Abstract
We report the case of a 48-year-old woman with treatment-resistant schizophrenia (TRS) and metabolic syndrome who completed a 5-week medical ketogenic diet (KD) in an inpatient setting. The patient was initially started on a 2.5:1 ratio of fat to combined carbohydrate and protein in grams, but her diet was modified on two occasions due to difficulty reaching consistent ketosis (βHB ≥0.5 mmol/L). Ketosis became more consistent throughout the study but was only fully maintained the last 5 days. Despite this, the patient had many clinically meaningful improvements including a 69% decrease in insulin-resistance (HOMA-IR), 41% decrease in C-peptide, and a 64% decrease in fasting insulin, despite minimal weight loss. Importantly, insulin-resistance moved from pre-diabetic to optimal levels. Fingerstick glucose also decreased. CRP reduced by 61%, suggesting movement from high to average cardiac risk. Extrapyramidal side effects (i.e., pseudoparkinsonism) improved dramatically (80% decrease), reaching almost full resolution. Global psychopathology ratings were not improved; however, the participant had only a few days consistently in ketosis and was facing significant personal stressors nearing the endpoint, which may have eclipsed clinical benefits. Despite this, we saw a hint of improvement in negative symptoms, which we point out as these are particularly problematic in TRS. These results are congruent with emerging data suggesting various health benefits of KD for people with schizophrenia, and we report for the first time its impact in TRS with long-term use of antipsychotic medications (clozapine, olanzapine) that contribute to metabolic syndrome, parkinsonian-like symptoms, and cardiac risk. Our results suggest that despite TRS, dual antipsychotic treatment and limited time in ketosis, a KD can reverse insulin resistance, greatly improve antipsychotic-associated pseudoparkinsonism, and reduce cardiac risk and inflammation. Thus, this diet may be a beneficial treatment alongside antipsychotic medication. We also suggest that well-controlled clinical trials longer than 5 weeks and with consistent ketosis are needed. Additionally, lower calories or a higher ratio of fat to combined protein and carbohydrate may be necessary to maintain ketosis for individuals with metabolic dysfunction who are taking antipsychotic medication.
Background
Schizophrenia (SZ) is a serious mental illness with positive symptoms (e.g., delusions, hallucinations), negative symptoms (e.g., alogia, blunted affect), and cognitive symptoms (e.g., poor attention, slow processing speed) (1). SZ affects 1% of the population (2, 3), and 5% of individuals with SZ die by suicide (4), making it a massive public health concern.
SZ is associated with metabolic complications including high insulin and glucose, insulin-resistance, metabolic syndrome, and diabetes (5, 6). SZ is also associated with an inflammatory state (7–11), which may drive SZ symptoms (9, 10, 12). Second-generation antipsychotics may exacerbate metabolic and inflammatory complications, with clozapine and olanzapine particularly implicated in insulin-resistance, poor glucose control, weight gain, type 2 diabetes mellitus (13), and increased C-reactive protein (CRP) and inflammatory cytokines (14, 15). Further, antipsychotics have serious adverse effects including extrapyramidal side effects (EPS) (e.g., pseudoparkinsonism) and sudden cardiac death, which reduce life quality and lifespan (13). Antipsychotics are also ineffective for negative symptoms, a domain which has no FDA-approved treatment (16). Therefore, alternative treatments, and ones that can potentially counter adverse effects of antipsychotic medications, are needed.
Dietary interventions have improved SZ symptoms. Our group found improvement in negative symptoms after a gluten-free diet in a sub-group of individuals with SZ with high IgG antibodies to gliadin (17). Several open label studies and case reports have shown robust benefits of a ketogenic diet (KD) for reducing metabolic complications and improving psychiatric symptoms in many psychiatric conditions (18–29), including SZ (30–34). A ketogenic diet as a medical treatment may, in part, work through bypassing glucose metabolism, impacting mitochondrial biogenesis, reducing systemic inflammation, and increasing gamma-aminobutyric acid (GABA) concentrations (35, 36). KD may also work through other biological processes relevant to neuropsychiatric illness, including decreasing oxidative stress (37), altering the gut microbiome (38), and decreasing glutamate excitotoxicity (39). We describe the case of a woman with treatment-resistant SZ (TRS) and metabolic syndrome on KD for 5 weeks and report metabolic, inflammatory, neurologic, and psychiatric outcomes in this patient.
Case report
The participant was a 48-year-old Black woman treated with KD for 5-weeks in an inpatient setting. She passed capacity assessment for consent and consented to publishing her individual case. The Institutional Review Board at the University of Maryland, data safety monitoring boards, and the hospital research committee oversaw this study.
The patient had a DSM-5 diagnosis of SZ confirmed using the Structured Clinical Interview (SCID) (40) and maintained an antipsychotic regimen without dose change for ≥14 days. She also met criteria for KD-implementation, including body mass index >18.5, not currently pregnant or lactating, and no insulin-dependent diabetes, eating disorders, heart failure, QTc prolongation ≥ 500 ms, significant kidney or liver disease, porphyria, genetic disorders that affect fat metabolism (e.g., Gaucher disease), carnitine deficiency, pyruvate kinase deficiency, gastroparesis, or preclusions from adhering to the intervention diet (e.g., refusal or prohibitive dietary allergies/restrictions).
