- 1Department of Veterinary Medicine and Animal Sciences (DIVAS), Università degli Studi di Milano, Milan, Italy
- 2Department of Agricultural and Environmental Sciences - Production, Landscape, Agroenergy, Università degli Studi di Milano, Milan, Italy
The preservation of testicular tissue and male germ cells represents a cutting-edge technique for safeguarding fertility, especially when sperm collection is not possible, such as in prepubertal animals, those that die unexpectedly or that receive gonadotoxic therapies after cancer detection, and in adult males suffering from some pathology related to azoospermia. Current methods under investigation include the optimization of cryopreservation protocols, as well as the development of culture platforms to enable in vitro spermatogenesis (IVS). Although these approaches are still in the research and development phase, they have shown promising potential for male fertility preservation. Cryopreservation is a common method for long-term in vitro storage of tissue and cells, which enables the maintenance of reproductive capacity across different animal species and contributes to the creation of gene banks for endangered species. Spermatogenic cells from cryopreserved testicular tissue can be cultured in vitro and resume their functions after thawing, contributing to the preservation of fertility and genetic resources in both small and large animals. The main challenges of IVS include providing a suitable microenvironment that mimics the testicular niche to support the survival and development of all the cell types, as well as to achieve complete differentiation toward spermatozoa. Therefore, there is a great interest in developing methods to study IVS, both for basic research and clinical application. Given the importance of this topic, this review aims to provide an overview of recent advancements in the cryopreservation and culture of both testicular tissue and cells for preserving male fertility in large and small domestic animals.
1 Introduction
Over the past few decades, significant advancements have been made in both cryopreservation and in vitro culture strategies aimed at preserving testicular tissues and maintaining the viability and functionality of spermatogonial stem cells (SSCs) across various animal species (1–3). This progress holds great promise for long-term fertility preservation and genetic conservation, particularly in prepubertal individuals or valuable breeding animals unable to produce mature sperm (1). However, the direct application of these technologies across different animal species remains challenging, often requiring protocol adjustments and further refinement (1). Therefore, this review aims to summarize and critically assess current cryopreservation and culture strategies for testicular tissues and cells across different animal models, discussing the methodologies, challenges, and progress in maintaining cellular integrity and promoting spermatogenic potential. By exploring these advancements in both small and large animals, this review highlights the current state of the art and outlines opportunities for further refinement and application. The manuscript is structured in two main sections: the first part provides an overview of cryopreservation techniques for testicular tissues and cells, including their principles, applications, and devices used; the second part focuses on in vitro culture systems, discussing their design, outcomes, and potential to support spermatogenic progression.
2 Cryopreservation strategies for testicular tissues and cells
Methods and protocols for storing male germplasms have been developed over the years to preserve fertility, promote the spread of specific genotypes and protect biodiversity in endangered breeds or species (4). Semen freezing is a widely recognized method for genome conservation and is commonly applied in infertility laboratories (5) but this method cannot be used for juvenile and pre-pubertal individuals whose gonads have not started producing spermatozoa. When semen preservation is not feasible, cryopreservation of testicular tissue fragments or testicular cell suspensions, containing early/immature germ cells, may potentially expand the range of biotechnological applications for germplasm and fertility preservation (6). These strategies have been applied in several animal models (7–15) and, even if the protocols are still experimental, they are very promising for application in assisted reproductive technologies (ARTs) (4). Testicular tissue cryopreservation enables the preservation of SSCs while maintaining critical cell–cell interactions and structural integrity, thus facilitating the restoration of gametogenesis through transplantation or IVS (16). This approach has been investigated in numerous studies focusing on the collection and preservation of testicular tissue from both sexually immature individuals (12, 17, 18) and adult animals (8, 19). However, testicular tissue is composed of various cell types, including germ cells, Sertoli cells, and Leydig cells, which work together to support gamete production and hormone secretion (20). Due to its complexity and cellular heterogeneity, testicular tissue cryopreservation is more challenging than preserving SSCs alone, as it requires maintaining intercellular interactions and ensuring post-thaw viability to restore spermatogenesis in vitro (6). Spermatogonial stem cells are responsible for the continuous production of spermatozoa through self-renewal and differentiation (21). Their cryopreservation, although technically demanding and still experimental, has been explored as an alternative strategy to tissue preservation, offering more control over cryopreservation parameters due to the cellular homogeneity (22). However, despite the development of different protocols in several animal species including goat (23), cattle (24) and horses (5), there are still no documented live birth resulting from cryopreserved SSCs in large animals (6).
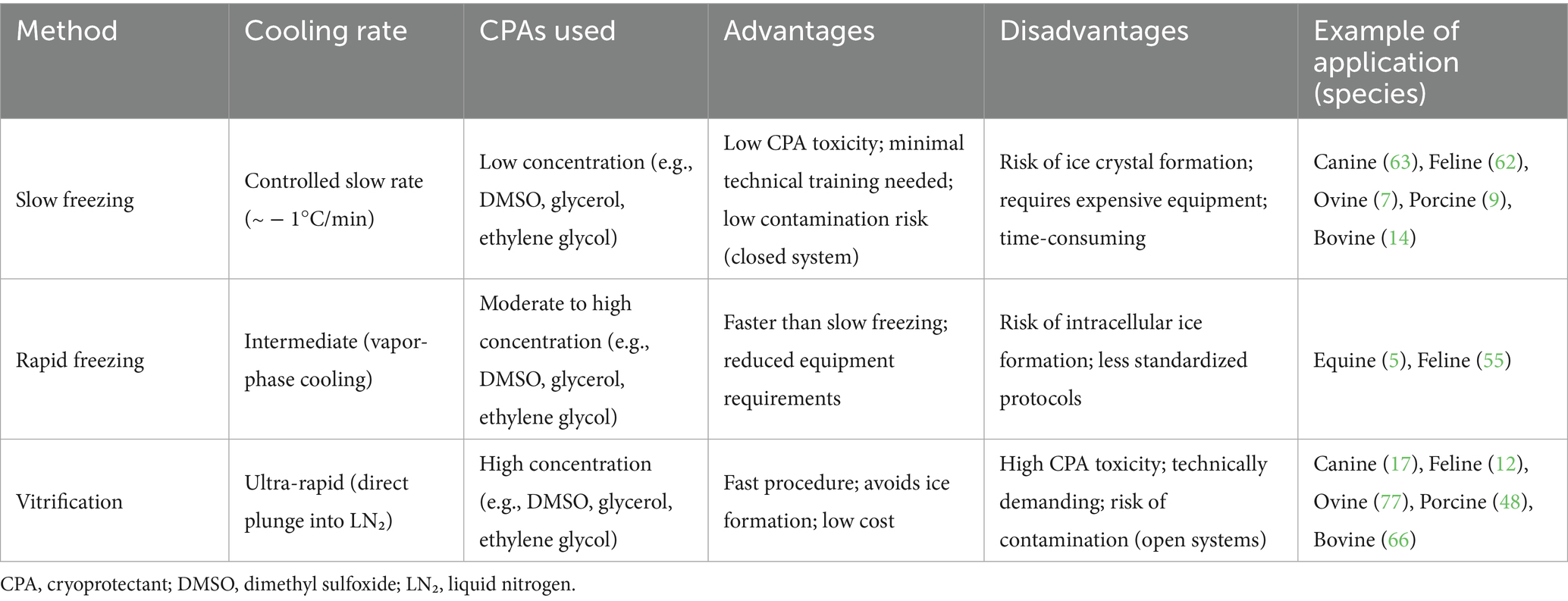
The two most widely used cryopreservation methods are slow freezing and vitrification. Few reports used a modified technique called rapid freezing (Table 1) (5, 25, 26).
Slow freezing, controlled using programmable freezers, involves the use of low concentrations of permeable cryoprotectants (CPAs) and gradual cooling (27). This approach allows for progressive cell dehydration and minimizes intracellular ice formation before storage in liquid nitrogen (1). However, it also carries a significant risk of freeze-induced injury and it is a time-consuming and costly process, as it requires expensive equipment (28).
Rapid freezing, or vapor fast freezing (VFF), involves sequential treatment with higher concentrations of CPAs, followed by exposure to liquid nitrogen vapors before immersion into liquid nitrogen (6, 28, 29). One of the main challenges of this technique is the risk of cryoinjury caused by intracellular ice formation, which can compromise cell viability (26).
Vitrification involves the transformation of water or water-based solutions into an amorphous, glass-like vitreous state without the formation of ice crystals (30). This method is generally faster than slow freezing and significantly reduces the formation of both intracellular and extracellular ice by utilizing high concentrations of CPAs and ultra-rapid cooling rates (28). To counteract CPAs toxicity, a preliminary equilibration with lower CPA levels is typically performed before exposing cells to the final vitrification solution (6).
One of the key advantages of vitrification is the minimal risk of freezing injury, resulting in a higher cell survival rate. However, this technique requires a high level of technical expertise (31). Moreover, despite its cost-effectiveness, the high CPAs concentrations necessary for vitrification may compromise the morphological and functional integrity of cells (32).
Given the complexity of preserving testicular tissue and cells, further optimization of processing and storage methods remains essential to improve cryopreservation outcomes (1, 30, 33).
2.1 Sample preparation
To provide optimal cryoprotection, CPAs must effectively diffuse in and out of the tissue to minimize cryoinjury. Therefore, the size of testicular tissue samples should be carefully considered (34). Particularly for vitrification, sample size plays a pivotal role in determining the likelihood of successful solidification of the aqueous environment of tissue and cells into a non-crystalline, glass-like state. Additionally, sample size has a role in the prevention of devitrification, which occurs during warming and is characterized by the formation of ice crystals (35).
