- 1Department of Psychiatry, College of Medicine, King Saud University, Riyadh, Saudi Arabia
- 2SABIC Psychological Health Research and Applications Chair (SPHRAC), Department of Psychiatry, College of Medicine, King Saud University, Riyadh, Saudi Arabia
- 3Department of Psychiatry, Eradah Mental Health Hospital, Al Kharj, Saudi Arabia
- 4College of Medicine, King Saud University, Riyadh, Saudi Arabia
Background: Nasal esketamine has demonstrated efficacy in the management of treatment-resistant depression and psychiatric emergency due to major depression. This study investigates acceptance and awareness of esketamine as a depression treatment option, focusing on factors that influence patients’ acceptance, including adherence to current medication regimens, regardless of prior esketamine exposure.
Methods: This cross-sectional study surveyed 283 adults with depression using a questionnaire and the Medication Adherence Rating Scale (MARS-10).
Results: 52.3% of participants were willing to receive esketamine, and 51.2% preferred its weekly or biweekly dosing over daily antidepressants; 79.5% reported cost as a potential barrier. Common concerns included medication unavailability (59.7%), fear of addiction (50.5%), anticipated stigma (24.4%), and first-month dosing frequency (21.2%). Regarding adherence, 77.4% were nonadherent to their current psychiatric medication regimen. Adherence to the current regimen was higher among patients with previous esketamine use (p <.001) and among those who had someone to stay with them during and after treatment (p = .047).
Conclusion: Patients are open to esketamine but have concerns that must be addressed. It also highlights non-adherence as a significant issue in patients with depression. These findings highlight the importance of patients’ education, family involvement, and logistical supports.
Introduction
Depression is a significant mental illness worldwide and a leading cause of disability (1). In Saudi Arabia, the Saudi National Mental Health Survey reported a lifetime prevalence for depression of 3% for Saudi males and 9% for females (2). Depression can significantly affect interpersonal, social, and functional domains (3). Although depression is a treatable condition (4), relevant knowledge of available treatments in the general population remains limited in Saudi Arabia, as highlighted in Aljadani et al.’s 2021 study (5).
One of the novel treatments for depression is ketamine. Ketamine is a non-barbiturate anesthetic used for induction and maintenance of anesthesia (6, 7) and for procedural sedation, acute and chronic pain, post-operative pain, and hemodynamic instability (7, 8). Its water and lipid solubility enable intravenous, intramuscular, and intranasal administration with high bioavailability (6, 7). By targeting various receptor channels, ketamine produces a range of potent central nervous system effects (9). Its antidepressant potential was recognized in the late 1990s (10). Bremen’s 2000 study reported that intravenous ketamine produced antidepressant effects within hours (10). Subsequent studies have corroborated ketamine’s benefits (11, 12). For instance, a study demonstrated that intravenous ketamine rapidly alleviates suicidal ideation with minimal and transient side effects (11).
Multiple definitions of treatment-resistant depression (TRD) have been proposed and appear in the literature. One definition entails two failed trials of two antidepressants from different classes with documentation regarding adequate dosage, duration, and compliance (13). Another TRD definition, from the Thesa and Rush model, stages TRD across five levels, describing nonresponse to multiple medication classes, as well as to electroconvulsive therapy (13). Approximately 15–33% of patients with depression experience TRD, whereas conventional antidepressants fail to provide the desired effects, highlighting the need for innovative treatment (14). In 2019, after trials demonstrated its efficacy (15–22), the U.S. FDA approved intranasal esketamine for TRD, making a significant advance in psychiatric care (20). Esketamine can reduce depressive symptoms significantly within 24 hours (19) and rapidly decrease suicidal ideation in acute settings, as shown in a randomized controlled trial by Canuso et al. (19). Another study corroborated this anti-suicide effect of nasal esketamine spray, particularly among patients with a history of prior attempts (22). However, its use is associated with side effects such as dissociation, dizziness, and the potential for abuse (23). The intranasal administration of esketamine is non-invasive and convenient, which may enhance patient adherence; it is favored over placebo due to its ease of use and rapid absorption (24). Ahmed et al. (2023) recently reviewed the latest clinical evidence and guidance on esketamine nasal spray for TRD, including its potential to meet unmet patient TRD needs in the Arabic Gulf countries (25). Other studies have also explored the topic. For instance, in the TRANSFORM-2 trial, combining esketamine with either a selective serotonin reuptake inhibitor (SSRI) or a serotonin norepinephrine reuptake inhibitor (SNRI) demonstrated improvements in depressive symptoms over a four-week trial versus a placebo (17). In another study, the SUSTAIN-2 trial, the authors concluded that the long-term use of esketamine with either SSRI or SNRI maintained significant improvement in depressive symptoms and was well tolerated (21). Other studies have also indicated the long-term effectiveness and safety of esketamine. For instance, the SUSTAIN-3 study followed 1148 adults with TRD for over 6.5 years; participants exhibited clear improvements in depressive symptoms during the first four weeks of treatment. About half of the participants achieved remission. Side effects such as headache, dizziness, and dissociation were usually mild, resolved the same day, and rarely led patients to discontinue treatment (26). Further, a 2023 review of five studies concluded that esketamine is effective and safe for maintaining antidepressant effects (27). Nonetheless, it is also essential to highlight that myths surround the use of esketamine for TRD, as per Di Vincenzo et al.’s (28) clinical review, in which the authors discussed some of the myths related to TRD, including side effects and related issues.
Indeed, many aspects shape patients’ perceptions of the treatments. Research has revealed that patients’ perception of new treatments and whether they trust the healthcare system or hold certain beliefs about medications deeply inform their willingness to embrace these therapies (29, 30). When patients are engaged and participate in treatment decision-making, they are more likely to comply with the management plan, and the clinical outcomes tend to be better overall (29, 30). Jilka et al. (2021) explored patient perspectives on ketamine as a treatment for depression. They reported that many approached the treatment with skepticism; however, as patients became more familiar with the treatment, they shifted toward a positive outlook, recognizing the benefits of ketamine (31). Jilka et al. (2021) also emphasized that educating patients, carefully monitoring, and addressing concerns are key factors in ensuring patients’ acceptance and adherence, as well as the effectiveness of the treatment (31). Fairchild et al. (2020) investigated patient preferences for ketamine in TRD and found that patients place a high value on symptom improvement with ketamine and accept significant risks for these benefits (32). Koss (2021) used an educational video to inform the public and healthcare providers about the benefits and safety of ketamine infusion, resulting in positive engagement and increased awareness (33). Another study examined patients’ perceptions and attitudes toward esketamine, noting that active education engagement and positive changes in their symptoms during the treatment shape perceptions and attitudes, resulting in better adherence (34). Notably, in Saudi Arabia, no studies have directly assessed the perception of esketamine; however, Al-Shareef et al. (2024) conducted a study in the eastern region to examine psychotropic medications. The authors found that patients who had moderate to high knowledge had previous experience with psychotropic medication, had a family member who suffered from a psychiatric illness, or suffered from psychiatric illness themselves (35).
The present study was conducted in response to the considerable burden that major depressive disorder causes (1, 36) and the need to explore new treatments in the Saudi context. A third motive was to explore esketamine in Saudi Arabia. Collectively, the study aims to evaluate the acceptance and awareness of esketamine among patients diagnosed with depression and to examine the factors that may affect treatment acceptance, including adherence to current medication regimens, regardless of patients’ prior esketamine exposure.
Material and methods
Study design, setting, and participants
This quantitative cross-sectional study involved adult patients diagnosed with depression and related disorders at King Khalid University Hospital (KKUH), Riyadh, Saudi Arabia. Specifically, inclusion criteria included adults aged 18–65 and diagnosed with major depressive disorder, persistent depressive disorder, and other specified and unspecified depressive disorders. Exclusion criteria included those with communication barriers, and those diagnosed with bipolar affective disorder, primary psychotic disorders, depressive disorder secondary to another medical condition, depressive disorder secondary to substance, depressive disorder secondary to medications, and substance use disorder. Participants who did not meet the inclusion criteria or did not fully complete the study instruments were excluded.
The research team distributed the study instrument among the participants from mid-April to mid-June 2025 through an electronic survey. The research team contacted participants by phone to explain the study and invite participation; the survey link (sent by WhatsApp/email) was provided to those who agreed.
KKUH’s IT department identified 505 eligible patients. Based on this number, we calculated the sample size using raosoft.com (http://www.raosoft.com/samplesize.html) with a 5% margin of error and a 95% confidence level. The calculated sample size was 219 patients. We added an additional 25% to the calculated sample size to account for non-respondents, yielding a total of 273 participants.
Survey instruments
The study instrument comprised three parts: (1) educational material about esketamine, (2) a questionnaire developed by the research team, and (3) the Medication Adherence Rating Scale (MARS-10).
The educational material summarized esketamine’s clinical indications, administration, pre-/post-treatment precautions, cost, and side effects. All participants read this section before proceeding, given esketamine’s limited use and recognition in Saudi Arabia. Moreover, the research team developed these materials based on what is known about esketamine in the literature (37, 38).
The research team developed the questionnaire to capture the participants’ sociodemographic characteristics (e.g., age, gender, comorbid mental illnesses, current psychotropic medications, and any previous experience with esketamine). Additionally, the questionnaire also entailed questions related to esketamine: (1) transportation, (2) administration setting, (3) medication-related concerns, and (4) depression-related concerns. Notably, we developed the questionnaire items based on the medication’s leaflet information, existing literature on esketamine, and relevant Saudi-specific contextual considerations that the research team deemed relevant.
