- 1Department of Animal Science, Universidad Nacional Agraria de la Selva, Tingo María, Peru
- 2Department of Animal and Public Health, Faculty of Veterinary Medicine, Universidad Nacional Mayor de San Marcos, Lima, Peru
- 3Postgraduate School, Universidad Nacional Agraria de la Selva, Tingo María, Peru
- 4Postgraduate School, Universidad Nacional Agraria La Molina, Lima, Peru
High-level use of antibiotics as grow promotors in animal nutrition in the last six decades has pushed to bacterial resistance to these molecules. The search for alternative ways including plants extracts, essential oils or phytochemicals to tackle this problem is increasing nowadays. This study aimed to evaluate the effects of Piper aduncum polyphenols (PaP) and flavonoids (PaF) on broiler chicken gut health. 396 Cobb 500 broiler chickens aged 1–33 d old were fed a base diet (BD). Birds were randomly divided into two control (C) and four supplementations (S) groups. C1 was fed with BD and C2 with BD + 50 ppm zinc bacitracin. S1 and S2 were supplemented with 17.5 and 35.0 ppm PaP, whereas S3 and S4 were supplemented with 17.5 and 35.0 ppm PaF of the diet, respectively and sub ministered in drinking water from 1–21 d of age. The in vivo gut microbiota at 21 and 28 d of age, gut villi histomorphometry at 7, 14, and 21 d and performance indices at 7, 21 and 33 d were evaluated. Data was processed using a general factorial arrangement. PaP and PaF supplementation, increased lymphocytes and globulins in chickens at 14 d of age (p < 0.05), at the same time erythrocytes, granulocytes, and ALT profiles decreased at 21 d of age (p < 0.05). Escherichia coli and Staphylococcus aureus abundance (log10 CFU/g) decreased, Lactobacillus sp. was enhanced in ileal mucosa and content of chickens at 21 d old on supplementation 35.0 ppm PaP, 17.5 and 35.0 ppm of PaF (p < 0.05) and villi length increased with the age of chickens supplemented 17.5 ppm of PaP, 17.5 and 35.0 ppm of PaF (p < 0.05). As a result, PaP and PaF maintain weight gain and feed conversion rate, reduce feed intake and improve carcass yield overall in the three stages of broiler chickens. In conclusion, PaP and PaF enhanced gut health, the immune and anti-inflammatory activity, and performance indices of broiler chickens.
1 Introduction
Residues from antibiotics, because of indiscriminate use in food animals mainly as growth promotors represent a risk in processing and profitability of livestock production. This adds antimicrobial resistance to certain microorganisms (1, 2), deterioration of the environment, and consequently, deterioration of public well-being and health (1, 3–6). This direct risk to public health has been documented in terms of cancerous properties, modification of microbiota, and potential allergies (7–11).These situations warrant the need to supply innocuous products for food and medication, guaranteeing animal and public health (12–15).
Enzymes, probiotics, prebiotics, herbs, amino acids, immunostimulants, organic acids, bacteriocins, and phytotherapeutic plants have been investigated as antibiotics growth promotors’ alternatives (16–21). These are the most common feed additives that acquire popularity in the poultry industry following the ban of antibiotic growth promoters (AGPs). They are commonly used worldwide because of their unique properties and positive impact on poultry production.
In the National Health Directorate, a variety of plants have been registered, which have been identified for the treatment of several diseases. These plants are known to have been used since ancestral times. Thus, the understanding of their diverse benefits as nutraceuticals or promoters of wellbeing and health (22, 23) is essential. Among these plants, Piper aduncum L. (Matico) contains phytochemical components, including polyphenols, flavonoids, triterpenes, alkaloids, and phenylpropanoids (24, 25). It also possesses antibacterial activity against pathogenic organisms (26). Polyphenols and flavonoids are phenol compounds that are extensively present in nature, which are responsible for the reliable performance of plants and their benefits to human health; they have been recognized in numerous studies (27–29).
One of the benefits of phenol compounds is their antioxidant activity (30–33). This eliminates free radicals generated by biomolecules, such as lipids, proteins, and nucleic acids under stressful conditions; this is the basic mechanism for protection against the development of cardiovascular and degenerative diseases, cancer, and diabetes in humans (29, 34, 35).
Another beneficial effect of polyphenols is their antimicrobial activity, wherein phenolic compounds, such as polyphenols and flavonoids reduce the colonization of bacteria with pathogenic potential in the gastrointestinal tract through diverse mechanisms (36–38).
In previous studies on polyphenols, such as curcumin, resveratrol, and epigallocatechin gallate, in broiler chickens, laying hens, and quails, promising results were obtained in terms of the performance of these birds (39–42).
The aim of the present study was to determine the effect of the polyphenol phytocompounds and flavonoids from P. aduncum leaves on the enhancing of gut health, as an alternative to antibiotic use as growth promotor in broiler chickens.
2 Materials and methods
2.1 Piper aduncum polyphenols and flavonoids
To obtain P. aduncum polyphenols and flavonoids in this study, first the leaves were collected from the plants, in the Luyando district in the Leoncio Prado province of the Huánuco region in Peru, located at 18 L 3981790, UTM 8973000 and 715 m.a.s.l. During the morning hours, the intermediate leaves were collected, carefully cleaned with distilled water and later dried in a stove at 40°C for 72 h. The leaves were ground in a Thomas Willey model 4 brand grinder (United States) until a thick powder was obtained. They were then sifted through a sieve to make the particle size homogeneous. Subsequently, the extract was obtained using the ethanolic extraction method, as described previously (43). This was followed by phytochemical shifting and division of the polyphenols using the Singleton and Rossi (44) colorimetric method and flavonoids according to the method of Kumazawa et al. (45).
2.2 Experimental design and broiler chickens
This study was reviewed and approved by the Ethics and Animal Wellbeing committee of the Veterinary Medicine School at the Universidad Nacional Mayor de San Marcos, with authorization, N° 2012–5. The location of this study was at 09° 17′58″ south latitude and 76° 01′07″ west longitude, at a height of 660 m.a.s.l., with an annual precipitation of 3,293 mm, an average annual temperature of 24.85°C, and a relative humidity of 80% (46).
Three hundred sixty Cobb 500 chickens aged 1 day, 40.0 ± 4.0 g weight, were used. The chickens were divided into six groups: C1, C2, S1, S2, S3, and S4, each with five replicates and 12 chickens per each. These birds received similar handling conditions and feeding, where a base diet (BD) was provided during the initial (1–7 d), growth (8–21 d), and finishing phase (22–33 d old). The chickens from C1 were fed with BD, those in C2 with BD + 50 ppm of zinc bacitracin (ZB) (Albac, Norway) in the diet, those in S1 and S2 were fed BD + 17.5 and 35 ppm of PaP, and those in S3 and S4 were fed BD + 17.5 and 35 ppm PaF of the diet via drinking water, from day 1 to 21 d of age. The PaP and PaF were formulated at a concentration of 10 mg/mL in a physiological serum dilutant. At this concentration, the solutions were separated into aliquots at the beginning of the experiment, according to the calculations at 17.5 ppm and 35 ppm of the chickens’ diet obtained for each day of the experiment. The aliquots were refrigerated at 4° C, to allow removal out daily for the volume that corresponded to each day for the S1-S4 groups of experimental chickens.
2.3 Experimental diet and nutrition
The diet was formulated in the Mixit-2 program, based on the tables by Rostagno et al. (47). For the preparation, a pre-mix of the micronutrients was made with raw insoluble fiber to obtain good homogenization for the feed. The final mix of the ingredients was made with a horizontal mixer with a capacity of 100 kg, for 10 min. Proximal analysis of the formulated experimental rations was performed for male broiler chickens during the initial, growth, and finishing phases (1–33 d of age). They were in line with the requirements for each phase (Table 1). The nutritional compositions of the initial, growth, and finishing stages were determined based on the requirements of each stage. With this goal, samples of the base diet and the diet supplemented with ZB were analyzed for proximal chemical composition in the Animal Nutrition Laboratory at the Universidad Nacional Agraria de la Selva.
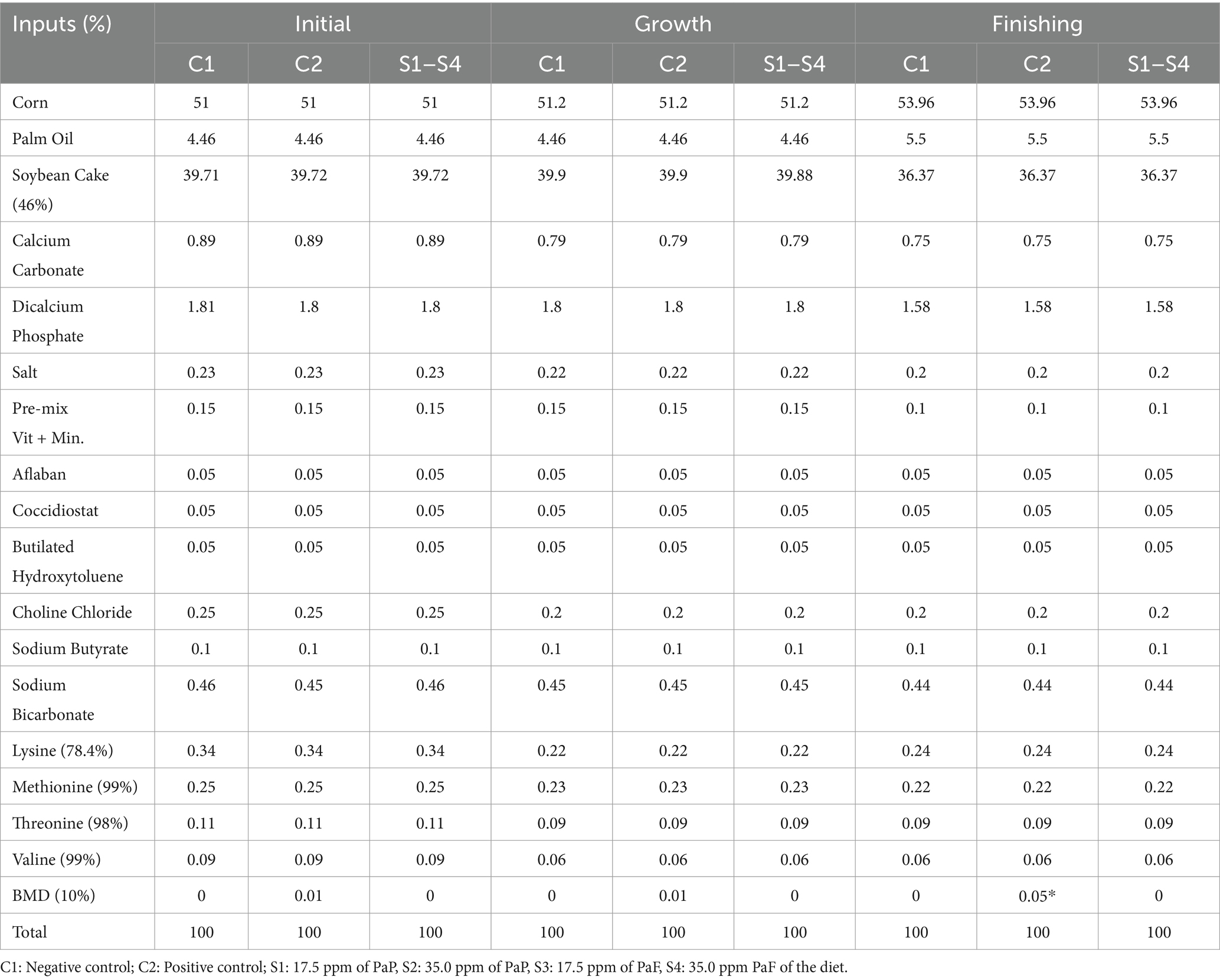
Table 1. Diets formulated for male broiler chickens during the initial phase (1–7 d of age), growth (8–21 d of age), and finishing (22–33 d of age).
2.4 Proximal chemical composition of diets
To determine dry matter (DM) content, the samples were dried in an air forced oven (Memmert, UN110 Plus, Germany) at 105°C for 4 h. The samples were analyzed for ashes after 12 h of combustion in a muffle furnace at 600°C (Linn Electro Therm, LM-312.06, Germany). Crude protein (CP) using a Kjeldahl Nitrogen Analyzer (Buchi Digestion Automat, K-438, and Buchi Distillation Unit K-350, Switzerland); Ether extract using an extractor (Aknom XT10, United States). Total fiber was determined by a semiautomatic fiber analyzer (Aknom 200, USA). The nitrogen- free extract was computed as the difference between DM and nutrients determined in the proximal analysis of the diets. The chemical analysis of diets is depicted in Table 2. The free extraction of nitrogen was determined from the difference between DM and nutrients from the proximal chemical analysis.