The participant began struggling with mental illness at age 18 and first experienced psychotic symptoms at age 21. During the 27 years since her first psychotic episode, her symptoms were unresponsive to several antipsychotic medications including haloperidol, risperidone, and aripiprazole, and only partially responsive to olanzapine before adding clozapine for her TRS. She had numerous prior hospitalizations and had been an inpatient for over 2 years prior to participation in this study. Additionally, she suffered from long-term unresponsive negative symptoms. She had a history of medication-induced type 2 diabetes and a metabolic syndrome diagnosis at KD initiation. She was also taking divalproex sodium (mood stabilization) (21 months), metformin (metabolic syndrome) (6 months), amlodipine (hypertension) (5 months), oxybutynin (enuresis) (3 months), propranolol (akathisia) (3 months), and a multivitamin, which was started alongside KD. Pro re nata (PRN) medications included lorazepam (akathisia, agitation, anxiety, insomnia), milk of magnesia (constipation), and acetaminophen (menstrual cramps). About 6 months prior to the study, a slow and cautious clozapine trial was initiated, given her limited response to olanzapine, which she had been on for 20 months before study initiation. When beginning clozapine, olanzapine was unable to be successfully tapered, despite slow and deliberate attempts, without worsening mood, disorganization, and psychosis. Thus, both clozapine and olanzapine were maintained. No antipsychotic changes occurred during the 5 weeks on KD to prevent introducing medication confounds. The only medication changes noted during the study were the addition of ipratropium bromide (sialorrhea) at the end of week 2, and ferrous sulphate (anemia) at week 5. Both were continued through the end of the study.
The patient was monitored by hospital staff for meal consumption on the in-patient unit and was reported to be adherent to KD, consuming only prescribed food, and finishing all or most of every meal (one-day menu in Appendix A). The participant was motivated to adhere to the diet, tolerated it well, and reported, “It’s something I have gotten used to and feel it made a positive change.”
Meals were planned by the hospital dietary department and were delivered to her room. The diet ratio began as 2.5:1 fat to combined protein and carbs in grams, ~2500–3800 calories, with a macronutrient breakdown of ~70% fat, ~20% protein, ≤10% carbohydrate. Twice daily β-hydroxybutyrate (βHB) and glucose levels were obtained using a KetoMojo® fingerstick (AM: fasting, PM: non-fasting). Following failure to reach consistent ketosis (βHB ≥0.5 mmol/L), her KD ratio was increased to 2.65:1 late in week 2 (macronutrient breakdown: ~73% fat, ~ 21% protein, ~7% carbohydrate), and a caloric reduction (daily <3000) occurred early in week 3 (See Figure 1). This improved ketosis consistency, but it was not fully met until the last 5 days (See Figures 1, 2). Overall, there was an upward trend in βHB, and glucose levels decreased over time (See Figure 2).
Figure 1
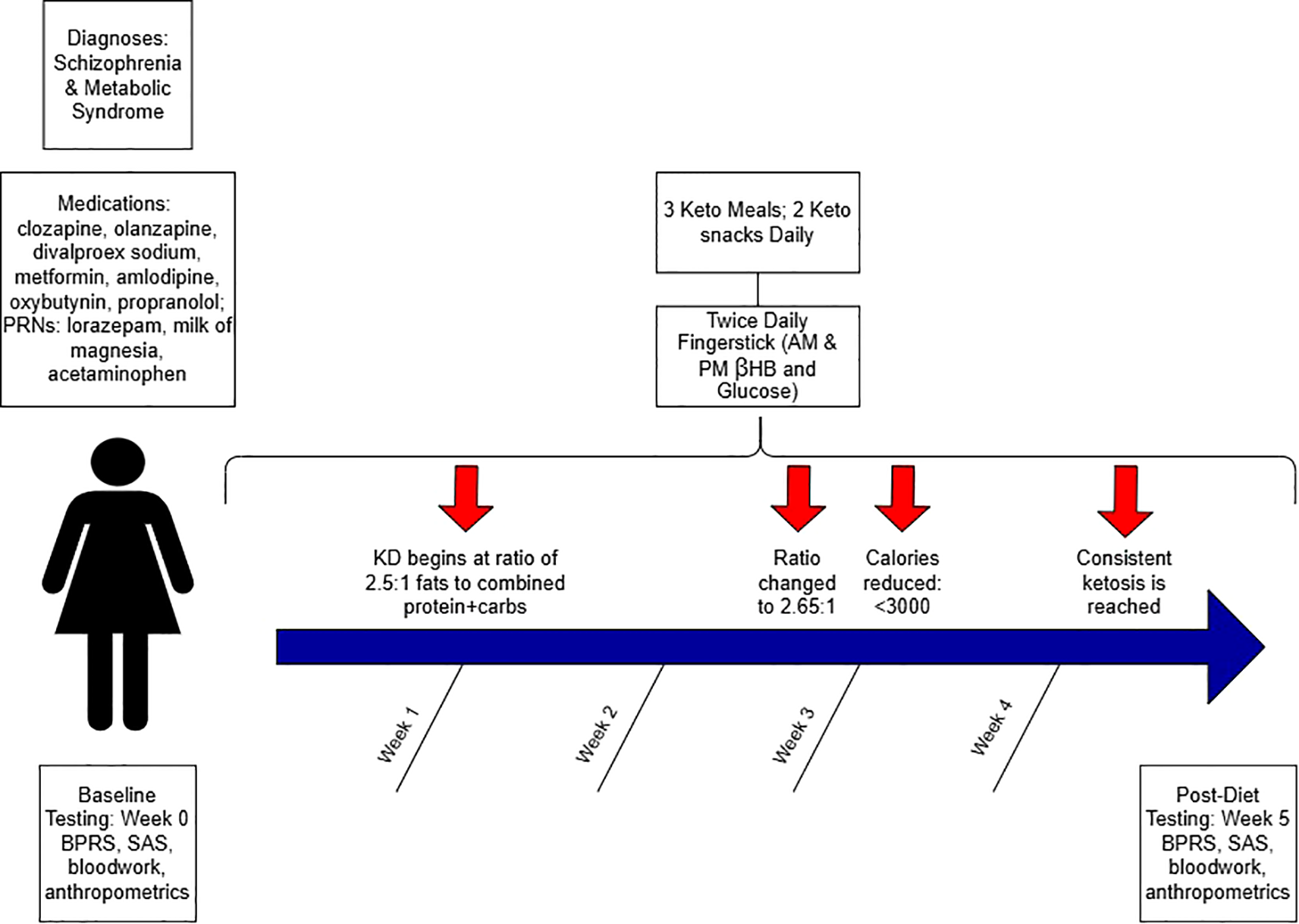
Schematic study diagram.
