- School of Life Sciences and Engineering, Northwest Minzu University, Lanzhou, China
This study investigated the effects of Glycyrrhiza uralensis extract (GUE), Lactobacillus acidophilus (Lac), and their combination on production performance, immune and antioxidant functions, and intestinal health in broilers. A total of 420 one-day-old male Liangfenghua broilers were randomly assigned to four groups and fed with basal diet, GUE diet with 0.1% GUE, Lac diet with 4.5 × 10⁷ CFU/kg Lac, or GUE+Lac diet with 0.1% GUE and 4.5 × 10⁷ CFU/kg Lac. The experiment lasted 84 days. The results demonstrated that both GUE and Lac enhanced health by increasing immune organ indices, serum levels of IFN-γ, IL-2, IgA, and IgG, and activities of GSH-Px and SOD (p < 0.05). They also improved intestinal morphology and barrier function by increasing villus height and the villus height to crypt depth ratio (VH/CD), upregulating expression of Zonula occludens-1 (ZO-1), mucin-2 (MUC2), and Occludin (OCLN), and reducing expression of IL-1β, TLR4, and TNF-α (p < 0.05), which increased nutrient metabolic rates (p < 0.05). These changes ultimately resulted in improved production performance, evidenced by increased body weight and decreased abdominal fat rate (p < 0.05). Notably, the combined GUE+Lac exhibited positive synergistic effects, leading to enhanced immune function as shown by increased serum levels of IL-2, IFN-γ, IgA, and IgG; improved antioxidant capacity indicated by elevated GSH-Px activity in serum and liver; and enhanced intestinal morphology and barrier function by increased villus height and VH/CD ratio, upregulated expression of ZO-1, MUC2, and OCLN, and reduced expression of IL-1β and TLR4, TNF-α (p < 0.05) compared to both GUE and Lac alone. This synergism between GUE and Lac significantly increased body weight and nutrient metabolic rates while decreasing abdominal fat rate in broilers. Altogether, we demonstrated the synergistic enhancement of production performance, immunity, antioxidation and intestinal health in broilers by combining GUE and Lactobacillus acidophilus. These findings provide robust scientific support for the future development and application of feed additive.
1 Introduction
High human demand for poultry meat has led to intensive production practices. Broilers raised under such a production system are susceptible to external factors including diseases, nutritional, and environmental challenges, which often result in poor health, such as enhanced stress response, and reduced immunity. In recent decades, antibiotic growth promoters have been widely used in the poultry industry to control diseases and enhance growth performance. However, the residues of antibiotics in animal derived foods and the development of drug-resistant bacteria pose health risks to consumers and the poultry industry. Therefore, it is essential to develop feed additives from natural sources such as medicinal plants and probiotics for poultry production that can both increase productive potential and maintain broiler health.
Glycyrrhiza uralensis is an herb from the leguminous family (1), and its rhizome is primarily used in traditional Chinese medicine (2). Glycyrrhiza uralensis extract (GUE) contains over 400 compounds, including triterpenoid saponins (glycyrrhizic acid), flavonoid glycosides (liquiritigenin, liquiritin) and polysaccharides. These compounds display diverse pharmacological effects such as anti-inflammatory, antioxidant, antibacterial, and antiviral (3, 4). Studies showed that Glycyrrhizic acid inhibited Mycoplasma gallisepticum (MG)-triggered inflammation and apoptosis by suppressing the MAPK pathway in chickens (5). Glycyrrhiza uralensis flavonoids reduced colonic myeloperoxidase activity and inflammatory cytokine production in mice with ulcerative colitis, effectively suppressing the inflammatory response (6). Glycyrrhiza chalcone A inhibited LPS-induced ROS production in murine monocyte macrophages in a dose-dependent manner (7). Glycyrrhiza polysaccharide, as a feed additive, enhanced growth performance and serum immunoglobulin level, increased cecum flora abundance and diversity in broilers (8). Additionally, Glycyrrhiza polysaccharide increased intestinal secretory immunoglobulin A (SIgA) level, promoted the secretion of goblet cells, and improved the intestinal barrier function in broilers (9).
Probiotics, a class of microorganisms, promote animal growth and health (10), with Lactobacillus being one of the most extensively studied and utilized probiotics in poultry production. Lactobacillus used fermentable carbohydrates to produce lactic acid, lowering intestinal pH, inhibiting harmful bacteria growth, improving the structure of intestinal mucosa and absorption of nutrients, which results in an increased growth performance in poultry (11, 12). Research has demonstrated that Lactobacillus plantarum enhances the growth performance, serum immunoglobulin content, and cecal flora in broilers (13). Lactobacillus acidophilus increased body weight gain and feed conversion ratio in Ross broilers (14). Furthermore, Lactobacillus promoted the recovery of intestinal mucosa and preserved the integrity of intestinal epithelial barrier by regulating the expression of tight junction proteins and inflammatory cytokines (15).
GUE, a water-soluble extract of Glycyrrhiza uralensis containing various bioactive components, promoted the colonization of beneficial bacteria while inhibiting the growth of harmful bacteria in the gut of broilers through its bioactive substances (16), thereby aiding probiotics like Lactobacillus in exerting their effects. Certain plant polysaccharides enhanced the growth of beneficial gut bacteria, while probiotics could also facilitate the absorption and utilization of active plant substances (17). Synbiotic benefits can also be driven by probiotic carbohydrate-metabolizing enzymes, enabling utilization of specific oligosaccharides and enhancing probiotic survival, as shown in epilactose-producing cellobiose 2-epimerase systems (18). Studies have confirmed the effects of Lactobacillus on growth performance in fast-growing broilers, as well as the synergistic benefits of synbiotics composed of probiotics and medicinal plants, such as Astragalus and their active components (19, 20). Our previous study also demonstrated that GUE effectively mitigated the damage caused by Deoxynivalenol (DON)-Zearalenone (ZEN) contamination (21); both GUE and Lactobacillus acidophilus as feed additives enhanced growth performance and improved the balance of intestinal microecology in the cecum, and their combined use showed an even more positive effect in Liangfenghua broilers (22), a medium-growing strain popular for its excellent meat quality. This study was conducted to further investigate their effects on carcass trait, meat quality, immune and antioxidant functions, and intestinal health, aiming to understand the mechanisms underlying their promotion of broiler production.
2 Materials and methods
The Glycyrrhiza uralensis extract (GUE; purity >98%; glycyrrhizin ≥10%; prepared from the root of Glycyrrhiza uralensis using water boiling extraction and ethanol precipitation methods; Yalan Pharmaceutical Co, Gansu, China) and the Lactobacillus acidophilus (Lac; Zhongxin Bio-Technology Co., Hebei, China; 3 × 109 CFU/g) used in the present study were commercial products. The number of viable Lactobacillus cells was quantified using the method described by Zeng et al. (23), and was consistent with product labels.
2.1 Ethics statement
All procedures involving in animals were performed following the Regulations for the Administration of Affairs Concerning Experimental Animals (Ministry of Science and Technology, China, 2004) and were approved and supervised by the Northwest Minzu University Animal Care and Use Committee (Permit No. xbmu-sm-20230513).
2.2 Experimental design, diets and management
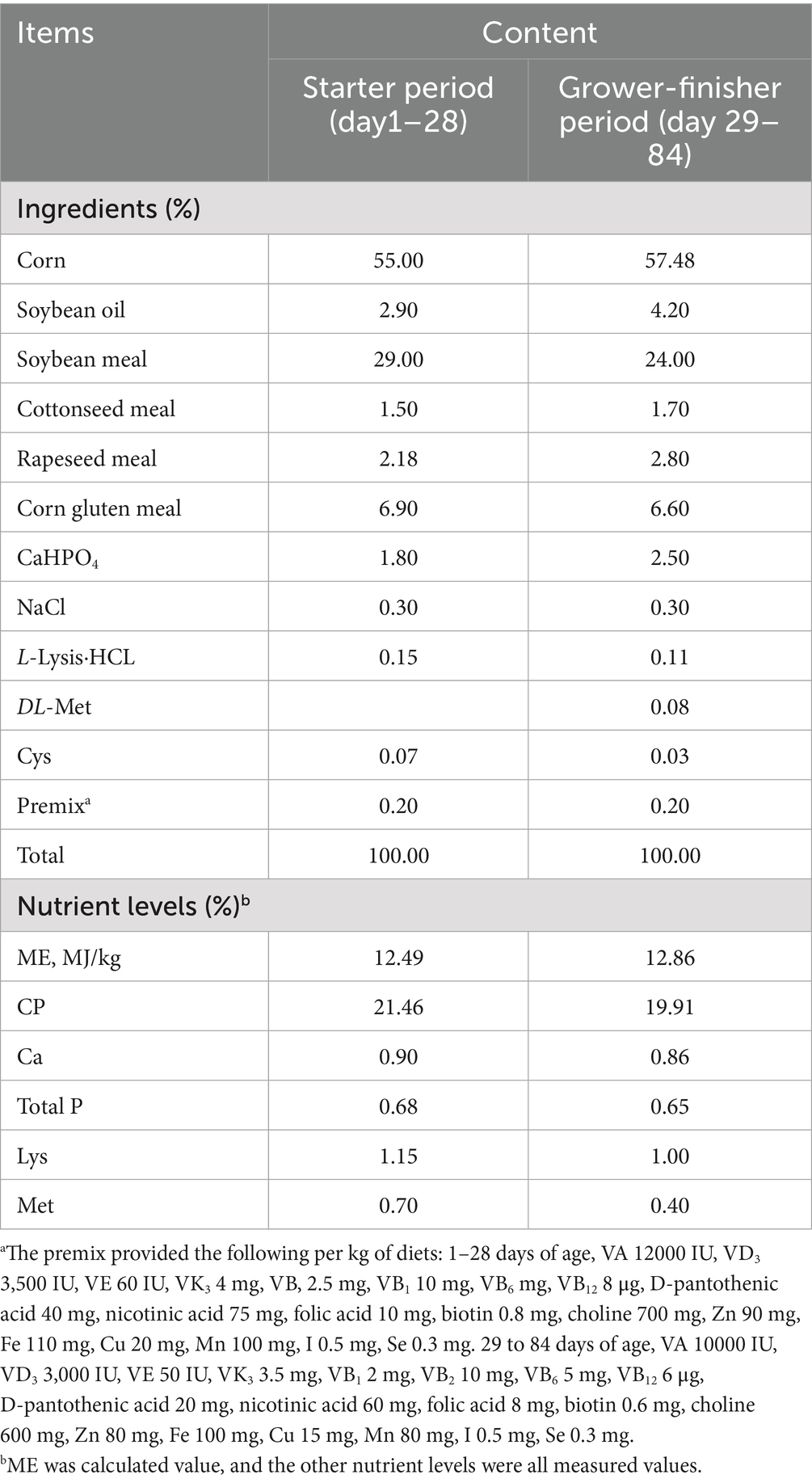
A total of 420 healthy one-day-old male Liangfenghua broilers were randomly divided into 4 groups, each with 7 replicates of 15 chickens, with no significant difference in initial body weight (40.26 ± 0.83) across groups. The broilers were allocated to the following dietary treatments: a basal diet (CON group), basal diet supplemented with 0.1% GUE (GUE group) (22, 24) basal diet supplemented with Lac at 4.5 × 10⁷ CFU/kg (Lac group), and basal diet supplemented with 0.1% GUE and 4.5 × 107 CFU/kg Lac (GUE+Lac group). The basal diet was formulated according to the broiler Feeding Standard in China (NY/T 33-2004) and was detailed in Table 1.
The trial was conducted at Gansu Agricultural Vocational Farm Co., Gansu, China. The chickens were housed in 3-layer ladder-type cages from 1 to 28 days old, and the nursery was preheated before the birds were introduced. The temperature inside the nursery was maintained at 34 °C with a relative humidity (RH) of 50% during the first week, gradually decreasing by 2 °C each week until the coop temperature reached 26 °C with RH at 45%. From 29 to 84 days old, the broilers were raised on the ground with the coop temperature at 26 °C and RH at 45%, ensuring the coop remained dry, hygienic, and well-ventilated. All the broilers were kept in a single room comprising four floor pens, each measuring 400 × 500 cm. Each pen had solid white plastic walls and was divided by wire mesh into 7 compartments. These compartments were equipped with a round feeder pan (diameter = 50 cm) and one nipple drinker. Cork shavings were used as bedding and were replaced every 3 days. The experiment lasted 84 days. During the test period, chickens had ad libitum access to feed and water. They were vaccinated with the Newcastle disease vaccine and the infectious bursal disease polyvalent vaccine at 7 and 14 days of age, respectively (21).
2.3 Sample collection and immune organ indices
On the last day of the starter stage (days 1–28), the grower stage (days 29–56), and the finisher stage (days 57–84), that was on day 28, 56 and 84 of the experiment, after a 12-h fasted feeding, 2 broilers were randomly selected from each replicate, with a total of 14 broilers per group for sampling. 5 mL of blood samples were collected from the wing vein of each individual, and serum was obtained by centrifuging at 3,000 rpm for 10 min. After that, the broilers were euthanized by severing the jugular vein and dissected immediately, and the thymus, bursa of Fabricius, and spleen were collected and weighed. The ratio of organ weight to body weight was used to determine relative organ weights (g/kg); approximately 5 g samples of liver, and breast muscle were collected; approximately 5 cm of intestinal segments from the middle of the jejunum (from the most distal insertion point of the duodenal mesentery to the junction with Meckel’s diverticulum) were excised. The jejunum samples were gently rinsed with ice-cold phosphate-buffered saline (PBS; pH 7.4) and split into two segments. One segment was immediately fixed in 10% paraformaldehyde solution for paraffin section, and the other was longitudinally cut and the mucosa gently scraped into a sterile tube using a sterilized glass slide for RT-PCR.
2.4 Apparent metabolic rate of nutrients
At 56 day of age, one broiler was chosen randomly from each replicate and housed in a metabolic cage with a tray for collecting excrement. All excreta were collected at 09:00 and 17:00 daily for three consecutive days. Following the addition of 10% sulfuric acid, the excreta was pooled per broiler and stored in a self-sealing bag at −20 °C until analysis.
