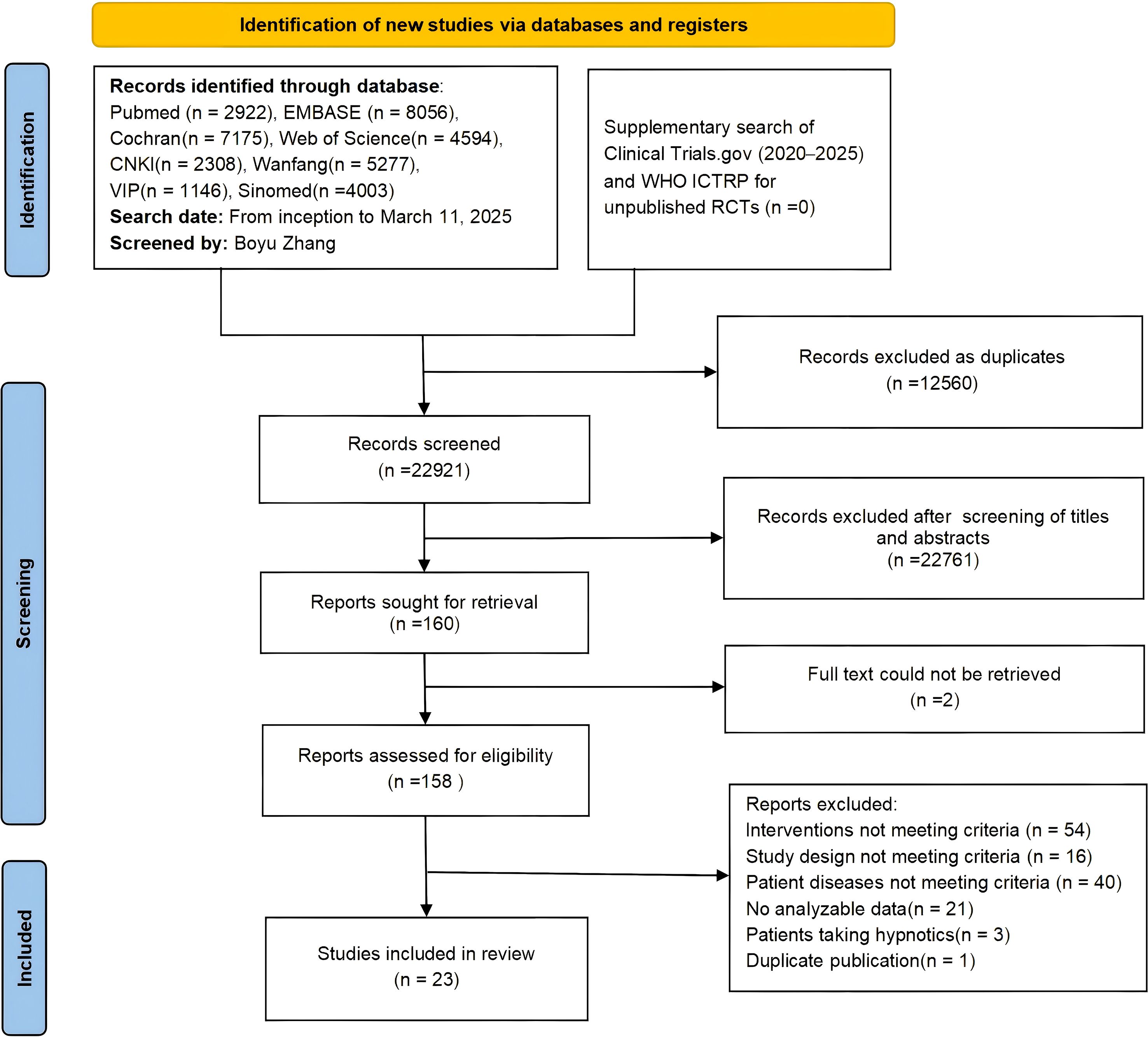
Figure 1. PRISMA 2020 flow diagram of literature screening process. Final included articles (n = 23) reporting on 24 two-arm RCTs (including 2 studies derived from one three-arm RCT).
3.2 Basic characteristics and quality assessment of included studies
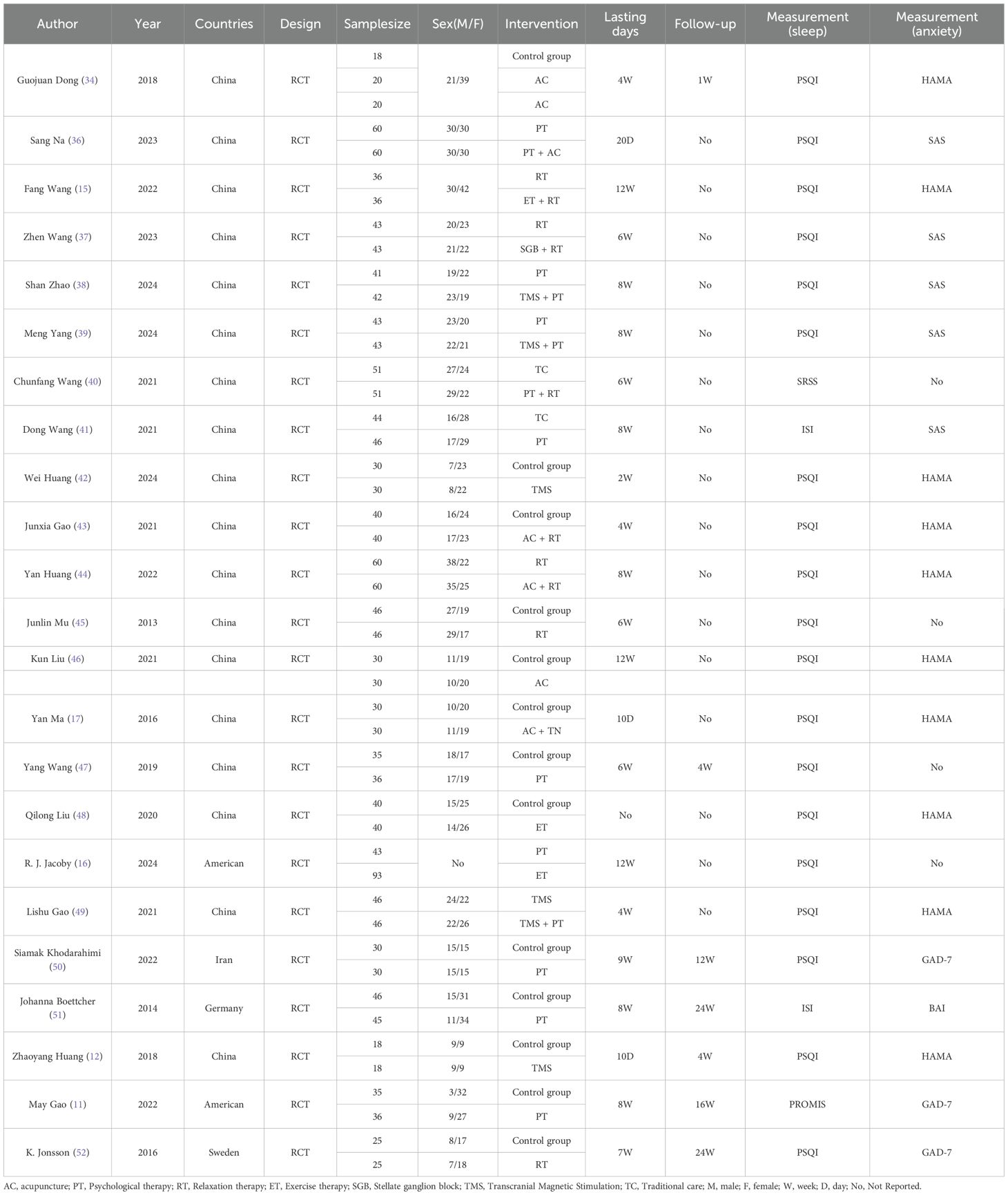
Of the included studies, 22 were two-arm RCTs and one was a three-arm RCT; these trials were conducted in five countries: China, the United States, Germany, Sweden, and Iran. A total of 1953 patients were involved in the study, with a mean age of 40.55 years and 57.03% of female participants. A total of 14 interventions were included in the study, with a minimum intervention duration of 10 days and a maximum intervention duration of 12 weeks. Regarding the assessment of sleep quality, the PSQI was used in 19 studies, the ISI in 2 studies, the SRSS in 1 study, and the PROMIS Sleep Disturbance short form in 1 study. As for assessing anxiety symptoms, HAMA was used in ten studies, SAS in five studies, GAD-7 in three studies, and BAI in one study. The basic information about the included studies is specified in Table 1.