Figure 2
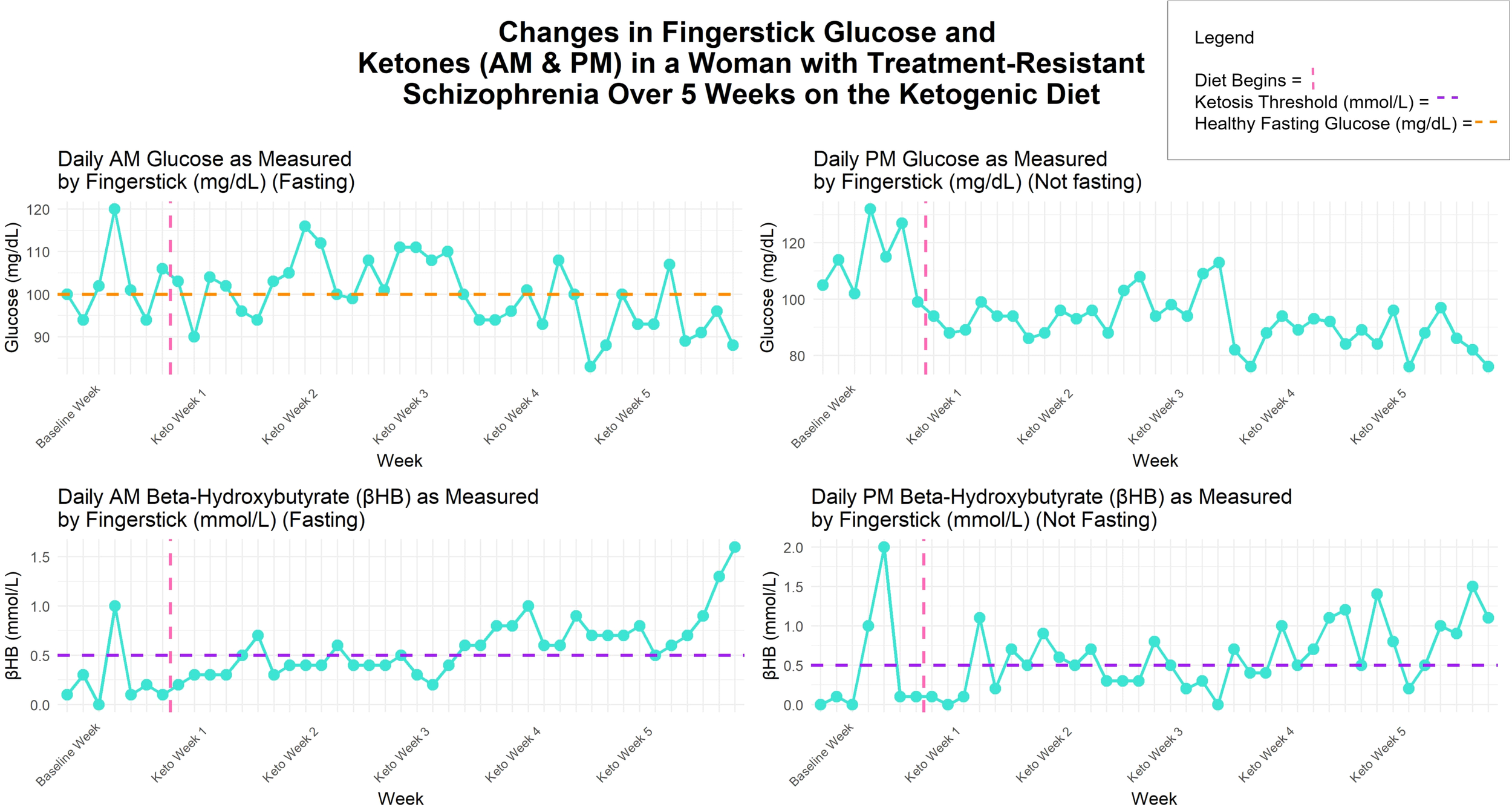
Changes in fingerstick βHB (mmol/L) and glucose (mg/dL) over 5 weeks on the ketogenic diet.
Laboratory results are reported in Table 1 where we report changes ≥20% as clinically meaningful (41). Insulin-resistance, measured by HOMA-IR, decreased by 69%, improving from prediabetic to optimal levels after 5 weeks (42). Additional metabolic markers improved, including C-peptide, which decreased by 41% as well as circulating fasting insulin, which decreased by 64%. Inflammatory markers also reduced, including IL-6, which reduced by 20%, and CRP, which reduced by 61%, the latter suggesting movement from high to average cardiac risk (43). Interestingly, these changes occurred independent of changes in weight or BMI.
