- 1Danisco Animal Nutrition & Health (IFF), Oegstgeest, Netherlands
- 2Danisco Nutrition and Biosciences (IFF), Brabrand, Denmark
Campylobacter jejuni remains a significant health concern in humans, with the consumption of contaminated poultry meat being the primary source. Nutritional virulence could be utilized to help reduce Campylobacter in poultry, specifically by reducing dietary iron (Fe) and phosphorus (P), which appear to be essential for Campylobacter growth and persistence, in combination with phytase supplementation to meet bird mineral requirements. We discuss the scientific basis of this hypothesis and present results of a small-scale proof-of-concept broiler study comprising: (1) control: mixed grain commercial diet; (2) added Fe- and inorganic phosphate (iP)-free premix: as control but formulated without Fe in the mineral premix and without added iP, with increased phytase [phytase unit (FTU)/kg per phase] and higher associated matrix values for Ca, metabolizable energy (ME), and digestible amino acids (AA) vs. control. Over the 42-day (d) trial, birds exhibited similar (non-significantly different) livability and growth performance. Average cecal Campylobacter loads were numerically 7.7-fold lower (87% reduction) in the added Fe- and iP-free treatment relative to the control [4.90 × 107 colony forming units (CFU)/g vs. 3.78 × 108 CFU/g, respectively; p-value = 0.12]. In addition, the range in recorded loads of Campylobacter was wide in both treatments, but the upper end of the range was 1 log10 units lower in added Fe- and iP-free vs. control (2.97 × 109 vs. 2.45 × 1,010), which suggests a reduced upper limit of colonization and reduction in average Campylobacter levels. Although caution is warranted on the numerical results, we believe they should encourage further ideations, investigations, and larger scale applications in the future.
1 Introduction
Campylobacteriosis, caused predominantly by the gram-negative bacterium C. jejuni, is the most reported gastrointestinal disease among humans in the EU: ~46.9 cases per 100,000 population were reported in 2022 (1). It is primarily a foodborne zoonotic disease acquired from the consumption of infected meat, especially poultry meat. Campylobacter is generally found in the avian gastrointestinal tract, residing predominantly and at high levels in the blind-ended caeca [Beery et al., 1988; (2)]. However, it can become pathogenic under certain situations, for example, among susceptible host populations (young or immunocompromised birds) under conditions of environmental stress or when birds are infected with certain Campylobacter species that are always pathogenic. Numerous on-farm postharvest and processing control strategies have been developed, including biosecurity measures, feed and water additives, bacteriocins, bacteriophage therapy, vaccination, and processing interventions (3), but they are not optimally effective, and flock colonization rates often remain high. New Campylobacter control approaches are continuously being sought, and those that build upon existing measures used for other purposes could be advantageous from a cost standpoint. This perspective article considers the concept of utilizing nutritional modifications as a potential approach to reduce C. jejuni in broilers.
2 Nutritional modification as a potential tool for reducing Campylobacter
Nutritional virulence is the exploitation of host nutritional resources by pathogens to support their existence. As research continues to bridge the gap between nutrition and health, focus on nutritional virulence is growing. To date, research has demonstrated that certain dietary nutrients, often oversupplied in commercial diets, promote Campylobacter survival and persistence in the poultry digestive tract. Specifically, it has been shown that Fe is essential for Campylobacter colonization, growth, and virulence. Ferrous (Fe2+) and ferric (Fe3+) Fe stimulate biofilm formation, which contributes to the persistence and survival of C. jejuni under stress conditions (4). Miller et al. (5) characterized the cellular uptake and processing mechanisms through which host Fe sources are sequestered and utilized by C. jejuni for survival and proliferation. These Fe sources include both direct sources, such as host heme compounds and transferrin, from which Fe is extracted by C. jejuni via contact-dependent or surface receptor binding process (6), as well as indirect sources, involving the sequestration of Fe from siderophores secreted by other bacteria residing in the gut microbiome (7). It is plausible that precise control of the Fe content in the diet to the required dose could reduce Campylobacter growth and persistence by reducing access to free Fe. Furthermore, P is another nutrient critical for Campylobacter growth and colonization. It is utilized for intestinal adhesion as part of the high-affinity phosphate transporter PstSCAB, which has been identified as a target for potential C. jejuni control methods (8). It is supplemented in conventional broiler diets in the form of iP, often at levels far higher than bird requirements, according to NRC recommendations (9). This raises the question as to whether better optimization of diets for P availability could also enable Campylobacter control.
