- 1College of Sports, Nanjing Tech University, Nanjing, China
- 2School of Athletic Performance, Shanghai University of Sport, Shanghai, China
Objective: This study aims to explore the relationship between nap duration, nighttime sleep, and depression among Chinese residents and determine recommended sleep durations to provide scientific evidence for the prevention and control of depression.
Methods: Based on the 2020 China Family Panel Studies, demographic data, health, and lifestyle information was obtained from the study subjects. A total of 6,795 valid samples were included. Logistic regression, restricted cubic splines, and stratified linear regression analyses were used to examine the associations between sleep behaviors and depression, including subgroup analyses by health status and age categories.
Results: A U-shaped dose–response relationship was observed between nighttime sleep and depressive symptoms (P-nonlinear < 0.001), with the lowest likelihood of depression occurring around 8.5 hours of sleep. A nap duration of 30–90 minutes was associated with a lower likelihood of depression, with no evidence of a nonlinear relationship (P-nonlinear = 0.889). Subgroup analyses revealed that nighttime sleep of 7–9 hours was protective against depression among individuals with self-rated general health or chronic diseases. Age-stratified analyses showed that sleep behaviors had stronger protective effects in young adults (<30 years), whereas depression in middle-aged and older adults (≥30 years) was more influenced by chronic disease status and education level.
Conclusion: Nighttime sleep of 7–9 hours and nap duration of 30–90 minutes are associated with reduced depressive symptoms; however, their effects vary across health and age subgroups. These findings highlight the importance of tailoring sleep recommendations to individual characteristics for effective mental health promotion.
1 Introduction
Depression is a widespread mental health disorder, affecting an estimated 280 million people globally (1). As one of the leading causes of disability, the prevention and intervention strategies for depression are a pressing focus in global public health. Notably, the prevalence of depression is not limited to middle-aged and elderly populations; its incidence among young adults is also significant (2). For instance, a survey conducted in the United States revealed that the prevalence of depressive symptoms among college students is as high as 85%, highlighting the severity and urgency of this issue, which requires further attention and research (3).
The global burden of depression has significantly increased during the COVID-19 pandemic. According to the latest data, the number of severe depression cases worldwide has risen by 52.3 million, representing an increase of 27.6% (4). Across the 204 countries and regions involved, the incidence of severe depression has risen to 3,152.9 cases per 100,000 people (5). Stress factors related to the pandemic, including unemployment, the death of loved ones, social isolation, and the compound effects of multiple stressors, have had a severe negative effect on global mental health (6). In the United States, the prevalence of depressive symptoms during COVID-19 tripled compared to pre-pandemic levels (7).
With hundreds of thousands of new COVID-19 cases being reported daily, the pandemic has profound affected human diet, daily outdoor activities, and overall lifestyle habits (8). Public health measures implemented to control the pandemic, such as advising individuals to avoid crowded public places (e.g., playgrounds, basketball courts, and football fields), inevitably led to reduced opportunities for physical exercise. Existing research has clearly indicated a close link between the frequency of physical exercise and the incidence of anxiety and depressive symptoms (9).
As the number of new COVID-19 cases continues to rise, necessary control measures such as lockdowns and restrictions on population movement have been implemented to curb the spread of the pandemic. However, these measures may exacerbate mental stress due to the restriction of personal freedom (10). Studies suggest that an individual’s geographical proximity to disaster events and the degree of personal effect experienced during natural disasters or epidemics may affect post-event mental health recovery. Furthermore, the influence of economic and social changes on mental health may be delayed (11).
Although direct exposure to the COVID-19 virus does not appear to have a significant direct effect on mental health symptoms, the long-term increase in COVID-19-related occupational and social barriers may lead to significant changes in mental health, particularly due to social disruption, work-related obstacles, and economic difficulties (12). Therefore, from a public health perspective, understanding and identifying modifiable risk factors for depression is crucial for developing effective prevention and intervention strategies.
Depression is recognized as a complex mental disorder, generally resulting from the combined interaction of genetic and environmental factors (13). Moreover, lifestyle is a key determinant of its occurrence (14). As a vital element of health, sleep has recently garnered increased attention in academic research. Studies have indicated that sleep duration correlates significantly with overall mortality, cardiovascular diseases, and the prevalence of chronic conditions such as metabolic syndrome (15, 16). Insufficient sleep duration and poor quality are considered major contributors to mental health disorders. Consequently, the relationship between sleep, as a modifiable lifestyle factor, and depression has been examined in numerous studies (17, 18).
Researchers have extensively explored the role of sleep duration in the development of depression. A prospective study found that short and long sleep durations are significantly associated with an increased risk of depression in adults (19). This perspective is corroborated by subsequent research, which suggests that insufficient or excessive sleep may increase the incidence of depression (20). However, the data collection for these studies was completed before the outbreak of the COVID-19 pandemic.
Therefore, this study utilizes the latest China Family Panel Studies (CFPS) data, which were obtained during the COVID-19 pandemic. This dataset was selected because it best represents the characteristics of depression among Chinese residents at this stage. This study aims to explore the relationship between nap duration, nighttime sleep, and depression among residents and determine recommended sleep durations. This will provide scientific evidence for the prevention and treatment of depression.
2 Methods
2.1 Study population
The CFPS is a nationwide, comprehensive project that collects individual, family, and community data via biennial follow-up surveys to track changes in China’s socioeconomic, demographic, educational, and health fields. Data for this study were derived from the 2020 CFPS project. The project employed computer-assisted survey techniques to meet diverse design needs, improve survey efficiency, and ensure data quality. Samples with missing demographic variables or missing Center for Epidemiologic Studies Depression Scale (CESD) scores were excluded. Ultimately, 6,795 samples were included in this study.
2.2 Outcome
Nighttime sleep duration was assessed using the question: “Excluding nap duration, how many hours do you usually sleep each day on average, representing a typical workday and rest day?” Based on established benefits of 7–9 hours of sleep (21), participants were categorized into three types groups: short nighttime sleep (<7 hours), moderate nighttime sleep (7–9 hours; reference group), and long nighttime sleep (>9 hours).
Nap duration was assessed using the question: “How many minutes do you usually nap?” Participants were categorized into four groups: no nap (0 minute), short nap (<30 minutes), moderate nap (30–90 minutes), and long nap (>90 minutes). These cut-points were selected by referencing epidemiological literature on daytime napping (22).