The dry matter (DM), crude protein (CP), crude ash (Ash), crude fat (ether extract, EE), and crude fiber (CF) contents in excreta samples and representative dietary samples collected were determined using the standard procedures of the Association of Official Analytical Chemists (AOAC; 2007), after which their apparent metabolic rates were calculated.
2.5 Carcass traits
On day 84, two broilers with a body weight close to the average in each replicate were selected and killed after fasting for 12 h. Following bleeding and plucking, the carcass was individually weighed. Subsequently, the birds were eviscerated, and eviscerated carcass weights were measured. The half-eviscerated carcass weight was calculated by excluding the trachea, esophagus, intestines, spleen, pancreas, gallbladder, reproductive organs, and gizzard contents and corneum from the carcass. The eviscerated weight was calculated by removing the heart, liver, proventriculus, gizzard, lungs, and abdominal fat from the half-eviscerated carcass. The rates of dressing, half-eviscerated carcass, eviscerated carcass, breast muscle, thigh muscle (thigh and drumstick), and abdominal fat (fat around the abdomen and gizzard) were calculated as relative weight to the live BW, following “Technical specification for performance testing of meat-type chicken” (NY/T828-2004, China). Moreover, meat of the right breast was sampled for meat quality assessment.
2.6 Meat quality
The pH at 45 min (pH45 min) and 24 h (pH24 h) post-mortem were measured at three sites in the breast muscles using a pH meter (HP818M; XIMA Instrument, China). Each sample was analyzed three times at various points, and the average values were used. Meat colors were measured according to the CIE (Commission Internationale de L’Eclairage) system [Hunter-L* (lightness), a* (redness), and b* (yellowness) values] with a colorimeter (CR-18, DOHO Co., Shenzhen, China) after 45-min postmortem. A skinless meat sample with approximately 1 cm thickness and 2.5 cm diameter was cooked to an internal temperature of 70 °C, and shear force was determined using a C-LM3B shear apparatus (Tenovo, Beijing, China). Meat samples with about 1 cm thick and 2.5 cm in diameter were wrapped in a layer of gauze, placed between filter papers, and subjected to a force of 35 kg for 5 min using a determinator (MAEC-18, MINGAO Instrument Co., Nanjing, China). The water loss rate was calculated by comparing the sample weight before and after squeezing, then dividing by the initial sample weight. To determine the cooking rate, meat samples were cut into approximately 3 × 3 cm pieces, steamed in a pot for 30 min, and re-weighed after cooling to room temperature. The cooking rate was calculated by dividing the sample weight after steaming by the initial sample weight.
Approximately 2.0 g meat sample was freeze-dried using a vacuum freeze dryer and then ground to a homogeneous powder. The fat (ether extract, EE) in the sample was extracted using a Soxhlet extractor (SOX406, Hanon Advanced Technology Group Co., Ltd., Shandong, China) (GB 5009.6–2016, China). The protein content was determined by Kjeldahl method using Kd780 Kjeldahl Nitrogen Analyzer (Peiou Analytical Instrument Co., Ltd., Shanghai, China). The contents of crude fat and crude protein are expressed as percentages in the fresh samples, respectively.
2.7 Biochemical and immune indices in serum
The activities of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALP), and the contents of total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), very low density lipoprotein cholesterol (VLDL-C) and urea nitrogen (UN) in serum were determined using an automatic biochemical analyzer (URIT-8021, Guilin Ulit Medical Electronic Co, Guilin, China).
The levels of interleukin-2 (IL-2), interferon-γ (IFN-γ), and immunoglobulin A and G (IgA and IgG) in serum were measured using commercial ELISA kits (Solarbio Science & Technology Co., Beijing, China) according to the manufacturer’s instructions.
2.8 Antioxidant indices in serum and liver
Nine volumes of cold saline was added to 0.5 g of liver sample, which were ground in a glass homogenizer to prepare a tissue homogenate. The homogenate was centrifuged at 2000 rpm for 15 min, and the supernatant was stored at −20 °C. The activities of glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD), and malondialdehyde (MDA) content in the supernatant and serum were measured using commercial assay kits (Servare Bioetechnology Co., Wuhan, China) according to the manufacturer’s instructions.
2.9 Jejunum morphological analysis
Jejunum samples fixed in paraformaldehyde were embedded in paraffin, sliced, dehydrated, and stained with hematoxylin and eosin. The histological changes in the jejunum were observed using a CX22 light microscope (OLMPUS, Tokyo, Japan) (24). Three fields were selected for each section, which contained 8 to 10 intact villi in each view. Images were captured using an Olympus microsystem (Tokyo, Japan), and the determination of villi height and crypt depth was performed using Image-Pro Plus image analysis software. Subsequently, the ratio of villus height to crypt depth (VH/CD) was calculated.
2.10 Real-time PCR analysis
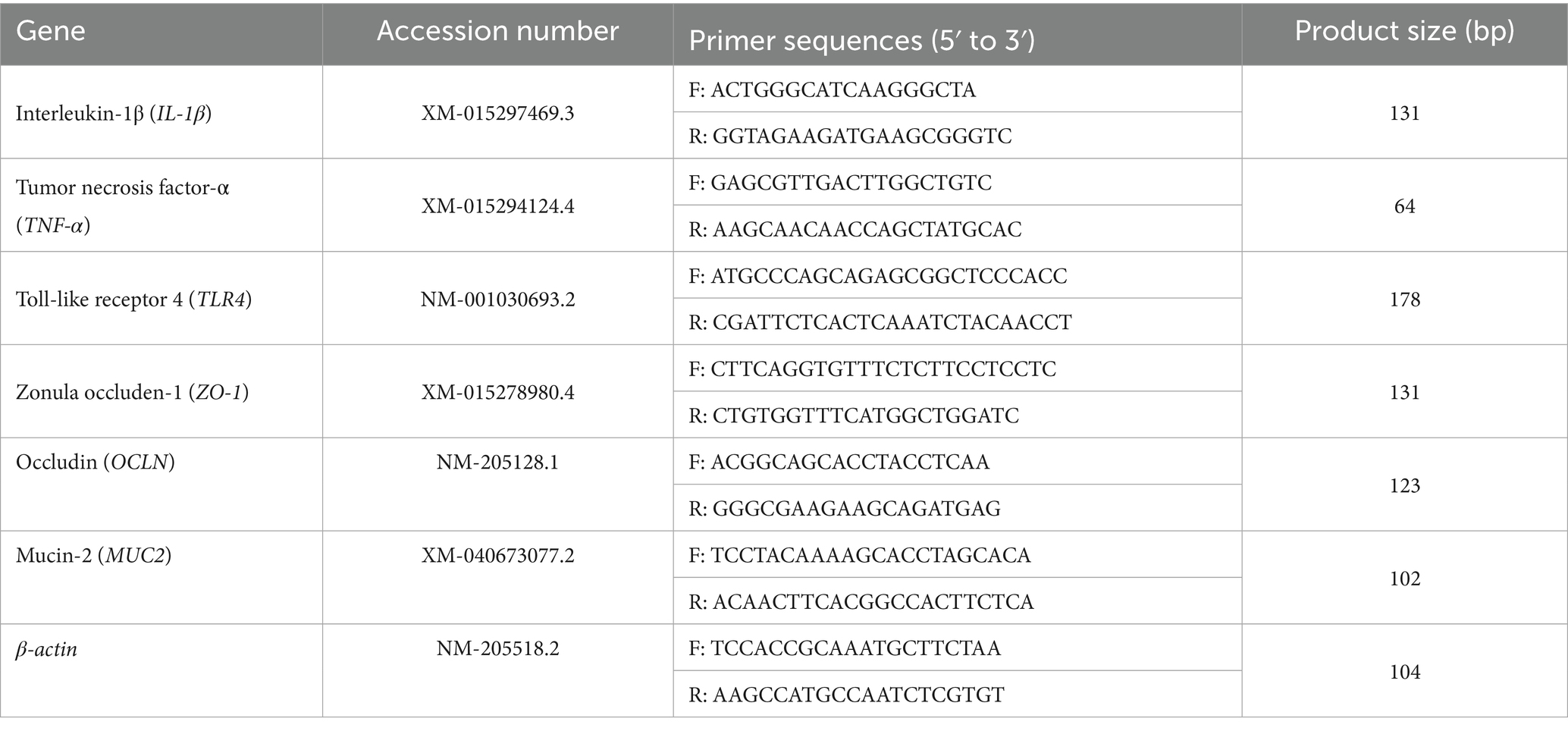
Total RNA was extracted from the jejunum mucosa using RNAiso Plus Kit (Accurate Biology, Changsha, China). The purity and concentration of RNA were determined using Nano drop One (Thermo, America). When the A260/A280 ratio of RNA falled within the range of 1.8–2.1, it was used to synthesize cDNA. The cDNA was synthesized with a reverse transcription kit (TaKaRa Biotechnology, Beijing, China) following the manufacturer’s instruction. The primer sequences used in Real-time fluorescence quantitative PCR were listed in Table 2. Amplification was performed in a total volume of 10 μL containing 5 μL of SYBR Green PCR Master Mix (TaKaRa Biotechnology, Beijing, China), 0.4 μL of each primer, 1 μL of cDNA, and 3.2 μL of ddH2O. The reaction conditions were as follows: denaturation at 95 °C for 30 s; followed by 40 cycles of 95 °C for 5 s, 60 °C for 30 s, and 72 °C for 30 s. The normalization stability of β-actin gene expression was tested, confirming it as an endogenous gene suitable for normalizing the target gene data. The relative fold changes in the expression of target genes were calculated by the 2−ΔΔCt method, with β-actin serving as the internal control gene to normalizing target gene expression.
2.11 Statistical analysis
The data were analyzed using SPSS software version 25.0 (IBM Corp., NY, USA). A two-way ANOVA (using the General Linear Model, GLM) was conducted to analyze the main effects of the GUE and Lac factors and their interaction. One-way analysis of variance (ANOVA) and post-hoc comparisons (Duncan’s test) were performed to assess differences among the GUE, Lac, and GUE+Lac groups. GraphPad Prism 9.0 (GraphPad Software Inc., CA, USA) was utilized for data visualization. Data variation was expressed as standard error of the mean (SEM), and the significance level was set at p < 0.05.
3 Results
3.1 Carcass characteristics and meat quality
The effects of the supplements on carcass traits and meat quality on d 84 were presented in Table 3. Broilers fed diets with GUE, Lac, and GUE+Lac showed a significantly higher live body weight (BW) and evisceration rate (p < 0.05), and a lower abdominal fat rate (p < 0.05) compared to the CON group. However, no significant differences (p > 0.05) were observed in the other traits tested. Furthermore, the combined GUE+Lac group showed significantly higher BW and lower abdominal fat rate compared to both the GUE and Lac groups, exhibiting a significant interaction effect on these measures (p < 0.05).
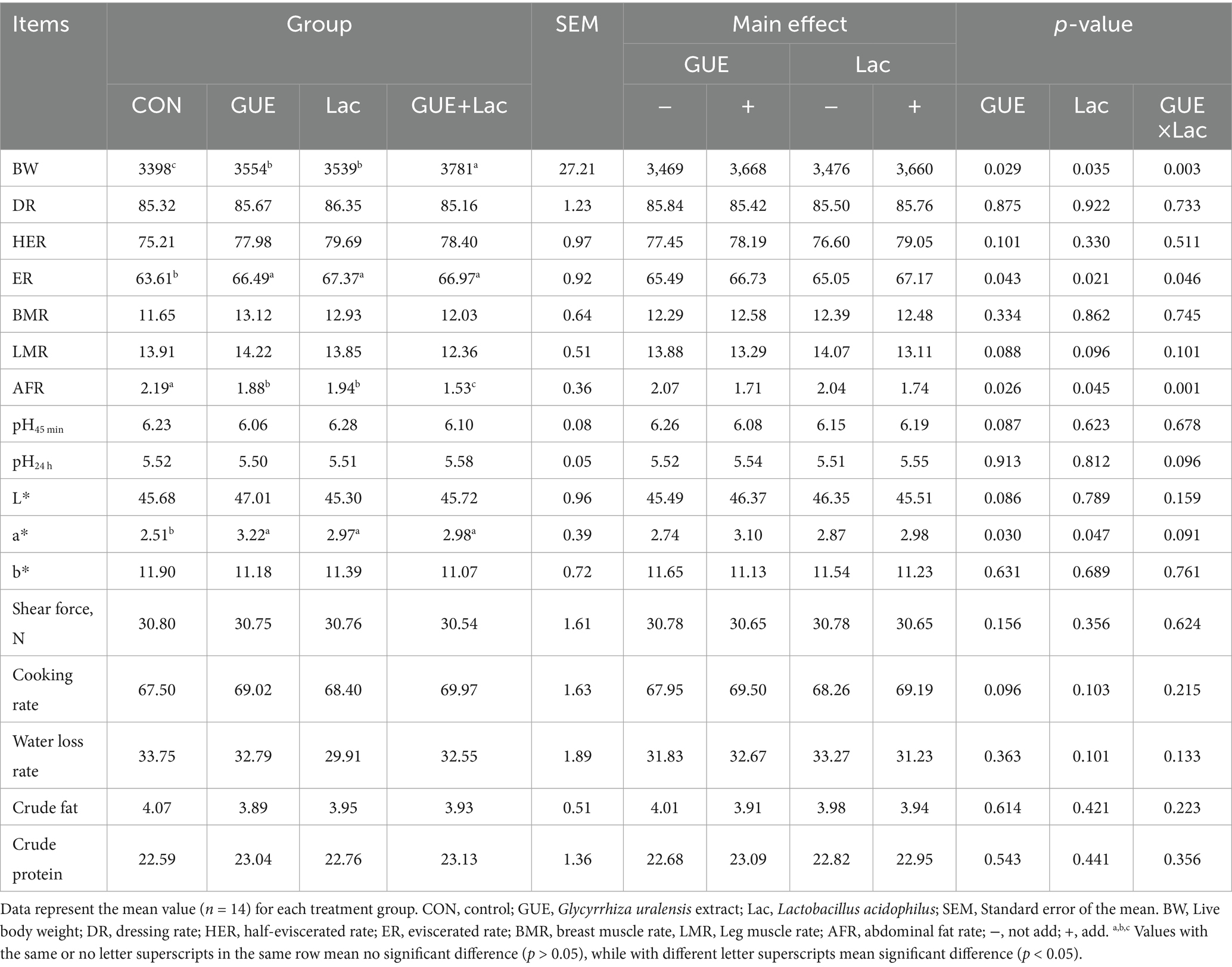
Table 3. Effects of GUE, Lac, and their combined supplementation on carcass characteristics and meat quality of broilers at 84 days of age (%).
