- 1Poultry Department, Faculty of Agriculture, Zagazig University, Zagazig, Egypt
- 2Department of Animal Nutrition and Clinical Nutrition, Faculty of Veterinary Medicine, New Valley University, New Valley, Egypt
- 3Department of Biology, College of Science, Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia
- 4Department of Biology, College of Science, King Khalid University, Abha, Saudi Arabia
- 5Department of Biological Sciences, Faculty of Science, King Abdulaziz University, Jeddah, Saudi Arabia
- 6Department of Pathology and Laboratory Diagnosis, College of Veterinary Medicine, Qassim University, Buraydah, Saudi Arabia
- 7Department of Biology, College of Science, United Arab Emirates University, Al Ain, United Arab Emirates
- 8Department of Agricultural Microbiology, Faculty of Agriculture, Zagazig University, Zagazig, Egypt
Introduction: Despite the widespread interest in using Bacillus spp. as a probiotic in poultry diets, no evidence has been found to support the use of Paenibacillus polymyxa in the diet of Japanese quails. This study examined the effects of supplementing growing Japanese quail with a mixture of Bacillus coagulans and P. polymyxa (Bc+Pp) on their growth performance, antioxidative activity, immunological status, digestive enzymes, caecal microbiota, and blood chemistry.
Methods: Two hundred 1-week-old meat-type quail chicks were divided into four groups at random; five pens, each containing ten birds. These birds were provided with a basic feed as a control group, or a feed diet treated with 0.5, 1.0, and 1.5 mg kg−1 of Bc+Pp mixture (1:1).
Results: According to the findings, the growing quail's growth performance was significantly (P < 0.05) enhanced by supplementing the Bc+Pp mixture. Body weight and body weight gain were boosted significantly (P = 0.0002, P = 0.0003) by Bc+Pp mixture supplementation at 5 weeks and 1–5 weeks. In contrast, feed consumption showed a non-significant difference (P = 0.8082) with the treatments within 1–5 weeks. Moreover, the feed conversion ratio was significantly (P < 0.05) boosted (P = 0.0137) with the supplementation of the Bc+Pp mixture. Furthermore, Bc+Pp mixture supplementation provided a significant boost in carcass traits, especially liver, gizzard, and giblet percentage (P = 0.0112, P = 0.0976, and P = 0.0028). The current result showed a significant (P < 0.05) increase in total protein, albumin, and globulin with supplementation of the Bc+Pp mixture. Moreover, the treatment significantly (P < 0.05) reduced total cholesterol, triglycerides, and low-density lipoprotein. Superoxide dismutase, total antioxidant capacity, reduced glutathione, and glutathione peroxidase were significantly (P < 0.05) improved by supplementation of the Bc+Pp mixture. Furthermore, the digestive enzymes were significantly (P < 0.05) improved, and the total bacterial and lactic acid bacteria counts were significantly (P < 0.05) augmented, whereas the counts of Salmonella spp., Escherichia coli, total coliform, and Enterococcus spp. were significantly (P < 0.05) decreased with dietary bacterial mixture treatments.
Discussion: In conclusion, supplementing growing Japanese quail with a mixture of Bc+Pp has a positive impact on their growth performance, antioxidative status, immunological response, digestive enzymes, and caecal microbiota.
Introduction
Probiotics have emerged as promising alternatives to antibiotic growth promoters in poultry, addressing concerns about antibiotic residues and antimicrobial resistance (1–5). This shift supports safer and sustainable poultry production (6, 7). One hopeful substitute for improving growth performance and producing safe products simultaneously is the use of probiotic bacteria (7–9). Probiotics are recognized as living microorganisms that boost healthy gut microbiota, improve gut barrier function, stimulate the secretion of digestive enzymes, increase nutritional absorption, and enhance the performance of broilers and quails (10, 11).
Moreover, probiotic administration improved feed consumption and growth rate in Japanese quail (12, 13). A further study indicated that probiotic administration lowered triglyceride and cholesterol levels, hence improving the serum lipid profile of broilers (14). By generating antibacterial compounds, probiotics also lessen harmful bacteria by competitive exclusion (15, 16). As a probiotic, Bacillus spp. has advantages over other types, such as the capacity to withstand low pH in the intestine and high temperatures during the pelleting process (17).
Additionally, Bacillus spp. has demonstrated a positive impact on improving gut function via multiple processes, including the synthesis of antimicrobial substances, activating the intestinal immune system, and diminishing harmful microorganisms by competitive exclusion (18, 19). Furthermore, poultry's immune response and antioxidative state were enhanced by dietary Bacillus species (20).
Commercial probiotics in poultry feed are frequently derived from Gram-positive, spore-forming bacteria, Bacillus coagulans, and Bacillus licheniformis (21). Due to their protective protein coating and additional characteristics, they can endure environmental threats during the pelletization process, packaging, and treatment (21), as well as withstand gastric acidity, subsequently reaching the intestine, where they germinate and proliferate without generating enterotoxins (22).
B. coagulans is a homofermentative type that efficiently utilizes sugars and has lately been regarded as an innovative and safer probiotic. B. coagulans generates L-lactic acid as the predominant derivative of sugar fermentation, constituting around 97% of the fermented ingredients, whereas acetic acid and succinic acid are produced as small byproducts (22). B. coagulans preserves the intestinal mucus membrane barrier by boosting gut microbiota, facilitating the renewal of broiler intestinal mucosa, and bolstering congenital immunity (23).
Because of its huge biotechnology potential in sustainable agriculture and several industrial processes, Paenibacillus polymyxa (formerly Bacillus polymyxa) has garnered significant attention (24, 25) due to its production of two types of antimicrobial peptides [ribosomally-synthesized bacteriocins (Lantibiotics and pediocins), and non-ribosomally synthesized peptides (Cyclic cationic lipopeptides or cyclic non-cationic lipopeptides)] (25). Due to the beneficial effects of P. polymyxa, it could improve growth performance and body health status of quail chicks through improving immune response, antioxidative status, increasing beneficial bacterial count, and reducing pathogenic bacterial count (26).
No evidence exists concerning the benefits of P. polymyxa in the diet of Japanese quails, despite the considerable interest in Bacillus spp. as a probiotic in poultry nutrition. Previous studies examined the effects of B. coagulans or P. polymyxa individually as dietary supplements in poultry diets; however, no research has been conducted on the combined usage of these bacteria. It is hypothesized that the dietary addition of B. coagulans and P. polymyxa (Bc+Pp) mixture is expected to exert beneficial effects on the growing quails. Therefore, this study aimed to assess the impact of dietary supplementation with a combination of Bc+Pp on growth performance, carcass characteristics, liver and kidney function, antioxidative capacity, caecal microbiota, digestive enzyme activities, and immune responses in growing quails.
Materials and methods
The present study adhered to the guidelines provided by the Local Experimental Animal Care Committee.
Isolation, screening, and identification of bacterial isolates
The current research aimed to use and identify safe Bacillus isolates exhibiting antioxidant and antibacterial characteristics. This investigation involved the collection of isolates from fresh chicken feces obtained from poultry farm cages.
The fecal samples were transferred to sterile containers and conveyed to the microbiology laboratory within 24 h of collection. A 10-g fecal sample was homogenized in 90 ml of peptone buffer to achieve a 10−1 dilution, followed by the preparation of successive dilutions up to 10−7. After each dilution, the samples were inoculated into Luria-Bertani medium (LB, Lab M Limited, Lancashire, UK) and incubated at 37°C for 24 h. The most promising isolates were chosen due to their robust antibacterial efficacy against Staphylococcus aureus, Salmonella typhi, and Pseudomonas aeruginosa (27).
Among the tested isolates, 20 exhibited inhibition zones ranging from 20 to 30 mm. B. coagulans BcMT15 had the most substantial inhibitory zones, measuring 30 mm against S. aureus, 28 mm against S. typhi, and 26 mm against P. aeruginosa. Consequent to these findings, B. coagulans BcMT15 was chosen for subsequent use in this study.
The preliminary identification of B. coagulans BcMT15 was conducted utilizing established methodologies, including Gram staining, spore production, culture characteristics, pigmentation, and an array of biochemical and physiological assays, as detailed by Sneath (28). Matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) was employed to validate the identification (29). B. coagulans BcMT15 isolate exhibited 99% similarity to B. coagulans DSM 32016.
The acid resistance test was conducted to demonstrate the probiotic capabilities of B. coagulans BcMT15, utilizing a spectrophotometer to measure the optical density of each sample at 650 nm hourly in triplicate (30). The bile salt tolerance test was conducted (30). One ml bacterial culture was inoculated into 9 ml of LB broth at pH 2.5 and incubated at 37°C for 3 h. Following the incubation, a spectrophotometer was employed to assess the optical density of each sample at 650 nm. The absorbance level (A650) has been calibrated to 0.08 ± 0.05 to standardize bacterial counts.
Every acid-tolerant isolate underwent screening for bile tolerance. One hundred μl of overnight-cultivated bacterial culture was inoculated into newly produced LB broth containing 0.3% bile salts (Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany). The vitality of bacteria in 0.3% bile was assessed by inoculating 100 μl of the bacterial sample onto the LB agar plate at time intervals of 0, 1, 2, 3, and 4 h. Plates devoid of bacterial colonies were classified as negative, while those containing colonies were classified as positive.
The acid tolerance and the survival rate were calculated using the following equation:
B. coagulans BcMT15 showed a higher survival rate of 85.2 ± 3.1 at low pH and 78.6 ± 2.8 at 0.3% bile salt. The safety of the chosen bacterial isolates was assessed by evaluating their potential for antibiotic resistance and hemolytic activity. To assess hemolytic activity, each isolate was cultivated on blood agar plates and incubated for 48 h at 42°C. Isolates were cultured on nutrient agar medium (Lab M Limited) at a final concentration of 106 colony-forming units (CFU) g−1 for antibiotic sensitivity testing. Standard antibiotic disks, including tetracycline, azithromycin, erythromycin, ceftriaxone, and gentamicin, were subsequently positioned on the medium. Results were documented during a 48 h incubation of the plates at 42°C.
The antioxidant and DNA-protective properties of the bacterial broth were assessed utilizing bacterial biosensors. The biosensor system employed Escherichia coli MG 1655 pRecA-lux (31), which incorporates luminescence genes regulated by a stress-inducible promoter and exhibits sensitivity to DNA damage. Following these evaluations, B. coagulans BcMT15 was identified as a promising probiotic strain for feed supplementation (32).
The safety assessment concentrated on hemolytic activity, mutagenicity (utilizing lux biosensors), and antibiotic resistance. The results indicated that none of the isolates had pro-mutagenic, hemolytic, or antibiotic-resistant characteristics. Of the 20 isolates evaluated, B. coagulans BcMT15 was selected for further investigation due to its superior combination of antimutagenic and antioxidant properties.
