- 1KVK-Fatehgarh Sahib, Punjab Agricultural University, Ludhiana, Punjab, India
- 2ICAR – Indian Institute of Agricultural Biotechnology, Ranchi, Jharkhand, India
- 3PG Department of Agriculture, Khalsa College Amritsar, Amritsar, Punjab, India
- 4Department of Botany, Punjab Agricultural University, Ludhiana, Punjab, India
- 5ICAR- Indian Agricultural Research Institute, Regional Station, Pusa, Samastipur, Bihar, India
Seaweeds are abundant and valuable marine resources that contain a diverse range of bioactive compounds, including lipids, minerals, phytohormones, amino acids, carbohydrates, osmo-protectants, and antibacterial substances. Historically, seaweeds have been widely used in food, feed, and medicine, but their agricultural significance has gained increasing recognition in recent years. With the growing shift toward organic and sustainable farming, seaweed extracts (SEs) have been explored as biofertilizers, soil conditioners, and natural biocontrol agents. They play a crucial role in enhancing soil health, improving plant growth, and increasing resistance against pests, diseases, and abiotic stressors such as drought, salinity, and extreme temperatures. Their ability to stimulate plant defense mechanisms and promote root development makes them an eco-friendly alternative to synthetic agrochemicals. Numerous studies have demonstrated the efficacy of seaweed extracts in boosting crop productivity while minimizing environmental impact. This review highlights recent advancements in seaweed-based agricultural applications, focusing on their benefits, mechanisms of action, and potential for integration into sustainable farming practices.
1 Introduction
The multicellular, microscopic algae known as seaweeds are found in maritime environments. Although there are more than 9 thousand macroalgae species in the waters, they can be generically categorized into three classes: Rhodophyta (red algae), Chlorophyta (green algae), and Phaeophyta (brown algae) (1). Seaweeds are naturally rich in nutrients, including beneficial lipids, enzymes, polysaccharides, proteins, and bioactive peptides (2). Additionally, they produce various stress-related bio-compounds to withstand and lessen various pressures in their natural habitats (3). Because of their rich resource content, seaweeds are useful as plant modifiers that can increase agricultural output. For many years, seaweeds have been employed in agriculture as bio-stimulants. According to the oldest reports, seaweeds were employed for centuries as soil conditioners before being used as biofertilizers and bio-stimulants (1). Seaweeds aren’t currently being used directly for either commercial or agricultural purposes. The best nutrient sources are seaweed extracts, full of the valuable micronutrients and macronutrients present in seaweeds (3). Since more than one-third of the global bio-regulator market is made up of seaweed extracts, their use as a preferred bio-regulator has caused a boom in the agribusiness sector (4). The growing urbanization, lack of groundwater, climate change, and a general reduction in arable land are some of the toughest challenges that the world’s agriculture is currently experiencing. Together, they represent a greater threat to meeting the world’s population’s rising food needs.
On the one hand, farmers and producers utilize excessive chemicals to increase crop output. On the other side, overusing these chemicals has resulted in several health risks and environmental safety concerns, as well as deteriorating quality of the rhizosphere (5). Therefore, it is urgent to find a strategy to reduce the use of harmful chemicals in agriculture if they are not eliminated. In this regard, the seaweed extract-based bio-stimulants provide a novel and substitute method for increasing agricultural crop productivity without using chemical fertilizers or pesticides (Shukla et al., 2019). Extract preparation from seaweeds can be directly applied onto the soil, just like chemical fertilizers or pesticides, or they can be used as foliar spray on plants (6). Applications made to the soil can create soil microflora, leading to soil retention and remediation (7). On plants, seaweed extracts can affect phytohormone homeostasis and reduce nutrient shortage (6). It is evident from the existing literature that seaweeds play a crucial role in both agriculture and daily life. Most seaweed deposits are found throughout the world’s coastlines and should be used wisely and effectively. Plants can benefit from the physiological processes of biostimulants, which can be synthetic or natural compounds (8). These processes include improved tolerance to various abiotic stressors and nutrient absorption and translocation (9). Plants can benefit from the physiological processes of biostimulants, which can be synthetic or natural compounds generated from microorganisms and/or plants. These processes include improved tolerance to various abiotic stressors and nutrient absorption and translocation (10). Creating bio-stimulants based on seaweed extract is one method of efficient use of seaweed biomass. However, farmers should be made aware of and encouraged to use these seaweed-based bio-regulators in their fields in place of standard chemical fertilizers and pesticides (11). Therefore, using seaweed-based solutions can be a sustainable way to enhance integrated pest management and organic farming (Wan et al., 2018). Even though there have been numerous reviews on its bio-regulator-based use in agricultural advancements, this review paper concentrates on a detailed understanding of the updates regarding the extraction methods for seaweed extract-based bio-regulator and their mechanisms of action for enhancing plant as well as soil health. We have also discussed the value of seaweed extracts in other fields, such as horticulture and floriculture. Additionally, the prospects for seaweed extract-based bio-stimulants are given in-depth, with a strong emphasis on the economic side.
2 Importance of seaweed
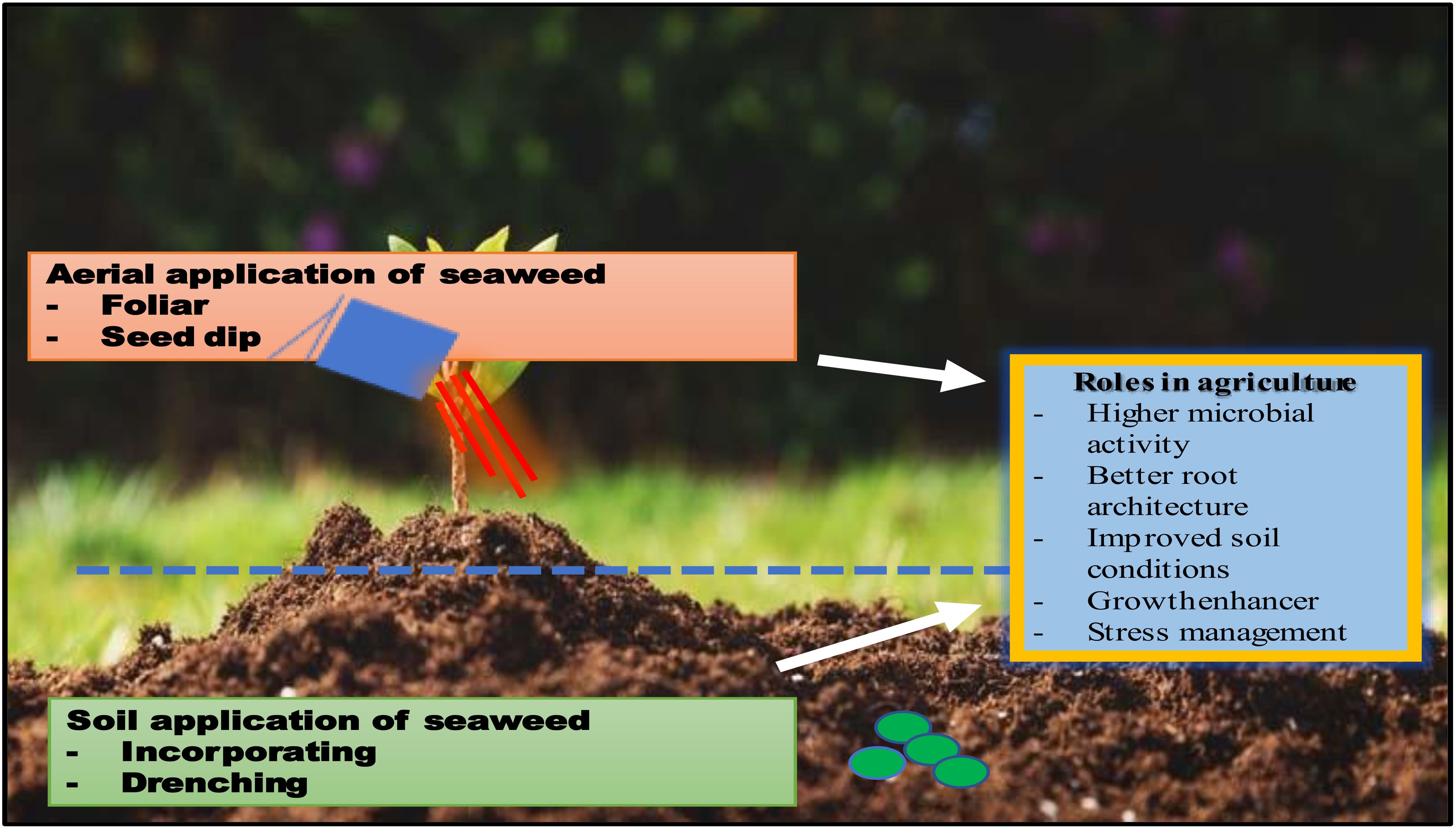
Rapid population expansion should lead to an increase in agro-based products. As one of the key components in increasing food production, the fertilizer industry will unavoidably grow alongside agro-based goods. There is now a 3–4 million tons fertilizer shortfall worldwide (12). Seaweeds are frequently used as manure throughout the world, particularly in regions close to the ocean. Seaweeds can be composted or used directly as organic manure (13). Two things determine how vital seaweed fertilizer is. First and foremost, seaweeds include significant concentrations of micronutrients besides Ca, N, P and K. Growth hormones like auxin, gibberellin, and cytokinin as well as oligo elements like Zn, Cu, etc. are also present (14). The presence of diverse mucopolysaccharides, which aid in soil conditioning by adding organic matter to the soil and increasing its moisture-retaining capacity, is a second distinguishing quality of seaweeds. Numerous seaweeds can be utilized as manure in all regions of the country, either directly or in the form of compost, due to their abundance of nutritional value for plant growth (Figure 1). When manure with seaweed compost was applied, efficient observations were also obtained with several crops such as cereals, fruits, and vegetables; crotons also thrived with its application; high nitrogen levels can also be maintained with its treatment.
