- 1Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, United States
- 2Johns Hopkins Center for Infectious Diseases in India, Pune, India
- 3BJ Government Medical College, Johns Hopkins Clinical Research Site, Pune, India
- 4Department of Global Health, Boston University School of Public Health, Boston, MA, United States
- 5BJ Government Medical College and Sassoon General Hospitals, Pune, India
- 6Indian Council of Medical Research, National Institute for Research in Tuberculosis, Chennai, India
- 7Dr. D. Y. Patil Medical College, Hospital and Research Centre, Pune, India
- 8M. V. Hospital for Diabetes, Chennai, India
- 9Department of Medicine, University of Massachusetts Chan Medical School, Worcester, MA, United States
- 10Center for Tuberculosis Research, School of Medicine, Johns Hopkins University, Baltimore, MD, United States
Some individuals with drug-susceptible pulmonary tuberculosis (PTB) experience tuberculosis recurrence. To evaluate the incidence of and risk factors for recurrence following completion of antituberculosis therapy, we pooled data from three prospective observational Indian PTB cohorts with 1,164 individuals ≥14 years old included in our analysis. Ninety-five (8%) experienced recurrence, with an 8.5 cases/100 person-years recurrence incidence rate (95% confidence interval 6.9–10.3) and a median time to recurrence of 6 months. From multivariable logistic regression, month 2 culture positivity (aHR 2.06; 95% CI 1.17–3.63), body mass index (BMI) < 17 mg/kg2 (aHR 1.7; 95% CI 1.1–2.8), and male sex (aHR 1.92; 95% CI 1.05–3.51) were independent recurrence risk factors. Understanding risk factors for TB recurrence could enable clinicians to identify patients at risk for recurrence during antituberculosis therapy and may be used to alter patient care strategies, such as more frequent monitoring post-treatment for high-risk individuals.
Introduction
Of the 7.5 million people diagnosed with tuberculosis (TB) globally in 2022, India had the highest proportion of TB, accounting for more than 30% of cases (1). Despite a ≥90% End TB Strategy goal treatment success rate (1, 2), programmatic settings often achieve suboptimal outcomes, including recurrence, which occurs after individuals have successfully completed antituberculosis therapy (ATT) and are deemed cured (3). The advent of genotyping of Mycobacterium tuberculosis (Mtb) has allowed for further classification of recurrence into either relapse of disease or reinfection (4), with reinfection more likely in hyperendemic areas (5, 6). Risk factors associated with TB recurrence include higher smear grade at baseline and month 2 of therapy (7, 8), HIV status (9), alcohol and tobacco use (10), month 2 culture positivity, and baseline chest xX-ray (CXR) cavitation (11, 12).
While studies have primarily focused on baseline risk factors, few have incorporated microbiological data throughout ATT and have not evaluated the timing of recurrence post-ATT completion. Identifying independent TB recurrence risk factors could enable clinicians to accurately identify these patients and augment treatment or post-treatment monitoring strategies (13). Using data from three Indian drug-sensitive pulmonary TB (PTB) observational cohorts, we evaluated recurrence incidence rates and risk factors, using data collected throughout ATT.
Materials and methods
Study design
We leveraged data from three Indian observational cohorts designed to assess the incidence rate of and risk factors for unfavorable TB treatment outcomes: Cohort for Tuberculosis Research by the Indo-US Medical Partnership (C-TRIUMPh) (14), The Effects of Diabetes on Tuberculosis Severity (EDOTS) (15), and The Impact of Diabetes on TB Treatment Outcomes (TB-DM) (16). These cohorts enrolled microbiologically confirmed or clinically diagnosed PTB; participants initiated standard of care ATT, consisting of 2 months isoniazid, rifampin, pyrazinamide, and ethambutol either thrice weekly or daily followed by 4 months of isoniazid and rifampin, according to India's National Tuberculosis Elimination Program (NTEP) guidelines (17). Before 2017, ATT was administered as thrice-weekly directly observed therapy; After 2017, NTEP guidelines shifted to daily self-administered regimens.
Study population
Participants were recruited from local tuberculosis units to four clinical research sites in Pune or Chennai. Those with newly diagnosed rifampin-susceptible PTB aged ≥ 14 years were included in this study. They had signs or symptoms consistent with TB disease: cough, hemoptysis, fever, weight loss, fatigue, night sweats, or pleuritic chest pain. They also had CXR findings consistent with TB, and/or were sputum smear positive by microscopy or by rapid diagnostic test (i.e., GeneXpert). Participants who had received 1 week (daily or intermittent doses) of any drugs with anti-TB activity within 30 days were excluded. TB-DM and EDOTS excluded people with HIV. Supplementary material include detailed information on enrollment dates, study sites, and cohort-specific inclusion and exclusion. All participants were followed during treatment and for up to 12 months following treatment completion. Standardized clinical assessments, demographics, sputum sampling, and outcome assessments were collected at baseline and months 2, 6, 12, and 18.
