- 1Guizhou Medical University, Guiyang, Guizhou, China
- 2Department of Urology, Guizhou Medical University Affiliated Hospital, Guiyang, Guizhou, China
Extracellular vesicles have been a hot research topic in recent years, and the diagnostic and therapeutic value of LprG and LAM, two key markers present in extracellular vesicles secreted by Mycobacterium tuberculosis or Mycobacterium tuberculosis-infected immune cells, in tuberculosis, has been widely emphasized in recent years. Genitourinary tuberculosis is a common form of extrapulmonary tuberculosis, and renal tuberculosis accounts for more than 20% of patients with Genitourinary tuberculosis. In this paper, we summarize the findings and research ideas of LprG and LAM in the diagnosis and treatment of renal tuberculosis in recent years and conclude that LprG and LAM have their unique diagnostic value in the intrapulmonary and extrapulmonary fields and can be used as a new potential idea for vaccine or immunotherapy in the future for research.
1 Research background and significance
In 2022, tuberculosis (TB) remained the second leading cause of death from a single infectious pathogen following COVID-19. According to the World Health Organization's statistics for 2022, ~1.3 million people worldwide died from tuberculosis, with China accounting for more than 7% of the global case burden (1). The high transmissibility and the prolonged treatment duration of tuberculosis result in higher costs for patients, leading to significant financial burdens for many families. Renal tuberculosis, as one of the common forms of extra pulmonary tuberculosis, is often overlooked by patients due to the difficulty in early diagnosis and atypical clinical manifestations. This results in renal tuberculosis patients seeking medical care at a later stage, making treatment more challenging and leading to poorer patient outcomes (2). With the increasing number of renal tuberculosis cases among children, the elderly, pregnant women, and individuals infected with HIV, the development of a strategy for early diagnosis and screening, as well as precise treatment for renal tuberculosis, has become increasingly important.
Renal tuberculosis is primarily a secondary condition resulting from the hematogenous dissemination of Mycobacterium tuberculosis from pulmonary tuberculosis lesions, with only a small proportion of cases being primary renal tuberculosis due to compromised immunity for various reasons. Urine acid-fast bacilli smear microscopy is a commonly used clinical method for detecting renal tuberculosis, similar to the World Health Organization (WHO)- recommended GeneXpert MTB/RIF test. Both methods have relatively poor sensitivity, with the former achieving a sensitivity of only 40% and the latter a positive detection rate of around 60% at best. Histopathological biopsy and serum or urine Mycobacterium tuberculosis culture remains the gold standard for diagnosing renal tuberculosis. However, the former is invasive, and the latter requires a long waiting time and is challenging to collect samples, making them not the preferred method for clinical diagnosis (3). With the advancement of imaging technology, a series of techniques, including cystoscopy, urethrography, B-ultrasound, CT scan, and MRI scan, have improved the detection rate of renal tuberculosis. However, imaging examinations cannot serve as the gold standard for diagnosing renal tuberculosis but rather as an indirect diagnostic method or for exclusionary purposes (4).
In recent years, technologies such as quantitative polymerase chain reaction (qPCR) gene detection have been used to analyze and assess the occurrence, development, and progression of tuberculosis (5). However, due to the complexity of tuberculosis in primary clinical settings, the diagnostic efficacy remains unsatisfactory. Therefore, the search for more specific DNA, RNA, or protein biomarkers of Mycobacterium tuberculosis (Mtb) has become a hot topic in recent research, and extracellular vesicles have naturally come into focus (6).
Extracellular Vesicles (EVs) are a class of tiny vesicles released by living cells, enclosed by a lipid bilayer that contains their contents and is unable to self-replicate. Depending on their size, they can be classified as ectosomes or exosomes. The contents they carry vary depending on the cell's life cycle and biological state (7), including proteins, nucleic acids, lipids, sugars, and other substances. They act independently or synergistically to mediate various biological functions in the body, such as cell-to-cell signaling, cell cycle regulation and differentiation, immune modulation, tissue repair, and cellular waste elimination (8).
In recent studies, it has been found that human infection with Mycobacterium tuberculosis (Mtb) results in the presence of EVs in the blood and secretions that carry Mtb fragments, specific proteins, and other substances, and there is hope that the contents within them can be used as biomarkers of tuberculosis or targets for drug guidance (9). As a result, the role of EVs in the diagnosis and treatment of tuberculosis, along with their associated clinical significance, is increasingly being accepted and recognized by the public. Research on using EVs as a new diagnostic method and treatment strategy for renal tuberculosis is receiving growing attention from the academic community.
The contents of EVs are highly complex, and extracting valuable biomarkers for the diagnosis and treatment of renal tuberculosis from them has been a significant challenge in recent research. Fortunately, studies have found that Lipoarabinomannan (LAM) and Lipoprotein G (LprG) are believed to have great potential. However, these two biomarkers differ in their biological functions and spatial structures, leading to differences in their roles and research value.
In this review, we will discuss recent literature on the changes in EVs secreted by hosts after Mycobacterium tuberculosis infection and summarize the potential and value of lipoarabinomannan (LAM) and lipoprotein (LprG) as new diagnostic markers and therapeutic targets for renal tuberculosis, hoping to provide new insights and references for future researchers in this field.
2 Lipoarabinomannan and Lipoprotein G in the bacterial extracellular vesicles of Mycobacterium tuberculosis
With the advancement of research on electric vehicles (EVs) in recent years, bacterial extracellular vesicles (bEVs) have garnered widespread attention from researchers. Depending on the bacterial species and the varying compositions of proteins, lipids, DNA, and RNA within bEVs, these bEVs often exhibit distinct biological activities. Zhao et al. summarized the characteristics of bEVs produced by known pathogens, including their ability to promote infection, protect the host, alter antibiotic or phage effects, regulate host immune function, and modulate cell proliferation (10, 11). In recent studies, EVs secreted by Mycobacterium tuberculosis have also been found to possess some of the characteristics above. Emilie et al.'s research on the components of bEVs secreted by Mycobacterium tuberculosis revealed that Mtb envelope lipoproteins, lipoglycans, and lipids exist in specific bEVs and can better reflect the characteristics of Mtb (12). Carolina et al.'s further research on bEVs secreted by M. tuberculosis (Mtb) found that the substances enclosed in these bEVs can regulate CD4+ T cells, which are then transferred to T cells to inhibit T cell responses, thereby allowing Mtb to evade the host's immune response. Moreover, LAM and LprG, substances present in these bEVs, are highly representative (13). Therefore, we speculate that M. tuberculosis bEVs carrying LAM and LprG may possess unique value.
2.1 Structure and spatial relationship of LAM and LprG
2.1.1 LprG
LprG is a relatively conserved lipoprotein found in all mycobacteria and is an essential component for maintaining the virulence of Mtb. In conditions of limited nutrients, the loss of LprG function results in delayed maturation of Mtb. In the study by Fukushi et al., it was demonstrated that LprG suppresses inflammation in macrophages by downregulating nitric oxide (NO), cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS), and pro-inflammatory cytokines through the nuclear factor κB (NF-κB), activator protein-1 (AP-1), and mitogen-activated protein kinase (MAPK) signaling pathways (14). The research design by Mariana et al. demonstrated at the genetic level that the presence or absence of LprG-related expression genes can influence the survival cycle of M. Tuberculosis in host cells (15). Therefore, the role of LprG in bEVs may be related to M. tuberculosis, as it reduces the host immune response and inflammatory reaction, thereby creating a more favorable cellular microenvironment. Additionally, LprG exhibits good stability even under certain extreme conditions, with minimal degradation by bacterial cells or reduction in its translation level (16, 17), suggesting its potential as a promising diagnostic marker for tuberculosis.
2.1.2 LAM
LAM is a type of arabinose carbohydrate found on the extracellular vesicle membranes of Mtb, accounting for about 15% of the weight of Mtb (18). It has a core of D-arabinan, an enantiomer containing L-arabinose (19), which is anchored to the cell membrane by phosphatidylinositol (PI). Due to its ability to be separated together with lipoproteins and its good surface exposure characteristics, LAM is also one of the essential pathotoxicological molecules of Mtb (20, 21). Mtb can alter the activity of LAM through various modifications (including acylation, phosphorylation, and nitrogen modification), enhancing or reducing its infectivity and ability to maintain cellular stability (22). Among these modifications, succinylation is relatively common, and related studies have shown that this feature is associated with Mtb's involvement in inflammation (22).
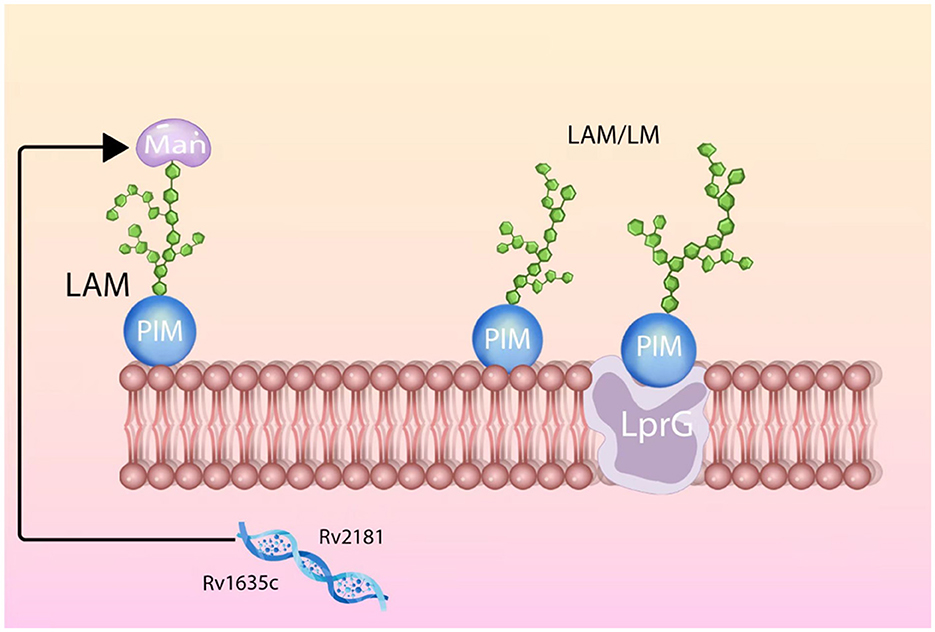
Additionally, due to the presence of 5-methylthio-D-xylose (MTX) sugar on LAM, as well as a D-mannose (Man) cap structure encoded and co-regulated by bacterial genes such as Rv1635c and Rv2181, LAM exhibits specific antioxidant properties (23–25). Meanwhile, the chemical composition of LAM varies among different Mtb strains. The fact that LAM is found in the serum of tuberculosis patients suggests that it may affect multiple host cell populations, thereby influencing a range of biological processes of Mtb within the host (216). Sparks et al. designed an experiment using gene knockout. They summarized previous experimental results, demonstrating that LAM has a certain degree of value in the growth of M. tuberculosis and in maintaining bacterial envelope integrity. They hypothesized that LAM could regulate the activity of septal hydrolase. Still, when they knocked down the septal hydrolase gene RipA, it did not alleviate the cell shape defects of LAM-deficient mycobacteria, thus negating this hypothesis (26). The presence of LAM on the surface of M. Tuberculosis allows it to mimic specific glycoproteins on the surface of human or other mammalian cells. Moreover, these mannose family substances on the cell wall of Mtb, including LAM, may also play a role in mitigating the host immune response. Therefore, LAM has a certain degree of value in the infection of host cells by Mtb and the maintenance of the stability of Mtb cell structure. Additionally, due to its location on the cell wall, it is more conducive to recognition by the human immune system and the elicitation of a series of immune responses, as well as to secretion outside the cell when included in bEVs.
Research has found that LAM and LprG have a very close spatial relationship. The hydrophobic pocket on LprG will interact with various types of LAM through acyl chains, and the mannose chains within LAM will also actively select to bind with LprG. This mutual selection binding mode not only stabilizes LAM on the cell membrane of Mtb but also facilitates the biological functions of LAM (27). Since LAM and LprG often co-occur, using LprG to localize LAM or identifying alternative methods to target LAM for diagnostic and therapeutic purposes has become a topic of interest. Based on the aforementioned related research, we have created a schematic diagram illustrating the spatial structural relationship between LAM and LprG, aiming to provide a visual explanation of this relationship (Figure 1).
Subsequently, a research team has publicly shared some vesicle proteins in the EVs secreted by Mtb through proteomic analysis (28) and summarized the currently identified protein composition in mycobacterial extracellular vesicles (29). It is foreseeable that more substances in the bEVs of Mtb will be discovered in the future. In recent years, several substances beyond LAM and LprG have been further confirmed to be related to the biological functions of Mtb in the host, including Cfp10, Cfp2, Mpt32, Mpt64, BfrB, LPqH, and LprA (13, 30–32). However, due to the properties of LAM and LprG, such as their resistance to mutation, good stability, and close spatial structure relationship, they hold greater significance in the diagnosis and treatment of tuberculosis.
2.2 LAM's immune response and cell signaling pathways
During the process of Mtb infection, LAM can regulate the host's immune response and infection progression through various cellular pathways. LAM primarily functions as an agonist of Toll-like receptor 2 (TLR2), directly or indirectly exerting its biological functions. It induces host cells to mount an immune response and produce inflammation that is favorable for the growth and reproduction of Mycobacterium tuberculosis. Through extensive summarization, we speculate that LAM plays a unique role throughout the entire process of Mycobacterium tuberculosis infection. The following are the main cellular pathways in which LAM is directly or indirectly involved, along with their respective roles.
2.2.1 Inflammatory activation and regulation of immune response
2.2.1.1 TLR2/MyD88/NF-κB pathway
The TLR2/MyD88/NF-κB pathway is a key inflammatory signaling pathway in the innate immune system, primarily involved in recognizing pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), thereby triggering inflammatory responses and regulating the immune system (33–35). Its core functions include activation of inflammatory responses, immune escape, and immune damage. The pathway is primarily initiated by TLR2 through the recognition of exogenous signals, including bacterial lipoproteins and certain fungal components (such as Aspergillus β-glucan), as well as endogenous signals (such as HMGB1), to initiate signal transduction. After activation by ligand binding, the TIR domain of TLR2 undergoes a conformational change, recruiting Myeloid Differentiation Factor 88 (MyD88) to form a complex, thereby initiating a series of downstream molecular effects (36–40).
As early as 20 years ago, Richard et al. demonstrated through relevant research that LAM is an agonist of TLR2 and can exert its biological effects by activating the TLR2/NF-κB pathway (41). In recent years, with the advancement of related research, we have learned that LAM can activate the Toll-like receptor 2 of cells, thereby activating the downstream MyD88-dependent pathway, triggering the nuclear translocation of NF-κB, and inducing the expression of immune response gene 1 (Irg1) to promote the release of cellular inflammatory mediators, such as interleukin-2 (IL-2), interleukin-4 (IL-4), interleukin-6 (IL-6), interleukin-10 (IL-10), interleukin-17A (IL-17A), tumor necrosis factor α (TNF-α), and granulocyte-macrophage colony-stimulating factor (GM-CSF), etc (42–45). LprG binds to TLR2 in the absence of TLR1, together with LAM and its precursors, but does not bind to TLR1 in the absence of TLR2 (218). At the same time, Mycobacterium tuberculosis can further modify LAM [e.g. by regulating protein O-mannosylation levels (220)] to inhibit the overactivation of TLR2 and further suppress the activation of related immune cells, such as T cells, thereby exerting its immune regulatory mechanisms to escape immune clearance (12, 46).
Furthermore, LAM is also released from Mycobacterium tuberculosis and can be detected in the intracellular or extracellular vesicles of infected cells. Therefore, the immunomodulatory properties of LAM are not limited to intact Mycobacterium tuberculosis but also apply to circulating LAM released in extracellular vesicles or apoptotic vesicles. This is the primary mechanism by which LAM exerts its immunomodulatory properties on infected or other cells (47).
The TLR2/MyD88/NF-κB pathway is a key link between innate immunity and inflammatory pathology. The type of ligand mainly influences the biological effects of its activation, but it primarily promotes inflammation and indirectly promotes fibrosis. Targeted therapy for key nodes in this pathway may provide new ideas and directions for the treatment of renal tuberculosis.
2.2.1.2 Mannose receptor (MR) pathway
The mechanism of the mannose receptor (MR) pathway in renal tuberculosis involves complex interactions in pathogen recognition, immune regulation, and host defense. However, the primary biological function of the MR pathway is to utilize a class of type II transmembrane glycoproteins primarily expressed on the surface of immune cells such as macrophages and dendritic cells. These glycoproteins recognize glycosylated structures (such as mannose) on the surface of pathogens through their extracellular domains, mediating phagocytosis and promoting the formation of phagosomes (48–52).
MR is an efficient endocytic receptor, most notably characterized by multiple C-type lectin domains (CTLDs) within a single peptide chain. Specifically, the CTLD4 domain can recognize specific components, thereby mediating cellular immune regulation (213).
LAM on Mtb and its EVs can bind to mannose receptors (such as CD206) on the surface of macrophages through its mannose structure, thereby facilitating the phagocytosis of Mtb by macrophages and its entry into the cell (53, 54). However, due to the unique immunomodulatory and immune evasion functions of various components, including LAM, on the Mtb membrane, MR-mediated phagocytosis can accelerate the infection of host cells by Mtb. These immunomodulatory and immune evasion functions include inhibiting host DNA repair, reducing the activation of reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and phagosome acidification, blocking the maturation of macrophage lysosomes, and aiding the survival of Mtb within phagosomes; inhibiting the expression of major histocompatibility complex (MHC) class I and II molecules, thereby reducing antigen presentation; binding to TLR2 to modulate the release of inflammatory cytokines and enhance Th2-type immune responses (55, 56). According to relevant studies, activation of the MR pathway can affect T cell activation response and create conditions for Mycobacterium tuberculosis survival in host cells by regulating TLR4 signal transduction. However, the interaction mechanism between MR and TLR4 remains unclear (212).