3 Nutrient composition
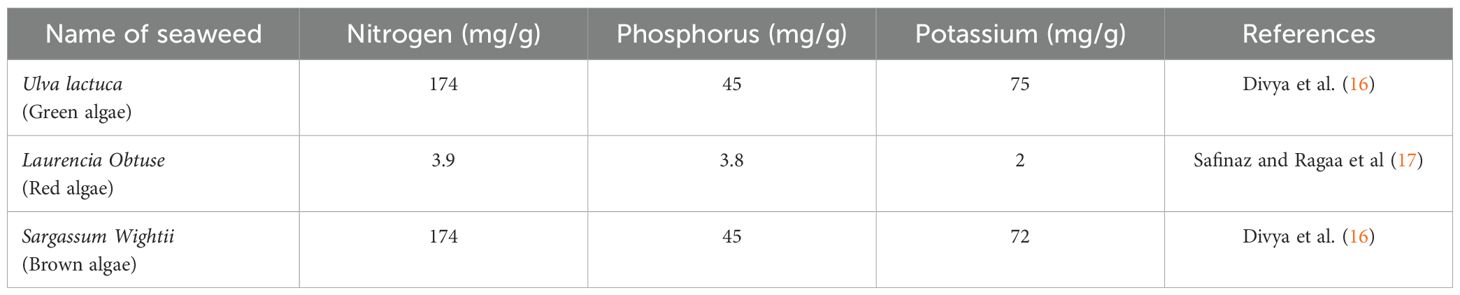
According to Mirparsa et al. (15), seaweeds are known to supplement the macronutrients (N, P, K, etc.) as well as a variety of micronutrients in amounts sufficient for plant growth (Table 1). The mineral content of various seaweed species from different taxonomic groupings, including red, green, and brown algae, was investigated. (Table 2). This indicated the presence of several mineral elements, including Ca, Mg, Na, K, Fe, Mn and Zn (19). In addition, several organic compounds, such as vitamins, cellulose, hemicelluloses, cellulose, fat, and amino acids, are rich in seaweed. It has also been shown that seaweeds have a greater mineral composition than terrestrial plants (20). Several researchers have identified a wide variety of polysaccharides as cell wall and storage component elements (21), indicating a high concentration of soluble and insoluble fibers. Seasonal variations, temperature, salinity, light, and the availability of nutrients are only a few examples of how the environment affects chemical composition (22). Between the wet winter and the warm summer, a considerable shift in composition was seen (23). More diverse amounts of phycobiliproteins, chlorophyll, and carotenoids were also detected (24).
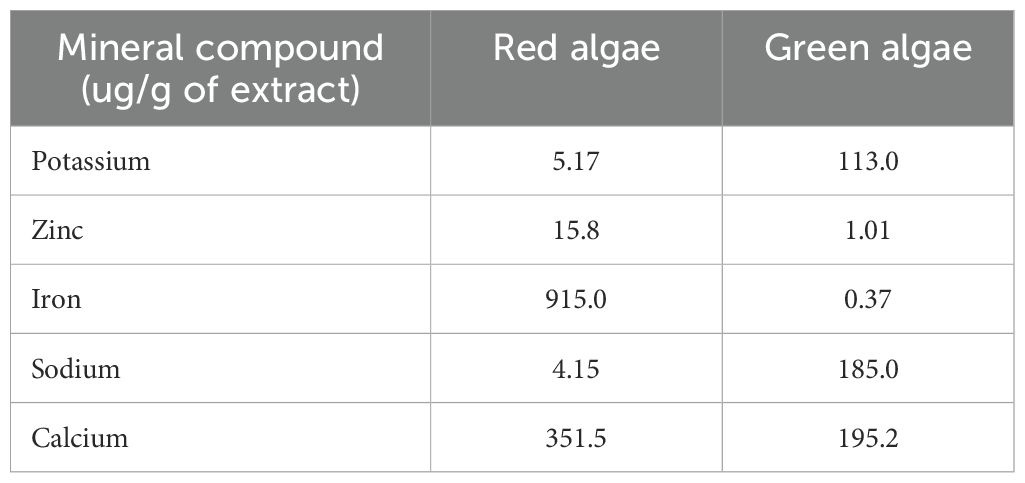
Table 2. Mineral composition of two genera of seaweed (18).
4 Chemical composition of seaweed
4.1 Growth hormones
The growth responses induced by seaweed extracts cannot be explained by the concentration of mineral nutritional components seen in commercial seaweed concentrations (SWCs). Seaweed concentrations (SWCs) may contain chemicals that regulate plant development, according to positive results seen in a variety of plant growth bioassays (25). Additionally, the wide variety of growth reactions brought on by its extracts suggests the existence of many hormones or chemicals that promote plant growth. Seaweed extracts and raw seaweed have both been found to contain cytokinins (26). Trans-zeatin and its dihydro derivatives, including trans-zeatin riboside are among the cytokinins found in seaweed compositions (27). Auxins have been found in other types of algae, such as Porphyra perforata. However, the amounts were small (28). The inactive conjugate of IAA with carboxyl groups, glycans, amino acids, and peptides is present in higher plants. Upon hydrolysis, these compounds are transformed into free active IAA (29). Ascophyllum nodosum and Laminaria digitata were used to extract water-soluble growth inhibitors that significantly slowed down the growth of lettuce’s hypocotyl (30). Studies using gas-liquid chromatography, thin-layer chromatography, and bioassays showed that one of these substances appeared to be connected to ABA. Others, including Tietz et al (31), have also reported finding ABA in seaweeds.
