- 1Laboratori Nazionali di Legnaro, Istituto Nazionale di Fisica Nucleare, Legnaro, Italy
- 2Politecnico di Milano, Dipartimento di energia, Milano, Italy
Microdosimetry measures the stochastic fluctuations of energy imparted by radiation at the micrometer level and can provide measurable quantities linked to biological effectiveness. Reference instruments are Tissue-Equivalent Proportional Counters, gas-filled detectors operated in single-event proportional mode with a chemical composition similar to biological materials. The Legnaro National Laboratories of INFN have extensive experience in the design and construction of miniaturized microdosimetric gas counters able to sustain the high fluence rates of hadron therapy beams without significant pile-up effects. This work discusses the current state-of-the-art detection technologies in microdosimetry for hadron therapy, with a focus on miniaturized gas counters. It describes in particular the development of engineered compact detectors optimized for use in clinics, featuring enhanced stability and reproducibility of response, carried out within INFN projects.
1 Introduction
The use of hadrons for cancer treatment is increasing worldwide due to their more favorable physical and biological properties over photon or electron beams. As of February 2025, more than 120 proton and 17 carbon-ion facilities are treating patients all over the world, and more than 30 are being constructed (Particle Therapy Co-Operative Group, 2025). However, the radiobiological effectiveness of ion beams can change significantly as they degrade in matter; in addition, carbon ions can undergo nuclear reactions which give rise to a build-up of secondary fragments travelling beyond the range of the primary beam. The clinical field at treatment depth comprises multiple components with varying biological effectiveness. This brings about the need for fast and accurate in-phantom monitoring not only of the physical dose, but also of additional physical quantities which are related to radiobiological effectiveness (the so-called radiation quality, determined by the type and energy spectrum of particles at the treatment depth). This is true not only for treatments with charged hadrons such as protons or carbon ions, but also for emerging binary therapies such as Boron Neutron Capture Therapy (BNCT).
Microdosimetry is a spectrometric technique which measures energy imparted by ionizing radiation in sensitive volumes of micrometric dimensions. It can provide an effective approach for radiation quality monitoring in hadron therapy: starting from microdosimetric quantities it is possible to derive radiation quality descriptors of clinical interest, such as the Linear Energy Transfer (LET) (Kellerer, 1972; Braby et al., 2023) or a physics-based estimation of the Relative Biological Effectiveness (RBE). The latter is based either on specific weighting functions, derived from iterative unfolding of radiobiological and microdosimetric measurements carried out in the same radiation field (Loncol et al., 1994; Tilikidis et al., 1996; Parisi et al., 2020), or on radiobiological models such as the Microdosimetric Kinetic Model and its various modifications (Hawkins, 2003; Bellinzona et al., 2021).
Microdosimetric measurements provide a fast characterization of the radiation field, which could be performed as a routine procedure. For this reason, its introduction into Quality Assurance (QA) procedures for hadron therapy is gaining increasing interest and has been recently recommended by the International Commission of Radiation Units (Braby et al., 2023). However, this requires the availability of validated instruments for the measurement of microdosimetric quantities.
2 Detectors for microdosimetry
Reference detectors for microdosimetry are the Tissue-Equivalent Proportional Counters (TEPCs), gas-filled counters working in the proportionality regime where the chemical composition of both the detector walls and the filling gas is chosen to be as similar as possible to that of human tissue. This ensures that both the mass stopping power and the secondary particle spectrum in the detector materials are approximately the same as those in tissue. The gas pressure inside the cavity is set by applying a density scaling principle: its thickness in mass per area must be the same as that of a micrometric tissue volume with density 1 g/cm3.
TEPCs generally have a spherical or cylindrical shape, with a cathode shell made of conductive plastic (typically A150, which is muscle-equivalent) and a central metallic anode wire. The filling gas is usually a methane- or propane-based tissue-equivalent mixture. If the voltage difference between cathode and anode is such that the counter works in proportional mode, each particle crossing the sensitive volume generates a signal pulse with an amplitude proportional to the initial ionization yield. The latter is then converted to energy imparted by means of a calibration factor, assuming that the W-value (mean energy to create an ion pair) does not depend on particle type and energy.
Commercial versions of TEPCs for microdosimetry were available on the market, most notably the FWT-LET1/2 counter manufactured by Far West Technology, Inc. This detector has a spherical sensitive volume with a diameter of 1.27 cm. The FWT-LET1/2 has been used in some clinical centers for a microdosimetric characterization of the radiation field, both for ion therapy (Coutrakon et al., 1997; Kase et al., 2006; Martino et al., 2010) and BNCT applications (Endo et al., 2004; Hu et al., 2020).
