Abstract
In this perspective article, we consider the pathway biochemical sensing will take as the huge businesses underpinning Big Data and the Internet of Things seek new layers of highly valuable information to integrate into our increasingly digitised world. Up to now, the complexity of biochemical sensing has limited its inclusion in a manner similar to more reliable and lower cost technologies based on physical transducers. At its core, this complexity arises from the fundamental need for biochemical sensors to interact intimately at the molecular level with one or more specific components (analytes) in samples that are often highly complex and hostile to the sensors. This limits the functional lifetime of biochemical sensors to at best days or weeks or most commonly single use, making long-term embedded use-models developed for Internet of Things applications beyond reach. Nevertheless, even single use sensors can lead to “big data”, if used in large enough scale (e.g., COVID-19 diagnostics), and progress in continuous is beginning to make headway towards longer-term use models in health and environmental monitoring. New concepts exploiting advanced materials and biomimetic concepts offer opportunities to further extend the lifetime of biochemical sensing devices.
1 Introduction
As our world emerges from the COVID-19 pandemic, one of the most striking outcomes has been the hugely positive role played by science in mitigating the scale of the potential disastrous impact of the virus on global society. This applies to the incredibly rapid development and use at huge scale of very effective vaccines, based on an ability to accurately map the 3D structure of the virus within weeks of the pandemic initiation, coupled with the development of molecular candidates that could block the virus’s mode of action, and their synthesis using newly developed RNA technologies. In parallel, accurate diagnostics based on PCR technology, along with less sensitive, but easier to use and much lower cost antigen-based lateral flow tests, provided a means to confirm infection and implement targeted responses to reduce viral spread among the general population. And while the twin approach of effective vaccination and accurate diagnosis undoubtedly significantly reduced the impact of the virus, the number infected is well over half a billion, with over six million deaths globally1. Many countries experienced a collapse in their PCR based diagnostics systems, due to the need to employ centralised laboratories with trained staff, and the time delay from sampling to reporting the outcome (typically from one to several days, or longer), which made rapid scale up to meet demand dynamics impossible. Furthermore, the much faster, lower cost antigen-based lateral flow tests were reluctantly embraced by the clinical establishment in many jurisdictions, as they were felt to be insufficiently sensitive and could be performed independently by the general population.
For those working and researching in diagnostics technologies, the lessons from the pandemic are manifold. Coupling of PCR accuracy and sensitivity with the ease of use, low cost and rapid turnaround of lateral flow platforms it a key requirement for developing effective responses that can be rapidly scaled up and implemented on a global scale, and advances in this regard are already being reported (Casati et al., 2022). The challenges of innovation in disease diagnostics highlight the complexity of detecting molecular targets compared to physical measurements, even though in this case only a YES/NO binary outcome is required. If we consider time-series measurements with biochemical sensors2, things get considerably more difficult, particularly if the measurements are to be made in an autonomous manner, in remote locations and challenging media, for a prolonged period of time (months, years).
It is sobering to consider that in the 40 years since the heady days of the 1980s, when all seemed possible (Garrett DeYoung, 1983), the current best available technology for arguably the most important chronic clinical condition, diabetes, is two-weeks continuous monitoring via a patch-based device. The rapid replacement of the pre-existing approach of a single-use glucose sensor coupled with finger-prick blood sampling has brought enormous benefit to millions of diabetics who now for the first time, can see the impact of lifestyle on glucose dynamics in the data, and take personalised actions to control their condition (Brown and Kelly, 2015). But clearly there are significant barriers yet to be overcome before technologies for longer-term autonomous biochemical sensing can become a reality.
Given the challenges of implementing long-term continuous monitoring with biochemical sensors, the possibility of using readily available and reliable sensors to provide indirect indication of clinical conditions has been the subject of increasing interest. For example, the potential exploitation of wearable fitness trackers has been investigated for early indication of COVID-19 infection. Expanding on previous studies, which demonstrate the ability for surveillance and real time tracking of influenza-like illnesses, several studies in the past 2 years have shown that commonly monitored data (such as heart rate, daily steps, and sleep time) can be used to predict pre-symptomatic cases (Miller et al., 2020; Mishra et al., 2020; Radin et al., 2020; Ates et al., 2021; Mason et al., 2022). Recent advances have also shown that inclusion of multimodal sensing, combined with algorithm development may lead to successful infection detection, but it must be noted that they are ultimately limited by their inability to distinguish between specific viral infections. Moreover, it cannot be overlooked that external contributing factors such as environment and behaviour are inextricably linked to these measurements. In reality these approaches are designed to provide an overall picture of personal condition rather than detection of a specific infection, but as personal condition is correlated with general health, they could provide early indication of infection across a population, and assist with strategic decision making regarding appropriate localised responses.