Primary outcomes
TB cure was defined as ≥2 consecutive respiratory sputum cultures negative for Mtb following the completion of 6 months of ATT with continued resolution of symptoms and negative mycobacteriology assessments (smear and culture). Following successful treatment completion, TB recurrence was defined as microbiological recurrence with Mtb growth on culture [either liquid Mycobacterium Growth Incubator Tube (MGIT) or solid Löwenstein-Jensen (LJ) culture media], or clinical recurrence based on symptoms suggestive of TB and acid-fast bacilli on smear microscopy. TB recurrence included cases of both reinfection and relapse, as whole genome sequencing of isolates to compare to baseline samples was not available.
Variable definitions
Smoking was defined as a history of ever smoking tobacco products (including current and former smokers), and alcohol use was defined as any reported history of alcohol use. Diabetes was defined as hemoglobin A1c > 6.5%, a random blood glucose > 200 mg/dL, or a prior diagnosis of diabetes on antidiabetic medication. A Timika CXR score was calculated as a summation of the percentage of lungs involved from 0 to 100% and an additional 40 points added for the presence of cavity (18).
Primary statistical analyses
The recurrence incidence rate was calculated as the number of individuals with recurrence divided by total person-time at risk per 100 person-years [Poisson exact 95% confidence intervals (CI)]. Variables associated with recurrence were evaluated using univariable Cox Proportional Hazards regression. To account for confounders identified a priori based on a literature review, we used multivariable Cox Proportional Hazards regression and visualized results using Forest plots. Differences between categorial variables (summarized as proportions) were assessed (Fisher's exact test). Continuous variables were summarized as medians with interquartile ranges (IQR) or means with standard deviation and were compared (Wilcoxon rank-sum test or t-test). The level of significance was assessed at 5%. Statistical analyses were conducted using Stata Version 17 (StataCorp, College Station, TX, USA) and RStudio Version 4.2.2 (Rstudio, Boston, MA, USA). To generate a recurrence prediction model, a priori we selected seven candidate predictors: age, alcohol use, sex, smoking history, month 2 culture positivity, month 2 smear positivity, and body mass index (BMI), based on prior TB prediction models (19) and co-author clinical input. Data was split into a training model (60%) and a validation model (40%). Model discrimination was evaluated by receiver operating characteristic (ROC) curve, area under the curve (AUC), and c-statistic.
Ethics statement
Ethics Committee/Institutional Review Board approval was obtained from each Indian participating site, Johns Hopkins University and the University of Massachusetts. Informed consent was obtained from all study participants.
Results
Of the 2,062 individuals enrolled in TB-DM, C-TRIUMPh, and EDOTS, 1,516 completed treatment and achieved cure; 1,164 individuals ≥14 years old were included in our analysis (Supplementary Figure 1), contributing a cumulative 1,118 person-years of follow-up. Individuals who experienced treatment failure, death, had baseline rifampin resistance, were lost to follow-up prior to 6 months, or withdrew, were excluded. Ninety-five (8%) experienced recurrence. Individuals who experienced recurrence were more likely males, from Pune, with a lower BMI, an alcohol use history, and had a positive month 2 smear or culture result (Table 1). The overall recurrence incidence rate was 8.5/100 person-years (95% CI 6.9–10.3), which occurred 179 days (median, IQR: 115–271) after ATT completion. Recurrence occurred in the following intervals after ATT completion: n = 18 at ≤ 3 months, n = 30 at >3– ≤ 6 months, n = 23 at >6– ≤ 9 months, and n = 24 at ≥9 months. Eighteen individuals experienced clinical recurrence, whereas 57 individuals experienced microbiologically confirmed recurrence (Supplementary Table 1).