Table 1
| Pre-Diet | Post-Diet | % Change | |
|---|---|---|---|
| Metabolic | |||
| HOMA-IR | 1.83 | 0.56 | -69.4% |
| C-peptide (ng/mL) | 1.7 | 1 | -41.2% |
| Fasting Insulin (µIU/mL) | 7.8 | 2.8 | -64.1% |
| Inflammatory | |||
| CRP (mg/L) | 7.25 | 2.86 | -60.6% |
| IL-6 (pg/mL) | 18.31 | 14.54 | -20.1% |
| Anthropometric | |||
| Weight (lbs) | 210.6 | 208.8 | -0.9% |
| BMI | 36.7 | 35.4 | -3.5% |
| Clinical Assessments | |||
| SAS | 15 | 3 | -80.0% |
| BPRS | 43 | 48 | +11.6% |
| Sub-domains: | |||
| Negative | 10 | 8 | |
| Positive | 6 | 7 | |
Pre- and post-diet outcomes in a woman with treatment-resistant schizophrenia and metabolic syndrome after a ketogenic diet for 5-weeks.
Antipsychotic-related EPS (i.e., pseudoparkinsonism), measured by the Simpson-Angus Scale (SAS) (44), reduced dramatically (15 to 3; -80%). Baseline score indicated a severe movement disorder, while post-diet level reflected a mild movement disorder, bordering on none. The Brief Psychiatric Rating Scale (BPRS) (45) total score was used to measure global psychopathology with symptom domain subscales calculated and included in Table 1. We noted a decrease in negative symptoms; however, her positive symptoms were rated higher at week 5 (physician and study team noted unit stressors potentially confounding the endpoint rating; more on this in the discussion), resulting in no dramatic change in total BPRS score (See Table 1). Clinical assessments were conducted by a blinded rater who was unaware of the participant's dietary intervention. Moderate side effects of tinnitus, hypersalivation, and stiffness were unlikely to be related to the diet but improved on KD. Keto flu symptoms were absent most weeks and were never rated above mild.
Discussion
This case describes metabolic, inflammatory, neurologic, and psychiatric outcomes in a woman with TRS and metabolic syndrome after 5 weeks on KD in an inpatient setting. Meals were curated by hospital dieticians, and the rater for clinical assessments was blinded to the participant's dietary intervention, mitigating bias.
This patient experienced improvement in metabolic markers of HOMA-IR, fasting insulin and C-peptide, and a downward trend throughout the diet in twice daily fingerstick glucose levels. This is notable given her history of type 2 diabetes and metabolic syndrome upon KD initiation and that she was only in ketosis consistently for 5 days. Additionally, inflammatory markers, CRP and IL-6, notably reduced, with the former bringing her risk for a cardiac event from high to average. IL-6 is implicated in SZ pathophysiology (46), and its reduction relates to successful SZ treatment (47). These are robust improvements despite a short time on diet therapy, inconsistent ketosis, and without a notable change in weight/BMI. Reducing several cardiac and metabolic risk factors could improve metabolic disease course and life expectancy in people taking antipsychotics.
Notably, EPS dramatically improved, aligning with reports on KD and Parkinson’s disease (48). If this holds in larger studies, KD could be extremely impactful for countering such a debilitating adverse effect of antipsychotic medications. It is possible that limited gluten consumption on KD contributed to improvements in neurological side effects, as there have been cases of neurologic improvements after a gluten-free diet in individuals with SZ (49). Neurologic issues such as gluten ataxia are seen in people with antibodies to gliadin, which is a protein within gluten, and the SZ population has high prevalence of these antibodies (50, 51). Also, KD is highly effective for epilepsy (52) and has shown efficacy in other neurologic diseases as well (53). Results from a Parkinsonian mouse model suggested that βHB, through its downstream conversion to succinate in the citric acid cycle, is neuroprotective through its ability to increase the rate of oxygen consumption through mitochondrial protein complex II and can improve parkinsonian-like motor deficits (54).
Given the participant's very short time consistently in ketosis (5 days), we did not expect to see significant improvement in her total BPRS score. While some improvements have been noted as early as two weeks (30, 34), other studies report maximum benefits over longer periods of time (31, 32). We did see a hint for decrease in negative symptoms, which is a notable improvement in this patient, who had not previously experienced relief in this domain, as is typical in TRS (55). Her week 5 positive symptom ratings were higher than baseline, which her psychiatrist and research staff believed was due to a series of upsetting altercations with another patient on the unit during the last two weeks of the study and menstruation during the last week, which is associated with worse psychosis in women (56, 57).
Despite endpoint stressors, our patient verbally reported many times that she benefitted from KD and stated she would like to try it again. From the reported data, it is noted that inconsistent ketosis, the short dietary duration, and the week 5 stressors precluded us from seeing global or positive symptom improvement that has been reported previously after KD in individuals with SZ (30–34). We acknowledge that these factors limit the generalizability of our case and comparability to other KD case studies and clinical trials. However, previous trials reported imperfect ketosis in their samples as well (32, 33), though to a lesser extent than our case. Ongoing clinical trials for KD in psychiatry are considering baseline ratings once the patient reaches ketosis, a design that mitigates intervention time spent out of ketosis and titration of diet during the intervention period, notable limitations of our study.
The delay in reaching βHB ≥ 0.5 mmol/L may be due to clozapine and olanzapine, which are known to induce insulin resistance, but also may be due to a high caloric diet at the beginning (some days > 3000 calories) (See Figure 2). However, even with inconsistent ketosis, our patient experienced large improvements in cardiometabolic and inflammatory markers and antipsychotic-associated Parkinsonian-like symptoms. This is intriguing as our data suggest that consistently meeting the ketosis threshold of βHB ≥ 0.5 mmol/L may not be necessary for certain metabolic, inflammatory, and neurological improvements, and that significant health benefits can occur on KD in a short amount of time. However, emerging clinical studies on KD for psychiatric symptom improvement suggest an optimal βHB range between 1–3 mmol/L (20). It remains unknown if the observed benefits, or additional ones, could occur in individuals with SZ in response to other low carbohydrate diets, such as the Mediterranean diet, which has been shown to improve depression (58) and relevant metabolic health markers (59) in samples without SZ.
