- 1Department of Otolaryngology-Head and Neck Surgery, The Third Affiliated Hospital, Sun Yat-sen University, Guangzhou, China
- 2Division of Pulmonary and Critical Care, Department of Internal Medicine, The Third Affiliated Hospital, Sun Yat-sen University, Guangzhou, China
- 3Department of Head and Neck, Sun Yat-sen University Cancer Center, Guangzhou, China
- 4State Key Laboratory of Oncology in South China, Guangzhou, China
- 5Collaborative Innovation Center for Cancer Medicine, Guangzhou, China
- 6Department of Urology, The Third Affiliated Hospital, Sun Yat-sen University, Guangzhou, China
- 7Department of Cell Biology & Institute of Biomedicine, National Engineering Research Center of Genetic Medicine, Guangdong Provincial Key Laboratory of Bioengineering Medicine, College of Life Science and Technology, Jinan University, Guangzhou, China
MicroRNAs (miRs) play important roles in tumor progression. miR-936 has been reported to suppress cell invasion and proliferation of glioma and non-small cell lung cancer. Nevertheless, the function of miR-936 in laryngeal squamous cell carcinoma (LSCC) remains undiscovered. Hence, our study was to investigate the role of miR-936 in LSCC. In our present research, we have testified that miR-936 was substantially downregulated in LSCC tissues compared with adjacent normal tissues. Furthermore, miR-936 could inhibit proliferation, migration and invasion, and improve the sensitivity to doxorubicin and cisplatin of LSCC cells. Additionally, luciferase reporter assays were performed to confirm that GPR78 was a novel target of miR-936, and the protein expression of GPR78 was obviously inhibited by miR-936 in LSCC cells. In summary, our study indicates that the miR-936/GPR78 axis could be both a diagnostic marker and a therapeutic target for LSCC.
Introduction
Laryngeal cancer ranks at the fourteenth most universal type of cancer in the world (1). It is estimated there over 13,000 new laryngeal cancer cases and 3,000 death cases took place in the United States, while 26,000 new cases and 14,000 deaths in China (1–3). Laryngeal squamous cell carcinoma (LSCC) occupies 85–90% of total malignant tumors in the larynx (4, 5). Despite advances in diagnosis and treatment, the long-term prognosis of LSCC patients has not been satisfactory in the past 20 years (6–8). Accordingly, a profound apprehension of the molecular biological mechanisms involving in LSCC tumorigenesis and development is imminently needed.
MicroRNAs (miRs) are a classical non-coding small RNA playing crucial regulatory roles in diverse pathological and physiological progresses by a posttranscriptional mechanism through binding the 3′-untranslated regions (3′-UTR) of target genes (9–12). And miRs can act as tumor oncogenes or suppressors through regulating their target genes that are usually dysregulated in cancer (13–15). In our current study, we have proven that miR-936 expression profile is meaningfully reduced in LSCC specimens, and overexpression of miR-936 suppresses LSCC cells proliferation migration and invasion. Moreover, our results have further exhibited that GPR78 is a direct target of miR-936.
Materials and Methods
Patients and Specimens
Twenty-five cases of LSCC and matching normal tissues were acquired from patients at the Department of Otolaryngology-Head and Neck Surgery, the Third Affiliated Hospital, Sun Yat-sen University and the Department of Head and Neck, Cancer Center, Sun Yat-sen University between December 2013 and February 2017. All patients were diagnosed as LSCC for the first time who underwent total or partial laryngectomy without chemical therapy or neoadjuvant radical before and after surgery. Signed informed approvals were acquired from patients, and the study was approved by the ethics committee of the Third Affiliated Hospital, Sun Yat-sen University.
Cell Culture and Reagents
The human LSCC cell line Hep-2, the normal bronchial epithelium cell line 16HBE and the HEK293T were ordered from China Center for Type Culture Collection (CCTCC). The human LSCC cell line KB-3-1 was kindly provided by Dr. Zhesheng Chen (St. John's University, USA). The cells were cultured at 37°C with 5% CO2 in a humidified atmosphere in Dulbecco's modified Eagle's medium (DMEM) containing 100 Unit/ml penicillin, 100 ng/ml streptomycin and 10% fetal bovine serum (FBS). Cell lines applied in this project were authenticated by short tandem repeat fingerprinting <3 months when this study was started. Anti-GPR78 (AB61731a) was from Sangon Biotech. The antibody of anti-GAPDH (KM9002) was purchased from Tianjin Sungene Biotech.
Plasmid
The synthesized precursor hsa-miR-936 was cloned into lentiviral vector pLKO.1-GFP to generate the hsa-miR-936 lentivirus construct. The GPR78 3′UTR fragment was cloned into a psiCHECK-2 dual luciferase reporter construct. Lentivirus was packaged with HEK293T cells and harvested from the supernatant of medium. Stable cell line was obtained through infecting lentivirus in Hep-2 or KB-3-1 cells and selecting with puromycin.
