Abstract
Necrotic enteritis (NE) is a severe gastrointestinal disease that poses a significant threat to the poultry industry. It leads to progressive damage to the small intestine, reduced performance, increased mortality rates, and substantial economic losses. With the removal of antimicrobial agents from chicken feed, there is an urgent need to find alternative approaches for NE control. Various approaches, including vaccination, prebiotics, probiotics, and plant-derived products, have been utilized to address NE in poultry management. To evaluate the efficacy of these preventive measures against NE, successful induction of NE is crucial to observe effects of these approaches in related studies. This study presents a comprehensive overview of the methods and approaches utilized for NE reproduction in related studies from 2004 to 2023. These considerations are the careful selection of a virulent Clostridium perfringens strain, preparation of challenge inoculum, choice of time and the route for challenge inoculum administration, and utilization of one or more predisposing factors to increase the rate of NE occurrence in birds under experiment. We also reviewed the different systems used for lesion scoring of NE-challenged birds. By gaining clarity on these fundamental parameters, researchers can make informed decisions regarding the selection of the most appropriate NE experimental design in their respective studies.
1 Introduction
Necrotic enteritis (NE) is a highly prevalent clostridial enteropathogenic ailment in poultry, with its initial documentation dating back to the 1950s in broiler chickens (1). NE poses a substantial challenge to the advancement of the poultry industry, particularly in nations with significant poultry production (2). It is widely recognized as a prominent limitation hindering the development and expansion of the poultry sector (2, 3). Based on estimations, NE disease outbreaks have had a considerable economic impact, reaching approximately $2 billion in 2000 and escalating to around $6 billion in 2015, corresponding to an approximate cost of $0.0625 per bird (4).
Necrotic enteritis is primarily caused by Clostridium perfringens (C. perfringens), an anaerobic gram-positive bacterium that forms spores (5). Clostridium perfringens is widely distributed in the environment and is commonly found in the gastrointestinal tract of various animal species, including poultry, livestock, and humans, both in healthy and diseased individuals (5, 6). Among the toxins associated with NE pathogenesis, α-toxin derived from C. perfringens has traditionally been identified as the primary toxin (7). However, the discovery of a novel toxin called necrotic enteritis toxin-B like (NetB) has attracted considerable attention due to its significant role in the development of NE (8). According to the established classification scheme based on toxin production, C. perfringens type G has emerged as a prominent causative agent of NE in poultry (9). This is attributed to its ability to produce both α-toxin and the NetB protein (9).
Necrotic enteritis in birds exhibits two distinct manifestations: clinical (acute) and subclinical (chronic) (1). The acute form is characterized by diarrhea, depression, sternal recumbency, and high flock mortality rates, which can reach up to 50% (10, 11). Pathologically, this form is associated with inflammation and extensive necrosis primarily observed in the small intestines of affected birds. In contrast, the chronic form of NE is characterized by mucosal damage in the small intestine and is marked by reduced weight gain at slaughter, decreased feed intake, and impaired growth performance (10, 11). NE typically affects birds in good body condition within the 2–6-week period, as maternal antibodies only provide protection for approximately 3 weeks (12–16). However, there have been reports of NE occurring in commercial layers older than 3 months of age (13).
Historically, antimicrobial agents were employed in chicken feed to enhance growth, modulate intestinal microbiota, and prevent the occurrence of NE (17). However, the use of these antibiotics resulted in the emergence of antibiotic resistance and posed risks to public health (18). As a response, the European Union implemented a prohibition on the inclusion of antibiotics in chicken feed in 2006, leading to a subsequent rise in NE cases (17). Consequently, alternative strategies have been implemented in poultry management to tackle NE, including the utilization of vaccination, prebiotics, probiotics, and plant-derived products (19). The reproduction of NE in experimental settings is an integral part of vaccine development studies and plays a crucial role in evaluating the effectiveness of preventive measures against NE. Several critical factors contribute to the successful induction of NE, including the careful selection of a virulent strain of C. perfringens capable of reproducing NE, precise preparation of the challenge inoculum, meticulous timing and route of infectious challenge inoculation, and consideration of relevant predisposing factors. Hence, it is imperative to thoroughly review the impact of these factors on NE reproduction and make informed decisions regarding their incorporation in the design of experimental NE disease. In the current study, we conducted a review of the methodologies employed for inducing NE disease during challenge experiments. Our objective was to provide a comprehensive summary of the experimental designs used to reproduce NE in related studies conducted between 2004 and 2023. The review highlights the need for further investigation and research in areas where ambiguity exists.
2 Selection of virulent challenge strains
Being the causative agent of avian NE, C. perfringens is recognized for its capacity to generate a diverse array of toxins (20, 21). The α-toxin from C. perfringens has traditionally been acknowledged as the principal virulence factor that elicits NE in birds (22). In the post-antibiotic era, extensive endeavors were directed toward developing effective vaccines for this particular toxin, with the aim of managing NE. Nonetheless, the importance of α-toxin in the development of NE was called into question following the revelation that pathogenicity persisted in chickens despite the absence of this toxin in a C. perfringens mutant (7). According to these findings, it was established that there were supplementary elements, apart from the α-toxin, that potentially contribute to the initiation of NE in birds. In a critical study, a novel protein, NetB, was isolated from a C. perfringens strain found in a bird afflicted with NE (22). Subsequently, it was discovered that C. perfringens netb knockout mutants were unable to induce NE, leading to the hypothesis that NetB may be the primary virulence factor involved in the pathogenesis of NE (22).
To effectively evaluate the efficacy of alternative approaches to combat NE in the post antibiotic era, it is imperative to induce the NE disease in experimental animal models. This highlights the importance of utilizing virulent strains of C. perfringens in infectious challenge studies to accurately induce the disease as it occurs in field conditions and to facilitate accurate assessments of the efficacy of the preventive measures. Since NetB toxin is the primary antigen responsible for causing NE (22), it becomes crucial to utilize C. perfringens strains that demonstrate a positive presence of the netb gene to reproduce NE in vivo. The development of NE-associated gut lesions is unattainable in the absence of netb gene. Although the presence of NetB as the major causative agent is critical and enough for reproduction of NE, other virulence factors from C. perfringens may contribute synergistically to intensify the severity of NE. In this regard, the co-presence of TpeL, another toxin from C. perfringens, along with NetB, has been demonstrated to potentially result in more severe intestinal lesions (23). Additional toxins and antigens derived from C. perfringens might potentially influence the pathogenesis of NE such as fructose 1, 6-biphosphate aldolase (FBA) (24), zinc metallopeptidase (ZMP) (25), perfringolysin O (PFO) (26), and pilin structural subunits (Cna, FimA, and FimB) (27, 28) (Figure 1). Despite the presence of these toxins and antigens, their involvement is not crucial for the experimental induction of NE.
Figure 1

The role of Clostridium perfringens antigens in NE pathogenesis. Numerous antigens and toxins produced by C. perfringens may play a role in the pathogenesis of NE, while their presence is not crucial for the development of NE. NetB serves as the principal toxin responsible for NE by forming pores and penetrating the intestinal mucosa. Other toxins, such as α-toxin and PFO, might play distinct roles in the pathogenesis of NE by causing mucosal degradation and forming pores in epithelial cells, respectively. Other antigens involved in NE pathogenesis may include those responsible for mucus-covered epithelial cell degradation (ZMP), C. perfringens attachment to intestinal lining cells (FBA), collagen interaction (Cna, FimA, and FimB), and mucosal damage leading to tissue degradation (TpeL).
The comprehensive overview of the challenge experiments conducted to induce NE in birds is represented in Table 1. Diverse virulent strains of C. perfringens have been demonstrated to induce NE in broiler chickens during experimental challenge studies (Figure 2). Among these strains, C. perfringens strains CP56, CP4, and EHE-NE18 are the most commonly used strains for NE experimental induction, followed by Del-1, CP58, CP1, and CP6. Other less frequently utilized strains include WER-NE36 and JGS4143. A great number of studies utilized C. perfringens type A strains derived from NE-affected flocks to induce NE infection in their challenge experiment. However, it is of utmost importance to acknowledge that strains exhibiting netb positivity were categorized under C. perfringens type A prior to the implementation of the novel C. perfringens classification system based on toxin types (9). Since numerous vaccine studies were conducted prior to the introduction of the current scheme, these investigations documented the utilization of C. perfringens type A strains in their experimental paradigm. Now we know that these strains belong to C. perfringens type G. The continued utilization of type A strains in recent studies is attributed to the prevailing absence of reclassification of these netb-positive strains within the G type category, as mandated by the updated toxinotyping scheme. In addition, several researchers employed unidentified strains of C. perfringens type G, which were isolated from afflicted birds during an outbreak of NE within a flock.
Table 1
| Challenge strain | Route of administration (CFU/ml or culture/feed Ratio (v/w)) |
Experiment program No. of days (days of age / No. of daily inoculations) |
Lesion score 1 | Scoring system | Predisposing factor(s) | Reference | ||
|---|---|---|---|---|---|---|---|---|
| CP4 | In-feed (2:1) | 5 CD 2 (23-27 / 2) | 2.09 | 0-4 | Protein-rich diet (20% to 28%) 3 Starvation (20 h) |
