Abstract
This study aimed to provide data on the subacute toxicity and fetotoxicity of aqueous and hydroethanolic extracts of Daniellia oliveri (Rolfe) Hutch. & Dalziel, Anacardium occidentale L., Diospyros mespiliformis Hochst. ex A. DC., Khaya senegalensis (Desv.) A. Juss., Ocimum gratissimum L., Vernonia amygdalina Delile, Pterocarpus erinaceus Poir., and Manihot esculenta Crantz used in the treatment of diarrheal diseases in Benin. For subacute toxicity, each male and female Wistar rat received by esophageal gavage a maximum of 1000 mg/kg over 28 days following the Organization for Economic Co-operation and Development (OECD) Guideline No. 407. Bodyweight changes, biochemical and hematological parameters were assessed. The fetotoxicity of the extracts was evaluated on Dutch Blue hen eggs at 100 mg/kg and 300 mg/kg. The hatching rate, biochemical and hematological constants were subsequently determined. The subacute toxicity data did not indicate any mortality or signs of toxicity. Also, no significant difference in the bodyweight of the rats and the hematological parameters was noted. Concerning fetotoxicity, a low hatching rate (varying from 20% to 50%) was observed in the batches treated with the extracts at 300mg/kg compared to the rate obtained in the negative control batch (80%). The batches that received the hydroethanolic extracts of Vernonia amygdalina and Manihot esculenta at the tested doses had a hatching rate of 0%. When looking at hematological parameters, no significant difference was noted for all batches. However, an increase in the Aspartate Amino-Transferase (ASAT) values was noted in the batches that received the hydroethanolic extract of Khaya senegalensis and the aqueous extract of Diospyros mespiliformis at 100 and 300 mg/kg. These results explicitly show that the hydroethanolic extracts of Manihot esculenta and Vernonia amygdalina are fetotoxic at the tested doses.
1 Introduction
For centuries, humans have been using plants for a variety of purposes, including food and medicine. Human believes that since plants are natural, their use is safe. However, many toxic secondary compounds are found in plants and must be taken into account (1). The active substances or secondary metabolite compounds contained in plant extracts can have physiological and pharmacological effects on humans (2). The ethnopharmacological use of plants involved in the treatment of various diarrheal diseases has been reported by previous studies (3). Among these plants, Diospyros mespiliformis and Anacardium occidentale are known for their involvement in the treatment of infectious diarrhea and malaria (4, 5). In addition, in Burkina Faso, the leaves of Daniellia oliveri are used in the treatment of gastrointestinal disorders (6). The anti-diarrheal properties of Pterocarpus erinaceus have been evaluated and reported by authors (7, 8). Despite the extensive use of medicinal plants in the treatment of many chronic and infectious diseases, studies have revealed that these plants are also involved in kidney and liver issues in humans (9, 10). The treatment of diarrheal diseases in Benin relies in some communities on the use of certain medicinal plants of the Beninese flora, such as Khaya senegalensis, Daniellia oliveri, Vernonia amygdalina, Manihot esculenta, Ocimum gratissimum, Diospyros mespiliformis, Pterocarpus erinaceus, and Anacardium occidentale (11). Our previous studies on these plants have revealed their antibacterial effect on bacterial strains isolated from diarrheic stools (12). Another study on these plants further showed that they were composed of tannins (11). Indeed, tannins have antimicrobial potential but also possess anti-nutritive properties by forming complexes with proteins, starch and digestive enzymes, causing a decrease in the nutritional value of food (13). The majority of medicinal plants contain dozens of different compounds, some of which are of great complexity. Secondary compounds such as mucilage, polysaccharides and tannins modulate and modify the effects of possible active molecules (14). Previous toxicity studies including acute, subchronic and subacute toxicity have been conducted in several countries (Nigeria, Portugal and Brazil) on a variety of plant extracts such as Diospyros mespiliformis, Daniellia oliveri and Anacardium occidentale (4, 15–17). In Benin, preliminary data on the acute toxicity of these plants on rats showed that no mortality was recorded during the 14 days of the experiment. Also, no signs of apparent toxicity were observed. The plants studied had an LD50 greater than 2000 mg/kg and are therefore considered non-toxic (11). However, there is no data on the long-term toxicity of these plant extracts from Benin, let alone their fetotoxic effects. Given the fact that the use of medicinal plants during pregnancy is increasingly considered a reasonable and safer alternative to conventional therapy due to their natural origin (15, 16), it is important to assess long-term toxicity and the potential effects of these medicinal plants on embryonic development. The present study aimed to evaluate in a holistic way the subacute toxicity and fetotoxicity of Beninese medicinal plants with antibacterial and antidiarrheal activities.
2 Material and Methods
2.1 Ethics Approval Statement
The Benin National Ethical Committee for Health Research has reviewed and approved this study (65/MS/DC/SGM/DRFMT/CNERS/SA). Moreover, this study received ethical approval from the Ethical Committee of the Research Unit in Applied Microbiology and Pharmacology of Natural Substances (035–19/URMAPHA/EPAC/UAC). All animal studies were performed according to the US National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of Lanzhou Institute of Husbandry and Pharmaceutical Science of Chinese Academy of Agricultural Sciences. The animals were examined and adapted to the new environmental conditions for one week before the experiment.
2.2 Plant’s Material
The plant material consisted of the leaves of Anacardium occidentale, Daniellia oliveri, Diospyros mespiliformis, Manihot esculenta, Ocimum gratissimum, Pterocarpus erinaceus, Vernonia amygdalina and bark of the stem of Khaya senegalensis. The plants were collected at different locations, taking into account the bioavailability of the collected species. The plant samples once collected were authenticated at the National Herbarium (Table 1). Figure 1 illustrates the collection sites of the medicinal plants studied.
Table 1
| Identification number | Scientific name | Botanical family | Used part | Collection area (municipality) | Collection period |
|---|---|---|---|---|---|
| YH 434/HNB | Anacardium occidentale L. | Anacadiaceae | Leaves | Abomey-Calavi | July 2020 |
| YH 436/HNB | Daniellia oliveri (Rolfe) Hutch. & Dalziel | Leguminosae | Leaves | Toffo | July 2020 |
| YH 438/HNB | Diospyros mespiliformis Hochst. ex A. DC. | Ebenaceae | Leaves | Toffo | July 2020 |
| YH 435/HNB | Khaya senegalensis (Desr.) A. Juss. | Meliaceae | Bark | Abomey-Calavi | July 2020 |
| YH 442/HNB | Manihot esculenta Crantz | Euphorbiaceae | Leaves | Abomey-Calavi | July 2020 |
| YH 437/HNB | Occimum gratissimum L. | Lamiaceae | Leaves | Abomey-Calavi | July 2020 |
| YH 440/HNB | Pterocarpus erinaceus Poir. | Euphorbiaceae | Leaves | Toffo | July 2020 |
| YH 439/HNB | Vernonia amygdalina Delile | Asteraceae | Leaves | Abomey-Calavi | July 2020 |
Information about in the selected medicinal plants.
Figure 1
Localization of plant collection sites.
2.3 Animal Material
The material consisted of albino Wistar rats and hen eggs. A total of 300 eggs were used for this study. Three months aged female and male rats weighing between 108 g and 160 g were obtained from the animal factory of the Institute of Applied Biomedical Sciences (ISBA) of the University of Abomey-Calavi for subacute toxicity tests. Female rats were nulliparous and non-pregnant. The animals were acclimated for at least one week before starting to be handled. The animals were fed with standard diets for rodents and water at will. The rats were maintained under laboratory conditions at 22°C (± 3°C) with a 12 h light and 12 h dark cycle (18). Animal care and handling were consistent with the guidelines accepted by the OECD Guidelines for Chemical Testing (19). For the assessment of fetotoxicity, Dutch blue hen eggs were used.
2.4 Preparation of Extracts
After collection, samples of the selected plants were cleaned of waste by washing with tap water and then dried in the shade at the Research Unit for Applied Microbiology and Pharmacology of Natural Substances at the laboratory’s ambient temperature. They were then reduced to powder using a Retsch SM 2000/1430/Upm/Smf electric mill.
The powders obtained were identified and stored in plastic vials at room temperature in the laboratory. From this powder, two types of extraction of each of the medicinal plants studied were carried out, following the methodology described by Legba et al. (2020) (20) for aqueous extraction and hydroethanolic extraction (50% water-ethanol; v/v). Briefly, fifty (50) grams of powder was macerated in 500 mL of solvent (water and water-ethanol). The mixture was stirred continuously for 72 hours at room temperature. The resulting homogenized mixture was filtered on absorbent cotton three times successively and finally filtered once on Wattman No. 1 paper. The obtained filtrates were then evaporated in ovens at either of 40°C (hydroethanolic filtrates) and 60°C (aqueous filtrates in order to avoid the growth of moulds) until dry masses were obtained, which represent the aqueous and hydroethanolic extracts. The extracts thus produced were placed in a refrigerator at 4°C and put back into solution during the different tests. Only extracts known for their proven antibacterial efficacy on strains involved in diarrheal infections were used in this study (12).
2.5 Procedure of Subacute Oral Toxicity Test
The repeated-dose oral toxicity study was conducted according to OECD guideline 407 (19). The animals were divided into twelve groups of 12 animals each (6 males and 6 females). The control group received distilled water. The test lots, namely the hydroethanolic extract of Daniellia oliveri (Dob), aqueous extract of Anacardium occidentale (AOa), hydroethanolic extracts of Anacardium occidentale (AOb), aqueous extract of Diospyros mespiliformis (DMa), hydroethanolic of Diospyros mespiliformis (DMb), aqueous extract of Ocimum gratissimum (OGa), hydroethanolic extract of Ocimum gratissimum (OGb), aqueous extract of Khaya senegalensis (KSa), hydroethanolic extract of Khaya senegalensis (KSb), hydroethanolic extract of Vernonia amygdalina (VAb), hydroethanolic extract of Pterocarpus erinaceus (PEb), and hydroethanolic extract of Manihot esculenta (MEb), all received the extract limit dose of 1000 mg/kg bodyweight daily for 28 days. In a recent study assessing a decoction of the mixture of plants Daniellia oliveri and Sarcocephalus latifolius, acute toxicity explored at doses of 10, 100, 1000, 1500, 2500 and 5000 mg/kg did not showed any signs of toxicity (16). In addition, Luka et al. (15) was evaluated the sub-acute toxicity of ethanolic extract of Diospyros mespiliformis at a maximum dose of 400mg/kg. Based on these data, and in accordance with the OECD protocol which states that, if after evaluation of other data from other results, no effects are expected, a limit test at a dose of 1000mg/kg bw/d, can be performed (19). Mortality, body weight, food and water consumption, and signs of general toxicity in animals were assessed daily when plant extracts were administered for 28 days.
2.5.1 Measurement of Biochemical and Hematological Parameters
Blood samples were collected in two types of tubes, one containing Ethylenediaminetetraacetic acid (EDTA) and the other without anticoagulant (dry tube). Samples from EDTA tubes were used for hematological analysis. The dry tubes were centrifuged at 4000 rpm for 10 minutes, and the resulting serum was stored at -20°C for biochemical analysis.
After sampling, two animals per batch were sacrificed with thiopental (30mg/kg), and vital organs such as the liver and kidney were collected. The harvested organs were rinsed with 0.9% saline and fixed in 10% buffered formalin.
The hematological analysis was performed using the SYSMEX KX 21N automaton using the protocol described by Sodipo and collaborators in 2012 (21). These examinations included red and white blood cell counts (RWBC), hemoglobin level (HB), hematocrit (HT), mean corpuscular volume (MCV), mean corpuscular hemoglobin content (MCH) and determination of mean corpuscular hemoglobin concentration (MCHC) and platelets (PLT). Biochemical tests consisted of the determination of urea, creatinine, aspartate aminotransferase (ASAT) and alanine aminotransferase (ALAT).