RNA Extraction and Real-Time Quantitative PCR (RT-qPCR)
Total RNAs were extracted from cells and tissues by applying HiPure Total RNA Mini Kit (Magen). Reverse transcription was performed with HiFi-script cDNA kit (Cwbio) according to the instruction. The BestarTM Real time PCR Master Mix was applied for RT-qPCR by SYER Green Method. All reactions were carried out in triplicate and repeated at least three independent times. The results of RT-qPCR were normalized to U6 by applying the 2−ΔΔCt method. The following primers were ordered from Sangon Biotech: miR-936 forward: 5′-AACGAGACGACGACAGAC-3′; miR-936 reverse: 5′-ACAGTAGAGGGAGGAATCGCAG-3′; U6 forward: 5′- GCGCGTCGTGAAGCGTTC-3′; U6 reverse: 5′- GTGCAGGGTCCGAGGT-3′ (16).
Western Blot Analysis
Cells were washed with PBS, resuspended and lysed in RIPA buffer containing protease inhibitors (0.03% aprotinin, 10 ng/ml PMSF, 1 μM sodium orthovanadate, 1% NP-40, 0.1% SDS and 0.5% sodium deoxycholate,) at 4°C for 30 min. After centrifuging at 14,000 × g for 10 min, lysate supernatants were collected and stored at −80°C. Proteins were isolated by 12% SDS-PAGE gels and transferred to the membrane of polyvinylidene difluoride. After that, membrane was blocked by 5% BSA for 1 h, then incubated with the primary antibody and secondary antibody, successively. According to instruction, signal was measured through the chemiluminescent gel imaging system of ChemiDoc XRS (Bio-RAD) (17, 18).
MTT Assay
Cells plated in 96-well plate were incubated for 0, 1, 2, 3 days, then add MTT 0.5 mg/ml. After 4 h incubation at 37°C, carefully absorb the culture medium in the well to prevent the cells from taking away formazan crystals, and dissolved crystals with 100 μl of DMSO. Multiscan Spectrum (Thermofisher) was used to measure the absorbance at 570 nm (19, 20).
Wound Healing Assay
Cells were cultured in 6-well plate. Until the cells reached 80–90% confluence, using a sterile 10 μl pipette tip to draw a straight mark on the cell monolayer. After drawing, the delineated cells were washed and incubated in serum-free medium. The gap of wounds were measured by microscopic photograph at a certain time (21).
Transwell Assay
Cells were plated in the upper compartment containing matrigel-coated polycarbonate membrane filter of a modified Boyden chamber (Corning), and the lower chamber was plated complete medium, and allowed to migrate for 24 h. Wiped the cells on the upper surface of membrane and fixed the cells on the lower surface of membrane by 4% paraformaldehyde and stained by 0.1% crystal violet staining solution (22).
Luciferase Reporter Assay
The GPR78 mutated and wild-type (WT) 3′-UTR fragments were cloned into psiCHECK-2 reporter. HEK293T cells were plated in 24-well plates and co-transfected with mutated or WT 3′-UTR luciferase reporter and pLKO.1-GFP-miR-936. After 24 h, according to a protocol, cell lysates were obtain, and Firefly/Renilla luciferases ratios were measured with the Dual Luciferase Reporter Assay Kit (Promega) (23).
Statistical Analysis
All statistics were analyzed by SPSS 20.0, and the results were shown as mean ± SD or median with the interquartile range. The Student's t-test and Mann–Whitney U-test were applied to analyze comparisons of two groups, and one-way ANOVA and Kruskal-Wallis test were applied to analyze comparisons of multiple groups. The P < 0.05 were considered statistically significant.
Results
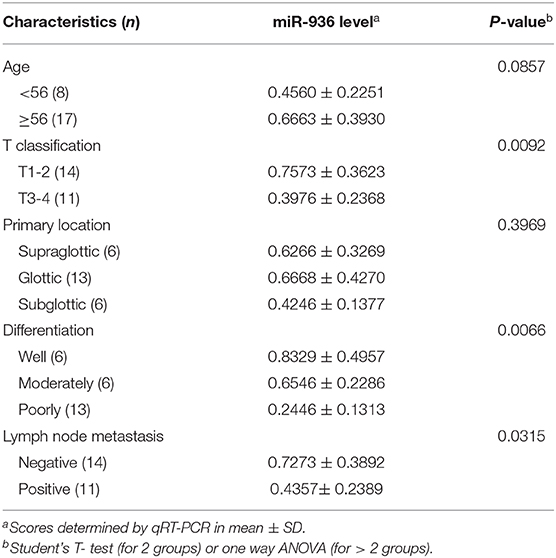
Downregulation of miR-936 in LSCC Is Correlated With Differentiation, Lymph Node Metastasis and T Stages
To explore miR-936 expression in the LSCC tissues, RT-qPCR was used to check with 25 pairs of laryngeal cancer and normal tissue. Results suggested that miR-936 expression was meaningfully downregulated in LSCC, with 72% (18/25) of the tumor tissues showing reduced expression compared to matched normal controls (Figure 1A). Further, we found that miR-936 expression was correlated with tumor grade, lymph node metastasis and T Stages, but not correlated with tumor primary locations and age. The expression of miR-936 in negative lymph node metastasis, well-differentiation and T1-2 groups were higher than that in positive lymph node metastasis, poor differentiation, and T3-4 groups respectively (Table 1 and Figures 1B–F). According to these data, the progression of LSCC may be associated with miR-936 expression.