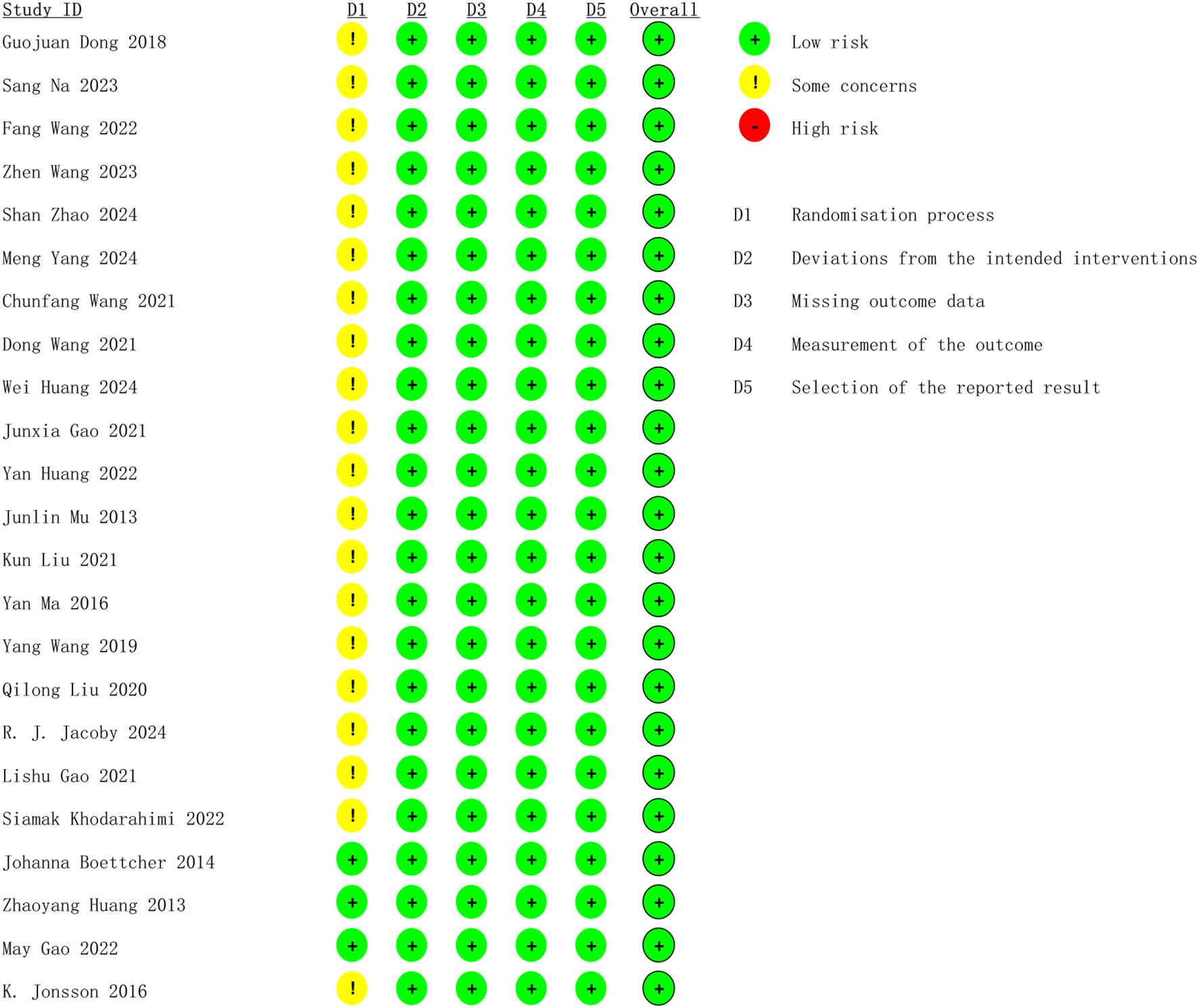
Concerning bias resulting from the random process, three studies were evaluated as low risk by clearly detailing how the randomized sequence was generated, how the group allocation was concealed, and the absence of baseline characteristics disparities between the two groups. The other 20 studies were classified as medium risk because of ambiguous randomization or inadequate allocation concealment. 23 studies were assessed as low risk for the other four possible aspects of bias. The overall bias risk of the included studies was assessed as low, suggesting that they were all high-quality studies. Figure 2 displays the methodological quality assessment results of the included studies.
3.3 NMA results
3.3.1 Network relationship diagram
In the network relationship diagram, each dot expresses an intervention, and the dot size shows a positive correlation with the number of studies involved in each intervention, with larger dots indicating a greater number of studies included; The line connecting two dots signifies a direct comparative study between these two interventions, while the line thickness indicates the number of studies comparing two regimens, with a thicker line indicating a greater number of relevant comparative studies. Figure 3 illustrate the detailed results. PSRF was calculated to determine the convergence of the results. The findings revealed that the PSRF values of all the outcome indicators were equal to 1 (Supplementary File S4), signifying complete convergence of the model. Node splitting was performed if there were closed loops. The results demonstrated that the P-values for all outcome indicators were greater than 0.05, indicating no local inconsistencies (Supplementary File S5). Furthermore, this study compared the fit of the consistency model with the inconsistency model using the Deviance Information Criterion (DIC). For the sleep scale, the DIC was 48.46361 for the consistency model and 48.18715 for the inconsistency model (difference < 5); for the anxiety scale, the DIC was 39.97305 for the consistency model and 39.86889 for the inconsistency model (difference < 5). As the DIC differences for both outcome measures were below the pre-specified threshold of 5, no significant global inconsistency was detected in the network.
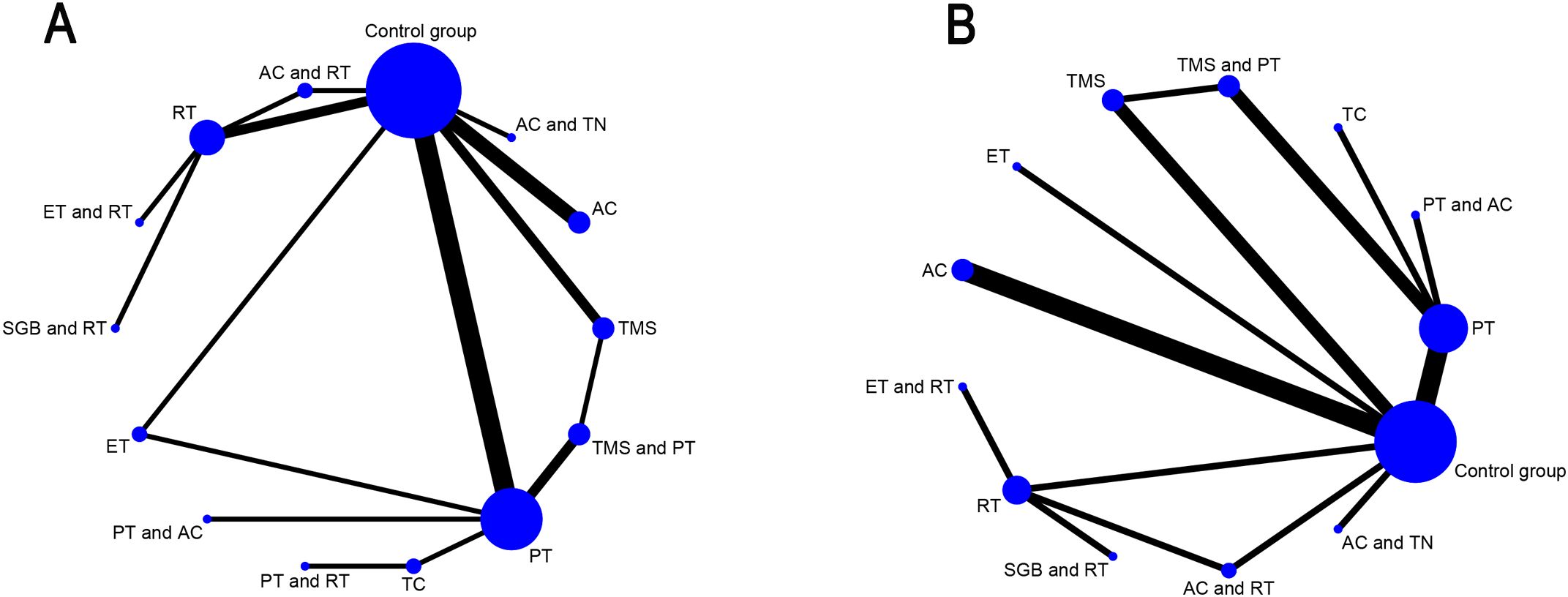
Figure 3. Network geometry of interventions for primary and secondary outcomes. (A) Sleep quality improvement in GAD patients; (B) Anxiety alleviation in GAD patients; AC, acupuncture; PT, Psychological therapy; RT, Relaxation therapy; ET, Exercise therapy; SGB, Stellate ganglion block; TMS, Transcranial Magnetic Stimulation; TC, Traditional care.