2 Building an internet of biochemical things
The term “Internet of Things” (IOT) was first introduced by Kevin Ashton in 1999, then working for Procter and Gamble, to describe how RFID short-range wireless communications technologies could be used to communicate with objects (packages, instruments, equipment) for tracking assets, and monitoring production lines and distribution chains. IOT involves embedding sensing and wireless communications into objects, integrating them into the internet, rendering them to some extent “smart” and “self-aware”, capable of communicating aspects of their condition (location, temperature, vibration/noise status, whether damaged or opened, etc.) within local or large-scale control networks. In concept, it is closely related to wireless sensor networks (WSNs), in that the fundamental building blocks of the IOT are suitable sensors (low-cost, reliable, robust, long use-time, easy or zero service requirement etc., such as thermistors, photodetectors, acoustic wave devices) and wireless communications. Important markets include smart-home technologies, wearable devices for tracking personal health and fitness, production line monitoring, asset tracking, energy monitoring, and supply chain monitoring. Considering why biochemical sensors cannot meet the requirements of the IOT is an interesting exercise, as it highlights one of the most formidable challenges for autonomous biochemical sensors—how to dramatically extend their useful lifetime to months and years. To move forward, the fundamental barrier(s) to progress with biochemical sensors compared to physical transducers must be identified and, if possible, overcome. Arguably, the greatest challenge can be summarised as the “chemical sensor paradox” (
Byrne and Diamond, 2006) in that these devices typically require;
• A selectively responsive surface to transduce a molecular binding event between a specific target species in a sample;
• A surface that does not change over time, retaining its original characteristics and response behaviour.
Unlike physical transducers like thermistors, these devices must interact intimately with the sample via a selective molecular binding process on the responsive surface that generates the analytical signal. However, at the same time, this sensitive surface must be able to remain unchanged over time so that calibration issues are minimised. Of course, these surfaces do change over time, due to the formation of biofilms or other passivating surface effects like oxidation of catalytic sites or (bio)receptor degradation, leading to loss of selectivity and sensitivity. Hence biochemical sensors need regular calibration to monitor and compensate for these effects. Calibration in turn requires some means for regularly exposing the sensor surface to calibrants, wash/condition solutions, and the sample; i.e., a plumbing system with integrated solution reservoirs and waste containment is required to support the sensor function. This degradation of the sensor surface over time is the reason why, after 40 years of intense research, the entire field of biochemical sensing is still incompatible with long-term use models.
3 Strategies for extending biochemical sensor lifetime
Integrating sensing mechanisms within a lab-on-chip (LOC) microfluidics environment can dramatically reduce sensing time and sample volumes, while simultaneously increasing sensitivity compared to bench-based analytical techniques. Through integrated micropumps and valves, microfluidics can incorporate calibration and washing routines, enabling a host of sensing motifs and assays to be implemented. For biosensing, the advantages offered by microfluidics such as device portability and sample miniaturization, coupled with a range of different transduction mechanisms, such as optical, fluorescent, electrochemical, represent a particularly attractive proposition. Microfluidic devices have been extensively investigated for making biochemical measurements in a variety of biological fluids, such as urine, saliva, ocular fluid, and sweat. Analysis of ocular fluid and sweat is rather a new phenomenon, especially for continuous sensing. Recent advances in this regard, have demonstrated ways to quantify concentrations of electrolytes (sodium, potassium) and metabolites (urea, glucose) in these sample fluids using wearable type platforms employing microfluidic components (
Gao et al., 2016;
Glennon et al., 2016;
Currano et al., 2018;
Xiao et al., 2019;
Xuan et al., 2021). While these advances are welcome, the use-model remains frustratingly short-term (days at best in most cases), and the way forward towards longer-term monitoring requires more radical approaches. One strategy is to mimic approaches seen in natural biosystems, as these offer many novel strategies for long-term biosensing that are demonstrably successful. Stimuli-responsive molecular and polymeric materials are at the core of many natural biosensing systems, as they can facilitate on-demand changes in key characteristics like conductivity, wetting behaviour, colour, solubility, metal ion binding/release capabilities, cell adhesion, surface morphology, flexibility, colloidal system stability and membrane permeability. Several of these materials have been used as coatings for surface modification, in the form of monolayers, polymer brushes, layer-by-layer coatings, block copolymer assembly and many others. Surface modification enabled control of wetting behaviour, surface tension and liquid transport even against gravity (
Chaudhury and Whitesides, 1992). Furthermore, advances in materials chemistry have enables advanced functionalities such as liquid movement, cargo transport, sensing and self-healing to be embedded into fluidic channels and platforms. This means that typically inactive fluidic components, such as pumps, valves, channel walls and filter membranes, can be empowered with active characteristics,
viabio-inspired approaches (
Figure 1). Examples reported recently in the literature include;
• Microfluidic-integrated passive pumps that promote enhanced liquid flow by using the inherent wicking behaviour of hydrogels and ionogels (Akyazi et al., 2018; Seo et al., 2019; Alvarez-Braña et al., 2021).
• Advances in digital microfluidics where electrowetting has been used for precise manipulation of nanolitre droplets used for the transport, mixing, splitting and recombination, analysis and assays of a wide range of samples and analytes, including live cells (Wheeler, 2008; Mair et al., 2019).
• Chemotactic, electrotactic and phototactic control of discrete droplets to allow programmed movement of microvehicles to a specific location in a microfluidic system, without the need to pump or move the bulk fluid (Figure 1A) (Florea et al., 2014; Francis et al., 2015; Lach et al., 2016; Xiao et al., 2018). Such attempts are often bioinspired by movement of biological organisms, bacteria or cells capable of travelling in chemical gradients (Ding et al., 2016; Tokárová et al., 2021; Arya et al., 2022).
• On-demand patterned expansion/contraction of features in channel walls for control of mixing behaviour (Figure 1B) (Stumpel et al., 2014; ter Schiphorst et al., 2018).
• Soft stimuli-responsive polymer actuators for controlling flow direction and flow rate (Figure 1C) (Delaney et al., 2017; Saez et al., 2018).