3 Supplemental phytase
More precise diet formulation, in which diets are optimized to meet but not exceed bird nutritional requirements, is a major area of current research among poultry nutritionists. This involves careful consideration of how diets are formulated (for example, the levels of total, available, or digestible nutrients) and the ingredients and feed additive biotechnologies to be included in the diet. Such research could be linked to the concept of reducing nutritional virulence. For example, microbially derived phytase is added routinely to broiler diets, primarily to increase the bioavailability of P, which is otherwise primarily bound within plant-based ingredients in the form of phytate (IP6, hexakisphosphate). The biochemical properties and activity for hydrolyzing phytate varies from one type to another (10), but its efficacy in broilers for increasing the digestibility of P and certain other nutrients is well documented [recently reviewed by (11)]. This efficacy enables the digestible P content of the diet [and that of other “released” nutrients, including calcium (Ca), AA, energy, and sodium (Na)] to be reduced to prescribed amounts (known as “matrix values”) and accounts for the expected contribution of the enzyme. If added iP could be totally removed from the diet and replaced by phytase, as demonstrated in a proof-of-concept study involving a phytase produced by Trichoderma reesei [PhyG, (12)], it might help reduce Campylobacter colonization, due to the bacteria’s dependence on P for successful colonization. Furthermore, phytate has a strong affinity for trace minerals at intestinal pH, forming difficult-to-digest complexes (13). This reduces the bioavailability of trace minerals in feed raw materials, even when present in quantities that would otherwise meet bird requirements. A fast-acting phytase with high activity at low pH can prevent trace mineral-phytate complex formation, thus reducing the need to supplement trace minerals in the diet (14). Hence, it is hypothesized that the reduction or removal of added Fe from an all-vegetable diet supplemented with phytase may also help reduce Campylobacter levels.
4 Early research findings
A small-scale research study conducted by the present authors to test the hypothesis that dietary removal of added Fe and iP, alongside appropriate phytase supplementation and matrix application to an all-vegetable diet, could lower cecal C. jejuni colonization without affecting the growth of broilers over 42 d. A completely randomized design with two treatments and three dietary phases (1 to 14, 15 to 28, and 29 to 42 ds of age) was employed. Treatments comprised: (1) commercial control: corn-wheat-soybean meal-based diet with phytase supplemented at 1,000 FTU/kg during all phases and with associated matrix values applied for available P, Ca, digestible AA, and ME; (2) added Fe- and iP-free: formulated without Fe in the mineral premix and without added iP, with phytase added at 3,000, 2,000, and 2,000 FTU/kg per phase, and associated higher matrix values applied for Ca, ME, and digestible AA. Diets were fed ad libitum to 500 Ross 308 male broilers housed in 20 floor pens with 25 birds/pen and 10 pens/treatment. The phytase was a bacterial 6-phytase variant produced in T. reesei, supplied by Danisco Animal Nutrition & Health (IFF, Oegstgeest, Netherlands). The calculated and analyzed nutrient composition of the diet ingredients has been listed in Table 1. Birds were batch-tested for Campylobacter negativity prior to randomization, and all birds were exposed to the bacteria through oral challenge with ~1.0 × 106 CFU/mL (~1.0 × 105 CFU/bird) of C. jejuni (JB strain, isolated from poultry) on d 14. Levels of C. jejuni in the ceca of birds at 42 ds of age (CFU/g) were determined by culture-dependent methods. All data were analyzed using JMP 16 software. Performance data (available upon request) were analyzed using one-way ANOVA with mean value segregation using Tukey’s Honest Significant Difference (HSD) test. The significance was defined as p-value < 0.05. Due to the nonparametric nature of microbial data, the Wilcoxon rank-sum test was used (Table 2).