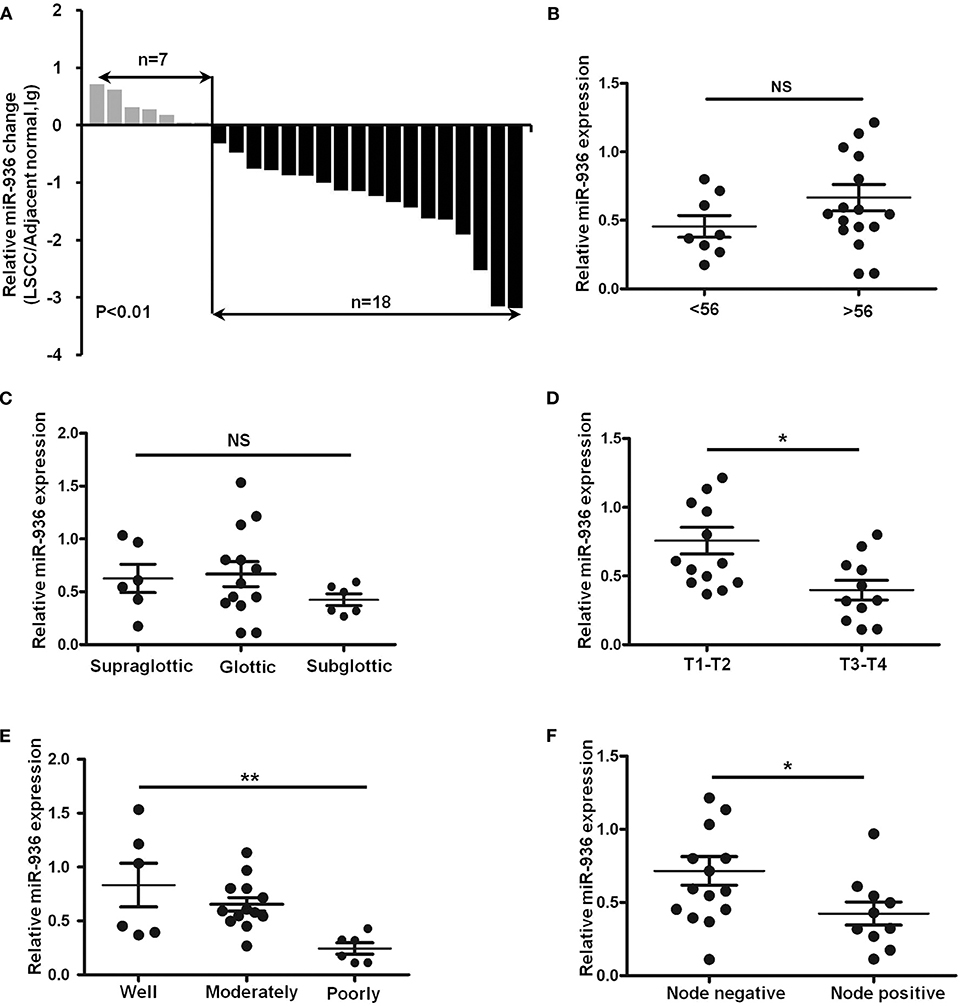
Figure 1. Downregulation of miR-936 in LSCC is correlated with T stages, differentiation and lymph node metastasis. (A) Expression of miR-936 in 25 pairs of LSCC tissues and adjacent normal tissues was detected using RT-qPCR. The relative miR-936 expression in two groups of LSCC tissues classified by age (B), T stage (D), and lymph node metastasis (F) were analyzed with Mann-Whitney U-test. The relative miR-936 expression in three groups of LSCC tissues classified by differentiation (C) and primary location (E) were analyzed with Kruskal-Wallis test. Data are presented as mean ± SD or median with the interquartile range. *p < 0.05; **p < 0.01; NS, no statistical significance.
Overexpression of miR-936 Suppresses the Proliferation of LSCC Cells
To investigate the function of miR-936 in LSCC, Hep-2 and KB-3-1 cells were infected with lentivirus expressing precursor miR-936, which successfully upregulated miR-936 in the cells (Figure 2A). The growth curves determined by MTT assay indicates that the proliferation abilities of Hep-2 and KB-3-1 cells were significantly attenuated when miR-936 was overexpressed (Figure 2B).