2.6 Evaluation of Fetotoxicity
The methodology used for this study was subdivided into four steps.
2.6.1 First Step: Formation of Egg Batches
Eggs (nonelair ® breed eggs) were arranged and classified in the incubator according to weight. All plant extracts were tested at 100 mg/kg and 300 mg/kg egg weight (22). Batches of 10 eggs were formed for each extract. The constituted batches were as follows:
Lot 1: control batch that received nothing; Lot 2: NaCl lot with NaCl suspension; Lot 3: hydroethanolic extract of Daniella oliveri at 100mg/kg; Lot 4: hydroethanolic extract of Daniella oliveri at 300mg/kg; Lot 5: aqueous extract of Anacardium occidentale at 100mg/kg; Lot 6: aqueous extract of Anacardium occidentale at 300mg/kg; Lot 7: hydroethanolic extract of Anacardium occidentale at 100 mg/kg; Lot 8: hydroethanolic extract of Anacardium occidentale at 300 mg/kg; Lot 9: aqueous extract of Diospyros mespiliformis at 100mg/kg; Lot 10: aqueous extract of Disopyros mespiliformis at 300mg/kg; Lot 11: hydroethanolic extract of Diopyros mespiliformis at 100 mg/kg; Lot 12: hydroethanolic extract of Diospyros mespiliformis at 300 mg/kg; Lot 13: aqueous extract Khaya senegalensis at 100mg/kg; Lot 14: aqueous extract of Khaya senegalensis at 300mg/kg; Lot 15: hydroethanolic extract of Khaya senegalensis at 100 mg/kg; Lot 16: hydroethanolic extract of Khaya senegalensis at 300mg/kg; Lot 17: aqueous extract of Ocimum gratissimum at 100mg/kg; Lot 18: aqueous extract of Ocimum gratissimum at 300mg/kg; Lot 19: hydroethanolic extract of Ocimum gratissimum at 100mg/kg; Lot 20: hydroethanolic extract of Ocimum gratissimum at 300mg/kg; Lot 21: hydroethanolic extract of Vernonia amygdalina at 100 mg/kg; Lot 22: hydroethanolic extract of Vernonia amygdalina at 300mg/kg; Lot 23: hydroethanolic extract of Pterocarpus erinaceus at 100 mg/kg; Lot 24: hydroethanolic extract of Pterocarpus erinaceus at 300 mg/kg; Lot 25: hydroethanolic extract of Manihot esculenta at 100mg/kg and Lot 26: hydroethanolic extract of Manihot esculenta at 300mg/kg.
2.6.2 Second Step: Inoculation of Plant Extracts
The 6th day of incubation, all the eggs were candied with the electric candle to discriminate fertile eggs from those who were not. Fertile eggs were used later in the study (23, 24). The injection of plant extracts was carried out according to the method described by Tavakkoli and collaborators in 2020 (22). Briefly, the injection or inoculation of the plant extracts was carried out after 7 days of incubation under normal conditions in an air chamber (37.7°C; 55% relative humidity; 0.06% CO2; 1/60 min of turning). All eggs were then incubated under the same incubation conditions. Before inoculation, the larger eggs were disinfected with 70% ethanol, and the shells were pierced to inoculate the plant extract into the air chamber. The pierced part was closed with sterile adhesive tape or sterilized adhesive plaster. On the 14th day, the same protocol was followed.
2.6.3 Third Step: Transfer the Eggs to the Hatcher
On the 18th day, all eggs were again examined with a flashlight to discriminate eggs that continued organogenesis from those that were not going to be transferred to the hatchery. It should be noted that, at each stage, non-fertile eggs were broken to determine the types of mortality (early or late). On day 21, embryonic lethality was determined and surviving chicks were evaluated for malformations (including head, eye, beak, body wall, and limbs), pericardium and subcutaneous edema, and gross liver lesions (25). Chick quality was assessed according to the Tona score (Table 2) (26).
Table 2
| Parameters | Characteristics | Score |
|---|---|---|
| Activities | Good | 6 |
| Low | 0 | |
| Fluff and appearance | Clean and dry | 10 |
| Wet | 8 | |
| Dirty and wet | 0 | |
| Resorption of the yolk sac | Chicks with a normal abdomen | 12 |
| Chicks with a large abdomen and | 0 | |
| Eyes | fairly hard to the touch | 16 |
| Open and shiny | 8 | |
| Open and not shiny | 0 | |
| Legs | Closed | 16 |
| Normal legs and toes | 8 | |
| One leg infected | 0 | |
| Umbilical | Both legs infected | 12 |
| Completely closed and clean | 6 | |
| Not completely closed and not discolored | 0 | |
| Remaining membrane | Not closed and discolored | 12 |
| No membrane | 8 | |
| Small membrane | 4 | |
| Large membrane | 0 | |
| Remaining vitellus | Very large membrane | 16 |
| No yolk | 12 | |
| Small yolk | 8 | |
| Large yolk | 0 |
Evaluation of the score of the different parameters determining the quality of the chicks.
Relative chick weight, organs removed, hatching and mortality rates were calculated (heart, liver and yolk sac) according to the formulas below:
2.6.4 Fourth Step: Measurement of Hematological and Biochemical Parameters
After the eggs hatched, a few chicks per batch were sacrificed and blood sample was collected from the neck in both EDTA and dry tubes. Hematological and biochemical parameters (urea, creatinemia, alanine aminotransferase and aspartate aminotransferase) were also determined. The leftover chicks were raised for a maximum of two weeks to observe the different malformations and other apparent deformities.
2.7 Statistical Analysis
Statistical analysis of data was done using one-way analysis of variance and multiparametric test (Student’s t-test) in SPSS 25.0 software. The graphs were carried out with Graph pad Prism 7. The following scale were used to assess the level of significance: *: p ≤ 0.05, significant; **: p ≤ 0.01, very significant; ***: p ≤ 0.001, highly significant; ****: p ≤ 0.0001, very highly significant.
3 Results
3.1 Subacute Toxicity
3.1.1 Clinical Observation and Body Weight
During the 28 days of oral administration, the extracts did not cause any behavioral changes in the test groups. A slight loss of appetite developed in the batch that was taking the hydroethanolic extract of Anacardium occidentale (Figure 2).
Figure 2
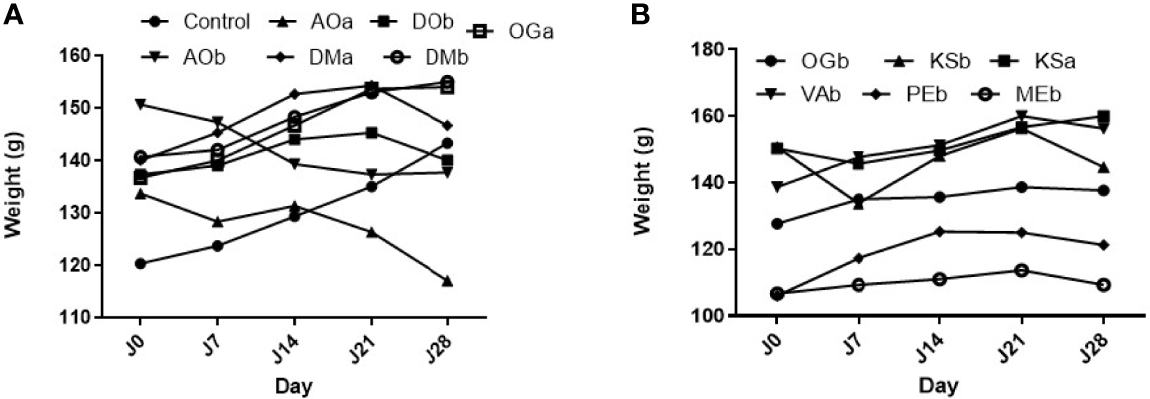
Effect of the extract plants on rats’ body weight. (A) Effect of the extract plants (AOa: Aqueous extract of A. occidentale, AOb: Hydroethanolic extract of A. occidentale, DOb: Hydroethanolic extract of D. oliveri, DMa: Aqueous extract of D. mespiliformis, DMb: Hydroethanolic extract of D. mespiliformis and OGa: Aqueous extract of O. gratissimum) on rats’ body weight ; (B) Effect of the extract plants (OGb: Hydroethanolic extract of O. gratissimum, KSa: Aqueous extract of K. senegalensis, KSb: Hydroethanolic extract of K. senegalensis, VAb: Hydroethanolic extract of V. amygdalina, MEb: Hydroethanolic extract of M. esculenta) on rats’ body weight.
3.1.2 Evaluation of Hematological Parameters
The treatments administered had no effect on the hematological parameters during the test period. No abnormal values were observed in comparison with the values of the control lots; however, a decrease in white blood cells was observed after 28 days of treatment (Table 3). Additionally, it should be noted that the average blood volume increased over time for the rats of the control lots, but this is not the case for the lots that received the extracts.