Our case shines light on other needed data in the field. The potential that KD could improve negative symptoms would be revolutionary as current treatments for negative symptoms are lacking. Also, testing the use of other ketosis-boosting strategies, such as exogenous ketones for SZ treatment, may be an alternative and has shown benefits in other psychiatric and neurologic conditions (60). Lastly, as we advance in precision medicine psychiatry (61), determining genetic variants or biomarkers associated with treatment response will help guide us in selecting which patients may best respond to KD. In the epilepsy field, genetic variants may help predict which patients will have the most robust treatment response to KD (61–64). Thus, a genetic-metabolic approach may help guide future treatments in nutritional psychiatry.
This is the first case to report therapeutic effects of KD on inflammatory markers and antipsychotic-associated EPS in a person with SZ, and the first to report effects of KD specifically in someone with TRS. We also hint at the possibility that negative symptom improvements may be seen even in people with TRS. These results may suggest KD as a potential adjunct treatment to antipsychotics to counter adverse effects, improve quality of life, and life expectancy, if positive data continues to accumulate. This case, as well as other studies (30–34), demonstrate diverse health benefits of KD for individuals with mental illness, suggesting the need for continued research in this area (65). We also point out the challenges in this study of reaching ketosis in a TRS patient on metabolically impactful antipsychotics and that even low levels of ketosis could vastly improve metabolic health. Well-controlled clinical trials with patients in therapeutic ketosis are needed to establish the effects of KD as a treatment for SZ and, more specifically, TRS, and on related biomarkers and health factors.
Statements
Data availability statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by University of Maryland Institutional Review Board; Spring Grove Hospital Center Research Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
SM: Data curation, Formal analysis, Visualization, Writing – original draft, Writing – review & editing. GV: Investigation, Methodology, Supervision, Writing – review & editing. EE: Data curation, Investigation, Methodology, Project administration, Writing – review & editing. DR: Conceptualization, Investigation, Methodology, Writing – review & editing. HA: Conceptualization, Investigation, Methodology, Writing – review & editing. VH: Conceptualization, Investigation, Methodology, Writing – review & editing. AK: Conceptualization, Investigation, Methodology, Project administration, Writing – review & editing. MG: Conceptualization, Data curation, Investigation, Methodology, Project administration, Writing – review & editing. AY: Investigation, Writing – review & editing. JC: Supervision, Writing – review & editing. SP: Supervision, Writing – review & editing. BB: Conceptualization, Methodology, Writing – review & editing. KS: Conceptualization, Investigation, Methodology, Writing – review & editing. CP: Conceptualization, Investigation, Methodology, Supervision, Writing – review & editing. DK: Conceptualization, Funding acquisition, Investigation, Methodology, Resources, Supervision, Writing – review & editing, Data curation.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This project was supported in part by unrestricted donations to the University of Maryland Baltimore (UMB) Foundation and an award from the Brain and Behavior Research Foundation (BBRF).
Acknowledgments
We would like to thank additional team members for their work on this including Mackenzie Cervenka, Jacob Nudelman, Sharon August, and Claribel Okafor. This project was supported in part by unrestricted donations to the University of Maryland Baltimore (UMB) Foundation and an award from the Brain and Behavior Research Foundation (BBRF). Microsoft 365 Copilot was used to assist with coding certain visual elements of the figure graphs in R.
Conflict of interest
DK has served as a consultant for Alkermes, Boehringer Ingelheim, Karuna, and Teva. BB is an employee of and receives funding from the Institute for Clinical and Translational Research, which is funded by the National Center for Advancing Translational Sciences through the Clinical & Translational Science Awards Program, grant number UL1TR003098. BB has received support from Nutricia, Vitaflo, and The Carson Harris Foundation and personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with Nutricia, BioMarin, and Therachon, and has received royalties from Demos/Springer Publishing Company.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. Microsoft 365 Copilot was used to assist with coding in R to create certain visual elements of the figure graphs including how to add the legend and place the graphs side by side.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Association AP . Diagnostic and statistical manual of mental disorders, 5th ed. (2013) Washington, D.C.
2
Saha S Chant D Welham J McGrath J . A systematic review of the prevalence of schizophrenia. PloS Med. (2005) 2:e141. doi: 10.1371/journal.pmed.0020141
3
Nucifora FC Woznica E Lee BJ Cascella N Sawa A . Treatment resistant schizophrenia: Clinical, biological, and therapeutic perspectives. Neurobiol Dis. (2019) 131:104257. doi: 10.1016/j.nbd.2018.08.016
4
Palmer BA Pankratz VS Bostwick JM . The lifetime risk of suicide in schizophrenia: a reexamination. Arch Gen Psychiatry. (2005) 62:247–53. doi: 10.1001/archpsyc.62.3.247
5
Mitchell AJ Vancampfort D Sweers K van Winkel R Yu W De Hert M . Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders–a systematic review and meta-analysis. Schizophr Bull. (2013) 39:306–18. doi: 10.1093/schbul/sbr148
6
Mori N McEvoy JP Miller BJ . Total and differential white blood cell counts, inflammatory markers, adipokines, and the metabolic syndrome in phase 1 of the clinical antipsychotic trials of intervention effectiveness study. Schizophr Res. (2015) 169:30–5. doi: 10.1016/j.schres.2015.10.001
7
Dickerson F Stallings C Origoni A Vaughan C Khushalani S Yang S et al . C-reactive protein is elevated in schizophrenia. Schizophr Res. (2013) 143:198–202. doi: 10.1016/j.schres.2012.10.041
8
Goldsmith DR Rapaport MH Miller BJ . A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. (2016) 21:1696–709. doi: 10.1038/mp.2016.3
9
Dunleavy C Elsworthy RJ Upthegrove R Wood SJ Aldred S . Inflammation in first-episode psychosis: The contribution of inflammatory biomarkers to the emergence of negative symptoms, a systematic review and meta-analysis. Acta Psychiatr Scand. (2022) 146:6–20. doi: 10.1111/acps.13416
10
Moga CI Pavăl D Micluția IV . Outlining the absence: from inflammation to A distinct endophenotype for the negative symptoms of schizophrenia. Psychiatr Danub. (2024) 36:161–73. doi: 10.24869/psyd.2024.161
11
Wang AK Miller BJ . Meta-analysis of cerebrospinal fluid cytokine and tryptophan catabolite alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder, and depression. Schizophr Bull. (2018) 44:75–83. doi: 10.1093/schbul/sbx035
12
Goldsmith DR Rapaport MH . Inflammation and negative symptoms of schizophrenia: implications for reward processing and motivational deficits. Front Psychiatry. (2020) 11:46. doi: 10.3389/fpsyt.2020.00046
13
Muench J Hamer AM . Adverse effects of antipsychotic medications. Am Fam Physician. (2010) 81:617–22.