(29) | ||
| In-feed (2:1) |
Mild
3 CD (28-30 / 2) |
1.55 | 0-5 | Wheat-based diet Protein-rich diet (20% to 28%) Starvation (20 h) |
(30) | |||
|
Moderate
5 CD (28-32 / 2) |
2.20 | |||||||
|
Severe
5 CD (28-32 / 2) |
2.68 | |||||||
| In-feed (2:1) | 5 CD (29-33 / 2) | 2.25 | 0-5 | Protein-rich diet (20% to 28%) | (31) | |||
| In-feed (2:1) | 5 CD (29-33 / 2) | 1.28 | 0-5 | Wheat-based diet Protein-rich diet (20% to 28%) Starvation (24 h) |
(32) | |||
| In-feed (2:1) | 5 CD (23-27 / 2) | 2.25 | 0-5 | Wheat-based diet Protein-rich diet (21% to 28%) Starvation (20 h) |
(33) | |||
| 2.76 | Turkey-based diet Protein-rich diet (21% to 28%) Starvation (20 h) |
|||||||
| Oral gavage + In-feed (1:1) | 5 CD (28-32 / 2) | 4.42 | 0-6 | Wheat-based diet Protein-rich diet (21% to 28%) Starvation (20 h) |
(34) | |||
| Oral gavage + In-feed (1:1) | 5 CD (28-32 / 2) | 4.21 | 0-6 | Wheat-based diet Protein-rich diet (21% to 28%) Starvation (15 h) |
(35) | |||
| Oral gavage + In-feed (1:1) | 5 CD (28-32 / 2) | 4 | 0-6 | Wheat-based diet Protein-rich diet (21% to 28%) Starvation (15 h) |
(24) | |||
| Oral gavage (108) | 3 CD (18-20 / 1) | 1.80 | 0-6 | E. maxima (day 14) | (36) | |||
| Oral gavage (108) | 3 CD (19-21 / 1) | 0.9 | 0-3 | E. maxima (day 14) | (37) | |||
| JGS4143 | Oral gavage + In-feed (1:1) | 4 CD | 3.3 4 | 0-5 | Wheat-based diet Protein-rich diet |
(38) | ||
| In-feed (3:4) | 4 CD (25-28 / 1) | 2.37 | 0-4 | Protein-rich diet (20% to 28%) | (39) | |||
| Oral gavage (109) | 3 CD (24-26 / 1) | 1.8 | 0-4 | Starvation (12 h) | (40) | |||
| CP56 | Oral gavage (4 × 108) | 4 CD (17-20 / 1) | ND 5 | 0-6 | Wheat/rye-based diet Protein-rich diet (to 30%) IBD vaccine (day 16) Anticoccidial vaccine (day 18) |
(41) | ||
| Oral gavage (4 × 108) | 4 CD (17-20 / 1) | 1.56 | 0-6 | Wheat/rye-based diet Protein-rich diet (to 30%) IBD vaccine (day 16) Anticoccidial vaccine (day 18) |
(42) | |||
| 4 CD (17-20 / 3) | 1.73 | |||||||
| 4 CD (17-20 / 1) | 3.46 | |||||||
| 4 CD (17-20 / 1) | 1.51 | |||||||
| CP56 | Oral gavage (4 × 108) | 4 CD (17-20 / 3) | 2.19 | 0-6 | Wheat/rye-based diet Protein-rich diet IBD vaccine (day 16) Anticoccidial vaccine (day 18) |
(43) | ||
| 2.98 | Wheat/rye-based diet Protein-rich diet IBD vaccine (day 16) Anticoccidial vaccine (day 18) Heat stress (35 ºC / day 17-35) |
|||||||
| Oral gavage (4 × 108) | 4 CD (17-20 / 1) | 1.07 | 0-6 | Wheat/rye-based diet Protein-rich diet (to 30%) IBD vaccine (day 16) Anticoccidial vaccine (day 18/20) |
(44) | |||
| Oral gavage (4 × 108) |
Mild
4 CD (17-20 / 1) |
0.75 | 0-6 | Wheat/rye-based diet Protein-rich diet (to 30%) IBD vaccine (day 16) Anticoccidial vaccine (day 18) |
(45) | |||
| In-feed (3:4) |
Severe
4 CD (19-22 / 2) |
1.70 | ||||||
| Oral gavage (4 × 108) | 4 CD (17-20 / 1) | 0.45 | 0-6 | Wheat-based diet Protein-rich diet (to 30%) IBD vaccine (day 16) |
(46) | |||
| 1.1 | Wheat-based diet Protein-rich diet (to 30%) IBD vaccine (day 16) Fusarium mycotoxin deoxynivalenol |
|||||||
| Oral gavage (4 × 108) | 4 CD (17-20 / 1) | 0.65 | 0-6 | Wheat-based diet Protein-rich diet (to 30%) IBD vaccine (day 16) |
(47) | |||
| 0.77 | Wheat-based diet Protein-rich diet (to 30%) IBD vaccine (day 16) Fumonisins mycotoxins |
Oral gavage (4 × 108) | 4 CD (17-20 / 3) | 2.18 | 0-6 | Wheat/rye-based diet Protein-rich diet IBD vaccine (day 16) Anticoccidial vaccine (day 18) |
(48) | |
| 3.20 | Wheat/rye-based diet Protein-rich diet IBD vaccine (day 16) Anticoccidial vaccine (day 18) High Stock density |
|||||||
| Oral gavage (4 × 108) | 4 CD (17-20 / 3) | 2.19 | 0-6 | Wheat/rye-based diet Protein-rich diet IBD vaccine (day 16) Anticoccidial vaccine (day 18) |
(49) | |||
| 3.79 | Wheat/rye-based diet Protein-rich diet IBD vaccine (day 16) Anticoccidial vaccine (day 18) Cold stress (15 ºC) |
|||||||
| Oral gavage (4 × 108) | 4 CD (17-20 / 3) | 4.6 | 0-6 | Wheat/rye-based diet Protein-rich diet IBD vaccine (day 16) E. maxima (day 18) |
(50) | |||
| Oral gavage (4 × 108) | 4 CD (17-20 / 3) | 2.19 | 0-6 | Wheat/rye-based diet Protein-rich diet IBD vaccine (day 16) Anticoccidial vaccine (day 18) |
(51) | |||
| CP58 | 1.26 | 0-5 | Protein-rich diet (20% to 40%) Starvation (18 h) |
(52) | ||||
| Oral gavage (2 × 107-108) + In-feed (1:10) | 5 CD (28-32 / 2) | 3.33 | 0-5 | Wheat-based diet Protein-rich diet (20% to 40%) Starvation (20 h) |
(53) | |||
| Oral gavage (109) + In-feed (1:2) | 4 CD (30-33 / 2) | 3 | 0-6 | Wheat-based diet Protein-rich diet (21.5% to 48%) Starvation (12 h) |
(54) | |||
| Oral gavage (109) + In-feed (1:2) | 4 CD (30-33 / 2) | 3 | 0-6 | Wheat-based diet Protein-rich diet (21.5% to 48%) Starvation (12 h) |
(55) | |||
| WER-NE36 | In-feed (3:4) |
Severe
2 CD (26-27 / 2) |
2.8 | 0-6 | Wheat-based diet Protein-rich diet (20% to 50%) |
(56) | ||
| In-feed (4:3) | 4 CD (19-22 / 1) | 4.25 | 0-6 | Wheat-based diet Protein-rich diet (20% to 50%) |
(57) | |||
| Oral gavage (108) | 2 CD (14-15 / 1) | 1 | 0-4 | Eimeria spp. (day 9) | (58) | |||
| EHE-NE18 | In-feed (3:4) | 2 CD (21-22 / 2) | 2.6 | 0-6 | Wheat-based diet Protein-rich diet (20% to 50%) |
(59) | ||
| 2 CD (14-15 / 2) | 3 | |||||||
| Oral gavage (109-1010) + In-feed (1:10) |
Mild
2 CD (24-25 / 2) |
1.9 | 0-6 | Wheat-based diet Protein-rich diet (20% to 50%) |
(56) | |||
| In-feed (3:4) |
Moderate
2 CD (26-27 / 2) |
2 | ||||||
| Oral gavage (108) | 2 CD (14-15 / 1) | 0.83 | 0-4 | Eimeria spp. (day 9) | (58) | |||
| Oral gavage (108-109) | Day 14 / 1 | 1.25 | 0-4 | Eimeria spp. (day 9) | (60) | |||
| Oral gavage (108) | 2 CD (14-15 / 1) | Exp. 1 | 1.5 | 0-4 | Anticoccidial vaccine (day 9) | (61) | ||
| Exp. 2 | 1.8 | |||||||
| Oral gavage (108-109) | 2 CD (14-15 / 1) | 1.3 | 0-4 | Eimeria spp. | (62) | |||
| 0.45 | Protein-rich diet (to 25%) | |||||||
| 1.4 |
Eimeria spp.
Protein-rich diet (to 25%) |
|||||||
| Oral gavage (108-109) | 2 CD (14-15 / 1) | 1.3 | 0-4 | Eimeria spp. (day 9) | (63) | |||
| 0.45 | Protein-rich diet (to 25%) | |||||||
| 1.4 |
Eimeria spp. (day 9) Protein-rich diet (to 25%) |
|||||||
| Oral gavage (108) | 2 CD (14-15 / 1) | 0.8 | 0-6 | Eimeria spp. (day 9) | (64) | |||
| Oral gavage (108) | 2 CD (14-15 / 1) | 0.8 | 0-6 | Eimeria spp. (day 9) | (65) | |||
| Oral gavage (108) | 2 CD (14-15 / 1) | 0.68 | 0-6 | Anticoccidial vaccine (day 9) | (66) | |||
| Del-1 | Oral gavage (109) | Day 18 | 3 | 0-4 | Protein-rich diet (17% to 24%) E. maxima strain 41A |
(67) | ||
| Oral gavage (109) | Day 18 | 2.5 | 0-4 | Protein-rich diet (18% to 24%) E. maxima (day 14) |
(68) | |||
| Oral gavage (109) | Day 21 / 1 | 3.17 | 0-4 | Protein-rich diet (18% to 24%) E. maxima (day 18) |
(69) | |||
| Day 18 / 1 | 0.87 | Protein-rich diet (21% to 24%) E. maxima (day 14) |
||||||
| Oral gavage (109) | Day 18 | Cobb | ≈ 3.5 | 0-4 | Protein-rich diet (17% to 24%) E. maxima (day 14) |
(70) | ||
| Ross | ≈ 3.3 | |||||||
| Hubbard | ≈ 2.8 | |||||||
| Oral gavage (109) | Day 18 | 2.9 | 0-4 | Protein-rich diet (17% to 24%) E. maxima (day 14) |
(71) | |||
| CP61 | Oral gavage (4 × 108) | 4 CD (17-20 / 1) | ND | 0-6 | Wheat/rye-based diet Protein-rich diet (to 30%) IBD vaccine (day 16) Anticoccidial vaccine (day 18) |
(41) | ||
| CP1 | In-feed (2:1) | 5 CD (29-33 / 1) | 1.63 | 0-5 | Wheat-based diet Protein-rich diet (20% to 28%) Starvation (24 h) |
(32) | ||
| In-feed (2:1) | 2 CD (28-29 / 2) | 2.87 | 0-6 | Protein-rich diet (20% to 28%) Starvation (24 h) | (72) | |||
| 2 CD (26-27 / 2) | 3.66 | |||||||
| Oral gavage (2.5 × 108) | 3 CD (14-16 / 1) | 0.95 | 0-6 | - | (73) | |||
| 4 CD (15-18 / 1) | 1.16 | Wheat-based diet Protein-rich diet (18% to 60%) |
||||||
| 4 CD (15-18 / 1) | 1.58 | Wheat-based diet Protein-rich diet (18% to 60%) Anticoccidial vaccine (day 10) |
||||||
| Oral gavage (1-2 × 108) | 2 CD (12-13 / 1) | 0.83 | 0-6 | - | (74) | |||
| 2.66 | Corticosterone in feed (day 11) | |||||||
| CP6 | Oral gavage (108) | 3 CD (19-21 / 1) | 0.9 | 0-3 | E. maxima (day 14) | (37) | ||
| Oral gavage (108) | 2 CD (19-20 / 1) | Trial 1 | 1.27 | 0-3 | E. maxima (day 14) | (75) | ||
| Trial 2 | 1 | |||||||
| In-feed | 3 CD (13-15 / 1) | Trial 3 | 0.92 | - | ||||
| Oral gavage (108) | 3 CD (18-20 / 1) | 0.37 | 0-3 |
E. maxima (day 14) Starvation (2-3 h) |
(76) | |||
| Oral gavage (108) | 3 CD (19-21 / 1) | 1.11 | 0-3 | E. maxima (day 14) | (77) | |||
| CP13 | Oral gavage (107) | 5 CD (15-19 / 2) | 2.89 | 0-3 | Wheat-based diet Anticoccidial vaccine (day 13) |
(78) | ||
| 3.45 | Wheat-based diet IBD vaccine (day 14) |
|||||||
| 1.67 | Wheat-based diet Anticoccidial vaccine (day 13) IBD vaccine (day 14) |
|||||||
| CP14 | 2.78 | Wheat-based diet Anticoccidial vaccine (day 13) |
||||||
| 2.56 | Wheat-based diet IBD vaccine (day 14) |
|||||||
| 1.78 | Wheat-based diet Anticoccidial vaccine (day 13) IBD vaccine (day 14) |
|||||||
| CP03 | 3.34 | Wheat-based diet Anticoccidial vaccine (day 13) |
||||||
| 2.23 | Wheat-based diet IBD vaccine (day 14) |
|||||||
| 2.43 | Wheat-based diet Anticoccidial vaccine (day 13) IBD vaccine (day 14) |
|||||||
| Type A | In-feed (2:1) | 3 CD (35-37 / 1) | ND | 0-4 | ND | (79) | ||
| In-drinking water (1:2) | ||||||||
| Oral gavage (4 × 108) | 2 CD (14-15 / 1) | 3 | 0-6 | ND | (80) | |||
| Oral gavage (108) | 2 CD (23-24 / 1) | 1.97 | 0-4 |
E. maxima (day 18) Salmonella Typhimurium (day 1) |
(81) | |||
| Oral gavage (2.2 × 108) | 5 CD (18-22 / 1) | ND | ND | Anticoccidial vaccine (day 15) | (82) | |||
| Oral gavage (108) | 3 CD (19-21 / 1) | 3.8 4 | 0-6 | Anticoccidial vaccine (day 12) | (83) | |||
| Oral gavage (2.2 × 107) | 3 CD (18-20 / 2) | 2.94 | 0-4 | Anticoccidial vaccine (day 14) IBD vaccine (day 14) |
(84) | |||
| Oral gavage (108) | Day 14 / 1 | Exp. 1 | 2.1 | 0-4 | E. maxima (day 9) | (85) | ||
| Exp. 2 | 1.6 | |||||||
| Oral gavage (6-8 × 108) | 4 CD (18-21 / 3) | 3 | 0-4 | IBD vaccine (day 16) Anticoccidial vaccine (day 19) |
(86) | |||
| Oral gavage (107) | 3 CD (17-19 / 2) | 1.25 | 0-4 | Wheat-based diet | (87) | |||
| 1.50 | Wheat-based diet Anticoccidial vaccine (day 14) |
|||||||
| 1.60 | Wheat-based diet IBD vaccine (day 14) |
|||||||
| 1.90 | Wheat-based diet Anticoccidial vaccine (day 14) IBD vaccine (day 14) |
|||||||
| Oral gavage (108) | 2 CD (22-23 / 1) | 2.10 | 0-4 | E. maxima (day 18) | (88) | |||
| 3.30 |
E. maxima (day 18) Salmonella Typhimurium (day 1) |
|||||||
| 2.20 |
E. maxima (day 18) Salmonella Typhimurium (day 17) |
|||||||
| Oral gavage (2.2 × 108) | 3 CD (18-20 / 1) | 1.33 |
Eimeria spp. (day 14) Starvation (8 h) |
(89) | ||||
| Oral gavage (4 × 108) | 3 CD (26-28 / 1) | 1.86 | 0-3 | Anticoccidial vaccine (day 23) | (90) | |||
| Oral gavage (109) | 7 CD (17-23 / 1) | 1.13 | 0-4 | E. maxima (day 12) | (91) | |||
| Oral gavage (2 × 108) | 7 CD (15-21 / 1) | 2.25 | 0-4 | ND | (92) | |||
| Oral gavage (2 × 108) | 4 CD (18-21 / 1) | 1.67 | 0-4 | Anticoccidial vaccine (day 14) Starvation (overnight) |
(93) | |||
| Oral gavage (3 × 108) | 3 CD (18-20 / 1) | 2.64 | 0-6 | Anticoccidial vaccine (day 14) | (94) | |||
| Oral gavage (5 × 108) | 3 CD (15-17 / 1) | 2.80 | 0-5 | E. acervolina (day 7) | (95) | |||
| Oral gavage (3.5 × 108) | 3 CD (14-16 / 1) | 3.18 | 0-4 |
Eimeria spp. (day 9) Protein-rich diet |
(96) | |||