4.2 Betaines
Betaines are a suitable solute that plants use to reduce osmotic stress brought on by salinity and drought stress. Several different betaines and betaine-like substances can be found in Ascophyllum nodosum preparations. Still, they may also play other roles, such as increasing the amount of chlorophyll in leaves after being treated with seaweed extracts (32). There may have been less chlorophyll breakdown, which would explain the rise in chlorophyll content (33). Due to the seaweed’s betaines’ effects on increased chlorophyll content in the leaves of numerous agricultural plants, yields from those plants have been found to increase. (34)
4.3 Sterols
Sterols are a crucial class of lipids in eukaryotic cells, as they are in many other eukaryotes. Typically, a plant cell contains a variety of sterols, including cholesterol, 24-methylenecholesterol, stigmasterol, and b-sitosterol (35). Brown seaweed primarily contains fucosterol and its derivatives, whereas red seaweed typically contains cholesterol and its derivatives. Ergosterol and 24-methylenecholesterol are the two primary lipids that green seaweed accumulates (35).
5 Application methods of seaweed
Seaweed biomass and seaweed meal have been applied to horticultural crops using a variety of application techniques. The type of seaweed product utilized determines the type of application. Seaweed species are found in tropical, temperate, and polar parts of the world’s coastal climates (Sarkar et al., 2016). Near the seaside, where seaweeds are abundant, the usage of whole seaweed biomass or meal is widespread. Whole seaweeds or seaweed meal are spread on the ground and usually incorporated into the soil to speed up the microbial decomposition of the seaweed. Because soil bacteria reduce nitrogen during the decomposition process, causing a temporary nutrient immobilization that may adversely affect plant growth, seaweeds are introduced into the soil well before planting crops. As an organic matter, the decomposed seaweed generally enhanced the soil’s physical and chemical characteristics, its ability to hold water and its microbial activity. Additionally, it shielded plants from environmental hazards including extreme temperatures and water stress (36).
Seaweed extracts, which are either in a soluble powder form or as liquid extracts, are readily available and are the most commonly utilized seaweed product on agricultural and horticultural crops. Applying drip irrigation to crops while mixing liquid extracts with irrigation water it is possible to apply extracts close to the plant’s roots. Seaweed extracts are also used as foliar sprays on various floral, vegetable, and tree crops (37). Leaf stomata opening in the morning appears to be the best time for foliar administration of seaweed extracts. The plant’s stage of growth has an impact on how effective seaweed extracts are. For instance, Dwelle and Hurley (38) discovered that seaweed extract had the most significant effect on potato yield when applied two weeks after tuber start.
6 Preparation of seaweed bioregulator
Seaweed extract includes vitamins, auxins, gibberellins, antibiotics, trace elements, and other chemical substances. To protect the crops, it is crucial to preserve some of the components as heat decomposes others. When making commercial seaweed extracts and evaluating the conflicting field trial findings that have been reported, it is known that several seaweed constituents experience significant seasonal fluctuations (12).
6.1 Extraction from brown seaweeds (Sargassum tenerrimum and Padina tetrastromatica)
For the preparation of seaweed extracts, species such as Sargassum tenerrimum and Padina tetrastromatica should be chopped into smaller pieces and boiled in purified water (39). After boiling, the mixture should be filtered to obtain the filtrate. This filtrate serves as the base for preparing different concentrations of seaweed extract, beginning with a 100% concentration.
6.2 Extraction from commercial seaweed meal (Ascophyllum nodosum and Fucus vesiculosus)
Challen and Hemingway (40) outlined a method using two commercial seaweed meal samples derived from Ascophyllum nodosum and Fucus vesiculosus. To prepare the extract, the seaweed meal should be diluted with water to match the total solids content of a commercial seaweed extract obtained from dried seaweed. Further dilution may be necessary. The process involves mixing the powdered seaweed meal with distilled water and allowing it to stand. The mixture should then be heated, cooled, and filtered through a fine screen to remove solids. The resulting liquid should undergo centrifugation, after which the separated solid fraction should be pressed to extract additional liquid. The pressed extract should be combined with the primary extract, and the mixture should be condensed under reduced pressure to yield a concentrated brown fluid.
7 Bioregulator nature
The positive properties of seaweed-based organic manure are attributed to the extensive spectrum of bioactive substances found in seaweed extract, including vitamins, auxins, gibberellins, antibiotics, trace elements, and amino acids. The species employed and the extraction technique may significantly impact the activity that promotes plant growth. Many seaweed components are said to experience seasonal fluctuations, and these variations are taken into account in the commercial manufacture and testing of organic seaweed manure. Surprisingly, despite the intended use of the extraction methods, there is a shortage of precise and thorough information about extraction techniques and procedures for agricultural purposes. This is partly because ownership dossiers for production and extraction techniques are rarely published and maintained (41). Extracts are frequently created by grinding at low temperatures, using water, acid, or alkali, or by physically disrupting the material (42). The most economical and practical approach for releasing micro- and macronutrients necessary for producing organic manure and bio-regulators appears to be the water-based method (43). Numerous publications indicate that water or an alkaline seaweed extract has a bio-stimulant impact on grains, legumes, vegetables, etc. (44). Species include Ascophyllum nodosum, Fucus vesiculata, Hypnea musciformis, Sargassum plagiophyllum, Ulva lactuca, Sargassum wightii, Laminaria saccharina, Padia tetrastomatica, and Ecklonia radiata have purportedly been used to manufacture liquid seaweed fertilizer (45).
8 Scope of seaweed in agriculture
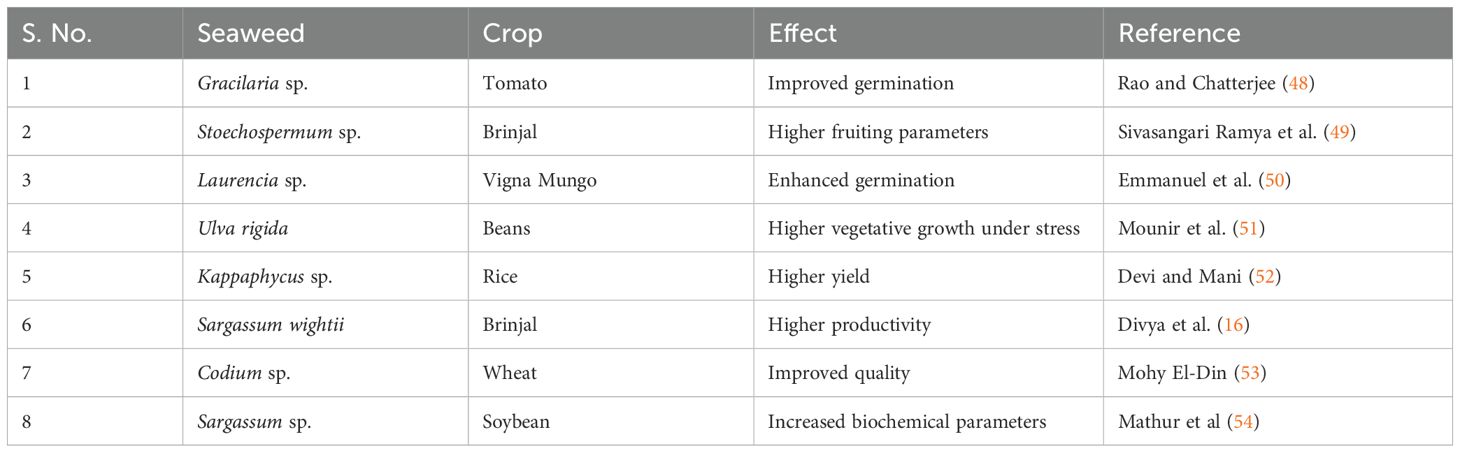
The right balance of phytohormones, humic acids, and phytonutrients in seaweed characterizes it as superior organic manure. In addition to boosting soil fertility, applying seaweed fertilizers improves the soil’s ability to retain moisture and provides sufficient micronutrients for plant growth (45). The development of liquid-based seaweed fertilizer is a result of current attention being paid to the spray application of plant nutrients, which improves nutrient absorption efficiency (46). Seaweed extracts are applied to the soil, utilized as a foliar spray, and used to soak seeds before planting. It has been suggested that using seaweed-based fertilizers will lower the nitrogen, phosphorus, and potash fertilizer doses. It is known that about 59 species of seaweed can increase crop output and growth, as well as seed germination and other growth factors, more effectively than artificial fertilizers (47) (Table 3).
