- 1Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden
- 2Department of Psychiatry, Icahn School of Medicine at Mount Sinai, New York, NY, United States
- 3Seaver Center for Autism Research and Treatment, Icahn School of Medicine at Mount Sinai, New York, NY, United States
- 4Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, United States
Objective: Postpartum depression (PPD) reportedly affects up to 20% of new mothers. While parental psychiatric history has been associated with an increased likelihood of neurodevelopmental conditions in the offspring, only a few studies of clinically diagnosed PPD exist exploring associated autism spectrum disorder (ASD) outcomes and no study to date has explored the contributions of paternal PPD with ASD risk or the combined influence.
Methods: A nationwide prospective cohort of all live births in Sweden from 1997 through 2021, followed up through December 31, 2022. Associations between parental PPD and ASD were quantified by hazard ratios and two-sided 95% confidence intervals (CIs) from Cox regressions.
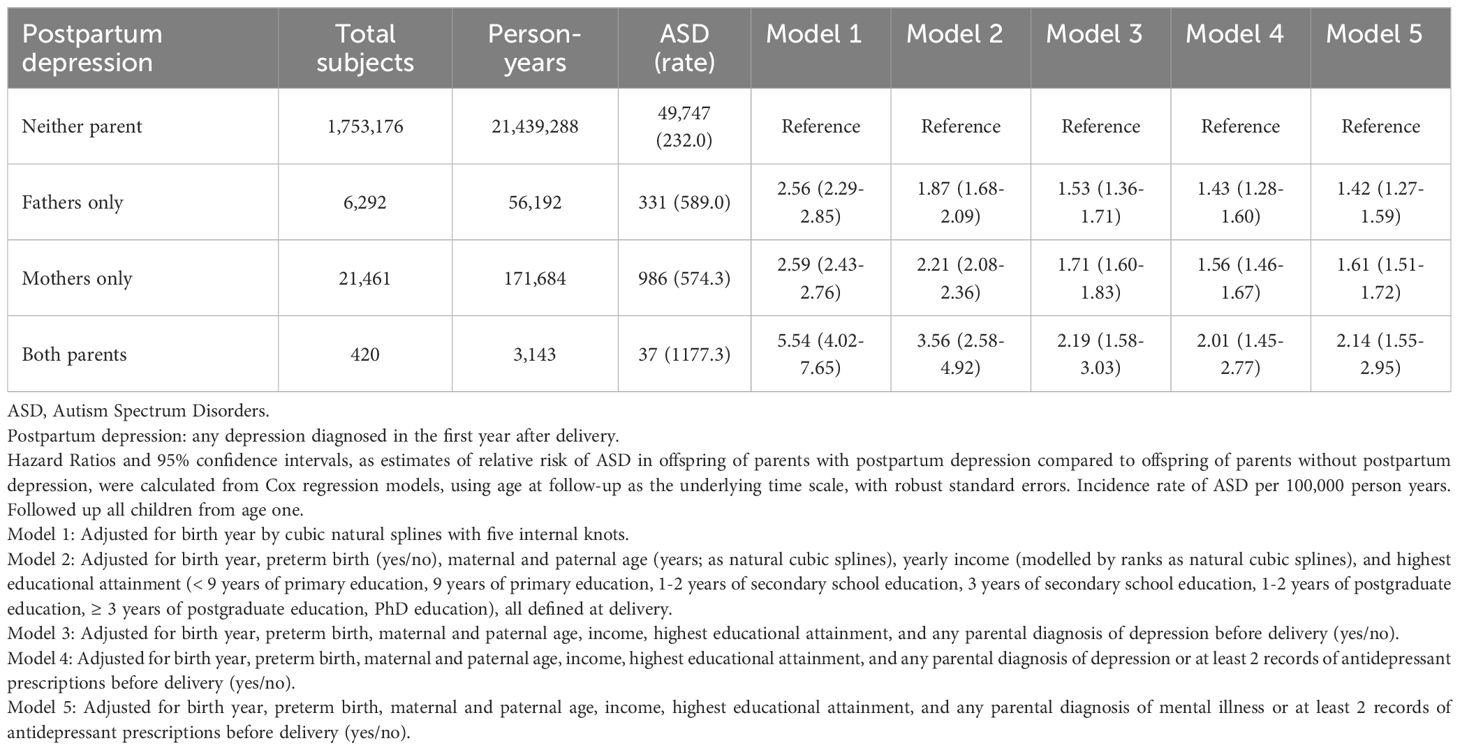
Results: Among 1,781,349 live-births, ASD was diagnosed in 986 (4.6%) children of 21,461 born to mothers with PPD (574.3 per 100,000 person-years), 331 (5.3%) of 6,292 born to fathers with PPD (589.0 per 100,000 person-years), and 37 (8.8%) of 420 when both parents had PPD (1177.3 per 100,000 person-years). The hazard ratio of ASD when the mother was diagnosed with PPD was 2.56[CI:2.29-2.85], for fathers 2.59[CI:2.43-2.76] and both 5.54[CI:4.02-7.65]. Adjustment for possible confounders and depression history provided similar trends (mother 1.53[CI:1.36-1.71], fathers 1.71[CI:1.60-1.83] and both 2.19[CI:1.58-3.03]).
Conclusion: Parental PPD was associated with an increased risk of ASD in the offspring, and this association was partially, though not fully, explained by depression history, antidepressant use, and other parental psychiatric factors. The magnitude of the association increased comparably when either parent was diagnosed with PPD and increased further when both parents were diagnosed, with a pattern indicative of shared genetic influences.
Introduction
Postpartum depression (PPD) reportedly affects 7-20% of new mothers depending largely on ascertainment methods (1). Characterized as a perinatal onset specifier of a unipolar major depressive disorder (2, 3), PPD is unusual among psychiatric disorders in that it is conditionally based on the timing of onset (after childbirth) as opposed to having unique symptomatology. This distinction has led some investigators to question whether PPD is unique compared to other major depressive disorders (4). Indeed, although traditionally attributed to biological factors, genetic, endocrinologic, or immunologic determinants associated with reproduction (5, 6), explorations into the genetic nature of PPD have revealed congruent underpinnings to other unipolar depressive disorders with respect to risk factors (7) and prevalence (8).