3.3.2 Primary outcome indicator: sleep quality
24 studies were included, including two two-arm RCTs (Guojuan Dong A and B) split from one three-arm RCT, and four self-rating sleep scales were utilized to assess post-intervention sleep quality. The scales used included the PSQI (23), ISI (24), PROMIS Sleep Disturbance short form (25), and SRSS (26). All scales were directionally consistent, where higher scores denoted poorer sleep quality.
NMA revealed that AC (SMD = 3.67, 95% CrI=1.34-6.09) and TMS+PT (SMD = 3.09, 95% CrI=0.32-5.89) significantly improved sleep quality in GAD patients compared with the control group, as shown in Table 2. The results of SUCRA probability ranking showed that AC (83.97%) > TMS and PT (76.29%) > PT and AC (64.80%) > AC and TN (61.59%). The greatest magnitude of improvement in patients’ sleep quality was achieved by AC alone, as shown in Figure 4.
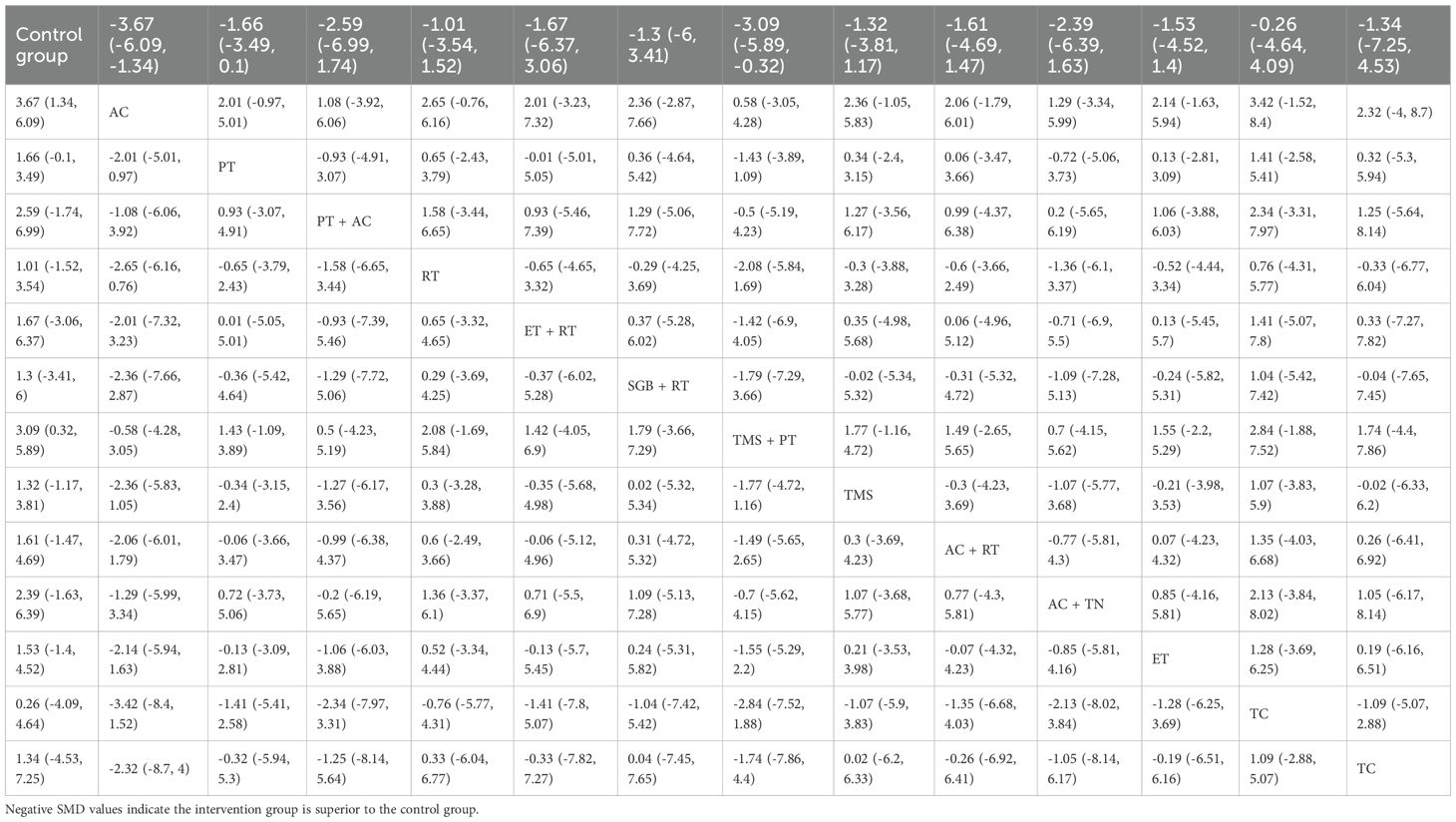
Table 2. Head-to-head comparisons of efficacy and acceptability of non-pharmacological interventions for insomnia.
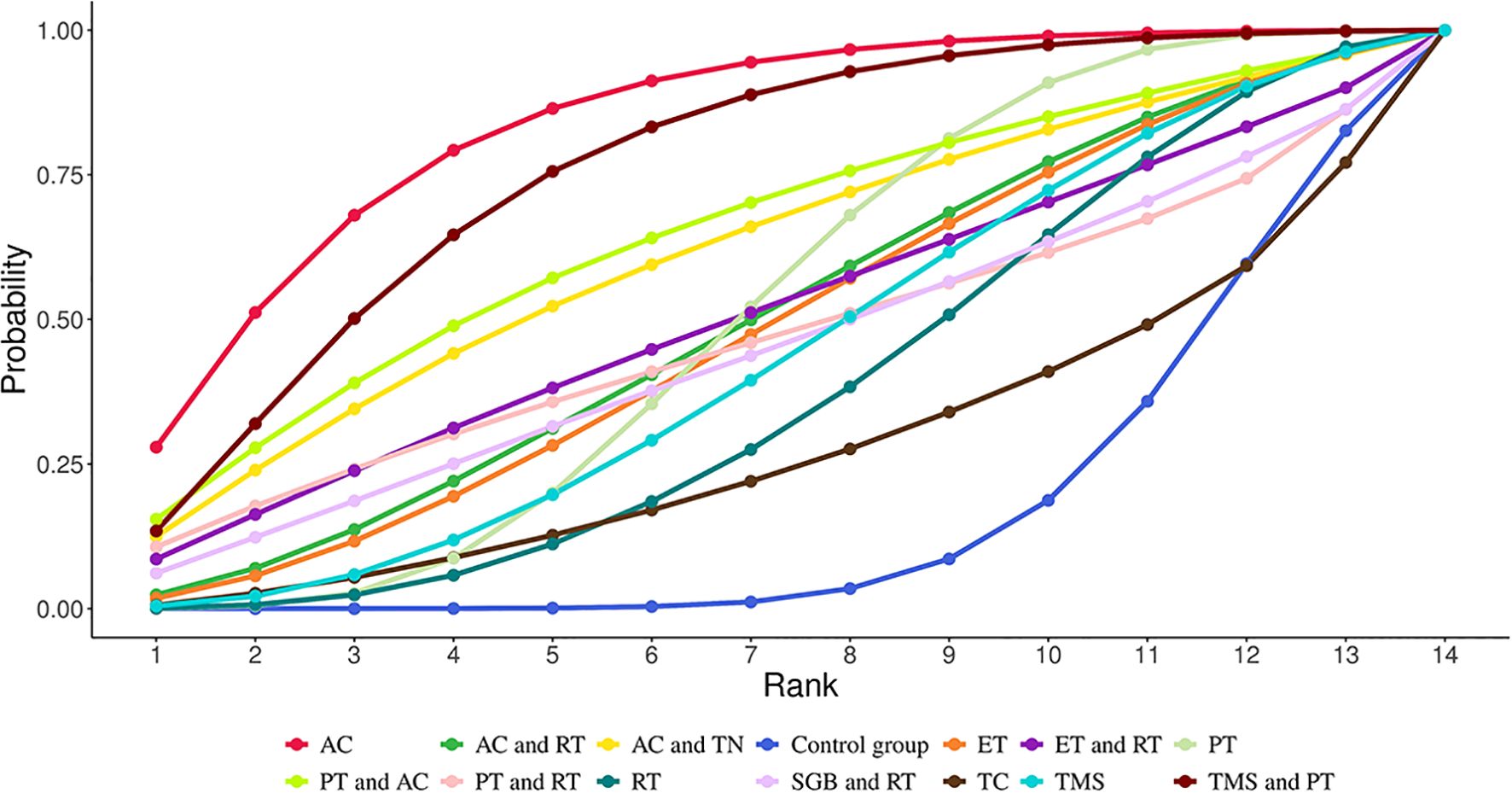
Figure 4. Cumulative Ranking Probabilities (SUCRA) of interventions for improving sleep quality in GAD patients. The Surface Under the Cumulative Ranking Curve (SUCRA) value represents the percentage of effectiveness, with 100% indicating the best intervention and 0% the worst.