| Oral gavage (107) | 3 CD (17-19 / 1) | 3.30 | 0-4 | Anticoccidial vaccine (day 14) | (97) | |||
| Type C | In-feed (2:1) | 3 CD (35-37 / 1) | ND | 0-4 | ND | (79) | ||
| In-drinking water (1:2) | ||||||||
| TpeL17 | Oral gavage (109) | 4 CD (23-26 / 1) | 3 | 0-4 | Protein-rich diet (16% to 24%) E. maxima |
(98) | ||
| CP5 | Intracloacal (5.8-8 × 108) | 4 CD (18-21 / 2) | 0.66 | 0-6 | Protein-rich diet (20% to 30%) | (99) | ||
| CP18 | 1.07 | |||||||
| CP26 | 1.5 | |||||||
| JRTK44 | Oral gavage (2 × 108) | Day 15 / 1 | 1.97 | 0-4 | Corn-based diet Anticoccidial vaccine (day 11) |
(100) | ||
| 0.57 | Wheat-based diet | |||||||
| 2.01 | Wheat-based diet Anticoccidial vaccine (day 11) |
|||||||
| Other type G isolates 6 | Oral gavage (2.5 × 108) | Day 20 / 1 | 2.16 | 0-4 | Anticoccidial vaccine (day 15) | (101) | ||
| Oral gavage (3 × 108) | 4 CD (17-20 / 3) | 2.07 | 0-6 | Wheat-based diet Protein-rich diet |
(102) | |||
| 5.07 | Wheat-based diet Protein-rich diet Anticoccidial vaccine (day 18) |
|||||||
| Drinking water (108) | 2 CD (19-20 / 1) | 0.5 | 0-3 |
E. maxima (day 14) Starvation (4 h) |
(103) | |||
| In-feed (2:5) | 3 CD (18-20 / 1) | 1.2 | Starvation (4 h) | |||||
| Oral gavage (108) | 3 CD (19-21 / 1) | 1.29 | E. maxima (day 14) | |||||
| In-feed (4:3) | 3 CD (21-23 / 1) | 4.3 | 0-6 | Wheat-based diet Protein-rich diet (20% to 50%) |
(104) | |||
| Oral gavage (108) | 2 CD (19-20 / 1) | 0.8 | 0-4 | E. maxima (day 14) | (105) | |||
| Oral gavage (109) | Day 32 / 1 | 3 | 0-4 |
E. brunetti
E. tenella |
(106) | |||
| Oral gavage (2.6 × 108) + In-feed (1:36) | 3 CD (17-19 / 2) | Exp. 1 | 3.8 | 0-4 | IBD vaccine (day 14) Protein-rich diet Starvation (24 h) |
(107) | ||
| Exp. 2 | 3.7 | |||||||
| Oral gavage (108) | 3 CD (19-21 / 1) | 1.46 | 0-3 | E. maxima (day 14) | (108) | |||
| Oral gavage (109) | 2 CD (18-19 / 1) | 2.04 | 0-4 |
E. maxima (day 13) Salmonella Typhimurium (day 1) |
(109) | |||
| Oral gavage (108) | 3 CD (18-20 / 1) | 1.37 | 0-3 | E. maxima (day 13) | (110) | |||
| Oral gavage (108) | 3 CD (17-19 / 1) | 2.31 | Used litter (day 4) | |||||
| Oral gavage (108) | 3 CD (19-21 / 1) | 3.28 | 0-3 | E. maxima (day 14) | (111) | |||
| Oral gavage (4 × 108) | 3 CD (19-21 / 1) | 3.33 | 0-6 | Eimeria spp. (day 14) | (112) | |||
| Oral gavage (108) | 3 CD (19-21 / 1) | 4.30 | 0-3 | E. maxima (day 14) | (113) | |||
| Oral gavage (5 × 108) | 3 CD (15-17 / 1) | 3 | 0-5 | E. acervolina (day 14) | (95) | |||
| Oral gavage (108) | 2 CD (18-19 / 1) | 1.8 | 0-4 | E. maxima (day 14) | (114) | |||
Summary of the C. perfringens challenge experiments used in NE vaccine studies
1 The highest lesion score observed through gross examination of the small intestine of challenged control birds is considered.
2 CD: consecutive days
3 The first and second values show the percentage of crude protein in the starter and grower diets, respectively.
4 Scoring was carried out based on lesions observed in histopathology.
5 ND: Not defined
6 Undefined strains of C. perfringens type G (netb-positive), mainly isolated from NE-affected flocks.
Figure 2

Virulent strains of Clostridium perfringens employed in experimental challenges to induce NE. Various strains of C. perfringens have been utilized for inducing in vivo NE infection in birds, with strains 56, 4, and EHE-NE18 being the most frequently employed in experimental challenges. A total of 93 studies that included at least one NE challenge experiment were considered in the analysis. The numerical values represent the relative proportion or percentage of studies in relation to the overall number of studies conducted. In certain studies, multiple strains were employed in the experimental design. The chart was generated using GraphPad Prism 9.0 (Graph-Pad Software, San Diego, CA, United States).
Consequently, the careful selection of a virulent strain that induces more severe NE lesions plays a crucial role in accurately assessing the effectiveness and protective capacity of NE vaccines. Recent research has provided evidence that the virulent C. perfringens strains TpeL17, WER-NE36, Del-1, and CP13 result in more severe NE infections, as indicated by higher mean lesion scores observed in the small intestine of challenged birds (Figure 3). However, these strains have been utilized in only a limited number of vaccine studies. In contrast, strains such as CP4 have been found to induce moderate to severe lesions in the intestinal tract of challenged birds and have also been employed in a greater number of studies. The mean lesion scores of the C. perfringens strains used in experimental NE challenge investigations are illustrated in Figure 3.
Figure 3

Lesion score ranges for virulent strains of Clostridium perfringens. The lesion scores documented in studies examining the effects of experimental NE infection in unvaccinated birds, caused by virulent strains of C. perfringens, have been recorded since 2004 until the present. The plotted values represent the mean NE lesion scores observed in unvaccinated birds challenged with virulent strains of C. perfringens. The analysis and visualization of the data were performed using GraphPad Prism 9.0 (Graph-Pad Software, San Diego, CA, United States).
It is important, however, to note that comparing the findings of NE infection studies is challenging due to differences in the experimental challenge infections. Numerous disparities concerning the administration and application of the virulent strains, as well as variations in the quantity and frequency of challenge inoculations, pose formidable obstacles when attempting to facilitate accurate and reliable comparisons among vaccine study results or other preventive measure investigations against NE.
3 NE challenge methods
3.1 Choice of culture media
Several culture media have been employed in the propagation of virulent strains of C. perfringens during challenge experiments. The selection of media has been based on a multitude of considerations, encompassing but not restricted to the simplicity of management and the efficient utilization of resources. Among the culture media employed for inducing NE in vitro through oral inoculation of birds or culture-infected feed or water, a fluid thioglycolate (FTG) medium is the most commonly used culture media (24, 33, 36, 37, 72, 73, 79, 87, 102, 105, 115, 116). This medium has the property of culturing under aerobic conditions due to the presence of sodium thioglycolate, a potent oxygen scavenger, in its composition. Other less commonly used media include Cooked Meat Medium (CMM) (24, 34, 38, 52, 80, 117), Brain Heart Infusion Broth (BHI) (10, 41, 42, 44–47, 53, 75, 78, 98), Tryptic Soy Broth (TSB) containing sodium thioglycolate (88, 118–120), and liver broth (85, 101). Many researchers initially cultivated C. perfringens in CMM before inoculating this medium into FTG medium, which is then employed for the challenge experiment by inoculating birds (24, 29, 30, 32, 34, 35, 38, 39, 53–59, 99, 104).
3.2 Preparation of challenge inoculum
The culture medium employed for the inoculation of birds undergoes incubation at a temperature of 37°C for a period ranging between 15 and 24 h. Research findings indicate that the 24-h culture of the FTG medium displays increased protease activity, leading to the degradation of toxins responsible for NE disease (121). Conversely, the 15-h broth cultures are observed to produce significantly higher levels of toxins in culture, which are considered essential in the formation of NE lesions, as opposed to toxins produced by vegetative C. perfringens in the intestinal tract post-inoculation (121). Additionally, the cultures with shorter incubation times have been shown to result in more severe lesions when compared to older cultures (24-h cultures) (29).
3.3 Time and route of challenge inoculation
The virulent strains utilized in challenge experiments have been administered to birds either directly through oral gavage into the crop, or indirectly by infecting feed or water with the bacterial culture (Table 1). Some researchers have employed a combination of both methods, administering the challenge inoculation through the oral route followed by an in-feed or-water challenge. In-feed inoculation possesses the characteristic of simplicity in application in contrast to the oral gavage, which induces stress associated with restraint. On the contrary, the amalgamation of broth culture and feed may result in a diminished level of the bacterium’s intended concentration (38), thereby necessitating a greater volume of broth cultures, consequently leading to an escalation in costs.
In the case of oral gavage, the challenge inoculum was composed of whole culture media containing approximately 108–109 bacteria per dose. The oral inoculation is usually carried out twice daily for 1 day or 5 consecutive days. A mixture of culture and feed or water at varying ratios has also been utilized twice per day for 2–5 consecutive days for the induction of NE. The ratios commonly employed for the mixture of culture and feed/water are 2:1 (v/w) (29–33, 72, 79), 3:4 (v/w) (39, 45, 56, 59), 1:1 (v/w) (24, 34, 35, 38), 2:5 (v/w) (103), 4:3 (v/w) (57, 104), and 1:2 (v/v or v/w) (54, 55, 79) for the challenge method involving the mixing of broth cultures with feed or water. Additionally, some investigators have utilized a combination of the oral route and in-feed challenge, employing cultures containing 108–1010 colony forming unit (CFU)/dose and a mixture of culture and feed with ratios of 1:10 (v/w) (53, 56), 1:1 (v/w) (24, 34, 38), and 1:2 (v/w) (54, 55). Birds are commonly deprived of food for a duration of 2–24 h prior to the initiation of the experimental challenge. This practice serves to facilitate the process of inoculation and also establishes a state of fasting-induced stress in broiler chickens.
4 Predisposing factors
Since the experimental induction of NE requires predisposing factors, empirical studies have identified a number of factors that can increase the likelihood and severity of NE in challenged birds (Table 1). Although a few studies induced NE without using any predisposing factors (92, 122), such predisposing factors are frequently employed in vaccine studies before or during the challenge experiment as a means of augmenting the risk of NE occurrence among birds. The factors under consideration include coccidial infections, nutritional factors such as diets containing high levels of indigestible carbohydrates and crude proteins, stress, and immunosuppression resulting from vaccination with other poultry vaccines either prior to or during the challenge experiment (17, 19, 21, 123, 124). As such, these factors have been considered important variables in avian NE disease research and management programs. The predisposing factors commonly employed in NE experimental infection are illustrated in Figure 4.
Figure 4

Requirements and key predisposing factors for induction of NE in birds. Successful in vivo induction of NE in birds necessitates careful consideration of various factors, such as the choice of virulent strains, the age and sex of the birds, and additional predisposing factors commonly associated with increased susceptibility to NE infection.