This leads to MR-mediated immune phagocytic function, which will facilitate the settlement and reproduction of Mtb within host cells. Therefore, using drugs or other means to inhibit the binding of MR and LAM or promote the secretion of other immune factors to enhance the antigen-presenting ability, immune response effect, and phagosome function of host cells can be used as an alternative adjuvant therapy for renal tuberculosis.
2.2.1.3 Mitogen-activated protein kinase (MAPK) pathway
MAPK pathway is a crucial intracellular signaling pathway. Although the composition of this pathway is complex, it is typically composed of several key cellular kinases or factors that form the central part of the pathway. These include extracellular signal-regulated kinases (ERK), c-Jun N-terminal kinases (JNK), and p38 mitogen-activated protein kinases (p38 MAPK). These members play critical roles in cellular signal transduction within the pathway, thereby regulating various physiological processes (57–60). Among these, ERK-mediated reactions are primarily involved in the transmission of signals for cell growth and proliferation, while JNK-mediated responses play essential roles in regulating apoptosis. p38 MAPK is typically associated with the expression of inflammatory factors and immune regulation (61–67).
Studies have shown that ERK1/2 is a conserved serine/threonine kinase, and its direct or indirect interactions with the PI3K/Akt, β-catenin, and Janus Kinase/Signal Transducer and Activator of Transcription (Jak/Stat) signaling pathways collectively mediate cell growth, regeneration, and proliferation. The activation of p38 MAPK can mediate the activation of NF-κB, thereby promoting the transcription and expression of inflammatory factors such as IL-6 and TNF-α, which in turn induces the organism's immune inflammatory response (68–70). Additionally, the JNK/MAPK pathway is closely linked to the apoptosis and autophagy processes of macrophages infected with Mycobacterium tuberculosis, and the activation of this pathway directly affects the host cell's immune defense capability against Mycobacterium tuberculosis (71).
LAM, a typical virulence factor of Mycobacterium tuberculosis, can mediate the MAPK pathway and induce the expression of IL-10 and IL-12 through the differential activation of p38 MAPK and ERK1/2, thereby synergistically regulating the inflammatory microenvironment of host cells in conjunction with TLR2 (44, 72). At the same time, although there have been no relevant studies in the past 5 years, based on pertinent previous studies, we speculate that LAM may mediate the phosphorylation of p47phox through the MAPK pathway and its related pathway crosstalk, thereby interfering with the activity of the NADPH oxidase complex and iNOS, reducing the production of reactive oxygen species (ROS) and nitric oxide (NO) (73–76). Although the effect of LAM on p47phond and whether it can affect p47phond through the MAPK pathway requires further research, the above studies still indicate that LAM can further reduce the bactericidal ability of macrophages and promote the survival of Mycobacterium tuberculosis by mediating the MAPK pathway.
2.2.1.4 JAK/STAT3 pathway
The JAK/STAT3 pathway is a classic example of cellular signal transduction. Current research suggests that the activation of this pathway is linked to significant biochemical processes, including inflammatory responses, lipid metabolism, cell autophagy, and the progression of fibrosis (77–81).
Existing research suggests that LAM can promote the secretion of IL-10 through the JAK/STAT3 pathway while simultaneously inhibiting the production of IL-12, resulting in cytokine polarization, a weakened Th1 immune response, and an enhanced Th2 immune response, thereby affecting the Th1/Th2 balance (82, 83). Since the Th1 immune response is a crucial component of the anti-tuberculosis immune response, inhibiting this biological effect will lead to an imbalance in the host's immune response during chronic tuberculosis infection.
On the other hand, after LAM activates the signal transducer and activator of transcription (STAT3), it induces a suppressor of cytokine signaling 3 (SOCS3), thereby altering the activity and function of macrophages (84). Combined with its differential effects on IL-10 and IL-12, this further influences the formation or function of various cytokines and chemokines in macrophages (85), regulating the balance between the host's pro-inflammatory and anti-inflammatory responses.
2.2.1.5 Mannose-binding lectin (MBL) pathway
The MBL pathway is a complement activation pathway that is triggered when foreign pathogens attack the body. It is typically activated by mannose and its analogs carried by pathogens, which bind to lectin receptors (such as the C-type lectin receptor Dectin-2) to activate complement pattern recognition molecules (PRMs), thereby activating this pathway (86–88).
Under conditions of Mtb infection, LAM can activate the complement system through the MBL pathway, making Mycobacterium tuberculosis highly susceptible to complement-mediated attack (89, 90). However, the Man cap and other structures on LAM can inhibit the formation of the complement membrane attack complex (MAC), and the sensitivity of LAM from different strains to receptors and the recognition of LAM by receptors vary, allowing Mycobacterium tuberculosis to evade complement-mediated lysis to a certain extent (91, 92).
The activation of the MBL pathway mediated by LAM can, to some extent, play a role in resisting Mtb's infection. Still, due to differences in receptor recognition, the MBL pathway often exhibits a certain activation lag, and LAM can regulate the inflammatory response through other pathways, thereby interfering with the progression of the immune response. Therefore, the complement immune function of this pathway may not be able to resist the invasion of Mtb fully but instead may induce the diseased tissue to enter a state of chronic infection.
2.2.2 Regulation of apoptosis and autophagy
2.2.2.1 Inhibition of apoptosis—PI3K/Akt pathway
Previous studies have shown that the virulence factor LAM of Mycobacterium tuberculosis can inhibit T cell migration to lymphoid tissues by interfering with the PI3K/AKT pathway, thereby reducing the initiation of Th1 effector responses in diseased tissues, leading to chronic infection (93). It can also interfere with calmodulin-dependent phosphatidylinositol 3-phosphate (PI3P) production, preventing phagosome maturation and thus ensuring the survival of Mycobacterium tuberculosis within the phagosome (94). These findings suggest that LAM can modulate the inflammatory response and enhance bacterial survival through the PI3K/Akt pathway.
As research teams explore the PI3K/Akt pathway, it has been found that LAM can not only induce infection and prevent phagosome maturation through the PI3K/Akt pathway but also regulate macrophage apoptosis infected with Mycobacterium tuberculosis and promote macrophage survival by promoting the phosphorylation of Bad, a pro-apoptotic protein in the B-cell lymphoma-2 (Bcl-2) family; regulating the activation of Caspase-3; and increasing the expression of the anti-apoptotic protein B-cell large lymphoma protein (Bcl-xL) (95–97).
This allows Mycobacterium tuberculosis to survive for a prolonged period in macrophages, providing a relatively safe breeding ground for the bacterium. However, in recent years, there have been few further studies on the regulation of the PI3K/Akt pathway by LAM, and additional research is still needed to explore whether the path mediated by LAM can affect macrophage apoptosis by modulating other cytokines and influence host apoptosis by interacting with other cellular pathways.
2.2.2.2 Inhibition of autophagy—mammalian target of rapamycin (mTOR) pathway
Recent studies have shown that LAM can inhibit the activation of the mTOR pathway and interfere with the downstream TCR-CD3, CD28, and interleukin-2R (IL-2R) signal transduction, thereby affecting the activation, proliferation, and interleukin-2 (IL-2) gene transcription and translation of T cells (especially CD4+ T cells), and further affecting the activation of T cells (98, 99).
At the same time, LAM can also inhibit the maturation of phagosomes in various ways by mediating the mTOR pathway. Although no relevant research reports have been seen in the past 5 years, we have summarized the appropriate research on mTOR and speculate that it may affect the function, growth, and morphology of phagosomes by inhibiting the downstream ULK1-Beclin1 molecules, thereby inhibiting autophagy (100–102). Further experiments are needed to elucidate the mechanisms by which LAM affects the mTOR pathway and other effects on phagosome formation, including how to target phagosome formation, how to interfere with phagosome function, and how to regulate downstream molecules.
LAM can modulate the autophagy process in all host cells, including macrophages, by interfering with the mTOR pathway and affecting the function of phagosomes, as well as the activation of T cells, thereby enabling Mtb to survive long-term within host cells.
2.2.3 Angiogenesis and fibrosis
2.2.3.1 Vascular formation and hyperplasia—vascular endothelial growth factor (VEGF)
VEGF is a cytokine that regulates vascular endothelial proliferation and is co-regulated or regulated by various upstream substances. During the prolonged course of Mtb infection, M. tuberculosis induces the aggregation of cytokines related to vascular proliferation and differentiation (such as IL-6, VEGF, and TNF-α) at the lesion site, thereby participating in the formation of granulomas, which are crucial in tuberculosis lesions, by promoting angiogenesis.
Current research suggests that LAM may increase vascular permeability through the TLR2/VEGF pathway, and the degree of vascular permeability increase is positively correlated with VEGF (103) and may promote angiogenesis through the HIF-1α/VEGF pathway directly or indirectly (104), which may indicate a close relationship between LAM and granuloma formation in renal tuberculosis. At the same time, due to the increase in VEGF caused by Mycobacterium tuberculosis, the newly formed blood vessels are unable to enhance local tissue blood supply, resulting in poor wound healing after tuberculosis resection (105).
Based on this connection, current research suggests that VEGF and its related pro-angiogenic cytokines can differentiate between latent and active Mycobacterium tuberculosis infection (106–108). In the future, we can explore whether LAM can also determine this infection by further studying the correlation between LAM and VEGF.
2.2.3.2 Fibrosis—transforming growth factor-β (TGF-β)
Fibrosis is a key feature of Mycobacterium tuberculosis infection. Current research has found that the levels of transforming growth factor-β (TGF-β), IL-10, matrix metalloproteinase-1, and other substances in the blood of tuberculosis patients are elevated. These substances mediate the progression of fibrosis at the lesion site (109, 110) and contribute to the formation of chronic inflammatory granulomas within the lesion (111).
Although LAM cannot directly activate TGF-β, we believe that LAM may indirectly activate the TGF-β/Smad pathway during long-term infection, thereby contributing to the tissue fibrosis process to a certain extent (for example, by activating TLR2 and utilizing its downstream molecules to influence the function of the TGF-β/Smad pathway) (112). The activation of TGF-β may be associated with the destruction of renal parenchyma and the development of ureteral stenosis in renal tuberculosis.
Current research has shown that TGF-β can be targeted by drugs to combat Mycobacterium tuberculosis infection (113). Therefore, we have reason to believe that regulating TGF-β levels may be a promising direction for future anti-tuberculosis fibrosis treatment (114).
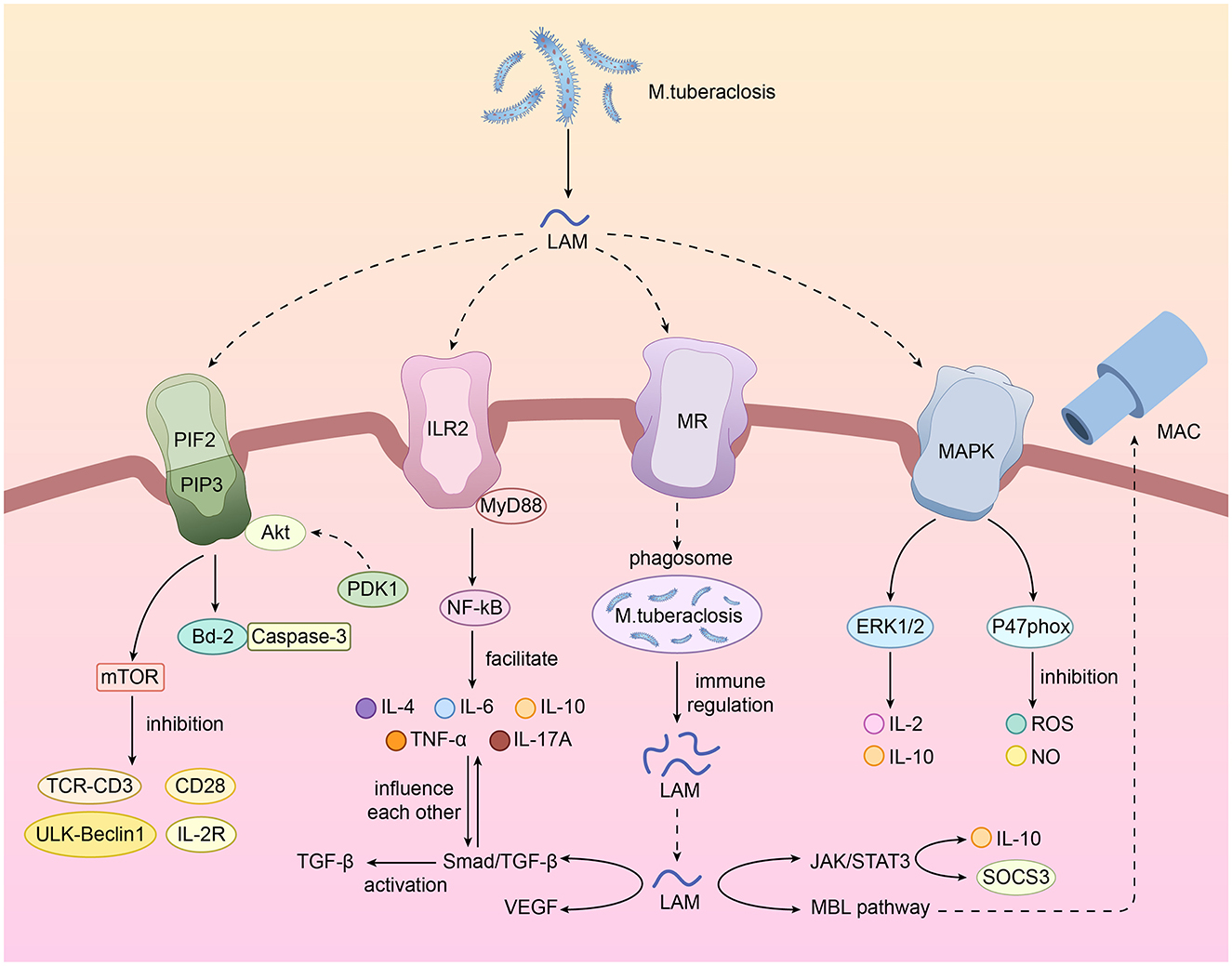
In summary, LAM plays a dual role in the cellular signaling pathways of renal tuberculosis. On the one hand, LAM can mediate immunity and enhance bacterial survival through pathways such as TLR2, the mannose receptor, the MAPK pathway, the MBL pathway, and the inhibition of apoptosis and autophagy. Secondly, LAM may cause damage to the renal structure by inducing angiogenesis (VEGF) and promoting fibrosis (TGF-β) directly or indirectly. We have compiled a summary table and drawn a relevant pathway diagram for quick reference (Table 1; Figure 2) of the cellular signaling pathways affected by LAM during the pathological process of renal tuberculosis. Further research on the cellular signaling pathways of LAM will help us better understand the entire pathophysiological process of renal tuberculosis and even urinary system tuberculosis.
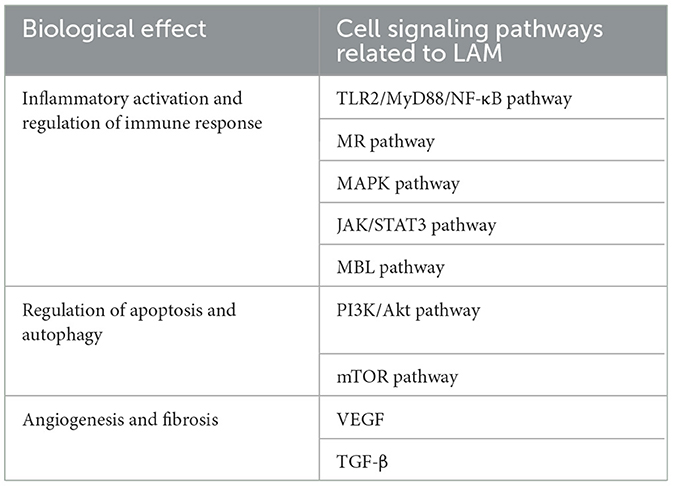
Table 1. Cell signaling pathways directly or incidentally related to LAM and their biological functions.
2.3 LprG's immune response and cell signaling pathways
During the process of Mtb's infection, LprG can influence the pathogenicity and drug resistance of Mtb by regulating cell wall permeability and inducing host immune evasion mechanisms. Our review of relevant studies has found that the relationship between LprG and LAM is more synergistic, where LprG facilitates the biological functions of LAM and further induces immunity, as well as maintains cell wall stability. The following describes the biological roles of LprG in immunity and its associated cellular signaling pathways.
