- 1Department of Physics and Astronomy, University of Calgary, Calgary, AB, Canada
- 2Institute for Quantum Science and Technology, University of Calgary, Calgary, AB, Canada
- 3Hotchkiss Brain Institute, University of Calgary, Calgary, AB, Canada
Electromagnetic radiation at telecommunication frequencies has been reported to have biological effects, particularly affecting the production of reactive oxygen species, raising the question of potential mechanisms. In this study, we explored whether the radical pair mechanism (RPM) could account for these effects. Given that telecommunication frequencies are much higher than those associated with typical hyperfine interactions in biological systems, any effects would necessarily be non-resonant. Our computational simulations confirm that the RPM cannot explain these effects under experimental conditions due to the negligible influence of low-amplitude oscillating fields. We find that observable effects on radical pairs at telecommunication frequencies would require hyperfine coupling constants that are precisely fine-tuned to values far exceeding those naturally occurring in biological systems. We conclude that another mechanism must be responsible for the effects of telecommunication frequency fields in biological systems.
1 Introduction
Reactive oxygen species (ROS) are crucial signaling molecules within cells, playing vital roles in various physiological processes Terzi and Suter (2020); Sies and Jones (2020); Sies et al. (2017); Gurhan and Barnes (2023). However, excessive accumulation of ROS can trigger oxidative stress, causing substantial damage to lipids, proteins, and DNA Imlay (2013). This damage compromises cell function and is associated with the onset of various pathologies, including cancer, cardiovascular diseases, and neurodegenerative conditions Jackson and Bartek (2009); Handy and Loscalzo (2017); Incalza et al. (2018); Münzel et al. (2017); Tarafdar and Pula (2018); Sbodio et al. (2019). Thus, the regulation of ROS levels is essential for maintaining cellular integrity and preventing the progression of these pathologies Sies and Jones (2020).
Experimental evidence, detailed in Table 1, shows that electromagnetic radiation at telecommunication frequencies, particularly within the ultra-high frequency (UHF) range, can influence ROS levels even at low amplitudes. Studies utilizing exposure to UHF electromagnetic fields have observed changes in ROS production within various cell types. For instance, Luukkonen et al. reported that exposure to 872 MHz radiation at a specific absorption rate (SAR) of 5 W/kg induced ROS production and DNA damage in human SH-SY5Y neuroblastoma cells Luukkonen et al. (2009). Similarly, other investigations have shown increased ROS levels in human peripheral blood mononuclear cells Kazemi et al. (2015), human HEK293 cells Pooam et al. (2022), and astrocytes Campisi et al. (2010) following exposure at frequencies relevant to mobile communication technologies. Moreover, the effects extend beyond cellular responses to affect whole organisms. Research has shown that Drosophila exposed to DECT (Digital Enhanced Cordless Telephone) base radiation 1.88–1.90 GHz exhibited elevated ROS levels in both bodies and ovaries Manta et al. (2014). In addition, studies on human spermatozoa exposed to 2.45 GHz Wi-Fi radiation revealed significant oxidative stress damage, including increased ROS levels, DNA fragmentation, and decreased sperm motility and vitality Ding et al. (2018).
Weak magnetic fields (MFs) have been known to influence chemical reactions. Numerous studies have documented the effects of static magnetic fields (SMFs) on ROS production in various systems, highlighting their biological significance. For instance, Calabrò et al. reported that exposure to a static magnetic field of 2.2 mT significantly increased ROS production in human SH-SY5Y neuroblastoma cells Calabrò et al. (2013). Bekhite et al. found that static magnetic fields ranging from 0.2 mT to 5 mT elevated ROS levels in mouse embryoid bodies, indicating the role of ROS in SMF-induced differentiation processes Bekhite et al. (2013). Additionally, Martino and Castello demonstrated that low-level magnetic fields (45
The radical pair mechanism (RPM) can explain static magnetic field effects on chemical reactions, originating from spin chemistry, with magnetoreception in avian species being a notable example Hayashi (2004); Hochstoeger et al. (2020); Huelga and Plenio (2013); Johnsen and Lohmann (2005); Wiltschko and Wiltschko (2023); Ritz et al. (2000), Ritz et al. (2004); Zadeh-Haghighi and Simon (2022a); Maeda et al. (2008); Kerpal et al. (2019); Mouritsen (2022); Xu et al. (2021). This model is based on the creation of radical pairs—molecules with unpaired electron spins in the presence of nearby nuclear spins—that are exquisitely sensitive to weak MFs Timmel and Hore (1996); Hore (2012); Hore et al. (2020). Recent studies have illustrated how RPM can influence ROS production in live cells. Usselman et al. demonstrated that coherent electron spin dynamics in radical pairs formed at flavoenzyme centers can modulate ROS levels through singlet-triplet interconversion, impacted by both static and oscillating magnetic fields Usselman et al. (2016). Superoxide radicals
Given the established effects of static magnetic fields on ROS through the RPM, it is pertinent to investigate whether the observed effects of telecommunication frequency radiation on ROS could be due to oscillating magnetic fields (OMFs) in the context of the RPM. While the RPM generally requires a resonance between the magnetic field frequency and the energy level differences in the radical pairs to have a pronounced effect, our study investigates whether telecommunication frequencies might still produce subtle non-resonant effects Timmel and Hore (1996); Rodgers et al. (2005); Hiscock et al. (2017); Woodward et al. (1997), Woodward et al. (2001); Henbest et al. (2004); Leberecht et al. (2022), Leberecht et al. (2023). Our investigation confirms that the RPM cannot account for the observed effects of radio frequencies typically found within telecommunication devices on ROS levels. This shows the need to look for alternative mechanisms, possibly involving the electric component of the UHF fields.