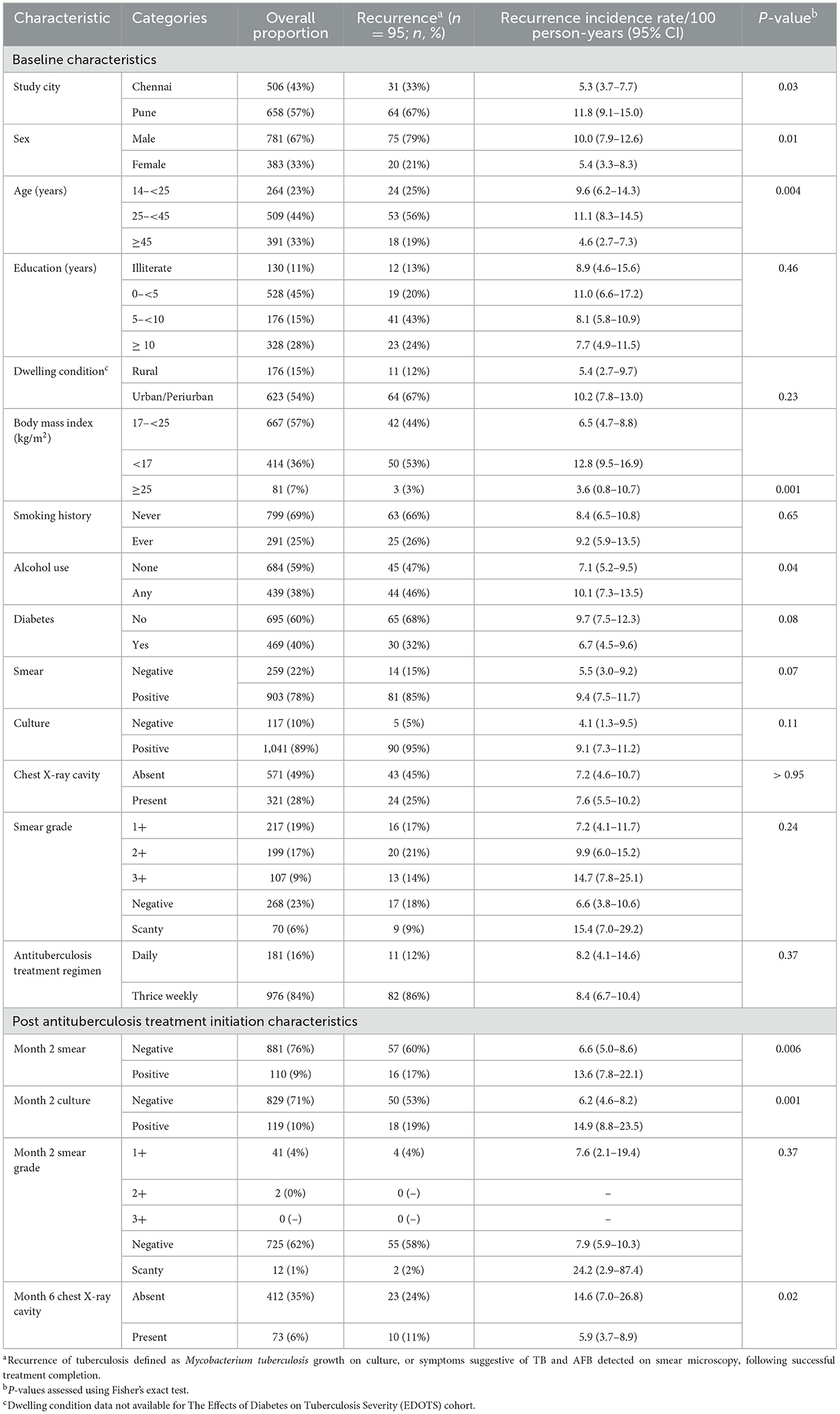
Table 1. Characteristics of the study population throughout antituberculosis therapy and incidence rate of recurrence by key characteristics.
Univariable regression analysis showed male sex, age > 45 years, BMI < 17 kg/m2, positive month 2 smear, positive month 2 culture, and month 6 cavity on CXR were significantly associated with increased TB recurrence hazards (Supplementary Table 2). Multivariable regression analysis identified male sex (aHR 1.92; 95% CI 1.05–3.51), BMI < 17 kg/m2 (aHR 1.72; 95% CI 1.1–2.8), and month 2 culture positivity (aHR 2.06; 95% CI 1.17–3.63) as independent risk factors for recurrence (Supplementary Table 2; Figure 1). When stratified by site, no city-specific associations emerged (Figure 1). A stratified analysis comparing males with and without recurrence revealed no differences in modifiable habits such as alcohol use or smoking history (Supplementary Table 3).