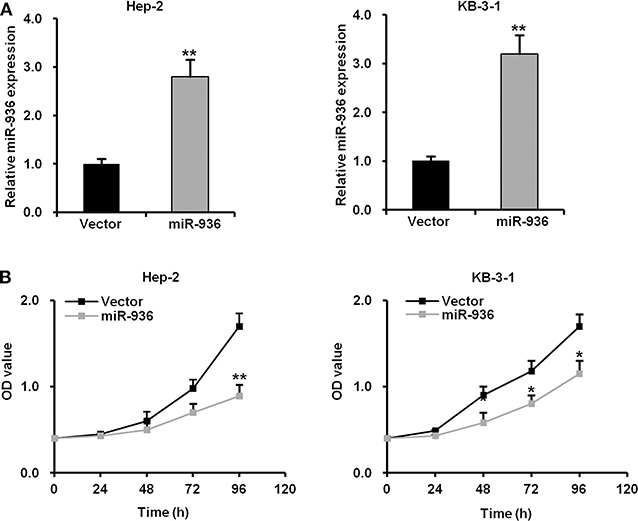
Figure 2. Overexpression of miR-936 suppresses the proliferation of LSCC cells. (A) RT-qPCR analysis of the relative miR-936 expression in Hep-2 and KB-3-1 cells expressed vector control and miR-936. (B) Cell proliferation of the two LSCC cell lines was measured using the MTT assay. OD values were measured every 24 h for 96 h with or without miR-936 transfection. Data are presented as mean ± SD. Student's t-test was used for statistical analysis. *p < 0.05; **p < 0.01.
Overexpression of miR-936 Suppresses the Migration and Invasion of LSCC Cells
To further verify whether miR-936 has an influence on the migration and invasion of LSCC cells, we performed wound healing and transwell assays in Hep-2 and KB-3-1 cells with miR-936 overexpression. The outcomes revealed that the migration and invasion of miR-936 overexpressing cells were importantly decreased when compared with control cells (Figures 3A–C).
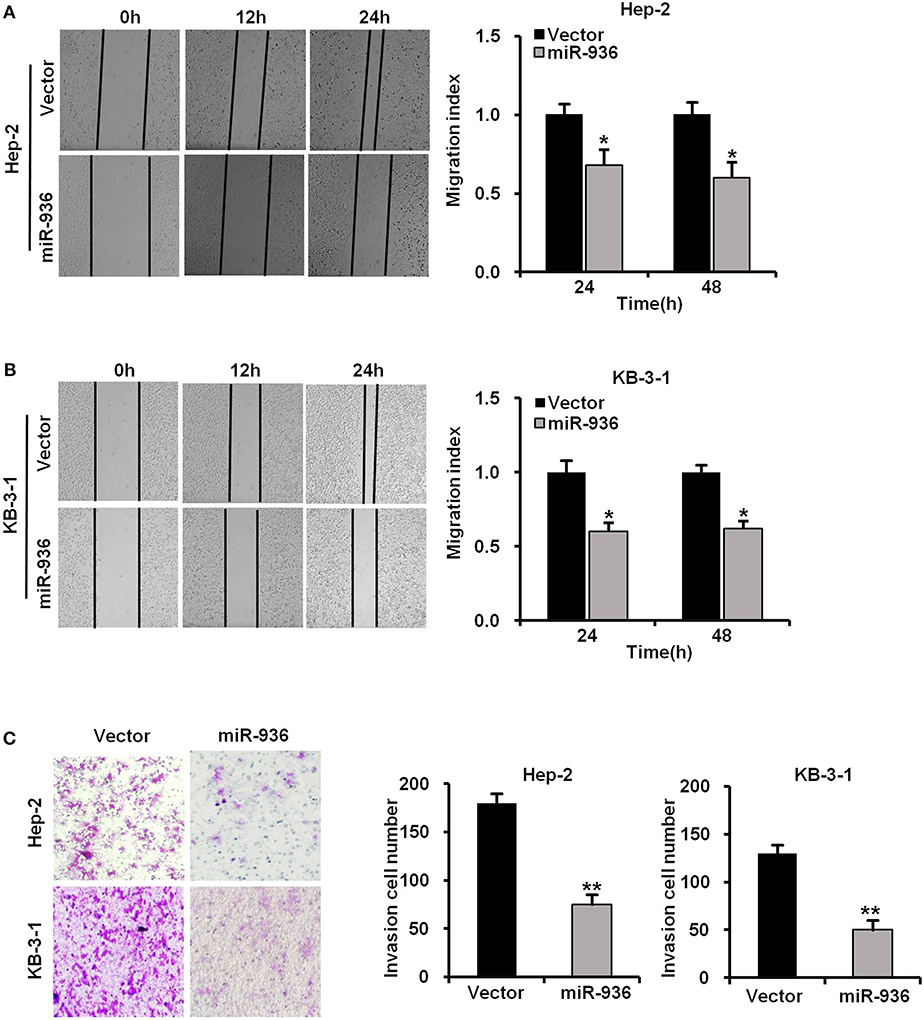
Figure 3. Overexpression of miR-936 suppresses the migration and invasion of LSCC cells. (A,B) Representative images and quantification of the indicated cells migration as determined with wound healing assay. (C) Representative images and quantification of the indicated cells invasion as determined with Transwell assay. Data are presented as mean ± SD. Student's t-test was used for statistical analysis. *p < 0.05; **p < 0.01.