2 Results
2.1 Radical pair mechanism
Radical pairs, formed during processes involving molecular bond breakage or electron transfer events, exhibit behavior dictated by their intrinsic angular momentum, represented by the spin quantum number
Hyperfine and Zeeman interactions. Radical pair dynamics are greatly influenced by Zeeman and hyperfine interactions. The Zeeman interaction aligns electron spins with an external magnetic field, altering energy states and influencing reaction pathways Improta and Barone (2004). This interaction is described by the following (Equation 1):
where
where
Spin dynamics and radical pair interconversion. The oscillation between the singlet and triplet states of radical pairs plays a crucial role in this dynamic Anisimov et al. (1983). These oscillations become pronounced when radicals are spatially separated enough to allow for coherent interconversion Hore (2021); Hore and Mouritsen (2016). The coherence in spin dynamics and the interconversion between singlet and triplet states can be mathematically described by the Liouville master equation, which governs the time evolution of the spin density matrix,
where the Liouvillian superoperator is defined as
where
Spin relaxation superoperator
where
Following insights from Timmel et al., the chemical fate of the radical pair is elucidated by considering separate spin-selective reactions for singlet and triplet pairs. These reactions are modelled with identical first-order rate constants,
and since
2.2 Specific absorption rate
The specific absorption rate is a key metric in assessing how electromagnetic fields affect biological tissues, particularly under radio frequency (RF) exposures. SAR measures the energy absorbed per unit mass of tissue, given in watts per kilogram (W/kg), and is vital for understanding the potential health impacts of RF radiation Poljak (2018). SAR provides a standardized way to compare exposure levels across different RF sources, making it important in regulatory settings. Experiments that investigate radio frequency effects on biological systems often report SAR values to quantify the intensity of exposure. For example, SAR data helps correlate RF exposure levels with biological outcomes like increased ROS production or DNA damage. To estimate the magnetic fields involved in these experiments, we use a conversion formula that relates SAR to magnetic field strengths, which assumes plane wave conditions. This estimation is crucial for aligning theoretical models with experimental conditions. The formula is as follows (Equation 7):
where
Given the experiment involving SH-SY5Y cells, where the SAR, dielectric constant
where
where
in which
2.3 Magnetic field effects on radical pair dynamics
By applying the principles discussed, we investigate how these fields influence the quantum states and reaction yields of radical pairs. The spin Hamiltonian for a typical radical pair system in the presence of a static magnetic field is expressed as Equation 11:
where
Focusing on ROS, we model a radical pair system comprising
Oscillating Magnetic Field. Oscillating magnetic fields introduce time-dependent dynamics that can alter radical pair processes, affecting the reactivity and yields of singlet and triplet products. We focus on the effects of radio frequency OMFs to assess whether the RPM can explain the observed ROS production under these conditions. The effect of an OMF on the system is incorporated into the Hamiltonian as follows (Equation 12):
where
For our calculations, we set the angle
In our model, the radical pair
Figure 1 illustrates how the triplet yield of our radical pair system responds to varying static magnetic field strengths and oscillating magnetic field frequencies, with an isotropic HFCC of
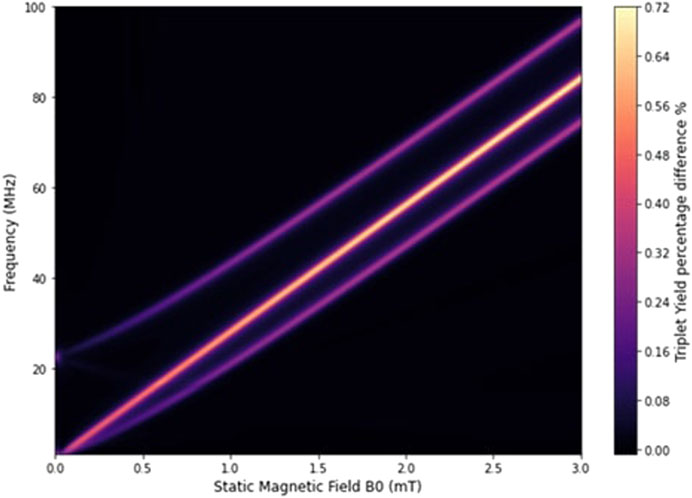
Figure 1. Variation in triplet yield with static magnetic field strength and radio frequency for a constant isotropic HFCC
where
One of these resonant peaks corresponds to the electron Larmor frequency, which is associated with transitions between specific energy levels of the radical pair system. For example, the transition from energy state
To investigate effects in higher-frequency fields, particularly those above 800 MHz, we studied the influence of the initial hyperfine coupling constant (
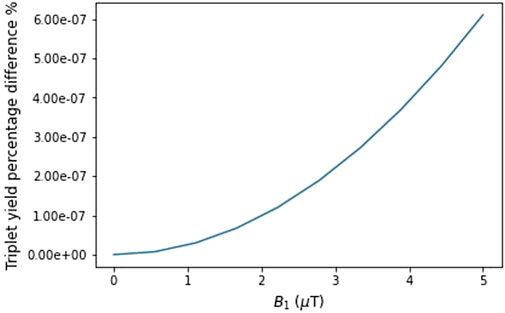
Figure 2. Effect of varying OMF amplitudes at 872 MHz from 0 to 5
The hyperfine coupling constant is a critical factor that modulates the response of radical pair systems to magnetic fields. It indicates a possible mechanism through which higher frequency fields can exert biological effects even when the static magnetic field aligns with Earth’s geomagnetic field strengths. Figure 3 addresses how varying the HFCC impact the triplet yield of a radical pair under a static magnetic field typical of the geomagnetic range.