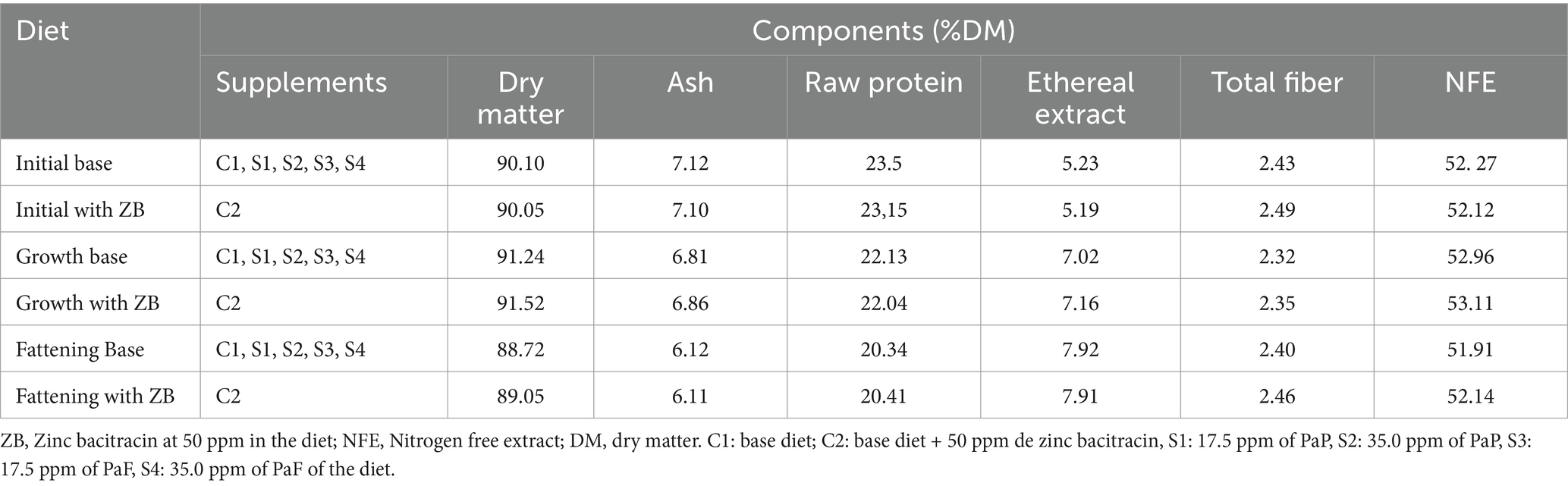
Table 2. Proximal analysis composition of diets for male broiler chickens during the initial, growth, and fattening stages (1–33 days of age).
2.5 Blood samples and hematological profiles
Blood samples were collected by puncturing the alar veins. Blood for the hematological profiles was obtained in 2-mL vacutainers that contained 2 mg of heparin. The blood for the blood metabolite profiles was obtained in 4-mL vacutainers, which were centrifuged at 1500 rpm for 5 min after coagulation. The serum was separated into 2-mL Eppendorf tubes and stored at −10°C until the analysis with a spectrophotometer. Samples were collected from 30 chickens, 5 chickens per each group of supplementation and controls at 7, 14, and 21 d of age.
The erythrocyte, total and differential leukocyte counts, hematocrit and hemoglobin were determined from whole blood. For the hematocrit, the microhematocrit method and hemoglobin levels, the cyanmethemoglobin method were carried out. Mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC) were also obtained (48).
The glucose level was determined from the blood serum using the glucosidase-peroxidase method. Total protein was obtained using the EDTA/Cu complex in sodium hydroxide. Albumin level was determined using tetrabrom-cresolsulfonphthalein (49, 50). Total cholesterol, alanine aminotransferase, aspartate aminotransferase, total bilirubin, and conjugated bilirubin (Laboratories QAC, España) levels were determined. Measurements were performed at 515 and 530 nm using an Auto Chemistry Analyzer-AS 830 spectrophotometers (Italy).
2.6 Intestinal content and microbial culture
Four chickens from each of the six supplementation groups were chosen at random at 21 and 28 days of age and sacrificed by atlanto-occipital dislocation. Their digestive tracts were immediately dissected and a segment of approximately 30 cm of the ileum, beside Meckel’s diverticulum, toward the blind (51, 52), was opened to obtain the intestinal content. This included scraping the mucosa and collecting it on sterile petri dishes.
Native species from the microbiota of broiler chickens, such as Escherichia coli, Lactobacillus sp., and Staphylococcus sp., were grown (51). For the cultivation of E. coli, MacConkey agar; for Lactobacillus sp., MRS agar, and for S. aureus, mannitol salt agar was used (Merck, Darmstadt, Germany). The culture petri dishes were incubated for 24 h at 37°C. The bacterial count was expressed as the base 10 logarithm of the number of colony-forming units per gram (log10CFU/g) of ileal content (51, 52).
2.7 Intestinal tissue and histomorphometry
Five chickens from each of the six treatments were randomly selected at 14, 21, and 28 d of age and sacrificed by atlanto-occipital dislocation. Their digestive tracts were immediately dissected. A segment approximately 5 cm from the middle part of the duodenum, jejunum, and ileum segments (51, 52) was taken, opened lengthwise, and transversely sectioned. They were removed and submerged three to four times in a sterile physiological serum solution to remove the intestinal content from the mucus. They were later attached with staples to sturdy posterboard bases to keep the segments straight. The three segments from each bird were stored in 100 mL of 10% formalin solution with physiological serum. The stored intestinal samples were processed using conventional histological methods and stained with hematoxylin and eosin (53).
Measurements of the length and width of the intestinal villi and depth of the crypts were taken by measuring 10 villi at 10X. The average of each intestinal segment, corresponding to each animal, was determined. A Leica® DM500 optical microscope and LAS EZ Leica® program, installed on a computer connected to the Leica® microscope were used. This also included a Leica® ICC50 camera. This system allows the distance between any pair of points chosen by the user to be determined. The villus length was measured from its apex to the apex of the Lieberkühn crypt entrance. The width of the villi was measured as the line perpendicular to the middle section of the villi. The depth of the Lieberkühn crypt was measured from the entrance to the crypt to its base zone. The measurements were recorded in micrometers.
2.8 Performance indices
At 7, 21, and 28 d of age, all chickens were weighed and feed consumption was recorded, and the following indices were determined:
• Feed intake (FI) was computed from the relationship between the total consumption of the lot and the number of birds in the lot.
• Weight gain (WG) was computed using the relationship between the final weight minus initial weight of the lot and number of birds in the lot.
• Feed conversion rate (FCR) was computed from the relationship between feed consumption and weight gain.
• Carcass yield: Was obtained from the relationship between weigh of carcass without any disposal and chicken live weight at 33 d old.
2.9 Statistical analyses
To evaluate the effect of PaP and PaF supplementation on the variables under study in relation to the age of the chicken, the data on blood metabolite profiles, measures of the villi, and Lieberkühn crypts in the intestinal segments were processed using a general factorial arrangement with three ages of chickens, two PaP and PaF levels + two controls, and for bacterial count, two ages of chickens were considered. The guide for statistical analysis was obtained from Pollesel et al. (54) and Bashir et al. (55). The data for the height and width of the villi, depth of the crypts, microbial abundance, hematological data, and the performance indices were primarily transformed using the square root, Box-Cox, or base 10 algorithms. Normality and homogeneity were later proven using the Shapiro–Wilk’s and Levene’s tests, respectively. A two tailed analysis of variance (ANOVA) was used to analyze the averages of the blood metabolites and hematological profiles, abundance of microbiota, and measurements of the villi and crypts. Tukey’s test was used to establish their significance (p ≤ 0.05). The Software Statistics Infostat (56) was used to process the data. The data for the productive indices were subjected to a completely randomized design, and the averages were compared using the student–Newman–Keuls (SNK) test (5%).
3 Results
3.1 Quantification of phenol compounds and flavonoids
In this study, in the P. aduncum leaves in a dry base, 12.5 mg of GAE/g of dry ethanol extract from phenol compounds and 0.2 mg of QE/g of dry extract from flavonoids were obtained. In this study, the mayor active components into these two groups of Piper aduncum phytocompounds have not being isolated. However, in previous studies dillapiod, asaricin, elemicin and myristicine as propenylphenols, pinostrobin and sakuranetin as flavonoids components have been identified in Piper aduncum (25, 57).
3.2 Hematology and blood metabolites profiles
The hematocrit, hemoglobin, and total erythrocyte profiles; MCV, MCH, and MCHC indices; and total leukocyte, lymphocyte, and granulocyte profiles of broiler chickens supplemented with PaP and PaF are depicted in Table 3. The hematocrit, hemoglobin, and total erythrocyte profiles, as well as the MCV and MCH indices, decreased in chickens supplemented with 17.5 and 35.0 ppm of PaP and 50 ppm of ZB, in comparison to those obtained for the C1 group chickens at 14 d (p < 0.05). The lymphocytes increased in the chickens because of supplementation with 35 ppm PaP, 17.5 and 35.0 ppm PaF, and C2 (p < 0.05) at 14 d of age (Table 3). Conversely, the granulocytes decreased in the chickens that were given supplements of 35 ppm PaP, 17.5, and 35 ppm PaF in the C2 group, in comparison to those in the C1 group at 14 d of age (p < 0.05).
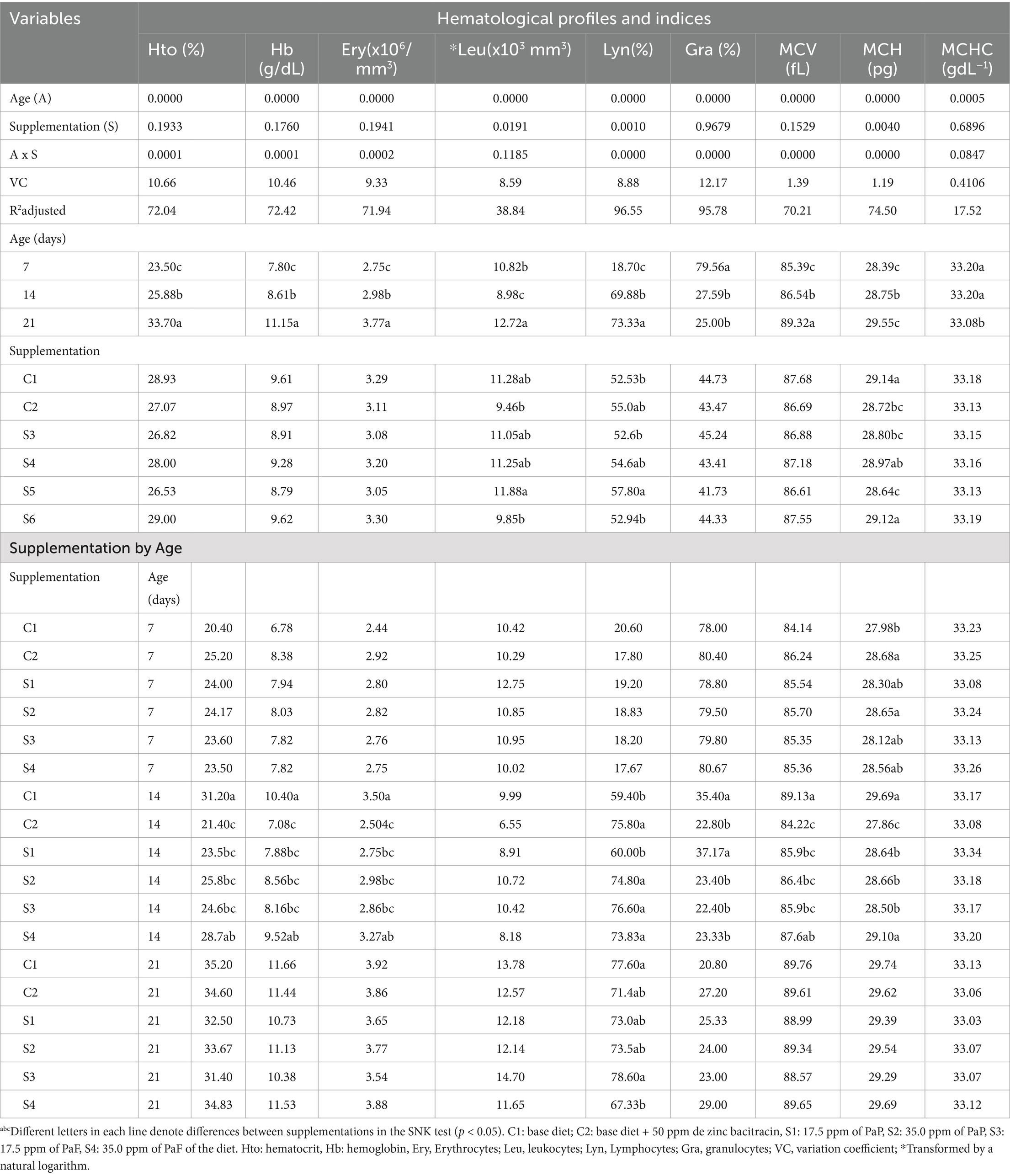
Table 3. Hematological profiles of broiler chickens supplemented with Piper aduncum polyphenols and flavonoids.