Of all the potential predisposing factors that have been identified for NE in birds, nutritional manipulation in chicken feed (high concentration of protein/carbohydrate), coccidiosis infection induction, and stress induction through deprivation of feed and water (starvation) are the most commonly utilized predisposing factors to induce the NE disease (Figure 5).
Figure 5
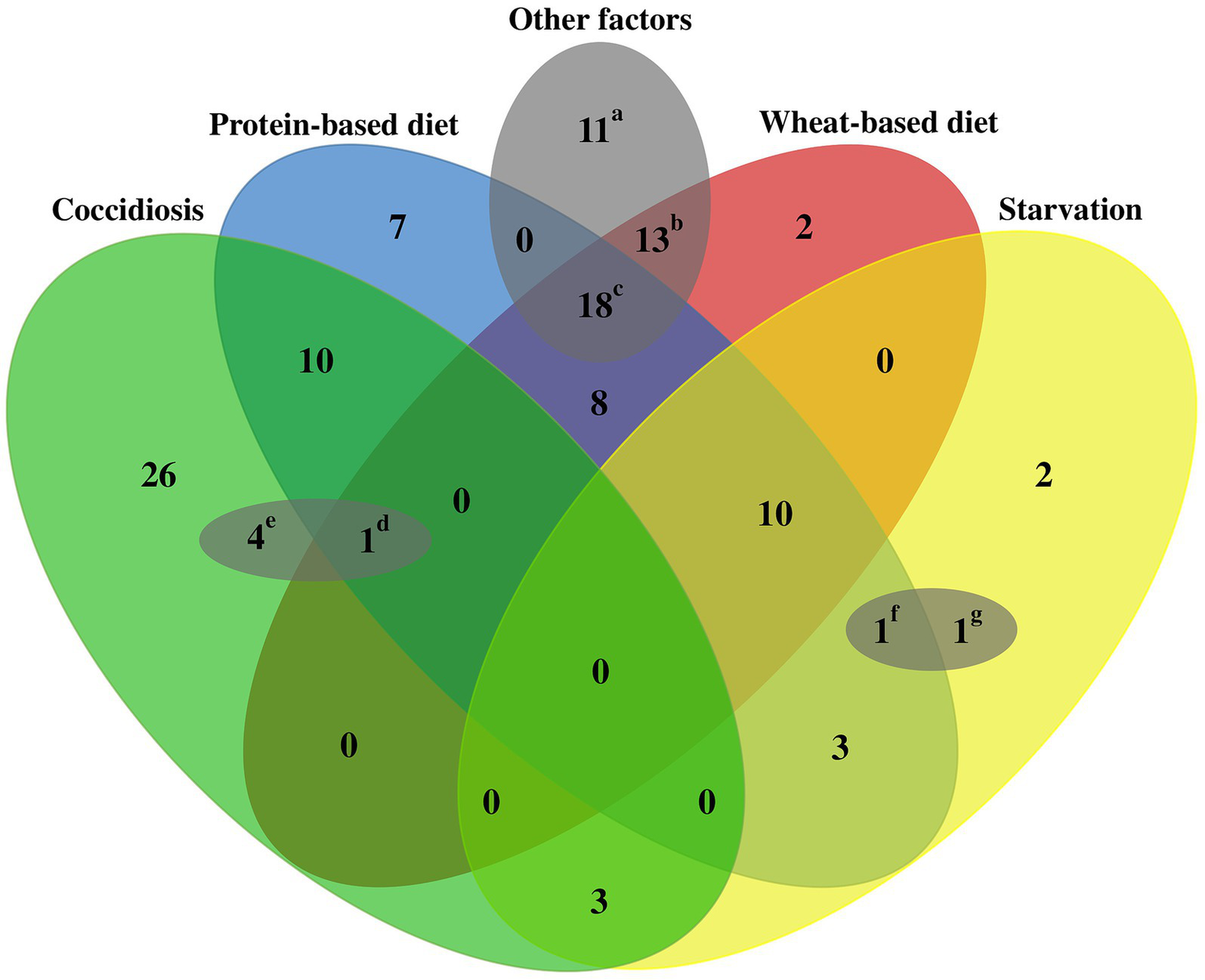
Schematic outline of common predisposing factors used for NE induction in experimental studies. A total of 120 challenge experiments were reviewed to evaluate the predisposing factors employed to induce NE in the respective infection experiments. Commonly utilized factors that predispose birds to NE include dietary factors (such as wheat-based and protein-rich diets), stress-induced starvation, and coccidiosis. In addition, several other less frequently encountered factors could also contribute to bird susceptibility, including, thermal stress variations, elevated stocking density, mycotoxin exposure, corticosterone levels, Salmonella species inoculation, and administration of anticoccidial and IBD vaccines in close temporal proximity to the challenge period. Each small letter includes at least one of the less frequent factors as follows: a: anticoccidial vaccines, IBD vaccines, and in-feed corticosterone; b: anticoccidial vaccines, IBD vaccines; c: anticoccidial vaccines, IBD vaccines, heat or cold stress, high stocking density, and mycotoxins; d: IBD vaccines, e: Salmonella typhimurium inoculation; f: IBD vaccines; and g: anticoccidial vaccines.
Coccidial infections have demonstrated a pivotal function in promoting the establishment and expansion of C. perfringens within the intestinal tract. It is hypothesized that the observed occurrence stems from the breakdown of the intestinal membrane, resulting in the formation of perforations in the epithelial tissue, thereby releasing plasma proteins into the intestinal lumen (21, 124). This serves as a copious nutrient source for C. perfringens propagation and toxin elaboration (17, 124). Coccidial infections can also induce mucogenesis, promoting the growth of C. perfringens (124). Several Eimeria species were indicated to predispose birds to NE in vaccine studies, with E. maxima being the most prevalent species (36, 37, 58, 67–69, 73, 75, 83–85, 98, 100, 102, 103, 105). However, it is important to note that coccidial infections are not always necessary for the occurrence of NE, as some researchers have successfully induced NE without utilizing this factor (24, 35, 52–55). Moreover, Eimeria infection has the potential to elicit a state of immunological stress, which may be unsuitable for conducting a vaccine study (56). This is due to the possibility of the infection-induced immune response confounding the desired immune response to the vaccine, resulting in potentially spurious or erroneous findings. As such, it is imperative to meticulously assess the immunological profile of the study participants and account for any pre-existing infections or immune perturbations when devising and executing vaccine trials.
The incidence of NE has also been revealed to be directly linked to the level of crude protein in the diet, as high protein levels can provide an optimal nutrient-rich environment for the proliferation of C. perfringens, thereby increasing the susceptibility of birds to NE (17, 125, 126). Fishmeal and soybean are the prevailing protein sources extensively incorporated into the diet of chickens during the challenge experiment. Additionally, cereal grains including wheat, rye, and barley could lead to an increment in the viscosity of the intestinal digesta, ultimately resulting in a prolonged bypass time (124, 127). As a result, the substrates produced by these non-starch polysaccharides could be more accessible to support the proliferation, growth, and toxin production of C. perfringens (100, 123, 126, 128). As such, this factor have been frequently utilized by many investigators to experimentally induce NE in birds (24, 29–35, 38, 39, 41, 42, 44, 45, 52–57, 59, 67–69, 72, 73, 98, 99, 104). The sudden implementation of these dietary modifications typically occurs during the transition from the starter diet to the grower diet, as evidenced by previous studies, thereby imposing an additional stressor on the birds involved in the experiment.
In addition to the factors mentioned above, some studies induced stress through the withdrawal of feed and water prior to the experimental infection to predispose birds to NE. The duration of the deprivation varies from a minimum of 2 h to a maximum of 24 h before the infectious challenge. Furthermore, apart from inducing stress, the state of starvation renders the process of conducting a challenge experiment more manageable, owing to the tendency of birds to consume contaminated feed post an extended period of fasting. Nonetheless, subjecting birds to intermittent periods of feed deprivation during the NE infection over consecutive days has shown the potential to mitigate the severity of gut lesions (51).
Several investigators have also employed immunosuppression induced by vaccination against several poultry diseases such as coccidiosis and infectious bursal disease (IBD) prior to the challenge experiment to predispose birds to NE (41, 42, 44, 45, 84) or inducing physiological stress using corticosterone in feed and water (74, 129, 130). Employing anticoccidial vaccines has exhibited divergent outcomes concerning NE incidence. The administration of commercial anticoccidial vaccines either immediately before or during in vivo NE infection for the purpose of immunosuppression may predispose birds to more pronounced NE lesions in the small intestine (78, 84, 87, 102). Conversely, the application of such vaccines during the initial day of life in chicks could potentially reduce the severity of NE lesions (50, 101, 106).
Other less common stressors demonstrated to contribute to NE-associated lesion severity. Heat and cold stresses, as environmental conditions, could play significant roles in the suppression of cellular and humoral immunity, leading to more severe gut lesions in birds (43, 49). Furthermore, elevated levels of glucocorticoids in the blood may arise as a result of heat stress and collaboratively add to the immunosuppressive impacts on heat-stressed birds (43). High body weights and fast growth also predispose birds to more severe NE disease (131). The rapid growth of birds has been elucidated to cause a transformation in the microbiota of the gastrointestinal tract and additionally resulted in the accumulation of a greater quantity of indigested or inadequately digested proteins within the gut, which consequently makes the gut a favorable environment for C. perfringens growth and proliferation (131, 132). This hypothesis aligns with the observation that NE typically manifests in birds exhibiting excellent body condition (11). Moreover, rearing birds in enclosures with a population that exceeds the normal stocking density (15 birds/m2 or 0.066 m2/bird) could induce stress, which suppresses the humoral immune responses, thereby increasing the likelihood of NE infection (48). Another concern that threatens overcrowded poultry farms is the elevated concentration of moisture and nitrogen emitted by the avian population, thereby diminishing the quality of the litter and creating a conducive habitat for microbial and coccidial proliferation (48). Additionally, the rivalry among birds raised in densely inhabited areas might potentially trigger heightened anxiety levels concerning nourishment intake, thus, concurrently intensifying the chances of occurring and spreading the NE infection throughout the group. Fungi may also contribute to the NE experimental model due to their ability to produce mycotoxins such as fumonisins and deoxynivalenol from Fusarium fungi (46, 47) or aflatoxin B1 from Aspergillus flavus (133). Oral administration of S. typhimurium strain in neonates is recognized as an additional contributing factor for the dependable NE induction as elucidated in some studies (81, 88, 109). The findings from these studies underscore the complex and multifaceted nature of NE pathogenesis. It is imperative to take this critical aspect into account when developing effective strategies for the prevention and control of the disease. The schematic outline of predisposing factors employed for inducing NE challenge is illustrated in Figure 5.
5 Other considerations
A few studies have shown the relationship between sex and breed with NE occurrence. Male birds, owing to their elevated degree of dietary intake and accelerated rate of growth, manifest a greater predisposition to NE when juxtaposed with their female counterparts (46, 134). Similarly, there have been reported breed-specific divergences in susceptibility to NE. Cobb chickens exhibit greater susceptibility to NE when contrasted with Ross and Hubbard chickens, thus manifesting more severe NE intestinal lesions subsequent to the infectious challenge experiment (70). Although the oral inoculation and/or in-feed administration of a virulent strain of C. perfringens is essential for the purpose of experimental NE infection, it is also feasible to induce NE without resorting to any form of inoculation through the utilization of previously contaminated/used bedding material that had been employed for NE-infected birds (110, 133, 135).
6 Scoring systems for NE lesions
After conducting the challenge experiment, the birds are humanely euthanized using approved methods, and subsequently undergo necropsy for additional pathological examination. For this purposes, birds are euthanized ethically through the utilization of CO2 inhalation, cervical dislocation, and electrical stunning, either on the last day of/or the day after the challenge experiment. A thorough inspection of the entire length of the small intestine is essential, and the gross pathological lesions should be evaluated using a scoring system formerly represented in the literature. The jejunum is considered to be the most impacted portion of the small intestine (58, 62, 66, 78, 84, 112, 136–140), whereas the duodenum has exhibited more pronounced lesions in certain research investigations (65, 90, 91, 141). Gross abnormalities were also apparent in the ileum and cecum, albeit to a lesser extent of involvement (2, 61–63, 65, 66, 84, 90, 91, 96, 136–138, 141). Various scoring systems have been developed to assess gross lesions associated with NE, with scales ranging from 0 to 3 (10, 12, 37, 75, 103, 142, 143), 0 to 4 (16, 29, 39, 40, 58, 67–71, 79, 85, 87, 98, 100, 101, 105, 144, 145), 0 to 5 (30–33, 38, 52, 53), and 0 to 6 (7, 22, 24, 34–36, 41, 42, 44, 45, 54–56, 59, 72, 80, 83, 102, 104, 146, 147). Although the scoring methodologies are derived from the visible lesions observed in the small intestines of birds, there have been instances where certain investigations employed scoring systems to assess the condition of the footpads in birds affected by NE (148). However, this methodology may not be entirely dependable owing to the enteropathogenic characteristics of the NE ailment. The systems that are extensively utilized for scoring NE lesions in experimental infection are depicted in Table 2.