Table 3
| Treatment | RBC (T/L) | HB(g/dl) | HT (%) | MCV (fl) | MCH (Pg) | MCHC (g/dl) | WBC (G/L) | PLT | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 28 | Day 0 | Day 28 | Day 0 | Day 28 | Day 0 | Day 28 | Day 0 | Day 28 | Day 0 | Day 28 | Day 0 | Day 28 | Day 0 | Day 28 | |
| Control | 7.93 ± 0.79 | 7.83 ± 0.18 | 15.7 ± 1.61 | 14.87 ± 0.09 | 41.57 ± 3.94 | 43.77 ± 1.44 | 52.6 ± 0.62 | 53 ± 0.76 |
19.8 ± 0.1 |
18.2 ± 0.51 | 37.6 ± 0.35 |
34.8 ± 0.72 |
7.37 ± 0.74 |
7.87 ± 0.15 |
224 ± 127 |
376.7 ± 22 |
| D. oliveri ETH2O | 8.16 ± 0.54 | 7.41 ± 0.24 | 15.33 ± 0.97 | 15.37 ± 0.43 | 40.93 ± 2.12 | 40.63 ± 0.63 | 50.3 ± 0.72 | 54.63 ± 1.13 |
18.8 ± 0.46 |
21.33 ± 0.55 | 24.07 ± 6.25 |
38.27 ± 0.62 | 6.83 ± 1.49 |
7.27 ± 0.19 |
699 ± 191 |
538.7 ± 61 |
| A. occidentale H2O | 7.7 ± 0.40 | 8.33 ± 0.38 | 15.23 ± 0.64 | 16.07 ± 0.29 | 40.17 ± 2.02 | 44.5 ± 1.16 | 52.2 ± 0.63 | 53.5 ± 1 |
19.87 ± 0.83 |
25.67 ± 6.02 | 31.33 ± 7.24 |
35.47 ± 0.6 |
9.03 ± 2.85 |
9.43 ± 1.17 |
177 ± 53.8 |
586 ± 95.3 |
| A. occidentale ETH2O | 7.96 ± 0.32 |
7.30 ± 0.14 | 15.27 ± 0.58 | 15.23 ± 0.47 | 41.83 ± 1.17 | 42.67 ± 1.09 | 52.57 ± 0.75 | 58.37 ± 0.45 |
19.17 ± 0.55 |
20.83 ± 0.26 | 36.43 ± 0.73 |
35.7 ± 0.25 | 9.27 ± 2.23 |
8.33 ± 1.08 |
481 ± 110 |
378 ± 56.7 |
| D. mespiliformis H2O | 8.59 ± 0.3 |
8.47 ± 0.12 | 16.13 ± 1.22 | 15.6 ± 0.26 |
42.93 ± 3 | 42.37 ± 1.99 | 49.9 ± 1.99 | 49.93 ± 1.59 |
18.73 ± 0.81 |
18.43 ± 0.18 | 37.5 ± 0.46 |
30.27 ± 7 |
10.13 ± 3.43 |
12.47 ± 0.64 |
504 ± 146 |
756 ± 132 |
| D. mespiliformis ETH2O | 7.49 ± 1.85 |
7.92 ± 0.23 | 14.67 ± 3.93 | 15.8 ± 0.1 |
40.3 ± 10.1 | 41.53 ± 0.42 | 53.7 ± 0.5 | 52 ± 1.15 |
19.23 ± 0.59 |
19.53 ± 0.68 | 35.87 ± 0.88 |
38.3 ± 0.47 |
7.23 ± 1.75 |
8.1 ± 0.11 |
453 ± 168 |
530 ± 41 |
| O. gratissimum H2O | 8.86 ± 0.24 | 7.59 ± 0.32 | 16.93 ± 0.22 | 15.27 ± 0.66 | 45.37 ± 0.43 | 39.43 ± 2.17 | 51.23 ± 1.52 | 54.43 ± 0.98 |
19.17 ± 0.71 |
20.37 ± 0.18 | 37.33 ± 0.24 |
37.07 ± 0.64 | 6 ± 1.15 |
6.57 ± 0.15 |
1037.7 ± 40.2 | 420 ±** 113 |
| O. gratissimum ETH2O | 8.58 ± 0.35 | 7.98 ± 0.24 | 17.08 ± 0.54 | 15.7 0.26 |
45.6 ± 1.68 | 42.7 ± 1.29 | 53.13 ± 0.23 | 51.93 ± 0.95 |
19.9 ± 0.15 |
19.53 ± 0.28 | 37.47 ± 0.17 |
35.67 ± 1.02 | 8.77 ± 2.63 |
9.33 ± 0.52 |
782.7 ± 92.1 |
856.7 ± 27.9 |
| K. senegalensis H2O | 8.16 ± 0.42 | 7.65 ± 0.24 | 15.9 ± 0.61 | 14.73 ± 0.28 | 42.3 ± 1.63 | 40.13 ± 1.08 | 51.97 ± 0.77 | 52.73 ± 0.58 |
19.5 ± 0.23 |
19.9 ± 0.15 | 37.53 ± 0.23 |
37.47 ± 0.49 | 5.33 ± 1.49 |
6.17 ± 0.26 |
502 ± 273 |
256 ± 63.6 |
| K. senegalensis ETH2O | 7.08 ± 1.38 | 7.62 ± 0.45 | 14.17 ± 2.03 | 22.77 ± 6.67 | 38.37 ± 5.64 | 41.63 ± 1.09 | 55.5 ± 3.51 | 54.87 ± 1.81 |
20.53 ± 1.38 |
21.3 ± 1.18 | 37 ± 0.17 |
38.7 ± 0.9 | 7.03 ± 1.13 |
7.53 ± 0.47 |
581 ± 197 |
815.3 ± 43.9 |
| V. amygdalina ETH2O | 9.28 ± 0.54 | 9.62 ± 0.28 | 17.23 ± 0.35 | 17.6 ± 0.81 |
47.5 ± 0.78 | 48.7 ± 1.16 | 51.5 ± 2.49 | 51.23 ± 0.54 |
18.67 ± 0.9 |
17.6 ± 0.84 | 36.3 ± 0.15 |
23.83 ± 5.58 | 4.67 ± 0.61 |
5.23 ± 0.59 |
707 ± 186 |
400.3 ± 99 |
| P. erinaceus ETH2O | 7.68 ± 0.64 | 7.16 ± 0.31 | 14.67 ± 1.11 | 14.23 ± 0.37 | 41.03 ± 3.27 | 38.87 ± 0.85 | 53.53 ± 0.43 | 54.97 ± 1.13 |
19.17 ± 0.29 |
20.67 ± 0.72 | 32.43 ± 3.52 |
37.37 ± 0.84 | 5.5 ± 0.63 |
6.4 ± 0.12 |
635 ± 155 |
637 ± 111 |
| M. esculenta ETH2O | 7.15 ± 1.72 | 7.54 ± 0.33 | 13.23 ± 3.27 | 15.63 ± 0.58 | 37.27 ± 9.15 | 41.8 ± 1.02 | 51.97 ± 0.37 | 54 ± 0.36 |
18.4 ± 0.15 |
20.87 ± 0.3 | 35.5 ± 0.15 |
38.5 ± 0.29 | 6.97 ± 0.50 |
6.87 ± 0.67 |
671 ± 222 |
521.3 ± 44 |
Effect of administered extracts on hematological parameters of Wistar rats.
H2O, Aqueous extract; ETH2O, Hydroethanolic extract; RBC, Red Blood Cell counts; HB, Hemoglobin level; HT, Hematocrit; MCV, Mean Corpuscular Volume; MCH, Mean Corpuscular Hemoglobin content; MCHC, Mean Corpuscular Hemoglobin Concentration; WBC, White Blood Cell counts; PLT, Platelets; A. occidentale: Anacardium occidentale L.; D. oliveri: Daniellia oliveri (Rolfe) Hutch. & Dalziel; K. senegalensis: Khaya senegalensis (Desv.) A.Juss; D. mespiliformis: Dyospiros mespiliformis Hochst. ex A.DC.; O. gratissimum: Ocimum gratissimum L.; P. erinaceus: Pterocarpus erinaceus Poir; M. esculenta: Manihot esculenta Crantz; V. amygdalina: Vernonia amygdalina Delile; The values of the hematological parameters of the Wistar rats are presented as Mean ± Standard Deviation. The values from the treated lots were compared with those from the control lot. Compared to the control lot, there was a decrease in the average platelet count from day 0 to day 28 in the rats of the lot treated with the aqueous extract of Ocimum gratissimum. This difference in mean platelets is very significant at the 5% level, (**): p < 0.01.
3.1.3 Evaluation of Biochemicals Parameters
The analysis of biochemical parameters did not show significant difference after 28 days of treatment, except for the batches that received the hydroethanolic extract of Manihot esculenta and Anacardium occidentale, where the level of creatinine and alanine amino transferase (ALAT) has increased respectively (Table 4).
Table 4
| Parameters | Urea (g/l) | Creatinine (mg/l) | ASAT (UI/L) | ALAT (UI/L) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Plants | Lots | Day 0 | Day 28 | Day 0 | Day 28 | Day 0 | Day 28 | Day 0 | Day 28 |
| Control | DW | 0.61 ± 0.05 | 0.68 ± 0.02 | 15.23 ± 2.81 | 18.45 ± 3.38 | 251.7 ± 75 | 250.33 ± 1.45 | 129.1 ± 46.4 | 81.61 ± 4.06 |
| D. oliveri | ETH2O | 0.74 ± 0.05 | 0.72 ± 0.01 | 14.85 ± 0.90 | 18.08 ± 1.07 | 253 ± 143 | 287 ± 19.3 | 92.99 ± 4.08 | 83.17 ± 2.29 |
| A. occidentale | H20 | 0.68 ± 0.07 | 0.82 ± 0.09 | 18.31 ± 1.44 | 14.06 ± 1.53 | 340 ± 43.7 | 339 ± 165 | 71.25 ± 6.48 | 160.66 ± 2.03 |
| ETH2O | 0.50 ± 0.12 | 0.93 ± 0.01 | 17.5 ± 0.56 | 15.83 ± 1.68 | 91 ± 11 | 409 ± 104 | 125.3 ± 24.6 | 426 ± 100*** | |
| D. mespiliformis | H2O | 0.69 ± 0.07 | 0.99 ± 0.04 | 11.46 ± 5.24 | 17.8 ± 2.14 | 160.7 ± 26.7 | 174.7 ± 40.7 | 77.48 ± 9.67 | 277 ± 174 |
| ETH2O | 0.61 ± 0.03 | 0.88 ± 0.08 | 14.37 ± 2.42 | 18.17 ± 0.33 | 139.3 ± 36 | 268.7 ± 27.1 | 95.34 ± 3.58 | 188.5 ± 59.3 | |
| O. gratissimum | H2O | 0.61 ± 0.03 | 0.87 ± 0.05 | 18.33 ± 1.08 | 17.7 ± 1.87 | 88 ± 8.08 | 197.33 ± 9.91 | 54.86 ± 0.21 | 100.29 ± 6.7 |
| ETH2O | 0.6 ± 0.01 | 0.63 ± 0.02 | 13.57 ± 3.55 | 17.68 ± 1.5 | 75.33 ± 4.26 | 201.3 ± 19.1 | 57.35 ± 2.14 | 60.77 ± 0.69 | |
| K. senegalensis | H2O | 0.41 ± 0.05 | 0.68 ± 0.02 | 9.58 ± 3.71 | 16.07 ± 1.75 | 97.7 ± 22.2 | 148.67 ± 5.49 | 58.91 ± 4.8 | 64.2 ± 6.18 |
| ETH2O | 0.51 ± 0.01 | 0.77 ± 0.06 | 11.16 ± 1.67 | 13.12 ± 3.74 | 83.3 ± 11.4 | 300 ± 4.04 | 58.51 ± 4.49 | 66.37 ± 2.14 | |
| V. amygdalina | ETH2O | 0.66 ± 0.10 | 0.88 ± 0.06 | 13.92 ± 1.94 | 16.55 ± 2.05 | 100.67 ± 8.19 | 246 ± 29.3 | 80.4 ± 15.5 | 158.8 ± 14.8 |
| P. erinaceus | ETH2O | 0.52 ± 0.07 | 0.74 ± 0.03 | 15.75 ± 2.68 | 14.31 ± 1.12 | 121.7 ± 10.4 | 219.7 ± 12.4 | 76.55 ± 6.22 | 83 ± 13.6 |
| M. esculenta | ETH2O | 0.54 ± 0.03 | 0.62 ± 0.03 | 17.01 ± 0.18 | 22.81 ± 1.76** | 91.67 ± 5.24 | 250.33 ± 1.45 | 57.35 ± 8.22 | 54.25 ± 7.41 |
Effect of administered extracts on biochemical parameters of Wistar rats.
H2O, Aqueous extract; ETH2O, Hydroethanolic extract; DW, Distilled water; ASAT, Aspartate aminotransferase; ALAT, Alanine aminotransferase; A. occidentale: Anacardium occidentale L.; D. oliveri: Daniella oliveri (Rolfe) Hutch. & Dalziel; K. senegalensis: Khaya senegalensis (Desv.) A.Juss; D. mespiliformis: Dyospiros mespiliformis Hochst. ex A.DC.; O. gratissimum: Ocimum gratissimum L.; P. erinaceus: Pterocarpus erinaceus Poir; M. esculenta: Manihot esculenta Crantz; V. amygdalina: Vernonia amygdalina Delile. The values of biochemical parameters of Wistar rats are presented as Mean ± Standard Deviation. The values of the treated batches were compared to those of the control batch. Compared to the control batch, there was a highly significant increase in the mean Alanine aminotransferase from day 0 to day 28 in the rats of the batch treated with the hydroethanolic extract of Anacardium occidentale, (***): p< 0.001. In addition, compared to the control batch there was a very significant increase in mean creatinine from day 0 to day 28 in rats from the batch treated with hydroethanolic extract of Manihot esculenta, (**): p< 0.01.
3.2 Fetotoxicity
3.2.1 Effect of Extracts on Hatching Rate
The batch of eggs receiving physiological solution (NaCl) showed a high hatching rate. The eggs of the batches receiving the hydroethanolic extracts at 100 mg/kg and 300 mg/kg V. amygdalina and M. esculenta did not hatch. The administered extracts thus prevented the evolution of embryogenesis (Figure 3).