2.3.1 Regulation of cell wall permeability
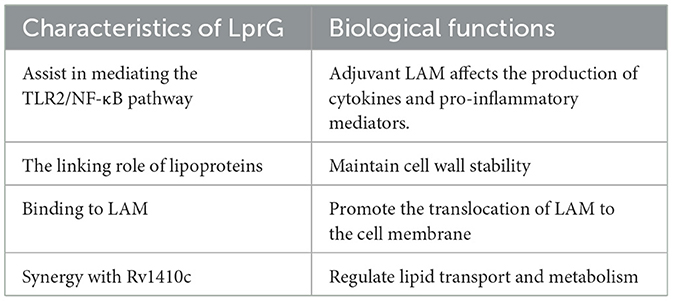
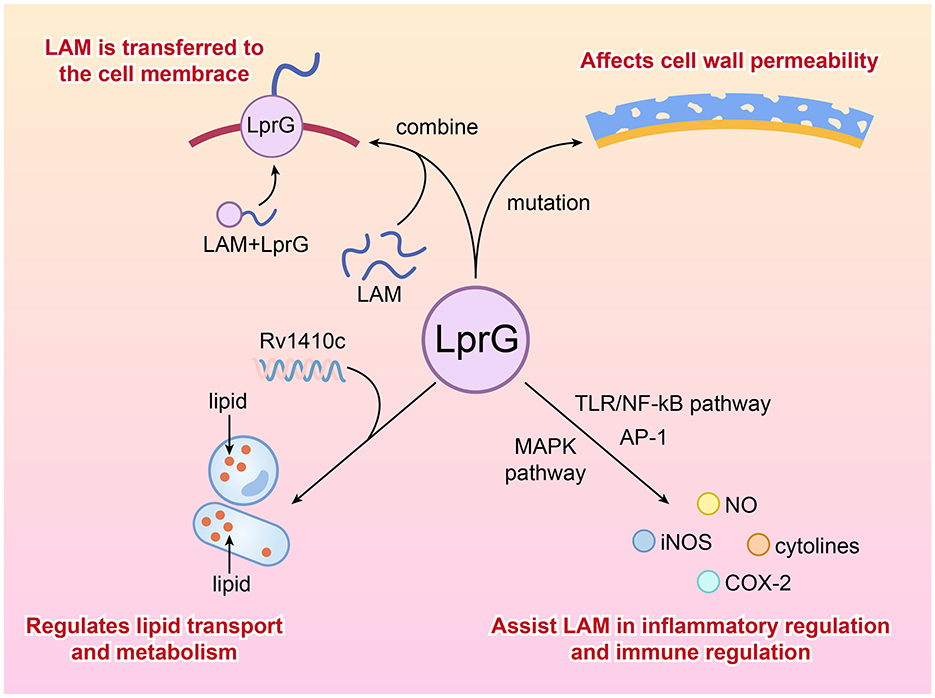
Previous studies have demonstrated that LprG is a crucial lipoprotein of Mycobacterium tuberculosis, which can bind to lipoarabinomannan (LAM) and facilitate the transport of LAM from the cytoplasm to the bacterial surface, thereby exerting its virulence functions. Additionally, LprG maintains the integrity of the cell wall through its role as a lipoprotein anchor (115).
Deletion or mutation of LprG can alter cell wall permeability, leading to changes in the sensitivity of bacteria to anti-tuberculosis drugs or anti-infective agents such as vancomycin and ethambutol (116, 117). Subsequent experiments with ethidium bromide (EtBr) permeability confirmed that the integrity of the cell membrane in LprG mutant strains was compromised, allowing drugs to enter the bacterial interior more easily (15) and making Mycobacterium tuberculosis more susceptible to killing by drugs and the host immune system.
Therefore, LprG can serve as a new target for anti-tuberculosis drugs, and its inhibitors, in combination with existing drugs, can enhance the bactericidal effect and shorten the treatment cycle (118).
2.3.2 Synergistic effects of LAM on host immune response
In the preceding text, we mentioned that LprG can influence the production of cytokines and pro-inflammatory mediators through various signaling pathways, such as the TLR2/NF-κB pathway, thereby modulating the host inflammatory response (14). The impact of LprG on immune and cellular signaling pathways primarily involves enhancing the function of LAM, facilitating its better release, and exerting its role in regulating inflammation, immune evasion, and immune homeostasis. This auxiliary and synergistic effect of LprG may promote the long-term latent infection of Mtb in the urinary system.
2.3.3 Auxiliary regulation of lipid metabolism
Lipid synthesis, accumulation, and subcellular distribution are crucial for the cellular homeostasis of Mycobacterium tuberculosis, and lipid metabolism is closely related to bacterial growth rate regulation, virulence, and antibiotic tolerance (211). LprG, together with Rv1410c from the same operon, can jointly regulate the transport and metabolism of lipids (especially triglycerides), which are essential sources for the synthesis of Mtb's cell wall components. It is important to note that LprG is anchored in the outer leaflet of the cytoplasmic membrane through three acyl chains. At the same time, Rv1410c functions to extract LprG from the membrane, allowing it to cross the cytoplasm and reach the outer membrane to transport lipids. Therefore, the absence of LprG may lead to disorders in lipid metabolism and transport, affecting the stability of the cell wall lipid layer and thereby increasing the sensitivity of bacteria to drugs (119–122). Drugs targeting the LprG-regulated lipid metabolism pathway may disrupt the cell wall structure of Mycobacterium tuberculosis, thereby enhancing the efficacy of existing drugs (123).
In summary, LprG primarily plays a role in Mycobacterium tuberculosis in assisting LAM in regulating immunity, lipid metabolism, and stabilizing the cell wall. The absence or inhibition of LprG can enhance the permeability of anti-tuberculosis drugs and improve the bactericidal effect. LprG can assist LAM in regulating the inflammatory response through the TLR2/NF-κB pathway, promoting the immune evasion of Mycobacterium tuberculosis. It also affects lipid metabolism and synergizes with LAM to enhance the pathogenicity of Mycobacterium tuberculosis. We have summarized the above content and created a summary table for reference (Table 2; Figure 3).
3 The value of LprG in the diagnosis of renal tuberculosis
In the pathophysiological process of various types of tuberculosis, the relationship between LprG and LAM is inseparable. However, in terms of diagnostic value, since LprG is not considered a major virulence factor of M. Tuberculosis, it currently primarily serves as a substance to aid in improving LAM detection. In patients with renal tuberculosis, LprG, as a relatively fixed component of M. Tuberculosis's extracellular vesicles (124), can be used to target and enrich the EVs secreted by M. Tuberculosis, thereby improving the detection rate of LAM. Meanwhile, due to its low toxicity, LprG has advantages over LAM in vaccine preparation (125, 126).
In some special populations, such as HIV patients and children, due to immune function defects or incomplete development, it is challenging to detect tuberculosis using conventional methods. In a study on the diagnostic efficacy of LprG and LAM in pediatric tuberculosis patients, LprG plays an important role in anchoring LAM to the cell membrane of Mtb and serves as a carrier protein for LAM. Therefore, using LprG for EV capture and LAM detection has the potential to become an emerging diagnostic technique for tuberculosis. In this experiment, researchers tested the levels of LprG and LAM in various samples, including urine samples closely related to the diagnosis of renal tuberculosis. The results showed that even in urine samples that had been repeatedly decomposed and filtered, both markers had higher detection rates for tuberculosis compared to the Xpert method. Moreover, since this detection method can use non-sputum samples, its detection rate for extrapulmonary tuberculosis, including renal tuberculosis, is also excellent (127).
To date, no studies have analyzed the potential of the single LprG molecule in the diagnosis of tuberculosis. Therefore, further in-depth research is still needed to investigate the role of single LprG in the diagnosis of both pulmonary and extrapulmonary tuberculosis.
4 The value of LAM in the diagnosis of renal tuberculosis and the exploration of novel LAM detection methods
4.1. New LAM detection methods and their significance in clinical practice
In a meta-analysis of HIV-infected individuals, researchers analyzed 844 records from 20 datasets and 10,202 participants, finding that Alere LAM had a higher detection rate among subjects with low CD4 counts and hospitalized patients. Alere LAM offers a rapid turnaround time and simplicity. Because almost all participants can provide urine samples, it has an advantage over Xpert in utilizing non-sputum specimens, regardless of patient symptoms, the presence or absence of sputum, or CD4 cell count (128, 129). Additionally, due to its higher sensitivity in diagnosing renal tuberculosis and ease of sample collection, Alere LAM is more acceptable to patient populations compared to Xpert. Its lower economic cost provides a unique advantage over invasive and expensive pathological and immunological tests. In another study of malnourished children and the use of LF-LAM for tuberculosis detection in HIV adult patients, LAM also showed promising detection rates (130, 131). In an interview study conducted in Ghana using Determine LAM, researchers found that despite some healthcare workers' concerns about confidentiality during the actual testing process, the quick and straightforward testing procedure and easy sample collection did not hinder patient diagnosis and treatment. Another prospective observational study using Determine LAM for tuberculosis detection in critically ill patients also showed that LAM testing maintained reasonable specificity even in severe cases (132). Therefore, selective use of LAM testing in critically ill patients can improve diagnostic rates to some extent (133).
Due to the fact that LAM testing can utilize urine samples, which are less constrained by time and environmental factors compared to blood or other body fluid samples (134), LAM determination is expected to replace Xpert as a new diagnostic criterion or be used in combination with other diagnostic markers for renal tuberculosis, especially in special populations and patients with severe diseases and complications (135, 136). With continuous technological advancements, emerging LAM detection methods have been successively introduced, offering improved detection rates and sensitivity, which has gradually gained public recognition and acceptance in the field of pulmonary and extrapulmonary diagnosis (137–139).
In recent years, research teams have attempted to explore the advantages and disadvantages of these novel or standard LAM detection methods in the diagnosis of pulmonary tuberculosis or extrapulmonary tuberculosis. Paul et al. conducted experiments to investigate the effect of LAM content in various samples on detection rates. The results showed that urine samples had a sensitivity of 62%, while serum or plasma samples had a sensitivity of 70% (140). The research team, led by Getachew et al., employed a systematic review and meta-analysis approach to assess the diagnostic value of the Alere LAM test, MTB-LAM-ELISA, and Fuji LAM test for pediatric tuberculosis. The results indicated that the sensitivity and specificity of the Alere LAM test were lower than those of the Fuji LAM test.
In comparison, the MTB-LAM-ELISA test exhibited higher specificity but lower sensitivity (141). Another prospective multicenter study and recent research on the Fuji LAM test for pediatric tuberculosis have further confirmed the test's better sensitivity and specificity (137, 138, 142, 143). Regarding the accuracy and cost-effectiveness of the Fuji LAM test, existing studies have demonstrated that its sensitivity is influenced by various factors, including batch number, sample size, and the number of CD4 (+) T cells, and that it can be used concurrently with the WHO-endorsed Xpert Ultra to reduce diagnostic costs for patients (144, 145).
Zhang et al. conducted a prospective observational study using a combined detection method of urine LAM testing (chemiluminescence method) and GeneXpert in HIV-infected tuberculosis patients. The results showed that the combination of Fuji LAM and GeneXpert methods could significantly improve detection rates (146). Another study incorporated bacterial culture testing and samples from extrapulmonary tuberculosis patients using this method, also achieving reasonable detection rates (147). A study conducted in Ethiopia demonstrated that the sensitivity of combined Xpert MTB/RIF and TB-LAM diagnostics for patients with extrapulmonary tuberculosis (EPTB) is superior to that of single tests (148). This suggests that the detection rate of LAM testing when combined with other diagnostic methods, may be superior to LAM testing alone.
A trial using molecular bacterial load assays for Mycobacterium tuberculosis in urine samples from HIV-infected patients with tuberculosis showed that the use of Mycobacterium tuberculosis testing could improve the diagnostic efficiency of tuberculosis as a supplementary test (149). Another study on oral swab PCR+LAM assays for the detection of tuberculosis in HIV patients also suggested that tongue swabs can be used as a supplementary test for LAM assays and other tuberculosis tests (150). The conclusions of these studies indicate that the current standard LAM testing combined with other testing methods will help improve the detection rate of tuberculosis and extrapulmonary tuberculosis, especially for patients with special populations such as HIV. Unfortunately, it also appears that standard LAM testing methods alone do not yield significant clinical benefits.
However, this does not mean that the effectiveness of the Fuji LAM test in clinical diagnosis is not recognized. Some scholars believe that the diagnosis of renal tuberculosis may be achieved by combining the Fuji LAM test with other examination methods (134, 151–155). However, currently, there are numerous LAM determination instruments on the market, with varying detection efficiencies and times. Therefore, we summarized the existing LAM detection methods mentioned in the above studies and compared their advantages and disadvantages (Table 3). Selecting an appropriate LAM detection method based on clinical needs or developing a more accurate and straightforward LAM detection method can reduce the time it takes for patients to start treatment to some extent (156).
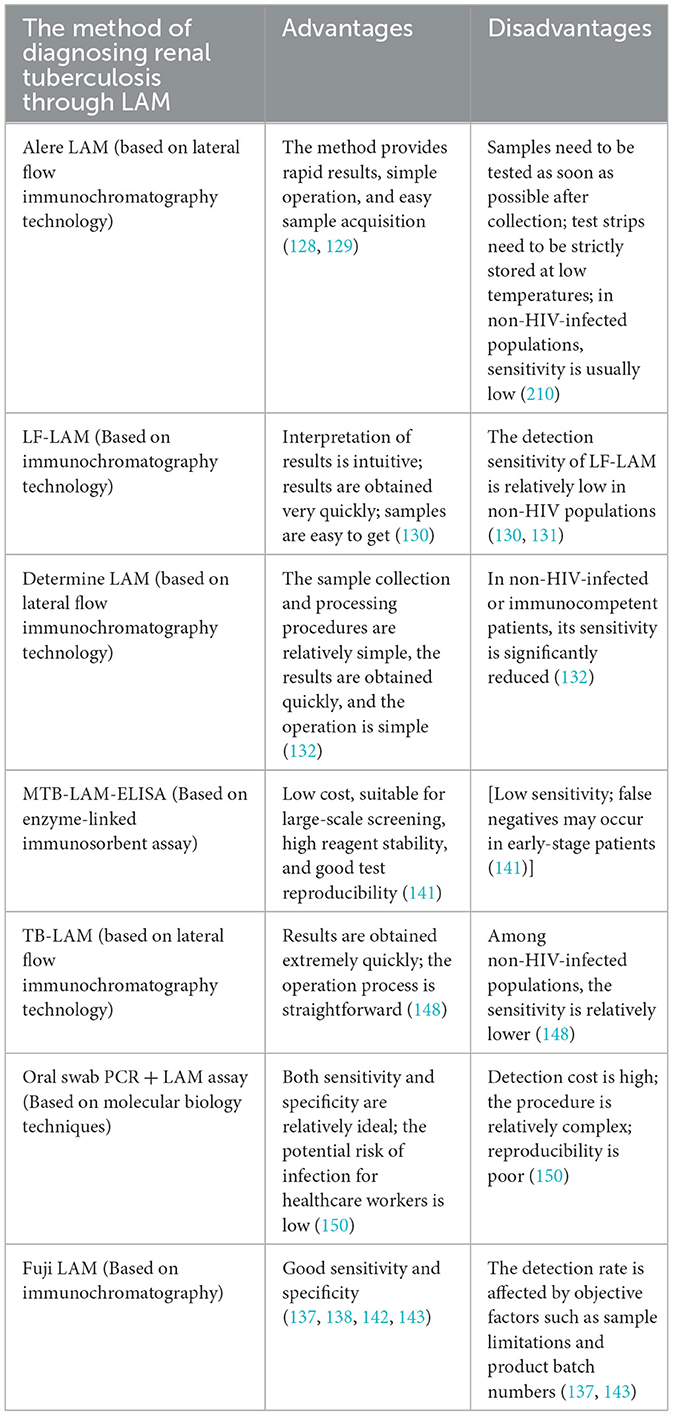
Table 3. Current diagnostic methods and advantages and disadvantages of renal tuberculosis through LAM.
4.2 Improved method for LAM detection—proteinase K
Currently, research teams have proposed and validated a scheme to optimize LAM testing using proteinase K. Recent studies have demonstrated that deproteinization of LAM with proteinase K can reduce the steric hindrance effect of LAM, thereby significantly improving the detectability of LAM (157–159). Huang et al. developed a novel LAM detection method using proteinase K-pretreated concanavalin A (ConA) to bind to LAM in urine for ELISA detection, finding that this method improves the sensitivity of LAM detection (160). Christopher et al. reported an automated detection platform that uses proteinase K-pretreated samples to determine LAM, allowing for determination with a reduced sample volume (161). The biological action of proteinase K can undoubtedly enhance the interaction between LAM and labeled antibodies, antigen-antibody complexes, and sensors, thereby improving the detection rate of LAM in urine samples. Since patients with renal tuberculosis often have very few urine samples collected due to various complications such as nephrectomy in the late stage, and after pretreatment of samples with proteinase K, fewer samples can be used for detection, so pretreatment of LAM with proteinase K is one of the optional schemes for LAM determination in the future.
4.3 Improved method for LAM detection—design of a novel sensor
In the design of new LAM determination sensors, Dinesh's research team developed a sensor composed of a nanocomposite synthesized from multi-walled carbon nanotubes and zinc oxide (MWCNT-ZnO) to enhance the binding efficiency of LAM and antibodies (162). Ubaid et al. designed a sensor based on phase shift cavity ring-down spectroscopy (PS-CRDS) and activated the surface of the tapered fiber in the sensor with the anti-LAM antigen CS-35, resulting in considerable sensitivity even when the sample concentration was as low as 10 pg/mL (163). In addition, research reports have been published on the determination of LAM by designing a plasma biosensor (P-FAB), which can be used alone or in combination with gold nanoparticles (AuNP) to detect LAM, thereby achieving the goal of reducing the sample size required for determination. At the same time, its sensor probes and AuNPs are also easy to preserve (164, 165), which enhances their operability in actual clinical work. At the same time, the current popular machine learning can also be applied to this type of detection (166). The research on various new sensors makes the detection samples of LAM more accurate and traceable, and more nanosensors may be designed in the future to facilitate the early screening of extrapulmonary tuberculosis, such as renal tuberculosis.
