- 1Department of Neonatology, Xinhua Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China
- 2Department of Neonatology, Children Hospital of Fudan University, Shanghai, China
- 3Department of Pediatric Infectious Diseases, Xinhua Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China
- 4Clinical Research Unit, Xinhua Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China
Objective: Neonatal bacterial meningitis is a severe infectious disease with a high risk of neurodevelopmental sequelae. The causative pathogens may be related to specific clinical features of the disease. Therefore, this study aimed at determining the pathogen-specific and clinical features of bacterial meningitis in full-term neonates.
Methods: We enrolled neonates from the Shanghai Neonate Meningitis Cohort (2005–2017), which is a multicenter retrospective cohort that recruits almost all full-term neonates in Shanghai who underwent lumbar puncture. Patient history and clinical examination results were extracted from the computer-documented information systems of four hospitals. The trends of pathogen distribution were analyzed and differences in the clinical manifestations, treatment, and clinical outcomes at discharge were compared according to the causative pathogen. Logistic regression was used to evaluate the pathogen-specific risk of neurological complications.
Results: In total, 518 cases of neonatal meningitis, including 189 proven cases, were included. Group B Streptococcus (GBS) and Escherichia coli (E. coli) were the leading pathogens in proven cases of early-onset and late-onset neonatal meningitis, respectively. The proportion of early-onset and late-onset GBS and late-onset E. coli meningitis cases increased gradually. GBS meningitis had the highest risk of neurological complications, whereas the overall incidence of hydrocephalus and brain abscess in E. coli was higher than that in GBS.
Conclusions: Rates of neonatal GBS and E. coli meningitis were high in 2005–2017 in Shanghai, and the risk of neurological complications was also high. Therefore, active prevention, rational use of antibiotics, and continuous monitoring of GBS and E. coli in neonates should be initiated in Shanghai.
Introduction
Despite advances in infant intensive care, neonatal meningitis remains a devastating disease. In some developed countries, Group B Streptococcus (GBS) and Escherichia coli (E. coli) were reported as the main pathogens of bacterial meningitis in young infants (1–4). However, in the developing world, the microbiology of bacterial meningitis in neonates varies geographically. A World Health Organization-supported study in four African countries showed that Klebsiella pneumoniae and E. coli were the most-prevalent causative pathogens in cases of neonatal meningitis (5). Reports from the central and western provinces of China showed that E. coli was a commonly isolated bacterium in cases of neonatal meningitis; other pathogens included Staphylococcus epidermidis, K. pneumonia, and GBS (6, 7). However, no large-scale study has examined the etiology of neonatal bacterial meningitis in the eastern regions of China.
The morbidity rate of neonatal meningitis, especially the incidence of neurological sequelae, is currently high worldwide (8, 9), and the clinical severity and prognosis of neonatal bacterial meningitis may be associated with the type of invading pathogen. Most studies have focused on the clinical features of a single pathogen. For instance, both hyper-, and hypothermia were reported as being the most frequent clinical signs of E. coli meningitis at admission (10). Further, GBS meningitis, an isolated illness caused by a gram-positive pathogen, was found to be characterized by the presence of specific signs of meningitis, such as lethargy and seizure (2, 11). Symptomatically, gram-negative pathogens are more likely to cause seizures as the initial manifestation of meningitis and are associated with higher cerebrospinal fluid (CSF) white blood cell counts than gram-positive pathogens (12). However, studies on the systematic identification of the clinical features of different pathogens are lacking. Selection bias in such comparative studies can only be reduced by simultaneously comparing the clinical differences of different pathogens in the same study.
Understanding the differences in the clinical manifestations of these pathogens is important for early identification of pathogens and the rational use of antibiotics. In addition, the lack of studies examining the etiology of neonatal bacterial meningitis in the eastern regions of China impedes the development of strategies for the prevention of neonatal bacterial meningitis in these regions. Therefore, this large-scale study aimed to identify the trends in bacterial infection among cases of meningitis in full-term neonates in Shanghai and explore the pathogen-specific and clinical features of bacterial meningitis among full-term neonates.
Materials and Methods
The Shanghai Neonate Meningitis Cohort is a multicenter retrospective cohort, including almost all full-term neonates who underwent lumbar puncture in Shanghai (2005–2017). It was conducted at neonatal wards of four tertiary class A pediatric hospitals in Shanghai that examined approximately 95% of the neonates with bacterial meningitis in Shanghai (Xinhua Hospital, affiliated to Shanghai Jiaotong University School of Medicine; Shanghai Children's Medical Center, affiliated to Shanghai Jiaotong University School of Medicine; Children's Hospital of Shanghai Jiaotong University, and Children Hospital of Fudan University).
The history and clinical examination results for all neonates were abstracted from the computer-documented hospital information systems. Approval for the study and sharing data with the coordinating institution was granted by Xinhua Hospital affiliated to Shanghai Jiaotong University School of Medicine and was consented by the other hospitals (No. XHEC-C-2017-084).