2.3 Explanatory variable
Depression was measured using the CESD. This scale, developed by Radloff at the National Institute of Mental Health, has been widely used to assess depression levels (23). The CFPS 2020 used the eight-item version of the CESD, which includes items such as: “I felt depressed,” “I felt happy,” and “I felt that life was not worth living.” Responses were scored as 0 = rarely or never, 1 = not often, 2 = sometimes or half the time, and 3 = most of the time, with reverse-scored items transposed. The summed scores of the eight items ranged from 5 to 24. Higher total scores indicate a greater risk of depression, with scores of ≥9 indicating clinically significant depressive symptoms (24). The CESD-8 is recognized as an effective and reliable tool for assessing depressive symptoms in adults (25). The reliability coefficient for the CESD-8 scale in this study was examined, yielding a Cronbach’s alpha greater than 0.7, which indicates good internal consistency.
2.4 Other covariates
The analysis incorporated a set of potential confounding variables.
Sociodemographic data included age, gender (female or male), education level (illiterate, primary school, middle school, or high school and above), region (urban or rural), and marital status (married or single).
Lifestyle factors included smoking (yes or no), drinking (yes or no), and frequency of physical activity (0 times a week, 1–2 times a week, 3–4 times a week, 5–6 times a week, or every day).
Health status included health self-assessment (bad, fair, or good) and chronic disease (no or yes).
2.5 Statistical methods
Statistical analyses were performed using R 4.2 software. Qualitative data were summarized using frequencies and percentages (%), with group comparisons conducted using the χ2 test or Fisher’s exact test. Quantitative data conforming to a normal distribution were expressed as mean ± standard deviation (x ± s), and group comparisons utilized the t-test. Logistic regression models were constructed, employing nap duration and nighttime sleep as the reference groups. The variance inflation factor was used to assess multicollinearity among independent variables, and the Box–Tidwell tested the linear relationship between continuous independent variables and the logit of depression. Three models were constructed. Model 1 included only nighttime and nap duration, Model 2 controlled for demographic variables, and Model 3 included all covariates. Restricted cubic spline plots were generated to explore the dose–response relationship between participants’ sleep duration and depression. Stratified logistic regression analysis and interaction effect analysis determined whether the association between sleep duration and depression was dependent on baseline or demographic factors. P-values were used for two-sided tests, with P < 0.05 considered statistically significant.
Additional stratified linear regression analyses were performed to further examine the relationship between sleep duration and depressive symptoms across nap and nighttime sleep subgroups, as well as age categories. In these models, which used CESD scores as the continuous dependent variables, each was adjusted for age, gender, education level, and chronic disease status. A total of 10 stratified models were constructed, with subgroup-specific regression coefficients, confidence intervals, and P-values reported. These models were designed to isolate the independent effects of sleep behaviors while controlling for key demographic and health-related covariates.
The primary analysis initially treated depression status as a binary variable, utilizing logistic regression models. However, when stratified analyses were performed by nap and nighttime sleep groups, several models encountered convergence failures or perfect separation. This was attributable to limited sample sizes or imbalanced group distributions within those specific strata. To address these methodological issues while preserving subgroup distinctions, the CESD total scores were subsequently used as continuous dependent variables, and linear regression models were employed. This change enhanced model stability and enabled a more nuanced interpretation of the relationship between sleep behaviors and depression severity.
For the age-stratified analyses, participants were divided into three groups based on the actual sample age distribution and developmental relevance: young adults (<30 years), middle-aged adults (30–44 years), and older adults (≥45 years). This classification ensured sufficient sample size and developmental relevance across subgroups.
3 Results
3.1 Participant characteristics
The valid sample consisted of 6795 participants, of which 3411 were female (50.20%). The average age was 31.80 ± 8.67 years, and 1353 participants (19.9%) exhibited depressive symptoms. Differences in nap sleep time, nighttime sleep, region, level of education, health self-assessment, chronic disease, and frequency of physical activity were statistically significant (P<0.05). Specific basic characteristics are shown in Table 1.
3.2 Logistic regression of the relationship between sleep duration and depression
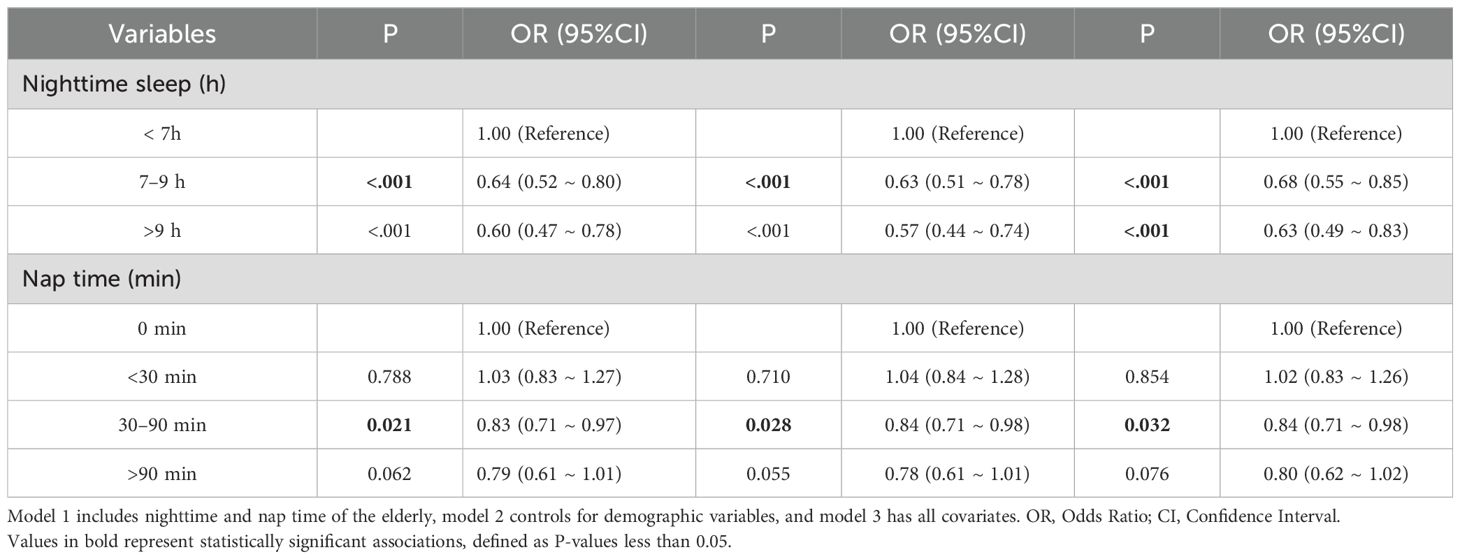
For the diagnosis of multicollinearity, we eliminated variables with VIF values greater than 10 to avoid multicollinearity. The Box-Tidwell test results showed that the continuous independent variables (nighttime sleep, nap sleep time) had a linear relationship with the dependent variable (depression) (P >0.05). The results of the logistic regression model analysis showed that, after controlling for all covariates in Model 3, participants with nighttime sleep ≥7 hours were found to have a protective factor against the likelihood of depression compared to those with nighttime sleep <7 hours. Compared to participants without a napping habit, those with nap times of 30–90 minutes were found to have a protective factor against the likelihood of depression (Table 2).