Table 2
| Lesion score | 0–6 | 0–5 | 0–4 | 0–3 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | (7) | (121) | (30) | (144) | (85) | (149) | (150) | (12) | (10) | (151) | |
| 0 | No gross lesions | ||||||||||
| 1 | Thin or friable walls | Thin or friable wall or very mild and superficial generalized inflammation | Thin or friable walls | 1 to 5 small white lesions (spots <1 mm in diameter) | Mild (slight mucus covering and loss of tone, thin wall, or friable) | 1 to 5 small (<1-mm diameter) lesions | < 10 focal gross lesions (Focal lesion definition: their maximum extension is less than the circumference of the gut mucosa) | Focal necrosis and ulceration | Slight mucus covering and loss of tone, thin wall or friable | ||
| Also diffuse superficial but removable fibrin | |||||||||||
| 2 | Focal necrosis or ulceration (1–5 foci) | Focal necrosis or ulceration | > 5 small white lesions (spots of <1 mm in diameter) or 1 to 5 larger lesions (spots of 1 to 2 mm in diameter) | Moderate (focal necrosis or ulceration) | > 5 small lesions but fewer than 5 larger (1 to 2-mm diameter) lesions | ≥ 10 focal gross lesions | Patches of necrosis 2 to 3 cm long | Focal necrosis or ulceration | |||
| Also non-removable fibrin deposit (1–5 foci) | |||||||||||
| 3 | Focal necrosis or ulceration (6–15 foci) | Large patches of necrosis | > 5 larger lesions (1 to 2 mm in diameter) or erosive zones | Marked (severe, sloughed mucosa with presence of blood in the lumen) | > 5 larger lesions and erosive zones | ≥ one lesion with a maximum extension larger than the circumference of the gut mucosa | Diffuse necrosis typical of field cases | Severe, sloughed mucosa with presence of blood in the lumen | |||
| Also non-removable fibrin deposit (6–15 foci) | |||||||||||
| 4 | Focal necrosis or ulceration (16 or more foci) | Severe or extensive necrosis typical of field cases | Death with positive NE diagnoses postmortem | NA1 | |||||||
| Also non-removable fibrin deposit (16 or more foci) | |||||||||||
| 5 | Patches of necrosis 2–3 cm long | Death during the experiment with lesion scores of 4 | NA | NA | |||||||
| 6 | Diffuse necrosis typical of field cases | NA | NA | NA | |||||||
Common scoring systems used in NE studies.
1Not assigned.
Owing to the variability of the systems employed for scoring NE lesions, there exists a challenge in comparing studies that adopt different scoring methods. Therefore, a standardized system for scoring NE lesions is imperative, enabling the convenient comparison of distinct groups of vaccinated birds concerning their protection against the challenge experiment. This standardized system should encompass a comprehensive range to facilitate statistical analysis, exhibit simplicity to permit the assessment of a large number of birds within a feasible time frame, maintain reproducibility among diverse observers, and also consider the severity of the disease generated under experimental conditions (121). Although it is necessary to select a broad system covering all NE lesions, the use of various antigens in immunization studies and different strains of C. perfringens in challenge experiments leads to variations in the gross lesions developed in NE disease (121).
7 Conclusion
The growing concern over restricted antibiotic usage in poultry production necessitates urgent evaluation of preventive strategies against NE. Experimental induction of NE in such studies becomes crucial for assessing the efficacy of these measures, ultimately benefiting the overall health and welfare of poultry. The development of NE is a consequence of the interplay of numerous contributing factors. These involve selecting a virulent C. perfringens strain, predisposing birds to NE through one or more predisposing factors, and choosing the most effective route of inoculation for optimal induction of NE disease in the challenge procedures. Selecting the ideal virulent strain containing the netb gene and developing an infection similar to field cases of NE is the first crucial step for experimental NE induction. Moreover, choosing among a variety of factors such as inducing coccidiosis infection, manipulating dietary protein or carbohydrates, and inducing stress conditions could raise the possibility of the NE occurrence in experimental challenges. When evaluating these factors, it is crucial to consider the potential drawbacks linked to these factors. For example, using coccidiosis infection as a predisposing factor to NE may introduce immunological alterations that could negatively impact vaccine research outcomes. Additionally, the ethical aspect of subjecting birds to stress must be considered throughout the research process. In conclusion, this review underscores the pivotal factors indispensable for the successful induction of NE. By elucidating these critical parameters, researchers can make well-informed choices when opting for the most appropriate NE experimental design.
Statements
Author contributions
MS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MG: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors express their sincere gratitude to all individuals who have significantly contributed to and enriched the research conducted. Their invaluable support has greatly facilitated the progress and findings of this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
C. perfringens, Clostridium perfringens; NE, Necrotic enteritis; NetB, Necrotic enteritis toxin β–like; FTG, Fluid thioglycolate; CMM, Cooked meat medium; BHI, Brain heart infusion; TSB, Tryptic soy broth; CFU, Colony forming unit; IBD, Infectious bursal disease; FBA, Fructose 1, 6-biphosphate aldolase; ZMP, Zinc metallopeptidase; PFO, Perfringolysin O; ND, Not defined; NA, Not assigned.
References
1.
Parish WE . Necrotic enteritis in the fowl (Gallus gallus domesticus): I. Histopathology of the disease and isolation of a strain of Clostridium welchii. J Comp Pathol Ther. (1961) 71:377–IN33. doi: 10.1016/S0368-1742(61)80043-X
2.
Van IF De BJ Pasmans F Huyghebaert G Haesebrouck F Ducatelle R . Clostridium perfringens in poultry: an emerging threat for animal and public health. Avian Pathol. (2004) 33:537–49. doi: 10.1080/03079450400013162
3.
Thomas S Abraham A Rodríguez-Mallon A Unajak S Bannantine JP . Challenges in veterinary vaccine development. Methods Mol Biol. (2022) 2411:3–34. doi: 10.1007/978-1-0716-1888-2_1
4.
Wade B Keyburn A . The true cost of necrotic enteritis. World Poult. (2015) 31:16–7.
5.
Keyburn AL Bannam TL Moore RJ Rood JI . Net B, a pore-forming toxin from necrotic enteritis strains of Clostridium perfringens. Toxins. (2010) 2:1913–27. doi: 10.3390/toxins2071913
6.
Mehdizadeh Gohari I Navarro AM Li J Shrestha A Uzal F McClane AB . Pathogenicity and virulence of Clostridium perfringens. Virulence. (2021) 12:723–53. doi: 10.1080/21505594.2021.1886777
7.
Keyburn AL Sheedy SA Ford ME Williamson MM Awad MM Rood JI et al . Alpha-toxin of Clostridium perfringens is not an essential virulence factor in necrotic enteritis in chickens. Infect Immun. (2006) 74:6496–500. doi: 10.1128/IAI.00806-06
8.
Rood JI Keyburn AL Moore RJ . Net B and necrotic enteritis: the hole movable story. Avian Pathol. (2016) 45:295–301. doi: 10.1080/03079457.2016.1158781
9.
Rood JI Adams V Lacey J Lyras D McClane BA Melville SB et al . Expansion of the Clostridium perfringens toxin-based typing scheme. Anaerobe. (2018) 53:5–10. doi: 10.1016/j.anaerobe.2018.04.011
10.
Gholamiandehkordi AR Timbermont L Lanckriet A Van Den BW Pedersen K Dewulf J et al . Quantification of gut lesions in a subclinical necrotic enteritis model. Avian Pathol. (2007) 36:375–82. doi: 10.1080/03079450701589118
11.
Opengart K Boulianne M . Necrotic enteritis In: SwayneDEBoulianneMLogueCMMcDougaldLRNairVSuarezDLet al, editors. Diseases of Poultry. Hoboken: John Wiley & Sons (2019). 972–6.
12.
Lovland A Kaldhusdal M Redhead K Skjerve E Lillehaug A . Maternal vaccination against subclinical necrotic enteritis in broilers. Avian Pathol. (2004) 33:81–90. doi: 10.1080/0379450310001636255
13.
To H Suzuki T Kawahara F Uetsuka K Nagai S Nunoya T . Experimental induction of necrotic enteritis in chickens by a net B-positive Japanese isolate of Clostridium perfringens. J Vet Med Sci. (2016) 79:350–8. doi: 10.1292/jvms.16-0500,
14.
Laishevtsev AI Kapustin AV Yakimova EA Danilyuk AV Gulyukin AM Belimenko VV . Necrotic enteritis of birds. IOP Conference Series: Earth and Environmental Science, IOP Publishing (2019). p. 022075.
15.
Behera D Pathak DC Sharma RK Dutta B Kalai K Sharma H et al . Study on incidence of necrotic enteritis of chicken in Assam. Ind Vet J. (2020) 97:20–2.
16.
Cooper KK Songer JG . Necrotic enteritis in chickens: a paradigm of enteric infection by Clostridium perfringens type a. Anaerobe. (2009) 15:55–60. doi: 10.1016/j.anaerobe.2009.01.006
17.
M’Sadeq SA Wu S Swick RA Choct M . Towards the control of necrotic enteritis in broiler chickens with in-feed antibiotics phasing-out worldwide. Anim Nutr. (2015) 1:1–11. doi: 10.1016/j.aninu.2015.02.004
18.
Casewell M Friis C Marco E McMullin P Phillips I . The European ban on growth-promoting antibiotics and emerging consequences for human and animal health. J Antimicrob Chemother. (2003) 52:159–61. doi: 10.1093/jac/dkg313
19.
Dahiya JP Wilkie DC Van Kessel AG Drew MD . Potential strategies for controlling necrotic enteritis in broiler chickens in post-antibiotic era. Anim Feed Sci Technol. (2006) 129:60–88. doi: 10.1016/j.anifeedsci.2005.12.003
20.
Li J Adams V Bannam TL Miyamoto K Garcia JP Uzal FA et al . Toxin plasmids of Clostridium perfringens. Microbiol Mol Biol Rev. (2013) 77:208–33. doi: 10.1128/MMBR.00062-12
21.
Fathima S Hakeem WGA Shanmugasundaram R Selvaraj RK . Necrotic enteritis in broiler chickens: A review on the pathogen, pathogenesis, and prevention. Microorganisms. (2022) 10:1958. doi: 10.3390/microorganisms10101958
22.
Keyburn AL Boyce JD Vaz P Bannam TL Ford ME Parker D et al . Net B, a new toxin that is associated with avian necrotic enteritis caused by Clostridium perfringens. PLoS Pathog. (2008) 4:e26. doi: 10.1371/journal.ppat.0040026
23.
Coursodon CF Glock RD Moore KL Cooper KK Songer JG . Tpe L-producing strains of Clostridium perfringens type a are highly virulent for broiler chicks. Anaerobe. (2012) 18:117–21. doi: 10.1016/j.anaerobe.2011.10.001
24.
Wilde S Jiang Y Tafoya AM Horsman J Yousif M Vazquez LA et al . Salmonella-vectored vaccine delivering three Clostridium perfringens antigens protects poultry against necrotic enteritis. PLoS One. (2019) 14:1–18. doi: 10.1371/journal.pone.0197721
25.
Wade B Keyburn AL Haring V Ford M Rood JI Moore RJ . Two putative zinc metalloproteases contribute to the virulence of Clostridium perfringens strains that cause avian necrotic enteritis. J Vet Diagn Invest. (2020) 32:259–67. doi: 10.1177/1040638719898689
26.
Verherstraeten S Goossens E Valgaeren B Pardon B Timbermont L Haesebrouck F et al . Perfringolysin o: the underrated Clostridium perfringens toxin?Toxins. (2015) 7:1702–21. doi: 10.3390/toxins7051702
27.
Wade B Keyburn AL Seemann T Rood JI Moore RJ . Binding of Clostridium perfringens to collagen correlates with the ability to cause necrotic enteritis in chickens. Vet Microbiol. (2015) 180:299–303. doi: 10.1016/j.vetmic.2015.09.019
28.