The MARS-10 is a self-administered, 10-item questionnaire to identify patient adherence to current medications (39, 40). The scale ranges from 0 to 10 (41). The MARS-10 reliability is evidenced by a Cronbach’s α coefficient of 0.75 (42). In the current study, the research team used the scale’s Arabic version after obtaining permission from its authors. The scale’s Arabic version was found to be reliable with a Cronbach’s α coefficient of 0.7 in psychiatric research assessing the adherence of schizophrenic patients (43) and 0.89 in a study assessing the adherence of medication among patients with chronic illnesses such as hypertension and diabetes mellitus (44). Consistent with previous studies, patients with a score ≤ 5 were classified as non-compliant, while those with a score > 5 were classified as compliant (45–48).
Ethical considerations
The Institutional Review Board of the College of Medicine at King Saud University (research project no. E-24-9385) approved the study. Participants reviewed an electronic informed-consent statement and selected “Next” to access the survey. The survey explained confidentiality, data anonymity, and information regarding the study’s scope and the principal investigator’s contact information. Consent to participate was indicated by clicking on the survey link.
Statistical data analysis
Data were analyzed using the Statistical Package for the Social Sciences (SPSS, v.26; IBM Corp., Chicago, IL, USA). Descriptive statistics summarized sociodemographic and clinical characteristics, as well as responses to the questionnaire and MARS-10 scale. To explore differences in medication adherence scores, independent samples t-tests compared means between two groups (e.g., presence vs. absence of comorbid mental illness, transportation availability vs. non-availability). One-way analysis of variance (ANOVA) compared adherence scores across groups (e.g., age categories, number of psychiatric medications, and types of comorbid psychiatric conditions). Associations between categorical variables (e.g., adherence status [compliant vs. non-compliant] and sociodemographic/clinical characteristics) were assessed using chi-square tests. Finally, a multiple linear regression analysis identified predictors of medication adherence scores. Independent variables included age, gender, presence of other mental illness, number of psychiatric medications, previous esketamine use, transportation availability, social support (i.e., the availability of someone to take the patient to the hospital, stay with them during/after therapy, or drive them home), cost concerns, and health insurance status. These variables were selected based on prior literature linking them to medication adherence among psychiatric patients. A statistical significance threshold was set at p ≤.05.
Results
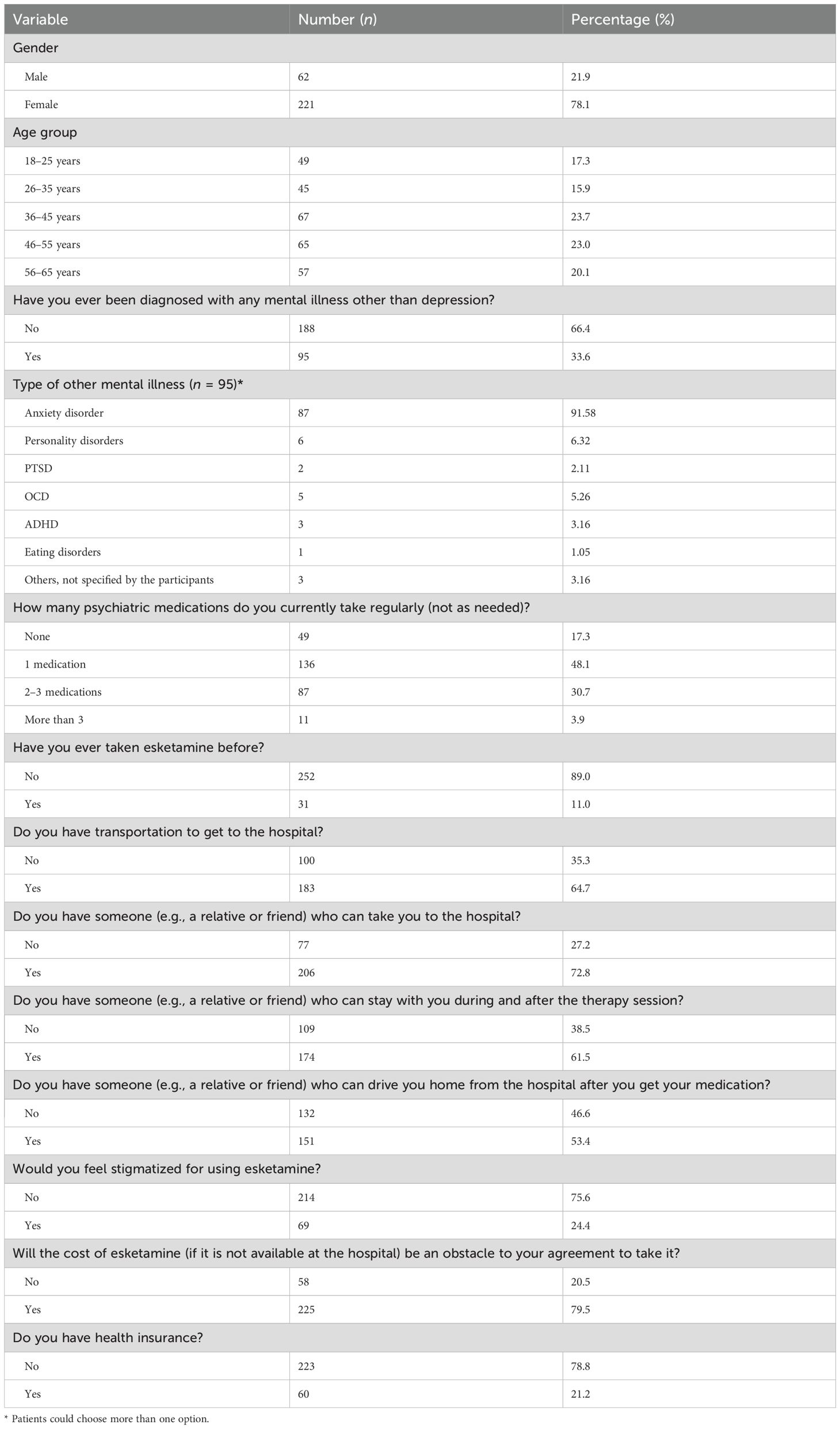
Baseline sociodemographic and clinical characteristics of the patients appear in Table 1. The sample included 283 patients with depression: 78% female (n = 221) and 21.9% male (n = 62). Age distribution was 18–25 years, 17.3% (n = 49); 26–35, 15.9% (n = 45); 36–45, 23.7% (n = 67); 46–55, 23.0% (n = 65); and 56–65, 20.1% (n = 57).
A third (33.6%, n = 95) of participants reported a diagnosis of another mental illness in addition to depression; the remaining two-thirds (66.4%, n = 188) did not. Among those with comorbid mental illnesses (n = 95, multiple responses allowed), anxiety disorder was most frequent (91.58%, n = 87), followed by personality disorders (6.32%, n = 6), obsessive-compulsive disorder (5.26%, n = 5), attention-deficit/hyperactivity disorder (3.16%, n = 3), unspecified other conditions (3.16%, n = 3), post-traumatic stress disorder (2.11%, n = 2), and eating disorders (1.05%, n = 1). Current psychiatric medication use was as follows: one medication, 48.1% (n = 136); two to three medications, 30.7% (n = 87); more than three medications, 3.9% (n = 11); none, 17.3% (n = 49).
Only 11.0% (n = 31) of the participants had ever used esketamine, whereas 89.0% (n = 252) had not. Regarding transportation access (noting that the research team asked about transportation in general without specifying the means of transportation, such as public, private, or both), 64.7% (n = 183) reported having access, while 35.3% (n = 100) did not.
Regarding social support, 72.8% (n = 206) had someone (e.g., a relative or friend) who could take them to the hospital; 61.5% (n = 174) had someone available to stay with them during and after therapy. Additionally, 53.4% (n = 151) had someone to drive them home after receiving the medication, while 46.6% (n = 132) did not.
Regarding perceived stigma, 24.4% (n = 69) of participants reported perceived stigma for using esketamine, while 75.6% (n = 214) reported no concern about stigma.
Financial considerations were also explored. A large proportion (79.5%, n = 225) stated that the cost of esketamine, if unavailable at the hospital, would be an obstacle to their agreement to use it. Finally, 78.8% (n = 223) of participants did not have health insurance, while 21.2% (n = 60) did.