Despite significant public health importance, research on PPD and the negative consequences on offspring outcomes (9, 10) has often included important methodological limitations, leaving aspects of the disorder and its impact incompletely characterized. Previous studies of PPD, for example, have primarily relied on symptom inventories rather than clinical diagnoses (11), leading to the overestimation of prevalence (12) and confounding correlations among offspring outcomes. Conversely, studies exploring clinically diagnosed PPD have generally depended on convenience rather than epidemiological samples, which are prone to sampling bias and for which outcomes are not normalizable (13). Finally, depression is understood to be a recurrent disorder, and unsurprisingly large population-based studies (14) and a recent meta-analysis (15) demonstrate that an individual’s depression history explains almost all PPD risk. Yet, most studies of PPD have either ignored or been unable to validate psychiatric history, relying instead on verbal reports, which are prone to bias and recall error thereby making it difficult to quantify the impact of prior psychiatric vulnerabilities. These methodologic limitations have made interpreting the impact of PPD on offspring outcomes challenging. What is therefore required is a systematic and prospective, large-scale, nationally inclusive epidemiological study that examines the impact of clinically diagnosed PPD on childhood outcomes using a complete depression history of both parents as well as outcome measures with complete offspring coverage.
Autism spectrum disorder (ASD) is a chronic neurodevelopmental disorder with high heritability that is generally believed to develop in utero secondary to genetic and environmental factors (16). Heterogenous in nature, ASD is defined by deficits in communication and learning, poor social reciprocity, and behavioral abnormalities. Although symptoms of ASD often appear in infancy, ASD is generally not diagnosed before the first two or three years of life (17) and while the causal factors for ASD remain mostly unknown, heredity explains a considerable amount of risk (18). Notably, as ASD has been shown to share genetic etiology with several psychiatric disorders (19, 20), it is unsurprising that studies exploring the association between parental psychiatric conditions whether during gestation (21) or lifetime history (22) and ASD offspring outcomes have observable relationships (23). Unfortunately, most of these earlier studies share limitations akin to the previously noted PPD literature thereby limiting statistical power and diagnostic precision. Recently, a multi-national, population-inclusive study with complete follow-up has highlighted the role of several parental psychiatric disorders in the mother, the father, and both parents before birth as risk factors for offspring ASD (20). Consistent with the “polygenic” basis of mental illness risk (24), ASD risk was observed to be high for pre-gestational maternal psychiatric disorders compared to paternal disorders, but highest when both parents had a psychiatric history (20).
The psychosocial adjustment specific to childrearing, after birth, and in the early postpartum impacts each parent and, for some, may represent a moment of increased vulnerability for a new or recurrent psychiatric illness, most commonly PPD (1, 4, 5). However, to our knowledge, how maternal or paternal PPD and the combined effect in both parents may relate to the observed risk of ASD outcomes has not yet been prospectively studied. Therefore, this study’s aim is to examine ASD risk in the offspring of parents who received a PPD diagnosis and to examine influences by parental psychiatric and antidepressant medication history, socioeconomic factors, offspring sex, and gestational age as well as changes due to temporal trends of birth year. While PPD may not be etiologically distinct from other depressive disorders (4, 8, 14, 15), its timing, heritability pattern, and developmental context make it an especially informative lens for studying the intergenerational transmission of risk for neurodevelopmental outcomes including ASD (9).
Methods
Data sources and study population
All live births in Sweden between January 1, 1997, and December 31, 2021, to parents (both) born in Sweden were assessed. Individual-level data from different Swedish registers was linked via the unique personal identity number of each resident. Biological parents were identified from the Swedish Medical Birth Register (MBR) and the Swedish Multi-generation Register. Vital status and emigration were derived from the Swedish Total Population Register. The study was approved by the National Swedish Ethics Review Board (2017/1875-31/1; 2018/1864-32; 2019-06314). Due to the nature of the study, no individual level consent was required.
Postpartum depression
In agreement with the Agency for Healthcare Research and Quality, and consistent with prior research, we defined PPD as any diagnoses of PPD, as well as any unipolar depression diagnoses occurring within the first postpartum year (13). The Swedish National Patient Register (NPR) was used to obtain depression diagnoses from specialist care according to the International Classification of Diseases (ICD, v8-v10; inpatient diagnoses since 1973 and outpatient diagnoses since 2001; see eTable 1 for the included ICD codes). Data quality of the NPR has been verified and validated for diagnoses of multiple psychiatric conditions (25).
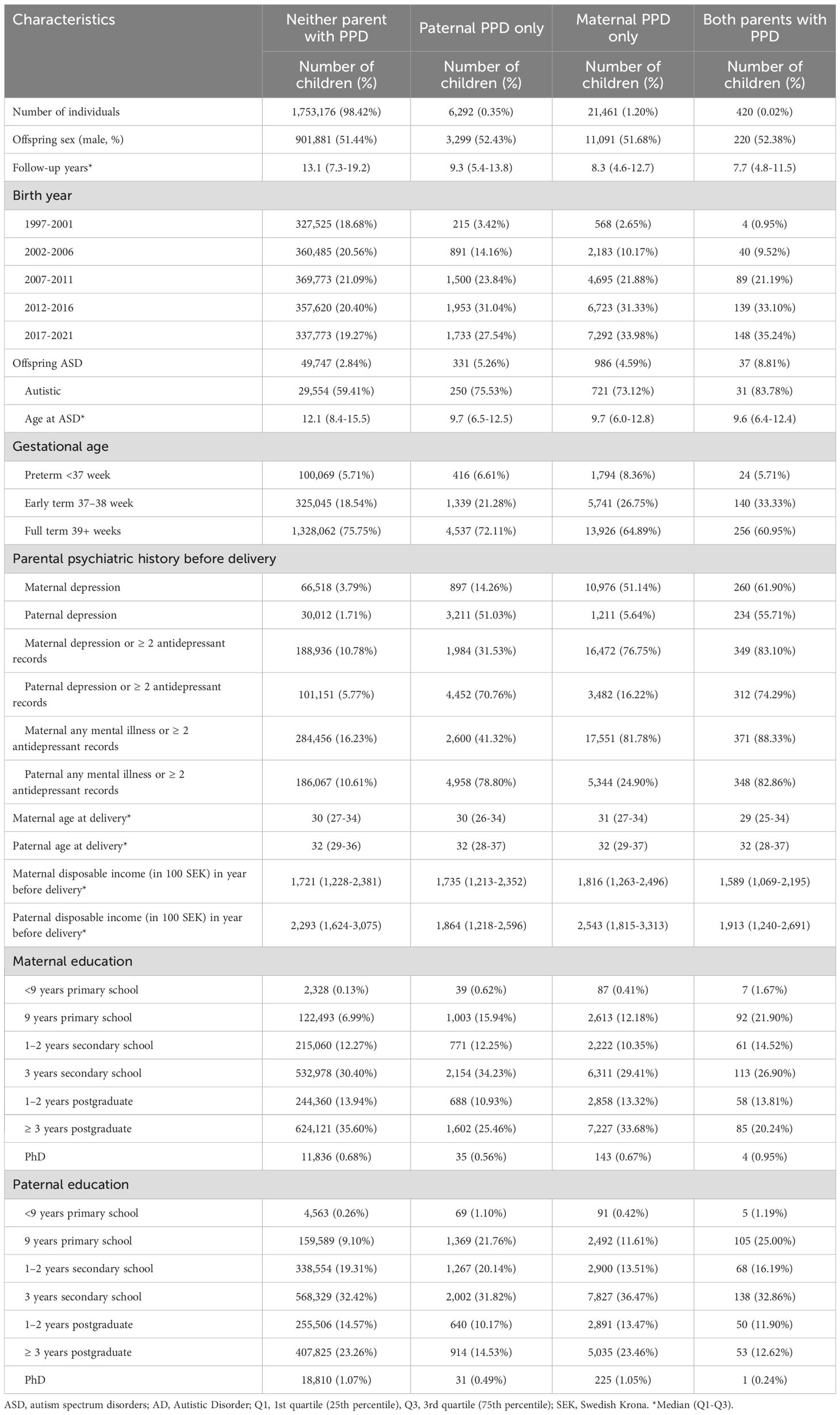
PPD was classified as ‘maternal only’ if present only in the gestational parent, ‘paternal only’ if present only in non-gestational parent, or ‘both’ if present in both parents within the first year of delivery, but not necessarily simultaneously (Table 1).