• Functionalised channels walls endowed with sensing capabilities for monitoring changes in pH, metal ion concentrations, solvent polarity, and biochemistry (Figure 1D) (Florea et al., 2012, 2013a, 2013b; Luka et al., 2015; Shakeri et al., 2022; Dunne et al., 2018).
• Surface tension/interfacial tension modulation for flow (regime) control and fluid mixing (Diguet et al., 2011; Venancio-Marques et al., 2013).
• Self-healing channels walls (Wang et al., 2022).
FIGURE 1
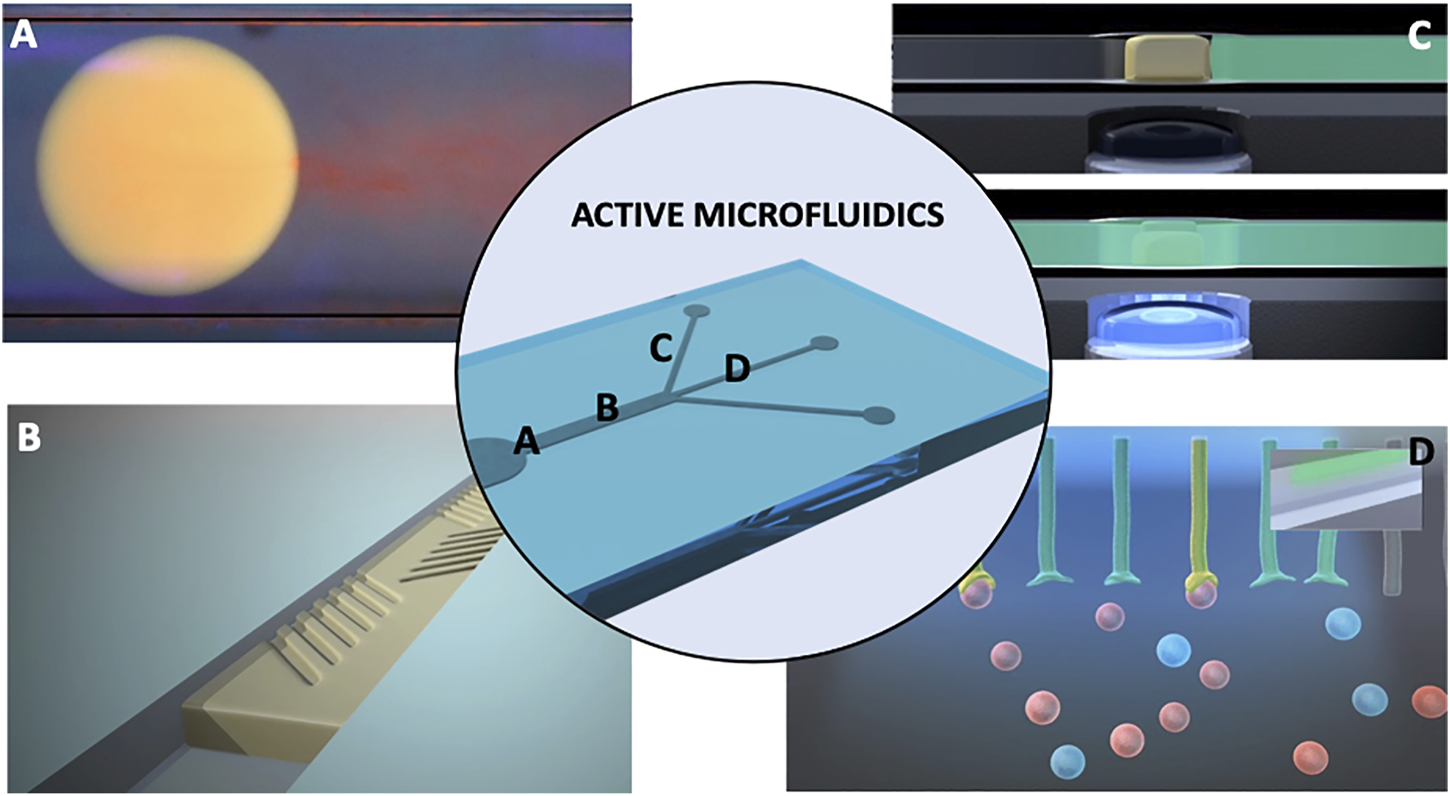
Graphical depiction of the active fluidics concept showing four functionalities that could be incorporated in to a single microfluidic devices: (A) energy-free transport of microdroplets travelling by chemotaxis in the left direction showing a red plume of expelled material from the right side; The droplet is based on the ionic liquid trihexyl(tetradecyl)phosphonium chloride powered by chemical gradients, as demonstrated by Francis et al., 2015; Image produced by Annael Sort-Montenegro and the authors, unpublished work; (B) Illustration showing on-demand generation of micro-topologies for control of mixing behaviour—envisioned angled features emerge from and converge into the surface under external photostimulation; such structures have been demonstrated by Stumpel et al., 2014; ter Schiphorst et al., 2018); (C) photo-responsive hydrogel microvalves showing that when the blue LED light is off, the micro-valve (depicted in yellow), stops the liquid flow (green), while switching the light on causes a shrinking of the photo-responsive hydrogel valve and the liquid flows through the channel; such photo-responsive microvalves have been demonstrated by Delaney et al., 2017; Saez et al., 2018; (D) Active channel walls that can be photoactivated to selectively bind and report chemical species presented in the flow (depicted here by a change in colour). The bound species can be subsequently released via removal of the photostimulation, which reverts the surface to the inactive non-binding form; such photo-activated coatings for metal ion accumulation, detection and release are described by Dunne et al., 2018. All images come from research projects led/supervised by the authors.
