- 1Unity of Child Neurology and Psychiatry, IRCCS Mondino Foundation, Pavia, Italy
- 2Department of Brain and Behavioral Sciences, University of Pavia, Pavia, Italy
- 3Scientific Institute, IRCCS E. Medea, Bosisio Parini (LC), Italy
- 4Department of Clinical and Experimental Sciences, University of Brescia, Brescia, Italy
- 5Unit of Child Neurology and Psychiatry, ASST Spedali Civili of Brescia, Brescia, Italy
- 6Department of Human and Social Sciences, Universitas Mercatorum, Roma, Italy
- 7Department of Human and Social Sciences, University of Bergamo, Bergamo, Italy
Introduction: Cerebral Palsy (CP) refers to a heterogeneous group of disorders resulting from early brain injury during development. The clinical and functional consequences are variable, but primarily characterized by motor and postural deficits that limit independence in activities of daily living, impacting child's and family's quality of life. There is consensus on the effectiveness of rehabilitative interventions when started early and administered intensively, leveraging neuronal plasticity. The Hand-Arm Bimanual Intensive Training Including Lower Extremities (HABIT-ILE) rehabilitation approach was developed to improve motor skills in children with CP, focusing on bimanual activities with integration of the lower limbs. The aim of this study is to present an intensive, individualized motor training protocol, based on HABIT-ILE principles, tailored for children and adolescents with CP.
Methods: To develop the protocol, we conducted a review of literature on HABIT-ILE applications. Additionally, we carried out multidisciplinary focus groups with professionals from three Italian Centers. These discussions focused on therapeutic setting, identifying materials, structuring play activities, to define strategies to enhance applicability and impact of the protocol.
Results: An intensive intervention protocol based on HABIT-ILE was developed. It consists of 30 h over 10 consecutive days, with daily sessions of 3 h. The intervention, structured around bimanual activities and lower limb involvement, was personalized according to clinical and motivational profile and conceived to be administered in pairs to children aged 6–17 years with CP and upper limb asymmetry. Sessions are divided into three components: bimanual tasks, occupational activities, and gross-motor activities, ensuring a global approach and enhancing neuroplasticity. Daily activities are selected by patients from a predetermined pool chosen by therapists, based on individual profiles and adapted progressively.
Discussion: The HABIT-ILE model represents an intensive and individualized approach for improving motor abilities in these patients. Our protocol, including personalization in an ecological context and pairwork, could increase motivation, adherence, and ultimately therapy effectiveness. We plan to verify feasibility, clinical effectiveness and sustainability of this model in multicenter contexts. Ongoing trials will provide evidence of applicability and efficacy, combined with non-invasive brain stimulation (NIBS) techniques such as transcutaneous vagus nerve stimulation or transcranial alternating current stimulation.
Clinical Trial Registration: ClinicalTrials.gov, Identifiers NCT06372028 and NCT06372041.
Introduction
Cerebral palsy (CP) is a group of disorders that affects an individual's movement, posture and balance (1) caused by early brain damage during development, affecting mainly motor development and overall functional independence. Moreover, motor disorders are often accompanied by impairments in sensation, perception, intelligence, communication, behavior, epilepsy, and secondary musculoskeletal problems (2).
CP has a significant impact on the quality of life (QoL) of children and their families, affecting a child's ability to manage daily self-care tasks and participate in recreational and learning activities (3) primarily because of reduced manual abilities and gross motor function (4).
Given its impact, CP represents one of the most common causes of childhood disability. A recent systematic review and meta-analysis by McIntyre and colleagues (5) provided an updated prevalence rate of CP: the authors outlined a slight decreasing trend in pre/perinatal cases, likely due to improvements in medical care, reporting a prevalence of 1.6 cases per 1,000 live births, while post-neonatal cases remained relatively stable at 0.8 per 10,000 live births.
Considering the complex nature of these conditions and the implications in daily activities, it is necessary to develop a multidimensional rehabilitative treatment tailored to individual needs (6, 7). Current approaches include pharmacological treatments and surgical interventions, complemented by innovative rehabilitation approaches (8). A growing body of evidence highlights the importance of intensive, early, and targeted rehabilitation interventions to promote neuroplasticity during critical developmental periods and improve motor and functional outcomes, achieving long-term benefits (7–10). Among the most effective experimental approaches, goal-directed training models have shown superior efficacy compared to simple isolated movement repetitions. These models encourage children to engage in complex motor activities that integrate into daily life, thereby enhancing functional autonomy (9, 11).
In recent years, intensive motor rehabilitation protocols for children with CP have achieved notable success, particularly those focusing on upper limb function, such as Constraint-Induced Movement Therapy (CIMT) and bimanual intensive therapy, which have gained traction for their demonstrated efficacy in improving upper-limb functionality in children with unilateral CP. These therapies emphasize the necessity of high-intensity, repetitive, goal-directed practice to foster motor learning and neural adaptations (12). Systematic reviews further support the idea that interventions combining intensity, task specificity, and individualized goals are more likely to achieve meaningful improvements in motor and functional outcomes (13).