Outcome: autism spectrum disorder
Diagnoses of ASD from age one onwards were obtained from the NPR using the ICD (eTable 1). In Sweden, all infants and preschoolers receive regular medical and developmental evaluations covering motor skills, language, cognitive abilities, and social development. Children suspected of ASD are referred to specialists for assessment. Previous publications document the diagnostic processes and validity of ASD diagnoses in Sweden (26).
Other covariates
Birth year, offspring sex, maternal age, and gestational age (weeks) data were extracted from the MBR. Paternal age was calculated by linking offspring to their biological fathers through the Multi-Generation Register. Paternal and maternal yearly disposable income (Swedish Krona) and educational attainment (completed school years) were obtained from the Swedish database for health insurance and labor market studies (LISA database). Parental history of antidepressant medication use was defined as at least two dispenses registered in the Swedish Prescribed Drug Register (available since July 2005) using the Anatomical Therapeutic Chemical classification code N06A. Depression and psychiatric history was defined as depression or any psychiatric diagnosis registered in the NPR. All covariates were defined before the birth of the child.
Statistical analyses
The relative risk of ASD in offspring of parents with PPD compared to offspring of parents without PPD was quantified by hazard ratios (HR) and associated two-sided 95% confidence intervals (CI) from Cox proportional hazards models, together with ASD incidence (cases per 100,000 person-years). The age at follow-up was the underlying time-scale. Offspring were followed from age one until ASD diagnosis, emigration, death, or December 31, 2022, whichever came first.
All statistical models were pre-specified before performing the analysis and based on prior literature (20). In Model 1 PPD in fathers, mothers, and both parents were adjusted for offspring birth year using natural cubic splines with five internal knots. Parental age (years), education (< 9 years primary education, 9 years primary education, 1-2 years secondary education, 3 years secondary education, 1-2 years postgraduate education, ≥ 3 years postgraduate education, PhD education), income (ordered into yearly ventiles), and preterm birth (yes/no) were additionally adjusted for in Model 2 to account for potential influences. Continuous variables were modeled using natural cubic splines (20). Parental depression diagnoses assigned before childbirth was additionally adjusted for in Model 3. Model 4 expanded Model 3 to include a history of >2 dispenses of antidepressant prescriptions, and Model 5 expanded Model 4 to adjust for any parental psychiatric diagnosis assigned before childbirth.
The proportional hazards assumption of the Cox regression was visually examined by weighted Schoenfeld residuals. Statistical tests were performed on the two-sided 5% level of significance. P-values were not adjusted for multiplicity of statistical tests, however, the primary research question is composed of only three statistical tests (PPD in the mother, the father, and in both parents, compared to neither parent with PPD). To address potential correlation between siblings, robust standard errors were applied.
Supplementary analyses
Psychiatric disorders before childbirth are associated with higher risk of preterm delivery (27), and increased risk of ASD in offspring (20). Whether ASD risk was modified by gestational age was explored by including an interaction term between PPD and gestational age (preterm term <37 weeks, early-term 37–38 weeks, full-term >39 weeks). ASD risk was also estimated separately by offspring sex. Because earlier PPD onset may represent a distinct etiological group, PPD was restricted to the first six weeks postpartum. To identify potential patients who seek help only in primary care, we expanded the definition of PPD to include those with either a depression diagnosis or the record of an antidepressant prescription during the postpartum period. We analyzed cases where PPD was the first instance of depression versus a recurrence. For first-ever depression, we included children of parents without prior depression or fewer than two antidepressant records before delivery. For recurrence depression, we included children of either parent with a depression history or at least two antidepressant records before delivery. We separately examined parents with an antidepressant prescription within one year before delivery. Due to the potential unreliability of ASD diagnoses before age two, we only considered ASD diagnoses after age two, and followed up with all children from age two onwards. Parents with ASD were excluded to test whether this risk was driven by the inherited ASD risk from parents.
The SAS software was used for all data management work and statistical analyses (Cox regression using Proc PHREG).
Results
The study population included 1,794,593 births in Sweden to Swedish-born parents between 1997 and 2021. Of these, 5,031 (0.28%) were excluded from the analysis due to death, and emigration prior to age one, and 8,213 (0.46%) were removed due to incomplete covariate data. The analytic cohort included 1,781,349 children (916,491 male, 864,858 female), contributing 21,670,307 person-years during an average follow-up of 12 years. In total, 6,292 (0.4%) of fathers only, 21,461 (1.2%) of mothers only, and 420 (0.02%) of both parents had a PPD diagnosis. Among 1,753,176 parents without a PPD diagnosis, 49,747 (2.8%) children had ASD (232 ASD per 100,000 person-years). In comparison, 331 (5.3%) children had a diagnosis for ASD (589 ASD per 200,000 person-years) when only the father was diagnosed with PPD, 986 (4.6%) children had ASD (574 ASD per 100,000 person-years) when only the mother was diagnosed with PPD, and 37 (8.8%) children had ASD (1177 ASD per 100,000 person-years) when both parents had PPD. For mothers diagnosed with PPD, the HR of an ASD outcome was 2.59[CI:2.43-2.76], for fathers 2.56[CI: 2.29-2.85], and both parents 5.54[CI: 4.02-7.65] (Table 2, Model 1). When parental depression history, parental age, education, income, and preterm delivery were controlled the HR of an ASD diagnosis when the mother was diagnosed with PPD was 1.71[CI:1.60-1.83], for fathers 1.53[CI:1.36-1.71] and both 2.19[CI:1.58-3.03] (Table 2, Model 3). Further adjustment for parental antidepressant use (Table 2, Model 4), followed by additional adjustment for any psychiatric history and pre-delivery antidepressant use (Table 2, Model 5) did not further reduce the magnitude of the association. No evidence for non-proportional hazards was detected (eFigure 1).