14
Li WT Huang XF Deng C Zhang BH Qian K He M et al . Olanzapine induces inflammation and immune response via activating ER stress in the rat prefrontal cortex. Curr Med Sci. (2021) 41:788–802. doi: 10.1007/s11596-021-2401-7
15
Leung JG Zhang L Markota M Ellingrod VL Gerberi DJ Bishop JR . A systematic review of clozapine-associated inflammation and related monitoring. Pharmacotherapy. (2023) 43:1364–96. doi: 10.1002/phar.2887
16
Chue P Lalonde JK . Addressing the unmet needs of patients with persistent negative symptoms of schizophrenia: emerging pharmacological treatment options. Neuropsychiatr Dis Treat. (2014) 10:777–89. doi: 10.2147/NDT.S43404
17
Kelly DL Demyanovich HK Rodriguez KM Ciháková D Talor MV McMahon RP et al . Randomized controlled trial of a gluten-free diet in patients with schizophrenia positive for antigliadin antibodies (AGA IgG): a pilot feasibility study. J Psychiatry Neurosci. (2019) 44:269–76. doi: 10.1503/jpn.180174
18
Chmiel I . Ketogenic diet in therapy of bipolar affective disorder - case report and literature review. Psychiatr Pol. (2022) 56:1345–63. doi: 10.12740/PP/OnlineFirst/136356
19
Decker DD Patel R Cheavens J Hayes SM Whitted W Lee AJ et al . A pilot study examining a ketogenic diet as an adjunct therapy in college students with major depressive disorder. Transl Psychiatry. (2025) 15:322. doi: 10.1038/s41398-025-03544-8
20
Campbell IH Needham N Grossi H Kamenska I Luz S Sheehan S et al . A pilot study of a ketogenic diet in bipolar disorder: clinical, metabolic and magnetic resonance spectroscopy findings. BJPsych Open. (2025) 11:e34. doi: 10.1192/bjo.2024.841
21
Edwards MGP Furuholmen-Jenssen T Søegaard EGI Thapa SB Andersen JR . Exploring diet-induced ketosis with exogenous ketone supplementation as a potential intervention in post-traumatic stress disorder: a feasibility study. Front Nutr. (2024) 11:1406366. doi: 10.3389/fnut.2024.1406366
22
Laurent N Bellamy EL Tague KA Hristova D Houston A . Ketogenic metabolic therapy for schizoaffective disorder: a retrospective case series of psychotic symptom remission and mood recovery. Front Nutr. (2025) 12:1506304. doi: 10.3389/fnut.2025.1506304
23
Laurent N . Retrospective case study: ketogenic metabolic therapy in the effective management of treatment-resistant depressive symptoms in bipolar disorder. Front Nutr. (2024) 11:1394679. doi: 10.3389/fnut.2024.1394679
24
Bellamy EL Laurent N . Transdiagnostic remission of psychiatric comorbidity in post-traumatic stress disorder, ADHD, and binge-eating disorder using ketogenic metabolic therapy: a retrospective case report. Front Nutr. (2025) 12:1600123. doi: 10.3389/fnut.2025.1600123
25
Calabrese L Frase R Ghaloo M . Complete remission of depression and anxiety using a ketogenic diet: case series. Front Nutr. (2024) 11:1396685. doi: 10.3389/fnut.2024.1396685
26
Calabrese L . Remission of OCD and ulcerative colitis with a ketogenic diet: Case Report. Front Psychiatry. (2025) 16:1541414. doi: 10.3389/fpsyt.2025.1541414
27
MacDonald AJ Palmer CM . Ketogenic diet as a therapeutic intervention for obsessive-compulsive disorder: a case series of three patients. Front Nutr. (2025) 12:1568076. doi: 10.3389/fnut.2025.1568076
28
Scolnick B Zupec-Kania B Calabrese L Aoki C Hildebrandt T . Remission from chronic anorexia nervosa with ketogenic diet and ketamine: case report. Front Psychiatry. (2020) 11:763. doi: 10.3389/fpsyt.2020.00763
29
Calabrese L Scolnick B Zupec-Kania B Beckwith C Costello K Frank GKW . Ketogenic diet and ketamine infusion treatment to target chronic persistent eating disorder psychopathology in anorexia nervosa: a pilot study. Eat Weight Disord. (2022) 27:3751–7. doi: 10.1007/s40519-022-01455-x
30
Palmer CM Gilbert-Jaramillo J Westman EC . The ketogenic diet and remission of psychotic symptoms in schizophrenia: Two case studies. Schizophr Res. (2019) 208:439–40. doi: 10.1016/j.schres.2019.03.019
31
Ford J Kyner J Mathalon D Sethi S Fryer S . Ketogenic diet improves metabolic and cognition function in psychotic spectrum disorders. Biol Psychiatry. (2025) 97:S1–73. doi: 10.1016/j.biopsych.2025.02.045
32
Sethi S Wakeham D Ketter T Hooshmand F Bjornstad J Richards B et al . Ketogenic diet intervention on metabolic and psychiatric health in bipolar and schizophrenia: A pilot trial. Psychiatry Res. (2024) 335:115866. doi: 10.1016/j.psychres.2024.115866
33