Testicular tissue sizes ranging from 0.3 to 1.5 mm3 are commonly used in cryopreservation across various animal species (36–39). Among testicular cells, SSCs are a rare subset of germ cells, representing only 0.2–0.3% of the germ cell population in bovine species (40). However, the proportion of SSCs in the testes of other livestock species, such as sheep, goats, pigs, and buffalo, remains undocumented (21). In the tissue, this heterogeneous cell population varies in function, size, water content, and membrane permeability, and the scarcity of SSCs further complicates the development of an efficient cryopreservation protocol (1).
The stem cells are typically isolated from testicular tissue through enzymatic digestion or mechanical isolation. Enzymatic digestion, using enzymes such as collagenase IV, trypsin, DNase I, and hyaluronidase, is commonly employed to dissociate testicular cells. Since no single enzyme is sufficient to isolate SSCs effectively, multi-step or sequential enzymatic digestion protocols are often used (41). The mechanical isolation method involves the removal of the tunica albuginea and visible connective tissues, followed by mechanical dissociation of seminiferous tubules using scissors and forceps, with subsequent filtration of the cells (42).
While enzymatic digestion generally yields higher numbers of SSCs compared to mechanical dissociation (43), both these methods may negatively affect the viability and functionality of germ cells, as well as alter their biophysical properties, leading to increased cellular sensitivity to freezing processes. Moreover, the disruption of cell-to-cell interactions may negatively affect cell proliferation and differentiation (44). In contrast, preserving testicular tissue maintains the in situ structure and cellular relationships, including the spatial arrangement of somatic cells and germ cells, which are essential for studying spermatogenesis and testicular function (43).
2.2 Cryopreservation carriers
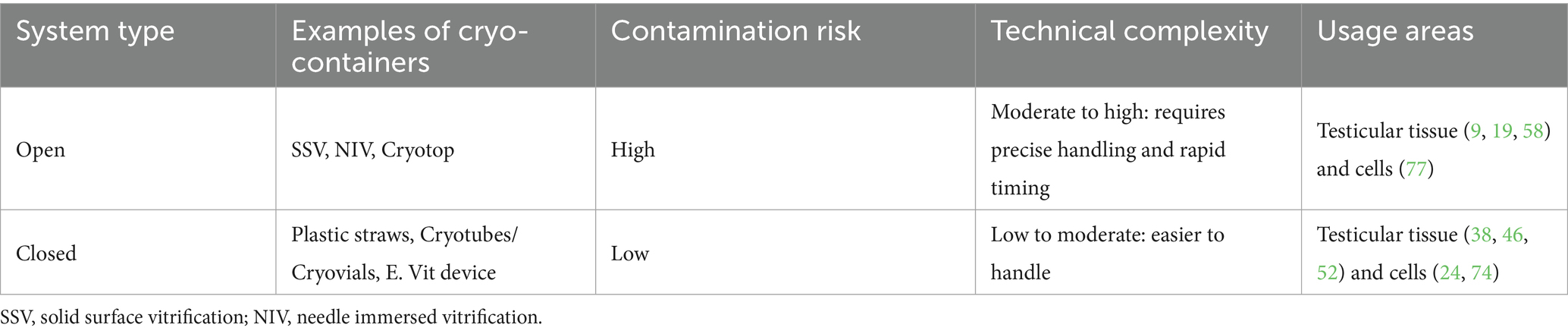
The device used for supporting the cryopreservation of samples can be an additional factor influencing the outcome of the process. While freezing of testicular tissue and cells is generally performed in cryovials or straws (45), studies on vitrification carefully select the appropriate vessel for the procedure, although plastic straws are also used in conventional vitrification due to their practicality, low cost, and space optimization within cryogenic cylinders (46). Vitrification methods can be classified as either “open vitrification” or “closed vitrification.” Open devices allow direct contact of the sample with liquid nitrogen for faster heat transfer (6). However, this direct contact introduces the risk of pathogen transmission to the sample during cooling and increases the potential for cross-contamination within the container (1). In contrast, closed systems prevent direct contact between the sample and the cooling solution during freezing or storage, thus addressing the contamination issue. A limitation of closed systems is that they result in slower cooling rates, requiring higher concentrations of CPAs to prevent ice crystal formation. This, in turn, increases the potential cytotoxicity of the CPAs, making the protocols more hazardous for cells (47). A summary of the main types of carriers used in cryopreservation, along with their characteristics, is provided in Table 2.
A simple and rapid vitrification method for testicular tissue has been reported in the ovine model using a novel device called E. Vit (FertileSafe, Ness Ziona, Israel), which enables all cryopreservation procedures to be performed within a straw. The device consists of a 0.3 mL straw with a 50 μm pore polycarbonate grid at one end, facilitating ultrarapid vitrification of tissues and cells with minimal volume. This design allows for the expulsion of excess CPAs while preventing sample loss (38). Using this device, ovine pre-pubertal testicular tissue (1 mm3) maintained plasma membrane integrity at 66.00% immediately after warming and 59.67% after 2 h of in vitro culture (IVC). However, extended culture up to 24 h post-warming led to a significant decrease in membrane integrity to 31.00%, and a stress response was observed (38).
Carrier-free systems, such as Solid Surface Vitrification (SSV), offer an alternative approach. Introduced in 2010, SSV is a containerless vitrification method that enables open vitrification of tissues and cells (36). The procedure involves immersing testicular tissue samples in a vitrification solution before placing them on a sterile aluminum boat floating on liquid nitrogen, then transferring them into precooled vials and submerging them in cryogenic storage (36). This method has proven to be an efficient method for vitrifying testicular biopsies in porcine models (36, 48, 49) and prepubertal domestic cats (12).
2.3 Warming rates
Whether freezing is allowed (as in conventional cryopreservation) or prevented (as in vitrification), the CPAs that have penetrated the internal compartments of a multicellular system must diffuse back through several membranes within the tissue during warming, with each membrane acting as a barrier (34).
Intracellular ice formation is considered the primary cause of cell damage induced by cryopreservation, even during the thawing process, when recrystallization may occur. Recrystallization refers to the growth of ice crystals, starting from small ice nuclei, into fully-fledged intra- and/or extracellular ice crystals, which increase in size as the temperature rises. During this process, cells must return to their original isotonic conditions, and uncontrolled water influx into the cells can generate osmotic stress and cellular swelling, leading to damage to the plasma membrane and subsequent cell lysis (27). To minimize osmotic shock, it is important to use a set of media with gradual decrease of the osmotic pressure (35).
Therefore, tissue and cell survival after freezing relies on effective thawing and CPAs removal to preserve integrity and minimize damage (50).
However, research addressing these issues has been limited. Tissues and cells are typically removed from storage by rapid warming followed by gradual removal of CPAs. The temperature during warming can significantly impact the outcome. For instance, thawing adult bovine tissue at 37°C and 97–100°C has been shown to result in better cell viability and spermatogonial cell survival compared to thawing at 4°C (51). Additionally, Lima et al. (52) demonstrated that warming at 50°C for 5 s can effectively ensure the reanimation and survival of vitrified testicular tissues from prepubertal domestic cats, but additional research is required to better understand the impact of warming protocols on avoiding devitrification and ice recrystallization and ensuring the optimal revival of tissue and cells (52).
3 Cryopreservation of testicular tissue and SSCs
3.1 Small animals
Relevant advancements in testicular tissue cryopreservation have been reported in small animal species, particularly cats and dogs, although at varying levels of experimental validation. The most significant achievement in this context was reported in the domestic cat, where spermatozoa retrieved from frozen–thawed testicular tissue were used for intracytoplasmic sperm injection (ICSI), resulting in the birth of live kittens after embryo transfer (53). Although this strategy did not involve grafting or IVS, it provided clear proof of functional sperm recovery leading to viable offspring. In contrast, earlier attempts involving xenografting of cryopreserved cat testicular tissue into immunodeficient mice did not yield successful preservation of germ cells (54), highlighting the challenges of this approach for restoring spermatogenesis in this species.
Comparative evaluations of cryopreservation techniques have further refined experimental protocols. In cats, rapid freezing - typically preceded by a pre-equilibration phase at 4–5°C - has consistently shown better preservation of sperm plasma membrane integrity and seminiferous epithelium compared to slow freezing (26, 55), while vitrification has emerged as a promising alternative, with several studies reporting good structural maintenance and reduced interstitial damage, indicating that ultra-rapid cooling may represent a valid alternative to conventional protocols (12, 19, 46, 56, 57). In dogs, needle-immersed vitrification (NIV) has been introduced to facilitate handling of small tissue fragments. Using NIV, higher preservation of undifferentiated germ cells was observed compared to slow freezing (58). However, findings in gray wolves (Canis lupus), a species closely related to domestic dogs, indicate species-specific variability, as slow freezing proved more effective than NIV (11).
The choice of CPAs also plays a critical role and appears to be species-specific. The combination of dimethyl sulfoxide (DMSO) and glycerol proved to be the most effective in the cat, ensuring superior preservation of the seminiferous epithelium and greater proliferative potential in both freezing and vitrification protocols (12, 37, 52, 56, 57). These compounds consistently maintained tubular architecture and cellular viability better than alternatives like ethylene glycol, which was associated with increased cytotoxicity and morphological disruption in several feline studies (46, 56). Conversely, in canine testicular tissue, favorable results were reported using DMSO in combination with EG in both pre- (17) and post- pubertal (58, 59) specimens, with preserved nuclear and tissue architecture despite some mild alterations like basement membrane detachment.
In cats, where more studies are available, tissue fragment size and warming rates have also been investigated. Larger tissue fragments (0.5 cm3) cryopreserved with glycerol showed better morphological features, although subsequent assessments of apoptosis and DNA integrity did not reveal significant differences between fragment sizes (37, 60). In prepubertal animals, exposure to 50°C for 5 s consistently resulted in better preservation of seminiferous tubule structure and enhanced somatic and germ cell viability compared to standard warming at 37°C (52) or higher temperatures such as 60°C (57). These results indicate the importance of integrating morphological and molecular assessments in protocol optimization and underscore the importance of fine-tuning warming conditions to maximize recovery after vitrification.