Supplementary results
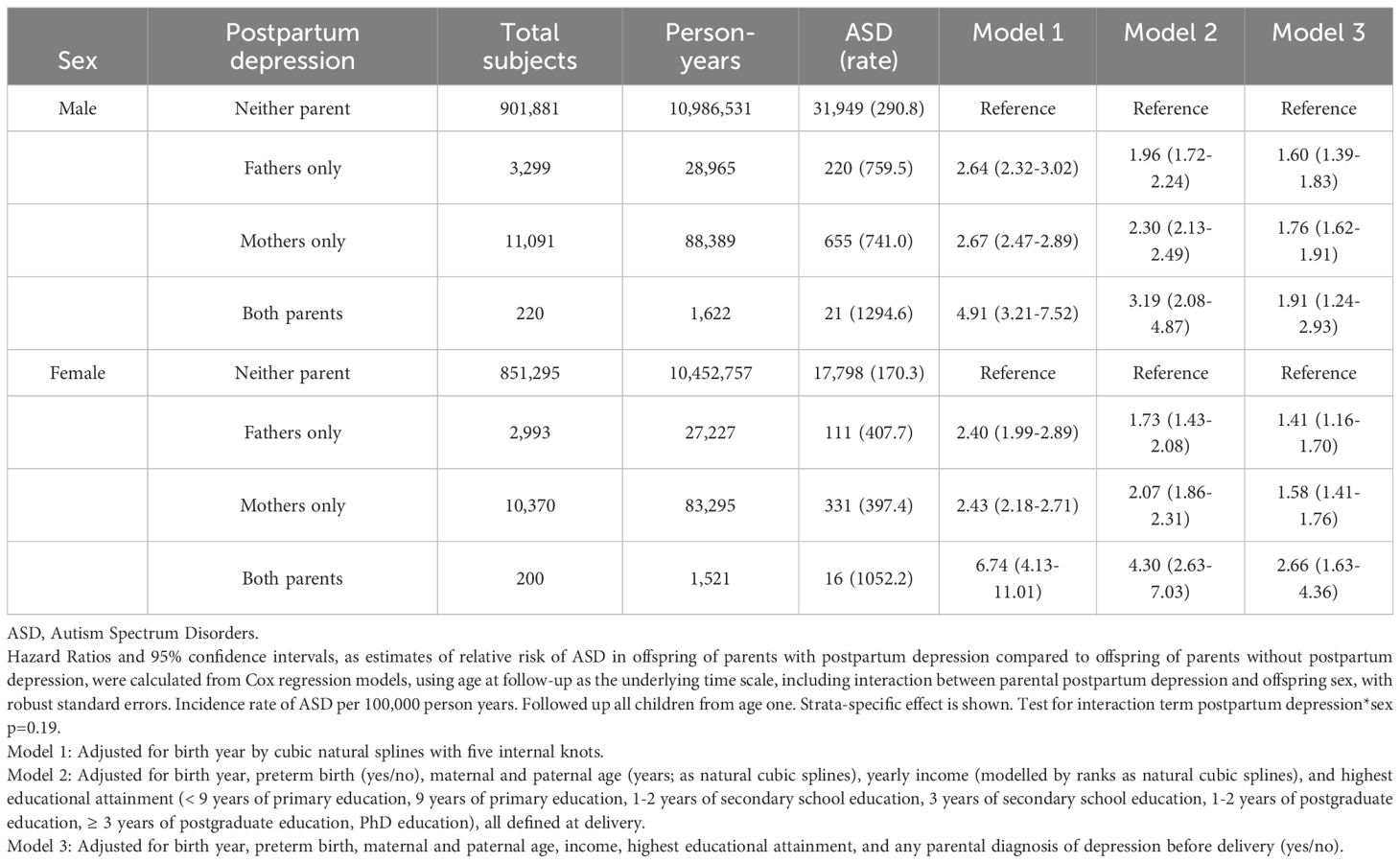
There was no support for modification by gestational age (Table 3) or offspring birth sex (Table 4). The timing of PPD, before or after 6 weeks post-delivery, did not influence the association with ASD (Table 5). When broadening the definition of PPD to include any diagnosis of depression or antidepressant prescription during the postpartum period, a total of 143,129 children were identified as having been exposed to parental PPD (when PPD was defined strictly through clinical depression diagnoses, 28,173 children were exposed). In models where history of depression and antidepressant prescription were adjusted for, the association between maternal PPD and offspring ASD remained comparable whereas the paternal association slightly declined (eTable 2). The strength of the association was similar when the PPD episode was a first depression or a recurrence (eTables 3, 4), and among parents who had been prescribed antidepressants within the year preceding childbirth (eTable 5). Furthermore, the risk of ASD was similar when children were followed up from age two (eTable 6), or after excluding parents with ASD diagnosis (eTable 7).