Within the most studied motor training models, a particular approach is represented by the Hand-Arm Bimanual Intensive Therapy (HABIT) model, currently integrated into clinical rehabilitation practice. Through intensive motor training, HABIT aims to improve coordination and functional use of the upper limbs (6). Given that children with unilateral CP often experience limitations not only in their upper limbs but also in their lower limbs, the HABIT model has been adapted over time to include lower-extremity training, leading to the development of Hand-Arm Bimanual Intensive Therapy Including Lower Extremities (HABIT-ILE). This approach integrates upper and lower limb exercises to improve postural control and walking abilities (14). The HABIT-ILE approach is grounded in motor learning principles and has been shown to significantly enhance motor skills across all four limbs while promoting greater functional independence (6, 15). It also leads to improvements in visuospatial skills and brain structure and connectivity, such as in corticospinal tract fibers or in the fronto-parietal network underlying motor skill learning (16–18). The development of these structures is highly dependent on activity. Structured and repetitive motor training, such as in the HABIT-ILE approach, improves synaptic refinement, strengthens appropriate connections, and facilitates motor cortex reorganization, with positive effects on brain plasticity and motor learning (19–21). By integrating bimanual coordination with lower extremity training, HABIT-ILE takes a comprehensive approach to motor rehabilitation, targeting both upper and lower limb functions to address motor control and functional limitations holistically (6).
Despite the proven efficacy of intensive motor training approaches, there remains a need for rehabilitation protocols that are both highly individualized and capable of addressing the complex motor impairments associated with CP. HABIT-ILE's unique integration of upper and lower limb training offers a promising avenue for enhancing neuroplasticity and functional outcomes. This study aims to develop a customized intensive motor training protocol inspired by the HABIT-ILE approach, specifically tailored for children with CP.
Materials and methods
Starting from an analysis and review of the literature concerning the HABIT-ILE approach, a multidisciplinary group of healthcare professionals, including rehabilitation therapists, child neuropsychiatrists, psychologists and researchers from three Italian Centers — I.R.C.C.S. Medea in Bosisio Parini (Lecco), I.R.C.C.S. Mondino in Pavia, and ASST Spedali Civili in Brescia — conducted focus groups to define materials, settings, and play-based tasks for different severity levels of motor functional impairments.
Throughout this process, the protocol was tailored for children and adolescents diagnosed with unilateral or bilateral spastic cerebral palsy, exhibiting significant upper limb involvement and asymmetry, and aged between 6 and 17 years.
More specifically, individuals with upper limb disabilities classified at levels I-III on the Manual Ability Classification System1 (MACS), and associated motor deficits classified at levels I-III on the Gross Motor Function Classification System2 (GMFCS) will be included.
Exclusion criteria will comprise children and adolescents classified at levels IV and V on the MACS and those with associated motor deficits classified at levels IV and V on the GMFCS. Furthermore, other exclusion criteria will include subjects with severe visual impairment, defined as levels IV and V on the Visual Function Classification System3 (VFCS), and those with severe cognitive disabilities, defined as an Intelligence Quotient (IQ) ≤ 50. We also defined that children will be treated in pairs and matched based on demographic and clinical-functional criteria, focusing on the severity of the upper limb impairment, intellectual functioning, and/or age, meeting at least two of these three criteria. These three aspects allow clinicians to propose rehabilitative activities that are adequate to clinical features and suitable for both the patients of the pair. Moreover, during the training period, each proposed activity can be further personalized based on individual interests. Participants' upper limb motor performance will be classified using MACS, with levels ranging from low to moderate impairment (I–II) or moderate to severe (II–III). Regarding gross motor abilities, they are considered in the patients’ selection and also in the clinical assessment of included participants but they don't represent a matching variable, according to the upper limb focus in our training protocol.
Results
The protocol, based on the principles of the HABIT-ILE model, focuses particularly on the involvement of the upper limbs in bimanual activities for patients with CP.
The rehabilitation intervention spreads over two consecutive weeks, with 5 weekly sessions, each lasting 3 h, for a total of 30 therapy hours. The training activities are led by a rehabilitation therapist (Neurodevelopmental Therapist, Occupational Therapist, or Physical Therapist), within a 1–2 therapist model, ensuring that the intervention remains personalized and goal-oriented.
The activities in the protocol are specifically designed and tailored to the clinical (MACS level) and demographic profiles of the participants, with the aim of improving treatment adherence and promoting greater engagement. This personalized approach ensures that each session is adjusted according to the individual needs of each patient, thus optimizing the overall effectiveness of the treatment. Each daily session is structured in three components: the first one involves fine motor activities, the second one focuses on daily life tasks chosen by the participants to stimulate motivation, and the third one includes gross motor activities, particularly adapted sports that promote postural control and the use of the lower limbs. These components are designed to offer a balanced and gradual approach, optimizing the benefits of rehabilitation. The rehabilitative activities designed in the study are based on principles of motor learning and problem-solving, with the patient being involved in finding strategies to reach a predefined goal. These strategies involve a complex interaction between perceptual, cognitive, and motor systems to carry out daily life activities. The repetition of actions and movements, along with a progressive increase in the complexity of tasks, stimulates mechanisms of brain plasticity, optimizing the benefits of the intervention and improving overall outcomes.