Cryopreservation of testicular cell suspensions, although still limited to unsorted populations, has shown promising results in both cats and dogs. In felines, slow freezing with 7.5% DMSO yielded the highest recovery rates (61), and one study reported that cell suspensions may better preserve sperm membrane integrity than tissue fragments (62). In canines, while SSC-specific cryopreservation protocols have not yet been developed, testicular cell suspensions have demonstrated the ability to colonize recipient testes after xenotransplantation into immunodeficient mice. However, no differentiation has been observed, likely due to the evolutionary distance between donor and recipient species (63, 64).
3.2 Large animals
In large animals, the efficacy of cryopreservation techniques for testicular tissue has shown species-specific trends.
In ovine species, slow freezing has proven more effective than vitrification in preserving immature testicular tissue integrity and functionality. These findings are supported by both in vivo data, demonstrating that slow freezing maintains seminiferous tubule architecture and spermatogenic activity after xenografting (7), and in vitro findings, which confirm better preservation of morphological features, extracellular matrix components, and gene expression profiles following cryopreservation by slow freezing, especially with 5 mm3 tissue fragments (18).
In porcine species, both slow freezing and vitrification have yielded comparable outcomes in terms of germ cell survival and DNA integrity, with several studies reporting no significant differences between the two methods (9, 36, 58). Notably, Kaneko et al. (48) demonstrated that vitrified immature testicular tissue retained functional germ cells, as evidenced by the birth of live piglets following xenografting.
In cattle, slow freezing is the most widely adopted method and has consistently provided reliable preservation of tissue structure and cell viability. Zhao et al. (65), for instance, reported that slow-frozen calf testicular tissues retained structural integrity and functional potential after xenotransplantation, with preserved seminiferous cords, angiogenesis, and increased expression of germline and somatic markers. However, a recent comparative study showed that vitrification, although associated with lower attachment of seminiferous tubules to the basement membrane, preserved germ and Sertoli cells, maintained membrane integrity, and reduced apoptosis—supporting germ cell viability and colony formation in short-term in vitro culture (66).
Among the various CPAs, DMSO remains the most effective for preserving testicular tissue structure and germ cell functionality across species. In ovine and equine models, DMSO-based slow freezing protocols have led to superior outcomes, including better seminiferous tubule integrity, reduced basement membrane disruption, and preserved germ cell viability and SSC marker expression (7, 67). When directly compared to other CPAs such as ethylene glycol, propylene glycol and glycerol, DMSO has also shown greater efficacy in porcine (48) and bovine (51) models. Notably, DMSO performance remained consistent regardless of animal tissue age (immature vs. adult) and cryopreservation strategy (slow freezing vs. vitrification), often outperforming alternative CPAs in preserving both cellular integrity and molecular functionality (36, 68–71). Additionally, recent studies suggest that DMSO may support DNA repair mechanisms post-thaw (71).
Cryopreservation outcomes have been further improved by supplementing DMSO-based media with protective additives. Knockout serum replacement (KSR), for example, has proven to be a valid alternative to fetal bovine serum (FBS), ensuring consistent cryoprotection and gonocyte recovery in both immature and adult bovine tissues (8, 39). Trehalose has also emerged as a particularly effective additive, showing consistent benefits across species. Its inclusion in cryopreservation protocols has been associated with improved antioxidant activity, enhanced cell viability and reduced apoptosis, supporting both the structural integrity and functional capacity of SSC-containing germ cells (8, 72, 73). Notably, trehalose-based vitrification strategies have also yielded promising results in the porcine model. In particular, the inclusion of trehalose in the vitrification medium was associated with preserved tissue viability after warming and, remarkably, enabled the generation of viable offspring from sperm retrieved from xenografted tissue, highlighting the long-term potential of trehalose in supporting germline functionality following cryopreservation (48).
The post-thaw recovery and transplantation efficiency have been reported to be higher when cryopreserving testicular tissue compared to isolated cells in both bovine and porcine species (15, 24). Nevertheless, the cryopreservation of isolated SSCs remains a promising strategy. In large animal species, slow freezing remains the most widely used technique for SSC cryopreservation, providing consistent results in terms of post-thaw viability and proliferative capacity. Studies conducted in sheep (74), cattle (14), pigs (75) and horses (5) have demonstrated that SSC-enriched suspensions preserved via slow freezing maintain cellular integrity and are capable of surviving and proliferating after thawing. Supporting this, Oatley et al. (76) showed that bovine SSCs cryopreserved using a simple slow freezing protocol retained their functional potential, as evidenced by their colonization of recipient mouse seminiferous tubules following transplantation.
Although vitrification is still underexplored in this context, promising results have been reported by Patra et al. (77) who showed that vitrified goat SSCs retained post-warming viability and colony-forming capacity, despite signs of oxidative stress and partial mitochondrial dysfunction. These findings suggest that vitrification may offer a viable alternative to slow freezing, though further refinement is needed to improve its consistency and efficacy.
As in testicular tissue, the choice and combination of CPAs plays a central role in SSC cryopreservation outcomes. Dimethyl sulfoxide remains the most widely used CPA across species, often combined with non-permeating agents such as sucrose or trehalose to enhance membrane protection during freezing and enhance post thaw outcomes. Consistent with findings in tissue cryopreservation, trehalose has been shown to enhance the viability, recovery, and proliferative capacity of SSCs in ovine (78), porcine (15), and bovine (24) models, with cryopreserved cells also demonstrating colony-forming potential after xenotransplantation. Similarly, sucrose has proven effective as an osmotic buffer and membrane stabilizer, with improved survival and proliferation of SSCs observed in sheep (79), cattle (14) and pigs (75) when added to DMSO-based media.
Beyond sugars, other strategies have aimed to mitigate oxidative damage associated with cryopreservation. In goats, the addition of melatonin (10−6 M) to the freezing medium improved mitochondrial function, antioxidant capacity, and overall cell viability while reducing apoptosis and autophagic activity (80). In cattle, a synergistic effect between DMSO and propanediol was also reported, leading to higher post-thaw viability and membrane integrity than either CPA alone (81). The robustness of DMSO-based protocols is also confirmed in equine species, where cryopreserved SSCs retained viability, metabolic activity, and expression of key stem cell markers (5).
Altogether, these findings highlight the need for species-specific and application-oriented protocols that consider the structural complexity of testicular tissue and the inherent sensitivity of SSCs, thereby supporting the development of effective fertility cryopreservation strategies both in small and large animals.
4 Recreation of spermatogenesis in vitro
Although cryopreservation is an effective strategy for fertility preservation, its combination with transplantation or in vitro culture techniques is essential to develop mature germ cells and obtain progeny. The culture of testicular cells, fresh or preserved, is aimed at achieving these results in vitro, without the use of experimental animals. In addition, recreating spermatogenesis in vitro in animal species holds significant importance for understanding reproductive biology, preserving endangered species, and advancing biotechnological applications such as ARTs and genetic conservation.
However, faithfully recreating the entire process in vitro remains a challenge. The main obstacles include the possibility to provide a suitable microenvironment that mimics the testicular niche to support the survival and development of all the cell types, as well as to achieve complete and functional spermatogenesis. In domestic species, several IVS approaches have been explored for propagation and differentiation of spermatogonia in vitro into mature sperm. These include the culture of testicular tissue explants, isolation and culture of SSCs and generation of three-dimensional (3D) culture platforms. Among these, the culture of SSCs using 3D culture models, such as organoids and decellularized tissue, is garnering increasing interest.
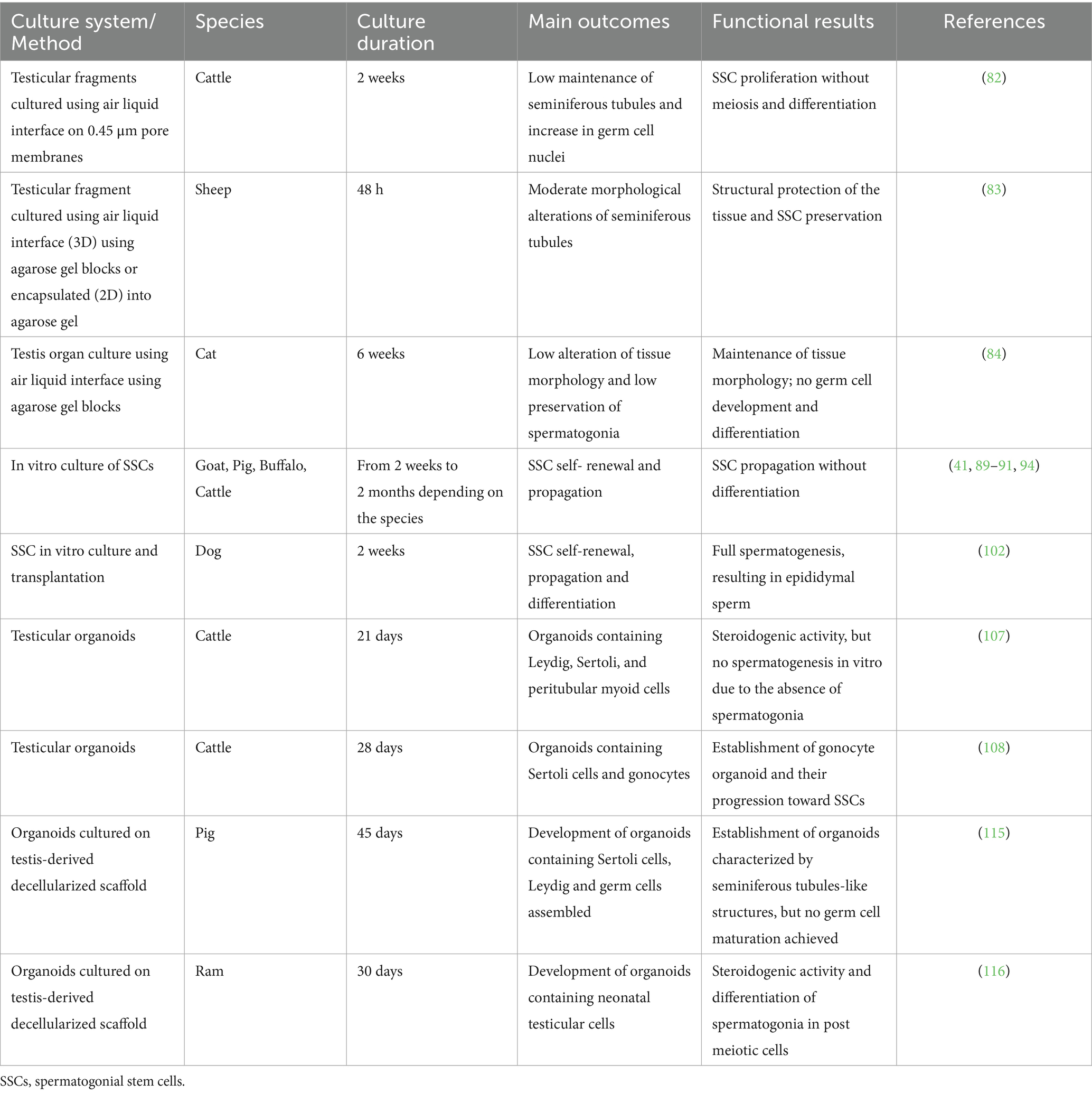
In this section, we will summarize the strategies developed for culturing testicular tissue and cells with the goal of recreating IVS in large and small animals (Table 3). Due to the limited literature available for both, the section is organized by techniques rather than by species, discussing the results across species when relevant.