3.3.3 Secondary outcome indicator: anxiety level
20 studies were included, including two two-arm studies (Guojuan Dong A and B) split from one three-arm studies, and four self-rating anxiety scales were utilized to assess post-intervention anxiety. The scales used included the HAMA (27), SAS (28), BAI (29), and GAD-7 (30). All scales were directionally consistent, where higher scores denoted more severe anxiety.
NMA showed that compared with the control group, AC (SMD = 2.20, 95% CrI=0.90-3.50), PT (SMD = 1.24, 95% CrI=0.10-1.24), AC+TN (SMD = 2.71, 95% CrI=0.48- 4.98), PT+AC (SMD = 3.91, 95% CrI=1.45-6.45), TMS+PT (SMD = 2.30, 95% CrI=0.72-3.94) significantly relieved anxiety of GAD patients, as illustrated in Table 3. The results of SUCRA probability ranking revealed that PT and AC (92.88%) > AC and TN (75.69%) > TMS and PT (69.87%) > AC (66.76%). PT+AC was the most effective for ameliorating patients’ anxiety, as shown in Figure 5.
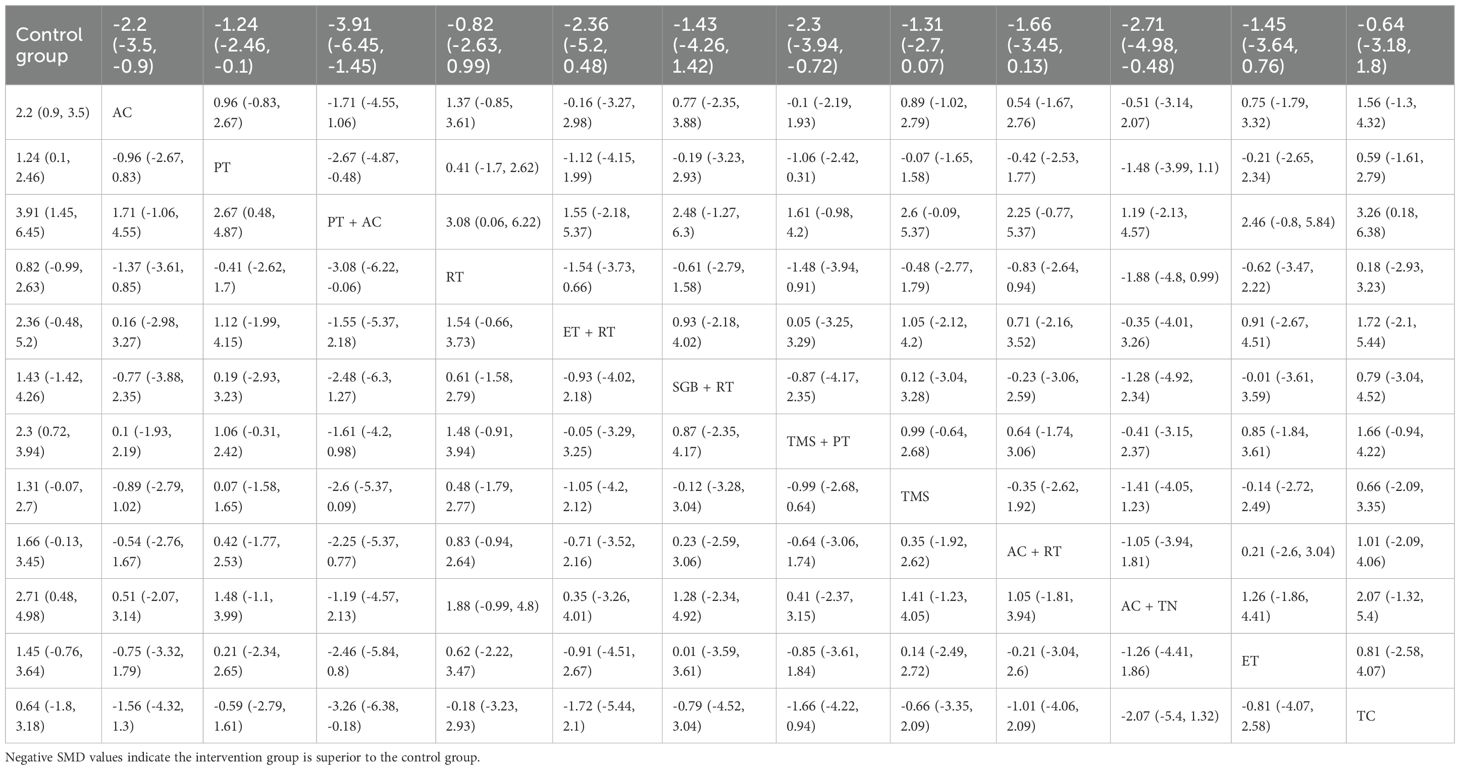
Table 3. Head-to-head comparisons of efficacy and acceptability of non-pharmacological interventions for anxiety.