In this study, the inclusion criteria for neonatal bacterial meningitis included one or more of the following: (a) isolation of a bacterial pathogen from CSF culture; (b) isolation of the same bacterial pathogen from blood drawn simultaneously at two different sites, with CSF pleocytosis (≥20 cells/mm3) (13–15); and (c) no pathogen isolated from either CSF or blood, with clinical symptoms and CSF pleocytosis (≥20 cells/mm3). Proven meningitis was defined in patients with either inclusion criterion (a) or (b). Clinically diagnosed meningitis was defined in patients with inclusion criterion (c). Exclusion criteria were as follows: (a) gestational age < 37 weeks, (b) onset of bacterial meningitis after >28 days of life, (c) history of severe neurological disease or ventricular drain, or (d) receipt of traumatic lumbar puncture (≥10,000 × 106/L red blood cells in CSF) (16) with negative CSF culture result.
Coagulase-negative staphylococci (CoNS), considered as being potential contaminants were further evaluated as follows: Three neonatologists gathered clinical data from the hospital's computerized information system, and pathogens were assessed to determine their clinical significance.
The following data were retrieved from the computer-documented hospital information systems: bacterial species, antimicrobial susceptibility testing, demographic information, clinical signs and symptoms, first laboratory examination of CSF, cranial magnetic resonance imaging (MRI) results during treatment, and clinical outcomes at discharge. Three independent neonatologists performed the data input and checked to avoid data mis-recording as much as possible.
The threshold for early-onset and late-onset diseases was seven days after birth (17, 18). Neonates presenting with symptoms of fever, lethargy, poor feeding, vomiting, cyanosis, and apnea were considered to have nonspecific symptoms. Neonates presenting with symptoms of seizures, dystonia, irritability, abnormal primitive reflexes, bulging fontanelle, and screaming were considered to have neurological symptoms. Neonates presenting with complications including omphalitis, pneumonia, skin infection, diarrhea, and urinary infection were considered to have non-neurological complications. Neurological complications were limited to abnormal cranial MRI results including ventriculitis, intracranial hemorrhage, subdural effusion, hydrocephalus, and brain abscess, which were confirmed by MRI experts. The clinical prognosis at discharge was evaluated using the Glasgow Outcome Scale (GOS) (1, death; 2, persistent vegetative state; 3, severe disability; 4, moderate disability; 5, good recovery). Moderate or severe disability was defined as any of the following conditions: spasticity, muscle weakness, and immobility in one or more limbs; microcephaly; hydrocephalus; seizure disorder; hearing loss. A GOS score of ≤ 4 was defined as poor prognosis (19). During the antibiotic treatment, some families decided to withdraw treatment. These neonates had been using antibiotics during hospitalization and did not complete antibiotic treatment before the families withdrew treatment. Once the families withdrew treatment, these neonates were discharged immediately. There were two reasons why the families withdrew treatment: (a) they can no longer afford hospitalization expenses; (b) the neonates had complications during treatment, or parents felt that the prognosis will be poor, and they lost confidence in the recovery of the neonates.
We calculated the median and interquartile range for continuous variables (as most data were not normally distributed), and frequency for categorical variables. Continuous variables were compared using nonparametric methods (Mann-Whitney U-test). Categorical variables were compared using the chi-square test or Fisher's exact test, where appropriate. Considering the multiple comparisons, we used the Bonferroni method to adjust for the type I error rate. Multivariate logistic regression models were used to evaluate risk factors for poor prognosis and neurological complications. All statistical analyses were performed using SPSS 17.0 (SPSS Inc, Chicago, IL, USA). The figures were produced using GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA, USA).
Results
Pathogen Distribution
We identified 518 cases of neonatal meningitis, including 189 proven cases and 329 clinically diagnosed cases. In the proven cases, gram-positive pathogens accounted for 116 cases (61.4%), and gram-negative pathogens accounted for the remaining 73 cases (38.6%). GBS (n = 55, 29.1%) and E. coli (n = 55, 29.1%) were the leading pathogens in the proven cases of neonatal meningitis. Other pathogens involved in < 5 cases included CoNS (n = 42, 22.2%), Enterobacteriaceae (except E. coli) (n = 11, 5.8%), and Enterococcus (n = 10, 5.3%) (Table 1).
Trends in Pathogen Distribution
Early-onset bacterial meningitis accounted for 31.2% of the proven cases among full-term neonates in Shanghai during 2005–2017. There was an upward trend in the proportions of both early-onset and late-onset GBS meningitis (Figure 1). Over the study duration, GBS was the leading pathogen in early-onset meningitis (21/49, 42.9%). The proportion of late-onset E. coli meningitis cases increased gradually, and E. coli was the most-common pathogen in cases of late-onset meningitis (45/140, 32.1%). The proportion of all other pathogens decreased gradually in cases of both early-onset and late-onset meningitis.