4.1 Testicular tissue explants
Testicular tissue fragments can be readily isolated from both immature and mature animals, both large and small, and cultured under controlled conditions to preserve cellular viability, proliferation, and differentiation. This in vitro culture aims to replicate the testicular microenvironment, which is essential for supporting spermatogenesis. In vivo, germ cells, Sertoli cells, peritubular cells, interstitial cells, and particularly Leydig cells must maintain normal morphology to synthesize autocrine and paracrine factors vital for spermatogenesis. Therefore, it is crucial that in vitro cultures preserve the testicular structure to maintain paracrine signaling, which is essential for germ cell proliferation and differentiation. Various culture systems for testicular tissue fragments from different species have been reported to enhance the stabilization of these fragments. An early study in cattle demonstrated the survival and proliferation of bovine SSCs during a two-week explant culture, where tissue fragments were cultured on the top of 0.45-mm pore membranes to create an air liquid interface culture system (82). Morphological analysis revealed maintenance of seminiferous tubular structure and a significant increase in germ cell nuclei per tubule compared to fresh tissue (82). However, no meiotic cells were observed, indicating spermatogonial proliferation without further differentiation (82). Despite these promising results, careful monitoring of the culture medium volume in each well is crucial when using transwell insert membranes. The medium level should be sufficient to contact the bottom of the insert without submerging the tissue. Hypoxia also limits the efficiency of organ culture. Recently, in sheep, culturing testicular fragments in agarose gel resulted in less cell loss and basement membrane disruption, suggesting structural protection of the tissue (83). This 3D system, however, may impede medium perfusion due to the rigidity of the agarose gel, which can affect both tissue architecture and function.
In domestic cats, testis organ culture does not progress as observed in other mammals. While some spermatogonia, potentially including SSCs, are maintained over extended periods, there is no advancement in germ cell development (84). This issue pertains not only to the initiation of spermatogenesis but also to its progression; even tissues containing more developed germ cells at the outset show no further differentiation. The complexity of spermatogenesis initiation and regulation in domestic cats appears to be greater than in other mammal species such as bull, buffalo, ram, goat, boar, wild boar, dog and rabbit (85). In particular, the overall rate of spermatogenesis in cats is lower compared to several other mammals, as indicated by a lower meiotic index (84). Additionally, abnormalities in the seminiferous epithelium are commonly observed in cats (84).
Most of these studies have been based on short-term culture time which is a limit if it is considered that spermatogenesis takes place in about 3 months in many species. Recently, it was demonstrated that small intact testicular tissue fragments cultured in knockout serum replacement could be effectively maintained in vitro for up to 4 weeks of culture (86). Testicular tissue integrity was dependent on fragment size and preparation method, where the smallest size and intact preparation method were advantageous.
Taken together, these findings indicate that for this type of culture platform culture time can be affected by several factors including tissue fragment size, preparation method, media supplements, matrix and serum sources (86). It is therefore important to consider all these factors to establish a long-term in vitro maintenance of the testicular tissue.
4.2 Culture and differentiation of spermatogonia in vitro
In vitro culture of spermatogonia, particularly SSCs, is essential for self-renewal, differentiation, and manipulation of testicular germ cells. Various culture systems and medium compositions have been developed to enhance SSC viability and proliferation (87, 88). However, long-term SSC cultures exceeding 2 months have not been established yet for domestic animals, with current efforts limited to short-term cultures in species such as goat (41), pig (89), buffalo (90) and cattle (91). To date, complete ex vivo spermatogenesis has been achieved in the murine models (92, 93), while, in domestic species, propagation of spermatogonia without effective meiotic division has only been reported.
Regarding media composition, two types, stempro-34 and DMEM supplemented with Fetal Bovine Serum (DMEM-FBS), have been utilized for SSC culture in domestic animals. Colony formation has been observed in SSCs culture of goats and pigs using DMEM-FBS medium, with these colonies containing PGP9.5-positive cells, a marker of undifferentiated spermatogonia, including SSCs (89, 94). Similarly, colonies formed in SSC cultures of piglets and calves using serum free stempro-34 medium contained Dolichos biflorus agglutinin (DBA)-positive cells (95, 96). In previous studies, spherical cell colonies (SDC) have been observed in porcine testicular cell culture containing PGP9.5-positive cells with stem and germ cell characteristics (97). Additionally, growth factors are essential to form the SDC in SSC cultures. In pig SSC cultures, epidermal growth factor (EGF) and fibroblast growth factor (FGF) positively influenced the number and size of SSC-like colonies, and their addition to primary cell cultures of neonatal pig testes influenced NANOG, PLZF, OCT4, and GATA4 transcript level (95). Furthermore, FGF2 has been shown to mediate mouse SSC self-renewal via up- regulation of Etv5 and Bcl6b through MAP2K1 activation (98). These findings suggest that SSC colonies can be formed in both stempro-34 and DMEM-FBS media and that FGF plays a significant role in SSC cultures. More recently, in dogs, colonies were observed in both media at day 7, and the addition of FGF significantly affected colony formation from two-month-old beagle’s testes (99). These results indicate that stempro-34 and DMEM-FBS media, supplemented with glial cell line-derived neurotrophic factor (GDNF) and FGF are well suited for deriving SDCs from neonatal beagle testes.
The use of DMEM-FBS and stempro-34 has not been the only approach used to culture spermatogonia. In cattle, spermatogonia were successfully cultured from cryopreserved testicular tissues using 2i medium (100). The obtained culture system resulted in enhanced proliferation, survival, anti- differentiation and apoptosis. These effects might be due to the attenuation of Suv39h1/2-mediated H3K9me3 level by 2i stimulation through MEK and GSK pathways (100).
In both small and large animals, advances have been made in SSC culture, demonstrating the potential of these cells to restore and produce germ cells in vitro (101). In dogs, SSCs were able to progress in vivo toward more differentiated testicular cell stages, including spermatocytes, spermatids, and sperm, following transplantation (102). Moreover, the in vitro generation of embryonic germ cell-like cells has also been reported in canines (101, 103). The canine model, in particular, offers promising opportunities to discover new signaling molecules, transcription factors, and mechanisms involved in self-renewal and differentiation processes critical for germ cell development.
On the other hand, in cats, Powell and colleagues (104) successfully isolated SSCs; however, no culture protocols have been developed so far. In the same study, they reported that markers commonly used for SSC identification in other species may be less reliable for isolating cat SSCs, whereas pluripotent markers, particularly SSEA-4, may provide more enriched SSC populations. SSCs are low in number in the testis, and the smallest subpopulation of spermatogonial cells identified was SSEA-4þ positive, expressing NANOG, POU5F1, and SOX2 (104).
Although several protocols to isolate and culture spermatogonia and SSCs have been developed in few domestic species, it is important to note that these culture systems consisted of multiple cell types. These included not only spermatogonia but also testicular somatic cells, such as Sertoli cells, suggesting that testicular cells rely on cell-to-cell interactions for establishing a reliable culture system that could restore spermatogenesis in vitro.
Last but not least, SSCs from domestic animals have been transplanted into the seminiferous tubules of germ cell-depleted infertile mice, although few of these experiments were able to restore spermatogenesis (63, 105, 106).
Overall results indicate that successful xenotransplantation is possible between SSCs and mice and demonstrate that this is a viable model that offers new insights for the treatment of infertility and understanding the mechanisms of spermatogenesis in domestic animals. However, improvement of the methodologies is necessary in future for completely restoring spermatogenesis in vitro.
4.3 Generation of 3D testicular culture models
Three-dimensional culture models have gained prominence in research due to their architectural and functional resemblance to native microenvironments. In domestic species, the generation of testicular organoids has become a valuable tool for studying testicular function and development. Notably, testicular organoids have been successfully established from testicular tissues, where cells were harvested using a two-step enzymatic digestion process. These organoids, characterized by an encapsulated shape, contained testis-specific cell types such as germ cells, Sertoli cells, Leydig cells, and peritubular myoid cells.