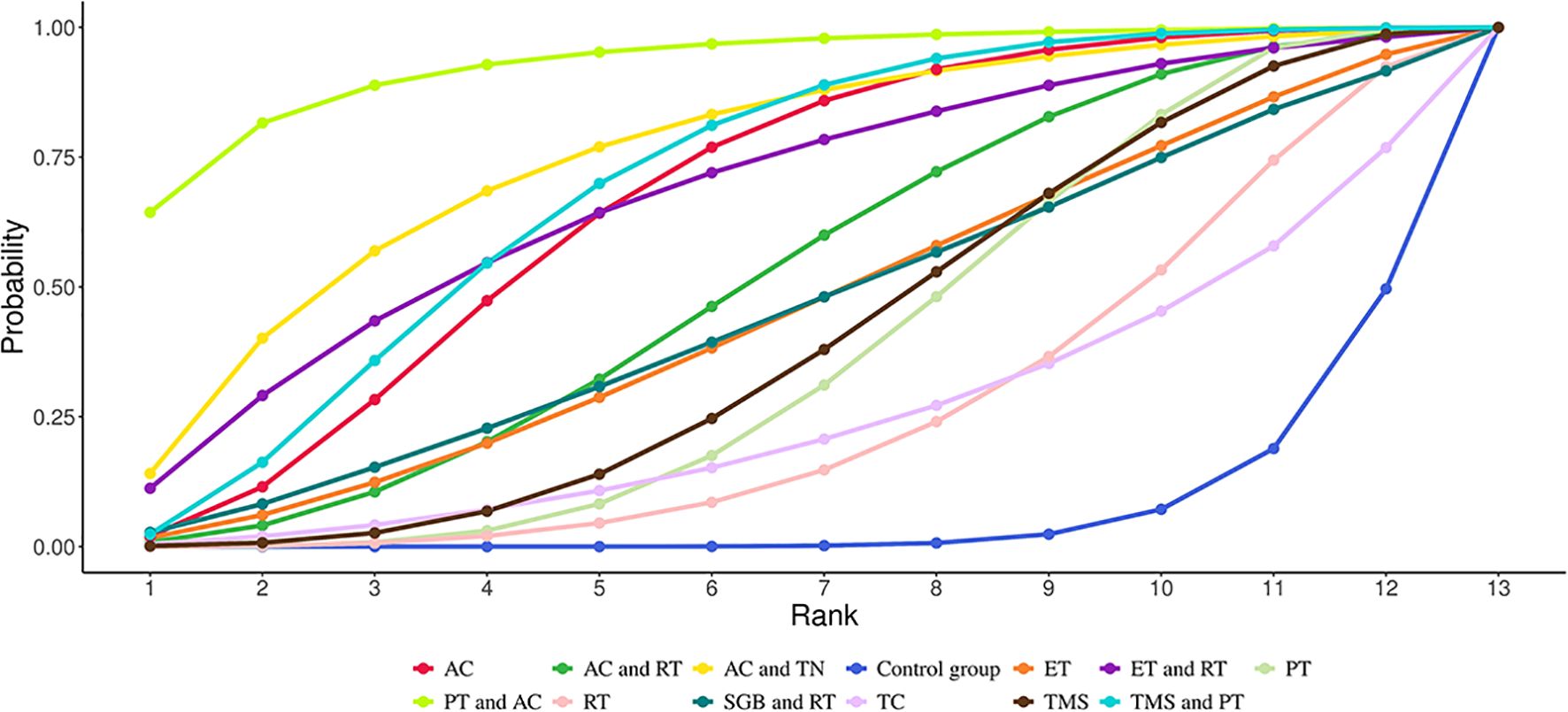
Figure 5. Cumulative Ranking Probabilities (SUCRA) of interventions for alleviating anxiety in GAD patients. The Surface Under the Cumulative Ranking Curve (SUCRA) value represents the percentage of effectiveness, with 100% indicating the best intervention and 0% the worst.
3.4 Publication bias
A correction-comparison funnel plot was utilized to test publication bias. The funnel plots were symmetrical, suggesting no publication bias, as shown in Figure 6. The results of egger’s test indicated no significant publication bias for both the primary outcome (sleep quality, P = 0.549) and the secondary outcome (anxiety severity, P = 0.467) (all P > 0.05), which is consistent with the funnel plot findings.
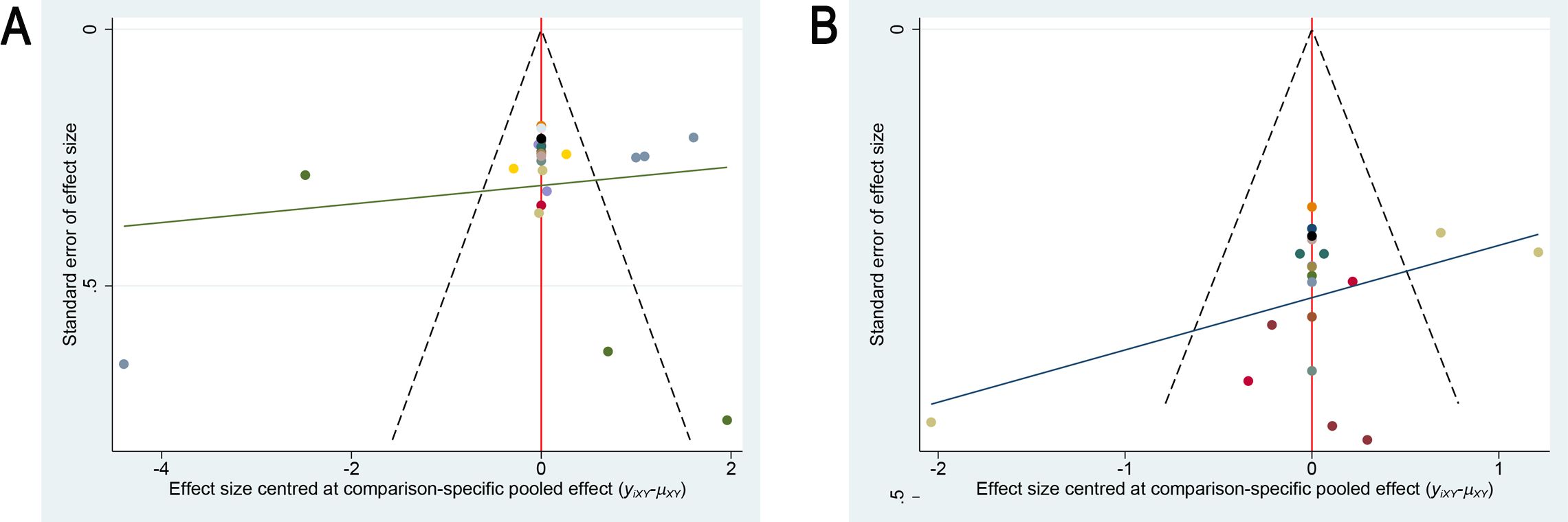
Figure 6. Comparison-adjusted funnel plots for publication bias assessment. (A) Sleep quality outcome; (B) Anxiety outcome.
4 Discussion
4.1 Summary
In NMA, 24 two-arm RCTs evaluating the efficacy of NIPs to improve insomnia symptoms in GAD patients were included, involving 14 interventions and 1,953 GAD patients with insomnia symptoms. Our meta-analysis demonstrated that compared to the control group, AC and TMS+PT significantly improved patients’ sleep quality; AC, PT, AC+PT, AC+TN, and TMS+PT significantly relieved patients’ anxiety. All differences were statistically significant. Results of the integrated evaluation of two outcome indicators indicated that AC and TMS+PT were the most effective interventions for GAD patients with insomnia symptoms.
4.2 Integrity and applicability of evidence
AC and TMS+PT have demonstrated significant superiority in improving insomnia symptoms in GAD patients.
AC has emerged as a promising technique, and a growing number of patients are strongly requesting AC therapies to treat sleep disorders (53, 54). AC refers to the practice of inserting sterile tiny needles or using infrared light generated by moxibustion to stimulate specific areas of human skin under the guidance of TCM theory. These areas are called acupoints, modulating qi and blood and harmonizing yin and yang. AC is safe, user-friendly, and tolerable with no side effects (55). A meta-analysis showed (56) that AC performed remarkably well in improving patients’ psychological symptoms, especially insomnia and anxiety, with significant reductions in PSQI, HAMD, and SAS, and mild adverse effects, compared with TAU or conventional medications. Another meta-analysis demonstrated (57) that AC presented a therapeutic advantage over benzodiazepines and sham AC in treating insomnia and anxiety in patients. Nevertheless, GAD patients were not the target of these studies. The evidence for AC for improving insomnia symptoms in GAD patients remains absent. This study employed NMA to ascertain the efficacy of 14 NIPs for insomnia in GAD patients, filling a knowledge gap in this area. It was found that AC exerted a significant superiority in ameliorating both insomnia symptoms and anxiety levels in GAD patients compared to the control group. Laboratory studies confirmed that AC, by modulating HPA axis activity (58), regulating SNS function (59), balancing circadian rhythms, and influencing the cytokines (e.g., IL-1, IL-6) and neurotransmitters (e.g., neuropeptide Y, 5-hydroxytryptophan, melatonin, dopamine, norepinephrine, γ-aminobutyric acid, and β-endorphin) (60–62), was beneficial in improving sleep quality and anxiety levels in patients. These targets highly matched the molecular mechanisms underlying the treatment of psychiatric disorders such as anxiety disorders (63). In terms of combination therapy, previous studies have mostly focused on CBT + medication, while the evidence for the effectiveness of non-pharmacological multimodal regimens is lacking. SUCRA values for PT+AC (64.80%) were found to be significantly higher than those for PT alone (50.43%), while RT+AC also had higher SUCRA values (49.55%) compared to RT alone (37.26%). This result aligned with the results of a prior meta-analysis (64), suggesting that AC may enhance the efficacy of NIPs through multi-targeted modulation.
