- 1Office of Research, Waterloo Regional Health Network, Kitchener, ON, Canada
- 2Department of Oncology, Waterloo Regional Health Network, Kitchener, ON, Canada
The standard of care for metastatic castrate sensitive prostate cancer (mCSPC) involves the use of doublet therapies, which prolong survival and delay disease progression. Doublet therapies include the addition of second-generation androgen receptor pathway inhibitors (ARPIs) to androgen deprivation therapy (ADT). ADT monotherapy has been associated with adverse effects on skeletal muscle morphology, muscle strength, and physical function. Our findings suggest that the addition of ARPIs to ADT may further exacerbate these adverse effects. This review provides an overview of the current evidence to initiate exercise during treatment as an intervention to mitigate these adverse effects. Despite growing research in exercise oncology, research on the effects of exercise in men with mCSPC treated with doublet therapy is lacking. Much of the current supporting evidence is based on men with metastatic castrate resistant prostate cancer. Nonetheless, this review examines the available research on the efficacy and benefits of participating in a regimented exercise program in men with metastatic prostate cancer. We highlight the emerging evidence that exercising during treatment has the potential to protect against the adverse effects of doublet therapy. Future research to uncover the effects of different doublet therapies on muscle health in mCSPC is needed. Moreover, an improved understanding of the optimal training dose and timing that would elicit the most optimal benefits on muscle health in men with mCSPC is required.
1 Introduction
Prostate cancer is one of the most prevalent malignancies in men worldwide. It is estimated that new cases will rise from 1.4 million in 2020 to 2.4 million by 2040 (1). Approximately one-third of prostate cancers are diagnosed as metastatic. Historically, androgen deprivation therapy (ADT) was the cornerstone of systemic treatment for metastatic prostate cancer. ADT, or castration therapy, inhibits gonadotropin release by suppressing circulating testosterone levels to achieve chemical castration. However, the standard of care for metastatic prostate cancer has evolved to include androgen receptor pathway inhibitor (ARPI) therapies in combination with ADT (also known as doublet therapy).
Doublet therapies combine ADT with hormonal drugs, such as enzalutamide, abiraterone, apalutamide, or darolutamide. Most ARPIs (enzalutamide, apalutamide, and darolutamide) inhibit ligand binding at the level of the androgen receptor, further preventing the upregulation of genes that promote invasiveness and metastatic potential (2). This therapeutic intensification has demonstrated superior efficacy in prolonging survival and delaying disease progression compared to ADT alone (2). However, the concurrent interference of the cellular pathways at the level of the androgen receptor with the suppression of testosterone can evoke multiple adverse side effects contributing to the reductions in muscle mass and strength, and subsequent impairments in physical function (3, 4). While ADT alone is known to negatively affect muscle health and reduce patient quality of life (5), the intensification with ARPIs further inhibits androgen signaling, potentially exacerbating the toxic effects of ADT.
This review evaluates the impact of doublet therapies on muscle health in metastatic castrate sensitive prostate cancer (mCSPC) patients. We specifically focus on mCSPC patients because it has received relatively less research attention than metastatic castrate resistant prostate cancer (mCRPC) patients. While mCSPC implies a disease that is sensitive to androgen ablation, nearly all patients treated with ADT will ultimately develop castrate resistant disease (6), which is more aggressive and has a poor prognosis (7). Given that the first ARPI (apalutamide) was approved for use in mCSPC patients in 2019 (8), the impact of these agents in this population is a relatively understudied research area. Assessments of muscle health in mCSPC can provide powerful clinical insights into patient survival (9, 10) and treatment response (11). They may be particularly relevant as many mCSPC patients are at risk of or have already developed sarcopenia before initiating ARPI treatment, a condition that can predispose them to an increased risk of falls and fractures, hospitalization, increased frailty, and other adverse events (9).
This review also explores the current evidence regarding whether initiating an exercise program before or during doublet therapy can mitigate treatment-induced toxicities on the muscle. It is important to acknowledge that a patient's response to ARPIs and exercise interventions can be influenced by factors such as age, baseline fitness level, and comorbidities, which are out of the scope of this review. The insights gained from this review will assist in ongoing improvement to the standard of care therapy for men with mCSPC by identifying ways to counteract the declines in muscle health. Ultimately, this review provides a foundation for future research investigating the impact of incorporating a supervised exercise program into the standard of care for mCSPC patients during treatment.
2 Overview of skeletal muscle function in the context of androgen-receptor signaling
Androgens promote myogenic differentiation by inducing the transition of mesenchymal pluripotent cells to myogenic cells. Specifically, serum testosterone and other androgens bind to intracellular androgen receptors within muscle cells. Upon binding, the androgen receptor moves from the cytoplasm into the nucleus where it binds to hormone response elements that upregulate the transcription of genes that promote myogenesis (12). In healthy individuals, skeletal muscle mass is maintained through the achievement of homeostasis between the androgen receptor–β-catenin and insulin-like growth factor I–Akt–mammalian target of rapamycin [mTOR] pathways that promote muscle growth and the TGF-β–SMAD, ubiquitin–proteasome, and autophagy pathways that promote muscle atrophy (13).
ADT, when delivered alone, decreases circulating testosterone levels, reducing the potential for muscle hypertrophy by decreasing the resting rate of muscle protein synthesis (14). It is known that decreasing androgen levels reduces the activation of androgen receptors, leading to a reduction in mTORC1 signaling. mTORC1 is a central regulator of muscle protein synthesis and was identified as a major contributor to skeletal muscle hypertrophy in response to an increased workload (15–17). Therefore, a reduction in mTORC1 signaling with ADT contributes to a loss in muscle mass. However, the relationship between ADT disruption in these signaling pathways and muscle loss is complex and not well understood (18).