Figure 3
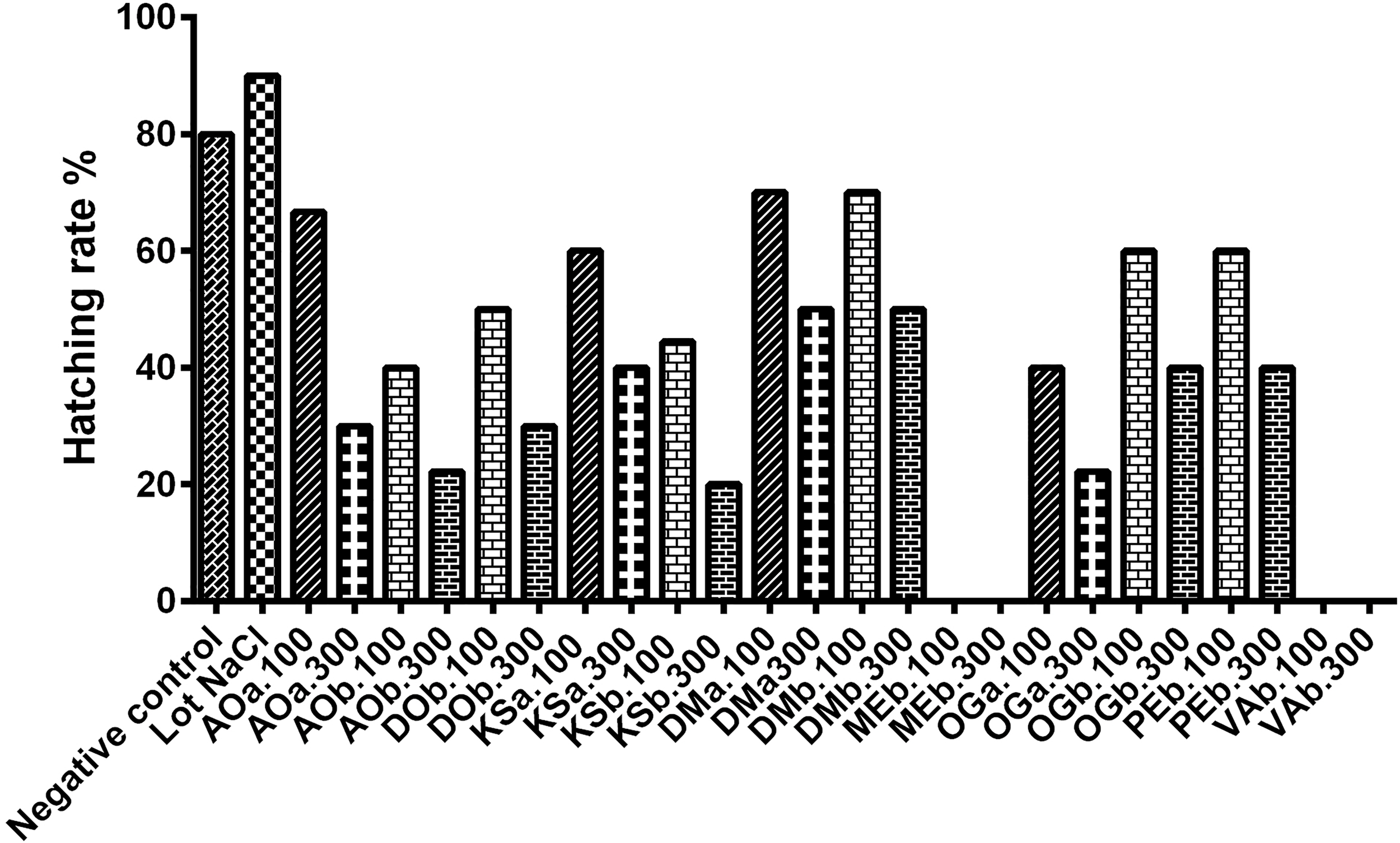
Hatching rate of incubated. AOa: Aqueous extract of A. occidentale, AOb: Hydroethanolic extract of A. occidentale, DOb: Hydroethanolic extract of D. oliveri, DMa: Aqueous extract of D. mespiliformis, DMb: Hydroethanolic extract of D. mespiliformis, OGa: Aqueous extract of O. gratissimum, OGb: Hydroethanolic extract of O. gratissimum, KSa: Aqueous extract of K. senegalensis, KSb: Hydroethanolic extract of K. senegalensis, VAb: Hydroethanolic extract of V. amygdalina, MEb: Hydroethanolic extract of M. esculenta ; 100: 100mg/kg; 300:300mg/kg.
3.2.2 Quality of Chick
After hatching, the quality of chicks was determined by the Tona score principle. All parameters were evaluated on 100%. The results showed that the chicks hatched were all of good quality, with a score varying between 80.75 ± 7.18 and 98.00 ± 2.31 (Figure 4).
Figure 4
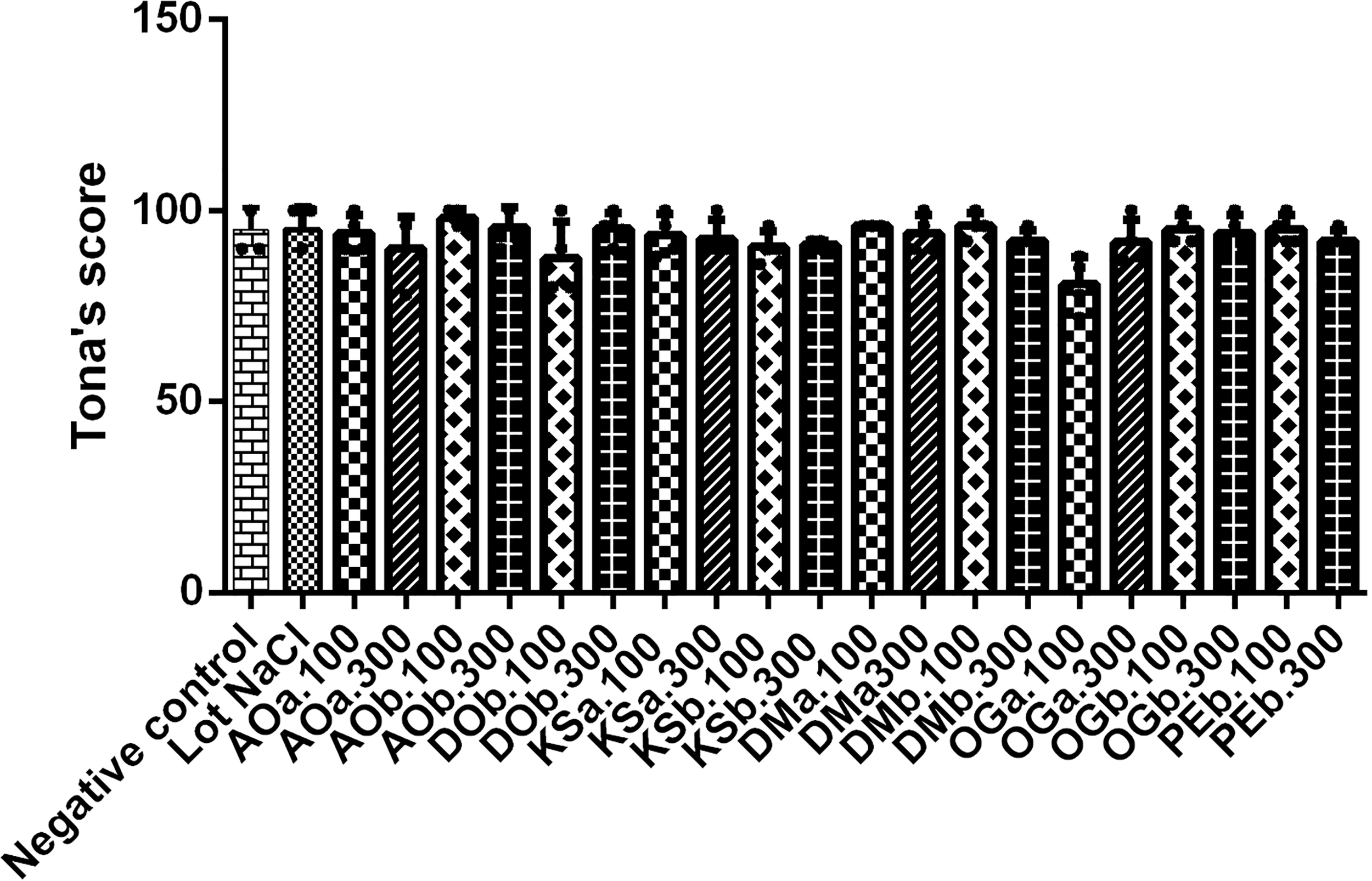
Determination of the quality of the chicks by the Tona score test. AOa: Aqueous extract of A. occidentale, AOb: Hydroethanolic extract of A. occidentale, DOb: Hydroethanolic extract of D. oliveri, DMa: Aqueous extract of D. mespiliformis, DMb: Hydroethanolic extract of D. mespiliformis, OGa: Aqueous extract of O. gratissimum, OGb: Hydroethanolic extract of O. gratissimum, KSa: Aqueous extract of K. senegalensis, KSb: Hydroethanolic extract of K. senegalensis, VAb: Hydroethanolic extract of V. amygdalina, MEb: Hydroethanolic extract of M. esculenta; 100: 100mg/kg; 300:300mg/kg.
3.2.3 Effect of Plant Extracts on Chick Weight and Organs
Following incubation of eggs, the weight of chicks produced varied from 28.78 ± 0.68 to 32.00 ± 0.99. The weights of chicks are naturally high compared to the weight without yolk sacs. The comparative analysis of the different parameters of the control and treated batches showed no significant difference between the weights of the chicks. However, a slight increase in the relative weight of chicks receiving the hydroethanolic extract of A. occidentale and the aqueous extract of D. mespiliformis was noticed. Moreover, a nonproportional decrease in the relative weights of the chicks compared to the initial weight of the treated chicks that received the hydroethanolic extracts of O. gratissimum at 100 mg/kg and 300 mg/kg was also observed (Table 5).
Table 5
| Chick weight | Chick weight SSV | Relative weight of chick SSV | |
|---|---|---|---|
| Negative control | 30.02± 0.64 | 26.25 ± 0.62 | 87.44 ± 1.18 |
| Lot NaCl | 28.78 ± 0.68 | 25.42 ± 0.30 | 88.43 ± 1.35 |
| A. occidentale H2Oa | 30.16 ± 0.32 | 26.32 ± 0.53 | 87.33 ± 2.44 |
| A. occidentale H2Ob | 29.80 ± 0.40 | 26.4 ± 0.20 | 88.62 ± 1.53 |
| A. occidentale ETH2Oa | 26.06 ± 0.06 | 23.74 ± 0.06 | 91.09 ± 0.22* |
| A. occidentale ETH2Ob | 26.07 ± 0.06 | 23.75 ± 0.06 | 91.10 ± 0.22* |
| D. oliveri ETH2Oa | 28.12 ± 0.06 | 23.03 ± 0.06 | 81.90 ± 0.04 |
| D. oliveri ETH2Ob | 28.13 ± 0.06 | 23.04 ± 0.06 | 81.90 ± 0.04 |
| K. senegalensis H2Oa | 28.88 ± 0.55 | 25.27 ± 1.09 | 87.40 ± 2.46 |
| K. senegalensis H2Ob | 28.89 ± 0.55 | 25.28 ± 1.09 | 87.40 ± 2.46 |
| K. senegalensis ETH2Oa | 29.30 ± 0.19 | 24.51 ± 0.06 | 83.65 ± 0.57 |
| K. senegalensis ETH2Ob | 29.11 ± 0.06 | 24.52 ± 0.06 | 84.23 ± 0.03 |
| D. mespiliformis H2Oa | 30.29 ± 0.92 | 25.09 ± 1.16 | 83.38 ± 6.22 |
| D. mespiliformis H2Ob | 28.68 ± 0.95 | 25.82 ± 0.75 | 90.40 ± 4.31* |
| D. mespiliformis ETH2Oa | 29.41 ± 1.87 | 26.39 ± 0.31 | 86.63 ± 1.78 |
| D. mespiliformis ETH2Ob | 30.07 ± 2.13 | 26.03 ± 0.57 | 88.11 ± 7.42 |
| O. gratissimum H2Oa | 29.71 ± 0.22 | 25.15 ± 0.11 | 84.66 ± 0.74 |
| O. gratissimum H2Ob | 29.72 ± 0.22 | 25.16 ± 0.11 | 84.66 ± 0.74 |
| O. gratissimum ETH2Oa | 32.00 ± 0.99 | 24.33 ± 1.54 | 76.18 ± 4.92*** |
| O. gratissimum ETH2Ob | 31.06 ± 0.86 | 24.46 ± 1.65 | 79.25 ± 6.98** |
| P. erinaceus ETH2Oa | 31.5 ± 0.50 | 26.06 ± 0.56 | 82.73 ± 0.99 |
| P. erinaceus ETH2Ob | 30.08 ± 0.93 | 24.49 ± 1.11 | 81.31 ± 1.50 |
Effects of plant extracts on relative chick weights.