Overexpression of miR-936 Improves the Drug Sensitivity of LSCC Cells to Doxorubicin and Cisplatin
To verify the effect of miR-936 on LSCC cells treated with chemotherapy drugs, we treated indicated cells with doxorubicin or cisplatin in different concentrations. As shown in Figures 4A–D, the drug resistance to doxorubicin or cisplatin was significantly lower in cells overexpressing miR-936 in comparison with control groups in Hep-2 and KB-3-1 cells. These data suggested that increasing miR-936 expression could improve the drug sensitivity of LSCC cells to chemotherapeutic drugs.
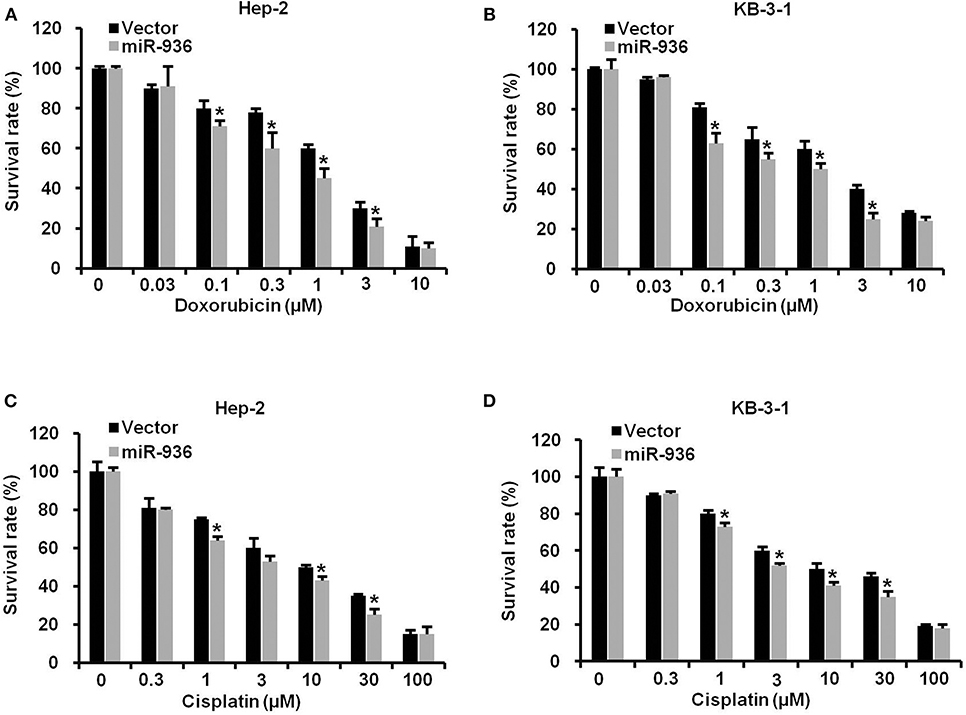
Figure 4. Overexpression of miR-936 improves the drug sensitivity of LSCC cells to doxorubicin and cisplatin. (A–D) Cell survival of the indicated cells treated with doxorubicin and cisplatin as determined with MTT assay. Data are presented as mean ± SD. Student's t-test was used for statistical analysis. *P < 0.05.
miR-936 Directly Targets GPR78
To understand the mechanism of miR-936 as a tumor suppressor in LSCC, we combined RNAhybird and PITA to search the new potential targets of miR-936. Both algorithms reveal that GPR78 was a downstream gene of miR-936. We then performed western blot analysis and found that overexpressing miR-936 in Hep-2 and KB-3-1 cells could decrease GPR78 protein levels notably (Figure 5A). The interaction between miR-936 and the 3′-UTR of GPR78 was illustrated in Figure 5B. And luciferase reporter assays were used in HEK293T cells to test whether miR-936 could directly interact with the 3′-UTR of GPR78. The ratio of fluorescence activity indicates the inhibitory effect of miR-936 on GPR78 in wild or mutant 3′-UTR. As exhibited in Figure 5C, overexpression of miR-936 markedly suppressed the luciferase activity of GPR78 WT 3′-UTR compared to mutant 3′-UTR. The result above indicated that miR-936 directly suppresses GPR78 expression through binding its 3′-UTR (2214nt~2222nt).