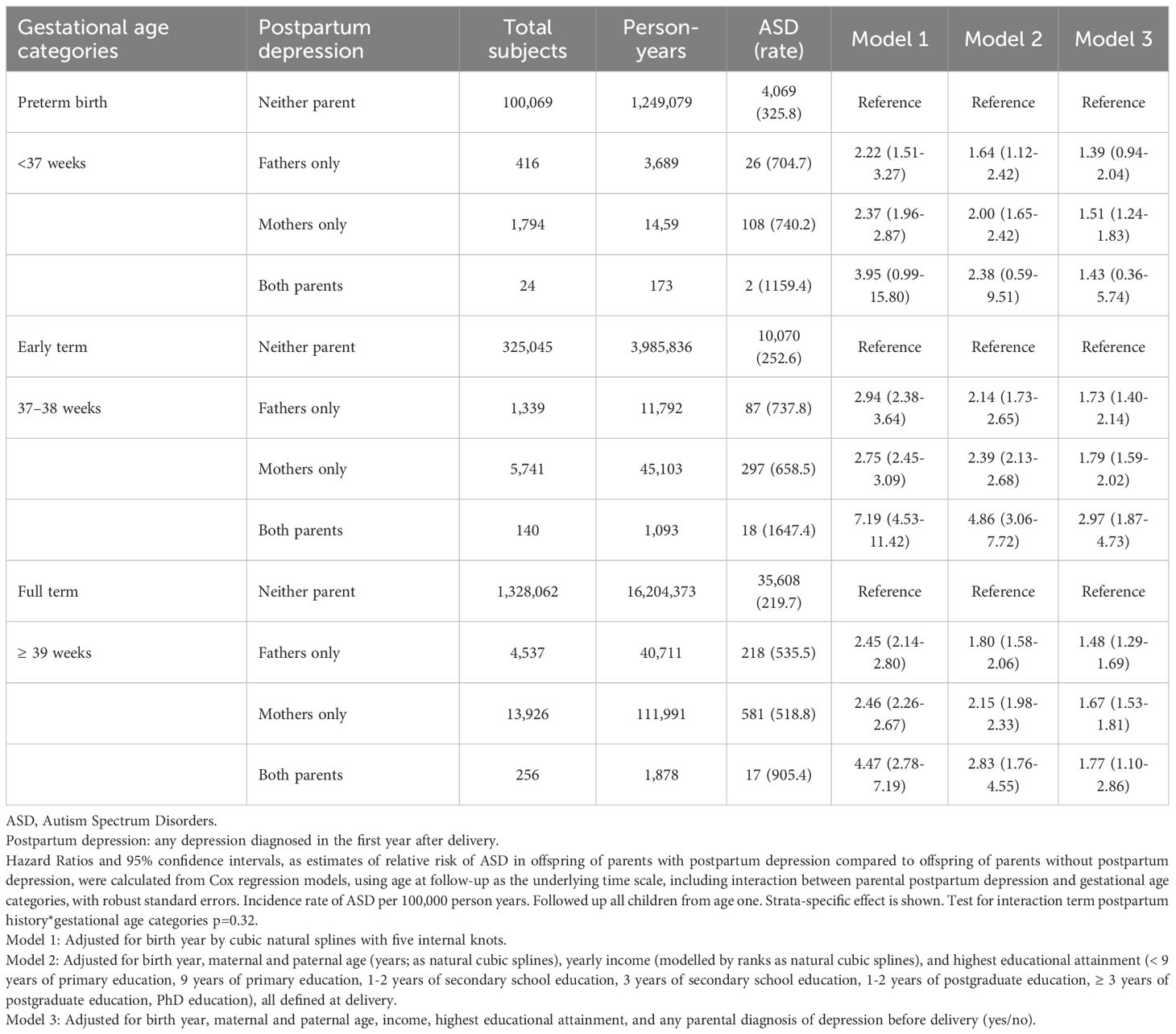
Table 3. Parental postpartum depression and relative risk of ASD in offspring by preterm, early term and full-term births.
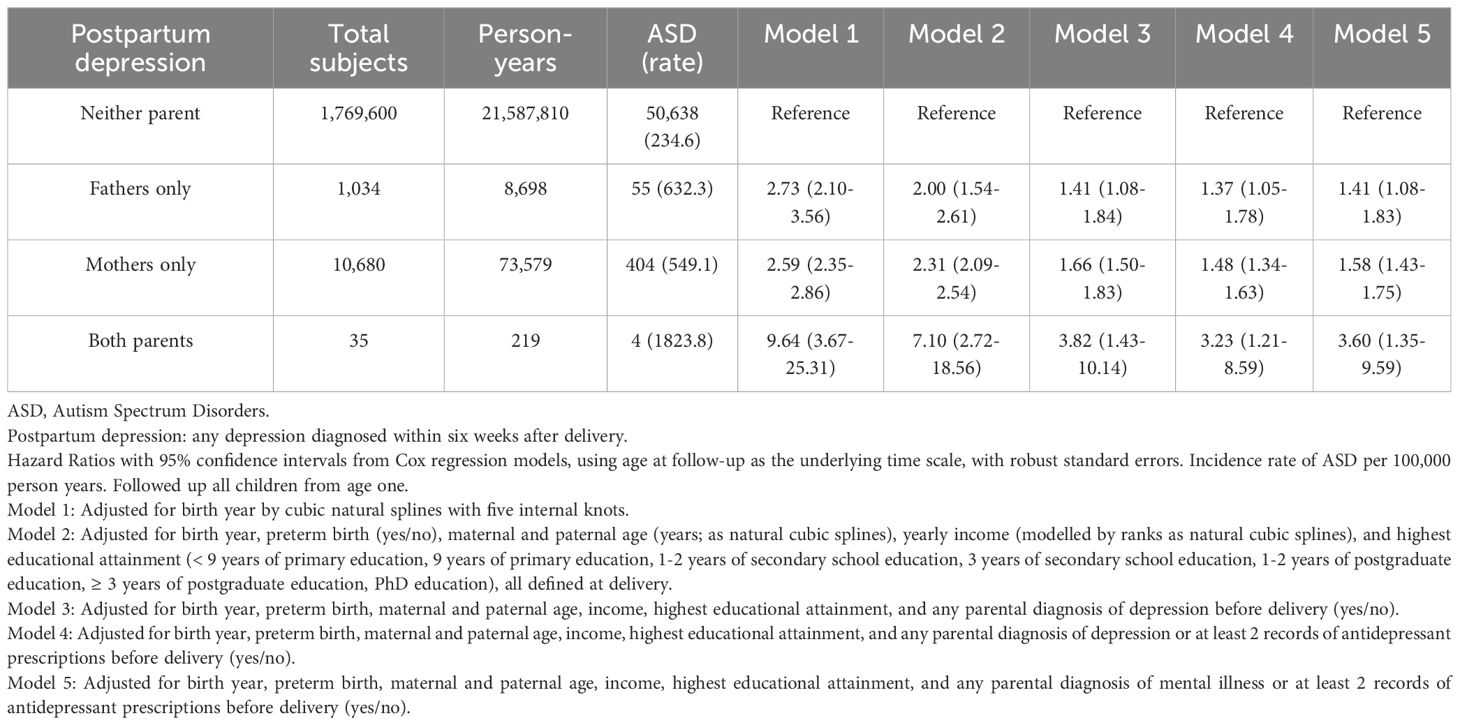
Table 5. Parental postpartum depression within six weeks after delivery and relative risk of ASD in offspring.
Discussion
Using the largest population-inclusive sample to date of clinically diagnosed PPD with psychiatric and offspring follow-up, this longitudinal study examined the relationship between PPD in either or both parents and the risk of ASD in the offspring. The magnitude of association between PPD and ASD in the offspring was similarly increased if either of the parents was diagnosed with PPD, and increased even further if both parents were diagnosed. After adjusting separately for parental depression history, antidepressant use, or any psychiatric history before delivery, this association was partially reduced.
Having a newborn is a stressful experience for most, if not all, new parents, and depressive symptomatology in the postpartum period is a regularly observed occurrence that may affect either parent. However, because diagnostic symptoms common to depression overlap with the normal early adjustment to the childrearing experience, studies limited to symptom inventories alone grossly overestimate PPD prevalence (12), whereas the incidence of clinically significant depression diagnoses in the postpartum are much less common (13). Interestingly, because depression is understood to be a recurrent disorder, it is not surprising that large epidemiological studies of PPD demonstrate that a depression history explains most risk (13, 28). By the same token, a unique pathogenesis of PPD in relation to other unipolar depressive disorders has yet to be established (4, 29).