To ensure the effectiveness of the treatment and maintain motivation, it is crucial that the environment in which it takes place is ecological, highly motivating, play-conducive, comfortable, and suitable for children and adolescents. On the first day of activities, there will be a 30-minutes session for caregivers and children. This session is aimed at identifying their main interests and the main difficulties they encounter in their daily lives that have the greatest impact on their quality of life. Patients will then select activities from a pool of options proposed by the therapist and tailored to their clinical characteristics.
It starts with a focus on bimanual activities and then gradually introduces exercises for the lower limbs, which require postural control, balance, and gross motor movements. During the two weeks of treatment, the activities are modified to maintain an appropriate level of difficulty, stimulating motivation without causing frustration or dissatisfaction. Each task is designed to give the child the opportunity to achieve success, encouraging participation.
The activities are personalized based on the functional limitations of the upper limbs and differentiated according to the child's MACS level, organized in increasing order of difficulty. This approach ensures a targeted and effective treatment, improving tolerability and overall outcomes. Moreover, to maintain high motivation, daily activities can be framed within a broader theme, such as a trip around the world or a magical adventure.
The components of the three-hour daily treatment are detailed below.
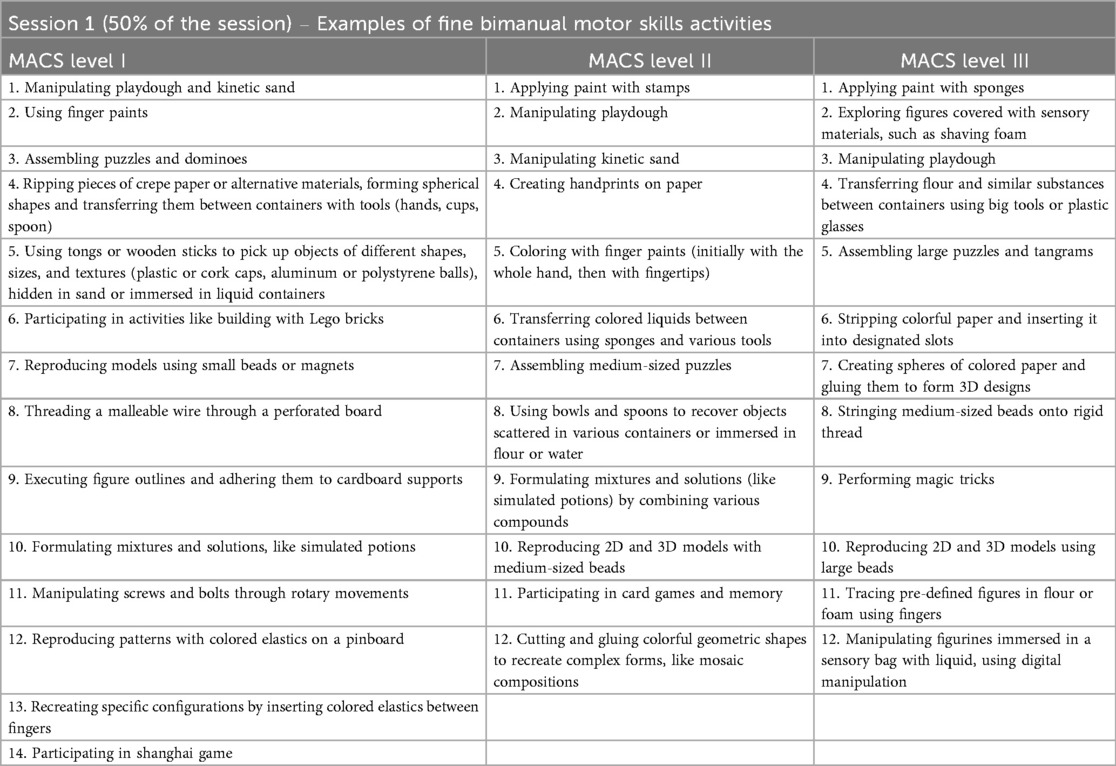
Component 1 - fine bimanual motor skills activities (50% of the daily session)
The first component of the daily session includes exercises of bimanual and fine motor tasks performed at a table, alternating complex tasks, such as cutting, with simpler repetitive movements, such as grasping an object with both hands. Depending on the patient's specific clinical characteristics and the progress observed, progressive integration of lower limb activities may occur from the early stages.
Examples of activities proposed for this session, and fully reported in Table 1, are subsequently adapted to the child's age, interests, motor functional and cognitive level, as well to the theme of the day.
For instance, the puzzle activity will be proposed using larger pieces for children with greater upper limb impairment, and progressively using smaller pieces for individuals with milder motor impairment. In cases of more severe limitation in upper limb use, the required task is to employ the most affected hand as a passive support to the activity, after which the task will require greater involvement of the hand in the manipulation and interlocking of the pieces.
For subjects in the lower age group, the puzzles will consist of colorful and engaging pictures and drawings, depicting animals, for example, while for older children more complex and realistic images may be employed.
Furthermore, the images to be reconstructed will be adapted to the chosen theme, for example, animals of the savannah, a seascape, or a famous monument for the world tour theme, or musical instruments for the music theme.
Another example of an adapted activity is dominoes, which can be modified into multiple themes in accordance with the child's interests and intellectual functioning, from cartoon characters, to superheroes, to syllables to form words.
According to the HABIT-ILE approach, the same task can be repeated over the course of the treatment weeks, varying the requests and the difficulty in terms of upper limb involvement, from a lower level, which involves its use as a passive stabilizer, to a gradually higher level, which encourages its more complex and active use.