9 Effect on soil health
9.1 Seaweed as a soil conditioner
To boost agricultural yield in coastal locations and to restore alkaline soils where deficiency diseases are common, seaweeds were utilized directly or through compost along with FYM in European countries starting in 1951. Since the ancient period, seaweeds have been employed by combining them with sand or soil or composting them with organic materials like peat, straw, etc. Due to the advantages of using seaweed as an organic source of nutrients, its use in agriculture has increased (55). Seaweeds add humic acid to clay soils that are impenetrable, devoid of crumby structure and have low quantities of organic matter. The polysaccharide alginates interact chemically with the soil’s metallic radicals or join up with larger clay aggregates to a greater extent to generate a crumby structure (12). Seaweeds were initially used as soil conditioners, but more recently, interest has grown in using them as organic or bio-fertilizers to increase plant nutrition (56). They are given out in various forms, such as manure, granules, powder, and foliar spray. (57).
9.2 Bio-remediation of polluted soils by seaweed
It is well known that by employing seaweed biomass as adsorbents, heavy metal ions like cadmium and lead are effectively removed (58). Seaweed may be used to remove heavy metal ions from aqueous solutions, according to investigations on heavy metal adsorption employing Kappaphycus sp (59). To reduce chromium toxicity, a mixture of green, red, and brown algae was created. Material analysis revealed two functional groups of seaweed surface polysaccharides that were involved in adsorption (60). Similar to this, investigations have shown that seaweed like Eucheuma denticulatum and Kappaphycus alvarezii is effective at removing lead (Pb) toxicity and cadmium (II) toxicity, respectively (61).
9.3 Rhizosphere microbes proliferation
The use of seaweed and seaweed extracts promotes the growth of beneficial soil microbes and the release of chemicals that condition the soil. Alginates influence soil characteristics and promote the development of helpful fungi, as was already indicated. In a study published in 2000, Ishii and colleagues discovered that alginate oligosaccharides, which are produced by the enzymatic breakdown of alginic acid, which is primarily extracted from brown algae, significantly induced hyphal growth and elongation in arbuscular mycorrhizal (AM) fungi and triggered their infectivity on trifoliate orange seedlings. Laminaria japonica Areschoug and Undaria pinnatifida (Harvey) Suringar, two different marine brown algae extracts, could be employed as an AM fungal growth stimulant (62).
10 Effect of seaweed on plant
10.1 Seaweed for crop production and soil fertility
To boost soil fertility and crop output, seaweed extract has been used directly or in compost form (41). In most cases, brown seaweeds like Ascophyllum nodosum, Fucus, Laminaria, Sargassum, and Turbinaria spp. Make commercial seaweed extract products (63). Seaweeds typically include a wide variety of uncommon minerals, including organic substances, plant hormones, and various polysaccharides (laminarin, fucoidan, and alginates), which are typically absent from terrestrial plants (64). Seaweed extracts applied topically to the leaves of various crops improve nutrient uptake, growth stimulation, and root development (65). These extracts function as chelators, improve the plant’s capacity to absorb minerals and nutrients and improve the soil’s structure and aeration, all of which encourage the development of roots (66). There are additional reports that applying algal extracts to the model plant Arabidopsis thaliana improves its root and shoot growth (67). Additionally, it increased the consumption of micronutrients like Mg, Zn, Mn, and Fe, as well as macronutrients like N, P, K, Ca, and S (68).
10.2 Phytohormones impact
Plant growth and stress tolerance have long been enhanced by using seaweeds and their extracts. According to Wally et al. (69), seaweed contains substances that promote plant growth, including auxins (IAA, IBA), gibberellins, cytokinins, etc. These substances also increase the growth and yield of various fruit and vegetable crops. The potential contribution of phytohormones in seaweed extract to plant growth has been extensively studied. According to Zodape et al. (68), cytokinin plays a part in controlling plant development. Various agricultural environments have used Ascophyllum nodosum seaweed extracts to increase production and productivity (70). The Ascophyllum nodosum extracts under study revealed a significant concentration of cytokinins (CKs), particularly trans-zeatin type CK and abscisic acid (69).
10.3 Antioxidant potential of seaweeds
Numerous studies have found a strong link between total phenolic content and antioxidant activity. Phlorotannins, a type of seaweed polyphenol chelate ferrous ions, scavenge free radicals like superoxide and peroxyl radicals, and neutralize nitric oxide (71). Phlorotannins including eckol, dieckol, phlorofucofuroeckol A, and 8,8′-bieckol from the Japanese brown algae Eisenia bicyclis, Ecklonia cava, and Ecklonia kurome exhibit 2-10 times more antioxidant activity when compared to catechin, tocopherol, and ascorbic acid. In a liposome system, these substances also demonstrated strong prevention of phospholipid peroxidation (72). According to Cho et al. (73), ethanol extracts of Sargassum siliquastrum have a 95% DPPH radical scavenging activity at 0.5 mg/ml or above.
10.4 Root development and mineral absorption
Seaweed products encourage the development and expansion of roots (74). When extracts were administered to maize at an early growth stage, the root-growth stimulatory impact was more prominent, and the reaction was comparable to that of auxin. This key hormone promotes root growth (74). In addition to other substances in the extracts, endogenous auxins may impact a stronger root system. Seaweed extracts increase the uptake of nutrients by roots (75), leading to root systems with higher water and nutrient efficiency, which in turn improves plant vigor and growth in general.
10.5 Post-harvest management
Numerous research on the impact of a commercial A. nodosum extract treatment on spinach revealed that it improved the leaf’s nutritional value and storage quality as well as the production of flavonoids (76). Once a month, 3–10 L/ha of A. nodosum extract was treated in the field to determine the extract’s phenolic and antioxidant content. The concentration of phenolic chemicals in cabbage, potato, and onion enhanced following the application of seaweed extract. The use of seaweed extract did not change yield but considerably raised the number of phenolics and flavonoids that are good for your health (77). Additionally, post-harvest treatments have been made using seaweed extracts. Post-harvest treatment of navel oranges with seaweed extract containing a mixture of Sargassum, Laminaria, and A. nodosum extracts significantly boosted post-harvest shelf-life and quality while maintained at room temperature or in cold storage.
11 Seaweed’s role in stress reduction
11.1 Abiotic stress reduction
Seaweeds can assist plants in coping with abiotic challenges and their bio-controlling abilities. Plants express a few proteins that are directly related to stress management when they are under stress. Osmoprotectants, transporter, and detoxifying enzymes are some of these. Some metabolisms can be changed to manage stress by creating regulating chemicals such as proline, salicylic acid, and abscisic acid (78). Plants release glycine betaine to stabilize protein structure and the cell wall, and they scavenge ROS to maintain turgor pressure. Research reveals that betaines and cytokinins are two bioactive components of seaweed that are important in stress management, yet the mechanism by which seaweeds help plants to tolerate stress is not fully understood (79).
Additionally, it was noted that plants’ endogenous levels of stress-related substances such as cytokinins, proline, and antioxidants increased when seaweed extract was present (76). However, seaweed polysaccharides have the capacity to stimulate plant defense mechanisms (80). Ascophyllum nodosum and their lipophilic fraction boost Arabidopsis’ capacity for cold tolerance by stabilizing membrane structure, reducing the expression of the chlorophyllase gene, and raising the expression of the cold tolerance gene. These actions also increase resistance to high-salt conditions, drought, and heat (81).