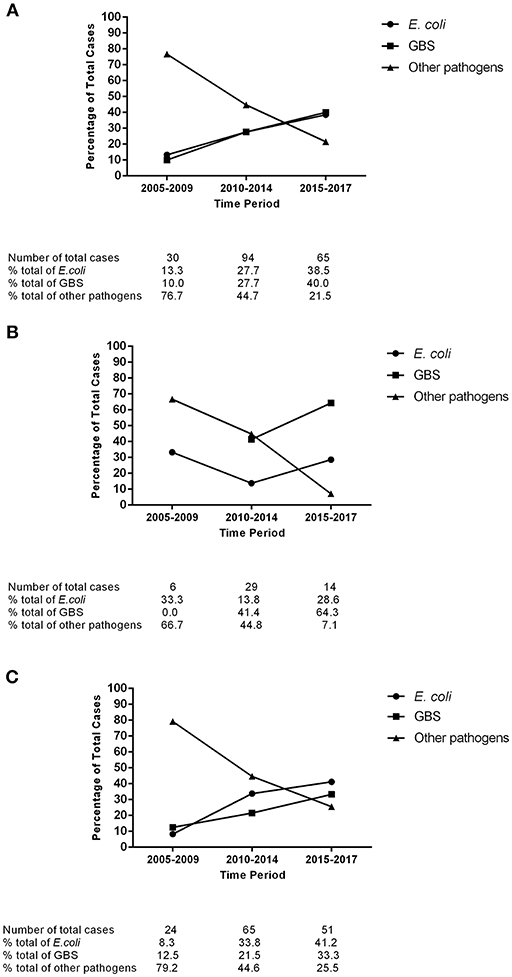
Figure 1. Trends in the pathogen distribution of full-term neonatal meningitis in Shanghai, 2005–2017. (A) All meningitis cases; (B) Early-onset meningitis cases; (C) Late-onset meningitis cases. E. coli, Escherichia coli; GBS, Group B Streptococcus.
Antibiotic Susceptibility
All GBS strains were sensitive to gentamicin, ampicillin, vancomycin, and linezolid while only 16.2% of GBS cases were sensitive to clindamycin (Table 2). Only one strain was resistant to penicillin. E. coli showed a high sensitivity to cefepime (88.6%) and meropenem (100.0%), a varying resistance to ampicillin, gentamicin, cefotaxime, and ceftriaxone (71.4, 36.8, 50.0, and 43.3%, respectively), and a high proportion of extended spectrum beta-lactamase (ESBL)-producing isolates (41.2%).
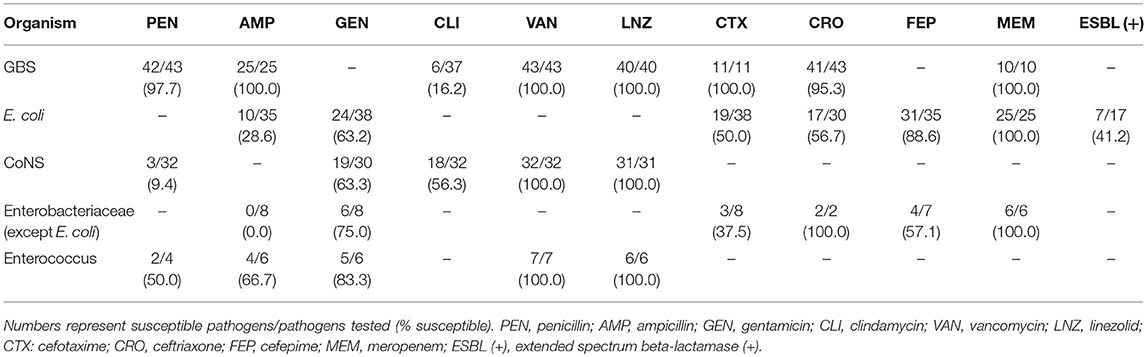
Table 2. In vitro antimicrobial susceptibility testing of common pathogens from full-term neonatal cases with proven meningitis.
Demographic Information and Clinical Symptoms
Of the 518 neonates, 307 (59.3%) were boys, 15 (2.9%) had low birth weights (< 2,500 g), and 344 (66.4%) were born by cesarean section. Fever was the predominant symptom in all cases, regardless of the etiology, and 197 cases (38.0%) showed neurological symptoms. Seizures (n = 83, 16.0%) were the leading presentation of neurological symptoms. Poor feeding, seizures, and irritability occurred more frequently in GBS cases than in the clinically diagnosed cases (P < 0.05). Incidence rates of the other symptoms did not differ among the clinically diagnosed, GBS and E. coli cases (Table 3).