In large animals, testicular organoids have been generated by isolating testicular cells from bovine testes and culturing them in ultra-low attachment plates with Matrigel (107). These organoids contained Leydig, Sertoli, and peritubular myoid cells, displaying specific localization and changes in number. The developed bovine testicular organoids exhibited steroidogenic activity, characterized by the production of testosterone into the culture media. However, these organoids lacked spermatogonia, limiting the ability to recreate spermatogenesis mechanisms in vitro.
Recently, Tang and colleagues (108) successfully established an in vitro 3D neonatal testicular organoid culture system containing bovine gonocytes that were cultured for a period of 28 days. Supplementation with GDNF, FGF2, and LIF helped maintain a high proportion of proliferating cells while promoting the transformation of gonocytes into SSCs (108). Additionally, FSH and testosterone were found to be beneficial for maintaining the viability and proliferation of cells in organoids (108). However, these testicular organoids did not exhibit critical testicular compartmentalization, and spermatogenesis was not studied.
Another significant advancement in the field of 3D culture platforms for domestic species is the development of testicular extracellular matrix (ECM)-derived scaffolds through tissue decellularization (109). This approach offers promising avenues for exploring cell-matrix interactions during spermatogenesis. By effectively removing cells and debris while preserving the native ECM composition, 3D structure, and biological activity, these scaffolds create a supportive environment for repopulation with SSCs and somatic cells (110). Tailored decellularization protocol using physical, chemical, or biological agents can be optimized for the unique characteristics of domestic animal tissues, thereby advancing applications such as artificial testis generation, fertility restoration, and drug screening in both large and small animals.
Vermeulen and colleagues were the first to apply and compare several decellularization protocols for prepubertal porcine testicular fragments (111). Following their work, decellularized testes have been obtained in cattle (112, 113) and sheep (114). The derived ECM can be used as 3D scaffolds or lyophilized.
Lyophilization of the decellularized testis allow for the preparation of a hydrogel. Recently, using this approach, porcine testicular organoids have been generated by encapsulating testicular cell suspensions (115). These organoids were maintained for 45 days in culture and consisted of tubule-like structures surrounded by interstitial cells; however, germ cell maturation was not achieved.
In another study, testis-derived scaffolds were fabricated from ram testicular tissue (116). These biological scaffolds were seeded with neonatal mouse testicular cells and supported the formation of organoids that, despite lacking the typical testicular architecture, were able to produce hormones and formed post-meiotic cells (116).
These findings indicate that the generation of 3D culture platform to recreate spermatogenesis in vitro is complex and involves well-orchestrated interactions among hormones, growth factors, cytokines, and ECM-derived biochemical and biomechanical cues and presents significant challenges. Additionally, the lack of knowledge regarding the niche microenvironment, nutritional requirements, and the regulatory mechanisms driving self-renewal, proliferation, and differentiation in domestic species has hindered progress in this field. Therefore, it is desirable to develop reliable 3D in vitro models that faithfully mimic the architecture and physiological microenvironment of native testicular tissue, bridging the gap between in vivo complexity and the oversimplified conventional two-dimensional in vitro cultures.
5 Future directions and conclusions
Despite significant progress has been made in the cryopreservation and culture of testicular tissue and cells, several challenges remain before these techniques can be translated into reliable veterinary applications. The optimization of cryopreservation protocols requires further refinement to ensure consistent preservation of tissue architecture, cell viability, and functionality across different species and developmental stages. Comparative studies investigating cryoprotectant combinations, fragment size, and warming strategies will be crucial for establishing standardized and effective methods. Concurrently, advancements in testicular tissue and SSCs culture systems must evolve toward more physiologically relevant platforms capable of supporting complete spermatogenesis. The implementation of biomimetic platforms such as testicular organoids, 3D scaffolds and ECM-derived hydrogels represents a promising direction for recreating the native testicular microenvironment. However, the lack of species-specific knowledge on somatic-germ cell interactions, hormonal regulation, and niche dynamics continues to hinder the full maturation of germ cells in culture. The integration of cryopreservation and long-term in vitro culture approaches, supported by molecular, epigenetic, and functional analyses, will be essential to assess the safety, efficiency, and reproducibility of these systems. Ultimately, the convergence of optimized cryopreservation and culture protocols could enable the development of species-specific fertility preservation strategies applicable to both domestic and wild animals, with implications for breeding management, biodiversity conservation, and ARTs in veterinary medicine.
Author contributions
VV: Investigation, Writing – original draft, Formal analysis, Conceptualization, Writing – review & editing, Methodology. MC: Formal analysis, Methodology, Conceptualization, Writing – review & editing. RP: Conceptualization, Writing – review & editing, Investigation, Writing – original draft, Formal analysis, Methodology. GL: Resources, Conceptualization, Funding acquisition, Project administration, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by “Piano di Sostegno alla Ricerca 2023 (Linea 2 Azione A),” Università degli Studi di Milano.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Onofre, J, Baert, Y, Faes, K, and Goossens, E. Cryopreservation of testicular tissue or testicular cell suspensions: a pivotal step in fertility preservation. Hum Reprod Update. (2016) 22:744–61. doi: 10.1093/humupd/dmw029
2. Silva, LDMD. Canine and feline testicular preservation. Animals. (2022) 12:124. doi: 10.3390/ani12010124
3. Silva, AMD, Pereira, AF, Comizzoli, P, and Silva, AR. Cryopreservation and culture of testicular tissues: an essential tool for biodiversity preservation. Biopreserv Biobank. (2020) 18:235–43. doi: 10.1089/bio.2020.0010
4. Luvoni, GC, and Colombo, M. Cold case: small animal gametes cryobanking. Theriogenology. (2020) 150:445–51. doi: 10.1016/j.theriogenology.2020.02.047
5. Costa, GMJ, Avelar, GF, Lacerda, SMSN, Figueiredo, AFA, Tavares, AO, Rezende-Neto, JV, et al. Horse spermatogonial stem cell cryopreservation: feasible protocols and potential biotechnological applications. Cell Tissue Res. (2017) 370:489–500. doi: 10.1007/s00441-017-2673-1
6. Patra, T, Pathak, D, and Gupta, MK. Strategies for cryopreservation of testicular cells and tissues in cancer and genetic diseases. Cell Tissue Res. (2021) 385:1–19. doi: 10.1007/s00441-021-03437-4
7. Pukazhenthi, BS, Nagashima, J, Travis, AJ, Costa, GM, Escobar, EN, França, LR, et al. Slow freezing, but not vitrification supports complete spermatogenesis in cryopreserved, neonatal sheep testicular xenografts. PLoS One. (2015) 10:e0123957. doi: 10.1371/journal.pone.0123957
8. Zhu, WQ, Cai, NN, Jiang, Y, Yang, R, Shi, JZ, Zhu, CL, et al. Survivable potential of germ cells after trehalose cryopreservation of bovine testicular tissues. Cryobiology. (2021) 101:105–14. doi: 10.1016/j.cryobiol.2021.05.001
9. Zeng, W, Snedaker, AK, Megee, S, Rathi, R, Chen, F, Honaramooz, A, et al. Preservation and transplantation of porcine testis tissue. Reprod Fertil Dev. (2009) 21:489–97. doi: 10.1071/rd08235
10. Lee, DR, Kaproth, MT, and Parks, JE. In vitro production of haploid germ cells from fresh or frozen-thawed testicular cells of neonatal bulls. Biol Reprod. (2001) 65:873–8. doi: 10.1095/biolreprod65.3.873
11. Andrae, CS, Oliveira, ECS, Ferraz, MAMM, and Nagashima, JB. Cryopreservation of grey wolf (Canis lupus) testicular tissue. Cryobiology. (2021) 100:173–9. doi: 10.1016/j.cryobiol.2021.01.010
12. Lima, D, Silva, T, Morais, GB, Aquino-Cortez, A, Evangelista, J, Júnior, FX, et al. Different associations of cryoprotectants for testicular tissue of prepubertal cats submitted to vitrification. Reprod Domest Anim. (2017) 52 Suppl 2:235–41. doi: 10.1111/rda.12833
13. Pothana, L, Makala, H, Devi, L, Varma, VP, and Goel, S. Germ cell differentiation in cryopreserved, immature, Indian spotted mouse deer (Moschiola indica) testes xenografted onto mice. Theriogenology. (2015) 83:625–33. doi: 10.1016/j.theriogenology.2014.10.028
14. Izadyar, F, Matthijs-Rijsenbilt, JJ, den Ouden, K, Creemers, LB, Woelders, H, and de Rooij, DG. Development of a cryopreservation protocol for type a spermatogonia. J Androl. (2002) 23:537–45. doi: 10.1002/j.1939-4640.2002.tb02276.x
15. Lee, YA, Kim, YH, Ha, SJ, Kim, KJ, Kim, BJ, Kim, BG, et al. Cryopreservation of porcine spermatogonial stem cells by slow-freezing testis tissue in trehalose. J Anim Sci. (2014) 92:984–95. doi: 10.2527/jas.2013-6843
16. Han, Z, Liu, X, Wang, H, Qazi, IH, Wang, L, Du, R, et al. Testicular tissue cryopreservation and transplantation as a strategy for feline conservation: a review of research advances. Front Vet Sci. (2025) 12:1572150. doi: 10.3389/fvets.2025.1572150
17. Teixeira, DO, Oliveira, ES, Fernandes, JS, Palomino, GJQ, Tabosa, BEA, Barbosa, HTS, et al. Histological evaluation of testicles from prepubertal dogs submitted to vitrification with different cryoprotectant associations. Res Soc Dev. (2021) 10:348101623864. doi: 10.33448/rsd-v10i16.23864
18. Gomes, FDR, Ñaupas, LVS, Palomino, GJQ, Celiz, RHY, Sá, NAR, Novaes, MAS, et al. Definition of protocols for cryopreservation and three-dimensional in vitro culture of prepubertal goat testicular tissue after histomorphological, ultrastructural, and functional analysis. Theriogenology. (2023) 211:151–60. doi: 10.1016/j.theriogenology.2023.08.015
19. Macente, BI, Fonseca-Alves, CE, Magalhães, GM, Tavares, MR, Mansano, CFM, Mouttham, L, et al. Influence of vitrification device, warming protocol, and subsequent in vitro culture on structural integrity of testicular fragments from adult domestic cats. Biopreserv Biobank. (2022) 20:392–400. doi: 10.1089/bio.2021.0092
20. Silva, AR, Pereira, AF, and Comizzoli, P. Biobanking and use of gonadal tissues - a promising strategy for conserving wildlife from the Caatinga biome. Anim Reprod. (2023) 19:e20220135. doi: 10.1590/1984-3143-AR2022-0135
21. Binsila, B, Selvaraju, S, Ranjithkumaran, R, Archana, SS, Krishnappa, B, Ghosh, SK, et al. Current scenario and challenges ahead in application of spermatogonial stem cell technology in livestock. J Assist Reprod Genet. (2021) 38:3155–73. doi: 10.1007/s10815-021-02334-7
22. Avarbock, MR, Brinster, CJ, and Brinster, RL. Reconstitution of spermatogenesis from frozen spermatogonial stem cells. Nat Med. (1996) 2:693–6. doi: 10.1038/nm0696-693
23. Shirazi, MS, Heidari, B, Naderi, MM, Behzadi, B, Sarvari, A, Borjian Boroujeni, S, et al. Transplantation of goat spermatogonial stem cells into the mouse rete testis. Int J Anim Biol. (2015) 1:61–8.