Plants function better under abiotic stressors thanks to bioactive chemicals found in seaweed extracts. Spraying extracts on plants has increased their resistance to the stress caused by freezing temperatures (81). Applying an A. nodosum extract formulation specifically improved the ability of grapes to withstand freezing because it reduced the leaves’ osmotic potential, which is a vital sign of osmotic tolerance. After nine days of seaweed extract treatment, the average osmotic potential of the treated plants was 1.57 MPa as opposed to 1.51 MPa in the untreated controls (82).
11.2 Biotic stress reduction
It has been demonstrated that seaweed extracts improve plant defense against pests and diseases. Seaweed products also affect the physiology and metabolism of plants, and they influence the microbial rhizosphere community, which benefits plant health. Various pest plants treated with seaweed extracts are typically avoided by aphids and other sap-feeding insects (83). Spraying apple plants with hydrolyzed seaweed extracts decreased red spider mite numbers (84), and 2-3 years of applying its extract resulted in a control similar to acaricides (84). Furthermore, it was demonstrated that using Maxicrop on strawberry plants (Fragaria sp.) greatly reduced the two-spotted red spider mite population (Tetranychus urticae) (83). The presence of chelated metals, which have been shown to reduce the population of red spider mites, in seaweed extracts has been hypothesized (85). Observations have shown the potential of its extracts in causing plant resistance to pests and diseases as they have become more popular as organic fertilizers or soil conditioners in the past. Numerous investigations have been carried out to assess the antibacterial properties of seaweed extracts. The antifungal activities of the extracts have been connected to terpenes (86). Numerous green algae, including Ulva fasciata and U. lactuca, have shown effectiveness against red cotton bug nymphs and adults (Dysdercus cingulatus).
Furthermore, many types of brown seaweeds are good at avoiding plant diseases (87). Extracts from various marine algae species, including Melanothamnus afaqhusainii, Sargassum tenerrimum, and Padina tetrastromatica, exhibited nematicidal activity against the root-knot nematode Meloidogyne javanica (88). The bioactive compounds obtained from green, brown, and red algae, such as polysaccharides, fatty acids, tannins, and halogenated compounds, have recently been found to be promising in suppressing fungus infects the roots of okra seedlings (87). With an improvement in strawberry output, foliar use of seaweed extracts was proven to reduce fruit rot (89). To establish direct pathogen inhibition and induce systemic resistance in the plants, thorough studies of the influence of seaweed extracts in suppressing pests and disease must be carried out.
Kahn et al. (90) discuss how seaweed products can reduce biotic stress. When sprayed to the leaves or absorbed into the soil at a 1:500 dilution, the seaweed extract Kelpak 66 has been demonstrated to decrease root damage caused by nematode (Meloidogyne incognita) predation in tomatoes (91). The roots, leaves, and fruits of very ill plants exhibited the most significant improvement with a sole soil application at the time of transplantation. Interestingly, when the seaweed extract was administered, the number of nematodes recovered from inside the roots was significantly less than that in the infected controls, even though the nematode count in the soil grew over the optimum point. When young nematodes were injected into Arabidopsis seedlings in a lab, the fertility of the root-knot nematode M. javanica decreased if the seedlings were treated with seaweed extract or an equivalent dosage of a betaine combination (92).
12 Physical environment interactions
Salt content, hydraulic conductivity, sunlight, temperature, and availability of nutrients are a few of the primary environmental elements that impact seaweeds. The relationships between sessile animals and their epiphytic bacteria, fungi, algae, and other sessile animals, as well as the connections between herbivores and plants and predators, including humans, are examples of biological interactions. All of these elements working together have an overall impact on the individual patterns of growth, morphology, and reproduction. The physicochemical environment of an organism, which is made up of all the external abiotic factors that affect it, is extremely complicated and continually changing. The saltwater density is influenced by temperature and salinity, mixing nutrient-rich bottom water with nutrient-depleted migration of epiphytic animal larvae. Turbidity, siltation, and the availability of nutrients can all be impacted by water velocity. These are illustrations of how one environmental factor influences another. The interaction of temperature and photoperiod controls growth and reproduction in many seaweed species. Additionally, interactions between physicochemical and biological components are more often than not. Factor interaction through sequential effects is the last type of interaction. Red algae may catabolize some phycobiliproteins due to a lack of nitrogen, lowering their capacity to absorb light. Therefore, many seaweed species and genera within seaweed floras inhabiting more or less constrained regions of the world’s seacoast are more or less identical.
13 Current status of seaweed fertilizer use
The United States, United Kingdom, Canada, New Zealand, Australia, Ireland, Scotland, England, France, and Spain are just a few of the nations that regularly employ seaweed fertilizers. Green, brown, and red seaweed extracts are offered for sale in South Africa to soil conditions. Hypnea, Ulva, and Enteromorpha are frequently employed in Brazil, while the Sargassum is utilized in China and the Hypnea in the West Indies.
14 Seaweed as a farming resource
As previously mentioned, coastal agricultural soils have been treated with seaweed for millennia, either in their fresh or sun-dried state. However, a variety of simple (compost, flour) or more complicated seaweed-based fertilizers are being offered commercially (bio-stimulant extracts). Since the middle of the 20th century, many types of extracts have replaced seaweed-based fertilizers as bio-stimulants in contemporary agriculture (93). The increased usage of extracts is likely due to their convenience in storage and transportation and the quick effects of their active ingredients on plants (94). The horticulture industry has already used seaweed extracts to a large extent (94), and horticultural crops have been the subject of the majority of studies on seaweed usage in agriculture. To enhance the economic and environmental balances, several seaweed extracts are utilized to boost the effectiveness of traditional fertilizer by decreasing doses. Through cryo processing, cell rupturing under high pressure, or extraction with water, acid, or an alkali, bioactive chemicals in seaweed can be recovered (42). All of these techniques prevent the target products from degrading.
15 Constraints
Long-term use and the high salt content of seaweeds may result in salinity issues. Utilizing purified preparations may help to mitigate this problem. These problems can be avoided by adopting sporadic pauses in the application of seaweed and allowing a rain rinse phase to minimize salt content. The use of seaweeds from contaminated locations as fertilizer or soil conditioner would increase the pollutants in soil and plants because seaweeds are efficient heavy metal accumulators in marine and other ecosystems (95). Seaweed contamination levels must therefore be assessed before application. Additionally, sulfides produced by the anaerobic breakdown of sulfur compounds in seaweed may cause soil acidification as they are microbially oxidized to sulfates (96). Carrageenans, laminarins, and ulvans are the distinctive organic components of seaweed that set them apart from other major plants’ polymeric carbon compounds like cellulose, hemicellulose, and lignin. The soil microbial community also experiences these novel compounds, which may be resistant to biodegradation (97). In such cases, a thorough analysis of the long-term effects of seaweed use on the local microbial population of the soil should be conducted. Additionally, seaweeds are known to support a variety of microbial communities that generate antibacterial chemicals (98). It is crucial to determine the depth to which this microbial community has established itself in the soil. The seaweed-associated microbial population that has been introduced may boost soil nutrient turnover, which could form the cornerstone of better plant and soil health.
16 Conclusion
This review highlights the significance of seaweed-based bio-regulators derived from red, brown, and green algae in agricultural disease management. Seaweed extracts (SEs) offer a natural and sustainable alternative to synthetic chemicals, reducing the environmental impact of conventional agrochemicals while enhancing plant health. Their bioactive compounds stimulate plant defense mechanisms by activating pathogen-responsive genes, transcription factors, and defense-related enzymes, thereby improving disease resistance. Standardizing extraction techniques, application rates, and treatment methods is crucial to maximizing the effectiveness of SEs, as variations in composition influence their efficacy against different plant diseases. Further research is needed to evaluate and compare diverse seaweed species to determine their optimal use in various agricultural conditions. By refining dosage, method, and application frequency, SEs can be integrated into modern farming systems to enhance plant growth, improve soil health, and boost stress tolerance. This approach will strengthen confidence in SEs as reliable bio-regulators, promoting their widespread adoption in sustainable agriculture and contributing to eco-friendly crop management strategies.