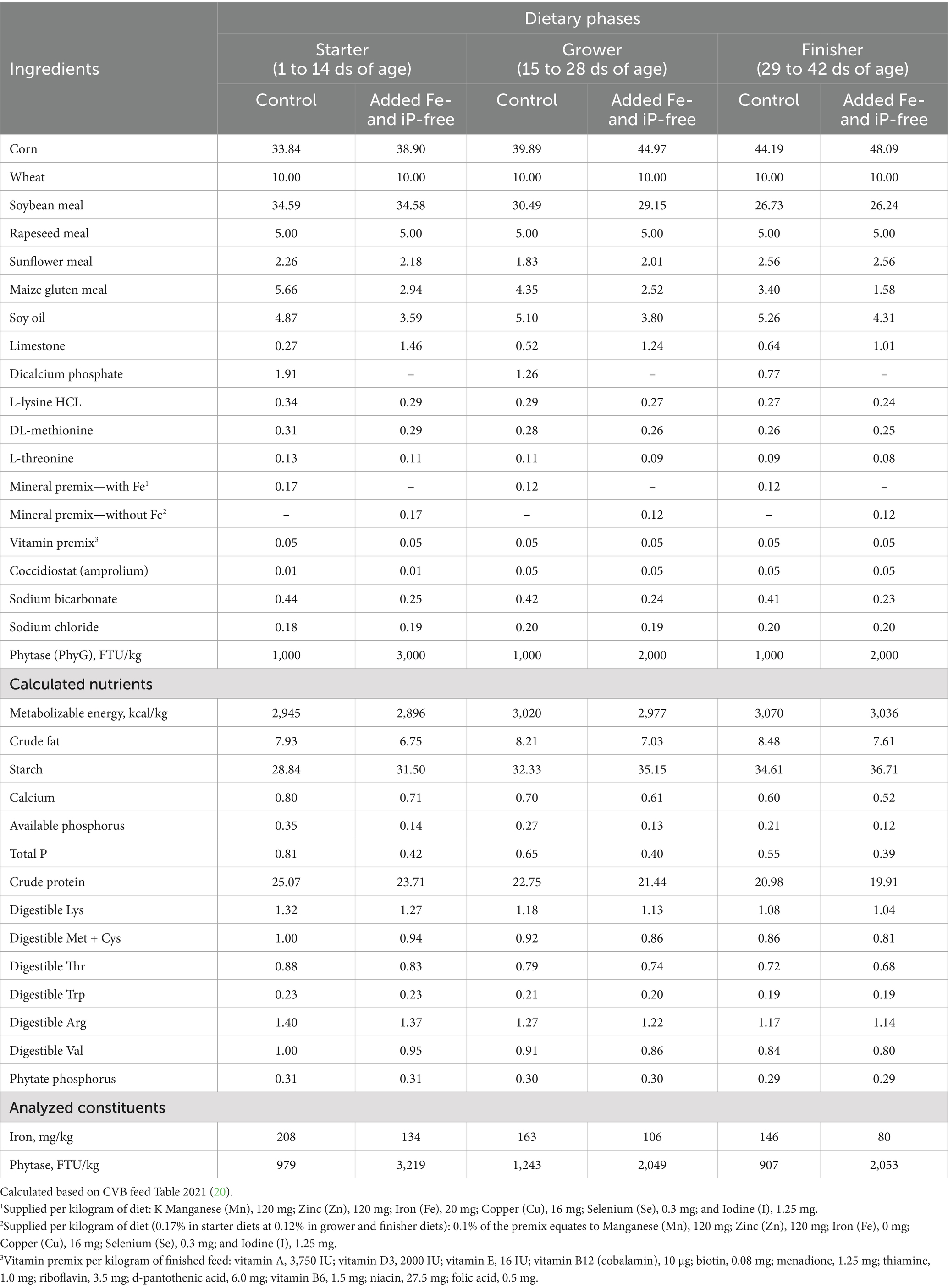
Table 1. Ingredients and calculated nutrient composition of the treatment diets, % as fed, unless otherwise stated.