24. Kim, KJ, Lee, YA, Kim, BJ, Kim, YH, Kim, BG, Kang, HG, et al. Cryopreservation of putative pre-pubertal bovine spermatogonial stem cells by slow freezing. Cryobiology. (2015) 70:175–83. doi: 10.1016/j.cryobiol.2015.02.007
25. Thuwanut, P, Srisuwatanasagul, S, Wongbandue, G, Tanpradit, N, Thongpakdee, A, Tongthainan, D, et al. Sperm quality and the morphology of cryopreserved testicular tissues recovered post-mortem from diverse wild species. Cryobiology. (2013) 67:244–7. doi: 10.1016/j.cryobiol.2013.07.002
26. Buarpung, S, Tharasanit, T, Comizzoli, P, and Techakumphu, M. Feline spermatozoa from fresh and cryopreserved testicular tissues have comparable ability to fertilize matured oocytes and sustain the embryo development after intracytoplasmic sperm injection. Theriogenology. (2013) 79:149–58. doi: 10.1016/j.theriogenology.2012.09.022
28. Lima, DBC, and Silva, LDMD. Cryopreservation of testicular tissue: an alternative to maintain the reproductive capacity in different animal species. Cienc Rural. (2017) 47:e20170135. doi: 10.1590/0103-8478cr20170135
29. Arciero, V, Ammar, O, Maggi, M, Vignozzi, L, Muratori, M, and Dabizzi, S. Vapour fast freezing with low semen volumes can highly improve motility and viability or DNA quality of cryopreserved human spermatozoa. Andrology. (2022) 10:1123–33. doi: 10.1111/andr.13208
30. Nikmahzar, A, Khadivi, F, Abbasi, M, Mahdavinezhad, F, Abbasi, Y, and Daneshi, E. Testicular tissue Vitrification: a promising strategy for male fertility preservation. Reprod Sci. (2023) 30:1687–700. doi: 10.1007/s43032-022-01113-8
31. Jang, TH, Park, SC, Yang, JH, Kim, JY, Seok, JH, Park, US, et al. Cryopreservation and its clinical applications. Integr Med Res. (2017) 6:12–8. doi: 10.1016/j.imr.2016.12.001
32. Baert, Y, Goossens, E, van Saen, D, Ning, L, in't Veld, P, and Tournaye, H. Orthotopic grafting of cryopreserved prepubertal testicular tissue: in search of a simple yet effective cryopreservation protocol. Fertil Steril. (2012) 97:e72:1152–7. doi: 10.1016/j.fertnstert.2012.02.010
33. Fahy, GM, MacFarlane, DR, Angell, CA, and Meryman, HT. Vitrification as an approach to cryopreservation. Cryobiology. (1984) 21:407–26. doi: 10.1016/0011-2240(84)90079-8
34. Honaramooz, A. Cryopreservation of testicular tissue In: I Katkov, editor. Current frontiers in cryobiology. Canada: University of Saskatchewan (2012)
35. Yavin, S, and Arav, A. Measurement of essential physical properties of vitrification solutions. Theriogenology. (2007) 67:81–9. doi: 10.1016/j.theriogenology.2006.09.029
36. Abrishami, M, Anzar, M, Yang, Y, and Honaramooz, A. Cryopreservation of immature porcine testis tissue to maintain its developmental potential after xenografting into recipient mice. Theriogenology. (2010) 73:86–96. doi: 10.1016/j.theriogenology.2009.08.004
37. Macente, BI, Toniollo, GH, Apparicio, M, Mansano, C, Thomé, HE, Canella, CL, et al. Evaluation of different fragment sizes and cryoprotectants for cryopreservation of feline testicular tissues. Reprod Domest Anim. (2017) 52:242–7. doi: 10.1111/rda.12828
38. Bebbere, D, Pinna, S, Nieddu, S, Natan, D, Arav, A, and Ledda, S. Gene expression analysis of ovine prepubertal testicular tissue vitrified with a novel cryodevice (E.Vit). J Assist Reprod Genet. (2019) 36:2145–4. doi: 10.1007/s10815-019-01559-x
39. Jiang, Y, Zhu, WQ, Zhu, XC, Cai, NN, Yang, R, Cai, H, et al. Cryopreservation of calf testicular tissues with knockout serum replacement. Cryobiology. (2020) 92:255–7. doi: 10.1016/j.cryobiol.2020.01.010
40. Narenji Sani, R, Tajik, P, Yousefi, MH, Movahedin, M, Qasemi-Panahi, B, Shafiei, S, et al. Follicle stimulating hormone increases spermatogonial stem cell colonization during in vitro co-culture. Vet Res Forum. (2013) 4:37–1.
41. Pramod, RK, and Mitra, A. In vitro culture and characterization of spermatogonial stem cells on Sertoli cell feeder layer in goat (Capra hircus). J Assist Reprod Genet. (2014) 31:993–1001. doi: 10.1007/s10815-014-0277-1
42. Han, SY, Gupta, MK, Uhm, SJ, and Lee, HT. Isolation and in vitro culture of pig spermatogonial stem cell. Asian Australas J Anim Sci. (2009) 22:187–93. doi: 10.5713/ajas.2009.80324
43. Yang, Y, Yarahmadi, M, and Honaramooz, A. Development of novel strategies for the isolation of piglet testis cells with a high proportion of gonocytes. Reprod Fertil Dev. (2010) 22:1057–65. doi: 10.1071/RD09316
44. Milazzo, JP, Vaudreuil, L, Cauliez, B, Gruel, E, Massé, L, Mousset-Siméon, N, et al. Comparison of conditions for cryopreservation of testicular tissue from immature mice. Hum Reprod. (2008) 23:17–28. doi: 10.1093/humrep/dem355
45. Travers, A, Milazzo, JP, Perdrix, A, Metton, C, Bironneau, A, Macé, B, et al. Assessment of freezing procedures for rat immature testicular tissue. Theriogenology. (2011) 76:981–90. doi: 10.1016/j.theriogenology.2011.04.025
46. Carvalho, JVG, Soares, ARB, Leão, DL, Reis, AN, Santos, RR, Rodrigues, APR, et al. Effect of different vitrification techniques on viability and apoptotic index of domestic cat testicular tissue cells. Animals. (2023) 13:2768. doi: 10.3390/ani13172768
47. Criado, E. The problem of contamination: open vs. closed vs. semi-closed Vitrification systems In: II Katkov, editor. Current Frontiers in cryopreservation. Spain: CERAM (2012)
48. Kaneko, H, Kikuchi, K, Nakai, M, Somfai, T, Noguchi, J, Tanihara, F, et al. Generation of live piglets for the first time using sperm retrieved from immature testicular tissue cryopreserved and grafted into nude mice. PLoS One. (2013) 8:e70989. doi: 10.1371/journal.pone.0070989
49. Kaneko, H, Kikuchi, K, Tanihara, F, Noguchi, J, Nakai, M, Ito, J, et al. Normal reproductive development of pigs produced using sperm retrieved from immature testicular tissue cryopreserved and grafted into nude mice. Theriogenology. (2014) 82:325–31. doi: 10.1016/j.theriogenology.2014.04.012
50. Bagchi, A, Woods, EJ, and Critser, JK. Cryopreservation and vitrification: recent advances in fertility preservation technologies. Expert Rev Med Devices. (2008) 5:359–70. doi: 10.1586/17434440.5.3.359
51. Wu, JJ, Hu, TJ, Guo, B, Yue, ZP, Yang, ZT, and Zhang, XM. Cryopreservation of adult bovine testicular tissue for spermatogonia enrichment. Cryo Lett. (2011) 32:402–9.