The time surrounding birth is a critical time for early developmental events and parental depression has been linked to adverse consequences for the offspring including impaired cognitive and language development (30), poor adjustment (31), behavioral problems (32), growth delay (33), fine motor skill (34), and neurodevelopmental challenges (35). However, whether these adverse outcomes are specific to PPD, or genetic factors associated with the parent’s depression or other psychiatric history remains unknown. Indeed, while genetic factors likely play a role in the development of some of these outcomes, non-genetic (environmental) risk factors and interactions between environment and genetics likely also contribute to some of these risks.
ASD represents a heterogeneous neurodevelopmental syndrome with deficits that fall into core domains and begin to emerge in early infancy (17). The genesis of ASD is believed to have both biological and environmental underpinnings with a shared genetic and metabolic etiology common to other psychiatric disorders including schizophrenia, bipolar disorder, and major depressive disorder (36). At the same time, genes inherited from parents are considered the major pathway for ASD; however given the numerous factors associated with healthy brain development, critical knowledge gaps remain. Indeed, an in-depth understanding of how ASD risk varies according to individual parent or total parental contributions (such as, genetic load), and how the various comorbid psychiatric histories may further inform ASD risk remains to be fully elucidated.
Although several large studies have examined the relationship between maternal PPD and ASD in offspring (35, 37, 38), and one has included both parents (22), none have done so prospectively. This is the first study to prospectively examine maternal, paternal, and combined parental PPD in relation to offspring ASD and PPD while also accounting for a broad range of covariates, including maternal and paternal autism and autism traits, disposable income, medication exposure, and educational attainment. Notably, while an association was observed between maternal PPD and ASD in the offspring, comparable to that found in the Australian population (38), paternal PPD was also found to be associated with increased ASD risk and greatest when both parents were diagnosed with PPD – similar to a pattern observed in Taiwan (22). This finding is also consistent with findings from Swedish and Finnish populations exploring the association of a wide array of pre-delivery psychiatric disorders with the risk of ASD in the offspring (20). The observation of increased ASD risk when only the father is diagnosed, and with the highest risk when both parents are diagnosed with PPD, may reflect a shared genetic etiology. That the combined effect of maternal and paternal PPD on ASD outcomes is additive, suggests that common genetic variants may be inherited from both parents (39). However, that this combined risk reduced when depression history in the parent was controlled may help inform future studies exploring underlying genetic and environmental mechanisms. Notably, although the relative risk increased two-fold to five-fold for children born to parents where both parents were diagnosed with PPD, the clinical implications and public impact are limited given the rarity of this observation.
The time surrounding childbirth is a time of significant vulnerability for the recurrence of various psychiatric disorders in the parents (40, 41). Importantly, although the timing of depression onset within the postpartum period may appear to be indicative of a biological etiology associated with the reproductive process, an earlier study exploring whether the period after childbirth was associated with depression revealed no differences in risk compared to a period following a randomly generated date (8). In this respect, studies into heritability and familial aggregation have pointed to PPD as being linked to genetic etiologies of unipolar depression, unrelated to reproduction, as well as polygenic risk scores nonspecific to childbirth (42). In the present study, after controlling for depression history, antidepressant use, and mental illness before delivery, the association between PPD and ASD remained. This suggests that a history of depression alone may not account for the association between parental psychopathology in the postpartum period and ASD. It may instead indicate that PPD occurring in parents without a prior history of depression reflects an enduring vulnerability to depressive illness which may have been triggered by the uniquely stressful conditions of early childrearing. However, it is equally important to note that because postpartum women are a medically captured population in Sweden available for PPD screening, the rate of treatment-seeking behaviors for a depressive illness before pregnancy is likely incomplete (43). In this respect, the observed association between PPD and ASD in the context of a psychiatric history and SSRI treatments likely represents an underestimation. Similarly, while the resolution of our data does not allow for further exploration of potential underlying influences, it would be inappropriate to imply that PPD causes ASD particularly given that PPD occurs after pregnancy, whereas ASD is thought to likely develop during the prenatal period (44, 45). Therefore, the observed association between PPD and ASD likely reflects shared genetic influences (pleiotropy), which may represent potential biological mechanisms (46). A genetic link between PPD and ASD is further supported by the observation of an increased risk of ASD in children born to fathers with PPD who are free from the biological factors specific to pregnancy and delivery.
This study comes with many strengths, including the large sample size and prospective design. The use of prospectively collected and clinically ascertained diagnoses of PPD/depression and ASD from within a publicly financed and universal health system with national coverage minimizes a wide range of potential biases. The extensive database and longitudinal design allow for detailed adjustment for potential confounding. Sweden’s public health system includes maternal care during and after pregnancy, including postpartum “in-home” visits with nearly 100% participation (47). This reduces the selection bias from a group of individuals with a higher frequency of treatment-seeking behavior.
The study also has limitations. The NPR does not include diagnoses from primary care. However, the availability of prescribed drug data captures those individuals treated in primary care who were prescribed antidepressants, and these cases were included in our analyses. We were not able to capture those with depression who do not visit health care and those who visit primary care but do not receive medications. We lacked information to distinguish between children raised by non-biological parents, but in Sweden, this accounts for only a small proportion of children (48). Because ASD diagnoses are only available in the ICD-9 and ICD-10 in the Swedish register, parents with ASD diagnosed exclusively prior to 1987 would not have been captured, and thus there remains a possibility of residual confounding from undetected parental ASD. Genetic confounding may also have occurred if parents with PPD are a specific group with a higher inherited risk of ASD. However, this genetic confounding could not fully account for the remaining association, because the results did not change after excluding parents diagnosed with ASD. Finally, as in any epidemiological study, we cannot rule out bias due to residual and unmeasured confounding. Therefore, our findings should be replicated in other populations and healthcare systems.
Conclusion
Using the largest nationally inclusive sample to date with clinically ascertained ASD and depression diagnoses, PPD was associated with an increased risk of ASD in the offspring, and this association was partially, though not fully, explained by depression history, antidepressant use, and other parental psychiatric factors. The magnitude of the association increased comparably when either parent was diagnosed with PPD and increased further when both parents were diagnosed - a pattern indicative of shared genetic influences.
Data availability statement
The datasets generated and analyzed during the current study are not publicly available due to patient protections, institutional and state policy. Data are available from the Swedish Government upon request to the Swedish Registers. Requests to access the datasets should be directed to cGF0aWVudHJlZ2lzdHJldEBzb2NhaWxzdHlyZWxzZW4uc2U=.
Ethics statement
The studies involving humans were approved by National Swedish Ethics Review Board, Sweden (2017/1875-31/1; 2018/1864- 32; 2019-06314). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
WY: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. AR: Writing – original draft, Writing – review & editing. SS: Data curation, Formal analysis, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. MS: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The study was supported by a grant to MS from the National Institutes of Health; Grant HD073010 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the Swedish Research Council (grant 2021-0214 to Drs Yin and Sandin).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2025.1693979/full#supplementary-material
References
1. Wang Z, Liu J, Shuai H, Cai Z, Fu X, Liu Y, et al. Mapping global prevalence of depression among postpartum women. Transl Psychiatry. (2021) 11:640. doi: 10.1038/s41398-021-01692-1
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Publishing (2013).