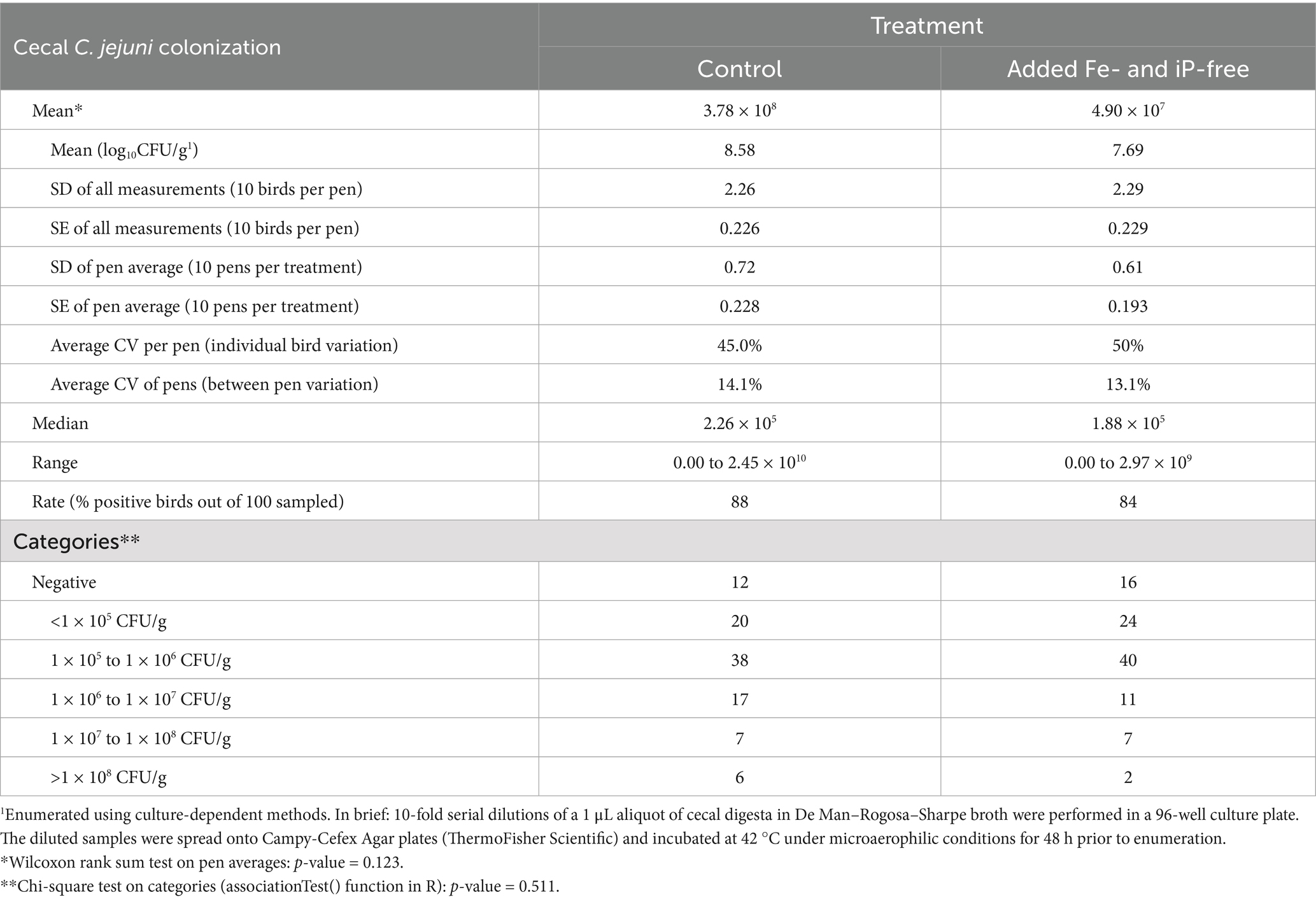
Table 2. Descriptive statistics on the effect of treatment on cecal colonization and categorized cecal loads** of Campylobacter jejuni at 42 ds of age.
Results from this small-scale experiment revealed that overall (0 to 42 d) growth performance—final body weight, body weight gain, mortality, and body weight-corrected feed conversion ratio—and livability did not differ significantly between the added Fe- and iP-free and control treatments (2,655 g/bird vs. 2,525 g/bird; 2,613 g/bird vs. 2,482 g/bird; 1.546 vs. 1.554; and 96.4% vs. 94.8%, respectively; p-value < 0.05 in all cases). Meanwhile, there was a 7.7-fold (87%) reduction in the average cecal C. jejuni load in the added Fe- and iP-free treatment relative to the control at 42 ds of age (4.90 × 107 CFU/g vs. 3.78 × 108 CFU/g, respectively; p = 0.12), as shown in Table 2. In addition, although the range in colonization loads was high (coefficient of variation was 45% among control-fed birds and 50% among birds fed the added Fe- and iP-free treatment), the upper end of the range was 1 log10 units lower in the added Fe- and iP-free treatment relative to the control 2.97 × 109 vs. 2.45 × 1010, respectively.