Concerns preventing esketamine use
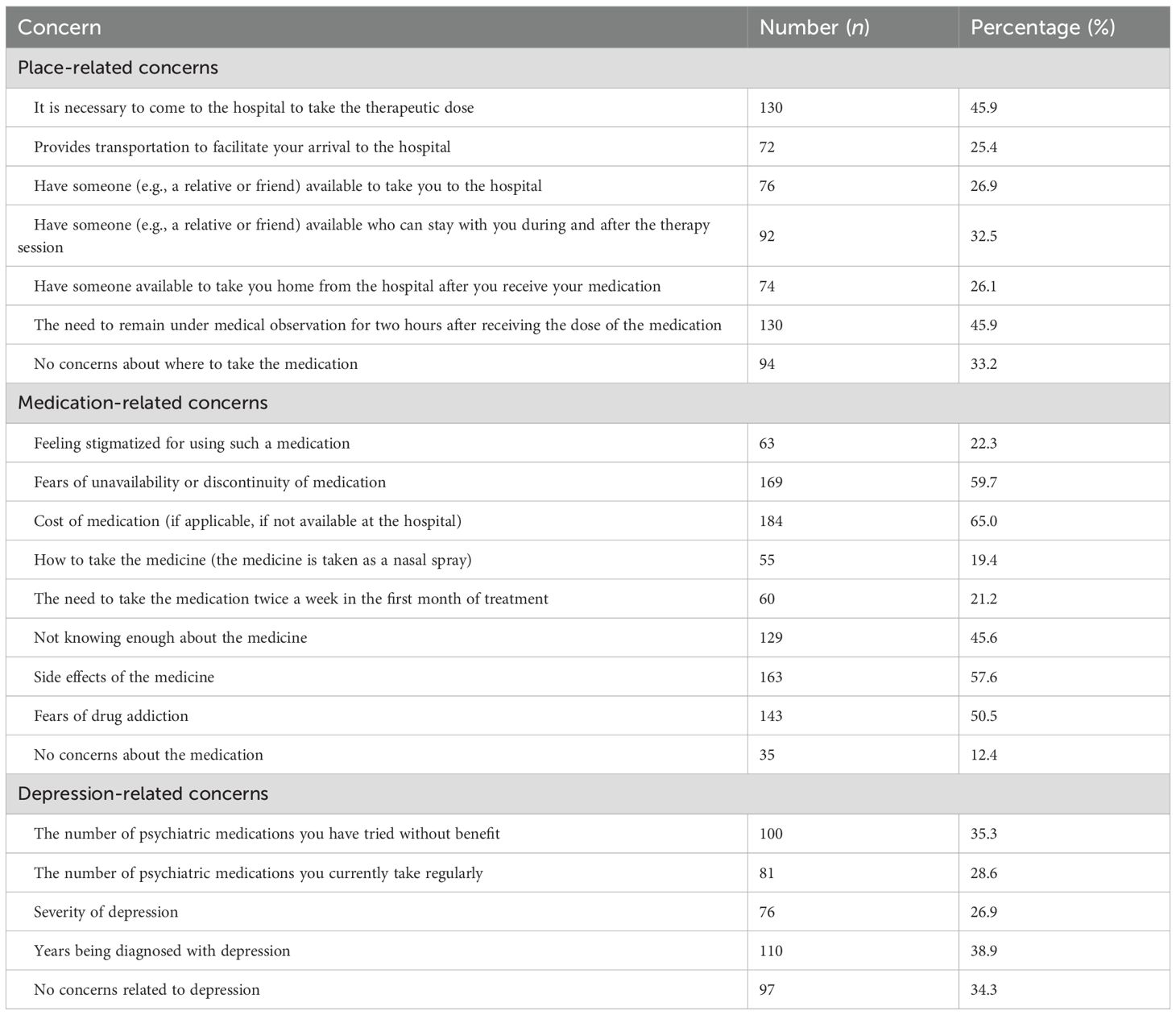
Participants identified several potential barriers to their acceptance or use of esketamine treatment (Table 2). Place-related concerns included the need to come to the hospital for each therapeutic dose (45.9%; n = 130). Similarly, 45.9% (n = 130) were concerned about the requirement for a two-hour post-dose observation. Additionally, 25.4% (n = 72) noted a need for transportation to facilitate hospital visits, while 26.9% (n = 76) reported needing someone to accompany them to the hospital. Approximately one-third (32.5%, n = 92) stated the need for someone to stay with them during and after the therapy session, and 26.1% (n = 74) mentioned the need for someone to take them home after receiving the medication. Despite these concerns, 33.2% (n = 94) reported no place-related concerns (Table 2).
Regarding medication-related concerns, 65.0% (n = 184) cited medication cost (if unavailable at the hospital) as a barrier, and 59.7% (n = 169) expressed fear about the unavailability or discontinuity of the medication. A majority of participants (57.6%; n = 163) reported concerns about potential side effects, while 50.5% (n = 143) feared the possibility addiction. A notable minority (45.6%; n = 129) cited a lack of sufficient knowledge about the medication. Other concerns included feeling stigmatized for using esketamine (22.3%, n = 63), concern about how to take the medication (nasal spray format; 19.4%, n = 55), and the need to take it twice per week during the first month of treatment (21.2%, n = 60). Only 12.4% (n = 35) reported no medication-related concerns (Table 2).
Regarding depression-related concerns, 35.3% (n = 100) reported that the number of previously tried psychiatric medications without benefit was a concern. Additionally, 28.6% (n = 81) expressed concern about the number of psychiatric medications they were currently taking. Depression severity was a concern for 26.9% (n = 76), while 38.9% (n = 110) indicated that the number of years since being diagnosed with depression contributed to their hesitation. Nonetheless, 34.3% (n = 97) reported having no depression-related concerns when considering esketamine use (Table 2).
Acceptance and preferences regarding esketamine
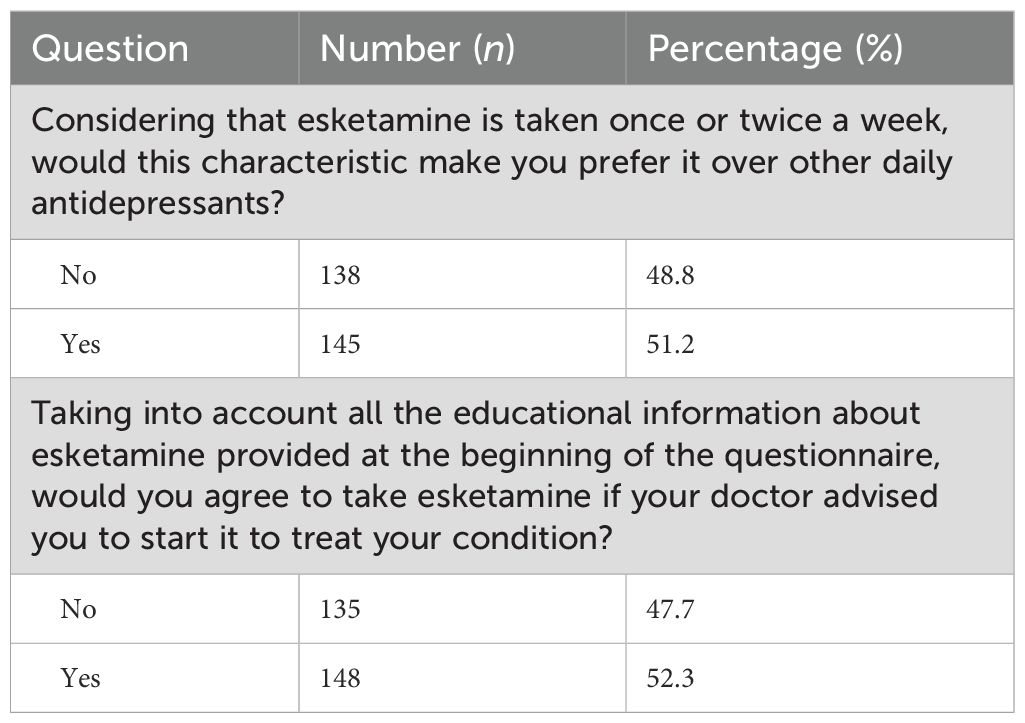
When asked whether the dosing schedule of esketamine—once or twice a week—would make them prefer it over other daily antidepressants, 51.2% (n = 145) of participants responded affirmatively, while 48.8% (n = 138) did not express a preference based on this characteristic (Table 3).
After reviewing the educational information about esketamine provided at the beginning of the survey, 52.3% (n = 148) of participants would agree to take esketamine if their doctor advised them to do so to treat their condition. In contrast, 47.7% (n = 135) indicated that they would not agree to take it even with medical advice (Table 3).
Responses to MARS-10 items
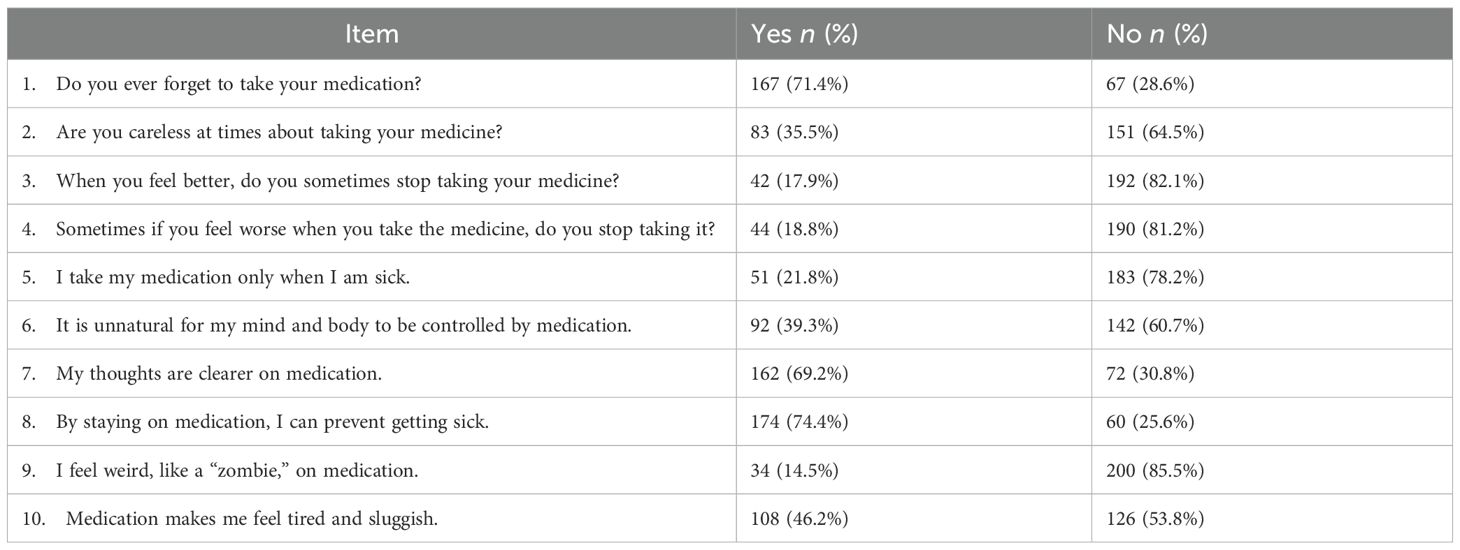
Among the 234 participants taking one or more psychiatric medications, MARS-10 responses reflected varied adherence behaviors (Table 4). Most participants (71.4%, n = 167) sometimes forgot to take their medication, while 28.6% (n = 67) did not forget. About one-third (35.5%, n = 83) admitted to being occasionally careless about taking their medication, while 64.5% (n = 151) disagreed (Table 4).