3.3 Dose–response relationship between nighttime sleep and depression
The dose-response relationship analysis between nighttime sleep and depression indicated a U-shaped relationship (P-nonlinear <0.001), with the likelihood of depression decreasing from 7.5 hours of sleep to about 8.5 hours (Figure 1). The lowest likelihood of depression was observed when participants had around 8.5 hours of sleep. The dose-response relationship analysis between nap sleep time and depression indicated no nonlinear relationship (P-nonlinear = 0.889) (Figure 1), also supported by the results in Table 2.

Figure 1. (A) Dose-response relationship between nighttime sleep and depression in older adults, (B) Dose-response relationship between nap time and depression in older adults. The x-axis represents sleep duration. The y-axis represents the OR values calculated by the model. The shaded area represents the 95% confidence interval (Overall trend test: P < 0.001; Nonlinear trend test: P < 0.001).
3.4 Subgroup analysis of the effect of sleep duration on depression
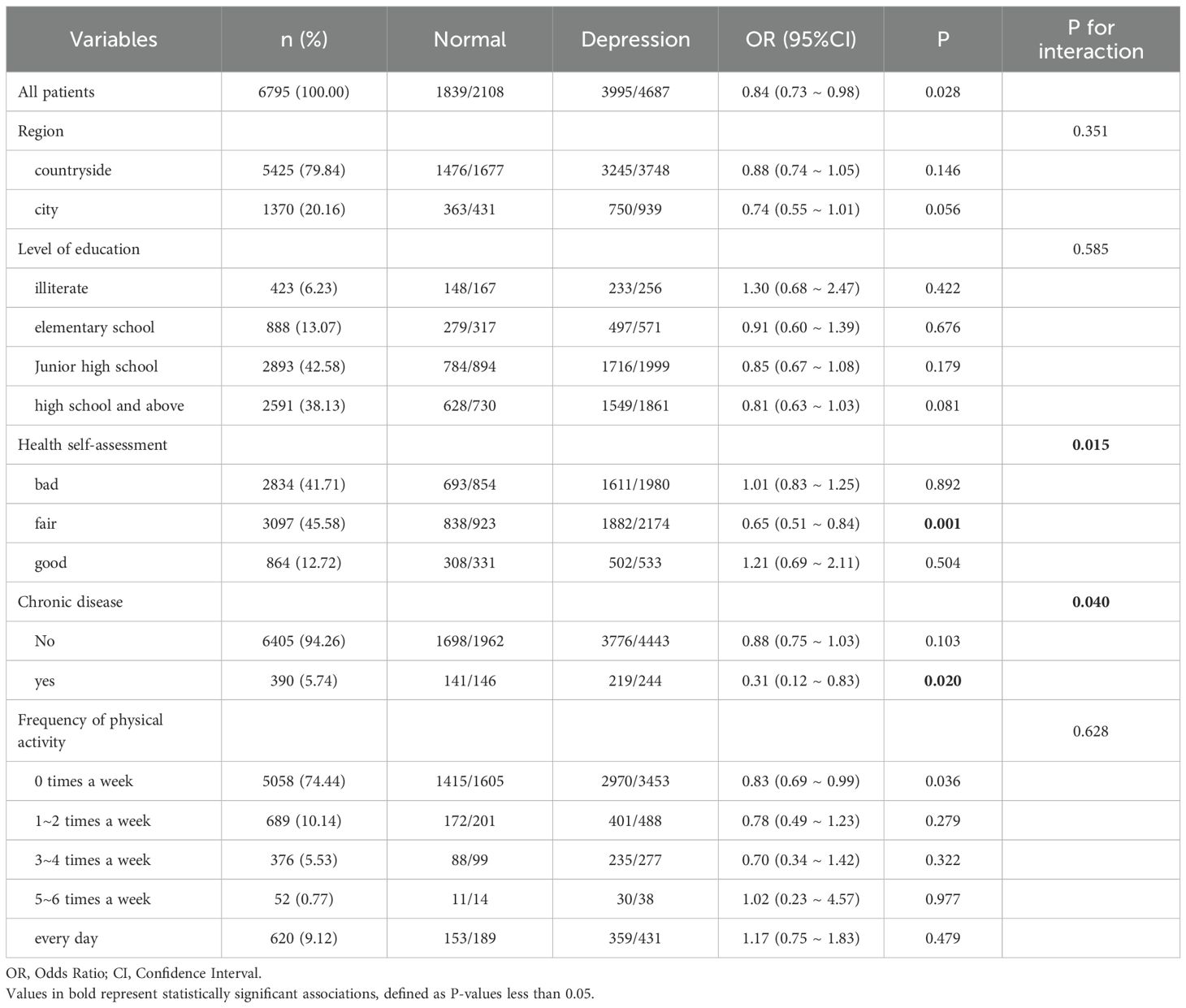
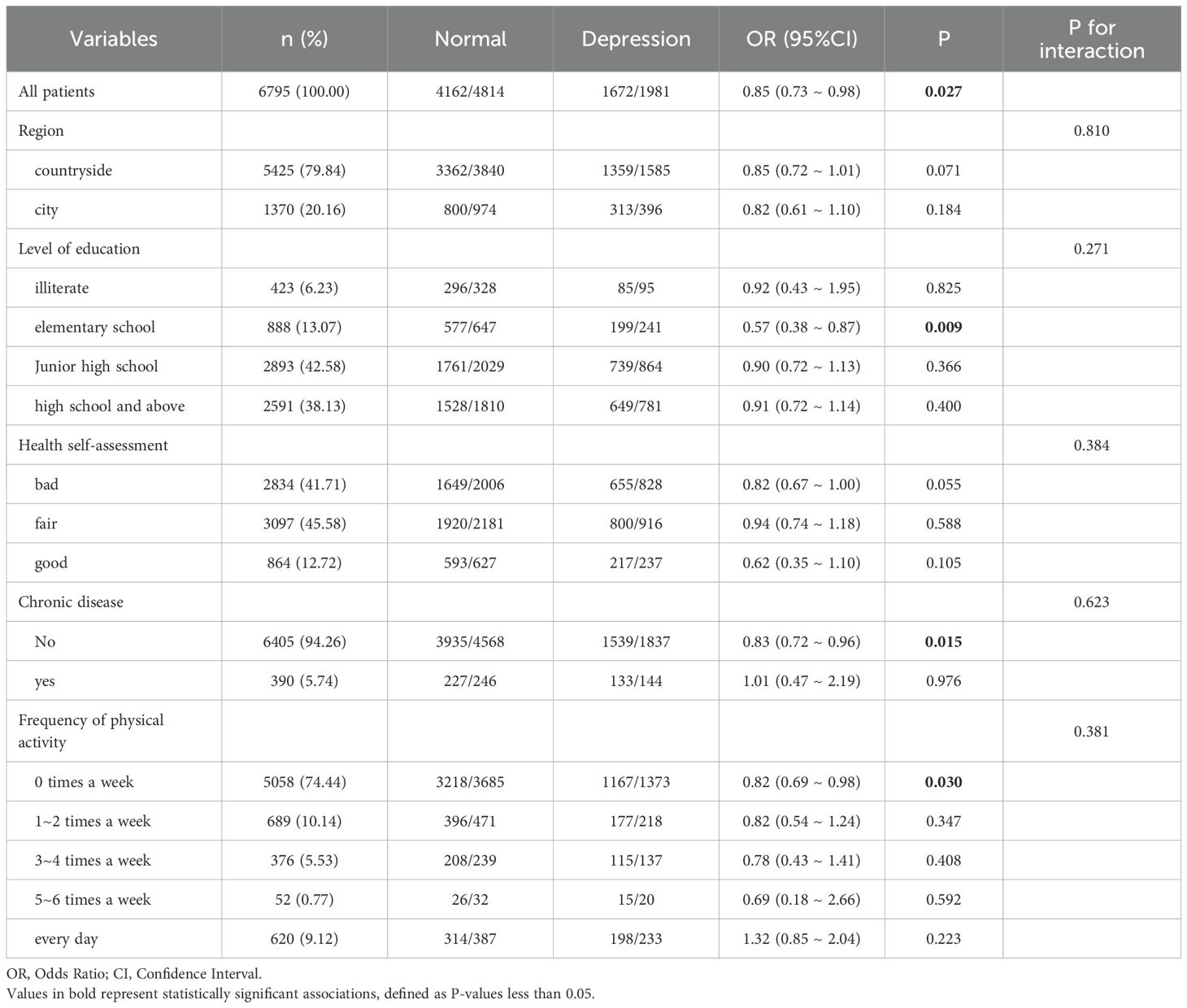
Stratified logistic regression analysis and interaction effect analysis were used to determine whether the association between sleep duration and depression depended on baseline or demographic factors. The association between nighttime sleep of 7–9 hours and depression demonstrated a significant interaction with