H2Oa: Aqueous extract at 100mg/kg, H2Ob: Aqueous extract at 300mg/kg, ETH2Oa: Hydroethanolic extract at 100mg/kg, ETH2Ob: Hydroethanolic extract at 300mg/kg, SSV : Without yolk sac; A. occidentale: Anacardium occidentale L. L.; D. oliveri: Daniellia oliveri (Rolfe) Hutch. & Dalziel; K. senegalensis: Khaya senegalensis (Desv.) A.Juss; D. mespiliformis: Dyospiros mespiliformis; O. gratissimum: Ocimum gratissimum L.; P. erinaceus: Pterocarpus erinaceus. Compared to the controls, there was a significant (*p < 0.05) increase in the mean weight of chicks whose eggs were treated with 100 and 300 mg/kg of hydroethanolic extract of Anacardium occidentale and 300 mg/kg of aqueous extract of Dyospiros mespiliformis. But, a highly significant decrease (***p < 0.001) and very significant decrease (**p < 0.01) in the mean weight of the chicks was observed in the eggs treated with 100 mg/kg of hydroethanolic extract of Ocimum gratissimum and 300 mg/kg of the same plant extract respectively.
Analysis of the effect of the extracts of medicinal plants used in this study on the relative weight of the vital organs of chicks, such as the heart, the liver and yolk sac indicated that the injection of these extracts did not cause a significant difference compared to the control batches (Table 6).
Table 6
| Relative weight of liver | Relative weight of heart | Relative weight of yolk sac | |
|---|---|---|---|
| Negative control | 2.84 ± 0.15 | 0.85 ± 0.04 | 8.41 ± 0.50 |
| Lot NaCl | 2.95 ± 0.21 | 0.75 ± 0.02 | 11.12 ± 0.15 |
| A. occidentale.H2Oa | 2.35 ± 0.03 | 0.78 ± 0.05 | 12.02 ± 0.61 |
| A. occidentale.H2Ob | 2.94 ± 0.07 | 0.73 ± 0.02 | 9.79 ± 0.95 |
| A. occidentale ETH2Oa | 2.62 ± 0.02 | 0.74 ± 0.02 | 7.73 ± 0.02 |
| A. occidentale ETH2O.b | 2.66 ± 0.02 | 0.78 ± 0.02 | 7.76 ± 0.02 |
| D.oliveri ETH2Oa | 2.98 ± 0.13 | 0.92 ± 0.05 | 7.27 ± 0.40 |
| D.oliveri ETH2Ob | 3.05 ± 0.11 | 0.90 ± 0.02 | 8.53 ± 0.98 |
| K. senegalensis H2Oa | 2.49 ± 0.09 | 0.79 ± 0.03 | 6.97 ± 0.05 |
| K. senegalensis H2Ob | 2.53 ± 0.10 | 0.83 ± 0.04 | 7.88 ± 0.78 |
| K. senegalensis ETH2Oa | 2.61 ± 0.03 | 0.83 ± 0.02 | 3.53 ± 0.04 |
| K. senegalensis ETH2Ob | 2.66 ± 0.02 | 0.87 ± 0.02 | 3.58 ± 0.02 |
| D. mespiliformis H2Oa | 2.60 ± 0.14 | 0.86 ± 0.06 | 4.89 ± 0.86 |
| D. mespiliformis H2Ob | 2.96 ± 0.18 | 0.95 ± 0.02 | 5.05 ± 0.81 |
| D. mespiliformis ETH2Oa | 3.08 ± 0.30 | 0.91 ± 0.08 | 6.77 ± 1.38 |
| D. mespiliformis ETH2Ob | 2.98 ± 0.34 | 0.91 ± 0.09 | 7.46 ± 1.20 |
| O. gratissimum H2Oa | 2.71 ± 0.13 | 0.76 ± 0.02 | 4.78 ± 0.30 |
| O. gratissimum H2Ob | 2.74 ± 0.13 | 0.75 ± 0.02 | 4.81 ± 0.30 |
| O. gratissimum ETH2Oa | 2.58 ± 0.10 | 0.77 ± 0.04 | 5.01 ± 0.81 |
| O. gratissimum ETH2Ob | 2.74 ± 0.20 | 0.81 ± 0.05 | 4.38 ± 0.49 |
| P. erinaceus ETH2Oa | 2.73 ± 0.086 | 0.81 ± 0.02 | 5.46 ± 1.07 |
| P. erinaceus ETH2Ob | 2.85 ± 0.11 | 0.82 ± 0.03 | 5.64 ± 1.02 |
Effect of the extracts of the plants studied on the relative weight of the organs of the hatched chicks.
H2Oa: Aqueous extract at 100mg/kg, H2Ob: Aqueous extract at 300mg/kg, ETH2Oa: Hydroethanolic extract at 100mg/kg, ETH2Ob: Hydroethanolic extract at 300mg/kg; A. occidentale: Anacardium occidentale L.; D. oliveri: Daniella oliveri (Rolfe) Hutch. & Dalziel; K. senegalensis: Khaya senegalensis (Desv.) A.Juss; D. mespiliformis: Dyospiros mespiliformis Hochst. ex A.DC.; O. gratissimum: Ocimum gratissimum L.; P. erinaceus: Pterocarpus erinaceus Poir.
3.2.4 Effect of Plant Extracts on Biochemical and Hematological Parameters of Chicks
The evaluation of the toxicological effect determined during embryogenesis and organogenesis of the aqueous and hydroethanolic extracts of the plants used on the biochemical and hematological parameters of the chicks obtained at hatching is shown in Tables 7, 8.
Table 7
| Parameters | Urea (g/L) | Creatinine (mg/L) | ALAT (UI/L) | ASAT (UI/L) | |
|---|---|---|---|---|---|
| Plants | Lots/extracts | Day 21 | Day 21 | Day 21 | Day 21 |
| Control négatif | Not treated | 0.29 ± 0.06 | 8.605 ± 1.05 | 8 ± 0.91 | 217.5 ± 8.70 |
| Control NaCl | NaCL | 0.23 ± 0.04 | 7.2425 ± 0.21 | 10 ± 0.40 | 222± 13.36 |
| A. occidentale | H2Oa | 0.385 ± 0.006 | 7.37± 0.28 | 6.5 ± 0.5 | 256± 23.81 |
| H2Ob | 0.425 ± 0.006 | 8.92 ± 0.60 | 7.75 ± 1.10 | 223.75 ± 18.48 | |
| ETH2Oa | 0.31 ± 0.01 | 6.87± 0.38 | 6.5 ± 0.28 | 214.5± 9.24 | |
| ETH2Ob | 0.30 ± 0.006 | 7.11 ± 0.25 | 7.5 ± 0.64 | 218.25± 11.53 | |
| D. oliveri | ETH2Oa | 0.27 ± 0.01 | 9.57± 0.16 | 8 ± 1.95 | 232± 18.73 |
| ETH2Ob | 0.28 ± 0.006 | 9.36 ± 0.03 | 7.75 ± 2.21 | 229.25± 22.29 | |
| K. senegalensis | H2Oa | 0.16 ± 0.004 | 16.02± 0.15 | 9 ± 0.91 | 239± 7.44 |
| H2Ob | 0.18 ± 0.006 | 16.21± 0.006 | 9.5 ± 1.04 | 208. 75± 27.86 | |
| ETH2Oa | 0.27 ± 0.006 | 14.02 ± 0.006 | 8 ± 0 | 311± 7** | |
| ETH2Ob | 0.31± 0.006 | 14.06 ± 0.006 | 8 ± 0 | 289± 0.57** | |
| D. mespiliformis | H2Oa | 0.18 ± 0.04 | 9.34± 1.04 | 8.25 ± 0.62 | 263.75 ± 12.05* |
| H2Ob | 0.34 ± 0.006 | 10.75 ± 0.31 | 8.25 ± 0.75 | 284± 6.94* | |
| ETH2Oa | 0.26 ± 0.05 | 6.91± 1.36 | 7.75 ± 1.10 | 226.25± 18.16 | |
| ETH2Ob | 0.2775 ± 0.05 | 6.87± 1.36 | 9 ± 0.91 | 241.75 ± 14.51 | |
| O. gratissimum | H2Oa | 0.3375 ± 0.01 | 11.11± 0.68 | 5.25 ± 0.47 | 222.25± 3.30 |
| H2Ob | 0.36 ± 0.006 | 11.11± 0.68 | 6.25 ± 0.47 | 271± 45.81* | |
| ETH2Oa | 0.28 ± 0.05 | 12.16 ± 0.13 | 6 ± 0.57 | 276.5± 22.59** | |
| ETH2Ob | 0.27 ± 0.04 | 12.15 ± 0.18 | 7.5 ± 0.5 | 286.5± 3.77** | |
| P. erinaceus | ETH2Oa | 0.36 ± 0.01 | 7.93 ± 1.45 | 7.5 ± 1.55 | 238.5± 24.99 |
| ETH2Ob | 0.38 ± 0.01 | 8.00 ± 0.49 | 7.25 ± 0.85 | 243.75 ± 28.83 | |
Effect of plant extracts on biochemical parametersof hatched chicks.
H2O, Aqueous extract; ETH2O, Hydro-ethanolic extract.; A. occidentale, Anacardium occidentale L.; D. oliveri, Daniella oliveri (Rolfe) Hutch. & Dalziel; K. senegalensis, Khaya senegalensis (Desv.) A.Juss; D. mespiliformis, Dyospiros mespiliformis Hochst. ex A.DC.; O. gratissimum, Ocimum gratissimum L.; P. erinaceus, Pterocarpus erinaceus Poir. a: 100mg/kg. b:300mg/kg; ASAT, Aspartate Aminotransferase; ALAT, Alanine Aminotransferase. Compared to controls, there was a very significant (**p < 0.01) increase in the mean value of aspartate aminotransferase in chicks whose eggs were treated with 100 and 300 mg/kg of hydroethanolic extract of Khaya senegalensis. The same observation was made in chicks whose eggs were treated with 100 and 300 mg/kg of hydroethanolic extract of Ocimum gratissimum. In chicks obtained from eggs treated with 100 and 300 mg/kg of aqueous extract of Dyospiros mespiliformis and with 300 mg/kg of aqueous extract of Ocimum gratissimum, the mean value of aspartate aminotransferase increased significantly (*p < 0.05) compared to controls.