Previous research has shown that ARPIs act through different biological mechanisms. Abiraterone prevents androgen synthesis through the inhibition of cytochrome P-450c17 (CYP17), a critical enzyme in androgen biosynthesis (19). In contrast, enzalutamide and apalutamide inhibit the signaling pathway, specifically by blocking androgens from binding to androgen receptors (20, 21). Darolutamide acts through a similar mechanism to enzalutamide and apalutamide but has a tighter bond to the androgen receptor allowing for stronger suppression of androgen induced cell growth (22). Unlike other ARPIs, darolutamide minimally crosses the blood-brain barrier (23). Given these distinct therapeutic mechanisms, it is plausible that each ARPI, when combined with ADT, has a different effect on androgen and androgen receptors. Studies have shown that in men with mCRPC, treatment with abiraterone leads to significant and rapid reduction in circulating androgens (24) and testosterone (25). This rapid reduction appears to lead to more rapid muscle atrophy compared to ADT alone (4). Similarly, enzalutamide has been shown to result in a more rapid loss of skeletal muscle compared to ADT (4). Consequently, these mechanistic differences may lead to varying effects on skeletal muscle morphology, strength, and overall physical function.
3 Effects of ADT on muscle health
Men undergoing ADT often experience a significant loss in muscle mass and strength, which can lead to sarcopenia and subsequent declines in physical function. Loss of muscle mass and strength typically manifests within 3–6 months of initiating ADT treatment (26) and continues over the years (27). The adverse effects of ADT on muscle health are summarized in Table 1.
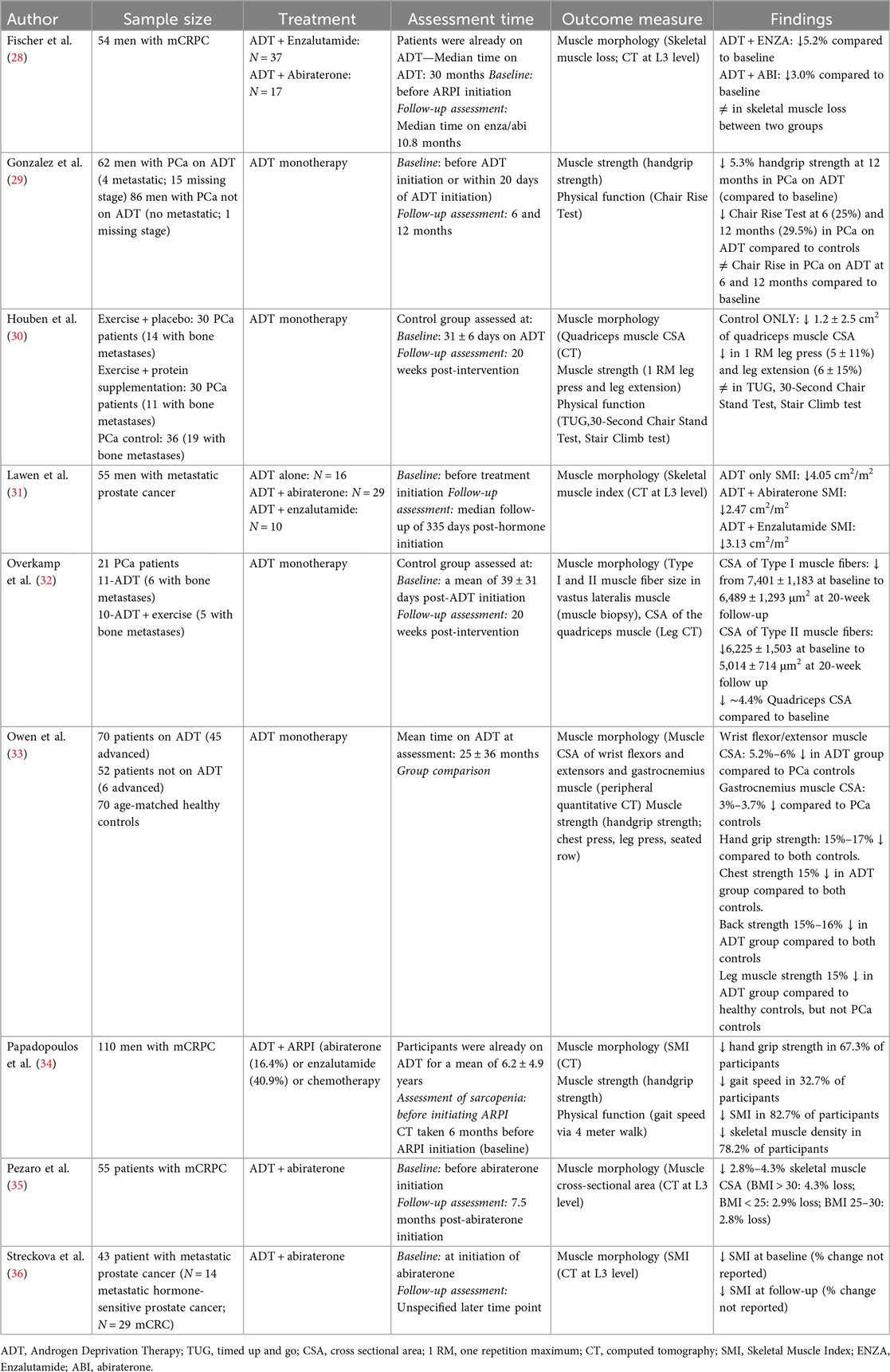
Table 1. A summary of published studies reporting changes in muscle morphology, strength, and physical function in men with prostate cancer receiving ADT only or doublet therapies.
There is evidence that ADT leads to muscle atrophy in metastatic cancer patients. For example, Overkamp et al. (32) assessed the impact of ADT on vastus lateralis muscle fibers, revealing a 12% and 17% decrease in vastus lateralis Type I and Type II muscle fiber cross-sectional area (CSA), respectively, after only 20 weeks of ADT treatment. Furthermore, studies have shown significant declines in muscle CSA in quadriceps (2% to 4.4%), wrist flexor and extensor (5.2%–6.0%), and gastrocnemius (3.0%–3.7%). ADT has also been shown to reduce skeletal muscle index (SMI) (9, 31) and skeletal muscle density (9).