health self-assessment and chronic disease (interaction P-values <0.05). This finding indicates that, for individuals with a general health self-assessment or chronic diseases, a 7–9 hour sleep duration may have a protective effect against the likelihood of depression. Nighttime sleep of 7–9 hours was thus only associated with the likelihood of depression in certain subgroups. These subgroup-specific associations are further supported by the logistic regression results shown in Table 3.
Conversely, the association between a nap duration of 30–90 minutes and depression showed no significant interaction with region, education level, health self-assessment, chronic disease status, or frequency of physical activity (interaction P-values >0.05). This suggests that the effect of nap duration on depression did not differ across these baseline or demographic factors; the association between appropriate nap duration and depression risk was consistent across all stratified subgroups, as shown in Table 4.
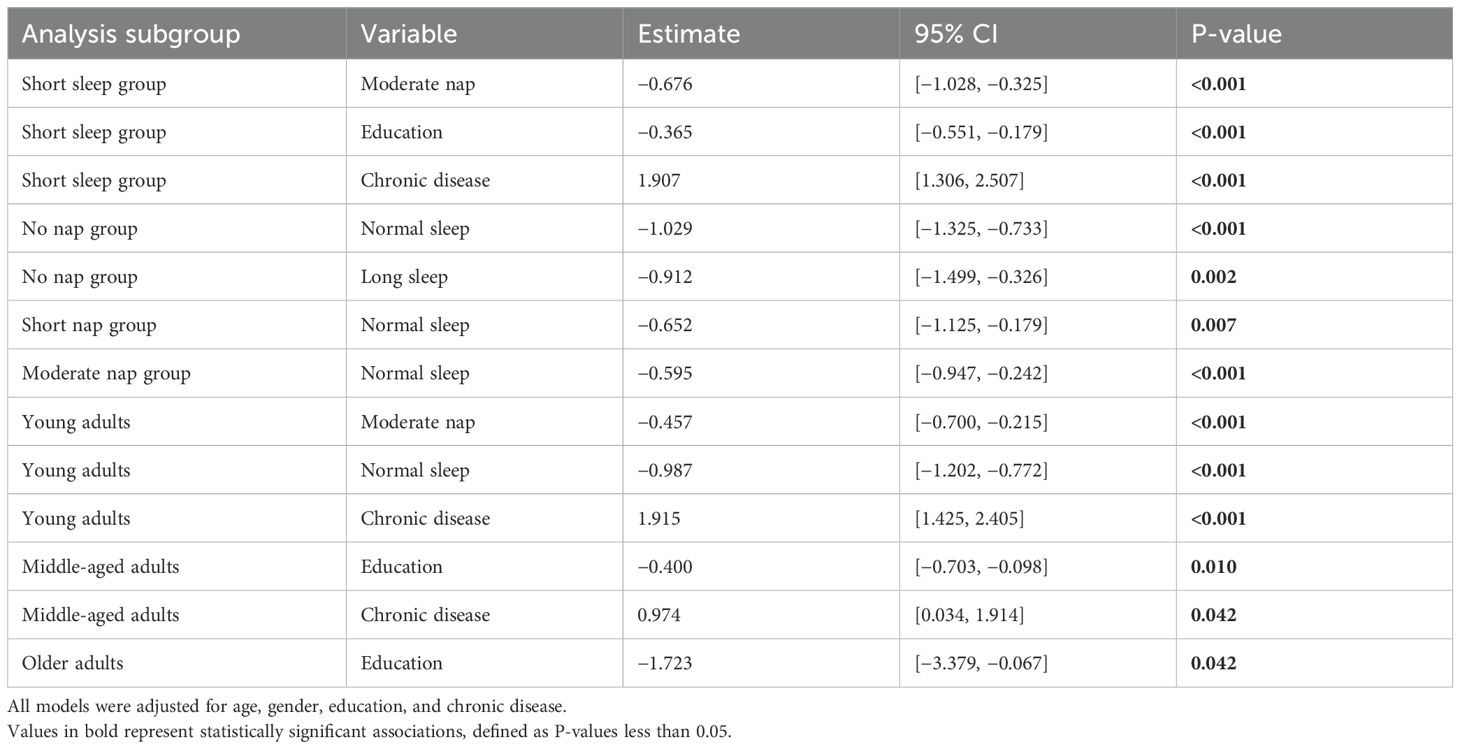
To gain a deeper understanding of the relationship between sleep duration and depressive symptoms, 10 stratified linear regression models were constructed. These models spanned various nap and nighttime sleep subgroups, in addition to age categories. CESD scores were utilized as the dependent variable in all models, which were adjusted for age, gender, education level, and chronic disease status.