All of these instances demonstrate the fundamental building blocks of fluidic systems that can monitor to some extent their functional condition, and repair localized instances of damage or restore adverse changes through programmed movement of microvehicles to damaged locations in response to variations in the chemistry of the local environment. This could be coupled with chemically triggered release of molecular cargoes to repair the damage at these locations, in a simplified analog of our body’s healing of minor cuts and abrasions. Other modes of movement might also be considered, for example, biohybrid microrobots incorporating bioflagella (Ahmad et al., 2021) or “spermbots” (Singh et al., 2020) designed to perform location specific repair functions in the human body, but modified to perform similar functions in an analytical fluidic system. Combined with channel walls that respond to molecular stimuli (expand, contract, open pores, change surface roughness), this could represent a transformational change in fluidics towards the creation of self-aware systems that have distributed biomimetic functionalities.
These advances are occurring in tandem with dramatic improvements in the range of technologies available for integrating stimuli-responsive materials into microfluidic systems. For many years, stereolithography and soft lithography has dominated microfluidic fabrication due to its early adoption of technologies and concepts developed for microelectronics. However, more recently, 3D printing technologies have become popular for the fabrication of fluidic platforms and functional components, due to low cost, ease of prototyping, and access to a much wider range of materials, including soft polymers and hydrogels. New materials and 3D printing technologies (The Hidden Project, 2022) adapted from bioengineering (often driven by the realization of functional tissue scaffolds or organ-on-a-chip devices) can also be used for creating sensing modules, for example inside channels or on channel walls, wherein the sensing element can be printed using the same fabrication technology in a single-step process (Figure 2A). Today, 3D printing technologies that can produce structures with sub-micron resolution are available, and are compatible with a wide range of materials and structure design, including complex 3D geometries and structure overhangs. However, breakthrough advances in fabrication technologies can often come with high equipment costs, which, at least in the initial phase, can delay widespread adoption and reduce impact. But already, advanced 3D printing technologies for microfabrication, including direct laser writing (DLW) by multi-photon polymerization are becoming widely used, and are opening up new ways to create electronic, optical and photonic functional microstructures directly inside microfluidic channels. (Carlotti and Mattoli, 2019; Mayer et al., 2019; Corrielli et al., 2021). Such an example is depicted in Figure 2B, showing flower-like micro-capsules fabricated by DLW that can open to release their content depending on the chemistry of the fluid passing through the channel. This could further involve the release of microdroplets that spontaneously move to specific sites in a fluidic system where damage has occurred, at which point they release their contents to repair or block the damaged channel (Figure 2C). (Francis et al., 2015; Diamond et al., 2019).
FIGURE 2
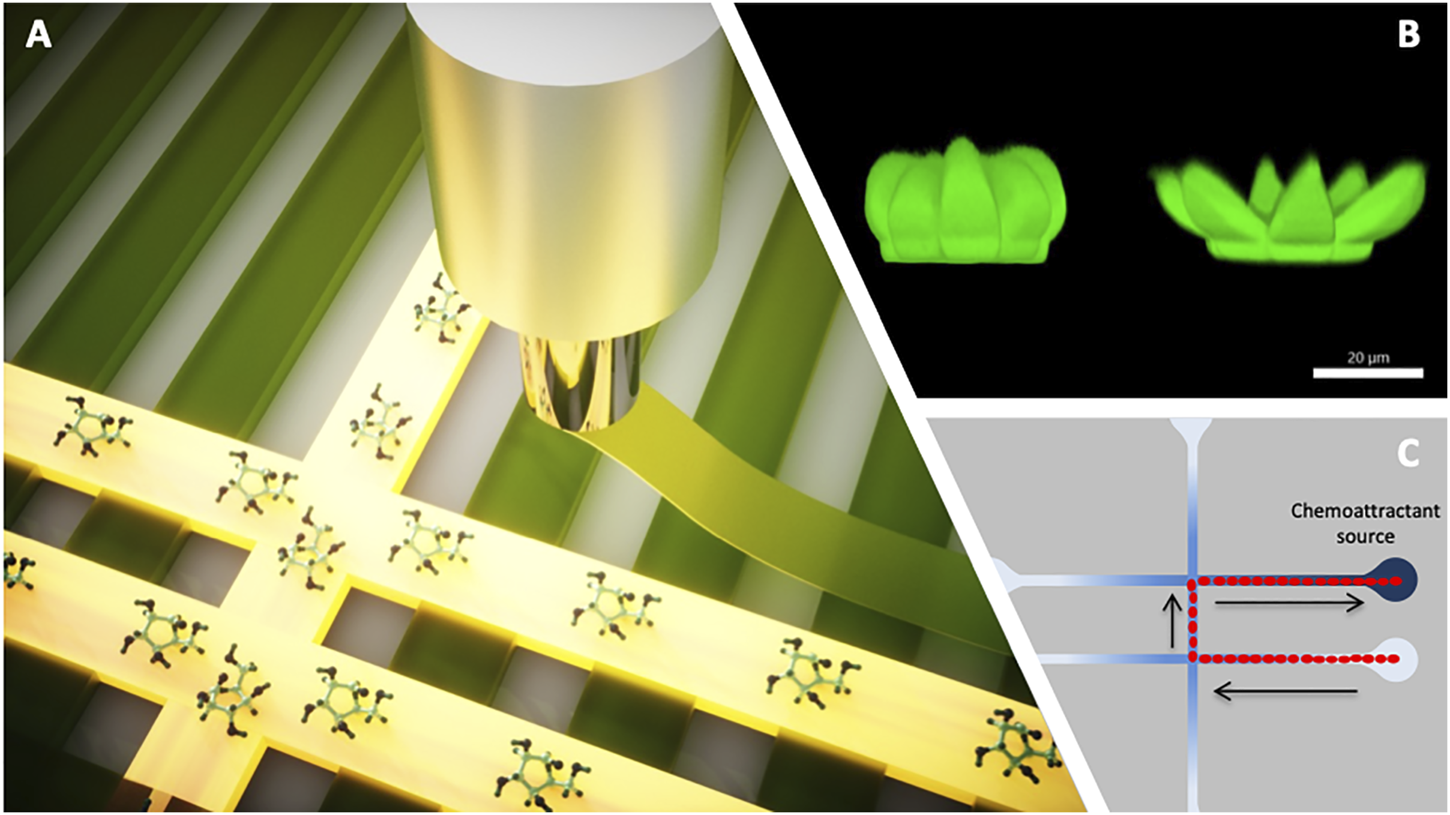
Graphical depiction of responsive microstructures and microvehicles that can be integrated in fluidic devices for functionality; (A) 3D printing by extrusion of a responsive hydrogel scaffold that fluoresces when interacting with the analyte of interest—such active components can be 3D printed as part of the 3D printing of the fluidic platform itself; a demonstration of such a scaffold is described by Bruen et al., 2020; (B) confocal image of flower-like microstructures in the passive (closed) and active (open) state—such active components can be fabricated inside the fluidic channel by direct laser writing and used to release active content when the chemistry of the fluid changes