There were no significant differences (p > 0.05) in pH45 min, pH24 h, lightness (L*) value, yellowness (b*) value, shear force, cooking rate, water loss rate, crude fat and crude protein contents of breast meat in broilers among the groups. Likewise, the GUE+Lac treatment did not yield reciprocal effects (p > 0.05). However, the redness (a*) values of breast meat was significantly higher (p < 0.05) in the GUE, Lac and GUE+Lac groups compared to CON group, although no interaction effect in the GUE+Lac group was observed (p > 0.05). These results suggested that both GUE and Lac supplementation enhanced growth and improved carcass quality, and their combined application had a synergistic effect, while having a limited effect on meat quality in broilers.
3.2 Apparent metabolic rate of nutrients
Compared to the CON group, the apparent metabolic rates of CP, EE, and CF in broilers significantly increased (p < 0.05) by 9.56, 3.18, and 6.45% with GUE; 7.31, 6.34, and 10.32% with Lac; and 13.53, 8.47, and 20.97% with GUE+Lac, respectively (Table 4). Notably, the combined GUE+Lac group exhibited the highest apparent metabolic rates of CP, EE, and CF, with a positive interactive effect (p < 0.05).
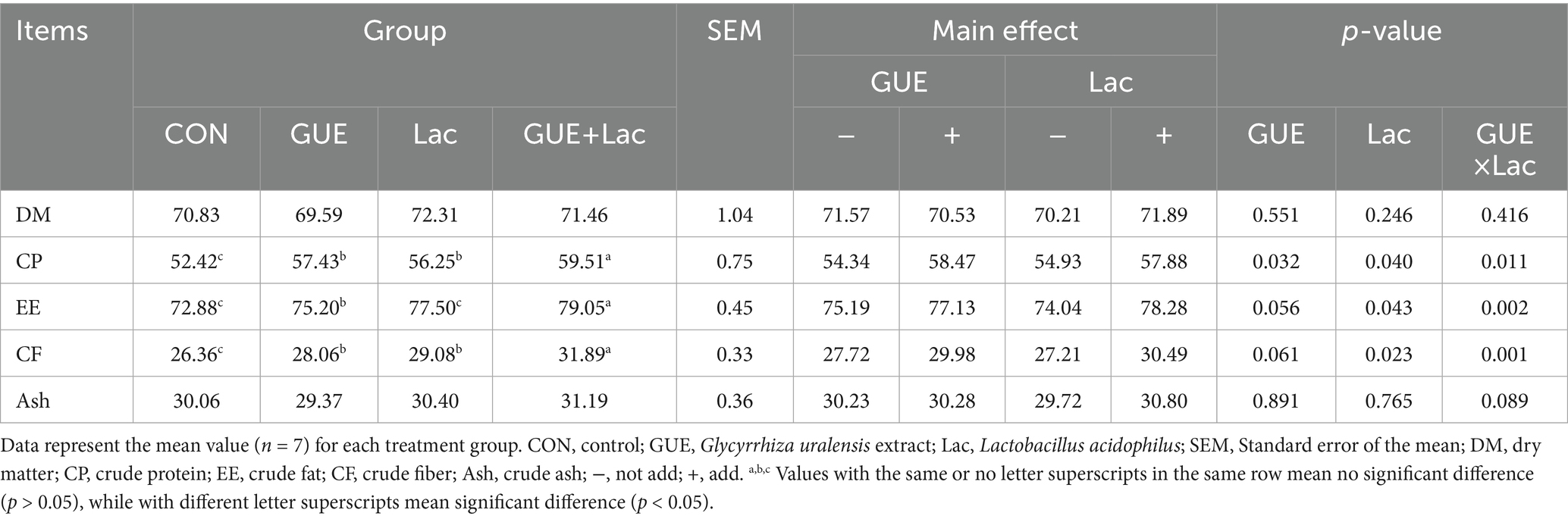
Table 4. Effects of GUE, Lac, and their combination on apparent metabolic rates of nutrients in broilers (%).
3.3 Immune organ indices
Lac supplementation increased (p < 0.05) the bursa index at 28 days and spleen index at 56 days. Supplementary GUE and GUE+Lac significantly increased the indices of thymus and bursa of Fabricius at 28 days and that of spleen at 56 days, and the combined application GUE+Lac exhibited a positive interactive effect (p < 0.05). No significant differences (p > 0.05) were observed in these indices at 84 days among groups (Table 5).
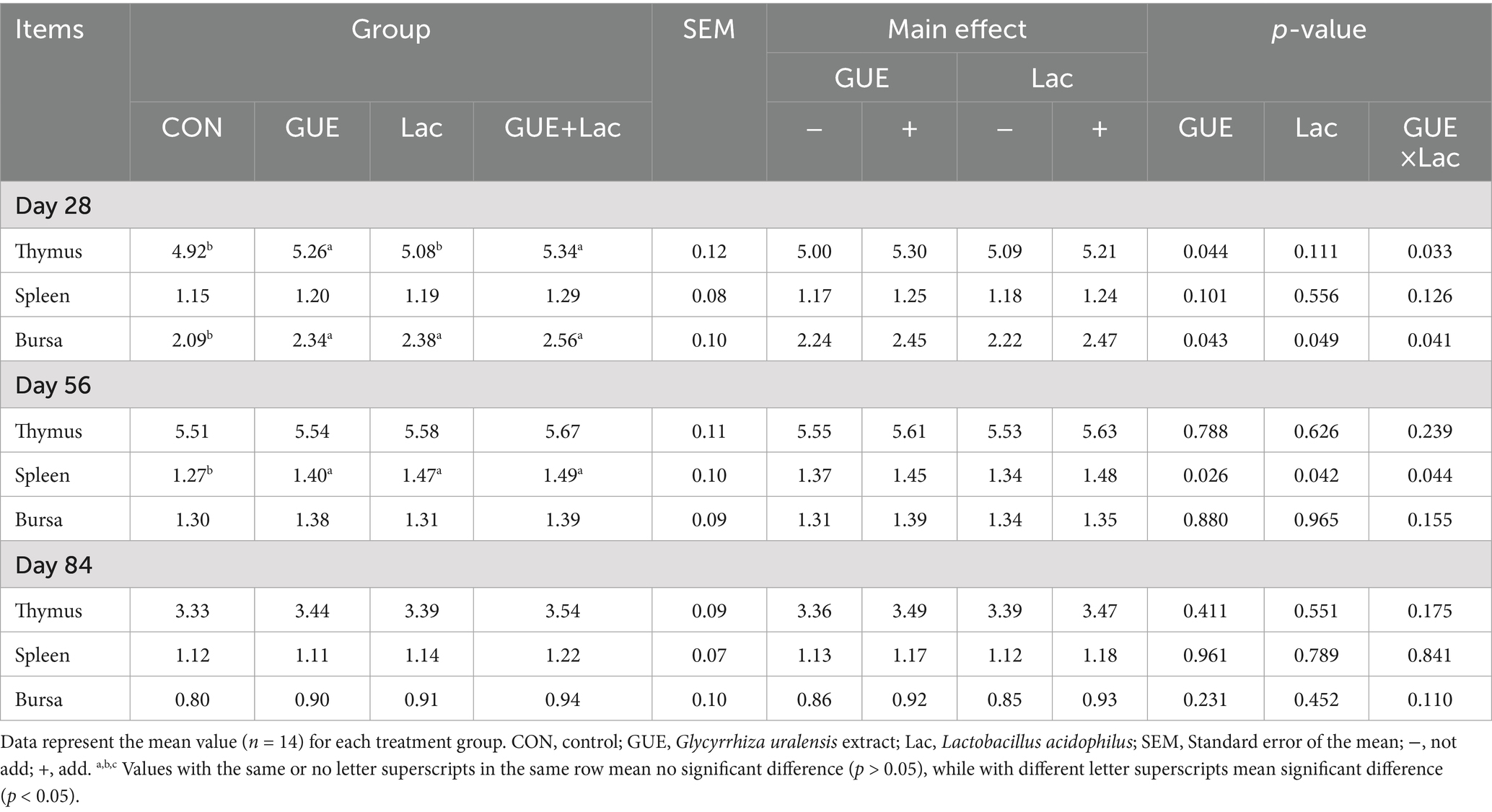
Table 5. Effect of GUE, Lac and their combined supplementation on immune organ indices of broilers (g/kg).
3.4 Serum biochemical parameters
The activity of ALT, AST, and ALP, as well as the content of HDL-C and UN in the serum of 28- and 84-day-old broilers, were not affected (p > 0.05) by any of the supplements. However, TC, TG and VLDL-C content in 28- and 84-day-old broilers and LDL-C content in 84-day-old broilers were significantly reduced (p < 0.05) by the supplements (Table 6). The GUE+Lac group showed a interactive effect (p < 0.05), leading to a significant elevation in serum LDL-C and TC levels compared to the other groups.
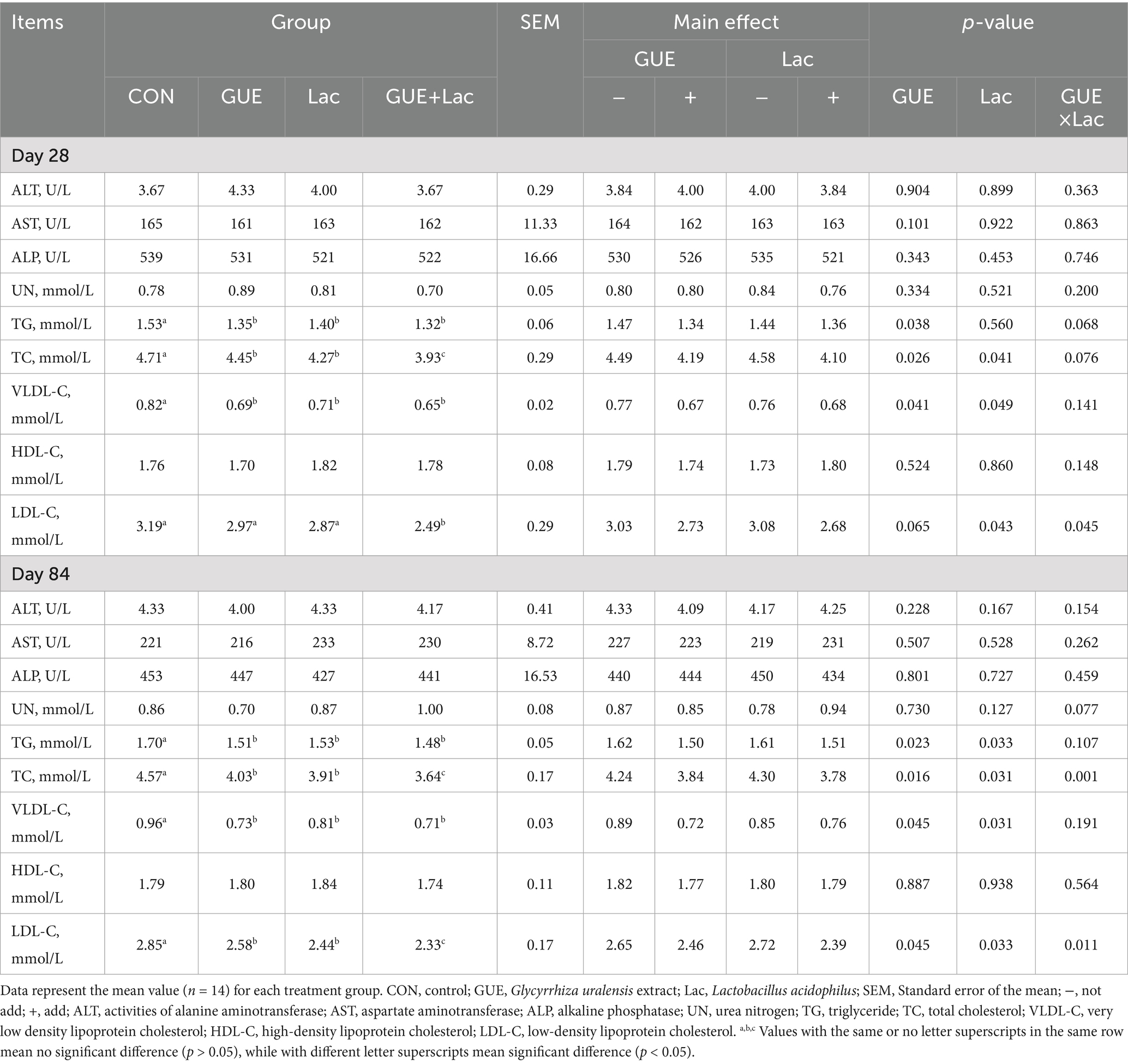
Table 6. Effects of GUE, Lac, and their combined supplementation on serum biochemical parameters of broilers.
3.5 Immune and inflammatory factors in serum
The levels of INF-γ and IgG in the serum of 28-day-old broilers were not affected (p > 0.05) by the supplements (Figure 1). However, Lac significantly increased IL-2 content, and the combined application of GUE+Lac exhibited a positive interactive effect, leading to increased IL-2 and IgA content compared to the CON group (p < 0.05). At 56 days of age, the serum content of INF-γ, IL-2, and IgA in broilers was significantly increased (p < 0.05) by the supplements. Additionally, the GUE+Lac treatment demonstrated a significant interaction effect, resulting in higher INF-γ and IgA levels compared to the other groups (p < 0.01). By 84 days of age, both GUE and Lac significantly increased (p < 0.05) the levels of IgA and IgG, and the GUE+Lac group showed a positive interactive effect, with the highest INF-γ, IgA and IgG levels among all groups (p < 0.05).