The main challenge in the development of microdosimetric counters for particle therapy is the high intensity of the radiation field. Both in therapy with charged hadrons and in BNCT, the incident flux is of the order of 107–1010 cm−2 s−1. The cross-sectional area of detectors developed for these applications must therefore be small enough to cope with such a high fluence rate without significant pile-up effects. To achieve this goal, two strategies have been developed: either to miniaturize TEPCs as much as possible, down to cavity sizes less than 1 mm in diameter, or to move to solid-state technology, with silicon- or diamond-based devices of physical micrometric size. Given its large cross area, measurements with the FWT-LET1/2 counter can be done using a particle fluence rate orders of magnitude lower than the therapeutic one.
2.1 Miniaturized gas detectors
The first miniaturized TEPC was developed by Kliauga (1990) at Columbia University: its sensitive volume is a right cylinder with diameter and height of only 0.5 mm. It was designed to work at simulated site sizes from 250 nm down to 5 nm, but with severe limitations on the region of proportionality at the smaller site sizes. This miniaturized TEPC worked only in gas-flow modality and its development was stopped in the mid-90s. It was mainly employed for measurements in photon and neutron fields. The same group developed also a wall-less version of the counter, with a larger sensitive cavity 3.2 mm in size (Kliauga, 1994), which was tested in research ion beams. None of these detectors were tested in therapeutic particle beams.
Other miniaturized counters were later developed (Gerlach et al., 2002; Tsuda et al., 2010; Tsuda et al., 2012; Burmeister et al., 2001; Burmeister et al., 2002), generally with larger sizes ranging from 1.5 to 3 mm. At GSI, (Gerlach et al., 2002) developed a miniaturized counter for carbon-ion therapy, aiming to compare LEM-model predictions with the microdosimetric RBE for clinical beams. The sensitive volume had a diameter of 3 mm and a length of 30 mm and was embedded in a Perspex plate. A wall-less cylindrical TEPC for carbon-ion beams was developed in Japan by Tsuda et al. (2010), Tsuda et al. (2012), with dimensions of 3 mm both in height and diameter, and tested in proton, helium and carbon-ion beams at HIMAC.
Two pairs of mini-TEPCs were developed by Burmeister et al. (2001), Burmeister et al. (2002) for BNCT applications, with sensitive volume dimensions of 2.5 mm and 1.5 mm, respectively. One detector in each pair had the tissue-equivalent wall doped with 200 ppm of 10B to enhance sensitivity to thermal neutrons, while the other had no boron doping. These detectors were tested in neutron beams with different energies both at MIT and at BNL.
Other approaches based on gas detectors were also tried to reduce the geometrical size of the sensitive volume. An example is the development of a multiple TEPC without a central anode which uses Gas Electron Multiplier (GEM) technology for the amplification stage (Farahmand et al., 2003). Devices of this type have been developed by several groups and tested in photon, neutron and carbon-ion fields (Farahmand et al., 2004; Byun et al., 2009; Orchard et al., 2011; De Nardo and Farahmand, 2016; De Nardo et al., 2017; Darvish-Molla et al., 2018). Individual sensitive volume dimensions are in the range 1–5 mm.
2.2 Solid-state devices
Solid-state microdosimeters are generally based on silicon or diamond detectors. They exploit the electric field in the depletion region of a p-n or p-i-n junction to detect charges generated by the passage of ionizing radiation. Given the absence of an amplification stage, the detection threshold is higher compared to gas detectors. Additionally, their response is direction-dependent, and correction factors are required to compare with biological data, as they are not tissue-equivalent.
The main advantage of solid-state devices over gas detectors is the possibility of minimizing the geometrical dimensions of the sensitive volume down to physical micrometric size. This allows for pile-up free measurements up to fluence rates 100 to 1,000 times higher. Telescope detectors can also be constructed by coupling the microdosimeter (ΔE stage) to an additional thick E stage made of the same material, allowing for the identification of incident particles that stop in it. This provides a more accurate correction for non-tissue-equivalence compared to using a single factor.
Silicon microdosimeters for particle therapy have been developed by several groups (Agosteo et al., 2006; Agosteo et al., 2008; Bradley et al., 1998; Rosenfeld, 2016; Tran et al., 2018; Guardiola et al., 2020; Prieto-Pena et al., 2019). In the pixelated versions, the detector is composed of an array of sensors arranged in planar geometries, either with a rectangular or cylindrical shape, coupled to a row-by-row or pixelated readout. The physical thickness of individual sensors is typically between 2 and 10 μm, corresponding to 4.6–23 µm when scaled at unit density, while their lateral dimensions range between 10 and 30 µm.