Significant associations emerged in multiple subgroups: Moderate nap duration was consistently associated with lower CESD scores in the short nighttime sleep group (β = −0.676, 95% CI: −1.028 to −0.325, p < 0.001) and among young adults (β = −0.457, 95% CI: −0.700 to −0.215, p < 0.001). Nighttime sleep duration showed protective effects in the no-nap group, with normal (β = −1.029, 95% CI: −1.325 to −0.733, p < 0.001) and long sleep durations (β = −0.912, 95% CI: −1.499 to −0.326, p = 0.002) linked to lower depression scores.
Education level was identified as a robust protective factor across multiple subgroups, including short nap (β = −0.395, p < 0.001), short nighttime sleep (β = −0.365, p < 0.001), middle-aged adults (β = −0.400, p = 0.010), and older adults (β = −1.723, p = 0.042). Conversely, chronic disease was positively associated with depressive symptoms in nearly all subgroups, with the strongest effect observed in the short nighttime sleep group (β = 1.907, 95% CI: 1.306 to 2.507, p < 0.001). The full results of the stratified linear regression models are presented in Table 5.
4 Discussion
This study revealed a U-shaped dose–response relationship between nighttime sleep and depressive symptoms. Specifically, the probability of depressive symptoms significantly decreased when nighttime sleep increased from 7.5 hours to 8.5 hours. This result aligns with a cross-sectional study involving 1,788 American adults (aged 19–89 years), which also showed a U-shaped association between sleep duration and depressive symptoms (26). Compared to those with nighttime sleep of less than 6 hours, individuals with sufficient nighttime sleep (not less than 6 hours) had a lower risk of depression. Furthermore, research conducted in southwestern China, involving 44,900 Han adults aged 30–79 years, similarly found that participants with nighttime sleep less than 7 hours had a higher odds ratio for depression (OR: 1.47, 95% CI: 1.31–1.65) compared to those with 7–8 hours of sleep. However, this Chinese study reported no significant association between nighttime sleep exceeding 9 hours and depressive symptoms in either the overall population or subgroup analyses (27)—a finding supported by other literature that suggests no clear link between long sleep duration and depression (28). Conversely, a meta-analysis of seven prospective studies indicated a significant association between short sleep duration and depression risk, with a risk ratio of 1.31 compared to normal sleep duration (29). Some cross-sectional studies have also identified associations between short (≤6 hours) and long sleep durations (≥9 hours) and an increased risk of depressive symptoms (30).
The observed inconsistency regarding the relationship between nighttime sleep and depressive symptoms across various studies may be attributed to several factors. (1) Differences in the methods used to adjust for potential confounding factors may introduce biased results. (2) Moreover, varied definition of sleep duration across studies may compromise the comparability of findings. (3) Differences in the age distribution of study subjects may also influence the observed relationship between sleep duration and depressive symptoms. (4) Furthermore, the relatively low proportion of participants with long sleep duration (14.94%) may result in insufficient statistical power to accurately assess the relationship between long sleep duration and depressive symptoms. (5) In addition, a potential bidirectional relationship between depression and sleep may cause differences between results from prospective cohort studies and cross-sectional studies. (6) Finally, urban–rural differences and socioeconomic status (SES) disparities may significantly affect the pathogenesis of depression. Given that sleep and depression are associated with specific health behaviors and SES, these factors may confound the sleep–depression association [e.g., long sleep duration is associated with lower SES, reduced physical activity, and obesity, all of which are risk factors for depression (31)]. Therefore, these potential confounding variables must be carefully considered when interpreting the relationship between sleep duration and depressive symptoms.
This study also found that participants with a nap duration of 30–90 minutes exhibited a lower likelihood of depression, which supports the potential role of napping in mood regulation. Previous studies have similarly indicated that short daytime naps are not significantly associated with depressive symptoms; however, excessively long nap duration have been identified as an independent risk factor for depression (32). Specifically, compared to moderate nappers, long nappers had a significantly higher incidence of depressive symptoms (OR = 1.32, 95% CI: 1.19–1.45) (33). This finding underscores the importance of nap duration in mental health and suggests that an appropriate nap duration may have a protective effect on emotional health.
Several possible explanatory mechanisms link sleep status and depressive symptoms. First, insufficient sleep may lead to daytime fatigue, subsequently causing excessive daytime sleepiness and the disruption of circadian rhythms, all of which may increase the risk of depressive symptoms. Individuals with shorter sleep durations may experience insufficient rest and increased perceived stress—an established risk factor for depressive symptoms (34). Second, inflammation is a key correlate of depression. Studies have shown that short sleep duration and insomnia symptoms are associated with elevated levels of inflammatory cytokines, such as C-reactive protein and interleukin-6 (35). Increasing evidence indicates that short and long sleep durations are associated with elevated levels of inflammation, a phenomenon particularly evident in individuals with depression (36). Chronic low-grade inflammation may therefore serve as an important biological pathway linking sleep and depressive symptoms. Notably, inflammation is not only associated with depression and stress disorders but also plays a crucial role in their pathophysiology (37). Increased inflammation may further promote the activation of the kynurenine pathway, resulting in reduced serotonin levels, which is closely related to the pathophysiological mechanisms of depression (38).