Depending on the difficulties exhibited by the child, a gradual involvement of the lower limbs can already be introduced in this first session, by altering the support on which the child will be sitting during bimanual tasks, from the chair or other stable support, to the more or less inflated fitness ball, to the roller, to the balance board. The upper limb activities in which the child demonstrates more difficulty will be introduced in a stable sitting position, while those in which the child appears and is perceived as more skilled and confident will be performed under more challenging conditions that also involve the lower limbs, to an increasing extent.
During the activities, the therapist should verbalize the movement as it is performed and the goal to be achieved. He should also provide feedback on patient's performance and encourage interaction with the physical and relational environment. This helps sustaining attention and motivation, ensuring that motor, sensory, cognitive and emotional-relational skills are all engaged in building the rehabilitation project.
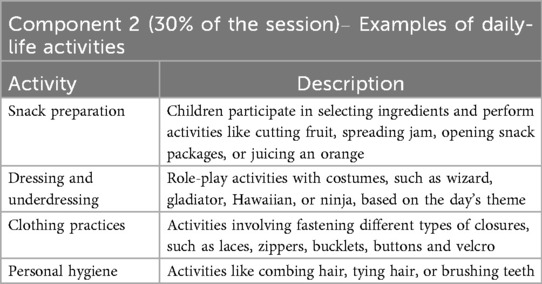
Component 2 - daily-life activities (30% of the daily session)
The activities in this second component are highly variable and selected together with the child and his/her family during the initial assessment. During this part of the daily training session, indeed, participants are involved in performing activities that promote the child's autonomy, chosen based on what they have identified as relevant and challenging. These tasks are designed to stimulate motivation and respond to the child's personal needs and are expected to be easily transferable to the child's daily life.
Some examples of this component's activities are listed in the Table 2.
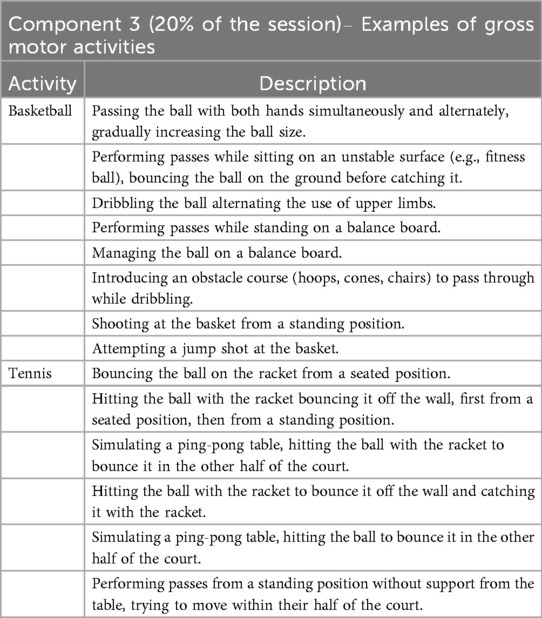
Component 3 - gross motor activities (20% of the daily session)
The third component of the daily training session consists of the execution of gross motor exercises, focusing on adapted sports activities (such as basketball, tennis, and golf) progressively involving a greater engagement of the lower limbs and requiring complex postural control.
A number of sports activities were designed and adapted for this session, including basketball, tennis, golf, darts, football, archery, and, additionally, exergames.
In Table 3 are described, as examples, the steps related to adapted basketball and tennis.
Regarding the progression of difficulty in lower limb involvement during the training sessions, we adopted the indicators proposed by the HABIT-ILE model, fully described by Bleyenheuft & Gordon (6).
For instance, if the daily theme is “trip around the world”, patients can take part in activities such as painting the flags of different countries as a bimanual activity, preparing typical meals from various cultures during an autonomy-related activity, and play a typical sport from a selected country (e.g., basketball for U.S.A. or soccer for Italy) at the end of the daily session.
The defined protocol, together with specific materials, was shared with all the participating centers to standardize training practices and mitigate potential biases associated with non-homogeneous interventions.
To improve the engagement and participation of children and families, thereby fostering adherence to the study, specialized informational materials were developed. These materials include a brochure and a video presentation, which provide a comprehensive and simplified explanation of the proposed trial and its phases.
Discussion
Cerebral Palsy is a leading cause of childhood disability, affecting motor function and overall independence. Given its lifelong impact, early and intensive interventions are critical in maximizing motor recovery and functional potential (25, 26). Furthermore, research by Novak and colleagues (8) highlights the necessity of combining high-intensity practice with targeted functional goals to induce neural adaptation and maximize therapeutic outcomes.
In this study, we presented a structured training protocol, created in a multicentre and multidisciplinary context which follows the well-established HABIT-ILE model, originally designed for unilateral CP rehabilitation. The combination of upper and lower limb training within daily functional tasks makes HABIT-ILE as an innovative approach in pediatric CP rehabilitation. It has shown evidence in promoting improvements in whole-body functioning, by incorporating lower-limb training and bimanual coordination (6). Our intensive rehabilitation program integrates upper- and lower-limb exercises into game-based and daily functional tasks, within a motivating and ecological framework, fostering both adherence and functional improvements.