With respect to silicon, diamond-based devices have improved tissue-equivalence and higher radiation hardness, and they are generally operated without voltage bias. Similar to silicon-based devices, these detectors have a physical thickness between 1 and 10 µm (corresponding to 3.5–35 μm at unit density). Despite being a less widespread technology compared to silicon, several detector designs have been reported, showing consistent response in both proton and carbon-ion beams (Rollet et al., 2012; Verona et al., 2018; Verona et al., 2020; Davis et al., 2017; Zahradnik et al., 2018).
3 The LNL mini-TEPCs
The Legnaro National Laboratories of INFN have extensive experience in developing miniaturized microdosimeters for hadron therapy. Since 1990, several devices were developed for research purposes, with varying cavity diameter, electrode design and external size. The first prototypes had a cylindrical cavity with a diameter and height of 1 mm and an A150 cathode with a thickness of 6 mm, for an overall external size of 15 mm (Cesari et al., 2001). One prototype was built with field tubes and another without, to quantify the impact of field distortions at the margins of the sensitive volume. The response of the two detectors was found to be almost the same (De Nardo et al., 2004a).
A second generation of prototypes was built with the aim of minimizing the counter external size. The smallest ones have a sensitive volume diameter of only 0.9 mm and an external diameter of 2.7 mm. The A150 cathode wall was 0.35 mm thick and surrounded by a Rexolite insulator. These detectors have been used to perform microdosimetric measurements at several clinical facilities, both in proton (De Nardo et al., 2004b; De Nardo et al., 2004c) and carbon-ion beams (Colautti et al., 2018a; Colautti et al., 2018b). The most recent version was designed with enlarged gas ducts to be operated without gas flow: when filled with pure propane, it was shown to provide a stable response for at least 1 year (Conte et al., 2019). The use of this gas instead of a tissue-equivalent mixture does not alter significantly the shape of microdosimetric spectra, if a proper scaling factor is applied (Chiriotti et al., 2015).
3.1 New mini-TEPCs for hadron therapy
The main drawbacks of these detectors were the difficult construction and assembly procedure, and the complex operation. A third generation of mini-TEPCs was therefore developed, initially in the framework of the CIMICE experiment, a Young Researchers’ Grant of the INFN Fifth Scientific Commission, and later in the Technology Transfer project 4MiCA. The design goal was to develop a compact, robust and easy-to-use detector, which could be used as a radiation quality monitor in routine clinical practice.
The main change introduced in the new version was the addition of guard tubes around the anode, kept at the same voltage. This third electrode resulted in a more stable response to ionizing radiation, because the removal of insulating materials around the anode minimizes the build-up of space charge at the edges of the sensitive volume, causing gas gain instabilities. Other relevant design changes included the use of a thicker anode wire and an increase in external size to provide structural robustness and simplify construction and assembly.
After successful tests of the first prototype (Bianchi et al., 2023), the engineering of the mini-TEPC design was carried out in the 4MiCA project. The aim was to simplify and standardize machining and assembly operations, to reduce overall construction time and cost, allow for easy replacement of failing components and ensure reproducibility of response between different detectors. A custom low-noise preamplifier optimized for high counting rate was also developed at Politecnico di Milano and integrated into the detector case together with a high-voltage filter. A photo of the engineered setup is shown in Figure 1.
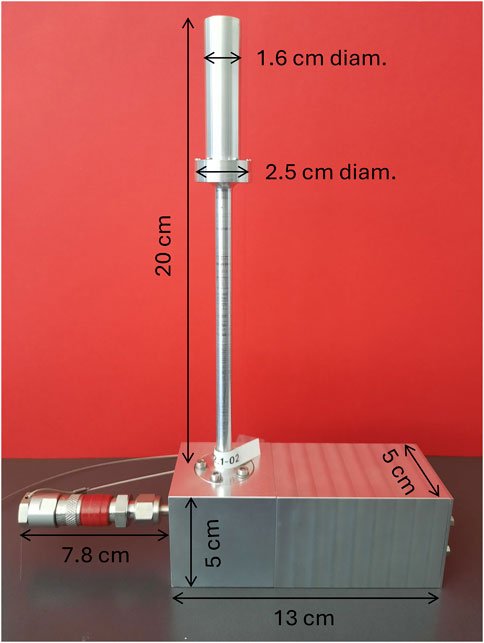
Figure 1. photo of the engineered setup, showing the sensitive sensor (top), the vacuum and gas-filling connector (bottom left) and the case for the integrated front-end electronics (bottom right). Dimensions of the components are shown.