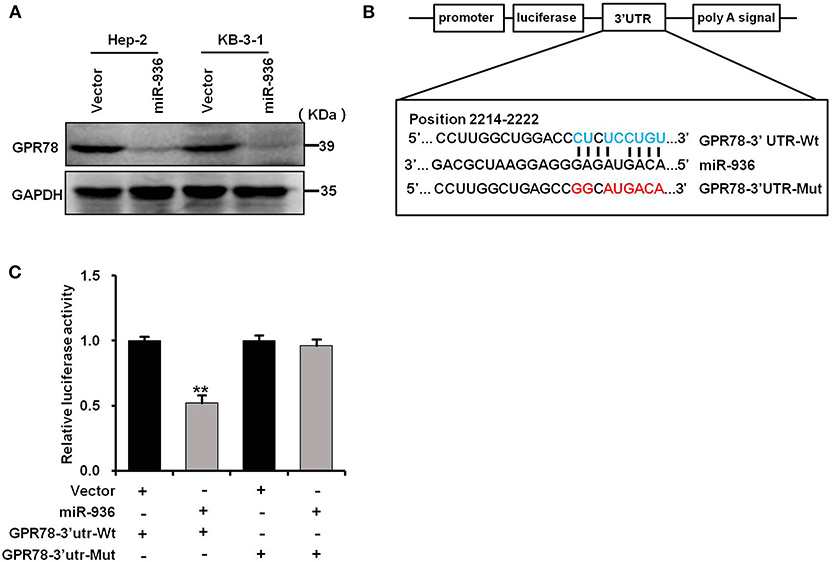
Figure 5. miR-936 directly targets GPR78. (A) Western blot analysis of GPR78 protein expressions in the indicated cells. GAPDH is the loading control. (B) A schematic diagram of the reporter constructs showed the wild type (Wt) and mutant (Mut) sequences of the miR-936 binding sites within human GPR78 3′-UTR. (C) Luciferase activity of reporters with GPR78 Wt or Mut 3′-UTR in the HEK293T cells. Data are presented as mean ± SD. Student's t-test was used for statistical analysis. **p < 0.01.
Discussion
The development of tumors is a synergistic process of tumor-associated activation and inhibition genes. A growing number of studies have shown that abnormal expression of miRs happened in most types of human malignancies, including LSCC (24). In our present study, miR-936 was downregulated in LSCC tissues in comparison with matching normal tissues, and correlated with poor clinical features, suggesting that miR-936 might be associated with tumor progression in LSCC. Biological function experiments indicated that overexpression of miR-936 meaningfully inhibited the proliferation, migration, and invasion of LSCC cells. Furthermore, overexpression of miR-936 improved the drug sensitivity of LSCC cells to doxorubicin and cisplatin which currently are used for LSCC chemotherapy in clinic, indicating the significance of the findings that miR-936 sensitized the anticancer effects of doxorubicin and cisplatin in LSCC. Moreover, computerized algorithm predicted that GPR78 was a direct downstream target of miR-936, and that overexpression of miR-936 can effectively reduce GPR78 protein expression in LSCC cells. Previous researches have manifested that down-regulated miRs are often described as biomarkers or therapeutic targets in laryngeal squamous cell carcinoma. For instance, we recently reported t miR-194 works as a tumor suppressor in LSCC through targeting Wee1 (23). The expression of miR-375 is also reduced in LSCC tissues, and overexpression of miR-375 could reduce LSCC cell proliferation, motility and invasion by targeting IGF1R (25). In addition, miR-34a/c is also downregulated in LSCC tissues and inhibits LSCC cells proliferation by inducing cell cycle arrest through directly suppressing GALNT7 (26). While miR-27a is upregulated in LSCC specimens, and overexpression of miR-27a enhances LSCC cells proliferation by targeting PLK2 (27). MiR-93 is also upregulated in LSCC tissues and promotes the proliferation, migration, and invasion by inhibiting cyclin G2 (28).
Previous studies have been reported that miR-936 were down-regulated in glioma and induced cell cycle arrest via targeting CKS1 (29). Another report has also demonstrated that miR-936 could directly target E2F2 to inhibit the invasion and proliferation of non-small cell lung cancer cell (30). However, the biological functions of miR-936 have not been described in other tumors, including LSCC. Our study confirmed that miR-936 expression profile was downregulated in LSCC tissues, which indicates that miR-936 may be related to the progression of LSCC. Searching miRNA target genes is fundamental to understanding its carcinogenic regulatory mechanism and the effective molecular therapeutic targets. By bioinformatics algorithm and experiment validation, we testified that GPR78 was a direct target of miR-936. GPR78, an orphan G-protein coupled receptor, is situated in an area of chromosome 4p where have been shown connection to schizophrenia and bipolar affective dysfunction (31). Recently, GPR78 was testified highly expressed in lung cancer cell, and knockdown of GPR78 prominently suppressed cell migration and metastasis (32). However, the expression and function of GPR78 in LSCC need to be further investigated in the future.