Beyond its effects on muscle morphology, ADT also negatively affects muscle strength and potentially physical function in men with metastatic prostate cancer. Previous findings have shown a reduction in handgrip strength (5.3% to 17%), leg press strength (5% to 15%), leg extension strength (6%), chest press strength (15%) and back strength (16%) (9, 29, 30, 33). The impact of ADT on physical function, on the other hand, is highly variable. Some studies have shown minimal declines in the chair rise test (0.6%–1.1%) (29), while others have shown no changes in Timed Up and Go and 30 s chair stand test (30). The variable effects of ADT on physical function may be attributed to differences in the duration of ADT, with declines in physical function more likely to be observed with longer periods of treatment. Collectively, these findings demonstrate a potential decline in muscle strength, which may be coupled with physical function impairments, underscoring the importance of monitoring and addressing muscle function in men with metastatic prostate cancer undergoing ADT.
4 Effects of doublet therapy (ADT and ARPI) on muscle health
Considering the adverse effects of ADT discussed previously, a logical extension is to hypothesize that the addition of ARPIs to ADT treatment would result in greater detriment to muscle morphology and subsequent declines in muscle strength and physical function. A few studies have investigated the effect of doublet therapies on these outcome measures, with most studies focusing on mCRPC (Table 1).
A limited number of studies have investigated the impact of doublet therapies on muscle morphology in metastatic prostate cancer patients. Lawen et al. (31) compared the effects of ADT alone to ADT + abiraterone or ADT + enzalutamide on SMI. In their study, SMI was quantified by computed tomography (CT) scan at the level of the L3 vertebrae at baseline and 6 months post-ARPI initiation. Although the authors did not specify the type of metastatic prostate cancer, they found that SMI decreased by 5%–7% across all groups after 6 months, irrespective of the treatment type. Similarly, Streckova et al. (36) investigated the effects of ADT and abiraterone on skeletal muscle mass quantified by CT scans at the level of the L3 vertebrae at the time of initiating abiraterone and at an unspecified time point during the study. The patient sample consisted of 29 patients with mCRPC and 14 patients with mCSPC. SMI was quantified from the combined estimate of the total cross-sectional area of the muscles at the L3 level. The authors reported a reduction in SMI at the time of abiraterone initiation and a further decrease in SMI at the unspecified later time point. Collectively, these limited findings suggest similar suppressive effects of treatments on SMI in a mixed group of metastatic prostate cancer patients.
Most of the available research has focused on investigating muscle health in patients with mCRPC, who have a poorer prognosis than mCSPC patients. Fischer et al. (28) investigated skeletal muscle mass in men who were receiving ADT and enzalutamide or ADT and abiraterone and prednisolone with a median ADT duration of 30 months. The authors reported a 5.2% decrease in skeletal muscle mass in men receiving ADT and enzalutamide and a 3.0% loss in those receiving ADT and abiraterone and prednisolone at a median of 10.8 months post-initiating ARPIs. The authors concluded that, irrespective of the hormonal drug used in doublet therapy, the effects on the muscle mass were equivalent. Similarly, Pezaro et al. (35) investigated how abiraterone affects muscle CSA at the level of the L3 vertebrae in men with mCRPC. They showed significant skeletal muscle loss (∼2.9% in BMI < 25 or 25–30; ∼4.3% in BMI > 30) after about 7.5 months of abiraterone use in mCRPC patients with the greatest loss observed in those with a BMI greater than 30. Collectively, these limited findings suggest that the addition of ARPIs impacts skeletal muscle morphology in metastatic prostate cancer patients, and the effects on muscle mass appear to be similar across different medications. However, more research is needed to elucidate the effects of different hormonal medications on muscle strength and physical function.
5 Initiating exercise to maintain muscle health
The benefits of exercise interventions in prostate cancer patients have been shown in several studies. Engaging in resistance training during ADT significantly improves muscle morphology (37–39), decreases prevalence of sarcopenia (37), improves physical function (30, 38, 39), and increases muscle strength (34, 37–40). Collectively, these studies support the inclusion of a supervised exercise program in the standard of care to counteract the negative effects of ADT on muscle health.
Most studies investigating the effects of exercise in men with metastatic prostate cancer have focused on safety, feasibility, and efficacy, showing that exercise is feasible and safe in this population, including those with bone metastases (41–46). While no studies have investigated the effects of exercise on muscle health in men with mCSPC, the knowledge gained from studies in men with mCRPC can be translated to this group. Therefore, this section will summarize the available literature (Table 2) pertaining to the effects of exercise on muscle health in metastatic prostate cancer patients without a specific focus on men with mCSPC.