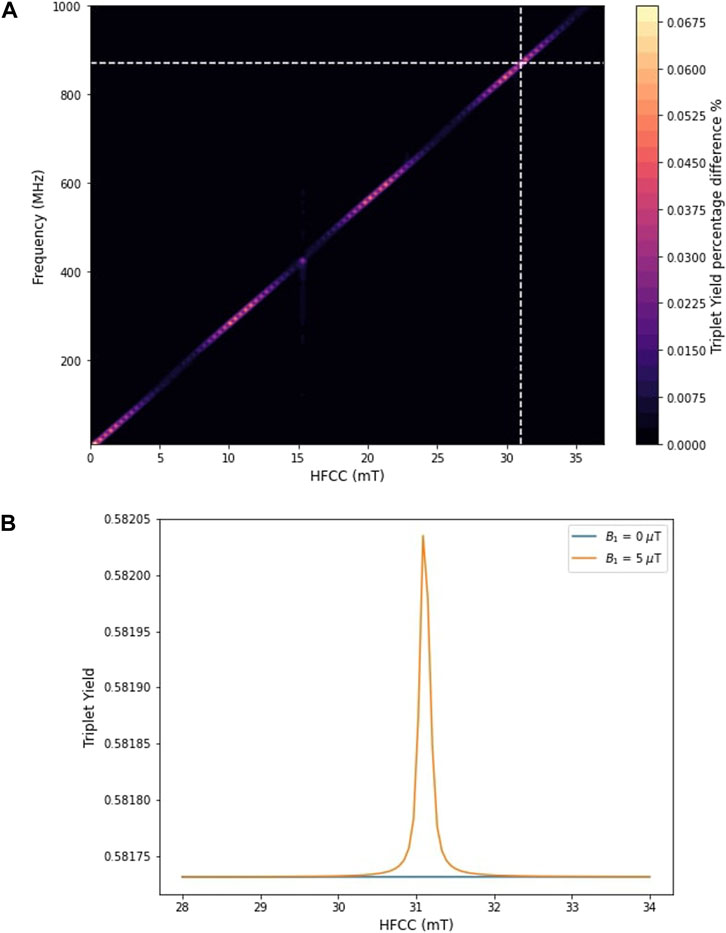
Figure 3. (A) Variation in triplet yield difference with and without OMF (
Our analysis reveals a direct correlation between the magnitude of the HFCC and the effect of an oscillating magnetic field on the triplet yield at higher frequencies. Notably, an HFCC of approximately 31.14 mT is required to observe an effect at a radio frequency of 872 MHz, shown as dashed white line on Figure 3A. Figure 3B further illustrates that the triplet yield varies significantly with HFCC, showing a narrow range around 31.14 mT where the effect of the oscillating magnetic field is maximized, which corresponds to a percentage difference of approximately 0.05% when comparing the triplet yield with and without the application of a 5
Nevertheless, given the minimal impact observed with the initial HFCC, we adjusted the HFCC to 31.14 mT to explore the influence of radio frequency on the triplet yield within a geomagnetic field range. We investigated the effects of varying reaction and relaxation rate constants while keeping the OMF amplitude fixed at 5
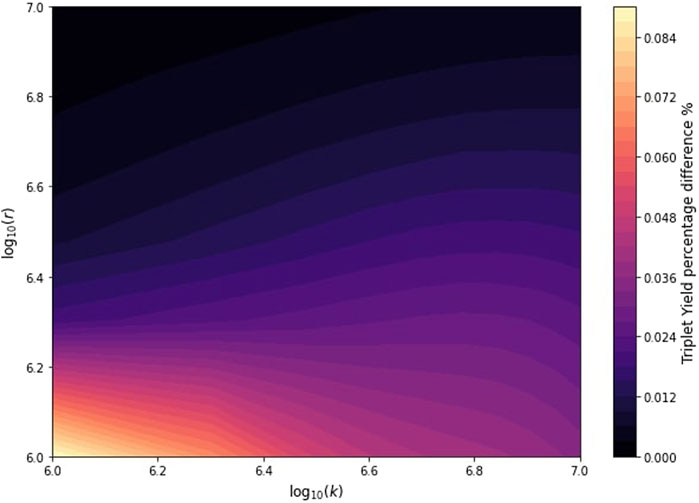
Figure 4. Analysis showing the percentage difference in triplet yield with and without an OMF at an amplitude of
3 Discussion
Consistent with simple resonance arguments, our study highlights the limitations of the radical pair mechanism in explaining the observed effects of radio frequency fields, particularly in the telecommunications range, such as 872 MHz. A critical finding of our analysis is the requirement for hyperfine coupling constants values, which are finely tuned and significantly higher than those observed in biological systems, to detect effects at these high frequencies under geomagnetic conditions Lee et al. (2014); Ban et al. (2013). Additionally, when we varied the OMF from 0 to 5
Therefore, while the RPM can well explain static magnetic field effects on ROS, it cannot account for the effects of telecommunication-frequency radiation on reactive oxygen species. Note that our modelling assumptions, such as utilizing a single nucleus H5, assuming an initial singlet state, and disregarding dipole-dipole and exchange interactions, have very likely led to an overestimation of the effects observed Efimova and Hore (2008); Kattnig and Hore (2017); Nohr et al. (2017); Babcock and Kattnig (2021); Luo (2023). Additionally, our choice of the spin relaxation rate constant
We also chose to focus on a radical pair system that directly involves
Several studies have described amplification routes that might enhance otherwise subtle RPM-based effects. For instance, a study demonstrated that an enzyme kinetic cycle could amplify small magnetic effects on radical pair recombination and Walleczek described magnetokinetic effects feeding into larger biochemical processes, including