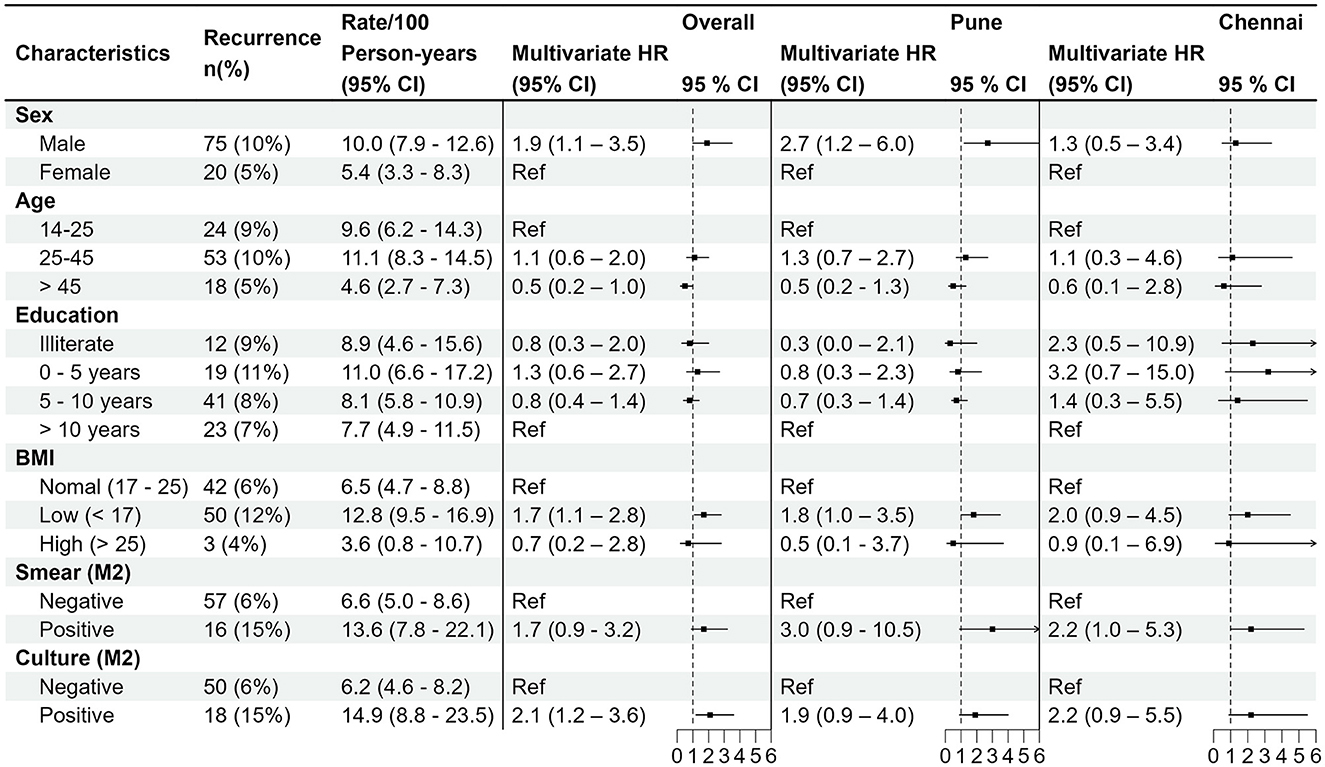
Figure 1. Forest plot showing the overall multivariable hazards of recurrence, adjusting for smoking history, alcohol use history, baseline smear result, baseline culture, baseline cavity, baseline X-ray score, antituberculosis therapy dosing regimen (thrice weekly vs. daily). City-specific stratification (Pune vs. Chennai) also shown. HR, hazard ratio; BMI, body mass index (kg/m2); M2, month 2.
We attempted to create a recurrence prediction model with age, alcohol use, sex, smoking history, month 2 culture positivity, month 2 smear positivity, and BMI as predictors, but the model performed poorly with an AUC of 0.66 (sensitivity 85%; specificity 37%).