The glucose, cholesterol, triglyceride, aspartate transaminase, protein, alanine transaminase, albumin, and globulin levels in broiler chickens are depicted in Table 4. At 14 d of age, the globulin profiles increased in the chickens supplemented with 17.5 and 35.0 ppm of PaF, in comparison to the obtained for those supplemented with PaP and the chickens from groups C1 and C2 (p < 0.05; Table 4). Conversely, the albumin profile of the chickens supplemented both levels of PaF decreased, in comparison to that obtained from the chickens in the groups supplemented PaP, C1, and C2 (p < 0.05; Table 4). Similarly, the triglyceride profile decreased in the chickens supplemented with the two levels of PaP and PaF and with C2, at 14 d of age (p < 0.05; Table 4) and at 21 d of age the ALT profiles also decreased in the chickens supplemented with 17.5 PaP and the two levels of PaF (p < 0.05; Table 4).
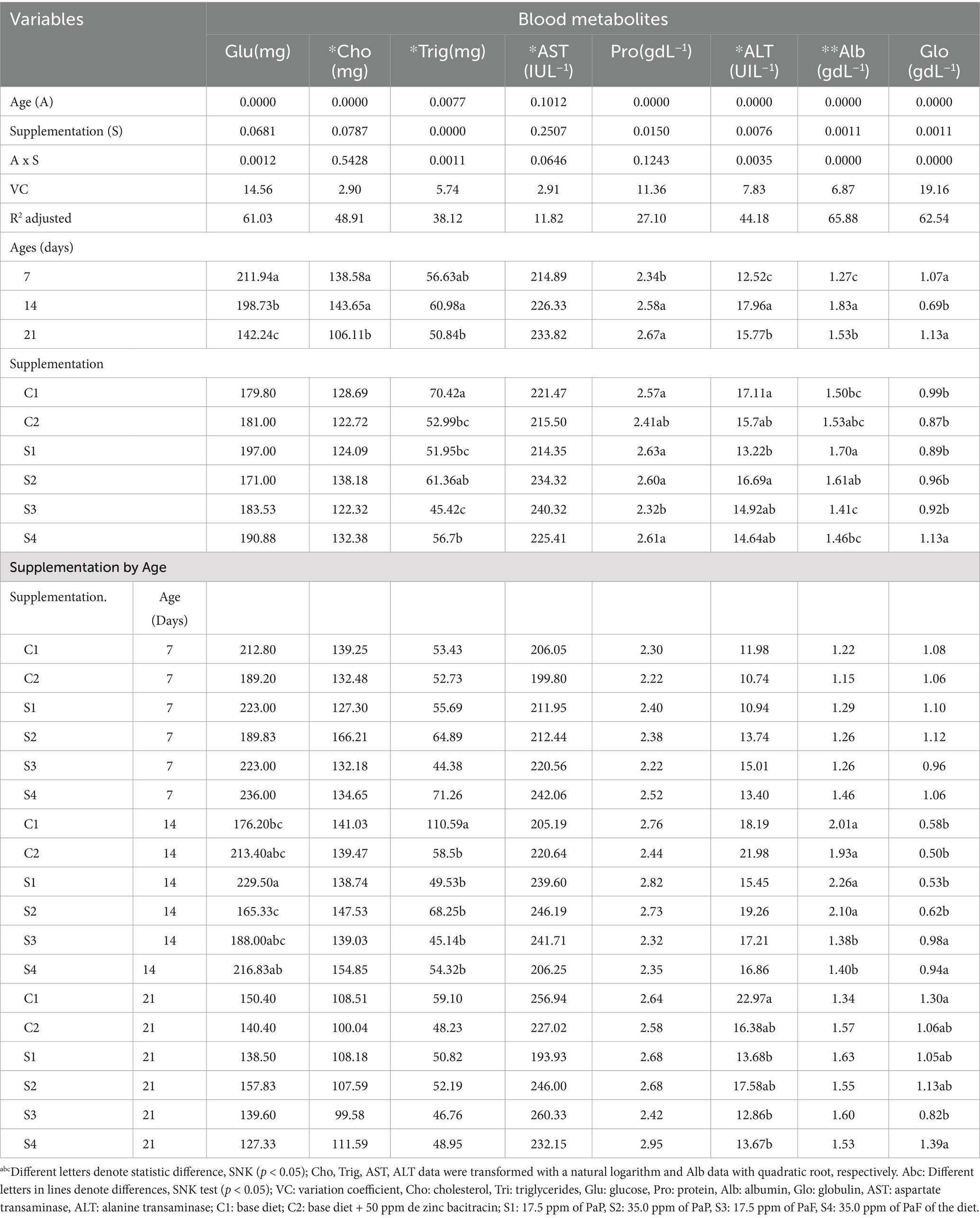
Table 4. Variance analysis of blood metabolites profiles of broiler chickens supplemented with P. aduncum polyphenols and flavonoids.
3.3 Antimicrobial activity
The microbial population obtained from the content and mucosa of the broiler chicken ileum at 21 and 28 d of age, as log10 CFU/g of fresh intestinal content, is depicted in Table 5 and Figure 1. The abundance (log10 CFU/g) of E. coli decreased in the groups of chickens that were supplemented with 35 ppm of PaP and 17.5 and 35 ppm of PaF, at 21 d of age, in comparison to the group C1 and C2 (p < 0.05; Table 5; Figure 1A). In addition, in the groups of chickens supplemented 17.5 and 35 ppm of PaF, the abundance was like that obtained at 28 d of age (p > 0.05; Figure 1A).
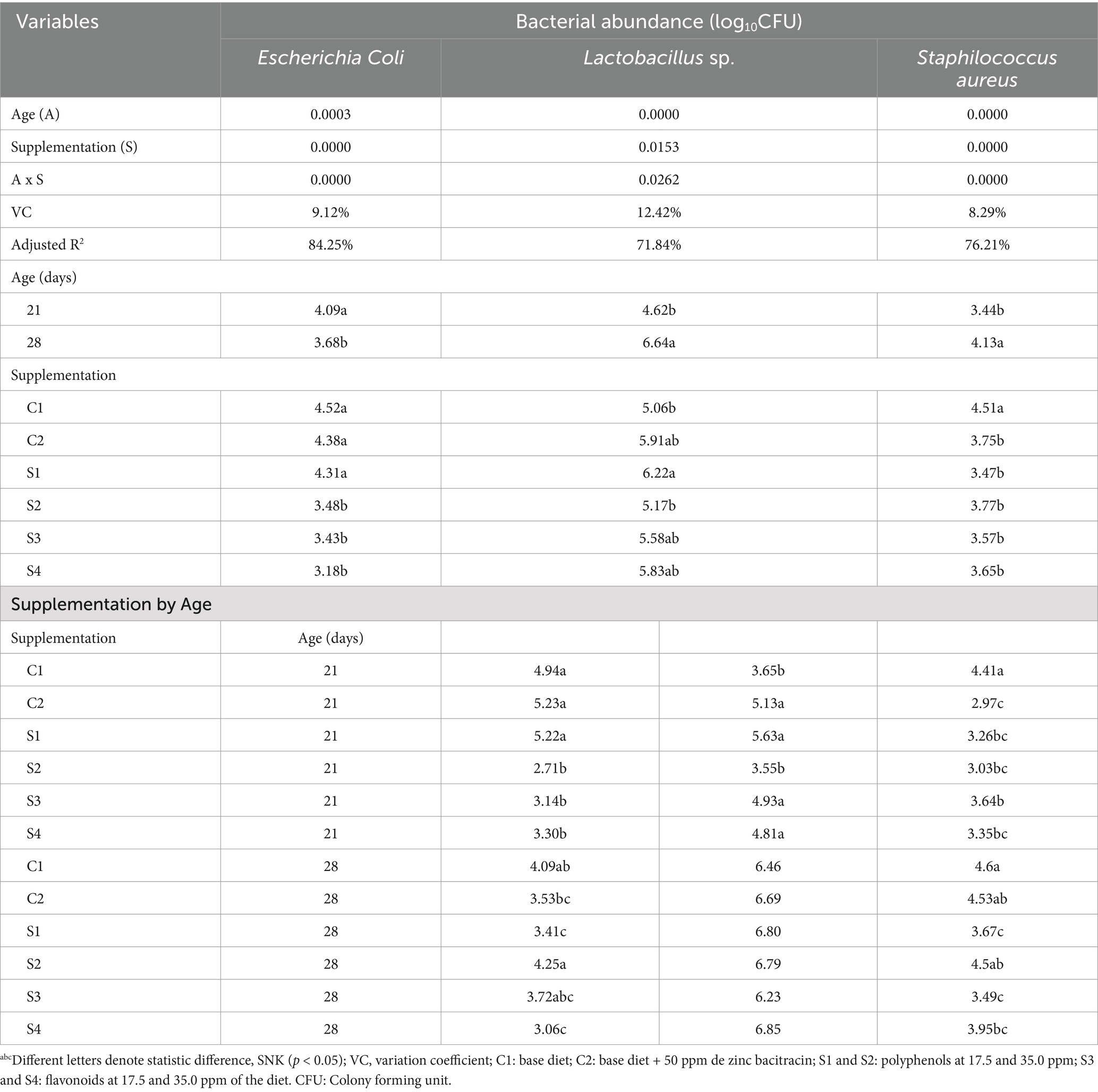
Table 5. Abundance (log10CFU/g) of Escherichia coli, Staphylococcus aureus and Lactobacillus sp. in the ileum mucosa content of broiler chickens supplemented with P. aduncum polyphenols and flavonoids.
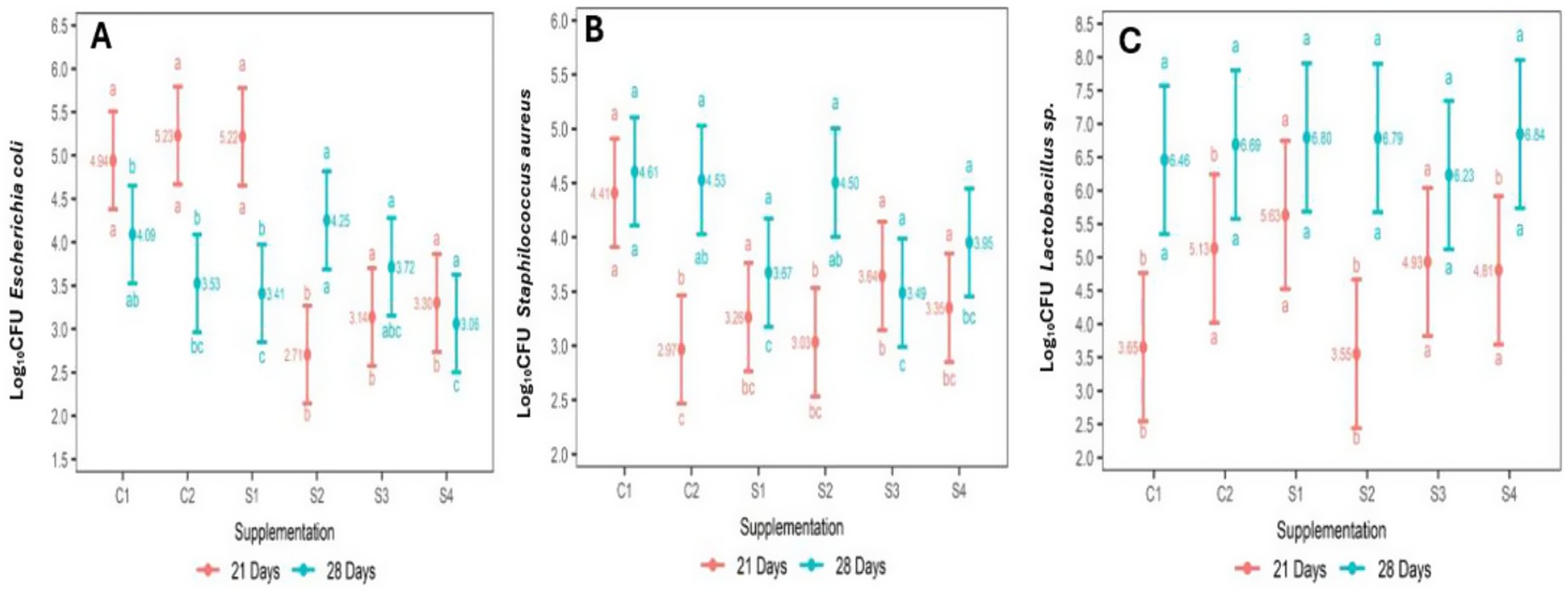
Figure 1. Interaction of PaP and PaF effect and Chickens age on Escherichia coli (A), Staphylococcus aureus (B) and Lactobacillus sp. abundance. (C) abc: different letters at the bottom of intervals denotes dependance of supplementations on chicken age, (SNK) test (p < 0.05). different letter at the top of intervals denotes dependance of age on PaP and PaF supplementation (SNK) test (p < 0.05). CFU: Colony forming units; C1 (−): Base diet, C2 (+): Base diet+ZB, S1: 17.5 ppm PPa, S2: 35 ppm PPa, S3: 17.5FPa, S4: 35 ppm FPa.