A detailed description of the final detector design can be found in (Bianchi et al., 2024; Selva et al., 2025). Briefly, the sensitive volume is a right cylinder with both diameter and height of 1 mm. The anode is a gold-plated tungsten wire 25 µm in diameter. The cathode is an A150 hollow cylinder with a length of 2 mm and a thickness of 0.9 mm, surrounded by a Rexolite insulator and an aluminum shell of 0.25 mm thickness. The total external diameter of the counter is 16 mm. Figure 2 shows a scheme of the core part of the detector.
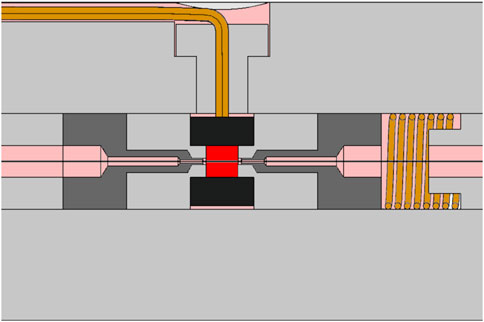
Figure 2. schematic drawing of the core part of the engineered mini-TEPCs. The sensitive volume is shown in red, the A150 cathode in black, gas ducts in pink and guard electrodes in dark gray. The anode wire is represented by the central line crossing horizontally the sensitive volume.
A first series of five identical new mini-TEPCs was constructed and tested in photon, neutron and proton radiation fields. These detectors showed a reproducible response within the overall measurement uncertainty and a pile-up free output up to 70 kHz. The new custom-made front-end electronics allows the collection of lineal energy events down to a threshold of 0.1 keV/μm. A detailed characterization of their performance is reported in (Bianchi et al., 2024).
3.2 Mini-TEPCs for BNCT
Miniaturized TEPCs for BNCT applications were also developed at the Legnaro National Laboratories of INFN. The first prototype device consisted of a twin miniaturized counter where two identical detectors were inserted into the same vacuum-tight sleeve (Moro et al., 2007; Moro et al., 2009). Only one of the two cathodes was loaded with 50 ppm of 10B, to discriminate the BNC dose component from the photon and neutron ones (Selva et al., 2022). The design of the twin TEPC is based on the second generation of LNL prototypes: also in this case, the external diameter is 2.7 mm, while the gas cavity has a diameter of 0.9 mm and is surrounded by a 0.35-mm-thick A150 wall. An upgraded version of this detector was also developed, with larger cavity dimensions (3 mm in diameter and height) and external size (Colautti et al., 2014). These counters were tested in high-intensity thermal neutron fields produced by nuclear reactors.
Similar to detectors developed for hadron therapy applications, these devices were very complex to build and assemble, and they worked only in gas-flow mode. To address these drawbacks, a new mini-TEPC with boron-doped cathode walls was developed in the framework of the Next-Generation EU project ANTHEM, based on the engineered design developed in the 4 MiCA project. To perform dose-components discrimination, this detector can be paired with another identical non-doped one, operated in the same conditions. The two measurements can be taken either simultaneously or sequentially. The latter choice avoids the need for duplicated HV power supply and read-out electronics.
The first pair of new mini-TEPCs for BNCT was recently characterized in a reactor-based thermal neutron field, at an intensity comparable to that of clinical treatments (109 cm−2 s−1) (Selva et al., 2025), showing consistent response without significant pile-up effects. Measurements are planned at several clinical BNCT centers both in-air and in-phantom.
4 Discussion and conclusion
The new engineered mini-TEPCs developed at LNL have several advantages over previous prototypes, making them attractive for use in clinical settings. The sensitive volume size of 1 mm is comparable to the dimensions of voxels used in treatment planning and allows measurements at fluence rates up to 107 cm−2 s−1 without severe pile-up distortions. The custom low-noise preamplifier has a dynamic range of more than four orders of magnitude, allowing to measure events in the entire lineal energy range of interest for both hadron therapy and BNCT. Since the anode and guard tubes are set to zero bias through the preamplifier, a high-voltage power supply with only one negative channel is needed, with very low power requirements (less than 100 mW).
The production time and overall cost have been significantly reduced thanks to serialized construction of components, improving also reproducibility of response between different sensors. The simplified assembly also allows for the replacement of individual detector components in case of failure (Bianchi et al., 2024). The detector case including front-end electronics can fit in a box with dimensions of 25 × 21 × 5 cm3 (see Figure 1), for an overall encumbrance comparable to silicon-based microdosimeters. The total weight of less than 1 kg and the sealed detector design allow easy transportation to clinical centers.