Discussion
This analysis of Indian adolescents and adults with drug-sensitive pulmonary tuberculosis, enrolled in three prospective observational Indian cohorts, provides key insights into the time to recurrence following treatment completion, as many previously published studies on recurrence do not include this information. This study also highlights differences among individuals who achieved a sustained cure following treatment completion compared to those who experienced recurrence. We observed that a high proportion (8%) experienced TB recurrence with an overall incidence rate of 8.5 cases/100 person-years (95% CI 6.9–10.3) and a 6-month median time to recurrence following treatment completion. Recurrence events occurred throughout ATT completion follow-up, without a 3-month timeframe containing the majority of events. Male sex, BMI < 17 kg/m2, and month 2 culture positivity were key independent recurrence risk factors. The association of male sex remained after accounting for confounding behavioral factors associated with increased risk of adverse TB treatment outcomes, such as smoking and alcohol use, habits which were far more common in men in this cohort (10, 20, 21). While the increased risk of tuberculosis disease in males is well-documented (22), these findings highlight the increased susceptibility of the male sex in a specific adverse TB treatment outcome: recurrence. Consistent with prior studies, month 2 culture positivity was also an important recurrence risk factor (23). With a low BMI cutoff of < 17 kg/m2, BMI was associated with recurrence on multivariable regression analysis. Undernutrition is a well-established risk factor for the development of TB disease (13, 24). There have been several studies suggesting that Asian Indians have more body fat relative to weight than their Caucasian counterparts. Thus, the conventional low BMI cut-off of < 18.5 kg/m2, derived from mortality statistics from primarily white populations, may poorly predict risk of disease in other groups (25–27). For diabetes, for example, a lower BMI cut-off point is recommended for screening for Asians by the American Diabetes Association (28). Our study suggests that use of a lower BMI cut-off when assessing for recurrence risk could be important in an Asian Indian population. We demonstrated some challenges with creating a recurrence prediction model despite a robust number of events. Given that recurrence occurred a median of 6 months after cure, it could be that traditional baseline epidemiologic risk factors are not always accurate predictors of recurrence, and more data at ATT completion is needed for improved prediction.
Our study has some limitations. We could not evaluate whether recurrence represented reinfection or relapse as genotyping comparing the Mtb strains at baseline and at the time of recurrence was not available (29). At the time the studies were started, thrice weekly ATT was the national program standard, and this shifted to daily ATT while the studies were being conducted. Although 85% received thrice weekly, rather than daily ATT, per the standard of care at the time of the study, we continued to observe increased recurrence hazards after adjusting for this variable; this could have contributed to our higher recurrence rates. While recurrence is known to occur more commonly in people with HIV, we were unable to assess this risk factor due to a low HIV prevalence (and exclusion by two cohorts). Finally, while we are unable to broadly extrapolate our findings to populations with recurrence of pulmonary TB outside of Pune and Chennai, India, given the nature of the cohorts, many of the risk factors found in our study are in line with previously published risk factors; a systematic meta-analysis including more heterogenous populations may yield different results.
In conclusion, we describe a high proportion of recurrence in our Indian cohort of individuals treated for drug-sensitive PTB. Interestingly, recurrence occurred throughout 9 months of post-ATT follow-up, suggesting that there is not a specific timeframe where monitoring for recurrence would be most useful. Given that India accounts for the highest burden of TB cases globally, a comprehensive understanding of risk factors for recurrence in Indian patients is critical, and our analysis could provide insights to clinicians to identify patients at risk of recurrence during ATT and may be used to alter patient care strategies, such as prolongation of ATT or more frequent monitoring post treatment for high-risk individuals, which could include clinical assessments, sputum culture, or imaging.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: Identifiable information. De-identified dataset available upon request. Requests to access these datasets should be directed to https://reportindia.org.
Ethics statement
The studies involving humans were approved by Johns Hopkins University, University of Massachusetts, Dr. D. Y. Patil Medical College (DYPMC), Byramjee-Jeejeebhoy Government Medical College-Sassoon General Hospitals (BJGMC-SGH), National Institute for Research in Tuberculosis, and Prof. M. V. Diabetes Research Center (MVDRC). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contributions
SK: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. NG: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing – review & editing. MP: Writing – review & editing, Data curation, Project administration. AGupte: Writing – review & editing, Methodology. MN: Writing – review & editing, Formal analysis. SR: Writing – review & editing, Data curation. NS: Writing – review & editing, Investigation. NP: Writing – review & editing, Investigation. SG: Writing – review & editing, Investigation. RK: Writing – review & editing, Investigation. RL: Writing – review & editing, Investigation. EH: Writing – review & editing, Investigation. KT: Writing – review & editing, Investigation. CP: Writing – review & editing, Investigation. TS: Writing – review & editing, Investigation. MB: Writing – review & editing, Investigation. AK: Writing – review & editing, Investigation. VV: Writing – review & editing, Investigation. HK: Writing – review & editing, Funding acquisition, Investigation. AGupta: Writing – review & editing, Conceptualization, Formal analysis, Funding acquisition, Investigation. JG: Investigation, Writing – review & editing, Funding acquisition. VM: Conceptualization, Funding acquisition, Investigation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Data in this manuscript were collected as part of the Regional Prospective Observational Research for Tuberculosis (RePORT) India Consortium. This project has been funded in whole or in part with Federal funds from the Government of India (GOI) Department of Biotechnology (DBT), the Indian Council of Medical Research (ICMR), the USA National Institutes of Health (NIH), the National Institute of Allergy and Infectious Diseases (NIAID), the Office of AIDS Research (OAR), and distributed in part by CRDF Global. This work was also supported by the National Institutes of Health (NIH R01AI097494 to JG), the NIH funded Johns Hopkins Baltimore-Washington-India Clinical Trials Unit for NIAID Networks (UM1AI069465 to VM, NG, AGupta, and SK), Ujala Foundation, Wyncote Foundation, and Gilead Foundation. SK was supported by the National Institutes of Health T32 AI007291-27 and the Johns Hopkins University Clinician Scientist Award.