TMS is a technique for modulating cerebral cortex activity through single or repetitive pulsed magnetic fields (65). TMS specifically induces sleep oscillations including slow waves and sleep spindle waves (65). For example, applying a single pulse of TMS at less than 1 Hz during non-rapid eye movement (NREM) sleep enhances slow wave activity (SWA), which is critical for brain function recovery. Due to the potential association of anxiety and related disorders with high reactivity to emotional stimuli (66), SWA-enriched sleep may function therapeutically by modulating such reactivity. In this study, TMS was not significantly different compared to control group and TAU. That is, TMS failed to significantly improve the sleep quality of GAD patients. This differs from the earlier review study results (67). Although clinical trials for GAD patients have demonstrated the potential of TMS in alleviating insomnia and anxiety symptoms, the effects of TMS need to be further corroborated by high-quality evidence. Notably, in terms of combination therapy, it was found that TMS+PT remarkably enhanced the sleep quality and anxiety of GAD patients in comparison with the control group, with significant differences.
Current PT for GAD is comprised of CBT, MT, and sleep hygiene education. CBT is the most well-researched and commonly used among them. PT eliminates or relieves patients’ fear of disease to a certain extent by enabling them to correctly understand the disease, further improving their confidence in treatment. Upon this basis, psychotherapists assist the patients in adjusting their work and rest, cultivating their concentration, sleep habits and so forth, enabling them to be more focused on falling asleep and integrating their fragmented light sleep time for the purpose of improving the patients’ sleep quality. Compared with the control group in this study, PT failed to exhibit a significant advantage in ameliorating sleep quality, though it significantly relieved anxiety in GAD patients. This result was in line with previous reviews. A meta-analysis evaluating the efficacy of MT for sleep disorders in adults demonstrated (68) that MT demonstrated no significant effect on ameliorating sleep quality. Another meta-analysis analyzing the influence of CBT on sleep disorders accompanying anxiety disorders also suggested (69) that CBT treatment for anxiety disorders provided only a moderate effect on improving insomnia symptoms (aggregated effect size of 0.527). Interestingly, this study found that neither PT nor TMS used alone was advantageous in improving sleep quality in GAD patients (both SUCRA values <50%). Nonetheless, the therapeutic effect is significantly enhanced when the two are applied in combination. TMS+PT demonstrated a remarkable advantage in improving both insomnia symptoms (SUCRA = 76.29%) and anxiety (SUCRA = 69.87%) second only to AC (SUCRA = 83.97%), with statistically significant effect sizes (SMD = 3.67, 95% CrI=1.34-6.09). This result challenges the conventional monotherapy-dominated therapeutic paradigm, indicating that combined interventions may address the complexity of GAD pathology through multi-target synergistic mechanisms. The synergistic effect of TMS and PT supports the scientific validity of “neuro-behavioral” integrated interventions, which provides a highly evidence-based strategy for the treatment of GAD comorbid insomnia. Future studies should further elucidate the molecular-loop mechanism and promote the clinical translation of the precise combination regimen.
4.3 Strengths and limitations
In terms of strengths, this study assessed for the first time the effectiveness of 14 NIPs on insomnia and anxiety symptoms in GAD patients through a Bayesian NMA, thereby addressing the lack of direct comparative evidence in this area. The MCMC algorithm was used to iteratively optimize the model and verify the convergence (PSRF = 1) and consistency (P>0.05 for node-splitting method) of the results, remarkably improving the robustness of the analysis. Furthermore, this study pioneers the prioritization of AC and combination therapies (such as TMS+PT) in improving insomnia symptoms in GAD patients (SUCRA >75%), providing a high-level evidence-based basis for clinical development of individualized treatment strategies.
However, this NMA retains several limitations that need to be identified. First, the type, composition, scoring criteria, and sensitivity of the insomnia and anxiety scales used in the included studies varied, potentially increasing between-study heterogeneity although SMD was applied as the effect size. Second, despite the acceptable overall heterogeneity of the studies (I² = 7%) under the random-effects model, the included studies differed significantly in the form of the intervention (e.g., types of Acupuncture, acupoint selection, TMS stimulation parameters, psychotherapeutic modalities) and session settings (10 days to 12 weeks), which may affect the incorporation and interpretation of effect sizes. Third, although this study identified several effective combined interventions, their specific protocols, such as the sequencing, duration, and integration of components, varied across the included trials. This heterogeneity limits our ability to formulate standardized clinical protocols. Fourth, as the included studies were dominated by Chinese populations, the efficacy of AC therapy, which has a rich cultural history and professional resources in China, may be affected by differences in cultural identity and operational standardization. Therefore, cross-cultural validation in Western populations is needed to clarify the generalizability of its efficacy. Finally, this NMA included a relatively limited number of studies, with some interventions represented in few papers, making these interventions under-researched. Finally, this study did not include several emerging non-pharmacological neuromodulation interventions, such as transcutaneous auricular vagus nerve stimulation (taVNS), due to reasons such as falling outside the predefined PICOS criteria or being published after the search cutoff date. A recent meta-analysis indicates that taVNS can significantly improve insomnia scores (70), however, the overall GRADE evidence quality remains very low, suggesting its potential while underscoring the need for higher-quality trials with longer-term follow-up. Subsequent research should consider incorporating interventions like taVNS into network meta-analyses.
4.4 Conclusion
This network meta-analysis showed that acupuncture (AC) and transcranial magnetic stimulation combined with psychotherapy (TMS+PT) produced the most pronounced improvements in sleep, PT+AC ranked among the top for reducing anxiety, and TMS+PT performed consistently across both outcomes, with statistically significant effect sizes. Notably, a considerable proportion of trials received RoB 2 judgments of “some concerns” for the randomization process, and long-term outcomes were infrequently reported, which lowers the certainty of the evidence. Future research should undertake larger, multicenter randomized controlled trials with rigorous allocation concealment and blinding where feasible, standardized intervention protocols and preregistered analyses, ≥6-month follow-up, and comprehensive safety reporting to evaluate the durability of benefits.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
B-YZ: Conceptualization, Data curation, Methodology, Writing – original draft, Writing – review & editing. YWL: Data curation, Methodology, Writing – original draft, Writing – review & editing. WC: Data curation, Methodology, Writing – review & editing. YuL: Data curation, Formal analysis, Supervision, Writing – review & editing. JZ: Data curation, Formal analysis, Supervision, Writing – review & editing. ZL: Data curation, Formal analysis, Writing – review & editing. YiL: Data curation, Formal analysis, Writing – review & editing. YC: Funding acquisition, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2025.1669888/full#supplementary-material
Supplementary File S1 | PRISMA 2020 Checklist.
Supplementary File S2 | PRISMA 2020 for Abstract Checklist.
Supplementary File S3 | Comprehensive Search Strategies for All Databases.
Supplementary File S4 | Potential Scale Reduction Factor (PSRF) Plots for Convergence Diagnosis (A) Sleep quality outcome (B) Anxiety outcome.
Supplementary File S5 | Node-Splitting Analysis for Local Inconsistency Assessment (A, B) Sleep quality outcome (C, D) Anxiety outcome.
References
1. Nepon J, Belik SL, Bolton J, and Sareen J. The relationship between anxiety disorders and suicide attempts: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Depress Anxiety. (2010) 27:791–8. doi: 10.1002/da.20674
2. Danielsson L, Hansson Scherman M, and Rosberg S. To sense and make sense of anxiety: physiotherapists' perceptions of their treatment for patients with generalized anxiety. Physiother Theory Pract. (2013) 29:604–15. doi: 10.3109/09593985.2013.778382
3. Association AP. Diagnostic and statistical manual of mental disorders: DSM-5. American psychiatric association: Washington, DC (2013).
