- 1Department of Radiation Oncology, University of Texas M.D. Anderson Cancer Center, Houston, TX, United States
- 2Department of Radiation Oncology, Allegheny General Hospital, Pittsburgh, PA, United States
We applaud the recent efforts of Palma et al. for prospectively addressing a novel issue regarding pathologic response following stereotactic ablative radiotherapy (SABR) for early-stage non-small cell lung cancer (1). This was a phase II trial evaluating the addition of neoadjuvant SABR prior to resection for purposes of evaluating the pathologic complete response (pCR) rate following SABR. This is of academic interest because the vast majority of SABR candidates are inoperable, and hence the pathologic response of SABR is largely not able to be assessed. The primary findings were a low (60%) pCR, highly discordant with the well-validated ≥90% local control (LC) in the irradiated lesion following modern, volumetric image-guided SABR (2–4). Although the low pCR may be concerning for many readers, there are several assuaging caveats worth highlighting.
First, the treatment paradigms of this study may allow for further optimization. For instance, SABR dosing in 25% of patients (e.g., 60 Gy/8 fractions) was of relatively lower biologically effective dose (BED) than employed at other institutions. This is noteworthy because BED can be further escalated by means of several treatment planning nuances, which is particularly important for lesions larger than 3 cm (5).
More importantly, the value and accuracy of histopathology at 10 weeks following SABR is evidently quite poor, and thus should not reflect the consistently high clinical LC with long-term follow up after SABR. This discrepancy is analogous to the situation regarding post-SABR positron emission tomography (PET), which may be unreliable even 1 year from SABR (6). Thoracic radiation oncologists are undoubtedly aware of scenarios (albeit uncommon) in which results of a 3–6-month post-SABR PET lead to a positive biopsy, but no treatment is delivered owing to comorbidities, and yet long-term follow-up reveals no recurrences (Figure 1). This may be explained by basic radiobiological principles; tumor cells can appear viable on histopathology but in fact are dead, dying, or senescent from lethal chromosomal damage. This is reflected by higher pCR rates with increasing time from hypofractionated/fractionated radiotherapy to resection of rectal and esophageal cancers (7–9). Moreover, the trial implies that LC is achieved not only by direct (SABR-mediated) tumoricide, but also lends credence to immune-based destruction as a vital component thereof. Because the latter can continue to occur past 10 weeks (the time of resection), the trial unfortunately eliminates this concept from the equation. Nevertheless, this issue will be better addressed (along with the preponderance of post-SABR out-of-field failures) by means of ongoing trials delivering concurrent and adjuvant immunotherapy with SABR (I-SABR, NCT03110978; NCT03446547; NCT03924869; NCT03833154). Although the observed pCR rate is perhaps anxiety-provoking, readers are recommended to continue to rely on the well-corroborated clinically-measured LC rates. Those findings should also not be a deterrent to enrollment onto important SABR trials such as VALOR, STABLE-MATES, SABRTooth, and POSITILV. Although academically interesting, performing two local therapies for an early-stage neoplasm for which either modality displays impressively high LC remains questionable. The rate of positive margins is exceedingly low in sublobar approaches (10) (and even lower with lobectomies) that neoadjuvant SABR would not provide a meaningful benefit thereof. Additionally, for reasons mentioned above, if the theoretical “selling point” of adding neoadjuvant SABR relates to promoting abscopal responses, then perhaps surgery should not be performed in the first place (11).
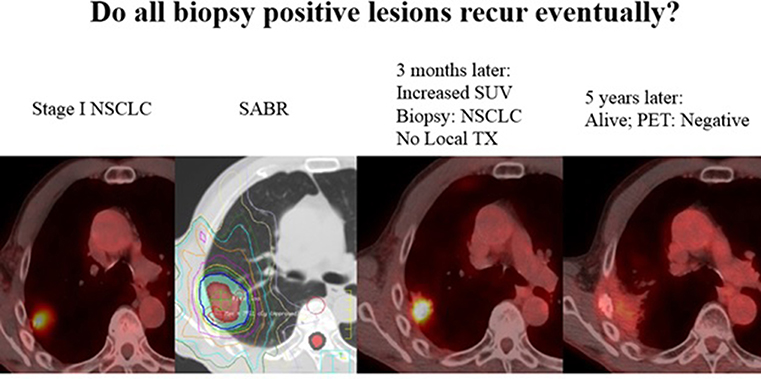
Figure 1. Not all “positive” biopsies months after SABR lead to recurrence. Here, a 71 year old male with stage I NSCLC was treated with SABR, 50Gy in 4 fractions. A routine surveillance PET/CT three months later after SABR showed increased SUV uptake. A biopsy was performed and was positive for “residual cancer.” The patient was sent for evaluation of surgery and thermal ablation but neither were recommended due to poor performance status. The patient was followed, and 5 years later his tumor has disappeared and he has been without disease or any signs of local recurrence. PET uptake has also resolved without any treatment. Although a biopsy and PET may suggest viable disease, tumor cells can often be dead/dying after SABR and lead to no recurrence.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Palma DA, Nguyen TK, Louie AV, Malthaner R, Fortin D, Rodrigues GB, et al. Measuring the integration of stereotactic ablative radiotherapy plus surgery for early-stage non-small cell lung cancer: a phase 2 clinical trial. JAMA Oncol. (2019). doi: 10.1001/jamaoncol.2018.6993. [Epub ahead of print].