While original HABIT-ILE model was designed for 60 h intervention, recent studies (21, 27, 28) have shown that clinical meaningful goals can be also achieved with lower dose intensive treatments. Specifically, in a review by Jackman et al. (29), the authors demonstrated that interventions characterized by individualization, patient-centred goals and active participation of children require fewer hours, with individual goals achievable within 14–25 h of practice. Furthermore, according to the authors (29), a threshold dose of 30–40 h can lead to improvements in unilateral motor skills. Given these evidences, we considered the dose of 30 h as a balance, ensuring at the same time clinical improvements and also training's feasibility and sustainability. However further research is necessary to investigate and define optimal intensity levels.
A fundamental aspect of the protocol is the high level of personalization in the proposed activities: the intervention is specifically tailored to the clinical and demographic profiles of each child, ensuring that tasks align with their motor abilities and cognitive capacities. By prioritizing motivation and personalization, as recommended by most recent evidence (30) and guidelines (31), our protocol not only enhances motor function but also fosters greater independence in daily activities, ultimately improving quality of life. This dual focus aligns with evidence supporting interventions that prioritize functional goals and environmental adaptation (32). Its emphasis on motivation-driven and personalized therapy ensures broad applicability and potential scalability across different, clinical and home-based, settings. The progression of motor request through the training period is also an important element to address neuroplasticity (18) and, at the same time, to maintain high levels of motivation and challenge.
While our protocol shows promise, its implementation faces several challenges. It is essential to involve specifically-trained practitioners to ensure accurate evaluation and consistent application of therapeutic techniques, thereby improving adherence to the protocol and maintaining constancy of the intervention. Furthermore, the intensive nature of the intervention may create logistical difficulties for families, as it requires balancing therapy with everyday and academic commitments. As many authors highlight, finding an equilibrium between therapy intensity and family needs demands careful planning and adaptability (33). In this regard, it will be important to consider patients' and their parents' feedback to evaluate the study's overall feasibility.
Group training are described in scientific literature as very useful, especially in promoting motivation (30, 34, 35), but the group setting may not always be sufficiently challenging or meet the specific needs of all children (34). Considering this, we hypothesize that a pair work training could be an optimal strategy to sustain motivational aspects and, simultaneously, guarantee an appropriate personalization of the treatment itself. We also believe that sharing activites between two individuals can be an effective way to motivate and support each other, and to make an intensive and demanding programme more feasible. In this respect, the compatibility in terms of personality traits and interaction styles within each couple, emotional and competition-related aspects may play a crucial role in ensuring a positive therapeutic experience. Therapists must cultivate an engaging and dynamic environment where activities are perceived as enjoyable and rewarding, thus promoting sustained participation both individually and in group settings. During pairwork sessions, elements of rivalry or frustration may emerge, necessitating therapist-led interventions to foster collaboration and encourage positive social interactions.
The three-hour daily session length may also pose a challenge, requiring thoughtful time management to sustain children's engagement and prevent drops in attention or motivation.
Importantly, to enhance the effects on motor plasticity, the HABIT-ILE protocol may be combined with Non-Invasive Brain Stimulation (NIBS) techniques, such as transcutaneous vagus nerve stimulation or transcranial alternating current stimulation, which stimulate the motor system respectively in a bottom-up and top-down fashion (3). The potential for innovations such as NIBS and digital tools to complement HABIT-ILE is an exciting avenue for exploration. Scientific evidence outlines the need for emerging technologies that could further enhance the efficacy and accessibility of intensive rehabilitation protocols, paving the way for broader implementation and greater impact (36).
Conclusion
In conclusion, the designed protocol represents an innovative and highly targeted approach for the treatment of children with CP, focusing on intensity, personalization, and functionality. Although the application results are beyond the scope of this study, its potential is significant, considering the growing body of evidence supporting the effectiveness of intensive and early rehabilitation interventions. We plan to verify feasibility, clinical effectiveness and sustainability of this model in multicenter contexts. The next step will be the conduction of clinical trials to validate the effectiveness of the protocol in practical settings, but the prospects for improving the quality of life for children and adolescents with CP are promising.
The continuous evolution of rehabilitation techniques, combined with more personalized approaches, could lead to a significant shift in the management of CP, enhancing not only children's motor skills but also their independence in daily activities, with a positive and lasting impact on their lives. The adoption of this protocol could become a crucial element in pediatric rehabilitation, helping to meet the needs of a growing population that faces challenges related to severe motor disabilities.
The future of rehabilitation lies at the intersection of targeted treatments, innovative technologies, and an increasing focus on the individual needs of patients and their families, paving the way for increasingly effective and accessible solutions.
Ethics statement
This study was approved by Comitato Etico I.R.C.C.S. Eugenio Medea. Prot. N. 66/22-CE; Comitato Etico Pavia, Prot. N. 0014336/23; Comitato Etico Brescia, Prot. N. 5673 and it will be conducted in accordance with the local legislation and institutional equirements. Written informed consent for participation in this study will be provided by the participants’ legal guardians/next of kin. Before the study begins, all participants and their parents or guardians will receive both oral and written descriptions of the protocols, including the potential risks and benefits. Three different versions of these descriptions (in Italian) will be provided to ensure comprehension: one for the parents, one for adolescents (12–17 years old), and one for children (6–11 years old). Written informed consent will be obtained from all parents. Minors aged 12 or older will be asked to give their written assent for participation. One copy of the consent form will be given to the participant, and another will be kept by the experimenter. Each recruitment center will be responsible for collecting and storing the informed consent of every patient recruited within their facility. Important protocol modifications will be reported to the Fondazione Regionale per la Ricerca Biomedica (FRRB) and clinicaltrials.gov, and approved by the Ethics Committees.