Additionally, sleep quality and duration may influence the composition of the gut microbiota (39). The microbiota–gut–brain axis is an interactive system implicated in various neurological diseases (40). Studies have shown that sleep disorders may alter the levels of brain-derived neurotrophic factor, which plays a key role in the pathophysiology of stress-related mood disorders (41). The hypothalamic–pituitary–adrenal (HPA) axis serves as an important pathway for understanding the neurobiology of stress responses (42). Activation of the HPA axis occurs when corticotropin-releasing hormone, released by the hypothalamus, stimulates the anterior pituitary to secrete adrenocorticotropic hormone, which, in turn, promotes the adrenal cortex to release cortisol into the bloodstream (43). Conversely, a decline in sleep quality may enhance the stress reactivity of the HPA axis.
Furthermore, adequate sleep helps in elevating melatonin levels, a molecule with multifaceted regulatory functions that can help alleviate depressive symptoms. Finally, research connecting sleep and the immune system suggests that sleep can optimize immune defense, and immune cell signaling may induce sleep. Immune activation and cytokines may play a role in the occurrence of depressive symptoms in certain individuals. These findings collectively underscore the important role of sleep in maintaining mental health and preventing depressive symptoms.
The study conducted a subgroup analysis of the sleep–depression relationship between to explore whether this association is influenced by baseline or demographic factors. Stratified logistic regression analysis and interaction effect analysis revealed a significant interaction between nighttime sleep of 7–9 hours and depression, particularly related to health self-assessment and chronic disease status. Specifically, for individuals with general health self-assessment or chronic diseases, maintaining 7–9 hours of nighttime sleep may have a protective effect, reducing the risk of depression. This result aligns with previous quantile regression studies, including 55,954 adults from two national surveys, which found that the association between short sleep duration and depression was stronger in individuals with chronic diseases (44). Thus, the association between short sleep duration and depression appears to be modified by chronic diseases. Sleep problems, chronic diseases, and depression are interrelated, with each increasing the risk of occurrence and worsening of the others. A review further indicated that alterations in mesolimbic dopaminergic function are neurobiological factors associated with this triad (45). However, the exact nature of these associations remains to be fully elucidated. Further research is necessary to clarify the underlying physiological mechanisms connecting sleep, depression, and chronic diseases. This finding suggests that the role of sleep duration in preventing depression may vary depending on an individual’s health status, emphasizing the importance of considering individual differences in clinical practice. The significant association between 7–9 hours of nighttime sleep and the likelihood of depression was observed only in certain subgroups, indicating that the effect of sleep on mental health is not universally applicable but is influenced by specific demographic characteristics. Therefore, future research and intervention strategies must consider the particularities of these subgroups to prevent and treat depression more effectively.
In addition to subgroup analyses by health status and nap duration, this study also performed stratified linear regression analyses across three age groups: young adults (<30 years), middle-aged adults (30–44 years), and older adults (≥45 years). The results demonstrated that the association between sleep duration and depressive symptoms varied significantly across age groups. Specifically, moderate nap duration (30–90 minutes) and normal nighttime sleep (7–9 hours) were strongly linked to lower CESD scores in young adults, whereas chronic disease status and education level showed a stronger association with depression in middle-aged and older adults.
These findings are consistent with prior research. A narrative review concluded that short and long sleep durations are risk factors for depression in older adults, often co-occurring with chronic diseases and cognitive decline (46). These age-related vulnerabilities may explain why sleep optimization shows stronger protective effects in younger populations, whereas the depressive symptoms of older adults are more influenced by comorbid conditions.
Age-related physiological changes, lifestyle patterns, and stress exposures likely contribute to these differences. For example, younger individuals often experience greater circadian instability, academic and occupational stress, and social irregularities, making them more sensitive to sleep deprivation and mood fluctuations. By contrast, older adults may suffer from fragmented sleep, reduced sleep efficiency, and a higher prevalence of chronic conditions, such as cardiovascular diseases, diabetes, and neurodegeneration, which can exacerbate depressive symptoms independently of sleep duration.
Therefore, sleep interventions should be tailored to developmental stages. Younger individuals may benefit more directly from behavioral sleep optimization, such as consistent sleep schedules and sleep hygiene education. By contrast, older adults likely require integrated approaches addressing sleep, chronic disease, and cognitive health simultaneously. These findings underscore the importance of age-specific strategies in clinical practice and public health planning. They also suggest that future research should further explore biological mechanisms—such as inflammation, HPA axis dysregulation, and neuroendocrine changes—that may mediate the sleep–depression relationship across the lifespan.
Conversely, this study demonstrated that the association between nap duration of 30–90 minutes and the risk of depression showed no interaction across subgroups defined by region, education level, health self-assessment, chronic disease status, and frequency of physical activity. This suggests that the beneficial effect of appropriate nap duration on reducing the risk of depression is consistent across different demographic characteristics and is not influenced by these factors. This finding supports the notion of napping as a universally beneficial health behavior and provides a theoretical basis for future sleep interventions. Overall, these findings offer new directions for future research, suggesting that sleep interventions must consider individual differences in health status and sleep habits. In addition, public health strategies should promote appropriate sleep habits to reduce the incidence of depression.
Despite providing valuable insights, this study has several limitations. First, due to the cross-sectional nature of the study design, the causal relationship between sleep duration and depressive symptoms cannot be determined; longitudinal designs are necessary to explore the temporal relationship. Second, the study’s reliance on self-reported sleep data introduces the risk of subjective bias. Future research should consider incorporating objective sleep monitoring techniques, such as polysomnography or wearable devices, to improve data accuracy. In addition, this study did not account for individual differences, such as genetic factors, life events, and personality traits, all of which may influence the sleep–depression relationship. Future research should therefore explore these potential mediating or moderating variables.
5 Conclusion
This study established a complex and nonlinear relationship between sleep duration and depressive symptoms among Chinese residents. A U-shaped dose–response pattern was observed for nighttime sleep, where a duration of 7–9 hours was associated with the lowest likelihood of depression. Furthermore, a nap duration of 30–90 minutes consistently exhibited a protective effect across all analyzed demographic and health subgroups. Importantly, stratified analyses revealed that the mental health benefits of sleep behaviors are age-dependent: younger adults experienced stronger protective effects from optimal sleep, whereas the presence of chronic disease and education level were more influential determinants of depression in middle-aged and older adults. These findings underscore the critical need for age-sensitive and health-specific sleep recommendations in mental health promotion. Public health strategies must prioritize nighttime sleep and nap hygiene, specifically tailoring interventions to individual characteristics to prevent depression more effectively.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.isss.pku.edu.cn/cfps/sjzx/gksj/index.htm.
Ethics statement
The CFPS project was approved by the Biomedical Ethics Committee of Peking University, approval number: IRB00001052-14010. Additionally, before the survey began, researchers first obtained informed consent from the subjects in written form. Therefore, at the start of this study, all data were completely anonymous. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YS: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Writing – original draft. YL: Methodology, Project administration, Resources, Software, Writing – review & editing. LL: Software, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the National Social Science Foundation of China (Youth Project No. 25CTY036).