Table 8
| Parameters | WBC (G/L) | RBC (T/L) | Hte (%) | Hb (g/dl) | MCV (fl) | TCMH(pg) | MCH(dl) | PLT | |
|---|---|---|---|---|---|---|---|---|---|
| Plants | Lots | Day 21 | Day 21 | Day 21 | Day 21 | Day 21 | Day 21 | Day 21 | Day 21 |
| Lot temoin | Not treated | 130.9± 6.11 | 2.15 ± 0.13 | 20.3 ± 3.56 | 22.15 ± 5.69 | 134.8 ± 2.78 | 62.5 ± 1.79 | 46.35 ± 1.31 | 72.5 ± 2.36 |
| Lot NaCl | NaCL | 129.6 ± 5.46 | 1.72 ± 0.42 | 28.9 ± 1.46 | 13.02 ± 0.36 | 130.9 ± 5.81 | 59.72 ± 1.29 | 45.821.62 | 64.75 ± 3.35 |
| A. occidentale | H2Oa | 138.75 ± 7.82 | 2.31 ± 0.09 | 28.8 ± 1.44 | 13.95 ± 0.72 | 124.15 ± 1.18 | 60.15 ± 0.66 | 48.45 ± 0.08 | 65.5 ± 10.68 |
| H2Ob | 128.05 ± 0.37 | 2.09 ± 0.03 | 26.9 ± 0 | 12.7 ± 0.05 | 128.7 ± 2.19 | 60.8 ± 0.75 | 47.3 ± 0.23 | 61.5 ± 3.17 | |
| ETH2Oa | 109.15 ± 2.75** | 2.05± 0.07 | 26.05± 0.65 | 12.45 ± 0.61 | 127.2 ± 2.62 | 60.62 ± 0.53 | 47.72 ± 1.40 | 46.75± 6.42 | |
| ETH2Ob | 88.6 ± 3.48 *** | 1.7 ± 0.05 | 25.75 ± 0.06 | 9.45 ± 0.45 | 148.1 ± 3.61 | 57.4 ± 0.51 | 37.1 ± 1.80 | 16.5 ± 4.51 | |
| D. oliveri | ETH2Oa | 112.32 ± 11.04 | 2.04 ± 0.17 | 26.075 ± 2.60 | 12.37 ± 1.16 | 127.6 ± 2.74 | 60.7 ± 1.26 | 32.57 ± 8.36 | 54.25 ± 9.75 |
| ETH2Ob | 121.1 ± 9.48 | 2.17± 0.15 | 28.4 ± 2.32 | 13.67± 0.53 | 127.57 ± 5.60 | 60.62 ± 1.31 | 47.72 ± 1.81 | 60 ± 8.11 | |
| K. senegalensis | H2Oa | 136.52 ± 3.09 | 2.28 ± 0.02 | 29.27 ± 0.99 | 14.22 ± 0.19 | 128.2 ± 4.32 | 62.4251.00 | 48.75 ± 2.28 | 86.5 ± 14.24 |
| H2Ob | 137 ± 1.21 | 2.28 ± 0.01 | 29.7 ± 0.57 | 14.3 ± 0.05 | 130.2 ± 2.54 | 62.70.34 | 48.25 ± 1.18 | 81 ± 8.08 | |
| ETH2Oa | 153.52 ± 4.75 | 2.66 ± 0.09 | 35.52 ± 1.48 | 16.45 ± 0.58 | 133.27 ± 1.25 | 61.87± 0.69 | 46.37 ± 0.30 | 67 ± 8.33 | |
| ETH2Ob | 155.45 ± 3.37 | 2.67± 0.09 | 36 ± 1.15 | 16.7 ± 0.40 | 134.7 ± 0.34 | 62.7 ± 0.63 | 46.55 ± 0.37 | 75.5 ± 0.28 | |
| D. mespiliformis | H2Oa | 128.37 ± 7.43 | 2.29 ± 0.09 | 28.35 ± 1.28 | 13.5 ± 0.64 | 123.52± 0.29 | 58.75 ± 0.72 | 47.57 ± 0.54 | 54 ± 3.39 |
| H2Ob | 125.42 ± 6.44 | 2.2125 ± 0.07 | 27.77 ± 1.05 | 13.25 ± 0.57 | 125.67 ± 1.31 | 59.9 ± 0.46 | 47.7 ± 0.47 | 53.25 ± 3.59 | |
| ETH2Oa | 137.77± 4.48 | 2.3475 ± 0.09 | 30.275 ± 1.49 | 14.15 ± 0.49 | 129.05 ± 2.13 | 60.325 ± 0.72 | 46.825 ± 1.12 | 65.25 ± 7.58 | |
| ETH2Ob | 148.97 ± 4.74 | 2.51 ± 0.10 | 31.77 ± 1.39 | 15.05 ± 0.41 | 126.52 ± 1.65 | 59.9 ± 0.88 | 47.375 ± 0.87 | 64.5 ± 7.84 | |
| O. gratissimum | H2Oa | 143.3 ± 6.82 | 2.4675 ± 0.14 | 32.225 ± 2.30 | 15.025 ± 0.95 | 130.075 ± 1.47 | 60.775 ± 0.27 | 46.625 ± 0.35 | 53 ± 7.77 |
| H2Ob | 135 ± 3.98 | 2.285 ± 0.10 | 29.4± 1.55 | 13.85 ± 0.66 | 128.3 ± 0.751 | 60.5 ± 0 | 47.05 ± 0.31 | 47.5 ± 3.17 | |
| ETH2Oa | 123.825 ± 5.53 | 2.07 ± 0.06 | 26.5 ± 1.04 | 12.85 ± 0.44 | 128 ± 3.05 | 61.925 ± 1.05 | 48.4 ± 0.37 | 59 ± 10.44 | |
| ETH2Ob | 131.4 ± 6.10 | 2.205 ± 0.10 | 28.025 ± 1.04 | 13.575 ± 0.55 | 127.225 ± 1.94 | 61.5 ± 0.80 | 48.35 ± 0.20 | 55.5 ± 3.27 | |
| P.erinaceus | ETH2Oa | 113.575 ± 1.65 | 1.97 ± 0.04 | 25.875 ± 0.71 | 12.3 ± 0.17 | 131.075 ± 2.00 | 62.525 ± 0.47 | 47.7 ± 0.77 | 61.75 ± 7.21 |
| ETH2Ob | 110.25 ± 3.73 | 1.865 ± 0.09 | 24.325 ± 1.63 | 11.55 ± 0.66 | 129.975 ± 2.59 | 62.075 ± 0.72 | 47.75 ± 0.78 | 60.25 ± 7.87 | |
Effect of extract plants on biochemical parameters on hatched chicks.
H2O, Aqueous extract; ETH2O, Hydro-ethanolic extract, A. occidentale, Anacardium occidentale L.; D. oliveri, Daniellia oliveri (Rolfe) Hutch. & Dalziel; K. senegalensis, Khaya senegalensis (Desv.) A.Juss; D. mespiliformis, Dyospiros mespiliformis Hochst. ex A.DC.; O. gratissimum, Ocimum gratissimum L.; P. erinaceus, Pterocarpus erinaceus Poir. a: 100mg/kg. b: 300mg/kg; RBC, Red Blood Cell counts; HB, Hemoglobin level; HT, Hematocrit; MCV, Mean CorpuscularVolume; MCH, Mean Corpuscular Hemoglobin content; MCHC, Mean Corpuscular Hemoglobin Concentration; WBC, White Blood Cell counts; PLT, Platelets. In chicks from eggs treated with 100 and 300 mg/kg of hydroethanolic extract of Anacardium occidentale, the mean value of white blood cell count increased significantly compared to controls, **p < 0.01, ***p < 0.001.
Regarding the biochemical parameters, no significant difference was noted for urea, creatinemia and ALAT. A slight increase in ASAT was noted for the batches that received the hydroethanolic extract of K. sengalensis at 100 and 300 mg/kg, the aqueous extract of D. mespiliformis at 100 and 300 mg/kg, the aqueous extract at 300 mg/kg and the hydroethanolic extract at 100 and 300 mg/kg of O. gratissimum (Table 7).
In general, none of the medicinal plant extracts used showed any effect on hematological parameters in the execution of white blood cell (WBC) and platelet values. A significant increase in the number of white blood cells (WBCs) was noted in the batches that received the hydroethanolic extracts of A. occidentale and K. senegalensis at 100 and 300 mg/kg (Table 8).
4 Discussion
The use of medicinal plants, especially in low and middle-income countries, is a primary health care alternative for the majority of the population. Despite being widely used, data on the toxicological profile of the plants used in traditional medicine are very scarce. This study was designed to evaluate the subacute toxicity and teratogenic effect of aqueous and hydroethanolic extracts of the leaves of Anacardium occidentale, Daniella oliveri, Diospyros mespiliformis, Manihot esculenta, Ocimum gratissimum, Pterocarpus erinaceus, Vernonia amygdalina and bark of the stem of Khaya senegalensis used in the traditional treatment of infectious diarrhea in Benin.
The results of the subacute toxicity experiment suggested that exposure to the different doses of some plant extracts assessed in this study could affect the weight growth and the hematological and biochemical parameters of the rats. Indeed, considerable weight gains were observed during the experiments for the majority of the study groups, except the groups treated with the aqueous and hydroethanolic extracts of A. occidentale and the hydroethanolic extract of K. senegalensis.
Weight loss observed in some animals could be related to the adverse effects of these extracts on the rats. The extracts would have affected the metabolism of the animals, which would have impacted their growth. Weight loss could also be related to the anorexia observed in these animals after gavage. Some toxicological studies have also reported weight loss in some animals treated with drug extracts. For instance, the weight loss of animals observed in the work of Agbodjento and collaborators in 2020 (27) on the larval and subacute toxicity of Gardenia ternifolia, Rourea coccinea and Cassytha filiformis, was justified by the anorexia status observed in some animals during the experiment. According to the study of Mukinda et al. (28), weight loss is correlated with the physiological state of the animal and can be explained not only by anorexia but also by altered metabolism in the animal.
For toxicological studies, hematological and biochemical parameters are important markers to evaluate the effect of the administered substance on the functioning of certain vital organs (29, 30). The hematopoietic system is one of the most sensitive parameters is seen as a potential risk for anemia and infections (31). Our data suggest that the extracts probably did not impact the hematopoietic system of the animals. Indeed, in our study, there was no significant variation between the hematological target of potentially toxic substances. Any alteration in erythrocyte and neutrophil parameters of the animals treated with the different extracts compared to the controls except in the case of platelets, for which a significant increase was observed in rats treated with the aqueous extract of O. gratissimum. Regarding the biochemical parameters, ALAT and ASAT, the two major markers of hepatic function were assessed (32). Under normal physiological conditions, these markers are present at low concentrations in serum. Elevated serum levels of these enzymes, particularly ALAT, are considered a sensitive marker of liver injury (33). Similarly, uremia and creatinine are markers of renal function (34). The hydroethanolic extract of M. esculenta caused a significant increase in creatinine in rats treated with this extract. Our data shows that aqueous extract of O. gratissimum and hydroethanolic extract of M. esculenta could affect the hepatic and renal functions of rats treated with these extracts. A subchronic toxicity study on M. esculenta revealed a significant increase in liver. Indeed, M esculenta could contain cyanide (35), this substance could be the basis of this increase in creatinine. In addition, hydroethanolic extract of A. occidentale significantly increased the ALAT value, this result was also observed in the study carried out by Tédong et al. (9), where the extract of A. occidentale increased significantly the biochemical parameters beyond 58 days of treatments.
Any increase in their serum levels reflects probable tissue damage to the kidneys (36, 37). However, comparison of the data obtained from this study with data from the literature shows little variation in relationships. Referring to the results of Onu et al. (38), no significant variation was noted for biochemical and hematological parameters. The latter worked on the effect of aqueous extract of bark and stem of Khaya senegalensis on some biochemical, hematological and histopathological parameters of rats. In Burkina-Faso, Kabore et al. (6) obtained similar results for D. oliveri and M. esculenta. The study that investigated the effects of cassava juice on renal and liver function and motor impairment in male rats indicates that chronic oral consumption of cassava juice caused renal and liver damage that correlated with impaired motor coordination in rats, similar to their major neurotoxic compound and linamarin. These observations differ from the data of our study. The differences obtained lie in the fact that these studies explored different effects related to the consumption of the plant. Looking at the abovementioned data, it is necessary to recommend the moderate use of all these plants, although no mortality was reported in this study.