4.4 Improvement of LAM detection method—application of nanoions
In recent years, the method of screening or detecting pathogens through nanoparticles has been evaluated as effective and highly stable by relevant experiments (167, 168). Meng et al. designed a portable instrument and used it to analyze urine samples from tuberculosis patients via copper ion probe, nano-ion cascade amplification, and stabilizer stabilization. The results showed 100% specificity and 90% sensitivity (169). Another experiment using copper nanoparticles to design a new LAM determination method also showed good diagnostic potential (170). This suggests that the improvement of LAM detection methods using nanoparticles can significantly enhance detection efficiency, which is of considerable clinical significance for diagnosing extrapulmonary tuberculosis with atypical clinical symptoms, such as renal tuberculosis.
4.5 Improvement of LAM detection method—design of new antibody
On the other hand, in the design and selection of new antibodies, due to the diversity of LAM antibodies, their ability to recognize and bind to LAM varies depending on the type of LAM antibody, and their impact on test results also varies (171–173). The PATHFAST TB LAM Ag assay is a new LAM assay designed based on chemiluminescent enzyme immunoassay. A research team has demonstrated through experiments that the sensitivity of this assay is 88.8%. The specificity is 100.0% (174), indicating that the PATHFAST TB LAM Ag assay has potential value for monitoring tuberculosis treatment and may be further applied in the field of extrapulmonary tuberculosis. Some research teams have developed or utilized high-affinity rabbit monoclonal antibodies to enhance detection methods, resulting in improved detection efficiency, whether through conventional ELISA experiments or other high-sensitivity detection methods (175–177). The use of reagents such as cyanobacterial lectin microvilli-N (MVN) to enhance the binding capacity between LAM and LAM antibodies in the design of magnetic bead ELISA experiments can also improve detection capabilities (178).
4.6 Summary of LAM detection methods
Detection methods based on ELISA for lipoarabinomannan (LAM) in extracellular vesicles have shown good diagnostic efficacy in pulmonary tuberculosis and extrapulmonary tuberculosis, including renal tuberculosis. Currently, numerous new LAM diagnostic methods are being developed. We have summarized these new and modified LAM diagnostic methods and their effects for reference (Table 4). It can be said that LAM detection is a landmark method in the diagnosis of tuberculosis. From the results, even in urine samples that have undergone multiple rounds of decomposition and filtration, any detection method for LAM in extracellular vesicles has a higher detection rate for tuberculosis than the Xpert method. However, current guidelines only recommend urine LAM testing for HIV patients who meet established criteria. Considering the superiority of LAM detection and other advantages, some research teams suggest expanding LAM testing to different populations, including non-HIV patients (179), or suggesting routine Fuji LAM testing for hospitalized HIV patients (180). This is particularly urgent in medical wards where patients often have multiple underlying diseases, and current treatment plans do not include specialized surgical treatments. Renal tuberculosis rarely shows clinical symptoms in the early stages or only manifests as common urinary tract infection symptoms. Moreover, HIV patients are not only more likely to develop renal tuberculosis, but their mortality rate is also higher (181). Therefore, LAM testing for HIV patients in medical wards can detect a large proportion of occult tuberculosis, such as renal tuberculosis or other urinary system tuberculosis patients, thereby further extending the patient's survival cycle.
5 The value of LAM and LprG in extracellular vesicles in the treatment of renal tuberculosis
In the course of research on anti-tuberculosis drugs and tuberculosis prevention, numerous studies have suggested that different treatment strategies can be designed based on the characteristics of LAM and LprG in Mycobacterium tuberculosis, thereby assisting in anti-tuberculosis treatment or the design of vaccines or vaccine adjuvants. These treatment strategies include traditional Chinese medicine prescriptions, new drugs, cell pathway targeting, and immune enhancement, among others. These treatment strategies and vaccine designs have unique features.
5.1 Traditional Chinese medicine and novel synthetic drugs
Drug design targeting LAM and LprG is primarily achieved by interfering with their effects on cell signaling pathways or disrupting their synthesis. Current research has found that it is relatively feasible for researchers to develop inhibitors targeting these pathways, such as the mTOR inhibitor sirolimus, traditional Chinese medicine Scutellaria baicalensis, and Sophora flavescens preparations, by inhibiting the activation of signaling pathways and interfering with the metabolism and function of Mycobacterium tuberculosis (182). However, designing drugs to reduce the toxicity of Mycobacterium tuberculosis or to kill it by disrupting the synthesis of both is relatively more challenging.
5.1.1 Inhibitioninhibition and immune regulation programs targeting TLR2-related pathways
Since LAM is an activator of TLR2, it can be used to treat tuberculosis by inhibiting the biological effects of this pathway. Current reports indicate that andrographolide can reduce inflammation by inhibiting the Notch1 pathway and down-regulating the phosphorylation of the NF-κB p65 subunit (183, 184). Other related substances in traditional Chinese medicine also have similar anti-inflammatory effects (185).
For TLR2 itself, current research techniques, such as gene silencing or TLR2 knockout, can improve inflammation and reduce vascular fibrosis to a certain extent (186). Transferring relevant research to the treatment of renal tuberculosis may be more conducive for clinicians to understand the role of TLR2 in the pathophysiology of renal tuberculosis.
5.1.2 Inhibitioninhibition and immune regulation programs targeting the MR pathway
Because Mycobacterium tuberculosis accelerates its intracellular colonization through the MR-mediated phagocytic process of host cells, the use of mannose analogs can block the binding between pathogens and host cells, thereby reducing infection. Additionally, monoclonal antibodies targeting MR can inhibit IL-4/IL-13-induced Th2-type responses and reverse immune escape (187, 188).
Or by inhibiting its binding to the receptor, such as the glucose mimetic 2-deoxy-D-glucose (2-DG), thereby preventing LAM from binding to the receptor to inhibit the pathway (189); polysaccharide immunostimulants (such as Poria cocos polysaccharides) can interact with MR (mannose receptor), increase the secretion and mRNA expression of TNF-α within cells, and inhibit tuberculosis infection (190).
Current research suggests that the treatments mentioned above can be combined with classic anti-tuberculosis drugs to form an anti-tuberculosis drug + immunomodulator regimen (such as the standard anti-tuberculosis regimen (e.g., 2HRZE/4HR) combined with the traditional Chinese medicine Poria cocos), which can increase the MTB negative conversion rate and improve the CD4+/CD8+ ratio. Additionally, immune enhancement methods can be employed to enhance antigen presentation through nanoparticles, nano-vaccines, and other carriers or by utilizing the mannose selectivity of MR to fabricate corresponding mannose into microfilms and assemble other drugs, thereby achieving an auxiliary anti-tuberculosis effect (191–197).
5.1.3 Inhibition of the mTOR pathway and immune modulation strategies
Recent studies have shown that baicalein, a compound found in the traditional Chinese medicine Scutellaria baicalensis, can inhibit the apoptosis of macrophages infected with Mycobacterium tuberculosis by suppressing the Akt/mTOR pathway, downregulating the assembly of absent in melanoma 2 (AIM2) and NOD-like receptor and pyrin domain-containing protein 3 (NLRP3) inflammasomes, and promoting autophagy, thereby making it difficult for Mycobacterium tuberculosis to spread further. Since LAM can act on the mTOR pathway, the inhibitory effect of baicalein on this pathway can also hinder the function of LAM (198).
5.1.4 Inhibition of the MAPK pathway and immune modulation strategies
The emergence of various inflammatory diseases in humans is closely related to the dysregulation of the MAPK signaling pathway. Current research suggests that tuberculosis, a disease characterized by inflammation, can be treated and regulated by inhibiting the MAPK pathway (59). LAM, as one of the mediators involved in the MAPK pathway, can also be prevented from exerting its inflammatory regulatory effects due to the inhibition of this pathway.
In the study of related drugs, isoliquiritigenin from licorice has been shown to exert its anti-inflammatory effects by inhibiting the activation of the MAPK signaling pathway (199). A new synthetic drug, 1,2-ethylenediamine (SQ109), can also assist in the treatment of tuberculosis by mediating the MAPK pathway to stimulate M1 macrophage polarization and induce the production of protective pro-inflammatory cytokines (57).
Currently, the design of drugs that target the MAPK pathway for Inhibition to combat the functions of LAM and LprG, thereby assisting in anti-tuberculosis treatment, is a hot topic in recent years. In the future, more drugs may be developed, but MAPK pathway inhibitors will remain key. This is not only related to the characteristics of LAM and LprG but also to the impact of the MAPK pathway on inflammatory mediators.
5.1.5 Inhibitioninhibition and drug design challenges for other potential pathways
LAM and LprG can have direct or indirect effects on various pathways within host cells. For the LAM-mediated MBL pathway, complement activators can be designed to enhance the role of complement immunity; however, there are currently few reports of similar drugs being used in clinical research for anti-tuberculosis drugs. In the field of regulating angiogenesis and mediating fibrosis, since LAM may only play an indirect role in these two pathophysiological processes, the treatment of these two pathological processes may not be achieved by a single inhibition of LAM and LprG, so there are also few similar research reports.
At present, while research on drugs that counter the functions of LAM and LprG and disrupt their synthesis is steadily progressing, it still faces many difficulties. For example, most studies remain at the level of in vitro experiments and lack in vivo verification. It is difficult to analyze the single-target effect of the synergistic action of multiple components in traditional Chinese medicine compounds, and the complex toxicology of Western medicine synthesis requires large-sample and multi-center clinical studies. At the same time, differences in the batches and purity of the raw materials used to manufacture medicinal drugs may affect their efficacy, among other factors. Therefore, there is still a lot of work to be done on the drug design of LAM and LprG.
Moreover, drug design targeting renal tuberculosis is not limited to LAM and LprG. Recent studies on bacterial urease C (UreC) have shown that urease inhibitors can disrupt the growth environment of Mtb and inhibit its reproduction by preventing UreC from interacting with RuvB-like protein 2 (RUVBL2) and impeding the formation of RUVBL1-RUVBL2-RAD51 DNA repair complex, thereby suppressing host DNA repair mechanisms. Therefore, drug design targeting LAM and LprG also needs to face challenges from other mechanisms of action and targeted drugs (219).
5.2 The relationship between LAM and LprG and vaccine preparation
An ideal tuberculosis vaccine would possess strong protective efficacy, stable and durable immunogenicity, and no adverse reactions. It could prevent tuberculosis through single or multiple immunizations. However, no vaccine with better protection or sustainability than Bacillus Calmette-Guérin (BCG) has emerged in clinical practice yet. Although some new candidate vaccines (e.g. Dendritic cell-targeted vaccines activate naive T cells to release cytokines and generate protective immunity) have shown specific effects in animal models, long-term experiments, follow-ups, and verifications are still needed to determine how to apply them to humans and whether they can replace BCG (215).
As one of the virulence factors of Mtb, LAM has the potential to become a vaccine. However, there is currently no consensus on how to reduce the virulence of LAM and how much attenuated LAM should be included. Moreover, the limited clinical trial data makes it difficult to support the effectiveness of this antigen against tuberculosis. Secondly, there are multiple issues in the development of LAM vaccines, including the lack of reliable preclinical evaluation indicators, suitable animal models for vaccine assessment, methods for simulating exposure, unified clinical trial endpoint standards, consistent evaluation environments, and complex immune populations (200).
Previous studies have indicated that due to the conservation of LprG, this protein is not easily degraded or subjected to structural and functional mutations. This makes it a potential key component for Mycobacterium vaccines, a hypothesis that has been further validated in subsequent experiments (201, 202). Moreover, even when used as an adjuvant rather than the primary immunogenic antigen in vaccines, LprG-based vaccines can remain relatively stable in vivo and effectively protect against Mycobacterium tuberculosis infection (203). These findings demonstrate that LprG, as a biomolecule, holds certain value in the study of immunotherapy for emerging renal tuberculosis.
In addition to LprG, LAM has unique biological characteristics that make it a potential new target for vaccine development. Studies have shown that antibodies against LAM can modulate the immune response to Mtb (204). Yan et al. constructed fusion proteins such as 38KD-MPT32-MPT64, CFP10-MTB81-EspC, and Ag85B-HBHA to improve the therapeutic effect by enhancing the antibody recognition ability of tuberculosis patients (205). However, as this study did not delve deeply into renal tuberculosis or extrapulmonary tuberculosis, further exploration is needed to construct fusion proteins and incorporate LAM to improve the diagnostic efficiency of renal tuberculosis. LAM can also serve as an adjuvant for BCG or as an immunogenicity modulator, becoming one of the intervention measures for tuberculosis treatment (206). Meanwhile, the technology of using LAM to prepare antibodies in vitro may become a new immunotherapy method for renal tuberculosis in the future (171).
At present, various new methods and ideas for vaccine research have been established. We believe that LAM will be utilized in the future for vaccine preparation research, enabling the conduct of clinical trials and marking a unique and exciting stage in tuberculosis vaccine research.
6 Summary
Renal tuberculosis, the second most significant infectious disease in the world with a high mortality rate, has the same characteristics as pulmonary tuberculosis but also has its characteristics in the field of urinary systems. Due to Mycobacterium tuberculosis's ability to effectively inhibit processes such as phagosome-lysosome fusion, phagosome acidification, and pro-inflammatory cytokine release, it prevents host cell death. Meanwhile, studies have shown that M. tuberculosis possesses certain mechanisms that can even acquire iron from host cells as a mode of host defense mechanism. Therefore, exploring new diagnostic and treatment approaches for tuberculosis is actually an exploration of the biological functional mechanisms of Mycobacterium tuberculosis (214). The exploration of new diagnoses and treatment ideas for pulmonary tuberculosis is, in fact, an extension of the exploration of diagnosis and treatment plans for renal tuberculosis and even urinary system tuberculosis. Extracellular vesicles can be found in various human body fluids and represent a type of cell secretion with high research potential. The research conclusions on its diagnosis and treatment of renal tuberculosis have more clinical guidance and practical effects.
In the research on the diagnosis of renal tuberculosis using LAM and LprG, these two biomarkers have their unique research value. LAM, as a characteristic virulence factor of Mycobacterium tuberculosis, can determine the presence and degree of infection. LprG, as the transport and riveting protein of LAM, can promote the function of LAM, but at the same time, it can also target and locate LAM through LprG. Due to the close relationship between LprG and LAM, utilizing LprG to identify and capture LAM has become a feasible diagnostic scheme design idea, thereby improving the detection rate.
Because LAM and LprG are biomarkers secreted by Mycobacterium tuberculosis (Mtb) and immune cells infected by Mtb, including macrophages, in extracellular vesicles. Therefore, the detection method using these two biomarkers effectively addresses the challenges encountered in traditional Xpert testing, including (1) The ability to detect only sputum samples; (2) Low sensitivity; (3) Long detection time, etc. At the same time, since exosomes can be found in various parts of the human body fluid, for patients with renal tuberculosis or other urinary system tuberculosis, and for patients with related immune deficiencies, tuberculosis detection through LAM and LprG is a suitable detection method.
In the therapeutic field, both of them serve as signature antigens of Mtb, showing promising prospects and research value in the development of vaccines, adjuvants, immunotherapy, and other treatment modalities. Moreover, relevant experimental results have been successfully implemented, yielding excellent outcomes.
The emergence of renal tuberculosis caused by drug-resistant Mycobacterium tuberculosis has further promoted research on extracellular vesicles and their contents in the diagnosis and treatment of renal tuberculosis. Although relevant experiments have been conducted on the use of extracellular vesicles as disease drug carriers and targeted therapies (207), targeted therapies for renal tuberculosis may be developed in the future. However, no related drugs have entered clinical trials, nor have their efficacy and economic benefits been widely accepted and recognized. Renal tuberculosis caused by multi-drug resistant Mycobacterium tuberculosis requires earlier diagnosis, treatment, and monitoring compared to ordinary renal tuberculosis to prevent severe sequelae and other adverse events (208, 209). The detection methods for LAM and LprG can utilize urine samples, which are easily obtained from patients with renal tuberculosis, thereby providing inherent advantages in early screening for renal tuberculosis or sputum-negative tuberculosis. Moreover, even for patients undergoing renal auto-nephrectomy, the detection of LAM and LprG can simplify disease detection due to their independence from sample timing and spatial constraints. The experiments summarized in this article involving children and HIV-infected tuberculosis patients have proven the above viewpoints. Meanwhile, some of the study populations selected also appear to suggest that the ability of LAM and LprG to accept non-sputum specimens is valuable in diagnosing renal tuberculosis.
At present, LprG is more commonly used as a means of localizing or capturing LAM in the detection of tuberculosis. The diagnostic capability of LprG alone for tuberculosis requires more experiments and research data for further evaluation. Moreover, the experimental designs of some researchers listed in this article have certain shortcomings, such as regional limitations or small sample sizes. Follow-up studies with multi-center, large sample sizes, and strict experimental operations are needed to confirm further the diagnostic and therapeutic value of LAM and LprG in pulmonary or extrapulmonary tuberculosis, including renal tuberculosis.
In the field of vaccine development for renal tuberculosis, both LAM and LprG have demonstrated sound biological and immunological effects in vivo, whether used as chimeric systems combined with other substances to prepare vaccines or as adjuvants for different vaccines. Moreover, even passive immunization with in vitro-prepared LAM antibodies can enable organisms to develop resistance against tuberculosis. We speculate that this may be related to the high conservation of LprG and its specific virulence factor role in Mycobacterium tuberculosis. These experiments suggest the possibility of conducting clinical research on LAM and LprG as new tuberculosis vaccines or immunotherapeutic agents in the future.