3. World Health Organization(WHO). The ICD-10 classification of mental and behavioural disorders. Geneva, Switzerland: World Health Organization (1993).
4. Batt MM, Duffy KA, Novick AM, Metcalf CA, and Epperson CN. Is postpartum depression different from depression occurring outside of the perinatal period? A review of the evidence. Focus (Am Psychiatr Publ). (2020) 18:106–19. doi: 10.1176/appi.focus.20190045
5. Mughal S, Azhar Y, and Siddiqui W. Postpartum depression. In: StatPearls. StatPearls Publishing, Treasure Island (FL (2023). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK519070/.
6. Couto TC, Brancaglion MY, Alvim-Soares A, Moreira L, Garcia FD, Nicolato R, et al. Postpartum depression: A systematic review of the genetics involved. World J Psychiatry. (2015) 5:103–11. doi: 10.5498/wjp.v5.i1.103
7. Guintivano J, Byrne EM, Kiewa J, Yao S, Bauer AE, Aberg KA, et al. Meta-analyses of genome-wide association studies for postpartum depression. Am J Psychiatry. (2023) 180:884–95. doi: 10.1176/appi.ajp.20230053
8. Silverman ME, Reichenberg A, Lichtenstein P, and Sandin S. Is depression more likely following childbirth? A population-based study. Arch Womens Ment Health. (2019) 22:253–8. doi: 10.1007/s00737-018-0891-5
9. Severo M, Ventriglio A, Bellomo A, Iuso S, and Petito A. Maternal perinatal depression and child neurocognitive development: A relationship still to be clarified. Front Psychiatry. (2023) 14:1151897. doi: 10.3389/fpsyt.2023.1151897
10. Netsi E, Pearson RM, Murray L, Cooper P, Craske MG, and Stein A. Association of persistent and severe postnatal depression with child outcomes. JAMA Psychiatry. (2018) 75:247–53. doi: 10.1001/jamapsychiatry.2017.4363
11. Levis B, Yan XW, He C, Sun Y, Benedetti A, and Thombs BD. Comparison of depression prevalence estimates in meta-analyses based on screening tools and rating scales versus diagnostic interviews: a meta-research review. BMC Med. (2019) 17:65. doi: 10.1186/s12916-019-1297-6
12. Thombs BD, Kwakkenbos L, Levis AW, and Benedetti A. Addressing overestimation of the prevalence of depression based on self-report screening questionnaires. CMAJ. (2018) 190:E44–9. doi: 10.1503/cmaj.170691
13. Gaynes BN, Gavin N, Meltzer-Brody S, Lohr KN, Swinson T, Gartlehner G, et al. Perinatal depression: prevalence, screening accuracy, and screening outcomes. Evid Rep Technol Assess (Summ). (2005) 119):1–8. doi: 10.1037/e439372005-001
14. Silverman ME, Reichenberg A, Savitz DA, Cnattingius S, Lichtenstein P, Hultman CM, et al. The risk factors for postpartum depression: A population-based study. Depress Anxiety. (2017) 34:178–87. doi: 10.1002/da.22597
15. Zacher Kjeldsen MM, Bricca A, Liu X, Frokjaer VG, Madsen KB, and Munk-Olsen T. Family history of psychiatric disorders as a risk factor for maternal postpartum depression: A systematic review and meta-analysis. JAMA Psychiatry. (2022) 79:1004–13. doi: 10.1001/jamapsychiatry.2022.2400
16. Lord C, Cook EH, Leventhal BL, and Amaral DG. Autism spectrum disorders. Neuron. (2000) 28:355–63. doi: 10.1016/S0896-6273(00)00115-X
17. National Institute of Mental Health. Autism Spectrum Disorder (2024). U.S. Department of Health and Human Services, National Institutes of Health. Available online at: https://www.nimh.nih.gov/health/topics/autism-spectrum-disorders-asd (Accessed January 25, 2024).
18. Sandin S, Yip BHK, Yin W, Weiss LA, Dougherty JD, Fass S, et al. Examining sex differences in autism heritability. JAMA Psychiatry. (2024) 81:673–80. doi: 10.1001/jamapsychiatry.2024.0525
19. Bai D, Yip BHK, Windham GC, Sourander A, Francis R, Yoffe R, et al. Association of genetic and environmental factors with autism in a 5-country cohort. JAMA Psychiatry. (2019) 76:1035–43. doi: 10.1001/jamapsychiatry.2019.1411
20. Yin W, Pulakka A, Reichenberg A, Kolevzon A, Ludvigsson JF, Risnes K, et al. Association between parental psychiatric disorders and risk of offspring autism spectrum disorder: a Swedish and Finnish population-based cohort study. Lancet Reg Health Eur. (2024) 40:100902. doi: 10.1016/j.lanepe.2024.100902
21. Avalos LA, Chandran A, Churchill ML, Gao X, Ames JL, and Nozadi SS. et al; Program Collaborators for Environmental influences on Child Health Outcomes. Prenatal depression and risk of child autism-related traits among participants in the Environmental influences on Child Health Outcomes program. Autism Res. (2023) 16:1825–35. doi: 10.1002/aur.2988
22. Chen LC, Chen MH, Hsu JW, Huang KL, Bai YM, Chen TJ, et al. Association of parental depression with offspring attention deficit hyperactivity disorder and autism spectrum disorder: A nationwide birth cohort study. J Affect Disord. (2020) 277:109–14. doi: 10.1016/j.jad.2020.07.059
23. Chien YL, Wu CS, Chang YC, Cheong ML, Yao TC, and Tsai HJ. Associations between parental psychiatric disorders and autism spectrum disorder in the offspring. Autism Res. (2022) 15:2409–19. doi: 10.1002/aur.2835
24. Kendall KM, Van Assche E, Andlauer TFM, Choi KW, Luykx JJ, Schulte EC, et al. The genetic basis of major depression. Psychol Med. (2021) 51:2217–30. doi: 10.1017/S0033291721000441
25. Byrne N, Regan C, and Howard L. Administrative registers in psychiatric research: a systematic review of validity studies. Acta Psychiatr Scand. (2005) 112:409–14. doi: 10.1111/j.1600-0447.2005.00663.x
26. Sandin S, Nygren KG, Iliadou A, Hultman CM, and Reichenberg A. Autism and mental retardation among offspring born after. Vitro fertilization. JAMA. (2013) 310:75–84. doi: 10.1001/jama.2013.7222
27. Yin W, Ludvigsson JF, Åden U, Risnes K, Persson M, Reichenberg A, et al. Paternal and maternal psychiatric history and risk of preterm and early term birth: A nationwide study using Swedish registers. PloS Med. (2023) 20:e1004256. doi: 10.1371/journal.pmed.1004256