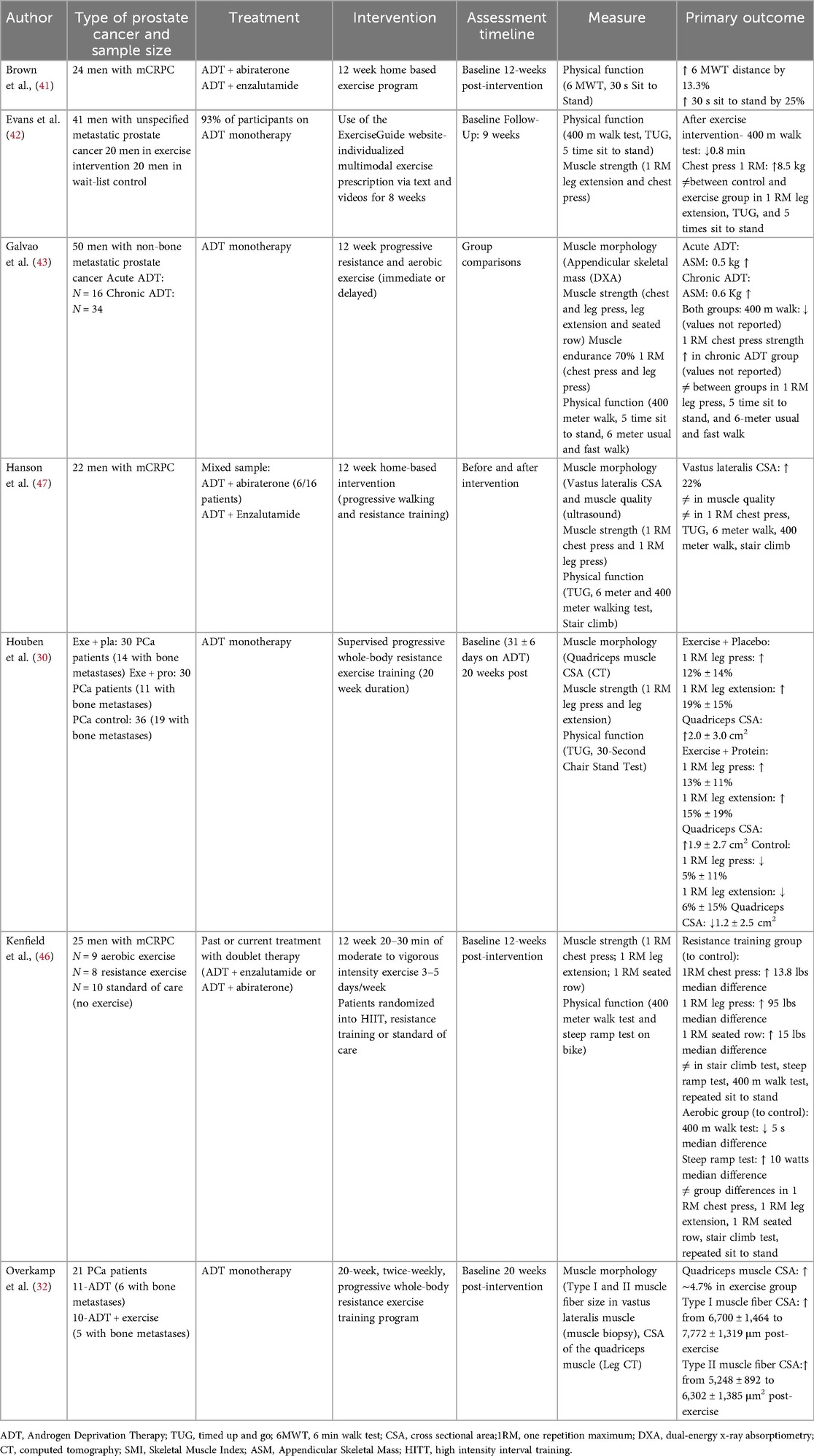
Table 2. A summary of published studies investigating exercise as an intervention to mitigate adverse effects on muscle function in men with prostate cancer receiving ADT or doublet therapy.
Several studies in men with metastatic prostate cancer on ADT monotherapy have shown positive effects of exercise on muscle health (Table 2). Exercise training has been shown to increase Type I and Type II muscle fiber CSA in the vastus lateralis by 16%–20% (32), and quadriceps CSA by approximately 5% (30, 32). Studies have also shown an increase in appendicular skeletal muscle mass by 0.5 kg to 0.6 kg (48), as well as muscle strength by 12% to 19% (30, 42, 48).
In contrast, the effects of exercise on physical function in men on ADT monotherapy are more variable. While some tests, particularly more endurance focused ones such as the 400-meter walk test, have shown improvements post-exercise (13–48 s time reduction) (42, 48), other tests, such as the Timed Up and Go and a 30 s stair stand test, have shown little to no change following exercise (30, 48). This discrepancy may be because the physical function tests are not sensitive enough to detect subtle changes, or may capture multiple domains including physical function, muscle strength, balance and coordination. Nonetheless, these preliminary findings suggest that improvements in muscle morphology and muscle strength might not always translate into improvements in physical function.
Other research has investigated the effects of exercise on muscle health in metastatic prostate cancer patients on doublet therapy (Table 2). The CHAMP study by Kenfield et al. (46) randomly assigned 25 men with mCRPC to either supervised remote high-intensity interval training (aerobic exercise; N = 9), supervised remote resistance training (N = 8), or a standard of care control group (N = 10) for 12 weeks. The treatments received by the participants in this study were heterogeneous, with 56% of patients on ADT + abiraterone, 28% on ADT + enzalutamide, and other antiandrogen therapies. Physical function (stair climb, 400-meter walk, and repeated sit-to-stand) and strength (chest-press, leg press or extension, and seated row) were measured at baseline and 12 weeks. The resistance exercise group had greater improvements in 1RM chest-press (13.8 lbs median difference), leg press (95 lbs median difference), and seated row (15 lbs median difference) compared to the control group. In contrast, the aerobic exercise group had greater improvements in steep ramp test (10 watts median difference) and 400-m walk times (5 s median difference) compared to the control group. These findings suggest divergent effects of different exercise modalities on strength and physical function. Similarly, Brown et al. (41) investigated the effect of a 12-week home-based exercise program in men with mCRPC receiving ADT + abiraterone acetate or ADT + enzalutamide. The exercise program included both resistance and aerobic components at moderate intensity. The authors assessed physical function using the 6-min walk test and the timed sit-to-stand test. They demonstrated that physical function improved significantly, with a 13.3% increase in 6-min walking distance and a 25% improvement in the timed sit-to-stand test.
However, other studies have reported variable results. For example, Hanson et al. (47) investigated the effects of a 12-week home-based exercise program in men with mCRPC on ADT + enzalutamide or ADT + abiraterone. The exercise program included progressive walking and resistance training. The authors observed a 22% increase in vastus lateralis CSA but no changes in muscle strength or physical function following exercise. Collectively, the findings from this small sample of studies suggest that home-based exercise programs can be effective in improving muscle morphology and preventing declines in physical function and muscle strength in men with mCRPC on doublet therapy. However, more studies are needed to confirm these findings.
6 Quality of life
While overall survival for men with metastatic prostate cancer continues to improve, optimizing health-related quality of life (HRQoL) for patients during and after treatment has become increasingly relevant. At least two studies assessed HRQoL in men with mCSPC receiving ADT + enzalutamide (49) or ADT + apalutamide (8). Based on the Functional Assessment of Cancer Therapy-General and Prostate-specific questionnaire (FACT-G and FACT-P) and the European Organization for Research Treatment of Cancer Quality of Life Questionnaires (EORTC QoL-C30), HRQoL was preserved in patients treated with doublet therapy (8, 49). This preservation in HRQoL has also been reported in mCRPC studies using the same measures (50, 51). Ronningas et al. (51) observed a significant association between a high physical symptom burden and HRQoL, reporting a correlation between low quality of life and high number of physical symptoms. Thus, investigating strategies aimed at mitigating these physical adverse effects, such as exercise, may be beneficial in enhancing HRQoL and patient care.