When asked about ceasing medication when feeling better, only 17.9% (n = 42) answered “Yes,” while most (82.1%, n = 192) continued their medication. Similarly, 18.8% (n = 44) reported stopping medication when they feel worse, and 21.8% (n = 51) stated that they take their medication only when sick (Table 4).
Regarding beliefs about medication, 39.3% (n = 92) felt that it was unnatural for medication to control their mind and body. However, a strong majority (69.2%, n = 162) believed their thoughts were clearer while on medication, and 74.4% (n = 174) agreed that staying on medication helps prevent illness (Table 4).
Few patients (14.5%, n = 34) reported feeling like a “zombie” on medication, with most (85.5%, n = 200) denying this experience. Additionally, 46.2% (n = 108) felt tired or sluggish from their medication, while 53.8% (n = 126) did not share this sentiment (Table 4).
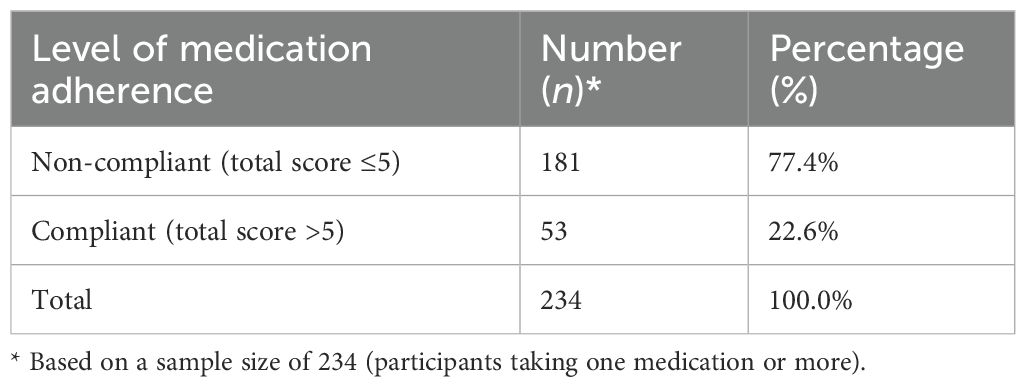
Medication adherence levels
Based on the responses to the MARS-10 scale, 77.4% (n = 181) were non-compliant, and 22.6% (n = 53) were compliant with their current medications (Table 5).
Differences in adherence scores by patient characteristics
An independent samples t-test examined whether medication adherence scores (as measured by the MARS-10) differed significantly across various patient characteristics (Table 6). There were no significant differences in adherence scores based on gender, t(281) = −0.47, p = .636, or transportation availability to the hospital, t(281) = 0.15, p = .878. Additionally, medication adherence did not significantly differ between participants with health insurance and those without, t(281) = −0.31, p = .759, nor between those who reported stigma and those who did not, t(281) = −0.61, p = .544 (Table 6).

Table 6. Independent samples t-test for differences in medication adherence based on the depression patients’ characteristics (n = 234).
However, three variables revealed statistically significant differences. Prior esketamine use was linked to significantly higher adherence scores (M = 5.78, SD = 2.34) versus no prior use (M = 3.87, SD = 2.03), t(281) = −4.51, p <.001. Having someone to stay during and after therapy was associated with higher adherence (M = 4.34, SD = 2.31) than not having such support (M = 3.78, SD = 1.90), t(281) = −2.00, p = .047. Furthermore, individuals who reported cost as a barrier had significantly higher adherence scores (M = 4.24, SD = 2.05) than those who did not report cost concerns (M = 3.46, SD = 2.46), t(281) = −2.24, p = .026 (Table 6).
Other comparisons, including having a mental illness other than depression (p = .092), having someone to take the patient to the hospital (p = .090), and having someone to drive the patient home after medication (p = .218), did not exhibit statistically significant differences (Table 6).
A one-way ANOVA assessed adherence based on age group, type of psychiatric diagnosis (in patients with comorbid conditions), and number of psychiatric medications currently taken (Table 7). The results indicated no significant difference in adherence scores across age groups, F(4, 278) = 0.19, p = .943. Participants aged 18–25 years reported the highest mean adherence score (M = 4.31, SD = 2.02), while those aged 36–45 years reported the lowest (M = 3.98, SD = 2.02); however, these differences were not statistically significant (Table 7).
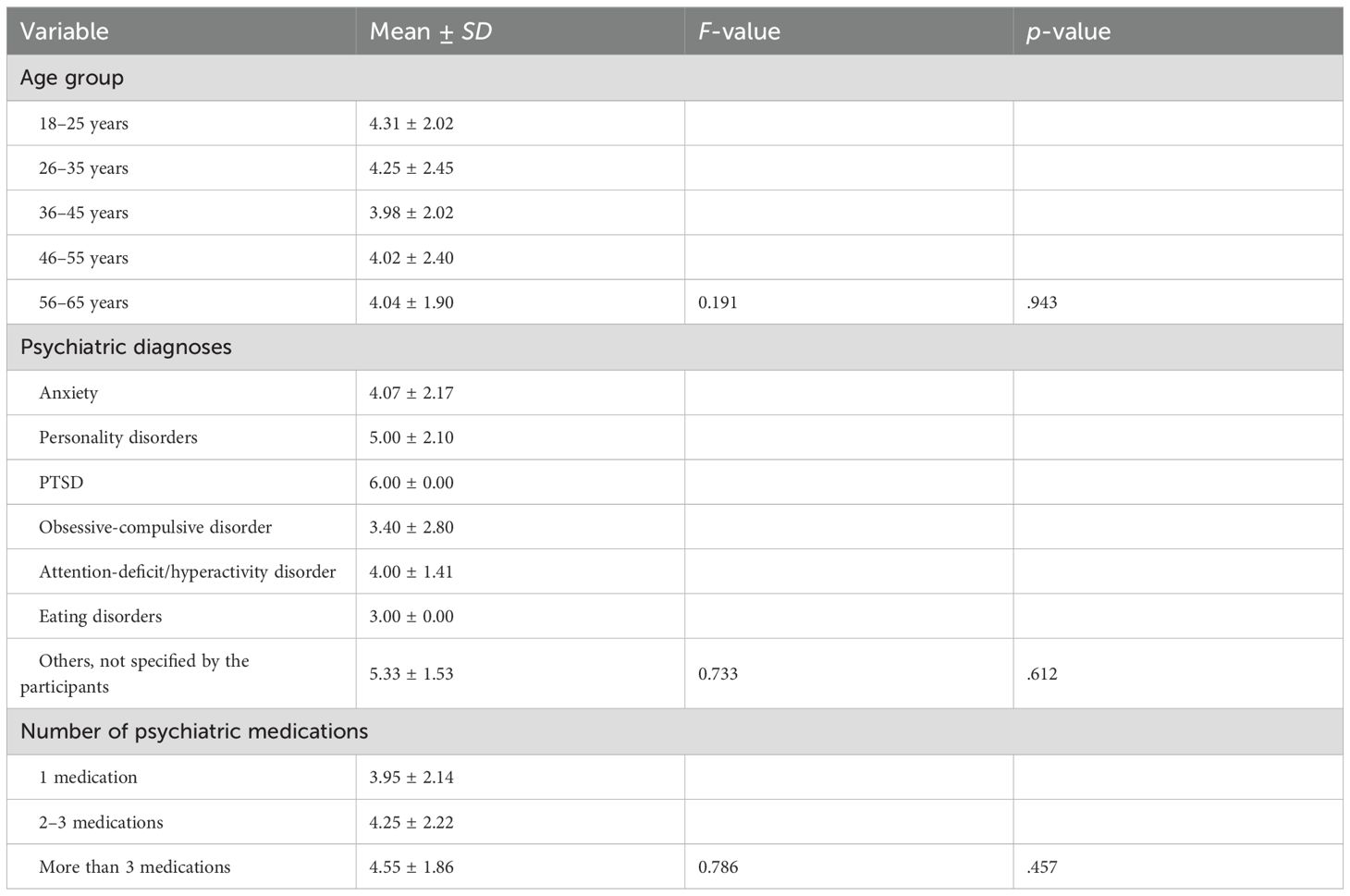
Table 7. One-way analysis of variance (ANOVA) test for differences in medication adherence based on the depression patients’ characteristics (n = 234).
Similarly, adherence did not significantly differ based on comorbid psychiatric diagnosis, F(6, 100) = 0.73, p = .612. Patients with PTSD (M = 6.00, SD = 0.00) and those with unspecified conditions (M = 5.33, SD = 1.53) had numerically higher adherence scores than other subgroups; however, these differences did not reach statistical significance (Table 7).
Adherence also did not significantly differ in terms of the number of psychiatric medications currently taken, F(2, 280) = 0.79, p = .457. Patients taking more than three medications had the highest mean adherence (M = 4.55, SD = 1.86), while those on one medication had the lowest (M = 3.95, SD = 2.14), though these differences were not statistically meaningful (Table 7).