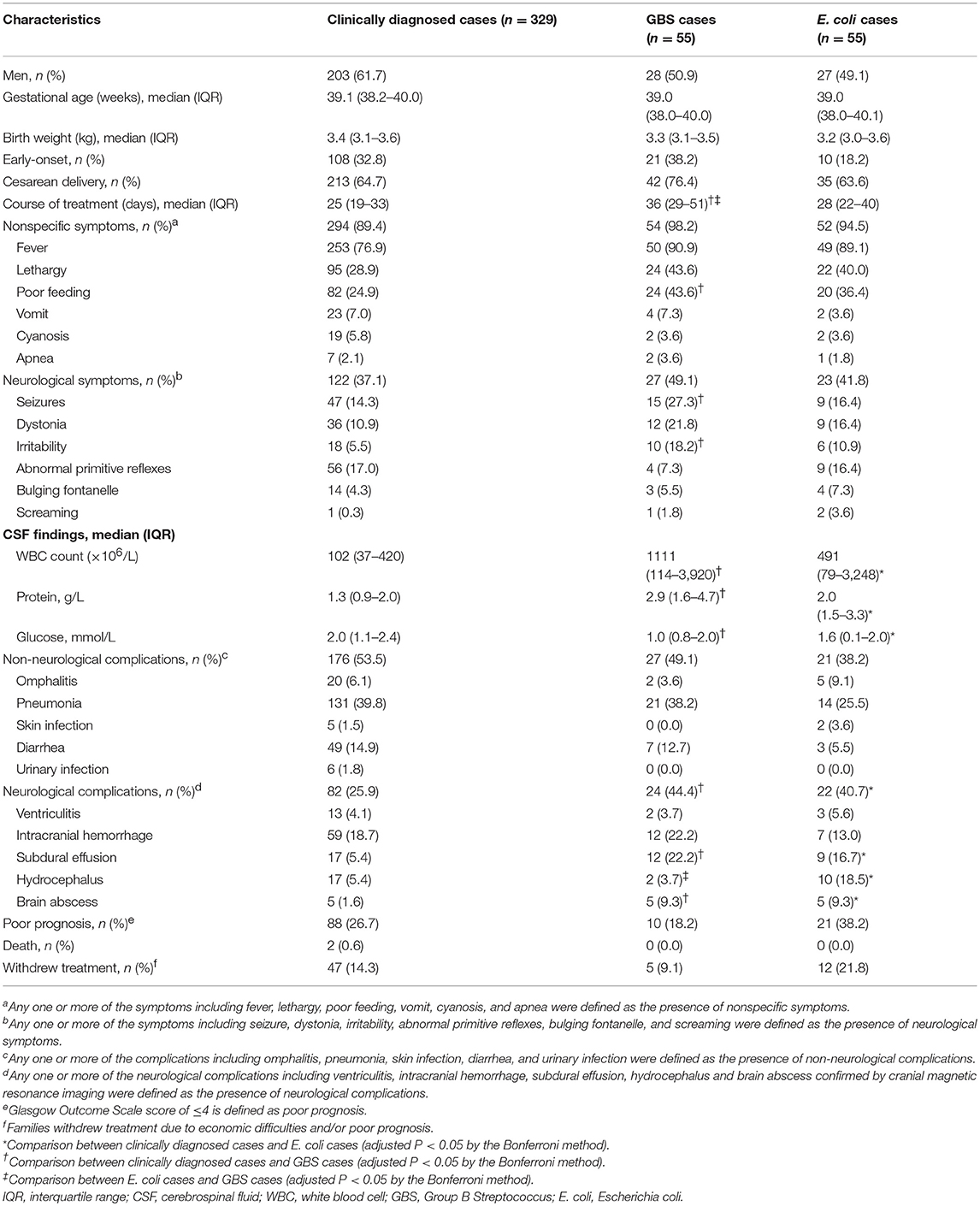
Table 3. Characteristics of full-term neonatal meningitis cases caused by the three most commonly cultured pathogens.
CSF Findings
Lumbar puncture was performed in all 518 cases. The GBS and E. coli cases showed higher CSF white blood cell counts, protein levels, and lower glucose levels than the clinically diagnosed cases (P < 0.05). There were no significant differences in CFS findings between the GBS and E. coli cases.
Clinical Outcomes at Discharge
A total of 3 neonates died in hospital, and 140 neonates (27.0%) had poor prognosis (GOS score ≤ 4) at discharge. Seventy-six neonates (14.7%) were discharged after families chose to withdraw treatment, in which 25 neonates received >2 weeks of treatment. The rates of in-hospital deaths and treatment withdrawal did not differ significantly between the clinically diagnosed, GBS, and E. coli meningitis cases. There was also no difference between the clinically diagnosed group and each proven group in terms of the incidence of poor prognosis. However, the rate of poor prognosis was significantly higher in E. coli cases than in GBS cases (P < 0.05), and this difference was consistent when we further adjusted for gender, birth weight, and early or late-onset pattern in the multivariate analyses (Supplementary Table 1).
Most neonates (501/518, 96.7%) underwent cranial MRI during treatment, and the results of 146 neonates (29.1%) were abnormal, including ventriculitis (21/501, 4.2%), intracranial hemorrhage (88/501, 17.6%), subdural effusion (41/501, 8.2%), hydrocephalus (33/501, 6.6%), and brain abscess (17/501, 3.4%). Neonates with GBS meningitis had a much higher risk of total neurological complications, especially subdural effusion and brain abscess, than clinically diagnosed cases (total neurological complications: odds ratio [OR], 2.3; 95% confidence interval [CI], 1.3–4.1; subdural effusion: OR, 5.0; 95% CI, 2.2–11.3; and brain abscess: OR, 6.3; 95% CI, 1.8–22.8). Neonates with E. coli meningitis had a relatively higher risk of total neurological complications, subdural effusion, hydrocephalus, and brain abscess than the clinically diagnosed cases (total neurological complications: OR, 2.0; 95% CI, 1.1–3.6; subdural effusion: OR, 3.5; 95% CI, 1.5–8.4; hydrocephalus: OR, 4.0; 95% CI, 1.7–9.3; and brain abscess: OR, 6.3; 95% CI, 1.8–22.8). Compared to neonates with GBS meningitis, those with E. coli meningitis showed a higher overall incidence of hydrocephalus and brain abscess.
Multivariate analysis of the neurological complications showed that neonates with GBS and E. coli meningitis had a significantly higher risk of neurological complications than those with clinically diagnosed meningitis (GBS: OR, 2.3; 95% CI, 1.3–4.3; E. coli: OR, 2.0; 95% CI, 1.1–3.6). There was no significant difference in the risk of neurological complications between the clinically diagnosed cases and the cases of the other pathogens (Table 4).