2. Sun B, Brooks ED, Komaki RU, Liao Z, Jeter M, McAleer M, et al. 7-year follow-up outcomes after stereotactic ablation radiotherapy for stage I NSCLC: results of a phase II clinical trial. Cancer. (2017) 123:3031–9. doi: 10.1002/cncr.30693
3. Schonewolf CA, Heskel M, Doucette A, Singhal S, Frick MA, Xanthopoulos EP, et al. Five-year long-term outcomes of stereotactic body radiation therapy for operable versus medically inoperable stage I non-small-cell lung cancer: analysis by operability, fractionation regimen, tumor size, and tumor location. Clin Lung Cancer. (2019) 20:e63–71. doi: 10.1016/j.cllc.2018.09.004
4. Timmerman RD, Paulus R, Pass HI, Gore EM, Edelman MJ, Galvin J, et al. Stereotactic body radiation therapy for operable early-stage lung cancer: findings from the NRG Oncology RTOG 0618trial. JAMA Oncol. (2018) 4:1263–6. doi: 10.1001/jamaoncol.2018.1251
5. Chang JY, Bezjak A, Mornex F IASLC Advanced Radiation Technology Committee. Stereotactic ablative radiotherapy for centrally located early stage non-small-cell lung cancer: what we have learned. J Thorac Oncol. (2015) 10:577–85. doi: 10.1097/JTO.0000000000000453
6. Henderson MA, Hoopes DJ, Fletcher JW, Lin PF, Tann M, Yiannoutsos CT, et al. A pilot trial of serial 18F-fluorodeoxyglucose positron emission tomography in patients with medically inoperable stage I non-small-cell lung cancer treated with hypofractionated stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. (2010) 76:789–95. doi: 10.1016/j.ijrobp.2009.02.051
7. Pettersson D, Lorinc E, Holm T, Iversen H, Cedermark B, Glimelius B, et al. Tumour regression in the randomized Stockholm III trial of radiotherapy regimens for rectal cancer. Br J Surg. (2015) 102:972–8. doi: 10.1002/bjs.9811
8. Shaikh T, Ruth K, Scott WJ, Burtness BA, Cohen SJ, Konski AA, et al. Increased time from neoadjuvant chemoradiation to surgery is associated with higher pathologic complete response rates in esophageal cancer. Ann Thorac Surg. (2015) 99:270–6. doi: 10.1016/j.athoracsur.2014.08.033
9. van der Werf LR, Dikken JL, van der Willik EM, van Berge Henegouwen MI, Nieuwenhuijzen GAP, Wijnhoven BPL, et al. Time interval between neoadjuvant chemoradiotherapy and surgery for oesophageal or junctional cancer: a nationwide study. Eur J Cancer. (2018) 91:76–85. doi: 10.1016/j.ejca.2017.12.009
10. Ajmani GS, Wang C, Kim KW, Howington JA, Krantz SB. Surgical quality of wedge resection affects overall survival in patients with early-stage non-small cell lung cancer. J Thorac Cardiovasc Surg. (2018) 156:380–91. doi: 10.1016/j.jtcvs.2018.02.095
Keywords: missile, lung cancer, NSCLC, SBRT, pathologic complete response, PCR, surgery, lobectomy
Citation: Brooks ED, Verma V and Chang JY (2019) Does Pathologic Response Equate to Clinical Response Following SABR for Early-Stage NSCLC? Front. Oncol. 9:551. doi: 10.3389/fonc.2019.00551
Received: 04 April 2019; Accepted: 06 June 2019;
Published: 21 June 2019.
Edited by:
Stephen V. Liu, Georgetown University, United StatesReviewed by:
Brendon Stiles, Cornell University, United StatesJonathan W. Lischalk, Georgetown University Medical Center, United States
Copyright © 2019 Brooks, Verma and Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joe Y. Chang, anljaGFuZ0BtZGFuZGVyc29uLm9yZw==
 Eric D. Brooks1
Eric D. Brooks1 Vivek Verma
Vivek Verma Joe Y. Chang
Joe Y. Chang