Associations between adherence and patient characteristics
Chi-square tests examined associations between medication adherence status (compliant vs. non-compliant) and various sociodemographic and clinical characteristics among patients with depression (Table 8). No statistically significant associations between medication adherence and gender emerged, χ²(1, N = 234) = 0.11, p = .451; age group, χ²(4, N = 234) = 3.23, p = .520; presence of a mental illness other than depression, χ²(1, N = 234) = 1.09, p = .296; number of psychiatric medications taken regularly, χ²(2, N = 234) = 1.45, p = .483; having someone to transport the patient to the hospital, χ²(1, N = 234) = 2.39, p = .122; having someone to drive them home, χ²(1, N = 234) = 1.54, p = .215; stigma perception, χ²(1, N = 234) = 0.35, p = .556; cost concerns, χ²(1, N = 234) = 0.31, p = .577; or health insurance status, χ²(1, N = 234) = 0.56, p = .456 (Table 8).
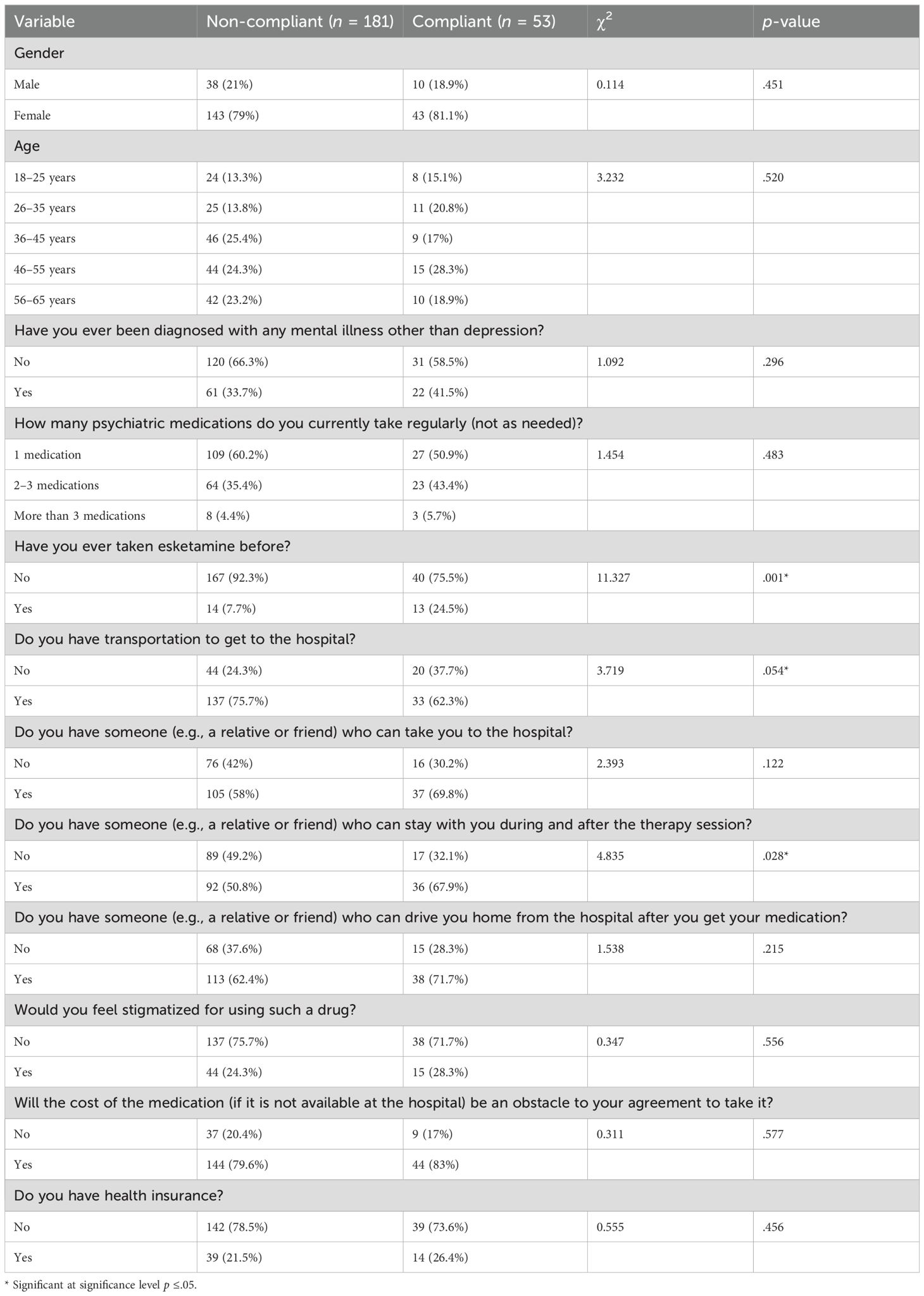
Table 8. Differences in the levels of medication adherence based on the depression patients’ characteristics (n = 234).
However, three variables were significant or near-significant. Patients who had previously taken esketamine were significantly more likely to be classified as adherent (24.5%) than non-adherent patients (7.7%), χ²(1, N = 234) = 11.33, p = .001 (Table 8). Similarly, having someone available to stay with the patient during and after therapy was significantly associated with adherence status. Among compliant patients, 67.9% had such support compared to 50.8% of non-compliant patients, χ²(1, N = 234) = 4.84, p = .028 (Table 8). Additionally, transportation availability approached statistical significance: a higher proportion of compliant patients lacked transportation (37.7%) compared to non-compliant patients (24.3%), χ²(1, N = 234) = 3.72, p = .054 (Table 8).
Predictors of medication adherence
A multiple linear regression analysis examined whether various demographic and clinical factors significantly predicted medication adherence scores among depression patients (Table 9). The model was statistically significant, F(10, 223) = 2.27, p <.05, and explained a substantial portion of the variance in medication adherence, R = .979, R² = .958, adjusted R² = .535. The standard error of the estimate was 1.43.
Despite the overall model reaching statistical significance, none of the individual predictors were statistically significant. Gender (β = .872, p = .422), age (β = –.962, p = .433), and having other mental illnesses (β = −.397, p = .683) did not significantly predict adherence. Similarly, the number of psychiatric medications taken (β = .406, p = .795), prior esketamine use (β = −.184, p = .775), and transportation availability (β = −.549, p = .592) were not significant (Table 9).
Additional factors, including having someone to take the patient to the hospital (β = −.149, p = .849), someone to stay with patients during and after therapy (β = .233, p = .794), cost as a barrier (β = −.008, p = .984), and health insurance (β = .258, p = .802), were also not significantly associated with adherence levels (Table 9).
Discussion
Approximately one-third of participants reported a comorbid mental illness, while two-thirds did not. Among those with comorbidity, anxiety disorder was the most common. This result aligns with other studies, such as a study from the Netherlands (49) and another from Australia (50), both indicating that anxiety is the most common comorbidity among patients with depression. Further, this high depression-anxiety comorbidity has been linked to more severe depressive symptoms (49), poorer treatment response (49), and increased risk of relapse (49). Therefore, recognizing this depression-anxiety comorbidity is critical, as early detection and simultaneous management of both conditions could significantly improve clinical outcomes in this population. Such a finding reflects the importance of routine screening for other mental illnesses, especially anxiety disorders, in this particular population.
In this study, financial considerations emerged as a significant barrier when considering esketamine treatment, with over three-quarters of the participants (79.5%) reporting esketamine’s cost as a potential barrier to use. This finding aligns with a U.S study (51), which reported that esketamine’s cost is a significant barrier to its use, finding that esketamine would only be cost-effective if the per-dose price were reduced by more than 40%. In another U.S. study (52), cost and insurance emerged as potential barriers to esketamine use. In Saudi Arabia, the single nasal spray bottle of esketamine costs around 952.90 SAR (53), which is considered high, suggesting that financial concerns may discourage treatment initiation (54) and limit accessibility and equitable use. Additionally, limited insurance coverage may further exacerbate these challenges in Saudi Arabia. The finding regarding patient perceptions of cost as a major barrier highlights the importance of establishing transparent pricing structures, expanding insurance coverage, and implementing sustainable reimbursement strategies to facilitate wider access to esketamine for Saudi patients with depression. Future research should evaluate financial models and policy interventions that reduce cost-related barriers, ensuring equitable access and improving adherence to esketamine treatment.
Nearly half of this study’s participants (45.9%) cited the need for hospital visits and a two-hour observation after the esketamine dose as deterrents. This result is consistent with other studies, such as one from the United Kingdom (31) and another from the U.S (55). These studies (31, 55) collectively affirm that mandatory therapy attendance and extended post-dose monitoring present significant barriers to esketamine treatment. Accordingly, to help patients overcome such a barrier, practical supportive measures could be considered (31, 55). Such measures could include providing transportation assistance, scheduling flexible therapy session times, and involving family members or caregivers to accompany patients, all of which may make attending more feasible (31, 55). Future research should also investigate the effect of such measures in reducing the logistical burden of esketamine treatment.
In this study, more than half (57.6%) of the patients reported concerns about potential side effects of esketamine treatment. These results align with studies conducted in China (23, 56), which found that concerns about side effects can directly affect treatment adherence, often leading to hesitation or discontinuation of esketamine therapy. Further, these studies (23, 56) found that commonly reported adverse effects among participants include dissociation, dizziness, nausea, and transient increases in blood pressure. Future research should explore ways to improve pre-treatment psychoeducation to help improve patients’ confidence and willingness to continue therapy, as well as practical strategies to manage specific side effects and make treatment easier to tolerate, ultimately supporting better adherence.
Moreover, about half of this study’s patients prefer to take esketamine once or twice a week over other daily antidepressants. This finding aligns with a U.S. study (57) that indicated a preference for esketamine treatment compared to the slower daily oral antidepressants. The U.S. study (57) further indicated that treatments with less-frequent dosing and faster onsets, such as esketamine, are generally better tolerated and more acceptable to patients compared to daily oral antidepressants. Future studies should examine approaches that prioritize patient convenience by optimizing dosing schedules and supporting adherence, particularly for therapies with less-frequent administration and rapid onset.