Acknowledgments
We would like to thank the participants and their families. We would also like to acknowledge all members of the c-TRIUMPh, TB-DM, and eDOTS Study Teams as follows: The TBDM-CTRIUMPH-Regional Prospective Observational Research for Tuberculosis (RePORT) India study team (lead author Amita Gupta) listed in alphabetical order: Aarti Kinikar, Akshay Gupte, Amita Gupta, Amita Nagraj, Anand Kumar, Andrea DeLuca, Anita More, Anju Kagal, Archana Gaikwad, Ashwini Nangude, Ayesha Momin, Balaji S., Beena Thomas, Bency Joseph, Bharath TK, Brindha B, Chhaya Valvi, Chandrasekaran Padmapriyadarsini, Deepak Pole, Deepanjali Biradar, Devanathan A., Devarajulu Reddy, Devi Sangamithrai M., Dhanaji Jagdale, Dileep Kadam, Divyashri Jain, Dolla C. K., S. Elilarasi, Gabriela Smit, Gangadarsharma R., Geetha Ramachandran, Hanumant Chaugule, Hari Koli, Hemanth Kumar, Jeeva J, Jessica Elf, Jonathan Golub, Jyoti Chandane, Kannan M., Kannan Thiruvengadam, Karthikesh M., Karunakaran S., Kelly Dooley, Lakshmi Murali, Lavanya M., Luke Hanna, Madasamy S., Madeshwaran A., Madhusudan Barthwal, Mageshkumar M., Mangaiyarkarasi S., Mahesh Gujare, Mandar Paradkar, Manoharan S., Michel Premkumar M., Mrunalini Kamble, Munivardhan P., Murugesan S., Gomathy N. S., Neeta Pradhan, Nikhil Gupte, Nishi Suryavanshi, Ponnuraja C., Poonam Patil, Prasad Deshpande, Prasanna Sahoo, Pratik Awale, Premkumar N., Rahul Lokhande, Rajkumar S., Ranganathan K., Rani S., Rani V., Renu Bharadwaj, Renu Madewar, Rengaraj R., Rewa Kohli, Robert Bollinger, Rosemarie Warlick, Rupak Shivakoti, Sachin Atre, Sahadev Javanjal, Samir Shaikh, Samyra Cox, Sandhya Khadse, Sanjay Gaikwad, Sathyamurthi P., Savita Kanade, Shalini Pawar, Shashank Hande, Shital Muley, Shital Sali, Shri Vijay Bala Yogendra Shivakumar, Shrinivasa B. M., Shyam Biswal, Silambu Chelvi K., Smita Nimkar, Sona Deshmukh, Soumya Swaminathan, Sriram Selvaraju, Subapriya K., Sunanda Kamble, Sushant Meshram, Surendhar S., Swapnil Raska, Swapnali Lakare, Syed Hissar, Uma Devi, Vandana Kulkarni, Vidula Hulyalkar, Vidya Mave, Vinod Taywade, Vrinda Bansode, Yogesh Daware, Zaheda Khan.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ftubr.2024.1433975/full#supplementary-material