The abundance (log10CFU) of S. aureus decreased in all groups of chickens supplemented with PaP and PaF, in comparison to the C1 group (p < 0.05) at 21 d of age (Table 5). Similarly, in chickens supplemented with 17.5 ppm PaP, 17.5 and, 35 ppm PaF, this abundance remained at the same level as that of the chickens at 28 d of age (p > 0.05; Figure 1B). Conversely, for the broiler chickens supplemented with 17.5 ppm of PaP and both levels of PaF, the abundance of Lactobacillus sp. increased in comparison to the C1 group at 21 d of age (p < 0.05; Table 5; Figure 1C). Notwithstanding, the abundance (log10 CFU) of Lactobacillus sp. in all groups of supplemented chickens was similar to that obtained in the C1 group at 28 d of age (p > 0.05; Table 5).
3.4 Intestinal histomorphometry
Table 6 and Figure 2 show the results of the measurements of the height and width of the duodenum, jejunum and ileum villi, depth of the Lieberkühn glands and the index for the height and depth of the crypts of broiler chickens supplemented with 17.5 and 35 ppm PaP and PaF. The height of the intestinal villi increased linearly with the age of the chickens and was dependent on the age of the broiler chickens under the effect of 17.5 ppm PaP and 17.5 and 35 ppm PaF (p < 0.05), compared to that in chickens in groups C1 and C2 (Figure 2A).
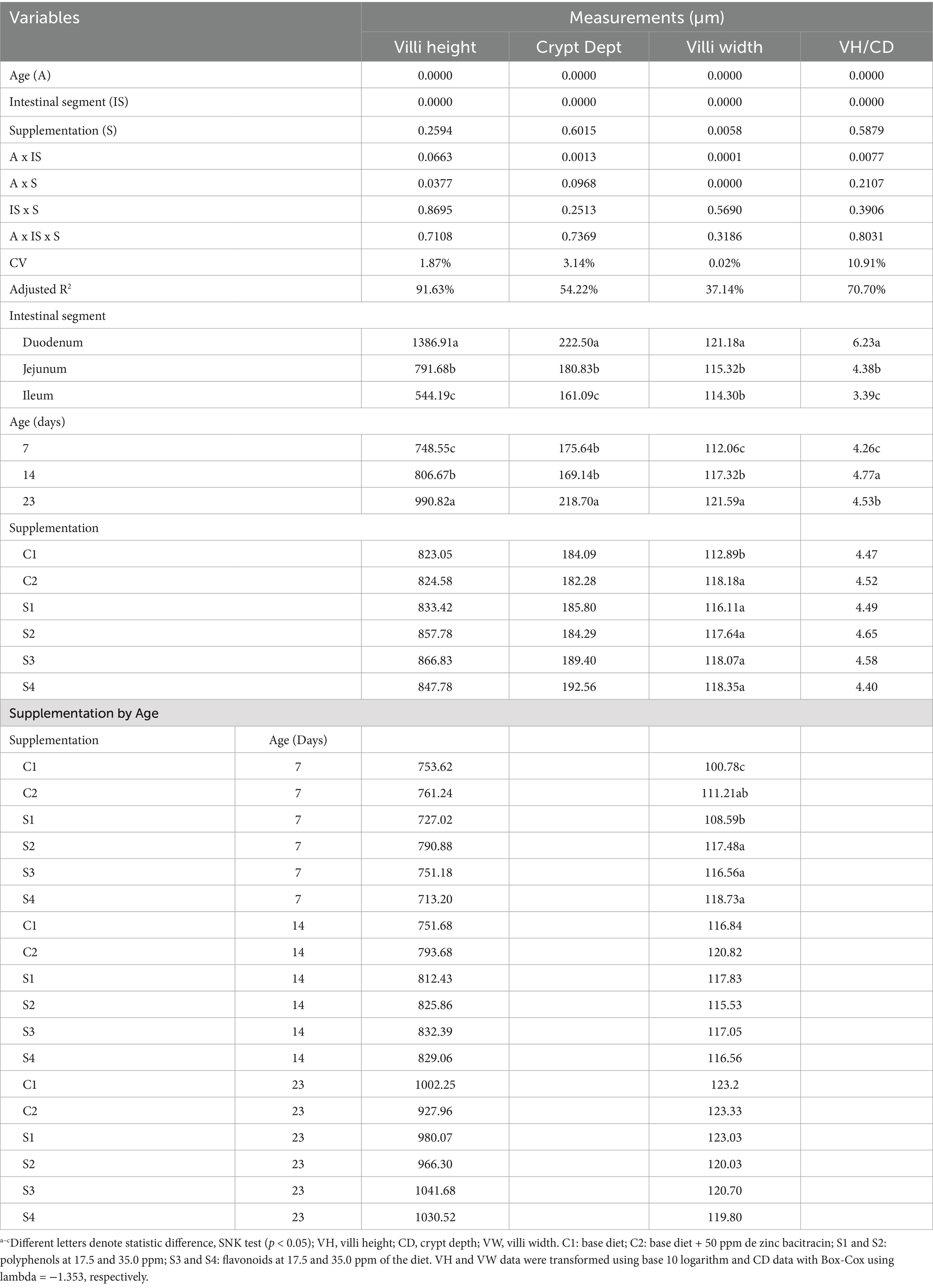
Table 6. Histomorphometry of the villi from duodenum, jejunum, and ileum of broiler chickens supplemented with P. aduncum polyphenols and flavonoids.
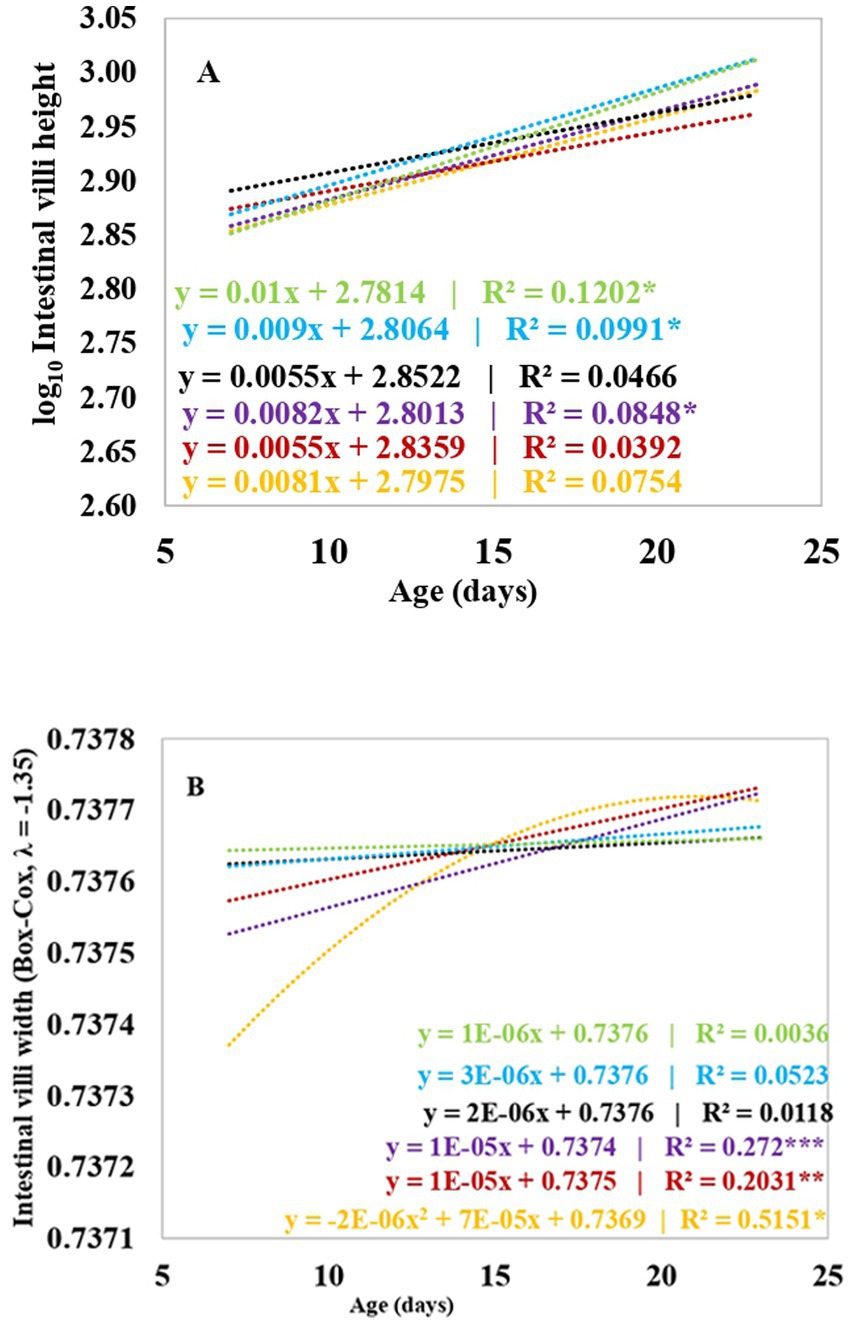
Figure 2. Regression analysis from the effect of the broiler’s chickens age on the villi height (A) and villi width (B) on supplementation with polyphenols and flavonoids from Piper aduncum leaves. *p < 0.05; **p < 0.01; ***p < 0.001. C1 (−): Base diet, C2 (+): Base diet+ZB, S1: 17.5 ppm PaP, S2: 35 ppm PaP, S3: 17.5 ppm PaF, S4: 35 ppm PaF.
Likewise, the width of the chicken villi increased with age, in a linear tendency, and was dependent on age on supplementation with 17.5 ppm PaP (p < 0.001; Table 6 and Figure 2B). Additionally, an increase in the width of the villi in chickens supplemented with 35 ppm PaP and 17.5 and 35 ppm PaF at 7 d of age was observed in comparison to that obtained for the chickens from group C1 (p < 0.05; Table 6).
3.5 Performance indices
Weight gain, feed conversion rate, and feed intake indices of the broiler chickens were evaluated as the principal indices for evaluating the performance of animal production during each of the stages: initial, growth, and fattening, as well as the total weight from the three phases (58). Table 7 depicts the results of these indices under the effect of supplementation 17.5 and 35 ppm PaP and PaF of the diet and supplemented in drinking water. PaP and PaF maintain weight gain and feed conversion rate (p > 0.05), reduce feed intake and improve carcass yield overall in the three stages of broiler chickens in comparison to that obtained for the C1 and C2 chickens’ groups (p < 0.05; Table 7).
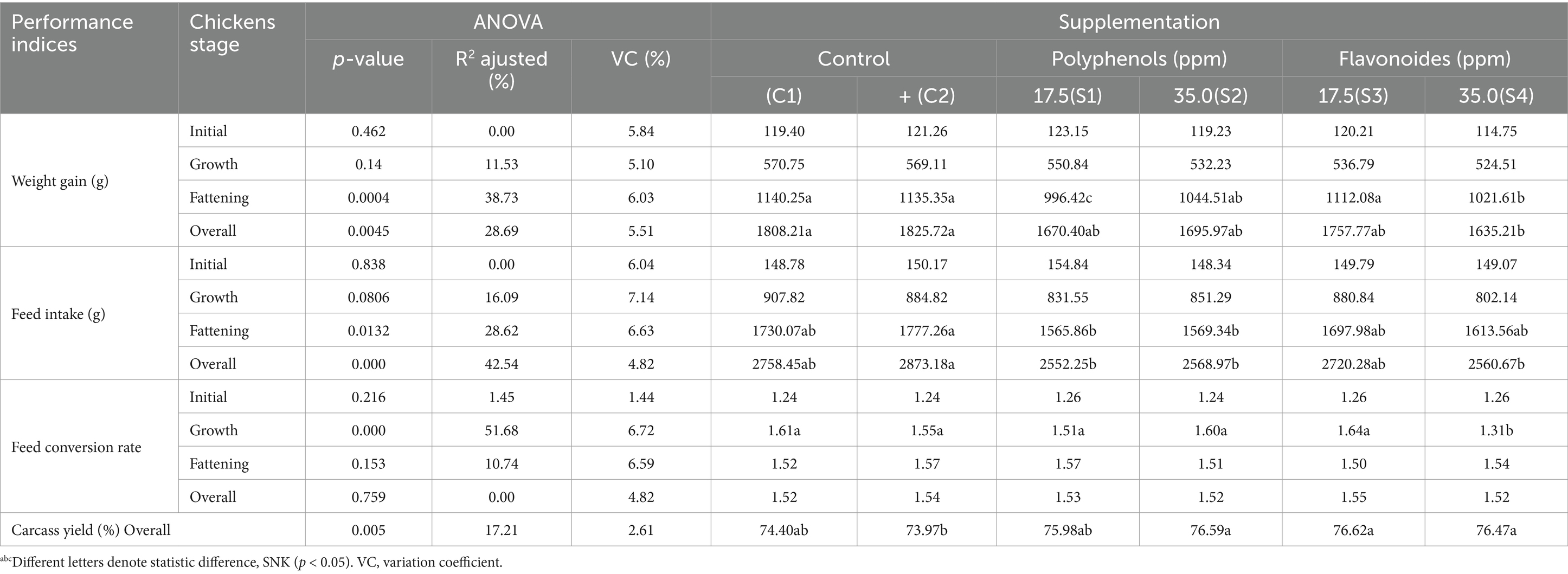
Table 7. Performance indices of broilers chickens supplemented with P. aduncum polyphenols and flavonoids.