5 Discussion
It is well known in the scientific community that C. jejuni counts in the broiler gut (especially in the ceca and feces) vary substantially between individual birds, even when birds are reared under controlled or standardized conditions (15). This often results in broad ranges or confidence intervals for group mean values, as observed in our study. Given this, it was considered that a 7.7-fold numerical reduction in mean cecal loads is of material interest. We also estimated that the average cecal C. jejuni load exhibited by the added Fe- and iP-free birds at 42 ds of age was below the level set by The European Food Safety Authority (EFSA) in 2011 as constituting an increased public health risk [>1,000 CFU/gram on broiler neck skin (16), equivalent to ~1 × 108 CFU/g in the ceca according to the studies by (17, 18)]. This highlights the need for exhaustive additional research to evaluate the effect of dietary Fe, P, and phytase intervention on Campylobacter colonization in a larger-scale setting. The study design limited the possibility of separating the effects of the individual components of the intervention (Fe reduction, iP removal, and phytase supplementation) on bird responses. However, a reduced capacity of C. jejuni to colonize the cecum when dietary Fe and P were reduced is consistent with the existing (aforementioned) research, implicating Fe and P as nutritional virulence factors for the bacterium. The maintenance of growth performance in the added Fe- and iP-free-fed birds at a level not significantly different from that achieved by control-fed birds was likely afforded through the application of a higher dose of phytase supplementation (tiered by phase to reflect the higher nutrient requirements of younger birds).
The study we have presented provides suggestive evidence that small modifications to the diet composition to lower nutritional virulence factors, in combination with phytase supplementation, can help reduce C. jejuni colonization. These results suggest that targeted nutritional modifications can be potentially used as an additional approach to improve food safety, which has been overlooked until now. The authors recognize that individual variation among animals and a combined strategy approach limit the study findings. This variation is common to Campylobacter studies, as seen in previous published work, including Humphrey et al. (19). Hence, increasing replication power and conducting larger field-scale studies would help advance the ideas proposed herein and enhance our understanding.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Ethical Committee on Animal Experiments at SPRG Inc. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
KG: Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. AB: Formal analysis, Writing – review & editing. SK: Data curation, Formal analysis, Writing – review & editing. CP: Methodology, Supervision, Writing – review & editing. KK: Writing – review & editing. LM: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors would like to thank Dr. Joelle Buck (Newbury, United Kingdom) for her assistance with the writing of this manuscript.
Conflict of interest
KG, AB, SK, CP, KK, and LM were employed by Danisco Animal Nutrition & Health (IFF), Oegstgeest, Netherlands, a global supplier of feed enzymes.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. ECDC. Campylobacteriosis: Annual Epidemiological Report for 2022. Stockholm, Sweden: European Centre for Disease Prevention and Control (2024).
2. Sahin, O, Morishita, TY, and Zhang, Q. Campylobacter colonization in poultry: sources of infection and modes of transmission. Anim Health Res Rev. (2002) 3:95–105. doi: 10.1079/ahrr200244
3. Zainol, MFA, Safiyanu, MB, Aziz, SA, Omar, AR, Chuang, KP, and Mariatulqabtiah, AR. Campylobacteriosis and control strategies against campylobacters in poultry farms. J Microbiol Biotechnol. (2024) 34:987–93. doi: 10.4014/jmb.2311.11045
4. Oh, E, Andrews, KJ, and Jeon, B. Enhanced biofilm formation by ferrous and ferric iron through oxidative stress in Campylobacter jejuni. Front Microbiol. (2018) 9:1204. doi: 10.3389/fmicb.2018.01204
5. Miller, CE, Williams, PH, and Ketley, JM. Pumping iron: mechanisms for iron uptake by Campylobacter. Microbiology. (2009) 155:3157–65. doi: 10.1099/mic.0.032425-0
6. Miller, CE, Rock, JD, Ridley, KA, Williams, PH, and Ketley, JM. Utilization of lactoferrin-bound and transferrin-bound iron by Campylobacter jejuni. J Bacteriol. (2008) 190:1900–11. doi: 10.1128/JB.01761-07
7. Palyada, K, Threadgill, D, and Stintzi, A. Iron acquisition and regulation in Campylobacter jejuni. J Bacteriol. (2004) 186:4714–29. doi: 10.1128/JB.186.14.4714-4729.2004
8. Sinha, R, LeVeque, RM, Bowlin, MQ, Gray, MJ, and DiRita, VJ. Phosphate transporter PstSCAB of Campylobacter jejuni is a critical determinant of lactate-dependent growth and colonization in chickens. J Bacteriol. (2020) 202:e00716–9. doi: 10.1128/JB.00716-19