Paenibacillus isolates were obtained from fresh chicken fecal samples taken from a poultry farm, stored in sterile containers, and processed in the laboratory within 24 h. A 10-g fecal sample was homogenized in 90 ml of peptone buffer, then undergoing repeated dilutions from 10−1 to 10−7. Each dilution was inoculated into LB medium and incubated at 37°C for 24 h. Fifteen isolates were chosen for their significant antibacterial efficacy against S. aureus, S. typhi, and P. aeruginosa. Screening indicated that all 15 isolates produced inhibition zones of between 25 and 36 mm. P. polymyxa PpMT37 demonstrated the most extensive inhibitory zones, measuring 36 mm against S. aureus, 29 mm against S. typhi, and 32 mm against P. aeruginosa, and was chosen for further investigation as the most promising isolate.
The identification of P. polymyxa PpMT37 was conducted through standard methodologies, encompassing Gram staining, spore morphology, evaluation of cultural characteristics, pigment production, and an extensive array of biochemical and physiological tests, as outlined by Sneath (28). This identification was additionally corroborated using MALDI-TOF MS, in accordance with the protocols established by Bille et al. (29). The isolate P. polymyxa PpMT37 exhibited 99% similarity to P. polymyxa DSM 365.
The acid resistance of P. polymyxa PpMT37 isolate was assessed by inoculating cultures into LB broth (pH 2.5), incubating at 37°C for 3 h, and periodically measuring optical density at 650 nm. Bile salt tolerance was evaluated by inoculating overnight cultures into newly produced LB broth with 0.3% bile salts, followed by plating samples onto LB agar at different time intervals to ascertain viability (30). P. polymyxa PpMT37 exhibited a survival rate of 82.4 ± 2.9 at low pH and 76.3 ± 2.7 in the presence of 0.3% bile salt.
For safety evaluation, each isolate underwent hemolytic activity testing by culturing on blood agar at 42°C for 48 h, and antibiotic resistance assessment utilizing standard antibiotic disks (tetracycline, azithromycin, erythromycin, ceftriaxone, and gentamicin) on nutrient agar medium (Lab M Limited) at 108 CFU g−1, with results documented after 48 h. The antioxidant and DNA-protective properties were assessed utilizing E. coli MG 1655 pRecA-lux biosensors, which react to DNA-damaging chemicals.
Of the 15 isolates assessed, P. polymyxa PpMT37 exhibited the most significant antimutagenic and antioxidant properties. All 15 Paenibacillus isolates showed an absence of pro-mutagenic, hemolytic, or antibiotic-resistant characteristics, while a few exhibited mild prooxidant activity. Biosensor tests were essential in finding isolates with enhanced antioxidant and antimutagenic properties, resulting in the selection of P. polymyxa PpMT37 for future investigation because of its exceptional bioactive profile.
Antimicrobial activity of B. coagulans BcMT15 and P. polymyxa PpMT37
To evaluate the antibacterial activity of B. coagulans BcMT15 and P. polymyxa PpMT37 (1.5 × 108 CFU ml−1) against different pathogenic Gram-positive and negative bacteria, four concentrations of 10%, 20%, 40%, and 80% were prepared. For each concentration, 8 mm disks were soaked for 30 min. The effectiveness of these disks was tested against several pathogenic bacteria that commonly infect poultry: S. aureus, Streptococcus pyogenes, Listeria monocytogenes, S. typhi, E. coli, and Klebsiella pneumoniae.
After the plates were inoculated with the bacteria, the saturated disks were placed on top. The plates were then incubated, and the resulting inhibition zones were measured in millimeters (33, 34).
Experimental design
Two hundred growing quails, averaging 27.95 ± 0.37 g at 1 week of age, were randomly divided into four equal groups, each including five replications of ten quails. This study continued for 5 weeks and dietary treatment groups were as follows: first group: control (basal diet without bacterial supplementation); second group (0.5 mg Bc+Pp; basal diet + 0.25 mg B. coagulans + 0.25 mg P. polymyxa kg diet−1); third group (1.0 mg Bc+Pp; basal diet + 0.5 mg B. coagulans + 0.5 mg P. polymyxa kg diet−1); and the fourth group (1.5 g Bc+Pp; basal diet + 0.75 mg B. coagulans + 0.75 mg P. polymyxa kg diet−1) and the concentrations of B. coagulans and P. polymyxa were 1.0 × 106 CFU g−1 and 1.5 × 108 CFU ml−1, respectively.
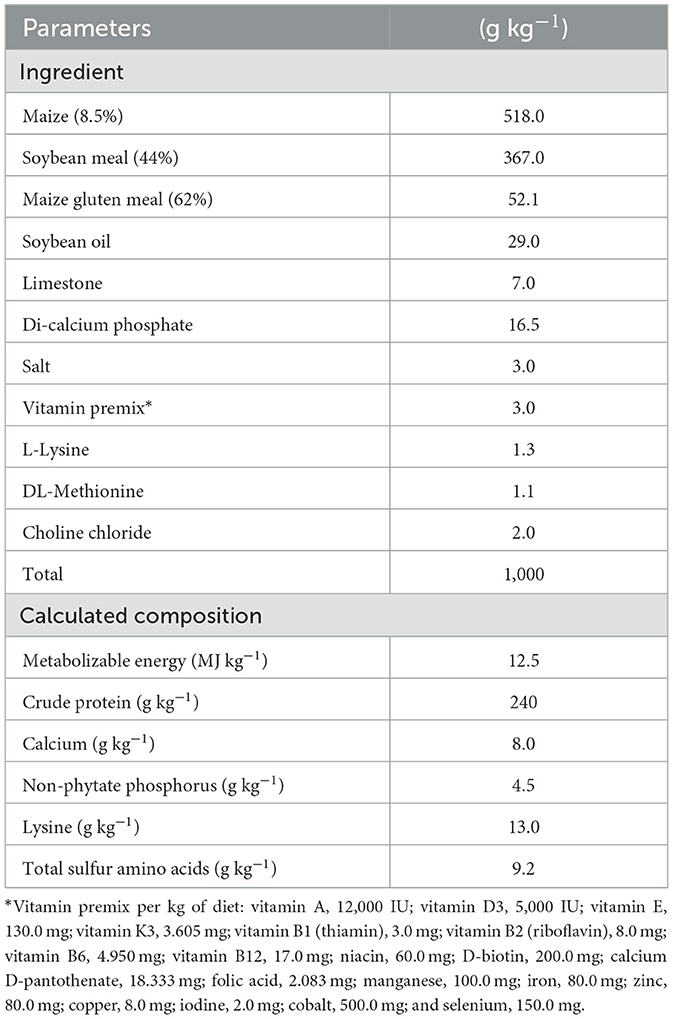
The standards outlined in (35) were utilized to design the basal diet to meet the requirements of quail throughout the quail phase (Table 1). A typical cage of 50 × 30 × 50 cm3 was employed for the rearing of quails. Water and nourishment were provided ad libitum.
Determination of growth performance and carcass characteristics
Quail weights were recorded at 1, 3, and 5 weeks of age to calculate body weight and body weight gain. During the trial, feed intake and feed conversion ratio were calculated. At week 5, six birds from each treatment group were randomly selected and euthanized for carcass analysis.
Assessment of blood biochemistry, digestive enzyme activity, immunological markers, and antioxidant activities in quails
Blood samples were obtained from the slaughtered birds directly into a tube containing the anticoagulant ethylene diamine tetraacetic acid (EDTA, Sigma-Aldrich). The serum was obtained using centrifugation of the blood sample at 4,000 rpm for 10 min, as recommended by Zhou et al. (36). The serum was transferred into a sterile tube, which was thereafter sealed and kept at −20°C until utilized.
The biochemical parameters of alanine aminotransferase, aspartate transaminase, lactate dehydrogenase, urea, creatinine, total cholesterol, high-density lipoprotein, low-density lipoprotein, and very low-density lipoprotein were quantified utilizing an automated analyzer and a Biodiagnostic commercial kit (Biodiagnostic, Giza, Egypt) in accordance with the manufacturer's guidelines (36). Creatinine, uric acid, and urea levels were measured using commercially available Quimica Clinica Aplicada kits (Quimica Clinica Aplicada, Tarragona, Spain).
Total protein was quantified with the Biuret method as described by Armstrong and Carr (37). Albumin concentrations were determined using a calorimetric technique (38). Globulin concentrations were determined by deducting albumin concentrations from total protein concentrations. Amylase activity was determined by the Somogyi method (39), and the technique recommended by Tietz and Fiereck (40) was used for the lipase enzyme. The methods described by Lynn and Clevette-Radford (41) were used to measure protease activity. Immunoglobulins were evaluated using enzyme-linked immunosorbent assay (ELISA) as described by Gao et al. (42).
Assessment of caecal microbial counts
To obtain the caecal content, three birds from each replication were slaughtered. Upon separating the caeca, the contents were meticulously collected in sterile cups to guarantee aseptic handling. The samples were preserved at 4°C until the quantification of the microbial population. A dilution factor of 10−6 was attained by a tenfold successive dilution of caecal material. Aliquots of 0.2 ml were dispensed using a sterile glass rod in sterile plastic Petri plates with a 90 mm diameter over different general-purpose and selective agar medium.
The selected organisms for enumeration and the corresponding media utilized were as follows: (i) total aerobic bacteria on nutrient agar medium (Lab M Limited, Product Code: LAB008); (ii) total yeasts and molds on Sabouraud dextrose agar (HiMedia Laboratories Pvt. Ltd., Mumbai, India, Product Code: MH063); (iii) E. coli on eosin methylene blue agar (Lab M Limited, Product Code: LAB061); (iv) total coliforms on MacConkey agar medium (Lab M Limited, Product Code: LAB045); (v) Salmonella spp. on xylose lysine decarboxylase agar (XLD agar; Lab M Limited, Product Code: LAB032); Enterococcus spp. on Enterococcus agar (HiMedia Laboratories, Product Code: MH2077); and (vi) lactic acid bacteria on MRS agar (Lab M Limited, Product Code: LAB223).
Following 20 min of drying in a laminar flow cabinet, the plates were incubated at 30°C in darkness for 3 days to enumerate total aerobic bacteria, total coliforms, E. coli, Salmonella spp., Enterococcus, and lactic acid bacteria. The plates were incubated in the dark at 28°C for 6 days to enumerate total yeasts and molds.
Six plates were made for each dilution for every sample and replication. Population densities were assessed by quantifying CFU per gram of dry caecal weight and thereafter expressing the results as log10 CFU (43, 44).
Statistical analysis
In accordance with Steel and Torrie (45), all microbiological tests were conducted in triplicate. Statistical analysis was performed using one-way analysis of variance (ANOVA). All statistical analyses were carried out using IBM SPSS 23 Statistics for Mac OS (Armonk, NY, USA). The least significant difference (LSD) test was used to compare all tested means (treatments) with a probability of P < 0.05.
The statistical model applied was: Yij = μ + Ti + eij where: Yij = an observation; μ = overall mean; Ti = the fixed effect of probiotic treatments; and eij = Random error. The pen served as the experimental unit for growth performance and carcass features, whereas the individual bird was designated as the experimental unit for biochemical, microbiological, enzymatic, and immunological parameters.