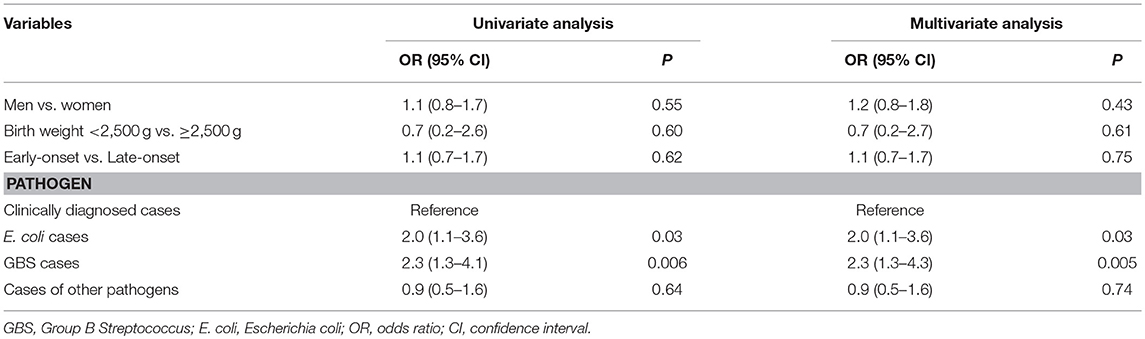
Table 4. Univariate and multivariate analyses of risk factors for neurological complications in full-term neonatal meningitis cases.
Discussion
To the best of our knowledge, this is the first long-term study of full-term neonatal meningitis with a large sample size in Shanghai, China. We found that GBS and E. coli were the leading pathogens in proven cases of neonatal meningitis, which was similar with that of the developed countries. GBS and E. coli were the most-common pathogens in early-onset and late-onset neonatal meningitis, respectively, both of which showed an increasing trend and had a high risk of neurological complications.
In some developed countries and regions, GBS is the most-prevalent pathogen causing meningitis in young infants, and is confined to this population (1, 2, 4). In the USA, approximately 16.7–22.7% of pregnant women showed GBS colonization (20). GBS prevention activities increased since the 1990s, and the first recommendation for universal screening was issued in 2002. This resulted in an estimated 80% decrease in early-onset GBS infection in the USA (21). In China, GBS infection occurs in 7.1–10.4% of pregnant women, a lower prevalence than that of the USA (22, 23). However, in our study, GBS was the leading pathogen in early-onset meningitis, and the prevalence of both early-onset and late-onset GBS meningitis has shown an increasing trend in recent years. Regarding antimicrobial susceptibility, neonates with GBS meningitis showed high sensitivity to penicillin and ampicillin, which remain the agents of choice for intrapartum antibiotic prophylaxis (IAP) (24, 25). These results may be partially related to the non-wide implementation of IAP in China.
In this study, neonates with GBS meningitis had a significantly higher incidence of poor feeding, seizures, and irritability than neonates with clinically diagnosed meningitis. In addition, the incidence of nonspecific and neurological symptoms of GBS and E. coli meningitis was both slightly higher than those of clinically diagnosed meningitis (although not significantly). As for the CSF findings, GBS and E. coli meningitis were significantly more serious than clinically diagnosed meningitis. Based on these, if a newborn presents with several clinical symptoms combined with severe CSF findings, we should be wary of GBS or E. coli meningitis.
The fact that neonatal GBS meningitis had the highest risk of neurological complications may be related to the virulence of GBS and the severe damage to the brain caused by its infection. In vitro and clinical studies have found that GBS can promote blood-brain barrier penetration (26–28) and may cause severe cerebrovascular diseases (29). Abnormal findings on full-term neonatal MRI were associated with a poor neurodevelopmental outcome (30). A meta-analysis on neurodevelopmental impairment after GBS disease in infants aged < 90 days showed that GBS meningitis was an important risk factor for moderate-to-severe neurodevelopmental impairment, affecting approximately 20% of survivors (31). Therefore, it is important to conduct brain imaging on neonates with GBS meningitis and to follow-up for long-term neurological sequelae. In addition, the implementation of IAP may be associated with a low mortality in cases of early-onset GBS disease, and mild late-onset GBS disease (32, 33). Currently, in China, only a few hospitals perform IAP by strictly following a risk-based strategy. Hence, IAP should be promoted and continuous monitoring of GBS should be initiated in China.
Similar to GBS, we also found that E. coli meningitis had a significantly higher risk of neurological complications than those of clinically diagnosed meningitis, especially subdural effusion, hydrocephalus, and brain abscess. As reported in other studies, neonatal E. coli meningitis may cause learning and memory impairments in adulthood (34), which may be related to the high incidence of E. coli neurological complications. Although E. coli meningitis had a similar incidence of neurological complications to GBS, the overall incidence of hydrocephalus and brain abscess was higher than the latter. As serious neurological complications, hydrocephalus and brain abscess were associated with significant long-term morbidity and mortality (35, 36). We further speculate that the high proportion of ESBL-producing isolates in E. coli cases increases the risk of serious neurological complications. The high proportion of ESBL-producing isolates in E. coli cases is similar to that reported in a large multicenter study in China, which may be related to the universal antibiotic overuse or misuse in China (37). At present, there is no national guideline for the treatment of neonatal meningitis in China, so the choice and course of antimicrobial treatment may vary in different hospitals. Therefore, a consensus guideline for treatment of neonatal meningitis should be established and better monitoring of drug-resistant bacteria should be encouraged.