A limited number of studies have investigated the impact of exercise on patient-reported HRQoL in patients with mCRPC. One study by Langlais et al. (52) examined the impact of a remotely monitored 12-week exercise program on HRQoL in men with mCRPC. The authors observed minimal changes in HRQoL, regardless of participation in an exercise program. These findings suggest that the duration or intensity of the exercise intervention may not have been sufficient to elicit an impact on HRQoL.
In contrast, other studies have shown more promising results. Dawson et al. (37) investigated changes in HRQoL following a 12-week exercise intervention in a mixed population of both early-stage and metastatic prostate cancer. Specifically, the exercise group had a 12.5% increase in general quality of life, while the control group had a 3% decrease. Similarly, prostate specific quality of life increased by 12.3% in the exercise group and decreased by 2.8% in the control group. Brown et al. (41) also reported improvements in patient-reported HRQoL in mCRPC patients who participated in a 12-week home-based exercise program. Collectively, these findings support the potential role of exercise in enhancing quality of life among a more advanced prostate cancer population. However, further research is needed to investigate the specific benefits of exercise in optimizing HRQoL in mCSPC patients.
7 Limitations of previous research
There are several limitations to the studies described in this review. The most significant limitation is the lack of rigorous and consistent assessment of sarcopenia. Most studies have only evaluated the effects of treatment or exercise on muscle CSA at the L3 vertebrae level without concomitant evaluations of muscle strength or physical function. However, the definition of sarcopenia was updated in 2010 to include a measure of muscle function in addition to muscle mass (53). The exclusion of functional muscle tests (e.g., muscle strength or physical function) can significantly alter the proportion of patients classified as sarcopenic (9). For example, Papadopoulos et al. (9) showed that using only muscle mass measurements in mCRPC resulted in a sarcopenia diagnosis in 82.7% of patients in their study. However, the number of patients diagnosed with sarcopenia significantly decreased to 27.3% when the muscle function tests were considered. Future research should also include muscle quality assessment, which is a part of the sarcopenia definition and influences muscle function.
Another significant limitation is the lack of information on the type of metastatic prostate cancer. Following the TITAN trial in 2019, apalutamide was the first ARPI to be FDA approved for the treatment of mCSPC (8) and as a result, very few studies have investigated the effects of ARPI treatment in this population. Distinguishing between mCSPC and mCRPC is important, as these populations have different prognoses, treatment durations, and exposure to various ARPIs. Therefore, the effects on muscle health may be different between these two populations. Furthermore, a lack of detailed patient characteristics, including ARPI type, comorbidities, age, and baseline physical activity levels, limits the ability to interpret findings and implement them in a clinic.
Lastly, future research in metastatic prostate cancer aiming to investigate the impact of treatment on muscle health should consider the addition of baseline assessments prior to or within the first few weeks of treatment initiation. This would allow for better insights into the longitudinal impact of different hormonal agents on muscle characteristics. Researchers should consider adopting a pragmatic longitudinal approach in order to preserve real-world feasibility as well as be able to capture the true effects of ADT prior to ARPI initiation to determine the veritable consequences of additive therapy with ARPIs on muscle morphology and physical function.
8 Clinical takeaways
Findings from this review suggest that rigorous evaluation of muscle health in men with metastatic prostate cancer may help to detect subclinical sarcopenia. The clinical harms induced by a sarcopenic phenotype have been well-established and include an increased risk of emergency department utilization (9), hospitalization (54), falls and fractures (55), frailty (56), accelerated rate of disease progression (9, 57, 58), and at least grade 3 (severe) treatment-related toxicity (9). Sarcopenia was also associated with decreased overall survival (9, 11). Therefore, the assessment of sarcopenia at baseline and during hormonal therapy can help identify patients at high risk for detrimental effects and poor outcomes. If a patient has sarcopenia, clinicians should consider referring them to an exercise program and other support services to improve their quality of life. Our review also highlights the significant potential of a well-designed exercise regimen to mitigate potential adverse effects associated with ADT monotherapy or doublet therapy. When introduced early in the treatment pathway, exercise may prevent a decline in muscle health, thereby counteracting the observed adverse effects of such agents.
Despite these promising findings, several areas require further research. Most studies to date have failed to comment on the underlying mechanisms contributing to changes in muscle health. Additionally, no studies have compared the effects of different ARPIs on muscle health, specifically in men with mCSPC. Moreover, the potential mitigation of adverse effects through exercise training in mCSPC patients remains understudied. Additional work is needed to identify optimal timing of exercise programs and how best to implement lifestyle modification for patients receiving doublet therapy. We hypothesize that early exercise intervention during the castration sensitive stage of the disease may have beneficial effects on musculoskeletal function relative to usual care.
Lastly, the majority of research to date has examined short-term musculoskeletal consequences in response to an exercise program that lasts 12–20 weeks. This brief intervention, followed by a short-term observation period, limits one's ability to draw conclusions regarding any sustainability in improvement or further decline in physical function beyond study timelines. Further research across these domains could help better understand longer-term consequences on muscle compartments and subsequently lead to the development of strategies to ensure lasting muscle and physical function. Finally, improved understanding of training dose, including optimal mode, duration and intensity of exercise, may ultimately translate to favourable muscle and physical function outcomes for men with metastatic prostate cancer.
9 Conclusions
This review examined emerging evidence supporting participating in a progressive resistance exercise program as a means to mitigate the adverse effects associated with doublet therapy. Evidence suggests that engaging in a progressive resistance training exercise program is not only safe in metastatic prostate cancer populations but serves to improve muscle morphology, strength and physical function throughout the course of treatment which has the potential to improve patient health-related quality of life regardless of which form of ARPIs is administered. Early initiation of an exercise program during the castration sensitive stage of disease may elicit beneficial effects or lessen the harm to musculoskeletal morphology and function. Further research should aim to establish the ideal training dose and sustainability after completion of the initial regimented exercise program.