Results
Isolation, screening, and identification of bacterial isolates
Of the 20 isolates evaluated, B. coagulans BcMT15 was selected for further investigation due to its superior combination of antibacterial, antimutagenic, and antioxidant properties (Supplementary Tables S1–S4). Furthermore, out of the 15 isolates assessed, P. polymyxa PpMT37 exhibited the most significant antibacterial, antimutagenic, and antioxidant properties and was selected for further investigation (Supplementary Tables S5–S8).
Antibacterial activity of B. coagulans BcMT15 and P. polymyxa PpMT37
Table 2 provides a comparative assessment of the antibacterial efficacy of B. coagulans BcMT15 and P. polymyxa PpMT37 against six pathogenic bacterial strains. P. polymyxa PpMT37 consistently showed superior antibacterial efficacy compared to B. coagulans BcMT15 against all evaluated pathogens (Supplementary Figure S1).
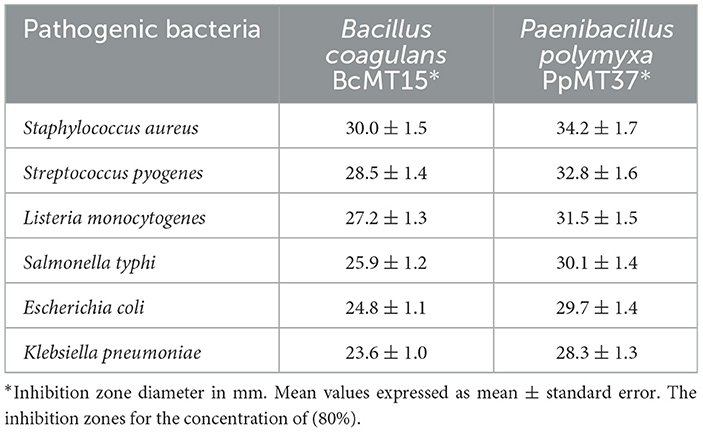
Table 2. Antibacterial activity of Bacillus coagulans BcMT15 and Paenibacillus polymyxa PpMT37 against pathogenic Gram-positive and Gram-negative bacteria.
B. coagulans BcMT15 (80%) had reduced, but nevertheless, notable, action, demonstrating the most substantial inhibition zone against S. aureus (30.0 ± 1.5 mm) and the least against K. pneumoniae (23.6 ± 1.0 mm; Table 2 and Supplementary Figure S1). In contrast, the most significant inhibition zone for P. polymyxa PpMT37 (80%) was recorded against S. aureus (34.2 ± 1.7 mm), followed by S. pyogenes (32.8 ± 1.6 mm), L. monocytogenes (31.5 ± 1.5 mm), S. typhi (30.1 ± 1.4 mm), E. coli (29.7 ± 1.4 mm), and K. pneumoniae (28.3 ± 1.3 mm; Table 2 and Supplementary Figure S1).
These findings indicated that P. polymyxa PpMT37 was more efficacious in suppressing the development of these pathogenic bacteria compared to B. coagulans BcMT15, as seen by the larger inhibition zones (Supplementary Figure S1). The uniformity of the results, indicated by the minimal standard deviations, emphasizes the dependability of the findings. This comparative study underscores the potential of P. polymyxa PpMT37 as a viable candidate for further investigation.
Effect of B. coagulans and P. polymyxa on the productive performance of Japanese quails
Table 3 illustrates the effects of a dietary Bc+Pp combination on the productive performance of meat-type quail. The results indicated a significant (P < 0.05) improvement in body weight throughout the trial duration, particularly at 3 and 5 weeks. The fourth group, administered 1.5 mg kg−1 of the bacterial combination, exhibited a significant (P < 0.05) rise in body weight (103.83 g) at the 3-week mark (P = 0.0077). The third group administered 1.0 mg kg−1 of bacterial combination had a significant (P < 0.05) increase (P = 0.0002) in body weight (230.38 g) at 5 weeks of age (Table 3).
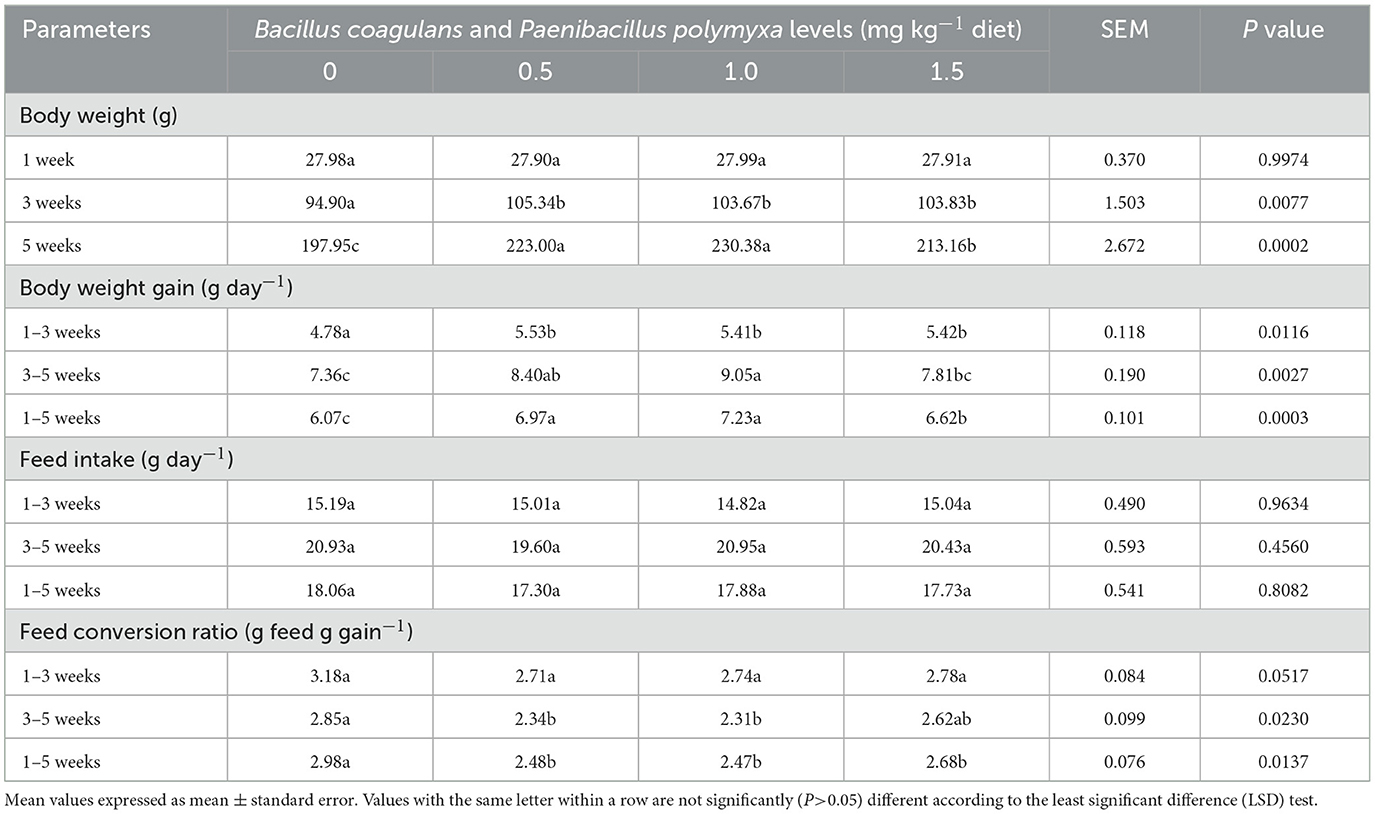
Table 3. Effect of dietary Bacillus coagulans and Paenibacillus polymyxa mixture on productive performance of growing quail.
Moreover, the bacterial mixture supplementation significantly (P < 0.05) enhanced (P = 0.0003) body weight gain throughout the trial period (weeks 1–5), with the third group (1.0 mg kg−1 Bc+Pp) demonstrating a superior body weight gain of 7.23 g from weeks 1 to 5 compared to the other groups and the control (Table 3). Furthermore, the bacterial combination treatment did not significantly (P>0.05) influence feed consumption during the study periods (weeks 1–5). The feed conversion ratio was significantly (P < 0.05) influenced by treatments throughout the trial duration, with the third group (1.0 mg kg−1 Bc+Pp) demonstrating superior values (P = 0.0230, P = 0.0137) during weeks 3–5 and 1–5, recording values of 2.31 and 2.47, respectively (Table 3).
Effect of B. coagulans and P. polymyxa on the carcass traits of Japanese quails
Table 4 displays the results of the interaction between the dietary Bc+Pp combination and the carcass characteristics of meat-type quails. The findings showed that there was no significant difference in the percentage of the carcass, gizzard, and heart (P = 0.2130, P = 0.0976, P = 0.3816) among all treatments (Table 4). Furthermore, the treatment with the bacterial combination for developing quail showed a significant (P < 0.05) rise in the percentage of the liver, giblets, and dressing (P = 0.0112, P = 0.0028, P = 0.0685). Additionally, the second group that was supplemented with a meal containing 0.5 mg kg−1 of the bacterial mixture showed an increased liver. As a result, the third group (1.0 mg kg−1 Bc+Pp) demonstrated an increase in the percentage of giblets and dressing (Table 4).
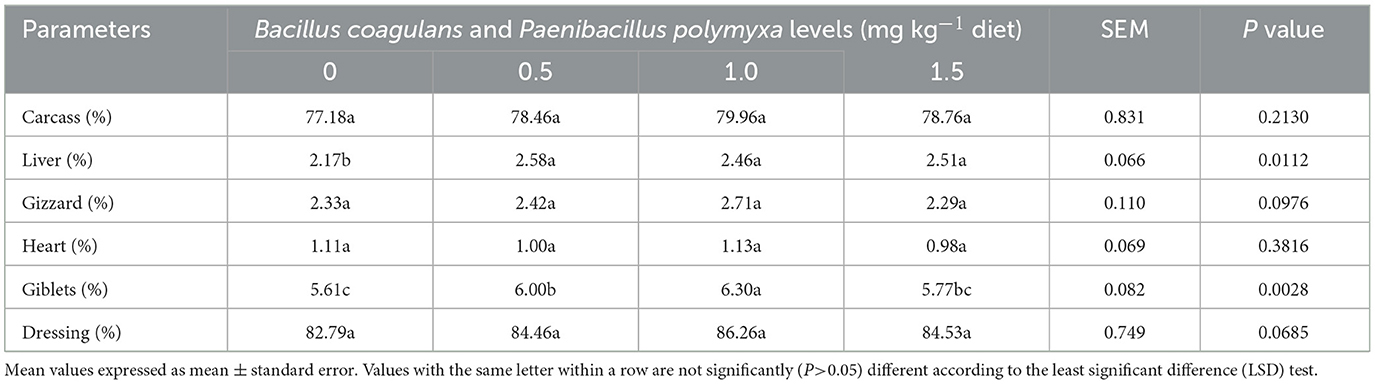
Table 4. Effect of dietary Bacillus coagulans and Paenibacillus polymyxa mixture on carcass traits and relative organ weights of growing quail.