above a certain threshold; image realized by Alexa Ennis and the authors, unpublished data; (C) Schematic illustration showing sequentially the positions of a droplet (depicted in red) that spontaneously moves from an initial location through microchannels via chemotaxis towards the source of the chemical attractant along a concentration gradient arising from localized damage. Upon arrival the droplets can be programmed to perform simple repair tasks (e.g., merging with droplets containing co-reactants for polymerisation); such chemotactic droplets have been demonstrated by Francis et al., 2015. All images come from the research resources of the authors.
4 The future
The key to future evolution of research outcomes into high impact use is to link emerging market opportunities, disruptive enabling research, and innovative technologies, to create solutions for societal needs. For the world of sensors, the value lies in the data they provide. The complexity of biochemical sensing compared to transducers has, up to now, inhibited their large-scale integration into an “Internet of Biochemical Things”, as this requires at its core, sensors that are inexpensive and reliable in long-term use. Natural systems, conversely, have solved these issues and offer concepts for researchers to learn from and adopt into devices. Characteristics of living entities like self-awareness (e.g., based on sensing of internal condition) and self-repair (e.g., based on triggered release of microvehicles/microdroplets capable of chemotactic movement to damage locations and performing repair tasks) provide the basis for extending functional lifetime, and reporting issues before they cause failure. Such concepts are already the subject of intense research, for example, in the major EU-project BATTERY 2030+, which seeks to dramatically improve the lifetime and efficiency of batteries through embedded sensors and materials that respond to prevent the growth of dendrites (The HIDDEN Project). In its ultimate instantiation, solutions based entirely on materials can be envisaged, without electronics for system operation and communications. Consider, for example, a material that swells and contracts in response to glucose concentration and using this response to control the release of insulin. Such materials are known (Bruen et al., 2020), but even if insulin control using a materials only approach can be demonstrated convincingly, can this be presented in a manner acceptable to society as the next step on the road towards the artificial pancreas which, in the heady excitement of the 1980s, appeared to be on the cusp of happening?
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
LF acknowledges funding from the European Research Council (ERC) Starting Grant (No. 802929—ChemLife), the European Horizon 2020 Research and Innovation Programme (No. 899349—5D NanoPrinting), Science Foundation Ireland (SFI), and European Regional Development Fund (ERDF) under grant number 12/RC/2278_P2. The TPP-DLW fabrication and the imaging used in some of the figures was carried out at the Additive Research Laboratory (AR-Lab) and the Advanced Microscopy Laboratory (AML), Trinity College Dublin, Ireland. The AR-Lab and AML are Science Foundation Ireland (SFI) supported centres, part of the CRANN Institute and affiliated to the AMBER centre. DD acknowledges funding from the European Commission through the Holifab project, Grant number 760927 (H2020NMBP-PILOTS-2017), and from SFI through the INSIGHT Center, Grant Number SFI/12/RC/ 2289-P2, co-funded by the European Regional Development Fund.
Acknowledgments
The authors would like to acknowledge support from Trinity College Dublin and Dublin City University, and the contributions of many researchers from these and other collaborating institutions that have enriched our research over many years. We would also like to acknowledge artwork from Mats Bjorklund (https://magipics.com.au), used in Figure 1B,C,D.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
1.^These numbers are continuing to increase and do not include many unregistered infections and deaths; see https://www.worldometers.info/coronavirus/.