Although the two biomarkers present in EVs listed in this article are of great clinical value, we must also be aware of their current limitations in clinical practical application. These limitations include difficulties in isolation and purification, heterogeneity and specificity that may vary significantly between individuals, limitations in detection techniques, a lack of clinical trials and standardization, and the fact that LAM and LprG in EVs may fluctuate with disease progression. These issues may require further research to improve isolation and purification techniques, enhance detection sensitivity and specificity, standardize operating procedures, strengthen clinical validation, and integrate multi-omics analysis to develop high-throughput technologies to improve the detection rates of LAM and LprG. Meanwhile, due to significant differences between the results of LAM and LprG-related tests and traditional detection methods, healthcare workers in clinical settings have refused to diagnose tuberculosis using LAM and LprG, thereby withholding early anti-tuberculosis treatment for patients with positive LAM test results (217).
In summary, LAM and LprG in renal tuberculosis extracellular vesicles not only have the potential to serve as diagnostic markers but also as relevant therapeutic targets for drugs. They also have the potential to be used as vaccine formulations or adjuvants in the prevention of renal tuberculosis. Future research should further explore the clinical application value of these approaches to provide new strategies for the early diagnosis and precise treatment of renal tuberculosis.
Author contributions
XP: Conceptualization, Supervision, Investigation, Writing – review & editing, Software, Methodology, Funding acquisition, Formal analysis, Visualization, Data curation, Writing – original draft. YL: Supervision, Writing – review & editing, Data curation, Investigation, Validation. SJ: Validation, Software, Writing – review & editing. QW: Project administration, Supervision, Conceptualization, Validation, Software, Investigation, Writing – review & editing, Resources.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The publication costs of this article are fully borne by XP.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Global tuberculosis report 2023. Geneva: World Health Organization; 2023. Licence: CC BY-NC-SA 3.0 IGO.
2. Jha SK, Rathish B. Genitourinary Tuberculosis. 2023 Apr 17. In: StatPearls. Treasure Island (FL): StatPearls Publishing (2024).
3. Rathish B, Wilson A, Pillay R, Warrier A, Philips G, A. Bundled approach to pulmonary tuberculosis testing: experience from a Tertiary Care Centre in South India. Cureus. (2019) 11:e6042. doi: 10.7759/cureus.6042
4. Muneer A, Macrae B, Krishnamoorthy S, Zumla A. Urogenital tuberculosis—epidemiology, pathogenesis and clinical features. Nat Rev Urol. (2019) 16:573–98. doi: 10.1038/s41585-019-0228-9
5. Kontsevaya I, Cabibbe AM, Cirillo DM, DiNardo AR, Frahm N, Gillespie SH, et al. Update on the diagnosis of tuberculosis. Clin Microbiol Infect. (2024) 30:1115–22. doi: 10.1016/j.cmi.2023.07.014
6. Sun X, Li W, Zhao L, Fan K, Qin F, Shi L, et al. Current landscape of exosomes in tuberculosis development, diagnosis, and treatment applications. Front Immunol. (2024) 15:1401867. doi: 10.3389/fimmu.2024.1401867
7. Couch Y, Buzàs EI, Di Vizio D, Gho YS, Harrison P, Hill AF, et al. brief history of nearly EV-erything—The rise and rise of extracellular vesicles. J Extracell Vesicles. (2021) 10:e12144. doi: 10.1002/jev2.12144
8. Rudraprasad D, Rawat A, Joseph J. Exosomes, extracellular vesicles and the eye. Exp Eye Res. (2022) 214:108892. doi: 10.1016/j.exer.2021.108892
9. Xi X, Wang B, Zhang R, Ling C. Serum exosome tRFs as a promising biomarker for active tuberculosis and latent tuberculosis infection. J Microbiol Methods. (2024) 222:106944. doi: 10.1016/j.mimet.2024.106944
10. Zhao G, Jones MK. Role of bacterial extracellular vesicles in manipulating infection. Infect Immun. (2023) 91:e0043922. doi: 10.1128/iai.00439-22
11. Gupta S, Bhagavathula M, Sharma V, Sharma N, Sharma N, Biswas A, et al. Dynamin-like proteins mediate extracellular vesicle secretion in Mycobacterium tuberculosis. EMBO Rep. (2023) 24:e55593. doi: 10.15252/embr.202255593
12. Layre E. Trafficking of Mycobacterium tuberculosis envelope components and release within extracellular vesicles: host-pathogen interactions beyond the wall. Front Immunol. (2020) 11:1230. doi: 10.3389/fimmu.2020.01230
13. Mehaffy C, Ryan JM, Kruh-Garcia NA, Dobos KM. Extracellular vesicles in mycobacteria and tuberculosis. Front Cell Infect Microbiol. (2022) 12:912831. doi: 10.3389/fcimb.2022.912831
14. Abekura F, Park J, Lim H, Kim HD, Choi H, Lee MJ, et al. Mycobacterium tuberculosis glycolipoprotein LprG inhibits inflammation through NF-κB signaling of ERK1/2 and JNK in LPS-induced murine macrophage cells. J Cell Biochem. (2022) 123:772–81. doi: 10.1002/jcb.30220
15. Viale MN, Colombatti Olivieri MA, Alonso N, Moyano RD, Imperiale B, Morcillo N, et al. Effect of the deletion of lprG and p55 genes in the K10 strain of Mycobacterium avium subspecies paratuberculosis. Res Vet Sci. (2021) 138:1–10. doi: 10.1016/j.rvsc.2021.05.019
16. Schirmer S, Rauh L, Alebouyeh S, Delgado-Velandia M, Salgueiro VC, Lerma L, et al. Immunogenicity of mycobacterial extracellular vesicles isolated from host-related conditions informs about tuberculosis disease status. Front Microbiol. (2022) 13:907296. doi: 10.3389/fmicb.2022.907296
17. Chiplunkar SS, Silva CA, Bermudez LE, Danelishvili L. Characterization of membrane vesicles released by Mycobacterium avium in response to environment mimicking the macrophage phagosome. Future Microbiol. (2019) 14:293–313. doi: 10.2217/fmb-2018-0249
18. Ortalo-Magné A, Dupont MA, Lemassu A, Andersen AB, Gounon P, Daffé M. Molecular composition of the outermost capsular material of the tubercle bacillus. Microbiology (Reading) (1995) 141:1609–20. doi: 10.1099/13500872-141-7-1609
19. Shimokawa M, Ishiwata A, Kashima T, Nakashima C, Li J, Fukushima R, et al. Identification and characterization of endo-α-, exo-α-, and exo-β-D-arabinofuranosidases degrading lipoarabinomannan and arabinogalactan of mycobacteria. Nat Commun. (2023) 14:5803. Erratum in: Nat Commun. (2023) 14:6299. doi: 10.1038/s41467-023-41431-2
20. Salgueiro VC, Passemar C, Vázquez-Iniesta L, Lerma L, Floto A, Prados-Rosales R. Extracellular vesicles in mycobacteria: new findings in biogenesis, host-pathogen interactions, and diagnostics. MBio. (2024) 15:e0255223. doi: 10.1128/mbio.02552-23
21. Corrigan DT, Ishida E, Chatterjee D, Lowary TL, Achkar JM. Monoclonal antibodies to lipoarabinomannan/arabinomannan—characteristics and implications for tuberculosis research and diagnostics. Trends Microbiol. (2023) 31:22–35. doi: 10.1016/j.tim.2022.07.001
22. Palčeková Z, Obregón-Henao A, De K, Walz A, Lam H, Philp J, et al. Role of succinyl substituents in the mannose-capping of lipoarabinomannan and control of inflammation in Mycobacterium tuberculosis infection. PLoS Pathog. (2023) 19:e1011636. doi: 10.1371/journal.ppat.1011636
23. Palčeková Z, De K, Angala SK, Gilleron M, Zuberogoitia S, Gouxette L, et al. Impact of methylthioxylose substituents on the biological activities of lipomannan and lipoarabinomannan in mycobacterium tuberculosis. ACS Infect Dis. (2024) 10:1379–90. doi: 10.1021/acsinfecdis.4c00079
24. De P, Amin AG, Flores D, Simpson A, Dobos K, Chatterjee D. Structural implications of lipoarabinomannan glycans from global clinical isolates in diagnosis of Mycobacterium tuberculosis infection. J Biol Chem. (2021) 297:101265. doi: 10.1016/j.jbc.2021.101265
25. Kaur D, Obregón-Henao A, Pham H, Chatterjee D, Brennan PJ, Jackson M. Lipoarabinomannan of Mycobacterium: mannose capping by a multifunctional terminal mannosyltransferase. Proc Natl Acad Sci U S A. (2008) 105:17973–7. doi: 10.1073/pnas.0807761105
26. Sparks IL, Kado T, Prithviraj M, Nijjer J, Yan J, Morita YS. Lipoarabinomannan mediates localized cell wall integrity during division in mycobacteria. Nat Commun. (2024) 15:2191. doi: 10.1038/s41467-024-46565-5
27. Shukla S, Richardson ET, Athman JJ, Shi L, Wearsch PA, McDonald D, et al. Mycobacterium tuberculosis lipoprotein LprG binds lipoarabinomannan and determines its cell envelope localization to control phagolysosomal fusion. PLoS Pathog. (2014) 10:e1004471. Erratum in: PLoS Pathog. (2014) 10:e1004596. doi: 10.1371/journal.ppat.1004471
28. Lee J, Kim SH, Choi DS, Lee JS, Kim DK, Go G, et al. Proteomic analysis of extracellular vesicles derived from Mycobacterium tuberculosis. Proteomics. (2015) 15:3331–7. doi: 10.1002/pmic.201500037
29. Palacios A, Gupta S, Rodriguez GM, Prados-Rosales R. Extracellular vesicles in the context of Mycobacterium tuberculosis infection. Mol Immunol. (2021) 133:175–81. doi: 10.1016/j.molimm.2021.02.010
30. Palacios A, Sampedro L, Sevilla IA, Molina E, Gil D, Azkargorta M, et al. Mycobacterium tuberculosis extracellular vesicle-associated lipoprotein LpqH as a potential biomarker to distinguish paratuberculosis infection or vaccination from tuberculosis infection. BMC Vet Res. (2019) 15:188. doi: 10.1186/s12917-019-1941-6
31. Pecora ND, Gehring AJ, Canaday DH, Boom WH, Harding CV. Mycobacterium tuberculosis LprA is a lipoprotein agonist of TLR2 that regulates innate immunity and APC function. J Immunol. (2006) 177:422–9. doi: 10.4049/jimmunol.177.1.422
32. Dahiya B, Khan A, Mor P, Kamra E, Singh N, Gupta KB, et al. Detection of Mycobacterium tuberculosis lipoarabinomannan and CFP-10 (Rv3874) from urinary extracellular vesicles of tuberculosis patients by immuno-PCR. Pathog Dis. (2019) 77:ftz049. Erratum in: Pathog Dis. (2019) 77:ftz056. doi: 10.1093/femspd/ftz049
33. O'Neill LA, Golenbock D, Bowie AG. The history of Toll-like receptors—redefining innate immunity. Nat Rev Immunol. (2013) 13:453–60. doi: 10.1038/nri3446
34. Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. (2011) 30:16–34. doi: 10.3109/08830185.2010.529976
35. Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. (2010) 140:805–20. doi: 10.1016/j.cell.2010.01.022
36. Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. (2017) 2:17023. doi: 10.1038/sigtrans.2017.23
37. Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol. (2010) 28:367–88. doi: 10.1146/annurev.immunol.021908.132603
38. Paarnio K, Väyrynen JP, Väyrynen SA, Kantola T, Karhu T, Tervahartiala T, et al. TLR2 and TLR4 in colorectal cancer: relationship to tumor necrosis and markers of systemic inflammation. Neoplasma. (2022) 69:1418–24. doi: 10.4149/neo_2022_220509N498
39. Guerrero-Romero F, Castellanos-Juárez FX, Salas-Pacheco JM, Morales-Gurrola FG, Salas-Leal AC, Simental-Mendía LE. Association between the expression of TLR4, TLR2, and MyD88 with low-grade chronic inflammation in individuals with metabolically healthy obesity. Mol Biol Rep. (2023) 50:4723–8. doi: 10.1007/s11033-023-08338-z
40. Huang D, Fang F, Xu F. Hyperoxia induces inflammation and regulates cytokine production in alveolar epithelium through TLR2/4-NF-κB-dependent mechanism. Eur Rev Med Pharmacol Sci. (2016) 20:1399–410.