4 Discussion
The objective of this study was to evaluate the effects of polyphenols and flavonoids from Piper aduncum on the intestinal health of broiler chickens. The polyphenols and flavonoids comprise phenol compounds that are extensively present in nature and are responsible for the good functioning of plants. Their benefits to human health have been recognized in diverse studies (27–29). Polyphenols have increased weight gain, decreased the feed conversion rate in broiler chickens, an increase in the production and quality of eggs in laying hens and quails (40–42). Previous studies have demonstrated that Piper aduncum contains diverse phytochemical compounds, including polyphenols and flavonoids, which possess antibacterial activities (24, 26). Supplementation of chickens with 17.5 and 35 ppm of PaP and FaP in this study increased the lymphocyte, globulin, height and width of the intestinal villi and abundance of Lactobacillus sp. However, the triglyceride, ALT, albumin, erythrocyte, MCV, MCH, granulocyte profiles and similarly, the abundance of E. coli and Staphylococcus aureus were reduced. Furthermore, PaP and PaF improve carcass yield, maintain weight gain and feed conversion rate and reduce feed intake overall in the three stages of broiler chickens. To our knowledge there is not information about whether Piper aduncum preparations are registered as feed supplements and are available on the market.
4.1 Quantification of phenol compounds and flavonoids
Most studies on the determination of the phytochemical composition of plants have focused on the determination of antimicrobial and antioxidant activities in vitro. Herein in this study, in vivo antimicrobial activity, changes in histomorphometry of the gut, and in the performance indices of broiler chickens by PaP and PaF were determined.
The P. aduncum phytocomponent groups obtained from the phytochemical screening in this study revealed components and gradations like those obtained in previous studies of this plant (59, 60). Nonetheless, the quantification of PaP and PaF obtained in this study has not been performed in previous studies (25, 59, 61, 62).
The total of polyphenols from 12.5 mg of GAE/g of dry ethanol extract and 0.2 mg of QE/g of flavonoids from dry extract proves that the P. aduncum leaves are an important source of polyphenols, and flavonoids. However, previous studies of these phytocompounds in other plants, such as Fragaria chiloensis spp. leaves have revealed 19.9 mg of GAE/g of dry methanol extract (DME) of polyphenols and 8.3 mg of QE/g of DME of flavonoids (33). In Murraya koenigii and Micromelum, 101 and 83 mg of GAE/g of dried ethanolic extract (DEE) of polyphenols, and 9.75 and 9.16 mg of QE/g of DEE of flavonoids have been obtained, respectively (63).
In general, the composition and content of polyphenols in plants depend on factors, such as species, genetics (variety and clones), and environmental factors, such as exposure to light, temperature, and soil. This includes farming practices, such as irrigation, nitrogen availability, and diverse management parameters for farming (64).
4.2 Hematology and blood metabolites profiles
To our knowledge, no previous studies have been carried out on the effects of supplementation with polyphenols and flavonoids from P. aduncum (PaP and PaF) on the hematological and blood metabolites profiles of broiler chickens.
Reduction in erythrocytes, hematocrit, hemoglobin profiles, and the MCV and MCH indices because of PaP, as well as the granulocytes because of PaF effect at 14 d of age in the present study is related to the results of our previous study in which the erythrocytes, hematocrit, hemoglobin and granulocytes profiles, and the MCV and MCH indices decreased and lymphocytes increased in chickens at 28 d old as the ethanolic extract of P. aduncum leaves increased from 0, 50 and 100 ppm of the diet. The erythrocytes of birds, like the erythrocytes of fish, are proposed to release cytokines and interferons (65–67), molecules with inflammatory activity that are released only by leukocytes. Therefore, the decrease in the erythrocytes and granulocytes of the experimental chickens at 14 days of age in this study (Table 3) could be associated with the anti-inflammatory activity of PaP through mechanisms that decrease the population of heterophils, erythrocytes and the expression of pro-inflammatory genes. These results could be associated to the fact that within the polyphenols from P. aduncum are found phytocomponents that have similar functions as dillapiole which has anti-inflammatory activity, identified in P. aduncum (68), and others attributed to P. umbellaceum (69), or like curcumin and resveratrol polyphenols. These polyphenols have been proven to have the characteristic of modulating the expression of pro-inflammatory genes, a decrease in heterophils, as well as the production of cytokines in previous studies in chickens (70–75).
In addition to the anti-inflammatory activity of PaP and PaF in this study, an increase in the levels of lymphocytes and globulins in broiler chickens at 14 d of age (Table 3) could also be associated with the immunostimulatory activity of the PaP phytocompounds. Lymphocyte proliferation and immunoglobulin titers are immunological markers that indicate humoral and cellular immune activity (76). Previous studies with other polyphenols, such as coumarin and resveratrol, have also proven to enhance the natural and acquired immunity of chickens and laying hens under stressful conditions because of heat or bacterial infection (71, 72, 77, 78).
Triglycerides are lipids that are synthesized by hepatic tissue and are present at the highest levels in vertebrates, including birds, and its main role is to serve as energy reserve (79). Around 50–70% of the fat in the diet is digested by pancreatic lipase, one of the main lipolytic enzymes, which also converts triglyceride substrata into free fatty acids and triglyceride monoesters (80, 81).
The decrease in blood lipids, such as triglycerides, in chickens supplemented with PaP and PaF at 14 d of age in this study (Table 4; Figure 2B) could be associated with the inhibition of pancreatic lipase by the PaP phytocompounds. These results align with those obtained in a previous study, wherein the ethanolic extract from P. aduncum decreased the cholesterol profiles in rats (60) and other studies that have demonstrated that the polyphenols derived from fruits and vegetables inhibit the activity of pancreatic lipase, as well as the cholesterol esterase (82). These results differ from those of a previous study, wherein the triglyceride profiles increased in broiler chickens at 28 d of age under the effect of P. aduncum ethanol extract (23). To our knowledge, this is the first study to report the modulation of erythrocyte, granulocyte, lymphocyte, globulin, and triglyceride profiles by PaP and PaF in chickens.
4.3 Antimicrobial activity
The chicken microbiota is made up of different phyla of microorganisms where commensal and symbiotic microorganisms and potential pathogens participate (83, 84). The microbial community is regulated by distinct groups of additives, including essential oils and extracts (85, 86). The antimicrobial activity of an extract or essential oil from a plant depends on diverse factors, among which are the chemical structure of its components (87, 88). In this manner, it is primarily attributed to the polyphenol content, and their concentration determines the antimicrobial potential of a plant (36, 89, 90). In this study the decrease in the population of gram-negative bacteria (log10CFU/g), such as E. coli and gram-positive, such as S. aureus in the intestinal content of broiler chickens because of the effect of 17.5 ppm and 35 ppm of PaP and PaF concurs with the results of the minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MBC) from our previous study. In this study, 6.25–25 mg/mL of five fractions from the P. aduncum ethanol extract inhibited the growth and demonstrated in vitro bactericidal action against E. coli ATCC 25922, S. aureus ATCC 25923, S. typhimurium ATCC 14028, and B. subtilis ATCC 6633.
Although previous studies have revealed that the intestinal microbiota in chickens primarily comprise gram-positive microorganisms (91), phytochemical compounds generally have greater antimicrobial activity against gram-positive bacteria than against gram-negative (88, 92, 93). Gram-negative bacteria release greater levels of endotoxins than gram-positive bacteria. These induce inflammation of the epithelium in the intestinal mucosa, affecting intestinal health to a greater extent.
The reduction in the E. coli and S. aureus population as log10Colony Forming units (log10CFU) in the intestinal microbiota of broiler chickens because of PaP and PaF in this study highlights the wide spectrum of antimicrobial activity of these phytocompounds. Similar results are non-existent, however, a reduction in the E. coli population in the intestinal contents of chickens has been observed in essential oils or phytogenic additives from other plants (51, 52, 94–96).
Polyphenols are phytocompounds that are found in edible vegetables, nutraceutical, and medicinal plants. They have been extensively studied and have received greater attention because of their antimicrobial activity for a wide range of microorganisms and the gastrointestinal microbiota (27, 28, 36). The reduction in the abundance of these groups of bacteria in the intestinal microbiota of chickens at 21 d of age in this study is aligned with the results obtained in mice, with polyphenols from tea (64) and itcould be associated with the phytocompounds from P. aduncum possessing antimicrobial action mechanisms. Asaricin, elemicin and myristicin have been identified as propenylphenols in Piper aduncum (25, 57). Previous studies with these three phytocompounds obtained from Piper sarmentosum and P. rivinoides, respectively, have revealed antimicrobial activity (97, 98). This antimicrobial activity would be associated with the chemical structure that characterizes these phytocompounds (37, 99). Antimicrobial mechanisms of phenols are linked to the hydrophobicity of the molecules, which enter the single membrane, thus disrupting permeability and homeostasis, resulting in a consequent loss of the cellular components and eventual cell death (88, 92, 93).
Nonetheless, PaP and PaF in this study also demonstrated an enhanced effect on the growth of the intestinal microbiota in chickens. It reveals an increase in the abundance of Lactobacillus sp. at 21 d of age, in comparison to group C1. This maintains a relationship with what was found in previous studies, wherein some polyphenols have proven to have beneficial influence on the beneficial bacteria in the microbiota, such as Bifidobacterium and Lactobacillus (27, 64, 99, 100). This highlights the variation in the ability of PaP and the compounds of these polyphenols to modify the intestinal microbiota of chickens, thereby strengthening their intestinal health. To the best of our knowledge, this is the first report on in vivo antimicrobial activity of polyphenols and flavonoids from P. aduncum.
4.4 Intestinal histomorphometry
Previous studies on the effects of polyphenols on gut histomorphometry are not found. However, previous studies have demonstrated that the height of intestinal villi increases because of extracts or essential oils from other plants (13, 15, 101–103). This facilitates the mechanisms for nutrient absorption as it does the mechanisms of the antibiotic as growth promoters, which also promotes an increase in the height of the intestinal villi (104).
The increase in the height and width of the intestinal villi with age, because of the effect of PaP and PaF in this study, can be elucidated by the two principal pharmacological activities recognized for polyphenols: the vast range of antimicrobial activities possessed by these phytocompounds (37, 99) and obtained in this study, as well as its antioxidant activity, observed in previous studies (30–33). This antioxidant activity is consistent with that obtained in a previous result of this study, where the ethanol extract from P. aduncum leaves inhibited the DPPH radicals at 65%, and this same extract proved to have a IC50 of 98.25 μg/mL. Another marker of the antioxidant activity of these fractions of P. aduncum in this study was the decrease in alanine transferase (ALT) profile at 21 d of age in chicken (Table 4). This enzyme is released by tissue cells mainly by hepatocytes and it increases when cells are injured or death (105), conversely it decreases when cells are enhanced by membrane protective chemicals as antioxidants do. The results of this study are aligned with those obtained in a previous study, wherein an increase in the depth of the Lieberkühn crypts and an increase in the width of the villi in relation to the age of the chickens under the effect of supplementing with 0.01% ethanol extract from P. aduncum leaves in the diets was reported. The increase in the width adopted a quadratic tendency (23), as well as other previous studies, where the modulation of the intestinal histomorphometry was obtained using sunflower flour, propolis extract, and integral propolis from bees (106–109).
The increase in the structures of the intestinal mucosa, such as the height and width of the villi, increases nutrient absorption and enzyme production owing to more dynamic replacement mechanisms from the enterocytes in the villi. This increase also promotes mechanisms that increase the population of goblet cells, which secrete mucus, and Paneth cells present in birds (110). Villi also secrete antimicrobial products, such as lysozymes and enteroendocrine cells, which secrete local hormones (111, 112) in a balanced manner and contribute to strengthening intestinal health.
4.5 Performance indices
Previous studies on the effects of PaP on weight gain, feed intake, and feed conversion rate in broiler chickens have not been conducted yet. Increasing carcass yield, maintaining weight gain and feed conversion rate, reducing feed intake overall in the three stages, in comparison to the chickens from groups C1 and C2, are consistent with the strengthening of gut health influenced by the results of the microbiota modulation, the increase in the height and width of the gut villi of the chickens supplemented with PaP and PaF in this study.
These results are consistent from previous studies using polyphenols from other plants, such as curcumin, resveratrol, and epigallocatechin gallate in broiler chickens, laying hens and quails, where an increase in the weight gain, a decrease in the feed conversion rate, an increase in the production and quality of eggs using mechanisms inherent to their antioxidant activity, have been reported (39–42).
Reduction of feed intake in the chickens supplemented PaP in the present study could be related to the fact that it contains phytochemical compounds which cause similar effects to those containing some phytogenic additives used in chicken diets which causes a modulation of anorexigenic hypothalamic neuropeptides responsible for higher feed efficiency by feed intake reduction and maintaining body weight (113).