To our knowledge, this is the first large-scale study of neonatal meningitis in Shanghai investigating pathogen trends in the past 13 years, and its results have furthered the understanding of the pathogen-specific and clinical features of bacterial meningitis in full-term neonates. However, there are some limitations. First, the meningitis cases in our study were diagnosed based on CSF pleocytosis, but the interpretation of CSF pleocytosis parameters are not yet standardized (38). This, together with the exclusion of infants who received traumatic lumbar punctures, may have led to underdiagnoses of meningitis in some cases and overdiagnoses in others, limiting this study's ability to estimate of overall burden of neonatal meningitis in Shanghai. Second, viral studies were not routinely considered without significant clinical signs and symptoms in neonates. However, CSF leukocytosis may also occur in neonatal viral meningitis. For example, a proportion of neonatal enteroviral meningitis can cause CSF leukocytosis (39, 40), thus the incidence of neonatal viral meningitis has likely been underestimated. Further, intracranial hemorrhage might also lead to CSF leukocytosis and be confused with clinically diagnosed cases. Third, although CoNS is sometimes a contaminant, it has increasingly been recognized as a cause of clinically significant nosocomial bloodstream infections, particularly in neonates (41). As a result, we did not rule out CoNS cases, which might introduce misdiagnosis; we only included cases with typical meningitis symptoms and signs, in addition to those with positive CSF culture or positive blood culture from blood drawn simultaneously at two different sites. Fourth, some families withdrew treatment, which increased the lost to follow-up rate. This might have introduced bias to the results, such as the incidence of neurological complications. To follow-up on this hospital-based retrospective survey, we plan to address these limitations by conducting a more detailed prospective study on neonatal meningitis in the future.
Rates of neonatal GBS and E. coli meningitis were high in 2005–2017 in Shanghai, and the risk of neurological complications was also high. Therefore, active prevention, rational use of antibiotics and continuous monitoring of GBS and E. coli in neonates should be initiated in Shanghai.
Data Availability Statement
The datasets for this manuscript are not publicly available because: part of the data is included in other manuscripts under preparation. Requests to access the datasets should be directed to Lisu Huang:aHVhbmdsaXN1QHhpbmh1YW1lZC5jb20uY24=.
Author Contributions
MX, LaH, YZ, CC, XZ, and LiH conceptualized and designed the study. HH, LW, and JT collected the data. MX, YZ, XZ, and LiH analyzed the data. MX drafted the initial manuscript. All authors contributed to the interpretation of the data and contributed to the final draft of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2019.00031/full#supplementary-material
Abbreviations
CoNS, Coagulase-negative staphylococci; E. coli, Escherichia coli; ESBL, extended spectrum beta-lactamase; GBS, Group B Streptococcus; GOS, Glasgow Outcome Scale; IAP, intrapartum antibiotic prophylaxis; CSF, cerebrospinal fluid.
References
1. Okike IO, Johnson AP, Henderson KL, Blackburn RM, Muller-Pebody B, Ladhani SN, et al. Incidence, etiology, and outcome of bacterial meningitis in infants aged < 90 days in the United kingdom and Republic of Ireland: prospective, enhanced, national population-based surveillance. Clin Infect Dis. (2014) 59:e150–7. doi: 10.1093/cid/ciu514
2. Gaschignard J, Levy C, Romain O, Cohen R, Bingen E, Aujard Y, et al. Neonatal bacterial meningitis: 444 cases in 7 years. Pediatr Infect Dis J (2011) 30:212–7. doi: 10.1097/INF.0b013e3181fab1e7
3. Ouchenir L, Renaud C, Khan S, Bitnun A, Boisvert AA, McDonald J, et al. The epidemiology, management, and outcomes of bacterial meningitis in infants. Pediatrics (2017) 140:e20170476. doi: 10.1542/peds.2017-0476
4. Thigpen MC, Whitney CG, Messonnier NE, Zell ER, Lynfield R, Hadler JL, et al. Bacterial meningitis in the United States, 1998-2007. N Engl J Med. (2011) 364:2016–25. doi: 10.1056/NEJMoa1005384