Given these limitations, it seems plausible that these effects may be more attributable to the electrical component of the electromagnetic field in the context of telecommunication devices. Electric fields directly interact with charged cellular structures, particularly voltage-gated ion channels and membrane-bound enzymes, providing a non-magnetic route to ROS modulation Panagopoulos et al. (2021); Bertagna et al. (2021); Liu et al. (2024). Friedman et al. (2007) demonstrated that 875 MHz RF exposure activates NADH oxidase, leading to ROS generation, matrix metalloproteinase (MMP) activation, and epidermal growth factor receptor (EGFR) signaling, ultimately stimulating the extracellular signal-regulated kinase (ERK) cascade. Subsequently, Georgiou and Margaritis (2021) proposed that exposure to 875 MHz RF (0.240
Our work highlights the need for a broader investigation to account for the observed effects. In general, electromagnetic field effects, including those related to ROS modulation, may intersect with signaling pathways fundamental to cellular processes. Future studies could explore the role of such interactions in more complex systems, including potential implications for electromagnetic influences on neuronal communication.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
OT: Conceptualization, Investigation, Methodology, Formal analysis, Software, Validation, Writing – original draft, Writing – review and editing. HZ-H: Conceptualization, Methodology, Validation, Supervision, Writing – review and editing. CS: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Natural Sciences and Engineering Research Council through its Discovery Grant program and the Alliance Quantum Consortia grant ‘Quantum Enhanced Sensing and Imaging (QuEnSI)’, as well as the National Research Council of Canada through its Quantum Sensing Challenge Program.
Acknowledgments
The authors would like to thank Rishabh for his valuable comments and insights.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agarwal, A., Desai, N. R., Makker, K., Varghese, A., Mouradi, R., Sabanegh, E., et al. (2009). Effects of radiofrequency electromagnetic waves (RF-EMW) from cellular phones on human ejaculated semen: an in vitro pilot study. Fertil. Steril. 92, 1318–1325. doi:10.1016/j.fertnstert.2008.08.022
Anisimov, O. A., Bizyaev, V. L., Lukzen, N. N., Grigoryants, V. M., and Molin, Y. N. (1983). The induction of quantum beats by hyperfine interactions in radical-ion pair recombination. Chem. Phys. Lett. 101, 131–135. doi:10.1016/0009-2614(83)87356-4
Atkins, P. W., and Friedman, R. (2005). Molecular quantum mechanics. New York: Oxford University Press.
Babcock, N. S., and Kattnig, D. R. (2021). Radical scavenging could answer the Challenge posed by electron–electron dipolar interactions in the cryptochrome compass model. JACS Au 1, 2033–2046. doi:10.1021/jacsau.1c00332
Ban, F., Gauld, J. W., Wetmore, S. D., and Boyd, R. J. (2013). The calculation of the hyperfine coupling tensors of biological radicals. Dordrecht: Springer Netherlands, 285–322.