At the same time, improvement in the feed efficiency in broilers chicken might be related to the anti-inflammatory effect of PaP obtained in the present study, and in this case at the hepatic tissue level, improving the hepatic metabolism interfering with the accumulation of lipids which are produced from high calorie diets during the fattening phase in broilers chicken (18, 114).
Even though polyphenols, in general, are well known for modulating the intestinal microbiota through their antimicrobial activity mechanisms (36–38), as well as for promoting the health of cells and tissues through their antioxidant activity (29, 34, 35), other studies have identified polyphenols because reduce activity on cellular metabolism through the inhibition of the lipase and cholesterol esterase activity (77, 81, 82), and it also by means of the inhibition of the amylase and glucosidase activity (115, 116).
Herein, inhibition mechanism of these enzymes because of the effect of PaP could primarily elucidate the reduction in triglyceride’s profiles in this study. This could have contributed, consequently, to maintaining weight gain and feed conversion rate and improving carcass yield overall in the three stages in the present study (Table 7). In general, beneficial effects of polyphenols depend on many factors, including plant species, their type, concentration, combination with other compounds, absorption and metabolic transformation, and the cell type or experimental animal species (29, 64, 117).
The results obtained in the present study support the grow promotor activity of PaP and PaF and therefore to be potentially used as an alternative of antibiotics as growth promotors in chickens. However, limitations associated with the practical application of plant extracts and essential oils in poultry arises (1) In general, variations in composition, relative proportions of bioactive phytocomponents as polyphenols in plants depend on factors, such as species, genetics (variety and clones), and environmental factors, such as exposure to light, temperature, and soil. This includes farming practices, such as irrigation, nitrogen availability, and diverse management parameters for farming (64). (2) The supplementation of phytocompounds in feed have some difficulties for the phytocompounds to homogenate, degradation in the feeders and low speed of being absorbed by the gut because of the very small quantities to be used (3) The supplementation in drinking water is easier for the phytocompounds to homogenate, fast in being absorbed by the gut but very difficult to manage the supplementation by itself. Nonetheless, further studies using physio pathological models and molecular techniques are essential to elucidate these nutraceutical effects of polyphenols from Piper aduncum.
5 Conclusion
Supplementation with 17.5 and 35 ppm of PaP and PaFdecreased the abundance of Staphylococcus aureus and Escherichia coli in the intestinal microbiota and increased Lactobacillus sp., villi length and width in the intestinal mucosa of broiler chickens, indicating improvement of intestinal health. In addition, 17.5 and 35 ppm of PaP and PaF supplementation increased lymphocytes, globulins and reduced ALT and granulocytes. Furthemore, PaP and PaF improve carcass yield, maintain weight gain and feed conversion rate and reduce feed intake overall in the three stages of broiler chickens. This study also showed that PaP and PaF did not have a detrimental effect on any of the parameters evaluated, therefore these phytocompounds enhanced gut health, immunity, anti-inflammatory activity and the performance indices in broiler chicken. In the short term, these results can be used in practical applications, such as (1) improving gut health by modulating the intestinal microbiota and enhancing intestinal villi growth, (2) enhancing immune and anti-inflammatory responses (3) reducing deposition of fat in carcass and obesity (4) using as growth promotors for replacing conventional antibiotics. However, more studies are essential to determine the type of phenols and flavonoids compounds in PaP and PaF and the mechanisms by which these phytocompounds promote these beneficial effects in physio pathological models.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
This study was reviewed and approved by the Ethics and Animal Wellbeing committee of the Veterinary Medicine School at the Universidad Nacional Mayor de San Marcos, with authorization, N° 2012–5.
Author contributions
DP-L: Conceptualization, Funding acquisition, Project administration, Writing – review & editing, Writing – original draft. RR-H: Supervision, Writing – review & editing, Investigation, Conceptualization. RP-C: Methodology, Investigation, Writing – review & editing. CA-S: Project administration, Writing – review & editing, Investigation, Methodology. JD-G: Visualization, Writing – review & editing, Methodology, Investigation. UA-P: Writing – review & editing, Software, Formal analysis, Data curation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study has been funded by PROCIENCIA.
Acknowledgments
We thank the PROCIENCIA grant N°200-2020-FONDECYT-DE: “Use of Natural Plant Extracts from the Peruvian Amazon to Replace Antibiotics as Growth Promotors in Broiler Chickens,” and the Universidad Nacional Agraria de la Selva, for the financing support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hou, J, Long, X, Wang, X, Li, L, Mao, D, Luo, Y, et al. Global trend of antimicrobial resistance in common bacterial pathogens in response to antibiotic consumption. J Hazard Mater. (2023) 442:130042. doi: 10.1016/j.jhazmat.2022.130042
2. Soni, K, Jyoti, K, Chandra, H, and Chandra, R. Bacterial antibiotic resistance in municipal wastewater treatment plant; mechanism and its impacts on human health and economy. Bioresource Technol Reports. (2022) 19:101080. doi: 10.1016/j.biteb.2022.101080
3. Abadi, ATV, Rizvanov, AA, Haertlé, T, and Blatt, NL. World Health Organization report: current crisis of antibiotic resistance. Bio Nano Sci. (2019) 9:778–88. doi: 10.1007/s12668-019-00658-422
4. Ayukekbong, JA, Ntemgwa, M, and Atab, AN. The threat of antimicrobial resistance in developing countries causes and control strategies. Antimicrob Resist Infect Control. (2017) 6:2–888. doi: 10.1186/s13756-017-0208-x
5. Kimera, ZI, Mshana, SE, Rweyemamu, MM, Mboera, LEG, and Matee, MIN. Antimicrobial use and resistance in food producing animals and the environment: an African perspective. Antimicrob Resist Infect Control. (2020) 9:37. doi: 10.1186/s13756-020-0697-x
6. Oloso, NA, Fagbo, S, Garbati, M, Olonitola, SO, Awosanya, EJ, Aworh, MK, et al. Antimicrobial resistance in food animals and the environment in Nigeria: a review. Int J Environ Res Public Health. (2018) 15:2–23. doi: 10.3390/ijerph15061284
7. Griboff, J, Carrizo, JC, Bonanse, RI, Valdés, ME, Wunderlin, DA, and Amé, MV. Multiantibiotic residues in commercial fish from Argentina. The presence of mixtures of antibiotics in edible fish, a challenge to health risk assessment. Food Chem. (2020) 332:127380. doi: 10.1016/j.foodchem.2020.127380
8. Menkem, ZE, Ngangom, BL, Tamunjoh, SSA, and Boyom, FF. Antibiotic residues in food animals: public health concern. Acta Ecol Sin. (2019) 39:411–5. doi: 10.1016/j.chnaes.2018.10.004
9. Merhia, A, El Khatib, S, Haddad, J, and Hassan, HF. A review of the antibiotic residues in food in the Arab countries. Applied. Food Res. (2023) 3:100332. doi: 10.1016/j.afres.2023.100332
10. Molognoni, L, Daguer, H, and Hoff, RB. Analysis of nitrofurans residues in foods of animal origin. Food Toxicol Forensics. (2021):379–419. doi: 10.1016/B978-0-12-822360-4.00015-7
11. Mouiche, MMM, Okah-Nnane, NH, Djibo, FMI, Mapiefou, NP, Mpouam, SE, Mfopit, SM, et al. Antibiotic residues in foods of animal origin in Cameroon: prevalence, consumers’ risk perceptions and attitudes. J Food Prot. (2024) 87:100237. doi: 10.1016/j.jfp.2024.100237
12. Paredes-López, DM, Robles-Huaynate, R, Beteta-Blas, X, and Aldava-Pardave, U. Effect of Morinda citrifolia fruit powder on physiological and productive performance of Cavia porcellus. Frontiers in veterinary science. Sec. Animal. Nutr Metab. (2023) 10:1–11. doi: 10.3389/fvets.2023.1134138
13. Stastník, O, Novotny, J, Roztocilov, A, Zálešáková, D, Řiháček, M, Horáková, L, et al. Caraway (Carum carvi L.) in fast-growing and slow-growing broiler chickens’ diets and its effect on performance, digestive tract morphology and blood biochemical profile. Poult Sci. (2022) 101:101980. doi: 10.1016/j.psj.2022.101980
14. Sugiharto, S. Role of nutraceuticals in gut health and growth performance of poultry. J Saudi Soc Agric Sci. (2016) 15:99–111. doi: 10.1016/j.jssas.2014.06.001
15. Toson, A, Abd El Latif, M, Mohamed, E, Gazwi, HSS, Saleh, M, Kokoszynski, D, et al. Efficacy of licorice extract on the growth performance, carcass characteristics, blood indices and antioxidants capacity in broilers. Animal. (2021) 17:100696. doi: 10.1016/j.animal.2022.100696
16. Abd El-Hack, M.E., El-Saadony, M.T., Shafi, M.E., and Qattan, S.Y.A., (2020). Probiotics in poultry feed: A comprehensive review. J. Anim. Physiol. Anim. Nutr. 104:1835–1850.
17. Abou-Kassem, DE, Mahrose, KM, El-Samahy, RA, Shafi, ME, El-Saadony, MT, Abd El-Hack, ME, et al. Influences of dietary herbal blend and feed restriction on growth, carcass characteristics and gut microbiota of growing rabbits. Ital J Anim Sci. (2021) 20:896–910. doi: 10.1080/1828051X.2021.1926348
18. Araujo, RGAC, Polycarpo, GV, Barbieri, A, Silva, KM, Ventura, G, and Polycarpo, VCC. Performance and economic viability of broiler chickens fed with probiotic and organic acids in an attempt to replace growth-promoting antibiotics. Brazilian J Poultry Sci. (2019) 21:02. doi: 10.1590/1806-9061-2018-0912
19. Arif, M, Baty, RS, Althubaiti, EH, Ijaz, MT, Fayyaz, M, Shafi, ME, et al. The impact of betaine supplementation in quail diet on growth performance, blood chemistry, and carcass traits. Saudi J Biology Sci. (2021) 29:1604–10. doi: 10.1016/j.sjbs.2021.11.002
20. Ashour, EA, Farsi, RM, Alaidaroos, BA, Abdel-Moneim, AME, El-Saadony, MT, Osman, AO, et al. Impacts of dietary supplementation of pyocyanin powder on growth performance, carcase traits, blood chemistry, meat quality and gut microbial activity of broilers. Ital J Anim Sci. (2021) 20:1357–72. doi: 10.1080/1828051X.2021.1924087
21. Llamas-Moya, S, Girdler, CP, Shalash, SMM, Atta, AM, Gharib, HB, Morsy, EA, et al. Effect of a multi-carbohydrase containing agalactosidase enzyme on the performance, carcass yield, and humoral immunity of broilers fed corn−soybean meal−based diets of varying energy density. J Appl Poult Res. (2019) 29:142–51. doi: 10.1016/j.japr.2019.10.001
22. Paredes López, D, Robles, HR, Cueva, CS, and Chacón, MS. Effect of Uncaria tomentosa aqueous extract on biochemical and hematological profiles and live performance parameters in broiler chickens. Livest Res Rural Dev. (2018) 7
23. Paredes-López, DM, Robles-Huaynate, RA, Vásquez-Soto, MR, Perales-Camacho, RA, Morales-Cauti, MS, Beteta-Blas, X, et al. Modulation of gut microbiota and morphometry, blood profiles, and performance of broiler chickens supplemented with Piper aduncum, Morinda citrifolia, and Artocarpus altilis leaves ethanolic extracts. Sec Animal Nutrit Metabolism. (2024) 11, 1–17. doi: 10.3389/fvets.2024.1286152
24. da Silva, AD, Matías, E, Rocha, J, Justino, AC, de Freitas, T, Campina, F, et al. Gas chromatography coupled to mass spectrometry (GC-MS) characterization and evaluation of antibacterial bioactivities of the essential oils from Piper arboreum Aubl., Piper aduncum L. e Piper gaudichaudianum Kunth. Z. Naturforsch. Zeitschrift für Naturforschung. (2021) 76:35–42. doi: 10.1515/znc-2020-0045
25. Parmar, VS, Jain, S, Gupta, S, and Talwar, S. Polyphenols and alkaloids from piper species. Phytochemistry. (1998) 49:1069–78.