Author contributions
SE: Writing – original draft, Methodology, Investigation, Conceptualization, Writing – review & editing. TL-K: Writing – review & editing, Supervision, Writing – original draft, Methodology, Investigation, Conceptualization. AB: Writing – review & editing, Conceptualization, Validation, Supervision, Methodology, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This project is funded in part by the Prostate Cancer Fight Foundation/Ride for Dad.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Schafer EJ, Laversanne M, Sung H, Soerjomataram I, Briganti A, Dahut W, et al. Recent patterns and trends in global prostate cancer incidence and mortality: an update. Eur Urol. (2025) 87(3):302–13. doi: 10.1016/j.eururo.2024.11.013
2. Batra A, Glick D, Valdes M. Contemporary systemic therapy intensification for prostate cancer: a review for general practitioners in oncology. Curr Oncol. (2024) 31(2):1047–62. doi: 10.3390/curroncol31020078
3. Alibhai SMH, Breunis H, Timilshina N, Naglie G, Tannock I, Krahn M, et al. Long-term impact of androgen-deprivation therapy on physical function and quality of life. Cancer. (2015) 121(14):2350–7. doi: 10.1002/cncr.29355
4. Blow TA, Murthy A, Grover R, Schwitzer E, Nanus DM, Halpenny D, et al. Profiling of skeletal muscle and adipose tissue depots in men with advanced prostate cancer receiving different forms of androgen deprivation therapy. Eur Urol Open Sci. (2023) 57:1–7. doi: 10.1016/j.euros.2023.09.004
5. Cheung AS, Zajac JD, Grossmann M. Muscle and bone effects of androgen deprivation therapy: current and emerging therapies. Endocr Relat Cancer. (2014) 21(5):R371–94. doi: 10.1530/ERC-14-0172
6. Mottet N, Bellmunt J, Briers E, Bolla M, Bourke L, Cornford P, et al. EAU-ESTRO-ESUR-SIOG guidelines on prostate cancer. Eur Assoc Urol. (2018).
7. Debes JD, Tindall DJ. Mechanisms of androgen-refractory prostate cancer. N Engl J Med. (2004) 351:1488–90. doi: 10.1056/NEJMp048178
8. Agarwal N, McQuarrie K, Bjartell A, Chowdhury S, Gomes AJPS, Chung BH. Health-related quality of life after apalutamide treatment in patients with metastatic castration-sensitive prostate cancer (TITAN): a randomized, placebo-controlled, phase 3 study. Lancet Oncol. (2019) 20(11):1518–30. doi: 10.1016/S1470-2045(19)30620-5
9. Papadopoulos E, Wong AKO, Law SCH, Zhang LZJ, Breunis H, Emmenegger U, et al. The impact of sarcopenia on clinical outcomes in men with metastatic castrate-resistant prostate cancer. PLoS One. (2023) 18(6):e0286381. doi: 10.1371/journal.pone.0286381
10. Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: a meta-analysis and systematic review. Eur J Cancer. (2016) 57:58–67. doi: 10.1016/j.ejca.2015.12.030
11. Huiskamp LFJ, Chargi N, Devriese LA, May AM, Huitema ADR, de Bree R. The predictive value of low skeletal muscle mass assessed on cross-sectional imaging for anti-cancer drug toxicity: a systematic review and meta-analysis. J Clin Med. (2020) 9(11):3780. doi: 10.3390/jcm9113780
12. Singh R, Artaza JN, Taylor WE, Gonzalez-Cadavid NF, Bhasin S. Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor-mediated pathway. Endocrinology. (2003) 144(11):5081–8. doi: 10.1210/en.2003-0741
13. Egerman MA, Glass DJ. Signaling pathways controlling skeletal muscle mass. Crit Rev Biochem Mol Biol. (2014) 49(1):59–68. doi: 10.3109/10409238.2013.857291
14. Hanson ED, Nelson AR, West DWD, Violet JA, O’Keefe L, Phillips SM, et al. Attenuation of resting but not load-mediated protein synthesis in prostate cancer patients on androgen deprivation. J Clin Endocrinol Metab. (2017) 102(3):1076–83. doi: 10.1210/jc.2016-3383
15. McGlory C, Phillips SM. Exercise and the regulation of skeletal muscle hypertrophy. Prog Mol Biol Tansl. (2015) 135:153–73. doi: 10.1016/bs.pmbts.2015.06.018
16. Goodman CA. The role of MTORC1 in regulating protein synthesis and skeletal muscle mass in response to Various mechanical stimuli. Rev Physiol Biochem Pharamcol. (2014) 166:42–95. doi: 10.1007/112_2013_17
17. Hornberg TA. Mechanotransduction and the regulation of MTORC1 signaling in skeletal muscle. Int J Biochem Cell Biol. (2011) 43:1267–76. doi: 10.1016/j.biocel.2011.05.007
18. de Rooy C, Grossman M, Zajac JD, Cheung AS. Targeting muscle signaling pathways to minimize adverse effects of androgen deprivation. Endocr Relat Cancer. (2016) 23:R15–26. doi: 10.1530/ERC-15-0232
19. O’Donnell A, Judson I, Dowsett M, Raynaud F, Dearnaley D, Mason M, et al. Hormonal impact of the 17alpha-hydroxylase/C(17,20)-lyase inhibitor Abiraterone acetate (CB7630) in patients with prostate cancer. Br J Cancer. (2004) 90(12):2317–25. doi: 10.1038/sj.bjc.6601879
20. Schalken J, Fitzpatrick JM. Enzalutamide: targeting the androgen signalling pathway in metastatic castration-resistant prostate cancer. BJU Int. (2016) 117(2):215–25. doi: 10.1111/bju.13123
21. Wang L, Li C, Zhao Z, Li X, Tang C, Guan Z, et al. Comparison of doublet and triplet therapies for metastatic hormone-sensitive prostate cancer: a systematic review and network meta-analysis. Front Oncol. (2023) 13:1104242. doi: 10.3389/fonc.2023.1104242
22. Crawford ED, Stanton W, Mandair D. Darolutamide: an evidenced-based review of its efficacy and safety in the treatment of prostate cancer. Cancer Manag Res. (2020) 12:5667–76. doi: 10.2147/CMAR.S227583