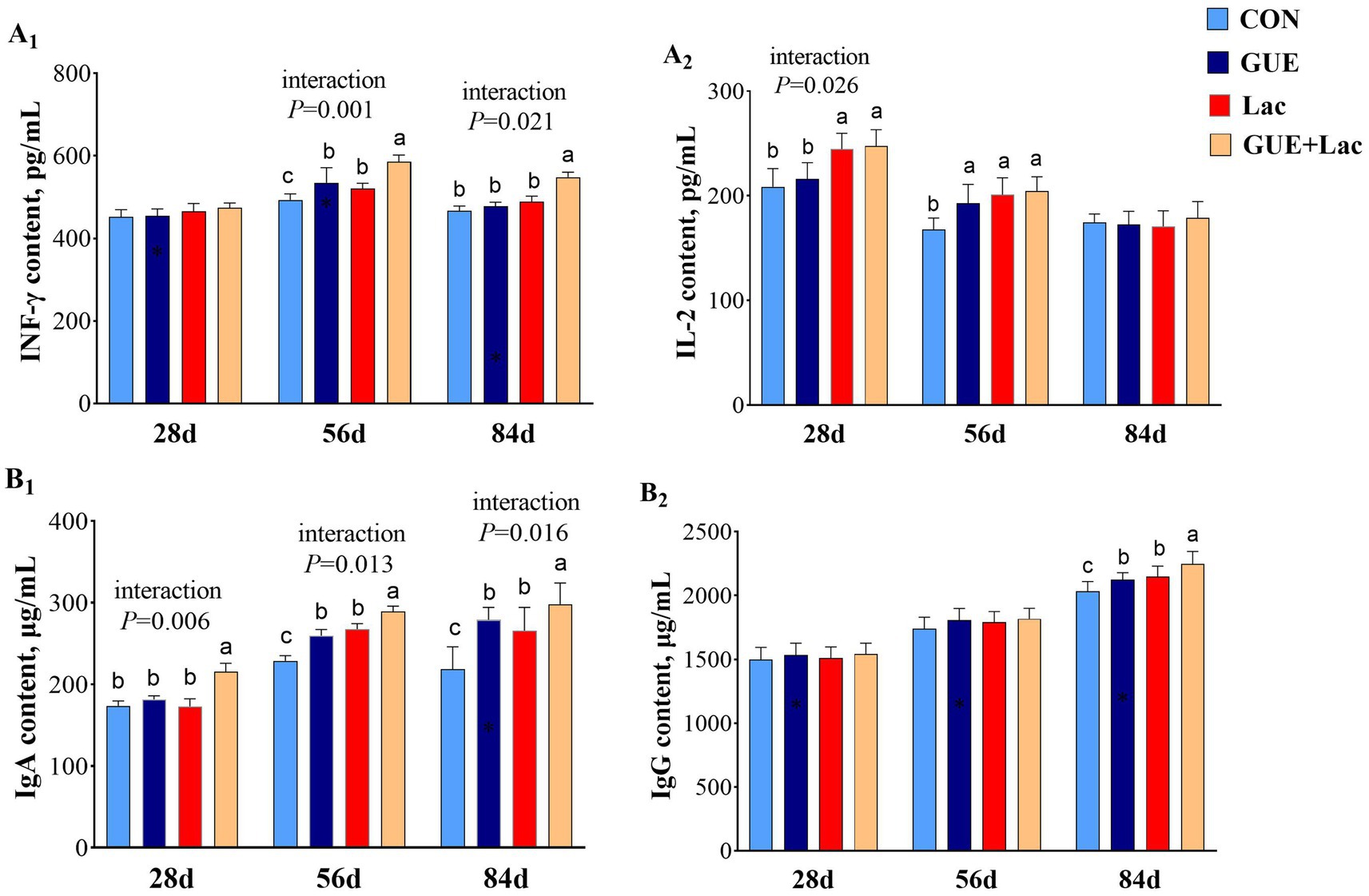
Figure 1. Effects of GUE, Lac, and their combined supplementation on serum immune parameters. CON: control; GUE, Glycyrrhiza uralensis extract; Lac, Lactobacillus acidophilus; IFN-γ, interferon-γ; IL-2, interleukin-2; IgA, immunoglobulin A; IgG, immunoglobulin G. Data are presented as mean ± SD (n = 14). a,b,cMeans denoted by different superscripts are significantly different (p < 0.05).
3.6 Serum and liver antioxidant parameters
Compared to the CON group, both the GUE and GUE+Lac groups significantly increased (p < 0.05) SOD and GSH-Px activities and reduced MDA content in the serum across all three age groups. Lac significantly increased (p < 0.05) the SOD and GSH-Px activities and reduced serum MDA content, but had no significant effect on SOD at day 28 (p > 0.05). Additionally, the GUE+Lac group showed an interactive effect on SOD and GSH-Px activities, resulting in significantly elevated GSH-Px activities at day 28 and 56 (p < 0.05) (Figure 2A1–A3). The effects of supplements on liver antioxidant indices in broilers were presented in Figure 2B1–B3. Both GUE and GUE+Lac supplementation significantly increased (p < 0.05) the activities of SOD and GSH-Px, while decreasing MDA content in the liver across all three age groups, but had no significant effect on SOD activity at day 28 (p > 0.05). Lac supplementation increased (p < 0.05) GSH-Px activity at day 28 and 56, SOD activity at day 84, and decreased (p < 0.05) MDA content at day 56 and 84. Additionally, the GUE+Lac group showed an interactive effect on GSH-Px at day 28, SOD and GSH-Px at day 28, and SOD at day 84, leading to significantly elevated activities of GSH-Px at day 28 and SOD at day 84 compared to the GUE and Lac groups (p < 0.05).
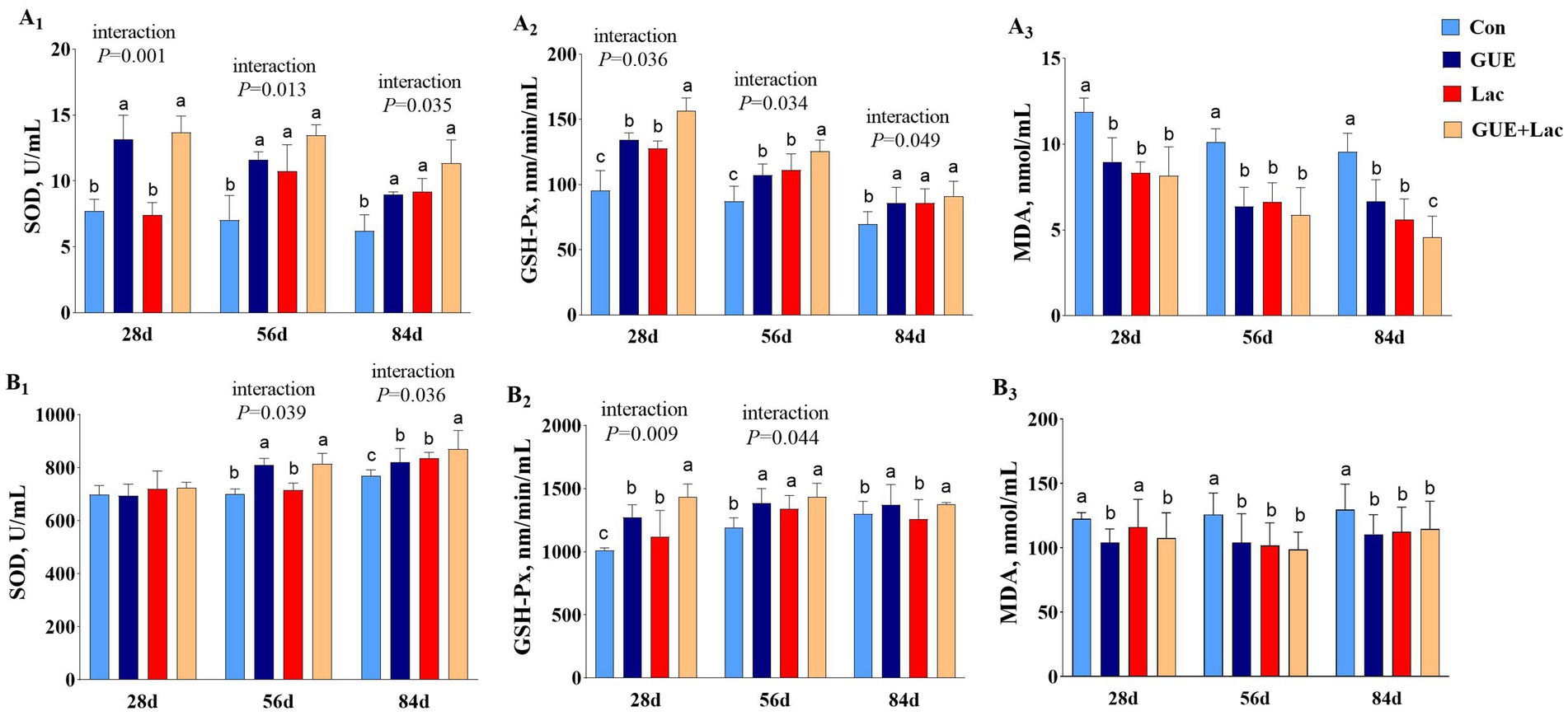
Figure 2. Effects of GUE, Lac, and their combined supplementation on serum (A1–A3) and live (B1–B3) antioxidant parameters. CON, control; GUE, Glycyrrhiza uralensis extract; Lac, Lactobacillus acidophilus; SOD, superoxide dismutase; GSH-Px, glutathione peroxidase; MDA, malondialdehyde. Data are presented as mean ± SD (n = 14). a,b,cMeans denoted by different superscripts are significantly different (p < 0.05).
3.7 Intestinal morphology in the jejunum
The supplements significantly increased (p < 0.01) the villus height and the ratio of villous height to crypt depth (VH/CD) in the jejunum of broilers at day 28, 56, and 84, with no significant effect (p > 0.05) on the crypt depth (Figure 3; Table 7). Furthermore, the GUE+Lac group significantly increased (p < 0.01) the villus height and VH/CD at day 28 and 56 compared to GUE and Lac groups, though no significant interaction effect was observed (p > 0.05).
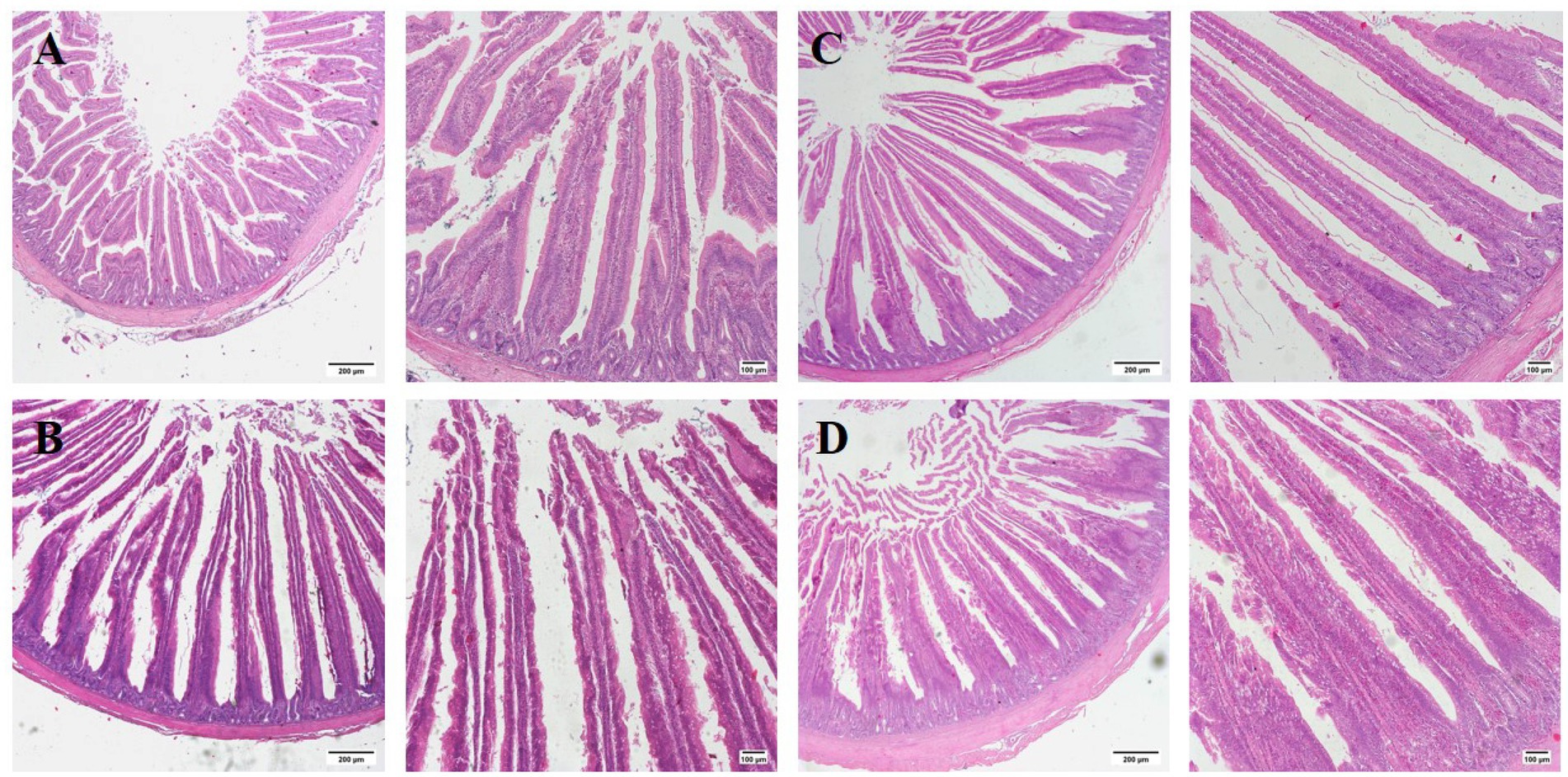
Figure 3. Histological representations of the H and E-stained jejunal sections of broilers at 28 days of age. (A) CON group; (B) GUE group; (C) Lac group; (D) GUE+Lac group. Scale bars represent 200 μm and 100 μm, respectively.