Author contributions
VV: Writing – original draft. BB: Conceptualization, Methodology, Writing – original draft. RN: Writing – original draft. EC: Writing – review & editing. SS: Writing – review & editing. VG: Writing – review & editing. AM: Writing – review & editing. VO: Writing – review & editing. JG: Writing – review & editing. CU: Writing – review & editing. ZC: Writing – review & editing. EF: Writing – review & editing. RB: Writing – review & editing. AF: Writing – review & editing. SO: Writing – original draft.
Boost working group
Unity of Child Neurology and Psychiatry, IRCCS Mondino Foundation, Pavia, Italy: B. Brafa, R. Borgatti, A. Ciricugno, B. De Falco, R. Nicotra, S. Orcesi, S. Signorini, V. Vacchini. Department of Brain and Behavioral Sciences, University of Pavia, Pavia, Italy: R. Borgatti, E. Capelli, A. Ciricugno, R. Nicotra, S. Orcesi. Department of Clinical and Experimental Sciences, University of Brescia, Brescia, Italy: E. Fazzi, J. Galli. Unit of Child Neurology and Psychiatry, ASST Spedali Civili of Brescia, Brescia, Italy: E. Fazzi, J. Galli, A. Morandi. Scientific Institute, IRCCS E. Medea, Bosisio Parini (LC), Italy: A. Finisguerra, V. Gasparroni, C. Maghini, A. Michelutti, V. Oldrati, L. Piccinini, C. Urgesi. Department of Human and Social Sciences, Universitas Mercatorum, Roma, Italy: C. Urgesi. Department of Human and Social Sciences, University of Bergamo, Bergamo, Italy: M. Arioli, Z. Cattaneo, M. Riva.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work is supported by Fondazione Regionale per la Ricerca Biomedica (Regione Lombardia), project FRRB 3438840 BOOST “Bottom-up and tOp-down neuromOdulation of motor plaSTicity in cerebral palsy”, and by grants from the Italian Ministry of Health (Ricerca Corrente 2024–2025, Scientific Institute, IRCCS E. Medea to AF).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Footnotes
1. ^Manual Ability Classification System (MACS) is a classification system developed to assess how children with cerebral palsy (CP) use their hands when handling objects in daily activities. It describes the child's typical manual performance rather than their maximal capacity, considering the collaborative use of both hands (22).
2. ^Gross Motor Function Classification System (GMFCS) is a five-level classification system used to describe global motor function limitations in children with CP (23).
3. ^Visual Function Classification System (VFCS) is a classification system to classify visual abilities of children with CP (24).
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CP, cerebral palsy; HABIT, Hand-Arm Bimanual Intensive Therapy; HABIT-ILE, Hand-Arm Bimanual Intensive Training Including Lower Extremities; NIBS, non-invasive brain stimulation; QoL: quality of life; CIMT, Constraint-Induced Movement Therapy; MACS, Manual Ability Classification System; GMFCS, Gross Motor Function Classification System; VFCS, Visual Function Classification System; IQ, intelligence quotient.
References
1. CDC. About Cerebral Palsy. Cerebral Palsy (CP) (2025). Available online at: https://www.cdc.gov/cerebral-palsy/about/index.html (Accessed March 31, 2025).