In conclusion, our results proved that miR-936 attenuated the proliferation, migration and invasion of LSCC cells and targeted GPR78. Moreover, high level of miR-936 could improve the sensitivity of LSCC cells to doxorubicin and cisplatin. The results manifests that the miR-936/GPR78 axis could play as a novel biomarker and a therapeutic target of LSCC.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the ethics committee of The Third Affiliated Hospital, Sun Yat-sen University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
X-JL, HL, PL, H-FW, ZS, and JY designed the projects, conducted the experiments, analyzed the results, and wrote the manuscript. A-KY, J-MD, Q-WJ, YY, J-RH, M-LY, Z-HX, M-NW, and YL conducted the experiments. All authors read and agreed the final manuscript.
Funding
This work was funded by grants from the National Natural Science Foundation of China Nos. 81772540 (ZS), 81772752 (J-MD), 81472760 (HL), and 81902771 (PL), the Science and Technology Program of Guangdong Nos. 2019A050510023 (ZS), 2017A020215122 (J-MD), 2014A030313057 (JY), and 2014A020212078 (HL), the Medical Scientific Research Foundation of Guangdong Province No. 2018118215914106 (PL), the Science and Technology Program of Guangzhou No 201709010038 (J-MD).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. (2019) 69:7–34. doi: 10.3322/caac.21551
2. Feng RM, Zong YN, Cao SM, Xu RH. Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer Commun. (2019) 39:22. doi: 10.1186/s40880-019-0368-6
3. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. (2016) 66:115–32. doi: 10.3322/caac.21338
4. Thompson LD. Laryngeal dysplasia, squamous cell carcinoma, and variants. Surg Pathol Clin. (2017) 10:15–33. doi: 10.1016/j.path.2016.10.003
5. Baugnon KL, Beitler JJ. Pitfalls in the staging of cancer of the laryngeal squamous cell carcinoma. Neuroimaging Clin N Am. (2013) 23:81–105. doi: 10.1016/j.nic.2012.08.008
6. Kolator M, Kolator P, Zatonski T. Assessment of quality of life in patients with laryngeal cancer: a review of articles. Adv Clin Exp Med. (2018) 27:711–5. doi: 10.17219/acem/69693
7. Garcia-Leon FJ, Garcia-Estepa R, Romero-Tabares A, Gomez-Millan Borrachina J. Treatment of advanced laryngeal cancer and quality of life. Systematic review. Acta Otorrinolaringol Esp. (2017) 68:212–9. doi: 10.1016/j.otoeng.2017.06.010
8. Obid R, Redlich M, Tomeh C. The treatment of laryngeal cancer. Oral Maxillofac Surg Clin North Am. (2019) 31:1–11. doi: 10.1016/j.coms.2018.09.001
9. Quevillon Huberdeau M, Simard MJ. A guide to microRNA-mediated gene silencing. FEBS J. (2019) 286:642–52. doi: 10.1111/febs.14666
10. Treiber T, Treiber N, Meister G. Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat Rev Mol Cell Biol. (2019) 20:5–20. doi: 10.1038/s41580-018-0059-1
11. Gebert LFR, MacRae IJ. Regulation of microRNA function in animals. Nat Rev Mol Cell Biol. (2019) 20:21–37. doi: 10.1038/s41580-018-0045-7
12. Duchaine TF, Fabian MR. Mechanistic Insights into MicroRNA-Mediated Gene Silencing. Cold Spring Harb Perspect Biol. (2019) 11:a032771. doi: 10.1101/cshperspect.a032771
13. Setijono SR, Kwon HY, Song SJ. MicroRNA, an antisense RNA, in sensing myeloid malignancies. Front Oncol. (2017) 7:331. doi: 10.3389/fonc.2017.00331
14. Kuninty PR, Schnittert J, Storm G, Prakash J. MicroRNA targeting to modulate tumor microenvironment. Front Oncol. (2016) 6:3. doi: 10.3389/fonc.2016.00003
15. Huang JT, Wang J, Srivastava V, Sen S, Liu SM. MicroRNA machinery genes as novel biomarkers for cancer. Front Oncol. (2014) 4:113. doi: 10.3389/fonc.2014.00113
16. Ye J, Zou MM, Li P, Lin XJ, Jiang QW, Yang Y, et al. Oxymatrine and cisplatin synergistically enhance anti-tumor immunity of CD8(+) T cells in Non-small cell lung cancer. Front Oncol. (2018) 8:631. doi: 10.3389/fonc.2018.00631
17. Huang JR, Qin WM, Wang K, Fu DR, Zhang WJ, Jiang QW, et al. Cyclin-dependent kinase 7 inhibitor THZ2 inhibits the growth of human gastric cancer in vitro and in vivo. Am J Transl Res. (2018) 10:3664–76.
18. Mei XL, Yang Y, Zhang YJ, Li Y, Zhao JM, Qiu JG, et al. Sildenafil inhibits the growth of human colorectal cancer in vitro and in vivo. Am J Cancer Res. (2015) 5:3311–24.