52. Lima, DBC, Silva, LDMD, and Comizzoli, P. Influence of warming and reanimation conditions on seminiferous tubule morphology, mitochondrial activity, and cell composition of vitrified testicular tissues in the domestic cat model. PLoS One. (2018) 13:e0207317. doi: 10.1371/journal.pone.0207317
53. Tharasanit, T, Buarpung, S, Manee-In, S, Thongkittidilok, C, Tiptanavattana, N, Comizzoli, P, et al. Birth of kittens after the transfer of frozen-thawed embryos produced by intracytoplasmic sperm injection with spermatozoa collected from cryopreserved testicular tissue. Reprod Domest Anim. (2012) 47:305–8. doi: 10.1111/rda.12072
54. Mota, PC, Ehmcke, J, Westernströer, B, Gassei, K, Ramalho-Santos, J, and Schlatt, S. Effects of different storage protocols on cat testis tissue potential for xenografting and recovery of spermatogenesis. Theriogenology. (2012) 77:299–310. doi: 10.1016/j.theriogenology.2011.07.042
55. Thuwanut, P, and Chatdarong, K. Cryopreservation of cat testicular tissues: effects of storage temperature, freezing protocols and cryoprotective agents. Reprod Domest Anim. (2012) 47:777–81. doi: 10.1111/j.1439-0531.2011.01967.x
56. Lima, DBC, Silva, TFPD, Aquino-Cortez, A, Leiva-Revilla, J, and Silva, LDMD. Vitrification of testicular tissue from prepubertal cats in cryotubes using different cryoprotectant associations. Theriogenology. (2018) 110:110–5. doi: 10.1016/j.theriogenology.2017.12.037
57. Fernandes, JS, Tabosa, BEA, Brito, BF, Silva, HVR, Lima, DBC, and Silva, LDMD. Influence of different warming temperatures on the vitrified testicular fragments from pre-pubertal cat. Reprod Domest Anim. (2021) 56:1342–8. doi: 10.1111/rda.13997
58. Picazo, CM, Castaño, C, Bóveda, P, Toledano-Díaz, A, Velázquez, R, Pequeño, B, et al. Cryopreservation of testicular tissue from the dog (Canis familiaris) and wild boar (Sus scrofa) by slow freezing and vitrification: differences in cryoresistance according to cell type. Theriogenology. (2022) 190:65–72. doi: 10.1016/j.theriogenology.2022.07.020
59. Santos, JFP, Siqueira, AKM, Silva, AV, Morte, RFB, Medeiros, HRR, Souza, WJS, et al. Comparison between two cryoprotectant agents for cryopreservation of domestic dog testicles: partial results. Rev Bras Reprod Anim. (2017) 41:546.
60. Macente, BI, Apparicio, M, Mansano, CFM, Tavares, MR, Fonseca-Alves, CE, Sousa, BP, et al. Effect of cryopreservation on sperm DNA fragmentation and apoptosis rates in the testicular tissue of domestic cats. Anim Reprod Sci. (2019) 211:106224. doi: 10.1016/j.anireprosci.2019.106224
61. Bashawat, M, Braun, BC, and Müller, K. Cell survival after cryopreservation of dissociated testicular cells from feline species. Cryobiology. (2020) 97:191–7. doi: 10.1016/j.cryobiol.2020.03.001
62. Chatdarong, K, Thuwanut, P, and Morrell, JM. The development of cat testicular sperm cryopreservation protocols: effects of tissue fragments or sperm cell suspension. Theriogenology. (2016) 85:200–6. doi: 10.1016/j.theriogenology.2015.09.030
63. Dobrinski, I, Avarbock, MR, and Brinster, RL. Transplantation of germ cells from rabbits and dogs into mouse testes. Biol Reprod. (1999) 61:1331–9. doi: 10.1095/biolreprod61.5.1331
64. Lee, KH, Lee, WY, Kim, DH, Lee, SH, Do, JT, Park, C, et al. Vitrified canine testicular cells allow the formation of spermatogonial stem cells and seminiferous tubules following their xenotransplantation into nude mice. Sci Rep. (2016) 6:21919. doi: 10.1038/srep21919
65. Zhao, Y, Zhu, W, Yang, R, Zhang, B, Tang, B, and Zhang, X. Xenotransplantation of cryopreserved calf testicular tissues. Vet Sci. (2025) 12:247. doi: 10.3390/vetsci12030247
66. Tang, S, Jones, C, Davies, J, Lane, S, and Coward, K. A comparative analysis of vitrification and two slow freezing methods for gonocyte-containing neonatal calf testicular tissue and subsequent in vitro culture. In vitro models. (2025) 4:1–13. doi: 10.1007/s44164-025-00085-8
67. Gómez, M, Alrashed, A, Su, CY, and Durrant, B. Cryopreservation of horse testicular tissue as a model for rhinoceros. Reprod Fertil Dev. (2019) 31:138. doi: 10.1071/RDv31n1Ab25
68. Wu, JY, Sun, YX, Wang, AB, Che, GY, Hu, TJ, and Zhang, XM. Effect of newborn bovine serum on cryopreservation of adult bovine testicular tissue. Andrologia. (2014) 46:308–12. doi: 10.1111/and.12084
69. Zhang, XG, Li, H, and Hu, JH. Effects of various cryoprotectants on the quality of frozen-thawed immature bovine (Qinchuan cattle) calf testicular tissue. Andrologia. (2017) 49:e12743. doi: 10.1111/and.12743
70. Chicaiza-Cabezas, N, Garcia-Herreros, M, and Aponte, PM. Germplasm cryopreservation in bulls: effects of gonadal tissue type, cryoprotectant agent, and freezing-thawing rates on sperm quality parameters. Cryobiology. (2023) 110:24–35. doi: 10.1016/j.cryobiol.2023.01.001
71. Kaya, C, Esin, B, Akar, M, Can, C, and Çevik, M. Investigation of the efficacy of different cryoprotectants in the freezing of testicular tissue and epididymal sperm: Spermatological parameters, tissue viability and PARP-1 gene expression. Cryobiology. (2024) 117:104982. doi: 10.1016/j.cryobiol.2024.104982
72. Zhang, XG, Wang, YH, Han, C, Hu, S, Wang, LQ, and Hu, JH. Effects of trehalose supplementation on cell viability and oxidative stress variables in frozen-thawed bovine calf testicular tissue. Cryobiology. (2015) 70:246–52. doi: 10.1016/j.cryobiol.2015.03.004
73. Li, H, Bian, YL, Schreurs, N, Zhang, XG, Raza, SHA, Fang, Q, et al. Effects of five cryoprotectants on proliferation and differentiation-related gene expression of frozen-thawed bovine calf testicular tissue. Reprod Domest Anim. (2018) 53:1211–8. doi: 10.1111/rda.13228
74. Binsila, B, Tomcy, TA, Krishnappa, B, Sadikh, M, Ramachandran, N, Kolte, AP, et al. Comparison of different freezing rates on post-thaw viability, proliferation, and stemness of sheep spermatogonial stem cells. Cryobiology. (2025) 118:105203. doi: 10.1016/j.cryobiol.2025.105203
75. Pan, CY, Yu, S, Zhang, PF, Wang, B, Zhu, ZD, Liu, YY, et al. Effect of sucrose on cryopreservation of pig spermatogonial stem cells. J Integr Agric. (2017) 16:1120–9. doi: 10.1016/S2095-3119(16)61489-2
76. Oatley, JM, Reeves, JJ, and McLean, DJ. Biological activity of cryopreserved bovine spermatogonial stem cells during in vitro culture. Biol Reprod. (2004) 71:942–7. doi: 10.1095/biolreprod.104.028894
77. Patra, T, and Gupta, MK. Solid surface vitrification of goat testicular cell suspension enriched for spermatogonial stem cells. Cryobiology. (2022) 104:8–14. doi: 10.1016/j.cryobiol.2021.11.177
78. Pramod, RK, Varughese, D, Jameel, AJ, Panda, BN, Goswami, S, and Mitra, A. Cryopreserved ovine spermatogonial stem cells maintain stemness and colony forming ability in vitro. Asian Pac J Reprod. (2023) 12:273–80. doi: 10.4103/2305-0500.390302
79. Qasemi-Panahi, B, Movahedin, M, Moghaddam, G, Tajik, P, Koruji, M, Ashrafi-Helan, J, et al. Isolation and proliferation of spermatogonial cells from Ghezel sheep. Avicenna J Med Biotechnol. (2018) 10:93–7.
80. Feng, TY, Li, Q, Ren, F, Xi, HM, Lv, DL, Li, Y, et al. Melatonin protects goat spermatogonial stem cells against oxidative damage during cryopreservation by improving antioxidant capacity and inhibiting mitochondrial apoptosis pathway. Oxidative Med Cell Longev. (2020) 2020:5954635. doi: 10.1155/2020/5954635
81. Barbosa, APM, Martins, C, and Sereno, JRB. Cryopreservation of bovine spermatogenic cells using different protective molecules. Arch Zootec. (2011) 60:293–6.