2. Bax M, Goldstein M, Rosenbaum P, Leviton A, Paneth N, Dan B, et al. Proposed definition and classification of cerebral palsy, April 2005. Dev Med Child Neurol. (2005) 47:571. doi: 10.1017/s001216220500112x
3. Oldrati V, Gasparroni V, Michelutti A, Ciricugno A, Borgatti R, Orcesi S, et al. Pairing transcutaneous vagus nerve stimulation with an intensive bimanual training in children and adolescents with cerebral palsy: study protocol of a randomized sham-controlled trial. Front Neurol. (2024) 15:1441128. doi: 10.3389/fneur.2024.1441128
4. Arnould C, Bleyenheuft Y, Thonnard J-L. Hand functioning in children with cerebral palsy. Front Neurol. (2014) 5:48. doi: 10.3389/fneur.2014.00048
5. McIntyre S, Goldsmith S, Webb A, Ehlinger V, Hollung SJ, McConnell K, et al. Global prevalence of cerebral palsy: a systematic analysis. Dev Med Child Neurol. (2022) 64:1494–506. doi: 10.1111/dmcn.15346
6. Bleyenheuft Y, Gordon AM. Hand-arm bimanual intensive therapy including lower extremities (HABIT-ILE) for children with cerebral palsy. Phys Occup Ther Pediatr. (2014) 34:390–403. doi: 10.3109/01942638.2014.932884
7. Novak I, Morgan C, Adde L, Blackman J, Boyd RN, Brunstrom-Hernandez J, et al. Early, accurate diagnosis and early intervention in cerebral palsy advances in diagnosis and treatment. JAMA Pediatr. (2017) 171:897–907. doi: 10.1001/jamapediatrics.2017.1689
8. Novak I, McIntyre S, Morgan C, Campbell L, Dark L, Morton N, et al. A systematic review of interventions for children with cerebral palsy: state of the evidence. Dev Med Child Neurol. (2013) 55:885–910. doi: 10.1111/dmcn.12246
9. Sakzewski L, Ziviani J, Abbott DF, Macdonell RA, Jackson GD, Boyd RN. Participation outcomes in a randomized trial of 2 models of upper-limb rehabilitation for children with congenital hemiplegia. Arch Phys Med Rehabil. (2011) 92:531–9. doi: 10.1016/j.apmr.2010.11.022
10. Sakzewski L, Ziviani J, Boyd RN. Efficacy of upper limb therapies for unilateral cerebral palsy: a meta-analysis. Pediatrics. (2014) 133:e175–204. doi: 10.1542/peds.2013-0675
11. Gillick B, Krach LE, Feyma T, Rich TL, Moberg K, Menk J, et al. Safety of primed repetitive transcranial magnetic stimulation and modified constraint-induced movement therapy in a randomized controlled trial in pediatric hemiparesis. Arch Phys Med Rehabil. (2015) 96:S104–13. doi: 10.1016/j.apmr.2014.09.012
12. Klevberg GL, Zucknick M, Jahnsen R, Eliasson A-C. Development of hand use with and without intensive training among children with unilateral cerebral palsy in scandinavia. Dev Neurorehabil. (2023) 26:163–71. doi: 10.1080/17518423.2023.2193256
13. Novak I, Morgan C, Fahey M, Finch-Edmondson M, Galea C, Hines A, et al. State of the evidence traffic lights 2019: systematic review of interventions for preventing and treating children with cerebral palsy. Curr Neurol Neurosci Rep. (2020) 20:3. doi: 10.1007/s11910-020-1022-z
14. Bleyenheuft Y, Ebner-Karestinos D, Surana B, Paradis J, Sidiropoulos A, Renders A, et al. Intensive upper- and lower-extremity training for children with bilateral cerebral palsy: a quasi-randomized trial. Dev Med Child Neurol. (2017) 59:625–33. doi: 10.1111/dmcn.13379
15. Gordon AM, Hung Y-C, Brandão MB, Ferre CL, Kuo H-C, Friel K, Petra E, Chinnan A, Charles JR. Bimanual training and constraint-induced movement therapy in children with hemiplegic cerebral palsy. Neurorehabil Neural Repair. (2011) 25:692–702. doi: 10.1177/1545968311402508
16. Saussez G, Brandão MB, Gordon AM, Bleyenheuft Y. Including a lower-extremity component during hand-arm bimanual intensive training does not attenuate improvements of the upper extremities: a retrospective study of randomized trials. Front Neurol. (2017) 8:495. doi: 10.3389/fneur.2017.00495
17. Bleyenheuft Y, Dricot L, Ebner-Karestinos D, Paradis J, Saussez G, Renders A, De Volder A, Araneda R, Gordon AM, Friel KM. Motor skill training may restore impaired corticospinal tract fibers in children with cerebral palsy. (2020) doi: 10.1177/1545968320918841
18. Araneda R, Klöcker A, Ebner-Karestinos D, Sogbossi ES, Renders A, Saussez G, et al. Feasibility and effectiveness of HABIT-ILE in children aged 1 to 4 years with cerebral palsy: a pilot study. Ann Phys Rehabil Med. (2021) 64:101381. doi: 10.1016/j.rehab.2020.03.006
19. Salimi I, Friel KM, Martin JH. Pyramidal tract stimulation restores normal corticospinal tract connections and visuomotor skill after early postnatal motor cortex activity blockade. J Neurosci. (2008) 28:7426–34. doi: 10.1523/JNEUROSCI.1078-08.2008
20. Friel K, Chakrabarty S, Kuo H-C, Martin J. Using motor behavior during an early critical period to restore skilled limb movement after damage to the corticospinal system during development. J Neurosci. (2012) 32:9265–76. doi: 10.1523/JNEUROSCI.1198-12.2012
21. Araneda R, Ebner-Karestinos D, Paradis J, Klöcker A, Saussez G, Demas J, et al. Changes induced by early hand-arm bimanual intensive therapy including lower extremities in young children with unilateral cerebral palsy. JAMA Pediatr. (2024) 178:19–28. doi: 10.1001/jamapediatrics.2023.4809