4. Marcks BA, Weisberg RB, Edelen MO, and Keller MB. The relationship between sleep disturbance and the course of anxiety disorders in primary care patients. Psychiatry Res. (2010) 178:487–92. doi: 10.1016/j.psychres.2009.07.004
5. Cox RC and Olatunji BO. A systematic review of sleep disturbance in anxiety and related disorders. J Anxiety Disord. (2016) 37:104–29. doi: 10.1016/j.janxdis.2015.12.001
6. Stein Murray B and Sareen J. Generalized anxiety disorder. New Engl J Med. (2015). 373:2059–68. doi: 10.1056/NEJMcp1502514
7. Li C-T, Bai Y-M, Lee Y-C, Mao W-C, Chen M-H, Tu P-C, et al. High dosage of hypnotics predicts subsequent sleep-related breathing disorders and is associated with worse outcomes for depression. Sleep. (2014) 37:803–9. doi: 10.5665/sleep.3594
8. Jun J, Kapella MC, and Hershberger PE. Non-pharmacological sleep interventions for adult patients in intensive care Units: A systematic review. Intensive Crit Care Nurs. (2021) 67:103124. doi: 10.1016/j.iccn.2021.103124
9. De Niet GJ, Tiemens BG, Kloos MW, and Hutschemaekers GJ. Review of systematic reviews about the efficacy of non-pharmacological interventions to improve sleep quality in insomnia. Int J Evidence-Based Healthcare. (2009) 7:233–42. doi: 10.1111/j.1744-1609.2009.00142.x
10. Geiger-Brown JM, Rogers VE, Liu W, Ludeman EM, Downton KD, and Diaz-Abad M. Cognitive behavioral therapy in persons with comorbid insomnia: a meta-analysis. Sleep Med Rev. (2015) 23:54–67. doi: 10.1016/j.smrv.2014.11.007
11. Gao M, Roy A, Deluty A, Sharkey KM, Hoge EA, Liu T, et al. Targeting anxiety to improve sleep disturbance: A randomized clinical trial of app-based mindfulness training. Psychosom Med. (2022) 84:632–42. doi: 10.1097/PSY.0000000000001083
12. Huang Z, Li Y, Bianchi MT, Zhan S, Jiang F, Li N, et al. Ouyang Q et al: Repetitive transcranial magnetic stimulation of the right parietal cortex for comorbid generalized anxiety disorder and insomnia: A randomized, double-blind, sham-controlled pilot study. Brain Stimul. (2018) 11:1103–9. doi: 10.1016/j.brs.2018.05.016
13. Nguyen VV, Zainal NH, and Newman MG. Why sleep is key: poor sleep quality is a mechanism for the bidirectional relationship between major depressive disorder and generalized anxiety disorder across 18 years. J Anxiety Disord. (2022) 90:102601. doi: 10.1016/j.janxdis.2022.102601
14. Li F, Fisher KJ, Harmer P, Irbe D, Tearse RG, and Weimer C. Tai chi and self-rated quality of sleep and daytime sleepiness in older adults: a randomized controlled trial. J Am Geriatr Soc. (2004) 52:892–900. doi: 10.1111/j.1532-5415.2004.52255.x
15. Wang F, Su WL, Xie XH, Liu MH, Chen JY, and Wen LX. Application of Baduanjin combined with TCM emotional regulation in patients with generalized anxiety and insomnia. Guiding J Traditional Chin Med Pharmacol. (2022) 28:75–9. doi: 10.13862/j.cn43-1446/r.2022.09.014
16. Jacoby RJ, Brown ML, Wieman ST, Rosenfield D, Hoeppner SS, Bui E, et al. Effect of cognitive behavioural therapy and yoga for generalised anxiety disorder on sleep quality in a randomised controlled trial: the role of worry, mindfulness, and perceived stress as mediators. J Sleep Res. (2024) 33:e13992. doi: 10.1111/jsr.13992
17. Ma Y, Liu JC, Liang XY, and Liu WC. Clinical study on massage combined with acupuncture for sleep disorders in generalized anxiety disorder. China J Traditional Chin Med Pharm. (2016) 31:4863–5. doi: CNKI:SUN:BXYY.0.2016-11-140
18. Lumley T. Network meta-analysis for indirect treatment comparisons. Stat Med. (2002) 21:2313–24. doi: 10.1002/sim.1201
19. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. Brennan SE et al: The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj. (2021) 372:n71. doi: 10.1136/bmj.n71
20. Psychiatry CSo. Chinese classification and diagnostic criteria of mental disorders (3rd ed.). Chin J Psychiatry. (2001) 3:59–63. doi: 10.3760/j:issn:1006-7884.2001.03.028
21. Vujnovic M, Manukhina O, Reed GM, Theodorakis PN, and Fountoulakis KN. ICD-11 revision of mental disorders: the global standard for health data, clinical documentation, and statistical aggregation. Consort Psychiatr. (2021) 2:3–6. doi: 10.17816/CP74
22. Lips P and Ooms ME. Non-pharmacological interventions. Best Pract Res Clin Endocrinol Metab. (2000) 14:265–77. doi: 10.1053/beem.2000.0073
23. Buysse DJ, Reynolds CF III, Monk TH, Berman SR, and Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. (1989) 28:193–213. doi: 10.1016/0165-1781(89)90047-4
24. Bastien CH, Vallières A, and Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. (2001) 2:297–307. doi: 10.1016/S1389-9457(00)00065-4
25. Yu L, Buysse DJ, Germain A, Moul DE, Stover A, Dodds NE, et al. Development of short forms from the PROMIS™ sleep disturbance and Sleep-Related Impairment item banks. Behav Sleep Med. (2011) 10:6–24. doi: 10.1080/15402002.2012.636266
26. Li JM. Introduction to self-rating scale of sleep (SRSS). China J Health Psychol. (2012) 20:1851–1. doi: 10.13342/j.cnki.cjhp.2012.12.040
27. Thompson E. Hamilton rating scale for anxiety (HAM-A). Occup Med. (2015) 65:601–1. doi: 10.1093/occmed/kqv054
28. Chiesi F, Primi C, and Carmona J. Measuring statistics anxiety: Cross-country validity of the Statistical Anxiety Scale (SAS). J psychoeducational Assess. (2011) 29:559–69. doi: 10.1177/0734282911404985
29. Beck AT, Epstein N, Brown G, and Steer R. Beck anxiety inventory. J consulting Clin Psychol. (1993). doi: 10.1037/t02025-000
31. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. Bmj. (2019) 366:l4898. doi: 10.1136/bmj.l4898
32. van Valkenhoef G, Lu G, de Brock B, Hillege H, Ades AE, and Welton NJ. Automating network meta-analysis. Res Synth Methods. (2012) 3:285–99. doi: 10.1002/jrsm.1054
33. van Valkenhoef G, Dias S, Ades AE, and Welton NJ. Automated generation of node-splitting models for assessment of inconsistency in network meta-analysis. Res Synth Methods. (2016) 7:80–93. doi: 10.1002/jrsm.1167
34. Dong GJ, Cao D, Dong Y, Zhang J, and Wang FC. Clinical study on scalp acupuncture for sleep disorders caused by pre-examination anxiety in college students. World J Acupuncture-Moxibustion. (2018) 28:156–60. doi: 10.1016/j.wjam.2018.09.003
35. Caldwell DM, Ades AE, and Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. Bmj. (2005) 331:897–900. doi: 10.1136/bmj.331.7521.897
36. Sang N and Chen C. Effect of moxibustion at Baihui (GV20) on emotion and sleep quality in patients with anxiety disorder. Hebei J Traditional Chin Med. (2023) 45:2075–8. doi: 10.3969/j.issn.1002-2619.2023.12.031
37. Wang Z, Du XY, and Yang Y. Effect of ultrasound-guided stellate ganglion block combined with biofeedback on moderate anxiety patients. J Int Psychiatry. (2023) 50:265–267,271. doi: 10.13479/j.cnki.jip.2023.02.017
38. Ding YM, Zhao S, and Huang J. Application of low-frequency repetitive transcranial magnetic stimulation combined with cognitive behavioral therapy in patients with anxiety disorder and sleep disorders. Shanghai Nurs. (2024) 24:30–3. doi: 10.3969/j.issn.1009-8399.2024.01.007
39. Yang M, Yang HM, Sun LL, and Bi YF. Application of low-frequency repetitive transcranial magnetic stimulation combined with cognitive behavioral therapy in patients with anxiety disorder and sleep disorders. World J Sleep Med. (2024) 11:873–5. doi: 10.3969/j.issn.2095-7130.2024.04.046