82. Oatley, JM, de Avila, DM, Reeves, JJ, and McLean, DJ. Testis tissue explant culture supports survival and proliferation of bovine spermatogonial stem cells. Biol Reprod. (2004) 70:625–31. doi: 10.1095/biolreprod.103.022483
83. Celiz, RH, Brito, DCC, Schiavo, M, Sá, N, Ñaupas, LVS, Mbemya, TG, et al. Two- and three-dimensional system in agarose matrix for in vitro culture of testicular fragments of adult sheep. Small Rumin Res. (2023) 224:106987. doi: 10.1016/j.smallrumres.2023.106987
84. Silva, AF, Escada-Rebelo, S, Amaral, S, Tavares, RS, Schlatt, S, Ramalho-Santos, J, et al. Can we induce spermatogenesis in the domestic cat using an in vitro tissue culture approach? PLoS One. (2018) 13:e0191912. doi: 10.1371/journal.pone.0191912
85. Hess, RA, and de Franca, RL. Spermatogenesis and cycle of the seminiferous epithelium. Adv Exp Med Biol. (2008) 636:1–15. doi: 10.1007/978-0-387-09597-4_1
86. Ibtisham, F, Cham, TC, Fayaz, MA, and Honaramooz, A. Long-term in vitro maintenance of piglet testicular tissue: effects of tissue fragment size, preparation method, and serum source. Animals. (2022) 13:128. doi: 10.3390/ani13010128
87. Fath-Bayati, L, Naserpour, L, Khoshandam, M, Jannatifar, R, and Fazaeli, H. Recent advances in developing 3D culture systems of spermatogonial stem cell preservation and differentiation: a narrative review. Int J Reprod Biomed. (2023) 21:681–96. doi: 10.18502/ijrm.v21i9.14397
88. van Maaren, J, Alves, LF, van Wely, M, van Pelt, AMM, and Mulder, CL. Favorable culture conditions for spermatogonial propagation in human and non-human primate primary testicular cell cultures: a systematic review and meta-analysis. Front Cell Dev Biol. (2024) 11:1330830. doi: 10.3389/fcell.2023.1330830
89. Zhao, X, Wan, W, Li, B, Zhang, X, Zhang, M, Wu, Z, et al. Isolation and in vitro expansion of porcine spermatogonial stem cells. Reprod Domest Anim. (2022) 57:210–20. doi: 10.1111/rda.14043
90. Xie, B, Qin, Z, Huang, B, Xie, T, Yao, H, Wei, Y, et al. In vitro culture and differentiation of buffalo (Bubalus bubalis) spermatogonia. Reprod Domest Anim. (2010) 45:275–82. doi: 10.1111/j.1439-0531.2008.01281.x
91. Oatley, MJ, Kaucher, AV, Yang, QE, Waqas, MS, and Oatley, JM. Conditions for long-term culture of cattle undifferentiated Spermatogonia. Biol Reprod. (2016) 95:14. doi: 10.1095/biolreprod.116.139832
92. Naeemi, S, Sabetkish, S, Kiani, MJ, Dehghan, A, and Kajbafzadeh, AM. Ex-vivo and In-vivo expansion of Spermatogonial stem cells using cell-seeded microfluidic testis scaffolds and animal model. Cell Tissue Bank. (2023) 24:153–66. doi: 10.1007/s10561-022-10024-6
93. Ishikura, Y, Ohta, H, Sato, T, Murase, Y, Yabuta, Y, Kojima, Y, et al. In vitro reconstitution of the whole male germ-cell development from mouse pluripotent stem cells. Cell Stem Cell. (2021) 28:2167–79.e9. doi: 10.1016/j.stem.2021.08.005
94. Heidari, B, Rahmati-Ahmadabadi, M, Akhondi, MM, Zarnani, AH, Jeddi-Tehrani, M, Shirazi, A, et al. Isolation, identification, and culture of goat spermatogonial stem cells using c-kit and PGP9.5 markers. J Assist Reprod Genet. (2012) 29:1029–38. doi: 10.1007/s10815-012-9828-5
95. Kuijk, EW, Colenbrander, B, and Roelen, BA. The effects of growth factors on in vitro-cultured porcine testicular cells. Reproduction. (2009) 138:721–31. doi: 10.1530/REP-09-0138
96. Aponte, PM, Soda, T, van de Kant, HJ, and de Rooij, DG. Basic features of bovine spermatogonial culture and effects of glial cell line-derived neurotrophic factor. Theriogenology. (2006) 65:1828–47. doi: 10.1016/j.theriogenology.2005.10.020
97. Luo, J, Megee, S, Rathi, R, and Dobrinski, I. Protein gene product 9.5 is a spermatogonia-specific marker in the pig testis: application to enrichment and culture of porcine spermatogonia. Mol Reprod Dev. (2006) 73:1531–40. doi: 10.1002/mrd.20529
98. Ishii, K, Kanatsu-Shinohara, M, Toyokuni, S, and Shinohara, T. FGF2 mediates mouse spermatogonial stem cell self-renewal via upregulation of Etv5 and Bcl6b through MAP2K1 activation. Development. (2012) 139:1734–43. doi: 10.1242/dev.076539
99. Lee, KH, Lee, R, Lee, WY, Kim, DH, Chung, HJ, Kim, JH, et al. Identification and in vitro derivation of spermatogonia in beagle testis. PLoS One. (2014) 9:e109963. doi: 10.1371/journal.pone.0109963
100. Cai, H, Jiang, Y, Zhang, S, Cai, NN, Zhu, WQ, Yang, R, et al. Culture bovine prospermatogonia with 2i medium. Andrologia. (2021) 53:e14056. doi: 10.1111/and.14056
101. de Souza, AF, Pieri, NCG, and Martins, DDS. Step by step about germ cells development in canine. Animals. (2021) 11:598. doi: 10.3390/ani11030598
102. Kim, Y, Turner, D, Nelson, J, Dobrinski, I, McEntee, M, and Travis, AJ. Production of donor-derived sperm after spermatogonial stem cell transplantation in the dog. Reproduction. (2008) 136:823–31. doi: 10.1530/REP-08-0226
103. de Souza, AF, Pieri, NCG, Roballo, KCS, Bressan, FF, Casals, JB, Ambrósio, CE, et al. Dynamics of male canine germ cell development. PLoS One. (2018) 13:e0193026. doi: 10.1371/journal.pone.0193026
104. Powell, RH, Galiguis, J, Biancardi, MN, Pope, CE, Leibo, SP, Wang, G, et al. Phenotypic and molecular characterization of domestic cat (Felis catus) Spermatogonial stem cells. Biol Reprod. (2016) 95:20. doi: 10.1095/biolreprod.115.134635
105. Zhao, X, Wan, W, Zhang, X, Wu, Z, and Yang, H. Spermatogonial stem cell transplantation in large animals. Animals. (2021) 11:918. doi: 10.3390/ani11040918
106. Pieri, NCG, Mançanares, ACF, de Souza, AF, Fernandes, H, Diaza, AMG, Bressan, FF, et al. Xenotransplantation of canine spermatogonial stem cells (cSSCs) regulated by FSH promotes spermatogenesis in infertile mice. Stem Cell Res Ther. (2019) 10:135. doi: 10.1186/s13287-019-1250-9
107. Cortez, J, Leiva, B, Torres, CG, Parraguez, VH, De Los Reyes, M, Carrasco, A, et al. Generation and characterization of bovine testicular organoids derived from primary somatic cell populations. Animals. (2022) 12:2283. doi: 10.3390/ani12172283
108. Tang, S, Jones, C, Mecca, R, Davies, J, Lane, S, and Coward, K. An in vitro three-dimensional (3D) testicular organoid culture system for efficient gonocyte maintenance and propagation using frozen/thawed neonatal bovine testicular tissues. Biomed Mater. (2024) 19:2709. doi: 10.1088/1748-605X/ad2709
109. De Kock, J, and Baert, Y. Decellularization of male reproductive tissue. Adv Exp Med Biol. (2021) 1345:161–4. doi: 10.1007/978-3-030-82735-9_14
110. Ashouri Movassagh, S, Ashouri Movassagh, S, Banitalebi Dehkordi, M, Pourmand, G, Gholami, K, Talebi, A, et al. Identification and differentiation of human spermatogonial cells on three-dimensional decellularized sheep testis. Acta Histochem. (2020) 122:151623. doi: 10.1016/j.acthis.2020.151623
111. Vermeulen, M, Del Vento, F, de Michele, F, Poels, J, and Wyns, C. Development of a Cytocompatible scaffold from pig immature testicular tissue allowing human Sertoli cell attachment, proliferation and functionality. Int J Mol Sci. (2018) 19:227. doi: 10.3390/ijms19010227
112. Khazaei, MR, Ami, Z, Khazaei, M, and Rezakhani, L. The decellularized calf testis: introducing suitable scaffolds for spermatogenesis studies. Int J Fertil Steril. (2023) 18:32–9. doi: 10.22074/ijfs.2023.1989173
113. Di Filippo, F, Brevini, TAL, Pennarossa, G, and Gandolfi, F. Generation of bovine decellularized testicular bio-scaffolds as a 3D platform for testis bioengineering. Front Bioeng Biotechnol. (2025) 12:1532107. doi: 10.3389/fbioe.2024.1532107
114. Akbarzadeh, A, Kianmanesh, M, Fendereski, K, Ebadi, M, Daryabari, SS, Masoomi, A, et al. Decellularised whole ovine testis as a potential bio-scaffold for tissue engineering. Reprod Fertil Dev. (2019) 31:1665–73. doi: 10.1071/RD19070
115. Vermeulen, M, Del Vento, F, Kanbar, M, Pyr Dit Ruys, S, Vertommen, D, Poels, J, et al. Generation of organized porcine testicular organoids in solubilized hydrogels from Decellularized extracellular matrix. Int J Mol Sci. (2019) 20:5476. doi: 10.3390/ijms20215476
Keywords: animals, freezing, in vitro spermatogenesis, stem cells, testicular tissue, vitrification
Citation: Vurchio V, Colombo M, Pasquariello R and Luvoni GC (2025) Cryopreservation and culture strategies for testicular tissue and cells in small and large animals. Front. Vet. Sci. 12:1638248. doi: 10.3389/fvets.2025.1638248
Edited by:
Stefano Romagnoli, University of Padua, ItalyReviewed by:
Gaffari Türk, Firat University, TürkiyeMustafa Yiğit Nizam, Dokuz Eylül University, Türkiye
Copyright © 2025 Vurchio, Colombo, Pasquariello and Luvoni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Martina Colombo, bWFydGluYS5jb2xvbWJvQHVuaW1pLml0
 Valeria Vurchio
Valeria Vurchio Martina Colombo
Martina Colombo Rolando Pasquariello
Rolando Pasquariello Gaia Cecilia Luvoni
Gaia Cecilia Luvoni