2.^We use the term “Biochemical Sensors” as a broad term to cover both “Chemical Sensors” and “Biosensors”; i.e., sensors that can detect and signal a target species in a sample solution, via a membrane/film surface that presents selective binding sites (ligands, enzymes, antibodies etc.) to the sample. This does not include non-contact approaches such spectroscopic methods. In Byrne and Diamond, 2006, the actual term used is “Chemical Sensor Paradox”, but the argument applies equally well to “biosensors” and hence “biochemical sensors”.
References
1
Ahmad R. Kleineberg C. Nasirimarekani V. Su Y.-J. Goli Pozveh S. Bae A. et al (2021). Light-powered reactivation of flagella and contraction of microtubule networks: Toward building an artificial cell. ACS Synth. Biol.10, 1490–1504. 10.1021/acssynbio.1c00071PubMed Abstract | CrossRef Full Text | Google Scholar
2
Akyazi T. Tudor A. Diamond D. Basabe-Desmonts L. Florea L. Benito-Lopez F. (2018). Driving flows in microfluidic paper-based analytical devices with a cholinium based poly(ionic liquid) hydrogel. Sensors Actuators B Chem.261, 372–378. 10.1016/j.snb.2018.01.154CrossRef Full Text | Google Scholar
3
Alvarez-Braña Y. Etxebarria-Elezgarai J. Ruiz de Larrinaga-Vicente L. Benito-Lopez F. Basabe-Desmonts L. (2021). Modular micropumps fabricated by 3D printed technologies for polymeric microfluidic device applications. Sensors Actuators B Chem.342, 129991. 10.1016/j.snb.2021.129991CrossRef Full Text | Google Scholar
4
Arya S. B. Chen S. Jordan-Javed F. Parent C. A. (2022). Ceramide-rich microdomains facilitate nuclear envelope budding for non-conventional exosome formation. Nat. Cell Biol.24, 1019–1028. 10.1038/s41556-022-00934-8PubMed Abstract | CrossRef Full Text | Google Scholar
5
Ates H. C. Yetisen A. K. Güder F. Dincer C. (2021). Wearable devices for the detection of COVID-19. Nat. Electron.4, 13–14. 10.1038/s41928-020-00533-1CrossRef Full Text | Google Scholar
6
Brown Adam Kelly Close (2015). Abbott’s FreeStyle libre – transforming glucose monitoring through utter simplicity, fingersticks aside. DiaTribe - Mak. Sense Diabetes, 35, 889-897. Available at:Accessed March 20, 2017] http://diatribe.org/abbott-freestyle-libre-transforming-glucose-monitoring-through-utter-simplicity-fingersticks.Google Scholar
7
Bruen D. Delaney C. Chung J. Ruberu K. Wallace G. G. Diamond D. et al (2020). 3D printed sugar-sensing hydrogels. Macromol. Rapid Commun.41, 1900610. 10.1002/marc.201900610CrossRef Full Text | Google Scholar
8
Byrne R. Diamond D. (2006). Chemo/bio-sensor networks. Nat. Mat.5, 421–424. 10.1038/nmat1661PubMed Abstract | CrossRef Full Text | Google Scholar
9
Carlotti M. Mattoli V. (2019). Functional materials for two‐photon polymerization in microfabrication. Small15, 1902687. 10.1002/smll.201902687CrossRef Full Text | Google Scholar
10
Casati B. Verdi J. P. Hempelmann A. Kittel M. Klaebisch A. G. Meister B. et al (2022). Rapid, adaptable and sensitive Cas13-based COVID-19 diagnostics using ADESSO. Nat. Commun.13, 3308. 10.1038/s41467-022-30862-yPubMed Abstract | CrossRef Full Text | Google Scholar
11
Chaudhury M. K. Whitesides G. M. (1992). How to make water run uphill. Science256, 1539–1541. 10.1126/science.256.5063.1539PubMed Abstract | CrossRef Full Text | Google Scholar
12
Corrielli G. Crespi A. Osellame R. (2021). Femtosecond laser micromachining for integrated quantum photonics. Nanophotonics10, 3789–3812. 10.1515/nanoph-2021-0419CrossRef Full Text | Google Scholar
13
Currano L. J. Sage F. C. Hagedon M. Hamilton L. Patrone J. Gerasopoulos K. (2018). Wearable sensor system for detection of lactate in sweat. Sci. Rep.8, 15890. 10.1038/s41598-018-33565-xPubMed Abstract | CrossRef Full Text | Google Scholar
14
Delaney C. McCluskey P. Coleman S. Whyte J. Kent N. Diamond D. (2017). Precision control of flow rate in microfluidic channels using photoresponsive soft polymer actuators. Lab. Chip17, 2013–2021. 10.1039/C7LC00368DPubMed Abstract | CrossRef Full Text | Google Scholar
15
Diamond D. Shinde A. Donohoe A. Barret R. McCaul M. (2019). Microfluidic platforms with bioinspired functionalities: New concepts for future devices. Sel. ?Lab-on-a-Chip & Microfluidics Europe 2019 Congress34, 573. Available at: http://doras.dcu.ie/23469/.Google Scholar
16
Diguet A. Li H. Queyriaux N. Chen Y. Baigl D. (2011). Photoreversible fragmentation of a liquid interface for micro-droplet generation by light actuation. Lab Chip11, 2666. 10.1039/c1lc20328bPubMed Abstract | CrossRef Full Text | Google Scholar
17
Ding Y. Qiu F. Casadevall I Solvas X. Chiu F. W. Y. Nelson B. J. Demello A. (2016). Microfluidic-based droplet and cell manipulations using artificial bacterial flagella. Micromachines7, 25. 10.3390/mi7020025PubMed Abstract | CrossRef Full Text | Google Scholar
18
Dunne A. Delaney C. Mckeon A. Nesterenko P. Paull B. Benito-Lopez F. et al (2018). Micro-capillary coatings based on spiropyran polymeric brushes for metal ion binding, detection, and release in continuous flow. Sensors18, 1083. 10.3390/s18041083PubMed Abstract | CrossRef Full Text | Google Scholar
19
The Hidden Project (2022). The Hidden Project. Available at https://hidden-project.eu/ (Accessed July 20, 2022).