Bedard, K., and Krause, K.-H. (2007). The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 87, 245–313. doi:10.1152/physrev.00044.2005
Bekhite, M. M., Figulla, H.-R., Sauer, H., and Wartenberg, M. (2013). Static magnetic fields increase cardiomyocyte differentiation of Flk-1+ cells derived from mouse embryonic stem cells via Ca2+ influx and ROS production. Int. J. Cardiol. 167, 798–808. doi:10.1016/j.ijcard.2012.02.020
Bertagna, F., Lewis, R., Silva, S. R. P., McFadden, J., and Jeevaratnam, K. (2021). Effects of electromagnetic fields on neuronal ion channels: a systematic review. Ann. N. Y. Acad. Sci. 1499, 82–103. doi:10.1111/nyas.14597
Buttiglione, M., Roca, L., Montemurno, E., Vitiello, F., Capozzi, V., and Cibelli, G. (2007). Radiofrequency radiation (900 MHz) induces Egr-1 gene expression and affects cell-cycle control in human neuroblastoma cells. J. Cell. Physiology 213, 759–767. doi:10.1002/jcp.21146
Calabrò, E., Condello, S., Currò, M., Ferlazzo, N., Caccamo, D., Magazù, S., et al. (2013). Effects of low intensity static magnetic field on FTIR spectra and ROS production in SH-SY5Y neuronal-like cells. Bioelectromagnetics 34, 618–629. doi:10.1002/bem.21815
Campisi, A., Gulino, M., Acquaviva, R., Bellia, P., Raciti, G., Grasso, R., et al. (2010). Reactive oxygen species levels and DNA fragmentation on astrocytes in primary culture after acute exposure to low intensity microwave electromagnetic field. Neurosci. Lett. 473, 52–55. doi:10.1016/j.neulet.2010.02.018
De Iuliis, G. N., Newey, R. J., King, B. V., and Aitken, R. J. (2009). Mobile phone radiation induces reactive oxygen species production and DNA damage in human spermatozoa in vitro. PLoS ONE 4, e6446. doi:10.1371/journal.pone.0006446
Ding, S.-S., Sun, P., Zhang, Z., Liu, X., Tian, H., Huo, Y. W., et al. (2018). Moderate dose of trolox preventing the deleterious effects of wi-fi radiation on spermatozoa in vitro through reduction of oxidative stress damage. Chin. Med. J. Engl. 131, 402–412. doi:10.4103/0366-6999.225045
Efimova, O., and Hore, P. J. (2008). Role of exchange and dipolar interactions in the radical pair model of the avian magnetic compass. Biophysical J. 94, 1565–1574. doi:10.1529/biophysj.107.119362
Eichwald, C., and Walleczek, J. (1996). Model for magnetic field effects on radical pair recombination in enzyme kinetics. Biophysical J. 71, 623–631. doi:10.1016/S0006-3495(96)79263-9
Friedman, J., Kraus, S., Hauptman, Y., Schiff, Y., and Seger, R. (2007). Mechanism of short-term erk activation by electromagnetic fields at mobile phone frequencies. Biochem. J. 405, 559–568. doi:10.1042/BJ20061653
Georgiou, C. D., and Margaritis, L. H. (2021). Oxidative stress and nadph oxidase: connecting electromagnetic fields, cation channels and biological effects. Int. J. Mol. Sci. 22, 10041. doi:10.3390/ijms221810041
Gerson, F., and Huber, W. (2003). Electron spin resonance spectroscopy of organic radicals. Weinheim: Wiley VCH.
Gurhan, H., and Barnes, F. (2023). Impact of weak radiofrequency and static magnetic fields on key signaling molecules, intracellular ph, membrane potential, and cell growth in HT-1080 fibrosarcoma cells. Sci. Rep. 13, 14223. doi:10.1038/s41598-023-41167-5
Handy, D. E., and Loscalzo, J. (2017). Responses to reductive stress in the cardiovascular system. Free Radic. Biol. Med. 109, 114–124. doi:10.1016/j.freeradbiomed.2016.12.006
Hayashi, H. (2004). Introduction to dynamic spin chemistry: magnetic field effects on chemical and biochemical reactions, vol. 8 (World Scientific)
Henbest, K. B., Kukura, P., Rodgers, C. T., Hore, P. J., and Timmel, C. R. (2004). Radio frequency magnetic field effects on a radical recombination reaction: a diagnostic test for the radical pair mechanism. J. Am. Chem. Soc. 126, 8102–8103. doi:10.1021/ja048220q
Hernansanz-Agustín, P., and Enríquez, J. A. (2021). Generation of reactive oxygen species by mitochondria. Antioxidants 10, 415. doi:10.3390/antiox10030415
Hiscock, H. G., Mouritsen, H., Manolopoulos, D. E., and Hore, P. J. (2017). Disruption of magnetic compass orientation in migratory birds by radiofrequency electromagnetic fields. Biophysical J. 113, 1475–1484. doi:10.1016/j.bpj.2017.07.031
Hochstoeger, T., Al Said, T., Maestre, D., Walter, F., Vilceanu, A., Pedron, M., et al. (2020). The biophysical, molecular, and anatomical landscape of pigeon cry4: a candidate light-based quantal magnetosensor. Sci. Adv. 6, eabb9110. doi:10.1126/sciadv.abb9110
Hogben, H. (2011). Coherent spin dynamics of radical pairs in weak magnetic fields. Oxford, United Kingdom: University of Oxford. Ph.D. thesis.