28. Munk-Olsen T, Laursen TM, Pedersen CB, Mors O, and Mortensen PB. New parents and mental disorders: a population-based register study. JAMA. (2006) 296:2582–9. doi: 10.1001/jama.296.21.2582
29. Viktorin A, Meltzer-Brody S, Kuja-Halkola R, Sullivan PF, Landén M, Lichtenstein P, et al. Heritability of perinatal depression and genetic overlap with nonperinatal depression. Am J Psychiatry. (2016) 173:158–65. doi: 10.1176/appi.ajp.2015.15010085
30. Santos IS, Blumenberg C, Munhoz TN, Matijasevich A, Salum C, Santos Júnior HG, et al. Maternal depression and child development at 3 years of age: a longitudinal study in a Brazilian child development promotion program. Pediatr Res. (2024) 95:1139–46. doi: 10.1038/s41390-023-02876-9
31. Elgar FJ, McGrath PJ, Waschbusch DA, Stewart SH, and Curtis LJ. Mutual influences on maternal depression and child adjustment problems. Clin Psychol Rev. (2004) 24:441–59. doi: 10.1016/j.cpr.2004.02.002
32. Charrois J, Côté SM, Paquin S, Séguin JR, Japel C, Vitaro F, et al. Maternal depression in early childhood and child emotional and behavioral outcomes at school age: examining the roles of preschool childcare quality and current maternal depression symptomatology. Eur Child Adolesc Psychiatry. (2020) 29:637–48. doi: 10.1007/s00787-019-01385-7
33. Avan B, Richter LM, Ramchandani PG, Norris SA, and Stein A. Maternal postnatal depression and children’s growth and behaviour during the early years of life: exploring the interaction between physical and mental health. Arch Dis Child. (2010) 95:690–5. doi: 10.1136/adc.2009.164848
34. Lubotzky-Gete S, Ornoy A, Grotto I, and Calderon-Margalit R. Postpartum depression and infant development up to 24 months: A nationwide population-based study. J Affect Disord. (2021) 285:136–43. doi: 10.1016/j.jad.2021.02.042
35. Peltier MR, Fassett MJ, Mensah NA, Khadka N, Yeh M, Chiu VY, et al. Postpartum depression increases the risk of autism diagnosis in the offspring. JAACAP Open. (2024) 3:232–44. doi: 10.1016/j.jaacop.2024.02.008
36. Kim Y, Vadodaria KC, Lenkei Z, Kato T, Gage FH, Marchetto MC, et al. Mitochondria, metabolism, and redox mechanisms in psychiatric disorders. Antioxid Redox Signal. (2019) 31:275–317. doi: 10.1089/ars.2018.7606
37. Chen MH, Pan TL, Bai YM, Huang KL, Tsai SJ, Su TP, et al. Postpartum depression and psychosis and subsequent severe mental illnesses in mothers and neurodevelopmental disorders in children: A nationwide study. J Clin Psychiatry. (2021) 82:20m13735. doi: 10.4088/JCP.20m13735
38. Tusa BS, Alati R, Betts K, Ayano G, and Dachew B. Associations of maternal perinatal depressive disorders with autism spectrum disorder in offspring: Findings from a data-linkage cohort study. Aust N Z J Psychiatry. (2025) 59:282–92. doi: 10.1177/00048674251315641
39. Gualtieri CT. Genomic variation, evolvability, and the paradox of mental illness. Front Psychiatry. (2021) 11:593233. doi: 10.3389/fpsyt.2020.593233
40. Abdelhafez MA, Ahmed KM, Ahmed NM, Ismail M, Mohd Daud MNB, Ping NPT, et al. Psychiatric illness and pregnancy: A literature review. Heliyon. (2023) 9:e20958. doi: 10.1016/j.heliyon.2023.e20958
41. Munk-Olsen T, Laursen TM, Mendelson T, Pedersen CB, Mors O, and Mortensen PB. Risks and predictors of readmission for a mental disorder during the postpartum period. Arch Gen Psychiatry. (2009) 66:189–95. doi: 10.1001/archgenpsychiatry.2008.528
42. Munk-Olsen T, Di Florio A, Madsen KB, Albiñana C, Mægbæk ML, Bergink V, et al. Postpartum and non-postpartum depression: a population-based matched case-control study comparing polygenic risk scores for severe mental disorders. Transl Psychiatry. (2023) 13:346. doi: 10.1038/s41398-023-02649-2
43. Herrman H, Patel V, Kieling C, Berk M, Buchweitz C, Cuijpers P, et al. Time for united action on depression: a Lancet-World Psychiatric Association Commission. Lancet. (2022) 399:957–1022. doi: 10.1016/S0140-6736(21)02141-3
44. Love C, Sominsky L, O’Hely M, Berk M, Vuillermin P, and Dawson SL. Prenatal environmental risk factors for autism spectrum disorder and their potential mechanisms. BMC Med. (2024) 22:393. doi: 10.1186/s12916-024-03617-3
45. Gardener H, Spiegelman D, and Buka SL. Prenatal risk factors for autism: comprehensive meta-analysis. Br J Psychiatry. (2009) 195:7–14. doi: 10.1192/bjp.bp.108.051672
46. Lee S, McAfee JC, Lee J, Gomez A, Ledford AT, Clarke D, et al. Massively parallel reporter assay investigates shared genetic variants of eight psychiatric disorders. Cell. (2025) 188(5):1409–1424. doi: 10.1016/j.cell.2024.12.022
47. Jansson A, Isacsson A, and Nyberg P. Help-seeking patterns among parents with a newborn child. Public Health Nurs. (1998) 15:319–28. doi: 10.1111/j.1525-1446.1998.tb00356.x
Keywords: postpartum depression (PPD), autism spectrum disorder (ASD), healthcare, parental mental health, cohort study, epidemiology
Citation: Yin W, Reichenberg A, Sandin S and Silverman ME (2025) The association between parental postpartum depression and offspring autism spectrum disorder. Front. Psychiatry 16:1693979. doi: 10.3389/fpsyt.2025.1693979
Received: 27 August 2025; Accepted: 17 October 2025;
Published: 11 November 2025.
Edited by:
Karen Tabb, University of Illinois at Urbana-Champaign, United StatesCopyright © 2025 Yin, Reichenberg, Sandin and Silverman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiyao Yin, d2VpeWFvLnlpbi4yQGtpLnNl
†ORCID: Weiyao Yin, orcid.org/0000-0002-9534-8207
 Weiyao Yin1*†
Weiyao Yin1*† Sven Sandin
Sven Sandin Michael E. Silverman
Michael E. Silverman