26. Debonsi, H, Morandim, A, Cavalheiro, M, Marques, M, Young, M, and kato, M. Composition and antifungal activity of essential oils from P. aduncum, P. Arboreum and P. tuberculatum. Química Nova. (2006) 29:467–70. doi: 10.1590/S0100-40422006000300012
27. Domínguez-Avila, JA, Villa-Rodriguez, JA, Montiel-Herrera, M, Pacheco-Ordaz, R, Roopchand, DE, Venema, K, et al. Phenolic compounds promote diversity of gut microbiota and maintain colonic health. Springer link. (2020) 66:3270–89. doi: 10.1007/s10620-020-06676-7
28. Espín, JC, González-Sarrías, A, and Tomás-Barberán, FA. The gut microbiota: a key factor in the therapeutic effects of (poly) phenols. Biochem Pharmacol. (2017) 139:82–93. doi: 10.1016/j.bcp.2017.04.033
29. Quideau, S, Deffieux, D, Douat-Casassus, C, and Pouységu, L. Plant polyphenols: chemical properties, biological activities, and synthesis. Angewandte International Edition Chemie. (2011) 50:586–621. doi: 10.1002/anie.201000044
30. Benvenuti, S, Pellati, F, Melegari, M, and Bertelli, D. Polyphenols, anthocyanins, ascorbic acid, and radical scavenging activity of Rubus, Ribes, and Aronia. J Food Sci. (2004) 69:164–9. doi: 10.1111/j.1365-2621.2004.tb13352.x
31. Chiva-Blancha, G, and Visiolib, F. Polyphenols and health: moving beyond antioxidants. J Berry Res. (2012) 2:63–71. doi: 10.3233/JBR-2012-028
32. Minatel, IO, Borges, CV, Ferreira, MI, Gomez, EH, Chung-Yen, OC, and Pereira Lima, GP. Phenolic compounds: functional properties, impact of processing and bioavailability. Livest Sci. (2017) 229:13–21. doi: 10.5772/66368
33. Simirgiotis, MJ, and Schmeda-Hirschmann, G. Determination of phenolic composition and antioxidant activity in fruits, rhizomes and leaves of the white strawberry (Fragaria chiloensis spp. Chiloensis form chiloensis) using HPLC-DAD–ESI-MS and free radical quenching techniques. J Food Compos Anal. (2010) 23:545–53. doi: 10.1016/j.jfca.2009.08.020
34. Nardini, M. Phenolic compounds in food: characterization and health benefits. Molecules. (2022) 27, 1–4. doi: 10.3390/molecules27030783
35. Rahman, M, Rahaman, S, Islam, R, Rahman, F, Mithi, FM, Alqahtani, T, et al. Role of phenolic compounds in human disease: current knowledge and prospects. Molecules. (2022) 27, 2–37. doi: 10.3390/molecules27010233
36. Daglia, M. Polyphenols as antimicrobial agents. Curr Opin Biotechnol. (2012) 23:174–81. doi: 10.1016/j.copbio.2011.08.007
37. Lima, EMF, Winans, SC, and Pinto, UM. Quorum sensing interference by phenolic compounds – a matter of bacterial misunderstanding. Heliyon. (2023) 9:e17657. doi: 10.1016/j.heliyon.2023.e17657
38. Olivero, JT, Pájaro, NP, and Stashenko, E. Antiquorum sensing activity of essential oils isolated from different species of genus piper. Vitae, Revista de la Facultad de Química Farmacéutica. (2011) 18:77–82. doi: 10.17533/udea.vitae.8781
39. Liu, LL, He, JH, Xie, HB, Yang, YS, Li, JC, and Zou, Y. Resveratrol induces antioxidant and heat shock protein mRNA expression in response to heat stress in black-boned chickens. Poult Sci. (2014) 93:54–62. doi: 10.3382/ps.2013-03423
40. Luo, J, Song, J, Liu, L, Xue, B, Tian, G, and Yang, Y. Effect of epigallocatechin gallate on growth performance and serum biochemical metabolites in heat-stressed broilers. Poult Sci. (2018) 97:599–606. doi: 10.3382/ps/pex353
41. Marchiori, MS, Oliveira, RC, Souza, CF, Baldissera, MD, Ribeiro, QM, Wagner, R, et al. Curcumin in the diet of quail in cold stress improves performance and egg quality. Anim Feed Sci Technol. (2019) 254:114192. doi: 10.1016/j.anifeedsci.2019.05.015
42. Reda, FM, El-Saadony, MT, Elnesr, SS, Alagawany, M, and Tufarelli, V. Effect of dietary supplementation of biological curcumin nanoparticles on growth and carcass traits, antioxidant status, immunity and caecal microbiota of japanese quails. Animals. (2020) 10:754. doi: 10.3390/ani10050754
43. Miranda, M. Métodos de análisis de drogas y extractos. Peru: Universidad Nacional Ciudad de la Habana, CUBA (2002).
44. Singleton, VL, and Rossi, JA. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic acid reagents. American Society Enol Viticul. (1965) 16:144–58. doi: 10.5344/ajev.1965.16.3.144
45. Kumazawa, S, Hamasaka, T, and Nakayama, T. Antioxidant activity of propolis of various geographic origins. Food Chem. (2004) 84:329–39. doi: 10.1016/S0308-8146(03)00216-4
46. Servicio Nacional de Meteorología e Hidrología (SENAMHI). (2023). Promedio de temperatura y precipitación normal para Tingo Maria. Available online at: https://www.senamhi.gob.pe/?p=pronostico-detalle&dp=10&localidad=0025 (Accessed November 15th, 2024).
47. Rostagno, H, Albino, L, Donzele, J, Gomes, P, De Oliveira, R, Lopez, D, et al. Tablas Brasileñas para aves y cerdos. Composición de alimentos y requerimientos nutricionales. 3a Edición. Viçosa, Brazil Universidad Federal de Viçosa–Departamento de Zootecnia (2024). 259 p.
48. Samour, J, Silvanose, C, and Pendl, H. Clinical and diagnostic procedures. J Avian Med. (2016) 73–178.
49. Eckersall, P. Proteins, proteomics, and dysproteinemias In: JJ Kaneko, JW Harvey, and ML Bruss, editors. Clinical biochemistry of domestic animals. 6th ed. US: Academic Press (2008). 117–55.
50. Watersson, C.L. (2009). Proteins. Animal clinical chemistry a practical guide for toxicologists and biomedical researchers by G.O Evans. Second Edition. CRC Press. FL, USA.
51. Ghazanfari, S, Mohammadi, Z, and Adib, MM. Effects of coriander essential oil on the performance, blood characteristics, intestinal microbiota and histological of broilers. Brazilian. J Poult Sci. (2015) 17:419–26. doi: 10.1590/1516-635X1704419-426
52. Hashemi, SR, Zulkifli, I, Davoodi, H, Zunita, Z, and Ebrahimi, M. Growth performance, intestinal microflora, plasma fatty acid profile in broiler chickens fed herbal plant (Euphorbia hirta) and mix of acidifiers. Anim Feed Sci Technol. (2012) 178:167–74. doi: 10.1016/j.anifeedsci.2012.09.006
53. Culling, C.S.A., and Allison, R.I., And Barr, W.T. (1985). Cellular pathology technique. Fourth edition, Butter Worth publishing, London. 642 pp.
54. Pollesel, M, Tassinari, M, Frabetti, A, Fornasini, D, and Cavallini, D. Effect of does parity order on litter homogeneity parameters. Ital J Anim Sci. (2020) 19:1188–94. doi: 10.1080/1828051X.2020.1827990
55. Bashir, MA, Khan, MH, Essa, M, Taj, MA, Fida, A, Samiullah, K, et al. Role of botanical leaves powder in the blood hematology of living organisms. J King Saud University Sci. (2022) 34:101789. doi: 10.1016/j.jksus.2021.101789
56. Software Statistics Infostat. (2022). Cordova, Dermatol Argent. Available at: https://www.infostat.com.ar/index.php?mod=page&id=15
57. Parmar, VS, Jain, SC, Bisht, KS, Jain, R, Taneja, P, Jha, A, et al. Phytochemistry of the genus Piper. Phytochemisrry. (1997) 46:597–673. doi: 10.1016/S0031-9422(97)00328-2
58. Ogbuewu, IP, Mokolopi, BG, and Mbajiorgu, CA. Meta-analysis of growth performance indices of broiler chickens in response to turmeric (Curcuma longa L.) supplementation. Anim Feed Sci Technol. (2022) 283:115155. doi: 10.1016/j.anifeedsci.2021.115155
59. Arroyo-Acevedo, J, Chávez-Asmat, RJ, Anampa-Guzmán, A, Donaires, R, and Ráez-Gonzáles, J. Protective effect of Piper aduncum capsule on DMBA induced breast Cancer in rats. Breast Cancer: Basic Clin Res. (2015) 9:41–8. doi: 10.4137/BCBCR.S24420
60. Rachmainia, F, Abdillaha, R, Oktavia, S, and Wahyuni, FS. Cholesterol lowering activity and vasorelaxant effect of ethanol extract Piper aduncum leaves in hypercholesterolemic wistar Kyoto rats. S Afr J Bot. (2024) 164:366–73. doi: 10.1016/j.sajb.2023.12.013
61. Peralta-Canchis, LP, Kroning, IS, Zandoná, GP, Kleinübing, NR, Oliveira, TL, Fiorentini, AM, et al. Chemical composition of Minthostachys setosa (Briquet) and Piper elongatum (Vahl) essential oils, antistaphylococcal activity and effect on Staphylococcus aureus biofilm removal. Biocatal Agric Biotechnol. (2024) 58:103170. doi: 10.1016/j.bcab.2024.103170
62. Potzernheim, MCL, Bizzo, HR, Silva, JP, and Vieira, RF. Chemical characterization of essential oil constituents of four populations of Piper aduncum L. from Distrito Federal. Brazil Biochem Systematics Ecol. (2012) 42:25–31. doi: 10.1016/j.bse.2011.12.025
63. Abeysinghe, DT, Kumara, KAH, Kaushalya, KAD, Chandrika, UG, and Alwis, DDH. Phytochemical screening, total polyphenol, flavonoid content, in vitro antioxidant, and antibacterial activities of Sri Lankan varieties of Murraya koenigii and Micromelum minutum leaves. Heliyon. (2021) 7:e07449. doi: 10.1016/j.heliyon.2021.e07449
64. Hussain, Shah M., Rafique, R., Rafique, T., Naseer, M., Khalil, U., and Rafique, R. (2021). Effect of climate change on polyphenols accumulation in grapevine. In: Phenolic compounds chemistry, synthesis, diversity, non-conventional industrial, pharmaceutical and therapeutic applications. By Farid A. Badria Mansoura University. Egypt. 452.
65. Paul, MS, Paolucci, S, Brajesh, N, Wood, RD, and Sharif, S. Chicken erythrocytes respond to toll-like receptor ligands by up-regulating cytokine transcripts. Res Vet Sci. (2013) 95:87–91. doi: 10.1016/j.rvsc.2013.01.024
66. Passantino, L, Massaro, MA, Jirillo, F, Modugno, DD, Ribaud, MR, Modugno, GD, et al. Antigenically activated avian erythrocytes release cytokine-like factors: a conserved phylogenetic function discovered in fish. Immunopharmacol Immunotoxicol. (2007) 29:141–52. doi: 10.1080/08923970701284664
67. Wakenell, P.S. (2010). Hematology in chicken and Turkey. In Schalm’s veterinary hematology 6th edition by Douglas J. Weiss and K. Jane Wardrop. Blackwell Publishing Ltd. IA, USA. Pp. 958–967
68. Parise-Filho, P, Pastrello, M, Pereira Camerlingo, CM, Silva, GN, Agostinho, LA, Thaís de Souza, FM, et al. The anti-inflammatory activity of dillapiole and some semisynthetic analogues. Pharm Biol. (2011) 49:1173–9. doi: 10.3109/13880209.2011.575793
69. Iwamoto, LH, Vendramini-Costa, DB, Araújo, MP, Gois Ruiz, ALT, de Oliveira Sousa, LM, Foglio, MA, et al. Anticancer and anti-inflammatory activities of a standardized dichloromethane extract from Piper umbellatum L. Leaves Evidence-Based Complementary and Alternative Medicine Evidence-Based Complement Alt Med. (2015) 2015:8. doi: 10.1155/2015/948737
70. Diaz-Gerevini, GT, Repossi, G, Dain, A, Tarres, MC, Das, UNFAMS, and Eynard, AR. Beneficial action of resveratrol: how and why? Nutrition. (2016) 32:174–8. doi: 10.1016/j.nut.2015.08.017
71. He, S, Yu, Q, He, Y, Hu, R, Xia, S, and He, J. Dietary resveratrol supplementation inhibits heat stress-induced high-activated innate immunity and inflammatory response in spleen of yellow-feather broilers. Poult Sci. (2019) 98:6378–87. doi: 10.3382/ps/pez471
72. Mohebodinia, H, Jazib, V, Bakhshalinejadc, R, Shabanib, A, and Ashayerizadeh, A. Effect of dietary resveratrol supplementation on growth performance, immune response, serum biochemical indices, cecal microflora, and intestinal morphology of broiler chickens challenged with Escherichia coli. Livest Sci. (2019) 229:13–21. doi: 10.1016/j.livsci.2019.09.008
73. Nawab, A, Tang, S, Li, G, An, L, Wu, J, Liu, W, et al. Dietary curcumin supplementation effects on blood immunological profile and liver enzymatic activity of laying hens after exposure to high temperature conditions. J Therm Biol. (2020) 90:102573. doi: 10.1016/j.jtherbio.2020.102573
74. Schwager, J, Richard, N, Widmer, F, and Rederstorff, D. Resveratrol distinctively modulates the inflammatory profiles of immune and endothelial cells. BMC Complement Med Therapies. (2017) 17:309. doi: 10.1186/s12906-017-1823-z
75. Zhong, X, Shi, Y, Chen, J, Xu, J, Wang, L, Beier, RC, et al. Polyphenol extracts from Punica granatum and Terminalia chebula are anti-inflammatory and increase the survival rate of chickens challenged with Escherichia coli. Biol Pharm Bull. (2014) 37:1575–82. doi: 10.1248/bpb.b14-00163
76. Plitt, T, and Faith, JJ. Seminars in immunology special issue: nutrition, microbiota, and immunity the unexplored microbes in health and disease. Semin Immunol. (2023) 66:101735. doi: 10.1016/j.smim.2023.101735
77. Liu, TT, Liu, XT, Chen, QX, and Shi, Y. Lipase inhibitors for obesity: a review. Biomed Pharmacother. (2020) 128:110314. doi: 10.1016/j.biopha.2020.110314
78. Nawab, A, Li, G, An, L, Wu, J, Chao, L, Xiao, M, et al. Effect of curcumin supplementation on TLR4 mediated non-specific immune responses in liver of laying hens under high-temperature conditions. J Therm Biol. (2019) 84:384–97. doi: 10.1016/j.jtherbio.2019.07.003
79. Stevens, L. Lipids and their metabolism In: Avian biochemistry and molecular biology. Ed. Lewis Stevens. Cambridge, UK: Cambridge University Press (1996). 46–64.