References
1. World Health Organization. Global Tuberculosis Report 2023. Geneva: World Health Organization (2023).
3. Lambert ML, Hasker E, Van Deun A, Roberfroid D, Boelaert M, Van der Stuyft P. Recurrence in tuberculosis: relapse or reinfection? Lancet Infect Dis. (2003) 3:282–7. doi: 10.1016/S1473-3099(03)00607-8
4. McIvor A, Koornhof H, Kana BD. Relapse, re-infection and mixed infections in tuberculosis disease. Pathog Dis. (2017) 75:ftx020. doi: 10.1093/femspd/ftx020
5. Wang J-Y, Lee L-N, Lai H-C, Hsu H-L, Liaw Y-S, Hsueh P-R, et al. Prediction of the tuberculosis reinfection proportion from the local incidence. J Infect Dis. (2007) 196:281–8. doi: 10.1086/518898
6. Uys P, Brand H, Warren R, van der Spuy G, Hoal EG, van Helden PD. The risk of tuberculosis reinfection soon after cure of a first disease episode is extremely high in a hyperendemic community. PLoS ONE. (2015) 10:e0144487. doi: 10.1371/journal.pone.0144487
7. Cudahy PGT, Wilson D, Cohen T. Risk factors for recurrent tuberculosis after successful treatment in a high burden setting: a cohort study. BMC Infect Dis. (2020) 20:789. doi: 10.1186/s12879-020-05515-4
8. Moosazadeh M, Bahrampour A, Nasehi M, Khanjani N. The incidence of recurrence of tuberculosis and its related factors in smear-positive pulmonary tuberculosis patients in Iran: a retrospective cohort study. Lung India. (2015) 32:557–60. doi: 10.4103/0970-2113.168113
9. Unis G, Ribeiro AW, Esteves LS, Spies FS, Picon PD, Costa ERD, et al. Tuberculosis recurrence in a high incidence setting for HIV and tuberculosis in Brazil. BMC Infect Dis. (2014) 14:548. doi: 10.1186/s12879-014-0548-6
10. Thomas BE, Thiruvengadam K, S R, Kadam D, Ovung S, Sivakumar S, et al. Smoking, alcohol use disorder and tuberculosis treatment outcomes: a dual co-morbidity burden that cannot be ignored. PLoS ONE. (2019) 14:e0220507. doi: 10.1371/journal.pone.0220507
11. Jo KW, Yoo JW, Hong Y, Lee JS, Lee SD, Kim WS, et al. Risk factors for 1-year relapse of pulmonary tuberculosis treated with a 6-month daily regimen. Respir Med. (2014) 108:654–9. doi: 10.1016/j.rmed.2014.01.010
12. Panjabi R, Comstock GW, Golub JE. Recurrent tuberculosis and its risk factors: adequately treated patients are still at high risk. Int J Tuberc Lung Dis. (2007) 11:828–37.
13. Bhargava A, Bhargava M, Meher A, Benedetti A, Velayutham B, Teja SG. Nutritional supplementation to prevent tuberculosis incidence in household contacts of patients with pulmonary tuberculosis in India (RATIONS): a field-based, open-label, cluster-randomised, controlled trial. Lancet. (2023) 402:627–40. doi: 10.1016/S0140-6736(23)01231-X
14. Gupte A, Padmapriyadarsini C, Mave V, Kadam D, Suryavanshi N, Shivakumar SVBY, et al. Cohort for Tuberculosis Research by the Indo-US Medical Partnership (CTRIUMPH): protocol for a multicentric prospective observational study. BMJ Open. (2016) 6:e010542. doi: 10.1136/bmjopen-2015-010542
15. Kornfeld H, West K, Kane K, Kumpatla S, Zacharias RR, Martinez-Balzano C, et al. High prevalence and heterogeneity of diabetes in patients with TB in South India: a report from the Effects of Diabetes on Tuberculosis Severity (EDOTS) Study. Chest. (2016) 149:1501–8. doi: 10.1016/j.chest.2016.02.675
16. Mave V, Meshram S, Lokhande R, Kadam D, Dharmshale S, Bharadwaj R, et al. Prevalence of dysglycemia and clinical presentation of pulmonary tuberculosis in Western India. Int J Tuberc Lung Dis. (2017) 21:1280–7. doi: 10.5588/ijtld.17.0474
18. Ralph AP, Ardian M, Wiguna A, Maguire GP, Becker NG, Drogumuller G, et al. A simple, valid, numerical score for grading chest x-ray severity in adult smear-positive pulmonary tuberculosis. Thorax. (2010) 65:863–9. doi: 10.1136/thx.2010.136242
19. Peetluk LS, Ridolfi FM, Rebeiro PF, Liu D, Rolla VC, Sterling TR. Systematic review of prediction models for pulmonary tuberculosis treatment outcomes in adults. BMJ Open. (2021) 11:e044687. doi: 10.1136/bmjopen-2020-044687
20. Neyrolles O, Quintana-Murci L. Sexual inequality in tuberculosis. PLoS Med. (2009) 6:e1000199. doi: 10.1371/journal.pmed.1000199
21. Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med. (2008) 3:17. doi: 10.1186/1751-0473-3-17
22. Nhamoyebonde S, Leslie A. Biological differences between the sexes and susceptibility to tuberculosis. J Infect Dis. (2014) 209(Suppl. 3):S100–6. doi: 10.1093/infdis/jiu147
23. Hamilton CD, Stout JE, Goodman PC, Mosher A, Menzies R, Schluger NW, et al. The value of end-of-treatment chest radiograph in predicting pulmonary tuberculosis relapse. Int J Tuberc Lung Dis. (2008) 12:1059–64.