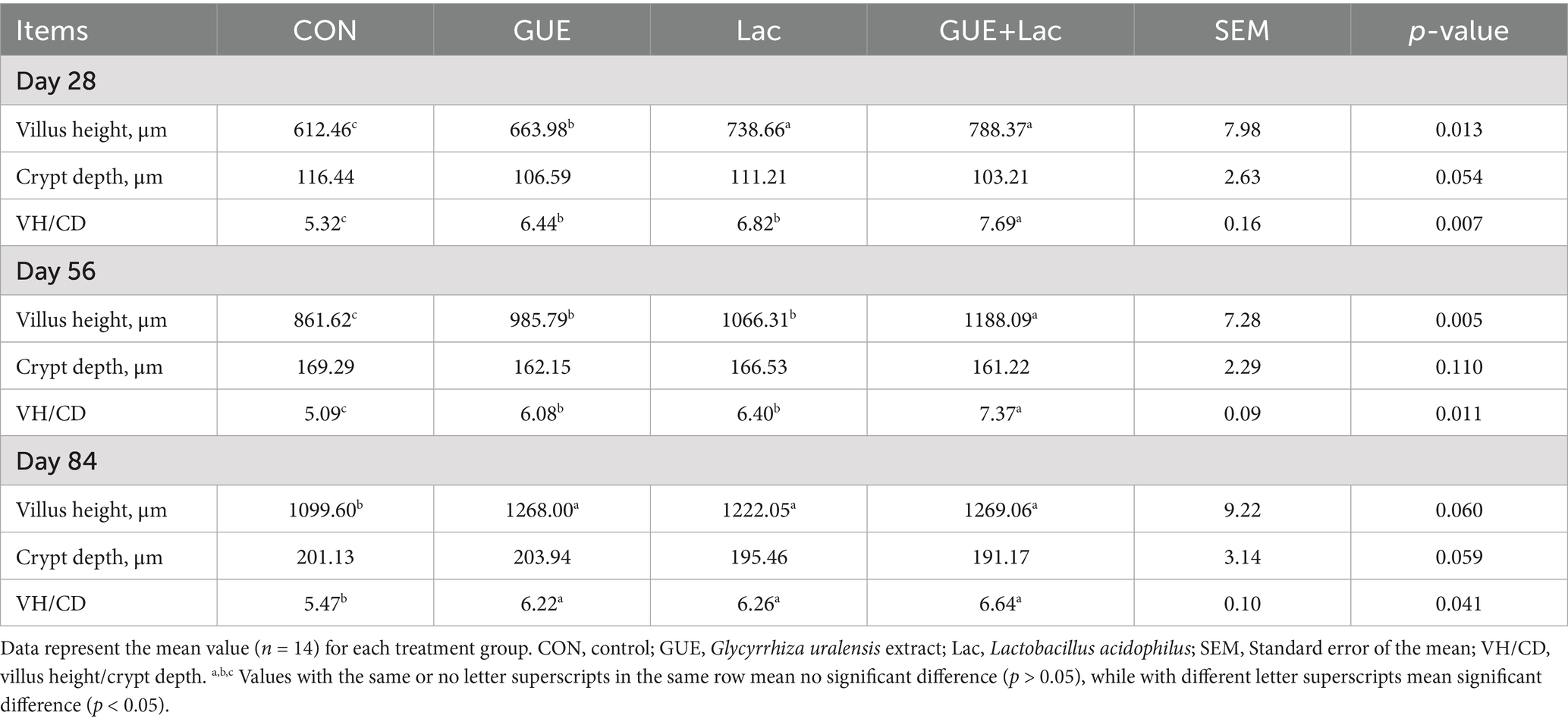
Table 7. Effects of GUE, Lac, and their combined supplementation on the jejunal morphology in broilers.
3.8 Expression of inflammatory cytokines and tight junction-related proteins in jejunal mucosa
Supplementation of both GUE and Lac reduced the expression of IL-1β and TLR4 in the jejunal mucosa of broilers at 28, 56 and 84 days, and the TNF-α expression at days 28 and 56. GUE+Lac showed an interactive effect, leading to further decreased the expression of IL-1β at day 28 and TNF-α and TLR4 at day 56 compared to the GUE and Lac groups (p < 0.05) (Figure 4A1–A3). Both GUE and Lac upregulated the expression of ZO-1 and MUC2 at days 28, 56 and 84, and the expression of OCLN at days 28 and 84 (p < 0.05). Additionally, the interaction of GUE+Lac was observed, which increased the expression of ZO-1 and OCLN at days 28 and 84 compared to the GUE and Lac groups (p < 0.05) (Figure 4B1–B3).
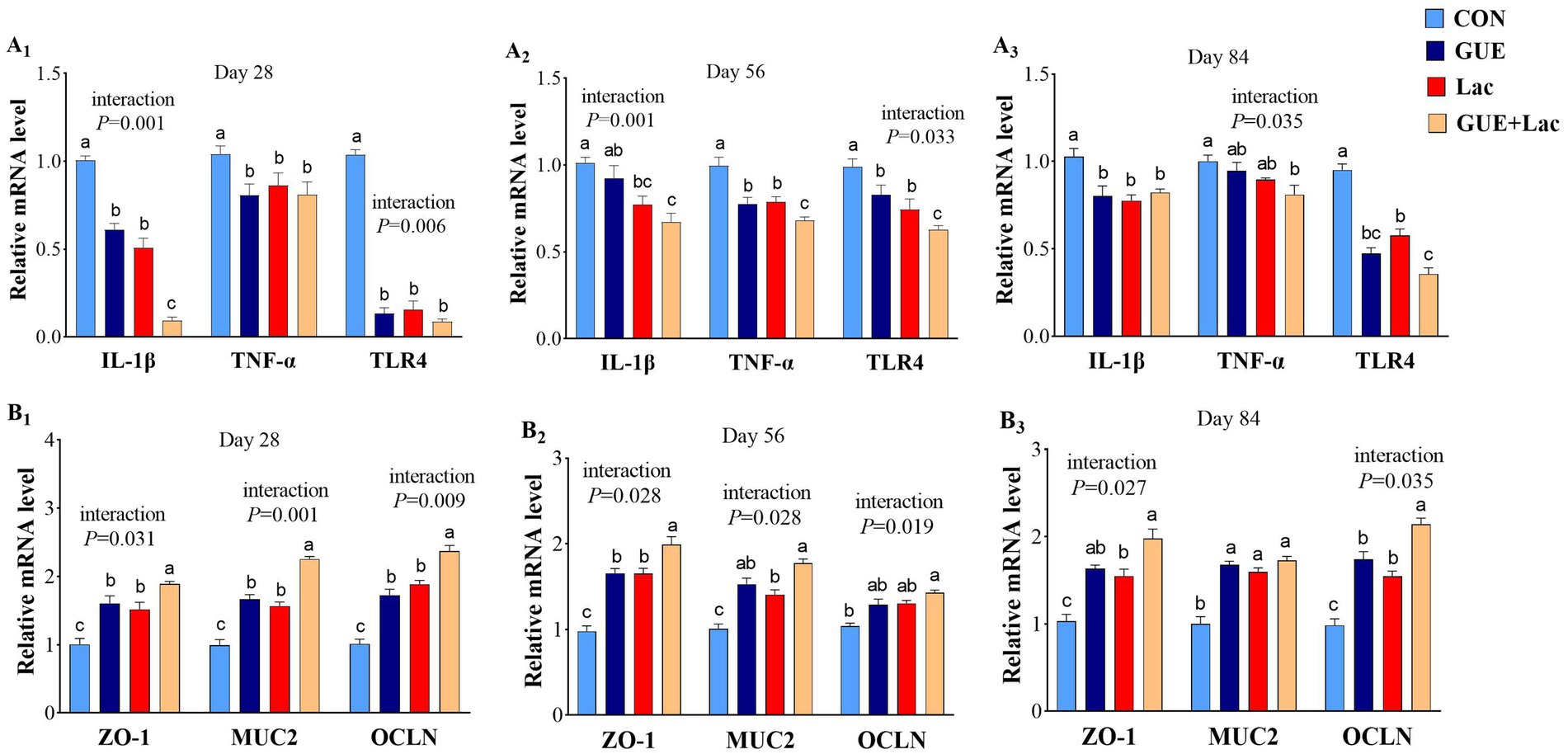
Figure 4. The mRNA expression of inflammatory cytokines (A1–A3) and tight junction-related proteins (B1–B3) in the jejunum. CON, control; GUE, Glycyrrhiza uralensis extract; Lac, Lactobacillus acidophilus; ZO-1, zonula occluden-1; MUC2, mucin-2; OCLN, occludin. Data are presented as mean ± SD (n = 14). a,b,cMeans denoted by different superscripts are significantly different (p < 0.05).
4 Discussion
Dietary supplementation with GUE increased BWG in broilers reared at high-density in both the grower and total phase (25), as well as FCR of broilers (26). L. acidophilus boosted feed efficiency and immune function in broilers by regulating nutrient metabolism and inflammation responses. Our previous study showed that supplementation with both GUE and L. acidophilus increased the ADG in grower-finisher phase, with their combined use yielding a more favorable effect in Liangfenghua broilers (22). Building on these findings, we conducted further investigations into the effects of GUE and Lactobacillus acidophilus, particularly, their combined supplementation on carcass performance, immunity, antioxidant capacity, and intestinal health in broilers.
4.1 Carcass characteristics and meat quality
Carcass traits encompass the quality and characteristics of animal carcasses after slaughter, serving as a comprehensive indicator for assessing production performance and economic value. In this study, both GUE and Lac supplementation increased the body weight and eviscerated percentage, and decreased the abdominal fat percentage of broilers. We also observed that GUE had no effects on other meat quality indicators of breast muscle but increased the a* value, indicating a slight improvement in meat color (27). Similarly, dietary GUE reduced abdominal fat rate but had no effect on breast muscle rate in broilers (28, 29). Dietary L. acidophilus increased the leg muscle rate and reduced the abdominal fat rate in broilers (30, 31). Lactobacillus cultures did not affect the a* and L* values of breast and leg muscle but increased the b* values of leg muscle in broilers (32). These findings suggested that both GUE and L. acidophilus supplementation could improve carcass quality to some extent by reducing abdominal fat and increasing total net carcass weight, without negatively affecting meat quality in broilers.
Additionally, both supplementary GUE and L. acidophilus increased the apparent metabolic rates of CP, EE, and CF in broilers. These findings indicated that, similar to fast-growing broilers (33), L. acidophilus enhanced production performance and feed utilization in medium-growing broilers by improving the digestion, absorption, and utilization of feed nutrients. Previous studies indicated that Lactobacillus enhanced the growth performance and the apparent metabolic rate of CP in broilers (20, 34). These effects can be related to the improvement of structure of intestinal flora (34), intestinal morphology, and the abundance of bacteria producing short-chain fatty acids (35). Glycyrrhiza uralensis alleviated intestinal inflammation by inhibiting pro-inflammatory factors (e.g., IL-6 and IL-1β) (36), and mitigated ulcerative colitis by repairing mitochondrial damage and increasing SOD, GSH-PX, and IL-10 levels in intestinal epithelial cells (37). These mechanisms collectively maintained the synthesis and activity of digestive enzyme by preventing inflammation and oxidative stress. Accordingly, both GUE and L. acidophilus supplementation could improve the production performance of broilers by promoting the digestion and absorption of nutrients (38).
While previous studies have explored the combined use of probiotics and medicinal plant extracts, the effects of their feeding effects appeared to be influenced by various factors, including bacterial strains, bioactive substances in medicinal plants, and the species and growth stages of animals. For example, Astragalus and L. plantarum together enhanced the growth performance of broilers (19), whereas the combination of complex probiotics with Astragalus polysaccharide did not show a similar effect in growing-finishing pigs (39). Our study revealed that the combination of GUE and Lac synergistically promoted the growth and improved the carcass traits by enhancing nutrient digestion and absorption and decreasing abdominal fat rate in broilers. The integration of plant extracts with probiotics enhanced the medicinal effects of plant extracts by leveraging the catabolic properties of probiotics (40). GUE increased the cecal abundance of Lactobacillus gallinarum and Lactobacillus reuteri in broilers (22). Human intestinal fungus metabolized 18β-glycyrrhetinic acid with low intestinal bioavailability into metabolites with inhibiting the activation of nuclear factor-kappa B (NF-κB) signaling pathway (41). Similarly, probiotic cocktails enhanced the effects of baicalin by accelerating its conversion into highly active compounds in the ileum, thereby increasing the abundance of short-chain fatty acid-producing bacteria in broilers (42).
4.2 Blood biochemistry parameters
Serum ALT, AST, and ALP activities reflect liver function, while UN content provides valuable insights into protein metabolism within the body. Our results showed that GUE, Lac and GUE+Lac did not significantly alter ALT, AST and ALP activities, nor UN and HDL-C levels in broiler serum. This indicated that these supplements are safe, do not impair liver function, and have no adverse effects on protein metabolism. In contrast, L. plantarum improved liver function by reducing ALT and AST levels in DON-challenged broiler chickens (43), while 18β-glycyrrhetinic acid alleviated DON-induced liver injury by inhibiting nuclear receptor coactivator 4-mediated ferritinophagy and ferroptosis (44).
Serum TG and TC levels serve as important indicators of lipid transport in the body. HDL is involved in cholesterol removal and transport triglycerides back to the liver, LDL is responsible for delivering cholesterol to cells, and VLDL transports triglycerides from the liver to other tissues. These lipoproteins offer an insight into overall lipid metabolism. The Glycyrrhiza uralensis supplementation lowered serum TC and TG levels (45), and GUE reduced serum TC and LDL levels in broilers (29). L. salivarius decreased the serum TC, LDL-C and TG levels of broilers (46). Lactobacillus mixture reduced serum TC and TG levels but did not affect serum HDL-C and LDL-C levels in broiler (34). In this study, both GUE and Lac significantly reduced the serum TC, TG and VLDL-C levels in broilers at days 28 and 84, as well as serum LDL-C level at day 84, while their combination GUE+Lac group showed a interactive effect, leading to a more significant elevation in serum LDL-C and TC levels. This suggested that they promoted cholesterol and lipid metabolism, with their combination proving more effective, which was consistent with the decreased abdominal fat rate in broilers. Although the exact mechanism is not fully understood, previous research suggests that glycyrrhizic acid inhibits lipid peroxide formation and facilitates the conversion of cholesterol into bile acids (1), and prevents abnormal lipid metabolism in the liver by modulation of gut microbiota in rats fed a high-fat diet (47). Similarly, plant-derived pectic polysaccharides, such as those from Rubus chingii Hu., have been shown to inhibit intestinal lipid absorption in vivo, contributing to improved lipid profiles and reduced fat deposition (48). Lactobacillus decomposed cholesterol in the digestive tract and lowered serum cholesterol levels (49). It also incorporated cholesterol into bacterial cells and inhibited cholesteryl ester synthesis by reducing the activity of acetyl-CoA carboxylase and 3-hydroxy-3-methylglutaryl-CoA reductase (34).