The fetotoxic study of the aqueous and hydroethanolic extracts of A. occidentale, O. gratissimum, D. mespiliformis and the hydroethanolic extracts of D. oliveri, P. erinaceus, V. amygdalina and M. esculenta on embryogenesis and organogenesis of hen eggs, showed a low hatching rate were treated with the 300 mg/kg extracts. Therefore, the injection of the aqueous and hydroethanolic extracts of the plants prevented the evolution of embryonic development of the incubated eggs. The destruction of the unhatched eggs shows blackish deposits found in the egg, indicating that the evolution of the embryos stopped immediately after the first injection dose on day 7. This could be explained by the metabolism of the extracts administered in the eggs constituting a closed space, which means that everything that is injected is metabolized and used. Indeed, the heart is the first functional organ from the fourth or fifth day of incubation, and the toxic effect of a plant extract can lead to the early embryonic deaths obtained in this study. The failure of eggs to hatch after receiving the hydroethanol extract of M. esculenta may be due to its probable content of cyanogenic compounds, according to a study in Uganda, where ingestion of cyanide contained in cassava (M. esculenta) flour was the cause of severe food poisoning (39). The mortalities that occurred in this study were mostly early, suggesting that the extracts tested in this study did not have sufficient effect on fetal viability after day 14.
Indeed, from the 10th day of incubation, embryonic development would be enough in the setting of the organs, which makes the fetus autonomous enough to fight against toxic products likely to hinder embryonic development (40). The abortifacient nature of the V. amygdalina aqueous extract in eggs observed in this study was also reported by Oyinleye et al. (41) and Zakaria et al. (42), where the hydroethanolic extract of V. amygdalina had an abortifacient effect on female rats. In this study, the batch of eggs treated with NaCl gave a better hatching rate than the control batch that had received nothing, which suggests that all the embryonic deaths would certainly not be due to the extracts of the plants evaluated, except for V. amygdalina and M. esculenta, but rather to other external factors not taken into account in this study. The chicks obtained after hatching did not present any apparent malformations and were judged to be of very good quality according to the Tona score test performed (26). Indeed, obtaining chicks of good quality is governed by several factors, such as the duration between the laying of the egg and the beginning of the incubation. Thus, we can see a decrease in albumin that should be used for the feeding of the embryo (43, 44). The evaluation of the toxicological effects of biological substances is mostly based on vital organs such as the liver and heart, particularly in this study of the yolk sac (38). At the level of the analysis of the weight of the chicks, no significant difference was noted, and all the weights of the chicks without the yolk sac were lower than those of the chicks. Nevertheless, a slight increase in the relative weight of chicks treated with hydroethanolic extracts of A. occidentale and D. mespiliformis at 300 mg/kg was noted. No significant difference was noted in the relative weights of the heart, liver and yolk sac of the treated batches compared to the data of the negative control batch. These data show that the aqueous and hydroethanolic extracts of A. occidentale, D. mespiliformis, K. senegalensis, O. gratissimum and the hydroethanolic extracts of D. oliveri. and P. erinaceus do not affect the organogenesis of these organs.
The effect of the aqueous and hydroethanolic extracts of the medicinal plants used was evaluated on the hematological and biochemical parameters of the chicks. In fact, the alterations of the chemical and biological parameters of the serum in a toxicological study are interpreted as being the toxic effect of a plant or a chemical substance (45). The absence of variation in red blood cells testifies to the absence of toxic effects of plant extracts at doses of 100 and 300 mg/kg (46). Regarding the hematological parameters, no significant variation was observed for red blood cells, hemoglobin level, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin concentration or mean corpuscular hemoglobin content except for an increase in the number of white blood cells, especially for the batches treated with the hydroethanolic extracts of A. occidentale and K. senegalensis at 100 mg/kg and 300 mg/kg. The results of a previous subacute toxicity study with the hydroethanolic extract of A. occidentale in rats also revealed an increase in white blood cells (17), which could be explained by the presence of an inflammatory or immune reaction following tissue damage. However, the increase in the number of white blood cells could be explained by the fact that at the beginning of a human life, this phenomenon is important to fight effectively against possible threats. Regarding the biochemical parameters exploring the effect of plant extracts on the liver and kidney, two vital organs are involved in the metabolism of humans. No statistically significant difference was noted in the levels of urea, creatinine and ALAT. A slight increase in ASAT was observed for all batches, particularly those that received the hydroethanolic extract of K. sengalensis at 100 and 300 mg/kg, the aqueous extract of D. mespiliformis at 100 and 300 mg/kg and the hydroethanolic extract at 100 and 300 mg/kg of O. gratissimum.
ASAT is not a specific marker of tissue lesions and is found in many other tissues (liver, heart muscle, skeletal muscle) (45). On the other hand, no significant difference was observed for ALAT, which is a liver-specific enzyme, and creatinine, which is a kidney metabolite, so it could be deduced that the plant extracts did not affect the structural organization of the liver and kidney of the chicks at the tested doses.
The absence of data on the fetotoxicity of the plant extracts used in this study prevents real confrontation with the data obtained concerning the evaluation of the biological parameters. Nevertheless, several studies evaluating the acute, subacute or subchronic toxicity of these extracts of plants reported that no significant difference was noted for the hematological and biochemical parameters carried out on the rats (2, 4, 6, 8).
5 Conclusion
In this study, the evaluation of subacute toxicity over 28 days and fetotoxicity on hen eggs provided information on the nontoxic effects of the studied plant extracts. However, the hydroethanolic extracts of M. esculenta and V. amygdalina are likely to have fetotoxic properties. Further toxicological and pharmacological studies need to be conducted in order to formulate an improved conventional drug for the benefit of the populations.
Funding
This research was financed by TWAS Research Grant Award_20-254 RG/BIO/AF/AC_G.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The Benin National Ethical Committee for Health Research has reviewed and approved this study (65/MS/DC/SGM/DRFMT/CNERS/SA). Moreover, this study received ethical approval from the Ethical Committee of the Research Unit in Applied Microbiology and Pharmacology of Natural Substances (035–19/URMAPHA/EPAC/UAC).
Author contributions
EH, PA, and RM wrote the protocol. The protocol was reviewed and validated by TD, CK, J-RK, and AD. TD obtained the funding. EH, TD, PA, RM, KF, ED, and AD participated in the experimentation. EH, TD, AA, and EA did the statistical analyses. EH, TD, and AA wrote the draft of the manuscript. TD, AA, J-RK, HB, and AD reviewed the manuscript. All authors read and approved the final manuscript. All authors have contributed to the article and approved the submitted version
Acknowledgments
The authors are very grateful to the World Academy of Sciences (TWAS) and the United Nations Educational, Scientific and Cultural Organization (UNESCO) which funded a part of this project under TWAS Research Grant Award_20-254 RG/BIO/AF/AC_G. The authors are also grateful to the Economic Community of West African States (ECOWAS), which, through its ambitious Research and Innovation Support Program, funded part of this study. The ECOWAS Research and Innovation Support Program is a competitive program that aims at selecting the best projects submitted by researchers through calls for applications. It is included in the ECOWAS Science, Technology and Innovation Policy (ECOPOST) Action Plan (Strategic Area 2: activities 2.1.4; Strategic Area 3: activities 3.1.13; 3.2.1 to 3.2.3; Strategic Area 7: activities 7.1.2; 7.1.8; Strategic Area 8: activities 8. 1.1; 8.1.2; 8.1.5) and is closely aligned with the ECOWAS Community Strategic Framework (CSF) 2016-2020, the African Union Strategy on Science, Technology and Innovation (STISA), the African Union Agenda 2063 and the Sustainable Development Goals (SDGs-30). These institutions reviewed the research project, provided technical advice on its implementation steps and validated the funding. They thank all the members of the Research Unit in Applied Microbiology and Pharmacology of natural substances, University of Abomey-Calavi (Benin) and those of the Laboratory of Pharmacology and Toxicology, Faculty of Health Sciences, University of Lome (Togo) for their assistance in the realization of this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fitd.2022.868645/full#supplementary-material
References
1
Albuquerque UP do Nascimento ALB Silva Chaves L Feitosa IS de Moura JMB Gonçalves PHS et al . The Chemical Ecology Approach to Modern and Early Human Use of Medicinal Plants. Chemoecology (2020) 30:89–102. doi: 10.1007/s00049-020-00302-8
2
Ojo O Oloyede O OIO Ojo A Basiru A Onikanni S . Toxicity Studies of the Crude Aqueous Leaves Extracts of Ocimum Gratissimum L.in Albino Rats. IOSR. J Environ Sci Toxicol Food Technol (IOSR-JESTFT) (2013) 5:34–9. doi: 10.9790/2402-0643439
3
Dougnon V Hounsa E Koudokpon H Legba BB Fabiyi K Sintondji K et al . A Literature Review—Khaya Senegalensis, Anacardium Ouest L., Cassia Sieberiana DC., Pterocarpus Erinaceus, Diospyros Mespiliformis, Ocimum Gratissimum, Manihot Esculenta, Vernonia Amygdalina Delile, Pseudocedrela Kotschyi and Daniellia Oliveri Possess Properties for Managing Infectious Diarrhea. Adv Biosci Biotechnol (2020) 11:457–73. doi: 10.4236/abb.2020.1110031
4
Ebbo AA Sani D Suleiman MM Ahmad A Hassan AZ . Acute and Sub-Chronic Toxicity Evaluation of the Crude Methanolic Extract of Diospyros Mespiliformis Hochst Ex a. Dc (Ebenaceae) and Its Fractions. Toxicol Rep (2020) 7:1138–44. doi: 10.1016/j.toxrep.2020.08.028
5
Encarnação S de Mello-Sampayo C Graça NAG Catarino L da Silva IBM Lima BS et al . Total Phenolic Content, Antioxidant Activity and Pre-Clinical Safety Evaluation of an Anacardium Occidentale Stem Bark Portuguese Hypoglycemic Traditional Herbal Preparation. Ind Crops Product (2016) 82:171–8. doi: 10.1016/j.indcrop.2015.11.001
6
Kabore A Meda R Kiendrebeogo M Gaston BAM Laya S . Phytochemical Analysis and Acute Toxicity of Two Medicinal Plants (Anogeissus Leiocarpus and Daniellia Oliveri) Used in Traditional Veterinary Medicine in Burkina Faso. Arch Appl Sci Res (2010) 6:47–52.
7
Tittikpina NK Nana F Fontanay S Philippot S Batawila K Akpagana K et al . Antibacterial Activity and Cytotoxicity of Pterocarpus Erinaceus Poir Extracts, Fractions and Isolated Compounds. J Ethnopharmacol (2018) 212:200–7. doi: 10.1016/j.jep.2017.10.020
8
Lajubutu BA Pinney RJ Roberts MF Odelola HA Oso BA . Antibacterial Activity of Diosquinone and Plumbagin From the Root Ofdiospyros Mespiliformis (Hostch) (Ebenaceae). Phytother Res (1995) 9:346–50. doi: 10.1002/ptr.2650090508
9
Tédong L Dzeufiet PDD Dimo T Asongalem EA Sokeng SN Flejou J-F et al . Acute and Subchronic Toxicity of Anacardium Occidentale Linn (Anacardiaceae) Leaves Hexane Extract in Mice. Afr J Tradit Complement Altern Med (2006) 4:140–7. doi: 10.4314/AJTCAM.V4I2.31194
10
Fouche G Adenubi OT Leboho T McGaw LJ Naidoo V Wellington KW et al . Acaricidal Activity of the Aqueous and Hydroethanolic Extracts of 15 South African Plants Against Rhipicephalus Turanicus and Their Toxicity on Human Liver and Kidney Cells. Onderstepoort J Vet Res (2019) 86(1):e1–7. doi: 10.4102/ojvr.v86i1.1665
11
Dougnon TV Hounsa E Agbodjento E Koudokpon H Legba B Fabiyi K et al . Toxicological Characterization of Ten Medicinal Plants of the Beninese Flora Used in the Traditional Treatment of Diarrheal Diseases. Evidence-Based Complement. Altern Med (2021) 2021:1–13. doi: 10.1155/2021/6676904
12
Dougnon V Hounsa E Agbodjento E Keilah LP Legba BB Sintondji K et al . Percentage Destabilization Effect of Some West African Medicinal Plants on the Outer Membrane of Various Bacteria Involved in Infectious Diarrhea. BioMed Res Int (2021) 2021:1–12. doi: 10.1155/2021/4134713
13
Chung KT Wong TY Wei CI Huang YW Lin Y . Tannins and Human Health: A Review. Crit Rev Food Sci Nutr (1998) 38:421–64. doi: 10.1080/10408699891274273
14
Nasri H Shirzad H . Toxicity and Safety of Medicinal Plants. J HerbMed. Pharmacol (2013) 2:2.