Effect of B. coagulans and P. polymyxa on biochemical parameters of Japanese quails
Table 5 illustrates the impact of a dietary combination of B. coagulans and P. polymyxa on the hepatic and renal functions of developing quail. The results indicated a significant (P < 0.05) rise in total protein, albumin, and globulin (P = 0.0027, P = 0.0283, and P = 0.0002) following bacterial combination treatments, with the third group (1.0 mg kg−1 Bc+Pp) exhibiting elevated values (4.39, 2.18, 2.22 g dl−1; Table 5).
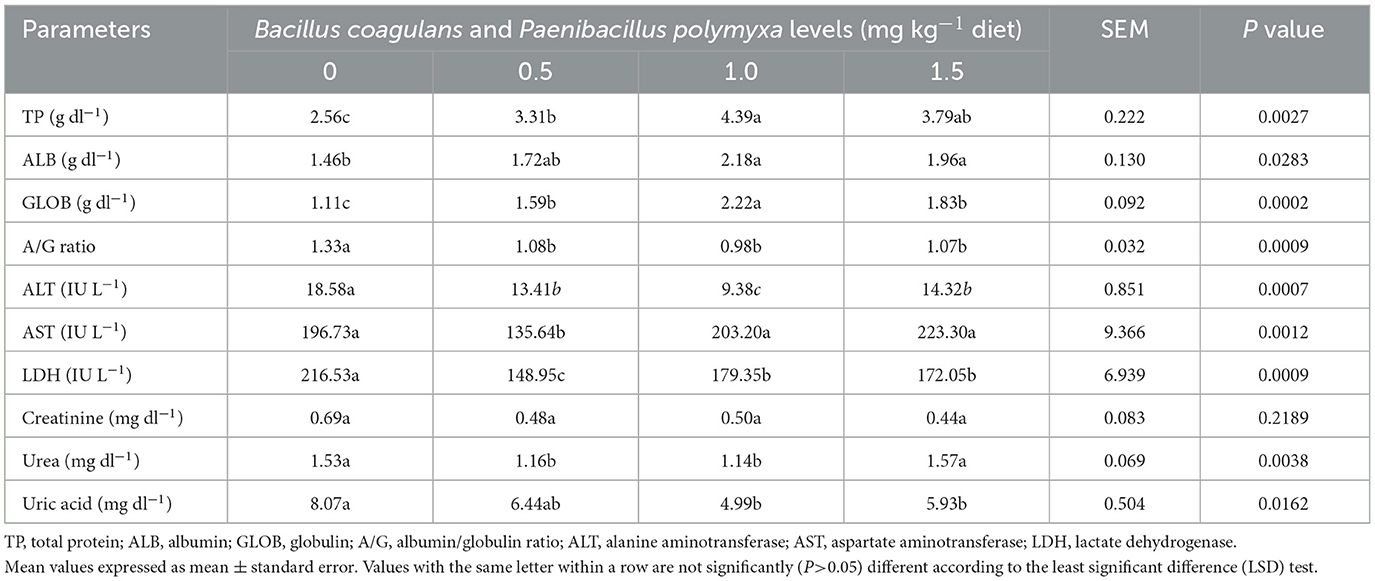
Table 5. Effect of dietary Bacillus coagulans and Paenibacillus polymyxa mixture on liver and kidney functions of growing quail.
The findings indicated a significant (P < 0.05) reduction in liver alanine aminotransferase, aspartate transaminase, and lactate dehydrogenase following bacterial combination treatments (P = 0.0007, P = 0.0012, and P = 0.0009), with the third group (1.0 mg kg−1 Bc+Pp) exhibiting decreased alanine aminotransferase levels (9.38 IU L−1; Table 5). Conversely, the second group (0.5 mg kg−1 Bc+Pp) had reduced aspartate transaminase and lactate dehydrogenase levels (135.64, 148.95) IU L−1 (Table 5).
The results also indicated a non-significant reduction in creatinine levels (P = 0.2189) following bacterial combination therapy, with the fourth group exhibiting decreased levels of 0.44 mg dl−1. The findings indicated a significant (P < 0.05) reduction in urea and uric acid levels (P = 0.0038, P = 0.0162), with the third group (1.0 mg kg−1 Bc+Pp) exhibiting decreased concentrations of 1.14 and 4.99 mg dl−1, respectively (Table 5).
Effect of B. coagulans and P. polymyxa on the lipid profile of Japanese quails
Table 6 presents the effects of the dietary Bc+Pp combination on the lipid profile of developing quail. The results indicated a significant (P < 0.05) decrease in total cholesterol, triglycerides, low-density lipoprotein, and very low-density lipoprotein (P < 0.0001, P = 0.0003, P < 0.0001, and P = 0.0003), with the second group treated with 0.5 mg kg−1 of the bacterial mixture exhibiting reduced levels of 184.35, 138.00, 92.53, and 27.60 mg dl−1, respectively (Table 6). The results also demonstrated that the third group (1.0 mg kg−1) significantly (P < 0.05) elevated high-density lipoprotein levels (68.10 mg dl−1) with bacterial mixture supplementation (Table 6).

Table 6. Effect of dietary Bacillus coagulans and Paenibacillus polymyxa mixture on lipid profile of growing quail.
Effect of B. coagulans and P. polymyxa on antioxidants and immunity status of Japanese quails
Table 7 illustrates the impact of a dietary combination of B. coagulans and P. polymyxa on the immunity and antioxidant levels of developing quail. The findings demonstrated a significant (P < 0.05) enhancement in antioxidant status following bacterial mixture supplementation, with the third group (1.0 mg kg−1 Bc+Pp) exhibiting significant (P < 0.05) increases in superoxide dismutase, reduced glutathione, and glutathione peroxidase levels (P = 0.0003, P = 0.0002, P = 0.0021; 0.41 U ml−1, 0.35 ng ml−1, 0.54 −1, respectively; Table 7).
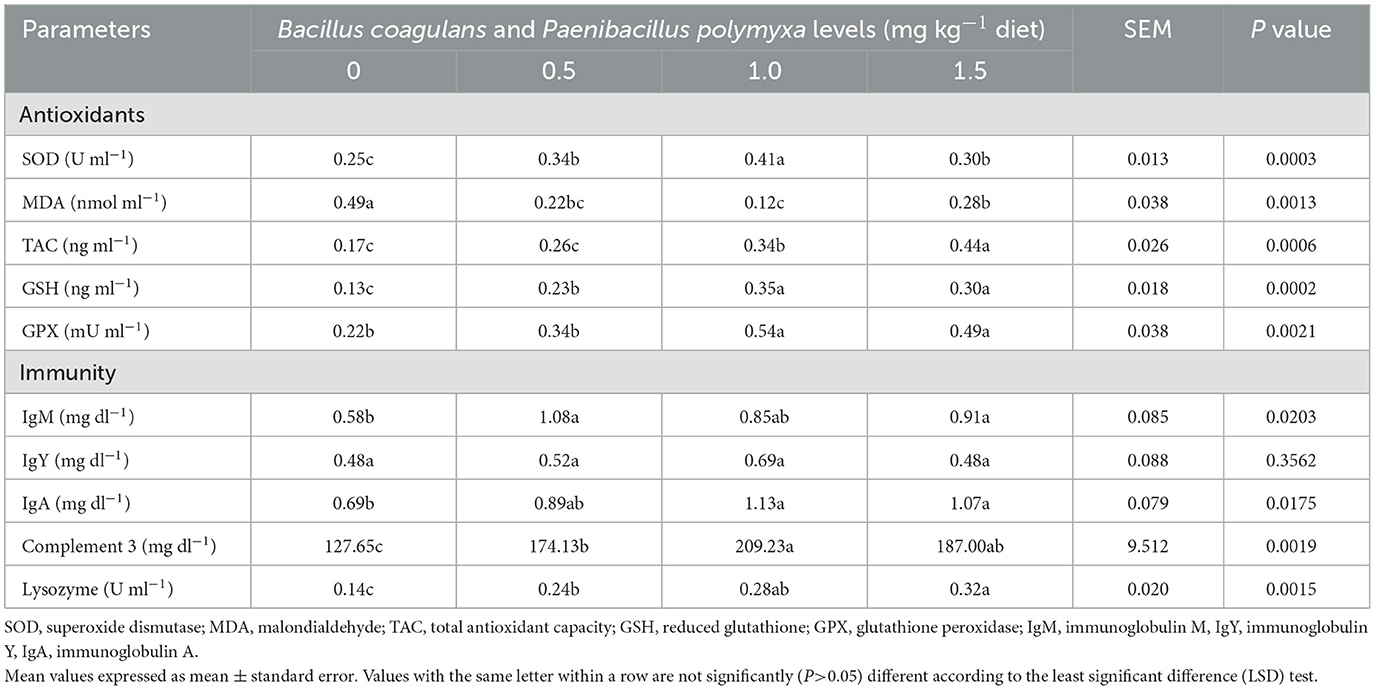
Table 7. Effect of dietary Bacillus coagulans and Paenibacillus polymyxa mixture on immunity and antioxidants of growing quail.
Moreover, the results indicated that the third group (1.0 mg kg−1 Bc+Pp) exhibited a significant (P < 0.05) reduction in malondialdehyde levels (0.12 nmol ml−1, P = 0.0013), while the fourth group (1.5 mg kg−1 Bc+Pp) had a significant (P < 0.05) rise in total antioxidant capacity concentration (0.44 ng ml−1, P = 0.0006; Table 7).
The findings also demonstrated a significant (P < 0.05) rise in IgM and IgA (P = 0.0203, and P = 0.0175), with the second group (0.5 mg kg−1 Bc+Pp) exhibiting a heightened IgM concentration (1.08 mg dl−1) and the third group (1.0 mg kg−1 Bc+Pp) showing elevated IgA levels (1.13 mg dl−1; Table 7). Furthermore, the introduction of a bacterial combination to the food of developing quail resulted in a non-significant elevation in IgY levels (P = 0.3562), with the third group exhibiting elevated levels (1.13 mg dl−1; Table 7).
Moreover, the introduction of a bacterial combination to the growth diet markedly enhanced levels of complement 3 and lysozymes (P = 0.0019, P = 0.0015). The third group (1.0 mg kg−1 Bc+Pp) exhibited elevated complement 3 levels (209.23 μ ml−1), while the fourth group (1.5 mg kg−1 Bc+Pp) had an increased lysozyme level (0.32 μ ml−1; Table 7).