Hogben, H. J., Efimova, O., Wagner-Rundell, N., Timmel, C. R., and Hore, P. (2009). Possible involvement of superoxide and dioxygen with cryptochrome in avian magnetoreception: origin of Zeeman resonances observed by in vivo EPR spectroscopy. Chem. Phys. Lett. 480, 118–122. doi:10.1016/j.cplett.2009.08.051
Hore, P. J. (2012). Are biochemical reactions affected by weak magnetic fields? Proc. Natl. Acad. Sci. U.S.A. 109, 1357–1358. doi:10.1073/pnas.1120531109
Hore, P. J. (2021). Radical quantum oscillations. Science 374, 1447–1448. doi:10.1126/science.abm9261
Hore, P. J. (2024). Spin chemistry in living systems. Natl. Sci. Rev. 11, nwae126. doi:10.1093/nsr/nwae126
Hore, P. J., Ivanov, K. L., and Wasielewski, M. R. (2020). Spin chemistry. J. Chem. Phys. 152, 120401. doi:10.1063/5.0006547
Hore, P. J., and Mouritsen, H. (2016). The radical-pair mechanism of magnetoreception. Annu. Rev. Biophysics 45, 299–344. doi:10.1146/annurev-biophys-032116-094545
Huelga, S. F., and Plenio, M. B. (2013). Vibrations, quanta and biology. Contemp. Phys. 54, 181–207. doi:10.1080/00405000.2013.829687
Imlay, J. A. (2013). The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat. Rev. Microbiol. 11, 443–454. doi:10.1038/nrmicro3032
Improta, R., and Barone, V. (2004). Interplay of electronic, environmental, and vibrational effects in determining the hyperfine coupling constants of organic free radicals. Chem. Rev. 104, 1231–1254. doi:10.1021/cr960085f
Incalza, M. A., D'Oria, R., Natalicchio, A., Perrini, S., Laviola, L., and Giorgino, F. (2018). Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharmacol. 100, 1–19. doi:10.1016/j.vph.2017.05.005
Jackson, S. P., and Bartek, J. (2009). The DNA-damage response in human biology and disease. Nature 461, 1071–1078. doi:10.1038/nature08467
Johnsen, S., and Lohmann, K. J. (2005). The physics and neurobiology of magnetoreception. Nat. Rev. Neurosci. 6, 703–712. doi:10.1038/nrn1745
Kattnig, D. R., Evans, E. W., Déjean, V., Dodson, C. A., Wallace, M. I., Mackenzie, S. R., et al. (2016). Chemical amplification of magnetic field effects relevant to avian magnetoreception. Nat. Chem. 8, 384–391. doi:10.1038/nchem.2447
Kattnig, D. R., and Hore, P. J. (2017). The sensitivity of a radical pair compass magnetoreceptor can Be significantly amplified by radical scavengers. Sci. Rep. 7, 11640. doi:10.1038/s41598-017-09914-7
Kazemi, E., Mortazavi, S. M. J., Ali-Ghanbari, A., Sharifzadeh, S., Ranjbaran, R., Mostafavi-Pour, Z., et al. (2015). Effect of 900 MHz electromagnetic radiation on the induction of ROS in human peripheral blood mononuclear cells. J. Biomed. Phys. Eng. 5, 105–114.
Kerpal, C., Richert, S., Storey, J. G., Pillai, S., Liddell, P. A., Gust, D., et al. (2019). Chemical compass behaviour at microtesla magnetic fields strengthens the radical pair hypothesis of avian magnetoreception. Nat. Commun. 10, 3707. doi:10.1038/s41467-019-11655-2
Leberecht, B., Kobylkov, D., Karwinkel, T., Döge, S., Burnus, L., Wong, S. Y., et al. (2022). Broadband 75–85 MHz radiofrequency fields disrupt magnetic compass orientation in night-migratory songbirds consistent with a flavin-based radical pair magnetoreceptor. J. Comp. Physiology A 208, 97–106. doi:10.1007/s00359-021-01537-8
Leberecht, B., Wong, S. Y., Satish, B., Döge, S., Hindman, J., Venkatraman, L., et al. (2023). Upper bound for broadband radiofrequency field disruption of magnetic compass orientation in night-migratory songbirds. Proc. Natl. Acad. Sci. U.S.A. 120, e2301153120. doi:10.1073/pnas.2301153120
Lee, A. A., Lau, J. C. S., Hogben, H. J., Biskup, T., Kattnig, D. R., and Hore, P. J. (2014). Alternative radical pairs for cryptochrome-based magnetoreception. J. R. Soc. Interface 11, 20131063. doi:10.1098/rsif.2013.1063
Liu, L., Huang, B., Lu, Y., Zhao, Y., Tang, X., and Shi, Y. (2024). Interactions between electromagnetic radiation and biological systems. iScience 27, 109201. doi:10.1016/j.isci.2024.109201
Liu, Y., Fiskum, G., and Schubert, D. (2002). Generation of reactive oxygen species by the mitochondrial electron transport chain. J. Neurochem. 80, 780–787. doi:10.1046/j.0022-3042.2002.00744.x
Luo, J. (2022). Spin dynamics of radical pairs in magnetic fields. Oxford, United Kingdom: University of Oxford. Ph.D. thesis.