15
Luka J Badau SJ Mbaya AW Gadzama JJ Kumshe HA . Acute Toxicity Study and Effect of Prolonged Administration (28 Days) of Crude Ethanolic Root Extract of Diospyros Mespiliformis Hochst (Ebenaceae) on Clinical, Haematological and Biochemical Parameters of Albino Rats. J Ethnopharmacol. (2014) 153:268–73. doi: 10.1016/j.jep.2014.02.033
16
Adeyanju O Olatoyinbo F . Toxicological Studies and Utilization of Daniella Oliveri Gum as Binder in Drug Formulation. J Pharm Appl Chem (2018) 4(3):169–79. doi: 10.18576/jpac/040302
17
Konan NA Bacchi EM Lincopan N Varela SD Varanda EA . Acute, Subacute Toxicity and Genotoxic Effect of a Hydroethanolic Extract of the Cashew (Anacardium Occidentale L. J Ethnopharmacol. (2007) 110:30–8. doi: 10.1016/j.jep.2006.08.033
18
Dougnon V Bankolé H Edorh P Klotoé JR Dougnon J Fah L et al . Acute Toxicity of Solanum Macrocarpon Linn (Solanaceae) on Wistar Rats: Study About Leaves and Fruits. Am J Biochem (2013) 3:84–8. doi: 10.5923/j.ajb.20130303.4
19
OECD (2008) , Test No. 407: Repeated Dose 28-day Oral Toxicity Study in Rodents, in OECD Guidelines for the Testing of Chemicals, Section 4. Paris: OECD Publishing. doi: 10.1787/9789264070684-en
20
Legba B Dougnon V Deguenon E Agbankpe J Senou M Aniambossou A et al . Toxicological Characterization of Six Plants of the Beninese Pharmacopoeia Used in the Treatment of Salmonellosis. J Toxicol (2019) 2019:1–12. doi: 10.1155/2019/3530659
21
Sodipo OA Abdulrahman FI Alemika TE Gulani IA . Chemical Composition and Biological Properties of the Petroleum Ether Extract of Solanum Macrocarpum L. (Local Name: Gorongo). Br J Pharm Res (2012) 21:108–28. doi: 10.9734/BJPR/2012/1207
22
Tavakkoli H Derakhshanfar A Moayedi J Poostforoosh Fard A . Utilization of a Chicken Embryo Membrane Model for Evaluation of Embryonic Vascular Toxicity of Dorema Ammoniacum. Avicenna. J Phytomed (2020) 10:152–60. doi: 10.22038/AJP.2019.13865
23
Oosterbaan AM Steegers EAP Ursem NTC . The Effects of Homocysteine and Folic Acid on Angiogenesis and VEGF Expression During Chicken Vascular Development. Microvas. Res (2012) 83:98–104. doi: 10.1016/j.mvr.2011.11.001
24
Georgescu N Apostol L Gherendi F . Inactivation of Salmonella Enterica Serovar Typhimurium on Egg Surface, by Direct and Indirect Treatments With Cold Atmospheric Plasma. Food Control. (2017) 76:52–61. doi: 10.1016/j.foodcont.2017.01.005
25
Elsayed M Mohamed N Hatab M Elaroussi M . Oxidative Stress of in-Ovo Ochratoxin A Administered During Chick Embryonic Development. Braz J Poult. Sci (2019) 21:eRBCA–2019-0637. doi: 10.1590/1806-9061-2017-0637
26
Tona K Bamelis F De Ketelaere B Bruggeman V Moraes V Buyse J et al . Effects of Egg Storage Time on Spread of Hatch, Chick Quality, and Chick Juvenile Growth. Poultry. Sci (2003) 82:736–41. doi: 10.1093/ps/82.5.736
27
Agbodjento E Klotoé JR Sacramento TI Dougnon TV Déguenon E Agbankpé J et al . Larval Cytotoxic and Subacute Toxicity of Gardenia Ternifolia, Rourea Coccinea, and Cassytha Filiformis Used in Traditional Medicine of Benin (West Africa). J Toxicol (2020) 2020:1–11. doi: 10.1155/2020/8843575
28
Mukinda JT Syce JA . Acute and Chronic Toxicity of the Aqueous Extract of Artemisia Afra in Rodents. J Ethnopharmacol (2007) 112:138–44. doi: 10.1016/j.jep.2007.02.011
29
Bagheri SM Yadegari M Mirjalily A Rezvani ME . Evaluation of Toxicity Effects of Asafetida on Biochemical, Hematological, and Histological Parameters in Male Wistar Rats. Toxicol Int (2015) 22(1):61–5. doi: 10.4103/0971-6580.172258
30
Porwal M Gautam SK Khan NA Maheshwari KK . Evaluation of Toxicity and Antihyperlipidemic Activity of Spondias Mombin L. Leaves Methanolic Extract in Laboratory Rats. Cardiovasc Hematol Disord Drug Targets (2020) 20(4):289–96. doi: 10.2174/1871529X20999201027232556
31
Betti AH Stein AC Dallegrave E Wouters ATB Watanabe TTN Driemeier D et al . Acute and Repeated-Doses (28 Days) Toxicity Study of Hypericum Polyanthemum Klotzsch Ex Reichardt (Guttiferare) in Mice. Food Chem Toxicol (2012) 50:2349–55. doi: 10.1016/j.fct.2012.04.012
32
Raghu C Ekena J Cullen JM Webb CB Trepanier LA . Evaluation of Potential Serum Biomarkers of Hepatic Fibrosis and Necroinflammatory Activity in Dogs With Liver Disease. J Vet Intern Med (2018) 32(3):1009–18. doi: 10.1111/jvim.15064
33
Niemelä O Alatalo P . Biomarkers of Alcohol Consumption and Related Liver Disease. Scand J Clin Lab Invest. (2010) 70(5):305–12. doi: 10.3109/00365513.2010.486442
34
Sueyoshi M Fukunaga M Mei M Nakajima A Tanaka G Murase T et al . Effects of Lactulose on Renal Function and Gut Microbiota in Adenine-Induced Chronic Kidney Disease Rats. Clin Exp Nephrol (2019) 23(7):908–19. doi: 10.1007/s10157-019-01727-4
35
Assih M Aboudoulatif D Dougnon V Kossi M Lawson-Evi P Kwashi E-G et al . Toxicological Study of Manihot Esculenta Crantz (Euphorbiaceae) Leaf Extracts. Br J Med Health Res (2021) 8:1–11. doi: 10.46624/bjmhr.2021.v8.i4.001
36
Manda P Manda O Manda MV . Etude Des Toxicités Aigue Et Subaiguë Du Remede Nature Utilise Dans Le Traitement Du Paludisme. Rev Ivoirienne Des Sci Technologie (2017) 29:145–458.
37
Yuliandra Y Armenia A Salasa AN Ismed F . Subchronic Toxicity of Ethanolic Extract of Cassytha Filiformis L. On the Renal Function of Rat. JSFK (2015) 2:54–9.
38
Onu A Saidu Y Ladan MJ Bilbis LS Aliero AA Sahabi SM . Effect of Aqueous Stem Bark Extract of Khaya Senegalensis on Some Biochemical, Haematological, and Histopathological Parameters of Rats. J Toxicol (2013) 2013:1–9. doi: 10.1155/2013/803835
39
Alitubeera PH Eyu P Kwesiga B Ario AR Zhu B-P . Outbreak of Cyanide Poisoning Caused by Consumption of Cassava Flour — Kasese District, Uganda, September 2017. MMWR Morb Mortal Wkly Rep (2019) 68:308–11. doi: 10.15585/mmwr.mm6813a3
40
Zahoor-ul- H Khan MZ Saleemi MK Khan A Javed I Bhatti SA et al . Toxico-Pathological Effects of In Ovo Inoculation of Ochratoxin A(OTA) in Chick Embryos and Subsequently in Hatched Chicks. Toxicol Pathol (2012) 40:33–9. doi: 10.1177/0192623311425058
41
Oyinleye OE Adeniran SA Ogunsuyi OM Oyeyemi IT Bakare AA . Genetic and Reproductive Toxicity of Aqueous Extracts of Telfairia Occidentalis (Hook F.), Vernonia Amygdalina and Their Combination on the Testicular Cells of Male Mice. Adv TRADIT Med (ADTM) (2020) 21:759–65. doi: 10.1007/s13596-020-00507-w
42
Zakaria Y Azlan NZ Nik NF Muhammad H . Phytochemicals and Acute Oral Toxicity Studies of the Aqueous Extract of Vernonia Amygdalina From State of Malaysia. J Med Plants Stud (2016) 5:1–05.
43
Lapão C Gama LT Soares MC . Effects of Broiler Breeder Age and Length of Egg Storage on Albumen Characteristics and Hatchability. Poult. Sci (1999) 78:640–5. doi: 10.1093/ps/78.5.640
44
Khan MJA Khan SH Bukhsh A Amin M . The Effect of Storage Time on Egg Quality and Hatchability Characteristics of Rhode Island Red (RIR) Hens. Vet Arhiv (2014) 14:291–303.
45
Ramaiah SK . A Toxicologist Guide to the Diagnostic Interpretation of Hepatic Biochemical Parameters. Food Chem Toxicol (2007) 45:1551–7. doi: 10.1016/j.fct.2007.06.007
46
Dossou-Yovo KM Diallo A Lawson-Evi P Kantati YT Darré T Bakoma B et al . A 90-Day Oral Toxicity of Hydroethanolic Root Extract of Carissa Spinarum in Wistar Rats. J Toxicol (2021) 2021:5570206. doi: 10.1155/2021/5570206
Summary
Keywords
subacute toxicity, fetotoxicity, medicinal plants, infectious diarrhea, Benin
Citation
Hounsa E, Dougnon TV, Agbankpe AJ, Assogba P, Koudokpon CH, Klotoe J-R, Moussa RT, Agbodjento E, Fabiyi K, Deguenon E, Bankole HS and Diallo A (2022) Fetotoxicity and Subacute Toxicity of Some Plants Involved in the Treatment of Infectious Diarrhea in Benin. Front. Trop. Dis 3:868645. doi: 10.3389/fitd.2022.868645
Received
03 February 2022
Accepted
17 May 2022
Published
22 June 2022
Volume
3 - 2022
Edited by
Daniel Gyamfi Amoako, National Institute for Communicable Diseases (NICD), South Africa
Reviewed by
Oluwafemi Adeleke Ojo, Bowen University, Nigeria; Abdel Jelil Njouendou, University of Buea, Cameroon
Updates
Copyright
© 2022 Hounsa, Dougnon, Agbankpe, Assogba, Koudokpon, Klotoe, Moussa, Agbodjento, Fabiyi, Deguenon, Bankole and Diallo.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tamegnon Victorien Dougnon, victorien.dougnon@gmail.com
This article was submitted to Antimicrobial Resistance, a section of the journal Frontiers in Tropical Diseases
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.