Author contributions
KS: Conceptualization, Writing – original draft, Writing – review & editing. AS: Methodology, Writing – original draft, Writing – review & editing. HC: Methodology, Software, Supervision, Writing – review & editing. HK: Conceptualization, Formal Analysis, Writing – review & editing. MH: Investigation, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Khan W, Rayirath UP, Subramanian S. Seaweed extracts as biostimulants of plant growth and development. J Plant Growth Regul. (2009) 28:386–99.
2. Okolie CL, Mason B, Critchley AT. Seaweeds as a source of proteins for use in pharmaceuticals and high-value applications. In: Novel proteins for food pharmaceuticals and agriculture. John Wiley & Sons Ltd, Chichester, UK (2018). p. 217–38.
3. Shukla PS, Borza T, Critchley AT, Prithiviraj B. Carrageenans from red seaweeds as promoters of growth and elicitors of defense response in plants. Front Mar Sci. (2016). doi: 10.3389/fmars.2016.00081
4. Al-Juthery HWA, Abbas Drebee H, Al-Khafaji BMK, Hadi RF. Plant biostimulants, seaweed extract as a model (article review). IOP Conf Ser Earth Environ Sci. (2020). doi: 10.1088/1755-1315/553/1/012015
5. Lin W, Lin M, Zhou H. The effects of chemical and organic fertilizer usage on rhizosphere soil in tea orchards. PloS One. (2019) 14:e0217018.
6. du Jardin P. Plant biostimulants: Definition, concept, main categories and regulation. Sci Horticult. (2015) 196:3–14. doi: 10.1016/j.scienta.2015.09.021
7. Chaudhary P, Xu M, Ahamad L, Chaudhary A, Kumar G, Adeleke BS, et al. Application of synthetic consortia for improvement of soil fertility, pollution remediation, and agricultural productivity: a review. Agronomy. (2023) 13:643. doi: 10.3390/agronomy13030643
8. Ngoroyemoto N, Kulkarni MG, Stirk WA, Gupta S, Finnie JF. Interactions between microorganisms and a seaweed-derived biostimulant on the growth and biochemical composition of Amaranthus hybridus L. Nat Prod Commun. (2020) 15:1–9. doi: 10.1177/1934578X20934228
9. Kakbra RF. Effect of seaweed, moringa leaf extract, and biofertilizer on cucumber growth, yield, and fruit quality under greenhouse conditions. arXiv Preprint arXiv. (2024) 2403:17984.
10. Li H, Penttinen P, Mikkonen A, Stoddard FL, Lindström K. Response of soil bacterial community diversity and composition to time, fertilization, and plant species in a sub-boreal climate. Front Microbiol. (2020) 11:1234. doi: 10.3389/fmicb.2020.01780
11. Ali O, Ramsubhag A, Jayaraman J. Biostimulatory activities of Ascophyllum nodosum extract in tomato and sweet pepper crops in a tropical environment. PloS One. (2019) 14:e0218353. doi: 10.1371/journal.pone.0216710
13. Cole AJ, Roberts DA, Garside AL, de Nys R, Paul NA. Seaweed compost for agricultural crop production. J Appl phycology. (2016) 28:629–42. doi: 10.1007/s10811-015-0544-2
14. Jahan S, Rautela S. Hazardous and trace materials in soil and plants. Cambridge, MA: Academic Press (2022). p. 231–46.
15. Mirparsa T, Ganjali HR, Dahmardeh M. The effect of biofertilizers on yield and yield components of sunflower oil seed and nut. Int J Agric Biosci. (2016) 5:46–9.
16. Divya K, Roja MN, Padal SB. Effect of seaweed liquid fertilizer of Sargassum wightii on germination, growth and productivity of brinjal. Int J Adv Res Sci Eng Technol. (2015) 2:868–71.
17. Safinaz AF, Ragaa AH. Effect of some red marine algae as biofertilizers on growth of maize (Zea mays L.) plants. Int Food Res J. (2013) 20:1629–32.
18. Aslam MN, Kreider JM, Paruchuri T, Bhagavathula N, DaSilva M, Zernicke RF, et al. A mineral-rich extract from the red marine algae Lithothamnion calcareum preserves bone structure and function in female mice on a western-style diet. Calcif Tissue Int. (2010) 86:313–24. doi: 10.1007/s00223-010-9340-9
19. Tuhy Ł, Samoraj M, Basadynska S, Chojnacka K. New micronutrient fertilizer biocomponents based on seaweed biomass. Pol J Environ Stud. (2015) 24:2213–21. doi: 10.15244/pjoes/39552
20. Kumar NJL, Kumar RN, Patel K, Viyol S, Bhoi R. Nutrient composition and calorific value of some seaweeds from Bet Dwarka, west coast of Gujarat, India. Our Nat. (2009) 7:18–25.
21. Heltan MM, Wakibia JG, Kenji GM, Mwasaru MA. Chemical composition of common seaweeds from the Kenya Coast. J Food Res. (2015) 4:28–38. doi: 10.5539/jfr.v4n6p28
22. Hanan MK, Shimaa ME. Seasonal variations in the biochemical composition of some common seaweed species from the coast of Abu Qir Bay, Alexandria, Egypt. Oceanologia. (2013) 55:435–52. doi: 10.5697/oc.55-2.435
23. Benjama O, Masniyom P. Nutritional composition and physicochemical properties of two green seaweeds (Ulva pertusa and U. intestinalis) from the Pattani Bay in Southern Thailand. Songklanakarin J Sci Technol. (2011) 33:575–83.
24. Chojnacka K, Saeid A, Witkowska Z, Tuhy L. Biological active compounds in seaweeds extracts—the prospects for the application. Open Conf Proc J. (2012) 3:20–8. doi: 10.2174/1876326X01203020020
25. Stirk WA, van Staden J. O. H. A. N. N. E. S. Seaweed products as biostimulants in agriculture. In: World seaweed resources [DVD-ROM]. ETI Information Services Lts, Univ, Amesterdam (2006), ISBN: ISBN, 907500080–4.
26. Brain KR, Chalopin MC, Turner TD, Blunden G, Wildgoose PB. Cytokinin activity of commercial aqueous seaweed extract. Plant Sci Lett. (1973) 1:241–5. doi: 10.1016/0304-4211(73)90026-6
27. Stirk WA, Staden J. Isolation and identification of cytokinins in a new commercial seaweed product made from Fucus serratus L. J Appl Phycol. (1997) 9:327–30. doi: 10.1023/A:1007910110045
28. Zhang W, Yamane H, Chapman DJ. The phyto-hormone profile of the red alga Porphyra perforata. Bot Mar. (1993) 36:257–66. doi: 10.1515/botm.1993.36.3.257
29. Bartel B. Auxin biosynthesis. Annu Rev Plant Physiol Plant Mol Biol. (1997) 48:51–66. doi: 10.1146/annurev.arplant.48.1.51
30. Hussain A, Boney AD. Hydrophilic growth inhibitors from Laminaria and Ascophyllum. New Phytol. (1973) 72:403–10. doi: 10.1111/j.1469-8137.1973.tb02048.x
31. Tietz A, Ruttkowski U, Kohler R, Kasprik W. Further investigations on the occurrence and the effects of abscisic acid in algae. Biochem Physiol Pflanzen. (1989) 184:259–66. doi: 10.1016/S0015-3796(89)80011-3
32. Blunden G, Jenkins T, Liu Y. Enhanced leaf chlorophyll levels in plants treated with seaweed extract. J Appl Phycol. (1997) 8:535–43. doi: 10.1007/BF02186333
33. Whapham CA, Blunden G, Jenkins T, Hankins SD. Significance of betaines in the increased chlorophyll content of plants treated with seaweed extract. J Appl Phycol. (1993) 5:231–4. doi: 10.1007/BF00004023
34. Genard H, Le Saos J, Billard J-P, Tremolieres A, Boucaud J. Effect of salinity on lipid composition, glycine betaine content and photosynthetic activity in chloroplasts of Suaeda maritima. Plant Physiol Biochem. (1991) 29:421–7.