Luo, J.-L. (2023). On the anisotropic weak magnetic field effect in radical-pair reactions. J. Chem. Phys. 158, 234302. doi:10.1063/5.0149644
Luukkonen, J., Hakulinen, P., Mäki-Paakkanen, J., Juutilainen, J., and Naarala, J. (2009). Enhancement of chemically induced reactive oxygen species production and DNA damage in human SH-SY5Y neuroblastoma cells by 872 MHz radiofrequency radiation. Mutat. Research/Fundamental Mol. Mech. Mutagen. 662, 54–58. doi:10.1016/j.mrfmmm.2008.12.005
Maeda, K., Henbest, K. B., Cintolesi, F., Kuprov, I., Rodgers, C. T., Liddell, P. A., et al. (2008). Chemical compass model of avian magnetoreception. Nature 453, 387–390. doi:10.1038/nature06834
Manta, A. K., Stravopodis, D. J., Papassideri, I. S., and Margaritis, L. H. (2014). Reactive oxygen species elevation and recovery in Drosophila bodies and ovaries following short-term and long-term exposure to DECT base EMF. Electromagn. Biol. Med. 33, 118–131. doi:10.3109/15368378.2013.791991
Martino, C. F., and Castello, P. R. (2011). Modulation of hydrogen peroxide production in cellular systems by low level magnetic fields. PLoS ONE 6, e22753. doi:10.1371/journal.pone.0022753
Moghadam, Z. M., Henneke, P., and Kolter, J. (2021). From flies to men: ROS and the NADPH oxidase in phagocytes. Front. Cell. Dev. Biol. 9, 628991. doi:10.3389/fcell.2021.628991
Mouritsen, H. (2022). “Magnetoreception in birds and its use for long-distance migration,” in Sturkie’s avian physiology. Editors C. G. Scanes, and S. Dridi, Seventh Edition (San Diego: Academic Press), 233–256.
Münzel, T., Camici, G. G., Maack, C., Bonetti, N. R., Fuster, V., and Kovacic, J. C. (2017). Impact of oxidative stress on the heart and vasculature: Part 2 of a 3-Part Series. J. Am. Coll. Cardiol. 70, 212–229. doi:10.1016/j.jacc.2017.05.035
Murphy, M. P. (2009). How mitochondria produce reactive oxygen species. Biochem. J. 417, 1–13. doi:10.1042/bj20081386
Nohr, D., Paulus, B., Rodriguez, R., Okafuji, A., Bittl, R., Schleicher, E., et al. (2017). Determination of radical–radical distances in light-active proteins and their implication for biological magnetoreception. Angew. Chem. Int. Ed. 56, 8550–8554. doi:10.1002/anie.201700389
Panagopoulos, D. J., Karabarbounis, A., Yakymenko, I., and Chrousos, G. P. (2021). Human-made electromagnetic fields: ion forced-oscillation and voltage-gated ion channel dysfunction, oxidative stress and dna damage (review). Int. J. Oncol. 59, 92. Epub 2021 Oct 7. doi:10.3892/ijo.2021.5272
Player, T. (2021). Spin dynamics calculations applied to systems of potential biological significance. Oxford, United Kingdom: University of Oxford. Ph.D. thesis.
Player, T. C., Baxter, E. D. A., Allatt, S., and Hore, P. J. (2021). Amplification of weak magnetic field effects on oscillating reactions. Sci. Rep. 11, 9615. doi:10.1038/s41598-021-88871-8
Player, T. C., and Hore, P. J. (2019). Viability of superoxide-containing radical pairs as magnetoreceptors. J. Chem. Phys. 151, 225101. doi:10.1063/1.5129608
Poljak, D. (2018). “Chapter 7 - on the use of boundary integral methods in bioelectromagnetics,” in Numerical methods and advanced simulation in biomechanics and biological processes. Editors M. Cerrolaza, S. J. Shefelbine, and D. Garzón-Alvarado (Academic Press), 119–143.
Pooam, M., Jourdan, N., Aguida, B., Dahon, C., Baouz, S., Terry, C., et al. (2022). Exposure to 1.8 ghz radiofrequency field modulates ros in human HEK293 cells as a function of signal amplitude. Commun. and Integr. Biol. 15, 54–66. doi:10.1080/19420889.2022.2027698
Rishabh, R., Zadeh-Haghighi, H., Salahub, D., and Simon, C. (2022). Radical pairs may explain reactive oxygen species-mediated effects of hypomagnetic field on neurogenesis. PLoS Comput. Biol. 18, e1010198. doi:10.1371/journal.pcbi.1010198
Rishabh, R., Zadeh-Haghighi, H., and Simon, C. (2023). Radical pairs and superoxide amplification can explain magnetic field effects on planarian regeneration. bioRxiv. doi:10.1101/2023.12.11.571125
Ritz, T., Adem, S., and Schulten, K. (2000). A model for photoreceptor-based magnetoreception in birds. Biophys. J. 78, 707–718. doi:10.1016/s0006-3495(00)76629-x
Ritz, T., Thalau, P., Phillips, J. B., Wiltschko, R., and Wiltschko, W. (2004). Resonance effects indicate a radical-pair mechanism for avian magnetic compass. Nature 429, 177–180. doi:10.1038/nature02534