19. Yuan ML, Li P, Xing ZH, Di JM, Liu H, Yang AK, et al. Inhibition of WEE1 suppresses the tumor growth in laryngeal squamous cell carcinoma. Front Pharmacol. (2018) 9:1041. doi: 10.3389/fphar.2018.01041
20. Yang Y, Qiu JG, Li Y, Di JM, Zhang WJ, Jiang QW, et al. Targeting ABCB1-mediated tumor multidrug resistance by CRISPR/Cas9-based genome editing. Am J Transl Res. (2016) 8:3986–94.
21. Luo Y, Wu JY, Hou GL, Lu MH, Shi Z, Di JM. ODAM is a predictor for biomedical recurrence and inhibits the migration and invasion of prostate cancer. Am J Transl Res. (2016) 8:670–9.
22. Luo Y, Jiang QW, Wu JY, Qiu JG, Zhang WJ, Mei XL, et al. Regulation of migration and invasion by Toll-like receptor-9 signaling network in prostate cancer. Oncotarget. (2015) 6:22564–74. doi: 10.18632/oncotarget.4197
23. Li P, Yang Y, Liu H, Yang AK, Di JM, Tan GM, et al. MiR-194 functions as a tumor suppressor in laryngeal squamous cell carcinoma by targeting Wee1. J Hematol Oncol. (2017) 10:32. doi: 10.1186/s13045-017-0402-6
24. Li P, Liu H, Wang Z, He F, Wang H, Shi Z, et al. MicroRNAs in laryngeal cancer: implications for diagnosis, prognosis and therapy. Am J Transl Res. (2016) 8:1935–44.
25. Luo J, Wu J, Li Z, Qin H, Wang B, Wong TS, et al. miR-375 suppresses IGF1R expression and contributes to inhibition of cell progression in laryngeal squamous cell carcinoma. Biomed Res Int. (2014) 2014:374598. doi: 10.1155/2014/374598
26. Li W, Ma H, Sun J. MicroRNA34a/c function as tumor suppressors in Hep2 laryngeal carcinoma cells and may reduce GALNT7 expression. Mol Med Rep. (2014) 9:1293–8. doi: 10.3892/mmr.2014.1929
27. Tian Y, Fu S, Qiu GB, Xu ZM, Liu N, Zhang XW, et al. MicroRNA-27a promotes proliferation and suppresses apoptosis by targeting PLK2 in laryngeal carcinoma. BMC Cancer. (2014) 14:678. doi: 10.1186/1471-2407-14-678
28. Xiao X, Zhou L, Cao P, Gong H, Zhang Y. MicroRNA-93 regulates cyclin G2 expression and plays an oncogenic role in laryngeal squamous cell carcinoma. Int J Oncol. (2015) 46:161–74. doi: 10.3892/ijo.2014.2704
29. Wang D, Zhi T, Xu X, Bao Z, Fan L, Li Z, et al. MicroRNA-936 induces cell cycle arrest and inhibits glioma cell proliferation by targeting CKS1. Am J Cancer Res. (2017) 7:2131–43.
30. Zhou X, Tao H. Overexpression of microRNA-936 suppresses non-small cell lung cancer cell proliferation and invasion via targeting E2F2. Exp Ther Med. (2018) 16:2696–702. doi: 10.3892/etm.2018.6490
31. Underwood SL, Christoforou A, Thomson PA, Wray NR, Tenesa A, Whittaker J, et al. Association analysis of the chromosome 4p-located G protein-coupled receptor 78 (GPR78) gene in bipolar affective disorder and schizophrenia. Mol Psychiatry. (2006) 11:384–94. doi: 10.1038/sj.mp.4001786
Keywords: laryngeal squamous cell carcinoma, miR-936, GPR78, proliferation, invasion, drug resistance
Citation: Lin X-J, Liu H, Li P, Wang H-F, Yang A-K, Di J-M, Jiang Q-W, Yang Y, Huang J-R, Yuan M-L, Xing Z-H, Wei M-N, Li Y, Shi Z and Ye J (2020) miR-936 Suppresses Cell Proliferation, Invasion, and Drug Resistance of Laryngeal Squamous Cell Carcinoma and Targets GPR78. Front. Oncol. 10:60. doi: 10.3389/fonc.2020.00060
Received: 27 June 2019; Accepted: 14 January 2020;
Published: 04 February 2020.
Edited by:
Chang Zou, Jinan University, ChinaCopyright © 2020 Lin, Liu, Li, Wang, Yang, Di, Jiang, Yang, Huang, Yuan, Xing, Wei, Li, Shi and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhi Shi, dHNoaXpoaUBqbnUuZWR1LmNu; Jin Ye, eWVqaW5AbWFpbC5zeXN1LmV1ZC5jbg==
†These authors have contributed equally to this work
 Xi-Jun Lin
Xi-Jun Lin Hui Liu2†
Hui Liu2† Qi-Wei Jiang
Qi-Wei Jiang Jia-Rong Huang
Jia-Rong Huang Meng-Ling Yuan
Meng-Ling Yuan Meng-Ning Wei
Meng-Ning Wei Zhi Shi
Zhi Shi