35. Nabil S, Cosson J. Seasonal variations in sterol composition of Delesseria sanGuinea (Ceramiales, Rhodophyta). Hydrobiologia. (1996) 326:511–4. doi: 10.1007/BF00047854
36. Anderson G. Seaweed extract shows improved fruit quality at McLaren Valevineyard trial. Aust N Z. Grapegrow. Winemak. (2009) 548:17–22.
37. Selvaraj R, Selvi M, Shakila P. Effect of seaweed liquid fertilizer on Abelmoschus esculentus and Lycopersicon esculentum. Seaweed Res Util. (2004) 26:121–3.
38. Dwelle RB, Hurley PJ. The effects of foliar application of cytokinins onpotato yields in southeastern Idaho. Ida. Agric Exp Stn. U. S. A. (1984), 293–9.
39. Bhosle NB, Untawale AG, Dhargalkar VK. Effect of seaweeds extract on the growth of Phaseolus vulgaris. Indian Mar Sci. (1975) 4:208–10.
40. Challen SB, Hemingway JC. Growth of higher plants in response to feeding with seaweed extracts. Proc Fifth Int Sea. Symp. (1966), 359–67. doi: 10.1016/B978-0-08-011841-3.50056-2
41. Craigie JS. Seaweed extract stimuli in plant science and agriculture. J Appl Phycol. (2011) 23:371–93. doi: 10.1007/s10811-010-9560-4
42. Sharma HS, Fleming C, Selby C, Rao JR, Martin T. Plant bio-stimulants: a review on the processing of macroalgae and use of extracts for crop management to reduce abiotic and biotic stresses. J Appl Phycol. (2014) 26:465–90.
43. Michalak I, Chojnacka K. Algae as production systems of bioactive compounds. Eng Life Sci. (2015) 15:160–76. doi: 10.1002/elsc.201400191
44. Kavipriya R, Dhanalakshmi PK, Jayashree S, Thangaraju N. Seaweed extract as a bio-stimulant for the legume crop green gram. J Ecobiotechnol. (2011) 3:16–9.
46. Srijaya TC, Pradeep PJ, Chtterji A. Effect of seaweed extract as an organic fertilizer on the growth enhancement of black mustard plant. J Coast Environ. (2010) 1:137–50.
47. Partani T. Determination of the effect rates of seaweed extract on growth and performance of corn (Sc704) in Gorgan. Int J Agric Crop Sci. (2013) 6:219–24.
48. Rao GMN, Chatterjee R. Effect of seaweed liquid fertilizer from Gracilaria textorii and Hypnea musciformis on seed germination and productivity of some vegetable crops. Univ J Plant Sci. (2014) 2115–20. doi: 10.13189/ujps.2014.020701
49. Sivasangari Ramya S, Vijayanand N, Rathinavel S. Foliar application of liquid biofertilizer of brown alga Stoehospermum marginatum on growth, biochemical and yield of Solanum melongena. Int J Recycl Org Waste Agric. (2015) 4:167–73.
50. Emmanuel JSS, Lakshmikandan M, Vasanthakumar P, Sivaraman K. Improved seedling growth and seed germination in legume crop Vigna mungo (L.) utilizing marine macro algal extracts. Proc Nat Acad Sci India Sec B Biol Sci. (2015) 85:643–51.
51. Mounir M, Halima C, Salma L, Abdelali B, Driss H, Mimoun EK. Seaweed extract effect on water deficit and antioxidative mechanisms in bean plants (Phaseolus vulgaris L.). J App Phys. (2015) 27:1689–98.
52. Devi NL, Mani S. Effect of seaweed saps Kappaphycus alvarezii and Gracilaria on growth, yield and quality of rice. Indian J Sci Technol. (2015) 8:74–84.
53. Mohy El-Din SM. Utilization of seaweed extracts as bio-fertilizers to stimulate the growth of wheat seedlings. Egypt J Exp Biol (Bot). (2015). 11:31–39.
54. Mathur C, Rai S, Sase N, Krish S, Jayasri MA. Enteromorpha intestinalis derived seaweed liquid fertilizers as prospective biostimulant for glycine max. Brazil Arch Biol Technol. (2015). 58:813–20. doi: 10.1590/S1516-89132015060304
56. Aitken JB, Senn TL. Seaweed products as a fertilizer and soil conditioner for horticultural crops. Bot Mar. (1964) 8:144–8.
57. Gharakhani H, Mirhadi SM, Yazdandoost M. The effect of different foliar application amounts and different times of seaweed using (Acadian) on potato yield and yield components. J Curr Res Sci. (2016) 1:23–7.
58. Vinoj Kumar V, Kaladharan P. Biosorption of metals from contaminated water using seaweed. Curr Sci. (2006) 90:1263–7.
59. Rahman MS, Sathasivam KV. Heavy metal adsorption onto Kappaphycus sp from aqueous solutions: the use of error functions for validation of isotherm and kinetics models. BioMed Res Int. (2015) 126298:1–13.
60. Abirami S, Srisudha S, Gunasekaran P. Comparative study of chromium biosorption using brown, red and green macroalgae. Int J Biol Pharm Res. (2013) 4:115–29.
61. Abdalla MM, El-Khoshiban N. The palliative effect of bioorganic fertilizer on lead pollution in Lycopersicum esculentum plants. J Basic Appl Sci. (2012) 8:399–410.
62. Kuwada K, Wamocho LS, Utamura M, Matsushita I, Ishii T. Effect of red and green algal extracts on hyphal growth of arbuscular fungi, and on mycorrhizal development and growth of papaya and passionfruit. Agron J. (2006) 98:1340–4. doi: 10.2134/agronj2005.0354
63. Sharma SHS, Lyons G, McRoberts C. Bio-stimulant activity of brown seaweed species from Strangford Lough: compositional analyses of polysaccharides and bioassay of extracts using mung bean (Vigna mungo L.) and pak choi (Brassica rapa chinensis L.). J Appl Phycol. (2012) 24:1081–91. doi: 10.1007/s10811-011-9737-5
64. Sivasankari S, Venkatesalu V, Anantharaj M, Chandrasekaran M. Effect of seaweed extracts on the growth and biochemical constituents of Vigna sinensis. Bioresour Technol. (2006) 97:1745–51. doi: 10.1016/j.biortech.2005.06.016
65. Mattner SW, Wite D, Riches DA, Porter IJ, Arioli T. The effect of kelp extract on seedling establishment of broccoli on contrasting soil types in southern Victoria. Aust Biol Agric Hortic. (2013) 29:258–70. doi: 10.1080/01448765.2013.830276
67. Rajasulochana N, Josmin LL, Leelavathy A. Effect of Ulva lactuca extract on the growth of Phaseolus mungo L., Brassica juncea Hook. F. and Thomas and Trigonella foenum graceum L. Indian Hydro. (2008) 11:275–9.
68. Zodape ST, Gupta A, Bhandari SC. Foliar application of seaweed sap as bio stimulant for enhancement of yield and quality of tomato (Lycopersicon esculentum Mill.). J Sci Ind Res. (2011) 70:215–9.