Danan A Westman EC Saslow LR Ede G . The ketogenic diet for refractory mental illness: A retrospective analysis of 31 inpatients. Front Psychiatry. (2022) 13:951376. doi: 10.3389/fpsyt.2022.951376
34
Pacheco A Easterling WS Pryer MW . A pilot study of the ketogenic diet in schizophrenia. Am J Psychiatry. (1965) 121:1110–1. doi: 10.1176/ajp.121.11.1110
35
Norwitz NG Sethi S Palmer CM . Ketogenic diet as a metabolic treatment for mental illness. Curr Opin Endocrinol Diabetes Obes. (2020) 27:269–74. doi: 10.1097/MED.0000000000000564
36
Brietzke E Mansur RB Subramaniapillai M Balanzá-Martínez V Vinberg M González-Pinto A et al . Ketogenic diet as a metabolic therapy for mood disorders: Evidence and developments. Neurosci Biobehav Rev. (2018) 94:11–6. doi: 10.1016/j.neubiorev.2018.07.020
37
Haces ML Hernández-Fonseca K Medina-Campos ON Montiel T Pedraza-Chaverri J Massieu L . Antioxidant capacity contributes to protection of ketone bodies against oxidative damage induced during hypoglycemic conditions. Exp Neurol. (2008) 211:85–96. doi: 10.1016/j.expneurol.2007.12.029
38
Allan NP Yamamoto BY Kunihiro BP Nunokawa CKL Rubas NC Wells RK et al . Ketogenic diet induced shifts in the gut microbiome associate with changes to inflammatory cytokines and brain-related miRNAs in children with autism spectrum disorder. Nutrients. (2024) 16, 1401. doi: 10.3390/nu16101401
39
Juge N Gray JA Omote H Miyaji T Inoue T Hara C et al . Metabolic control of vesicular glutamate transport and release. Neuron. (2010) 68:99–112. doi: 10.1016/j.neuron.2010.09.002
40
First MB Spitzer RL Miriam G Williams JBW Benjamin LS eds. Structured clinical interview for DSM-IV axis II personality disorders: SCID-II1997. Washington: American Psychiatric Press, Inc..
41
Kane J Honigfeld G Singer J Meltzer H . Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. (1988) 45:789–96. doi: 10.1001/archpsyc.1988.01800330013001
42
Severeyn E Velásquez J Perpiñán G Herrera H Wong S Díaz J et al eds. HOMA-IR assessment for impaired glucose tolerance, impaired fasting glucose and insulin resistance diagnosis. In: VIII Latin American Conference on Biomedical Engineering and XLII National Conference on Biomedical Engineering, vol. 2020 . Springer International Publishing, Cham.
43
LabCorp . C-reactive protein (CRP), high sensitivity. In: Cardiac Risk Assessment. Burlington, NC: Laboratory Corporation of America Holdings.
44
Simpson GM Angus JWS . A rating scale for extrapyramidal side effects. Acta Psychiatrica Scandinavica Supplementum. (1970) 212:11–9. doi: 10.1111/j.1600-0447.1970.tb02066.x
45
Overall JE Gorham DR . Brief psychiatric rating scale (BPRS). PsycTESTS. (1962). doi: 10.1037/t01554-000
46
Zhou X Tian B Han HB . Serum interleukin-6 in schizophrenia: A system review and meta-analysis. Cytokine. (2021) 141:155441. doi: 10.1016/j.cyto.2021.155441
47
Zhang L Zheng H Wu R Zhu F Kosten TR Zhang XY et al . Minocycline adjunctive treatment to risperidone for negative symptoms in schizophrenia: Association with pro-inflammatory cytokine levels. Prog Neuropsychopharmacol Biol Psychiatry. (2018) 85:69–76. doi: 10.1016/j.pnpbp.2018.04.004
48
Phillips MCL Murtagh DKJ Gilbertson LJ Asztely FJS Lynch CDP . Low-fat versus ketogenic diet in Parkinson’s disease: A pilot randomized controlled trial. Mov Disord. (2018) 33:1306–14. doi: 10.1002/mds.27390
49
Jackson J Eaton W Cascella N Fasano A Warfel D Feldman S et al . A gluten-free diet in people with schizophrenia and anti-tissue transglutaminase or anti-gliadin antibodies. Schizophr Res. (2012) 140:262–3. doi: 10.1016/j.schres.2012.06.011
50
Čiháková D Eaton WW Talor MV Harkus UH Demyanovich HK Rodriguez K et al . Gliadin-related antibodies in schizophrenia. Schizophr Res. (2018) 195:585–6. doi: 10.1016/j.schres.2017.08.051
51
Daniels EC Eaton WW Čiháková D Talor MV Lemke H Mo C et al . The relationship of peripheral inflammation with antibodies to gliadin (AGA IgG) in persons with schizophrenia. Schizophr Res. (2023) 256:50–1. doi: 10.1016/j.schres.2023.02.027
52
Sourbron J Klinkenberg S van Kuijk SMJ Lagae L Lambrechts D Braakman HMH et al . Ketogenic diet for the treatment of pediatric epilepsy: review and meta-analysis. Childs Nerv Syst. (2020) 36:1099–109. doi: 10.1007/s00381-020-04578-7
53
Pietrzak D Kasperek K Rękawek P Piątkowska-Chmiel I . The therapeutic role of ketogenic diet in neurological disorders. Nutrients. (2022) 14, 1952. doi: 10.3390/nu14091952
54