From a clinical perspective, the tissue-equivalent composition of TEPCs is an advantage over solid-state devices especially in mixed radiation fields: non-equivalent materials would require specific conversion factors for each particle type and energy, even though a unique average factor is generally applied with silicon or diamond microdosimeters. Mixed fields are always present in particle therapy, particularly beyond the Bragg peak or in out-of-field regions, where fragments generated by nuclear reactions can change significantly the radiation quality. This is also the case in BNCT, where the tissue-equivalence of the neutron converter is of critical importance for a correct assessment of all dose components.
Given their favorable features (low detection threshold, high dynamic range, tissue-equivalent response and high portability), LNL mini-TEPCs could be promising detectors for introducing microdosimetry in routine QA procedures in hadron therapy, following the recent recommendation of the International Commission of Radiation Units (Braby et al., 2023). Their main drawback is their relatively large cross-sectional area, which limits their performance at fluence rates higher than 107 cm−2 s−1. They can measure at clinical intensities in both carbon-ion and BNCT fields, but this is still not possible in proton beams where single-event measurements can be performed only at reduced intensity. However, other approaches can be implemented in clinical conditions, such as measuring the mean values of the distributions in a multi-event approach, similar to standard dosimetry, and applying the variance-covariance technique (Grindborg et al., 1995).
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
AS: Methodology, Writing – original draft, Investigation. AB: Investigation, Writing – review and editing, Methodology. AF: Investigation, Writing – review and editing, Methodology. VC: Supervision, Methodology, Investigation, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was funded by the fifth Scientific Commission and the Technological Transfer Commission of INFN.
Acknowledgments
The highly professional work of the LNL Technical Office and Mechanical Workshop in designing and constructing the various LNL mini-TEPCs is gratefully acknowledged.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agosteo, S., Colautti, P., Fazzi, A., Moro, D., and Pola, A. (2006). A solid state microdosimeter based on a monolithic silicon telescope. Radiat. Prot. Dosim. 122, 382–386. doi:10.1093/rpd/ncl468
Agosteo, S., Fallica, P., Fazzi, A., Introini, M., Pola, A., and Valvo, G. (2008). A pixelated silicon telescope for solid state microdosimetry. Radiat. Meas. 43, 585–589. doi:10.1016/j.radmeas.2007.12.053
Bellinzona, V. E., Cordoni, F., Missiaggia, M., Tommasino, F., Scifoni, E., La Tessa, C., et al. (2021). Linking microdosimetric measurements to biological effectiveness in ion beam therapy: a review of theoretical aspects of MKM and other models. Front. Phys. 8, 578492. doi:10.3389/fphy.2020.578492
Bianchi, A., Selva, A., Pasquato, F., Rossignoli, M., Minarello, A., Fazzi, A., et al. (2024). Microdosimetric measurements for LET monitoring in proton therapy. The development of engineered mini-TEPCs for clinical applications: first results. Radiat. Meas. 177, 107271. doi:10.1016/j.radmeas.2024.107271
Bianchi, A., Selva, A., Rossignoli, M., Pasquato, F., Missiaggia, M., Scifoni, E., et al. (2023). Microdosimetry with a mini-TEPC in the spread-out Bragg peak of 148 MeV protons. Radiat. Phys. Chem. 202, 110567. doi:10.1016/j.radphyschem.2022.110567
Braby, L. A., Conte, V., Dingfelder, M., Goodhead, D. T., Pinsky, L. S., Rosenfeld, A. B., et al. (2023). ICRU Report 98. Stochastic nature of radiation interactions: microdosimetry. J. Int. Comm. Radiat. Units Meas. 23, 1–168. doi:10.1177/14736691231211380
Bradley, P. D., Rosenfeld, A., Lee, K., Jamieson, D., Heiser, G., and Satoh, S. (1998). Charge collection and radiation hardness of a SOI microdosimeter for medical and space applications. IEEE Trans. Nucl. Sci. 45, 2700–2710. doi:10.1109/23.736518
Burmeister, J., Kota, C., Maughan, R. L., and Waker, A. J. (2001). Miniature tissue-equivalent proportional counters for BNCT and BNCEFNT dosimetry. Med. Phys. 28, 1911–1925. doi:10.1118/1.1398303
Burmeister, J., Kota, C., Maughan, R. L., and Waker, A. J. (2002). Characterization of miniature tissue-equivalent proportional counters for neutron radiotherapy applications. Phys. Med. Biol. 47, 1633–1645. doi:10.1088/0031-9155/47/10/302
Byun, S. H., Spirou, G. M., Hanu, A., Prestwich, W. V., and Waker, A. J. (2009). Simulation and first test of a microdosimetric detector based on a thick gas electron multiplier. IEEE Trans. Nucl. Sci. 563, 1108–1113. doi:10.1109/tns.2008.2009214
Cesari, V., Iborra, N., De Nardo, L., Querini, P., Conte, V., Colautti, P., et al. (2001). Microdosimetric measurements of the Nice therapeutic proton beam. Phys. Med. 16 (3), 76–82.