41. Tapping RI, Tobias PS. Mycobacterial lipoarabinomannan mediates physical interactions between TLR1 and TLR2 to induce signaling. J Endotoxin Res. (2003) 9:264–8. doi: 10.1177/09680519030090040801
42. Augenstreich J, Briken V. Host cell targets of released lipid and secreted protein effectors of mycobacterium tuberculosis. Front Cell Infect Microbiol. (2020) 10:595029. doi: 10.3389/fcimb.2020.595029
43. Bomfim CCB, Fisher L, Amaral EP, Mittereder L, McCann K, Correa AAS, et al. Mycobacterium tuberculosis Induces Irg1 in murine macrophages by a pathway involving both TLR-2 and STING/IFNAR signaling and requiring bacterial phagocytosis. Front Cell Infect Microbiol. (2022) 12:862582. doi: 10.3389/fcimb.2022.862582
44. Hook JS, Cao M, Weng K, Kinnare N, Moreland JG. Mycobacterium tuberculosis Lipoarabinomannan Activates Human Neutrophils via a TLR2/1 Mechanism Distinct from PamCSK. J Immunol. (2020) 204:671–81. 34 doi: 10.4049/jimmunol.1900919
45. Silva CS, Sundling C, Folkesson E, Fröberg G, Nobrega C, Canto-Gomes J, et al. High Dimensional immune profiling reveals different response patterns in active and latent tuberculosis following stimulation with mycobacterial glycolipids. Front Immunol. (2021) 12:727300. doi: 10.3389/fimmu.2021.727300
46. Athman JJ, Sande OJ, Groft SG, Reba SM, Nagy N, Wearsch PA, et al. Mycobacterium tuberculosis membrane vesicles inhibit t cell activation. J Immunol. (2017) 198:2028–37. doi: 10.4049/jimmunol.1601199
47. Correia-Neves M, Nigou J, Mousavian Z, Sundling C, Källenius G. Immunological hyporesponsiveness in tuberculosis: the role of mycobacterial glycolipids. Front Immunol. (2022) 13:1035122. doi: 10.3389/fimmu.2022.1035122
48. Lubow J, Virgilio MC, Merlino M, Collins DR, Mashiba M, Peterson BG, et al. Mannose receptor is an HIV restriction factor counteracted by Vpr in macrophages. Elife. (2020) 9:e51035. doi: 10.7554/eLife.51035
49. van der Zande HJP, Nitsche D, Schlautmann L, Guigas B, Burgdorf S. The mannose receptor: from endocytic receptor and biomarker to regulator of (meta)inflammation. Front Immunol. (2021) 12:765034. doi: 10.3389/fimmu.2021.765034
50. Rajaram MVS, Arnett E, Azad AK, Guirado E, Ni B, Gerberick AD, et al. Tuberculosis-Initiated human mannose receptor signaling regulates macrophage recognition and vesicle trafficking by FcRγ-Chain, Grb2, and SHP-1. Cell Rep. (2017) 21:126–40. doi: 10.1016/j.celrep.2017.09.034
51. Zhang Y, Xu D, Nie Q, Wang J, Fang D, Xie Y, et al. Macrophages exploit the mannose receptor and JAK-STAT1-MHC-II pathway to drive antigen presentation and the antimycobacterial immune response after BCG vaccination. Acta Biochim Biophys Sin. (2024) 56:1130–44. doi: 10.3724/abbs.2024100
52. Stavenhagen K, Mehta AY, Laan L, Gao C, Heimburg-Molinaro J, van Die I, et al. N-glycosylation of mannose receptor (CD206) regulates glycan binding by C-type lectin domains. J Biol Chem. (2022) 298:102591. doi: 10.1016/j.jbc.2022.102591
53. Suzuki Y, Shirai M, Asada K, Yasui H, Karayama M, Hozumi H, et al. Macrophage mannose receptor, CD206, predict prognosis in patients with pulmonary tuberculosis. Sci Rep. (2018) 8:13129. doi: 10.1038/s41598-018-31565-5
54. Cummings RD. The mannose receptor ligands and the macrophage glycome. Curr Opin Struct Biol. (2022) 75:102394. doi: 10.1016/j.sbi.2022.102394
55. Leddy O, White FM, Bryson BD. Immunopeptidomics reveals determinants of Mycobacterium tuberculosis antigen presentation on MHC class I. Elife. (2023) 12:e84070. doi: 10.7554/eLife.84070
56. Bo H, Moure UAE, Yang Y, Pan J, Li L, Wang M, et al. Mycobacterium tuberculosis-macrophage interaction: molecular updates. Front Cell Infect Microbiol. (2023) 13:1062963. doi: 10.3389/fcimb.2023.1062963
57. Singh M, Kumar S, Singh B, Jain P, Kumari A, Pahuja I, et al. The 1, 2-ethylenediamine SQ109 protects against tuberculosis by promoting M1 macrophage polarization through the p38 MAPK pathway. Commun Biol. (2022) 5:759. doi: 10.1038/s42003-022-03693-2
58. Zhao JW, Sun ZQ, Zhang XY, Zhang Y, Liu J, Ye J, et al. Mycobacterial 3-hydroxyacyl-l-thioester dehydratase Y derived from Mycobacterium tuberculosis induces COX-2 expression in mouse macrophages through MAPK-NF-κB pathway. Immunol Lett. (2014) 161:125–32. doi: 10.1016/j.imlet.2014.05.013
59. Saleem S. Targeting MAPK signaling: a promising approach for treating inflammatory lung disease. Pathol Res Pract. (2024) 254:155122. doi: 10.1016/j.prp.2024.155122
60. Sun Y, Liu WZ, Liu T, Feng X, Yang N, Zhou HF. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J Recept Signal Transduct Res. (2015) 35:600–4. doi: 10.3109/10799893.2015.1030412
61. Lv X, Zheng L, Zhang T, Wang W, Chen Y, Li J, et al. CLCA1 exacerbates lung inflammation via p38 MAPK pathway in acute respiratory distress syndrome. Exp Lung Res. (2024) 50:85–95. doi: 10.1080/01902148.2024.2334262
62. Bao J, He Y, Yang C, Lu N, Li A, Gao S, et al. Inhibition of mycobacteria proliferation in macrophages by low cisplatin concentration through phosphorylated p53-related apoptosis pathway. PLoS ONE. (2023) 18:e0281170. doi: 10.1371/journal.pone.0281170
63. Abo-Kadoum MA, Assad M, Uae M, Nzaou SAE, Gong Z, Moaaz A, et al. Mycobacterium tuberculosis RKIP (Rv2140c) dephosphorylates ERK/NF-κB upstream signaling molecules to subvert macrophage innate immune response. Infect Genet Evol. (2021) 94:105019. doi: 10.1016/j.meegid.2021.105019
64. Asl ER, Amini M, Najafi S, Mansoori B, Mokhtarzadeh A, Mohammadi A, et al. Interplay between MAPK/ERK signaling pathway and MicroRNAs: A crucial mechanism regulating cancer cell metabolism and tumor progression. Life Sci. (2021) 278:119499. doi: 10.1016/j.lfs.2021.119499
66. Hammouda MB, Ford AE, Liu Y, Zhang JY. The JNK signaling pathway in inflammatory skin disorders and cancer. Cells. (2020) 9:857. doi: 10.3390/cells9040857
67. Guo YJ, Pan WW, Liu SB, Shen ZF, Xu Y, Hu LL. ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med. (2020) 19:1997–2007. doi: 10.3892/etm.2020.8454
68. Surewicz K, Aung H, Kanost RA, Jones L, Hejal R, Toossi Z. The differential interaction of p38 MAP kinase and tumor necrosis factor-alpha in human alveolar macrophages and monocytes induced by Mycobacterium tuberculois. Cell Immunol. (2004) 228:34–41. doi: 10.1016/j.cellimm.2004.03.007
69. Liu B, Zhang Y, Yang Z, Liu M, Zhang C, Zhao Y, et al. ω-3 DPA protected neurons from neuroinflammation by balancing microglia M1/M2 polarizations through inhibiting NF-κB/MAPK p38 signaling and activating neuron-BDNF-PI3K/AKT pathways. Mar Drugs. (2021) 19:587. doi: 10.3390/md19110587
70. Wen X, Jiao L, Tan H. MAPK/ERK pathway as a central regulator in vertebrate organ regeneration. Int J Mol Sci. (2022) 23:1464. doi: 10.3390/ijms23031464
71. Ke Z, Lu J, Zhu J, Yang Z, Jin Z, Yuan L. Down-regulation of lincRNA-EPS regulates apoptosis and autophagy in BCG-infected RAW264. 7 macrophages via JNK/MAPK signaling pathway. Infect Genet Evol. (2020) 77:104077. doi: 10.1016/j.meegid.2019.104077
72. Bhattacharya P, Gupta G, Majumder S, Adhikari A, Banerjee S, Halder K, et al. Arabinosylated lipoarabinomannan skews Th2 phenotype towards Th1 during Leishmania infection by chromatin modification: involvement of MAPK signaling. PLoS ONE. (2011) 6:e24141. doi: 10.1371/journal.pone.0024141
73. Chan ED, Morris KR, Belisle JT, Hill P, Remigio LK, Brennan PJ, et al. Induction of inducible nitric oxide synthase-NO* by lipoarabinomannan of Mycobacterium tuberculosis is mediated by MEK1-ERK, MKK7-JNK, and NF-kappaB signaling pathways. Infect Immun. (2001) 69:2001–10. doi: 10.1128/IAI.69.4.2001-2010.2001
74. Yang CS, Shin DM, Lee HM, Son JW, Lee SJ, Akira S, et al. ASK1-p38 MAPK-p47phox activation is essential for inflammatory responses during tuberculosis via TLR2-ROS signalling. Cell Microbiol. (2008) 10:741–54. doi: 10.1111/j.1462-5822.2007.01081.x
75. Roach TI, Barton CH, Chatterjee D, Liew FY, Blackwell JM. Opposing effects of interferon-gamma on iNOS and interleukin-10 expression in lipopolysaccharide- and mycobacterial lipoarabinomannan-stimulated macrophages. Immunology. (1995) 85:106–13.
76. Morris KR, Lutz RD, Choi HS, Kamitani T, Chmura K, Chan ED. Role of the NF-kappaB signaling pathway and kappaB cis-regulatory elements on the IRF-1 and iNOS promoter regions in mycobacterial lipoarabinomannan induction of nitric oxide. Infect Immun. (2003) 71:1442–52. doi: 10.1128/IAI.71.3.1442-1452.2003
77. Wang T, Fahrmann JF, Lee H, Li YJ, Tripathi SC, Yue C, et al. JAK/STAT3-regulated fatty acid β-oxidation is critical for breast cancer stem cell self-renewal and chemoresistance. Cell Metab. (2018) 27:136–150.e5. Erratum in: Cell Metab. (2018) 27:1357. doi: 10.1016/j.cmet.2017.11.001
78. Lin P, Shi HY, Lu YY, Lin J. Centella asiatica alleviates psoriasis through JAK/STAT3-mediated inflammation: an in vitro and in vivo study. J Ethnopharmacol. 2023; 317:116746. doi: 10.1016/j.jep.2023.116746
79. Jiang H, Yang J, Li T, Wang X, Fan Z, Ye Q, et al. JAK/STAT3 signaling in cardiac fibrosis: a promising therapeutic target. Front Pharmacol. (2024) 15:1336102. doi: 10.3389/fphar.2024.1336102
80. Xu J, Zhang J, Mao QF, Wu J, Wang Y. The interaction between autophagy and JAK/STAT3 signaling pathway in tumors. Front Genet. (2022) 13:880359. doi: 10.3389/fgene.2022.880359
81. Zegeye MM, Lindkvist M, Fälker K, Kumawat AK, Paramel G, Grenegård M, et al. Activation of the JAK/STAT3 and PI3K/AKT pathways are crucial for IL-6 trans-signaling-mediated pro-inflammatory response in human vascular endothelial cells. Cell Commun Signal. (2018) 16:55. doi: 10.1186/s12964-018-0268-4
82. Pan Q, Yan J, Liu Q, Yuan C, Zhang XL, A. single-stranded DNA aptamer against mannose-capped lipoarabinomannan enhances anti-tuberculosis activity of macrophages through downregulation of lipid-sensing nuclear receptor peroxisome proliferator-activated receptor γ expression. Microbiol Immunol. (2017) 61:92–102. doi: 10.1111/1348-0421.12470
83. Yuan C, Qu ZL, Tang XL, Liu Q, Luo W, Huang C, et al. Mycobacterium tuberculosis mannose-capped lipoarabinomannan induces IL-10-producing B cells and hinders CD4Th1 immunity. iScience. (2019) 11:13–30. doi: 10.1016/j.isci.2018.11.039
84. Qasimi P, Ming-Lum A, Ghanipour A, Ong CJ, Cox ME, Ihle J, et al. Divergent mechanisms utilized by SOCS3 to mediate interleukin-10 inhibition of tumor necrosis factor alpha and nitric oxide production by macrophages. J Biol Chem. (2006) 281:6316–24. doi: 10.1074/jbc.M508608200
85. Dennis V, Kaushal D, Dixit S, Mehra S, Singh S, et al. SOCS3 and IL-10 anti-inflammatory activity in Lyme disease. FASEB J. (2008) 22:860.17. doi: 10.1096/fasebj.22.1_supplement.860.17
86. Swierzko AS, Bartłomiejczyk MA, Brzostek A, Łukasiewicz J, Michalski M, Dziadek J, et al. Mycobacterial antigen 85 complex (Ag85) as a target for ficolins and mannose-binding lectin. Int J Med Microbiol. (2016) 306:212–21. doi: 10.1016/j.ijmm.2016.04.004
87. Yonekawa A, Saijo S, Hoshino Y, Miyake Y, Ishikawa E, Suzukawa M, et al. Dectin-2 is a direct receptor for mannose-capped lipoarabinomannan of mycobacteria. Immunity. (2014) 41:402–13. doi: 10.1016/j.immuni.2014.08.005
88. Goyal S, Klassert TE, Slevogt H. C-type lectin receptors in tuberculosis: what we know. Med Microbiol Immunol. (2016) 205:513–35. doi: 10.1007/s00430-016-0470-1
89. Kang PB, Azad AK, Torrelles JB, Kaufman TM, Beharka A, Tibesar E, et al. The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis. J Exp Med. (2005) 202:987–99. doi: 10.1084/jem.20051239
90. Bartlomiejczyk MA, Swierzko AS, Brzostek A, Dziadek J, Cedzynski M. Interaction of lectin pathway of complement-activating pattern recognition molecules with mycobacteria. Clin Exp Immunol. (2014) 178:310–9. doi: 10.1111/cei.12416
91. Kang BK, Schlesinger LS. Characterization of mannose receptor-dependent phagocytosis mediated by Mycobacterium tuberculosis lipoarabinomannan. Infect Immun. (1998) 66:2769–77. doi: 10.1128/IAI.66.6.2769-2777.1998
92. Schlesinger LS, Kaufman TM, Iyer S, Hull SR, Marchiando LK. Differences in mannose receptor-mediated uptake of lipoarabinomannan from virulent and attenuated strains of Mycobacterium tuberculosis by human macrophages. J Immunol. (1996) 157:4568–75. doi: 10.4049/jimmunol.157.10.4568
93. Richmond JM, Lee J, Green DS, Kornfeld H, Cruikshank WW. Mannose-capped lipoarabinomannan from Mycobacterium tuberculosis preferentially inhibits sphingosine-1-phosphate-induced migration of Th1 cells. J Immunol. (2012) 189:5886–95. doi: 10.4049/jimmunol.1103092
94. Vergne I, Chua J, Deretic V. Tuberculosis toxin blocking phagosome maturation inhibits a novel Ca2+/calmodulin-PI3K hVPS34 cascade. J Exp Med. (2003) 198:653–9. doi: 10.1084/jem.20030527
95. Maiti D, Bhattacharyya A, Basu J. Lipoarabinomannan from Mycobacterium tuberculosis promotes macrophage survival by phosphorylating Bad through a phosphatidylinositol 3-kinase/Akt pathway. J Biol Chem. (2001) 276:329–33. doi: 10.1074/jbc.M002650200
96. Bohsali A, Abdalla H, Velmurugan K, Briken V. The non-pathogenic mycobacteria M. smegmatis and M fortuitum induce rapid host cell apoptosis via a caspase-3 and TNF dependent pathway. BMC Microbiol. (2010) 10:237. doi: 10.1186/1471-2180-10-237
97. Chattree V, Khanna N, Bisht V, Rao DN. Inhibition of apoptosis, activation of NKT cell and upregulation of CD40 and CD40L mediated by M. leprae antigen(s) combined with Murabutide and Trat peptide in leprosy patients. Mol Cell Biochem. (2008) 309:87–97. doi: 10.1007/s11010-007-9646-8
98. Dominguez-Villar M, Gautron AS, de Marcken M, Keller MJ, Hafler DA. TLR7 induces anergy in human CD4(+) T cells. Nat Immunol. (2015) 16:118–28. doi: 10.1038/ni.3036
99. Karim AF, Sande OJ, Tomechko SE, Ding X, Li M, Maxwell S, et al. Proteomics and network analyses reveal inhibition of Akt-mTOR signaling in CD4+ T cells by mycobacterium tuberculosis mannose-capped lipoarabinomannan. Proteomics. (2017) 17:1700233. doi: 10.1002/pmic.201700233
100. Li Z, Miao Z, Ding L, Teng X, Bao J. Energy metabolism disorder mediated ammonia gas-induced autophagy via AMPK/mTOR/ULK1-Beclin1 pathway in chicken livers. Ecotoxicol Environ Saf. (2021) 217:112219. doi: 10.1016/j.ecoenv.2021.112219
101. Cao Y, Han J, Xiao Y, Wang Z, Zhang H, Fang R, et al. Xiao-Er-Kang-Du capsules regulate autophagy against the influenza B virus (Victoria strain) through the mTOR/ULK1/Beclin1/VPS34 pathway. J Ethnopharmacol. 2025; 337(Pt 2):118872. doi: 10.1016/j.jep.2024.118872
102. Deesrisak K, Yingchutrakul Y, Krobthong S, Roytrakul S, Chatupheeraphat C, Subkorn P, et al. Bioactive peptide isolated from sesame seeds inhibits cell proliferation and induces apoptosis and autophagy in leukemic cells. EXCLI J. (2021) 20:709–21. doi: 10.17179/excli2021-3406
103. Chen WL, Lee KL, Lai KS, Tsai JH, Hsiao SH, Chung CL. Toll-like receptor 2 mediates VEGF overexpression and mesothelial hyperpermeability in tuberculous pleural effusion. Int J Mol Sci. (2023) 24:2846. doi: 10.3390/ijms24032846
104. Liu J, Zeng Y, Ma W, Chen S, Zheng Y, Ye S, et al. Preliminary investigation of the clinical value of vascular endothelial growth factor and hypoxia-inducible factor-1alpha in pericardial fluid in diagnosing malignant and tuberculous pericardial effusion. Cardiology. (2010) 116:37–41. doi: 10.1159/000313465
105. Wang Y, Wang L, Wen Z, Wang J, Zhu Y, Chen H, et al. High IL-6 and VEGF-A levels correlate with delayed wound healing in cervical lymph node tuberculosis patients. Int J Tuberc Lung Dis. (2018) 22:1227–32. doi: 10.5588/ijtld.18.0027