Lepp D Zhou Y Ojha S Mehdizadeh Gohari I Carere J Yang C et al . Clostridium perfringens produces an adhesive pilus required for the pathogenesis of necrotic enteritis in poultry. J Bacteriol. (2021) 203:e00578–20. doi: 10.1128/JB.00578-20
29.
Thompson DR Parreira VR Kulkarni RR Prescott JF . Live attenuated vaccine-based control of necrotic enteritis of broiler chickens. Vet Microbiol. (2006) 113:25–34. doi: 10.1016/j.vetmic.2005.10.015
30.
Kulkarni RR Parreira VR Sharif S Prescott JF . Immunization of broiler chickens against Clostridium perfringens-induced necrotic enteritis. Clin Vaccine Immunol. (2007) 14:1070–7. doi: 10.1128/CVI.00162-07
31.
Kulkarni RR Parreira VR Sharif S Prescott JF . Oral immunization of broiler chickens against necrotic enteritis with an attenuated Salmonella vaccine vector expressing Clostridium perfringens antigens. Vaccine. (2008) 26:4194–203. doi: 10.1016/j.vaccine.2008.05.079
32.
Jiang Y Kulkarni RR Parreira VR Prescott JF . Immunization of broiler chickens against Clostridium perfringens-induced necrotic enteritis using purified recombinant immunogenic proteins. Avian Dis. (2009) 53:409–15. doi: 10.1637/8656-021109-Reg.1
33.
Kulkarni RR Parreira VR Jiang YFF Prescott JF . A live oral recombinant Salmonella enterica serovar typhimurium vaccine expressing Clostridium perfringens antigens confers protection against necrotic enteritis in broiler chickens. Clin Vaccine Immunol. (2010) 17:205–14. doi: 10.1128/CVI.00406-09
34.
Jiang Y Mo H Willingham C Wang S Park J Yeun Y et al . Protection against necrotic enteritis in broiler chickens by regulated delayed lysis Salmonella vaccines. Avian Dis. (2015) 59:475–85. doi: 10.1637/11094-041715-Reg
35.
Hunter JGLL Wilde S Tafoya AM Horsman J Yousif M Diamos AG et al . Evaluation of a toxoid fusion protein vaccine produced in plants to protect poultry against necrotic enteritis. Peer J. (2019) 2019:1–19. doi: 10.7717/peerj.6600
36.
Gangaiah D Ryan V Van Hoesel D Mane SP Mckinley ET Lakshmanan N et al . Recombinant Limosi Lactobacillus (Lactobacillus) delivering nanobodies against Clostridium perfringens net B and alpha toxin confers potential protection from necrotic enteritis. Microbiology. (2022) 11:e1270. doi: 10.1002/mbo3.1270
37.
Wang S Hofacre CL Wanda SYY Zhou J Callum RA Nordgren B et al . A triple-sugar regulated Salmonella vaccine protects against Clostridium perfringens-induced necrotic enteritis in broiler chickens. Poult Sci. (2022) 101:101592. doi: 10.1016/j.psj.2021.101592
38.
Zekarias B Mo H Curtiss R Curtiss R III . Recombinant attenuated Salmonella enterica serovar typhimurium expressing the carboxy-terminal domain of alpha toxin from Clostridium perfringens induces protective responses against necrotic enteritis in chickens. Clin Vaccine Immunol. (2008) 15:805–16. doi: 10.1128/CVI.00457-07
39.
Cooper KK Trinh HT Songer JG . Immunization with recombinant alpha toxin partially protects broiler chicks against experimental challenge with Clostridium perfringens. Vet Microbiol. (2009) 133:92–7. doi: 10.1016/j.vetmic.2008.06.001
40.
Valipouri AR Rahimi S Karkhane AA Torshizi MAKK Mobarez AM Grimes JL . Immunization of broiler chickens with recombinant alpha-toxin protein for protection against necrotic enteritis. J Appl Poult Res. (2022) 31:100299. doi: 10.1016/j.japr.2022.100299
41.
Lanckriet A Timbermont L Eeckhaut V Haesebrouck F Ducatelle R Van Immerseel F . Variable protection after vaccination of broiler chickens against necrotic enteritis using supernatants of different Clostridium perfringens strains. Vaccine. (2010) 28:5920–3. doi: 10.1016/j.vaccine.2010.06.035
42.
Mot D Timbermont L Delezie E Haesebrouck F Ducatelle R van Immerseel F . Day-of-hatch vaccination is not protective against necrotic enteritis in broiler chickens. Avian Pathol. (2013) 42:179–84. doi: 10.1080/03079457.2013.778955
43.
Tsiouris V Georgopoulou I Batzios C Pappaioannou N Ducatelle R Fortomaris P . Heat stress as a predisposing factor for necrotic enteritis in broiler chicks. Avian Pathol. (2018) 47:616–24. doi: 10.1080/03079457.2018.1524574
44.
da Costa SPF Mot D Bokori-Brown M Savva CG Basak AK Van Immerseel F et al . Protection against avian necrotic enteritis after immunisation with net B genetic or formaldehyde toxoids. Vaccine. (2013) 31:4003–8. doi: 10.1016/j.vaccine.2013.05.063
45.
Fernandes da Costa SP Mot D Geeraerts S Bokori-Brown M Van Immerseel F Titball RW . Variable protection against experimental broiler necrotic enteritis after immunization with the C-terminal fragment of Clostridium perfringens alpha-toxin and a non-toxic net B variant. Avian Pathol. (2016) 45:381–8. doi: 10.1080/03079457.2015.1129663
46.
Antonissen G Van Immerseel F Pasmans F Ducatelle R Haesebrouck F Timbermont L et al . The mycotoxin deoxynivalenol predisposes for the development of Clostridium perfringens-induced necrotic enteritis in broiler chickens. PLoS One. (2014) 9:1–8. doi: 10.1371/journal.pone.0108775
47.
Antonissen G Croubels S Pasmans F Ducatelle R Eeckhaut V Devreese M et al . Fumonisins affect the intestinal microbial homeostasis in broiler chickens, predisposing to necrotic enteritis. Vet Res. (2015) 46:1–11. doi: 10.1186/s13567-015-0234-8
48.
Tsiouris V Georgopoulou I Batzios C Pappaioannou N Ducatelle R Fortomaris P . High stocking density as a predisposing factor for necrotic enteritis in broiler chicks. Avian Pathol. (2015) 44:59–66. doi: 10.1080/03079457.2014.1000820
49.
Tsiouris V Georgopoulou I Batzios C Pappaioannou N Ducatelle R Fortomaris P . The effect of cold stress on the pathogenesis of necrotic enteritis in broiler chicks. Avian Pathol. (2015) 44:430–5. doi: 10.1080/03079457.2015.1083094
50.
Tsiouris V Georgopoulou I Batzios C Pappaioannou N Diakou A Petridou E et al . The role of an attenuated anticoccidial vaccine on the intestinal ecosystem and on the pathogenesis of experimental necrotic enteritis in broiler chickens. Avian Pathol. (2013) 42:163–70. doi: 10.1080/03079457.2013.776161
51.
Tsiouris V Georgopoulou I Batzios C Pappaioannou N Ducatelle R Fortomaris P . Temporary feed restriction partially protects broilers from necrotic enteritis. Avian Pathol. (2014) 43:139–45. doi: 10.1080/03079457.2014.889278
52.
Katalani C Ahmadian G Nematzadeh G Amani J Ehsani P Razmyar J et al . Immunization with oral and parenteral subunit chimeric vaccine candidate confers protection against necrotic enteritis in chickens. Vaccine. (2020) 38:7284–91. doi: 10.1016/j.vaccine.2020.09.047
53.
Hoseini ZS Hajizade A Razmyar J Ahmadian G Arpanaei A . Mesoporous silica nanoparticles-based formulations of a chimeric proteinous vaccine candidate against necrotic enteritis disease. Mater Sci Eng C. (2021) 128:112316. doi: 10.1016/j.msec.2021.112316
54.
Shamshirgaran MA Golchin M Mohammadi E . Lactobacillus casei displaying Clostridium perfringens net B antigen protects chickens against necrotic enteritis. Appl Microbiol Biotechnol. (2022) 106:6441–53. doi: 10.1007/s00253-022-12155-y
55.
Shamshirgaran MA Golchin M Salehi M Kheirandish R . Evaluation the efficacy of oral immunization of broiler chickens with a recombinant Lactobacillus casei vaccine vector expressing the Carboxy-terminal fragment of α-toxin from Clostridium perfringens. BMC Vet Res. (2023) 19:1–9. doi: 10.1186/s12917-023-03566-8
56.
Keyburn AL Portela RW Sproat K Ford ME Bannam TL Yan X et al . Vaccination with recombinant net B toxin partially protects broiler chickens from necrotic enteritis. Vet Res. (2013) 44:1–8. doi: 10.1186/1297-9716-44-54
57.
Stanley D Keyburn AL Denman SE Moore RJ . Changes in the caecal microflora of chickens following Clostridium perfringens challenge to induce necrotic enteritis. Vet Microbiol. (2012) 159:155–62. doi: 10.1016/j.vetmic.2012.03.032
58.
Gharib-Naseri K Kheravii SK Keerqin C Morgan N Swick RA Choct M et al . Two different Clostridium perfringens strains produce different levels of necrotic enteritis in broiler chickens. Poult Sci. (2019) 98:6422–32. doi: 10.3382/ps/pez480
59.
Keyburn AL Portela RW Ford ME Bannam TL Yan XX Rood JI et al . Maternal immunization with vaccines containing recombinant net B toxin partially protects progeny chickens from necrotic enteritis. Vet Res. (2013) 44:1–7. doi: 10.1186/1297-9716-44-108
60.
Xue GD Barekatain R Wu SB Choct M Swick RA . Dietary L-glutamine supplementation improves growth performance, gut morphology, and serum biochemical indices of broiler chickens during necrotic enteritis challenge. Poult Sci. (2018) 97:1334–41. doi: 10.3382/ps/pex444
61.
Keerqin C Wu SB Svihus B Swick R Morgan N Choct M . An early feeding regime and a high-density amino acid diet on growth performance of broilers under subclinical necrotic enteritis challenge. Anim Nutr. (2017) 3:25–32. doi: 10.1016/j.aninu.2017.01.002
62.
Stanley D Wu SB Rodgers N Swick RA Moore RJ . Differential responses of cecal microbiota to fishmeal, Eimeria and Clostridium perfringens in a necrotic enteritis challenge model in chickens. PLoS One. (2014) 9:e104739. doi: 10.1371/journal.pone.0104739
63.
Rodgers NJ Swick RA Geier MS Moore RJ Choct M Wu SB . A multifactorial analysis of the extent to which Eimeria and fishmeal predispose broiler chickens to necrotic enteritis. Avian Dis. (2015) 59:38–45. doi: 10.1637/10774-011614-Reg.1
64.
Hilliar M Keerqin C Girish CK Barekatain R Wu SB Swick RA . Reducing protein and supplementing crystalline amino acids, to alter dietary amino acid profiles in birds challenged for subclinical necrotic enteritis. Poult Sci. (2020) 99:2048–60. doi: 10.1016/j.psj.2019.11.042
65.
Keerqin C McGlashan K Van TTH Chinivasagam HN Moore RJ Choct M et al . A lytic bacteriophage isolate reduced Clostridium perfringens induced lesions in necrotic enteritis challenged broilers. Front Vet Sci. (2022) 9:1058115. doi: 10.3389/fvets.2022.1058115
66.
Daneshmand A Sharma NK Kheravii SK Hall L Wu SB . Buffered formic acid and a monoglyceride blend improve performance and modulate gut bacteria and immunity gene expression in broilers under necrotic enteritis challenge. Poult Sci. (2023) 102:102978. doi: 10.1016/j.psj.2023.102978
67.
Jang SI Lillehoj HS Lee SH Lee KW Lillehoj EP Hong YH et al . Vaccination with Clostridium perfringens recombinant proteins in combination with Montanide™ ISA 71 VG adjuvant increases protection against experimental necrotic enteritis in commercial broiler chickens. Vaccine. (2012) 30:5401–6. doi: 10.1016/j.vaccine.2012.06.007
68.
Lillehoj HS Jang SI Panebra A Lillehoj EP Dupuis L Ben AJ et al . In ovo vaccination using Eimeria profilin and Clostridium perfringens net B proteins in Montanide IMS adjuvant increases protective immunity against experimentally-induced necrotic enteritis. Asian-Australasian. J Anim Sci. (2017) 30:1478–85. doi: 10.5713/ajas.17.0053
69.