24. Lonnroth K, Williams BG, Cegielski P, Dye C. A consistent log-linear relationship between tuberculosis incidence and body mass index. Int J Epidemiol. (2010) 39:149–55. doi: 10.1093/ije/dyp308
25. Deurenberg-Yap M, Schmidt G, van Staveren WA, Deurenberg P. The paradox of low body mass index and high body fat percentage among Chinese, Malays and Indians in Singapore. Int J Obes Relat Metab Disord. (2000) 24:1011–7. doi: 10.1038/sj.ijo.0801353
26. Banerji MA, Faridi N, Atluri R, Chaiken RL, Lebovitz HE. Body composition, visceral fat, leptin, and insulin resistance in Asian Indian men. J Clin Endocrinol Metab. (1999) 84:137–44. doi: 10.1210/jc.84.1.137
27. Dudeja V, Misra A, Pandey RM, Devina G, Kumar G, Vikram NK, et al. does not accurately predict overweight in Asian Indians in northern India. Br J Nutr. (2001) 86:105–12. doi: 10.1079/BJN2001382
28. Misra A. Ethnic-specific criteria for classification of body mass index: a perspective for Asian Indians and American Diabetes Association Position Statement. Diabetes Technol Ther. (2015) 17:667–71. doi: 10.1089/dia.2015.0007
Keywords: tuberculosis, recurrence, risk factors, adverse outcome, epidemiology (EPI)
Citation: Krishnan S, Gupte N, Paradkar M, Gupte A, Naik M, Raskar S, Suryavanshi N, Pradhan N, Gaikwad S, Karyakarte R, Lokhande R, Hanna Luke E, Thiruvengadam K, Padmapriyadarsini C, Sahasrabudhe T, Barthwal M, Kakrani A, Viswanathan V, Kornfeld H, Gupta A, Golub JE and Mave V (2024) Clinical and laboratory risk factors for pulmonary tuberculosis recurrence in three pooled Indian cohorts. Front. Tuberc. 2:1433975. doi: 10.3389/ftubr.2024.1433975
Received: 16 May 2024; Accepted: 27 June 2024;
Published: 12 July 2024.
Edited by:
Fraser Wares, KNCV TB Foundation, NetherlandsReviewed by:
Vineet Bhatia, World Health Organization, IndiaChristina G. Rivera, Mayo Clinic, United States
Copyright © 2024 Krishnan, Gupte, Paradkar, Gupte, Naik, Raskar, Suryavanshi, Pradhan, Gaikwad, Karyakarte, Lokhande, Hanna Luke, Thiruvengadam, Padmapriyadarsini, Sahasrabudhe, Barthwal, Kakrani, Viswanathan, Kornfeld, Gupta, Golub and Mave. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sonya Krishnan, c2tyaXNoMjVAamhtaS5lZHU=
†These authors have contributed equally to this work
 Sonya Krishnan
Sonya Krishnan Nikhil Gupte2,3†
Nikhil Gupte2,3† Mandar Paradkar
Mandar Paradkar Akshay Gupte
Akshay Gupte Mrunmayi Naik
Mrunmayi Naik Swapnil Raskar
Swapnil Raskar Rajesh Karyakarte
Rajesh Karyakarte Elizabeth Hanna Luke
Elizabeth Hanna Luke Chandrasekaran Padmapriyadarsini
Chandrasekaran Padmapriyadarsini ArjunLal Kakrani
ArjunLal Kakrani Vijay Viswanathan
Vijay Viswanathan Hardy Kornfeld
Hardy Kornfeld Amita Gupta
Amita Gupta Jonathan E. Golub
Jonathan E. Golub Vidya Mave
Vidya Mave