Effect of B. coagulans and P. polymyxa on the status of digestive enzymes of Japanese quails
The impact of a dietary combination of B. coagulans and P. polymyxa on the digestive enzymes of developing quail is presented in Table 8. The results indicated a significant (P < 0.05) increase in amylase, lipase, and protease enzyme levels (P = 0.0005, P = 0.0013, and P = 0.0006) due to bacterial mixture supplementation, with the fourth group (1.5 mg kg−1 Bc+Pp) exhibiting elevated amylase levels (16.22 U L−1). The third group (1.0 mg kg−1 Bc+Pp) had elevated levels of lipase and protease enzymes (12.84, 1.56 U L−1) compared to the control and other groups (Table 8).
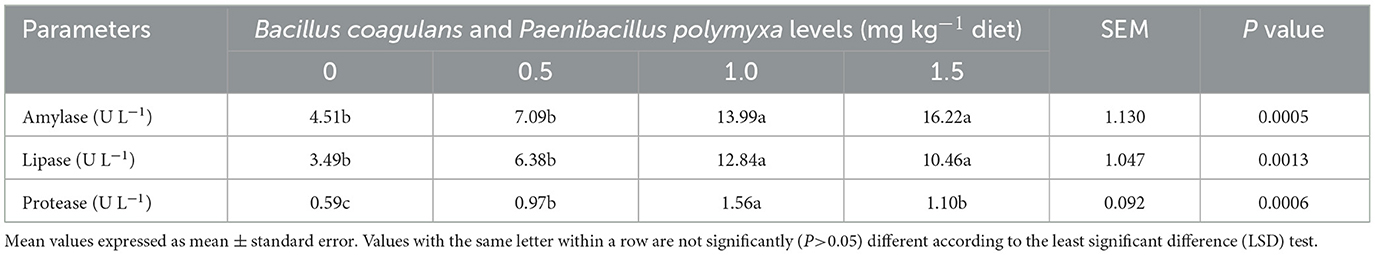
Table 8. Effect of dietary Bacillus coagulans and Paenibacillus polymyxa mixture on digestive enzymes of growing quail.
Effect of B. coagulans and P. polymyxa on caecal bacterial count of Japanese quails
The effect of a dietary combination of B. coagulans and P. polymyxa on the caecal bacterial count (Log10 CFU g−1) in developing quail is presented in Table 9. The results demonstrated a significant (P < 0.05) rise (P = 0.0002) in total bacterial count due to bacterial mixture supplementation, with the third group (1.0 mg kg−1 Bc+Pp) exhibiting elevated levels (Table 9). Furthermore, the supplementation of bacterial mixture significantly (P < 0.05) reduced the overall counts of yeasts and molds, as well as E. coli, total coliforms, Salmonella spp., and Enterococcus spp. Conversely, there was a significant (P < 0.05) difference in the counts of lactic acid bacteria, with the third group (1.0 mg kg−1 Bc+Pp) exhibiting enhanced outcomes (Table 9).
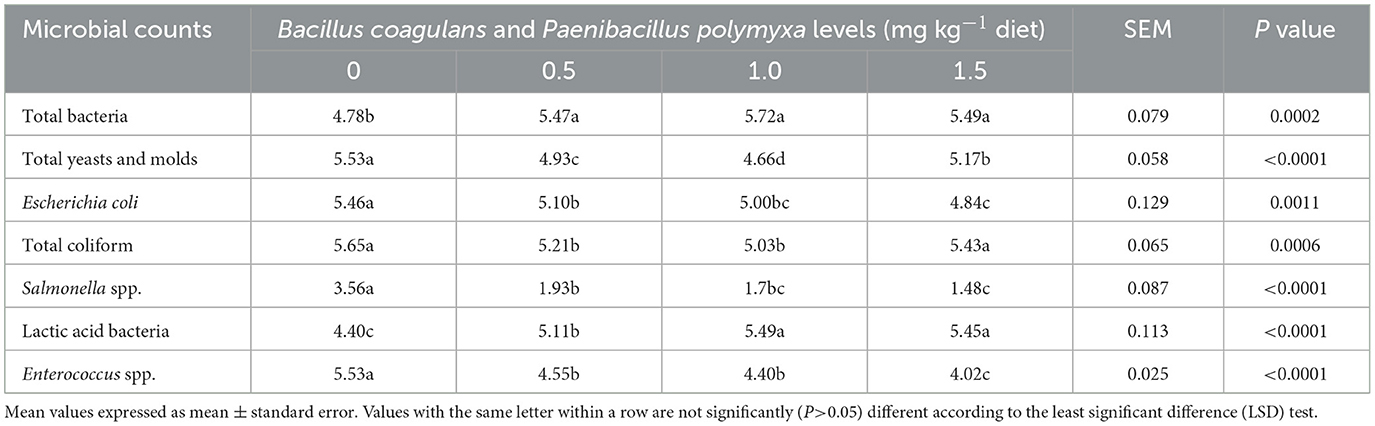
Table 9. Effect of dietary Bacillus coagulans and Paenibacillus polymyxa mixture on caecal bacterial count (Log10 CFU g−1) of growing quail.
Discussion
Growth performance of meat-type quail
Bacillus strains enhance the productive performance of hens (46). Nevertheless, information regarding the advantageous effects of P. polymyxa on poultry is limited (9). Furthermore, no research exists concerning the influence of the Bc+Pp on Japanese quail. Consequently, our research sought to examine the advantageous impact of the Bc+Pp on the diets of meat-type quails. Wu et al. (9) previously investigated the advantageous effects of P. polymyxa on broiler chickens, revealing that those fed diets supplemented with P. polymyxa showed significant (P < 0.05) improvements in overall health by enhancing gut barrier function, decreasing cell apoptosis, and increasing antioxidative capacity (9). In the same context, economic efficiency of broiler chickens was improved by using P. polymyxa in their diet, which also improved their growth rate, carcass dressing and the composition of the gut microbiota (47). Similarly, P. polymyxa 10 slightly improved the growth performance of broiler chickens during the starter phase (48).
Probiotics made from the bacteria Paenibacillus xylanexedens ysm1 have recently been shown to increase broiler chickens productivity (8, 49). The addition of Bacillus subtilis spores to the diet of Japanese quail reduced their feed conversion ratio and increased their body weight and body weight gain (50). Additionally, quail health and development performance were also improved by these probiotics (11).
Moreover, chickens receiving B. coagulans diets showed a significant (P < 0.05) increase in body weight, body weight gain to feed intake ratio from day 15 to 21 (51). The incorporation of a dietary blend of B. coagulans and P. polymyxa improved body weight and body weight gain in meat-type quail. The highest body weight and body weight gain were seen in the 1.0 mg kg−1 dietary group. Moreover, the administration of a B. coagulans and P. polymyxa combination enhanced the feed conversion ratio, with the most effective dosage being 1 mg kg−1 of feed. These results correspond with the findings of Seifi et al. (52). Their results showed that administering single-dose probiotics improves quails' body weight increase and feed intake. Gupta et al. (53), found that dietary interventions with probiotics improved the performance of Japanese quail. The use of probiotics improves growth performance by increasing beneficial gut flora. Enhancing gut barrier function enhances the release of digestive enzymes, hence augmenting nutrient absorption and facilitating body weight and body weight gain (12, 54).
Additionally, Flores et al. (55) demonstrated that broilers administered Bacillus strains exhibited enhanced feed intake, feed efficiency, and body weight gain. The enhancement of feed efficiency in groups treated with a mixture of B. coagulans and P. polymyxa may result from the promotion of gut health through the augmentation of beneficial microflora, the suppression of harmful bacteria via competitive elimination, and the synthesis of antibacterial agents (16), or from increased nutrient digestibility resulting in improved feed conversion ratio (56). Conversely, several studies have revealed that the incorporation of probiotics in broiler diets does not affect productive efficiency (20, 57). The discrepancies in the results may arise from variations in experimental methodologies, including probiotic strains, doses, avian age, and breed (58).
In the current study, the inclusion of a B. coagulans and P. polymyxa mixture at 1 mg kg−1 in the diet enhanced productive performance qualities and increased giblet percentage in growing quail, with no significant variations observed in carcass features. The observed increase in giblet percentage, particularly liver weight, in quails supplemented with B. coagulans and P. polymyxa may reflect a physiological adaptation associated with enhanced growth performance and improved nutrient utilization (Table 3), as well as improved liver function (Table 5).
Probiotics have been reported to stimulate hepatic function through the upregulation of protein synthesis, lipid metabolism, and detoxification pathways, which could result in slight organ enlargement within a healthy range (51). The inclusion of probiotics in broiler diets did not result in substantial variations in dressing and carcass percentages (59) or carcass yield (51). Conversely, Kaushal et al. (60) reported that the inclusion of probiotics in the broiler's diet positively influenced dressing yield.
Blood biochemical parameters
The current study demonstrated that a mixture of B. coagulans and P. polymyxa significantly (P < 0.05) elevated total protein and globulin concentration while exhibiting no variation in albumin, thereby resulting in a decreased albumin/globulin ratio. These findings are consistent with prior research on meat-type quails (61, 62) and poultry (63). The observed impacts may result from enhanced dietary protein utilization in groups supplemented with a mixture of B. coagulans and P. polymyxa achieved by inhibiting pathogen proliferation, which diminishes protein degradation into nitrogen, enhances dietary protein metabolism and expands the surface area for utilization of digested feed (63), or by augmenting the absorption power of the intestinal villi (64).
In our investigation, the dietary addition of a mixture of B. coagulans and P. polymyxa decreased blood aspartate aminotransferase and lactate dehydrogenase levels, particularly in the 0.5 mg kg−1 diet group. The reduction in aspartate aminotransferase corresponds with the results of Kasmani et al. (61) and Abramowicz et al. (65), suggesting that the combination of Bc+Pp provides a protective role in the liver by reducing pathogen translocation, thereby decreasing serum transaminase levels (66).
Lactate dehydrogenase is acknowledged as an indicator of cellular toxicity (67), and a decrease in lactate dehydrogenase levels in groups treated with a mixture of B. coagulans and P. polymyxa suggests a reduction in cellular damage (65). Concerning renal function, there was no notable reduction in creatinine levels, whereas there was a significant (P < 0.05) decrease in urea and uric acid attributable to dietary supplementation with a mixture of B. coagulans and P. polymyxa. Our findings support a previous study (20) suggesting that dietary probiotics reduce urea and creatinine levels by improving protein digestion in Japanese quail.
Our current results demonstrated that the administration of B. coagulans and P. polymyxa mixture in the diet of meat-type quails resulted in a hypolipidemic effect, as indicated by significantly (P < 0.05) reduced levels of total cholesterol, triglycerides, low-density lipoprotein, and very low-density lipoprotein. The 1.0 mg kg−1 feed had the highest efficacy among the supplemented groups. The reduction in cholesterol and triglycerides due to probiotic inclusion corresponds with prior studies on developing quail and chicken. In contrast, the administration of P. polymyxa had no significant effect on cholesterol and triglyceride levels in fowl (9).