5. Furyk JS, Swann O, Molyneux E. Systematic review: neonatal meningitis in the developing world. Trop Med Int Health (2011) 16:672–9. doi: 10.1111/j.1365-3156.2011.02750.x
6. The Collaborative Group For Neonatal Meningitis Study TC, Liu CQ. Epidemiology of neonatal purulent meningitis in Hebei Province, China: a multicenter study. Zhongguo Dang Dai Er Ke Za Zhi (2015) 17:419–24. doi: 10.7499/j.issn.1008-8830.2015.05.001
7. Jiang H, Su M, Kui L, Huang H, Qiu L, Li L, et al. Prevalence and antibiotic resistance profiles of cerebrospinal fluid pathogens in children with acute bacterial meningitis in Yunnan province, China, 2012-2015. PloS ONE (2017) 12:e0180161. doi: 10.1371/journal.pone.0180161
8. Harvey D, Holt DE, Bedford H. Bacterial meningitis in the newborn: a prospective study of mortality and morbidity. Semin Perinatol. (1999) 23:218–25. doi: 10.1016/S0146-0005(99)80066-4
9. Stevens JP, Eames M, Kent A, Halket S, Holt D, Harvey D. Long term outcome of neonatal meningitis. Arch Dis Child Fetal Neonatal Ed. (2003) 88:F179–84. doi: 10.1136/fn.88.3.F179
10. Houdouin V, Bonacorsi S, Bidet P, de La Rocque F, Cohen R, Aujard Y, et al. Clinical outcome and bacterial characteristics of 99 Escherichia coli meningitis in young infants. Arch Pediatr. (2008) 15(Suppl. 3):S138–47. doi: 10.1016/S0929-693X(08)75497-6
11. Baud O, Aujard Y. Neonatal bacterial meningitis. Handb Clin Neurol. (2013) 112:1109–13. doi: 10.1016/B978-0-444-52910-7.00030-1
12. Smith PB, Cotten CM, Garges HP, Tiffany KF, Lenfestey RW, Moody MA, et al. A comparison of neonatal Gram-negative rod and Gram-positive cocci meningitis. J Perinatol. (2006) 26:111–4. doi: 10.1038/sj.jp.7211438
13. Garges HP, Moody MA, Cotten CM, Smith PB, Tiffany KF, Lenfestey R, et al. Neonatal meningitis: what is the correlation among cerebrospinal fluid cultures, blood cultures, and cerebrospinal fluid parameters? Pediatrics (2006) 117:1094–100. doi: 10.1542/peds.2005-1132
14. Byington CL, Kendrick J, Sheng X. Normative cerebrospinal fluid profiles in febrile infants. J Pediatr. (2011) 158:130–4. doi: 10.1016/j.jpeds.2010.07.022
15. Kestenbaum LA, Ebberson J, Zorc JJ, Hodinka RL, Shah SS. Defining cerebrospinal fluid white blood cell count reference values in neonates and young infants. Pediatrics (2010) 125:257–64. doi: 10.1542/peds.2009-1181
16. Lyons TW, Cruz AT, Freedman SB, Neuman MI, Balamuth F, Mistry RD, et al. Interpretation of cerebrospinal fluid white blood cell counts in young infants with a traumatic lumbar puncture. Ann Emerg Med. (2017) 69:622–31. doi: 10.1016/j.annemergmed.2016.10.008
17. May M, Daley AJ, Donath S, Isaacs D. Australasian study group for neonatal infections. Early onset neonatal meningitis in Australia and New Zealand, 1992-2002. Arch Dis Child Fetal Neonatal Ed. (2005) 90:F324–7. doi: 10.1136/adc.2004.066134
18. Heath PT, Nik Yusoff NK, Baker CJ. Neonatal meningitis. Arch Dis Child Fetal Neonatal Ed. (2003) 88:F173–8. doi: 10.1136/fn.88.3.F173
19. Tan J, Kan J, Qiu G, Zhao D, Ren F, Luo Z, et al. Clinical prognosis in neonatal bacterial meningitis: the role of cerebrospinal fluid protein. PLoS ONE (2015) 10:e0141620. doi: 10.1371/journal.pone.0141620
20. Kwatra G, Cunnington MC, Merrall E, Kwatra G, Cunnington MC, Merrall E, et al. Prevalence of maternal colonisation with group B streptococcus: a systematic review and meta-analysis. Lancet Infect Dis. (2016) 16:1076–84. doi: 10.1016/S1473-3099(16)30055-X
21. Phares CR, Lynfield R, Farley MM, Mohle-Boetani J, Harrison LH, Petit S, et al. Epidemiology of invasive group B streptococcal disease in the United States, 1999-2005. JAMA (2008) 299:2056–65. doi: 10.1001/jama.299.17.2056
22. Lu B, Li D, Cui Y, Sui W, Huang L, Lu X. Epidemiology of Group B streptococcus isolated from pregnant women in Beijing, China. Clin Microbiol Infect. (2014) 20:O370–3. doi: 10.1111/1469-0691.12416
23. Tsui MH, Ip M, Ng PC, Sahota DS, Leung TN, Lau TK. Change in prevalence of group B Streptococcus maternal colonisation in Hong Kong. Hong Kong Med J. (2009) 15:414–9.
24. Verani JR, McGee L, Schrag SJ. Prevention of perinatal group B streptococcal disease–revised guidelines from CDC, 2010. MMWR Recomm Rep. (2010) 59:1–36.