4.3 Immunity
The immune system directly influences animal growth efficiency, metabolic balance, and overall health by defending against pathogens and maintaining physiological homeostasis. The immune organ index reflects the development and functional status of immune organs in animals. Supplementary GUE increased the indices of the thymus and bursa of Fabricius at day 28 and the spleen at day 56, while Lac increased that of bursa of Fabricius at day 28 and the spleen at day 56, suggesting that these supplements could promote the development of immune organs in broiler chickens during early growth. However, both GUE and Lac had no significant effect on the immune organ index at day 84. This suggests that under normal conditions, the immune organs of Liangfenghua broilers at day 84 are likely fully developed and no longer influenced by external factors.
Immune factors play a critical role in the regulation of immune responses. Serum levels of IgA and IgG are considered indicators of humoral immunity. Studies showed that GUE enhanced antibody titers against specific and non-specific antigens, thereby improving broilers’ humoral immunity (26). Glycyrrhiza uralensis increased serum IgG levels in quail (50) and serum monocyte and granulocyte levels, enhancing innate or specific immune responses in laying hens (51). Some active components in GUE exhibit immune-enhancing and anti-inflammatory effects. Glycyrrhizic polysaccharides raised the serum IgA, IgG and IgM levels in broilers (8). Glycyrrhizic flavonoids increased the serum IgG content and improved the immune function in piglets (52). Additionally, L. plantarum increased serum levels of IgA and IgG in Daheng broilers (53) and serum levels of IgA and IgM in Cobb broilers (54). L. acidophilus increased the serum IgG level in broilers (33). The probiotic genus Lactobacillus exerts positive immunomodulatory effects on multiple immune responses, including cellular immunity, humoral immunity, and intestinal mucosal immunity (55). Our study demonstrates that the inclusion of GUE or Lac in the diet can enhance the broiler’s immunity, evidenced by increased serum levels of IgA at day 56 and IgA and IgG at day 84.
Cytokines have various functions, including regulating immune and participating in inflammatory responses. Serum levels of INF-γ and IL-2 are commonly used as indicators of immune strength (56). INF-γ serves as an immunomodulatory molecule, promoting the maturation of cytotoxic T lymphocytes, B cell proliferation, and antibody production (57). IL-2 enhanced the activities of T cells and stimulated B cells to produce antibodies, serving as a pivotal mediator in immune responses triggered by inflammation (58). Our study observed that GUE increased the serum levels of INF-γ and IL-2 at 56, and Lac increased the serum levels of IL-2 at day 28 and both INF-γ and IL-2 at 56, enhanced humoral immunity and anti-inflammatory capacity in broilers. Likewise, Glycyrrhizic polysaccharides raised the increased the immune organ index and serum cytokine IL-2 levels in mice (59). Glycyrrhizic flavonoids increased the serum IgG content and improved the immune function in piglets (52). L. plantarum increased serum levels of IL-2 and IFN-γ in Daheng broilers (53). L. acidophilus increased the serum levels of IL-2 and IL-4 in broilers (33).
Furthermore, the combined application GUE+Lac demonstrated a positive interaction effect, resulting in increased serum levels of IL-2 and IgA at day 28, INF-γ and IgA at day 56, and INF-γ, IgA and IgG at 84. These results suggested GUE and L. acidophilus could synergistically boost the immunity by stimulating the secretion of immune factors and anti-inflammatory cytokines in broilers. This could be interpreted as L. acidophilus accelerating the biotransformation and absorption of the bioactive components in GUE by utilizing the catabolic properties of probiotics, while GUE promotes the proliferation of probiotics, thereby building a mutually reinforcing mechanism. However, further research is needed to fully understand this mechanism.
4.4 Antioxidation
The systemic antioxidant capacity is crucial for the health and growth of broilers. An increase in antioxidant capacity is associated with improved immune function and reduced inflammation and oxidative stress. The liver, as a key metabolic organ, is highly susceptible to oxidative stress due to its role in detoxification and biotransformation of xenobiotics that generate reactive oxygen species (ROS). Oxidative stress disrupts redox balance, promoting inflammation and impairing liver function (60). Antioxidant mechanisms included enzymes such as SOD, GSH-Px, and catalase (CAT), along with non-enzymatic molecules like glutathione (GSH), which are essential for neutralizing ROS and maintaining cellular homeostasis (61). Given the persistent challenges posed by oxidative stress, there is an urgent need for exogenous antioxidants that can bolster cellular defenses and support the immune system. GUE, as a natural antioxidant, inhibited mitochondrial lipid peroxidation, scavenged free radicals, and enhanced antioxidant enzyme activity through its active ingredients (62). GUE supplementation enhanced growth performance and increased the activities of SOD, CAT (63) and GSH-Px, while reducing MDA content in serum of broilers (64). Glycyrrhiza polysaccharide raised the serum levels of GSH and SOD in broilers, while simultaneously reducing the MDA level (65). Dietary GUE has also demonstrated benefits in enhancing liver antioxidant capacity of broilers. GUE reduced hepatic triglycerides and oxidative stress markers in broilers, as shown by decreased plasma cholesterol and improved lipid profiles (64). In this study, GUE significantly increased the activities of SOD and GSH-Px while decreasing MDA content in the serum and liver of broilers, indicating that GUE enhanced the systemic and hepatic antioxidant capacity by activating the antioxidant enzyme system, thereby alleviating oxidative stress and its harmful effects on health.
We also observed that Lac increased GSH-Px and SOD activities while reducing MDA content in the serum of broilers. It also enhanced hepatic GSH-Px activity at day 28 and 56, and SOD activity at day 84, while decreasing MDA content. These results indicated the beneficial effects of L. acidophilus supplementation on systemic and hepatic antioxidant capacity in broilers. Similarly, studies found that Lactobacillus casei effectively increased the levels of T-AOC and SOD while reducing the MDA level in broilers (66). Lactobacillus reuteri mitigated diquat-induced hepatic oxidative stress and inflammation in hens (67). Lactobacillus plantarum upregulated the expression of the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway in the liver, a key regulator of antioxidant responses (68). Lactobacillus decreased hepatic MDA level and increased total antioxidant capacity (TAC) by upregulating SOD and GSH-Px in broilers (69). However, Yu et al. (54) reported that dietary Bacillus coagulans, but not Lactobacillus plantarum, increased activities of GSH-Px and SOD, and decreased MDA level in the serum of broilers challenged by lipopolysaccharide. This indicates that Lactobacillus, as a probiotic, has the potential to act as a natural antioxidant in broilers; however, this effect depends on the strain type.
Additionally, the combination of GUE and Lac demonstrated significant interaction effects on SOD and GSH-Px, elevating serum GSH-Px activity at days 28 and 56, as well as hepatic GSH-Px activity at day 28 and SOD activity at day 84. GUE contains various bioactive components that can exert antioxidant and anti-inflammatory effects through multiple shared pathways. Glycyrrhetinic acid suppressed the expression of inflammatory factors by blocking NF-κB signaling pathway (70). Glycyrrhiza polysaccharides promoted the maturation and cytokine IL-12 secretion of dendritic cells (DCs) through toll-like receptor 4 (TLR4) and down-stream p38 and NF-κB signaling pathways (71). Glycyrrhizin maintained intracellular redox balance and eliminated mitochondrial ROS by enhancing the activities of SOD, CAT, and GSH-Px through downregulating high-mobility group box-1 protein (HMGB1)/TLR5 signaling (72). It also reduced inflammatory mediators such as NF-κB p65, IL-1β, and IL-18 by suppressing the HMGB1/TLR4 pathway (73). Glycyrrhiza flavonoids affected the levels of SOD, CAT, MDA, and inflammatory factors TNF-α, IL-6, and IL-1β by activating the PI3K-Akt signaling pathway (74). Furthermore, the bioactive components of Glycyrrhiza uralensis can undergo biotransformation by microorganisms to produce derivatives with enhanced pharmacological activity (41, 75). 18 β-glycyrrhetinic acid, a metabolite of glycyrrhetinic acid, exhibited pronounced inhibitory activity on the production of intracellular nitric oxide (76). Therefore, it can be inferred that supplementary Lac and intestinal probiotics, such as Lactobacillus augmented by GUE (22), enhanced the biotransformation and utilization of bioactive components of GUE (41), which synergistically improved antioxidant and anti-inflammatory capacity in broilers.
4.5 Intestinal health
Our previous study found that both GUE and L. acidophilus supplementation enhanced gut antioxidant capacity, promoted sIgA secretion, and modulated the cecal microbial community composition (22). Additionally, GUE effectively mitigated the damage to growth performance and intestinal health caused by deoxynivalenol (DON) and zearalenone (ZEN) contamination in broilers (21). In this study, we observed that supplementation with GUE and Lac significantly increased villus height, the VH/CD ratio in the jejunal mucosa of broilers. The intestinal villi serve as the primary site for nutrient absorption, and the structural integrity of the intestinal morphology significantly influences animal health. Increased villus height provide a larger the absorptive area, promoting nutrient uptake and optimizing production performance in broilers (77).
The intestinal barrier permits the uptake of essential nutrients and immune sensing while restricting pathogenic molecules and bacteria. Both structural and molecular components of the intestinal tract work together to perform this complex yet essential function. The integrity of the intestinal epithelium acts as a physical barrier against enteric pathogen invasion and ensures optimal nutrient absorption (78). Tight junctions (TJs) are the core structures that maintain intestinal barrier function, primarily composed of key proteins such as OCLN and ZO-1. MUC2 is the main component of the intestinal mucus layer, serving as the first physical and chemical barrier of the intestinal defense system. Upregulated TJ proteins improved barrier function and permeability, thereby reducing inflammation and oxidative stress (79). Our result indicated both GUE and Lac upregulated the mRNA expression of of ZO-1 and MUC2 in the jejunal mucosa at days 28, 56 and 84, as well as the expression of OCLN at days 28 and 84. Thus, the intestinal barrier function was enhanced. Several studies have documented the effects of Glycyrrhiza uralensis and its active components on the intestinal health of animals. Ibrahim et al. (16) found that GUE promoted the expression of junctional adhesion molecule 2 (JAM-2) and MUC-2 in the jejunal mucosa of broilers. Glycyrrhiza polysaccharide increased the villus height, the VH/CD ratio, and the expression of Ocludin, Claudin-1, and MUC2 in the mucosa of jejunum, ileum, and duodenum of broilers (8). Glycyrrhiza flavonoids improved the morphological structure of the intestine by increasing villus height and VH/CD in duodenum in piglets (80). Additionally, L.acidophilus has been shown to enhance intestinal health and protect against enteric pathogen invasion by increasing the VH/CD ratio and the expression of intestinal barrier function proteins in broilers (65, 81). L. plantarum enhanced the digestive enzyme secretion and increased nutrient digestibility by the increasing VH/CD ratio (82), and improved intestinal barrier function and permeability by upregulating the expression of MUC2, Occludin, and sIgA in the jejunum of broilers (83). These results suggest a promoting effect of GUE and Lac on intestinal development and functional improvement under normal conditions, consistent with the increased nutrient metabolism rate.
Cytokines are critical in regulating intestinal immune function and maintaining immune balance. GUE reduced the expression of TLR4 and IL-1β in the jejunal mucosa of broilers (16), and improved the microstructure of colonic mucosa and reduced the expression of TNF-α and IL-6 in rats (84). Glycyrrhiza chalcone inhibited TNF-α, IL-1β, and IL-6 expressions in the colon tissue of mice with colitis (85). Furthermore, L. acidophilus alleviated inflammatory responses by downregulating the expression of inflammatory cytokine IL-1β and IL-8 in the jejunum of broilers infected with Clostridium perfringens (81). In acute colitis mice, L. acidophilus reduced intestinal inflammation by suppressing the production of pro-inflammatory cytokine IL-6, TNF-α and IL-1β in colonic tissue (86). In this study, both GUE and Lac reduced the expression of IL-1β and TLR4 in the jejunal mucosa of broilers at 28, 56 and 84 days, and the TNF-α expression at days 28 and 56. This suggested that under normal conditions, GUE and Lac can improve the intestinal immune function in broilers.
The combined supplementation with GUE and Lac increased the villus height and VH/CD at days 28 and 56 compared to GUE and Lac groups. Furthermore, the interaction between GUE and Lac further upregulated expression of ZO-1 and OCLN at days 28 and 84, and decreased the expression of IL-1β at day 28 and TNF-α and TLR4 at day 56. This finding suggests that the selected combination of GUE and L. acidophilus more effectively enhances the intestinal morphology and promotes intestinal health in broilers. As mentioned previously, the combination could establish a mutually reinforcing mechanism: the active components in GUE promote the growth of probiotics, while the probiotics, in turn, enhance the host’s absorption and utilization of these active substances by improving intestinal barrier function and permeability. This aligns with evidence that microbial metabolites, such as butyric acid, regulate intestinal barrier integrity by modulating post-translational modifications of epithelial proteinsepithelial protein, including GAPDH lactylation and butyrylation, thereby influencing tight junction stability (87).