Chiriotti, S., Moro, D., Colautti, P., Conte, V., and Grosswendt, B. (2015). Equivalence of pure propane and propane-TE gases for microdosimetric measurements. Radiat. Prot. Dosim. 166, 242–246. doi:10.1093/rpd/ncv293
Colautti, P., Conte, V., Selva, A., Chiriotti, S., Pola, A., Bortot, D., et al. (2018a). Microdosimetric study at the CNAO active-scanning carbon-ion beam. Radiat. Prot. Dosim. 180, 157–161. doi:10.1093/rpd/ncx217
Colautti, P., Conte, V., Selva, A., Chiriotti, S., Pola, A., Bortot, D., et al. (2018b). Miniaturized microdosimeters as LET monitors: first comparison of calculated and experimental data performed at the 62 MeV/u 12C beam of INFN-LNS with four different detectors. Phys. Med. 52, 113–121. doi:10.1016/j.ejmp.2018.07.004
Colautti, P., Moro, D., Chiriotti, S., Conte, V., Evangelista, L., Altieri, S., et al. (2014). Microdosimetric measurements in the thermal neutron irradiation facility of LENA reactor. Appl. Radiat. Isot. 88, 147–152. doi:10.1016/j.apradiso.2014.01.005
Conte, V., Bianchi, A., Selva, A., Petringa, G., Cirrone, G., Parisi, A., et al. (2019). Microdosimetry at the CATANA 62 MeV proton beam with a sealed miniaturized TEPC. Phys. Med. 62, 114–122. doi:10.1016/j.ejmp.2019.06.011
Coutrakon, G., Cortese, J., Ghebremedhin, A., Hubbard, J., Johanning, J., Koss, P., et al. (1997). Microdosimetry spectra of the Loma Linda proton beam and relative biological effectiveness comparisons. Med. Phys. 24, 1499–1506. doi:10.1118/1.598038
Darvish-Molla, S., Prestwich, W. V., and Byun, S. H. (2018). Development of an advanced two-dimensional microdosimetric detector based on THick Gas Electron Multipliers. Med. Phys. 453, 1241–1254. doi:10.1002/mp.12750
Davis, J. A., Ganesan, K., Prokopovich, D. A., Petasecca, M., Lerch, M. L. F., Jamieson, D. N., et al. (2017). A 3D lateral electrode structure for diamond based microdosimetry. Appl. Phys. Lett. 110, 013503. doi:10.1063/1.4973628
De Nardo, L., Dal Corso, F., and Pegoraro, M. (2017). Microdosimetric measurements in gamma and neutron fields with a tissue equivalent proportional counter based on a gas electron multiplier. Radiat. Prot. Dosim. 1752, 260–266. doi:10.1093/rpd/ncw294
De Nardo, L., Cesari, V., Donà, G., Magrin, G., Colautti, P., Conte, V., et al. (2004a). Mini TEPCs for radiation therapy. Radiat. Prot. Dosim. 108, 345–352. doi:10.1093/rpd/nch023
De Nardo, L., Cesari, V., Iborra, N., Conte, V., Colautti, P., Hérault, J., et al. (2004b). Microdosimetric assessment of Nice therapeutic proton beam biological quality. Phys. Med. 20, 71–77.