20
Florea L. Fay C. Lahiff E. Phelan T. O’Connor N. E. Corcoran B. et al (2013a). Dynamic pH mapping in microfluidic devices by integrating adaptive coatings based on polyaniline with colorimetric imaging techniques. Lab Chip13, 1079. 10.1039/c2lc41065fPubMed Abstract | CrossRef Full Text | Google Scholar
21
Florea L. Hennart A. Diamond D. Benito-Lopez F. (2012). Synthesis and characterisation of spiropyran-polymer brushes in micro-capillaries: Towards an integrated optical sensor for continuous flow analysis. Sensors and Actuators B Chemical175, 92–99. 10.1016/j.snb.2011.12.055CrossRef Full Text | Google Scholar
22
Florea L. McKeon A. Diamond D. Benito-Lopez F. (2013b). Spiropyran polymeric microcapillary coatings for photodetection of solvent polarity. Langmuir29, 2790–2797. 10.1021/la304985pPubMed Abstract | CrossRef Full Text | Google Scholar
23
Florea L. Wagner K. Wagner P. Wallace G. G. Benito-Lopez F. Officer D. L. et al (2014). Photo-chemopropulsion - light-stimulated movement of microdroplets. Adv. Mat.26, 7339–7345. 10.1002/adma.201403007PubMed Abstract | CrossRef Full Text | Google Scholar
24
Francis W. Fay C. Florea L. Diamond D. (2015). Self-propelled chemotactic ionic liquid droplets. Chem. Commun.51, 2342–2344. 10.1039/C4CC09214GPubMed Abstract | CrossRef Full Text | Google Scholar
25
Gao W. Emaminejad S. Nyein H. Y. Y. Challa S. Chen K. Peck A. et al (2016). Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature529, 509–514. 10.1038/nature16521PubMed Abstract | CrossRef Full Text | Google Scholar
26
Garrett DeYoung H. (1983). Biosensors - the mating of biology and electronics. High Technology, 41–49. Google Scholar
27
Glennon T. O’Quigley C. McCaul M. Matzeu G. Beirne S. Wallace G. G. et al (2016). ‘SWEATCH’: A wearable platform for harvesting and analysing sweat sodium content. Electroanalysis28, 1283–1289. 10.1002/elan.201600106CrossRef Full Text | Google Scholar
28
Lach S. Yoon S. M. Grzybowski B. A. (2016). Tactic, reactive, and functional droplets outside of equilibrium. Chem. Soc. Rev.45, 4766–4796. 10.1039/C6CS00242KPubMed Abstract | CrossRef Full Text | Google Scholar
29
Luka G. Ahmadi A. Najjaran H. Alocilja E. DeRosa M. Wolthers K. et al (2015). Microfluidics integrated biosensors: A leading technology towards lab-on-a-chip and sensing applications. Sensors15, 30011–30031. 10.3390/s151229783PubMed Abstract | CrossRef Full Text | Google Scholar
30
Mair B. Aldridge P. M. Atwal R. S. Philpott D. Zhang M. Masud S. N. et al (2019). High-throughput genome-wide phenotypic screening via immunomagnetic cell sorting. Nat. Biomed. Eng.3, 796–805. 10.1038/s41551-019-0454-8PubMed Abstract | CrossRef Full Text | Google Scholar
31
Mason A. E. Hecht F. M. Davis S. K. Natale J. L. Hartogensis W. Damaso N. et al (2022). Detection of COVID-19 using multimodal data from a wearable device: Results from the first TemPredict study. Sci. Rep.12, 3463. 10.1038/s41598-022-07314-0PubMed Abstract | CrossRef Full Text | Google Scholar
32
Mayer F. Richter S. Westhauser J. Blasco E. Barner-Kowollik C. Wegener M. (2019). Multimaterial 3D laser microprinting using an integrated microfluidic system. Sci. Adv.5, eaau9160. 10.1126/sciadv.aau9160PubMed Abstract | CrossRef Full Text | Google Scholar
33
Miller D. J. Capodilupo J. V. Lastella M. Sargent C. Roach G. D. Lee V. H. et al (2020). Analyzing changes in respiratory rate to predict the risk of COVID-19 infection. PLoS ONE15, e0243693. 10.1371/journal.pone.0243693PubMed Abstract | CrossRef Full Text | Google Scholar
34
Mishra T. Wang M. Metwally A. A. Bogu G. K. Brooks A. W. Bahmani A. et al (2020). Pre-symptomatic detection of COVID-19 from smartwatch data. Nat. Biomed. Eng.4, 1208–1220. 10.1038/s41551-020-00640-6PubMed Abstract | CrossRef Full Text | Google Scholar
35
Radin J. M. Wineinger N. E. Topol E. J. Steinhubl S. R. (2020). Harnessing wearable device data to improve state-level real-time surveillance of influenza-like illness in the USA: A population-based study. The Lancet Digital Health2, e85–e93. 10.1016/S2589-7500(19)30222-5PubMed Abstract | CrossRef Full Text | Google Scholar