The hypocholesterolemic effect of the B. coagulans and P. polymyxa mixture may arise from the modulation of lipid metabolism through the deconjugation of bile salts and the quantity of cholesterol absorbed from the gastrointestinal tract, as the co-precipitation of intestinal cholesterol interacts with deconjugated bile salts, thereby inhibiting the synthesis of the intestinal cholesterol transporter (68). The increase of high-density lipoprotein and the decrease of low-density lipoprotein and very low-density lipoprotein in the B. coagulans and P. polymyxa mixture-treated groups correspond with earlier studies (50, 65). Ognik et al. (69) reported that the dietary inclusion of probiotics reduces oxidative processes inside cells, resulting in lower levels of total cholesterol, triglycerides, and low-density lipoprotein.
Antioxidative capacity and immune responses
The current findings indicated that a dietary Bc+Pp enhanced the antioxidant capacity of meat-type quail by considerably reducing malondialdehyde levels and increasing superoxide dismutase and total antioxidant capacity concentrations. The optimal dosage was 1.0 mg kg−1 diet. The introduction of P. polymyxa in broiler diets enhanced antioxidant capability by reducing malondialdehyde levels and elevating reduced glutathione and glutathione peroxidase activity (9). The inclusion of dietary Bacillus enhanced the actions of superoxide dismutase and glutathione peroxidase in poultry. The findings align with those of Abdel-Moneim et al. (50), who demonstrated that the incorporation of Bacillus-based probiotics in the diet of Japanese quail boosts antioxidant capacity by elevating glutathione levels and catalase activity while decreasing malondialdehyde (50).
The reduction in malondialdehyde content signifies an enhancement in antioxidant defense and mitigation of oxidative stress, thereby safeguarding biological components from peroxidation and oxidation, as evidenced by prior research (69, 70). The enhancement of antioxidant capacity in the treated groups in the present study may result from probiotic bacteria utilizing their natural resources to neutralize extra free radicals and bolster the host's antioxidant capacity (71, 72).
The combination of B. coagulans and P. polymyxa enhanced antioxidant capacity and significantly (P < 0.05) improved immunological response in the supplemented groups. Probiotic dietary supplementation enhanced antibody production against sheep red blood cells and elevated antibody titers against Newcastle disease virus in meat-type quail (61). The outcomes indicated that administering a Bc+Pp in the quail diet enhanced globulin, serum immunoglobulin, and C3 levels. This aligns with Wu et al. (9), who showed that dietary P. polymyxa stimulates an immunological state by preserving equilibrium between pro-inflammatory and anti-inflammatory responses, thereby safeguarding intestinal homeostasis (9). Moreover, feeding B. subtilis increases IgM levels, with no observed variations in IgA and IgG levels in poultry breeders (73). Our results concur with those of Fathi et al. (20), who indicated that supplementing B. subtilis positively influences IgM levels in poultry subjected to heat stressors (20).
Additionally, probiotic administration enhanced humoral immunity by elevating serum IgA and IgM levels in poultry (74). Bai et al. (75) observed that the incorporation of probiotics derived from B. subtilis fmbJ into broiler feeds resulted in a significant (P < 0.05) rise in blood levels of both IgA and IgG. The elevated immunoglobulin levels in the treated groups may result from the immunomodulatory effects of P. polymyxa (24). The increased levels of IgM, IgA, and complement 3 in probiotic-supplemented groups suggest an enhancement of systemic humoral immunity in Japanese quails. IgM is typically associated with primary immune responses, while IgA plays a role in both mucosal and systemic immunity. The elevation in complement 3 further supports enhanced innate immune activity. The capacity of P. polymyxa to augment immunological state efficacy provides compelling justification for its application as an antibiotic alternative to improve animal well being and efficiency (20).
Digestive enzymes and caecal microbiota
The prescription of probiotics improves growth performance by increasing the population of beneficial bacteria in the gut. The reduction in total coliform counts and the increase in lactic acid bacteria may indicate a shift toward a more balanced microbial profile in the cecum. According to Salah et al. (12), enhancing the function of the gut barrier promotes the production of digestive enzymes, which ultimately leads to an increase in the absorption of nutrients and helps to promote body weight and body weight gain, which aligns with our results.
Dietary supplementation with P. polymyxa 10 (BSC10) improved gut status by augmenting gut barrier function and enhancing the immunity of broilers (9). Dietary supplementation with Paenibacillus xylanexedens, as a probiotic, improved gut shape and decreased E. coli levels in the cecum of poultry (8). Prior research indicates that the administration of Bacillus spp. enhances gut microbial profile (19), promotes the colonization of beneficial bacteria in poultry (76), alters the caecal bacteria of broilers to a healthier equilibrium by providing helpful microorganisms and reducing potentially pathogenic microorganisms, and improves the interaction dynamics within the gastrointestinal microbiome (77).
The recent in vitro findings indicated that the isolated P. polymyxa LM31 has antibacterial properties through the production of antibiotics such as penicillin and polymyxin. Our findings indicated that the dietary mixture of B. coagulans and P. polymyxa elicited significant (P < 0.05) alterations in caecal microbiota, resulting in a decline in the amount of caecal E. coli and Enterococcus spp. with an increase in the count of caecal lactic acid bacteria. P. polymyxa has shown antibacterial properties against microorganisms using diffusible metabolites and bacterial volatiles as plant elicitors (78).
The inclusion of B. subtilis markedly decreased intestinal coliforms and E. coli in laying Japanese quails (79). The native Lactobacillus strains (150 g ton−1 food) included as probiotics markedly diminished E. coli proliferation, while Lactobacillus spp. proliferated in Japanese quail (62). Supplementation of Bacillus spp. reduced caecal E. coli, although caecal lactobacilli counts rose in poultry (80). Supplementation with B. subtilis C-3102 decreased caecal E. coli levels and elevated caecal lactobacilli counts in broiler chicks (81). Comprehensive research has been undertaken to clarify the pathogenic inhibitory effect of probiotics, yet the precise mechanism remains incompletely understood (2). Bacillus spp. may reduce infections through direct suppression, the synthesis of antimicrobial peptides, or by strengthening the mucosal layer of the intestine to obstruct microbial diffusion across the membrane (19).
Pathogenic management is a crucial concern for poultry producers and consumers, with significant economic and public health ramifications (19). The current findings demonstrated that a dietary combination of B. coagulans and P. polymyxa suppressed E. coli and Enterococcus spp. while concurrently promoting lactic acid bacteria. The suppression of detrimental bacteria may lead to a more resilient intestinal milieu, optimizing nutrient absorption, augmenting productivity, enhancing immunological function, and strengthening antioxidative capabilities.
Conclusion
In summary, dietary supplementation with a probiotic combination of B. coagulans and P. polymyxa at a dosage of 1 mg kg−1 significantly (P < 0.05) enhanced growth performance, feed conversion efficiency, and various health-related metrics, encompassing liver and kidney function biomarkers, lipid profile, antioxidant capacity, immunological indicators, digestive enzyme activity, and caecal microbial load. The findings indicate that low-dose probiotic combinations may function as efficient natural performance enhancers for the growth of meat-type quail; nevertheless, more study is necessary to verify long-term safety and refine dosage methodologies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal studies were approved by the investigational measures followed the Local Experimental Animal Care Committee's established protocols. The code for ethical approval is (ZU-IACUC/2/F/313/2023). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
FR: Software, Writing – original draft, Writing – review & editing. MA: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. AS: Writing – original draft, Writing – review & editing. LA: Writing – original draft, Writing – review & editing. MAA: Writing – original draft, Writing – review & editing. SAA: Writing – original draft, Writing – review & editing. SA: Writing – original draft, Writing – review & editing. KE-T: Conceptualization, Writing – original draft, Writing – review & editing. ME-S: Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2025R457), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. King Khalid University for funding this work through Large Research Project under grant number (R.G.P2/581/46). This project was supported by the UAEU program of Advanced Research (Grant number: 12S169).
Acknowledgments
The authors gratefully acknowledge Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2025R457), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. The authors extend their appreciation to the deanship of King Khalid University for supporting this work under the large group of research number (R.G.P2/581/46).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2025.1639681/full#supplementary-material
References
1. Rezaeipour V, Valizadeh A, Abdullahpour R, Sadeghi AR. Effects of dietary threonine and a multi strains probiotic (Primalac) supplementation on growth performance, blood metabolites and carcass characteristics in Japanese quails. Poult Sci J. (2015) 3:135–41.
2. Hernandez-Patlan D, Solis-Cruz B, Hargis BM, Tellez G. The use of probiotics in poultry production for the control of bacterial infections and aflatoxins. In:Franco-Robles E, Ramírez-Emiliano J, , editors. Prebiotics and Probiotics—Potential Benefits in Nutrition and Health. London: IntechOpen (2019). p. 1–21. doi: 10.5772/intechopen.88817
3. Alagawany M, Abd El-Hack ME. Natural Feed Additives Used in The Poultry Industry. Sharjah, UAE: Bentham Science Publishers (2020). 285 p. doi: 10.2174/9789811488450120010020
4. Reda FM, Salah AS, Attia YA, Alhotan RA, Mahmoud MA, Di Cerbo A, et al. Use of black pepper oil in growing-quail diets and its impact on growth, carcass measurements, intestinal microbiota, and blood chemistry. Arch Anim Breed. (2024) 67:445–54. doi: 10.5194/aab-67-445-2024
5. Mohamed SM, Alagawany M, El-Kholy MS, El-Mekkawy MM, Salah AS, Attia YA, et al. Effect of dietary microalgae on growth performance and health in meat-type quails. Poult Sci. (2025) 104:104709. doi: 10.1016/j.psj.2024.104709
6. El-Nile AE, Sallam SM, Abd El-Hack ME, Salah AS, Alagawany M. Nanoclay in animal diets: properties, structure, applications, and toxicity. In: Alagawany M, Sallam SM, Abd El-Hack ME, , editors. Organic Feed Additives for Livestock. Amsterdam, Netherlands: Academic Press (2025). pp. 139–47. doi: 10.1016/B978-0-443-13510-1.00009-8
7. Salah AS, Lestingi A, El-Tarabany MS, Mostafa M, Zaki RS, Azzam MM, et al. Effect of Spirulina supplementation on growth, immunity, antioxidant status and pathomorphological perspectives in broilers exposed to dietary aflatoxin B1. J Appl Poult Res. (2025) 34:100519. doi: 10.1016/j.japr.2025.100519
8. Ekim B, Calik A, Ceylan A, Saçakli P. Effects of Paenibacillus xylanexedens on growth performance, intestinal histomorphology, intestinal microflora, and immune response in broiler chickens challenged with Escherichia coli K88. Poult Sci. (2020) 99:214–23. doi: 10.3382/ps/pez460
9. Wu Y, Wang B, Zeng Z, Liu R, Tang L, Gong L, et al. Effects of probiotics Lactobacillus plantarum 16 and Paenibacillus polymyxa 10 on intestinal barrier function, antioxidative capacity, apoptosis, immune response, and biochemical parameters in broilers. Poult Sci. (2019) 98:5028–39. doi: 10.3382/ps/pez226
10. Salah AS, Ahmed-Farid OA, El-Tarabany MS. Carcass yields, muscle amino acid and fatty acid profiles, and antioxidant indices of broilers supplemented with synbiotic and/or organic acids. J Anim Physiol Anim Nutr. (2019) 103:41–52. doi: 10.1111/jpn.12994
11. Soomro RN, Abd El-Hack ME, Shah SS, Taha AE, Alagawany M, Swelum AA, et al. Impact of restricting feed and probiotic supplementation on growth performance, mortality and carcass traits of meat-type quails. Anim Sci J. (2019) 90:1388–95. doi: 10.1111/asj.13290
12. Salah AS, El-Tarabany MS, Mostafa M, Zaki RS, Azzam MM, El Euony OI, et al. Impact of dietary Spirulina on performance, antioxidant status, carcass traits and pathological alteration in broilers exposed to ochratoxin A stress. Front Vet Sci. (2025) 11:1532353. doi: 10.3389/fvets.2024.1532353
13. Hazrati S, Rezaeipour V, Asadzadeh S. Effects of phytogenic feed additives, probiotic and mannan-oligosaccharides on performance, blood metabolites, meat quality, intestinal morphology, and microbial population of Japanese quail. Br Poult Sci. (2020) 61:132–9. doi: 10.1080/00071668.2019.1686122
14. Joya M, Ashayerizadeh O, Dastar B. Effects of Spirulina (Arthrospira) platensis and Bacillus subtilis PB6 on growth performance, intestinal microbiota and morphology, and serum parameters in broiler chickens. Anim Prod Sci. (2020) 61:390–8. doi: 10.1071/AN20218
15. Gadde U, Oh ST, Lee YS, Davis E, Zimmerman N, Rehberger T, et al. The effects of direct-fed microbial supplementation, as an alternative to antibiotics, on growth performance, intestinal immune status, and epithelial barrier gene expression in broiler chickens. Probiotics Antimicrob Proteins. (2017) 9:397–405. doi: 10.1007/s12602-017-9275-9
16. Momtazan R, Moravej H, Zaghari M, Taheri HR. A note on the effects of a combination of an enzyme complex and probiotic in the diet on performance of broiler chickens. Ir J Agric Food Res. (2011) 50:249–54.