5 Conclusion
In conclusion, dietary supplementation with both GUE and Lactobacillus acidophilus enhanced systemic and hepatic antioxidant capacity, immunity, and intestinal health, while improving production performance by increasing digestion and absorption of feed nutrients and eviscerated carcass weight and reducing the abdominal fat rate in broilers. The combined use of GUE and Lactobacillus acidophilus showed significant interaction effects, further enhancing the production and health in broilers by building a mutually reinforcing mechanism. These findings demonstrate the promising potential of plant extract-probiotics combinations as feed additives.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by All procedures involving in animals were performed following the Regulations for the Administration of Affairs Concerning Experimental Animals (Ministry of Science and Technology, China, 2004) and were approved and supervised by the Northwest Minzu University Animal Care and Use Committee (Permit No. xbmu-sm-20230513). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
JL: Conceptualization, Data curation, Formal analysis, Investigation, Software, Validation, Writing – original draft, Writing – review & editing. SL: Validation, Writing – original draft, Methodology, Resources. JW: Data curation, Methodology, Validation, Writing – original draft. YC: Software, Visualization, Writing – original draft. SJ: Data curation, Methodology, Writing – original draft. GZ: Conceptualization, Funding acquisition, Investigation, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported and funded by the Fundamental Research Funds for the Central Universities (No. 31920250072).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Alagawany, M, Elnesr, SS, Farag, MR, Abd El-Hack, ME, Khafaga, AF, Taha, AE, et al. Use of Licorice (Glycyrrhiza Glabra) herb as a feed additive in poultry: current knowledge and prospects. Animals (Basel). (2019) 9:536. doi: 10.3390/ani9080536
2. Nomura, T, Fukai, T, and Akiyama, T. Chemistry of phenolic compounds of licorice (Glycyrrhiza species) and their estrogenic and cytotoxic activities. Pure Appl Chem. (2002) 74:1199–206. doi: 10.1351/pac200274071199
3. Tang, S, Cai, S, Ji, S, Yan, X, Zhang, W, Qiao, X, et al. Isoangustone a induces autophagic cell death in colorectal cancer cells by activating Ampk Signaling. Fitoterapia. (2021) 152:104935. doi: 10.1016/j.fitote.2021.104935
4. Yi, Y, Li, J, Lai, X, Zhang, M, Kuang, Y, Bao, YO, et al. Natural triterpenoids from Licorice potently inhibit Sars-Cov-2 infection. J Adv Res. (2022) 36:201–10. doi: 10.1016/j.jare.2021.11.012
5. Yu, JY, Ha, JY, Kim, KM, Jung, YS, Jung, JC, and Oh, S. Anti-inflammatory activities of Licorice extract and its active compounds, Glycyrrhizic acid, Liquiritin and liquiritigenin, in Bv2 cells and mice liver. Molecules (Basel, Switzerland). (2015) 20:13041–54. doi: 10.3390/molecules200713041
6. Guo, A, He, D, Xu, HB, Geng, CA, and Zhao, J. Promotion of regulatory T cell induction by immunomodulatory herbal medicine Licorice and its two constituents. Sci Rep. (2015) 5:14046. doi: 10.1038/srep14046
7. Fu, Y, Chen, J, Li, YJ, Zheng, YF, and Li, P. Antioxidant and anti-inflammatory activities of six flavonoids separated from Licorice. Food Chem. (2013) 141:1063–71. doi: 10.1016/j.foodchem.2013.03.089
8. Srinual, O, Moonmanee, T, Lumsangkul, C, Doan, HV, Punyatong, M, Yachai, M, et al. Can red yeast (Sporidiobolus Pararoseus) be used as a novel feed additive for mycotoxin binders in broiler chickens? Toxins (Basel). (2022) 14:678. doi: 10.3390/toxins14100678
9. Wu, Y, Wu, C, Che, Y, Zhang, T, Dai, C, Nguyen, AD, et al. Effects of Glycyrrhiza polysaccharides on chickens' intestinal health and homeostasis. Front Vet Sci. (2022) 9:891429. doi: 10.3389/fvets.2022.891429
10. Neveling, DP, and Dicks, LMT. Probiotics: an antibiotic replacement strategy for healthy broilers and productive rearing. Probiotics Antimicrob Proteins. (2021) 13:1–11. doi: 10.1007/s12602-020-09640-z
11. Al-Khalaifa, H, Al-Nasser, A, Al-Surayee, T, Al-Kandari, S, Al-Enzi, N, Al-Sharrah, T, et al. Effect of dietary probiotics and prebiotics on the performance of broiler chickens. Poult Sci. (2019) 98:4465–79. doi: 10.3382/ps/pez282
12. Chen, Y, Cheng, Y, Wen, C, Kang, Y, Wang, A, and Zhou, Y. Effects of dietary synbiotic supplementation as an alternative to antibiotic on the growth performance, carcass characteristics, meat quality, immunity, and oxidative status of Cherry Valley ducks. J Poult Sci. (2018) 55:182–9. doi: 10.2141/jpsa.0170128
13. Humam, AM, Loh, TC, Foo, HL, Samsudin, AA, Mustapha, NM, Zulkifli, I, et al. Effects of feeding different postbiotics produced by Lactobacillus Plantarum on growth performance, carcass yield, intestinal morphology, gut microbiota composition, immune status, and growth gene expression in broilers under heat stress. Animals (Basel). (2019) 9:644. doi: 10.3390/ani9090644
14. De Cesare, A, Sirri, F, Manfreda, G, Moniaci, P, Giardini, A, Zampiga, M, et al. Effect of dietary supplementation with Lactobacillus Acidophilus D2/Csl (Cect 4529) on Caecum Microbioma and productive performance in broiler chickens. PLoS One. (2017) 12:e0176309. doi: 10.1371/journal.pone.0176309
15. Wang, L, Li, L, Lv, Y, Chen, Q, Feng, J, and Zhao, X. Lactobacillus Plantarum restores intestinal permeability disrupted by Salmonella infection in newly-hatched chicks. Sci Rep. (2018) 8:2229. doi: 10.1038/s41598-018-20752-z
16. Ibrahim, D, Sewid, AH, Arisha, AH, Abd El-Fattah, AH, Abdelaziz, AM, Al-Jabr, OA, et al. Influence of Glycyrrhiza Glabra extract on growth, gene expression of gut integrity, and Campylobacter Jejuni colonization in broiler chickens. Front Vet Sci. (2020) 7:612063. doi: 10.3389/fvets.2020.612063
17. Qiao, Y, Liu, C, Guo, Y, Zhang, W, Guo, W, Oleksandr, K, et al. Polysaccharides derived from Astragalus Membranaceus and Glycyrrhiza Uralensis improve growth performance of broilers by enhancing intestinal health and modulating gut microbiota. Poult Sci. (2022) 101:101905. doi: 10.1016/j.psj.2022.101905
18. Chen, Z, Song, YY, Yan, YH, Chen, W, Ren, T, Ma, AJ, et al. Characterization of an epilactose-producing cellobiose 2-epimerase from Clostridium sp. TW13 and reutilization of waste milk. Food Chem. (2025) 480:143948. doi: 10.1016/j.foodchem.2025.143948
19. Wei, F, Abulahaiti, D, Tian, CC, Chen, Y, Jiang, SS, Lu, JX, et al. Effects of dietary Astragalus mongholicus, Astragalus polysaccharides and Lactobacillus on growth performance, immunity and antioxidant status in Qingjiaoma finishing broilers. Czeh J Anim Sci. (2022) 67:275–85. doi: 10.17221/12/2022-cjas
20. Wu, XZ, Wen, ZG, and Hua, JL. Effects of dietary inclusion of Lactobacillus and inulin on growth performance, gut microbiota, nutrient utilization, and immune parameters in broilers. Poult Sci. (2019) 98:4656–63. doi: 10.3382/ps/pez166
21. Chen, Y, Zhang, G, Li, J, Li, X, Jiang, S, Zha Xi, Y, et al. Glycyrrhiza uralensis extract supplementation mitigated the negative effects of prolonged low-dose exposure to deoxynivalenol and zearalenone on growth performance and intestinal health of broiler chickens. Front Vet Sci. (2025) 12:1570265. doi: 10.3389/fvets.2025.1570265
22. Li, X, Li, J, Yuan, H, Chen, Y, Li, S, Jiang, S, et al. Effect of supplementation with Glycyrrhiza uralensis extract and Lactobacillus Acidophilus on growth performance and intestinal health in broiler chickens. Front Vet Sci. (2024) 11:1436807. doi: 10.3389/fvets.2024.1436807
23. Zeng, M, Zou, YZ, Shi, ZG, Wang, JT, Yang, Y, Bai, YB, et al. A broad-spectrum broth rapidly and completely repairing the sublethal injuries of Escherichia coli caused by freezing and lactic acid alone or in combination for accurate enumeration. LWT. (2024) 201:116219. doi: 10.1016/j.lwt.2024.116219
24. Rashidi, N, Ghorbani, MR, Tatar, A, and Salari, S. Response of broiler chickens reared at high density to dietary supplementation with licorice extract and probiotic. J Anim Physiol Anim Nutr. (2019) 103:100–7. doi: 10.1111/jpn.13007
25. Stenzinger, A, Alber, M, Allgäuer, M, Jurmeister, P, Bockmayr, M, Budczies, J, et al. Artificial intelligence and pathology: from principles to practice and future applications in histomorphology and molecular profiling. Semin Cancer Biol. (2022) 84:129–43. doi: 10.1016/j.semcancer.2021.02.011
26. Jagadeeswaran, A, and Selvasubramanian, S. Effect of supplementation of Licorice root (Glycyrrhiza Glabra L.) extracts on immune status in commercial broilers. Int J Adv Vet Sci Technol. (2014) 3:88–92. doi: 10.23953/cloud.ijavst.190
27. Li, XK, Wang, JZ, Wang, CQ, Zhang, CH, Li, X, Tang, CH, et al. Effect of dietary phosphorus levels on meat quality and lipid metabolism in broiler chickens. Food Chem. (2016) 205:289–96. doi: 10.1016/j.foodchem.2016.02.133
28. Shaban, E, Bouyeh, M, and Seidavi, A. The effect of high levels of Glycyrrhiza glabra powder on performance, carcass characteristics, blood parameters, immunity, intestinal microbial flora, intestinal morphology and breast muscle fatty acid profile in broilers. J Hellenic Vet Med Soc. (2024) 75:7943–56. doi: 10.12681/jhvms.36094
29. Sedghi, M, Golian, A, Kermanshahi, H, and Ahmadi, H. Effect of dietary supplementation of licorice extract and a prebiotic on performance and blood metabolites of broilers. South Afr J Anim Sci. (2011) 40:371–80. doi: 10.4314/sajas.v40i4.65259
30. Qiu, K, Wang, X, Zhang, H, Wang, J, Qi, G, and Wu, S. Dietary supplementation of a new probiotic compound improves the growth performance and health of broilers by altering the composition of Cecal microflora. Biology (Basel). (2022) 11:633. doi: 10.3390/biology11050633
31. Dev, K, Begum, J, Biswas, A, Kannoujia, J, Mir, NA, Sonowal, J, et al. Dietary Lactobacillus acidophilus and mannan-oligosaccharides alter the lipid metabolism and health indices in broiler chickens. Probiotics Antimicrob Proteins. (2021) 13:633–46. doi: 10.1007/s12602-020-09717-9
32. Zhu, NH, Zhang, RJ, Wu, H, and Zhang, B. Effects of Lactobacillus cultures on growth performance, xanthophyll deposition, and color of the meat and skin of broilers. J Appl Poult Res. (2009) 18:570–8. doi: 10.3382/japr.2009-00012
33. Liu, J, Gu, H, Jia, R, Li, S, Chen, Z, Zheng, A, et al. Effects of Lactobacillus Acidophilus on production performance and immunity of broiler chickens and their mechanism. Front Vet Sci. (2025) 12:1554502. doi: 10.3389/fvets.2025.1554502
34. Salehizadeh, M, Modarressi, MH, Mousavi, SN, and Ebrahimi, MT. Effects of probiotic lactic acid bacteria on growth performance, carcass characteristics, hematological indices, humoral immunity, and Igf-I gene expression in broiler chicken. Trop Anim Health Prod. (2019) 51:2279–86. doi: 10.1007/s11250-019-01935-w
35. Wang, B, Gong, L, Zhou, Y, Tang, L, Zeng, Z, Wang, Q, et al. Probiotic Paenibacillus Polymyxa 10 and Lactobacillus Plantarum 16 enhance growth performance of broilers by improving the intestinal health. Anim Nutr. (2021) 7:829–40. doi: 10.1016/j.aninu.2021.03.008
36. Kong, J, Xiang, Q, Shi, G, Xu, Z, Ma, X, Wang, Y, et al. Licorice protects against ulcerative colitis via the Nrf2/PINK1-mediated mitochondrial autophagy. Immun Inflamm Dis. (2023) 11:e757. doi: 10.1002/iid3.757
37. Kong, J, Xiang, Q, Ge, W, Wang, Y, Xu, F, and Shi, G. Network pharmacology mechanisms and experimental verification of Licorice in the treatment of ulcerative colitis. J Ethnopharmacol. (2024) 324:117691. doi: 10.1016/j.jep.2023.117691
38. Ocampo, CL, Gómez-Verduzco, G, Tapia-Perez, G, Gutierrez, OL, and Sumano, LH. Effects of glycyrrhizic acid on productive and immune parameters of broilers. Rev Bras Ciênc Avíc. (2016) 18:435–42. doi: 10.1590/1806-9061-2015-0135
39. Zhang, Y, Lyu, H, Xu, S, Fang, Z, Feng, B, Che, L, et al. Effects of compound probiotics and Astragalus polysaccharide on growth performance, serum biochemical indices and Fecal microorganism of growing-finishing pigs. Anim Nutr Inst. (2021) 33:3542–53. doi: 10.3969/j.issn.1006-267x.2021.06.056
40. Li, SP, Zhao, XJ, and Wang, JY. Synergy of Astragalus polysaccharides and probiotics (Lactobacillus and Bacillus cereus) on immunity and intestinal microbiota in chicks. Poult Sci. (2009) 88:519–25. doi: 10.3382/ps.2008-00365