Goo D Gadde UDD Kim WKK Gay CGG Porta EWW Jones SWW et al . Hyperimmune egg yolk antibodies developed against Clostridium perfringens antigens protect against necrotic enteritis. Poult Sci. (2023) 102:102841. doi: 10.1016/j.psj.2023.102841
70.
Jang SI Lillehoj HS Lee SHH Lee KW Lillehoj EP Hong YH et al . Relative disease susceptibility and clostridial toxin antibody responses in three commercial broiler lines coinfected with Clostridium perfringens and Eimeria maxima using an experimental model of necrotic enteritis. Avian Dis. (2013) 57:684–7. doi: 10.1637/10496-011813-ResNote.1
71.
Kim DK Lillehoj HS Jang SI Lee SH Hong YH Cheng HH . Transcriptional profiles of host-pathogen responses to necrotic enteritis and differential regulation of immune genes in two inbreed chicken lines showing disparate disease susceptibility. PLoS One. (2014) 9:1–20. doi: 10.1371/journal.pone.0114960
72.
Lepp D Ojha S Gohari IM Chakravarty B Prescott JF Gong J . Immunization with subunits of a novel pilus produced by virulent Clostridium perfringens strains confers partial protection against necrotic enteritis in chickens. Vet Microbiol. (2019) 230:7–13. doi: 10.1016/j.vetmic.2019.01.005
73.
Yang WY Lee YJ Lu HY Branton SL Chou CH Wang C . The net B-positive Clostridium perfringens in the experimental induction of necrotic enteritis with or without predisposing factors. Poult Sci. (2019) 98:5297–306. doi: 10.3382/ps/pez311
74.
Zaytsoff SJM Boras VF Uwiera RRE Inglis GD . A stress-induced model of acute necrotic enteritis in broiler chickens using dietary corticosterone administration. Poult Sci. (2022) 101:101726. doi: 10.1016/j.psj.2022.101726
75.
Jaramillo-Jaramillo AS Coulson TJDD Hofacre C Jones M O’Neill L Nguyen N et al . Effect of in-water administration of quorum system inhibitors in broilers’ productive performance and intestinal microbiome in a mild necrotic enteritis challenge. Avian Pathol. (2023) 52:309–22. doi: 10.1080/03079457.2023.2224260
76.
Hofacre CL Reynolds DJ Mathis GF Lumpkins BS Ollis N Smith JA et al . Effect of a competitive exclusion culture in a necrotic enteritis challenge model in broilers. J Appl Poult Res. (2019) 28:350–5. doi: 10.3382/japr/pfy078
77.
Shah BR Hakeem WA Shanmugasundaram R Selvaraj RK . Effect of synbiotic supplementation on production performance and severity of necrotic enteritis in broilers during an experimental necrotic enteritis challenge. Poult Sci. (2023) 102:102959–15. doi: 10.1016/j.psj.2023.102959
78.
Justino L Baptista AASS de Souza M Menck-Costa MF Pires BG Cicero CE et al . Evaluation of predisposing factors of necrotic enteritis in experimentally challenged broiler chickens. Animals. (2022) 12:1880. doi: 10.3390/ani12151880
79.
Saleh N Fathalla SI Nabil R Mosaad AA . Clinicopathological and immunological studies on toxoids vaccine as a successful alternative in controlling clostridial infection in broilers. Anaerobe. (2011) 17:426–30. doi: 10.1016/j.anaerobe.2011.04.019
80.
Salem HM Ismael E Shaalan M . Evaluation of the effects of silver nanoparticles against experimentally induced necrotic enteritis in broiler chickens. Int J Nanomedicine. (2021) 16:6783–96. doi: 10.2147/IJN.S319708
81.
Latorre JD Adhikari B Park SH Teague KD Graham LE Mahaffey BD et al . Evaluation of the epithelial barrier function and ileal microbiome in an established necrotic enteritis challenge model in broiler chickens. Front Vet Sci. (2018) 5:199. doi: 10.3389/fvets.2018.00199
82.
Zhao Y Zeng Y Zeng D Wang H Sun N Xin J et al . Dietary probiotic supplementation suppresses subclinical necrotic enteritis in broiler chickens in a microbiota-dependent manner. Front Immunol. (2022) 13:855426. doi: 10.3389/fimmu.2022.855426
83.
Abd El-Ghany WA Abdel-Latif MA Hosny F Alatfeehy NM Noreldin AE Quesnell RR et al . Comparative efficacy of postbiotic, probiotic, and antibiotic against necrotic enteritis in broiler chickens. Poult Sci. (2022) 101:101988. doi: 10.1016/j.psj.2022.101988
84.
Lee JH Lee B Rousseau X Gomes GA Oh HJ Kim YJ et al . Stimbiotic supplementation modulated intestinal inflammatory response and improved broilers performance in an experimentally-induced necrotic enteritis infection model. J Anim Sci Biotechnol. (2022) 13:1–17. doi: 10.1186/s40104-022-00753-9
85.
Lensing M Van der Klis JD Fabri T Cazemier A Else AJ . Efficacy of a lactylate on production performance and intestinal health of broilers during a subclinical Clostridium perfringens infection. Poult Sci. (2010) 89:2401–9. doi: 10.3382/ps.2010-00942
86.
Jerzsele A Szeker K Csizinszky R Gere E Jakab C Mallo JJ et al . Efficacy of protected sodium butyrate, a protected blend of essential oils, their combination, and Bacillus amyloliquefaciens spore suspension against artificially induced necrotic enteritis in broilers. Poult Sci. (2012) 91:837–43. doi: 10.3382/ps.2011-01853
87.
McReynolds JL Byrd JA Anderson RC Moore RW Edrington TS Genovese KJ et al . Evaluation of immunosuppressants and dietary mechanisms in an experimental disease model for necrotic enteritis. Poult Sci. (2004) 83:1948–52. doi: 10.1093/ps/83.12.1948
88.
Shivaramaiah S Wolfenden RE Barta JR Morgan MJ Wolfenden AD Hargis BM et al . The role of an early Salmonella Typhimurium infection as a predisposing factor for necrotic enteritis in a laboratory challenge model. Avian Dis. (2011) 55:319–23. doi: 10.1637/9604-112910-ResNote.1
89.
Pham VH Kan L Huang J Geng Y Zhen W Guo Y et al . Dietary encapsulated essential oils and organic acids mixture improves gut health in broiler chickens challenged with necrotic enteritis. J Anim Sci Biotechnol. (2020) 11:1–18. doi: 10.1186/s40104-019-0421-y
90.
Aljumaah MR Alkhulaifi MM Abudabos AM Aljumaah RS Alsaleh AN Stanley D . Bacillus subtilis PB6 based probiotic supplementation plays a role in the recovery after the necrotic enteritis challenge. PLoS One. (2020) 15:1–18. doi: 10.1371/journal.pone.0232781
91.
Kan L Guo F Liu Y Pham VH Guo Y Wang Z . Probiotics Bacillus licheniformis improves intestinal health of subclinical necrotic enteritis-challenged broilers. Front Microbiol. (2021) 12:623739. doi: 10.3389/fmicb.2021.623739
92.
Yuan L Li M Qiao Y Wang H Cui L Wang M . The impact of Berberine on intestinal morphology, microbes, and immune function of broilers in response to necrotic enteritis challenge. Biomed Res Int. (2021) 2021:1–9. doi: 10.1155/2021/1877075
93.
Wang Y Xu Y Xu S Yang J Wang K Zhan X . Bacillus subtilis DSM29784 alleviates negative effects on growth performance in broilers by improving the intestinal health under necrotic enteritis challenge. Front Microbiol. (2021) 12:1723187. doi: 10.3389/fmicb.2021.723187
94.
Guo S He L Zhang Y Niu J Li C Zhang Z et al . Effects of vitamin a on immune responses and vitamin a metabolism in broiler chickens challenged with necrotic enteritis. Lifestyles. (2023) 13:1122. doi: 10.3390/life13051122
95.
Zheng C Xiao G Yan X Qiu T Liu S Ou J et al . Complex of lauric acid monoglyceride and cinnamaldehyde ameliorated subclinical necrotic enteritis in yellow-feathered broilers by regulating gut morphology, barrier, inflammation and serum biochemistry. Animals. (2023) 13:516. doi: 10.3390/ani13030516
96.
Vidanarachchi JK Mikkelsen LL Constantinoiu CC Choct M Iji PA . Natural plant extracts and prebiotic compounds as alternatives to antibiotics in broiler chicken diets in a necrotic enteritis challenge model. Anim Prod Sci. (2013) 53:1247–59. doi: 10.1071/AN12374
97.
Swaggerty CL Byrd JA Arsenault RJ Perry F Johnson CN Genovese KJ et al . A blend of microencapsulated organic acids and botanicals reduces necrotic enteritis via specific signaling pathways in broilers. Poult Sci. (2022) 101:101753. doi: 10.1016/j.psj.2022.101753
98.
Yuan B Sun Z Lu M Lillehoj H Lee Y Liu L et al . Immunization with pooled antigens for Clostridium perfringens conferred partial protection against experimental necrotic enteritis in broiler chickens. Vaccine. (2022) 10:979. doi: 10.3390/vaccines10060979
99.
Gaghan C Gorrell K Taha-Abdelaziz K Sharif S Kulkarni RR . Intracloacal inoculation of broiler chickens with Clostridium perfringens strains: evaluation of necrotic enteritis disease development and lymphoid immune responses. Microorganisms. (2023) 11:771. doi: 10.3390/microorganisms11030771
100.
Daneshmand A Kermanshahi H Mohammed J Sekhavati MH Javadmanesh A Ahmadian M et al . Intestinal changes and immune responses during Clostridium perfringens-induced necrotic enteritis in broiler chickens. Poult Sci. (2022) 101:101652. doi: 10.1016/j.psj.2021.101652
101.
van Eerden E Santos RR Molist F Dardi M Pantoja-Millas LA Molist-Badiola J et al . Efficacy of an attenuated vaccine against avian coccidiosis in combination with feed additives based on organic acids and essential oils on production performance and intestinal lesions in broilers experimentally challenged with necrotic enteritis. Poult Sci. (2022) 101:101848. doi: 10.1016/j.psj.2022.101848
102.
Fatemi Motlagh M Mousavi Gargari SL . A bivalent vaccine against avian necrotic enteritis and coccidiosis. J Appl Microbiol. (2022) 132:113–25. doi: 10.1111/jam.15178
103.
Bortoluzzi C Vieira BS Hofacre C Applegate TJ . Effect of different challenge models to induce necrotic enteritis on the growth performance and intestinal microbiota of broiler chickens. Poult Sci. (2019) 98:2800–12. doi: 10.3382/ps/pez084
104.
Huang T Peng XY Gao B Wei QL Xiang R Yuan MG et al . The effect of Clostridium butyricum on gut microbiota, immune response and intestinal barrier function during the development of necrotic enteritis in chickens. Front Microbiol. (2019) 10:2309. doi: 10.3389/fmicb.2019.02309
105.
Fries-Craft K Graham D Hargis BM Bobeck EA . Evaluating a Salmonella Typhimurium, Eimeria maxima, and Clostridium perfringens coinfection necrotic enteritis model in broiler chickens: repeatability, dosing, and immune outcomes. Poult Sci. (2023) 102:103018. doi: 10.1016/j.psj.2023.103018
106.
Bangoura B Alnassan AA Lendner M Shehata AA Krüger M Daugschies A . Efficacy of an anticoccidial live vaccine in prevention of necrotic enteritis in chickens. Exp Parasitol. (2014) 145:125–34. doi: 10.1016/j.exppara.2014.08.004
107.
Shojadoost B Peighambari SM Nikpiran H . Effects of virginiamycin against experimentally induced necrotic enteritis in broiler chickens vaccinated or not with an attenuated coccidial vaccine. J Appl Poult Res. (2013) 22:160–7. doi: 10.3382/japr.2012-00541
108.
Bortoluzzi C Vieira BS Lumpkins B Mathis GF King WD Graugnard D et al . Can dietary zinc diminish the impact of necrotic enteritis on growth performance of broiler chickens by modulating the intestinal immune-system and microbiota?Poult Sci. (2019) 98:3181–93. doi: 10.3382/ps/pez045
109.
Hernandez-Patlan D Solis-Cruz B Pontin KP Hernandez-Velasco X Merino-Guzman R Adhikari B et al . Impact of a Bacillus direct-fed microbial on growth performance, intestinal barrier integrity, necrotic enteritis lesions, and Ileal microbiota in broiler chickens using a laboratory challenge model. Front Vet Sci. (2019) 6:108. doi: 10.3389/fvets.2019.00108
110.