De Nardo, L., and Farahmand, M. (2016). Operation of gas electron multiplier (GEM) with propane gas at low pressure and comparison with tissue-equivalent gas mixtures. Nucl. Instrum. Meth. A 819, 154–162. doi:10.1016/j.nima.2016.02.096
De Nardo, L., Moro, D., Colautti, P., Conte, V., Tornielli, G., and Cuttone, G. (2004c). Microdosimetric investigation at the therapeutic proton beam facility of CATANA. Radiat. Prot. Dosim. 110, 681–686. doi:10.1093/rpd/nch111
Endo, S., Onizuka, Y., Ishikawa, M., Takada, M., Sakurai, Y., Kobayashi, T., et al. (2004). Microdosimetry of neutron field for boron neutron capture therapy at Kyoto University Reactor. Radiat. Prot. Dosim. 110, 641–644. doi:10.1093/rpd/nch150
Farahmand, M., Bos, A. J. J., De Nardo, L., and van Eijk, C. W. E. (2004). First microdosimetric measurements with a TEPC based on a GEM. Radiat. Prot. Dosim. 110, 839–843. doi:10.1093/rpd/nch144
Farahmand, M., Bos, A., Huizenga, J., De Nardo, L., and van Eijk, C. (2003). Design of a new tissue-equivalent proportional counter based on a gas electron multiplier. Nucl. Instrum. Meth. A 509, 262–267. doi:10.1016/s0168-9002(03)01636-x
Gerlach, R., Roos, H., and M. Kellerer, A. (2002). Heavy ion RBE and microdosimetric spectra. Radiat. Prot. Dosim. 99, 413–418. doi:10.1093/oxfordjournals.rpd.a006821
Grindborg, J. E., Samuelson, G., and Lindborg, L. (1995). Variance-covariance measurements in photon beams for simulated nanometre objects. Radiat. Prot. Dosim. 61, 193–198. doi:10.1093/oxfordjournals.rpd.a082782
Guardiola, C., Fleta, C., Quirion, D., Pellegrini, G., and Gómez, F. (2020). Silicon 3D microdetectors for microdosimetry in hadron therapy. Micromachines 11, 1053. doi:10.3390/mi11121053
Hawkins, R. B. (2003). A microdosimetric-kinetic model for the effect of non-Poisson distribution of lethal lesions on the variation of RBE with LET. Radiat. Res. 160, 61–69. doi:10.1667/rr3010
Hu, N., Tanaka, H., Takata, T., Okazaki, K., Uchida, R., and Sakurai, Y. (2020). Microdosimetric quantities of an accelerator-based neutron source used for boron neutron capture therapy measured using a gas-filled proportional counter. J. Radiat. Res. 61, 214–220. doi:10.1093/jrr/rrz101
Kase, Y., Kanai, T., Matsumoto, Y., Furusawa, Y., Okamoto, H., Asaba, T., et al. (2006). Microdosimetric measurements and estimation of human cell survival for heavy-ion beams. Radiat. Res. 166, 629–638. doi:10.1667/rr0536.1
Kellerer, A. M. (1972). An algorithm for LET analysis. Phys. Med. Biol. 17, 232–240. doi:10.1088/0031-9155/17/2/009
Kliauga, P. (1990). Measurement of single event energy deposition spectra at 5 nm to 250 nm simulated site sizes. Radiat. Prot. Dosim. 31, 119–123. doi:10.1093/oxfordjournals.rpd.a080650
Kliauga, P. (1994). Nanodosimetry of heavy ions using a miniature cylindrical counter of wall-less design. Radiat. Prot. Dosim. 52, 317–321. doi:10.1093/rpd/52.1-4.317
Loncol, T., Cosgrove, V., Denis, J., Gueulette, J., Mazal, A., Menzel, H., et al. (1994). Radio-biological effectiveness of radiation beams with broad LET spectra: microdosimetric analysis using biological weighting functions. Radiat. Prot. Dosim. 52, 347–352. doi:10.1093/rpd/52.1-4.347
Martino, G., Durante, M., and Schardt, D. (2010). Microdosimetry measurements characterizing the radiation fields of 300 MeV/u 12C and 185 MeV/u 7Li pencil beams stopping in water. Phys. Med. Biol. 55, 3441–3449. doi:10.1088/0031-9155/55/12/011
Moro, D., Colautti, P., Gualdrini, G., Masi, M., Conte, V., De Nardo, L., et al. (2007). Two miniaturised TEPCS in a single detector for BNCT microdosimetry. Radiat. Prot. Dosim. 122, 396–400. doi:10.1093/rpd/ncl484
Moro, D., Colautti, P., Lollo, M., Esposito, J., Conte, V., De Nardo, L., et al. (2009). BNCT dosimetry performed with a mini twin tissue-equivalent proportional counters (TEPC). Appl. Radiat. Isot. 67, S171–S174. doi:10.1016/j.apradiso.2009.03.042
Orchard, G. M., Chin, K., Prestwich, W., Waker, A., and Byun, S. (2011). Development of a thick gas electron multiplier for microdosimetry. Nucl. Instrum. Meth. A 6381, 122–126. doi:10.1016/j.nima.2011.01.179
Parisi, A., Sato, T., Matsuya, Y., Kase, Y., Magrin, G., Verona, C., et al. (2020). Development of a new microdosimetric biological weighting function for the RBE10assessment in case of the V79 cell line exposed to ions from1H to238U. Phys. Med. Biol. 65, 235010. doi:10.1088/1361-6560/abbf96
Particle Therapy Co-Operative Group (2025). Particle therapy Co-operative group. Available online at: https://www.ptcog.site/.