106. Martins CP, Carvalho FR, Faustino R, Medeiros T, Rosário NFD, Schmidt CM, et al. Vascular endothelial growth factor (VEGF) and interleukin-1 receptor antagonist (IL-1Ra) as promising biomarkers for distinguishing active from latent tuberculosis in children and adolescents. Tuberculosis (Edinb). 2022; 134:102205. doi: 10.1016/j.tube.2022.102205
107. Delemarre EM, van Hoorn L, Bossink AWJ, Drylewicz J, Joosten SA, Ottenhoff THM, et al. Serum biomarker profile including CCL1, CXCL10, VEGF, and adenosine deaminase activity distinguishes active from remotely acquired latent tuberculosis. Front Immunol. (2021) 12:725447. doi: 10.3389/fimmu.2021.725447
108. Saghazadeh A, Rezaei N. Vascular endothelial growth factor levels in tuberculosis: a systematic review and meta-analysis. PLoS ONE. (2022) 17:e0268543. doi: 10.1371/journal.pone.0268543
109. Isiguzo G, Du Bruyn E, Howlett P, Ntsekhe M. Diagnosis and management of tuberculous pericarditis: what is new? Curr Cardiol Rep. (2020) 22:2. doi: 10.1007/s11886-020-1254-1
110. Santoso A, Rasiha R, Zainal ATF, Khairunnisa IN, Fais MK, Gunawan AMAK. Transforming growth factor-β and matrix metalloproteinases as potential biomarkers of fibrotic lesions induced by tuberculosis: a systematic review and meta-analysis. BMJ Open. (2023) 13:e070377. doi: 10.1136/bmjopen-2022-070377
111. McCaffrey EF, Donato M, Keren L, Chen Z, Delmastro A, Fitzpatrick MB, et al. The immunoregulatory landscape of human tuberculosis granulomas. Nat Immunol. (2022) 23:318–29. Erratum in: Nat Immunol. (2022) 23:814. doi: 10.1038/s41590-021-01121-x
112. Wang J, Xue T, Ye H, Sang C, Wu S, Li S. Study of the common activating mechanism of apoptosis and epithelial-to-mesenchymal transition in alveolar type II epithelial cells. Respir Physiol Neurobiol. (2021) 284:103584. doi: 10.1016/j.resp.2020.103584
113. Yang Z, Lou C, Wang X, Wang C, Shi Z, Niu N. Preparation, characterization, and in-vitro cytotoxicity of nanoliposomes loaded with anti-tubercular drugs and TGF-β1 siRNA for improving spinal tuberculosis therapy. BMC Infect Dis. (2022) 22:824. doi: 10.1186/s12879-022-07791-8
114. Singh M, Vaughn C, Sasaninia K, Yeh C, Mehta D, Khieran I, et al. Understanding the relationship between glutathione, TGF-β, and vitamin D in combating mycobacterium tuberculosis infections. J Clin Med. (2020) 9:2757. doi: 10.3390/jcm9092757
115. Gaur RL, Ren K, Blumenthal A, Bhamidi S, González-Nilo FD, Jackson M, et al. LprG-mediated surface expression of lipoarabinomannan is essential for virulence of Mycobacterium tuberculosis. PLoS Pathog. (2014) 10:e1004376. Erratum in: PLoS Pathog. (2014) 10:e1004489. Erratum in: PLoS Pathog. (2014) 10:e1004494. Gibbs, Sara [corrected to González-Nilo, Fernando D]. Erratum in: PLoS Pathog. (2015) 11:e1005336. doi: 10.1371/journal.ppat.1004376
116. Hohl M, Remm S, Eskandarian HA, Dal Molin M, Arnold FM, Hürlimann LM, et al. Increased drug permeability of a stiffened mycobacterial outer membrane in cells lacking MFS transporter Rv1410 and lipoprotein LprG. Mol Microbiol. (2019) 111:1263–82. doi: 10.1111/mmi.14220
117. Shi W, Chen J, Zhang S, Zhang W, Zhang Y. Identification of Novel Mutations in LprG (rv1411c), rv0521, rv3630, rv0010c, ppsC, and cyp128 associated with pyrazinoic acid/pyrazinamide resistance in mycobacterium tuberculosis. Antimicrob Agents Chemother. (2018) 62:e00430–18. doi: 10.1128/AAC.00430-18
118. Machaba KE, Mhlongo NN, Soliman MES. Induced mutation proves a potential target for TB therapy: a molecular dynamics study on LprG. Cell Biochem Biophys. (2018) 76:345–56. doi: 10.1007/s12013-018-0852-7
119. Nisbett LM, Previti ML, Seeliger JC, A. Loss of function in LprG-Rv1410c homologues attenuates growth during biofilm formation in Mycobacterium smegmatis. Pathogens. (2023) 12:1375. doi: 10.3390/pathogens12121375
120. Touchette MH, Van Vlack ER, Bai L, Kim J, Cognetta AB. 3rd, Previti ML, Backus KM, Martin DW, Cravatt BF, Seeliger JC. A screen for protein-protein interactions in live mycobacteria reveals a functional link between the virulence-associated lipid transporter LprG and the mycolyltransferase antigen 85A. ACS Infect Dis. (2017) 3:336–48. doi: 10.1021/acsinfecdis.6b00179
121. Martinot AJ, Farrow M, Bai L, Layre E, Cheng TY, Tsai JH, et al. Mycobacterial metabolic syndrome: LprG and Rv1410 regulate triacylglyceride levels, growth rate and virulence in mycobacterium tuberculosis. PLoS Pathog. (2016) 12:e1005351. doi: 10.1371/journal.ppat.1005351
122. Remm S, De Vecchis D, Schöppe J, Hutter CAJ, Gonda I, Hohl M, et al. Structural basis for triacylglyceride extraction from mycobacterial inner membrane by MFS transporter Rv1410. Nat Commun. (2023) 14:6449. doi: 10.1038/s41467-023-42073-0
123. Bai L, Parkin LA, Zhang H, Shum R, Previti ML, Seeliger JC. Dimethylaminophenyl hydrazides as inhibitors of the lipid transport protein LprG in mycobacteria. ACS Infect Dis. (2020) 6:637–48. doi: 10.1021/acsinfecdis.9b00497
124. Ryan JM, Shelton K, Dzieciatkowska M, Kruh-Garcia N, Dobos KM. Establishment of minimum protein standards for Mycobacterium tuberculosis-derived extracellular vesicles through comparison of EV enrichment methods. Mycobacteria. (2025) 1:3. doi: 10.1186/s44350-025-00003-8
125. Espinosa-Cueto P, Magallanes-Puebla A, Mancilla R. Phosphate starvation enhances phagocytosis of Mycobacterium bovis/BCG by macrophages. BMC Immunol. (2020) 21:34. doi: 10.1186/s12865-020-00364-x
126. Nain Z, Karim MM, Sen MK, Adhikari UK. Structural basis and designing of peptide vaccine using PE-PGRS family protein of Mycobacterium ulcerans-An integrated vaccinomics approach. Mol Immunol. (2020) 120:146–63. doi: 10.1016/j.molimm.2020.02.009
127. Zheng W, LaCourse SM, Song B, Singh DK, Khanna M, Olivo J, et al. Diagnosis of paediatric tuberculosis by optically detecting two virulence factors on extracellular vesicles in blood samples. Nat Biomed Eng. (2022) 6:979–91. doi: 10.1038/s41551-022-00922-1
128. Zhang TH, Ma ZC, Liu RM, Shang YY, Ma LP, Han M, et al. [Evaluation of the efficacy of urine-based lipoarabinomannan antigen test in the diagnosis of pulmonary tuberculosis]. Zhonghua Jie He He Hu Xi Za Zhi. (2024) 47:132–6. doi: 10.3760/cma.j.cn112147-20230814-00074
129. Sossen B, Ryan A, Bielawski J, Greyling R, Matthews G, Hurribunce-James S, et al. Urine lipoarabinomannan for rapid tuberculosis diagnosis in HIV-infected adult outpatients in Khayelitsha. South Afr J HIV Med. (2021) 22:1226. doi: 10.4102/sajhivmed.v22i1.1226
130. Osório DV, Munyangaju I, Muhiwa A, Nacarapa E, Nhangave AV, Ramos JM. Lipoarabinomannan Antigen Assay (TB-LAM) for diagnosing pulmonary tuberculosis in children with severe acute malnutrition in Mozambique. J Trop Pediatr. (2021) 67:fmaa072. doi: 10.1093/tropej/fmaa072
131. Mangu C, Cossa M, Ndege R, Khosa C, Leukes V, de la Torre-Pérez L, et al. Expanding Xpert MTB/RIF Ultra® and LF-LAM testing for diagnosis of tuberculosis among HIV-positive adults admitted to hospitals in Tanzania and Mozambique: a randomized controlled trial (the EXULTANT trial). BMC Infect Dis. (2024) 24:831. doi: 10.1186/s12879-024-09651-z
132. de Vasconcellos K, Ramjathan P, Singh D. The utility of point-of-care urinary lipoarabinomannan testing for the diagnosis of tuberculosis in critically ill patients: a prospective observational study. BMC Infect Dis. (2021) 21:281. doi: 10.1186/s12879-021-05979-y
133. Chernick L, Kalla IS, Venter M. Clinical, radiological, and laboratory predictors of a positive urine lipoarabinomannan test in sputum-scarce and sputum-negative patients with HIV-associated tuberculosis in two Johannesburg hospitals. South Afr J HIV Med. (2021) 22:1234. doi: 10.4102/sajhivmed.v22i1.1234
134. Bjerrum S, Åhsberg J, Szekely R, Opintan J, Lartey M, Shah M, et al. Diagnostic accuracy of urine lipoarabinomannan testing in early morning urine versus spot urine for diagnosis of tuberculosis among people with HIV. Microbiol Spectr. (2022) 10:e0020822. doi: 10.1128/spectrum.00208-22
135. Kamra E, Prasad T, Rais A, Dahiya B, Sheoran A, Soni A, et al. Diagnosis of genitourinary tuberculosis: detection of mycobacterial lipoarabinomannan and MPT-64 biomarkers within urine extracellular vesicles by nano-based immuno-PCR assay. Sci Rep. (2023) 13:11560. doi: 10.1038/s41598-023-38740-3
136. Wobudeya E, Nanfuka M, Ton Nu Nguyet MH, Taguebue JV, Moh R, Breton G, et al. Effect of decentralising childhood tuberculosis diagnosis to primary health centre versus district hospital levels on disease detection in children from six high tuberculosis incidence countries: an operational research, pre-post intervention study. EClinicalMedicine. (2024) 70:102527. doi: 10.1016/j.eclinm.2024.102527
137. Nicol MP, Schumacher SG, Workman L, Broger T, Baard C, Prins M, et al. Accuracy of a novel urine test, Fujifilm SILVAMP tuberculosis lipoarabinomannan, for the diagnosis of pulmonary tuberculosis in children. Clin Infect Dis. (2021) 72:e280–8. doi: 10.1093/cid/ciaa1052
138. Comella-Del-Barrio P, Molina-Moya B, Gautier J, Villar-Hernández R, Doresca MJC, Sallés-Mingels B, et al. Diagnostic performance of the Fujifilm SILVAMP TB-LAM in children with presumptive tuberculosis. J Clin Med. (2021) 10:1914. doi: 10.3390/jcm10091914
139. Chen YL, Zhu MM, Guan CP, Zhang YA, Wang MS. Diagnostic value of the cerebrospinal fluid lipoarabinomannan assay for tuberculous meningitis: a systematic review and meta-analysis. Front Public Health. (2023) 11:1228134. doi: 10.3389/fpubh.2023.1228134
140. Drain PK, Niu X, Shapiro AE, Magcaba ZP, Ngcobo Z, Ngwane MW, et al. Real-world diagnostic accuracy of lipoarabinomannan in three non-sputum biospecimens for pulmonary tuberculosis disease. EBioMedicine. (2024) 108:105353. doi: 10.1016/j.ebiom.2024.105353
141. Seid G, Alemu A, Tsedalu T, Dagne B. Value of urine-based lipoarabinomannan (LAM) antigen tests for diagnosing tuberculosis in children: systematic review and meta-analysis. IJID Reg. (2022) 4:97–104. doi: 10.1016/j.ijregi.2022.06.004
142. Székely R, Sossen B, Mukoka M, Muyoyeta M, Nakabugo E, Hella J, et al. Prospective multicentre accuracy evaluation of the FUJIFILM SILVAMP TB LAM test for the diagnosis of tuberculosis in people living with HIV demonstrates lot-to-lot variability. PLoS ONE. (2024) 19:e0303846. doi: 10.1371/journal.pone.0303846
143. Sood M, Sharma S, Sood S, Sharma V. Diagnostic accuracy of urine based lipoarabinomannan point of care tuberculosis diagnostic test in HIV negative children: a systematic review and meta-analysis. Diagn Microbiol Infect Dis. (2023) 105:115879. doi: 10.1016/j.diagmicrobio.2022.115879
144. Huerga H, Bastard M, Lubega AV, Akinyi M, Antabak NT, Ohler L, et al. Novel FujiLAM assay to detect tuberculosis in HIV-positive ambulatory patients in four African countries: a diagnostic accuracy study. Lancet Glob Health. (2023) 11:e126–35. doi: 10.1016/S2214-109X(22)00463-6
145. Fekadu G, Wang Y, You JHS. Standard diagnostics with and without urine-based lipoarabinomannan testing for tuberculosis disease in HIV-infected patients in a high-burden setting-A cost-effectiveness analysis. PLoS ONE. (2023) 18:e0288605. doi: 10.1371/journal.pone.0288605
146. Zhang Y, Chen S, Wei H, Zhong Q, Yuan Y, Wang Y, et al. Breakthrough of chemiluminescence-based LAM urine test beyond HIV-positive individuals: Clinical diagnostic value of pulmonary tuberculosis in the general population. Medicine (Baltimore). (2023) 102:e36371. doi: 10.1097/MD.0000000000036371
147. Gao M, Wu Q, Wang X, Sun X, Li M, Bai G. Advancements in LAM-based diagnostic kit for tuberculosis detection: enhancing TB diagnosis in HIV-negative individuals. Front Microbiol. (2024) 15:1367092. doi: 10.3389/fmicb.2024.1367092
148. Simieneh A, Tadesse M, Kebede W, Gashaw M, Abebe G. Combination of Xpert® MTB/RIF and DetermineTM TB-LAM Ag improves the diagnosis of extrapulmonary tuberculosis at Jimma University Medical Center, Oromia, Ethiopia. PLoS ONE. (2022) 17:e0263172. doi: 10.1371/journal.pone.0263172
149. Mapamba DA, Sauli E, Lalashowi J, Buza J, John J, Mwaisango Z, et al. Performance of tuberculosis molecular bacterial load assay compared to Alere TB-LAM in urine of pulmonary tuberculosis patients with HIV co-infections. Int J Mol Sci. (2023) 24:3715. doi: 10.3390/ijms24043715
150. Shapiro AE, Olson AM, Kidoguchi L, Niu X, Ngcobo Z, Magcaba ZP, et al. Complementary nonsputum diagnostic testing for tuberculosis in people with HIV using oral swab PCR and urine lipoarabinomannan detection. J Clin Microbiol. (2022) 60:e0043122. doi: 10.1128/jcm.00431-22
151. Broger T, Nicol MP, Sigal GB, Gotuzzo E, Zimmer AJ, Surtie S, et al. Diagnostic accuracy of 3 urine lipoarabinomannan tuberculosis assays in HIV-negative outpatients. J Clin Invest. (2020) 130:5756–64. doi: 10.1172/JCI140461
152. Broger T, Muyoyeta M, Kerkhoff AD, Denkinger CM, Moreau E. Tuberculosis test results using fresh versus biobanked urine samples with FujiLAM. Lancet Infect Dis. (2020) 20:22–3. doi: 10.1016/S1473-3099(19)30684-X
153. Nkereuwem E, Togun T, Gomez MP, Székely R, Macé A, Jobe D, et al. Reach4KidsAfrica (R4KA) Consortium. Comparing accuracy of lipoarabinomannan urine tests for diagnosis of pulmonary tuberculosis in children from four African countries: a cross-sectional study. Lancet Infect Dis. (2021) 21:376–84. doi: 10.1016/S1473-3099(20)30598-3
154. Yin X, Ye QQ, Wu KF, Zeng JY Li NX, Mo JJ, Huang PY, et al. Diagnostic value of Lipoarabinomannan antigen for detecting Mycobacterium tuberculosis in adults and children with or without HIV infection. J Clin Lab Anal. (2022) 36:e24238. doi: 10.1002/jcla.24238
155. Burke RM, Nyirenda S, Twabi HH, Nliwasa M, Joekes E, Walker N, et al. Design and protocol for a cluster randomised trial of enhanced diagnostics for tuberculosis screening among people living with HIV in hospital in Malawi (CASTLE study). PLoS ONE. (2022) 17:e0261877. doi: 10.1371/journal.pone.0261877
156. Lyu M, Zhou Y, Chen Y, Lai H, Wang Y, Cheng Y, et al. Exploring the eligibility of all reported lipoarabinomannan-testing assays in different clinical situations: a systematic review and meta-analysis of 97 articles. Int J Infect Dis. (2022) 125:19–34. doi: 10.1016/j.ijid.2022.10.015
157. Panraksa Y, Amin AG, Graham B, Henry CS, Chatterjee D. Immobilization of Proteinase K for urine pretreatment to improve diagnostic accuracy of active tuberculosis. PLoS ONE. (2021) 16:e0257615. doi: 10.1371/journal.pone.0257615
158. Clarke E, Robinson R, Laurentius LB, Porter MD. Proteinase K Pretreatment for the quantitative recovery and sensitive detection of the tuberculosis biomarker mannose-capped lipoarabinomannan spiked into human serum. Anal Chem. (2023) 95:9191–98. doi: 10.1021/acs.analchem.3c00214
159. Amin AG De P, Graham B, Calderon RI, Franke MF, Chatterjee D. Urine lipoarabinomannan in HIV uninfected, smear negative, symptomatic TB patients: effective sample pretreatment for a sensitive immunoassay and mass spectrometry. Sci Rep. (2021) 11:2922. doi: 10.1038/s41598-021-82445-4
160. Huang H, Qu R, Wu K, Xu J, Li J, Lu S, et al. Proteinase K-pretreated ConA-based ELISA assay: a novel urine LAM detection strategy for TB diagnosis. Front Microbiol. (2023) 14:1236599. doi: 10.3389/fmicb.2023.1236599
161. Lambert CJ, Clarke E, Patel D, Laurentius LB, Gale BK, Sant HJ, et al. Microfluidic platform for the enzymatic pretreatment of human serum for the detection of the tuberculosis biomarker mannose-capped lipoarabinomannan. Anal Methods. (2024) 16:5475–81. doi: 10.1039/D4AY00772G