The present study found that about half (52.3%) of the patients would agree to take esketamine under a doctor’s advisement to treat their condition, while the remaining indicated they would not agree to take it even with medical advice. This result aligns with another U.S. study (58) that found that over half of the participants were likely to follow a physician’s advice to take esketamine, with the other half refusing to initiate treatment even with a medical recommendation. Future studies should investigate the effectiveness of structured psychoeducation programs in addressing patient hesitancy toward esketamine, particularly for those who may refuse treatment even when recommended by a physician (59). Such programs could include counseling on treatment benefits, side-effect management, and the expected course of therapy.
Moreover, in this study, the majority of participants (71.4%) reported that they sometimes forget to take medication. This finding corresponds to a U.S. study (60), which found that simply forgetting was the main reason for patient non-adherence. Additionally, another Canadian study (61) reported that forgetfulness was the most common reason for noncompliance. Given that forgetfulness is a frequently reported issue in patients’ adherence, we recommend the routine use of reminders, such as phone alarms and medication apps, as well as family involvement, to support adherence to antidepressant treatment.
In this sample, over three-quarters of patients with depression (77.4%) were non-adherent to their current psychiatric medications, exceeding the more than half (~50%) non-adherence rates commonly reported among international psychiatric populations (31, 34). Several factors may underlie this study’s elevated non-adherence rate, including forgetfulness and lack of illness insight, which are frequently cited in the literature as barriers to regular medication intake (60, 61). In addition, stigma associated with psychiatric disorders, limited health literacy, and negative beliefs about psychotropic medications can all contribute to non-adherence (35). In the Saudi context, cultural dynamics may also play a role, such as patients sometimes relying more on family decision-making or traditional beliefs, which may interfere with long-term adherence (35). Together, these elements highlight the multifaceted nature of poor adherence in patients with depression, highlighting the imperative to employ comprehensive interventions. Structured education programs, consistent follow-up visits, and motivational interviewing approaches can help overcome these barriers, while digital reminders and family-based support strategies may address practical issues such as forgetfulness.
The findings revealed higher medication adherence among patients with prior esketamine exposure and among patients with someone to accompany them to and from therapy sessions. These findings align with past research that has demonstrated the positive impact of treatment experience and support systems on adherence to psychiatric medication (31, 34). Importantly, we hypothesize that this difference is unlikely to be explained by a lingering pharmacological effect of esketamine. Rather, we hypothesize that it reflects the influence of treatment experience and perceived benefit—factors that prior studies have emphasized as key adherence drivers (31, 34). Another plausible explanation is that these patients were more likely to have TRD, had experienced genuine symptom improvement with esketamine, and were, therefore, more motivated to maintain care. Experiencing benefit from an otherwise treatment-resistant illness may have strengthened patients’ belief in the value of psychiatric care, which in turn reinforced adherence. Esketamine trial data support this explanation. For instance, Pepe et al. (34) found that patients who responded to intranasal esketamine were more likely to demonstrate long-term adherence and emphasized the predictive value of treatment familiarity and the perceived benefit of adherence behavior. Similarly, qualitative research on ketamine has established a link between perceived treatment benefits and a motivation to continue (31). At the same time, another study cautions that treatment adherence in TRD can be low despite exposure to new treatments, given the chronic and relapsing nature of the illness (62). Accordingly, future research should use longitudinal designs and large, diverse samples to determine whether the higher adherence observed in this study’s patients regarding esketamine exposure can be sustained over the long term.
Additionally, social and logistical support were critical factors in medication adherence in this study. Having a companion during treatment sessions or for the trip home improved adherence, and transportation availability also approached statistical significance as an adherence predictor. These findings support previous research demonstrating that family support and logistical facilitation are key determinants of adherence in psychiatric care (31, 34). The logistical aspect is also noteworthy: transportation difficulties may limit patients’ ability to attend regular treatment sessions, especially in cases where treatment requires frequent hospital visits, such as with esketamine. In Saudi Arabia, where travel distances can be considerable and public transportation is limited, transportation barriers are particularly relevant. Therefore, addressing transportation barriers—through healthcare-provided transport services, community initiatives, or insurance coverage—may significantly improve adherence. In addition, we recommend that future care plans proactively involve family members in ongoing support, as well as to overcome logistical barriers as part of a comprehensive adherence-enhancing strategy.
Regarding demographic and clinical factors using ANOVA, this study found no significant differences in medication adherence based on age, type of psychiatric comorbidity, or the number of medications taken. This finding aligns with earlier studies, which reported that younger age and fewer medications are associated with better adherence (29, 30) and that certain psychiatric comorbidities, such as anxiety and obsessive-compulsive disorder, are often linked to lower adherence (62). One possible explanation is that strong family involvement in the Saudi population may buffer the effects of age or comorbidity, leading to a more uniform adherence pattern across demographic subgroups. Future qualitative Saudi studies are needed to gain better insight into these probable underlying factors.
Finally, although the regression model was statistically significant overall, no individual predictor—such as gender, age, social support, insurance status, or history of esketamine use—was significant. This finding diverges from previous reports where demographic and psychosocial variables were noted to predict adherence (29, 30). Therefore, further Saudi research should be conducted to compare results in the Saudi context.
Strengths and limitations
This study has several strengths. It is among the first in Saudi Arabia to investigate esketamine and patient perceptions, addressing a critical gap in the regional literature. This study also examined barriers and obstacles that patients could face during esketamine treatment, providing a foundation for future research and interventions and potentially leading to the successful adoption of esketamine treatment in psychiatric care. In addition, the sample size was sufficient for statistically meaningful conclusions.
However, this study also has limitations. First, the cross-sectional design precludes a direct causal inference. Hence, future Saudi studies using a longitudinal design to better explore causal relationships over time are warranted. Second, self-reported data are susceptible to recall issues or participants giving socially desirable answers, requiring the consideration of objective measures or combining self-reports with clinician assessments to improve accuracy. For instance, comorbid psychiatric conditions were self-reported, which may have led to the underestimation or overestimation of the actual figures; future studies should use more rigorous methods to assess such comorbidities, such as corroborating with medical records or clinical assessments. Third, although the research team phrased the study tool questions as clearly as possible, some participant responses should be interpreted with caution, as participants may vary in their understanding of questions, potentially leading to misleading answers and, hence, results. For example, regarding the question asking the participants whether the severity of depression was a concern, some participants might have understood that their depression was too severe to respond to treatment, while others might have understood that their depression was mild; therefore, they do not need a newer treatment such as esketamine. Fourth, specific questions in the study merit greater specificity and analytic depth, which future studies could explore to inform the development of targeted interventions. For instance, in this study, questions related to transportation were phrased without specifying the availability of public, private, or both public and private means of transportation. Exploring these various modes of transportation in more depth and developing interventions to facilitate transportation to receive the esketamine could help overcome the transportation-related concern. Additionally, regarding the study tools, another limitation is that the study did not employ a standardized scale to assess depression diagnosis and its severity, as the study’s aim was to evaluate the perception and attitude toward esketamine among those already known to suffer from depression, regardless of the severity of the illness, noting that the participants were being followed in psychiatric clinics and were already diagnosed with depression. However, future Saudi research should consider using structured or semi-structured interviews or a standardized scale to further confirm the accuracy and severity of the illness, as this may lead to more accurate and reflective results. Participant-related constraints further limited generalizability. The sample included participants diagnosed with depression irrespective of TRD status, which is the main indication for using esketamine per international guidelines (63). Thus, this study’s participants may not fully reflect those who benefit from esketamine, namely those with TRD. Therefore, future studies in Saudi Arabia should specifically target populations relevant to esketamine usage. Another participant-related limitation is that the sample may not fully reflect the broader population of patients with depression in Saudi Arabia, thereby limiting the generalizability of the findings. Including participants from different regions and healthcare settings would help improve representativeness.