23. Moilanen AM, Riikonen R, Oksala R, Ravanti L, Aho E, Wohlfahrt G, et al. Discovery of ODM-201, a new-generation androgen inhibitor targeting resistance mechanisms to androgen signaling-directed prostate cancer therapies. Sci Rep. (2015) 5:12007. doi: 10.1038/srep12007
24. Yan CJ, Peng W, Kheoh T, Welkowsky E, Hagg CM, Chandler DW, et al. Androgen dynamics and serum PSA in patients treated with Abiraterone acetate. Prostate Cancer Prostatic Dis. (2014) 17:192–8. doi: 10.1038/pcan.2014.8
25. Shaver AL, Nikita N, Sharma S, Keith SW, Zarrabi KK, Kelly WK, et al. The safety of Abiraterone acetate in patients with metastatic castration-resistant prostate cancer: an individual-participant data meta-analysis based on 14 randomized clinical trials. Cancers (Basel). (2025) 17(17):2747. doi: 10.3390/cancers17172747
26. Smith MR, Saad F, Egerdie B, Sieber PR, Tammela TLJ, Ke C, et al. Sarcopenia during androgen-deprivation therapy for prostate cancer. J Clin Oncol. (2012) 30(26):3271–6. doi: 10.1200/JCO.2011.38.8850
27. van Londen GJ, Levy ME, Perera S, Nelson JB, Greenspan SL. Body composition changes during androgen deprivation therapy for prostate cancer: a 2-year prospective study. Crit Rev Oncol Hematol. (2008) 68(2):172–7. doi: 10.1016/j.critrevonc.2008.06.006
28. Fischer S, Clements S, McWilliam A, Green A, Descamps T, Oing C, et al. Influence of Abiraterone and enzalutamide on body composition in patients with metastatic castration resistant prostate cancer. Cancer Treat Res Commun. (2020) 25:100256. doi: 10.1016/j.ctarc.2020.100256
29. Gonzalez BD, Jim HSL, Small BJ, Sutton SK, Fishman MN, Zachariah B, et al. Changes in physical functioning and muscle strength in men receiving androgen deprivation therapy for prostate cancer: a controlled comparison. Support Care Cancer. (2016) 24(5):2201–7. doi: 10.1007/s00520-015-3016-y
30. Houben LHP, Overkamp M, Van Kraaij P, Trommelen J, Van Roermund JGH, De Vries P, et al. Resistance exercise training increases muscle mass and strength in prostate cancer patients on androgen deprivation therapy. Med Sci Sports Exerc. (2023) 55(4):614–24. doi: 10.1249/MSS.0000000000003095
31. Lawen T, Masoumi-Ravandi K, Rendon RA, Connor L, Mason RJ. A comparison of the sarcopenic effect of androgen receptor-axis-targeted agents vs. Androgen deprivation alone in patients with metastatic prostate cancer. Can Urol Assoc J. (2023) 17(8):274–9. doi: 10.5489/cuaj.8245
32. Overkamp M, Houben LHP, Aussieker T, van Kranenburg JMX, Pinckaers PJM, Mikkelsen UR, et al. Resistance exercise counteracts the impact of androgen deprivation therapy on muscle characteristics in cancer patients. J Clin Endocrinol Metab. (2023) 108(10):e907–15. doi: 10.1210/clinem/dgad245
33. Owen PJ, Daly RM, Dalla Via J, Mundell NL, Livingston PM, Rantalainen T, et al. The clinical relevance of adiposity when assessing muscle health in men treated with androgen deprivation for prostate cancer. J Cachexia Sarcopenia Muscle. (2019) 10(5):1036–44. doi: 10.1002/jcsm.12446
34. Papadopoulos E, Gillen J, Moore D, Au D, Kurgan N, Klentrou P, et al. High-intensity interval training or resistance training versus usual care in men with prostate cancer on active surveillance: a 3-arm feasibility randomized controlled trial. Appl Physiol Nutr Metab. (2021) 46(12):1535–44. doi: 10.1139/apnm-2021-0365
35. Pezaro C, Mukherji D, Tunariu N, Cassidy AM, Omlin A, Bianchini D, et al. Sarcopenia and change in body composition following maximal androgen suppression with Abiraterone in men with castration-resistant prostate cancer. Br J Cancer. (2013) 109:325–31. doi: 10.1038/bjc.2013.340
36. Streckova E, Stejskal J, Kuruczova D, Svobodnik A, Stepanova R, Buchler T. Skeletal muscle loss during treatment with Abiraterone in patients with metastatic prostate cancer. Prostate Cancer. (2025) 2025(1):1468262. doi: 10.1155/proc/1468262
37. Dawson JK, Dorff TB, Schroeder TE, Lane CJ, Gross ME, Dieli-Conwright CM. Impact of resistance training on body composition and metabolic syndrome variables during androgen deprivation therapy for prostate cancer: a pilot randomized controlled trial. BMC Cancer. (2018) 18(1):368–15. doi: 10.1186/s12885-018-4306-9
38. Galvão DA, Nosaka K, Taaffee DR, Spry N, Kristjanson LJ, McGuigan MR, et al. Resistance training and reduction of treatment Side effects in prostate cancer patients. Med Sci Sports Exerc. (2006) 38(12):2045–52. doi: 10.1249/01.mss.0000233803.48691.8b
39. Hanson ED, Sheaff AK, Sood S, Ma L, Francis JD, Goldberg AP, et al. Strength training induces muscle hypertrophy and functional gains in black prostate cancer patients despite androgen deprivation therapy. J Gerontol A Biol Sci Med Sci. (2013) 68(4):490–8. doi: 10.1093/gerona/gls206
40. Newton RU, Galvão DA, Spry N, Joseph D, Chambers SK, Gardiner RA, et al. Timing of exercise for muscle strength and physical function in men initiating ADT for prostate cancer. Prostate Cancer Prostatic Dis. (2020) 23(3):457–64. doi: 10.1038/s41391-019-0200-z
41. Brown M, Murphy MH, McAneney H, McBride K, Crawford F, Cole A, et al. Feasibility of home-based exercise training during adjuvant treatment for metastatic castrate-resistant prostate cancer patients treated with an androgen receptor pathway inhibitor (EXACT). Support Care Cancer. (2023) 31(7):442. doi: 10.1007/s00520-023-07894-1