25. Tunkel AR, Hartman BJ, Kaplan SL, Kaufman BA, Roos KL, Scheld WM, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. (2004) 39:1267–84. doi: 10.1086/425368
26. Magalhaes V, Andrade EB, Alves J, Ribeiro A, Kim KS, Lima M, et al. Group B Streptococcus hijacks the host plasminogen system to promote brain endothelial cell invasion. PLoS ONE (2013) 8:e63244. doi: 10.1371/journal.pone.0063244
27. van Sorge NM, Quach D, Gurney MA, Sullam PM, Nizet V, Doran KS. The group B streptococcal serine-rich repeat 1 glycoprotein mediates penetration of the blood-brain barrier. J Infect Dis. (2009) 199:1479–87. doi: 10.1086/598217
28. Cutting AS, Del Rosario Y, Mu R, Rodriguez A, Till A, Subramani S, et al. The role of autophagy during group B Streptococcus infection of blood-brain barrier endothelium. J Biol Chem. (2014) 289:35711–23. doi: 10.1074/jbc.M114.588657
29. Tibussek D, Sinclair A, Yau I, Teatero S, Fittipaldi N, Richardson SE, et al. Late-onset group B streptococcal meningitis has cerebrovascular complications. J Pediatr. (2015) 166:1187–92.e1. doi: 10.1016/j.jpeds.2015.02.014
30. Massaro AN. MRI for neurodevelopmental prognostication in the high-risk term infant. Semin Perinatol. (2015) 39:159–67. doi: 10.1053/j.semperi.2015.01.009
31. Kohli-Lynch M, Russell NJ, Seale AC, Dangor Z, Tann CJ, Baker CJ, et al. Neurodevelopmental impairment in children after group B streptococcal disease worldwide: systematic review and meta-analyses. Clin Infect Dis. (2017) 65:S190–9. doi: 10.1093/cid/cix663
32. Edmond KM, Kortsalioudaki C, Scott S, Schrag SJ, Zaidi AK, Cousens S, et al. Group B streptococcal disease in infants aged younger than 3 months: systematic review and meta-analysis. Lancet (2012) 379:547–56. doi: 10.1016/S0140-6736(11)61651-6
33. Berardi A, Rossi C, Lugli L, Creti R, Bacchi Reggiani ML, Lanari M, et al. Group B streptococcus late-onset disease: 2003-2010. Pediatrics (2013) 131:e361–8. doi: 10.1542/peds.2012-1231
34. Barichello T, Dagostim VS, Generoso JS, Simoes LR, Dominguini D, Silvestre C, et al. Neonatal Escherichia coli K1 meningitis causes learning and memory impairments in adulthood. J Neuroimmunol. (2014) 272:35–41. doi: 10.1016/j.jneuroim.2014.05.003
35. Tully HM, Dobyns WB. Infantile hydrocephalus: a review of epidemiology, classification and causes. Eur J Med Genet. (2014) 57:359–68. doi: 10.1016/j.ejmg.2014.06.002
36. Felsenstein S, Williams B, Shingadia D, Coxon L, Riordan A, Demetriades AK, et al. Clinical and microbiologic features guiding treatment recommendations for brain abscesses in children. Pediatr Infect Dis J. (2013) 32:129–35. doi: 10.1097/INF.0b013e3182748d6e
37. Quan J, Zhao D, Liu L, Chen Y, Zhou J, Jiang Y, et al. High prevalence of ESBL-producing Escherichia coli and Klebsiella pneumoniae in community-onset bloodstream infections in China. J Antimicrob Chemother. (2017) 72:273–80. doi: 10.1093/jac/dkw372
38. Srinivasan L, Harris MC, Shah SS. Lumbar puncture in the neonate: challenges in decision making and interpretation. Semin Perinatol. (2012) 36:445–53. doi: 10.1053/j.semperi.2012.06.007
39. Seiden JA, Zorc JJ, Hodinka RL, Shah SS. Lack of cerebrospinal fluid pleocytosis in young infants with enterovirus infections of the central nervous system. Pediatr Emerg Care (2010) 26:77–81. doi: 10.1097/PEC.0b013e3181ce2fad
40. Tan NW, Lee EY, Khoo GM, Tee NW, Krishnamoorthy S, Choong CT. Cerebrospinal fluid white cell count: discriminatory or otherwise for enteroviral meningitis in infants and young children? J Neurovirol. (2016) 22:213–7. doi: 10.1007/s13365-015-0387-2
Keywords: pathogens, clinical features, neonatal bacterial meningitis, Group B Streptococcus, Escherichia coli, neurological complications
Citation: Xu M, Hu L, Huang H, Wang L, Tan J, Zhang Y, Chen C, Zhang X and Huang L (2019) Etiology and Clinical Features of Full-Term Neonatal Bacterial Meningitis: A Multicenter Retrospective Cohort Study. Front. Pediatr. 7:31. doi: 10.3389/fped.2019.00031
Received: 22 September 2018; Accepted: 24 January 2019;
Published: 13 February 2019.
Edited by:
Eric Giannoni, Lausanne University Hospital (CHUV), SwitzerlandReviewed by:
Kirsty Le Doare, Imperial College London, United KingdomPhilipp Agyeman, University of Bern, Switzerland
Copyright © 2019 Xu, Hu, Huang, Wang, Tan, Zhang, Chen, Zhang and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chao Chen, Y2hlbjYwMTBAMTYzLmNvbQ==
Xi Zhang, emhhbmd4aUB4aW5odWFtZWQuY29tLmNu
Lisu Huang, aHVhbmdsaXN1QHhpbmh1YW1lZC5jb20uY24=
†These authors have contributed equally to this work and are first authors
 Min Xu
Min Xu Lan Hu
Lan Hu Heyu Huang
Heyu Huang Liping Wang
Liping Wang Jintong Tan1
Jintong Tan1 Yongjun Zhang
Yongjun Zhang Chao Chen
Chao Chen Xi Zhang
Xi Zhang Lisu Huang
Lisu Huang