36
Saez J. Glennon T. Czugala M. Tudor A. Ducreé J. Diamond D. et al (2018). Reusable ionogel-based photo-actuators in a lab-on-a-disc. Sensors and Actuators B Chemical257, 963–970. 10.1016/j.snb.2017.11.016CrossRef Full Text | Google Scholar
37
Seo J. Wang C. Chang S. Park J. Kim W. (2019). A hydrogel-driven microfluidic suction pump with a high flow rate. Lab Chip19, 1790–1796. 10.1039/C9LC00062CPubMed Abstract | CrossRef Full Text | Google Scholar
38
Shakeri A. Jarad N. A. Khan S. F Didar T. (2022). Bio-functionalization of microfluidic platforms made of thermoplastic materials: A review. Analytica Chimica Acta1209, 339283. 10.1016/j.aca.2021.339283PubMed Abstract | CrossRef Full Text | Google Scholar
39
Singh A. Ansari M. Mahajan M. Srivastava S. Kashyap S. Dwivedi P. et al (2020). Sperm cell driven microrobots—emerging opportunities and challenges for biologically inspired robotic design. Micromachines11, 448. 10.3390/mi11040448CrossRef Full Text | Google Scholar
40
Stumpel J. E. Ziółkowski B. Florea L. Diamond D. Broer D. J. Schenning A. P. H. J. (2014). Photoswitchable ratchet surface topographies based on self-protonating spiropyran–NIPAAM hydrogels. ACS Appl. Mat. Interfaces6, 7268–7274. 10.1021/am500542fPubMed Abstract | CrossRef Full Text | Google Scholar
41
ter Schiphorst J. Melpignano G. G. Amirabadi H. E. Houben M. H. J. M. Bakker S. den Toonder J. M. J. et al (2018). Photoresponsive passive micromixers based on spiropyran size-tunable hydrogels. Macromol. Rapid Commun.39, 1700086. 10.1002/marc.201700086CrossRef Full Text | Google Scholar
42
Tokárová V. Sudalaiyadum Perumal A. Nayak M. Shum H. Kašpar O. Rajendran K. et al (2021). Patterns of bacterial motility in microfluidics-confining environments. Proc. Natl. Acad. Sci. U. S. A.118, e2013925118. 10.1073/pnas.2013925118PubMed Abstract | CrossRef Full Text | Google Scholar
43
Venancio-Marques A. Barbaud F. Baigl D. (2013). Microfluidic mixing triggered by an external LED illumination. J. Am. Chem. Soc.135, 3218–3223. 10.1021/ja311837rPubMed Abstract | CrossRef Full Text | Google Scholar
44
Wang H. Vu S. Pignanelli J. Abdel Fatah T. Trant J. F. Mahshid S. et al (2022). Fabrication and characterization of autonomously self-healable and stretchable soft microfluidics. Advanced Sustainable Systems6, 2100074. 10.1002/adsu.202100074CrossRef Full Text | Google Scholar
45
Wheeler A. R. (2008). Putting electrowetting to work. Science322, 539–540. 10.1126/science.1165719PubMed Abstract | CrossRef Full Text | Google Scholar
46
Xiao J. Liu Y. Su L. Zhao D. Zhao L. Zhang X. (2019). Microfluidic chip-based wearable colorimetric sensor for simple and facile detection of sweat glucose. Anal. Chem.91, 14803–14807. 10.1021/acs.analchem.9b03110PubMed Abstract | CrossRef Full Text | Google Scholar
47
Xiao Y. Zarghami S. Wagner K. Wagner P. Gordon K. C. Florea L. et al (2018). Moving droplets in 3D using light. Adv. Mat.30, 1801821. 10.1002/adma.201801821PubMed Abstract | CrossRef Full Text | Google Scholar
48
Xuan X. Pérez-Ràfols C. Chen C. Cuartero M. Crespo G. A. (2021). Lactate biosensing for reliable on-body sweat analysis. ACS Sens.6, 2763–2771. 10.1021/acssensors.1c01009PubMed Abstract | CrossRef Full Text | Google Scholar
Summary
Keywords
chemical sensors, biosensors, autonomous, IOT, microfluidics, stimuli-responsive materials, biomimetic
Citation
Florea L and Diamond D (2022) Sensors and “The internet of biochemical things”. Front. Sens. 3:1010212. doi: 10.3389/fsens.2022.1010212
Received
02 August 2022
Accepted
20 September 2022
Published
06 October 2022
Volume
3 - 2022
Edited by
Chatchawal Wongchoosuk, Kasetsart University, Thailand
Reviewed by
Cong Wang, China University of Geosciences Wuhan, China
Kagan Kerman, University of Toronto, Canada
Updates
Copyright
© 2022 Florea and Diamond.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dermot Diamond, dermot.diamond@dcu.ie
This article was submitted to Chemical Sensors, a section of the journal Frontiers in Sensors
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.