69. Wally OS, Critchley AT, Hiltz D, Craigie JS, Han X, Zaharia LI, et al. Regulation of phytohormone biosynthesis and accumulation in Arabidopsis following treatment with commercial extract from the marine macroalga Ascophyllum nodosum. J Plant Growth Regul. (2012) 32:324–39. doi: 10.1007/s00344-012-9301-9
70. Rayorath P, Jithesh MN, Farid A, Khan W, Palanisamy R, Hankins SD, et al. Rapid bioassays to evaluate the plant growth promoting activity of Ascophyllum nodosum (L.) Le Jol. using a model plant, Arabidopsis thaliana (L.) Heynh. J Appl Phycol. (2008) 20:423–9. doi: 10.1007/s10811-007-9280-6
71. Valentão P, Trindade P, Gomes D, de Pinho PG, Mouga T, Andrade PB. Codium tomentosum and Plocamium cartilagineum: Chemistry and antioxidant potential. Food Chem. (2010) 119:1359–68. doi: 10.1016/j.foodchem.2009.09.015
72. Shibata T, Ishimaru K, Kawaguchi S, Yoshikawa H, Hama Y. Antioxidant activities of phlorotannins isolated from Japanese Laminariaceae. J Appl Phycology. (2008) 20:705–11. doi: 10.1007/s10811-007-9254-8
73. Cho SH, Kang SE, Cho JY, Kim AR, Park SM, Hong YK. The antioxidant properties of brown seaweed (Sargassum siliquastrum) extracts. J Medicinal Food. (2007) 10:479–85. doi: 10.1089/jmf.2006.099
74. Jeannin I, Lescure JC, Morot-Gaudry JF. The effects of aqueous seaweed sprays on the growth of maize. Bot Mar. (1991) 34:469–73. doi: 10.1515/botm.1991.34.6.469
75. Crouch IJ, Beckett RP, Staden J. Effect of seaweed concentrate on the growth and mineral nutrition of nutrient stressed lettuce. J Appl Phycol. (1990) 2:269–72. doi: 10.1007/BF02179784
76. Fan D, Hodges DM, Critchley AT, Prithiviraj B. A commercial extract of Brown Macroagla (Ascophyllum nodosum) affects yield and the nutritional quality of spinach in vitro. Commun Soil Sci Plant Anal. (2013) 44:1873–84. doi: 10.1080/00103624.2013.790404
77. Lola-Luz T, Hennequart F, Gaffney M. Effect on yield total phenolic, totalflavonoid and total isothiocyanate content of two broccoli cultivars (Brassicaoleraceae var italica) following the application of a commercial brown seaweed extract (Ascophyllum nodosum). Agric Food Sci. (2014) 23:28–37. doi: 10.23986/afsci.8832
78. Zhang X, Wang K, Ervin EH. Optimizing dosages of seaweed extract-based cytokinins and zeatin biocide for improving creeping bent grass heat tolerance. Crop Sci. (2010) 50:316–20. doi: 10.2135/cropsci2009.02.0090
79. Zhang X, Ervin EH. Cytokinin-containing seaweed and humic acid extracts associated with creeping bent grass leaf cytokinins and drought resistance. Crop Sci. (2004) 44:1737–45. doi: 10.2135/cropsci2004.1737
80. Ben Salah I, Aghrouss S, Douira A, Aissam S, El Alaoui-Talibi Z, Filali-Maltouf A, et al. Seaweed polysaccharides as bio-elicitors of natural defenses in olive trees against verticillium wilt of olive. J Plant Interact. (2018) 13:248–55. doi: 10.1080/17429145.2018.1471528
81. Mancuso S, Azzarello E, Mugnai S, Briand X. Marine bioactive substances(IPA extract) improve foliar ion uptake and water stress tolerance in pottedVitis vinifera plants. Adv Hortic Sci. (2006) 20:156–61.
82. Wilson S. Frost management in cool climate vineyards. Final Report to Grape and Wine Research and Development Corporation (2001).
83. Hankins SD, Hockey HP. The effect of a liquid seaweed extract from Ascophyllum nodosum (Fucales, Phaeophyta) on the two spotted red spider mite Tetranychus urticae. Hydrobiologia. (1990) 204:555–9. doi: 10.1007/BF00040286
84. Stephenson WM. The effect of hydrolyzed seaweed on certain plant pests and diseases. Proc Int Seaweed Symp. (1966) 5:405–15.
85. Abetz P. Seaweed extracts: have they a place in Australian agriculture or horticulture? J Austral Inst Agric Sci. (1980) 46:23–9.
86. Paulert R, Talamini V, Cassolato JEF, Duarte MER, Noseda MD, Smania AJ, et al. Effects of sulfated polysaccharide and alcoholoic extracts from green seaweed Ulva fasciata on anthracnose severity and growth of common bean (Phaseolus vulgaris L.). J Plant Dis Prot. (2009) 6:263–70. doi: 10.1007/BF03356321
87. Peres JCF, De Carvalho LR, Gonçalez E, Berian LOS, D’arc Felicio J. Evaluation of antifungal activity of seaweed extracts. Ciênc Agrotec Lavras. (2012) 36:294–9. doi: 10.1590/S1413-70542012000300004
88. Khan SA, Abid M, Hussain F. Nematicidal activity of seaweeds against Meloidogyne javanica. Pak J Nematol. (2015) 33:195–203.
89. Washington WS, Engleitner S, Boontjes G, Shanmuganathan N. Effect of fungicides, seaweed extracts, tea tree oil, and fungal agents on fruit rot and yield in strawberry. Aust J Exp Agric. (1999) 39:487–94. doi: 10.1071/EA98164
90. Kahn W, Rayirath UP, Subramanian S, Jithesh MN, Rayorath P, Hodges DM, et al. Seaweed extracts as bio-stimulants of plant growth and development. J Plant Growth Regul. (2009) 27:270–9.
91. Featonby-Smith BC, van Staden J. The effect of seaweed concentrate on the growth of tomato plants in nematode-infested soil. Sci Hortic. (1983) 20:137–46. doi: 10.1016/0304-4238(83)90134-6
92. Wu Y, Jenkins T, Blunden G, von Mende N, Hankins SD. Suppression of fecundity of the root-knot nematode, Meloidogyne javanica, in monoxenic cultures of Arabidopsis thaliana treated with an alkaline extract of Ascophyllum nodosum. J Appl Phycol. (1998) 10:91–4. doi: 10.1023/A:1008067420092
93. Battacharyya D, Babgohari MZ, Rathor P, Prithiviraj B. Seaweed extracts as bio-stimulants in horticulture. Sci Hortic. (2015) 196:39–48. doi: 10.1016/j.scienta.2015.09.012
94. McHugh DJ. A guide to the seaweed industry. In: FAO fish tech pap 441. Rome,Italy: FAO (2003). p. 105.
95. Wosnitza TMA, Barrantes JG. Utilization of seaweed Ulva sp. in Paracas Bay (Peru): experimenting with compost. J Appl Phycol. (2003) 18:27–31. doi: 10.1007/s10811-005-9010-x
96. Brady NC, Weil R. The nature and properties of soils. 14th edn. Upper Saddle River: Pearson Prentice Hall (2008).
97. Jaulneau V, Lafitte C, Jacquet C, Fournier S, Salamagne S, Brian X, et al. Ulvan, a sulfated polysaccharide from green algae, activates plant immunity through the jasmonic acid signaling pathway. J BioMed Biotechnol. (2010). doi: 10.1155/2010/52529
Keywords: seaweed extracts, biofertilizers, biostimulants, plant growth promotion, sustainable agriculture, soil health, organic farming, abiotic stress tolerance
Citation: Singh A, Sharma K, Chahal HS, Kaur H and Hasanain M (2025) Seaweed-derived plant boosters: revolutionizing sustainable farming and soil health. Front. Soil Sci. 5:1504045. doi: 10.3389/fsoil.2025.1504045
Received: 30 September 2024; Accepted: 26 March 2025;
Published: 24 April 2025.
Edited by:
Amer Morsy Abdelaziz, Al-Azhar University, EgyptReviewed by:
Kubavat Denish, Council of Scientific and Industrial Research (CSIR), IndiaMohamed Sharaf, Al-Azhar University, Egypt
Copyright © 2025 Singh, Sharma, Chahal, Kaur and Hasanain. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kartik Sharma, a2FydGlrLmljYXJAZ21haWwuY29t
 Amanpreet Singh
Amanpreet Singh Kartik Sharma
Kartik Sharma Harmandeep Singh Chahal3
Harmandeep Singh Chahal3