40. Wang CF, Duan N, and Guo ZY. Effects of relaxation training combined with willpower motivation on sleep and quality of life in patients with anxiety-induced sleep disorders. J Clin Psychosomatic Dis. (2021) 27:81–84,108. doi: 10.3969/j.issn.1672-187X.2021.03.018
41. Wang D. Effect of cognitive behavioral intervention on sleep quality and anxiety symptoms in patients with generalized anxiety disorder. World J Sleep Med. (2021) 8:1309–11. doi: 10.3969/j.issn.2095-7130.2021.08.003
42. Huang W, Zheng Z, Zou K, and Li J. Randomized controlled study of low-frequency repetitive transcranial magnetic stimulation over the right parietal lobe for anxiety-related insomnia. J Psychiatry. (2024) 37:1–5. doi: 10.3969/j.issn.2095-9346.2024.01.001
43. Gao JX. Effects of TCM characteristic nursing intervention combined with relaxation training on anxiety symptoms, sleep quality and quality of life in patients with anxiety disorder. Pract Clin J Integrated Traditional Chin Western Med. (2021) 21:158–9. doi: 10.13638/j.issn.1671-4040.2021.20.075
44. Huang Y. Clinical study on auricular point sticking combined with relaxation training for anxiety disorder. New Chin Med. (2022) 54:147–50. doi: 10.13457/j.cnki.jncm.2022.04.038
45. Mu JL, Zhang CH, Zhang N, and Du HR. Effect of relaxation therapy on sleep function in patients with generalized anxiety disorder. Chin J Phys Med Rehabil. (2013) 35:390–3. doi: 10.3760/cma.j.issn.0254-1424.2013.05.016
46. Liu K and Li Q. Study on clinical effect of acupuncture in treatment of generalized anxiety disorder. China Pract Med. (2021) 16:167–9. doi: 10.14163/j.cnki.11-5547/r.2021.09.071
47. Wang Y and Chi XH. Effect of TIP emotional intervention on quality of life in elderly patients with anxiety-induced insomnia. Chin J Gerontology. (2019) 39:3806–9. doi: 10.3969 /j.issn.1005-9202.2019.15.064
48. Liu QL and Huang HL. Therapeutic effect of physical exercise on elderly patients with mild to moderate generalized anxiety disorder. Chin J Gerontology. (2020) 40:3704–6. doi: 10.3969/j.issn.1005-9202.2020.17.038
49. Gao L, Xie J, Huang T, Shang Y, and Gao Z. Effects of mindfulness decompression therapy combined with transcranial magnetic stimulation in generalized anxiety disorder. Am J Transl Res. (2021) 13:6827–36. doi: 10.7150/ijtr.0129487
50. Khodarahimi S, Mirderikvand F, and Amraei K. The efficacy of affective and sensory psychotherapy module for sleep disturbances in generalized anxiety disorder. Explore (NY). (2022) 18:17–24. doi: 10.1016/j.explore.2020.11.011
51. Boettcher J, Aström V, Påhlsson D, Schenström O, Andersson G, and Carlbring P. Internet-based mindfulness treatment for anxiety disorders: a randomized controlled trial. Behav Ther. (2014) 45:241–53. doi: 10.1016/j.beth.2013.11.003
52. Jonsson K and Kjellgren A. Promising effects of treatment with flotation-REST (restricted environmental stimulation technique) as an intervention for generalized anxiety disorder (GAD): a randomized controlled pilot trial. BMC Complementary Altern Med. (2016) 16:108. doi: 10.1186/s12906-016-1089-x
53. Su D and Li L. Trends in the use of complementary and alternative medicine in the United States: 2002-2007. J Health Care Poor Underserved. (2011) 22:296–310. doi: 10.1353/hpu.2011.0002
54. Pei W, Peng R, Gu Y, Zhou X, and Ruan J. Research trends of acupuncture therapy on insomnia in two decades (from 1999 to 2018):a bibliometric analysis. BMC Complement Altern Med. (2019) 19:225. doi: 10.1186/s12906-019-2606-5
55. Lin YF, Liu ZD, Ma W, and Shen WD. Hazards of insomnia and the effects of acupuncture treatment on insomnia. J Integr Med. (2016) 14:174–86. doi: 10.1016/S2095-4964(16)60248-0
56. Fangfang MA, Hewei Z, Bingxue LI, Peiyu C, Mingwei YU, and Xiaomin W. Acupuncture and moxibustion for Malignant tumor patients with psychological symptoms of insomnia, anxiety and depression: a systematic review and Meta-analysis. J Tradit Chin Med. (2023) 43:441–56. doi: 10.19852/j.cnki.jtcm.20230313.001
57. Zhang X, Wang X, Chen H, Li X, Gao Y, Zuo G, et al. Acupuncture treatment for improving anxiety status in patients with primary insomnia: a systematic review and meta-analysis. J Acupuncture Tuina Sci. (2024) 22:423–34. doi: 10.1007/s11726-024-1463-z
58. Park HJ, Park HJ, Chae Y, Kim JW, Lee H, and Chung JH. Effect of acupuncture on hypothalamic-pituitary-adrenal system in maternal separation rats. Cell Mol Neurobiol. (2011) 31:1123–7. doi: 10.1007/s10571-011-9718-x
59. Kim SK and Bae H. Acupuncture and immune modulation. Autonomic Neurosci. (2010) 157:38–41. doi: 10.1016/j.autneu.2010.03.010
60. Chae Y, Hong M-S, Kim G-H, Hahm D-H, Park H-J, Ha E, et al. Protein array analysis of cytokine levels on the action of acupuncture in carrageenan-induced inflammation. Neurological Res. (2007) 29:55–8. doi: 10.1179/016164107X172365
61. Hirsch D and Zukowska Z. NPY and stress 30 years later: the peripheral view. Cell Mol Neurobiol. (2012) 32:645–59. doi: 10.1007/s10571-011-9793-z
62. Huang W, Kutner N, and Bliwise DL. Autonomic activation in insomnia: the case for acupuncture. J Clin Sleep Med. (2011) 7:95–102. doi: 10.5664/jcsm.28048
63. Samuels N, Gropp C, Singer SR, and Oberbaum M. Acupuncture for psychiatric illness: a literature review. Behav Med. (2008) 34:55–64. doi: 10.3200/BMED.34.2.55-64
64. Kutana S, Mao JJ, and Garland SN. Acupuncture as an adjunct treatment to cognitive-behavioral therapy for insomnia. Sleep Med Clinics. (2023) 18:113–22. doi: 10.1016/j.jsmc.2022.10.005
65. Massimini M, Ferrarelli F, Esser SK, Riedner BA, Huber R, Murphy M, et al. Triggering sleep slow waves by transcranial magnetic stimulation. Proc Natl Acad Sci U.S.A. (2007) 104:8496–501. doi: 10.1073/pnas.0702495104
66. Shin LM and Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. (2010) 35:169–91. doi: 10.1038/npp.2009.83
67. Chellappa SL and Aeschbach D. Sleep and anxiety: From mechanisms to interventions. Sleep Med Rev. (2022) 61:101583. doi: 10.1016/j.smrv.2021.101583
68. Kim SM, Park JM, Seo HJ, Kim J, Noh JW, and Kim HL. Effects of mindfulness-based stress reduction on adults with sleep disturbance: an updated systematic review and meta-analysis. BMJ Open. (2022) 12:e058032. doi: 10.1136/bmjopen-2021-058032
69. Belleville G, Cousineau H, Levrier K, St-Pierre-Delorme ME, and Marchand A. The impact of cognitive-behavior therapy for anxiety disorders on concomitant sleep disturbances: a meta-analysis. J Anxiety Disord. (2010) 24:379–86. doi: 10.1016/j.janxdis.2010.02.010
Keywords: non-pharmacological interventions, sleep, insomnia, anxiety, GAD, network meta-analysis
Citation: Zhang B, Li Y, Chu W, Li Y, Zhang J, Lv Z, Luo Y and Chen Y (2025) Efficacy of non-pharmacological interventions for alleviating insomnia in individuals with generalized anxiety disorder: systematic evaluation and net meta-analysis. Front. Psychiatry 16:1669888. doi: 10.3389/fpsyt.2025.1669888
Received: 20 July 2025; Accepted: 15 October 2025;
Published: 31 October 2025.
Edited by:
Redhwan Ahmed Al-Naggar, National University of Malaysia, MalaysiaReviewed by:
Xin Liu, Guangzhou University of Chinese Medicine, ChinaHelen Michaela De Oliveira, Universidade Federal de Mato Grosso do Sul, Brazil
Li Sheng, Southwest Medical University, China
Copyright © 2025 Zhang, Li, Chu, Li, Zhang, Lv, Luo and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Chen, OTUyMDI2MDE1QHFxLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Boyu Zhang
Boyu Zhang Yiwen Li1†
Yiwen Li1† Wenming Chu
Wenming Chu