Acknowledgments
The authors would like to thank China Social Science Survey Center (ISSS) at Peking University for providing the CFPS data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. World Health Organization. Depressive disorder (depression) (2023). Available online at: https://www.who.int/news-room/fact-sheets/detail/depression (Accessed January 5, 2024).
2. Bayram N and Bilgel N. The prevalence and socio-demographic correlations of depression, anxiety and stress among a group of university students. Soc Psychiatry Psychiatr Epidemiol. (2008) 43:667–72. doi: 10.1007/s00127-008-0345-x
3. Garlow SJ, Rosenberg J, Moore JD, Haas AP, Koestner B, Hendin H, et al. Depression, desperation, and suicidal ideation in college students: results from the American Foundation for Suicide Prevention College Screening Project at Emory University. Depress Anxiety. (2008) 25:482–8. doi: 10.1002/da.20321
4. GBD 2019 Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. (2022) 9:137–50. doi: 10.1016/S2215-0366(21)00395-3
5. COVID-19 Mental Disorders Collaborators. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet. (2021) 398:1700–12. doi: 10.1016/S0140-6736(21)02143-7
6. Fernández-Alcántara M, Kokou-Kpolou CK, Cruz-Quintana F, and Pérez-Marfil MN. Editorial: new perspectives in bereavement and loss: complicated and disenfranchised grief along the life cycle. Front Psychol. (2021) 12:691464. doi: 10.3389/fpsyg.2021.691464
7. Ettman CK, Abdalla SM, Cohen GH, Sampson L, Vivier PM, and Galea S. Prevalence of depression symptoms in US adults before and during the COVID-19 pandemic. JAMA Netw Open. (2020) 3:e2019686. doi: 10.1001/jamanetworkopen.2020.19686
8. Romero-Blanco C, Rodríguez-Almagro J, Onieva-Zafra MD, Parra-Fernández ML, Prado-Laguna MDC, and Hernández-Martínez A. Physical activity and sedentary lifestyle in university students: changes during confinement due to the COVID-19 pandemic. Int J Environ Res Public Health. (2020) 17:6567. doi: 10.3390/ijerph17186567
9. Ogawa S, Kitagawa Y, Fukushima M, Yonehara H, Nishida A, Togo F, et al. Interactive effect of sleep duration and physical activity on anxiety/depression in adolescents. Psychiatry Res. (2019) 273:456–60. doi: 10.1016/j.psychres.2018.12.085
10. Pierce M, Hope H, Ford T, Hatch S, Hotopf M, John A, et al. Mental health before and during the COVID-19 pandemic: a longitudinal probability sample survey of the UK population. Lancet Psychiatry. (2020) 7:883–92. doi: 10.1016/S2215-0366(20)30308-4
11. Jeong H, Yim HW, Song YJ, Ki M, Min JA, Cho J, et al. Mental health status of people isolated due to Middle East Respiratory Syndrome. Epidemiol Health. (2016) 38:e2016048. doi: 10.4178/epih.e2016048
12. Batterham PJ, Calear AL, McCallum SM, Morse AR, Banfield M, Farrer LM, et al. Trajectories of depression and anxiety symptoms during the COVID-19 pandemic in a representative Australian adult cohort. Med J Aust. (2021) 214:462–8. doi: 10.5694/mja2.51043
13. Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M, et al. Major depressive disorder. Nat Rev Dis Primers. (2016) 2:16065. doi: 10.1038/nrdp.2016.65
14. Opie RS, Itsiopoulos C, Parletta N, Sanchez-Villegas A, Akbaraly TN, Ruusunen A, et al. Dietary recommendations for the prevention of depression. Nutr Neurosci. (2017) 20:161–71. doi: 10.1179/1476830515Y.0000000043
15. Liu TZ, Xu C, Rota M, Cai H, Zhang C, Shi MJ, et al. Sleep duration and risk of all-cause mortality: A flexible, non-linear, meta-regression of 40 prospective cohort studies. Sleep Med Rev. (2017) 32:28–36. doi: 10.1016/j.smrv.2016.02.005
16. Smiley A, King D, and Bidulescu A. The association between sleep duration and metabolic syndrome: the NHANES 2013/2014. Nutrients. (2019) 11:2582. doi: 10.3390/nu11112582
17. Dong L, Xie Y, and Zou X. Association between sleep duration and depression in US adults: A cross-sectional study. J Affect Disord. (2022) 296:183–8. doi: 10.1016/j.jad.2021.09.075
18. Jing R, Xu T, Rong H, Lai X, and Fang H. Longitudinal association between sleep duration and depressive symptoms in Chinese elderly. Nat Sci Sleep. (2020) 12:737–47. doi: 10.2147/NSS.S269992
19. Vanden Eng JL, Chan A, Abílio AP, Wolkon A, Ponce de Leon G, Gimnig J, et al. Bed net durability assessments: exploring a composite measure of net damage. PloS One. (2015) 10:e0128499. doi: 10.1371/journal.pone.0128499
20. Sun Y, Shi L, Bao Y, Sun Y, Shi J, and Lu L. The bidirectional relationship between sleep duration and depression in community-dwelling middle-aged and elderly individuals: evidence from a longitudinal study. Sleep Med. (2018) 52:221–9. doi: 10.1016/j.sleep.2018.03.011
21. Chaput JP, Dutil C, and Sampasa-Kanyinga H. Sleeping hours: what is the ideal number and how does age impact this? Nat Sci Sleep. (2018) 10:421–30. doi: 10.2147/NSS.S163071
22. Li J, Cacchione PZ, Hodgson N, Riegel B, Keenan BT, Scharf MT, et al. Afternoon napping and cognition in chinese older adults: findings from the China health and retirement longitudinal study baseline assessment. J Am Geriatr Soc. (2017) 65:373–80. doi: 10.1111/jgs.14368
23. Radloff LS. The CES-D scale A self-report depression scale for research in the general population. Appl psychol Measurement. (1977) 1:385–401. doi: 10.1177/014662167700100306
24. Wu Y, Su B, Chen C, Zhao Y, Zhong P, and Zheng X. Urban-rural disparities in the prevalence and trends of depressive symptoms among Chinese elderly and their associated factors. J Affect Disord. (2023) 340:258–68. doi: 10.1016/j.jad.2023.07.117