Tieu K Perier C Caspersen C Teismann P Wu DC Yan SD et al . D-beta-hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. J Clin Invest. (2003) 112:892–901. doi: 10.1172/JCI200318797
55
Lee MA Cola P Jayathilake K Meltzer HY . Long-term outcome of clozapine in treatment-resistant schizophrenia. J Clin Psychopharmacol. (2023) 43:211–9. doi: 10.1097/JCP.0000000000001671
56
Mazza M Marano G . Unmasking the cycle: Premenstrual and menstrual exacerbation of psychiatric disorders and impact on female mental health. World J Psychiatry. (2025) 15:107132. doi: 10.5498/wjp.v15.i8.107132
57
Seeman MV . Menstrual exacerbation of schizophrenia symptoms. Acta Psychiatr Scand. (2012) 125:363–71. doi: 10.1111/j.1600-0447.2011.01822.x
58
Jacka FN O’Neil A Opie R Itsiopoulos C Cotton S Mohebbi M et al . A randomized controlled trial of dietary improvement for adults with major depression (the ‘SMILES’ trial). BMC Med. (2017) 15:23. doi: 10.1186/s12916-017-0791-y
59
Dayi T Ozgoren M . Effects of the Mediterranean diet on the components of metabolic syndrome. J Prev Med Hyg. (2022) 63:E56–64. doi: 10.15167/2421-4248/jpmh2022.63.2s3.2747
60
Mohib O Bomans S Jimenez Garcia B Leemans L Ligneel C De Waele E et al . Clinical benefits of exogenous ketosis in adults with disease: A systematic review. Nutrients. (2025) 17, 3125. doi: 10.3390/nu17193125
61
Liu Y Fan L Yang H Wang D Liu R Shan T et al . Ketogenic therapy towards precision medicine for brain diseases. Front Nutr. (2024) 11:1266690. doi: 10.3389/fnut.2024.1266690
62
Wang D Liu Y Fan L Yang H Pan M Shi J et al . Improvement on BAF53B mutation caused developmental and epileptic encephalopathy with a ketogenic diet. Res Square. (2022). doi: 10.21203/rs.3.rs-1921317/v1
63
Ko A Jung DE Kim SH Kang HC Lee JS Lee ST et al . The efficacy of ketogenic diet for specific genetic mutation in developmental and epileptic encephalopathy. Front Neurol. (2018) 9:530. doi: 10.3389/fneur.2018.00530
64
Hsieh TY Su TY Hung KY Hsu MS Lin YJ Kuo HC et al . Ketogenic diet effectiveness is superior for drug resistant epilepsy with causative genetic mutation than those without genetic etiology. Epilepsy Behav. (2024) 161:110052. doi: 10.1016/j.yebeh.2024.110052
65
Kelly DL Kearns A Eberhardt E Vyas G Roche DJO Harrington V et al . Advancing the Study of Ketogenic Diets in Schizophrenia: Results of a Change.org Support Petition. Chicago, Illinois: Schizophrenia International Research Society (SIRS (2025). March 29, 2025 - April 2, 2025.
Appendix A
| Meal | Food items |
|---|---|
| Breakfast | Sausage & eggs |
| Lunch | Seasoned ground beef; green peppers; salad |
| 2 pm Snack | Pecans & cashews |
| Dinner | Chicken with green beans; macadamia nuts; salad |
| 8 pm Snack | KetoCal® 4:1 formula - vanilla/chocolate |
Example ketogenic diet menu (1 Day; 2855 calories).
Summary
Keywords
ketogenic diet, schizophrenia, metabolic syndrome, inflammation, insulin-resistance, extrapyramidal side effects
Citation
Murray SL, Vyas G, Eberhardt E, Roche DJO, Adams HA, Harrington V, Kearns A, Glassman M, Yuen A, Chiappelli J, Pugh S, Barron B, Sandow K, Palmer CM and Kelly DL (2025) Case Report: Metabolic, inflammatory, and neurological improvements after a ketogenic diet in a woman with treatment-resistant schizophrenia and metabolic syndrome. Front. Psychiatry 16:1710785. doi: 10.3389/fpsyt.2025.1710785
Received
22 September 2025
Accepted
30 October 2025
Published
20 November 2025
Volume
16 - 2025
Edited by
Declan McKernan, University of Galway, Ireland
Reviewed by
Carlo Ignazio Cattaneo, Novara Medical School, Italy
Yang Liu, University of Technology Sydney, Australia
Karin Huizer, Parnassia Group, Netherlands
Updates
Copyright
© 2025 Murray, Vyas, Eberhardt, Roche, Adams, Harrington, Kearns, Glassman, Yuen, Chiappelli, Pugh, Barron, Sandow, Palmer and Kelly.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sidney L. Murray, sidney.murray@som.umaryland.edu; Deanna L. Kelly, dlkelly@som.umaryland.edu
ORCID: Sidney L. Murray, orcid.org/0000-0002-6752-8438; Deanna L. Kelly, orcid.org/0000-0002-2176-518X; Deanna L. Kelly, orcid.org/0000-0002-2176-518X; Joshua Chiappelli, orcid.org/0000-0001-6121-7439; Daniel J. O. Roche, orcid.org/0000-0001-6298-1519; Valerie Harrington, orcid.org/0000-0001-7699-4089; Christopher M. Palmer, orcid.org/0000-0001-6104-7943; Heather A. Adams, orcid.org/0009-0005-5741-3652; Bobbie Barron, orcid.org/0000-0001-5390-794X
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.