Conclusion
This study aimed to assess the awareness and acceptance of esketamine as a treatment option and explored factors potentially influencing acceptance among patients with depression. Key barriers regarding the acceptance of esketamine included the need to come to the hospital and undergo observation after treatment. To overcome these concerns, we suggest providing social support to the patients to facilitate their acceptance of the medication. Another concern was the cost associated with esketamine. To reduce the cost-related concern, we recommend that insurance cover esketamine and that government hospitals make it readily available or perhaps support its local production, if feasible. Furthermore, this study found that patients’ adherence to their current medication regimen was significantly associated with having previous esketamine experience. Therefore, we recommend providing the patients with related educational material to improve their awareness and, hence, facilitate their acceptance of esketamine as a therapeutic option when clinically indicated. Furthermore, social support was associated with adherence to the current medication regimen. This reflects the importance of multidisciplinary team involvement, including social workers, to provide the needed social support to patients and their families. Collectively, these strategies may enhance equitable access, acceptance, and adherence within the Saudi context.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Institutional Review Board of the College of Medicine at King Saud University (research project no. E-24-9385). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AhA: Validation, Writing – review & editing, Software, Resources, Methodology, Data curation, Project administration, Funding acquisition, Conceptualization, Visualization, Writing – original draft, Formal Analysis, Supervision. AyA: Validation, Resources, Formal Analysis, Funding acquisition, Project administration, Writing – review & editing, Methodology, Supervision, Software, Conceptualization, Data curation, Visualization. FA: Supervision, Conceptualization, Writing – original draft, Validation, Methodology. ASA: Conceptualization, Validation, Methodology, Project administration, Writing – original draft. AB: Methodology, Conceptualization, Project administration, Validation, Visualization, Writing – original draft. ZA: Validation, Project administration, Writing – original draft. LA: Writing – original draft, Project administration, Validation. AAA: Validation, Data curation, Writing – original draft, Project administration. AM: Writing – original draft, Project administration, Methodology, Conceptualization. MA: Funding acquisition, Conceptualization, Supervision, Writing – review & editing, Methodology, Validation, Software, Resources.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was funded by the Deanship of Scientific Research, King Saud University, through the Vice Deanship of Scientific Research Chairs; SABIC Psychological Health Research and Applications Chair (SPHRAC), Department of Psychiatry, College of Medicine, King Saud University, Riyadh, Saudi Arabia. The funding source had no involvement in the study.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research, King Saud University, for funding through the Vice Deanship of Scientific Research Chairs; SABIC Psychological Health Research and Applications Chair (SPHRAC), Department of Psychiatry, College of Medicine, King Saud University, Riyadh, Saudi Arabia.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rong J, Wang X, Cheng P, Li D, and Zhao D. Global, regional and national burden of depressive disorders and attributable risk factors, from 1990 to 2021: Results from the 2021 Global Burden of Disease Study. Br J Psychiatry. (2025) 227:688–97. doi: 10.1192/bjp.2024.266
2. Al-Subaie AS, Al-Habeeb A, and Altwaijri YA. Overview of the Saudi national mental health survey. Int J Methods Psychiatr Res. (2020) 29:e1835. doi: 10.1002/mpr.1835
3. Duggal D, Fertuck EA, and Huprich SK. The domain of social dysfunction in complex depressive disorders. In: de la Parra G, Dagnino P, and Behn A, editors. Depression and Personality Dysfunction Depression and Personality. Springer International Publishing, Cham (2021). p. 123–44. doi: 10.1007/978-3-030-70699-9_5
4. Citrome L. Management of depression. Current options for this highly treatable disorder. Postgrad Med. (1994) 95:137–42. doi: 10.1080/00325481.1994.11945787
5. AlJadani AH, Alshammari SN, Alshammari KA, Althagafi AA, and AlHarbi MM. Public awareness, beliefs and attitude towards depressive disorders in Saudi Arabia. Saudi Med J. (2021) 42:1117–24. doi: 10.15537/smj.2021.42.10.20210425
6. Sinner B and Graf BM. Ketamine. In: Schüttler J and Schwilden H, editors. Modern Anesthetics, vol. 182 Handbook Exp Pharmacol. Springer, Berlin (2008). p. 313–33. doi: 10.1007/978-3-540-74806-9_15
7. Li L and Vlisides PE. Ketamine: 50 years of modulating the mind. Front Hum Neurosci. (2016) 10:612/full. doi: 10.3389/fnhum.2016.00612/full
8. Abdollahpour A, Saffarieh E, and Zoroufchi BH. A review on the recent application of ketamine in management of anesthesia, pain, and health care. J Family Med Prim Care. (2020) 9:1317–24. doi: 10.4103/jfmpc.jfmpc_875_19
9. Hustveit O, Maurset A, and Oye I. Interaction of the chiral forms of ketamine with opioid, phencyclidine, sigma and muscarinic receptors. Pharmacol Toxicol. (1995) 77:355–9. doi: 10.1111/j.1600-0773.1995.tb01041.x
10. Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. (2000) 47:351–4. doi: 10.1016/S0006-3223(99)00230-9
11. Abbar M, Demattei C, El-Hage W, Llorca PM, Samalin L, Demaricourt P, et al. Ketamine for the acute treatment of severe suicidal ideation: double-blind, randomised placebo-controlled trial. BMJ. (2022) 25:e067194. doi: 10.1136/bmj-2021-067194
12. Alshammari TK. The ketamine antidepressant story: New insights. Molecules. (2020) 25:5777. doi: 10.3390/molecules25235777
13. McIntyre RS, Alsuwaidan M, Baune BT, Berk M, Demyttenaere K, Goldberg JF, et al. Treatment-resistant depression: definition, prevalence, detection, management, and investigational interventions. World Psychiatry. (2023) 22:394–412. doi: 10.1002/wps.21120
15. Van De Loo AJAE, Bervoets AC, Mooren L, Bouwmeester NH, Garssen J, Zuiker R, et al. The effects of intranasal esketamine (84 mg) and oral mirtazapine (30 mg) on on-road driving performance: a double-blind, placebo-controlled study. Psychopharmacol (Berl). (2017) 234:3175–83. doi: 10.1007/s00213-017-4706-6
16. Daly EJ, Singh JB, Fedgchin M, Cooper K, Lim P, Shelton RC, et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. (2018) 75:139. doi: 10.1001/jamapsychiatry.2017.3739
17. Popova V, Daly EJ, Trivedi M, Cooper K, Lane R, Lim P, et al. Efficacy and safety of flexibly dosed esketamine nasal spray combined with a newly initiated oral antidepressant in treatment-resistant depression: a randomized double-blind active-controlled study. Am J Psychiatry. (2019) 176:428–38. doi: 10.1176/appi.ajp.2019.19020172
18. Targum SD, Daly E, Fedgchin M, Cooper K, and Singh JB. Comparability of blinded remote and site-based assessments of response to adjunctive esketamine or placebo nasal spray in patients with treatment-resistant depression. J Psychiatr Res. (2019) 111:68–73. doi: 10.1016/j.jpsychires.2019.01.017
19. Canuso CM, Singh JB, Fedgchin M, Alphs L, Lane R, Lim P, et al. Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Am J Psychiatry. (2018) 175:620–30. doi: 10.1176/appi.ajp.2018.17060720
20. Daly EJ, Trivedi MH, Janik A, Li H, Zhang Y, Li X, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. (2019) 76(9):893–903. doi: 10.1001/jamapsychiatry.2019.1189
21. Wajs E, Aluisio L, Holder R, Daly EJ, Lane R, Lim P, et al. Esketamine nasal spray plus oral antidepressant in patients with treatment-resistant depression: assessment of long-term safety in a phase 3, open-label study (SUSTAIN-2). J Clin Psychiatry. (2020) 81:19m12891. doi: 10.4088/JCP.19m12891
22. Canuso CM, Ionescu DF, Li X, Qiu X, Lane R, Turkoz I, et al. Esketamine nasal spray for the rapid reduction of depressive symptoms in major depressive disorder with acute suicidal ideation or behavior. J Clin Psychopharmacol. (2021) 41:516–24. doi: 10.1097/JCP.0000000000001465
23. Yang S, Wang J, Li X, Wang T, Xu Z, Xu X, et al. Adverse effects of esketamine for the treatment of major depression disorder: findings from randomized controlled trials. Psychiatr Q. (2022) 93:81–95. doi: 10.1007/s11126-020-09871-x
24. Bahk WM, Pae CU, Woo YS, Lim HK, Han C, Choi JS, et al. Rapid onset of intranasal esketamine in patients with treatment-resistant depression and major depression with suicidal ideation: a meta-analysis. Clin Psychopharmacol Neurosci. (2021) 19:341–54. doi: 10.9758/cpn.2021.19.2.341
25. Ahmed NN, Albishi F, Khan SA, Alsayegh A, Stip E, and Makhoul S. Management of treatment-resistant depression with esketamine nasal spray: clinical questions for daily practice in Gulf Cooperation Council countries. Middle East Curr Psychiatry. (2023) 30:99. doi: 10.1186/s43045-023-00369-3
26. Zaki N, Chen LN, Lane R, Doherty T, Drevets WC, Morrison RL, et al. Safety and efficacy with esketamine in treatment-resistant depression: long-term extension study. Int J Neuropsychopharmacol. (2025) 28(6):pyaf027. doi: 10.1093/ijnp/pyaf027
27. Herrera-Imbroda J. Changing the paradigm in treatment-resistant depression: a review of long-term efficacy and tolerability of esketamine nasal spray. Exp Clin Psychopharmacol. (2023) 31:1092–101. doi: 10.1037/pha0000650
28. Di Vincenzo M, Martiadis V, Della Rocca B, Arsenio E, D’Arpa A, Volpicelli A, et al. Facts and myths about use of esketamine for treatment-resistant depression: a narrative clinical review. Front Psychiatry. (2024) 15:1394787. doi: 10.3389/fpsyt.2024.1394787
29. Medlinskiene K, Tomlinson J, Marques I, Richardson S, Stirling K, and Petty D. Barriers and facilitators to the uptake of new medicines into clinical practice: a systematic review. BMC Health Serv Res. (2021) 21:1198. doi: 10.1186/s12913-021-07196-4
30. Lublóy Á. Factors affecting the uptake of new medicines: a systematic literature review. BMC Health Serv Res. (2014) 14:469. doi: 10.1186/1472-6963-14-469
31. Jilka S, Odoi CM, Wilson E, Meran S, Simblett S, and Wykes T. Ketamine treatment for depression: qualitative study exploring patient views. BJPsych Open. (2021) 7:e32. doi: 10.1192/bjo.2020.165
32. Fairchild AO, Katz EG, Reed SD, Johnson FR, DiBernardo A, Hough D, et al. Patient preferences for ketamine-based antidepressant treatments in treatment-resistant depression: results from a clinical trial and panel. Neurol Psychiatry Brain Res. (2020) 37:67–78. doi: 10.1016/j.npbr.2020.05.003
33. Koss TB. Ketamine: safe and effective treatment for severe depression. J Am Psychiatr Nurses Assoc. (2021) 27:502–6. doi: 10.1177/10783903211033023
34. Pepe M, Bartolucci G, Marcelli I, Pesaresi F, Brugnami A, Caso R, et al. The patient’s perspective on the effects of intranasal esketamine in treatment-resistant depression. Brain Sci. (2023) 13:1494. doi: 10.3390/brainsci13101494
35. Al-Shareef EM, Kadah Salim AM, Al-Farrah NM, Al-Murad BM, Moallem AA, Radwan MA, et al. Knowledge and perception towards psychotropic drugs among the general population in Saudi Arabia. Psychol Res Behav Manage. (2024) 17:3543–53. doi: 10.2147/PRBM.S485798
36. World Health Organization. Depression(2019). Available online at: https://www.who.int/health-topics/depressiontab=tab_2 (Accessed June 28, 2025).