17. Shivaramaiah S, Pumford NR, Morgan MJ, Wolfenden RE, Wolfenden AD, Torres-Rodríguez A, et al. Evaluation of Bacillus species as potential candidates for direct-fed microbials in commercial poultry. Poult Sci. (2011) 90:1574–80. doi: 10.3382/ps.2010-00745
18. Abd El-Moneim AE, Sabic EM. Beneficial effect of feeding olive pulp and Aspergillus awamori on productive performance, egg quality, serum/yolk cholesterol and oxidative status in laying Japanese quails. J Anim Feed Sci. (2019) 28:52–61. doi: 10.22358/jafs/105537/2019
19. Grant AQ, Gay CG, Lillehoj HS. Bacillus spp. as direct-fed microbial antibiotic alternatives to enhance growth, immunity, and gut health in poultry. Avian Pathol. (2018) 47:339–51. doi: 10.1080/03079457.2018.1464117
20. Fathi MM, Ebeid TA, Al-Homidan I, Soliman NK, Abou-Emera OK. Influence of probiotic supplementation on immune response in broilers raised under hot climate. Br Poult Sci. (2017) 58:512–6. doi: 10.1080/00071668.2017.1332405
21. Bonos E, Giannenas I, Sidiropoulou E, Stylianaki I, Tzora A, Skoufos I, et al. Effect of Bacillus pumilus supplementation on performance, intestinal morphology, gut microflora and meat quality of broilers fed different energy concentrations. Anim Feed Sci Technol. (2021) 274:114859. doi: 10.1016/j.anifeedsci.2021.114859
22. Xing SC, Chen JY, Cai YF, Huang CB, Liao XD Mi JD. Bacillus coagulans R11 consumption influenced the abundances of cecum antibiotic resistance genes in lead-exposed laying hens. Environ Pollut. (2021) 274:116562. doi: 10.1016/j.envpol.2021.116562
23. Liu C, Radebe SM, Zhang H, Jia J, Xie S, Shi M, et al. Effect of Bacillus coagulans on maintaining the integrity intestinal mucosal barrier in broilers. Vet Microbiol. (2022) 266:109357. doi: 10.1016/j.vetmic.2022.109357
24. Lal S, Tabacchioni S. Ecology and biotechnological potential of Paenibacillus polymyxa: a minireview. Indian J Microbiol. (2009) 49:2–10. doi: 10.1007/s12088-009-0008-y
25. Grady EN, MacDonald J, Liu L, Richman A, Yuan ZC. Current knowledge and perspectives of Paenibacillus: a review. Microb Cell Fact. (2016) 15:203. doi: 10.1186/s12934-016-0603-7
26. Alagawany M, Madkour M, El-Saadony MT, Reda FM. Paenibacillus polymyxa (LM31) as a new feed additive: antioxidant and antimicrobial activity and its effects on growth, blood biochemistry, and intestinal bacterial populations of growing Japanese quail. Anim Feed Sci Technol. (2021) 276:114920. doi: 10.1016/j.anifeedsci.2021.114920
27. Alagawany M, El-Saadony MT, Elnesr SS, Farahat M, Attia G, Madkour M, et al. Use of lemongrass essential oil as a feed additive in quail's nutrition: its effect on growth, carcass, blood biochemistry, antioxidant and immunological indices, digestive enzymes and intestinal microbiota. Poult Sci. (2021) 100:101172. doi: 10.1016/j.psj.2021.101172
28. Sneath PH. Endospore-forming Gram-positive rods and cocci. In:Sneath PH, Mair NS, Sharpe ME, and Holt JG, , editors. Bergey's Manual of Systematic Bacteriology, Vol. 2. Philadelphia, PN: Williams and Wilkins (1986). pp. 1104–207. doi: 10.1515/9783112581704-021
29. Bille E, Dauphin B, Leto J, Bougnoux ME, Beretti JL, Lotz A, et al. MALDI-TOF MS Andromas strategy for the routine identification of bacteria, mycobacteria, yeasts, Aspergillus spp. and positive blood cultures. Clin Microbiol Infect. (2012) 18:1117–25. doi: 10.1111/j.1469-0691.2011.03688.x
30. Sahadeva RPK, Leong SF, Chua KH, Tan CH, Chan HY, Tong EV, et al. Survival of commercial probiotic strains to pH and bile. Int Food Res J. (2011) 18:1515–22.
31. Chistyakov VA, Prazdnova EVE, Mazanko MS, Bren AB. The use of biosensors to explore the potential of probiotic strains to reduce the SOS response and mutagenesis in bacteria. Biosensors. (2018) 8:25. doi: 10.3390/bios8010025
32. Algburi A, Al-Hasani HM, Ismael TK, Abdelhameed A, Weeks R, Ermakov AM, et al. Antimicrobial activity of Bacillus subtilis KATMIRA1933 and Bacillus amyloliquefaciens B-1895 against Staphylococcus aureus biofilms isolated from wound infection. Probiotics Antimicrob Proteins. (2021) 13:125–34. doi: 10.1007/s12602-020-09673-4
33. El-Saadony MT, Saad AM, Taha TF, Najjar AA, Zabermawi NM, Nader MM, et al. Selenium nanoparticles from Lactobacillus paracasei HM1 capable of antagonizing animal pathogenic fungi as a new source from human breast milk. Saudi J Biol Sci. (2021) 28:6782–94. doi: 10.1016/j.sjbs.2021.07.059
34. Saad AM, Sitohy MZ, Ahmed AI, Rabie NA, Amin SA, Aboelenin SM, et al. Biochemical and functional characterization of kidney bean protein alcalase-hydrolysates and their preservative action on stored chicken meat. Molecules. (2021) 26:4690. doi: 10.3390/molecules26154690
35. National Research Council. Nutrient Requirements of Poultry: 1994. Washington, DC: National Academies Press (1994). 157 p.
36. Zhou Z, Xu X, Luo D, Zhou Z, Zhang S, He R, et al. Effect of dietary supplementation of Lactiplantibacillus plantarum N-1 and its synergies with oligomeric isomaltose on the growth performance and meat quality in Hu sheep. Foods. (2023) 12:1858. doi: 10.3390/foods12091858
37. Armstrong WD, Carr CW. Physiological Chemistry: Laboratory Directions. 3rd. ed. Minneapolis, MN: Burgess Publishing Co. (1964). 150 p.
39. Somogyi M. Modification of two methods for the assay of amylase. Clin Chem. (1960) 6:23–35. doi: 10.1093/clinchem/6.1.23
40. Tietz NW, Fiereck EA. A specific method for serum lipase determination. Clin Chim Acta. (1966) 13:352–8. doi: 10.1016/0009-8981(66)90215-4
41. Lynn KR, Clevette-Radford NA. Purification and characterization of hevain, a serine protease from Hevea brasiliensis. Phytochemistry. (1984) 23:963–4. doi: 10.1016/S0031-9422(00)82592-3
42. Gao D, Yu J, Dai X, Tian Y, Sun J, Xu X, et al. Development and evaluation of an indirect enzyme-linked immunosorbent assay based on a recombinant SifA protein to detect Salmonella infection in poultry. Poult Sci. (2023) 102:102513. doi: 10.1016/j.psj.2023.102513
43. Hallmann J, Quadt-Hallmann A, Mahaffee WF, Kloepper JW. Bacterial endophytes in agricultural crops. Can J Microbiol. (1997) 43:895–914. doi: 10.1139/m97-131
44. Bamedi A, Salari S, Baghban F. Changes in performance, cecal microflora counts and intestinal histology of Japanese quails fed diets containing different fibre sources. Vet Anim Sci. (2024) 25:100386. doi: 10.1016/j.vas.2024.100386
45. Steel RGD, Torrie JH. Principles and Procedures of Statistics with Special Reference to the Biological Sciences. New York, NY: McGraw-Hill (1960). 481 p.