25. Briggs R, Carey D, O’Halloran AM, Kenny RA, and Kennelly SP. Validation of the 8-item Centre for Epidemiological Studies Depression Scale in a cohort of community-dwelling older people: data from The Irish Longitudinal Study on Ageing (TILDA). Eur Geriatr Med. (2018) 9:121–6. doi: 10.1007/s41999-017-0016-0
26. Watson NF, Harden KP, Buchwald D, Vitiello MV, Pack AI, Strachan E, et al. Sleep duration and depressive symptoms: a gene-environment interaction. Sleep. (2014) 37:351–8. doi: 10.5665/sleep.3412
27. Gao Y, Tang W, Mao D, Chen L, and Ding X. Association between Nocturnal Sleep Duration and Insomnia symptoms with depressive symptoms among 44,900 Chinese Han adults aged 30–79 in Southwest China. BMC Psychiatry. (2023) 23:127. doi: 10.1186/s12888-023-04601-6
28. Paudel M, Taylor BC, Ancoli-Israel S, Blackwell T, Maglione JE, Stone K, et al. Sleep disturbances and risk of depression in older men. Sleep. (2013) 36:1033–40. doi: 10.5665/sleep.2804
29. Zhai L, Zhang H, and Zhang D. Sleep duration and depression among adults: A meta-analysis of prospective studies. Depress Anxiety. (2015) 32:664–70. doi: 10.1002/da.22386
30. Sun X, Zheng B, Lv J, Guo Y, Bian Z, Yang L, et al. Sleep behavior and depression: Findings from the China Kadoorie Biobank of 0.5 million Chinese adults. J Affect Disord. (2018) 229:120–4. doi: 10.1016/j.jad.2017.12.058
31. Whinnery J, Jackson N, Rattanaumpawan P, and Grandner MA. Short and long sleep duration associated with race/ethnicity, sociodemographics, and socioeconomic position. Sleep. (2014) 37:601–11. doi: 10.5665/sleep.3508
32. Zhang X, Li G, Shi C, and Sun Y. Associations of sleep duration, daytime napping, and snoring with depression in rural China: a cross-sectional study. BMC Public Health. (2023) 23:1530. doi: 10.1186/s12889-023-16479-w
33. Li Y, Wu Y, Zhai L, Wang T, Sun Y, and Zhang D. Longitudinal association of sleep duration with depressive symptoms among middle-aged and older Chinese. Sci Rep. (2017) 7:11794. doi: 10.1038/s41598-017-12182-0
34. Racic M, Todorovic R, Ivkovic N, Masic S, Joksimovic B, and Kulic M. Self- perceived stress in relation to anxiety, depression and health-related quality of life among health professions students: A cross-sectional study from Bosnia and Herzegovina. Zdr Varst. (2017) 56:251–9. doi: 10.1515/sjph-2017-0034
35. Kim S and Yoon H. Volunteering, subjective sleep quality, and chronic inflammation: A 5-year follow-up of the national social life, health, and aging project. Res Aging. (2020) 42:291–9. doi: 10.1177/0164027520922624
36. Prather AA, Vogelzangs N, and Penninx BW. Sleep duration, insomnia, and markers of systemic inflammation: results from the Netherlands Study of Depression and Anxiety (NESDA). J Psychiatr Res. (2015) 60:95–102. doi: 10.1016/j.jpsychires.2014.09.018
37. Osimo EF, Baxter LJ, Lewis G, Jones PB, and Khandaker GM. Prevalence of low-grade inflammation in depression: a systematic review and meta-analysis of CRP levels. Psychol Med. (2019) 49:1958–70. doi: 10.1017/S0033291719001454
38. Haroon E, Welle JR, Woolwine BJ, Goldsmith DR, Baer W, Patel T, et al. Associations among peripheral and central kynurenine pathway metabolites and inflammation in depression. Neuropsychopharmacology. (2020) 45:998–1007. doi: 10.1038/s41386-020-0607-1
39. Matenchuk BA, Mandhane PJ, and Kozyrskyj AL. Sleep, circadian rhythm, and gut microbiota. Sleep Med Rev. (2020) 53:101340. doi: 10.1016/j.smrv.2020.101340
40. Kelly JR, Keane VO, Cryan JF, Clarke G, and Dinan TG. Mood and microbes: gut to brain communication in depression. Gastroenterol Clin North Am. (2019) 48:389–405. doi: 10.1016/j.gtc.2019.04.006
41. Rahmani M, Rahmani F, and Rezaei N. The brain-derived neurotrophic factor: missing link between sleep deprivation, insomnia, and depression. Neurochem Res. (2020) 45:221–31. doi: 10.1007/s11064-019-02914-1
42. Sapolsky RM, Romero LM, and Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. (2000) 21:55–89. doi: 10.1210/edrv.21.1.0389
43. van Dalfsen JH and Markus CR. The influence of sleep on human hypothalamic-pituitary-adrenal (HPA) axis reactivity: A systematic review. Sleep Med Rev. (2018) 39:187–94. doi: 10.1016/j.smrv.2017.10.002
44. Pan L, Huang C, Liu Y, Peng J, Lin R, Yu Y, et al. Quantile regression to explore association of sleep duration with depression among adults in NHANES and KNHANES. J Affect Disord. (2024) 345:244–51. doi: 10.1016/j.jad.2023.10.126
45. Finan PH and Smith MT. The comorbidity of insomnia, chronic pain, and depression: dopamine as a putative mechanism. Sleep Med Rev. (2013) 17:173–83. doi: 10.1016/j.smrv.2012.03.003
Keywords: COVID-19 pandemic, depression, nap time, nighttime sleep, health promotion
Citation: Song Y, Liu Y and Liu L (2025) Nonlinear associations of nighttime sleep and protective napping with depressive symptoms: a cross-sectional analysis from the China Family Panel Studies. Front. Psychiatry 16:1680714. doi: 10.3389/fpsyt.2025.1680714
Received: 06 August 2025; Accepted: 06 October 2025;
Published: 24 October 2025.
Edited by:
Elke Humer, University of Continuing Education Krems, AustriaReviewed by:
Arcady A. Putilov, Institute of Higher Nervous Activity and Neurophysiology (RAS), RussiaMuhammad Aqeel, Foundation University Rawalpindi, Pakistan
Copyright © 2025 Song, Liu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Liu, bGl1bGlAc3VzLmVkdS5jbg==
†ORCID: Yanliqing Song, orcid.org/0009-0000-3725-714X
Li Liu, orcid.org/0009-0006-1153-4675
 Yanliqing Song
Yanliqing Song Yue Liu
Yue Liu Li Liu
Li Liu