37. Janssen Pharmaceuticals, Inc. SPRAVATO. Prescribing information. Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2025/211243s016lbl.pdf (Accessed September 12, 2024).
38. European Medicines Agency (EMA). Spravato. European Medicines Agency (EMA). Available online at: https://www.ema.europa.eu/en/medicines/human/EPAR/spravat (Accessed September 12, 2024).
39. Horne R and Weinman J. Self-regulation and self-management in asthma: exploring the role of illness perceptions and treatment beliefs in explaining non-adherence to preventer medication. Psychol Health. (2002) 17:17–32. doi: 10.1080/08870440290001502
40. Hughes I, Hill B, and Budd R. Compliance with antipsychotic medication: from theory to practice. J Ment Health. (1997) 6:473–90. doi: 10.1080/09638239718572
41. Fialko L, Garety PA, Kuipers E, Dunn G, Bebbington PE, Fowler D, et al. A large-scale validation study of the Medication Adherence Rating Scale (MARS). Schizophr Res. (2008) 100:53–9. doi: 10.1016/j.schres.2007.10.029
42. Thompson K, Kulkarni J, and Sergejew AA. Reliability and validity of a new Medication Adherence Rating Scale (MARS) for the psychoses. Schizophr Res. (2000) 42:241–7. doi: 10.1016/S0920-9964(99)00130-9
43. Ali MM, Taha MM, Ahmed AE, Ali S, Baiti MA, Alhazmi AA, et al. Psychotropic medication adherence and its associated factors among schizophrenia patients: exploring the consistency of adherence scales. Cureus. (2023) 15:e46118. doi: 10.7759/cureus.46118
44. Al-Qerem W, Al Bawab AQ, Abusara O, Alkhatib N, and Horne R. Validation of the Arabic version of medication adherence report scale questionnaire and beliefs about medication-specific questionnaire: a factor analysis study. PloS One. (2022) 17:e0266606. doi: 10.1371/journal.pone.0266606
45. Prabahar K, Albalawi MA, Almani L, and Alenizy S. Assessment of medication adherence in patients with chronic diseases in Tabuk, Kingdom of Saudi Arabia. J Res Pharm Pract. (2021) 9:196–201. doi: 10.4103/JRPP.JRPP_20_97
46. Christudas M, Gupta B, Undela K, Isaac N, Ram D, and Ramesh M. Assessment of impact of pharmacophilia and pharmacophobia on medication adherence in patients with psychiatric disorders: a cross-sectional study. Indian J Pharmacol. (2016) 48:701. doi: 10.4103/0253-7613.194858
47. Sowunmi O and Onifade P. Psychometric evaluation of Medication Adherence Rating Scale (MARS) among Nigerian patients with schizophrenia. Niger J Clin Pract. (2019) 22:1281. doi: 10.4103/njcp.njcp_325_18
48. Wang D, Ross B, Xi C, Pan Y, Zhou L, Yang X, et al. Medication adherence and its correlates among patients affected by schizophrenia with an episodic course: a large-scale multi-center cross-sectional study in China. Asian J Psychiatr. (2020) 53:102198. doi: 10.1016/j.ajp.2020.102198
49. Lamers F, van Oppen P, Comijs HC, Smit JH, Spinhoven P, van Balkom AJ, et al. Comorbidity patterns of anxiety and depressive disorders in a large cohort study: the Netherlands Study of Depression and Anxiety (NESDA). J Clin Psychiatry. (2011) 72:341–8. doi: 10.4088/JCP.10m06176blu
50. Almeida OP, Draper B, Pirkis J, Snowdon J, Lautenschlager NT, Byrne G, et al. Anxiety, depression, and comorbid anxiety and depression: risk factors and outcome over two years. Int Psychogeriatr. (2012) 24:1622–32. doi: 10.1017/S104161021200107X
51. Ross EL and Soeteman DI. Cost-effectiveness of esketamine nasal spray for patients with treatment-resistant depression in the United States. Psychiatr Serv. (2020) 71:988–97. doi: 10.1176/appi.ps.201900625
52. Brendle M, Robison RJ, and Malone DC. Cost-effectiveness of esketamine nasal spray compared to intravenous ketamine for patients with treatment-resistant depression in the US utilizing clinical trial efficacy and real-world effectiveness estimates. J Affect Disord. (2022) 319:388–96. doi: 10.1016/j.jad.2022.09.083
53. Saudi Food & Drug Authority. Spravato [drug listing]. Saudi FDA – Drugs List. Available online at: https://www.wideip.sfda.gov.sa/en/drugs-list?TradeName=spravato (Accessed September 13, 2025).
54. Wilkinson ST, Howard DH, and Busch SH. Psychiatric practice patterns and barriers to the adoption of esketamine. JAMA. (2019) 322:1039–40. doi: 10.1001/jama.2019.10728
55. Staff Writer. Weekly Mind Reader: identifying barriers to esketamine nasal treatment (2024). Psychiatrist.com. Available online at: https://www.psychiatrist.com/news/weekly-mind-reader-identifying-barriers-to-esketamine-nasal-treatment/ (Accessed July 10, 2025).
56. Guo H, Wang B, Yuan Q, Wu S, Liu J, He M, et al. Neurological adverse events associated with esketamine: a pharmacovigilance study using the FDA Adverse Event Reporting System. Front Pharmacol. (2022) 13:849758. doi: 10.3389/fphar.2022.849758
57. Katz EG, McNulty P, Levitan B, Treichler P, Martynowicz J, and Jamieson CUS. Food and Drug Administration’s Patient-Focused Drug Development Initiative: experience with integration of patient-experience data in a New Drug Application for esketamine nasal spray plus a newly initiated oral antidepressant for treatment-resistant depression. Ther Innov Regul Sci. (2022) 56:38–46. doi: 10.1007/s43441-021-00340-6
58. Joshi K, Liberman JN, Parab P, Darer JD, and Harding L. Barriers to esketamine nasal spray treatment among adults with treatment-resistant depression. J Clin Psychiatry. (2024) 85:23m15102. doi: 10.4088/JCP.23m15102
59. Bahredar MJ, Asgharnejad Farid AA, Ghanizadeh A, and Birashk B. The efficacy of psycho-educational group program on medication adherence and global functioning of patients with bipolar disorder type I. Int J Community Based Nurs Midwifery. (2014) 2:12–9. doi: 10.30476/IJCBNM.2014.267729
60. Sansone RA and Sansone LA. Antidepressant adherence: are patients taking their medications? Innov Clin Neurosci. (2012) 9(5-6):41–6.
61. Bulloch AG, Adair CE, and Patten SB. Forgetfulness: a role in noncompliance with antidepressant treatment. Can J Psychiatry. (2006) 51:719–22. doi: 10.1177/070674370605101110
62. Litz M and Leslie D. The impact of mental health comorbidities on adherence to buprenorphine: a claims-based analysis. Am J Addict. (2017) 26:859–63. doi: 10.1111/ajad.12644
Keywords: depression, esketamine, medication adherence, perspectives, Saudi Arabia
Citation: Almadani AH, Alghamdi AH, Alfahad FB, Alibrahim AS, Binbakhit AI, Alenazi ZB, Alruwaili LM, Alshahwan AA, Muhnna AK and Aljaffer MA (2025) The perspectives of patients with depression toward esketamine, and the influence of their medication adherence on their viewpoints: a Saudi cross-sectional study. Front. Psychiatry 16:1678119. doi: 10.3389/fpsyt.2025.1678119
Received: 01 August 2025; Accepted: 08 October 2025;
Published: 29 October 2025.
Edited by:
Glenn Hartelius, Attention Strategies Institute, United StatesReviewed by:
Anna Julia Krupa, Jagiellonian University Medical College, PolandMatteo Di Vincenzo, University of Campania Luigi Vanvitelli, Italy
Jesús Herrera-Imbroda, Andusian Health Service, Spain
Copyright © 2025 Almadani, Alghamdi, Alfahad, Alibrahim, Binbakhit, Alenazi, Alruwaili, Alshahwan, Muhnna and Aljaffer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ahmad H. Almadani, YWhhbG1hZGFuaUBrc3UuZWR1LnNh
†ORCID: Ahmad H. Almadani, orcid.org/0000-0003-4490-3220
Ayedh H. Alghamdi, orcid.org/0009-0009-5452-0961
Fahad B. Alfahad, orcid.org/0000-0002-8290-1835
Abdullah S. Alibrahim, orcid.org/0009-0005-4091-5621
Abdulrahman I. Binbakhit, orcid.org/0009-0000-0645-049X
Ziyad B. Alenazi, orcid.org/0009-0007-5335-5330
Lama M. Alruwaili, orcid.org/0009-0006-7496-8147
Abdulrahman A. Alshahwan, orcid.org/0009-0004-3398-4461
Abdullah K. Muhnna, orcid.org/0009-0009-3689-6201
Mohammed A. Aljaffar, orcid.org/0000-0002-0345-5567
 Ahmad H. Almadani
Ahmad H. Almadani Ayedh H. Alghamdi
Ayedh H. Alghamdi Fahad B. Alfahad3†
Fahad B. Alfahad3† Mohammed A. Aljaffer
Mohammed A. Aljaffer