22. Eliasson A-C, Krumlinde-Sundholm L, Rösblad B, Beckung E, Arner M, Öhrvall A-M, et al. The Manual Ability Classification System (MACS) for children with cerebral palsy: scale development and evidence of validity and reliability. Dev Med Child Neurol. (2006) 48:549–54. doi: 10.1111/j.1469-8749.2006.tb01313.x
23. Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. (1997) 39:214–23. doi: 10.1111/j.1469-8749.1997.tb07414.x
24. Baranello G, Signorini S, Tinelli F, Guzzetta A, Pagliano E, Rossi A, et al. Visual function classification system for children with cerebral palsy: development and validation. Dev Med Child Neurol. (2020) 62:104–10. doi: 10.1111/dmcn.14270
25. Reid LB, Rose SE, Boyd RN. Rehabilitation and neuroplasticity in children with unilateral cerebral palsy. Nat Rev Neurol. (2015) 11:390–400. doi: 10.1038/nrneurol.2015.97
26. Taub E, Uswatte G. Importance for CP rehabilitation of transfer of motor improvement to everyday life. Pediatrics. (2014) 133:e215–7. doi: 10.1542/peds.2013-3411
27. Chen C, Kang L, Hong W-H, Chen F-C, Chen H-C, Wu C. Effect of therapist-based constraint-induced therapy at home on motor control, motor performance and daily function in children with cerebral palsy: a randomized controlled study. Clin Rehabil. (2013) 27:236–45. doi: 10.1177/0269215512455652
28. Hwang YS, Kwon J-Y. Effects of modified constraint-induced movement therapy in real-world arm use in young children with unilateral cerebral palsy: a single-blind randomized trial. Neuropediatrics. (2020) 51:259–66. doi: 10.1055/s-0040-1702220
29. Jackman M, Lannin N, Galea C, Sakzewski L, Miller L, Novak I. What is the threshold dose of upper limb training for children with cerebral palsy to improve function? A systematic review. Aust Occup Ther J. (2020) 67:269–80. doi: 10.1111/1440-1630.12666
30. Miller L, Ziviani J, Ware RS, Boyd RN. Does context matter? Mastery motivation and therapy engagement of children with cerebral palsy. Phys Occup Ther Pediatr. (2016) 36:155–70. doi: 10.3109/01942638.2015.1076556
31. La Riabilitazione nella PC Care Pathways. Available online at: https://sinpia.eu/wp-content/uploads/2023/10/La-Riabilitazione-nella-PC-Care-Pathways-04.07.pdf (Accessed March 31, 2025).
32. Morgan C, Novak I, Badawi N. Enriched environments and motor outcomes in cerebral palsy: systematic review and meta-analysis. Pediatrics. (2013) 132:e735–46. doi: 10.1542/peds.2012-3985
33. Zvolanek KM, Goyal V, Hruby A, Ingo C, Sukal-Moulton T. Motivators and barriers to research participation for individuals with cerebral palsy and their families. PLoS One. (2022) 17:e0262153. doi: 10.1371/journal.pone.0262153
34. Crompton J, Imms C, McCoy AT, Randall M, Eldridge B, Scoullar B, et al. Group-based task-related training for children with cerebral palsy: a pilot study. Phys Occup Ther Pediatr. (2007) 27:43–65. doi: 10.1300/J006v27n04_04
35. Butler LP, Walton GM. The opportunity to collaborate increases preschoolers’ motivation for challenging tasks. J Exp Child Psychol. (2013) 116:953–61. doi: 10.1016/j.jecp.2013.06.007
36. Bayón C, Martín-Lorenzo T, Moral-Saiz B, Ramírez Ó, Pérez-Somarriba Á, Lerma-Lara S, et al. A robot-based gait training therapy for pediatric population with cerebral palsy: goal setting, proposal and preliminary clinical implementation. J Neuroeng Rehabil. (2018) 15:69. doi: 10.1186/s12984-018-0412-9
Keywords: neuroplasticity, children, adolescents, cerebral palsy, intensive training, HABIT-ILE, quality of life, upper limb motor deficit
Citation: Vacchini V, Brafa B, Nicotra R, Capelli E, Signorini S, Gasparroni V, Michelutti A, Oldrati V, Galli J, Urgesi C, Cattaneo Z, Fazzi EM, Borgatti R, Finisguerra A, Orcesi S and Boost Working Group (2025) Improving neuroplasticity and Quality of Life in children with Cerebral Palsy: a customized intensive motor training protocol integrating the HABIT-ILE approach. Front. Rehabil. Sci. 6:1613103. doi: 10.3389/fresc.2025.1613103
Received: 1 June 2025; Accepted: 23 September 2025;
Published: 13 October 2025.
Edited by:
Stephanie C. DeLuca, Virginia Tech, United StatesReviewed by:
Emerson Hart, Hawaii Pacific University, United StatesAngela Rittller, The Ohio State University, United States
Copyright: © 2025 Vacchini, Brafa, Nicotra, Capelli, Signorini, Gasparroni, Michelutti, Oldrati, Galli, Urgesi, Cattaneo, Fazzi, Borgatti, Finisguerra, Orcesi and Boost Working Group. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Benedetta Brafa, YmVuZWRldHRhLmJyYWZhQG1vbmRpbm8uaXQ=
†These authors share first authorship
 Valeria Vacchini
Valeria Vacchini Benedetta Brafa
Benedetta Brafa Roberta Nicotra1,2
Roberta Nicotra1,2 Elena Capelli
Elena Capelli Sabrina Signorini
Sabrina Signorini Verusca Gasparroni
Verusca Gasparroni Arianna Michelutti
Arianna Michelutti Viola Oldrati
Viola Oldrati Jessica Galli
Jessica Galli Cosimo Urgesi
Cosimo Urgesi Elisa Maria Fazzi
Elisa Maria Fazzi Renato Borgatti
Renato Borgatti Alessandra Finisguerra
Alessandra Finisguerra Simona Orcesi
Simona Orcesi