Sokale AO Menconi A Mathis GF Lumpkins B Sims MD Whelan RA et al . Effect of Bacillus subtilis DSM 32315 on the intestinal structural integrity and growth performance of broiler chickens under necrotic enteritis challenge. Poult Sci. (2019) 98:5392–400. doi: 10.3382/ps/pez368
111.
Akerele G Al Hakeem WG Lourenco J Selvaraj RK . The effect of necrotic enteritis challenge on production performance, Cecal microbiome, and Cecal tonsil transcriptome in broilers. Pathogens. (2022) 11::839. doi: 10.3390/pathogens11080839
112.
Lv H Chen P Wang Y Xu L Zhang K Zhao J et al . Chlorogenic acid protects against intestinal inflammation and injury by inactivating the mt DNA–cGAS–STING signaling pathway in broilers under necrotic enteritis challenge. Poult Sci. (2024) 103:103274. doi: 10.1016/j.psj.2023.103274
113.
Moritz AH Lumpkins B Mathis GF Bridges WC Wilson S Blair ME et al . Comparative efficacy of tannin-free grain sorghum varieties for the control of necrotic enteritis caused by Clostridium perfringens in broiler chickens. Poult Sci. (2023) 102:102300. doi: 10.1016/j.psj.2022.102300
114.
Fries-Craft K Bobeck EA . Early Salmonella Typhimurium inoculation may obscure anti-interleukin-10 protective effects on broiler performance during coccidiosis and necrotic enteritis challenge. Poult Sci. (2024) 103:103187. doi: 10.1016/j.psj.2023.103187
115.
McReynolds JL Byrd JA Genovese KJ Poole TL Duke SE Farnell MB et al . Dietary lactose and its effect on the disease condition of necrotic enteritis. Poult Sci. (2007) 86:1656–61. doi: 10.1093/ps/86.8.1656
116.
McReynolds J Waneck C Byrd J Genovese K Duke S Nisbet D . Efficacy of multistrain direct-fed microbial and phytogenetic products in reducing necrotic enteritis in commercial broilers. Poult Sci. (2009) 88:2075–80. doi: 10.3382/ps.2009-00106
117.
Dahiya JP Hoehler D Van Kessel AG Drew MD . Dietary encapsulated glycine influences Clostridium perfringens and lactobacilli growth in the gastrointestinal tract of broiler chickens. J Nutr. (2007) 137:1408–14. doi: 10.1093/jn/137.6.1408
118.
Duff AF Vuong CN Searer KL Briggs WN Wilson KM Hargis BM et al . Preliminary studies on development of a novel subunit vaccine targeting Clostridium perfringens mucolytic enzymes for the control of necrotic enteritis in broilers. Poult Sci. (2019) 98:6319–25. doi: 10.3382/ps/pez448
119.
Wang H Latorre JD Bansal M Abraha M Al-Rubaye B Tellez-Isaias G et al . Microbial metabolite deoxycholic acid controls Clostridium perfringens-induced chicken necrotic enteritis through attenuating inflammatory cyclooxygenase signaling. Sci Rep. (2019) 9:14541. doi: 10.1038/s41598-019-51104-0
120.
Fu Y Bansal M Alenezi T Almansour A Wang H Sun X . Vaccines using Clostridium perfringens sporulation proteins reduce necrotic enteritis in chickens. Microorganisms. (2022) 10:1–15. doi: 10.3390/microorganisms10061110
121.
Shojadoost B Vince AR Prescott JF . The successful experimental induction of necrotic enteritis in chickens by Clostridium perfringens: a critical review. Vet Res. (2012) 43:1–12. doi: 10.1186/1297-9716-43-74
122.
Jin X Huang G Luo Z Hu Y Liu D . Oregano (Origanum vulgare l.) essential oil feed supplement protected broilers chickens against Clostridium perfringens induced necrotic enteritis. Agriculture. (2022) 12:18. doi: 10.3390/agriculture12010018
123.
Moore RJ . Necrotic enteritis predisposing factors in broiler chickens. Avian Pathol. (2016) 45:275–81. doi: 10.1080/03079457.2016.1150587
124.
Riaz A Umar S Munir MT Tariq M . Replacements of antibiotics in the control of necrotic enteritis: a review. Sci Lett. (2017) 5:208–16.
125.
Abid SA Azeem T Chaudhary ZI Rehman ZU Umar S . Emerging threat of necrotic enteritis in poultry and its control without use of antibiotics: a review. J Anim Plant Sci. (2016) 26:1556–1567.
126.
Adhikari P Kiess A Adhikari R Jha R . An approach to alternative strategies to control avian coccidiosis and necrotic enteritis. J Appl Poult Res. (2020) 29:515–34. doi: 10.1016/j.japr.2019.11.005
127.
Paiva D McElroy A . Necrotic enteritis: applications for the poultry industry. J Appl Poult Res. (2014) 23:557–66. doi: 10.3382/japr.2013-00925
128.
Zou J Zheng P Zhang K Ding X Bai S . Effects of exogenous enzymes and dietary energy on performance and digestive physiology of broilers. J Anim Sci Biotechnol. (2013) 4:1–9. doi: 10.1186/2049-1891-4-14
129.
Zaytsoff SJM Montina T Boras VF Brassard J Moote PE Uwiera RRE et al . Microbiota transplantation in day-old broiler chickens ameliorates necrotic enteritis via modulation of the intestinal microbiota and host immune responses. Pathogens. (2022) 11:972. doi: 10.3390/pathogens11090972
130.
Zaytsoff SJM Lyons SM Garner AM Uwiera RRE Zandberg WF Abbott DW et al . Host responses to Clostridium perfringens challenge in a chicken model of chronic stress. Gut Pathog. (2020) 12:1–16. doi: 10.1186/s13099-020-00362-9
131.
Dierick E Hirvonen OP Haesebrouck F Ducatelle R Van Immerseel F Goossens E . Rapid growth predisposes broilers to necrotic enteritis. Avian Pathol. (2019) 48:416–22. doi: 10.1080/03079457.2019.1614147
132.
Nakamura YK Omaye ST . Metabolic diseases and pro-and prebiotics: mechanistic insights. Nutr Metab (Lond). (2012) 9:1–9. doi: 10.1186/1743-7075-9-60
133.
Liu N Wang JQ Jia SC Chen YK Wang JP . Effect of yeast cell wall on the growth performance and gut health of broilers challenged with aflatoxin B 1 and necrotic enteritis. Poult Sci. (2018) 97:477–84. doi: 10.3382/ps/pex342
134.
Droual R Farver TB Bickford AA . Relationship of sex, age, and concurrent intestinal disease to necrotic enteritis in turkeys. Avian Dis. (1995) 39:599–605. doi: 10.2307/1591814
135.
Abdelli N Pérez JF Vilarrasa E Luna IC Melo-Duran D D’angelo M et al . Targeted-release organic acids and essential oils improve performance and digestive function in broilers under a necrotic enteritis challenge. Animals. (2020) 10:259. doi: 10.3390/ani10020259
136.
Xue W Zhao Q Li P Zhang R Lan J Wang J et al . Identification and characterization of a novel nanobody against duck hepatitis a virus type 1. Virology. (2019) 528:101–9. doi: 10.1016/j.virol.2018.12.013
137.
Daneshmand A Kermanshahi H Sekhavati MH Javadmanesh A Ahmadian M Alizadeh M et al . Effects of cLFchimera peptide on intestinal morphology, integrity, microbiota, and immune cells in broiler chickens challenged with necrotic enteritis. Sci Rep. (2020) 10:1–11. doi: 10.1038/s41598-020-74754-x
138.
Rodrigues I Svihus B Bedford MR Gous R Choct M . Intermittent lighting improves resilience of broilers during the peak phase of sub-clinical necrotic enteritis infection. Poult Sci. (2018) 97:438–46. doi: 10.3382/ps/pex315
139.
Wu SB Stanley D Rodgers N Swick RA Moore RJ . Two necrotic enteritis predisposing factors, dietary fishmeal and Eimeria infection, induce large changes in the caecal microbiota of broiler chickens. Vet Microbiol. (2014) 169:188–97. doi: 10.1016/j.vetmic.2014.01.007
140.
Keerqin C Morgan NK Wu SB Swick RA Choct M . Dietary inclusion of arabinoxylo-oligosaccharides in response to broilers challenged with subclinical necrotic enteritis. Br Poult Sci. (2017) 58:418–24. doi: 10.1080/00071668.2017.1327705
141.
Calik A Omara II White MB Evans NP Karnezos TP Dalloul RA . Dietary non-drug feed additive as an alternative for antibiotic growth promoters for broilers during a necrotic enteritis challenge. Microorganisms. (2019) 7:257. doi: 10.3390/microorganisms7080257
142.
Collier CT Hofacre CL Payne AM Anderson DB Kaiser P Mackie RI et al . Coccidia-induced mucogenesis promotes the onset of necrotic enteritis by supporting Clostridium perfringens growth. Vet Immunol Immunopathol. (2008) 122:104–15. doi: 10.1016/j.vetimm.2007.10.014
143.
He W Goes EC Wakaruk J Barreda DR Korver DR . A poultry subclinical necrotic enteritis disease model based on natural Clostridium perfringens uptake. Front Physiol. (2022) 13:788592. doi: 10.3389/fphys.2022.788592
144.
Prescott JF Sivendra R Barnum DA . The use of bacitracin in the prevention and treatment of experimentally-induced necrotic enteritis in the chicken. Can Vet J. (1978) 19:181–3. PMID:
145.
Cooper KK Theoret JR Stewart BA Trinh HT Glock RD Songer JG . Virulence for chickens of Clostridium perfringens isolated from poultry and other sources. Anaerobe. (2010) 16:289–92. doi: 10.1016/j.anaerobe.2010.02.006
146.
Timbermont L Lanckriet A Gholamiandehkordi AR Pasmans F Martel A Haesebrouck F et al . Origin of Clostridium perfringens isolates determines the ability to induce necrotic enteritis in broilers. Comp Immunol Microbiol Infect Dis. (2009) 32:503–12. doi: 10.1016/j.cimid.2008.07.001
147.
Timbermont L Lanckriet A Dewulf J Nollet N Schwarzer K Haesebrouck F et al . Control of Clostridium perfringens-induced necrotic enteritis in broilers by target-released butyric acid, fatty acids and essential oils. Avian Pathol. (2010) 39:117–21. doi: 10.1080/03079451003610586
148.
Whelan RA Doranalli K Rinttilä T Vienola K Jurgens G Apajalahti J . The impact of Bacillus subtilis DSM 32315 on the pathology, performance, and intestinal microbiome of broiler chickens in a necrotic enteritis challenge. Poult Sci. (2019) 98:3450–63. doi: 10.3382/ps/pey500
149.
Hofacre CL Froyman R Gautrias B George B Goodwin MA Brown J . Use of Aviguard and other intestinal bioproducts in experimental Clostridium perfringens-associated necrotizing enteritis in broiler chickens. Avian Dis. (1998) 42:579–84. doi: 10.2307/1592685
150.
Collier CT Van der Klis JD Deplancke B Anderson DB Gaskins HR . Effects of tylosin on bacterial mucolysis, Clostridium perfringens colonization, and intestinal barrier function in a chick model of necrotic enteritis. Antimicrob Agents Chemother. (2003) 47:3311–7. doi: 10.1128/AAC.47.10.3311-3317.2003
151.
Richardson K Hofacre C Mathis G Lumpkins B Phillips R . Impact of controlling bacteria in feed on broiler performance during a Clostridial challenge. Avian Dis. (2017) 61:453–6. doi: 10.1637/11616-022817-Reg.1
Summary
Keywords
necrotic enteritis, Clostridium perfringens , disease induction, experimental challenge, predisposing factors, poultry
Citation
Shamshirgaran MA and Golchin M (2024) A comprehensive review of experimental models and induction protocols for avian necrotic enteritis over the past 2 decades. Front. Vet. Sci. 11:1429637. doi: 10.3389/fvets.2024.1429637
Received
08 May 2024
Accepted
07 June 2024
Published
24 July 2024
Volume
11 - 2024
Edited by
Juan D. Latorre, University of Arkansas, United States
Reviewed by
Kenneth James Genovese, United States Department of Agriculture, United States
Katarzyna B. Miska, Agricultural Research Service (USDA), United States
Updates
Copyright
© 2024 Shamshirgaran and Golchin.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mehdi Golchin, golchin@uk.ac.ir
†ORCID: Mohammad Ali Shamshirgaran, orcid.org/0000-0002-1199-4464
Mehdi Golchin, orcid.org/0000-0002-0969-4921
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.