9. NRC. Nutrient requirements of poultry. 9th Revised ed. Washington, DC: The National Academies Press (1994).
10. Menezes-Blackburn, D, Gabler, S, and Greiner, R. Performance of seven commercial phytases in an in vitro simulation of poultry digestive tract. J Agric Food Chem. (2015) 63:6142–9. doi: 10.1021/acs.jafc.5b01996
11. Selle, PH, Macelline, SP, Chrystal, PV, and Liu, SY. The contribution of phytate-degrading enzymes to chicken-meat production. Animals. (2023) 13:603. doi: 10.3390/ani13040603
12. Marchal, L, Bello, A, Sobotik, EB, Archer, G, and Dersjant-Li, Y. A novel consensus bacterial 6-phytase variant completely replaced inorganic phosphate in broiler diets, maintaining growth performance and bone quality: data from two independent trials. Poult Sci. (2021) 100:100962. doi: 10.1016/j.psj.2020.12.059
13. Philippi, H, Sommerfeld, V, Monteiro, A, Rodehutscord, M, and Olukosi, O. Impact of trace mineral source and phytase supplementation on prececal phytate degradation and mineral digestibility, bone mineralization, and tissue gene expression in broiler chickens. Biol Trace Elem Res. (2024) 202:5235–50. doi: 10.1007/s12011-024-04076
14. Dersjant-Li, Y, Kwakernaak, C, Bello, A, and Marchal, L. A novel consensus bacterial 6-phytase variant supplemented to an all-vegetable broiler diet totally replaced added trace minerals including zinc, iron, copper and manganese in two experiments. Poult Sci. (2025) 104:104610. doi: 10.1016/j.psk.2024.104610
15. Bahrndorff, S, Garcia, AB, Vigre, H, Nauta, M, Heegaard, PMH, Madsen, M, et al. Intestinal colonization of broiler chickens by Campylobacter spp. in an experimental infection study. Epidemiol Infect. (2014) 143:2381–9. doi: 10.1017/S0950268814003239
16. EFSA. Scientific opinion on Campylobacter in broiler meat production: control options and performance objectives and/or targets at different stages of the food chain. EFSA J. (2011) 9:2105. doi: 10.2903/j.efsa.2011.2105
17. Brena, MC. Effect of different poultry production methods on Campylobacter incidence and transmission in the broiler meat food chain, PhD thesis. Liverpool: University of Liverpool (2013).
18. Emanowicz, M, Meade, J, Bolton, D, Golden, O, Gutierrez, M, Byrne, W, et al. The impact of key processing stages and flock variables on the prevalence and levels of Campylobacter on broiler carcasses. Food Microbiol. (2021) 95:103688. doi: 10.1016/j.fm.2020.103688
19. Humphrey, S, Chaloner, G, Kemmett, K, Davidson, N, Williams, N, Kipar, A, et al. Campylobacter jejuni is not merely a commensal in commercial broiler chickens and affects bird welfare. MBio. (2014) 5:e01364–14. doi: 10.1128/mBio.01364-14
20. CVB (2021). CVB Feed Table 2021: Chemical composition and nutritional values of feedstuffs. Available online at: https://www.cvbdiervoeding.nl/bestand/10741/cvb-feed-table-2021.pdf.ashx (Accessed May 1, 2025)
Keywords: broiler, Campylobacter, food safety, Iron, nutritional virulence, phosphorus, phytase
Citation: Gibbs K, Bello A, van der Klein S, Poulsen C, Kragh K and Marchal L (2025) Considering the concept of nutritional modification coupled with phytase supplementation for reducing Campylobacter jejuni in broilers. Front. Vet. Sci. 12:1686545. doi: 10.3389/fvets.2025.1686545
Edited by:
Adrian Macri, University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca, RomaniaReviewed by:
Gelana Urgesa Ayana, Jimma University, EthiopiaHafsan Hafsan, Universitas Islam Makassar, Indonesia
Copyright © 2025 Gibbs, Bello, van der Klein, Poulsen, Kragh and Marchal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kirsty Gibbs, a2lyc3R5LkdpYmJzQGlmZi5jb20=
 Kirsty Gibbs
Kirsty Gibbs Abiodun Bello1
Abiodun Bello1 Charlotte Poulsen
Charlotte Poulsen