41. Zhang, M, Zhang, J, Wang, C, Yan, JK, Yi, J, Ning, J, et al. Biotransformation of 18β-Glycyrrhetinic acid by human intestinal fungus Aspergillus Niger Rg13b1 and the potential anti-inflammatory mechanism of its metabolites. J Agric Food Chem. (2022) 70:15104–15. doi: 10.1021/acs.jafc.2c05455
42. Gao, M, Liao, C, Fu, J, Ning, Z, Lv, Z, and Guo, Y. Probiotic cocktails accelerate baicalin metabolism in the ileum to modulate intestinal health in broiler chickens. J Anim Sci Biotechnol. (2024) 15:25. doi: 10.1186/s40104-023-00974-6
43. Azizi, T, Daneshyar, M, Alimehr, M, Shalizar-Jalali, A, Tukmechi, A, and Khalilvandi-Behroozyar, H. Effect of Lactobacillus sp. and yeast supplementation on performance and some blood attributes in deoxynivalenol-challenged broiler chickens. Res Vet Sci. (2023) 159:35–43. doi: 10.1016/j.rvsc.2023.04.003
44. Jiang, J, Zhou, X, Chen, H, Wang, X, Ruan, Y, Liu, X, et al. 18β-glycyrrhetinic acid protects against deoxynivalenol-induced liver injury via modulating ferritinophagy and mitochondrial quality control. J Hazard Mater. (2024) 471:134319. doi: 10.1016/j.jhazmat.2024.134319
45. Rezaei, M, Kalantar, M, and Nasr, J. Thymus vulgaris L., Glycyrrhiza glabra, and combo enzyme in corn or barley-basal diets in broiler chickens. Int J Plant Anim Environ Sci. (2014) 4:418–23. doi: 10.12980/JCLM.2.201414J54
46. Shokryazdan, P, Faseleh Jahromi, M, Liang, JB, Ramasamy, K, Sieo, CC, and Ho, YW. Effects of a Lactobacillus Salivarius mixture on performance, intestinal health and serum lipids of broiler chickens. PLoS One. (2017) 12:e0175959. doi: 10.1371/journal.pone.0175959
47. Wang, S, Li, XY, Ji, HF, and Shen, L. Modulation of gut microbiota by glycyrrhizic acid may contribute to its anti-NAFLD effect in rats fed a high-fat diet. Life Sci. (2022) 310:121110. doi: 10.1016/j.lfs.2022.121110
48. Wang, X, Liu, Y, Xu, Y, Gao, S, Xu, Q, Gong, H, et al. Structural characterization of a pectic polysaccharide from Rubus chingii Hu. Unripe fruits and its efficacy in inhibiting intestinal lipid absorption in vivo. Carbohydr Polym. (2025) 363:123728. doi: 10.1016/j.carbpol.2025.123728
49. Frappier, M, Auclair, J, Bouasker, S, Gunaratnam, S, Diarra, C, and Millette, M. Screening and characterization of some lactobacillaceae for detection of cholesterol-lowering activities. Probiotics Antimicrob Proteins. (2022) 14:873–83. doi: 10.1007/s12602-022-09959-9
50. Reda, FM, El-Saadony, MT, El-Rayes, TK, Farahat, M, Attia, G, and Alagawany, M. Dietary effect of Licorice (Glycyrrhiza Glabra) on quail performance, carcass, blood metabolites and intestinal microbiota. Poult Sci. (2021) 100:101266. doi: 10.1016/j.psj.2021.101266
51. Dorhoi, A, Dobrean, V, Zăhan, M, and Virag, P. Modulatory effects of several herbal extracts on avian peripheral blood cell immune responses. Phytother Res. (2006) 20:352–8. doi: 10.1002/ptr.1859
52. Bennour, I, Haroun, N, Sicard, F, Mounien, L, and Landrier, JF. Vitamin D and obesity/adiposity-a brief overview of recent studies. Nutrients. (2022) 14:2049. doi: 10.3390/nu14102049
53. Song, X, Lin, Z, Yu, C, Qiu, M, Peng, H, Jiang, X, et al. Effects of Lactobacillus Plantarum on growth traits, slaughter performance, serum markers and intestinal bacterial community of Daheng broilers. J Anim Physiol Anim Nutr. (2022) 106:575–85. doi: 10.1111/jpn.13621
54. Yu, Y, Li, Q, Zeng, X, Xu, Y, Jin, K, Liu, J, et al. Effects of probiotics on the growth performance, antioxidant functions, immune responses, and caecal microbiota of broilers challenged by lipopolysaccharide. Front Vet Sci. (2022) 9:846649. doi: 10.3389/fvets.2022.846649
55. Rastogi, S, and Singh, A. Gut microbiome and human health: exploring how the probiotic genus Lactobacillus modulate immune responses. Front Pharmacol. (2022) 13:1042189. doi: 10.3389/fphar.2022.1042189
56. Kveler, K, Starosvetsky, E, Ziv-Kenet, A, Kalugny, Y, Gorelik, Y, Shalev-Malul, G, et al. Immune-centric network of cytokines and cells in disease context identified by computational Mining of Pubmed. Nat Biotechnol. (2018) 36:651–9. doi: 10.1038/nbt.4152
57. Gocher, AM, Workman, CJ, and Vignali, DAA. Interferon-Γ: teammate or opponent in the tumour microenvironment? Nat Rev Immunol. (2022) 22:158–72. doi: 10.1038/s41577-021-00566-3
58. Hernandez, R, Põder, J, LaPorte, KM, and Malek, TR. Engineering il-2 for immunotherapy of autoimmunity and cancer. Nat Rev Immunol. (2022) 22:614–28. doi: 10.1038/s41577-022-00680-w
59. Chen, J, Zhu, XQ, Yang, L, Luo, Y, Wang, MY, Liu, XT, et al. Effect of Glycyrrhiza uralensis Fisch polysaccharide on growth performance and immunologic function in mice in Ural City, Xinjiang. Asian Pac J Trop Med. (2016) 9:1078–83. doi: 10.1016/j.apjtm.2016.08.004
60. Allameh, A, Niayesh-Mehr, R, Aliarab, A, Sebastiani, G, and Pantopoulos, K. Oxidative stress in liver pathophysiology and disease. Antioxidants (Basel). (2023) 12:1653. doi: 10.3390/antiox12091653
61. Sharma, P, Nandave, M, Nandave, D, Yadav, S, Vargas-De-La-Cruz, C, Singh, S, et al. Reactive oxygen species (Ros)-mediated oxidative stress in chronic liver diseases and its mitigation by medicinal plants. Am J Transl Res. (2023) 15:6321–41.
62. Sohail, M, Rakha, A, Butt, MS, and Asghar, M. Investigating the antioxidant potential of licorice extracts obtained through different extraction modes. J Food Biochem. (2018) 42:e12466. doi: 10.1111/jfbc.12466
63. Toson, E, Abd El Latif, M, Mohamed, A, Gazwi, HSS, Saleh, M, Kokoszynski, D, et al. Efficacy of licorice extract on the growth performance, carcass characteristics, blood indices and antioxidants capacity in broilers. Animal. (2023) 17:100696. doi: 10.1016/j.animal.2022.100696
64. Abo-Samaha, MI, Alghamdi, YS, El-Shobokshy, SA, Albogami, S, El-Maksoud, EMA, Farrag, F, et al. Licorice extract supplementation affects antioxidant activity, growth-related genes, lipid metabolism, and immune markers in broiler chickens. Life (Basel). (2022) 12:914. doi: 10.3390/life12060914
65. Wu, Z, Yang, K, Zhang, A, Chang, W, Zheng, A, Chen, Z, et al. Effects of Lactobacillus Acidophilus on the growth performance, immune response, and intestinal barrier function of broiler chickens challenged with Escherichia Coli O157. Poult Sci. (2021) 100:101323. doi: 10.1016/j.psj.2021.101323
66. Gyawali, I, Zeng, Y, Zhou, J, Li, J, Wu, T, Shu, G, et al. Effect of novel Lactobacillus paracaesi microcapsule on growth performance, gut health and microbiome community of broiler chickens. Poult Sci. (2022) 101:101912. doi: 10.1016/j.psj.2022.101912
67. Zhou, X, Hu, M, Luo, J, Xie, B, Ma, P, Wu, G, et al. Resistant effects determination of Lactobacillus supplementation on broilers to consecutive hydrogen sulfide exposure. Poult Sci. (2023) 102:103102. doi: 10.1016/j.psj.2023.103102
68. Zhan, S, Wu, L, Lv, Y, Huang, W, Ge, C, Hu, Z, et al. Lactobacillus Reuteri alleviates diquat induced hepatic impairment and mitochondrial dysfunction via activation of the Nrf2 antioxidant system and suppression of Nf-Κb inflammatory response. Poult Sci. (2025) 104:104997. doi: 10.1016/j.psj.2025.104997
69. Hashemitabar, SH, and Hosseinian, SA. The comparative effects of probiotics on growth, antioxidant indices and intestinal histomorphology of broilers under heat stress condition. Sci Rep. (2024) 14:23471. doi: 10.1038/s41598-024-66301-9
70. Song, Y, Xing, X, Shen, J, Chen, G, Zhao, L, Tian, L, et al. Anti-inflammatory effect of glycyrrhetinic acid in IL-1β-induced Sw982 cells and adjuvant-induced arthritis. Heliyon. (2023) 9:e15588. doi: 10.1016/j.heliyon.2023.e15588
71. Aipire, A, Mahabati, M, Cai, S, Wei, X, Yuan, P, Aimaier, A, et al. The immunostimulatory activity of polysaccharides from Glycyrrhiza uralensis. PeerJ. (2020) 8:e8294. doi: 10.7717/peerj.8294
72. Zhou, XR, Wang, XY, Sun, YM, Zhang, C, Liu, KJ, Zhang, FY, et al. Glycyrrhizin protects submandibular gland against radiation damage by enhancing antioxidant defense and preserving mitochondrial homeostasis. Antioxid Redox Signal. (2024) 41:723–43. doi: 10.1089/ars.2022.0183
73. Sun, Q, Li, L, Li, J, Li, SY, Zhang, Y, Chen, XS, et al. Glycyrrhizin alleviates brain injury in necrotizing enterocolitis model mice by suppressing Hmgb1/Tlr4 pathway. Int Immunopharmacol. (2025) 150:114294. doi: 10.1016/j.intimp.2025.114294
74. Pei, H, He, L, Shi, M, Guo, X, Chen, W, Li, J, et al. Pi3k-Akt Signaling pathway based on network pharmacology for the anti-Alzheimer's disease effect of Licorice stem flavonoids. Aging. (2023) 15:3381–93. doi: 10.18632/aging.204536
75. Xie, Z, Gu, X, Dekebo, A, Wei, S, and Hu, X. Two new compounds from biotransformation of glycyrrhetinic acid. Nat Prod Res. (2025) 39:2603–7. doi: 10.1080/14786419.2024.2306918
76. Fan, B, Jiang, B, Yan, S, Xu, B, Huang, H, and Chen, G. Anti-inflammatory 18β-glycyrrhetinin acid derivatives produced by biocatalysis. Planta Med. (2019) 85:56–61. doi: 10.1055/a-0662-0296
77. Duangnumsawang, Y, Zentek, J, and Goodarzi Boroojeni, F. Development and functional properties of intestinal mucus layer in poultry. Front Immunol. (2021) 12:745849. doi: 10.3389/fimmu.2021.745849
78. Vancamelbeke, M, and Vermeire, S. The intestinal barrier: a fundamental role in health and disease. Expert Rev Gastroenterol Hepatol. (2017) 11:821–34. doi: 10.1080/17474124.2017.1343143
79. Zhang, J, Fang, Y, Fu, Y, Jalukar, S, Ma, J, Liu, Y, et al. Yeast polysaccharide mitigated oxidative injury in broilers induced by mixed mycotoxins via regulating intestinal mucosal oxidative stress and hepatic metabolic enzymes. Poult Sci. (2023) 102:102862. doi: 10.1016/j.psj.2023.102862
80. You, T, Tang, J, Yin, S, Jia, G, Liu, G, Tian, G, et al. Effect of dietary Licorice flavonoids powder on performance, intestinal immunity and health of weaned piglets. J Anim Physiol Anim Nutr. (2023) 107:147–56. doi: 10.1111/jpn.13694
81. Li, Z, Wang, W, Liu, D, and Guo, Y. Effects of Lactobacillus Acidophilus on the growth performance and intestinal health of broilers challenged with Clostridium Perfringens. J Anim Sci Biotechnol. (2018) 9:25. doi: 10.1186/s40104-018-0243-3
82. Pham Thi, HH, Phan Thi, TV, Pham Huynh, N, Doan, V, Onoda, S, and Nguyen, TL. Therapeutic effect of heat-killed Lactobacillus Plantarum L-137 on the gut health and growth of broilers. Acta Trop. (2022) 232:106537. doi: 10.1016/j.actatropica.2022.106537
83. Chang, HM, Loh, TC, Foo, HL, and Lim, ETC. Lactiplantibacillus plantarum postbiotics: alternative of antibiotic growth promoter to ameliorate gut health in broiler chickens. Front Vet Sci. (2022) 9:883324. doi: 10.3389/fvets.2022.883324
84. Zargari-Samadnejad, A, Mehrvarz, S, Allizadeh-Naeini, S, and Tanideh, N. Healing effect of licorice extract in acetic acid-induced ulcerative colitis in rat. Res Pharm Sci. (2012) 7:837. doi: 10.1007/s00580-011-1249-9
85. Liu, D, Huo, X, Gao, L, Zhang, J, Ni, H, and Cao, L. Nf-Κb and Nrf2 pathways contribute to the protective effect of Licochalcone A on dextran sulphate sodium-induced ulcerative colitis in mice. Biomed Pharmacother. (2018) 102:922–9. doi: 10.1016/j.biopha.2018.03.130
86. Park, JS, Choi, JW, Jhun, J, Kwon, JY, Lee, BI, Yang, CW, et al. Lactobacillus Acidophilus improves intestinal inflammation in an acute colitis mouse model by regulation of Th17 and Treg cell balance and fibrosis development. J Med Food. (2018) 21:215–24. doi: 10.1089/jmf.2017.3990
Keywords: Glycyrrhiza uralensis extract, Lactobacillus acidophilus , production performance, immunity, antioxidation, intestinal health, broiler
Citation: Lu JX, Li SB, Wu JH, Chen Y, Jiang SS and Zhang GH (2025) Effect of Glycyrrhiza uralensis extract, Lactobacillus acidophilus and their combined supplementation on production performance, immunity, antioxidation and intestinal health in broilers. Front. Vet. Sci. 12:1675593. doi: 10.3389/fvets.2025.1675593
Edited by:
Adrian Macri, University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca, RomaniaReviewed by:
Baseer Ahmad, Muhammad Nawaz Shareef University of Agriculture, PakistanMaria Fernanda Peralta, National University of Río Cuarto, Argentina
Tiyu Li, Inner Mongolia Minzu University, China
Copyright © 2025 Lu, Li, Wu, Chen, Jiang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: GuoHua Zhang, MjgwMTEyMTAzQHhibXUuZWR1LmNu
 JianXiong Lu
JianXiong Lu ShuaiBing Li
ShuaiBing Li Yan Chen
Yan Chen GuoHua Zhang
GuoHua Zhang