Prieto-Pena, J., Gomez, F., Fleta, C., Guardiola, C., Pellegrini, G., Donetti, M., et al. (2019). Microdosimetric spectra measurements on a clinical carbon beam at nominal therapeutic fluence rate with silicon cylindrical microdosimeters. IEEE Trans. Nucl. Sci. 66, 1840–1847. doi:10.1109/tns.2019.2921453
Rollet, S., Angelone, M., Magrin, G., Marinelli, M., Milani, E., Pillon, M., et al. (2012). A novel microdosimeter based upon artificial single crystal diamond. IEEE Trans. Nucl. Sci. 59, 2409–2415. doi:10.1109/tns.2012.2209677
Rosenfeld, A. B. (2016). Novel detectors for silicon based microdosimetry, their concepts and applications. Nucl. Instrum. Meth. A 809, 156–170. doi:10.1016/j.nima.2015.08.059
Selva, A., Bellan, L., Bianchi, A., Giustiniani, G., Colautti, P., Fagotti, E., et al. (2022). Microdosimetry of an accelerator based thermal neutron field for boron neutron capture therapy. Appl. Radiat. Isot. 182, 110144. doi:10.1016/j.apradiso.2022.110144
Selva, A., Bianchi, A., Rossignoli, M., Bortolussi, S., Postuma, I., Pisent, A., et al. (2025). A new boron-doped gas microdosimeter for clinical Boron Neutron Capture Therapy. Appl. Radiat. Isot. 220, 111721. doi:10.1016/j.apradiso.2025.111721
Tilikidis, A., Lind, B., Näfstadius, P., and Brahme, A. (1996). An estimation of the relative biological effectiveness of 50 MV bremsstrahlung beams by microdosimetric techniques. Phys. Med. Biol. 41, 55–69. doi:10.1088/0031-9155/41/1/005
Tran, L. T., Bolst, D., Guatelli, S., Biasi, G., Fazzi, A., Sagia, E., et al. (2018). High spatial resolution microdosimetry with monolithic ΔE-E detector on12C beam: monte Carlo simulations and experiment. Nucl. Instrum. Meth. A 887, 70–80. doi:10.1016/j.nima.2017.12.079
Tsuda, S., Sato, T., Takahashi, F., Satoh, D., Endo, A., Sasaki, S., et al. (2010). Measurement of microdosimetric spectra with a wall-less tissue-equivalent proportional counter for a 290 MeV/u 12C beam. Phys. Med. Biol. 55, 5089–5101. doi:10.1088/0031-9155/55/17/013
Tsuda, S., Sato, T., Takahashi, F., Satoh, D., Sasaki, S., Namito, Y., et al. (2012). Systematic measurement of lineal energy distributions for proton, He and Si ion beams over a wide energy range using a wall-less tissue equivalent proportional counter. J. Radiat. Res. 53, 264–271. doi:10.1269/jrr.11135
Verona, C., Cirrone, G. A. P., Magrin, G., Marinelli, M., Palomba, S., Petringa, G., et al. (2020). Microdosimetric measurements of a monoenergetic and modulated Bragg Peaks of 62 MeV therapeutic proton beam with a synthetic single crystal diamond microdosimeter. Med. Phys. 47, 5791–5801. doi:10.1002/mp.14466
Verona, C., Magrin, G., Solevi, P., Bandorf, M., Marinelli, M., Stock, M., et al. (2018). Toward the use of single crystal diamond based detector for ion-beam therapy microdosimetry. Radiat. Meas. 110, 25–31. doi:10.1016/j.radmeas.2018.02.001
Keywords: microdosimetry, tissue-equivalent proportional counters, hadron therapy, BNCT, LET, radiation quality
Citation: Selva A, Bianchi A, Fazzi A and Conte V (2025) Miniaturized microdosimeters for hadron therapy. Front. Sens. 6:1597929. doi: 10.3389/fsens.2025.1597929
Received: 21 March 2025; Accepted: 26 June 2025;
Published: 07 July 2025.
Edited by:
Alberto Quaranta, University of Trento, ItalyReviewed by:
Yuqin Shang, Arizona State University, United StatesCopyright © 2025 Selva, Bianchi, Fazzi and Conte. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anna Selva, YW5uYS5zZWx2YUBsbmwuaW5mbi5pdA==
 Anna Selva
Anna Selva Anna Bianchi
Anna Bianchi Alberto Fazzi2
Alberto Fazzi2 Valeria Conte
Valeria Conte