42. Evans HEL, Galvão DA, Forbes CC, Girard D, Vandelanotte C, Newton RU, et al. Acceptability and preliminary efficacy of a web- and telephone-based personalised exercise intervention for individuals with metastatic prostate cancer: the exercise guide pilot randomised controlled trial. Cancers (Basel). (2021) 13(23):5925. doi: 10.3390/cancers13235925
43. Galvão DA, Taaffe DR, Cormie P, Spry N, Chambers SK, Peddle-McIntyre C, et al. Efficacy and safety of a modular multi-modal exercise program in prostate cancer patients with bone metastases: a randomized controlled trial. BMC Cancer. (2011) 11(1):517. doi: 10.1186/1471-2407-11-517
44. Galvão DA, Taaffe DR, Spry N, Cormie P, Joseph D, Chambers SK, et al. Exercise preserves physical function in prostate cancer patients with bone metastases. Med Sci Sports Exerc. (2017) 50(3):393–9. doi: 10.1249/MSS.0000000000001454
45. Hart NH, Newton RU, Spry NA, Taaffe DR, Chambers SK, Feeney KT, et al. Can exercise suppress tumour growth in advanced prostate cancer patients with sclerotic bone metastases? A randomized, controlled study protocol examining feasibility, safety and efficacy. BMJ Open. (2017) 7:e014458. doi: 10.1136/bmjopen-2016-014458
46. Kenfield SA, Van Blarigan EL, Panchal N, Bang A, Zhang L, Graff RE, et al. Feasibility, safety, and acceptability of a remotely monitored exercise pilot CHAMP: a clinical trial of high-intensity aerobic and resistance exercise for metastatic castrate-resistant prostate cancer. Cancer Med. (2021) 10(22):8058–70. doi: 10.1002/cam4.4324
47. Hanson ED, Alzer M, Carver J, Stopforth CK, Lucas AR, Whang YE, et al. Feasibility of home-based exercise training in men with metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. (2023) 26(2):302–8. doi: 10.1038/s41391-022-00523-8
48. Galvão DA, Taaffe DR, Spry N, Joseph D, Newton RU. Acute versus chronic exposure to androgen suppression for prostate cancer: impact on the exercise response. J Urol. (2011) 186(4):1291–7. doi: 10.1016/j.juro.2011.05.055
49. Stenzl A, Szmulewitz RZ, Petrylak D, Holzbeierlein J, Villers A, Azad A, et al. The impact of enzalutamide on quality of life in men with metastatic hormone-sensitive prostate cancer based on prior therapy, risk, and symptom subgroups. Prostate. (2022) 82(13):1237–47. doi: 10.1002/pros.24396
50. Dearden L, Shalet N, Artenie C, Mills A, Jackson C, Grant L. Fatigue, treatment satisfaction and health-related quality of life among patients receiving novel drugs suppressing androgen signaling for the treatment of metastatic castrate-resistant prostate cancer. Eur J Cancer Care (Engl). (2018) 28(1):e12949. doi: 10.1111/ecc.12949
51. Ronningas U, Holm M, Fransson P, Beckman L, Wennman-Larsen A. Symptoms and quality of life among men starting treatment for metastatic castration-resistant prostate cancer-a prospective multicenter study. BMC Palliat Care. (2024) 23(1):80. doi: 10.1186/s12904-024-01410-w
52. Langlais CS, Chen Y-H, Van Blarigan EL, Chan JM, Ryan CJ, Zhang L, et al. Quality of life for men with metastatic castrate-resistant prostate cancer participating in an aerobic and resistance exercise pilot intervention. Urol Oncol. (2023) 41(3):146.e1–11. doi: 10.1016/j.urolonc.2022.11.016
53. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: european consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing. (2010) 39(4):412–23. doi: 10.1093/ageing/afq034
54. Zhang X, Zhang W, Wang C, Tao W, Dou Q, Yang Y. Sarcopenia as a predictor of hospitalization among older people: a systematic review and meta-analysis. BMC Geriatr. (2018) 18(1):188. doi: 10.1186/s12877-018-0878-0
55. Yeung SSY, Reijnierse EM, Pham VK, Trappenburg MC, Lim WK, Meskers CGM, et al. Sarcopenia and its association with falls and fractures in older adults: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. (2019) 10(3):485–500. doi: 10.1002/jcsm.12411
56. Cesari M, Landi F, Vellas B, Bernabei R, Marzetti E. Sarcopenia and physical frailty: two sides of the same coin. Front Aging Neurosci. (2014) 6:192. doi: 10.3389/fnagi.2014.00192
57. Pak S, Kim MS, Park EY, Kim SH, Lee KH, Joung JY. Association of body composition with survival and treatment efficacy in castration-resistant prostate cancer. Front Oncol. (2020) 10:558. doi: 10.3389/fonc.2020.00558
Keywords: metastatic prostate cancer, metastatic castrate sensitive, muscle morphology and function, muscle strength, physical function, exercise
Citation: Edwards S, Lulic-Kuryllo T and Batra A (2025) The impact of second-generation androgen receptor pathway inhibitors on skeletal muscle morphology and strategies to mitigate their effects in prostate cancer patients. Front. Rehabil. Sci. 6:1655422. doi: 10.3389/fresc.2025.1655422
Received: 27 June 2025; Accepted: 22 September 2025;
Published: 3 October 2025.
Edited by:
André R. Nelson, Victoria University, AustraliaReviewed by:
Erik D. Hanson, University of North Carolina at Chapel Hill, United StatesDr Abhishek Kumar, University of Alabama at Birmingham, United States
Copyright: © 2025 Edwards, Lulic-Kuryllo and Batra. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anupam Batra, YW51cGFtLmJhdHJhQHdyaG4uY2E=
 Sarah Edwards1
Sarah Edwards1 Tea Lulic-Kuryllo
Tea Lulic-Kuryllo