46. Wang WC, Yan FF, Hu JY, Amen OA, Cheng HW. Supplementation of Bacillus subtilis-based probiotic reduces heat stress-related behaviors and inflammatory response in broiler chickens. J Anim Sci. (2018) 96:1654–66. doi: 10.1093/jas/sky092
47. Zhou L, Abouelezz K, Momenah MA, Bajaber MA, Baazaoui N, Taha TF, et al. Dietary Paenibacillus polymyxa AM20 as a new probiotic: improving effects on IR broiler growth performance, hepatosomatic index, thyroid hormones, lipid profile, immune response, antioxidant parameters, and caecal microorganisms. Poult Sci. (2024) 103:103239. doi: 10.1016/j.psj.2023.103239
48. Wang B, Gong L, Zhou Y, Tang L, Zeng Z, Wang Q, et al. Probiotic Paenibacillus polymyxa 10 and Lactobacillus plantarum 16 enhance growth performance of broilers by improving the intestinal health. Anim Nutr. (2021):829–40. doi: 10.1016/j.aninu.2021.03.008
49. Alsulami MN, El-Saadony MT. Supplementing broiler diets with bacterial selenium nanoparticles enhancing performance, carcass traits, blood indices, antioxidant status, and caecal microbiota of Eimeria tenella-infected broiler chickens. Poult Sci. (2023) 102:103111. doi: 10.1016/j.psj.2023.103111
50. Abdel-Moneim AE, Selim DA, Basuony HA, Sabic EM, Saleh AA, Ebeid TA. Effect of dietary supplementation of Bacillus subtilis spores on growth performance, oxidative status, and digestive enzyme activities in Japanese quail birds. Trop Anim Health Prod. (2020) 52:671–80. doi: 10.1007/s11250-019-02055-1
51. Zhen W, Shao Y, Gong X, Wu Y, Geng Y, Wang Z, et al. Effect of dietary Bacillus coagulans supplementation on growth performance and immune responses of broiler chickens challenged by Salmonella enteritidis. Poult Sci. (2018) 97:2654–66. doi: 10.3382/ps/pey119
52. Seifi K, Karimi Torshizi MA, Rahimi S, Kazemifard M. Efficiency of early, single-dose probiotic administration methods on performance, small intestinal morphology, blood biochemistry, and immune response of Japanese quail. Poult Sci. (2017) 96:2151–8. doi: 10.3382/ps/pew446
53. Gupta NK, Shrivastava AK, Praveen PK, Ganguly S. Effect of feed supplement and probiotics on growth performance of Japanese quails. Indian J Anim Nutr. (2016) 33:486–9. doi: 10.5958/2231-6744.2016.00087.6
54. Halder N, Sunder J, De AK, Bhattacharya D, Joardar SN. Probiotics in poultry: a comprehensive review. J Basic Appl Zool. (2024) 85:23. doi: 10.1186/s41936-024-00379-5
55. Flores C, Williams M, Pieniazek J, Dersjant-Li Y, Awati A, Lee JT. Direct-fed microbial and its combination with xylanase, amylase, and protease enzymes in comparison with AGPs on broiler growth performance and foot-pad lesion development. J Appl Poult Res. (2016) 25:328–37. doi: 10.3382/japr/pfw016
56. Murugesan GR, Persia ME. Influence of a direct-fed microbial and xylanase enzyme on the dietary energy uptake efficiency and performance of broiler chickens. J Sci Food Agric. (2015) 95:2521–7. doi: 10.1002/jsfa.6984
57. Martínez EA, Babot JD, Lorenzo-Pisarello MJ, Apella MC, Chaia AP. Feed supplementation with avian Propionibacterium acidipropionici contributes to mucosa development in early stages of rearing broiler chickens. Benef Microbes. (2016) 7:687–98. doi: 10.3920/BM2016.0077
58. Jha R, Das R, Oak S, Mishra P. Probiotics (direct-fed microbials) in poultry nutrition and their effects on nutrient utilization, growth and laying performance, and gut health: a systematic review. Animals. (2020) 10:1863. doi: 10.3390/ani10101863
59. Sarangi NR, Babu LK, Kumar A, Pradhan CR, Pati PK, Mishra JP. Effect of dietary supplementation of prebiotic, probiotic, and synbiotic on growth performance and carcass characteristics of broiler chickens. Vet World. (2016) 9:313–8. doi: 10.14202/vetworld.2016.313-319
60. Kaushal S, Sharma RK, Singh DV, Shukla SK, Kumar S, Palod J, et al. Performance, carcass characteristics and economics of broiler chickens fed dietary enzymes and probiotic. Iran J Vet Res. (2019) 20:293–8.
61. Kasmani FB, Torshizi MAK, Allameh A, Shariatmadari F. A novel aflatoxin-binding Bacillus probiotic: performance, serum biochemistry, and immunological parameters in Japanese quail. Poult Sci. (2012) 91:1846–53. doi: 10.3382/ps.2011-01830
62. Siadati SA, Ebrahimnezhad Y, Salehi Jouzani GH, Shayegh J. Evaluation of probiotic potential of some native Lactobacillus strains on the growth performance and serum biochemical parameters of Japanese quails (Coturnix coturnix japonica) during rearing period. Rev Bras Cienc Avic. (2017) 19:399–408. doi: 10.1590/1806-9061-2016-0393
63. Yazhini P, Visha P, Selvaraj P, Vasanthakumar P, Chandran V. Dietary encapsulated probiotic effect on broiler serum biochemical parameters. Vet World. (2018) 11:1344–8. doi: 10.14202/vetworld.2018.1344-1348
64. Aliakbarpour HR, Chamani M, Rahimi G, Sadeghi AA, Qujeq D. The Bacillus subtilis and lactic acid bacteria probiotics influences intestinal mucin gene expression, histomorphology and growth performance in broilers. Asian-Australas J Anim Sci. (2012) 25:1285–91. doi: 10.5713/ajas.2012.12110
65. Abramowicz K, Krauze M, Ognik K. The effect of a probiotic preparation containing Bacillus subtilis PB6 in the diet of chickens on redox and biochemical parameters in their blood. Ann Anim Sci. (2019) 19:433–51. doi: 10.2478/aoas-2018-0059
66. Rishi P, Mavi SK, Bharrhan S, Shukla G, Tewari R. Protective efficacy of probiotic alone or in conjunction with a prebiotic in Salmonella-induced liver damage. FEMS Microbiol Ecol. (2009) 69:222–30. doi: 10.1111/j.1574-6941.2009.00703.x
67. Dostal A, Gagnon M, Chassard C, Zimmermann MB, O'Mahony L, Lacroix C. Salmonella adhesion, invasion and cellular immune responses are differentially affected by iron concentrations in a combined in vitro gut fermentation-cell model. PLoS ONE. (2014) 9:e93549. doi: 10.1371/journal.pone.0093549
68. Zarezadeh M, Musazadeh V, Faghfouri AH, Roshanravan N, Dehghan P. Probiotics act as a potent intervention in improving lipid profile: an umbrella systematic review and meta-analysis. Crit Rev Food Sci Nutr. (2023) 63:145–58. doi: 10.1080/10408398.2021.2004578
69. Ognik K, Krauze M, Cholewińska E, Abramowicz K. The effect of a probiotic containing Enterococcus faecium DSM 7134 on redox and biochemical parameters in chicken blood. Ann Anim Sci. (2017) 17:1075–88. doi: 10.1515/aoas-2016-0097
70. Zhang L, Bai K, Zhang J, Xu W, Huang Q, Wang T. Dietary effects of Bacillus subtilis fmbj on the antioxidant capacity of broilers at an early age. Poult Sci. (2017) 96:3564–73. doi: 10.3382/ps/pex172
71. Amaretti A, Di Nunzio M, Pompei A, Raimondi S, Rossi M, Bordoni A. Antioxidant properties of potentially probiotic bacteria: in vitro and in vivo activities. Appl Microbiol Biotechnol. (2013) 97:809–17. doi: 10.1007/s00253-012-4241-7
72. Wang Y, Wu Y, Wang Y, Xu H, Mei X, Yu D, et al. Antioxidant properties of probiotic bacteria. Nutrients. (2017) 9:521. doi: 10.3390/nu9050521
73. Liu X, Peng C, Qu X, Guo S, Chen JF, He C, et al. Effects of Bacillus subtilis C-3102 on production, hatching performance, egg quality, serum antioxidant capacity and immune response of laying breeders. J Anim Physiol Anim Nutr. (2019) 103:182–90. doi: 10.1111/jpn.13022
74. Zhang ZF, Kim IH. Effects of multistrain probiotics on growth performance, apparent ileal nutrient digestibility, blood characteristics, cecal microbial shedding, and excreta odor contents in broilers. Poult Sci. (2014) 93:364–70. doi: 10.3382/ps.2013-03314
75. Bai K, Huang Q, Zhang J, He J, Zhang L, Wang T. Supplemental effects of probiotic Bacillus subtilis fmbJ on growth performance, antioxidant capacity, and meat quality of broiler chickens. Poult Sci. (2017) 96:74–82. doi: 10.3382/ps/pew246
76. Al-Fataftah A-R, Abdelqader A. Effects of dietary Bacillus subtilis on heat-stressed broilers performance, intestinal morphology and microflora composition. Anim Feed Sci Technol. (2014) 198:279–85. doi: 10.1016/j.anifeedsci.2014.10.012
77. Ma Y, Wang W, Zhang H, Wang J, Zhang W, Gao J, et al. Supplemental Bacillus subtilis DSM 32315 manipulates intestinal structure and microbial composition in broiler chickens. Sci Rep. (2018) 8:15358. doi: 10.1038/s41598-018-33762-8
78. Phi QT, Park YM, Seul KJ Ryu CM, Park SH, Kim JG, Ghim SY. Assessment of root-associated Paenibacillus polymyxa groups on growth promotion and induced systemic resistance in pepper. J Microbiol Biotechnol. (2010) 20:1605–13.
79. Manafi M, Khalaji S, Hedayati M. Assessment of a probiotic containing Bacillus subtilis on the performance and gut health of laying Japanese quails (Coturnix coturnix Japonica). Rev Bras Cienc Avic. (2016) 18:599–606. doi: 10.1590/1806-9061-2016-0220
80. Wealleans A, Sirukhi M, Egorov I. Performance, gut morphology and microbiology effects of a Bacillus probiotic, avilamycin and their combination in mixed grain broiler diets. Br Poult Sci. (2017) 58:523–9. doi: 10.1080/00071668.2017.1349298
Keywords: antimicrobial activity, biochemical parameters, carcass characteristics, caecal microbiota, growth performance, immune response, organic poultry, probiotics
Citation: Reda FM, Alagawany M, Salah AS, Almutairi LA, Alqahtani MA, Alamoudi SA, Altuwaijri S, El-Tarabily KA and El-Saadony MT (2025) Harnessing functional feed additives for sustainable production: the role of Bacillus coagulans and Paenibacillus polymyxa mixture in improving production and health of meat-type quails. Front. Vet. Sci. 12:1639681. doi: 10.3389/fvets.2025.1639681
Received: 02 June 2025; Accepted: 27 August 2025;
Published: 24 September 2025.
Edited by:
Mierlita Daniel, University of Oradea, RomaniaReviewed by:
Yueh-Sheng Lee, Chiaho Agriscience Co., Ltd, TaiwanMuhittin Zengin, University of Wisconsin-Madison, United States
Copyright © 2025 Reda, Alagawany, Salah, Almutairi, Alqahtani, Alamoudi, Altuwaijri, El-Tarabily and El-Saadony. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Khaled A. El-Tarabily, a3RhcmFiaWx5QHVhZXUuYWMuYWU=
 Fayiz M. Reda1
Fayiz M. Reda1 Mahmoud Alagawany
Mahmoud Alagawany Khaled A. El-Tarabily
Khaled A. El-Tarabily