162. Rotake DR, Zalke JB, Gechode HV, Peshkar SM, Singh SG. Cost-effective chemiresistive biosensor with MWCNT-ZnO nanofibers for early detection of tuberculosis (TB) lipoarabinomannan (LAM) antigen. Mikrochim Acta. (2024) 191:714. doi: 10.1007/s00604-024-06780-9
163. Ullah U, Saleem S, Farooq M, Yameen B, Cheema MI. Lipoarabinomannan-based tuberculosis diagnosis using a fiber cavity ring down biosensor. Biomed Opt Express. (2024) 15:1428–36. doi: 10.1364/BOE.516892
164. M D, Bandaru R, Janakiraman V, Sai VVR. A plasmonic fiberoptic absorbance biosensor for mannose-capped lipoarabinomannan based tuberculosis diagnosis. Biosens Bioelectron. 2020; 167:112488. doi: 10.1016/j.bios.2020.112488
165. Wood A, Barizuddin S, Darr CM, Mathai CJ, Ball A, Minch K, et al. Ultrasensitive detection of lipoarabinomannan with plasmonic grating biosensors in clinical samples of HIV negative patients with tuberculosis. PLoS ONE. (2019) 14:e0214161. doi: 10.1371/journal.pone.0214161
166. Huang Y, Darr CM, Gangopadhyay K, Gangopadhyay S, Bok S, Chakraborty S. Applications of machine learning tools for ultra-sensitive detection of lipoarabinomannan with plasmonic grating biosensors in clinical samples of tuberculosis. PLoS ONE. (2022) 17:e0275658. doi: 10.1371/journal.pone.0275658
167. Lam B, Amer L, Thompson E, Clark AE, Garretson AF, Carlin AF, et al. Matrix-insensitive sensor arrays via peptide-coated nanoparticles: rapid saliva screening for pathogens in oral and respiratory diseases. ACS Appl Mater Interfaces. (2024) 16:67362–72. doi: 10.1021/acsami.4c15662
168. Pan B, He Q, Yu X, De Choch D, Lam KS, Hammock BD, et al. Versatility and stability of melamine foam-based biosensors (f-ELISA) using antibodies, nanobodies, and peptides as sensing probes. Talanta. (2024) 279:126634. doi: 10.1016/j.talanta.2024.126634
169. Meng Y, Wang Y, Zhan Z, Chen Y, Zhang C, Peng W, et al. Fructose@histone synergistically improve the performance of DNA-templated Cu NPs: rapid analysis of LAM in tuberculosis urine samples using a handheld fluorometer and a smartphone RGB camera. J Mater Chem B. (2024) 12:6668–77. doi: 10.1039/D4TB00693C
170. Chen P, Meng Y, Liu T, Peng W, Gao Y, He Y, et al. Sensitive urine immunoassay for visualization of lipoarabinomannan for noninvasive tuberculosis diagnosis. ACS Nano. (2023) 17:6998–7006. doi: 10.1021/acsnano.3c01374
171. Cantera JL, Lillis LM, Peck RB, Moreau E, Schouten JA, Davis P, et al. Performance of novel antibodies for lipoarabinomannan to develop diagnostic tests for Mycobacterium tuberculosis. PLoS ONE. (2022) 17:e0274415. Erratum in: PLoS ONE. 2024; 19:e0297828. doi: 10.1371/journal.pone.0274415
172. Hoa NB, Fajans M, Nguyen Van H, Vu Ngoc B, Nguyen Viet N, et al. Urine lipoarabinomannan concentrations among HIV-negative adults with pulmonary or extrapulmonary tuberculosis disease in Vietnam. PLOS Glob Public Health. (2024) 4:e0003891. doi: 10.1371/journal.pgph.0003891
173. Magni R, Rruga F, Alsaab FM, Sharif S, Howard M, Espina V, et al. Lipoarabinomannan antigenic epitope differences in tuberculosis disease subtypes. Sci Rep. (2020) 10:13944. Erratum in: Sci Rep. (2021) 11:19546. doi: 10.1038/s41598-021-98304-1
174. Akinaga A, Takahashi M, Yamazaki T, Chikamatsu K, Matsushita S, Hashimoto Y, et al. Development and preliminary evaluation toward a new tuberculosis treatment monitoring tool: the PATHFAST TB LAM Ag assay. J Clin Microbiol. (2024) 62:e0062924. doi: 10.1128/jcm.00629-24
175. Li Y, Ru Z, Wei H, Wu M, Xie G, Lou J, et al. Improving the diagnosis of active tuberculosis: a novel approach using magnetic particle-based chemiluminescence LAM assay. BMC Pulm Med. (2024) 24:100. doi: 10.1186/s12890-024-02893-2
176. Yan Z, Wang J, Pang Y, Wang X, Yi L, Wei P, et al. Immunoassay with Novel Paired Antibodies for Detection of Lipoarabinomannan in the Pleural Fluid and Plasma of Patients with Tuberculous Pleurisy. Microorganisms. (2023) 11:2259. doi: 10.3390/microorganisms11092259
177. Yan ZH, Zhao B, Pang Y, Wang XJ Yi L, Wang HL, Yang B, et al. Generation of mycobacterial lipoarabinomannan-specific monoclonal antibodies and their ability to identify mycobacterium isolates. J Microbiol Immunol Infect. (2021) 54:437–46. doi: 10.1016/j.jmii.2020.02.005
178. van der Horst M, Karamchand L, Bauer WS, Nel AJM, Blackburn JM, Wright DW. The cyanobacterial lectin, microvirin-N, enhances the specificity and sensitivity of lipoarabinomannan-based TB diagnostic tests. Analyst. (2021) 146:1207–15. doi: 10.1039/D0AN01725F
179. Ketema W, Woubishet K, Tesfaye S, Gutema S, Taye K, Shibeshi MS, et al. Breakthrough in the challenges of tuberculosis diagnosis: lateral flow urine Lipoarabinomannan (LAM) assay for the diagnosis of active tuberculosis in a subset of Human Immuno Deficiency Virus (HIV) patients at Hawassa university comprehensive specialized hospital, Hawassa, Ethiopia. Int Med Case Rep J. (2022) 15:393–7. doi: 10.2147/IMCRJ.S373197
180. Dhana A, Hamada Y, Kengne AP, Kerkhoff AD, Broger T, Denkinger CM, et al. Diagnostic accuracy of WHO screening criteria to guide lateral-flow lipoarabinomannan testing among HIV-positive inpatients: a systematic review and individual participant data meta-analysis. J Infect. (2022) 85:40–8. doi: 10.1016/j.jinf.2022.05.010
181. Huerga H, Mathabire Rucker SC, Bastard M, Mpunga J, Amoros Quiles I, Kabaghe C, et al. Urine lipoarabinomannan testing for All HIV patients hospitalized in medical wards identifies a large proportion of patients with tuberculosis at risk of death. Open Forum Infect Dis. (2020) 8:ofaa639. doi: 10.1093/ofid/ofaa639
182. Lin M, Zhou H, Li R, Quan LL, Jin Z, Tong XW. Analysis of the mechanism of action of kushen in the treatment of tuberculosis based on network pharmacology. Altern Ther Health Med. (2023) 29:155–61.
183. He W, Sun J, Zhang Q, Li Y, Fu Y, Zheng Y, et al. Andrographolide exerts anti-inflammatory effects in Mycobacterium tuberculosis-infected macrophages by regulating the Notch1/Akt/NF-κB axis. J Leukoc Biol. (2020) 108:1747–64. doi: 10.1002/JLB.3MA1119-584RRR
184. Fu Y, Shen J, Liu F, Zhang H, Zheng Y, Jiang X. Andrographolide suppresses pyroptosis in mycobacterium tuberculosis-infected macrophages via the microRNA-155/Nrf2 Axis. Oxid Med Cell Longev. (2022) 2022:1885066. doi: 10.1155/2022/1885066
185. Cebani L, Mvubu NE. Can we exploit inflammasomes for host-directed therapy in the fight against Mycobacterium tuberculosis Infection? Int J Mol Sci. (2024) 25:8196. doi: 10.3390/ijms25158196
186. Nama MA. Gene silencing of toll-like receptor 2 gene expression as a tactic to control mycobacterium tuberculosis and granuloma formation. Arch Razi Inst. (2022) 77:827–34. doi: 10.22092/ARI.2022.357152.1984
187. Prabhu P, Fernandes T, Damani M, Chaubey P, Narayanan S, Sawarkar S. 2 Receptor specific ligand conjugated nanocarriers: an effective strategy for targeted therapy of tuberculosis. Curr Drug Deliv. (2022) 19:830–45. doi: 10.2174/1567201819666211216141942
188. Pawde DM, Viswanadh MK, Mehata AK, Sonkar R, Narendra, Poddar S, et al. Mannose receptor targeted bioadhesive chitosan nanoparticles of clofazimine for effective therapy of tuberculosis. Saudi Pharm J. (2020) 28:1616–25. doi: 10.1016/j.jsps.2020.10.008
189. Dingare C, Cao D, Yang JJ, Sozen B, Steventon B. Mannose controls mesoderm specification and symmetry breaking in mouse gastruloids. Dev Cell. (2024) 59:1523–37.e6. doi: 10.1016/j.devcel.2024.03.031
190. Zhang W, He J, Zheng D, Zhao P, Wang Y, Zhao J, et al. Immunomodulatory activity and its mechanisms of two polysaccharides from Poria cocos. Molecules. (2023) 29:50. doi: 10.3390/molecules29010050
191. Zhao P, Fan Y, Wang Z, Tang H, Tian Y, Zhang Y. Enhanced cancer immunotherapy by bacterial cytoplasmic membranes coated nanovaccines for co-delivery of ovalbumin antigen and immune adjuvants to dendritic cells in lymph nodes. Int J Nanomedicine. (2025) 20:2289–304. doi: 10.2147/IJN.S496873
192. Feng H, Feng Y, Lin L, Wu D, Liu Q, Li H, et al. Mannose receptor-mediated carbon nanotubes as an antigen delivery system to enhance immune response both in vitro and in vivo. Int J Mol Sci. (2022) 23:4239. Erratum in: Int J Mol Sci. (2025) 26:3975. doi: 10.3390/ijms26093975
193. Costa A, Sarmento B, Seabra V. Mannose-functionalized solid lipid nanoparticles are effective in targeting alveolar macrophages. Eur J Pharm Sci. (2018) 114:103–13. doi: 10.1016/j.ejps.2017.12.006
194. Truzzi E, Nascimento TL, Iannuccelli V, Costantino L, Lima EM, Leo E, et al. In Vivo biodistribution of respirable solid lipid nanoparticles surface-decorated with a mannose-based surfactant: a promising tool for pulmonary tuberculosis treatment? Nanomaterials. (2020) 10:568. doi: 10.3390/nano10030568
195. Wang Y, Liu Y, Long M, Dong Y, Li L, Zhou X. Nanoparticles target M2 macrophages to silence kallikrein-related peptidase 12 for the treatment of tuberculosis and drug-resistant tuberculosis. Acta Biomater. (2024) 188:358–73. doi: 10.1016/j.actbio.2024.09.026
196. Durán V, Grabski E, Hozsa C, Becker J, Yasar H, Monteiro JT, et al. Fucosylated lipid nanocarriers loaded with antibiotics efficiently inhibit mycobacterial propagation in human myeloid cells. J Control Release. (2021) 334:201–12. doi: 10.1016/j.jconrel.2021.04.012
197. Vieira ACC, Chaves LL, Pinheiro M, Lima SAC, Ferreira D, Sarmento B, et al. Mannosylated solid lipid nanoparticles for the selective delivery of rifampicin to macrophages. Artif Cells Nanomed Biotechnol. (2018) 46:653–63. doi: 10.1080/21691401.2018.1434186
198. Ning B, Shen J, Liu F, Zhang H, Jiang X. baicalein Suppresses NLRP3 and AIM2 inflammasome-mediated pyroptosis in macrophages infected by mycobacterium tuberculosis via induced autophagy. Microbiol Spectr. (2023) 11:e0471122. doi: 10.1128/spectrum.04711-22
199. Sun J, Zhang Q, Yang G, Li Y, Fu Y, Zheng Y, et al. The licorice flavonoid isoliquiritigenin attenuates Mycobacterium tuberculosis-induced inflammation through Notch1/NF-κB and MAPK signaling pathways. J Ethnopharmacol. (2022) 294:115368. doi: 10.1016/j.jep.2022.115368
200. Li J, Zhao A, Tang J, Wang G, Shi Y, Zhan L, et al. Tuberculosis vaccine development: from classic to clinical candidates. Eur J Clin Microbiol Infect Dis. (2020) 39:1405–25. doi: 10.1007/s10096-020-03843-6
201. Ishwarlall TZ, Adeleke VT, Maharaj L, Okpeku M, Adeniyi AA, Adeleke MA. Identification of potential candidate vaccines against Mycobacterium ulcerans based on the major facilitator superfamily transporter protein. Front Immunol. (2022) 13:1023558. doi: 10.3389/fimmu.2022.1023558
202. Martinot AJ, Blass E, Yu J, Aid M, Mahrokhian SH, Cohen SB, et al. Protective efficacy of an attenuated Mtb ΔLprG vaccine in mice. PLoS Pathog. (2020) 16:e1009096. doi: 10.1371/journal.ppat.1009096
203. Kumar A, Sharma P, Arun A, Meena LS. Development of peptide vaccine candidate using highly antigenic PE-PGRS family proteins to stimulate the host immune response against Mycobacterium tuberculosis H37Rv: an immuno-informatics approach. J Biomol Struct Dyn. (2023) 41:3382–404. doi: 10.1080/07391102.2022.2048079
204. Correia-Neves M, Sundling C, Cooper A, Källenius G. Lipoarabinomannan in active and passive protection against tuberculosis. Front Immunol. (2019) 10:1968. doi: 10.3389/fimmu.2019.01968
205. Yan Z, Wang X, Yi L, Yang B, Wei P, Ruan H, et al. Enhanced serum IgG detection potential using 38KD-MPT32-MPT64, CFP10-Mtb81-EspC fusion protein and Lipoarabinomannan (LAM) for human tuberculosis. Pathogens. (2022) 11:1545. doi: 10.3390/pathogens11121545
206. Zhou KL Li X, Zhang XL, Pan Q. Mycobacterial mannose-capped lipoarabinomannan: a modulator bridging innate and adaptive immunity. Emerg Microbes Infect. (2019) 8:1168–77. doi: 10.1080/22221751.2019.1649097
207. Dai J, Su Y, Zhong S, Cong L, Liu B, Yang J, et al. Exosomes: key players in cancer and potential therapeutic strategy. Signal Transduct Target Ther. (2020) 5:145. doi: 10.1038/s41392-020-00261-0
208. Mishra S, Gala J, Chacko J. Factors affecting mortality in critically Ill patients with tuberculosis: a systematic review and meta-analysis. Crit Care Med. (2024) 52:e304-e313. doi: 10.1097/CCM.0000000000006226
209. Akalu TY, Clements ACA, Wolde HF, Alene KA. Prevalence of long-term physical sequelae among patients treated with multi-drug and extensively drug-resistant tuberculosis: a systematic review and meta-analysis. EClinicalMedicine. (2023) 57:101900. doi: 10.1016/j.eclinm.2023.101900
210. Broger T, Koeppel L, Huerga H, Miller P, Gupta-Wright A, Blanc FX, et al. Diagnostic yield of urine lipoarabinomannan and sputum tuberculosis tests in people living with HIV: a systematic review and meta-analysis of individual participant data. Lancet Glob Health. (2023) 11:e903–16. doi: 10.2139/ssrn.4243688
211. Ehrt S, Schnappinger D, Rhee KY. Metabolic principles of persistence and pathogenicity in Mycobacterium tuberculosis. Nat Rev Microbiol. (2018) 16:496–507. doi: 10.1038/s41579-018-0013-4
212. Salazar F, Hall L, Negm OH, Awuah D, Tighe PJ, Shakib F, et al. The mannose receptor negatively modulates the Toll-like receptor 4-aryl hydrocarbon receptor-indoleamine 2,3-dioxygenase axis in dendritic cells affecting T helper cell polarization. J Allergy Clin Immunol. (2016) 137:1841–51.e2. doi: 10.1016/j.jaci.2015.10.033
213. Ouyang A, Wang H, Su J, Liu X. Mannose receptor mediates the activation of chitooligosaccharides on blunt snout bream (Megalobrama amblycephala) macrophages. Front Immunol. (2021) 12:686846. doi: 10.3389/fimmu.2021.686846
214. Krishnan V, Nath S, Nair P, Das B. Mycobacterium tuberculosis and its clever approaches to escape the deadly macrophage. World J Microbiol Biotechnol. (2023) 39:300. doi: 10.1007/s11274-023-03735-9
215. Kim H, Shin SJ. Pathological and protective roles of dendritic cells in Mycobacterium tuberculosis infection: Interaction between host immune responses and pathogen evasion. Front Cell Infect Microbiol. (2022) 12:891878. doi: 10.3389/fcimb.2022.891878
216. Torrelles JB, Chatterjee D. Collected Thoughts on Mycobacterial Lipoarabinomannan, a Cell Envelope Lipoglycan. Pathogens. (2023) 12:1281. doi: 10.3390/pathogens12111281
217. Åhsberg J, Tersbøl BP, Puplampu P, Kwashie A, Commey JO, Adusi-Poku Y, et al. Use of the urine determine LAM test in the context of tuberculosis diagnosis among inpatients with HIV in Ghana: a mixed methods study. Front Public Health. (2024) 11:1271763. doi: 10.3389/fpubh.2023.1271763
218. Shukla S, Richardson ET, Drage MG, Boom WH, Harding CV. Mycobacterium tuberculosis lipoprotein and lipoglycan binding to toll-like receptor 2 correlates with agonist activity and functional outcomes. Infect Immun. (2018) 86:e00450–18. doi: 10.1128/IAI.00450-18
219. Liu S, Guan L, Peng C, Cheng Y, Cheng H, Wang F, et al. Mycobacterium tuberculosis suppresses host DNA repair to boost its intracellular survival. Cell Host Microbe. (2023) 31:1820–36.e10. doi: 10.1016/j.chom.2023.09.010
Keywords: extracellular vesicles, Mycobacterium tuberculosis, renal tuberculosis, LAM, LprG, diagnosis and treatment
Citation: Peng X, Li Y, Jin S and Wang Q (2025) The value of LAM and LprG in extracellular vesicles in the diagnostic and therapeutic field of renal tuberculosis. Front. Tuberc. 3:1608780. doi: 10.3389/ftubr.2025.1608780
Received: 09 April 2025; Accepted: 23 June 2025;
Published: 12 August 2025.
Edited by:
Novel N. Chegou, Stellenbosch University, South AfricaReviewed by:
Gayathri Ramasubban, EMPE Diagnostics Private Limited, IndiaHanan Afzal, University of Central Punjab, Pakistan
Copyright © 2025 Peng, Li, Jin and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiang Wang, Z3ltbndxQDE2My5jb20=
 Xuefeng Peng
Xuefeng Peng Yue Li1
Yue Li1