80. Liu, M, Lu, Y, Gao, P, Xie, X, Li, D, Yu, D, et al. Effect of curcumin on laying performance, egg quality, endocrine hormones, and immune activity in heat-stressed hens. Poult Sci. (2020) 99:2196–202. doi: 10.1016/j.psj.2019.12.001
81. Yu, B, Tang, Q, Fu, C, Regenstein, J, Huang, J, and Wang, L. Effects of different particle-sized insoluble dietary fiber from citrus peel on adsorption and activity inhibition of pancreatic lipase. Food Chem. (2022) 398:133834. doi: 10.1016/j.foodchem.2022.133834
82. He, X, Chen, L, Pu, Y, Wang, H, Cao, J, and Jiang, J. Fruit and vegetable polyphenols as natural bioactive inhibitors of pancreatic lipase and cholesterol esterase: inhibition mechanisms, polyphenol influences, application challenges. Food Biosci. (2023) 55:103054. doi: 10.1016/j.fbio.2023.103054
83. Pan, D, and Yu, Z. Intestinal microbiome of poultry and its interaction with host and diet. Special Focus on Microbial Interactions. (2014) 5:108–19. doi: 10.4161/gmic.26945
84. Sowmiya, S, Jasmine, R, Mohan, S, Santhanam, R, Prathiviraj, R, Kiran, GS, et al. Analysis of the gut microbiota of healthy CARI-Nirbheek (Aseel cross) chickens: a metagenomic approaches. Environ Advan. (2022) 9:100304. doi: 10.1016/j.envadv.2022.100304
85. Clavijo, V, and Vives, MJ. The gastrointestinal microbiome and its association with the control of pathogens in broiler chicken production: a review. Poult Sci. (2018) 97:1006–21. doi: 10.3382/ps/pex359
86. Oakley, BB, Lillehoj, HS, Kogut, MH, Kim, WK, Maurer, JJ, Pedroso, A, et al. The chicken gastrointestinal microbiome. FEMS Microbiol Lett. (2014) 360:100–12. doi: 10.1111/1574-6968.12608
87. Skoufos, I, Bonos, E, Anastasiou, I, Tsinas, A, and Tzora, A. The effects of phytobiotics in health or disease challenged animals. Feed Additives Aromatic Plants Herbs Animal Nutrit Health. (2020):311–37. doi: 10.1016/B978-0-12-814700-9.00018-2
88. Socaci, SA, Farcas, CA, and Tofana, M. Functional ingredients derived from aromatic plants. Feed Additives Aromatic Plants Herbs Animal Nutrit Health. (2020):133–46. doi: 10.1016/B978-0-12-814700-9.00008-X
89. Cabarkapa, I, Puvaca, N, Popovic, S, Colovi, D, Kostadinovi, L, Tatham, EK, et al. Aromatic plants and their extracts pharmacokinetics and in vitro/in vivo mechanisms of action. Feed Additives Aromatic Plants Herbs Animal Nutrit Health. (2020):75–88. doi: 10.1016/B978-0-12-814700-9.00005-4
90. Soleimani, M, Arzani, A, Arzani, V, and Roberts, TH. Phenolic compounds and antimicrobial properties of mint and thyme. J Herbal Med. (2022) 36:100604. doi: 10.1016/j.hermed.2022.100604
91. Gong, J, Forster, RJ, Yu, J, Chambers, JR, Sabour, PM, Wheatcroft, R, et al. Diversity and phylogenetic analysis of bacteria in the mucosa of chicken ceca and comparison with bacteria in the cecal lumen. FEMS Microbiol Lett. (2002) 208:1–7. doi: 10.1111/j.1574-6968.2002.tb11051.x
92. Giannenas, I, Bonos, E, Christaki, E, and Florou-Paneri, P. Essential oils and their applications in animal nutrition. Medicinal and Aromatic Plants, (2013). 2:2–12. doi: 10.4172/2167-0412.1000140
93. O’Bryan, CA, Pendleton, SJ, Crandall, PG, and Ricke, SC. Potential of plant essential oils and their components in animal agriculture – in vitro studies on antibacterial mode of action. Front Vet Sci. (2015) 2:35. doi: 10.3389/fvets.2015.00035
94. Jang, I.S., Ko, Y.H., Kang, S.Y., and Lee, S.Y. (2017). Effect of a commercial essential oil on growth performance, digestive enzyme activity and intestinal microflora population in broiler chicken. Animal Feed Science and Technology. 134:304–314.
95. Murugesan, GR, Syed, B, Haldar, S, and Pender, C. Phytogenic feed additives as an alternative to antibiotic growth promoters in broiler chickens. Front Vet Sci. (2015) 2:1–6. doi: 10.3389/fvets.2015.00021
96. Si, W, Gong, J, Tsao, R, Zhou, T, Yu, H, Poppe, C, et al. Antimicrobial activity of essential oils and structurally related synthetic food additives towards selected pathogenic and beneficial gut bacteria. J Appl Microbiol. (2006) 100:296–305. doi: 10.1111/j.1365-2672.2005.02789.x
97. Fonseca, VFA, Castro dos Santos, S, Pereira Carneiro, JN, Lucas dos Santos, AT, de Freitas, MA, et al. Characterization and analysis of the bioactivity of the Piper rivinoides Kunth essential oil and its components myristicin and elemicin, against opportunistic fungal pathogens. Microb Pathog. (2025) 199:107242. doi: 10.1016/j.micpath.2024.107242
98. Masuda, T, Inazumi, A, Yamada, Y, Padolina, WG, Kikuzaki, H, and Nakatani, N. Antimicrobial phenylpropanoids from Piper sarmentosum. Phytochmistry. (1991) 30:3227–8. doi: 10.1016/0031-9422(91)83180-S
99. Makarewicz, M, Drozdz, I, Tarko, T, and Duda-Chodak, A. The interactions between polyphenols and microorganisms, especially gut microbiota. Antioxidants. (2021) 10:1–70. doi: 10.3390/antiox10020188
100. Man, AWC, Zhou, Y, Xia, N, and Li, H. Involvement of gut microbiota, microbial metabolites and interaction with polyphenol in host immunometabolism. Nutrients. (2020) 12:1–29. doi: 10.3390/nu12103054
101. Islam, R, Sultana, S, Bhakta, S, Haque, Z, Hasan, AL, Siddique, MP, et al. Modulation of growth performance, gut morphometry, and cecal microbiota in broilers by clove (Syzygium aromaticum) and tulsi (Ocimum sanctum) supplementation. Poult Sci. (2023) 102:102266. doi: 10.1016/j.psj.2022.102266
102. Kiczorowska, B, Al-Yasiry, ARM, Samolińska, M, Marek, AR, and Pyzik, E. The effect of dietary supplementation of the broiler chicken diet with Boswellia serrata resin on growth performance, digestibility, and gastrointestinal characteristics, morphology, and microbiota. Livest Sci. (2016) 191:117–24. doi: 10.1016/j.livsci.2016.07.019
103. Ji, L-Z, Dersjant-Li, Y, and Giannenas, I. Application of aromatic plants and their extracts in diets of broiler chickens. Feed Additives Aromatic Plants Herbs Animal Nutrit Health. (2020):159–85. doi: 10.1016/B978-0-12-814700-9.00010-8
104. Miles, RD, Butcher, GD, Henry, PR, and Littell, RC. Effect of antibiotic growth promotors on broiler performance, intestinal growth parameters, and quantitative morphology. Poult Sci. (2000) 85:476–85. doi: 10.1093/ps/85.3.476
105. Kim, WR, Flamm, SL, Di Bisceglie, AM, and Bodenheimer, HC. Serum activity of alanine aminotransferase (ALT) as an Indicator of health and disease. Hepatology. (2008) 47:1363–70. doi: 10.1002/hep.22109
106. Eyng, C, Murakami, AE, Duarte, CRA, and Santos, TC. Effect of dietary supplementation with an ethanolic extract of propolis on broiler intestinal morphology and digestive enzyme activity. Animal Physiol Animal Nutrit. (2014) 98:393–401. doi: 10.1111/jpn.12116
107. Hamedi, S, Rezaian, M, and Shomali, T. Histological changes of small intestinal mucosa of cocks due to sunflower meal single feeding. Am J Anim Vet Sci. (2011) 6:171–5. doi: 10.3844/ajavsp.2011.171.175
108. Prakatur, I, Miskulin, M, Pavic, M, Marjanovic, K, Blazicevic, V, Miskulin, I, et al. Intestinal morphology in broiler chickens supplemented with propolis and bee pollen. Animals. (2019) 9:1–12. doi: 10.3390/ani9060301
109. Wang, J, Li, S, Wang, Q, Xin, B, and Wang, H. Trophic effect of bee pollen on small intestine in broiler chickens. J Med Food. (2007) 10:276–80. doi: 10.1089/jmf.2006.215
110. Wang, L, Li, J, Li J., Jr JLi, RX, Li, CF, Li, S, et al. Identification of the Paneth cells in chicken small intestine. Poult Sci. (2016) 95:1631–5. doi: 10.3382/ps/pew079
111. Barker, N, van Oudenaarden, A, and Clevers, H. Identifying the stem cell of the intestinal crypt: strategies and pitfalls. Cell Stem Cell. (2012) 11:452–60. doi: 10.1016/j.stem.2012.09.009
112. Yang, C, and Otteman, KM. Control of bacterial colonization in the glands and crypts. Curr Opin Microbiol. (2019) 47:38–44. doi: 10.1016/j.mib.2018.11.004
113. Flees, J, Greene, E, Gangul, B, and Dridi, S. Phytogenic feed and water-additives improve feed efficiency in broilers via modulation of (an) orexigenic hypothalamic neuropeptide expression. Neuropeptides. (2020) 81:102005. doi: 10.1016/j.npep.2020.102005
114. Loayza-Besares, J, Hernandez-Guevara, E, Gutierrez-Rengifo, FM, and Paredes-Lopez, DM. Effect of the Uncaria tomentosa leaves hydroalcoholic extract on physiological parameters and performance indices of broiler chickens. Folia Amazonica. (2024) 33:e33786–15. doi: 10.24841/fa.v33i2.786
115. Kan, L, Monic, EC, Tomassen, MM, Fogliano, V, and Oliviero, T. Inhibition of α-glucosidases by tea polyphenols in rat intestinal extract and Caco-2 cells grown on Transwell. Food Chem. (2021) 361:130047. doi: 10.1016/j.foodchem.2021.130047
116. Krishnan, V, Verma, P, Saha, S, Singh, B, Vinutha, T, Kumara, RR, et al. Biocatalysis and agricultural. Biotechnology. (2022) 43. doi: 10.1016/j.bcab.2022.102411
Keywords: gut health, histomorphometry, microbiota, Piper aduncum, polyphenols
Citation: Paredes-Lopez DM, Robles-Huaynate RA, Perales-Camacho RA, Alania-Santiago CV, Diaz-Gonzales JP and Aldava-Pardave U (2025) Piper aduncum polyphenols and flavonoids enhance gut health, immune and anti-inflammatory activity and performance indices of broiler chickens. Front. Vet. Sci. 12:1597948. doi: 10.3389/fvets.2025.1597948
Edited by:
Adrian Macri, University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca, RomaniaReviewed by:
Joanna Wojtacka, University of Warmia and Mazury in Olsztyn, PolandAdam Kerek, University of Veterinary Medicine Budapest, Hungary
Copyright © 2025 Paredes-Lopez, Robles-Huaynate, Perales-Camacho, Alania-Santiago, Diaz-Gonzales and Aldava-Pardave. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: D. M. Paredes-Lopez, ZGFuaWVsLnBhcmVkZXNAdW5hcy5lZHUucGU=
†ORCID: R. A. Perales-Camacho, https://orcid.org/0000-0002-3390-1388
U. Aldava-Pardave, orcid.org/0000-0001-8298-5445
 D. M. Paredes-Lopez1*
D. M. Paredes-Lopez1* R. A. Robles-Huaynate
R. A. Robles-Huaynate