Rodgers, C. T., Henbest, K. B., Kukura, P., Timmel, C. R., and Hore, P. J. (2005). Low-field optically detected EPR spectroscopy of transient photoinduced radical pairs. J. Phys. Chem. A 109, 5035–5041. doi:10.1021/jp050765z
Sbodio, J. I., Snyder, S. H., and Paul, B. D. (2019). Redox mechanisms in neurodegeneration: from disease outcomes to therapeutic opportunities. Antioxid. Redox Signal. 30, 1450–1499. doi:10.1089/ars.2017.7321
Sefidbakht, Y., Moosavi-Movahedi, A. A., Hosseinkhani, S., Khodagholi, F., Torkzadeh-Mahani, M., Foolad, F., et al. (2014). Effects of 940 MHz EMF on bioluminescence and oxidative response of stable luciferase producing HEK cells. Photochem Photobiol. Sci. 13, 1082–1092. doi:10.1039/c3pp50451d
Sies, H., Berndt, C., and Jones, D. P. (2017). Oxidative stress. Annu. Rev. Biochem. 86, 715–748. doi:10.1146/annurev-biochem-061516-045037
Sies, H., and Jones, D. P. (2020). Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell. Biol. 21, 363–383. doi:10.1038/s41580-020-0230-3
Tarafdar, A., and Pula, G. (2018). The role of NADPH oxidases and oxidative stress in neurodegenerative disorders. Int. J. Mol. Sci. 19, 3824. doi:10.3390/ijms19123824
Terzi, A., and Suter, D. M. (2020). The role of NADPH oxidases in neuronal development. Free Radic. Biol. Med. 154, 33–47. doi:10.1016/j.freeradbiomed.2020.04.027
Timmel, C. R., and Hore, P. J. (1996). Oscillating magnetic field effects on the yields of radical pair reactions. Chem. Phys. Lett. 257, 401–408. doi:10.1016/0009-2614(96)00466-6
Timmel, C. R., Till, U., Brocklehurst, B., Mclauchlan, K. A., and Hore, P. J. (1998). Effects of weak magnetic fields on free radical recombination reactions. Mol. Phys. 95, 71–89. doi:10.1080/002689798167395
Usselman, R. J., Chavarriaga, C., Castello, P. R., Procopio, M., Ritz, T., Dratz, E. A., et al. (2016). The quantum biology of reactive oxygen species partitioning impacts cellular bioenergetics. Sci. Rep. 6, 38543. doi:10.1038/srep38543
Usselman, R. J., Hill, I., Singel, D. J., and Martino, C. F. (2014). Spin biochemistry modulates reactive oxygen species (ROS) production by radio frequency magnetic fields. PLOS ONE 9, e93065. doi:10.1371/journal.pone.0093065
Walleczek, J. (1995). Magnetokinetic effects on radical pairs: a paradigm for magnetic field interactions with biological systems at lower than thermal energy, 395, 420. doi:10.1021/ba-1995-0250.ch022
Wiltschko, R., and Wiltschko, W. (2023). Animal navigation: how animals use environmental factors to find their way. Eur. Phys. J. Spec. Top. 232, 237–252. doi:10.1140/epjs/s11734-022-00610-w
Woodward, J., Jackson, R., Timmel, C., Hore, P., and McLauchlan, K. (1997). Resonant radiofrequency magnetic field effects on a chemical reaction. Chem. Phys. Lett. 272, 376–382. doi:10.1016/s0009-2614(97)00542-3
Woodward, J. R., Timmel, C. R., McLauchlan, K. A., and Hore, P. J. (2001). Radio frequency magnetic field effects on electron-hole recombination. Phys. Rev. Lett. 87, 077602. doi:10.1103/physrevlett.87.077602
Xu, J., Jarocha, L. E., Zollitsch, T., Konowalczyk, M., Henbest, K. B., Richert, S., et al. (2021). Magnetic sensitivity of cryptochrome 4 from a migratory songbird. Nature 594, 535–540. doi:10.1038/s41586-021-03618-9
Zadeh-Haghighi, H., and Simon, C. (2022a). Magnetic field effects in biology from the perspective of the radical pair mechanism. J. R. Soc. Interface 19, 20220325. doi:10.1098/rsif.2022.0325
Zadeh-Haghighi, H., and Simon, C. (2022b). Radical pairs can explain magnetic field and lithium effects on the circadian clock. Sci. Rep. 12, 269. doi:10.1038/s41598-021-04334-0
Keywords: radical pair mechanism (RPM), reactive oxygen species (ROS), electromagnetic fields, telecommunication frequency, oscillating magnetic field (OMF)
Citation: Talbi O, Zadeh-Haghighi H and Simon C (2025) The radical pair mechanism cannot explain telecommunication frequency effects on reactive oxygen species. Front. Quantum Sci. Technol. 4:1544473. doi: 10.3389/frqst.2025.1544473
Received: 13 December 2024; Accepted: 25 March 2025;
Published: 09 April 2025.
Edited by:
Marco Cavaglià, Polytechnic University of Turin, ItalyReviewed by:
Abdulbast A. Abushgra, Ashland University, United StatesRichard H. W. Funk, Technical University Dresden, Germany
Copyright © 2025 Talbi, Zadeh-Haghighi and Simon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Owaiss Talbi, b3dhaXNzLnRhbGJpQHVjYWxnYXJ5LmNh; Christoph Simon, Y3NpbW9AdWNhbGdhcnkuY2E=
 Owaiss Talbi
Owaiss Talbi Hadi Zadeh-Haghighi
Hadi Zadeh-Haghighi Christoph Simon1,2,3*
Christoph Simon1,2,3*